Abstract
The aim of the study was to explore a propriety standardized ethanolic extract from leaves of Orthosiphon stamineus Benth in improving impairments in short-term social memory in vivo, possibly via blockade of adenosine A2A receptors (A2AR). The ethanolic extract of O. stamineus leaves showed significant in vitro binding activity of A2AR with 74% inhibition at 150 μg/ml and significant A2AR antagonist activity with 98% inhibition at 300 μg/mL. A significant adenosine A1 receptor (A1R) antagonist activity with 100% inhibition was observed at 300 μg/mL. Its effect on learning and memory was assessed via social recognition task using Sprague Dawley rats whereby the ethanolic extract of O. stamineus showed significant (p < 0.001) change in recognition index (RI) at 300 mg/kg and 600 mg/kg p.o and 120 mg/kg i.p., respectively, compared to the vehicle control. In comparison, the ethanolic extract of Polygonum minus aerial parts showed small change in inflexion; however, it remained insignificant in RI at 200 mg/kg p.o. Our findings suggest that the ethanolic extract of O. stamineus leaves improves memory by reversing age-related deficits in short-term social memory and the possible involvement of adenosine A1 and adenosine A2A as a target bioactivity site in the restoration of memory.
1. Background
Orthosiphon stamineus Benth (Lamiaceae) is a herbaceous perennial plant, widely distributed throughout the tropical regions, especially in Southeast Asia. It is commonly known as cat's whiskers. It is also known as misai kuching in Malaysia and kumis kuching in Indonesia [1]. It is referred to as java tea and consumed as an herbal tea in Europe for urinary flushing (European Herbal Pharmacopoeia). The leaves of O. stamineus are traditionally used in South East Asia for a variety of ailments such as bladder and kidney disease (due to its strong diuretic effect), detoxification, relieving joint stiffness and inflammation including arthritis and rheumatism, gout, treating catarrh of the bladder, eliminating stones from the bladder, and treating diabetes mellitus [2, 3]. Scientific studies have further reported the herb to possess anti-inflammatory [4], antioxidant [5, 6], antibacterial [7], hepatoprotective [8], diuretic [9], antihypertensive [10], and hypoglycemic effects [11].
Several classes of bioactive compounds such as flavonoids, diterpenes, triterpenes, saponins, sterols organic acids, caffeic acids derivatives, chromenes, and oleanic and ursolic acid are known for O. stamineus [12–16]. Recent studies have emerged on the flavonoids of O. stamineus possessing antagonist activity on adenosine A1 receptors (A1R) [17]. While the study focused more on the role of the receptors in diuretic activity, adenosine receptors in the central nervous system have also been implicated in the modulation of cognitive functions [18]. While the A1R antagonist activity has been reported in O. stamineus, A2AR antagonist activity was not.
The adenosine receptors have been associated with sleep and arousal, cognition, and memory and with protecting from neuronal damage and degeneration as well as influencing neuronal maturation [19]. Endogenous adenosine is generally known to modulate cognition through the activation of adenosine A1 receptors. Evidence is now emerging on a possible role of A2A receptors in learning and memory [20]. The adenosine receptors A1 and A2A belong to the G-protein-coupled receptor family [18] and antagonist actions on these receptors produced CNS-enhancing effects. Selective blockade of A1 and A2A receptors were shown to facilitate learning and memory in vivo [21, 22]. They might also protect against memory dysfunction shown in experimental models of aging such as Alzheimer's disease.
The social recognition test (SRT) has been used in studies with caffeine, an adenosine A2A receptor antagonist, in reversing cognitive decline in age-related deficits in olfactory discrimination, Parkinson's disease, and attention deficit hyperactivity disorder (ADHD) [21, 23]. Using the social recognition test, an adenosine A2A receptor antagonist demonstrated the ability to reverse short-term memory loss in Spontaneously Hypertensive Rats (SHR) which have impairments across several cognitive domains such as attention, short-term memory, and spatial reference memory [20].
The social recognition test was first introduced by Thor and Holloway [24] and is based on the premise that rodents spend more time with unfamiliar juveniles than familiar ones. Memory-enhancing drugs are used in this model to investigate whether the duration of investigation is reduced when the juvenile rat is presented twice. The social recognition test in rats has become increasingly popular for the pharmaceutical industry as a tool to evaluate compounds for procognitive activity. This memory test probes short-term recognition/working memory to investigate novel target mechanisms relevant to cognitive impairment including neuropsychiatric disorders such as dementia, Alzheimer's disease (AD), schizophrenia, and Parkinson's disease (PD). Importantly the test uses spontaneous naturalistic behavior of an adult rat when exposed to a juvenile conspecific on two occasions to access cognition, where the output measured (recognition index (RI)/ratio of investigation duration between the two sessions) involves an assessment of social exploration, strongly influenced by an olfactory component. As a result, SRT animal model was selected in this study.
Antagonists to A2AR are not the only target when seeking cognition enhancing treatment. The inhibitory effects on other target sites such as acetylcholinesterase and serotonin have shown improvement in memory and cognition. One such plant preparation shown to possess antiacetylcholinesterase activity, a neurotransmitter related to learning and memory, is the standardized extract of Ginkgo biloba. Standardised extracts of G. biloba were shown to improve memory and normalized cognitive deficits in animal models [25, 26]. Meanwhile, leaves of another Malaysian herb, Polygonum hydropiper, have been reported to also possess antiacetylcholinesterase activity and recently its related species P. minus demonstrated enhanced memory in rats study using the Barnes maze test and demonstrated anticholinesterase activity [27]. The purpose of this study is to evaluate O. stamineus leaf ethanolic extract for cognition-improving benefits and adenosine A2A receptor as a possible target. The effect is compared with G. biloba and P. minus extract, for memory improvement in an SRT animal model.
2. Materials and Methods
2.1. Extract and Drug
2.1.1. O. stamineus Leaves
O. stamineus leaves, of white flower variety, procured from Biotropics Malaysia Berhad, Malaysia, were harvested at maturity approximately 3 months after planting. The plant material was identified on the basis of exomorphic characters and literature review by a taxonomist from the Institute of Bioscience, Universiti Putra Malaysia (UPM). The voucher specimen of O. stamineus (SK 2083/12) was deposited in the Herbarium, Institute of Bioscience, UPM of Malaysia.
2.1.2. Ethanolic Extract of O. stamineus Leaves
1000 g of O. stamineus leaves was dried by oven at a temperature of 40°C for 48 hours and ground into a fine powder using a lab mill (Retsch ZM200, Haan, Germany) and was extracted twice with 2 L and 1.5 L of 70% ethanol in water (v/v) using ultrasonic treatment for a period of 30 min at room temperature. The solution was separated from the remaining material. The organic solvent was removed under reduced pressure at 40°C and dried.
2.1.3. HPLC Analysis of O. stamineus Ethanolic Extract
The extract was characterized using HPLC techniques based on seven known compounds of O. stamineus used as reference standards [28]. The compounds were 3′-hydroxy-4′,5,6,7-tetramethoxyflavone, sinensetin, orthosiphol B, orthosiphol A, staminol A, orthosiphonone A, and ombuin (3,3′,5-trihydroxy-4′,7-dimethoxyflavone). HPLC analysis of the extract was performed using Agilent 1200 Liquid Chromatography (LC) with a photodiode array detector on Zorbax Eclipse XDB-C18, 4.6 × 150 mm, 5 μm column. The mobile phase consisted of solvent A: water and solvent B: acetonitrile. The following gradient was used: 0–8 min, 70% A; 8–15 min, 70–53% A; 15–30 min, 53–49% A, hold for 10 min; 40–42 min, 49–0% A, hold for 4 min; 46–48 min, 0% A for final washing and equilibrium of the column for the next run. Operating conditions were set at flow rates of 1 mL/min, column temperature at 25°C, UV detection at 230 nm, and injection volume of 5 uL. The extract at the concentration of 50 mg/mL was first injected followed by the mixture of the standards. Identification of the marker compounds was achieved by comparing with retention times of reference standards and their UV spectra.
2.1.4. Aqueous Extract of P. minus
1000 g of aerial parts including stem and leaves of the plant was harvested at maturity approximately 2 months after planting and was dried by oven drying at the temperature of 40°C for 48 hours and shredded to 2 to 5 cm in size. The dried leaves were extracted according to the method described in George et al. [27]. The dried leaves were then subjected to percolation using purified water and extracted at a temperature of about 80°C with an extraction ratio of approximately 1 : 10. The extract was further filtered, concentrated using rotary evaporator with the water bath temperature of 65°C, and freeze-dried. The voucher specimen of the plant (SK 2077/12) was deposited in the Herbarium, Institute of Bioscience, UPM, Malaysia.
2.2. In Vitro Adenosine Receptors A2A and A1 Assays
The adenosine A2A receptor (A2AR) and A1 receptor (A1R) assays were performed to determine test item's A2AR and A1R blockade activity. O. stamineus extract was tested at 15 and 150 μg/mL for A2A binding assay, and the method employed was adapted from the one described by Varani et al. [29]. Adenosine A2A and adenosine A1 functional assays were performed at 3,30 and 300 μg/mL and the method was adapted from Paucher et al. [30] and Taylor et al. [31], respectively. Adenosine A2A binding assay, selective adenosine A2A, and adenosine A1 antagonist assays were conducted by Eurofin Panlabs (previously known as Ricerca) with test catalog numbers of 200610, 300500, and 401000, respectively. Reference standards were run as an integral part of all three assays to ensure the validity of the results. The assays were performed under conditions described in Tables 1 –3.
Table 1.
Adenosine receptor A2A binding assay parameters.
| Adenosine A2A | |
|---|---|
| Source | Human recombinant HEK-293 cells |
| Ligand | 0.05 μM [3] CGS-21680 |
| Vehicle | 1% DMSO |
| Incubation time/temp. | 90 minutes at 25°C |
| Incubation buffer | 50 mM Tris-HCl, pH 7.4, 10 mM MgCl2, 1 mM EDTA, 2 U/mL adenosine Deaminase |
| Nonspecific ligand | 50 μM NECA (5-N-ethylcarboxamide adenosine) |
| KD | 0.064 μM |
| B max | 7 pmole/mg protein |
| Specific binding | 85% |
| Quantitation method | Radioligand binding |
| Significance criteria | ≥50% of max stimulation |
| Reference | CGS-21680 |
Table 2.
Adenosine receptor A2A functional assay parameters.
| Adenosine A2A adenylyl cyclase | |
|---|---|
| Source | Human recombinant HEK-293 cells |
| Control | 0.1 µM NECA |
| Vehicle | 0.40% DMSO |
| Incubation time/temp. | 10 minutes at 37°C |
| Incubation buffer | Modified Hank's balanced salt solution (HBSS) pH 7.4 |
| Quantitation method | HTRF quantitation of cAMP accumulation |
| Significant criteria for agonist | ≥50% increase in cAMP relative to NECA response |
| Significant criteria for antagonist | ≥50% inhibition of NECA-induced cAMP increase |
Table 3.
Adenosine receptor A1 functional assay parameter.
| Adenosine A1 | |
|---|---|
| Source | Wistar rat vas deferens |
| Control | 0.3 μM CHA (N6-cyclohexyladenosine) |
| Vehicle | 0.10% DMSO |
| Incubation time/temp. | 5 minutes at 32°C |
| Incubation buffer | KREBS pH 7.4 |
| Quantitation method | Isometric (gram changes) |
| Significant criteria for agonist | ≥50% reduction of neurogenic twitch relative to 0.3 μM CHA response |
| Significant criteria for antagonist | ≥50% inhibition of 0.3 μM CHA-induced relaxation |
2.3. Animals
Ninety adult male SD rats (3-month-old, 200–250 g) and juvenile male rats of the same strain (35–40-day-old, 75–100 g) from the National Institute of Nutrition, Tarnaka, Hyderabad, were used as described in Table 4. The diet comprised standard pellet diet by Provimi (Nutrilab Rodent). Juvenile rats were kept in groups of ten per cage and served as social stimuli for the adult rats. The animals were maintained in a room under controlled temperature (22 ± 2°C), with relative humidity of between 50 and 70% and were subjected to a 12 h light cycle (lights on 8:00 a.m.) with free access to food and water. All the experimental procedures (IAEC/CPCSEA approval number 1412/a/11 in February 2012) were performed according to the guidelines on animal care of the OECD Principles of Good Laboratory Practice, as revised in 1997 and adopted on November 26th, 1997, by decision of the OECD Council [C(97)186/Final].
Table 4.
Animal grouping according to test materials, dose, and route of administration.
| Group number | Dose level | Route | Number of animals |
|---|---|---|---|
| 1 | Vehicle control | p.o. | 10 |
| 2 | BT 00119 (200 mg/kg) | p.o. | 10 |
| 3 | BT 00119 (300 mg/kg) | p.o. | 10 |
| 4 | BT 00119 (600 mg/kg) | p.o. | 10 |
| 5 | PME 00012 (200 mg/kg) | p.o. | 10 |
| 6 | GBE 000120 (120 mg/kg) | p.o. | 10 |
| 7 | Donepezil (3 mg/kg) | i.p. | 10 |
| 8 | BT 00119 (60 mg/kg) | i.p. | 10 |
| 9 | BT 00119 (120 mg/kg) | i.p. | 10 |
2.4. Treatment
The plant extract of O. stamineus (doses 60, 120, 200, 300, and 600 mg/kg b.w.), a commercial extract of G. biloba (120 mg/kg, standardised to 27.25% Ginkgo flavonglycosides, 6% Terpene lactones, and ≤ 5 ppm ginkgolic acid determined through HPLC methods), water extract of P. minus (200 mg/kg), and the drug donepezil (ARICEPT tablet, Zydus Cadila Ltd., 3 mg/kg) were dissolved in distilled water. The control solution consisted of distilled water (vehicle). The extract of O. stamineus was tested i.p. and orally. Extracts of O. stamineus at doses of 60 and 120 mg/kg b.w. and donepezil at 3 mg/kg b.w. were administered i.p. for a direct comparison to donepezil activity, 120 min before the second encounter C2. In addition, extracts of O. stamineus at doses of 200, 300, and 600 mg/kg b.w., G. biloba extract at a dose of 120 mg/kg, a concentration derived from past animal studies of G. biloba in cognition-related investigations [32], and 200 mg/kg water extract of P. minus (as a direct comparison with the lower dose of the test extract) and vehicle were administered orally, 120 min before the second encounter C2.
2.5. Social Recognition Test
Short-term social memory was assessed with the SRT described by Mondadori et al. [33]. Nine groups of rats, each consisting of 10 males, were used for the study. Adult Sprague Dawley (SD) rats were housed individually in polycarbonate cages and they were used only after at least 7 days of habituation to their new environment. The test was scored in a consistent manner in an observation room, where the rats had been habituated for at least 1 h before the beginning of the test. All juveniles were isolated in individual cages for 30 min prior to the beginning of the experiment. The SRT consisted of two successive presentations (5–10 min each) separated by a short period of time where a juvenile rat was placed in the home cage of the adult rat and the time (s) spent by the adult in investigating the juvenile (nosing, sniffing, grooming, or pawing) was recorded (C1). At the end of the first presentation, the juvenile was removed and kept in an individual cage during the delay period and reexposed to the adult rat after 120 min and time (s) spent by the adult in investigating the juvenile was recorded (C2). In this paradigm, a reduction in the investigation time during the second encounter reflects the recognition ability of the adult rat. A pretest was performed for verification that the test compounds themselves do not have effects on social investigation per se. In this experiment, a different juvenile to the one used in the first presentation was exposed to the adult rat during the second encounter, with a similar duration of social investigation time being expected. RI was calculated using the formula (RI = C2/C1) for social recognition assay.
All values are expressed as means ± SEM (n equals the number of rats included in each analysis). The RI (RI = C2/C1) was calculated for social recognition assay. The data was analyzed by comparing control versus treatment and standard and changes in activity before and after treatment (C1 versus C2) and RI versus control, standard, and treatment using Student's t-test by Graph Pad Prism 4.0 software.
3. Result
3.1. Characterization of O. stamineus Ethanolic Extract
Chromatographic profile of O. stamineus ethanolic extract composition and reference compounds are as shown in Figures 1 and 2, respectively. The peaks corresponding to selected seven compounds were identified based on retention time against reference standards, and the UV spectrum. The peaks of ombuin (3,3′,5-trihydroxy-4′,7-dimethoxyflavone) (0.14%), 3′-hydroxy-4′,5,6,7-tetramethoxyflavone (0.10%), sinensetin (0.07%), orthosiphol B (0.26%), orthosiphol A (0.67%), staminol A (0.45%), and orthosiphonone A (0.12%) are eluted at retention times 7.675 min, 11.220 min, 15.554 min, 32.391 min, 35.911 min, 37.196 min, and 39.604 min, respectively. The resulting standardized extract is based on the group of marker compounds.
Figure 1.
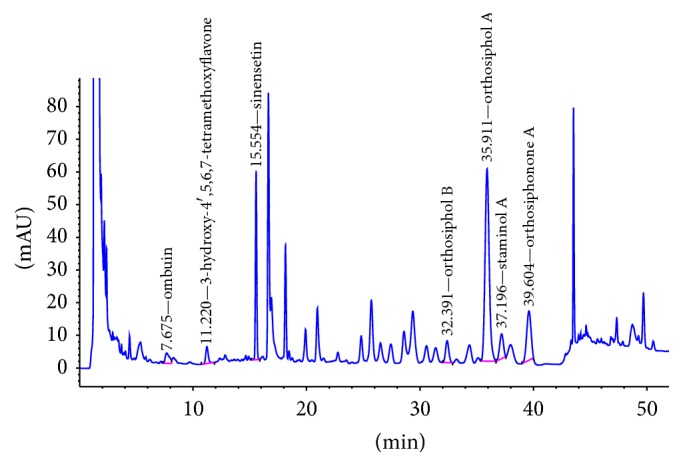
HPLC chromatograms of O. stamineus leaf ethanolic extract.
Figure 2.
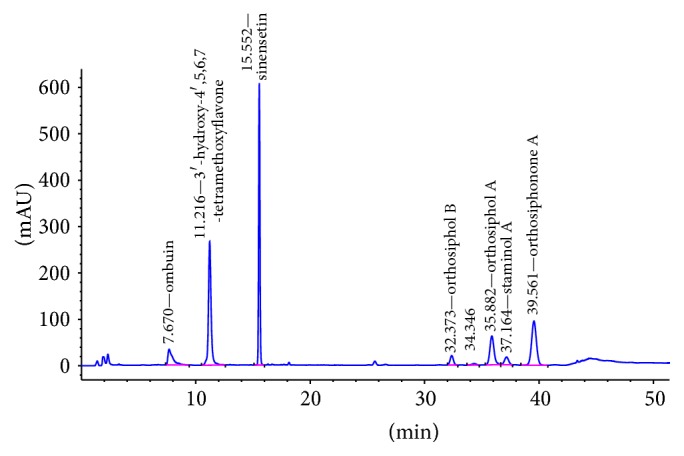
HPLC chromatograms of reference standard compounds. The identified peaks are ombuin (3,3′,5-trihydroxy-4′,7-dimethoxyflavone), 3′-hydroxy-4′,5,6,7-tetramethoxyflavone, sinensetin, orthosiphol B, orthosiphol A, staminol A, and orthosiphonone A.
3.2. In Vitro Adenosine A2A Receptor (A2AR) and Adenosinse A1 Receptor (A1R) Assays
The ethanolic extract of O. stamineus leaves showed significant binding activity with 74% inhibition of A2AR at a dose of 150 μg/mL and antagonist activity in the A2A functional assay at 300 μg/mL with 98% inhibition of cAMP response induced by NECA (Table 5). The extract showed similar activity in A1R inhibition, with an antagonist activity at 300 μg/mL where the extract displayed 100% inhibition of response induced by cyclohexyladenosine (CHA). The antagonist activity of the O. stamineus leaves ethanolic extract to adenosine A2A and adenosine A1 receptors suggests the biological activity of O. stamineus in an in vitro system. The IC50 for A2AR binding activity is estimated at 60.07 μg/mL and determined with nonlinear regression analysis by Inplot GraphPad Prism, San Diego, CA, computer program. The IC50 for A1R antagonist is 95.1 μg/mL (Figure 3). The IC50 for A2AR antagonist based on the response curve is 51.5 μg/mL (Figure 4). The Ki value for the A2AR binding assay is calculated using the Cheng-Prusoff equation (1973) and is estimated at 33.72 mM.
Table 5.
Results of in vitro adenosine A2A and adenosine A1 assays.
| Assay | Concentration (μg/mL) | Inhibition (%) | IC50 (μg/mL) |
|---|---|---|---|
| Adenosine A2A binding assay | 15 | 17 | 60.07 |
| 150 | 74 | ||
|
| |||
| Adenosine A2A functional assay antagonist | 3 | 14 | 51.5 |
| 30 | 26 | ||
| 300 | 98 | ||
|
| |||
| Increase in cAMP (%) | |||
| Adenosine A2A functional assay agonist | 3 | −1 | — |
| 30 | −3 | ||
| 300 | −8 | ||
|
| |||
| Adenosine A1 functional assay antagonist activity | 3 | 0 | 95.1 |
| 30 | 0 | ||
| 300 | 100 | ||
|
| |||
| Reduction in neurogenic twitch (%) | |||
| Adenosine A1 functional assay agonist activity | 3 | 5 | — |
| 30 | 12 | ||
| 300 | 29 | ||
Figure 3.
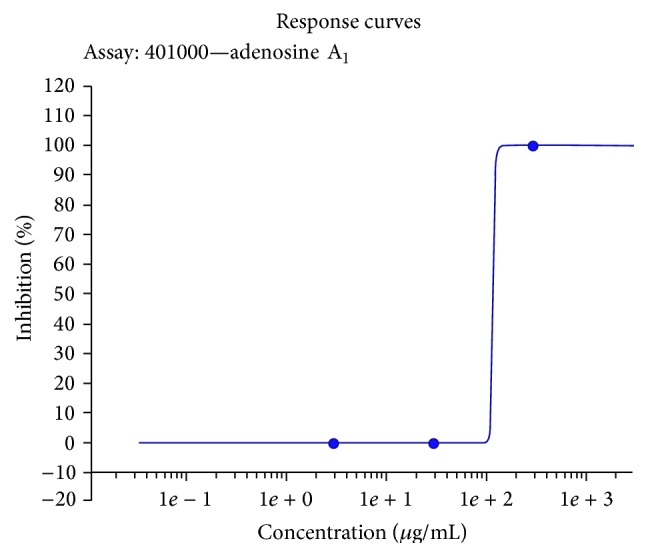
Response curve for adenosine A1 antagonist assay. ∗The IC50 of adenosine A1 antagonist assay for O. stamineus ethanolic extract is 95.1 μg/mL.
Figure 4.
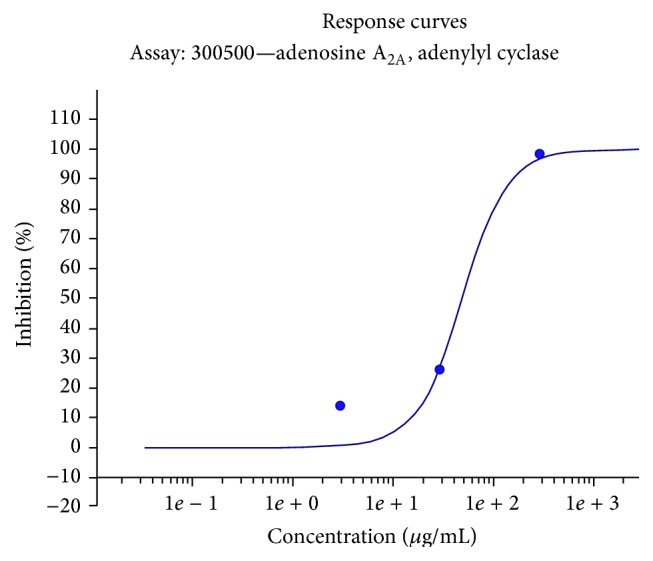
Response curve for adenosine A2A antagonist assay. ∗The IC50 of adenosine A2A antagonist assay for O. stamineus ethanolic extract is 51.5 μg/mL.
3.3. Social Recognition Test
In the SRT procedure, SD rats presented a clear impairment of the juvenile recognition ability (recognition index) in comparison to control rats (p < 0.001), since control group spent as much time investigating the juvenile rat during the second encounter as they did on the first exposure. The difference between treated and control groups on juvenile recognition ability is showed with more details in Table 6, with detailed analysis of the investigation time. The investigatory behaviour of the adult SD rats was concentrated in the first 5 min of the juvenile presentation, with a significant reduction in the investigation time during the second encounter 120 min later. The effects of the administration of acute doses of O. stamineus extract (200, 300, and 600 mg/kg, p.o., and 60, 120 mg/kg, i.p.), P. minus (200 mg/kg, p.o.), G. biloba (120 mg/kg, p.o.), donezepil (3 mg/kg, i.p.), and the vehicle (p.o.) in the SD rats social investigation time are given in Table 6. O. stamineus extract has shown significant (p < 0.001) change in RI compared to vehicle control at an oral dose of 300 mg/kg and 600 mg/kg, respectively. It also exerted significant (p < 0.001) change in RI at a dose of 120 mg/kg i.p. compared to vehicle control. However, 200 mg/kg oral and 60 mg/kg i.p. dose remained insignificant for O. stamineus extract. The reduction in inflexion was further confirmed with significant (p < 0.05, p < 0.001, and p < 0.05) change in activity before (C1) and after (C2) treatment for O. stamineus extract group, at oral doses of 300 mg/kg, 600 mg/kg, and 120 mg/kg i.p., respectively (Table 4, C1 versus C2 significance). The extract of P. minus and G. biloba has shown small change in inflexion; however it remained insignificant for RI compared to vehicle, at an oral dose of 200 mg/kg and 120 mg/kg. The standard drug donepezil dosed at 3 mg/kg i.p. has shown change in inflexion but no significant change in RI as compared to vehicle control.
Table 6.
Effect of O. stamineus (BT 00119), P. minus (PM 00012), G. biloba (GBE 00110), and donepezil on recognition index with respect to duration of interactions in social recognition test in the SD rats.
| Treatment (mg/kg) p.o./i.p., immediately after C1 |
Investigation duration (seconds) | C2 versus C1 | Recognition index (C2/C1) | |||||
|---|---|---|---|---|---|---|---|---|
| Route | First contact (C1) | Second contact (C2) 120 min after C1 | ||||||
| Mean ± SEM | p value | Mean ± SEM | p value | p value | Mean ± SEM | p value | ||
| Vehicle | p.o. | 22.00 ± 11.59 | — | 25.00 ± 13.0 | — | 0.1777 | 1.107 ± 0.20 | — |
| BT 00119 (200) | p.o. | 28.67 ± 5.36 | 0.3146 | 14.33 ± 8.51 | 0.2656 | 0.1273 | 0.5033 ± 0.24 | 0.0661 |
| BT 00119 (300) | p.o. | 57.33 ± 7.53a∗ | 0.0315 | 14.33 ± 2.90 | 0.2348 | 0.0119b∗ | 0.2400 ± 0.02 | 0.0068c∗∗∗ |
| BT 00119 (600) | p.o. | 71.00 ± 7.81a∗ | 0.0124 | 8.333 ± 1.85 | 0.1374 | 0.0051b∗∗∗ | 0.1133 ± 0.017 | 0.0042c∗∗∗ |
| PME 00012 (200) | p.o. | 48.67 ± 6.88 | 0.0595 | 31.00 ± 5.68 | 0.3475 | 0.1419 | 0.6833 ± 0.18 | 0.0986 |
| GBE 00110 (120) | p.o. | 52.67 ± 5.69a∗ | 0.0382 | 33.67 ± 2.84 | 0.2759 | 0.0775 | 0.6600 ± 0.11 | 0.0651 |
| Donepezil (3) | i.p. | 30.67 ± 1.20 | 0.2492 | 18.33 ± 4.33 | 0.3266 | 0.0621 | 0.5500 ± 0.11 | 0.0593 |
| BT 00119 (60) | i.p. | 26.67 ± 4.80 | 0.3644 | 8.667 ± 5.23 | 0.1550 | 0.1801 | 0.3867 ± 0.28 | 0.0539 |
| BT 00119 (120) | i.p. | 51.00 ± 2.51a∗ | 0.0354 | 14.00 ± 2.51 | 0.2272 | 0.0136b∗ | 0.2733 ± 0.05 | 0.0086c∗∗∗ |
p.o. = per oral, i.p. = intraperitoneal, and SEM = standard error mean.
a∗ p < 0.05 indicates the significance of first contact in comparison with vehicle control for all groups.
b∗ p < 0.05 and b∗∗∗ p < 0.001 indicate the significance in comparing the change in activity before and after treatment.
c∗∗ p < 0.01 and c∗∗∗ p < 0.001 indicate the significant changes of RI when compared with vehicle control.
4. Discussion
The chemical constituents and the A2AR binding activity of O. stamineus extract have demonstrated that, with a single treatment of O. stamineus leaves extract after C1, the time spent in scrutinizing the same partner at a second meeting, 120 min later, is shortened. The extract-induced reduction of the exploration time can be attributed to learning of the specific information of the partner retained from the first meeting that reduced the need for new information. The assumption that specific attributes of a particular partner were remembered is strengthened by the significant RI (p < 0.001) seen with the test extract. The ethanolic extract of O. stamineus leaves showed significant activity with 74% inhibition of A2AR at a dose of 150 μg/mL. Therefore, the study suggests the possible binding of the O. stamineus extract to A2AR, attributing the social recognition task with this biological activity.
The present results demonstrate that the SD rats present a significant impairment of short-term social memory in SRT in the vehicle group as the RI of more than 1 signifies no improvement in recognition (RI should be <1). In fact, a longer time to recognize juvenile rat (C2) was observed for the vehicle group. The findings also suggest the involvement of the adenosine receptors in this response, since the acute administration of O. stamineus leaves extract reversed this social memory deficit in SD rats. Several studies have demonstrated that the selective blockade of adenosine A1 and adenosine A2A receptors facilitates learning and memory in rodents models [22, 34]. The Ki value of the extract in this experiment was 33.72 mM. Caffeine and theophylline, another naturally occurring xanthine mainly found in tea, are nonselective AR antagonists. Their stimulating properties are associated with micromolar range affinities for the A2AR. Although caffeine and theophylline have similar in vitro affinities for the A2A receptor, caffeine has a higher stimulating effect due to a higher brain unbound fraction wt a Ki value of 23400 nM [35]. Though caffeine derivatives possess stronger A2AR binding activities, the multicompounds that exist in Orthosiphon stamineus extract including flavonoids may have affected memory more than one way possibly also via other receptors such as the inhibition of acetylcholinesterase, thereby enhancing cognition [36]. Furthermore, the extract tested in this study also possessed additional A1R antagonist activity, as previously reported in O. stamineus [17]. From the SRT, promnesic property of O. stamineus leaves extract was observed in SD rats at dose dependent manner of 300 mg/kg and 600 mg/kg p.o. and 60 mg/kg and 120 mg/kg i.p. This study revealed that the O. stamineus leaves extract (300 and 600 mg/kg, p.o.; 60 and 120 mg/kg i.p.) exerted significant activity when compared to standard donepezil (3 mg/kg i.p.) statistically reaching significance in all except at 60 mg/kg i.p. where RI was only almost significant (p < 0.0539). The O. stamineus leaves extract appears to prevent the amnesic effect of the long delay (120 minutes) where such a preventive effect may be deduced as promnesic. The extract at 120 mg/kg i.p. was comparable to 300 mg/kg and 600 mg/kg orally dosed in RI, signifying greater bioavailability when administered i.p. at only one-third of the oral dosage.
As for G. biloba extract, a 120 mg/kg oral dose failed to demonstrate significant activity in this study but in another study, a single i.p. injection of G. biloba extract at 120 mg/kg dose demonstrated improvement in recognition performances in young rats in a similar olfactory animal model study [32]. This may be due to G. biloba having a lower bioavailability when administered through nonintravenous route (as observed in this study). The herb P. minus on the other hand has been shown to possess antiacetylcholinesterase activity in a recent study [27] although in the current study improvement in recognition index was not significant. The promnesic effects are probably more apparent in a model that tests attention rather than learning and memory from olfactory cues. This is in parallel to findings by Blokland [37] where it was suggested that the role of acetylcholine in learning and memory processes was still not conclusive, rendering its role more important in attention processes. A different animal model such as Barnes maze that tests spatial learning and memory instead of memory by olfactory and social cues, such as in this study, may have been a better model to investigate P. minus [27].
Based on the mean of RI for the extracts given i.p., donepezil fared better (in terms of lower mean) than the plant extract of G. biloba at their tested dosage though the route of administration was different. A2AR activity has never been known for G. biloba though anticholinesterase activity has been reported [25] and in vivo memory improvement has been documented for G. biloba extracts [26]. Donepezil, a reversible inhibitor of cholinesterase which is clinically used for treatment of dementia, showed slightly weak (RI at p < 0.0593) cognition enhancing properties at 3 mg/kg i.p. The effect of 3 mg/kg donepezil is similar to 60 mg/kg i.p. of O. stamineus extract based on the mean at C1 and having changes to RI after treatment at p < 0.0593 and p < 0.0539, respectively. This may be due to it being a popular acetylcholinesterase inhibitor for memory impairment which is age-related and long term such as Alzheimer and dementia. The short reaction time (120 minutes) and the use of adult but not aged rats to impart a significant effect in the RI may have contributed to the weaker response in the Donepezil group.
Neurodegenerative disease can be the result of neuronal cell death caused by oxidative stress, apoptosis, and inflammation. Apart from A2AR activity, alcohol extracts of O. stamineus leaves may possess other biological activities that are neuroprotective. They have been reported to possess antiapoptotic effects in a H2O2 (a potent free radical) induced cell apoptosis [5]. The antioxidant properties of O. stamineus in addition may play a positive role in the prevention of neurogeneration caused by damaging free radicals [7]. The O. stamineus is known to contain several classes of bioactive compounds such as flavonoids, diterpenes, triterpenes, saponins, sterols organic acids, caffeic acids derivatives, chromenes, and oleanic and ursolic acid, known for [12–16]. Flavonoids have been shown to possess antioxidative and anti-inflammatory effects that suggest neuroprotective property [36]. Oleanic acid which has been isolated from O. stamineus has been reported to protect against neuronal death induced by beta-amyloid in cultured rat cortical neurons and improve beta-amyloid induced memory deficit in mice [38]. Ursolic acid reduced the production of proinflammatory cytokines and neurotoxic reactive oxygen species, thus possibly leading to an additional neuroprotective effect [39].
Caffeine is another example of adenosine A2A receptor antagonist that modulates the release of different neurotransmitters in the olfactory bulb of rodents [40] known to play a role in social olfactory recognition [41]. It appears to be that O. stamineus extract behaves similarly to caffeine in improving short-term memory and alertness. O. stamineus, however, does not contain caffeine but is rich in terpenoids and flavonoids. Terpenoids from natural products such as G. biloba and Asian ginseng (Panax ginseng) are currently being investigated as potential therapeutics in Alzheimer's disease, already showing some promise [42]. Terpenoids were identified in the O. stamineus extract used, that is, orthosiphol B and orthosiphol F, staminol A, and orthosiphonone A, which makes this extract a potential candidate for further investigation in the area of cognition disorder.
5. Conclusion
Our findings suggest that the propriety standardized ethanolic extract of O. stamineus may reverse age-related deficits in short-term social memory and can be considered to prevent or decrease the rate of neurodegeneration. The further investigation of not only adenosinergic but also other neurotransmitters in producing improvements in cognition should be evaluated in the future. The involvement of A1 and A2A blockade in the social memory deficit can be further clarified in their role in the Orthosiphon stamineus effects along with selective A1 and A2A antagonist assayed in a social recognition tests for confirmation of target.
Acknowledgment
The authors would like to thank Biotropics Malaysia Bhd for funding the study.
Conflict of Interests
The authors declare that Annie George, Sasikala Chinnappan, and Hoi Jin Wong are employees of Biotropics Berhad Malaysia which funded this study. Biotropics Malaysia Berhad has filed a patent based on this discovery (WO 2011/078652 A1). Yogendra Choudhary, Vandana Kotak Choudhary, and Praveen Bommu have no conflict of interests in this study.
Authors' Contribution
Annie George and Sasikala Chinnappan were responsible for the conception of the study and participated in the in vivo design and worked on drafting of the paper. Vandana Kotak Choudhary carried out the monitoring and coordination of the study, Yogendra Choudhary interpreted the data and participated in literature search and drafting of the paper. Praveen Bommu carried out the experimental work and collected and interpreted the data. Hoi Jin Wong worked on the standardisation of the herbal extract used in this study. All authors read and approved the final paper. All authors contributed equally to this work.
References
- 1.Burkill H. I. A Dictionary of the Economic Products of the Malay Peninsula. Vol. 2. Kuala Lumpur, Malaysia: Ministry of Agriculture & Cooperatives; 1966. [Google Scholar]
- 2.Wan Hassan W. E., Mustaffa M. Healing Herbs of Malaysia. Federal Land Development Authority (FELDA); 2006. [Google Scholar]
- 3.Jaganath I. B., Ng L. T. The Green Pharmacy of Malaysia. Kuala Lumpur, Malaysia: Vinpress Sdn Bhd; 2000. [Google Scholar]
- 4.Awale S., Tezuka Y., Banskota A. H., Kadota S. Siphonols A–E: novel nitric oxide inhibitors from Orthosiphon stamineus of Indonesia. Bioorganic & Medicinal Chemistry Letters. 2003;13(1):31–35. doi: 10.1016/s0960-894x(02)00854-5. [DOI] [PubMed] [Google Scholar]
- 5.Abdelwahab S. I., Mohan S., Elhassan M. M., et al. Antiapoptotic and antioxidant properties of Orthosiphon stamineus benth (Cat's Whiskers): intervention in the Bcl-2-mediated apoptotic pathway. Evidence-Based Complementary and Alternative Medicine. 2011;2011:10. doi: 10.1155/2011/156765.156765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akowuah G. A., Zhari I., Norhayati I., Sadikun A., Khamsah S. M. Sinensetin, eupatorin, 3′-hydroxy-5, 6, 7, 4′-tetramethoxyflavone and rosmarinic acid contents and antioxidative effect of Orthosiphon stamineus from Malaysia. Food Chemistry. 2004;87(4):559–566. doi: 10.1016/j.foodchem.2004.01.008. [DOI] [Google Scholar]
- 7.Ho C.-H., Noryati I., Sulaiman S.-F., Rosma A. In vitro antibacterial and antioxidant activities of Orthosiphon stamineus Benth. extracts against food-borne bacteria. Food Chemistry. 2010;122(4):1168–1172. doi: 10.1016/j.foodchem.2010.03.110. [DOI] [Google Scholar]
- 8.Han C. J., Hussin A. H., Ismail S. Effect of methanol leaf extract of Orthosiphon stamineus benth. on hepatic drug metabolizing enzymes in Sprague Dawley (SD) rats. Journal of Biosciences. 2008;19(1):21–23. [Google Scholar]
- 9.Arafat O. M., Tham S. Y., Sadikun A., Zhari I., Haughton P. J., Asmawi M. Z. Studies on diuretic and hypouricemic effects of Orthosiphon stamineus methanol extracts in rats. Journal of Ethnopharmacology. 2008;118(3):354–360. doi: 10.1016/j.jep.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 10.Ohashi K., Bohgaki T., Shibuya H. Antihypertensive substance in the leaves of kumis kucing (Orthosiphon aristatus) in Java island. Yakugaku Zasshi. 2000;120(5):474–482. doi: 10.1248/yakushi1947.120.5_474. [DOI] [PubMed] [Google Scholar]
- 11.Sriplang K., Adisakwattana S., Rungsipipat A., Yibchok-Anun S. Effects of Orthosiphon stamineus aqueous extract on plasma glucose concentration and lipid profile in normal and streptozotocin-induced diabetic rats. Journal of Ethnopharmacology. 2007;109(3):510–514. doi: 10.1016/j.jep.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 12.Malterud K. E., Hanche-Olsen I. M., Smith-Kielland I. Flavonoids from Orthosiphon spicatus . Planta Medica. 1989;55(6):569–570. doi: 10.1055/s-2006-962099. [DOI] [PubMed] [Google Scholar]
- 13.Sumaryono W., Proksch P., Wray V., Witte L., Hartmann T. Qualitative and quantitative analysis of the phenolic constituents from Orthosiphon aristatus . Planta Medica. 1991;57(2):176–180. doi: 10.1055/s-2006-960060. [DOI] [PubMed] [Google Scholar]
- 14.Masuda T., Masuda K., Nakatani N. Orthosiphol A, a highly oxygenated diterpene from the leaves of Orthosiphon stamineus . Tetrahedron Letters. 1992;33(7):945–946. doi: 10.1016/s0040-4039(00)91583-1. [DOI] [Google Scholar]
- 15.Olah N.-K., Radu L., Mogoşan C., Hanganu D., Gocan S. Phytochemical and pharmacological studies on Orthosiphon stamineus Benth. (Lamiaceae) hydroalcoholic extracts. Journal of Pharmaceutical and Biomedical Analysis. 2003;33(1):117–123. doi: 10.1016/s0731-7085(03)00227-9. [DOI] [PubMed] [Google Scholar]
- 16.Hossain M. A., Ismail Z. Isolation and characterization of triterpenes from the leaves of Orthosiphon stamineus . Arabian Journal of Chemistry. 2013;6(3):295–298. doi: 10.1016/j.arabjc.2010.10.009. [DOI] [Google Scholar]
- 17.Yuliana N. D., Khatib A., Link-Struensee A. M. R., et al. Adenosine A1 receptor binding activity of methoxy flavonoids from Orthosiphon stamineus . Planta Medica. 2009;75(2):132–136. doi: 10.1055/s-0028-1088379. [DOI] [PubMed] [Google Scholar]
- 18.Fredholm B. B., Bättig K., Holmén J., Nehlig A., Zvartau E. E. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacological Reviews. 1999;51(1):83–133. [PubMed] [Google Scholar]
- 19.Chen J.-F., Sonsalla P. K., Pedata F., et al. Adenosine A2A receptors and brain injury: broad spectrum of neuroprotection, multifaceted actions and ‘fine tuning’ modulation. Progress in Neurobiology. 2007;83(5):310–331. doi: 10.1016/j.pneurobio.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Prediger R. D., Batista L. C., Takahashi R. N. Caffeine reverses age-related deficits in olfactory discrimination and social recognition memory in rats. Involvement of adenosine A1 and A2A receptors. Neurobiology of Aging. 2005;26(6):957–964. doi: 10.1016/j.neurobiolaging.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi R. N., Pamplona F. A., Prediger R. D. Adenosine receptor antagonists for cognitive dysfunction: a review of animal studies. Frontiers in Bioscience. 2008;1(13):2614–2632. doi: 10.2741/2870. [DOI] [PubMed] [Google Scholar]
- 22.Pereira G. S., Mello e Souza T., Vinadé E. R. C., et al. Blockade of adenosine A1 receptors in the posterior cingulate cortex facilitates memory in rats. European Journal of Pharmacology. 2002;437(3):151–154. doi: 10.1016/s0014-2999(02)01307-9. [DOI] [PubMed] [Google Scholar]
- 23.Gomes C. V., Kaster M. P., Tomé A. R., Agostinho P. M., Cunha R. A. Adenosine receptors and brain diseases: neuroprotection and neurodegeneration. Biochimica et Biophysica Acta. 2011;1808(5):1380–1399. doi: 10.1016/j.bbamem.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Thor D. H., Holloway W. R. Social memory of the male laboratory rat. Journal of Comparative and Physiological Psychology. 1982;96(6):1000–1006. doi: 10.1037/0735-7036.96.6.1000. [DOI] [Google Scholar]
- 25.Stackman R. W., Eckenstein F., Frei B., Kulhanek D., Nowlin J., Quinn J. F. Prevention of age-related spatial memory deficits in a transgenic mouse model of Alzheimer's disease by chronic Ginkgo biloba treatment. Experimental Neurology. 2003;184(1):510–520. doi: 10.1016/s0014-4886(03)00399-6. [DOI] [PubMed] [Google Scholar]
- 26.Walesiuk A., Trofimiuk E., Braszko J. J. Ginkgo biloba extract diminishes stress-induced memory deficits in rats. Pharmacological Reports. 2005;57(2):176–187. [PubMed] [Google Scholar]
- 27.George A., Ng C. P., O'Callaghan M., Jensen G. S., Wong H. J. In vitro and ex-vivo cellular antioxidant protection and cognitive enhancing effects of an extract of Polygonum minus Huds (Lineminus) demonstrated in a Barnes Maze animal model for memory and learning. BMC Complementary and Alternative Medicine. 2014;14, article 161 doi: 10.1186/1472-6882-14-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adyana I. K., Finna S., Muhammad I. From the ethnopharmacology to clinical study of Orthosiphon stamineus Benth. Journal of Pharmacy and Pharmaceutical Sciences. 2013;5(3):66–73. [Google Scholar]
- 29.Varani K., Gessi S., Dalpiaz A., Borea P. A. Pharmacological and biochemical characterization of purified A2a adenosine receptors in human platelet membranes by [3H]-CGS 21680 binding. British Journal of Pharmacology. 1996;117(8):1693–1701. doi: 10.1111/j.1476-5381.1996.tb15341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poucher S. M., Keddie J. R., Singh P., Caulkett P. W. R., Jones G., Collis M. G. The in vitro pharmacology of ZM 241385, a potent, non-xanthine, A2a selective adenosine receptor antagonist. British Journal of Pharmacology. 1995;115(6):1096–1102. doi: 10.1111/j.1476-5381.1995.tb15923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor D. A., Wiese S., Faison E. P., Yarbrough G. G. Pharmacological characterization of purinergic receptors in the rat vas deferens. Journal of Pharmacology and Experimental Therapeutics. 1983;224(1):40–45. [PubMed] [Google Scholar]
- 32.Sylvia W., Stemmelin J., Will B., Christen Y., Di Scala G. Facilitative effects of egb 761 on olfactory recognition in young and aged rats. Pharmacology Biochemistry and Behavior. 2000;65(2):321–326. doi: 10.1016/s0091-3057(99)00188-4. [DOI] [PubMed] [Google Scholar]
- 33.Mondadori C., Moebius H.-J., Zingg M. CGP 36 742, an orally active GABAB receptor antagonist, facilitates memory in a social recognition test in rats. Behavioural Brain Research. 1996;77(1-2):227–229. doi: 10.1016/0166-4328(95)00226-x. [DOI] [PubMed] [Google Scholar]
- 34.Angelucci M. E. M., Cesário C., Hiroi R. H., Rosalen P. L., Da Cunha C. Effects of caffeine on learning and memory in rats tested in the Morris water maze. Brazilian Journal of Medical and Biological Research. 2002;35(10):1201–1208. doi: 10.1590/s0100-879x2002001000013. [DOI] [PubMed] [Google Scholar]
- 35.Ruiz M. D. L., Lim Y. H., Zheng J. Adenosine A2A receptor as a drug discovery target. Journal of Medicinal Chemistry. 2014;57(9):3623–3650. doi: 10.1021/jm4011669. [DOI] [PubMed] [Google Scholar]
- 36.Uriarte-Pueyo I., Calvo M. I. Flavonoids as acetylcholinesterase inhibitors. Current Medicinal Chemistry. 2011;18(34):5289–5302. doi: 10.2174/092986711798184325. [DOI] [PubMed] [Google Scholar]
- 37.Blokland A. Acetylcholine: a neurotransmitter for learning and memory? A review. Brain Research Reviews. 1996;21:285–300. doi: 10.1016/0165-0173(95)00016-x. [DOI] [PubMed] [Google Scholar]
- 38.Cho S. O., Ban J. Y., Kim J. Y., et al. Anti-ischemic activities of aralia cordata and its active component, oleanolic acid. Archives of Pharmacal Research. 2009;32(6):923–932. doi: 10.1007/s12272-009-1615-1. [DOI] [PubMed] [Google Scholar]
- 39.Wilkinson K., Boyd J. D., Glicksman M., Moore K. J., El Khoury J. A high content drug screen identifies ursolic acid as an inhibitor of amyloid beta protein interactions with its receptor CD36. The Journal of Biological Chemistry. 2011;286(40):34914–34922. doi: 10.1074/jbc.m111.232116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hadfield M. G. Caffeine and the olfactory bulb. Molecular Neurobiology. 1997;15(1):31–39. doi: 10.1007/BF02740614. [DOI] [PubMed] [Google Scholar]
- 41.Prediger R. D. S., Batista L. C., Miyoshi E., Takahashi R. N. Facilitation of short-term social memory by ethanol in rats is mediated by dopaminergic receptors. Behavioural Brain Research. 2004;153(1):149–157. doi: 10.1016/j.bbr.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 42.Yoo K.-Y., Park S.-Y. Terpenoids as potential anti-alzheimer's disease therapeutics. Molecules. 2012;17(3):3524–3538. doi: 10.3390/molecules17033524. [DOI] [PMC free article] [PubMed] [Google Scholar]


