Abstract
Objective
To characterize clinical and radiological features associated with biliary cystic tumors (BCTs) of the liver, and to define recurrence-free and overall survival.
Background
Biliary cystadenoma (BCA) and biliary cystadenocarcinoma (BCAC) are rare tumors that arise in the liver.
Methods
Between 1984 and 2013, 248 patients who underwent surgical resection of BCA or BCAC were identified. Clinical and outcome data were analyzed.
Results
Median total bilirubin, CA19–9, and carcinoembryonic antigen (CEA) levels were 0.6 mg/dL, 15.0 U/mL, and 2.7 ng/mL, respectively. Preoperative imaging included computed tomography only (62.5%), magnetic resonance imaging only (6.9%), or CT + MRI (18.5%). Features on cross-sectional imaging included multiloculation (56.9%), mural nodularity (16.5%), and biliary ductal dilatation (17.7%). The presence of these factors did not reliably predict BCAC versus BCA (sensitivity, 81%; specificity, 21%). Median biliary cyst size was 10.0 cm (interquartile range, 7–13 cm). Operative interventions included unroofing/partial excision of the lesion (14.1%), less than hemihepatectomy (48.8%), or hemi-/extended hepatectomy (36.3%). On pathology most lesions were BCA (89.1%), whereas 27 (10.9%) were BCAC. At last follow-up, there were 46 (18.3%) recurrences; 2 patients who initially had BCA recurred with BCAC. Median overall survival was 18.1 years; 1-year, 3-year, and 5-year survival was 95.0%, 86.8%, and 84.2%, respectively. Long-term outcomes were associated with BCAC versus BCA, as well as the presence of spindle cell/ovarian stroma (both P < 0.05).
Conclusions
Among patients undergoing surgery for BCT, associated malignancy was uncommon (10%) and no preoperative findings reliably predicted underlying BCAC. After excision of BCA, long-term outcomes were good; however, patients with BCAC had a worse long-term prognosis.
Keywords: biliary cystadenoma, biliary cystadenocarcinoma, cyst, liver
With the proliferation of cross-sectional imaging, an increasing number of hepatic cystic lesions are being discovered. In fact, it is estimated that up to 20% of the general population have a cystic lesion in the liver.1–3 Although the overwhelming majority of these lesions are benign simple cysts, a small subset (3%–5%) of hepatic cysts may represent biliary cystic tumors (BCT).4 BCT arise from the biliary epithelium and can be classified as either biliary cystadenoma (BCA) or biliary cystadenocarcinoma (BCAC). The first report of BCT was by Heuter in 1887, with the first reported resection 5 years later by Keen.5,6 Subsequently, in 1958 Edmondson7 described the pathognomonic pathologic features of BCT as a multilocular lesion lined by columnar epithelium with an accompanying dense cellular “ovarian-like” stroma. More recently, the light microscopic and immunohistochemical features of hepatobiliary BCT have been further defined and characterized.8 Despite this, fewer than 250 cases of BCT have been reported in the world literature.4
Although rare, the management of BCT is important as these lesions are believed to be premalignant and may have a risk of malignant transformation as high as 20% to 30%.8,9 As such, accurate diagnosis of BCT is critical. Two previous studies have suggested certain clinical factors and radiological characteristics that may help differentiate BCA from BCAC.9,10 Deriving clinically meaningful conclusions from these 2 studies is difficult, however, as these reports included only 20 and 30 patients, respectively.9,10 In general, several investigators have suggested that anechoic lesions with internal septations characterized by septal thickening, papillary projections, and mural nodules may help differentiate BCA from BCAC.11–15 However, the different clinical and radiological characteristics of BCA versus BCAC have never been rigorously investigated. In addition, the optimal management of patients with BCA and BCAC remains poorly described. The various management options previously reported for BCT have included percutaneous aspiration, sclerosis, and surgical procedures such as unroofing/fenestration or partial hepatic resection.2,16–18 Given the malignant potential of BCT, inappropriate therapeutic management of these lesions may lead to an increase in the incidence of postprocedure recurrence, as well as adverse long-term outcomes. Unfortunately, virtually all previous reports on the management of BCT have been single-institution case reports or small series consisting of 30 or fewer patients. Current data on the surgical management and outcomes of patients with BCT is therefore largely anecdotal leaving minimal data to guide clinical practice.
Given the limited sample sizes and single-center nature of previously published reports, we sought to utilize data from an international, multicenter database derived from 10 major hepatobiliary centers to better characterize the features, management, and outcomes of patients with biliary cystic lesions of the liver. The purpose of the current study was to elucidate the clinical presentation and surgical management of patients with BCT. Specifically, we sought to evaluate and validate previous clinical and radiological characteristics purported to differentiate BCA from BCAC.9,10 In addition, we wanted to define the recurrence-free survival (RFS) and overall survival (OS) of patients with BCT relative to the type of surgical procedure performed (eg, unroofing/fenestration vs hepatic resection), as well as the underlying histology (eg, BCA vs BCAC).
METHODS
Patient Population
Using an international, multi-institutional hepatobiliary database from 10 major centers in North America, Europe, and Australia [Johns Hopkins Hospital, Baltimore, MD (n = 38); Emory University, Atlanta, GA (n = 25); Medical College of Wisconsin, WI (n = 12); University of Pittsburgh Medical Center, Pittsburgh, PA (n = 30); University of Virginia, Charlottesville, VA (n = 9); Mayo College of Medicine, Rochester, MN (n = 77); Ohio State=University, OH (n = 14); Institute for Digestive Diseases and Liver Transplantation Fundeni, Bucharest, Romania (n = 11); Royal Prince Alfred Hospital, Sydney, Australia (n = 28); Hospital Curry Cabral, Lisbon, Portugal (n = 4)], 248 patients who were treated for either BCA or BCAC between 1984 and 2013 were identified (1983–1993: 30, 1994–2003: 64, 2004–2013: 154). Each patient was diagnosed with BCA or BCAC on the basis of pathological examination at the respective institution. The institutional review board of each participating center approved the study protocol.
Data Collection
Standard demographic variables including age, gender, and race as well as baseline comorbidities such as coronary artery disease, hypertension, and diabetes mellitus were collected from the medical chart. Data on underlying liver disease, as well as presenting symptoms and liver function values, were recorded. Details on all preoperative imaging studies including ultrasound, computed tomo-graphic (CT) scans, and magnetic resonance imaging (MRI) were collected. Each institution rereviewed the case images for the features designated in the database. In addition, preoperative procedures such as biopsies, percutaneous cyst drainage, endoscopic retrograde cholangiopancreatography with or without endobiliary stenting, and percutaneous transhepatic cholangiography were reviewed.
Data on operative treatment were also collected, including the approach and extent of surgical resection as well as estimated blood loss. Resections were categorized as unroofing/fenestration or formal hepatic resection; hepatic resection was categorized as less than hemihepatectomy (2 segments or less) versus hemi- or extended hepatectomy (at least 3 segments). Lymphadenectomy, if completed, was documented. Anatomic considerations were identified including the location, as well as size of the BCT within the liver. When performed, results of intraoperative frozen sections were reviewed and compared with permanent pathology reports. The epithelium of BCT was classified as either mucinous or serous and the presence of ovarian-like stroma was documented, as well as the presence of atypia. Pathological assessment of the specimen was made at each center, and central review of pathology was not performed.
Postoperative complications were assessed until 30 days after the operation and were graded according to the Clavien-Dindo classification system; a major complication was defined as grade III or higher.19 Perioperative mortality was calculated on the basis of the number of patients who expired within 90 days of the operation.20 Long-term outcomes were examined, including the incidence of recurrence, treatment of recurrent disease, and OS at last follow-up.
Statistical Analysis
Continuous variables were presented as the mean ± standard deviation (SD) or with the median and interquartile range (IQR). Categorical variables were displayed as whole numbers and percentages. Baseline characteristics of the study population were summarized according to tumor type (ie, BCA vs BCAC). Comparative analysis of continuous variables was performed using Wilcoxon test for parametric and nonparametric data and 1-way analysis of variance, as appropriate. Fisher exact test or χ2 test was used for comparing categorical variables. Assessment of certain variables was performed utilizing receiver operating characteristic (ROC) curves according to the previously proposed cutoff values suggested by Wang et al.10
RFS and OS were estimated using the method of Kaplan-Meier, and differences in survival were examined with the log-rank test. A multivariable Cox proportional hazards regression model was used to identify predictors of the development of BCAC. The most parsimonious model was created using a stepwise approach that included factors statistically significant on univariate analysis (ie, P < 0.20). For statistical analyses, P values less than 0.05 (2-tailed) were deemed significant. Hazard ratios (HRs) were presented with 95% confidence intervals (CI). All analyses were carried out with STATA version 12.0 (StataCorp LP, College Station, TX).
RESULTS
Patient, Presentation, and Disease Characteristics
The clinicopathological features of the 248 patients included in the study cohort are presented in Table 1. The median age at diagnosis was 54 years (range, 14–83). Patients were overwhelmingly female (86.7%) and white (88.3%). Most patients initially presented with abdominal pain (57.3%), whereas other patients complained of abdominal fullness (20.2%), early satiety (12.5%), or weight loss (3.6%). Interestingly, 13.7% patients were completely asymptomatic, whereas a small subset of patients (11.3%) presented with jaundice. Most patients, however, presented with normal laboratory values including bilirubin (median, 0.6 mg/dL) and Alanine Aminotransferase (median, 27 U/L), as well as tumor markers including CA19–9 (median, 15 U/mL) and carcinoembryonic antigen (CEA) (2.7 ng/mL) levels. The preoperative clinical characteristics of patients with BCA versus BCAC were comparable, except BCAC patients were more likely to be male and older (both P < 0.05).
TABLE 1.
Characteristics of the Patients With Biliary Cystadenoma and Cystadenocarcinoma
| Total (n = 248) | BCA (n = 221, 89.1%) | BCAC (n = 27,10.9%) | P | |
|---|---|---|---|---|
| Demographics | ||||
| Age, mean (SD), yr | 52.1 (15.1) | 51.2 (15.1) | 58.9 (13.3) | 0.012 |
| Female, n (%) | 215 (86.7) | 199 (90.0) | 16 (59.3) | <0.001 |
| Race, White, n (%) | 219 (88.3) | 193 (87.3) | 26 (96.3) | 0.346 |
| Symptom, n (%) | 214 (86.3) | 189 (85.5) | 25 (92.6) | 0.313 |
| Abdominal pain | 142 (57.3) | 128 (57.9) | 14 (51.9) | 0.547 |
| Abdominal fullness | 50 (20.2) | 40 (18.1) | 10 (37.0) | 0.021 |
| Early satiety | 31 (12.5) | 29 (13.1) | 2 (7.4) | 0.397 |
| Weight loss | 9 (3.6) | 8 (3.6) | 1 (3.7) | 0.982 |
| Jaundice | 28 (11.3) | 25 (11.3) | 3 (11.1) | 0.975 |
| Asymptomatic | 34 (13.7) | 32 (14.5) | 2 (7.4) | 0.313 |
| Preoperative laboratory data | ||||
| Total Bilirubin (mg/dL), median (IQR) | 0.6 (0.4–0.8) | 0.6 (0.4–0.7) | 0.7 (0.5–1.0) | 0.844 |
| CA 19–9 (U/mL), median (IQR) (n = 210) | 15 (7.1–94.0) | 15 (6.0–63.1) | 210 (37.1–280.0) | 0.647 |
| CEA (ng/mL), median (IQR) (n = 210) | 2.7 (1.0–4.6) | 2.4 (0.9–4.6) | 4.3 (1.9–90.0) | <0.001 |
| ALT (U/L), median (IQR) | 27.0 (17.5–45.5) | 27.0 (18.0–48.0) | 20.5 (17.0–37.0) | 0.355 |
| Preoperative imaging, n (%) | ||||
| CT | 155 (62.5) | 137 (62.0) | 18 (66.7) | 0.175 |
| MRI | 17 (6.9) | 17 (7.7) | 0 (0) | |
| CT + MRI | 46 (18.5) | 39 (17.6) | 7 (25.9) | |
| USG | 145 (58.5) | 128 (57.9) | 17 (63.0) | 0.373 |
| Endoscopic retrograde pancreatography | 40 (16.1) | 36 (16.3) | 4 (14.8) | 0.872 |
| Preoperative image finding features (any of below), n (%) | 197 (79.4) | 175 (79.2) | 22 (81.5) | 0.781 |
| Multilocular cyst | 141 (56.9) | 123 (55.7) | 18 (66.7) | 0.093 |
| Septa | 163 (65.7) | 145 (65.6) | 18 (66.7) | 0.913 |
| Mural nodularity | 41 (16.5) | 24 (10.9) | 17 (63.0) | <0.001 |
| Calcification | 25 (10.1) | 16 (7.2) | 9 (33.3) | <0.001 |
| Hypervascular | 19 (7.7) | 10 (4.5) | 9 (33.3) | <0.001 |
| Enhancement after contrast | 42 (16.9) | 31 (14.0) | 11 (40.7) | <0.001 |
| Biliary ductal dilatation | 44 (17.7) | 36 (16.3) | 8 (29.6) | 0.087 |
| Primary cystic tumor | ||||
| Number of cystic lesions, mean (SD) | 1.3 (0.8) | 1.4 (0.8) | 1.5 (0.8) | 0.445 |
| Biliary cyst size (cm), median (IQR) | 10 (7–13) | 10 (7–12) | 10.5 (7–15) | 0.338 |
| Intrahepatic Location | ||||
| Left | 108 (43.5) | 98 (44.3) | 10 (37.0) | 0.033 |
| Right | 76 (30.6) | 62 (28.1) | 14(51.9) | |
| Both | 50 (20.2) | 48 (21.7) | 2 (7.4) |
Bold values highlight comparisons which reached statistical significance since p < .05.
Although most patients underwent an ultrasonography (58.5%), additional cross-sectional imaging was obtained in the overwhelming majority of patients (87.9%) (Fig. 1). In most instances, cross-sectional imaging involved CT alone (62.5%), whereas fewer patients underwent MRI alone (6.9%) or CT + MRI (18.5%). There were no differences in the type of cross-sectional imaging utilized among patients with BCA versus BCAC (all P > 0.05). Preoperative radiological examinations noted that BCTs were more often located in the left hemiliver (43.5%) compared with the right (30.6%) or central (20.2%) liver (P = 0.033). In the majority of cases, the lesion was solitary (73.4%); median size was 10 cm (IQR, 7–13 cm). The majority of BCTs were multilocular (56.9%) and had septations (65.7%), whereas fewer lesions had mural nodularity (16.5%), biliary ductal dilatation (17.7%), or calcification (10.1%).
FIGURE 1.
Example of a (A) coronal and (B) axial MRI depicting a large lobulated cystic mass with enhancing thick septations (arrow) and nodularity (asterisks) suspicious for biliary cystadenoma/cystadenocarcinoma. Final pathology revealed biliary cystadenoma.
Preoperative interventions included endoscopic retrograde pancreatography (16.1%), cyst aspiration (19.8%), sclerosis (2.0%), or fine-needle biopsy (FNB) (20.6%). Among all patients who underwent FNB (n = 52), 5 patients who had a BCAC on final pathology had a preoperative FNB that was read as nondiagnostic, consistent with BCA, or “benign cyst.” In contrast, FNB suggested adenocarcinoma in 6 patients of whom 5 later had BCAC confirmed on final pathology. As such, the sensitivity and specificity of FNB to detect BCAC accurately was 50% and 97.6%, respectively.
Treatment, Perioperative Outcomes, and Pathological Findings
Treatment characteristics and details of the surgical procedures are displayed in Table 2. Most patients underwent an open procedure (n = 191); 10 patients had an initial minimally invasive approach that was converted to a laparotomy, whereas 45 had a fully laparoscopic surgical approach; all laparoscopic procedures occurred after 2000. At the time of surgery, the operative intervention consisted of unroofing/fenestration (14.1%), less than a hemihepatectomy (48.8%) or hemi-/extended hepatectomy (36.3%). Compared with BCA, patients with a BCAC were less likely to undergo an unroofing/fenestration (15.4% vs 3.7%, respectively) and more likely to undergo a major hepatectomy (31.7% vs 74.1%, P < 0.001). Lymphadenectomy was performed in 6.4% of patients (BCA 2.7% vs BCAC 37.0%). The estimated median blood loss was 300 mL. There were no postoperative deaths within 90 days of surgery. A total of 62 patients (25.0%) experienced a postoperative complication. Most complications were minor (51.6%) grade I-II complications, whereas 45.2% of patients had more serious grade III-IV complications that required an intervention. Complications were more common following a major hepatectomy (39.8%) versus an unroofing/fenestration or a minor resection (13.6%) (P < 0.05). Overall length of stay was 6 days (IQR, 4–7).
TABLE 2.
Treatment, Pathological Findings, and Perioperative Outcomes
| Total (n = 248) | BCA (n = 221, 89.1%) | BCAC (n = 27, 10.9%) | P | |
|---|---|---|---|---|
| Operation | <0.001 | |||
| Unroofing/partial excision | 35 (14.1) | 34 (15.4) | 1 (3.7) | |
| Less than hemi-hepatectomy | 121 (48.8) | 116 (52.5) | 5 (18.5) | |
| Hemi-/extended hepatectomy | 90 (36.3) | 70 (31.7) | 20 (74.1) | |
| Liver transplant | 2 (0.8) | 1 (0.4) | 1 (3.7) | |
| Surgical approach (n = 247) | 0.008 | |||
| Laparoscopic | 45 (18.2) | 43 (19.5) | 2 (7.4) | |
| Hand-assisted | 1 (0.4) | 0 (0) | 1 (3.7) | |
| Laparoscopic converted to open | 10 (4.0) | 10 (4.6) | 0 (0) | |
| Open | 191 (77.4) | 167 (75.9) | 24 (88.9) | |
| Pathology | ||||
| Spindle cell/Ovarian-like stroma | 84 (33.9) | 76 (34.4) | 8 (29.6) | 0.655 |
| Epithelium | 0.330 | |||
| Mucinous | 147 (59.3) | 129 (58.4) | 18 (66.7) | |
| Serous | 54 (21.8) | 50 (22.6) | 4 (14.8) | |
| Margin, R0 (n = 209) | 187 (89.5) | 161 (88.5) | 26 (96.3) | 0.007 |
| Adjuvant chemotherapy | 7 (2.8) | 0 (0) | 7 (25.9) | <0.001 |
| Volume of blood loss, median (IQR), mL | 300 (150–700) | 300 (150–600) | 500 (300–1800) | 0.026 |
| Length of hospitalization, median (IQR), d | 6 (4–7) | 5 (4–7) | 7 (6–10) | 0.388 |
| Complication within 30 days | 62 (25.0) | 50 (22.6) | 12 (44.4) | 0.011 |
Bold values highlight comparisons which reached statistical significance since P < .05.
On final pathological assessment, most BCT lesions were noted to be BCA (89.1%), whereas 27 (10.9%) were BCAC. Among patients with BCA, 8.6% had evidence of atypia. Overall, roughly one third of all BCT pathological specimens had evidence of spindle cell/ovarian stroma (33.3%) with this pathological feature being much more common among female patients (P < 0.001). Among the 248 patients, pathological margin status was available on 209 patients (84.3%). While most patients (89.5%) had a negative surgical margin (R0), a subset was noted to have microscopic/macroscopic disease (R1/R2) (10.5%). Among patients who underwent a formal resection (eg, not an unroofing/fenestration), 94.6% of patients had an R0 margin and 5.4% had an R1/R2 margin.
Differentiating BCA From BCAC
Among the 248 patients with BCT, the ability of preoperative cross-sectional imaging to predict BCAC relative to BCA was assessed. Specifically, the presence of “high risk” features such as multinodularity, septa, mural nodule, solid component, papillary projection, calcification, hypervascularity, cyst wall enhancement after contrast injection, and biliary ductal dilatation were examined. On the basis of the presence of any one of these features, the sensitivity and specificity of preoperative cross-sectional imaging to predict BCAC was 81% and 21%, respectively; the positive and negative predictive values were 11% and 91%, respectively. In addition to radiologic features, certain clinical and tumor features, as well as laboratory values that had previously been proposed as a means to differentiate BCA from BCAC, were examined (Table 3).9,10 Age and sex were both associated with an increased risk of BCAC relative to BCA on univariate analyses. When assessed using ROC, the ability of tumor size to discriminate BCA from BCAC was poor (area under the curve [AUC] 0.532). Other factors such as age (AUC 0.647) as well as serum CEA (AUC 0.690) and CA19–9 (0.690) were only moderately good predictors of BCAC. In contrast, tumor location in the right hemiliver was more strongly associated with risk of BCAC relative BCA (OR 2.77, 95% CI 0.16–0.83; P = 0.01).
TABLE 3.
Statistical Analyses of Clinical Characteristics Between Biliary Cystadenoma and Cystadenocarcinoma
| Variable | BCA (n = 221, 89.1%) | BCAC (n = 27, 10.9%) | P |
|---|---|---|---|
| Age, yr | |||
| Mean (SD) | 51.2 (15.1) | 58.9 (13.3) | 0.386 |
| A1 ≤60 | 151 | 14 | 0.087 |
| A2 >60 | 70 | 13 | |
| A1’ ≤50 | 101 | 5 | 0.007 |
| A2’ >50 | 120 | 22 | |
| Gender | |||
| F | 198 | 16 | <0.001 |
| M | 22 | 11 | |
| CA 19–9 (n = 210) | |||
| Normal (<37) | 117 | 7 | 0.022 |
| Elevated | 67 | 19 | |
| CEA (n = 210) | |||
| Normal (<2.5) | 93 | 8 | 0.136 |
| Elevated | 88 | 21 | |
| Location | |||
| Left* | 146 | 12 | 0.014 |
| Non Left | 62 | 14 | |
| Size | |||
| Median (IQR) | 10 (7–12) | 10.5 (7–15) | 0.056 |
| ≤8 cm | 83 | 11 | 0.747 |
| >8 cm | 138 | 16 | |
| ≤11 cm | 76 | 9 | 0.913 |
| >11 cm | 145 | 18 |
Bold values highlight comparisons which reached statistical significance since p < .05.
Includes patients who had disease in both left and right hemiliver.
Prognosis: RFS and OS
Among the 248 patients, there were 46 (18.5%) recurrences. Median RFS was 12.1 years (95% CI 6.5–18.1), whereas 1-year, 3-year, and 5-year RFS was 89.1%, 72.6%, and 61.4%, respectively (Fig. 2). An increased likelihood of recurrence was associated with the type of surgical procedure performed. Specifically, the incidence of recurrence was 48.6% among patients who underwent an unroofing/fenestration versus 15.7% and 10%, respectively, for patients who had a partial or major hepatectomy (P < 0.001). Among the patients who underwent an unroofing/fenestration, 16 of 35 (45.6%) patients with BCA and the 1 patient with BCAC (100%) recurred. As most unroofing/fenestration procedures were performed laparoscopically, a minimally invasive approach was also noted to be associated with a higher proportion of recurrences (laparoscopic 33.3% vs open 14.7%; P = 0.033). The incidence of recurrence was 9.5% among patients with an R0 resection versus 59.1% among patients with an R1/R2 resection. Among the subset of patients who had an R0 laparoscopic resection, the overall recurrence was 20.7% (BCA: 14.8%; BCAC: 100%). Of note, the overall incidence of recurrence was generally higher among patients with BCAC (33.3%) versus BCA (16.7%) (P = 0.04), regardless of the procedure performed. The other factor associated with risk of recurrence was presence of spindle cell/ovarian stroma. In fact, patients with spindle cell/ovarian stroma had a recurrence rate of 26.3% versus only 10.7% for patients who did not have this pathological feature (P = 0.003). Of note, 2 patients (0.9%) who initially had BCA recurred with BCAC; these 2 patients had undergone a left hepatectomy and an unroofing/fenestration, respectively.
FIGURE 2.
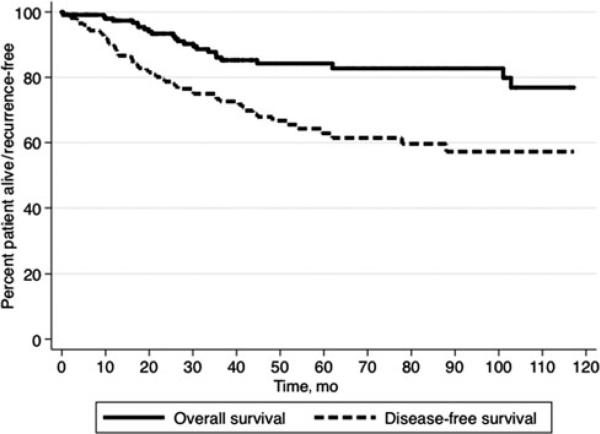
In examining the entire cohort, after surgical resection, the median RFS was 12.1 years (95% CI 6.5–18.1), whereas 1-year, 3-year, and 5-year RFS was 89.1%, 72.6%, and 61.4%, respectively. Median OS was not reached; 1-year, 3-year, and 5-year OS was 95.0%, 86.8%, and 84.2%, respectively.
In examining the entire cohort, median OS was not reached; 1-year, 3-year, and 5-year OS was 95.0%, 86.8%, and 84.2%, respectively. Median OS of patients with BCA was not reached versus 8.4 years for patients with BCAC (HR 3.91, P = 0.01) (Fig. 3). Among patients with BCAC, the presence of spindle cell/ovarian stroma was also associated with survival (median survival: spindle cell/ovarian stroma, 8.3 years vs no spindle cell/ovarian stroma, not reached; P = 0.003). On univariate and multivariate analyses, no other factor was associated with long-term prognosis (Table 4).
FIGURE 3.
After excision, long-term outcomes were better among patients with BCA versus BCAC. Specifically, median OS of patients with BCA was not reached versus 8.4 years for patients with BCAC (HR 3.91, P = 0.01).
TABLE 4.
Cox Regression Analyses of Variables Associated With OS in Patients With Biliary Cystadenoma and Cystadenocarcinoma
| Variable | Univariate Analysis |
Multivariate Analysis |
||||
|---|---|---|---|---|---|---|
| Prognostic Factor | HR | 95% CI | P | HR | 95% CI | P |
| Male sex | 2.29 | 1.06–4.95 | 0.04 | 1.49 | 0.54–4.06 | 0.44 |
| Patient symptomatic | 2.34 | 0.70–7.76 | 0.12 | 2.20 | 0.44–10.92 | 0.34 |
| Preoperative total bilirubin | 1.00 | 0.84–1.20 | 0.99 | — | — | — |
| Elevated serum CA 19–9 (≥37) | 5.00 | 0.67–37.02 | 0.12 | 2.82 | 0.35–22.46 | 0.33 |
| Elevated serum CEA (≥2.5) | 4.69 | 0.63–34.67 | 0.13 | 2.24 | 0.28–17.89 | 0.45 |
| Preoperative image finding | 0.82 | 0.38–1.77 | 0.61 | — | — | — |
| Multilocular cyst | 2.15 | 0.79–5.91 | 0.12 | |||
| Septa | 0.88 | 0.43–1.79 | 0.72 | |||
| Mural nodule/solid component/papillary projection | 4.20 | 1.87–9.39 | 0.001 | |||
| Calcification | 3.13 | 1.02–9.61 | 0.07 | |||
| Hypervascular | 3.34 | 1.08–10.36 | 0.06 | |||
| Cyst wall enhancement | 2.60 | 0.96–7.10 | 0.08 | |||
| Biliary ductal dilatation | 0.88 | 0.34–2.28 | 0.79 | — | ||
| Tumor type (BCA vs BCAC) | 5.95 | 2.66–13.3 | <0.001 | 3.91 | 1.39–11.02 | 0.01 |
| Number of cystic lesions | 0.77 | 0.40–1.49 | 0.43 | — | — | — |
| Size of largest cyst | 1.04 | 0.98–1.10 | 0.16 | 1.01 | 0.96–1.07 | 0.63 |
| The left lobe involved | 1.25 | 0.59–2.64 | 0.56 | — | — | — |
| Spindle cell/ovarian like stroma | 1.10 | 0.52–2.36 | 0.80 | — | — | — |
| Margin | 1.28 | 0.38–4.33 | 0.70 | — | — | — |
| Type of operative management | ||||||
| Unroofing/partial excision | — | Reference | — | — | — | |
| Less than hemihepatectomy | 0.59 | 0.16–2.20 | 0.43 | — | — | — |
| Hemi-/extended hepatectomy | 1.88 | 0.54–6.57 | 0.33 | — | — | — |
| Complications within 30 days | 2.21 | 1.06–4.63 | 0.04 | 1.34 | 0.56–3.22 | 0.51 |
| Length of hospitalization | 1.01 | 0.99–1.02 | 0.26 | — | — | — |
Of the 46 patients who recurred, 33 (78.6%) underwent repeat resection. Repeat surgery involved partial hepatic resection (33.3%) or hemi-/extended hepatic resection (66.7%). At last follow-up, 26 of these 46 patients (56.5%) were free of disease with a median follow-up of 18.5 months from the time of the second surgery.
DISCUSSION
Hepatic cysts are diagnosed with increasing frequency, and a subset of these lesions will be potentially categorized as BCT. Although these lesions may represent premalignant (BCA) or frank malignancy (BCAC), data on the presentation, management, and prognosis of BCT are scarce. In fact, the available data are solely limited to single-center case reports or case series.2,6,9,10,12,14,18,21,22 Furthermore, data on the long-term outcomes of patents with BCT after surgery remain poorly defined. Although OS data have been reported, information on overall recurrence and factors associated with recurrence have not been reported. The current study is valuable because we examined a large, multi-institutional cohort of patients with BCT and identified certain factors that influenced RFS and OS. Specifically, we noted that OS was 84.2%. Perhaps more interestingly, we found that recurrence following surgical resection of BCT was relatively high—with nearly 1 in 5 patients experiencing a recurrence (18.4%). Furthermore, we identified certain factors to be associated with the risk of recurrence including operative intervention (ie, unroofing/fenestration vs formal hepatic resection) and pathological findings (ie, presence vs absence of spindle cell/ovarian stroma). In addition, while male sex was associated with the risk of BCAC, no specific radiographic marker could reliably differentiate BCA from BCAC.
Data from the current study confirms the strong predilection of BCT to occur in middle-aged women. However, it is important to note that both BCA and BCAC occurred in men. Other investigators have suggested that a BCT in a male patient is associated with a higher possibility of BCAC relative to BCA.10 We corroborated this finding as male sex was associated with an increased risk of BCAC in our study (Table 3). In their report on 20 cases of BCA and 10 cases of BCAC, Wang and colleagues also suggested that patient age and tumor size were helpful in discriminating BCA from BCAC. However, in our much larger cohort of patients, we were unable to validate these findings. In fact, we noted that tumor size had a poor ability to distinguish BCAC from BCA, whereas elevation of tumor markers such as CA19–9 was only moderately good. Other investigators have suggested that certain radiological features such as a mural nodule, solid component, or hypervascularity were helpful in distinguishing BCAC from BCA.9,10 Interestingly, we found that the presence of these features was associated with a high negative predictive value (91%), but a low positive predictive value. In other words, the absence of all these features strongly suggested BCA rather than BCAC; however, the presence of any one of these features did not necessarily imply that the lesion would indeed be BCAC. It is also important to note that a subset of patients underwent either CT or MRI alone, which may not have allowed for full evaluation of the lesion. For example, calcifications when present are often thin and septal and therefore may be missed by MRI. Given the challenges in diagnosing BCT preoperatively, it may be a prudent approach to perform a frozen section examination at the time of performing an unroofing/fenestration for a presumed simple hepatic cyst to exclude BCT.23
Although patients with BCT had a long-term 5-year survival of almost 85%, the recurrence rate was relatively high at 18.4%. Not surprisingly, recurrence was considerably higher among patients who underwent an unroofing/fenestration compared to a formal hepatic resection. Other authors have noted that the potential for curative treatment is strongly associated with complete removal of the BCT lesion.2,8,9,24 Although in the current series, a laparoscopic approach was also associated with recurrence, this was undoubtedly because of the fact that most laparoscopic procedures involved incomplete excision of the lesion with an unroofing/fenestration. Although recurrence can often be treated with an additional operation, it is also important to note that 2 patients who initially had BCA recurred with BCAC. Given the worse prognosis associated with BCAC (Fig. 3), the chance of recurrence should obviously be avoided, or at least minimized to the greatest extent possible. As such, on the basis of our data in conjunction with other reports, complete excision is mandatory for BCT lesions regardless of operative approach.
The other factor associated with risk of recurrence and worse outcome was the presence of spindle cell/ovarian stroma. In fact, patients with spindle cell/ovarian stroma had a 2-fold higher incidence of recurrence compared with patients who did not have this pathological feature. Although the presence of spindle cell/ovarian stroma was initially believed by some investigators to be an essential feature of BCA and BCAC tumors, it has been recognized by others that this is not necessarily the case. For example, in the series reported by Devaney and colleagues,8 15% to 30% of BCA/BCAC lesions had evidence of ovarian-like stroma. In the current study, we similarly noted that about one-third of patients had this pathological feature. However, unlike the Devaney series, which noted that this distinctive feature occurred only in female patients, we found that a subset of specimens from male patients had evidence of ovarian-like stroma—although the feature was much more common among female patients. Others have pointed out that the ovarian-like stroma found in BCTs is similar in appearance to the stromal cells seen in the developing hepatobiliary tract and thus its presence is not necessarily restricted to female patients.8,25,26
This study had several limitations. Despite being the largest series of BCTs to be reported in the literature, the current study still had a relatively small sample size. For example, there were only 27 BCAC cases. The current series was, however, several fold larger than any previously published study on BCT. Although the multi-institutional study design offered the benefits of higher statistical power and generalizability of the results, collaborating with multiple institutions did limit the ability to easily standardize all diagnostic and treatment criteria. For example, all pathological specimens were not reviewed centrally. This may explain in part the disparate incidence of ovarian stroma and serous findings that we report compared with previous studies.4 There are several reasons why ovarian stroma might have been underreported including that it is notoriously focal, that BCA tend to be large tumors and complete histological analysis of the entire surface area is often not performed, as well as the fact that ovarian stroma can be difficult to recognize and immunohistochemical markers have not been widely used or known until the last 10 years. The reasons for “serous” to appear in the pathology report are also multifactorial. Normal biliary epithelium varies from low cuboidal in the periphery to columnar in the hilum. The cells in the cyst can show similar variation across cases and cause an observer to only refer to the columnar epithelial versions as mucinous and those with low cuboidal epithelium as serous because the mucin is less evident in low cuboidal biliary epithelium. Many observers also use a mucin stain to demonstrate cellular mucin in the apical region of the epithelium; however, secondary changes in the cyst such as epithelial sloughing and focal flattening could account for an erroneously negative mucin stain. In such cases, it is often assumed that a negative mucin stain must mean that the lesion is serous. In addition, pyloric and foveolar metaplasia is common and can be misinterpreted as a type of serous epithelium because it contains a mucin that is a different, lighter color on routine stains and is reactive at a different pH on mucin stains than the more common intestinal type of mucin.
CONCLUSIONS
BCTs most commonly presented as large cysts in women, although about 15% of patients were male. Although underlying malignancy (BCAC) was uncommon (≈ 10%) among patients who underwent surgery for a BCT, it did appear that male patients with a BCT were more likely to have a BCAC versus a BCA. Other clinical (age, tumor size) and radiological (nodularity, solid component, hypervascularity) factors did not reliably predict underlying BCAC. After excision of BCA, long-term outcomes were good; however, patients with BCAC had a worse long-term prognosis. In addition, recurrence occurred in up to 1 in 5 patients and nearly half of patients who had an incomplete excision experienced a recurrence. As such, surgeons should routinely seek complete excision of the lesion during the initial procedure. In addition, long-term surveillance of all patients—both BCA and BCAC—is required because of the risk of recurrence.
ACKNOWLEDGMENTS
The authors thank Donielle Neal for her help in the preparation of the manuscript.
Footnotes
Disclosure: The authors declare no conflicts of interest with respect to the authorship and/or publication of this article. The authors received no financial support for the research and/or authorship of this article.
REFERENCES
- 1.Carrim ZI, Murchison JT. The prevalence of simple renal and hepatic cysts detected by spiral computed tomography. Clin Radiol. 2003;58:626–629. doi: 10.1016/s0009-9260(03)00165-x. [DOI] [PubMed] [Google Scholar]
- 2.Vogt DP, Henderson JM, Chmielewski E. Cystadenoma and cystadenocarcinoma of the liver: a single center experience. J Am Coll Surg. 2005;200:727–733. doi: 10.1016/j.jamcollsurg.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Walt AJ. Cysts and benign tumors of the liver. Surg Clin North Am. 1977;57:449–464. doi: 10.1016/s0039-6109(16)41194-1. [DOI] [PubMed] [Google Scholar]
- 4.Soares KC, Arnaoutakis DJ, Kamel I, et al. Cystic neoplasms of the liver: biliary cystadenoma and cystadenocarcinoma. J Am Coll Surg. 2014;218:119–128. doi: 10.1016/j.jamcollsurg.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henson SW, Jr, Gray HK, Dockerty MB. Benign tumors of the liver. VI. Multilocular cystadenomas. Surg Gynecol Obstet. 1957;104:551–554. [PubMed] [Google Scholar]
- 6.Short WF, Nedwich A, Levy HA, et al. Biliary cystadenoma. Report of a case and review of the literature. Arch Surg. 1971;102:78–80. doi: 10.1001/archsurg.1971.01350010080021. [DOI] [PubMed] [Google Scholar]
- 7.Edmondson H. Atlas of Tumour Pathology: Tumors of the Liver and Intrahepatic Bile Ducts. Armed Forces Institute of Pathology; Washington, DC: 1958. [Google Scholar]
- 8.Devaney K, Goodman ZD, Ishak KG. Hepatobiliary cystadenoma and cystadenocarcinoma. A light microscopic and immunohistochemical study of 70 patients. Am J Surg Pathol. 1994;18:1078–1091. [PubMed] [Google Scholar]
- 9.Teoh AY, Ng SS, Lee KF, et al. Biliary cystadenoma and other complicated cystic lesions of the liver: diagnostic and therapeutic challenges. World J Surg. 2006;30:1560–1566. doi: 10.1007/s00268-005-0461-7. [DOI] [PubMed] [Google Scholar]
- 10.Wang C, Miao R, Liu H, et al. Intrahepatic biliary cystadenoma and cystadenocarcinoma: an experience of 30 cases. Dig Liver Dis. 2012;44:426–431. doi: 10.1016/j.dld.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Xu HX. Contrast-enhanced ultrasound in the biliary system: potential uses and indications. World J Radiol. 2009;1:37–44. doi: 10.4329/wjr.v1.i1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu FC, Chen JH, Yang KC, et al. Hepatobiliary cystadenoma: a report of two cases. J Gastrointestin Liver Dis. 2008;17:203–206. [PubMed] [Google Scholar]
- 13.Xu HX, Lu MD, Liu LN, et al. Imaging features of intrahepatic biliary cystadenoma and cystadenocarcinoma on B-mode and contrast-enhanced ultrasound. Ultraschall Med. 2012;33:E241–E249. doi: 10.1055/s-0031-1299276. [DOI] [PubMed] [Google Scholar]
- 14.Korobkin M, Stephens DH, Lee JK, et al. Biliary cystadenoma and cystadenocarcinoma: CT and sonographic findings. AJR Am J Roentgenol. 1989;153:507–511. doi: 10.2214/ajr.153.3.507. [DOI] [PubMed] [Google Scholar]
- 15.Buetow PC, Buck JL, Pantongrag-Brown L, et al. Biliary cystadenoma and cystadenocarcinoma: clinical-imaging-pathologic correlations with emphasis on the importance of ovarian stroma. Radiology. 1995;196:805–810. doi: 10.1148/radiology.196.3.7644647. [DOI] [PubMed] [Google Scholar]
- 16.Emre A, Serin KR, Ozden I, et al. Intrahepatic biliary cystic neoplasms: surgical results of 9 patients and literature review. World J Gastroenterol. 2011;17:361–365. doi: 10.3748/wjg.v17.i3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akwari OE, Tucker A, Seigler HF, et al. Hepatobiliary cystadenoma with mesenchymal stroma. Ann Surg. 1990;211:18–27. doi: 10.1097/00000658-199001000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koffron A, Rao S, Ferrario M, et al. Intrahepatic biliary cystadenoma: role of cyst fluid analysis and surgical management in the laparoscopic era. Surgery. 2004;136:926–936. doi: 10.1016/j.surg.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 19.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayo SC, Shore AD, Nathan H, et al. Refining the definition of perioperative mortality following hepatectomy using death within 90 days as the standard criterion. HPB (Oxford) 2011;13:473–482. doi: 10.1111/j.1477-2574.2011.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsiftsis D, Christodoulakis M, de Bree E, et al. Primary intrahepatic biliary cystadenomatous tumors. J Surg Oncol. 1997;64:341–346. doi: 10.1002/(sici)1096-9098(199704)64:4<341::aid-jso17>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 22.Kubota E, Katsumi K, Iida M, et al. Biliary cystadenocarcinoma followed up as benign cystadenoma for 10 years. J Gastroenterol. 2003;38:278–282. doi: 10.1007/s005350300048. [DOI] [PubMed] [Google Scholar]
- 23.Simo KA, McKillop IH, Ahrens WA, et al. Invasive biliary mucinous cystic neoplasm: a review. HPB (Oxford) 2012;14:725–740. doi: 10.1111/j.1477-2574.2012.00532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sang X, Sun Y, Mao Y, et al. Hepatobiliary cystadenomas and cystadenocarcinomas: a report of 33 cases. Liver Int. 2011;31:1337–1344. doi: 10.1111/j.1478-3231.2011.02560.x. [DOI] [PubMed] [Google Scholar]
- 25.Compagno J, Oertel JE. Mucinous cystic neoplasms of the pancreas with overt and latent malignancy (cystadenocarcinoma and cystadenoma). A clinicopathologic study of 41 cases. Am J Clin Pathol. 1978;69:573–580. doi: 10.1093/ajcp/69.6.573. [DOI] [PubMed] [Google Scholar]
- 26.Soini Y, Autio-Harmainen H, Miettinen M. Immunoreactivity for laminin and type IV collagen in malignant and benign fibrous histiocytoma. J Pathol. 1989;158:223–228. doi: 10.1002/path.1711580309. [DOI] [PubMed] [Google Scholar]