Abstract
A systematic review of the literature on the management of peritoneal carcinomatosis (PC) from colon cancer with cytoreductive surgery (CRS) and intraperitoneal chemotherapy (IPC) was undertaken using OVID Medline. Forty-six relevant studies were reviewed. Mean weighted overall morbidity following CRS and IPC was 49% (range 22–76%) and mortality was 3.6% (range 0–19%). Median overall survival ranged from 15 to 63 months, and 5-year overall survival ranged from 7 to 100%. This represents an improvement over historical treatment with systemic chemotherapy alone, even in the era of modern chemotherapeutic agents. Quality of life following surgery is initially decreased but improves with time and approaches baseline. Available data appear to support the treatment of PC from colon cancer with CRS and IPC. There is a large amount of variability among studies and few high-quality studies exist. Further studies are needed to standardize techniques.
Keywords: colorectal cancer, carcinomatosis, intraperitoneal chemotherapy, outcomes
Colon cancer presents with synchronous peritoneal spread in 5 to 10% of patients, and up to 20 to 50% of patients with recurrent disease will develop metachronous peritoneal disease.1 2 3 4 Peritoneal carcinomatosis (PC) from colon cancer has traditionally been viewed as distant metastatic disease, with only a 12-month median survival even with systemic chemotherapy.5 Long-term survival or cure in such cases was essentially unheard of. With advances in systemic chemotherapy, median survivals of 15 to 24 months have been achieved.6 7 8 Nevertheless, cure does not appear to be attainable with systemic treatments alone.
A paradigm shift occurred when peritoneal disease was viewed as regional disease rather than diffuse metastatic disease, analogous to colorectal liver metastases in which local treatment can lead to long-term survival and even cure.9 Cytoreductive surgery (CRS) and intraperitoneal chemotherapy (IPC) have been used in the setting of other peritoneal malignancies for such purpose with promising results. These techniques have been implemented in the management of PC secondary to colon cancer.
This review aims to summarize the current evidence for CRS and IPC in the management of PC from colon cancer.
Methods
A systematic literature search was performed using OVID Medline. The following medical subject terms and keywords and their combinations were used: Peritoneal Neoplasms (MeSH) (subheadings therapy, surgery, secondary, drug therapy), AND Colorectal Neoplasms (Mesh) (subheadings drug therapy, surgery, therapy, pathology), AND keywords cytoreductive OR cytoreduction, AND hyperthermia OR hyperthermic OR IPC. The search was limited to English language studies.
A total of 217 articles were identified. The titles and abstracts from the initial search were identified and reviewed for relevance. Review articles, editorials, and case reports were excluded. Studies were also excluded if most patients in the study had a primary malignancy other than colorectal cancer, namely those of appendiceal origin, as those were outside the scope of this review. Four full-text articles were unavailable and were excluded. Articles prior to the year 2000 were excluded to ensure contemporary data. Articles focusing on liver metastases in addition to PC were included and analyzed separately.
Weighted means were calculated for morbidity, and mortality and were weighted sample size.
Definitions
Cytoreductive Surgery
CRS for peritoneal disease refers to the surgical extirpation of all visible intraperitoneal tumor deposits. To accomplish this, the involved peritoneum is stripped and visceral resections may be performed. The procedure has been previously described in detail by Sugarbaker.10
Intraperitoneal Chemotherapy
IPC refers to the administration of chemotherapy directly into the peritoneal cavity. This can be performed intra- and/or postoperatively through a variety of techniques described as follows:
Hyperthermic Intraperitoneal Chemotherapy (HIPEC): This technique refers to the administration of a heated chemotherapeutic agent to the peritoneal cavity intraoperatively, generally following complete CRS.
Early Postoperative Intraperitoneal Chemotherapy (EPIC): This technique refers to the administration of a chemotherapeutic agent to the peritoneal cavity in the immediate postoperative period via an intraperitoneal catheter. It can be used alone or in combination with HIPEC.
Sequential Postoperative Intraperitoneal Chemotherapy (SPIC): This technique refers to the administration of a chemotherapeutic agent to the peritoneal cavity in repeated cycles as an adjuvant treatment.
Peritoneal Cancer Index
The Peritoneal Cancer Index (PCI) is the most accepted metric to quantify the extent of peritoneal disease. It is most accurately assessed at the time of surgery, as the sensitivity in detecting peritoneal disease by computed tomographic (CT) scan has been shown to be 41.1% and the specificity 89%.11 PCI is calculated by evaluating the size of peritoneal lesions in each of 13 abdominopelvic regions.12 Lesion size (LS) is scored in each of the 13 regions (Fig. 1) and summed to yield a score from 0 to 39.10
Fig. 1.
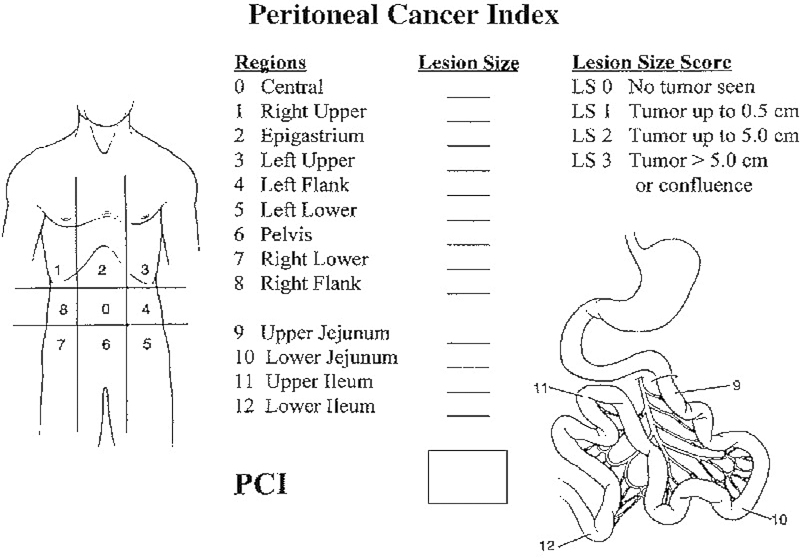
Peritoneal Cancer Index (PCI) calculation for patients with peritoneal carcinomatosis.
Completeness of Cytoreduction
The completeness of cytoreduction (CC) score provides an assessment of the amount of disease remaining after CRS. CC0 indicates that no macroscopic disease remains at the end of an operation. CC1 indicates that tumor nodules less than 2.5 mm in greatest diameter remain at the end of an operation. CC2 indicates that tumor nodules between 2.5 mm and 2.5 cm in greatest diameter remain. CC3 indicates that tumor nodules greater than 2.5 cm in greatest diameter remain.
The CC score is more commonly used in PC than the R-status that is traditionally used for primary malignancies. In PC, it is generally believed that an R0 status cannot be achieved, and therefore CC0 is equivalent to R1 (no gross residual disease). R2a indicates that minimal tumor nodules less than 5 mm remain. R2b indicates that gross tumor nodules greater than 5 mm and up to 2 cm remain. R2c indicates that extensive disease over 2 cm remains.
The definition of complete cytoreduction varies among studies. It includes CC0 and R0 but may also include CC1, R1, and/or R2a.
Results
A total of 217 articles were identified. Following title and abstract review for exclusion criteria, a total of 46 articles were reviewed. Forty-three articles were included focusing on PC secondary to colon cancer, whereas three articles focused on patients with both PC and liver metastases secondary to colon cancer. The studies addressing liver metastases were reviewed separately below. Few level 1 or 2 studies (according to the Oxford Centre for Evidence-Based Medicine classification of levels of evidence)13 have been undertaken and there is extensive variation among studies in terms of study design, patient selection, and operative and adjuvant treatments. Approximately 80% of the reviewed studies provide only level 4 evidence and consist of mainly retrospective case series. Patients range in terms of age, extent of disease, and anticipated treatment goals. Treatment options described included systemic chemotherapy, CRS, HIPEC, EPIC, SPIC, or combinations of these. The patient and treatment details from the reviewed studies are summarized in Table 1. The level of evidence of each study is also indicated. Overall outcomes and those specific to higher-quality studies are discussed in the following text.
Table 1. Summary of studies of patients with peritoneal carcinomatosis secondary to colon cancer treated with CRS and/or IPC identified from systematic review.
| Author | Year | n | Level of evidence | Groups | Type of IPC | Agent for IPC | |
|---|---|---|---|---|---|---|---|
| 1 | Pestieau and Sugarbaker33 | 2000 | 104 | 4 | HIPEC | MMC | |
| 2 | Elias et al34 | 2001 | 64 | 4 | HIPEC and EPIC | MMC ± cisplatin for HIPEC, MMC and 5-FU for EPIC | |
| 3 | Pilati et al35 | 2003 | 34 | 4 | HIPEC | MMC and cisplatin | |
| 4 | Verwaal et al5 | 2003 | 105 | 1b | 51 control | HIPEC vs. none | MMC |
| 54 experimental | |||||||
| 5 | Elias et al21 | 2004 | 35 | 2b | 16 EPIC group | EPIC vs. none | MMC and 5-FU |
| 19 non-EPIC group | |||||||
| 6 | Glehen et al36 | 2004 | 53 | 4 | HIPEC | MMC | |
| 7 | Glehen et al37 | 2004 | 506 | 4 | HIPEC (53.5%), EPIC (24.3%), or both (22.2%) | ||
| 8 | Shen et al38 | 2004 | 77 | 4 | HIPEC | MMC | |
| 9 | Verwaal et al39 | 2004 | 102 | 4 | HIPEC | MMC | |
| 10 | Kecmanovic et al 40 | 2005 | 18 | 4 | HIPEC and EPIC | MMC for HIPEC, 5-FU for EPIC | |
| 11 | Verwaal et al41 | 2005 | 117 | 4 | HIPEC | MMC | |
| 12 | Cavaliere et al42 | 2006 | 120 | 4 | HIPEC | MMC and cisplatin or oxaliplatin and 5-FU and leucovorin | |
| 13 | da Silva and Sugarbekar43 | 2006 | 70 | 4 | HIPEC and/or EPIC | MMC for HIPEC, 5-FU ± MMC for EPIC | |
| 14 | Füzün et al44 | 2006 | 29 | 4 | HIPEC and EPIC | 5FU for HIPEC, 5-FU for EPIC | |
| 15 | Zanon et al45 | 2006 | 25 | 4 | HIPEC | MMC | |
| 16 | Bijelic et al46 | 2007 | 49 | 4 | HIPEC and/or EPIC | MMC for HIPEC, 5-FU ± MMC EPIC | |
| 17 | Elias et al15 | 2007 | 46 | 3b | 23 HIPEC | HIPEC vs. EPIC | oxaliplatin and 5-FU and leucovorin for HIPEC, MMC and 5FU for EPIC |
| 23 EPIC | |||||||
| 18 | Piso et al47 | 2007 | 32 | 4 | HIPEC and EPIC | MMC and doxorubicin for HIPEC, 5-FU for EPIC | |
| 19 | Franko et al48 | 2008 | 65 | 4 | HIPEC | MMC | |
| 20 | Verwaal et al49 | 2008 | 105 | 1b | 51 control | HIPEC vs. none | MMC |
| 54 experimental | |||||||
| 21 | Yan and Morris50 | 2008 | 50 | 4 | HIPEC and EPIC | MMC for HIPEC, 5-FU for EPIC | |
| 22 | Elias et al7 | 2009 | 3b | 48 HIPEC | HIPEC vs. none | Oxaliplatin and 5-FU and leucovorin | |
| 48 control | |||||||
| 23 | Pelz et al51 | 2009 | 40 | 4 | HIPEC | MMC | |
| 24 | Swellengrebel et al52 | 2009 | 92 | 4 | HIPEC | ||
| 25 | Bretcha-Boix et al53 | 2010 | 20 | 4 | HIPEC and EPIC | MMC or oxaliplatin and 5-FU for HIPEC, 5-FU for EPIC | |
| 26 | Chua et al54 | 2010 | 56 | 4 | HIPEC and EPIC (59%) | MMC | |
| 27 | Elias et al55 | 2010 | 523 | 4 | HIPEC (84%) or EPIC (16%) | MMC ± cisplatin or oxaliplatin ± irinotecan and 5-FU and leucovorin for HIPEC and MMC and 5-FU for EPIC | |
| 28 | Franko et al8 | 2010 | 3b | 67 HIPEC | HIPEC versus none | MMC | |
| 29 | Saxena et al56 | 2010 | 63 | 4 | HIPEC (19%), EPIC (27%), or both (54%) | MMC for HIPEC, 5-FU for EPIC | |
| 30 | Vaira et al57 | 2010 | 40 | 4 | HIPEC | MMC ± cisplatinum or oxaliplatin and 5-FU | |
| 31 | Cavaliere et al58 | 2011 | 146 | 4 | HIPEC | Cisplatin ± MMC or oxaliplatin and 5-FU and leucovorin | |
| 32 | Chua et al59 | 2011 | 110 | 4 | HIPEC (50%), EPIC (17%), or both (33%) | ||
| 33 | Hill et al22 | 2011 | 62 | 4 | HIPEC | MMC | |
| 34 | Klaver et al60 | 2011 | 21 | 4 | HIPEC (50%), EPIC (25%), or both (21%) | 5-FU for EPIC | |
| 35 | Quenet et al19 | 2011 | 146 | 2b | 103 oxaliplatin/irinotecan | HIPEC | Oxaliplatin ± irinotecan and 5-FU and leucovorin |
| 43 oxaliplatin | |||||||
| 36 | Stojadinovic et al61 | 2011 | 53 | 4 | HIPEC | MMC | |
| 37 | Cashin et al14 | 2012 | 151 | 4 | 69 HIPEC | HIPEC vs. SPIC (or none) | MMC ± 5-FU or oxaliplatin and irinotecan for HIPEC, 5-FU and leucovorin for SPIC |
| 57 SPIC | |||||||
| 38 | Cashin et al62 | 2012 | 32 | 3b | 16 HIPEC | HIPEC ± EPIC (56%) versus SPIC | oxaliplatin for HIPEC, 5-FU and leucovorin for EPIC |
| 16 SPIC | |||||||
| 39 | Hompes et al63 | 2012 | 48 | 4 | HIPEC | oxaliplatin and 5FU | |
| 40 | Klaver et al64 | 2012 | 24 | 4 | HIPEC (50%), EPIC (25%), or both (20.8%) | MMC or oxaliplatin for HIPEC, 5FU for EPIC | |
| 41 | Passot et al65 | 2012 | 120 | 4 | HIPEC | MMC ± irinotecan or oxaliplatin | |
| 42 | Goéré et al66 | 2013 | 107 | 4 | HIPEC (72%) or EPIC (28%) | oxaliplatin +/− irinotecan and 5-FU and leucovorin for HIPEC, MMC and 5-FU or cisplatin and doxorubicin for EPIC | |
| 43 | Yonemura et al67 | 2013 | 142 | 4 | HIPEC | MMC and cisplatin |
Abbreviations: CRS, cytoreductive surgery; EPIC, early postoperative intraperitoneal chemotherapy; 5-FU, 5-fluorouracil; HIPEC, hyperthermic intraperitoneal chemotherapy; IPC, intraperitoneal chemotherapy; MMC, Mitomycin C; SPIC, sequential postoperative intraperitoneal chemotherapy.
Short-Term Outcomes
Overall morbidity following CRS and IPC ranged from 22 to 76% with a weighted mean of 49%. Major morbidity (including only studies reporting severe and grades 3–4 toxicity) ranged from 18 to 51% with a weighted mean of 24%. Perioperative mortality ranged from 0 to 19% with a weighted mean of 3.6%. The mortality and morbidity outcomes from the reviewed studies are summarized in Table 2.
Table 2. Mortality and morbidity of patients with peritoneal carcinomatosis secondary to colon cancer treated with CRS and/or IPC identified from systematic review.
| Author | Subgroup | Mortality (%) | Morbidity (%) | Grades 1–2 morbidity | Grades 3–4 morbidity | |
|---|---|---|---|---|---|---|
| 1 | Pestieau and Sugarbaker33 | |||||
| 2 | Elias et al34 | 9.3 | 65.6 | |||
| 3 | Pilati et al35 | 0 | 35 | |||
| 4 | Verwaal et al5 | 8 | ||||
| 5 | Elias et al21 | EPIC | 18.8 | |||
| 6 | Glehen et al36 | 4 | 23 | |||
| 7 | Glehen et al37 | 4 | 22.9 | |||
| 8 | Shen et al38 | 12 | 30 | |||
| 9 | Verwaal et al39 | |||||
| 10 | Kecmanovic et al40 | 0 | 44.4 | |||
| 11 | Verwaal et al41 | 6 | ||||
| 12 | Cavaliere et al42 | 22.5 | ||||
| 13 | da Silva and Sugarbekar43 | |||||
| 14 | Füzün et al44 | 0 | 41 | |||
| 15 | Zanon et al45 | 4 | 24 | |||
| 16 | Bijelic et al46 | |||||
| 17 | Elias et al15 | EPIC | 8.7 | 56.5 | ||
| HIPEC | 0 | 47.8 | ||||
| 18 | Piso et al47 | 0 | 34 | |||
| 19 | Franko et al48 | 1 | 60 | |||
| 20 | Verwaal et al49 | HIPEC | 7.4 | |||
| 21 | Yan and Morris50 | 0 | 76 | 46 | 18 | |
| 22 | Elias et al7 | |||||
| 23 | Pelz et al51 | |||||
| 24 | Swellengrebel et al52 | |||||
| 25 | Bretcha-Boix et al53 | 2.5 | 40 (grades 2–4) | |||
| 26 | Chua et al54 | |||||
| 27 | Elias et al55 | 3.3 | 31 | |||
| 28 | Franko et al8 | |||||
| 29 | Saxena et al56 | 0 | 52 | 31 | ||
| 30 | Vaira et al57 | 2.5 | 55 | |||
| 31 | Cavaliere et al58 | 2.7 | 27.4 | |||
| 32 | Chua et al59 | |||||
| 33 | Hill et al22 | 48 | ||||
| 34 | Klaver et al60 | |||||
| 35 | Quenet et al19 | All patients | 4.1 | 47.2 | ||
| Oxaliplatin | 2.3 | 34.9 | ||||
| Oxaliplatin/irinotecan | 4.9 | 52.4 | ||||
| 36 | Stojadinovic et al61 | |||||
| 37 | Cashin et al14 | HIPEC | 3 | 28 | ||
| SPIC | 2 | 17 | ||||
| 38 | Cashin et al62 | HIPEC | 6 | 37 | ||
| SPIC | 6 | 19 | ||||
| 39 | Hompes et al63 | 0 | 52.1 | |||
| 40 | Klaver et al64 | 0 | 62 | |||
| 41 | Passot et al65 | 3.8 | 21.8 | |||
| 42 | Goéré et al66 | |||||
| 43 | Yonemura et al67 | 0.7 | 42.9 | 25.4 | 17.6 |
Abbreviations: CRS, cytoreductive surgery; EPIC, early postoperative intraperitoneal chemotherapy; HIPEC, hyperthermic intraperitoneal chemotherapy; IPC, intraperitoneal chemotherapy; SPIC, sequential postoperative intraperitoneal chemotherapy.
To date, only one randomized clinical trial (RCT) on this topic has been completed and published. In 2003, Verwaal et al published an RCT comparing systemic chemotherapy with CRS and HIPEC plus systemic chemotherapy for PC from colorectal cancer.5 This study randomized a total of 105 patients with colorectal or appendiceal adenocarcinoma to either standard treatment with systemic intravenous (IV) 5-fluorouracil (5-FU) and leucovorin (or irinotecan if prior 5-FU had been given) or to the investigational arm with CRS and HIPEC with intraperitoneal (IP) MMC for 90 minutes, followed by systemic chemotherapy as per the standard treatment. There were 51 patients assigned to the standard treatment arm, 44 of whom started treatment, and 54 patients assigned to the experimental arm, 49 of whom underwent surgery and 33 of whom started adjuvant chemotherapy. In terms of cytoreduction, 41% had a complete cytoreduction (R1/CC0). An 8% mortality rate was observed as a result of abdominal sepsis or pulmonary embolism. Grade 3 toxicity (according to the WHO scale) occurred in 66.7% of patients undergoing surgery, and grade 4 toxicity occurred in 45.8%, with leukopenia and small bowel fistula/leakage occurring most frequently.
Long-Term Outcomes
Mean weighted median overall survival for all studies reviewed was 27 months with a range of 15 to 63 months, and mean weighted 5-year overall survival was 27% with a range of 7 to 100%. Among studies reporting results after complete cytoreduction, mean weighted median survival was 31 months with a range of 12 to 48 months, and mean weighted 5-year overall survival was 31% with a range of 22 to 45%. The survival outcomes from the reviewed studies are summarized in Table 3.
Table 3. Survival outcomes of patients with peritoneal carcinomatosis secondary to colon cancer treated with CRS and/or IPC identified from systematic review.
| Author | Subgroup | Median survival (mo) | Overall survival (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 y | 2 y | 3 y | 4 y | 5 y | 10 y | ||||
| 1 | Pestieau and Sugarbaker33 | Synchronous | NR | 100 | |||||
| Metachronous | 24 | 30 | |||||||
| 2 | Elias et al34 | 60.1 | 47.1 | 36 | 27.4 | ||||
| 3 | Pilati et al35 | 18 | 31 | ||||||
| 4 | Verwaal et al5 | HIPEC | 22.4 | ||||||
| 5 | Elias et al21 | 60 | |||||||
| 6 | Glehen et al36 | All patients | 12.8 | 55 | 32 | 11 | |||
| CC0 | 32.9 | 85 | 54 | 22 | |||||
| 7 | Glehen et al37 | All patients | 19.2 | 72 | 39 | 19 | |||
| CC0 | 32.4 | 87 | 47 | 31 | |||||
| 8 | Shen et al38 | All patients | 16 | 25 | 17 | ||||
| R0/R1 | 28 | ||||||||
| 9 | Verwaal et al39 | 19.9 | |||||||
| 10 | Kecmanovic et al40 | All patients | 15 | ||||||
| CC0 | 19.9 | ||||||||
| 11 | Verwaal et al41 | All patients | 21.8 | 75 | 28 | 19 | |||
| R1 | 42.9 | 94 | 56 | 43 | |||||
| 12 | Cavaliere et al42 | All patients | 19 | 25.8 | |||||
| CC0 | 33.5 | ||||||||
| 13 | da Silva and Sugarbekar43 | 33 | 88 | 44 | 32 | ||||
| 14 | Füzün et al44 | All patients | 21 | 72 | 13 | 7 | |||
| CC0 | 87 | 37 | 25 | ||||||
| 15 | Zanon et al45 | 30.3 | 64 | 40 | |||||
| 16 | Bijelic et al46 | CC0/1 | 30 | 17 | |||||
| 17 | Elias et al15 | HIPEC | 54 | ||||||
| EPIC | 28 | ||||||||
| 18 | Piso et al47 | 96 | |||||||
| 19 | Franko et al48 | All patients | 15.3 | ||||||
| R0/R1 | 20.2 | ||||||||
| 20 | Verwaal et al49 | HIPEC with R1 | 48 | 45 | |||||
| 21 | Yan and Morris50 | 29 | 79 | 67 | 39 | ||||
| 22 | Elias et al7 | HIPEC | 62.7 | 81 | 51 | ||||
| 23 | Pelz et al51 | ||||||||
| 24 | Swellengrebel et al52 | All patients | 25.6 | ||||||
| R1 | 26.2 | ||||||||
| 25 | Bretcha-Boix et al53 | 36 | |||||||
| 26 | Chua et al54 | 38 | 85 | 66 | 48 | ||||
| 27 | Elias et al55 | 30.1 | 81 | 41 | 27 | ||||
| 28 | Franko et al8 | HIPEC | 34.7 | ||||||
| 29 | Saxena et al56 | ||||||||
| 30 | Vaira et al57 | 43 | |||||||
| 31 | Cavaliere et al58 | All patients | 21 | 45 | |||||
| CC0 | 25 | 50 | |||||||
| 32 | Chua et al59 | All patients | 38 | 92 | 55 | 30 | |||
| CC0 | 46 | ||||||||
| 33 | Hill et al22 | All patients | 18 | 71.3 | 45.4 | ||||
| R0/R1 | 34 | 96.4 | 72.4 | ||||||
| 34 | Klaver et al60 | 28 | 71 | 43 | |||||
| 35 | Quenet et al19 | Oxaliplatin | 40.8 | 41.8 | |||||
| Oxaliplatin/irinotecan | 47 | 42.4 | |||||||
| 36 | Stojadinovic et al61 | CC0/1 | 12 | ||||||
| 37 | Cashin et al14 | HIPEC | 34 | 40 | |||||
| CC0 with HIPEC | 39 | ||||||||
| SPIC | 25 | 18 | |||||||
| CC0 with SPIC | 32 | ||||||||
| 38 | Cashin et al62 | HIPEC | 36.5 | ||||||
| SPIC | 23.9 | ||||||||
| 39 | Hompes et al63 | 97.9 | 88.7 | ||||||
| 40 | Klaver et al64 | 35 | 83 | ||||||
| 41 | Passot et al65 | 36.2 | 77 | 51 | 33 | ||||
| 42 | Goéré et al66 | 35 | 15 | ||||||
| 43 | Yonemura et al67 | All patients | 24.4 | ||||||
| CC0 | 25.9 | ||||||||
Abbreviations: CRS, cytoreductive surgery; EPIC, early postoperative intraperitoneal chemotherapy; HIPEC, hyperthermic intraperitoneal chemotherapy; IPC, intraperitoneal chemotherapy; SPIC, sequential postoperative intraperitoneal chemotherapy.
In the RCT by Verwaal et al, median survival following systemic chemotherapy alone was 12.6 months, compared with 22.2 months following CRS, HIPEC, and adjuvant systemic chemotherapy (p = 0.028).5 Progression-free survival was 12.6 months in the HIPEC arm compared with 7.7 months in the standard arm (p = 0.02). In patients who underwent complete cytoreduction, median survival was 45 months and 5-year survival was 45%. While this study provides level 1 evidence for CRS and HIPEC over systemic chemotherapy, several considerations must be noted in its application to patients with PC from colon cancer. The study included patients with colorectal primaries, but 17.1% of all patients included in the study had appendiceal primaries and 11.4% had rectal primaries, which may have different outcomes than colon cancer alone. Patients eligible for the study also represented a highly selected group of patients fit for major surgery. In addition, CC0 status was not achievable in most patients, a factor that was significantly associated with survival outcomes. These issues underscore the importance of patient selection for such procedures.
Overall, the study by Verwaal et al presents the highest-quality evidence currently available, but a major critique of the RCT was the use of 5-FU and leucovorin in the systemic chemotherapy arm, which was the standard of care at the time, rather than modern chemotherapy such as FOLFIRI/FOLFOX and bevacizumab. More recent retrospective studies have compared modern systemic chemotherapy with CRS and HIPEC. In a retrospective cohort study by Elias et al, 48 patients undergoing complete CRS (tumor deposits < 1 mm) and HIPEC (bidirectional chemotherapy with IV 5-FU and leucovorin and IP oxaliplatin) were matched to 48 patients who had systemic chemotherapy alone (control group).7 An overall 5-year survival of 51% was observed in the HIPEC group compared with 13% in the control group (p < 0.05). The median survival was 62.7 months in the HIPEC group compared with 23.9 months in the control group (p < 0.05). Using a similar study design, Franko et al also found that patients receiving HIPEC had a significantly higher median survival than patients receiving only systemic chemotherapy (34.7 vs. 16.8 months, p < 0.001).8 There remains no consensus on the chemotherapeutic regimen used, and indeed the role of IPC itself over CRS alone.
Outcomes by Intraperitoneal Chemotherapy Variables
A small number of studies with levels 2 to 4 evidence have investigated the variables associated with the administration and technique for IPC.
Type of Intraperitoneal Chemotherapy
A few studies have compared the different methods of IPC administration, including HIPEC, EPIC, and SPIC, or a combination of these. In a retrospective cohort study by Cashin et al, 151 patients were identified with peritoneal disease from colorectal cancer.14 Of those patients, 69 underwent CRS and HIPEC (with IP MMC, oxaliplatin, or oxaliplatin and irinotecan) and 57 underwent CRS and SPIC (with IP 5-FU). Grades 3 to 4 90-day mortality occurred in 40.6% of HIPEC patients and 29.8% of SPIC patients (p = 0.02). The 90-day mortality was 4.3% in the HIPEC patients and 3.5% in the SPIC patients (p = 0.98). Patients in the HIPEC group improved overall survival with a median of 34 months and 5-year survival of 40%, compared with 25 months and 18%, respectively, in the SPIC group (p = 0.01). Among patients with CC0 resections only, the median survival was 39 months in HIPEC patients and 32 months in SPIC patients (p = 0.3). On multivariate analysis, the type of IPC was an independent prognostic factor, with improved outcomes in patients who received HIPEC compared with SPIC.
In a retrospective cohort study by Elias et al in 2007, 23 patients who underwent complete CRS and HIPEC with IP oxaliplatin and IV 5-FU/leucovorin for colorectal PC (HIPEC group) were compared with a matched group of 23 patients who underwent complete CRS with IP (normothermic) MMC and EPIC with IP 5-FU up to postoperative day 4 (EPIC group).15 Mortality was 0% in the HIPEC group and 8.7% in the EPIC group, although this difference was not statistically significant. Overall morbidity was comparable, but on subgroup analysis a significant difference was noted in the rate of enteric fistulas (0% in the HIPEC group vs. 26% in the EPIC group, p = 0.02). Overall 5-year survival was 54% in the HIPEC group compared with 28% in the EPIC group. Although this difference was not statistically significant (p = 0.22), the power of the study may have been a contributing factor. Over a median follow-up period of 113 months, peritoneal recurrence occurred in 26% in the HIPEC group and 57% in the EPIC group (p = 0.03).
Although the role of hyperthermia was not specifically tested in these two studies, the improved outcomes with HIPEC over other types of IPC administered postoperatively without hyperthermia suggests that hyperthermia may play a role in improving the penetration of the IPC, as demonstrated in animal studies,16 17 18 in the treatment of peritoneal disease.
Chemotherapeutic Agent for Intraperitoneal Chemotherapy
There was only one study identified that specifically investigated the chemotherapeutic agent(s) used for IPC for PC from colon cancer. Quenet et al conducted a bi-institutional prospective study on 146 patients who underwent CRS and HIPEC for colorectal PC.19 Forty-three patients received IP oxaliplatin alone for HIPEC and 103 patients received IP oxaliplatin and irinotecan. All patients received intraoperative IV 5-FU and leucovorin following CRS. Although 90.4% of all patients received a CC0 resection, there was a significant difference between groups with 25.6% of patients in the oxaliplatin alone group compared with 2.9% of patients in the oxaliplatin/irinotecan group achieving CC1 or CC2 status (p = 0.001). An overall 30-day or in-hospital mortality of 4.1% was observed, with no difference between groups. However, the overall morbidity was 34.9% in the oxaliplatin alone group and 52.4% in the oxaliplatin/irinotecan group (p = 0.05). On multivariate analysis, the chemotherapeutic agent(s) used for HIPEC was associated with morbidity (OR = 2.35 for oxaliplatin/irinotecan vs. oxaliplatin alone). The 5-year and median overall survival rates were comparable between groups, at 41.8% and 40.8 months, respectively, for oxaliplatin alone, and 42.4% and 47 months, respectively, for oxaliplatin/irinotecan. The significantly increased morbidity, with no associated improvement in overall survival, suggests that irinotecan should not be added to oxaliplatin for IP administration.
Although there appears to be no benefit in adding irinotecan to oxaliplatin, several IPC regimens exist that have not been investigated or compared. Given the lack of studies specific to chemotherapeutic agents used for IPC for colon cancer, relevant data have to be extrapolated from studies with other primary malignancies. In a prospective cohort study, McConnell et al studied complications of patients with PC from a variety of primary sites (including 33% from colorectal cancer) following IPC with HIPEC and/or EPIC and different chemotherapeutic agents.20 Eighty-five patients received HIPEC with IP MMC and EPIC with IP 5-FU (HIPEC and EPIC group) and 113 received HIPEC alone with IP oxaliplatin (HIPEC group). Significantly more grade III/IV complications (defined by the Clavien-Dindo grading system) occurred in the combined HIPEC and EPIC group compared with the HIPEC alone group (44.7 vs. 31% respectively, p = 0.047). While this study focused on the type of IPC (HIPEC and EPIC versus HIPEC alone), the two arms also differed in the chemotherapeutic agent used, and therefore it is difficult to conclude whether that group had a higher complication rate due to the type of IPC or the use of MMC rather than oxaliplatin. It also does not address survival outcomes among various chemotherapeutic agents. To date, no studies have specifically compared the use of MMC versus oxaliplatin.
Role of Intraperitoneal Chemotherapy
Because most studies have shown improved outcomes associated with a complete cytoreduction rather than from variations in IPC technique, the added value of IPC in addition to CRS has been questioned. Elias et al attempted to complete an RCT comparing patients who underwent complete CRS and then received either adjuvant systemic chemotherapy alone (control group) or EPIC and adjuvant systemic chemotherapy (EPIC group).21 The EPIC regimen comprised IP MMC followed by IP 5-FU up to postoperative day 5. Unfortunately, only 35 patients were accrued, with 19 in the control group and 16 in the EPIC group. The recruited patients were analyzed and a 2-year overall survival of 60% was observed in both groups. However, there were three perioperative deaths in the EPIC group and none in the control group, although more patients in the EPIC group underwent simultaneous liver resections for liver metastases and had more extensive PC. Given the limited sample size and follow-up, it is difficult to make definitive conclusions on the specific role of IPC after complete CRS in the treatment of PC from colon cancer, and therefore larger and more rigorous studies are needed.
Quality of Life
Few studies have focused on quality of life following CRS and IPC. Only one study specifically addressed quality of life following surgery for PC of colonic origin. Hill et al prospectively identified 62 such patients undergoing CRS and HIPEC.22 Emotional well-being, according to the Functional Assessment of Cancer Therapy Colon Scale (FACT-C), was significantly improved from baseline at 3 months postoperatively and remained above baseline at 6 and 12 months. The mean physical and functional well-being decreased to below baseline at 3 months but returned to near or above baseline at 6 and 12 months following surgery. A significant perceived decrease in role limitations due to physical health, as per the Short Form assessment (SF-36), occurred at 3 months, but this also returned to baseline by 6 and 12 months. Pain decreased from 3 months onward postoperatively. The incidence of depressive symptoms and depression tended to decrease over time from surgery, as measured by the Center for Epidemiologic Studies Depression Scale (CES-D). Pain interference with functioning, as per the Brief Pain Inventory (BPI), increased above baseline at 3 months, but was decreased by 6 months and significantly below baseline by 12 months. Forty-seven percent of patients stated that they had returned to normal activity by 1 year following surgery. Sixty-one percent of patients reported that their health was much or somewhat better by 12 months, whereas 17% reported that it was worse or much worse. Quality of life appeared to decrease initially but recovered by 6 to 12 months postoperatively.
Among studies looking at quality of life following CRS and HIPEC for all primary malignancies, similar outcomes have been shown. In a prospective study by Tsilimparis et al, health-related quality of life was studied in 90 patients undergoing CRS and HIPEC for a variety of primary malignancies, 21% of which were colorectal cancer.23 Most quality-of-life outcomes decreased in the initial postoperative period and took approximately 24 months to return back or close to baseline. Symptoms were worse in the postoperative period, but pain, appetite, and constipation improved to near baseline by 1 month. Fatigue and diarrhea persisted for at least 6 months but improved by 24 to 36 months. Mean global health status, which represents a subjective perception of health, returned to baseline at 6 months and was greater than baseline at 24 months. Physical function recovered at 6 months and was greater than baseline at 36 months. Emotional function was at baseline by 12 months. Similarly, McQuellon et al found that quality of life and self-reported performance status decreased postoperatively following CRS and HIPEC in 64 patients, but showed improvement over the first year to at or above baseline.24 Macri et al also found that physical and functional well-being were decreased at 3 months but were back at baseline by 6 months in 17 patients undergoing CRS and HIPEC.25
Treatment of Peritoneal Carcinomatosis with Synchronous Liver Metastases
A few studies have investigated the role of CRS and IPC in patients with colorectal cancer liver metastases in addition to PC. The studies are summarized in Table 4. Two level 4 studies have found that patients treated for PC with synchronous liver metastases had no worse survival than patients treated for PC without liver metastases with a median survival of 36 months and 2-year survival of 65%.26 27 Morbidity of 31 to 39% and mortality of 0 to 2.3% were observed. However, there were differences between groups and not all patients underwent synchronous liver resections.
Table 4. Summary of studies of patients with peritoneal carcinomatosis and liver metastases secondary to colon cancer treated with CRS and/or IPC identified from systematic review.
| Author | Year | n | Level of evidence | Subgroups | Type of IPC | Agents for IPC | |
|---|---|---|---|---|---|---|---|
| 1 | Kianmanesh et al26 | 2007 | 43 | 4 | HIPEC | MMC and cisplatin | |
| 2 | Chua et al27 | 2009 | 55 | 4 | HIPEC (12%), EPIC (22%), or both (67%) | MMC for HIPEC, 5-FU for EPIC | |
| 3 | Maggiori et al28 | 2013 | 98 | 3b | 37 liver metastases | HIPEC (43%), EPIC (49%), or both (8%) | oxaliplatin for HIPEC, MMC and 5-FU for EPIC |
| 61 no liver metastases | HIPEC (80%), EPIC (18%), or both (2%) |
Abbreviations: CRS, cytoreductive surgery; EPIC, early postoperative intraperitoneal chemotherapy; 5-FU, 5-fluorouracil; HIPEC, hyperthermic intraperitoneal chemotherapy; IPC, intraperitoneal chemotherapy; MMC, mitomycin C.
A level 3b cohort study by Maggiori et al matched patients with PC and liver metastases from colorectal cancer undergoing CRS, IPC (with HIPEC, EPIC, or both), and synchronous liver resection to those with only PC undergoing CRS and IPC.28 Morbidity and mortality were similar between groups, but there was a trend toward increased perioperative mortality in the liver resection group (0 vs. 8% for the peritoneal disease only group vs. liver metastases group, respectively; p = 0.051). There was a significant decrease in overall survival in the PC plus liver metastases group compared with the PC alone group (median survival 32 vs. 49 months, 3-year survival 40 vs. 66%, and 5-year survival 26 vs. 43%, p = 0.042). Increased PCI was the main prognostic factor, followed by the synchronous resection of liver metastases. The worst survival was seen in patients with either a PCI of 12 or greater or the presence of three or more liver metastases. Though there was no difference in the number of peritoneal and distant metastases, the liver metastases group had significantly more recurrent liver metastases (61 vs. 12%, p < 0.001). CRS and IPC combined with liver resection may have a role in highly selected patients with a low burden of both peritoneal and liver disease.
Discussion
PC secondary to colorectal cancer has been traditionally viewed nihilistically. However, advances in the treatment of such patients have occurred with improved systemic chemotherapy and the application of CRS and IPC to PC of colorectal origin. Long-term survival has been demonstrated in patients with PC secondary to colon cancer undergoing CRS and IPC in multiple case series, cohort studies, and a randomized trial, with 5-year survival rates of up to 45% being achieved in patients undergoing complete cytoreduction and intraperitoneal chemotherapy.
Many aspects of CRS and IPC need to be further elucidated. A lack of standardization exists among treatment protocols and therefore a great degree of variability among studies on the topic. It is also important to recognize the generally low quality of studies in the literature on this topic. Further studies are needed to determine which patients benefit most from CRS and IPC and the optimal techniques for such procedures. Also, further data are needed specifically for patients with liver metastases in addition to PC.
In the past, several randomized trials have been attempted to answer some of these important questions, including the study by Elias et al in 200421 and an ACOSOG/USMCI trial by Stojadinovic.29 Unfortunately, these studies were closed early due to poor accrual. Fortunately, several clinical trials are currently underway. A Swedish randomized study comparing systemic chemotherapy with CRS and EPIC with IP 5-FU and IV Isovorin in colorectal cancer has completed patient recruitment,30 and a French multicenter randomized trial also comparing systemic chemotherapy with CRS and HIPEC with IP oxaliplatin and IV 5FU/LV has been initiated.31 A randomized trial from Memorial Sloan Kettering comparing HIPEC with EPIC following CRS for colorectal and appendiceal primaries is also in process.32 Though CRS and IPC have been gaining widespread interest, and even acceptance, this may also make it difficult to accrue patients for randomized trials, because both physicians and patients may be more reluctant to participate in studies where all patients are not offered CRS and/or IPC.
Low mortality and acceptable morbidity with an apparent improvement in long-term survival and possible cure following CRS and IPC for colon cancer have led to increasing acceptance among surgeons and patients of this treatment with an otherwise poor prognosis. Currently, support for CRS and IPC is increasing among both physicians and patients because PC from colon cancer in selected patients in whom a complete cytoreduction can be achieved. Ongoing studies will be necessary to standardize the procedure and optimize variables that currently exist among centers.
References
- 1.Jayne D G, Fook S, Loi C, Seow-Choen F. Peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2002;89(12):1545–1550. doi: 10.1046/j.1365-2168.2002.02274.x. [DOI] [PubMed] [Google Scholar]
- 2.Glehen O Osinsky D Beaujard A C Gilly F N Natural history of peritoneal carcinomatosis from nongynecologic malignancies Surg Oncol Clin N Am 2003123729–739., xiii [DOI] [PubMed] [Google Scholar]
- 3.Sugarbaker P H, Cunliffe W J, Belliveau J. et al. Rationale for integrating early postoperative intraperitoneal chemotherapy into the surgical treatment of gastrointestinal cancer. Semin Oncol. 1989;16(4) 06:83–97. [PubMed] [Google Scholar]
- 4.Minsky B D, Mies C, Rich T A, Recht A, Chaffey J T. Potentially curative surgery of colon cancer: patterns of failure and survival. J Clin Oncol. 1988;6(1):106–118. doi: 10.1200/JCO.1988.6.1.106. [DOI] [PubMed] [Google Scholar]
- 5.Verwaal V J, van Ruth S, de Bree E. et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21(20):3737–3743. doi: 10.1200/JCO.2003.04.187. [DOI] [PubMed] [Google Scholar]
- 6.Meyerhardt J A, Mayer R J. Systemic therapy for colorectal cancer. N Engl J Med. 2005;352(5):476–487. doi: 10.1056/NEJMra040958. [DOI] [PubMed] [Google Scholar]
- 7.Elias D, Lefevre J H, Chevalier J. et al. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J Clin Oncol. 2009;27(5):681–685. doi: 10.1200/JCO.2008.19.7160. [DOI] [PubMed] [Google Scholar]
- 8.Franko J, Ibrahim Z, Gusani N J, Holtzman M P, Bartlett D L, Zeh H J III. Cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion versus systemic chemotherapy alone for colorectal peritoneal carcinomatosis. Cancer. 2010;116(16):3756–3762. doi: 10.1002/cncr.25116. [DOI] [PubMed] [Google Scholar]
- 9.Tomlinson J S, Jarnagin W R, DeMatteo R P. et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25(29):4575–4580. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 10.Sugarbaker P H. Strategies for the prevention and treatment of peritoneal carcinomatosis from gastrointestinal cancer. Cancer Invest. 2005;23(2):155–172. [PubMed] [Google Scholar]
- 11.de Bree E, Koops W, Kröger R, van Ruth S, Verwaal V J, Zoetmulder F AN. Preoperative computed tomography and selection of patients with colorectal peritoneal carcinomatosis for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Eur J Surg Oncol. 2006;32(1):65–71. doi: 10.1016/j.ejso.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 12.Harmon R L, Sugarbaker P H. Prognostic indicators in peritoneal carcinomatosis from gastrointestinal cancer. Int Semin Surg Oncol. 2005;2(1):3. doi: 10.1186/1477-7800-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phillips B Ball C Sackett D et al. 2009. Available at: http://www.cebm.net/. Accessed on February 2014
- 14.Cashin P H, Graf W, Nygren P, Mahteme H. Cytoreductive surgery and intraperitoneal chemotherapy for colorectal peritoneal carcinomatosis: prognosis and treatment of recurrences in a cohort study. Eur J Surg Oncol. 2012;38(6):509–515. doi: 10.1016/j.ejso.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Elias D, Benizri E, Di Pietrantonio D, Menegon P, Malka D, Raynard B. Comparison of two kinds of intraperitoneal chemotherapy following complete cytoreductive surgery of colorectal peritoneal carcinomatosis. Ann Surg Oncol. 2007;14(2):509–514. doi: 10.1245/s10434-006-9167-9. [DOI] [PubMed] [Google Scholar]
- 16.Shiu M H, Fortner J G. Intraperitoneal hyperthermic treatment of implanted peritoneal cancer in rats. Cancer Res. 1980;40(11):4081–4084. [PubMed] [Google Scholar]
- 17.Los G, Smals O A, van Vugt M J. et al. A rationale for carboplatin treatment and abdominal hyperthermia in cancers restricted to the peritoneal cavity. Cancer Res. 1992;52(5):1252–1258. [PubMed] [Google Scholar]
- 18.Los G, van Vugt M J, Pinedo H M. Response of peritoneal solid tumours after intraperitoneal chemohyperthermia treatment with cisplatin or carboplatin. Br J Cancer. 1994;69(2):235–241. doi: 10.1038/bjc.1994.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quenet F, Goéré D, Mehta S S. et al. Results of two bi-institutional prospective studies using intraperitoneal oxaliplatin with or without irinotecan during HIPEC after cytoreductive surgery for colorectal carcinomatosis. Ann Surg. 2011;254(2):294–301. doi: 10.1097/SLA.0b013e3182263933. [DOI] [PubMed] [Google Scholar]
- 20.McConnell Y J, Mack L A, Francis W P, Ho T, Temple W J. HIPEC + EPIC versus HIPEC-alone: differences in major complications following cytoreduction surgery for peritoneal malignancy. J Surg Oncol. 2013;107(6):591–596. doi: 10.1002/jso.23276. [DOI] [PubMed] [Google Scholar]
- 21.Elias D, Delperro J R, Sideris L. et al. Treatment of peritoneal carcinomatosis from colorectal cancer: impact of complete cytoreductive surgery and difficulties in conducting randomized trials. Ann Surg Oncol. 2004;11(5):518–521. doi: 10.1245/ASO.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Hill A R, McQuellon R P, Russell G B, Shen P, Stewart J H IV, Levine E A. Survival and quality of life following cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis of colonic origin. Ann Surg Oncol. 2011;18(13):3673–3679. doi: 10.1245/s10434-011-1793-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsilimparis N, Bockelmann C, Raue W. et al. Quality of life in patients after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: is it worth the risk? Ann Surg Oncol. 2013;20(1):226–232. doi: 10.1245/s10434-012-2579-9. [DOI] [PubMed] [Google Scholar]
- 24.McQuellon R P, Loggie B W, Fleming R A, Russell G B, Lehman A B, Rambo T D. Quality of life after intraperitoneal hyperthermic chemotherapy (IPHC) for peritoneal carcinomatosis. Eur J Surg Oncol. 2001;27(1):65–73. doi: 10.1053/ejso.2000.1033. [DOI] [PubMed] [Google Scholar]
- 25.Macrì A, Maugeri I, Trimarchi G. et al. Evaluation of quality of life of patients submitted to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinosis of gastrointestinal and ovarian origin and identification of factors influencing outcome. In Vivo. 2009;23(1):147–150. [PubMed] [Google Scholar]
- 26.Kianmanesh R, Scaringi S, Sabate J-M. et al. Iterative cytoreductive surgery associated with hyperthermic intraperitoneal chemotherapy for treatment of peritoneal carcinomatosis of colorectal origin with or without liver metastases. Ann Surg. 2007;245(4):597–603. doi: 10.1097/01.sla.0000255561.87771.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chua T C, Yan T D, Zhao J, Morris D L. Peritoneal carcinomatosis and liver metastases from colorectal cancer treated with cytoreductive surgery perioperative intraperitoneal chemotherapy and liver resection. Eur J Surg Oncol. 2009;35(12):1299–1305. doi: 10.1016/j.ejso.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Maggiori L, Goéré D, Viana B. et al. Should patients with peritoneal carcinomatosis of colorectal origin with synchronous liver metastases be treated with a curative intent? A case-control study. Ann Surg. 2013;258(1):116–121. doi: 10.1097/SLA.0b013e3182778089. [DOI] [PubMed] [Google Scholar]
- 29.Stojadinovic A Standard therapy with or without surgery and Mitomycin C in treating patients with advanced limited peritoneal dissemination of colon cancer Clinical trial NCT01167725. Available at: http://clinicaltrials.gov/show/NCT01167725. Accessed on February 2014
- 30.Graf W Cytoreduction and intraperitoneal chemotherapy versus systemic chemotherapy in colorectal peritoneal carcinomatosis Clinical trial NCT01524094. Available at: http://clinicaltrials.gov/show/NCT01524094. Accessed on February 2014
- 31.Quenet F Systemic chemotherapy with or without intraperitoneal chemohyperthermia in treating patients undergoing surgery for peritoneal carcinomatosis from colorectal cancer Clinical trial NCT00769405. Available at: http://clinicaltrials.gov/ct2/show/NCT00769405. Accessed on February 2014
- 32.Nash G ICARuS post-operative intraperitoneal chemotherapy (EPIC) and hyperthermic intraperitoneal chemotherapy (HIPEC) after optimal cytoreductive surgery (CRS) for neoplasms of the appendix, colon or rectum with isolated peritoneal metastasis Clinical trial NCT01815359. Available at: http://clinicaltrials.gov/ct2/show/NCT01815359. Accessed on February 2014
- 33.Pestieau S R Sugarbaker P H Treatment of primary colon cancer with peritoneal carcinomatosis: comparison of concomitant vs. delayed management Dis Colon Rectum 200043101341–1346., discussion 1347–1348 [DOI] [PubMed] [Google Scholar]
- 34.Elias D, Blot F, El Otmany A. et al. Curative treatment of peritoneal carcinomatosis arising from colorectal cancer by complete resection and intraperitoneal chemotherapy. Cancer. 2001;92(1):71–76. doi: 10.1002/1097-0142(20010701)92:1<71::aid-cncr1293>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 35.Pilati P, Mocellin S, Rossi C R. et al. Cytoreductive surgery combined with hyperthermic intraperitoneal intraoperative chemotherapy for peritoneal carcinomatosis arising from colon adenocarcinoma. Ann Surg Oncol. 2003;10(5):508–513. doi: 10.1245/aso.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 36.Glehen O, Cotte E, Schreiber V, Sayag-Beaujard A C, Vignal J, Gilly F N. Intraperitoneal chemohyperthermia and attempted cytoreductive surgery in patients with peritoneal carcinomatosis of colorectal origin. Br J Surg. 2004;91(6):747–754. doi: 10.1002/bjs.4473. [DOI] [PubMed] [Google Scholar]
- 37.Glehen O, Kwiatkowski F, Sugarbaker P H. et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol. 2004;22(16):3284–3292. doi: 10.1200/JCO.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 38.Shen P, Hawksworth J, Lovato J. et al. Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy with mitomycin C for peritoneal carcinomatosis from nonappendiceal colorectal carcinoma. Ann Surg Oncol. 2004;11(2):178–186. doi: 10.1245/aso.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 39.Verwaal V J, van Tinteren H, van Ruth S, Zoetmulder F A. Predicting the survival of patients with peritoneal carcinomatosis of colorectal origin treated by aggressive cytoreduction and hyperthermic intraperitoneal chemotherapy. Br J Surg. 2004;91(6):739–746. doi: 10.1002/bjs.4516. [DOI] [PubMed] [Google Scholar]
- 40.Kecmanovic D M, Pavlov M J, Ceranic M S, Sepetkovski A V, Kovacevic P A, Stamenkovic A B. Treatment of peritoneal carcinomatosis from colorectal cancer by cytoreductive surgery and hyperthermic perioperative intraperitoneal chemotherapy. Eur J Surg Oncol. 2005;31(2):147–152. doi: 10.1016/j.ejso.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 41.Verwaal V J, van Ruth S, Witkamp A, Boot H, van Slooten G, Zoetmulder F A. Long-term survival of peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol. 2005;12(1):65–71. doi: 10.1007/s10434-004-1167-z. [DOI] [PubMed] [Google Scholar]
- 42.Cavaliere F Valle M De Simone M et al. 120 peritoneal carcinomatoses from colorectal cancer treated with peritonectomy and intra-abdominal chemohyperthermia: a S.I.T.I.L.O. multicentric study In Vivo 200620(6A):747–750. [PubMed] [Google Scholar]
- 43.da Silva R G, Sugarbaker P H. Analysis of prognostic factors in seventy patients having a complete cytoreduction plus perioperative intraperitoneal chemotherapy for carcinomatosis from colorectal cancer. J Am Coll Surg. 2006;203(6):878–886. doi: 10.1016/j.jamcollsurg.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 44.Füzün M, Sökmen S, Terzi C, Canda A E. Cytoreductive approach to peritoneal carcinomatosis originated from colorectal cancer: Turkish experience. Acta Chir Iugosl. 2006;53(2):17–21. doi: 10.2298/aci0602017f. [DOI] [PubMed] [Google Scholar]
- 45.Zanon C, Bortolini M, Chiappino I. et al. Cytoreductive surgery combined with intraperitoneal chemohyperthermia for the treatment of advanced colon cancer. World J Surg. 2006;30(11):2025–2032. doi: 10.1007/s00268-005-0486-y. [DOI] [PubMed] [Google Scholar]
- 46.Bijelic L, Yan T D, Sugarbaker P H. Failure analysis of recurrent disease following complete cytoreduction and perioperative intraperitoneal chemotherapy in patients with peritoneal carcinomatosis from colorectal cancer. Ann Surg Oncol. 2007;14(8):2281–2288. doi: 10.1245/s10434-007-9410-z. [DOI] [PubMed] [Google Scholar]
- 47.Piso P, Dahlke M H, Ghali N. et al. Multimodality treatment of peritoneal carcinomatosis from colorectal cancer: first results of a new German centre for peritoneal surface malignancies. Int J Colorectal Dis. 2007;22(11):1295–1300. doi: 10.1007/s00384-007-0313-z. [DOI] [PubMed] [Google Scholar]
- 48.Franko J, Gusani N J, Holtzman M P. et al. Multivisceral resection does not affect morbidity and survival after cytoreductive surgery and chemoperfusion for carcinomatosis from colorectal cancer. Ann Surg Oncol. 2008;15(11):3065–3072. doi: 10.1245/s10434-008-0105-x. [DOI] [PubMed] [Google Scholar]
- 49.Verwaal V J, Bruin S, Boot H, van Slooten G, van Tinteren H. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2008;15(9):2426–2432. doi: 10.1245/s10434-008-9966-2. [DOI] [PubMed] [Google Scholar]
- 50.Yan T D, Morris D L. Cytoreductive surgery and perioperative intraperitoneal chemotherapy for isolated colorectal peritoneal carcinomatosis: experimental therapy or standard of care? Ann Surg. 2008;248(5):829–835. doi: 10.1097/SLA.0b013e31818a15b5. [DOI] [PubMed] [Google Scholar]
- 51.Pelz J O, Stojadinovic A, Nissan A, Hohenberger W, Esquivel J. Evaluation of a peritoneal surface disease severity score in patients with colon cancer with peritoneal carcinomatosis. J Surg Oncol. 2009;99(1):9–15. doi: 10.1002/jso.21169. [DOI] [PubMed] [Google Scholar]
- 52.Swellengrebel H A, Zoetmulder F A, Smeenk R M, Antonini N, Verwaal V J. Quantitative intra-operative assessment of peritoneal carcinomatosis—a comparison of three prognostic tools. Eur J Surg Oncol. 2009;35(10):1078–1084. doi: 10.1016/j.ejso.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 53.Bretcha-Boix P, Farré-Alegre J, Sureda M, Dussan C, Pérez Ruixo J J, Brugarolas Masllorens A. Cytoreductive surgery and perioperative intraperitoneal chemotherapy in patients with peritoneal carcinomatosis of colonic origin: outcomes after 7 years' experience of a new centre for peritoneal surface malignancies. Clin Transl Oncol. 2010;12(6):437–442. doi: 10.1007/s12094-010-0531-7. [DOI] [PubMed] [Google Scholar]
- 54.Chua T C, Morris D L, Esquivel J. Impact of the peritoneal surface disease severity score on survival in patients with colorectal cancer peritoneal carcinomatosis undergoing complete cytoreduction and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2010;17(5):1330–1336. doi: 10.1245/s10434-009-0866-x. [DOI] [PubMed] [Google Scholar]
- 55.Elias D, Gilly F, Boutitie F. et al. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol. 2010;28(1):63–68. doi: 10.1200/JCO.2009.23.9285. [DOI] [PubMed] [Google Scholar]
- 56.Saxena A, Yan T D, Morris D L. A critical evaluation of risk factors for complications after cytoreductive surgery and perioperative intraperitoneal chemotherapy for colorectal peritoneal carcinomatosis. World J Surg. 2010;34(1):70–78. doi: 10.1007/s00268-009-0206-0. [DOI] [PubMed] [Google Scholar]
- 57.Vaira M, Cioppa T, D'Amico S. et al. Treatment of peritoneal carcinomatosis from colonic cancer by cytoreduction, peritonectomy and hyperthermic intraperitoneal chemotherapy (HIPEC). Experience of ten years. In Vivo. 2010;24(1):79–84. [PubMed] [Google Scholar]
- 58.Cavaliere F, De Simone M, Virzì S. et al. Prognostic factors and oncologic outcome in 146 patients with colorectal peritoneal carcinomatosis treated with cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy: Italian multicenter study S.I.T.I.L.O. Eur J Surg Oncol. 2011;37(2):148–154. doi: 10.1016/j.ejso.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 59.Chua T C, Morris D L, Saxena A. et al. Influence of modern systemic therapies as adjunct to cytoreduction and perioperative intraperitoneal chemotherapy for patients with colorectal peritoneal carcinomatosis: a multicenter study. Ann Surg Oncol. 2011;18(6):1560–1567. doi: 10.1245/s10434-010-1522-1. [DOI] [PubMed] [Google Scholar]
- 60.Klaver Y L, de Hingh I H, Boot H, Verwaal V J. Results of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy after early failure of adjuvant systemic chemotherapy. J Surg Oncol. 2011;103(5):431–434. doi: 10.1002/jso.21836. [DOI] [PubMed] [Google Scholar]
- 61.Stojadinovic A, Nissan A, Eberhardt J, Chua T C, Pelz J O, Esquivel J. Development of a Bayesian Belief Network Model for personalized prognostic risk assessment in colon carcinomatosis. Am Surg. 2011;77(2):221–230. [PubMed] [Google Scholar]
- 62.Cashin P H, Graf W, Nygren P, Mahteme H. Intraoperative hyperthermic versus postoperative normothermic intraperitoneal chemotherapy for colonic peritoneal carcinomatosis: a case-control study. Ann Oncol. 2012;23(3):647–652. doi: 10.1093/annonc/mdr301. [DOI] [PubMed] [Google Scholar]
- 63.Hompes D, D'Hoore A, Van Cutsem E. et al. The treatment of peritoneal carcinomatosis of colorectal cancer with complete cytoreductive surgery and hyperthermic intraperitoneal peroperative chemotherapy (HIPEC) with oxaliplatin: a Belgian multicentre prospective phase II clinical study. Ann Surg Oncol. 2012;19(7):2186–2194. doi: 10.1245/s10434-012-2264-z. [DOI] [PubMed] [Google Scholar]
- 64.Klaver Y L, Chua T C, de Hingh I H, Morris D L. Outcomes of elderly patients undergoing cytoreductive surgery and perioperative intraperitoneal chemotherapy for colorectal cancer peritoneal carcinomatosis. J Surg Oncol. 2012;105(2):113–118. doi: 10.1002/jso.22019. [DOI] [PubMed] [Google Scholar]
- 65.Passot G, Vaudoyer D, Cotte E. et al. Progression following neoadjuvant systemic chemotherapy may not be a contraindication to a curative approach for colorectal carcinomatosis. Ann Surg. 2012;256(1):125–129. doi: 10.1097/SLA.0b013e318255486a. [DOI] [PubMed] [Google Scholar]
- 66.Goéré D, Malka D, Tzanis D. et al. Is there a possibility of a cure in patients with colorectal peritoneal carcinomatosis amenable to complete cytoreductive surgery and intraperitoneal chemotherapy? Ann Surg. 2013;257(6):1065–1071. doi: 10.1097/SLA.0b013e31827e9289. [DOI] [PubMed] [Google Scholar]
- 67.Yonemura Y, Canbay E, Ishibashi H. Prognostic factors of peritoneal metastases from colorectal cancer following cytoreductive surgery and perioperative chemotherapy. ScientificWorldJournal. 2013;2013:978394. doi: 10.1155/2013/978394. [DOI] [PMC free article] [PubMed] [Google Scholar]


