Abstract
Robotic surgery is an emerging field in colorectal surgery and may overcome the limitations of conventional laparoscopic surgery, such as rigid instrumentation, poor ergonomics, and assistant-dependent camera movements and retraction. In addition, robotic-assisted colectomy appears to offer comparable outcomes to laparoscopic colectomy with limited long-term outcomes data. Prolonged operating time, increased costs and learning curve are the major drawbacks of robotic colectomy for colon cancer. Although new robotic platforms promise improved ingenuity through developing technology, the role of the robot in colon cancer surgery is still unclear.
Keywords: robotic surgery, colectomy, colon cancer
The aim of surgical treatment of colorectal cancer is to remove the primary tumor, including lymphatic drainage, with clear surgical margins.1 Results of the Clinical Outcomes of Surgical Therapy Study Group (COST) trial in 2004 showed that laparoscopy has comparable long-term oncological outcomes to open colectomy in the treatment of colon cancer.2 To date, utilization of the laparoscopic technique in colon cancer surgery has been increasing.3
However, laparoscopy has important drawbacks, including lack of three-dimensional visualization, limited maneuverability because of rigid instrumentation, poor ergonomics, amplified impact of physiological tremors, and assistant-dependent camera movements and retraction. Robotic surgery was developed to overcome the technical difficulties of conventional laparoscopy (Fig. 1).4 5 The initial experiences with robotic colectomy (for both benign and malignant tumors) were reported in the early 2000s, mostly as case series and case reports.6 7 8 The consensus was that robotic colectomy was safe and feasible, but had longer operative times and higher costs than conventional laparoscopy.7
Fig. 1.
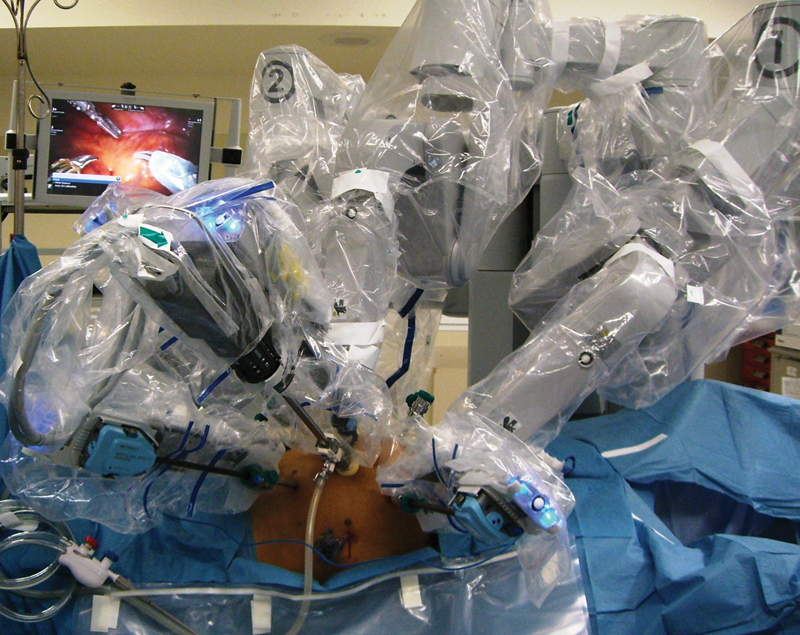
The introduction of the da Vinci robotic surgical system (Intuitive Surgical, Inc., Sunnyvale, CA) has revolutionized the field of minimally invasive surgery. This robotic system provides high-definition, three-dimensional vision, surgeon-motion filtration, articulating movements of the instruments, stable camera control and retraction, and better ergonomics. The fatigue that is associated with unnatural positions during laparoscopy was eliminated by the use of robotic technology.5
In this article, the authors will review the outcomes, advantages and disadvantages, and future clinical implementations of robotic colon cancer surgery compared with conventional laparoscopy and open surgery.
Robotic Right Colectomy for Cancer
Commonly, a 12-mm incision is made left to the umbilicus for initial access and is used as the camera site. The preferred trocar placement for robotic right colectomy is shown in Fig. 2 and the operating room design is shown in Fig. 3. The reported port number for robotic right colectomy for cancer varies from 4 to 5 trocars.9 10 11 12 13 14 The basic concept in port placement is to place the camera in the middle, with one working arm superior and one inferior to the camera. One 5-mm assistant port is added in the left abdomen for additional retraction. An additional third arm is usually placed on the right side of the abdomen to improve traction/counter-tractions. Both an inferior-to-superior9 10 12 13 and medial-to-lateral11 dissection techniques can be performed during robotic right colectomy.15 The ileocolic pedicle can be taken with Hem-o-Lok (Weck Surgical Instruments, Teleflex Medical, Durham, NC) clips or any vessel-sealing energy devices. The colon is then dissected from the fascia of Gerota and retroperitoneum; the duodenum, ureters, and gonadal vessels are preserved. Finally, take down of the hepatic flexure is performed using sharp dissection.
Fig. 2.
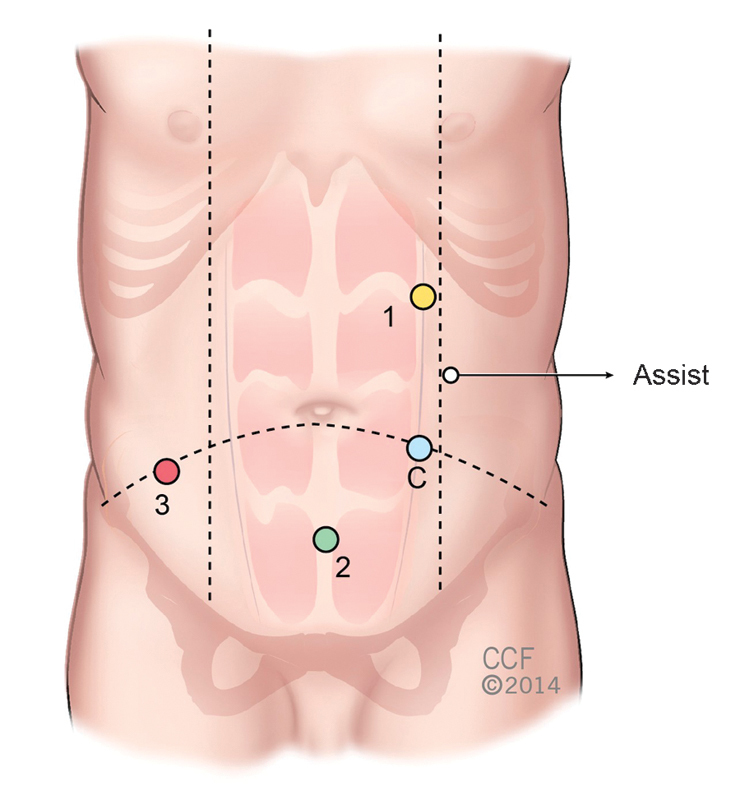
Trocar placement for robotic right colectomy.
Fig. 3.
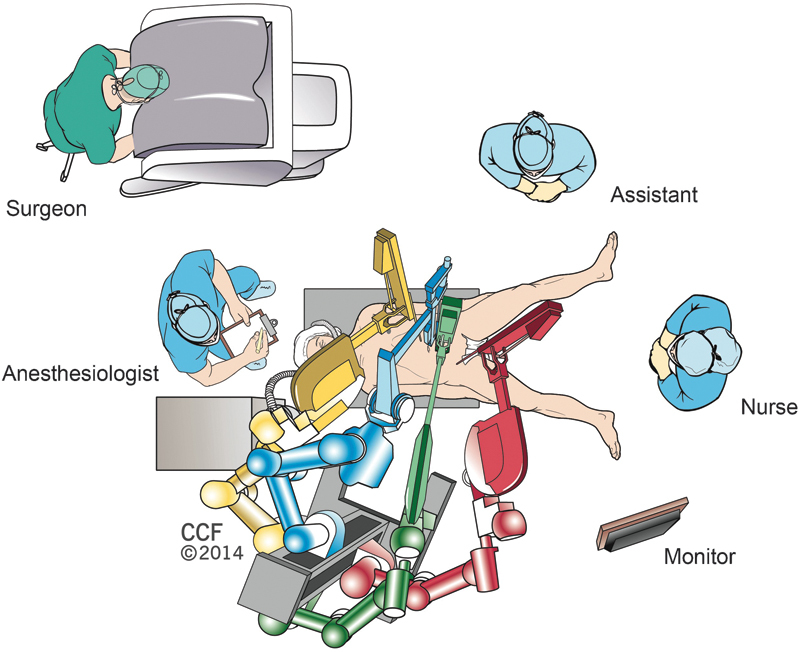
Operating room design for robotic right colectomy.
Prolonged operating time is one of the major drawbacks of robotic surgery. The only randomized clinical study comparing robotic and conventional laparoscopic right colectomy in colon cancer showed that the operative time was significantly longer in the former group.9 Similarly, robotic right colectomy was associated with a longer operating time than open right colectomy for colon cancer.11 However, the study comparing the first 30 laparoscopic and robotic right colectomies of the same surgeon and institute suggested statistically comparable operating times for both the groups.12 Previous studies, including patients with both benign and malign disease, reported either prolonged16 or comparable17 operating times for robotic right colectomy. D'Annibale et al reported docking time, surgeons' experience (place on the learning curve), and intracorporeal creation of anastomosis as factors influencing the prolonged operating time for robotic right colectomy. In addition, operating time gradually decreased as the number of robotic right colectomy cases increased,10 18 suggesting that as the surgeon and surgical team gain experience, operating time shortens.
Previous studies showed similar blood loss during robotic and laparoscopic right colectomy for cancer.9 12 On the other hand, robotic surgery was found to be associated with significantly reduced blood loss when compared with open right colectomy.11 Reduced blood loss is an advantage of robotic colectomy over open surgery, but laparoscopic colectomy offers comparable outcomes in this regard. Because intracorporeal anastomosis is challenging with nonarticulated rigid instruments, extracorporeal creation of anastomosis during conventional laparoscopic colectomy is preferred. Intracorporeal anastomosis creation is not only possible with robotic surgery but is also safe19 and associated with fewer wounds and anastomotic complications.20 Although the reported conversion rates were similar for robotic and laparoscopic procedures,9 12 there were no conversions to open surgery in 106 robotic right colectomies, which suggests that robotic surgery is associated with better ergonomics as well as visualization and dexterity.
Another benefit of minimally invasive surgery is that the length of hospital stay is shorter than that of open surgery. This holds true for robotic right colectomy for colon cancer as well.11 However, length of hospital stay was comparable between robotic and laparoscopic right colectomy.9 12 In addition, robotic right colectomy provides similar overall morbidity and mortality rates with open11 and conventional laparoscopic right colectomy.9 12
New techniques and devices have reduced the number of necessary ports and improved cosmesis while decreasing abdominal wall and body trauma. Robotic single-port colorectal surgery has been introduced with right colectomy.21 22 23 Single-incision robotic colectomy can also offer an advantage in terms of specimen extraction and improved cosmesis.
Robotic right colectomy for colon cancer is feasible and does not compromise oncological principles.9 11 12 A disease-free survival rate of 90% and an overall survival rate of 92% were reported after robotic right colectomy for colon cancer at a median follow-up time of 36 months (6–96 months).10 Previously reported 30-month overall survival rates by the American Joint Committee on Cancer (AJCC) were 89.2% for stage II and 72.7% for stage III colon cancer.24 These results suggest that robotic right colectomy may offer the same 3-year survival rate for patients with colon cancer as open and conventional laparoscopic surgery.
Intraoperative, pathological, and short-term postoperative outcomes for robotic right colectomy are shown in Table 1.
Table 1. Outcomes of robotic right colectomy for colon cancer.
| No. of patients | Operative time (min) | Blood loss (mL) | Conversion | No. of harvested lymph nodes | Anastomosis leakage | Length of stay (d) | |
|---|---|---|---|---|---|---|---|
| Park et al 2012 | 35 | 195 | 35.8 | 0 | 29.9 | 1 | 7.9 |
| Shin 2012 | 6 | 342.5 | 185 | 0 | 25.8 | 0 | 10.7 |
| Park et al 2012 | 15 | 201.4 | 41.7 | 0 | 24.2 | 0 | 7 |
| Luca et al 2011 | 33 | 191.7 | 6.1 | N/Aa | 26.6 | 0 | 5 |
| D'Annibale et al 2010 | 50 | 223.5 | 20 | 0 | 18.8 | 0 | 7 |
No data included for conversion.
Robotic Left Colectomy for Cancer
To perform a robotic left colectomy, five to six ports are required including camera and assistant ports.25 Fig. 4 shows the port placement for three robotic arms, camera, and assistant ports. A 12-mm camera port is placed in the supraumbilical area using an open technique. The camera is inserted and additional trocars are placed as shown in Fig. 4. A right upper quadrant robotic port (port #3) is used for splenic flexure mobilization and left colectomy. Once the robot is redocked for the pelvis, the port in the right upper quadrant is released from the robotic arm, and it can be used as an assistant port. An additional robotic port (port #3P) is placed in the left-mid abdomen, lateral to the edge of the rectus muscle and at an equal distance from the right upper and lower quadrant trocars, when pelvic dissection is necessary (anterior resection, low anterior resection, and abdominoperineal resection procedures). The assistant port can be used for small bowel/colon retraction and suction irrigation.
Fig. 4.
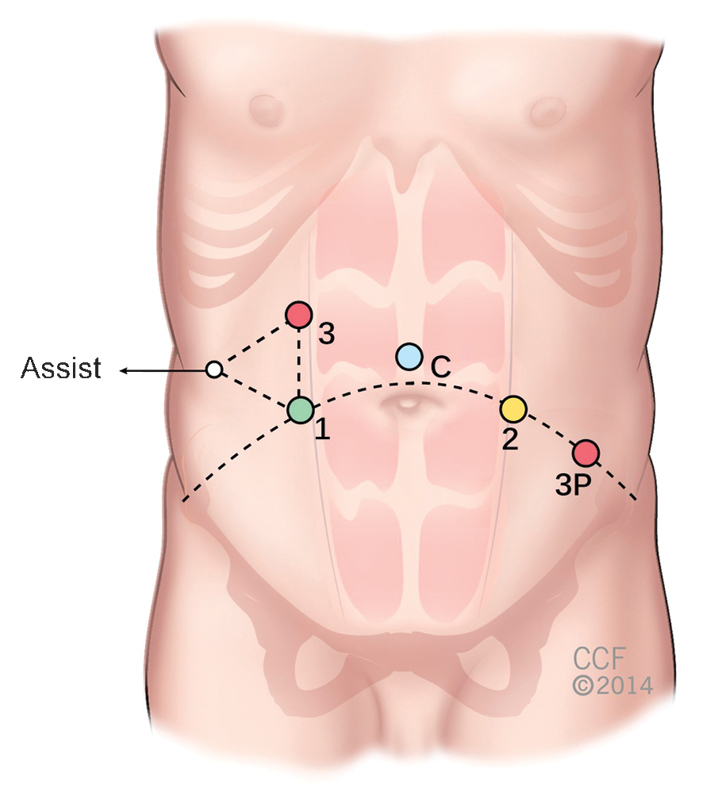
Port placement for robotic left colectomy and rectal resections.
There are several described techniques for robotic left colectomy: hybrid (with laparoscopic splenic flexure mobilization), single docking (mobilizing the second and third robotic arms for different parts of the surgery), and double docking (first docking from the left upper quadrant for splenic flexure mobilization and then docking to the left lower quadrant for the rest of the procedure).26 Recently described “single-position flip arm technique” allows performing splenic flexure mobilization and low anterior resection with only one docking position.27 We use single docking technique for isolated left colectomy procedure and double docking technique for splenic flexure mobilization requiring procedures in our current practice. The operating room design during robotic left colectomy and splenic flexure mobilization is shown in Fig. 5. We prefer to dock robot at a 45-degree angle from the patient's left side for procedures requiring pelvic dissection (Fig. 6).
Fig. 5.
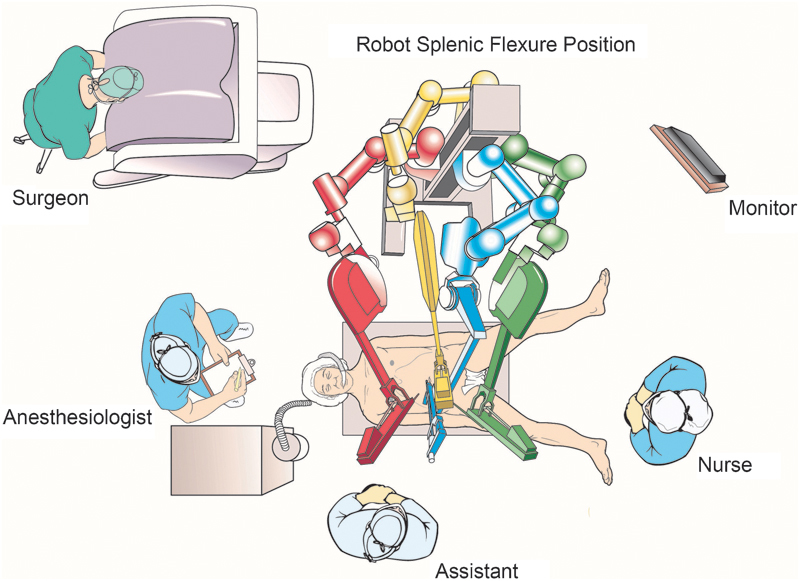
Operating room design for left colectomy and splenic flexure mobilization.
Fig. 6.
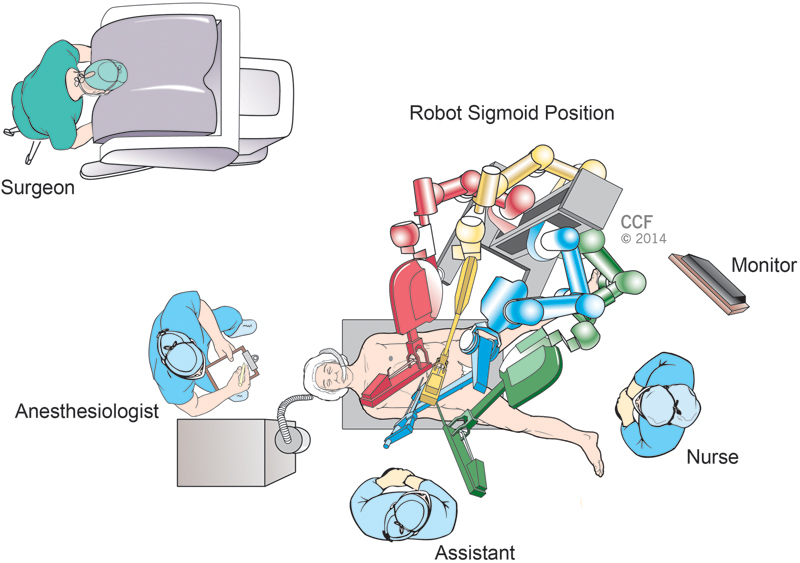
Operating room design for sigmoid colon and rectal resections.
One study that compared laparoscopic and robotic anterior resection for sigmoid colon cancer found that the robotic procedure was associated with a significantly longer operative time.28 Shin12 reported comparable operative times for robotic and laparoscopic resection of left-sided colon cancer. On the other hand, comparable operating times were reported for patients who underwent laparoscopic or robotic colectomy for colon cancer.29 In addition, a study comparing laparoscopic and robotic left colectomies for either benign or malignant disease of the colon reported a longer operating time for the robotic cases.17 Comparing operative times between studies and procedures is difficult because of the lack of a uniform study design for evaluating the operative time during robotic colectomy. However, the presence of a trained and experienced robotic surgical team in the operating room may be as important as the experience of the surgeon.
Reported blood loss and conversion rates during robotic cases were comparable with those of laparoscopic left colectomy.12 28 On the other hand, either a comparable or shorter length of hospital stay was reported for robotic left colectomy for cancer.12 28 Similarly, outcomes in terms of blood loss and length of hospital stay were comparable between robotic and laparoscopic left colectomy for benign and malignant disease of the colon.17
It has been hypothesized that image-guidance technology may help in reducing rates of anastomotic leak. One of the main causes of this complication is thought to be poor perfusion of the intestinal stump/segment. Evaluating colonic segment perfusion is usually subjective, but the near-infrared camera of the robotic platform (firefly) allows surgeons to visualize the vascular structure of the colon after indocyanine green (ICG) injection. Fluorescence imaging has been shown to reduce the anastomotic leak rate after low anterior resection and may also help to identify sentinel lymph nodes.30
No severe complications or mortalities were reported after robotic anterior resection for sigmoid colon cancer, and postoperative morbidity and mortality rates were similar to those of the laparoscopy group.28 Similarly, a 92% overall and an 89% disease-free 3-year survival rate were reported after robotic sigmoid colectomy, which were comparable to those in the laparoscopy group.28
Intraoperative, pathological, and short-term postoperative outcomes for robotic left colectomy are shown in Table 2.
Table 2. Outcomes of robotic left and sigmoid colectomy for cancer.
| No. of patients | Operative time (min) | Blood loss (mL) | Conversion | No. of harvested lymph nodes | Anastomosis leakage | Length of stay (d) | |
|---|---|---|---|---|---|---|---|
| Lim et al 2013 | 34 | 252.5 | 60.3 | 0 | 12 | 0 | 5.5 |
| Helvind et al 2013 | 101a | 243 | N/Ab | 5 | 23.4 | 5c | 6.4 |
| Shin 2012 | 7 | 337.1 | 105.7 | 0 | 16.9 | 0 | 9.1 |
| Luca et al 2009 | 55d | 290 | 68 | 0 | 18.5 | 7e | 7.5 |
Cohort includes 44 left colectomies.
No data for blood loss.
Anastomotic leak for all colectomies.
Cohort includes 27 left colectomies.
Two out of seven anastomotic leaks were after colectomy.
Our Experience with Robotic Colorectal Surgery
In total, 99 robotic colorectal resections were performed in our department between October 2010 and July 2014 (Table 3). Primary surgical indications were colorectal cancer, rectal prolapse, ulcerative colitis, Crohn disease, diverticular disease, constipation, and endometriosis (Table 4). Our recently published case-matched study comparing outcomes of robotic and laparoscopic procedures that were performed in our institution between October 2010 and July 2014 showed comparable outcomes, with the exception of operating time.31 Operating time was significantly longer in patients with robotic surgery (185 vs. 267 minutes, p < 0.0001).
Table 3. Robotic colorectal procedures that were performed at our institution.
| Procedure performed | N (%) |
|---|---|
| Anterior proctosigmoidectomy | 32 (32.3) |
| Abdominoperineal resection | 15 (15.2) |
| Rectopexy for prolapse | 12 (12.1) |
| Completion proctectomy with IPAA | 9 (9.1) |
| Resection rectopexy for prolapse | 9 (9.1) |
| Sigmoid colectomy | 6 (6.1) |
| Completion proctectomy with end-ileostomy | 4 (4) |
| Ileocecectomy | 2 (2) |
| Right hemicolectomy | 2 (2) |
| Total abdominal colectomy | 2 (2) |
| Total proctocolectomy | 2 (2) |
| Pouch excision with end-ileostomy | 1 (1) |
| Total proctocolectomy with IPAA | 1 (1) |
| Other | 2 (2) |
Abbreviation: IPAA, Ileal pouch anal anastomosis.
Table 4. Primary diagnoses of patients who underwent robotic colorectal procedures at our institution.
| Diagnosis | N (%) |
|---|---|
| Colorectal cancer | 49 (49.5) |
| Rectal prolapse | 22 (22.2) |
| Ulcerative colitis | 13 (13.1) |
| Crohn disease | 7 (7.1) |
| Diverticular disease | 5 (5.1) |
| Constipation | 2 (2) |
| Endometriosis | 1 (1) |
Recently, we compared our results for robotic versus conventional laparoscopic rectal cancer surgery in obese patients. Obesity adds to the technical difficulty of laparoscopic colorectal surgery. Robotic approach has the potential to overcome this limitation because of its proposed technical advantages over laparoscopy. However, it is unknown if robotic approach would actually serve as a better option in obese patients. The aim of this study was to compare the short-term outcomes of robotic surgery versus conventional laparoscopy surgery in this patient population. In this unpublished series, there were 29 robotic- and 27 laparoscopic proctectomy patients. The perioperative parameters, oncologic findings, and postoperative 30-day short-term outcomes of these patients were compared between the robotic surgery and laparoscopic surgery groups. Both groups were comparable in terms of patient demographics, body mass index (34.9 ± 7.2 vs. 35.2 ± 5.0 kg/m2, p = 0.71), comorbidities, and surgical and tumor characteristics except for histologic differentiation (p = 0.007). Comparison of the intraoperative findings revealed no significant differences between the two groups, including operative time (329.0 ± 102.2 vs. 294.6 ± 81.1 minutes, p = 0.13), blood loss (434.0 ± 612.4 vs. 339.4 ± 271.9 mL, p = 0.68), tumor distance to distal margin (3.9 ± 2.1 vs. 3.2 ± 2.0 cm, p = 0.17), resection margin involvement (2 [6.9%] vs. 2 [7.4%], p = 0.99), conversions (2 [6.9%] vs. 5 [18.5%], p = 0.24), and intraoperative complications (2 [6.9%] vs. 0 [0%], p = 0.49]. Regarding postoperative outcomes, there were no significant differences in complications, including surgical site infections, sepsis, ileus, and urinary complications. No anastomotic leak and no ureter injury were observed in both the groups. Other outcomes were also similar except that robotic surgery was associated with quicker return of bowel function (3.2 ± 1.9 vs. 4.3 ± 2.3 days, p = 0.01) and shorter hospital stay (6.4 ± 4.2 vs. 8.4 ± 4.4 days, p = 0.02). In conclusion, our own results suggest that robotic surgery compared with laparoscopy has similar short-term outcomes with an additional benefit of quicker return of bowel function and shorter hospital stay in obese patients undergoing rectal cancer surgery.
Cost of Robotic Colectomy
The cost of a robotic system, including its yearly maintenance fees and disposables, can represent a significant cost to hospitals and health systems. This is compounded by the lack of reimbursements by payers. Currently, robotic prostatectomy is the only procedure with an associated Current Procedural Terminology code. However, the reimbursements remain the same for laparoscopic and robotic cases. Initial studies reported that robotic colorectal surgery is associated with an additional $350 direct equipment cost per case.7 Despite the increasing clinical implementation of robotic colectomy, it is still more expensive than conventional laparoscopic procedures9 32 as well as open surgery.33 To date, there are no published reports that have established the cost-effectiveness of robotic colorectal surgery. Expected improvements in technology and potential competitions may reduce the cost of robotic surgery in the future.
Learning Curve
A few studies in the literature have investigated the learning curve for robotic colorectal surgery,34 35 36 37 38 39 including resection procedures. Specified learning curve values range from 15 to 35 cases. A study investigating learning curve of robotic total mesorectal excision showed that even a surgeon without laparoscopy experience could adapt robotic surgery after 20 cases in terms of operating time.39 However, further studies are warranted to determine the learning curve for robotic colon resection for cancer.
Advantages of Robotic Colectomy
Three-dimensional high-definition video imaging
Image magnification
Filtration of physiological tremor
Better ergonomics
Articulating robotic instruments
Intracorporeal anastomosis
Opportunity to perform remote surgery regardless of distance
Disadvantages of Robotic Colectomy
Prolonged operating time
Increased cost
Need of double docking in left colectomy requiring splenic flexure mobilization
Learning curve and need for specialized surgical team
Future Directions
The da Vinci Xi System (Intuitive Surgical Inc. Sunnyvale, CA) has just been made available to surgeons and it offers several potential advantages as compared to the previous versions of the da Vinci system. The major advancement with this new system is the ability to perform multiquadrant surgeries without repositioning the robot because of its overhead instrument arm design. In addition, it has improved three-dimensional visualization and smaller and thinner robotic arms which offer increased range of motion.
Conclusion
In the scope of colectomy for cancer, robotic surgery appears to offer short-term outcomes that are comparable to those of conventional laparoscopy in terms of length of hospital stay, morbidity, and mortality. In addition, robotic colectomy can be performed without compromising oncological principles, but data for long-term outcomes are still limited. Prolonged operating time, increased costs, and learning curve are the major drawbacks. Furthermore, the most commonly used robotic platform is very large and has a limited intracorporeal range of motion. Therefore, it poorly fits in efficient traction of the colon and multiquadrant operations. Newer generation machines offer new opportunities for complex colorectal surgery; however, the value of robotic-assisted surgery compared with conventional laparoscopic surgery remains unknown.
References
- 1.Park I J, Choi G S, Kang B M, Lim K H, Jun S H. Lymph node metastasis patterns in right-sided colon cancers: is segmental resection of these tumors oncologically safe? Ann Surg Oncol. 2009;16(6):1501–1506. doi: 10.1245/s10434-009-0368-x. [DOI] [PubMed] [Google Scholar]
- 2.Clinical Outcomes of Surgical Therapy Study Group . A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350(20):2050–2059. doi: 10.1056/NEJMoa032651. [DOI] [PubMed] [Google Scholar]
- 3.Steele S R Stein S L Bordeianou L G Johnson E Herzig D O Champagne B J; American Society of Colon and Rectal Surgeons' Young Surgeons Committee. The impact of practice environment on laparoscopic colectomy utilization following colorectal residency: a survey of the ASCRS Young Surgeons Colorectal Dis 2012143374–381. [DOI] [PubMed] [Google Scholar]
- 4.Baek S K, Carmichael J C, Pigazzi A. Robotic surgery: colon and rectum. Cancer J. 2013;19(2):140–146. doi: 10.1097/PPO.0b013e31828ba0fd. [DOI] [PubMed] [Google Scholar]
- 5.Alasari S, Min B S. Robotic colorectal surgery: a systematic review. ISRN Surg. 2012;2012:293894. doi: 10.5402/2012/293894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weber P A Merola S Wasielewski A Ballantyne G H Telerobotic-assisted laparoscopic right and sigmoid colectomies for benign disease Dis Colon Rectum 200245121689–1694., discussion 1695–1696 [DOI] [PubMed] [Google Scholar]
- 7.Delaney C P, Lynch A C, Senagore A J, Fazio V W. Comparison of robotically performed and traditional laparoscopic colorectal surgery. Dis Colon Rectum. 2003;46(12):1633–1639. doi: 10.1007/BF02660768. [DOI] [PubMed] [Google Scholar]
- 8.D'Annibale A, Morpurgo E, Fiscon V. et al. Robotic and laparoscopic surgery for treatment of colorectal diseases. Dis Colon Rectum. 2004;47(12):2162–2168. doi: 10.1007/s10350-004-0711-z. [DOI] [PubMed] [Google Scholar]
- 9.Park J S, Choi G S, Park S Y, Kim H J, Ryuk J P. Randomized clinical trial of robot-assisted versus standard laparoscopic right colectomy. Br J Surg. 2012;99(9):1219–1226. doi: 10.1002/bjs.8841. [DOI] [PubMed] [Google Scholar]
- 10.D'Annibale A, Pernazza G, Morpurgo E. et al. Robotic right colon resection: evaluation of first 50 consecutive cases for malignant disease. Ann Surg Oncol. 2010;17(11):2856–2862. doi: 10.1245/s10434-010-1175-0. [DOI] [PubMed] [Google Scholar]
- 11.Luca F, Ghezzi T L, Valvo M. et al. Surgical and pathological outcomes after right hemicolectomy: case-matched study comparing robotic and open surgery. Int J Med Robot. 2011;7(3):298–303. doi: 10.1002/rcs.398. [DOI] [PubMed] [Google Scholar]
- 12.Shin J Y. Comparison of short-term surgical outcomes between a robotic colectomy and a laparoscopic colectomy during early experience. J Korean Soc Coloproctol. 2012;28(1):19–26. doi: 10.3393/jksc.2012.28.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park S Y, Choi G S, Park J S, Kim H J, Choi W H, Ryuk J P. Robot-assisted right colectomy with lymphadenectomy and intracorporeal anastomosis for colon cancer: technical considerations. Surg Laparosc Endosc Percutan Tech. 2012;22(5):e271–e276. doi: 10.1097/SLE.0b013e31826581bd. [DOI] [PubMed] [Google Scholar]
- 14.Anvari M, Birch D W, Bamehriz F, Gryfe R, Chapman T. Robotic-assisted laparoscopic colorectal surgery. Surg Laparosc Endosc Percutan Tech. 2004;14(6):311–315. doi: 10.1097/01.sle.0000148473.05042.8f. [DOI] [PubMed] [Google Scholar]
- 15.Ballantyne G H, Ewing D, Pigazzi A, Wasielewski A. Telerobotic-assisted laparoscopic right hemicolectomy: lateral to medial or medial to lateral dissection? Surg Laparosc Endosc Percutan Tech. 2006;16(6):406–410. doi: 10.1097/01.sle.0000213732.03204.50. [DOI] [PubMed] [Google Scholar]
- 16.deSouza A L, Prasad L M, Park J J, Marecik S J, Blumetti J, Abcarian H. Robotic assistance in right hemicolectomy: is there a role? Dis Colon Rectum. 2010;53(7):1000–1006. doi: 10.1007/DCR.0b013e3181d32096. [DOI] [PubMed] [Google Scholar]
- 17.Deutsch G B, Sathyanarayana S A, Gunabushanam V. et al. Robotic vs. laparoscopic colorectal surgery: an institutional experience. Surg Endosc. 2012;26(4):956–963. doi: 10.1007/s00464-011-1977-6. [DOI] [PubMed] [Google Scholar]
- 18.Lujan H J, Maciel V H, Romero R, Plasencia G. Laparoscopic versus robotic right colectomy: A single surgeon's experience. J Robotic Surg. 2013;7(2):95–102. doi: 10.1007/s11701-011-0320-5. [DOI] [PubMed] [Google Scholar]
- 19.Trastulli S, Desiderio J, Farinacci F. et al. Robotic right colectomy for cancer with intracorporeal anastomosis: short-term outcomes from a single institution. Int J Colorectal Dis. 2013;28(6):807–814. doi: 10.1007/s00384-012-1604-6. [DOI] [PubMed] [Google Scholar]
- 20.Morpurgo E, Contardo T, Molaro R, Zerbinati A, Orsini C, D'Annibale A. Robotic-assisted intracorporeal anastomosis versus extracorporeal anastomosis in laparoscopic right hemicolectomy for cancer: a case control study. J Laparoendosc Adv Surg Tech A. 2013;23(5):414–417. doi: 10.1089/lap.2012.0404. [DOI] [PubMed] [Google Scholar]
- 21.Ostrowitz M B, Eschete D, Zemon H, DeNoto G. Robotic-assisted single-incision right colectomy: early experience. Int J Med Robot. 2009;5(4):465–470. doi: 10.1002/rcs.281. [DOI] [PubMed] [Google Scholar]
- 22.Morelli L, Guadagni S, Caprili G, Di Candio G, Boggi U, Mosca F. Robotic right colectomy using the Da Vinci Single-Site® platform: case report. Int J Med Robot. 2013;9(3):258–261. doi: 10.1002/rcs.1488. [DOI] [PubMed] [Google Scholar]
- 23.Wong M T, Ng K H, Ho K S, Eu K W. Single-incision laparoscopic surgery for right hemicolectomy: our initial experience with 10 cases. Tech Coloproctol. 2010;14(3):225–228. doi: 10.1007/s10151-010-0596-x. [DOI] [PubMed] [Google Scholar]
- 24.O'Connell J B, Maggard M A, Ko C Y. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst. 2004;96(19):1420–1425. doi: 10.1093/jnci/djh275. [DOI] [PubMed] [Google Scholar]
- 25.Parra-Davila E, Diaz-Hernandez J J. Totally robotic left colectomy. J Robot Surg. 2011;5(1):57–64. doi: 10.1007/s11701-011-0254-y. [DOI] [PubMed] [Google Scholar]
- 26.Parra-Davila E M. Ortiz-Ortiz C M Robotic left colectomy In: Robotics in General Surgery New York: Springer; 2014203–212. [Google Scholar]
- 27.Obias V, Sanchez C, Nam A, Montenegro G, Makhoul R. Totally robotic single-position ‘flip’ arm technique for splenic flexure mobilizations and low anterior resections. Int J Med Robot. 2011;7(2):123–126. doi: 10.1002/rcs.375. [DOI] [PubMed] [Google Scholar]
- 28.Lim D R, Min B S, Kim M S. et al. Robotic versus laparoscopic anterior resection of sigmoid colon cancer: comparative study of long-term oncologic outcomes. Surg Endosc. 2013;27(4):1379–1385. doi: 10.1007/s00464-012-2619-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Helvind N M, Eriksen J R, Mogensen A. et al. No differences in short-term morbidity and mortality after robot-assisted laparoscopic versus laparoscopic resection for colonic cancer: a case-control study of 263 patients. Surg Endosc. 2013;27(7):2575–2580. doi: 10.1007/s00464-013-2792-z. [DOI] [PubMed] [Google Scholar]
- 30.Buchs N. New trends in robotic colorectal surgery. Adv Robot Autom. 2014;3:e117. [Google Scholar]
- 31.Gorgun E Aytac E Gurland B Costedio M M Robotic versus laparoscopic colorectal surgery: A case matched study from a tertiary referral center Surg Endosc 201428(1, Suppl):441 [Google Scholar]
- 32.Tyler J A, Fox J P, Desai M M, Perry W B, Glasgow S C. Outcomes and costs associated with robotic colectomy in the minimally invasive era. Dis Colon Rectum. 2013;56(4):458–466. doi: 10.1097/DCR.0b013e31827085ec. [DOI] [PubMed] [Google Scholar]
- 33.Bertani E, Chiappa A, Biffi R. et al. Assessing appropriateness for elective colorectal cancer surgery: clinical, oncological, and quality-of-life short-term outcomes employing different treatment approaches. Int J Colorectal Dis. 2011;26(10):1317–1327. doi: 10.1007/s00384-011-1270-0. [DOI] [PubMed] [Google Scholar]
- 34.Sng K K, Hara M, Shin J W, Yoo B E, Yang K S, Kim S H. The multiphasic learning curve for robot-assisted rectal surgery. Surg Endosc. 2013;27(9):3297–3307. doi: 10.1007/s00464-013-2909-4. [DOI] [PubMed] [Google Scholar]
- 35.D'Annibale A, Pernazza G, Monsellato I. et al. Total mesorectal excision: a comparison of oncological and functional outcomes between robotic and laparoscopic surgery for rectal cancer. Surg Endosc. 2013;27(6):1887–1895. doi: 10.1007/s00464-012-2731-4. [DOI] [PubMed] [Google Scholar]
- 36.Bokhari M B, Patel C B, Ramos-Valadez D I, Ragupathi M, Haas E M. Learning curve for robotic-assisted laparoscopic colorectal surgery. Surg Endosc. 2011;25(3):855–860. doi: 10.1007/s00464-010-1281-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiménez-Rodríguez R M, Díaz-Pavón J M, de la Portilla de Juan F, Prendes-Sillero E, Dussort H C, Padillo J. Learning curve for robotic-assisted laparoscopic rectal cancer surgery. Int J Colorectal Dis. 2013;28(6):815–821. doi: 10.1007/s00384-012-1620-6. [DOI] [PubMed] [Google Scholar]
- 38.Park J S, Choi G S, Lim K H, Jang Y S, Jun S H. S052: a comparison of robot-assisted, laparoscopic, and open surgery in the treatment of rectal cancer. Surg Endosc. 2011;25(1):240–248. doi: 10.1007/s00464-010-1166-z. [DOI] [PubMed] [Google Scholar]
- 39.Kim Y W, Lee H M, Kim N K, Min B S, Lee K Y. The learning curve for robot-assisted total mesorectal excision for rectal cancer. Surg Laparosc Endosc Percutan Tech. 2012;22(5):400–405. doi: 10.1097/SLE.0b013e3182622c2d. [DOI] [PubMed] [Google Scholar]


