Background
There is considerable interest in investigating the role of the microbiota in various diseases, including transplant rejection. Germ-free (GF) and gnotobiotic mice are powerful models for this line of investigation, but performing surgery within the confines of a sterile housing isolator is exceptionally challenging. Development of rigorous protocols to be able to remove axenic mice from their sterile isolator for surgical intervention in a class II biological safety cabinet (BSC) without compromising sterility would give many investigators access to this model and broaden possible studies. However, it is assumed that GF animals will most often become colonized with environmental microbiota on leaving the isolator. In this study, we tested whether applying sterile techniques for animal transport out of the isolator and skin transplantation in a class II BSC could maintain animal sterility.
Methods
Quantitative polymerase chain reaction of the bacterial 16S ribosomal RNA gene, and cultures in various aerobic and anaerobic conditions were used to probe for bacterial contamination before and after transplantation.
Results
Of 28 surgeries performed, only 3 mice acquired bacterial contamination coincident with a transient shutdown of the ventilation system in the BSC.
Conclusions
Our results indicate that skin transplantation can be successfully performed in GF mice using sterile conditions for transport and surgery in a class II BSC, but requires continuous positive airflow. Our approach paves the way to investigating the role of the microbiota in modulating immune responses to skin allografts as a first model of solid organ transplantation in GF mice.
Orthotopic free skin grafting has been a standard procedure to study transplantation tolerance and rejection since the groundbreaking work developed by Peter Medawar and colleagues in the 1940s.1 In the early 1960s, the development of the flexible film isolator by Philip Trexler at the Laboratories of Bacteriology at Notre Dame coupled with the use of peracetic acid to provide cold chemical sterilization unleashed the ability to maintain axenic and gnotobiotic animals with relative ease and reduced budget compared to the contemporary standard practice requiring stainless steel isolators and large bulk autoclave units.2 Commercial rodent vendors used the new flexible film isolator and cold chemical sterilant technology to rederive existing rodent stocks and start an era of specific pathogen-free (SPF) rodent colonies.2,3 During the decades between the introduction of flexible film isolator technology and the NIH Human Microbiome roadmap initiative in 2005, the laboratory animal industry moved away from flexible film isolator housing of research colonies due to inherent limitations and expense of maintaining mice in this rigorous housing environment. Initially static plastic “shoe box” cages with microisolation lids were developed to house SPF mice. To assist in protecting the mice from adventitious pathogen exposure, laminar flow hoods and biological safety cabinets (BSCs) were used for open cage handling and procedures. In the late 1990s, individually ventilated cages (IVC) housed on ventilated racks began to replace static microisolation housing to enhance colony protection from adventitious pathogen exposure. Currently, this housing strategy has become somewhat standardized at research institutions housing large colonies of SPF rodents, particularly mice. In the early years of this millennium, the vast majority of academic research institutions had abandoned the use of sterile or gnotobiotic isolator housing for either static microisolation or IVC housing.
Currently, with renewed interest in using axenic and gnotobiotic mice to study relationships between host microbiota and host physiologic responses, academic and research institutions have re-established sterile isolator housing. Some institutions have existing programs, personnel with memory of isolator housing programs, but no recent experience, or are having to train personnel and build programs where they did not exist. Similarly, few institutions have available equipment to support sterile surgical manipulations of axenic animals. Limitations in using germ-free (GF) mice for transplantation studies include restriction to procedures that can be readily performed within a sterile isolator housing unit or a class III BSC. Indeed, previous reports of skin grafting in mice described the use of sterile flexible film isolator for axenic mouse transplant.4 However, modern class II BSCs designed to maintain product sterility within the cabinet's workspace are in common use in most research facilities maintaining SPF mice on IVC racks and could be used for surgery in GF mice if transportation from and back to the sterile isolator could reliably protect from external microbial contaminants. To our knowledge, there is no convenient and reliable procedure described for using a class II BSC to perform sterile surgery on GF mice. In fact, the prevailing tenet posits that once out of the sterile protective environment of the isolator, GF mice are readily contaminated. We hypothesized that with preparation of a BSC in a manner akin to preparing for sterile surgery, animal, personnel, and equipment sterility could be maintained. Here, we describe the procedures necessary and sufficient to allow for sterile orthotopic free skin graft surgeries to be performed within a class II BSC on GF mice with reintroduction to sterile isolator housing while maintaining sterile GF status.
MATERIALS AND METHODS
Chemical Sterilants
Chlorine dioxide (Pharmacal Clidox-S 1:3:1 solution) prepared at ratio of 1 part Clidox-S base, 3 parts water and 1 part Clidox-S activator was prepared manually at least 30 minutes before being used for chemical sterilization. Once prepared, the solution must be used or discarded within a 24-hour period as per product label specifications. Cidex Plus glutaraldehyde sterilization solution was prepared according to product label specifications.
Sterile Preparation of Dry Goods
All dry goods were rendered sterile by autoclaving at 250°F and 15 pounds per square inch for 40 minutes. Items were double wrapped with paper sterile field material (Busse Hospital Disposables) or instant sealing sterilization pouches (Fisherbrand).
Preparation of Surgical Assistant
A surgical assistant assumes responsibility for preparing the BSC before the start of each experiment and assisting the surgeon during the experimental procedures. The surgical assistant first washed their hands with surgical scrub and water. Hands were dried with sterile towels wrapped with the surgical gown. Once the surgical assistant had dried their hands, an autoclaved surgical gown was donned. Next, the surgical assistant donned a pair of sterile surgical gloves using closed gloving technique.5-7 Donning of the surgical gown and gloves occurred within the procedure space. The surgical assistant proceeded to the BSC and took a seat. Before introducing gloved and gowned arms and hands into the BSC, an assistant sprayed the surgical gloves with 1:3:1 chlorine dioxide.
BSC Advanced Preparation
A NuAire class II type B2 BSC was used for all surgical procedures. The blower and florescent light for the BSC were switched on. The back wall, sides, horizontal working surface, and inner protective shield were sprayed with 1:3:1 chlorine dioxide and allowed to air dry. Once the chlorine dioxide had air-dried within the BSC, the surgical assistant proceeded with setting up the surgical workspace. Sterile paper surgical drapes cut to the dimension of the inner BSC work surface were opened at the interface of the sash of the BSC. As a matter of standard practice, the left side of the BSC was used for material introduction and later considered the higher-risk area of the working space for potential contamination. Metal working surfaces of the BSC though sprayed with 1:3:1 chlorine dioxide were considered contaminated surfaces. Surgical drapes were placed to cover the entire horizontal working space without touching surgical gloves to the metal surface.
Sterile Container and Cold Pack Preparation
An autoclavable beaker containing a rubber coated lead weight was sterilized in double-wrapped surgical drape paper. The sterile beaker and weight were introduced into the BSC after the working surface had been draped. The beaker was then brought to the inner edge of the air grate of the BSC and an assistant poured prepared Cidex-Plus into the beaker. Using sterile forceps to hold a reusable cold pack, a nonsterile reusable cold pack with waterproof outer wrap was placed into the beaker and held down using the sterile lead weight. After 10 hours of soak in Cidex-Plus, an assistant wearing sterile gown and gloves removed the reusable cold pack with sterile forceps. The cold pack with external surface now rendered sterile was placed into a sterilized instant sealing sterilization pouch and sealed. The sealed pouch was then placed into a second sterilized instant sealing sterilization pouch, sealed, and removed from the BSC. The sealed pouches were then placed in a holding container within a 20°C freezer for a minimum of 12 hours.
Preparation of Surgeon
The surgeon donned their sterile attire following the same procedures as the surgical assistant and an assistant sprayed the surgical gloves with 1:3:1 chlorine dioxide before introducing gloved and gowned arms into the prepared BSC.The surgical assistant then placed a binocular magnifier (OptiVISOR 1¾× at 14") on the surgeon's head in preparation for surgical procedure.
Skin Transplantation Equipment and Preparation
Working from the left side of the BSC to the middle, the surgical assistant passed sterile materials to the surgeon at the BSC/sash interface using aseptic techniques. Materials included frozen ice pack, surgical instruments, orthopedic stockinette (Tex-Care medical Stockinette 91319-325), bookends used as a fiber optic light stand, aluminum foil, umbilical tape, syringes, needles, and surgical gauze. The surgeon drew sterile saline, ketamine, xylazine, and buprenorphine from sterile dispensing vials at the BSC/sash interface. To prepare fiber optic light for use within the BSC, bookends were placed at the right end of the BSC working space. The surgical assistant passed the fiber optic into the BSC, which was intercepted by the surgeon using an orthopedic stockinette. The orthopedic stockinette was unrolled over the length of the fiber optic. An additional layer of protection was provided by overlaying aluminum foil on the stockinette and securing with umbilical tape.
Mice
Axenic C57BL/6 mice were bred and maintained in The University of Chicago Gnotobiotic Research Animal Facility (GRAF). Mice were housed in positively pressured flexible film isolators and fed autoclaved 5K67 LabDiet. Mice were provided with autoclaved water and pine shaving bedding. Light cycles were maintained at 12:12, and environmental parameters of temperature and humidity were kept within the range required by The Guide for the Care and Use of Laboratory Animals, Eighth Edition.8 Male mice, 6 to 8 weeks of age, were used as tail skin allograft donors, and female mice, 6 to 8 weeks of age, were used as allograft recipients.
Transfer of Mice From Sterile Isolator Housing to BSC
A transport sleeve connecting the housing isolator to an autoclaved sterilization cylinder containing microisolation cage setups and sealed with Mylar was secured no less than 5 hours before the removal of mice. The transport sleeve and transfer port were fogged with 1:3:1 chlorine dioxide. The isolator inner port cap was removed, the mylar seal of the sterilization cylinder was punctured, and the cage setups introduced into the isolator. Mice were placed into the microisolation cage setups and the cages placed back into the sterilization cylinder. The inner port cap was replaced and the transport sleeve disconnected from the outer isolator transfer port using a twisting and scrunching motion. The sterilization cylinder containing the mice was then moved and positioned just outside of the BSC. The exposed end of the transfer sleeve was sprayed with 1:3:1 chlorine dioxide and opened at the interface of the BSC just above the air intake grate. Using care not to touch the sides of the transport sleeve, the surgeon reached into the cylinder and removed the cages containing the mice.
Skin Graft Harvest and Preparation
Axenic donors were anesthetized using ketamine (65-80 mg/kg) and xylazine (5-8 mg/kg), administered by intraperitoneal injection and euthanized by cervical dislocation in accordance with AVMA guidelines on Euthanasia, 2013 Edition. To harvest tail skin, the tail of the donor mouse was amputated at the base after euthanasia. A single midventral incision was made cranial caudal the length of the tail. Using thumb forceps, the tail skin was peeled from the underlying tissues. A single drop of 0.9% NaCl was placed on the ventral surface of the harvested tissue. The tissue was then placed on the inner lid surface of a sterile petri dish. The inner bottom portion of the petri dish was lined with sterile surgical gauze and moistened with sterile saline. The lid of the petri dish was then placed onto the bottom of the dish and the petri dish placed on a sterile ice pack while the donor mouse was prepared.
Skin Transplant Procedure
Recipient mice were anesthetized using ketamine/xylazine as described for donor preparation. Hair was removed from the dorsal thoracic region using dressing forceps and retrograde plucking. Lifting the skin with forceps, a rectangular area of skin was gently cut from the rostral mid-dorsal thoracic region with straight scissors. A drop of sterile saline was placed on the exposed tissue. Using forceps and scissors, a piece of donor tissue was trimmed to match the area of the recipient transplant site and then placed flat onto the tissue deficit. A sterile nonadhering dressing (Albahealth LLC, Cat. no. 220) was placed over the grafted tissue followed by 2 sterile adhesive dressings (Coverlet adhesive dressing, list no. 00231) which secured the dressing to the hair peripheral to the graft. Postoperative analgesia (buprenorphine, 0.05-0.1 mg/kg) was administered by subcutaneous injection. Mice were monitored in recovery cages within the BSC until conscious and maintaining sternal recumbency before transport to postsurgical sterile flexible film isolator.
Transferring Mice From the BSC to an Isolator
A 2-L wide mouth Nalgene container was introduced into the BSC using aseptic technique. Using large dressing forceps to lift the transplanted mice by the tail, each mouse was placed into the sterile Nalgene container. The lid to the container was sealed, and the container placed within one of the empty sterile cage setups with the wire-bar removed. The cage was then handed to the surgical assistant outside of the BSC. The cage was brought to the recovery isolator and the outer port cap removed from the sterile transfer port. The lid was removed from the sterile transport cage and an assistant wearing sterile gloves removed the sterile Nalgene container with mice from the cage. The outer surface of the Nalgene was sprayed with 1:3:1 chlorine dioxide and placed into the transfer port. The outer port cap was replaced and the transfer port fogged with 1:3:1 chlorine dioxide atomized with pressurized air. After no longer than 30 minutes, the inner port cap was removed, and the mice was brought into the sterile recovery isolator and housed.
Monitoring Mice for Skin Graft Rejection
Mice were monitored periodically until lights off for continued recovery from surgery and comfort. Mice were then monitored daily for continued stability and bandage assessment. On day 8 after transplantation, the mice were removed from the recovery isolator, transferred to the BSC, anesthetized, and bandages were removed. Mice were then recovered and transported back to the same recovery isolator. All support procedures and anesthesia were performed as described above and as outlined in Table 1. After bandage removal, the mice were monitored daily for graft rejection.
TABLE 1.
Chronological preparations for sterile skin transplantation in GF mice
Monitoring Mice for Sterility
All housing isolators were monitored for sterility following standard operating procedures. Briefly, random fecal samples were collected and subjected to cultivation in nutrient broth, Sabouraud Dextrose broth, and BBL brain heart infusion–prepared culture media. Culture conditions were 37°C aerobic and anaerobic and 42°C aerobic and were monitored at 24, 48, 72 hours and again at 5 days. Additionally, fecal samples were screened for contamination by DNA extraction and quantitative real-time polymerase chain reaction (PCR) using universal bacterial primers for the 16S RNA-encoding gene (IDT, 8F was 5′-AGA GTT TGA TCC TGG CTC AG-3′, and 338R was 5′-TGC TGC CTC CCG TAG GAG T-3′). After skin transplantation, each mouse had fecal material collected on a weekly basis and analyzed as described until removed from study.
RESULTS
Adapting Sterile Surgical Procedures to BSC Use
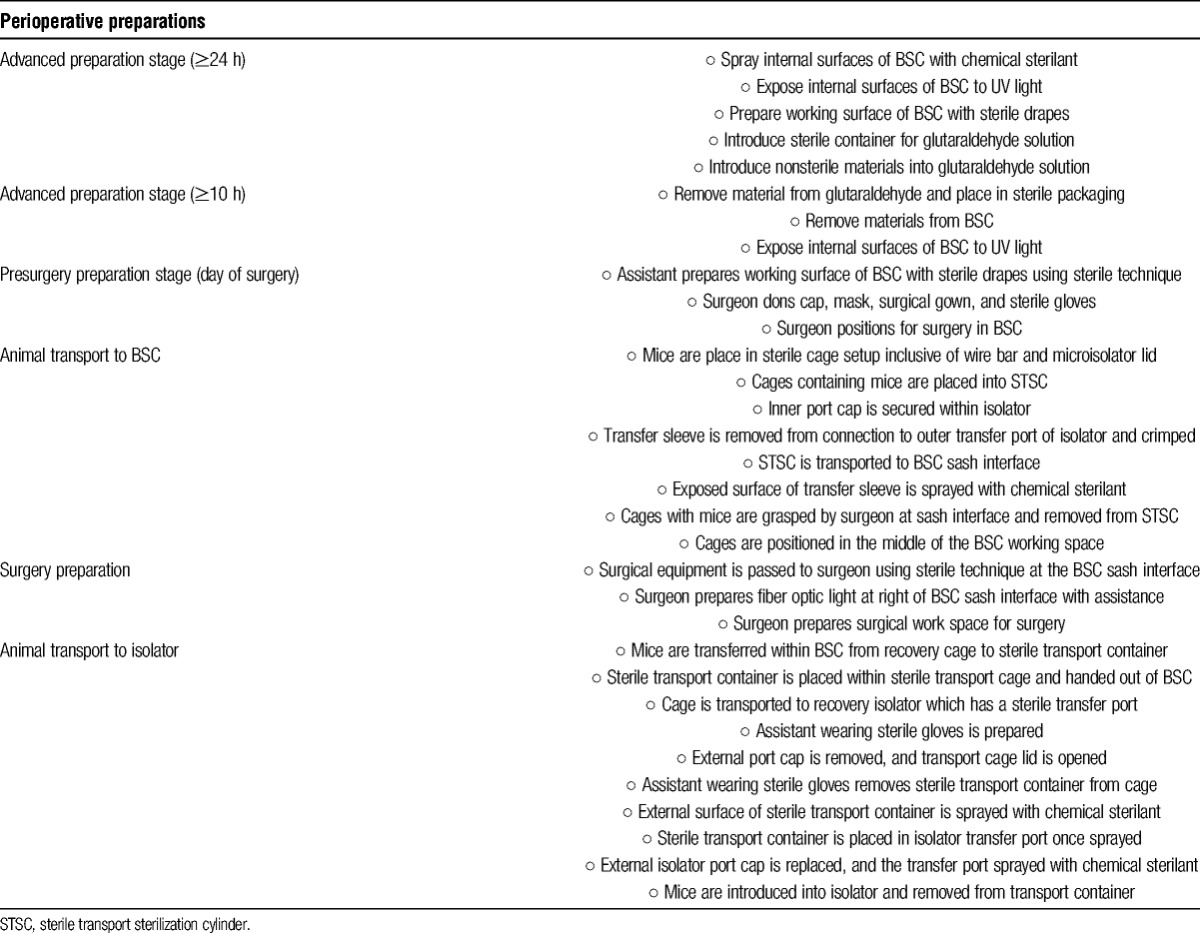
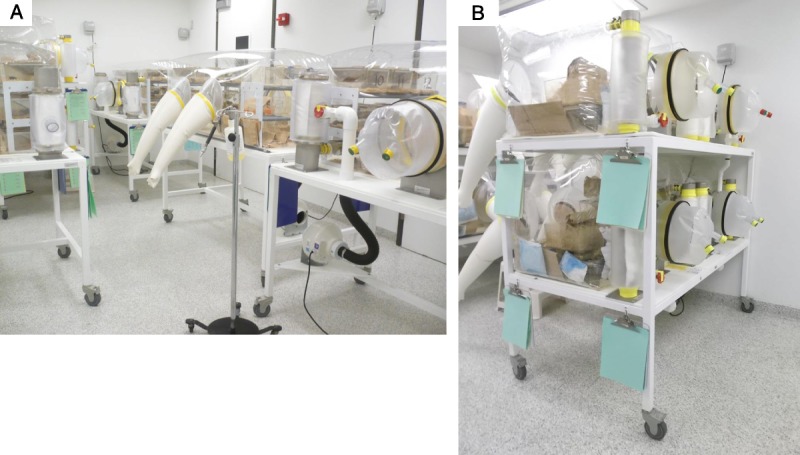
The GRAF housing our colony of axenic mice was built specifically to house GF and gnotobiotic mice in flexible film isolators (Figure 1). Included in facility design was a class II B2 BSC; however, no surgical isolator was available. With this limitation, we set about determining if skin transplantation studies using axenic mice could be achieved using the available class II B2 BSC (Figure 2A). In support of this consideration and cognizant of the aim of maintaining transplant recipient sterility, adaptation of sterile techniques rather than simple use of aseptic techniques was required. To achieve this goal, advanced planning in coordination with the GRAF personnel was required including staging of BSC preparation, preparation of materials requiring cold sterilization, animal transport, and surgeon preparation (Table 1). The procedures described in this study to create a sterile surgical area included using a class II B2 BSC, which had 100% high-efficiency particulate arrestance filtered air delivered to the working area of the BSC. This air was expected to be free of microbial contaminants. Once the BSC blower had been turned on, the interior surfaces were sprayed with a sterilizing concentration of chlorine dioxide. This process in itself was not considered sufficient to render the sprayed inner surfaces sterile; however, this process was consistent with high-level disinfection of the surface area. To enhance inner BSC surface disinfection, the use of an ultraviolet light with greater than 12-hour exposure was used. To limit exposure of materials introduced into the BSC to room air and to similarly limit exposure of the inner working area of the BSC to nonsterile surfaces or materials, sterile materials were passed into the BSC at the air curtain interface of the left or right working area of the BSC. All undraped stainless steel surfaces of the inner BSC, inclusive of the side and back walls as well as the air grate, were considered nonsterile (Figure 2B). All materials subsequently used within the BSC were permitted to touch the sterile drape material only. Any contact of materials with wall or grate surfaces was considered to represent a breach of sterility, and those items were immediately handed out of the BSC with care not to allow the contaminated surface to touch any area of the sterile working surface or the surgeon's or assistant's sterile gloves.
FIGURE 1.
Flexible film isolator housing areas of the Gnotobiotic Research Animal Facility. A, Single level tables with single large isolators. B, Double level tables with four isolators.
FIGURE 2.
Class II type B2 BSC. A, BSC with fluorescent lights on. White arrow shows hard duct connection of cabinet to room exhaust. B, Interior of BSC with sterile paper surgical drapes positioned over work surface. Although sprayed with chemical sterilant all exposed stainless steel surfaces are considered nonsterile.
Preparation of the Surgical Work Area ‘Within the BSC
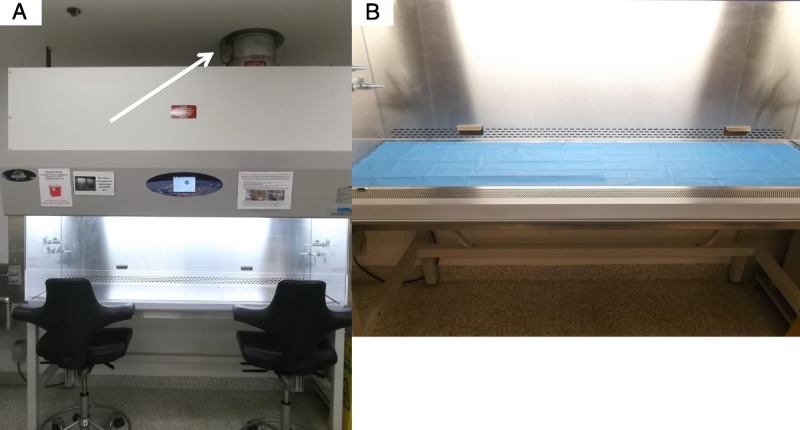
After the initial preparation, the inner working surface of the BSC was considered to have 3 virtual working zones (Figure 3). Zones 1 and 3 were areas to the left and right, respectively, of the central surgical area (zone 2) within the BSC. All sterile inanimate materials were double wrapped and passed into the BSC in zone 1 (Figure 4A) or zone 3 by unsealing the outer wrap and allowing the sterile gloved surgeon or assistant working in the BSC to remove the inner wrapped materials. Once the inner wrapped materials were brought into the BSC, they were removed from the inner wrap and the wrapping material passed out of the BSC to the nonsterile assistant. Similarly, when the GF mice were transported to the BSC within a sterile sterilization cylinder, they were introduced into the BSC through zone 1 and brought toward the area of zone 2 (Figure 4B, C). Using techniques adapted from orthopedic surgical procedures, any materials that could not be rendered sterile but rather only surface decontaminated were handed to the surgeon in zone 3. The surgeon intercepted these items using sterile materials to grasp the items so as to protect their surgical gloves. Such grasping sterile materials included a sterile surgical pouch for receiving the ice pack and a sterile orthopedic stockinette for receiving the fiber optic illuminator. To enhance the barrier between the sterile stockinette surface and the inner decontaminated fiber optic coil, sterile aluminum foil was wrapped over the stockinette containing the fiber optic coil and secured with sterile umbilical tape tied tightly onto the foil (Figure 4G). All anesthesia, surgical, and bandage removal procedures were performed in zone 2 by the surgeon (Figure 4D, E, F). To further mitigate risk of contamination within the BSC or of the GF mice, arm movements by the sterile assistant or surgeon within the BSC were minimized. Rather than reaching a distance to zone 1 or zone 3, the assistant or surgeon moved the wheeled chair they were sitting on to the specific work area while keeping their arms at the same distance within the BSC. Similarly, rather than reaching deep into the BSC which would necessitate bringing contaminated areas of the forearm into the BSC, reach was extended by use of 12-in sterile dressing forceps. Whenever possible, the mice were not handled or touched directly by the assistant's or surgeon's gloves, but rather by the sterile surgical instruments or forceps. In practice, handling of the sterile mice directly was limited to administration of injections, placement of free skin graft’, and bandage wrapping and removal.
FIGURE 3.
Sterility zones within the BSC. BSC with blue surgical drapes creating a sterile working surface. Zone 1 depicted by yellow arrows represents the region of the BSC where sterile materials are passed to the surgeon. Zone 2 depicted by white arrows represents the region of the BSC where procedures are performed. Zone 3 depicted by red arrows represents the region of the BSC where nonsterile materials protected by sterile external covering are positioned.
FIGURE 4.
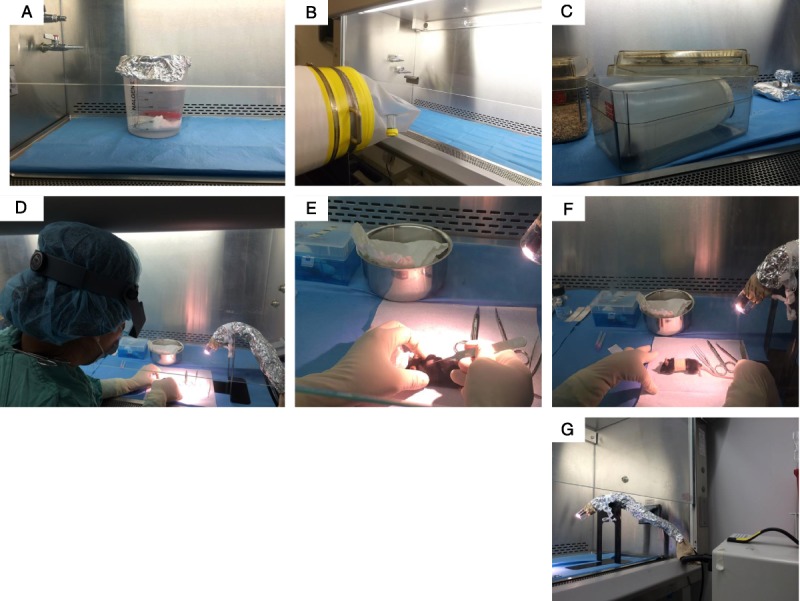
Skin transplantation procedure in GF mice. A, Zone 1 with reusable cold pack submerged in a beaker of gluteraldehyde secured with rubber coated lead weight. B, Sterilization cylinder with transport sleeve positioned to introduce GF mice and sterile caging material from flexible film isolator into zone 1 of BSC. C, Sterile cage containing a sterile wide mouth Nalgene bottle introduced into zone 1/zone 2 used to transport postoperative mice from BSC to isolator. D, Surgeon wearing magnification visor preparing surgical site at left edge of zone 2. E, Placement of skin graft on dorsal thorax of recipient GF mouse. F, Bandaged, anesthetized, postoperative recipient mouse. G, Fiber optic light positioned within the BSC in zone 3 with sterile mylar film over light source attached to sterile orthopedic stockinette, covered with sterile aluminum foil and secured with sterile umbilical tape to sterile metal stand.
Skin Transplantation Procedures
Three to 4 mice received skin transplants per surgery day. The period in which the GF mice were maintained within the BSC was less than 4 hours. Twenty-eight skin transplants have been performed by a dedicated microsurgeon (Table 2). Two recipient mice experienced graft failure due to technical reasons. Twenty-six free graft transplant recipients demonstrated successful engraftment as evidenced by primary intention healing of the grafted skin edges and observation of tail hair regrowth under bright light illumination and magnification. Three recipient mice became contaminated after an unfortunate rare event when transient airflow failure of the BSC occurred during the surgical interval.
TABLE 2.
Outcomes of Skin Transplantation in GF Mice

Monitoring for Sterility
Isolator and animal sterility were monitored on a weekly basis both before and after skin transplantation. With the exception of the mice contaminated during surgery due to transient BSC airflow failure, all transplanted mice remained sterile during the study interval as determined by 16S RNA quantitative PCR monitoring of bacterial load (Figure 5) and cultivation (Figure 6). For those mice contaminated by BSC failure, observation of culture-positive contamination was evident at 24 hours after culture incubation in all aerobic media conditions. Detection of culture positive contamination was determined by visual inspection and compared to positive and negative reference culture tubes prepared with each batch of test cultures (Figure 6).
FIGURE 5.
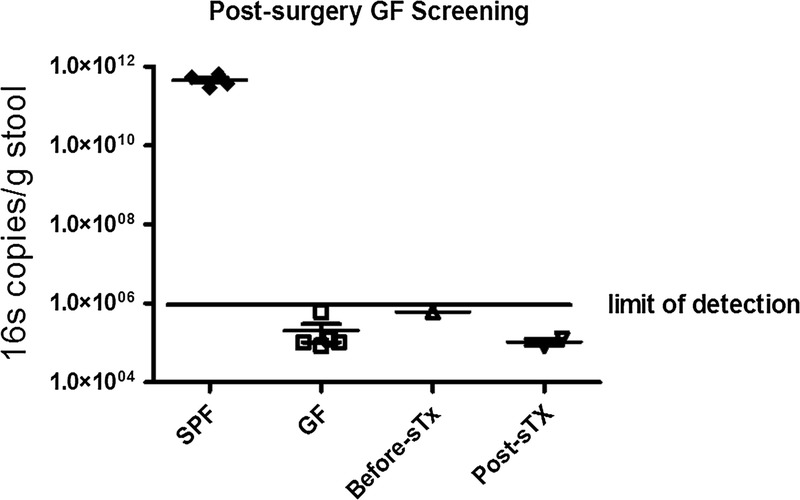
Absence of bacterial contamination of GF mice after skin transplantation. DNA was extracted from fresh stool and universal primers for the bacterial 16S ribosomal RNA gene were used to detect bacterial DNA quantitatively at day 7 before and after sTx. Results were normalized per gram of stool. sTx indicates skin transplantation.
FIGURE 6.

Monitoring for the presence of culturable bacteria in the stool of GF mice. Example of positive control tubes of Nutrient Broth, Sabouraud Dextrose Broth, and BBL Brain Heart Infusion Broth (red bracket group on left) and of negative control tubes Nutrient Broth, Sabouraud Dextrose Broth, and BBL Brain Heart Infusion Broth (yellow bracket group on right) at day 5 after culture setup.
DISCUSSION
Our results indicate that the procedures described here for (1) sterile use of a class II B2 BSC, (2) free skin graft transplant sterile surgery procedures, (3) sterile transport of GF mice in and out of a sterile flexible film isolator, and (4) to and from the isolator and BSC were sufficient to maintain sterility of the transplanted mice and their respective housing isolator for the duration of the planned experiments.
Skin transplantation into GF mice has been described in the 1960s and 1970s.9,10 However, the difficulty of performing surgery within the sterile isolator restricted the number of groups capable of expanding this line of research. Moreover, the limitation of techniques to monitor the continuing sterility of the animals after transplantation cautioned interpretation of the results. The current availability of quantitative real-time PCR to assess bacterial load longitudinally in individual animals before and after transplantation in an inexpensive and rapid manner is key to evaluating the success of the sterile techniques and to permitting subsequent interpretation of the data.
In the techniques described in this article, loss of sterility is most likely to occur during the surgical procedure, if the surgeon were to bring into the BSC some portions of the arms contaminated outside of the BSC and place them over the sterile working field. Another critical step is the return of the animals to the isolator as fog time for the port when using chlorine dioxide is 5 hours for complete sterilization, but only 30 minutes are used during the transfer of the mice for animal comfort. Contamination is mitigated by ensuring the port is sterile before transfer and by using sterile gloves and technique when transferring the transportation container into the port. The BSC contamination occurred on 1 single surgery day during our studies and was due to a BSC air curtain failure secondary to building exhaust failure, an unfortunate rare event. Indeed, type B2 class II BSCs use hard ducting and direct exhaust out of the facility. Because of operational design intended to protect the user from potential biological and chemical hazards within the BSC working area, when the BSC air curtain fails, the BSC internal blowers shut off.11 This design doubles the risk of contamination of GF mice not only if the BSC itself fails but also if the building exhaust fails. Provided the research does not involve biohazardous pathogens or chemicals, it is likely that type A2 class II BSCs, which are designed to have recirculated air rather than exterior exhaust, may also be appropriate for surgical manipulation of GF mice, though this would need to be tested.
For skin transplantation experiments, the maximum time that our animals spent outside of the sterile isolators was 4 hours. Our techniques can be applied to transplantation of both minor-mismatched and major-mismatched skin grafts, provided that genetically distinct strains are available in GF housing. Although any strain can be rederived as GF, through sterile extraction of pups by cesarean section and fostering by GF females, not every institution has rederivation capabilities. Also, whether the techniques described in this article will be sufficient to maintain sterility for surgical procedures that require longer periods in the BSC remains to be established. It is likely that each type of new procedure will present new challenges that will need to be uniquely overcome.
As a measure of precaution, all our transplanted animals were returned into a different isolator than that used to keep breeders and animals before surgery. The availability of separate breeding and return isolators ensures that potential contamination is not transferred to nonsurgery animals.
In conclusion, sterile surgical techniques in a class II type B2 BSC and sterile animal transport from and to the isolator housing unit are sufficient to maintain sterility of GF mice during skin transplantation, provided positive air flow is continuously maintained in the BSC. These optimized techniques will enable future probing of how absence of microbiota in axenic mice or presence of select microbial species in gnotobiotic mice affect alloimmunity to a transplanted organ.
ACKNOWLEDGMENTS
The authors thank Kristin Kolar from the GRAF for exceptional technical help.
Footnotes
B.T. and Y.W. are co-first authors. C.B. and M.-L.A. are co-last authors.
This work was supported by NIH/NIAID RO1 AI115716 to MLA.
The authors declare no conflicts of interest.
B.T. designed the sterile approach and wrote the article. Y.W. performed the microsurgery and helped plan the sterile approach. L.C. and C.B. helped design the sterile approach and assisted during the surgery. A.V. helped design the sterile approach. M.L.A. designed the project and edited the article.
REFERENCES
- 1. Billingham RE, Medawar PB. The technique of free skin grafting in mammals. J Exp Biol. 1951; 28: 385– 402. [Google Scholar]
- 2. Lindsey JR, Baker HJ. Historical Foundations. In: Suckow MA, Weisbroth SH, Franklin CL, editors. The Laboratory Rat. Burlington, MA: Elsevier Academic Press; 2006: 1– 47. [Google Scholar]
- 3. Rahija RJ. Gnotobiotics. In: Fox JG, Barthold SW, Davisson MT, et al. The Mouse in Biomedical Research. Vol 3 2nd ed Burlington, MA: Elsevier Academic Press; 2007: 217– 233. [Google Scholar]
- 4. Seibert K, Pollard M. A simple method for skin grafting in germfree mice. Lab Anim Sci. 1973; 23: 256– 258. [PubMed] [Google Scholar]
- 5. Roush JK. Infection Control. In: Harari J, editor. Small Animal Surgery. Media, PA: Williams & Wilkins; 1996: 23– 31. [Google Scholar]
- 6. Knecht CD, Allen AR, Williams DJ, et al. Fundamental Techniques in Veterinary Surgery. 2nd ed Philadelphia, PA: W. B. Saunders Company; 1981. [Google Scholar]
- 7. Holmberg D. Surgical Methods. In: Slatter D, editor. Textbook of Small Animal Surgery 2nd ed Philadelphia, PA: W. B. Saunders Company; 1993. [Google Scholar]
- 8.National Research Council. Guide for the care and use of Laboratory Animals 8th ed Washington, DC: The National Academies Press; 2011. [Google Scholar]
- 9. Miller JF, Dukor P, Grant G. The immunological responsiveness of germ-free mice thymectomized at birth. I. Antibody production and skin homograft rejection. Clin Exp Immunol. 1967; 2: 531– 542. [PMC free article] [PubMed] [Google Scholar]
- 10. Smith CS, Pilgriim HI, Steinmuller D. The survival of skin allografts and xenografts in germ-free mice. Transplantation. 1972; 13: 38– 41. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention; National Institutes of Health. Biosafety in Microbiological and Biomedical Laboratories. 5th ed Washington, DC: U.S. Government Printing Office; 2009. [Google Scholar]


