Abstract
The kainate-type of ionotropic glutamate receptors are assembled from a combination of five different pore-forming subunits (GluK1-5), which confer distinct functional and pharmacological properties. These receptors are also modulated by co-assembly with the auxiliary subunits Neto1 and Neto2. To determine the impact of variation in subunit composition on the functional interaction between kainate receptors and Neto subunits, the Neto subunits were combined with either GluK1 or GluK2 in HEK-293T cells and responses to glutamate examined through patch-clamp recordings. Co-expression of GluK1 with either Neto1 or Neto2 caused a substantial increase in glutamate sensitivity and a slowing of the onset of desensitization at low agonist concentrations. However, at higher glutamate concentrations the primary effect of Neto2 was to slow the onset of desensitization, while that of Neto1 was to increase recovery from desensitization. In contrast, co-expression of Neto2 with GluK2 homomeric receptors had only modest effects on glutamate sensitivity, but increased the rate of recovery from desensitization as well as slowing its onset at all agonist concentrations. The properties of chimeric Neto1/Neto2 subunits suggested that the extracellular N-terminal region including the two CUB domains was largely responsible for the distinct regulatory effects of Neto1 and Neto2 on the desensitization properties of GluK1 homomeric receptors. These results further demonstrate that the functional effects of Neto subunits depend upon the subunit identity of both the auxiliary and the pore-forming subunits.
1. Introduction
Many ion channels are regulated by assembly with auxiliary subunits, which unlike the pore-forming subunits are not required for channel formation or activity, but are able to alter function and localization in a wide variety of ways (Yu et al., 2005). Within the family of ligand-gated channels, the AMPA and kainate types of ionotropic glutamate receptors have been found to associate with auxiliary subunits. While the AMPA receptors are regulated by a large and diverse group of auxiliary subunits (Guzman & Jonas, 2010; Jackson & Nicoll, 2011), only the Neto1 and Neto2 subunits have so far met all the criteria for auxiliary subunits of the kainate receptors (Copits & Swanson, 2012).
Structurally the two Neto subunits are highly homologous, but they have very different expression patterns as well as distinct functional effects. The Neto1 subunit is found at high levels predominantly in the CA3 region of the hippocampus while Neto2 is broadly distributed throughout the mammalian brain (Ng et al., 2009; Straub et al., 2011a). Both Neto subunits have been demonstrated to associate with neuronal kainate receptors and modify their functional responses to glutamate (Zhang et al. 2009; Tang et al, 2011; Straub et al., 2011a). Neto1 appears to be critical for the slow decay kinetics that are characteristic of post-synaptic currents mediated by kainate receptors at the mossy fiber-CA3 synapse, and which are lost with genetic deletion of Neto1, but not Neto2 (Tang et al., 2011; Straub et al., 2011a).
The Neto subunits may interact in distinct ways with different kainate receptor subunits. Tetrameric kainate receptors assemble from a combination of five pore-forming subunits (GluK1-GluK5). The GluK1-3 subunits are able to form functional homomeric receptors while the GluK4-5 subunits (formerly KA1 and KA2) require co-assembly with GluK1-3 (Lerma & Marques, 2013). Most post-synaptic kainate receptors are likely to be heteromeric and to contain GluK1 or GluK2 subunits in combination with GluK4 or GluK5 (Herb et al., 1992; Contractor et al., 2003; Darstein et al., 2003; Fernandes et al., 2009; Palacios-Filardo et al. 2015). In adult rodents, the GluK2 subunit is found at high levels throughout the brain, while the GluK1 subunit is more restricted, and is located predominantly in hippocampal interneurons and cerebellar Purkinje cells (Bahn et al., 1994). However, the GluK1 subunit is highly expressed in the immature brain, where it may be important for synaptic development (Vesikansa et al., 2012; Lauri et al., 2005, 2006; Carta et al., 2014). In the absence of Neto subunits, GluK1 and GluK2 each form homomeric receptors with similar functional properties, characterized by relatively low sensitivity to glutamate and rapid and complete onset of desensitization with slow recovery from desensitization (Sommer et al, 1992; Heckmann et al,. 1996; Paternain et al., 1998; Fisher & Fisher, 2014).
Coexpression of either Neto1 or Neto2 with recombinant kainate receptors has been shown to change the rates of deactivation as well as onset and recovery from desensitization although the relative amagnitudes of these effects are quite different and may be subunit-dependent (Copits & Swanson, 2012). Neto1 enhances recovery from desensitization for GluK1, GluK2 and GluK2/5 receptors, but slows the onset of desensitization in response to saturating agonist concentrations only for GluK2 homomers (Copits et al., 2011; Fisher & Mott, 2013; Palacios-Filardo et al., 2015). In contrast, Neto2 has been reported to slow the onset of desensitization for both GluK1 and GluK2 homomers, speed recovery for GluK2 receptors (Zhang et al., 2009) and either enhance (Straub et al., 2011b) or have no effect (Copits et al., 2011) on recovery of GluK1 homomers. In addition to their effects on gating kinetics, both Neto1 and Neto2 have been suggested to enhance membrane trafficking and synaptic localization of some kainate receptors, although these effects may be region-specific (Copits et al, 2011; Straub et al., 2011a; Tang et al., 2011; Tang et al., 2012; Wyeth et al., 2014).
These previous results describing the functional effects of the Neto subunits on recombinant receptors have largely been obtained using applications of saturating agonist concentrations. Comparing the responses of receptors over a variety of activation levels can provide insight into the regulatory actions of auxiliary subunits (Fisher & Mott, 2013). Therefore, we examined the impact of variations in both kainate receptor subunit and auxiliary subunit composition on the response of GluK1 or GluK2 homomers to a range of glutamate concentrations. Whole cell recordings from transfected HEK-293T cells were used to determine glutamate EC50s while rapid application recordings from excised patches were used to quantify onset of and recovery from desensitization. The results demonstrate that the effects of Neto1 and Neto2 auxiliary subunits on glutamate sensitivity and concentration-dependent desensitization properties are quite distinct, and are influenced by the subunit composition of the kainate receptors.
2. Materials and Methods
2.1. Transient transfection of HEK-293T cells
HEK-293T cells (GenHunter, Nashville TN) were maintained in Dulbecco’s modified Eagle medium (DMEM) with 10% fetal bovine serum and antibiotics (100 IU/ml penicillin, 100 μg/ml streptomycin). Cells were transfected using calcium-phosphate precipitation with full-length cDNAs for rat GluK1-2a(Q) (obtained from Dr. C. Mulle, Univ. Bordeaux, France), rat GluK2(Q)(from Dr. S. Heinemann, Salk Institute, San Diego CA), mouse Neto1 (from Dr. M. Salter, Univ Toronto, Canada), human Neto1 and/or rat Neto2 (both from Dr. S. Tomita, Yale University, New Haven CT) in mammalian expression vectors. Human Neto1 was sub-cloned into the same vector as Neto2 (pCDNA3.1) to facilitate generation of the chimeric subunits. Plasmids were added at a 1:4 ratio (1 μg GluK1/2:4 μg Neto1/2) to increase incorporation of the Neto subunit into the receptor complexes (Fisher & Mott, 2012). To isolate the transfected cells, 1 μg of the plasmid pHook™-1 (Invitrogen Life Technologies, Grand Island NY) was also included (Chesnut et al., 1996). The selection procedure for the surface antibody encoded by pHook was performed 18-28 hrs following transfection. Cells were detached by a 2 min treatment with 0.025% trypsin, resuspended in DMEM, and incubated with antigen-coated magnetic beads for 30-60 min at 37°C (Chesnut et al., 1996). Cells bound to beads were then isolated with a magnetic stand, plated onto collagen-coated glass coverslips and incubated in supplemented DMEM overnight.
2.2. Electrophysiological recordings
Cells bound to at least 3 magnetic beads were utilized for electrophysiological recordings. Patch pipettes (resistance of 5-10 M) were manufactured from borosilicate glass (World Precision Instruments, Sarasota FL) using a dual-stage micropipette puller (Narishige, Japan). Cells were continually perfused with an external bath solution consisting of (in mM): 150 NaCl, 3 KCl, 10 HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid), 1 CaCl2 and 0.4 MgCl2 with pH 7.4 and osmolarity adjusted to 295–305 mOsm. Recording electrodes were filled with a solution containing (in mM); 130 CsGluconate, 5 CsCl, 0.5 CaCl2, 10 HEPES, 5 CsBapta, 2 MgCl2, 2 MgATP and 0.3 NaGTP adjusted to pH 7.4 and 290–300 mOsm. Glutamate was diluted into the bath solution from freshly made or frozen stocks in water. For whole-cell recordings a computer-driven solution application system was utilized (SF-77B, Harvard Apparatus, Holliston, MA) with an open-tip exchange time of <50 msec. For rapid application recordings, the cell membrane was excised immediately after achieving the whole-cell configuration to produce outside-out patches and the glass applicator modified to reduce transition time. The open-tip exchange time for these recordings was <400 μsec.
2.3. Chimera construction
The Neto1/Neto2 chimeric subunits were created by generation of unique restriction sites using site-directed mutagenesis (QuikChange, Agilent Technologies, Santa Clara CA). A new restriction site was introduced into Neto1 and Neto2 sequences to permit a splice site within the extracellular domain of the subunits, at a location following the two CUB domains (between F258 and Q259 of Neto1 and F259 and R260 of Neto2 (numbering from the mature sequence)). The products from the restriction digest were then gel-purified, exchanged, and re-ligated. Any sequence changes resulting from the introduction of the restriction site were reversed with site-directed mutagenesis following re-ligation. Oligonucleotide primers were synthesized by Integrated DNA Technologies (Coralville, IA) and constructs were verified by DNA sequencing (University of South Carolina Environmental Genomics core, Columbia, SC).
2.4. Data Analysis and statistical tests
Currents were analyzed using the programs Clampfit (pClamp10.3 suite, Axon Instruments, Foster City, CA) and Prism (Graphpad, San Diego, CA). Concentration-response data was fit with a four-parameter logistic equation (Current = [minimum current + (maximum current - minimum Current)]/1+(10^(log EC50 – log [agonist])*n) where n represents the Hill number. These fits were made to normalized data with the peak current measured at a given agonist concentration expressed as a percentage of the maximum response for each cell. Decay rates were fit with the Levenberg-Marquardt least squares method and the number of exponentials required for the fit determined through F test of the sum of squared residuals (Clampfit). Unpaired t-tests, ANOVA, and post-hoc Tukey-Kramer multiple comparisons tests of current amplitudes, time constants, %areas, or logEC50 values were performed using the Instat program (Graphpad) with a significance level of p<0.05.
3. Results
3.1. Neto1 and Neto2 increase glutamate sensitivity of homomeric GluK1 receptors
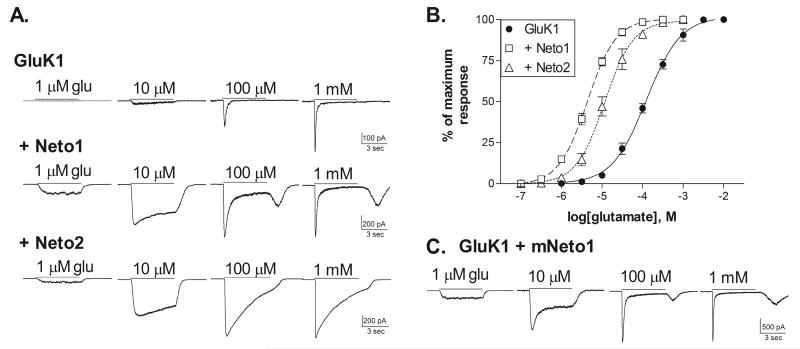
The GluK1 kainate receptor subunits are able to form functional homomeric channels that respond to glutamate with rapidly desensitizing currents (Sommer et al., 1992; Swanson & Heinemann, 1998; Fisher & Fisher, 2014). We examined the impact of Neto1 and Neto2 on the characteristics of the whole-cell responses of GluK1 homomers (Figure 1). The glutamate EC50 of the GluK1 receptors in the absence of Neto averaged 124.6±16.2 μM (n=5) (Figure 1B). Addition of either Neto subunit greatly increased glutamate sensitivity, with average EC50s of 4.4±0.4 (n=7) with Neto1 and 13.7±4.2 μM (n=6) with Neto2 (both p≤0.001 compared to GluK1 alone). These large shifts in glutamate sensitivity are in contrast to the effects of Neto subunits at homomeric GluK2 receptors, where they have only modest (2-3×) impact (Zhang et al., 2009; Fisher & Mott, 2013) and are comparable to the findings of Palacios-Filardo et al. (2015). The characteristics of the glutamate-activated whole-cell currents with and without Neto were also distinctive (Figure 1A). With either Neto1 or Neto2, low agonist concentrations (up to 10-30 μM) produced little desensitization during the 5 second application period. At higher concentrations, the GluK1+Neto1 responses began to show desensitization, and a rebound current was evident following agonist removal. This suggests that recovery from desensitization was faster than deactivation and/or agonist washout under these recording conditions. In contrast, the GluK1+Neto2 receptors continued to exhibit very slow and incomplete desensitization even in response to mM levels of glutamate. The properties of the GluK1+Neto2 receptors are similar to those previously reported for responses to saturating glutamate concentrations (Straub et al., 2011b; Copits et al. 2011). However, the appearance of a rebound current has not been noted for the combination of GluK1+Neto1, although this phenomenon was also observed with addition of Neto1 to heteromeric, GluK5-containing receptors (Fisher & Mott, 2013), and can be seen in responses to glutamate of retinal neurons expressing Neto1 (Puthussery et al., 2014). The present studies were performed with a human Neto1 subunit while the initial report used a mouse clone (Copits et al. 2011). To determine if species variations might be responsible for these different observations, we co-transfected GluK1 with mouse Neto1 and examined the whole-cell responses to glutamate (Figure 1C). The glutamate EC50 for GluK1+mNeto1 averaged 3.7±0.6 μM (n=6) (not shown), and was not significantly different from GluK1+hNeto1 (p>0.05). In addition, the whole-cell current traces showed similar characteristics, including the occurrence of a rebound current at high glutamate concentrations. Thus, no substantial functional differences were apparent between the mouse and human Neto1 subunits in their interaction with GluK1 under these recording conditions.
Figure 1. Co-expression of Neto1 or Neto2 with GluK1 alters glutamate sensitivity.
A. Whole-cell current traces from HEK-293T cells transiently transfected with GluK1 alone, or in combination with either human Neto1 or rat Neto2. Cells were voltage-clamped at −70 mV in the whole-cell configuration. Glutamate was applied for 5 sec (solid bar) at the concentration indicated.
B. Glutamate concentration-response relationships were constructed by normalizing the peak amplitude of the current response to the maximum response to saturating agonist levels for each cell. Symbols represent mean ± SEM. Averaged data was fit with a sigmoidal curve (solid or dashed lines). The glutamate EC50s for the fits shown were 124.1 μM (GluK1, n=5), 4.3 μM (+Neto1, n=7) and 11.7 μM (+Neto2, n=6).
C. Representative whole-cell current traces from cells transfected with GluK1 and mouse Neto1. Responses obtained as described in A.
3.2. Neto1 and Neto2 have distinct effects on the kinetics of desensitization
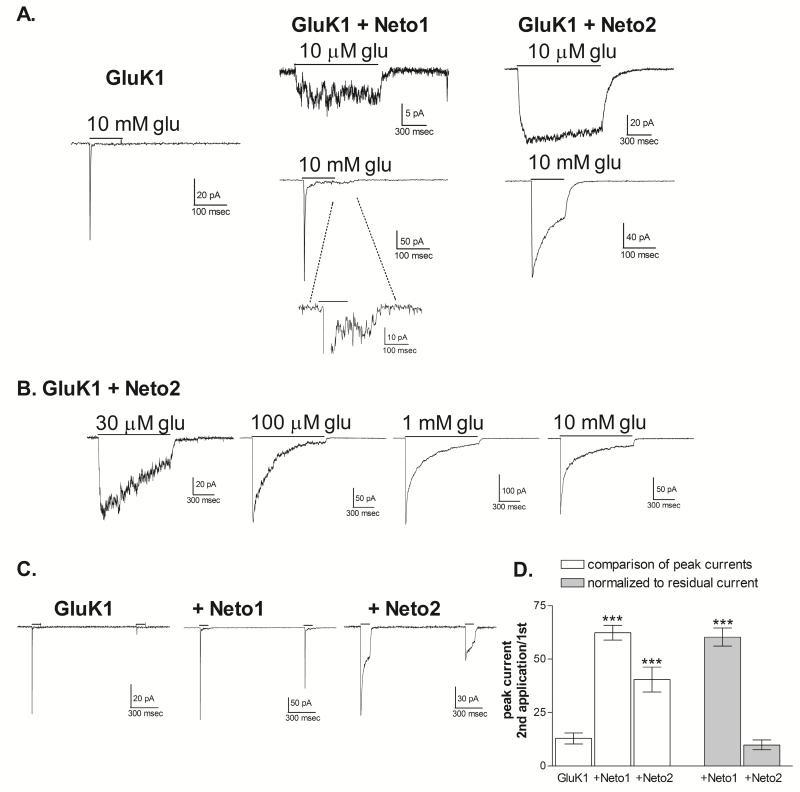
To compare the macroscopic kinetic properties of desensitization, outside-out patches were excised from transfected cells. Onset of desensitization was initially examined using 1 sec applications of 10 μM glutamate and 100 msec applications of 10 mM glutamate (Figure 2A). For receptors containing Neto1 or Neto2, 10 μM glutamate, is a level near the EC50 concentration (Figure 1), and produced responses that showed little macroscopic desensitization in outside-out patches. As previously reported in studies using lifted whole-cell recordings (Copits et al., 2011), we found that the rate of desensitization in response to a saturating agonist concentration was slowed by Neto2, but not by Neto1. 10 mM glutamate produced complete desensitization of homomeric GluK1 receptors without Neto1 within the 100 msec period and the decay was fit with a single exponential, with an average τ of 6.73 ± 2.38 msec (n=11). With the addition of Neto1, desensitization remained rapid, with an average τ of 2.59 ± 0.50 msec (n=4, p>0.5 compared to GluK1 alone), but was incomplete, with a residual current averaging 3.1 ± 0.6% of the peak current. A small rebound current could be observed even with the rapid solution exchange conditions provided by the excised patch recordings (Figure 2A). These results are consistent with previous findings that Neto1 speeds desensitization of GluK1 homomeric receptors when the agonist sites are saturated (Copits et al., 2011). However, the response to 10 μM glutamate demonstrates that macroscopic desensitization of partially occupied receptors is clearly reduced by Neto1, consistent with the observations using whole-cell recordings (Figure 1A). Addition of Neto2 to GluK1 dramatically slowed the onset of desensitization to both low and high agonist concentrations, and the current decay of the response to 10 mM glutamate was best fit with the sum of two exponential components. The weighted mean τ averaged 62.4±18.4 msec (n=5, p≤0.001 compared to GluK1 alone), within the range of previous reports (Copits et al., 2011; Straub et al., 2011b). Desensitization was not complete within the 100 msec application period, with a residual current averaging 39.1±4.1% of the peak response.
Figure 2. Effect of Neto1 and Neto2 on macroscopic desensitization kinetics of GluK1 homomers.
A. Outside-out patches were excised from HEK-293T cells transiently transfected as indicated. 10 mM glutamate (100 msec) or 10 μM glutamate (1 sec) was applied (solid line) to patches voltage-clamped at −70 mV. Traces shown are averages of 3-5 applications to the same patch. Inset for GluK1+Neto1 response to 10 mM glutamate shows rebound current following agonist removal.
B. Cells were transiently transfected with GluK1 and Neto2. Responses from outside-out patches were obtained with 1 sec applications of glutamate at the concentration indicated. All traces shown were obtained from different patches. The currents displayed are normalized to the same relative peak amplitude to facilitate comparison of the decay properties.
C. Paired applications of 10 mM glutamate (100 msec) were given 1 sec apart to outside-out patches voltage-clamped at −70 mV. Cells were transfected as indicated.
D. The % recovery was calculated by dividing the peak amplitude of the second response by that of the first and multiplying by 100. To normalize the effect of the non-desensitized receptors as represented by the residual current, the amplitude of the current at the end of the first 100 msec application was subtracted from both responses. *** (p≤0.001) represents a significant difference from GluK1 receptors without Neto.
The large current amplitude associated with the GluK1+Neto2 receptors permitted a fuller characterization and quantification of their concentration-dependent desensitization properties (Figure 2B, Table 1). Glutamate concentrations from 10 μM to 10 mM were applied for 1 sec to outside-out patches (Figure 2B). In general, the characteristics were consistent with the qualitative observations from the whole-cell recordings. While 10 μM glutamate produced little to no desensitization over the application period the decay with 30 μM glutamate was fit with a single exponential component (designated as the slower component τ2 in Table 1). At glutamate concentrations of 100 μM and higher, a faster component (designated τ1) appeared, and the contribution (% area) of this fast component increased as the glutamate concentration increased. The time constants of the two components became briefer with increasing glutamate concentrations up to 1 mM glutamate, but 10 mM glutamate did not produce any additional change. The residual current measured at the end of the 1 sec application remained at near 10% of the peak current for concentrations of 100 μM and higher.
Table 1.
Desensitization onset summary (1 second application to excised patches)
| Receptor isoform | 30 μM glutamate |
100 μM glutamate |
1 mM glutamate |
10 mM glutamate |
|
|---|---|---|---|---|---|
| GluK1 + Neto2 | τ1 (ms) | - | 44.2 ± 11.8 | 24.7 ± 6.4 | 27.2 ± 2.2 |
| τ1 area | 0% | 35.7 ± 4.9% | 41.8 ± 7.0% | 52.6 ± 4.8% | |
| τ2 | 854.3 ± 406.2 | 411.0 ± 135.4 | 282.7 ± 3.2 | 284.1 ± 56.6 | |
| residual (% of peak) | 3 3.3 ± 2.3% (n=4) |
9.5 ± 1.5% (n=7) |
9.5 ± 3.3% (n=3) |
8.6 ± 0.7% (n=5) |
|
|
| |||||
| GluK2 | τ1 | 35.5 ± 0.4 | 11.4 ± 0.5 | 5.5 ± 0.6 | 5.5 ± 0.9 |
| τ1 area | 87.5 ± 4.1% | 98.9 ± 0.6% | 100% | 100% | |
| τ2 | 150.6 ± 20.3 | 120.9 ± 48.1 | - | - | |
| residual | 1.4 ± 0.2% (n=8) |
0.4 ± 0.1% (n=8) |
0.07 ± 0.04% (n=4) |
0.1 ± 0.04% (n=4) |
|
|
| |||||
| GluK2 + Neto2 | τ1 | - | 60.1 ± 15.3 | 36.4 ± 6.5 | 30.4 ±3.7 |
| τ1 area | 0% | 69.3 ± 5.1% | 80.6 ± 2.3% | 84.2 ± 4.8% | |
| τ2 | 424.8 ± 66.5 | 247.7 ± 55.8 | 244.4 ± 24.5 | 246.5 ± 24.0 | |
| residual | 26.9 ± 2.5% (n=9) |
4.3 ± 0.3% (n=4) |
2.4 ± 0.4% (n=5) |
2.7 ± 1.0% (n=9) |
|
τ area represents % contribution to the 2-component exponential fit. Residual current is measured at the end of the 1 second application.
Earlier work showed that addition of Neto1 speeds recovery from desensitization for GluK1 or GluK2-containing receptors (Copits et al., 2011; Fisher & Mott, 2013), while the effects of Neto2 on this characteristic are less clear (Zhang et al., 2009; Copits et al., 2011; Straub et al., 2011b). To compare the effect of Neto1 and Neto2 on the recovery of homomeric GluK1 receptors, 10 mM glutamate was applied for 100 msec with a 1 second interval between applications (Figure 2C). The response of GluK1 receptors showed little recovery at this time point, consistent with previous reports (Swanson & Heinemann, 1998; Copits et al., 2011; Straub et al., 2011a). Addition of Neto1 significantly increased the peak amplitude of the second response relative to the first (Figures 2C, 2D), indicating a substantial increase in the recovery from desensitization. Addition of Neto2 also appeared to increase recovery, when only the peak amplitudes are considered (Figures 2C, 2D). However, because desensitization was incomplete, a substantial population of the receptors remained open, rather than desensitized, at the end of the 100 msec application. When this residual current was subtracted from both responses, the actual recovery from desensitization was not significantly different from that of GluK2 alone (Figure 2D). Since the addition of Neto1 produced a much smaller residual current compared to Neto2, normalization for this current had little effect on the % recovery of GluK1+Neto1 receptors (Figure 2D). Therefore, these results are in agreement with the conclusion of Copits et al. (2011) that Neto2 has minimal impact on the recovery of GluK1 receptors from desensitization, while Neto1 speeds recovery. However, these results are inconsistent with the report from Straub et al. (2011b) which found that Neto2 generated a fast phase of recovery for GluK1-containing recombinant receptors and showed a significantly higher response with a 1 sec. interval between applications.
3.3. Effect of Neto2 on homomeric GluK2 receptors
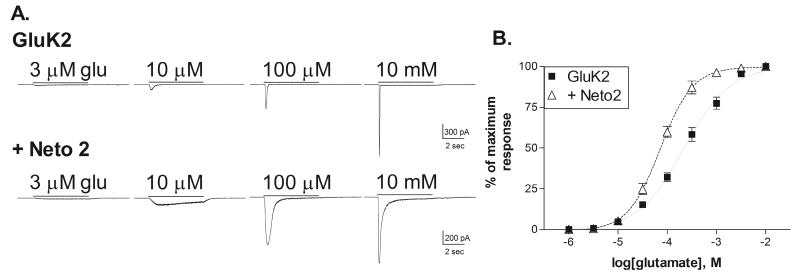
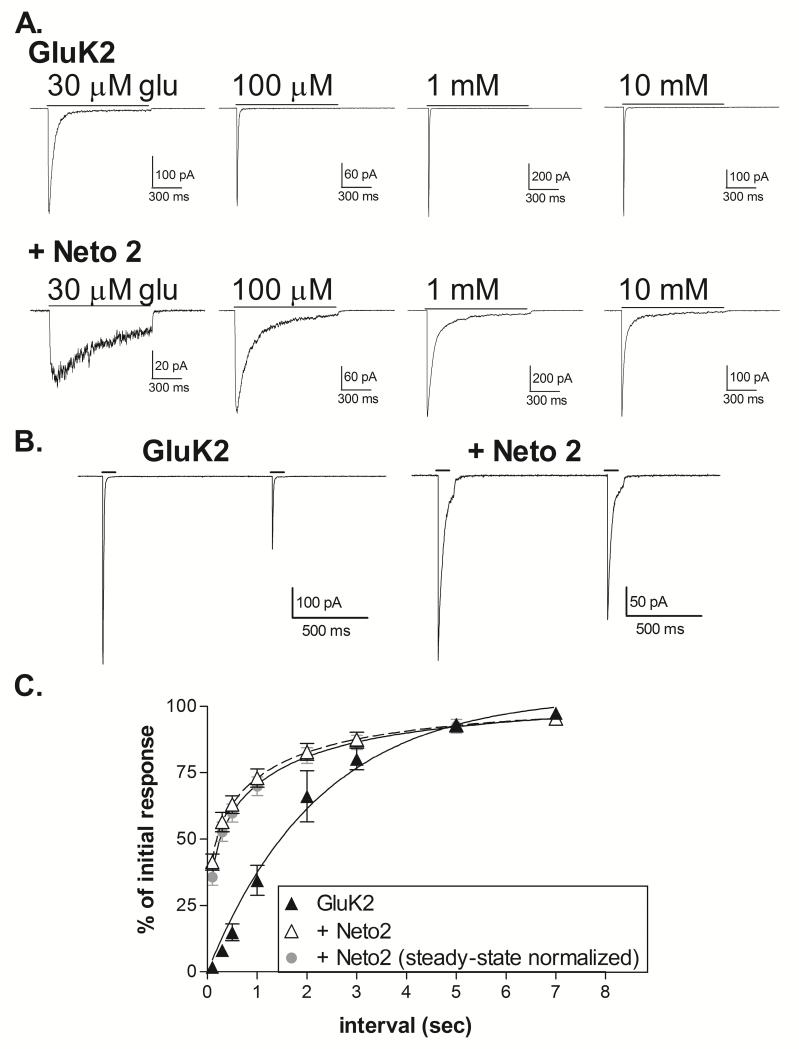
The characteristics of GluK1+Neto1 receptors are substantially different from those of GluK2+Neto1 receptors (Fisher & Mott, 2013), suggesting that the effects of the Neto subunits can be influenced by the identity of the kainate receptor subunit. Therefore, we also compared the impact of Neto2 on GluK2 homomers. In whole-cell recordings, the addition of Neto2 slowed the onset of desensitization at all glutamate concentrations, and caused a modest increase in glutamate sensitivity (Figure 3). The average glutamate EC50 was 233.9 ± 36.8 μM for GluK2 homomers (n=5) and 77.2 ± 9.3 μM for GluK2+Neto2 (n=6) (p<0.001, 2-tailed t-test). This effect was comparable in magnitude to that produced by Neto1 at GluK2 homomers (Fisher & Mott, 2013), and in contrast to the more substantial impact of both Neto1 and Neto2 on the glutamate EC50 of GluK1 homomers (Figure 1). The desensitization properties were quantified using rapid application of glutamate to outside-out patches (Figure 4). As previously reported (Heckmann et al., 1996), the onset of desensitization showed modest concentration-dependence for homomeric GluK2 receptors (Figure 4A, Table 1). The decay was fit with two exponential components at concentrations below the glutamate EC50, although the fast component was predominant. The time constant of the fast component was reduced by increasing glutamate levels up to 1 mM glutamate. Addition of Neto2 to GluK2 caused a slowing of desensitization at all concentrations (Figure 4A, Table 1). At concentrations of 100 μM and above, the decay was fit with the sum of two exponential components and the relative area of the slow component (τ2) decreased with increasing glutamate concentrations, although the time constant was largely unaffected. When comparing the responses to 10 mM glutamate, the primary difference between GluK1+Neto2 and GluK2+Neto2 was in the relative areas of the two components, rather than the time constants. At lower concentrations (30 and 100 μM glutamate) however, τ2 was slower for GluK1+Neto2, even though these receptors have significantly greater glutamate sensitivity than the GluK2+Neto2 combination. Thus, although the effect of Neto2 on desensitization onset of GluK1 and GluK2 receptors is qualitatively similar, the magnitude of this effect is much greater at GluK1 receptors.
Figure 3. Effect of Neto2 on the glutamate sensitivity of GluK2 homomeric receptors.
A. Representative current traces from HEK-293T cells transiently transfected with GluK2 alone, or GluK2+Neto2. Cells were voltage-clamped at −70 mV in the whole-cell configuration. Glutamate was applied for 5 sec (solid bar) at the concentration indicated. For each subunit combination, all traces shown were obtained from the same cell.
B. Glutamate concentration-response relationships were constructed by normalizing the peak amplitude of the current response to the maximum response to saturating agonist levels for each cell. Symbols represent mean ± SEM. Averaged data was fit with a sigmoidal curve (solid or dashed lines). The glutamate EC50s for the fits shown were 218.2 μM (GluK2, n=5) and 72.5 μM (+Neto2, n=6).
Figure 4. Effect of Neto2 on desensitization onset and recovery of GluK2 homomeric receptors.
A. Outside-out patches were excised from HEK-293T cells transiently transfected with GluK2 alone or GluK2+Neto2 and voltage-clamped at −70 mV. Traces represent the averages of 3-5 applications of glutamate at the concentration indicated applied for 1 sec.
B. Paired applications of 10 mM glutamate (100 msec) were given to outside-out patches at 1 sec intervals.
C. Recovery was calculated by dividing the peak amplitude of the second response by that of the first and multiplying by 100. To normalize the effect of the non-desensitized receptors, the amplitude of the current at the end of the first application was subtracted from both responses. Symbols represent mean ± SEM. For the GluK1 homomers, the averaged data shown was fit with a single exponential with τ = 1.56 sec. For GluK1+Neto2, the non-normalized data was fit with the sum of two exponentials (dashed line) with τ1 = 0.042 sec (51.7%) and τ2 = 1.33 sec.
We found that Neto2 did not appear to alter recovery of desensitized GluK1 homomers, at least with a 1 sec. interval between glutamate applications (Figure 2), consistent with a previous report which examined a full range of time intervals (Copits et al., 2011; but see Straub et al., 2011b). In contrast, addition of Neto2 to GluK2 homomers enhanced the rate of recovery from desensitization, even when the current amplitudes were normalized to account for the residual non-desensitized population (Figure 4B, 4C). In the absence of Neto, the recovery of GluK2 was fit with a single exponential component averaging 1.72±0.43 sec (n=3), within the range of previous reports (Heckmann et al., 1996; Mott et al., 2010; Straub et al., 2011a; Fisher & Mott, 2013). With the addition of Neto2, the data was best fit with a sum of two components for most patches (3 of 5), with an average weighted mean of 0.591 ± 0.234 sec (n=5). For the data fit with two components, the average τ values were 0.041±0.007 sec (46.1±5.5%) and 1.57±0.50 sec (n=3). These results are consistent with the addition of a new, faster phase of recovery (Straub et al., 2011b) and confirm the observation that Neto2 has differential, subunit-dependent effects on recovery from desensitization.
3.4. Functional effect of chimeric Neto1/ Neto2 subunits
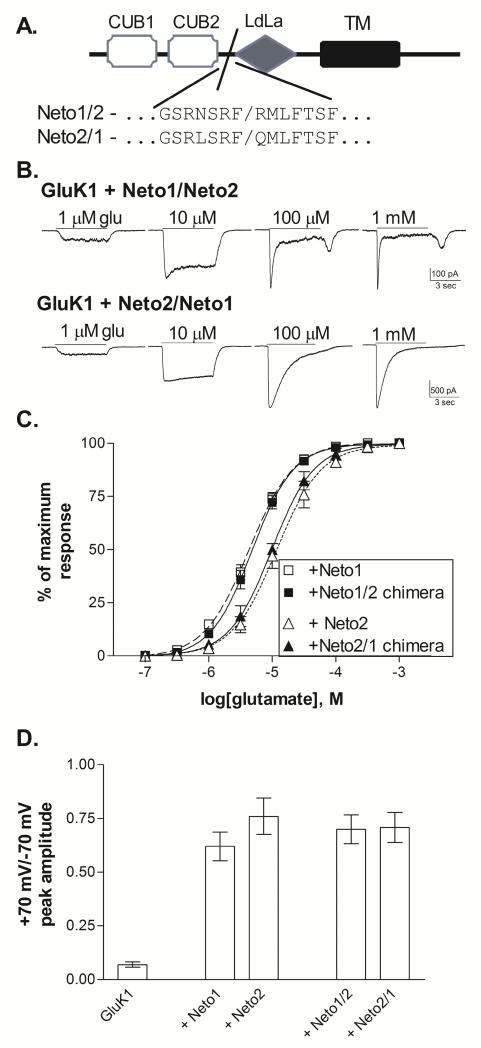
Although Neto1 and Neto2 are highly homologous (Stohr et al., 2002; Zhang et al., 2009), their effects on the functional properties of GluK1 homomeric receptors are clearly distinct. To begin to identify the general structural differences between the Neto subtypes responsible for their unique effects, chimeric subunits were created (Figure 5A). The extracellular domain of the Neto proteins, which makes up about 60% of the structure, contains two CUB (complement C1r/C1s, Uegf and Bmp1) domains and a region homologous to the LdLa (low-density lipoprotein class A) domain (Stohr et al. 2002). Mutations within the LdLa domain can eliminate the ability of the Neto proteins to regulate the kinetics of kainate receptors without preventing their interaction (Zhang et al., 2009; Fisher & Mott, 2012). Although the intracellular domain is important for some aspects of Neto function, deletion of this region did not prevent either Neto1 or Neto2 from altering desensitization of GluK2 receptors (Fisher & Mott, 2012), indicating that the extracellular and/or transmembrane domains primarily mediate this functional effect. In many proteins, the CUB domains are responsible for protein-protein interactions, and in the Neto subunits these regions are necessary for their assembly with ionotropic glutamate receptors (Ng et al., 2009; Tang et al., 2011). Therefore, the chimeric subunits were designed to exchange the N-terminal domains of Neto1 and Neto2 through both CUB domains, separating this region from the LdLa domain (Figure 5A).
Figure 5. Functional effects of Neto1/Neto2 chimeric subunits.
A. Schematic representation of Neto subunit structure. The extracellular N-terminal sequence contains two CUB domains and one region with homology to the LDL-receptor class A repeat (LDLa) (Stöhr et al., 2002). The single transmembrane domain (TM) is followed by an intracellular C-terminal domain. The chimeric splice site is located at the end of the second CUB domain, prior to the LDLa domain. Neto1/2 chimeras contain Neto1 structure from the N-terminal domain up to the splice site, and Neto2 structure beyond the splice site while Neto 2/1 chimeras contain the reverse.
B. Representative whole-cell currents from transiently transfected HEK-293T cells. Glutamate was applied for 5 sec (solid line) at the concentration indicated to cells voltage-clamped at −70 mV.
C. Glutamate concentration-response relationships were constructed by normalizing the current amplitude to the peak response to a saturating concentration for each cell. Symbols represent mean±SEM and averaged data were fit with a sigmoidal function (lines). GluK1+Neto1 and GluK1+Neto2 data is repeated from Figure 1. Glutamate EC50s from the fits shown to the averaged data were 4.8 μM (Neto1/2, n=9) and 9.8 μM (Neto2/1, n=4).
D. Peak current amplitudes were measured in response to 5 sec. applications of 100 μM glutamate at holding potentials of +70 mV and −70 mV. The reversal potential was near 0 mV for all subunit combinations. Bars represent mean ± SEM (n=5 for all combinations). All Neto-containing receptors were significantly different (p≤0.001) from GluK1 without Neto, but were not different from one another (p>0.5).
Both chimeric constructs produced functional effects when co-expressed with GluK1 (Figure 5). In whole-cell recordings, the GluK1+Neto1/Neto2 receptors exhibited rapid desensitization in response to high (≥100 μM) glutamate concentrations, with a rebound current apparent following agonist removal (Figure 5B). These characteristics are comparable to those seen with GluK1+Neto1 (Figure 1). The glutamate EC50 for these receptors averaged 5.0±0.6 μM (n=9), and was significantly different from GluK1 (p≤0.001), but not GluK1+Neto1. In contrast, the GluK1+Neto2/Neto1 receptors showed slow desensitization in whole-cell recordings, even in response to saturating agonist concentrations (Figure 5B), comparable to the responses of GluK1+Neto2 (Figure 1). The glutamate EC50 averaged 9.9±0.9 μM (n=4, p>0.5 compared to GluK1+Neto2). These results suggest that structural differences within the extracellular N-terminal region containing the two CUB domains are responsible for at least some of the differential functional properties of the Neto1 and Neto2 subunits. However, the magnitude of the effect on whole-cell desensitization of the Neto2/Neto1 chimera did not appear to fully replicate the impact of the Neto2 subunit. Thus, other regions of the Neto2 subunit including the LdLa, transmembrane, and intracellular domains could play an important role. Alternatively, the Neto chimeras might have deficits in their ability to interact with kainate subunits. To determine whether the chimeric constructs readily assembled with the GluK1 receptors, the rectification of the current was examined by comparing the response to 100 μM glutamate at −70 mV and +70 mV. Outward current through GluK1(Q) receptors is blocked by cytoplasmic polyamines, producing an inward rectification that can by relieved by either Neto1 or Neto2 (Fisher & Mott, 2012). Therefore, the +70/−70 current ratio provides a functional indication of Neto assembly with the kainate receptor. Both Neto1/2 and Neto2/1 constructs reduced inward rectification to the same extent as wild-type Netos (Figure 5D), demonstrating that the chimeric subunits were effectively incorporated into the receptor complex. This suggests that the smaller impact of the Neto2/1 chimera on whole-cell desensitization was not due to less efficient assembly of this construct compared to Neto2. However, the stoichiometric relationships for these different functional interactions (i.e. the number of Neto subunits required for full kinetic effects vs. reduction of polyamine block) are not known. Therefore, these results may only indicate that at least one of the chimeric subunits is generally associated with a given kainate receptor in these experiments. Further studies will be necessary to determine the specific amino acid residues that confer each of the distinct functional effects associated with Neto1 and Neto2 as well as the structures that determine their interactions with different kainate receptor subunits.
4. Discussion
Structural heterogeneity of kainate receptors can be achieved by changes in composition of both their pore-forming (GluK1-GluK5) and auxiliary subunits (Neto1 and Neto2). These studies show that the effects of the two Neto subunits are both distinct from one another and dependent upon the identity of the pore-forming subunits. Recombinant kainate receptors containing either GluK1 or GluK2 subunits have very similar functional characteristics (Fisher & Fisher, 2014). However, with the addition of Neto1 or Neto2 subunits these receptors behave quite differently from one another. The Neto subunits greatly increased glutamate sensitivity for GluK1 receptors (~10-30×), with only modest effects at GluK2 receptors (~3×) (Fisher & Mott, 2013; Palacios-Filardo et al., 2015). While both Neto1 and Neto2 slowed the onset of desensitization of GluK1 homomers when non-saturating concentrations of glutamate were applied, only Neto2 slowed desensitization of these receptors with saturating agonist levels. The effect on desensitization was greater for Neto2 compared to Neto1, and the effect of Neto2 was greater at GluK1 receptors compared to GluK2. While Neto1 enhanced recovery from desensitization for both GluK1 and GluK2 receptors, we found that Neto2 had this effect only for GluK2. Thus, the properties of GluK1 and GluK2 homomeric receptors can be very distinct depending upon their association with Neto1 or Neto2.
A key difference between the actions of Neto1 and Neto2 at GluK1 receptors is that Neto1 slows onset of desensitization only under non-saturating conditions, while Neto2 influences even fully occupied receptors. It was previously reported that Neto1 speeds desensitization of GluK1 receptors in response to very high glutamate concentrations (Copits et al., 2011). However, our results demonstrate that Neto1 also increases the glutamate sensitivity of these receptors. Comparing the whole-cell responses of GluK1 and GluK1+Neto1 at glutamate concentrations that produce equivalent activation (i.e. an EC50 concentration for each) suggest that Neto1 is able to slow desensitization of partially occupied receptors Although further studies with rapid application recordings will be needed to quantify these properties, the concentration-dependent effects of Neto1 on GluK1 are comparable to those at GluK2/K5 heteromeric receptors, in which the rate of desensitization onset is altered only with non-saturating agonist levels (Fisher & Mott, 2013). Together these results are consistent with the suggestion that Neto1 may have differential effects on at least two phases of desensitization, which are controlled by binding site occupancy (Bowie & Lange, 2002; Robert & Howe, 2003; Schauder et al., 2013; Fisher & Mott, 2013; Reiner & Isacoff, 2014). Addition of Neto2 to GluK2 increased the contribution from the slower phase of desensitization and generated a new slow phase of recovery, perhaps related to two different modes of gating of the receptors (Zhang et al., 2014). The substantial effect of Neto1 and Neto2 on the glutamate EC50 of GluK1 receptors might be related to modulation of desensitization. If desensitization is typically faster than activation at low agonist concentrations, then interventions that reduce the rate of desensitization can appear to enhance agonist affinity (Perrais et al., 2009). However, it may also be that the Neto subunits directly increase glutamate affinity through a unique interaction with GluK1 subunits.
Two prior publications, Copits et al., 2011 and Straub et al., 2011b, have examined the desensitization properties of GluK1+Neto2 receptors in response to saturating levels of glutamate. The current findings are most consistent with those of the Copits et al. (2011) which reported a steady state current of about 40% of the peak current and rates of recovery from desensitization similar to the Neto-less receptors. In contrast, the responses observed by Straub et al. (2011b) show much more complete desensitization (residual current of about 12% of the peak) and appearance of a faster phase of recovery. There are no obvious methodological differences between these three studies to account for this variation in results, although there may be variations in the cloned subunits or in the stoichiometry of the expressed receptors. The observations of more complete desensitization and more rapid recovery from desensitization may be related. Thus, Neto2 may speed recovery from one phase of desensitization but not both, as is observed with GluK2. Thus, under conditions which produce more complete desensitization (Straub et al, 2011), faster recovery may also occur. Further studies will be needed to clarify the mechanisms underlying these differing observations.
GluK1+Neto1 responses were characterized by a rebound current following agonist removal. Evidence from neuronal recordings support these observations from recombinant receptors as Puthussery et al., (2014) reported rebound currents in retinal neurons which were associated both with Neto1 expression and high sensitivity to the GluK1-selective antagonist ACET. A number of potential mechanisms might underlie this phenomenon. In some cases this current is produced by when receptors contain subunits with differing agonist sensitivities. Heteromeric kainate receptors containing the high-affinity GluK4 or GluK5 subunits show this behavior, and the rebound current is more pronounced when the affinity difference between the subunits is enhanced, as with mutations within the agonist binding site (Mott et al., 2010; Fisher & Mott 2011; Fisher & Fisher, 2014). For these receptors, the current appears to be generated when the low-affinity sites release the agonist while the high-affinity sites remain bound, briefly resulting in an activated, non-desensitizing receptor (Mott et al., 2010). Addition of Neto1 increased the rebound current associated with these heteromeric receptors, possibly by reducing the desensitization of partially occupied receptors (Fisher & Mott, 2013). Although the homomeric GluK1 receptors would not be expected to have subunits with differing affinities, Neto1 may not associate with all four subunits within the receptor. As the Neto subunits have a substantial impact on glutamate sensitivity, the rebound current could potentially arise from differential activation and deactivation of Neto1-bound and Neto1-less subunits within the same receptor. The rebound current could also be a consequence of both the rapid recovery from desensitization and the non-desensitizing response to low agonist concentrations, which are observed in GluK1+Neto1 receptors. This combination of effects may explain why this current is not seen for GluK1+Neto2 receptor, which have a slower phase of recovery from desensitization, or in GluK2+Neto1 receptors, in which glutamate sensitivity is only slightly enhanced (Fisher & Mott, 2013). Alternative mechanisms could also be considered, including the possibility of an agonist-induced open channel block, which can produce similar kinetic properties.
Studies of auxiliary subunits that modulate AMPA receptors suggest that multiple domains within both the pore-forming and auxiliary subunits can play important roles in the subunit-subunit interaction (Jackson & Nicoll, 2011; Shanks et al., 2014; Cais et al., 2014) and this is likely to be true for the Neto subunits as well. The CUB and LdLa domains within both Neto proteins are known to be important for their ability to assemble with and modulate kainate receptors (Zhang et al., 2009; Tang et al., 2011),and the intracellular C-terminal domain regulates voltage-dependent block (Fisher & Mott, 2012). However, the structural variations between Neto1 and Neto2 responsible for their distinct effects have not been identified. Our results suggest that the extracellular N-terminus, including the two CUB domains, contributes to some, but not all, of these functional differences. CUB domains are found in a wide variety of otherwise unrelated proteins, and often mediate protein-protein interaction (Bork & Beckmann,1993). These domains in the Neuropilin proteins, from which the Netos derive part of their name, have been shown to be critical for their ligand-binding capabilities (Nakamura et al., 1998; Gagnon et al., 2000). These relatively small domains (112-116 residues each) are highly homologous, sharing about 70% sequence identity between Neto1 and Neto2 (Stohr et al., 2002).
This study demonstrates that co-assembly of recombinant kainate receptors with the Neto auxiliary subunits alters their onset and recovery from desensitization in a subunit-dependent manner. At the excitatory synapse, these effects may increase the ability of some post-synaptic receptors to continue to respond under conditions of rapid neuronal firing, enhancing summation. In addition, both Neto subunits greatly increase the sensitivity of GluK1 receptors to glutamate and reduce macroscopic desensitization in response to submaximal agonist concentrations. The combination of these functional changes may permit activation of extrasynaptic receptors by low ambient glutamate levels. In addition to effects on kinetic properties, the Neto subunits may also influence membrane trafficking and synaptic localization of kainate receptors (Copits & Swanson, 2012; Tang et al., 2012; Wyeth et al., 2014; Palacios-Filardo et al., 2015). Further work will be needed to characterize the full range of functional roles for Neto1 and Neto2 in neurons expressing distinct complements of pore-forming kainate receptor subunits.
Highlights.
Both Neto1 and Neto2 increase glutamate sensitivity of GluK1 receptors
Neto1 slows onset of desensitization of GluK1 receptors in response to sub-maximal but not saturating glutamate levels
Neto2 slows onset of desensitization of GluK1 and GluK2 receptors at all levels of activation
The differing effects of Neto1 and Neto2 at GluK1 receptors are associated with their extracellular CUB domains
Acknowledgements
Thanks to Dr. David Mott (University of South Carolina School of Medicine) for helpful comments regarding this study and to Dr. Paul Housley (University of South Carolina School of Medicine) for assistance with construction of the chimeric subunits.
Funding
This work was supported by grants from NIH-NINDS (R01-NS065869 and R03-NS088858) and an ASPIRE grant from the Office of the Vice President for Research at the University of South Carolina. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health or other sponsors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests
The author has no conflicts of interest to report.
REFERENCES
- Bahn S, Volk B, Wisden W. Kainate receptor gene expression in the developing rat brain. J Neurosci. 1994;14:5525–5547. doi: 10.1523/JNEUROSCI.14-09-05525.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bork P, Beckmann G. The CUB domain. A widespread module in developmentally regulated proteins. J Mol Biol. 1993;231:539–545. doi: 10.1006/jmbi.1993.1305. [DOI] [PubMed] [Google Scholar]
- Bowie D, Lange GD. Functional stoichiometry of glutamate receptor desensitization. J Neurosci. 2002;22:3392–3403. doi: 10.1523/JNEUROSCI.22-09-03392.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cais O, Herguedas B, Krol K, Cull-Candy SG, Farrant M, Greger IH. Mapping the interaction sites between AMPA receptors and TARPs reveals a role for the receptor N-terminal domain in channel gating. Cell Reports. 2014;9:728–740. doi: 10.1016/j.celrep.2014.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta M, Fievre S, Gorlewicz A, Mulle C. Kainate receptors in the hippocampus. Eur J Neurosci. 2014;39:1835–1844. doi: 10.1111/ejn.12590. [DOI] [PubMed] [Google Scholar]
- Chesnut JD, Baytan AR, Russell M, Chang MP, Bernard A, Maxwell IH, Hoeffler JP. Selective isolation of transiently transfected cells from a mammalian cell population with vectors expressing a membrane anchored single-chain antibody. J Immunol Methods. 1996;193:17–27. doi: 10.1016/0022-1759(96)00032-4. [DOI] [PubMed] [Google Scholar]
- Contractor A, Sailer AW, Darstein M, Maron C, Xu J, Swanson GT, Heinemann SF. Loss of kainate receptor-mediated heterosynaptic facilitation of mossy-fiber synapses in KA2 −/− mice. J Neurosci. 2003;23:422–429. doi: 10.1523/JNEUROSCI.23-02-00422.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copits BA, Robbins JS, Frausto S, Swanson GT. Synaptic targeting and functional modulation of GluK1 kainate receptors by the auxiliary neuropilin and tolloid-like (NETO) proteins. J Neurosci. 2011;31:7334–7340. doi: 10.1523/JNEUROSCI.0100-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copits BA, Swanson GT. Dancing partners at the synapse: auxiliary subunits that shape kainate receptor function. Nature Rev Neurosci. 2012;13:675–686. doi: 10.1038/nrn3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darstein M, Petralia RS, Swanson GT, Wenthold RJ, Heinemann SF. Distribution of kainate receptor subunits at hippocampal mossy fiber synapses. J Neurosci. 2003;23:8013–8019. doi: 10.1523/JNEUROSCI.23-22-08013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes HB, Catches JS, Petralia RS, Copits BA, Xu J, Russell TA, Swanson GT, Contractor A. High-affinity kainate receptor subunits are necessary for ionotropic but not metabotropic signaling. Neuron. 2009;63:818–829. doi: 10.1016/j.neuron.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher MT, Fisher JL. Contributions of different kainate receptor subunits to the properties of recombinant homomeric and heteromeric receptors. Neurosci. 2014;278:70–80. doi: 10.1016/j.neuroscience.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JL, Mott DD. Distinct functional roles of subunits within the heteromeric kainate receptor. J Neurosci. 2011;31:17113–17122. doi: 10.1523/JNEUROSCI.3685-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JL, Mott DD. The auxiliary subunits Neto1 and Neto2 reduce voltage-dependent inhibition of recombinant kainate receptors. J Neurosci. 2012;32:12928–12933. doi: 10.1523/JNEUROSCI.2211-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JL, Mott DD. Modulation of homomeric and heteromeric kainate receptors by the auxiliary subunit Neto1. J Physiol. 2013;591:4711–4724. doi: 10.1113/jphysiol.2013.256776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon ML, Bielenberg DR, Gechtman Z, Miao HQ, Takashima S, Soker S, Klagsbrun M. Identification of a natural soluble neuropilin-1 that binds vascular endothelial growth factor: In vivo expression and antitumor activity. Proc Natl Acad Sci. 2000;97:2573–2578. doi: 10.1073/pnas.040337597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman SJ, Jonas P. Beyond TARPs: the growing list of auxiliary AMPAR subunits. Neuron. 2010;66:8–10. doi: 10.1016/j.neuron.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Heckmann M, Bufler J, Franke C, Dudel J. Kinetics of homomeric GluR6 glutamate receptor channels. Biophys J. 1996;71:1743–1750. doi: 10.1016/S0006-3495(96)79375-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herb A, Burnashev N, Werner P, Sakmann B, Wisden W, Seeburg PH. The KA-2 subunit of excitatory amino acid receptors shows widespread expression in brain and forms ion channels with distantly related subunits. Neuron. 1992;8:775–785. doi: 10.1016/0896-6273(92)90098-x. [DOI] [PubMed] [Google Scholar]
- Jackson AC, Nicoll RA. The expanding social network of ionotropic glutamate receptors: TARPs and other transmembrane auxiliary subunits. Neuron. 2011;70:178–199. doi: 10.1016/j.neuron.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauri SE, Segerstrale M, Vesikansa A, Maingret F, Mulle C, Collingridge GL, Isaac JTR, Taira T. Endogenous activation of kainate receptors regulates glutamate release and network activity in the developing hippocampus. J Neurosci. 2005;25:4473–4484. doi: 10.1523/JNEUROSCI.4050-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauri SE, Vesikansa A, Segerstrale M, Collingridge GL, Isaac JTR, Taira T. Functional maturation of CA1 synapses involves activity-dependent loss of tonic kainate receptor-mediated inhibition of glutamate release. Neuron. 2006;50:415–429. doi: 10.1016/j.neuron.2006.03.020. [DOI] [PubMed] [Google Scholar]
- Lerma J, Marques JM. Kainate receptors in health and disease. Neuron. 2013;80:292–311. doi: 10.1016/j.neuron.2013.09.045. [DOI] [PubMed] [Google Scholar]
- Mott DD, Rojas A, Fisher JL, Dingledine RJf, Benveniste M. Subunit-specific desensitization of heteromeric kainate receptors. J Physiol. 2010;588:683–700. doi: 10.1113/jphysiol.2009.185207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura F, Tanaka M, Takahasi T, Kalb RG, Strittmatter SM. Neuropilin-1 extracellular domains mediate Semaphorin D/III-induced growth cone collapse. Neuron. 1998;21:1093–1100. doi: 10.1016/s0896-6273(00)80626-1. [DOI] [PubMed] [Google Scholar]
- Ng D, Pitcher GM, Szilard RK, Sertié A, Kanisek M, Clapcote SJ, Lipina T, Kalia LV, Joo D, McKerlie C, Cortez M, Roder JC, Salter MW, McInnes RR. Neto1 is a novel CUB-domain NMDA receptor-interacting protein required for synaptic plasticity and learning. PLOS Biol. 2009;7:e41. doi: 10.1371/journal.pbio.1000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios-Filardo J, Aller MI, Lerma J. Synaptic targeting of kainate receptors. Cerebral Cortex. 2015 doi: 10.1093/cercor/bhu244. DOI: 10.1093/cercor/bhu244. [DOI] [PubMed] [Google Scholar]
- Paternain AV, Rodriguez-Moreno A, Villarroel A, Lerma J. Activation and desensitization properties of native and recombinant kainate receptors. Neuropharmacol. 1998;37:1249–1259. doi: 10.1016/s0028-3908(98)00098-7. [DOI] [PubMed] [Google Scholar]
- Perrais D, Coussen F, Mulle C. Atypical functional properties of GluK3-containing kainate receptors. J Neurosci. 2009;29:15499–15510. doi: 10.1523/JNEUROSCI.2724-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthussery T, Percival KA, Venkataramani S, Gayet-Primo J, Grunert U, Taylor WR. Kainate receptors mediate synaptic input to transient and sustained OFF visual pathways in primate retina. J Neurosci. 2014;34:7611–7621. doi: 10.1523/JNEUROSCI.4855-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A, Isacoff EY. Tethered ligands reveal glutamate receptor desensitization depends upon subunit occupancy. Nature Chem Biol. 2014;10:273–280. doi: 10.1038/nchembio.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert A, Howe JR. How AMPA receptor desensitization depends upon receptor occupancy. J Neurosci. 2003;23:847–858. doi: 10.1523/JNEUROSCI.23-03-00847.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauder DM, Kuybeda O, Zhang J, Klymko K, Bartesaghi A, Borgnia MJ, Mayer ML, Subramaniam S. Glutamate receptor desensitization is mediated by changes in quaternary structure of the ligand binding domain. Proc Natl Acad Sci. 2013;110:5921–5926. doi: 10.1073/pnas.1217549110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks NF, Cais O, Maruo T, Savas JN, Zaika EI, Azumaya CM, Yates JR, Gerger I, Nakagawa T. Molecular dissection of the interaction between the AMPA receptor and cornichon homolog-3. J Neurosci. 2014;34:12104–12120. doi: 10.1523/JNEUROSCI.0595-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer B, Burnashev N, Verdoorn TA, Keinanen K, Sakmann B, Seeburg PH. A glutamate receptor channel with high affinity for domoate and kainate. EMBO J. 1992;11:1651–1656. doi: 10.1002/j.1460-2075.1992.tb05211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub C, Hunt DL, Yamasaki M, Kim KS, Watanabe M, Castillo PE, Tomita S. Distinct functions of kainate receptors in the brain are determined by the auxiliary subunit Neto 1. Nature Neurosci. 2011a;14:866–873. doi: 10.1038/nn.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub C, Zhang W, Howe JR. Neto2 modulation of kainate receptors with different subunit compositions. J Neurosci. 2011b;31:8078–8082. doi: 10.1523/JNEUROSCI.0024-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohr H, Berger C, Frohlich S, Weber BH. A novel gene encoding a putative transmembrane protein with two extracellular CUB domains and a low-density lipoprotein class A module: isolation of alternatively spliced isoforms in retina and brain. Gene. 2002;286:223–231. doi: 10.1016/s0378-1119(02)00438-9. [DOI] [PubMed] [Google Scholar]
- Swanson GT, Heinemann SF. Heterogeneity of homomeric GluR5 kainate receptor desensitization expressed in HEK293 cells. J Physiol. 1998;513:639–646. doi: 10.1111/j.1469-7793.1998.639ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M, Pelkey KA, Ng D, Ivakine E, McBain CJ, Salter MW, McInnes RR. Neto1 is an auxiliary subunit of native synaptic kainate receptors. J Neurosci. 2011;31:10009–10018. doi: 10.1523/JNEUROSCI.6617-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M, Ivakine E, Mahadevan V, Salter MW, McInnes RR. Neto2 interacts with the scaffolding protein GRIP and regulates synaptic abundance of kainate receptors. PLoS One. 2012;7:e51433. doi: 10.1371/journal.pone.0051433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesikansa A, Sakha P, Kuja-Panula J, Molchanova S, Rivera C, Huttunen HJ, Rauvala H, Taira T, Lauri SE. Expression of GluK1c underlies the developmental switch in presynaptic kainate receptor function. Sci Rep. 2012;2:310–321. doi: 10.1038/srep00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyeth MS, Pelkey KA, Petralia RS, Salter MW, McInnes RR, McBain CJ. Neto auxiliary protein interactions regulate kainate and NMDA receptor subunit localization at mossy fiber-CA3 pyramidal cell synapses. J Neurosci. 2014;34:622–628. doi: 10.1523/JNEUROSCI.3098-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FH, Yarov-Yarovoy V, Gutman GA, Catterall Overview of molecular relationships in the voltage-gated ion channel superfamily. Pharmacol Rev. 2005;57:387–395. doi: 10.1124/pr.57.4.13. [DOI] [PubMed] [Google Scholar]
- Zhang W, St-Gelais F, Grabner CP, Trinidad JC, Sumicka A, Morimoto-Tomita M, Kim KS, Straub C, Burlingame AL, Howe JR, Tomita S. A transmembrane accessory subunit that modulates kainate-type glutamate receptors. Neuron. 2009;61:385–396. doi: 10.1016/j.neuron.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Devi SP, Tomita S, Howe JR. Auxiliary proteins promote modal gating of AMPA- and kainate-type glutamate receptors. Eur J Neurosci. 2014;39:1138–1147. doi: 10.1111/ejn.12519. [DOI] [PMC free article] [PubMed] [Google Scholar]