Abstract
Background
Internal tandem duplication (ITD) of the FLT3 gene is associated with poor prognosis in acute myeloid leukemia (AML) patients with a normal karyotype. The current standard PCR assay for FLT3/ITD detection is not sufficiently sensitive to monitor minimal residual disease (MRD). Clone-specific assays may have sufficient sensitivity but are not practical to implement, since each clone-specific primer/probe requires clinical validation.
Objective
To develop an assay for clinical molecular diagnostics laboratories to monitor MRD in FLT3/ITD AMLs.
Methods
We designed a simple novel assay, tandem duplication PCR (TD-PCR), and tested its sensitivity, specificity and clinical utility in FLT3/ITD AML patients.
Results
TD-PCR was capable of detecting a single ITD molecule and was applicable to 75% of ITD mutants tested. TD-PCR detected MRD in bone marrow prior to patient relapse. TD-PCR also identified low level ITD mutants not only in FLT3/ITD AMLs but also in initial diagnostic specimens reportedly negative by the standard assay in patients who progressed with the same ITDs detected by the TD-PCR assay.
Conclusion
Detection of MRD by TD-PCR may guide patient selection for early clinical intervention. In contrast to clone-specific approaches, TD-PCR assay can be more easily validated for MRD detection in clinical laboratories due to standardized primers and a universal positive control. In addition, our results on multi-clonality and low-level ITDs suggest that further studies are warranted to elucidate their clinical/biological significance.
1 Introduction
Internal tandem duplication (ITD) mutations in the juxtamembrane domain of the FMS-like tyrosine kinase (FLT3) gene occur in 20–30% of patients with acute myeloid leukemia (AML). In AML patients with a normal karyotype, the presence of FLT3/ITD mutations carries a markedly increased rate of relapse and an inferior disease free survival [1–4]. FLT3/ITD AML patients, therefore, commonly undergo allogeneic hematopoietic cell transplantation (HCT) [5, 6]. As with other AML patients, relapse following transplantation occurs frequently [7, 8]. ITD mutations provide a potentially useful molecular marker for monitoring minimal residual disease (MRD). FLT3/ITD AML patients with persistent MRD may benefit from HCT. In addition, early detection of MRD followed by donor lymphocyte infusion and/or FLT3 inhibition in the post-transplant setting could potentially improve disease-free survival.
ITD mutations of the FLT3 gene typically result from head-to-tail insertion of a duplicated portion of the juxtamembrane region [9]. ITD mutations occur almost exclusively within exon 14 at random positions. Longer ITDs may extend to involve intron 14 or even exon 15. The current standard-of-care PCR assay (hereafter, the “standard assay”) detects ITD mutations with primers that straddle the duplicated portion of the juxtamembrane region, resulting in a lengthened product [10]. The limit of detection (or analytic sensitivity) of this assay is approximately 1–5% mutant alleles in clinical diagnostic laboratories, limiting its utility for detection of MRD. Individualized, clone-specific primers/probes have been designed to improve sensitivity in a strategy similar to clone-specific immunoglobulin gene rearrangement analysis for monitoring MRD of lymphoid malignancies [11–16]. These FLT3 assays have not been adopted by clinical laboratories, in part because sequence constraints at the ITD junction may limit sensitivity and because validation of each clone-specific primer/probe—which is required for a Clinical Laboratory Improvement Amendments (CLIA)-certified laboratory in the United States—is not practical in term of time and expense [17, 18]. In this study, we developed a simple ultra-sensitive assay, tandem duplication polymerase chain reaction (TD-PCR), that allows clinical MRD monitoring in FLT3/ITD AMLs by using a standardized set of primer pairs and a universal positive control.
2 Materials and Methods
2.1 Patients
Three groups of samples were studied. The first group consisted of peripheral blood or bone marrow specimens from a cohort of 58 ITD-positive AML patients examined by the standard PCR assay at initial diagnosis or relapse in 2009 and 2010 [10]. Only specimens with greater than 20% blasts were included. Among these 58 ITD-positive patients, the standard assay yielded one (53 specimens), two (4 specimens) and three (1 specimen) ITDs. These 64 ITDs defined the applicability of TD-PCR for MRD detection. The second group consisted of patients with serial ITD-positive specimens at presentation, ITD-negative bone marrow specimens in clinical remission, and ITD-positive specimens at relapse submitted to our molecular diagnostics laboratory for monitoring ITD mutations by the standard assay since 2002. By excluding patients who underwent hematopoietic cell transplantation, 11 patients were identified. This group was used to test if minimal residual ITDs can be detected by TD-PCR and how many genomes are required to demonstrate MRD in bone marrow specimens from FLT3/ITD AML patients who achieved remission after standard chemotherapy but relapsed with the same ITD later. The third group was also selected from our laboratory archives. We identified 3 patients who did not have ITD mutations detected at diagnosis, but had newly emerging ITD mutants detected by the standard assay later in their disease course.
2.2 Tandem Duplication-PCR
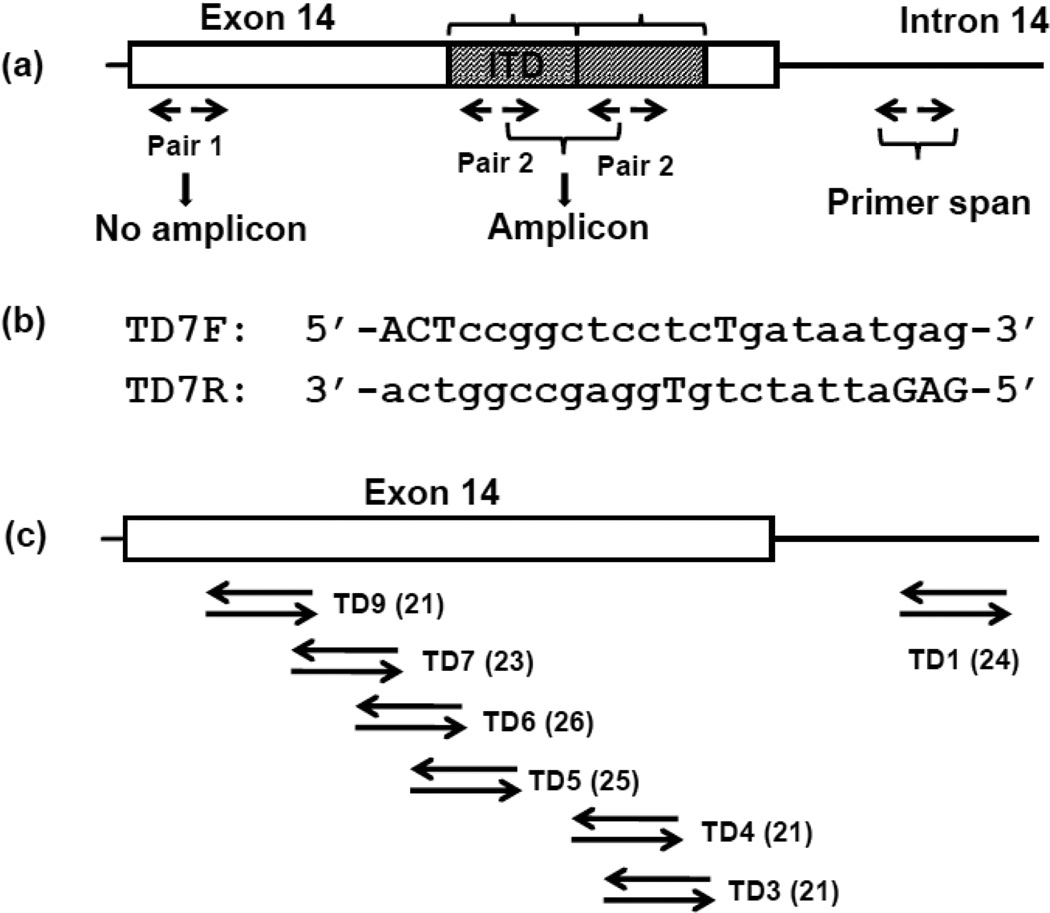
The principle of TD-PCR has been described previously [19]. Briefly, TD-PCR uses a primer pair facing outward in contrast to a primer pair flanking the ITD in the standard assay (Fig. 1a). Amplification only occurs when both primers are spanned by the duplicated sequence. The original TD-PCR assay was designed to confirm a minor ITD mutant that was isolated unexpectedly by our DNA fraction collection tool [19, 20]. These TD-PCR primers were located within intron 14 and were applicable to only a minority of long ITDs for MRD detection. In the current design, the primers overlap almost completely (Fig. 1b), thus shortening the primer span (Fig. 1c). TD-PCR was performed using 6 primer pairs tiled across exon 14 and 1 pair within intron 14 (Fig. 1c) [Supplementary Table S1].
Fig 1. TD-PCR.
In contrast to standard PCR, primers of TD-PCR are oriented away from each other so that a product forms only when 2 sets of primers are juxtaposed because of an ITD (a). The primer span is defined as the length between the 3’ ends of the reverse and forward primers. The sequences of forward and reverse primers are complementary to each other to reduce primer span (c, primer pair TD7). Mismatched nucleotides (capitalized) were introduced at the 5’end and the middle portion of the primers. Seven primer pairs are distributed across exon 14 and intron 14 (c). The primer span of each pair is in parentheses.
2.3 Polymerase chain reaction
The standard assay was performed as described previously [10]. The TD-PCR reactions were performed separately for each primer pair; the construction of the assay prohibits multiplpexing. TD-PCR was performed in a 10 µL volume containing 1 ng or 50 ng DNA, 5 pmol of each forward and reverse primer, 1.5 mM MgCl2, 0.2 mM each deoxyribonucleotide, 0.5 units of AmpliTaq Gold DNA polymerase and 1 µL buffer, or in a 25 µL volume containing 250 ng DNA, 12.5 pmol of each forward and reverse primers, 1.5 mM MgCl2, 0.2 mM each deoxyribonucleotide, 1.25 units of AmpliTaq Gold DNA polymerase and 2.5 µL buffer (Applied Biosystems, Foster City, CA). Samples were subjected to 40 cycles of denaturation (95oC, 30 sec), annealing (57°C, 30 sec) and extension (72oC, 60 sec). Capillary electrophoresis was performed and analyzed as described previously [21].
2.4 Confirmation of analytic specificity of ITDs detected by TD-PCR
Long ITDs detected by TD-PCR were confirmed by two methods. First, the sizes of TD-PCR amplicons from adjacent but independently-analyzed primer pairs were compared. ITDs with longer duplication segments are expected to be amplifiable by 2 or more adjacent primer pairs. Amplification by two or more adjacent primer pairs with amplicons of the appropriate sizes indicates duplication-specific amplification from an ITD mutant and is not observed in negative controls (Supplementary Fig. S1). Second, the size of the TD-PCR amplicon was compared to the size detected by the standard assay (Supplementary Fig. S2). For those short ITDs amplifiable by only one primer pair or ITDs with a low level of allelic burden, duplication-specific amplification was confirmed by Sanger sequencing of TD-PCR amplicons.
2.5 Sanger Sequencing
TD-PCR products were reamplified using overlapping internal primers tagged with M13F and M13R sequences, TD3F3seq3M13F (5’-tgtaaaacgacggccagtGTaatgggTgtttccaagag-3’) and TD3R3Seq3M13R (5'-caggaaacagctatgaccACgaaTctcccatttgagatc-3') for TD3 TD-PCR products or primers TD7F3Seq2M13F (5'-tgtaaaacgacggccagttcctcTgataatgagtacttctac-3') and TD7R3Seq2M13R (5'-caggaaacagctatgaccTggagccggtcacctgtacca-3') for TD7 TD-PCR products. The capitalized nucleotides in the primer sequences are nucleotides altered to introduce mismatches. The M13F and M13R sequences are underlined. PCR products were purified using USB ExoSapit (GE Healthcare, Uppsala, Sweden) and cycle sequenced using the BigDye Terminator version 3.1 cycle sequencing kit according to the manufacturer’s protocol and resolved on an ABI 3500xL sequencer (Applied Biosystems) as described previously [22]. Sequences were analyzed using Sequencher software (Gene Codes Corp, Inc., Ann Arbor, MI).
3 Results
3.1 TD-PCR has single-molecule analytic sensitivity
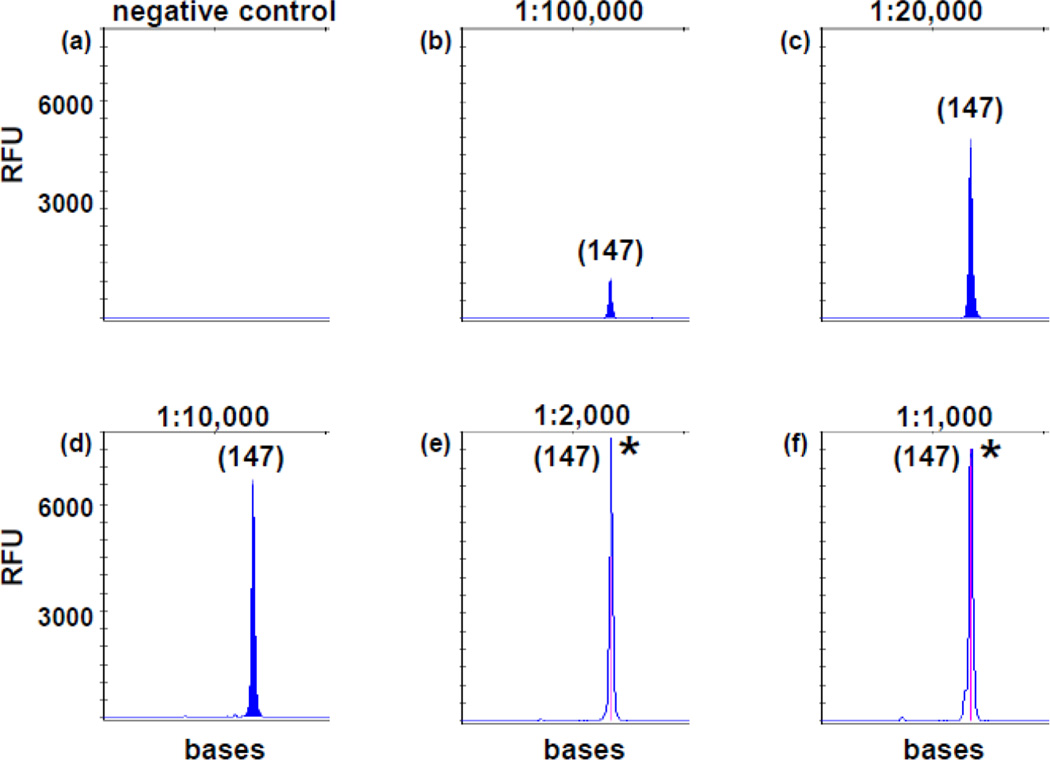
We selected an acute myelomonocytic leukemia specimen with a single dominant heterozygous ITD mutant migrating at 456 bases by the standard assay. This marrow was almost entirely packed with blasts and immature myeloid cells. Sanger sequencing of amplicons from both standard PCR and TD-PCR confirmed an ITD of 129 bases (c.1715_1837+6dup) encompassing the sequences of primers pairs TD3, 4, 5, 6, 7 and 9. TD-PCR was conducted in replicates with 50 ng (equivalent to approximately 8,000 cells assuming each cell contains 6–7 pg DNA) or 250 ng DNA (equivalent to approximately 40,000 cells) in each reaction. The expected amplicons were detected in 1 of 1 reaction at dilutions of 10−3, 5×10−4 and 10−4 and in 2 to 4 of 4 replicates at a dilution of 5×10−5 of 250 ng DNA (equivalent to 2 mutant cells per reaction) (Fig. 2). Primer pairs TD3, 5, 7 and 9 detected the ITD in 1 of 4 replicates at 10−5 dilution of 250 ng DNA (equivalent to 0.4 mutant cells per reaction) (Fig. 2) and in 4–6 of 20 replicates at 5×10−5 dilution of 50 ng DNA (0.4 mutant cells per reaction), indicating TD-PCR is capable of detecting a single ITD molecule. Primer pairs TD4 and TD6 had slightly lower sensitivity as amplicons were not detected in all 4 replicates at 10−5 dilution of 250 ng DNA.
Fig 2. Analytic sensitivity of TD-PCR for primer pair TD3.
DNA from a diagnostic FLT3/ITD AML of nearly 100% leukemia cells was serially diluted with normal DNA: 1 in 1,000 (10−3 in f), 1 in 2,000 (5×10−4 in e), 1 in 10,000 (10−4 in d), 1 in 20,000 (5×10−5 in c) and 1 in 100,000 (10−5 in b). (b) is equivalent to approximately 0.4 mutant cell genomes in 250 µg DNA. (a) is normal DNA. PCR products from (e) and (f) were diluted 10-fold before electrophoresis. “*” indicates off-scale peaks. Sizes in bases are in parentheses. RFU: relative fluorescence unit.
3.2 TD-PCR is applicable for MRD detection in 75% of ITDs
We examined 64 ITDs from 58 newly diagnosed or relapsed FLT3/ITD AML patients using 50 ng DNA for each of 7 primers pairs. Forty-eight ITDs (75%) with sizes of duplication segments ranging from 36 to more than 170 bases and all larger ITDs (≥ 42 bases) were amplifiable by one or more primers pairs. Amplification of the corresponding ITDs detected by the standard assay was supported by a consistent linear correlation between the estimated TD-PCR amplicon size calculated from the amplicon size determined by the standard assay and the observed TD-PCR amplicon size (Supplementary Fig. S2 and Fig. S3). Sanger sequencing of selected amplicons also confirmed the duplication-specific amplification by TD-PCR. Forty-one ITDs (64%) were amplifiable by either TD3 or TD7 or both. Sixteen ITDs with a duplication segment of 18 to 42 bases were not amplifiable by any of the 7 primer pairs. Low-level ITD mutants, undetectable by the standard assay, were observed in 17 specimens. This includes 12 of the 43 specimens whose dominant mutants (defined as ITDs detected by the standard assay) were amplifiable by TD-PCR as well as 5 of the 15 specimens whose dominant mutants were not amplifiable by TD-PCR (Supplementary Fig. S4).
3.3 TD-PCR detects MRD in remission
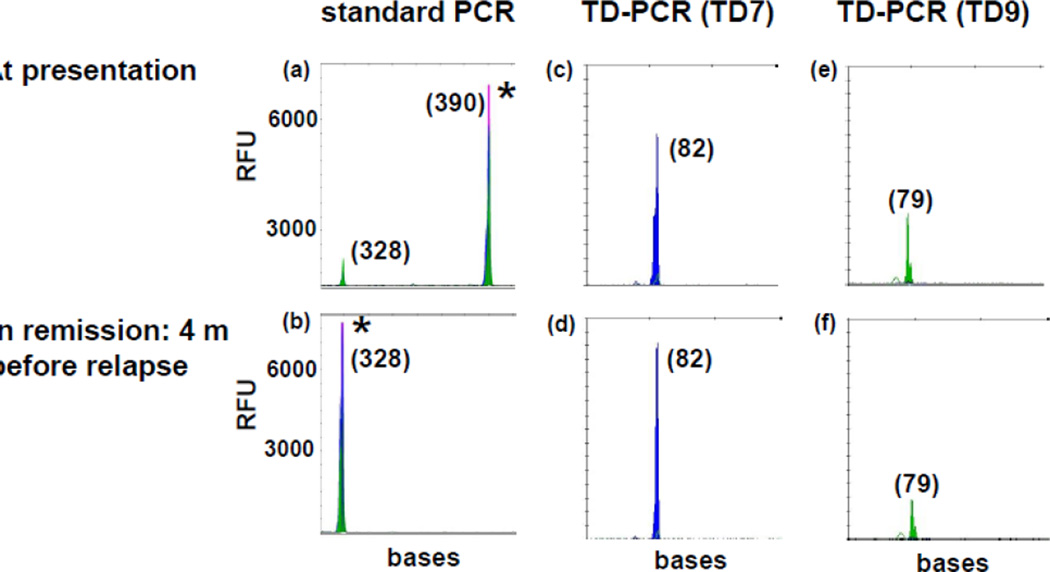
One ng of DNA from specimens at presentation was screened by 7 TD-PCR primer pairs in 11 patients who relapsed after standard chemotherapy to identify informative pairs. Seven patients with one or more informative primer pairs were examined in remission. Twelve available marrows reportedly ITD-negative by the standard assay from these 7 patients were tested by TD3 or TD7 primer pair, 1–14 months before relapse with the same ITD [Table 1]. The TD-PCR assay was conducted with a sequential increase of DNA input for negative specimens until all 12 morrows had MRD detected. Eight samples required 100 ng DNA to detect a signal, 3 samples required 600 ng, and 1 sample required 1.1 µg (Fig. 3). The results suggest that a minimum of 1 µg DNA (equivalent to 160,000 cells) is needed to detect MRD.
Table 1.
Detection of MRD in remission samples by TD-PCR
| TD-PCRb | ||||
|---|---|---|---|---|
| Specimen | Interval before relapse | 50 ng | 250 ng | 250 ng |
| 1 | 6 months | 0/2 | 2/2 | |
| 2 | 4 months | 0/2 | 0/2 | 1/2 |
| 3A | 4 months | 2/2 | ||
| 3B | 5 months | 2/2 | ||
| 4A | 14 months | 0/2 | 1/2 | |
| 4Ba | 5 months | 2/2 | ||
| 4Ca | 4 months | 0/2 | 1/2 | |
| 4Da | 1 month | 2/2 | ||
| 5 | 3 months | 1/2 | ||
| 6 | 9 months | 2/2 | ||
| 7A | 5 months | 2/2 | ||
| 7B | 2 months | 2/2 |
suspicious for early relapse by flow cytometry.
TD-PCR was conducted with a sequential increase of DNA input for negative specimens until all 12 marrows had MRD detected (50 ng, 250 ng, and additional 250 ng DNA in duplicates) using primer pair TD3 or TD7. The numerator represents the replicate number of positive TD-PCR assays, and the denominator represents the total number of assays.
Fig 3. TD-PCR detected MRD at 4 months before relapse.
Standard PCR showed an ITD mutant migrating at 390 bases at both presentation (a) and relapse (not shown), but not at 4 months before relapse (b). TD-PCR using primer pair TD7 (c and d) detected MRD in remission (d). The ITD mutant was detected in 1 of 4 replicates of 250 ng DNA per reaction and in 1 of 20 replicates of 50 ng DNA per reaction (data not shown), indicating MRD at a level of approximately one in 160,000 cells. Duplication-specific amplification was also confirmed by another primer pair TD9 (e and f). TD-PCR products at presentation (c and e) were diluted 100 fold before electrophoresis. ‘*” indicates off-scale peaks. Sizes in bases are in parentheses. RFU: relative fluorescence unit.
3.4 TD-PCR detects low-level mutants in “ITD-negative” patients who progressed with “newly evolving” ITDs
Two AML patients with ITDs detected by the standard assay in the relapsed specimens but not in the initial diagnostic specimens and one patient with an ITD mutant in the AML specimen but not the initial myelodysplastic syndrome/myeloproliferative disorder (MDS/MPD) specimen were studied to evaluate if the newly evolving ITD mutants were present in the initial diagnostic specimens [Supplementary Table S2]. All 3 patients who were “ITD negative” by the standard assay but positive by TD-PCR in the initial specimens relapsed/progressed with the TD-PCR FLT3/ITD as a major clone.
3.5 FLT3/ITD mutations occur in healthy individuals
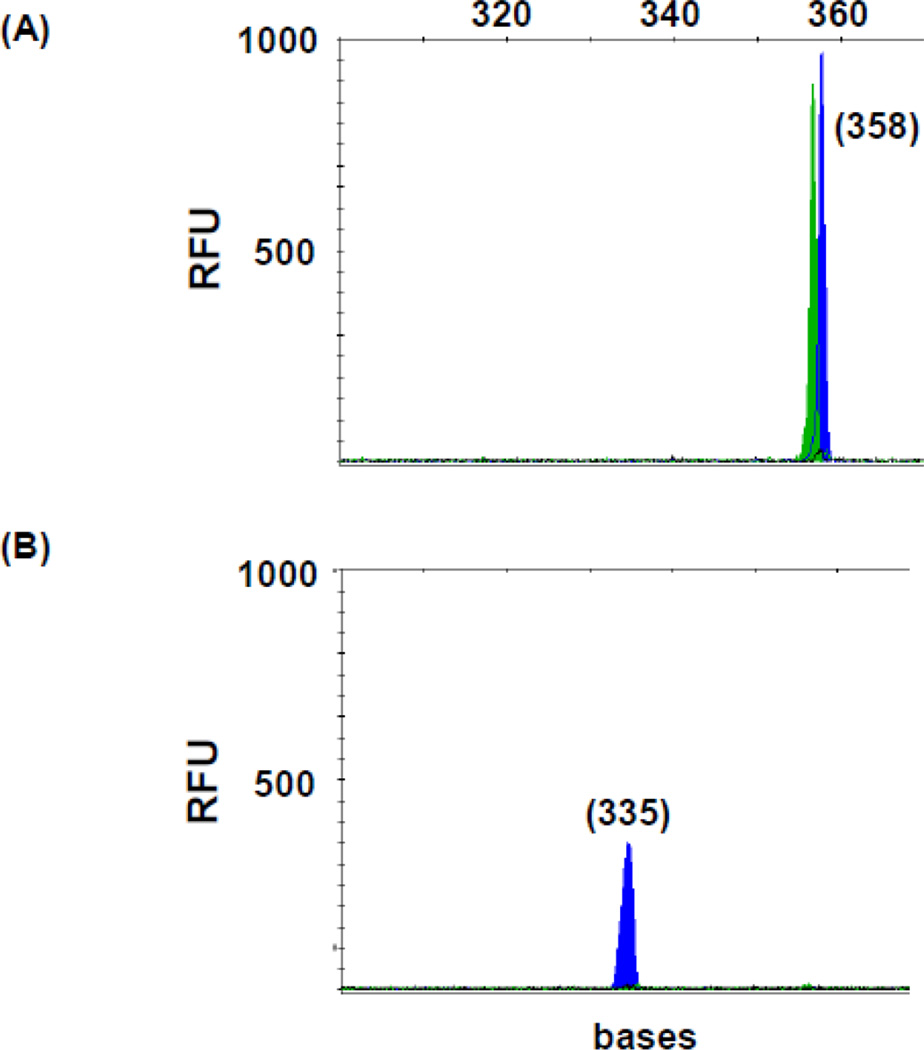
Bone marrows of 24 healthy transplant donors were tested by TD-PCR with TD3 and TD7 primers (2–15.5 µg from each individual or 105.5 µg across all individuals, equivalent to approximately 14 million cells, in aggregate, examined for each primer pair). One ITD was detected by TD3 and one by TD7 from two individuals (Fig. 4). These required 2.4 million cells (15.5 µg DNA) and 1.5 million cells (9.5 µg DNA) per individual, respectively. Interestingly, the duplications, 227 bases (c.1705-14_1838-11dup) and 335 bases (c.1598-3_1837+6dup), are out-of-frame (to our knowledge, not reported before) and longer than the usual AML-associated ones.
Fig 4.
FLT3/ITD mutation in normal marrow. TD7 TD-PCR detected an ITD migrating at 358 bases (A). Duplication-specific amplification was confirmed by ScaI restriction enzyme digestion with a peak migrating at 335 bases (B) and by Sanger sequencing. Sizes in bases are in parentheses. RFU: relative fluorescence unit.
4. Discussion
We have developed an ultrasensitive TD-PCR assay that is amenable to clinical laboratory monitoring of MRD in FLT3/ITD AML patients. This addresses an unmet need, since there is no practicable assay currently available for MRD of FLT3/ITD in clinical molecular diagnostics laboratories. The limit of detection is at least 1 in 100,000 and is limited by the total input of DNA rather than the dilution factor since the wild-type allele is not competitive for amplification. The analytic specificity was demonstrated by duplication-specific amplification, defined as the presence of amplification by two or more adjacent primer pairs with amplicons of the appropriate size (Supplementary Fig. S1). It was also confirmed by Sanger sequencing of the TD-PCR amplicons. With the improved design in this study, TD-PCR is applicable for MRD detection in approximately 75% of ITD mutants. A minimum of 1 µg of DNA is recommended for MRD detection in remission bone marrow specimens. One ITD mutation was detected in an aggregate of 105.5 µg DNA from normal donors, suggesting an approximate 99% clinical specificity if 1 µg DNA is used for MRD detection. However, these “false positive” amplicons from normal individuals have unique characteristics which distinguish them from “true” ITDs: they are long compared to AML-associated ones and may not be in frame. They occur at very low levels and may not be detectable in subsequent samples so are unlikely to be confused with true ITD mutations; however, we do not have follow-up specimens from these normal donors to confirm this.
This assay has also been applied to a cohort of FLT3/ITD AML patients after allogeneic hematopoietic cell transplant [23]. In that study, TD-PCR was applicable for MRD detection in 28 (76%) of 37 patients. TD-PCR predicted relapse with remarkable accuracy using the day 60 post-transplant marrow as analytes. Of note, the day 60 post-transplant marrows were all negative by the standard assay. Six (86%) of 7 patients with MRD by TD-PCR have relapsed compared with only 2 (10%) of 21 patients who were negative for MRD. Among these 2 “false negative” patients, one patient relapsed with no ITD detected and one patient relapsed with the same ITD at 28 months post-transplant. MRD was detected by TD-PCR in a marrow sample collected at 12 months post-transplant from the second patient. Seven of 27 specimens collected within 2 months prior to transplant showed no MRD by TD-PCR. All these 7 patients were negative by TD-PCR at day 60 post-transplant and did not relapse with a follow-up of 12–102 months. TD-PCR may be useful to identify patients who may not need HCT and to identify patients who will benefit from post-transplant immunotherapy using donor lymphocyte infusion or maintenance therapy using tyrosine kinase inhibitors.
Clone-specific PCR has been a sensitive assay for detection of FLT3/ITD mutational status and lymphoid malignancies [11–16]. The analytic sensitivity, however, varied depending on the context of the junctional sequences. The advantage of TD-PCR is the use of common primers rather than customized, clonal-specific primers. Up to 60% of ITD mutants can be analyzed by TD-PCR using only primer pair 3 or primer pair 7. This has practical implications for testing: the U.S. CLIA requires a laborious validation process to confirm the analytic and clinical performance characteristics of each custom primer set [17]. We are unaware of a single clinical laboratory offering clone-specific FLT3 PCR in the United States.
TD-PCR is designed for MRD detection. The conventional PCR assay is still the standard-of-care for ITD detection in newly diagnosed AMLs [10]. In this study, we designed the forward and reverse primers using nearly complimentary sequences to reduce the primer span, and we introduced mismatched nucleotides within the 5’end and/or middle portion of primers to reduce annealing of primer pairs to each other. We thereby successfully validated the revised TD-PCR assays with broader applicability, while keeping its ultra-high sensitivity. TD-PCR, however, is still only applicable to ITDs longer than approximately 40 bases. On the other hand, very long ITD’s appear to be a challenge for next generation sequencing (NGS) methods to detect, requiring specialized bioinformatics or possibly longer sequencing technology [24]. A recent MRD study reported successful NGS detection of all ITD’s tested (< 100 bases) except for an 183-base ITD [25]. While a universal solution may ultimately be provided by NGS [26], a current feasible and comprehensive MRD assay could be based upon NGS for short ITD detection and TD-PCR for longer ITD detection. In support of this approach, our preliminary NGS data shows a limit of detection of 10−5 for the canonical 30-base ITD of the MV4-11 cell-line.
The clinical application of TD-PCR is also limited by the instability of FLT3/ITD status. On average, 17% (6–33%) of FLT3/ITD patients relapse without any ITD mutation, but 14% (7–27%) of AML patients without mutation by the standard assay relapse with an ITD mutation (a particular drawback for clone-specific primer approaches) [Table 2] [1, 27–31]. The incidence of newly emerging ITD mutants is likely influenced by the analytic sensitivity of assays used to detect ITD mutations at diagnosis. TD-PCR found low-level ITDs undetectable by the standard assay not only in FLT3/ITD AMLs but also in AML patients reportedly negative for ITD by the standard assay. We demonstrated that those so-called newly evolving ITD mutants were indeed present at very low levels in the initial diagnostic specimens. This has also been demonstrated by using clone-specific PCR [32], suggesting a need for identifying ITD mutations undetectable by conventional PCR assays. While the clone-specific assay can only be retrospectively applied to this group of patients, TD-PCR can be prospectively applied to approximately 60–70% of patients without knowing the ITD sequences by using, for example, primer pairs 3 and 7.
Table 2.
Instability of ITD status at presentation and relapse
| ITD statusa | Neg/ | Neg/Neg | Neg/Pos | Pos/ | Pos/Neg | Pos/Pos |
|---|---|---|---|---|---|---|
| Nakano et al. [27] | 22 | 16 | 6 (27%) | 6 | 1 (17%) | 5 |
| Kottaridis et al. [1]b | 26 | 23 | 3 (12%) | 18 | 6(33%)c | 12 |
| Shih et al. [28] | 91 | 83 | 8 (9%) | 17 | 1 (6%) | 16 |
| Schnittger et al. [29] | 55 | 51 | 4 (7%) | 42 | 4 (10%) | 38 |
| Cloos et al. [30] | 66 | 54 | 12 (18%) | 16 | 4 (25%) | 12 |
| Nazha et al. [31] | 37 | 29 | 8 (22%) | 16 | 3 (19%) | 13 |
| Total | 297 | 256 | 41 (14%) | 116 | 20 (17%) | 96 |
Neg/, no ITD at presentation; Pos/, ITD at presentation; Neg/Pos, no ITD at presentation and ITD at relapse; Pos/Neg, ITD at presentation and no ITD at relapse.
The two cases with Neg/Pos for D835 mutation and the 2 cases with Pos/Pos for D835 mutation were counted as Neg/Neg for ITD mutational status.
including one case with a new ITD and absence of the original ITD.
Multiple ITD mutations may be more common than previously believed. In this study, we demonstrated multiple minor ITD mutants that were undetectable by the standard PCR assay in FLT3/ITD AML patients. The clinical significance of multiple ITD mutations, however, is controversial [1, 33, 34], partly because the definition of multiple ITD mutations varies depending on the analytic sensitivity of assays. By using TD-PCR and our DNA fraction collection tool, we have identified FLT3/ITD AML patients with 5 or more ITD mutants [20]. The presence of multiple ITD mutations suggests that ITD is a later event rather than an initiating driver mutation for leukemogenesis in a portion of FLT3/ITD AMLs [35, 36].
5. Conclusion
TD-PCR is a simple ultra-sensitive assay for monitoring MRD in a large portion of FLT3/ITD AML patients. In contrast to assays using clone-specific primers/probes, TD-PCR is amenable for clinical validation in molecular diagnostics laboratories by using a standard set of primer pairs and a universal positive control. TD-PCR also detected cases of low level multi-clonality of ITD mutants.
Supplementary Material
Key points.
TD-PCR is a simple ultra-sensitive assay to detect minimal residual disease of AML patients with FLT3/ITD mutations.
In contrast to assays using clone-specific primers/probes, TD-PCR is amenable for clinical validation in molecular diagnostics laboratories by using a standard set of primer pairs and a universal positive control.
TD-PCR also detected cases of low level multi-clonality of ITD mutants.
Acknowledgments
Funding The authors, MTL and CDG, gratefully acknowledge the funding received from National Institutes of Health (R21HG004315 and R21HG005745 to CGD) and the National Cancer Institute at the National Institutes of Health (1UM1CA186691-01 to MTL and CGD) of the United States.
Footnotes
Compliance with Ethical Standards
Ethical approval and informed consent The Johns Hopkins Medicine institutional review board granted approval to this study.
Conflict of interest The authors, MTL, LHT, JCD, SR, HT, GZ, KWP, MJL and CGD, report that they have no conflicts of interests.
References
- 1.Kottaridis PD, Gale RE, Frew ME, Harrison G, Langabeer SE, Belton AA, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: Analysis of 854 patients from the united kingdom medical research council AML 10 and 12 trials. Blood. 2001;98(6):1752–1759. doi: 10.1182/blood.v98.6.1752. [DOI] [PubMed] [Google Scholar]
- 2.Thiede C, Steudel C, Mohr B, Schaich M, Schakel U, Platzbecker U, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: Association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99(12):4326–4335. doi: 10.1182/blood.v99.12.4326. [DOI] [PubMed] [Google Scholar]
- 3.Schnittger S, Schoch C, Dugas M, Kern W, Staib P, Wuchter C, et al. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: Correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood. 2002;100(1):59–66. doi: 10.1182/blood.v100.1.59. [DOI] [PubMed] [Google Scholar]
- 4.Lazenby M, Gilkes AF, Marrin C, Evans A, Hills RK, Burnett AK. The prognostic relevance of flt3 and npm1 mutations on older patients treated intensively or non-intensively: a study of 1312 patients in the UK NCRI AML16 trial. Leukemia. 2014;28(10):1953–1959. doi: 10.1038/leu.2014.90. [DOI] [PubMed] [Google Scholar]
- 5.Meshinchi S, Arceci RJ, Sanders JE, Smith FO, Woods WB, Radich JP, et al. Role of allogeneic stem cell transplantation in FLT3/ITD-positive AML. Blood. 2006;108(1):400. doi: 10.1182/blood-2005-12-4938. [DOI] [PubMed] [Google Scholar]
- 6.DeZern AE, Sung A, Kim S, Smith BD, Karp JE, Gore SD, et al. Role of allogeneic transplantation for FLT3/ITD acute myeloid leukemia: Outcomes from 133 consecutive newly diagnosed patients from a single institution. Biol Blood Marrow Transplant. 2011;17(9):1404–1409. doi: 10.1016/j.bbmt.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunet S, Labopin M, Esteve J, Cornelissen J, Socié G, Iori AP, et al. Impact of FLT3 internal tandem duplication on the outcome of related and unrelated hematopoietic transplantation for adult acute myeloid leukemia in first remission: a retrospective analysis. J Clin Oncol. 2012;30(7):735–741. doi: 10.1200/JCO.2011.36.9868. [DOI] [PubMed] [Google Scholar]
- 8.Sengsayadeth SM, Jagasia M, Engelhardt BG, Kassim A, Strickland SA, Goodman S, et al. Allo-SCT for high-risk AML-CR1 in the molecular era: Impact of FLT3/ITD outweighs the conventional markers. Bone Marrow Transplant. 2012;47(12):1535–1537. doi: 10.1038/bmt.2012.88. [DOI] [PubMed] [Google Scholar]
- 9.Nakao M, Yokota S, Iwai T, Kaneko H, Horiike S, Kashima K, et al. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia. 1996;10(12):1911–1918. [PubMed] [Google Scholar]
- 10.Murphy KM, Levis M, Hafez MJ, Geiger T, Cooper LC, Smith BD, et al. Detection of FLT3 internal tandem duplication and D835 mutations by a multiplex polymerase chain reaction and capillary electrophoresis assay. J Mol Diagn. 2003;5(2):96–102. doi: 10.1016/S1525-1578(10)60458-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stirewalt DL, Willman CL, Radich JP. Quantitative, real-time polymerase chain reactions for FLT3 internal tandem duplications are highly sensitive and specific. Leuk Res. 2001;25(12):1085–1088. doi: 10.1016/s0145-2126(01)00087-x. [DOI] [PubMed] [Google Scholar]
- 12.Scholl S, Loncarevic IF, Krause C, Clement JH, Hoffken K, Sayer HG. Analyses of minimal residual disease based on Flt3 mutations in allogeneic peripheral blood stem cell transplantation. J Cancer Res Clin Oncol. 2005;131(5):279–283. doi: 10.1007/s00432-004-0660-x. [DOI] [PubMed] [Google Scholar]
- 13.Chou WC, Hou HA, Liu CY, Chen CY, Lin LI, Huang YN, et al. Sensitive measurement of quantity dynamics of FLT3 internal tandem duplication at early time points provides prognostic information. Ann Oncol. 2011;22(3):696–704. doi: 10.1093/annonc/mdq402. [DOI] [PubMed] [Google Scholar]
- 14.Abdelhamid E, Preudhomme C, Helevaut N, Nibourel O, Gardin C, Rousselot P, et al. Minimal residual disease monitoring based on FLT3 internal tandem duplication in adult acute myeloid leukemia. Leuk Res. 2012;36(3):316–323. doi: 10.1016/j.leukres.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Schiller J, Praulich I, Krings Rocha C, Kreuzer KA. Patient-specific analysis of FLT3 internal tandem duplications for the prognostication and monitoring of acute myeloid leukemia. Eur J Haematol. 2012;89(1):53–62. doi: 10.1111/j.1600-0609.2012.01785.x. [DOI] [PubMed] [Google Scholar]
- 16.Brüggemann M, Gökbuget N, Kneba M. Acute lymphoblastic leukemia: monitoring minimal residual disease as a therapeutic principle. Semin Oncol. 2012;39(1):47–57. doi: 10.1053/j.seminoncol.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Jennings L, Van Deerlin VM, Gulley ML College of American Pathologists Molecular Pathology Resource Committee. Recommended principles and practices for validating clinical molecular pathology tests. Arch Pathol Lab Med. 2009;133(5):743–755. doi: 10.5858/133.5.743. [DOI] [PubMed] [Google Scholar]
- 18.Mattocks CJ, Morris MA, Matthijs G, Swinnen E, Corveleyn A, Dequeker E, et al. A standardized framework for the validation and verification of clinical molecular genetic tests. Eur J Hum Genet. 2010;18(12):1276–1288. doi: 10.1038/ejhg.2010.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin MT, Tseng LH, Beierl K, Hsieh A, Thiess M, Chase N, et al. Tandem duplication PCR: An ultrasensitive assay for the detection of internal tandem duplications of the FLT3 gene. Diagn Mol Pathol. 2013;22(3):149–155. doi: 10.1097/PDM.0b013e31828308a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin MT, Rich RG, Shipley RF, Hafez MJ, Tseng LH, Murphy KM, et al. A molecular fraction collecting tool for the ABI 310 automated sequencer. J Mol Diagn. 2007;9(5):598–603. doi: 10.2353/jmoldx.2007.070022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin MT, Tseng LH, Kamiyama H, Kamiyama M, Lim P, Hidalgo M, et al. Quantifying the relative amount of mouse and human DNA in cancer xenografts using species-specific variation in gene length. BioTechniques. 2010;48(3):211–218. doi: 10.2144/000113363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dudley J, Tseng LH, Rooper L, Harris M, Haley L, Chen G, Gocke CD, Eshleman JR, Lin MT. Challenges posed to pathologists in the detection of KRAS mutations in colorectal cancers. Arch Pathol Lab Med. 2015;139(2):211–218. doi: 10.5858/arpa.2013-0649-OA. [DOI] [PubMed] [Google Scholar]
- 23.Grunwald MR, Tseng LH, Lin MT, Pratz KW, Eshleman JR, Levis MJ, et al. Improved FLT3/ITD PCR assay predicts outcome following allogeneic transplant for AML. Biol Blood Marrow Transplant. 2014;20(12):1989–1995. doi: 10.1016/j.bbmt.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spencer DH, Abel HJ, Lockwood CM, Payton JE, Szankasi P, Kelley TW, et al. Detection of FLT3 internal tandem duplication in targeted, short-read-length, next-generation sequencing data. J Mol Diagn. 2013;15(1):81–93. doi: 10.1016/j.jmoldx.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Bibault JE, Figeac M, Helevaut N, Rodriguez C, Quief S, Sebda S, et al. Next-generation sequencing of FLT3 internal tandem duplications for minimal residual disease monitoring in acute myeloid leukemia. Oncotarget. 2015 Jun 2; doi: 10.18632/oncotarget.4333. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiba K, Shiraishi Y, Nagata Y, Yoshida K, Imoto S, Ogawa S, et al. Genomon ITDetector: a tool for somatic internal tandem duplication detection from cancer genome sequencing data. Bioinformatics. 2015;31(1):116–118. doi: 10.1093/bioinformatics/btu593. [DOI] [PubMed] [Google Scholar]
- 27.Nakano Y, Kiyoi H, Miyawaki S, Asou N, Ohno R, Saito H, et al. Molecular evolution of acute myeloid leukaemia in relapse: unstable N-ras and FLT3 genes compared with p53 gene. Br J Haematol. 1999;104(4):659–664. doi: 10.1046/j.1365-2141.1999.01256.x. [DOI] [PubMed] [Google Scholar]
- 28.Shih LY, Huang CF, Wu JH, Lin TL, Dunn P, Wang PN, et al. Internal tandem duplication of FLT3 in relapsed acute myeloid leukemia: A comparative analysis of bone marrow samples from 108 adult patients at diagnosis and relapse. Blood. 2002;100(7):2387–2392. doi: 10.1182/blood-2002-01-0195. [DOI] [PubMed] [Google Scholar]
- 29.Schnittger S, Schoch C, Kern W, Hiddemann W, Haferlach T. FLT3 length mutations as marker for follow-up studies in acute myeloid leukaemia. Acta Haematol. 2004;112(1–2):68–78. doi: 10.1159/000077561. [DOI] [PubMed] [Google Scholar]
- 30.Cloos J, Goemans BF, Hess CJ, van Oostveen JW, Waisfisz Q, Corthals S, et al. Stability and prognostic influence of FLT3 mutations in paired initial and relapsed AML samples. Leukemia. 2006;20(7):1217–1220. doi: 10.1038/sj.leu.2404246. [DOI] [PubMed] [Google Scholar]
- 31.Nazha A, Cortes J, Faderl S, Pierce S, Daver N, Kadia T, et al. Activating internal tandem duplication mutations of the fms-like tyrosine kinase-3 (FLT3-ITD) at complete response and relapse in patients with acute myeloid leukemia. Haematologica. 2012;97(8):1242–1245. doi: 10.3324/haematol.2012.062638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ottone T, Zaza S, Divona M, Hasan SK, Lavorgna S, Laterza S, et al. Identification of emerging FLT3 ITD-positive clones during clinical remission and kinetics of disease relapse in acute myeloid leukaemia with mutated nucleophosmin. Br J Haematol. 2013;161(4):533–540. doi: 10.1111/bjh.12288. [DOI] [PubMed] [Google Scholar]
- 33.Gale RE, Green C, Allen C, Mead AJ, Burnett AK, Hills RK, et al. The impact of FLT3 internal tandem duplication mutant level, number, size, and interaction with NPM1 mutations in a large cohort of young adult patients with acute myeloid leukemia. Blood. 2008;111(5):2776–2784. doi: 10.1182/blood-2007-08-109090. [DOI] [PubMed] [Google Scholar]
- 34.Meshinchi S, Stirewalt DL, Alonzo TA, Boggon TJ, Gerbing RB, Rocnik JL, et al. Structural and numerical variation of FLT3/ITD in pediatric AML. Blood. 2008;111(10):4930–4933. doi: 10.1182/blood-2008-01-117770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelly LM, Kutok JL, Williams IR, Boulton CL, Amaral SM, Curley DP, et al. PML/RARalpha and FLT3-ITD induce an APL-like disease in a mouse model. Proc Natl Acad Sci U S A. 2002;99(12):8283–8288. doi: 10.1073/pnas.122233699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Welch JS, Ley TJ, Link DC, Miller CA, Larson DE, Koboldt DC, et al. The origin and evolution of mutations in acute myeloid leukemia. Cell. 2012;150(2):264–278. doi: 10.1016/j.cell.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.