Abstract
Primary dilated cardiomyopathy (DCM) is a non-ischemic heart disease with impaired pumping function of the heart. In this study, we used human induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs) from a healthy volunteer and a primary DCM patient to investigate the impact of DCM on iPSC-CMs’ responses to different types of anisotropic strain. A bioreactor system was established that generates cardiac-mimetic forces of 150 kPa at 5% anisotropic cyclic strain and 1 Hz frequency. After confirming cardiac induction of the iPSCs, it was determined that fibronectin was favorable to other extracellular matrix protein coatings (gelatin, laminin, vitronectin) in terms of viable cell area and density, and was therefore selected as the coating for further study. When iPSC-CMs were exposed to three strain conditions (no strain, 5% static strain, and 5% cyclic strain), the static strain elicited significant induction of sarcomere components in comparison to other strain conditions. However, this induction occurred only in iPSC-CMs from a healthy volunteer (“control iPSC-CMs”), not in iPSC-CMs from the DCM patient (“DCM iPSC-CMs”). The donor type also significantly influenced gene expressions of cell-cell and cell-matrix interaction markers in response to the strain conditions. Gene expression of connexin-43 (cell-cell interaction) had a higher fold change in healthy versus diseased iPSC-CMs under static and cyclic strain, as opposed to integrins α-5 and α-10 (cell-matrix interaction). In summary, our iPSC-CM-based study to model the effects of different strain conditions suggests that intrinsic, genetic-based differences in the cardiomyocyte responses to strain may influence disease manifestation in vivo.
Keywords: anisotropic strain, dilated cardiomyopathy, iPSC, cardiac maturation, cell-cell interaction
1. Introduction
Primary dilated cardiomyopathy (DCM) is a leading cause of heart failure, second only to ischemic heart disease (Sehnert et al., 2002; Sun et al., 2012). A common pathophysiology of primary DCM is thought to involve compromised contractility of cardiomyocytes (CMs), leading to impaired systolic function of the heart. As heart failure progresses, the ventricles remodel, dilate and their walls thin (Marian and Roberts, 1994; Morita et al., 2005; Willott et al., 2010). Although primary DCM is classically considered idiopathic, a significant fraction of the cases is now known to involve specific gene mutations affecting the CM structure and function (Herman et al., 2012).
In the healthy adult heart, CMs are characterized by their interactions between neighboring cells as well as the surrounding matrix. In CMs, gap junctions are responsible for mechanical and electrical coupling between cells and action potential transmission (Christoforou et al., 2013; Simon et al., 1998; Willecke et al., 2002). In particular, among the connexin gene family that is responsible for development of gap junction in CMs (Christoforou et al., 2013; Palatinus et al., 2012), connexin-43 (Cx43) is the most abundant in the heart and ventricular muscle, and low levels of its protein expression have been previously associated with ventricular arrhythmia in DCM patients (Kitamura et al., 2002; Severs et al., 2008). On the other hand, cell-matrix interactions, mediated by integrins, are also critical for the maturation and function of CMs. Of the 16 α-subunits in mammalian cells, only seven subunits, α1, α3, α5, α6, α7, α9, and α10, are expressed in CMs (Ross and Borg, 2001). Fetal and neonatal CMs contain the α1, α3, α5 subunits, whereas healthy adult CMs lack α1 and α5 subunits (Terracio et al., 1991). This suggests that these subunits can be used as markers of CM immaturity.
In order to better elucidate and ultimately regulate the cellular mechanisms that cause DCM, in vitro models are needed that employ a cell source recapitulating the behavior of diseased CMs. Previous studies have shown that iPSCs raised from healthy and diseased donors can be used to generate in vitro models of DCM in iPSC-derived CMs (iPSC-CMs) (Rajala et al., 2011; Schwartz et al., 1995). In this study, CMs were derived from iPSCs of both a healthy donor and a 7 month old male patient with congenital DCM, a rare but fatal disorder. This DCM patient has no family history of the disease and its etiology is also unknown. CMs derived from iPSCs of both cell sources were exposed to three different conditions of anisotropic strain (no strain, 5% static strain, and 5% cyclic strain) to study how these different culture conditions influence the structure and function of CMs by altering cell-cell and cell-matrix interactions.
2. Materials and Methods
2.1 Reprogramming, Passaging, and Cardiac Differentiation of iPSCs
Human skin fibroblasts were obtained from either a healthy donor or a DCM patient (7 month old male) at the Vanderbilt University Medical Center following appropriate consent under the guideline of an approved IRB protocol (Vanderbilt #080369). Cells from only one patient with congenital DCM were obtained due to the rarity of this disorder. The patient has no family history of DCM and its etiology is unknown. Human iPSC lines were reprogrammed from skin fibroblasts by established methods (Aboud et al., 2014a; Okita et al., 2011; Wang et al., 2015; Yang et al., 2014). Specifically, vector pEP4EO2SCK2MEN2L (OCT4/SOX2/KLF4/MYC/NANOG/LIN28) and pEP4EO2SET2K (OCT4/SOX2/LT/KLF4) were co-transfected into 1.0 × 106 human skin fibroblasts via nucleofection (VPD-1001 with program U-23, Amaxa, MD). Transfected fibroblasts were plated directly to Matrigel-coated dishes in fibroblast medium. On day one post-transfection, fibroblast medium were replaced with reprogramming medium consisting of DMEM/F12 culture medium supplemented with N-2 supplement (Invitrogen), B27 (Invitrogen), 0.1 mM nonessential amino acids, 1 mM GlutaMAX, 0.1 mM β-mercaptoethanol, PD0325901 (P, 0.5 mM), CHIR99021(C, 3 mM), A-83-01(A, 0.5 mM) (all from Stemgent, San Diego, CA), hLIF (L, 1000 U/ml, Millipore) and HA-100 (H, 10 mM). Culture media were refreshed every other day. On day 13 post-transfection, the transfected cells were cultured with mTeSR1 to expand iPSCs.
These iPSC lines were cultured and maintained following the previously published methods (Aboud et al., 2012; Aboud et al., 2014b; Okita et al., 2011). Upon 80~90% confluence, cardiac induction of these cells was conducted by the “Matrigel sandwich” method as described previously (Zhang et al., 2012). The cardiac differentiated cells were maintained in RPMI/B27 without insulin until day 10–11. The culture media was then changed every other day for one week with RPMI/B27 media containing neither insulin nor glucose. The iPSC-CMs were maintained in this RPMI/B-27 supplement until after cardiac differentiation on day 30.
Characterization of iPSC-CMs
Cardiac differentiation of iPSC-CMs was evaluated by Flow Cytometry (FACS, BD FACSAria III). ~106 cells were fixed in 4% paraformaldehyde and permeabilized by a FACS buffer with 0.1% saponin. Troponin T mouse monoclonal antibodies (Santa Cruz Biotechnology) diluted in FACS buffer (1:50) as primary antibodies and the primary IgG isotype (Donkey anti-mouse, Invitrogen) as secondary antibodies were used for FACS analysis. The resulting data were analyzed using FlowJo v8.5.2.
For immunostaining, fixed, permeabilized cells were blocked and treated with Troponin T mouse monoclonal antibodies (Santa Cruz Biotechnology) and α-actinin mouse monoclonal antibodies (Sigma), followed by incubation with with IgG secondary antibodies and counter staining with DAPI. Slides were viewed with a confocal microscope (Olympus FV-1000) and the image analysis was done with NIS-Elements BR3.0 software.
2.2 Fabrication of PDMS devices
A silicone elastomer was created using a 1:10 ratio of catalyst to elastomer base by mass (Sylgard 184, Dow Corning). The resulting poly-dimethyl siloxane (PDMS) solution was poured into a pre-fabricated mold and degassed under vacuum for 15 minutes to remove air bubbles. Large rectangular glass coverslips were clamped onto the PDMS molds to provide a flat surface and the molds were cured overnight at 70°C. The following day, PDMS molds (overall thickness of 1/8 of an inch) were removed and placed in 70% ethanol for several days to remove any unreacted catalyst and ultimately placed in a 90°C oven for 30 minutes to evaporate the ethanol. The device contains a cell culture channel with two wells measuring 10 mm × 10 mm × 1 mm (L x W x H), in which the cells were seeded and cultured. A fiberglass embedding technique was used to integrate actuator harnesses into the stretcher device, allowing easy loading of the PDMS device onto the bioreactor system. The elastic modulus of the device was measured in a parallel plate configuration on a rheometer (RA2000, TA Instrument) in oscillation mode with a frequency of 1 Hz and 5% strain at 37°C.
2.3 Bioreactor system
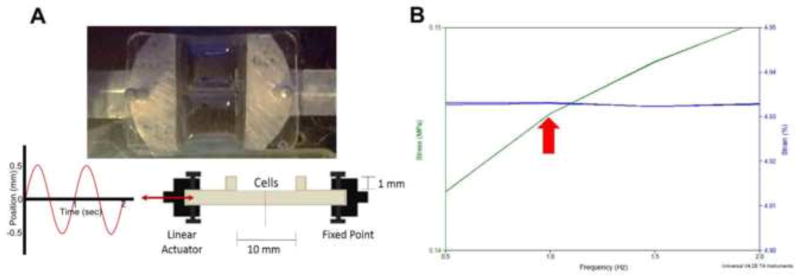
In order to control the strain dynamics on the PDMS device, a design integrating a fixed point and an opposing micro-positioning linear actuator were chosen. The actuators used in the bioreactor system are Physik Instrumente M-235.5DD high power direct drive DC actuators with a ballscrew driving mechanism (Physik Instrumente, GmbH). The actuators have a total travel length of 50 mm. Ballscrew actuators were chosen to reduce the backlash when performing cyclic motion. Direct drive DC-driven linear actuators were chosen to achieve the high speeds and accelerations necessary for 1 Hz cyclic motion akin to human physiology and produce smooth displacement curves. In addition, these actuators provide forces of over 120 N and incremental motion of 0.5 μm with 0.5 μm repeatability. The actuators are equipped with rotary encoders for feedback during the control process. Control of the bioreactor system was achieved through a PCI bus card integrated into the control computer and using Labview. Before bioreactor operation, the actuators were first referenced to non-contact hall-effect limit switches and then moved into position to load the PDMS devices to the system. A MATLAB program was used to write the motion profile instructions for the actuators and was capable of writing any arbitrary waveform to a set of motion instructions for the actuators. Additional parameters were tested, such as 1, 5, 7, and 10% strain (data not shown), frequency of strain (0.5 to 2 Hz using 0.5 increments (Figure 1), and length of exposure to strain was also extended past 48 hours (data not shown). These parameters tested were based on previous works (Clements et al., 1997; Govoni et al., 2013b; Hoit, 2011; Nguyen et al., 2013b; Shimko and Claycomb, 2008a; Yamamoto et al., 2001).
Figure 1.
(A) Top and side view of PDMS stretcher device. Arrow: stretching direction. (B) Force that cells experience/cm2 in the stretcher device. Arrow: 150 kPa at 1 Hz and an approximate 5% strain.
2.4 Protein coating
Clean and dry PDMS molds were placed in Harrick Plasma PDC-002 under vacuum. The unit was set to high and the samples were then treated with plasma for 90 seconds. The molds were removed and immediately coated with a pre-mixed solution of an extracellular matrix protein (5μg/cm2) in serum-free medium. Four protein coatings were tested; gelatin as it increases beating behavior and functions of cardiomyocytes (Koch-Schneidemann et al., 1994; Miskon et al., 2009), laminin as it improves cell attachment of adult cardiomyocytes (Koch-Schneidemann et al., 1994), fibronectin as it enhances the functions of cardiomyocytes derived from stem cells (Burridge et al., 2014; Gandaglia et al., 2012), and vitronectin because it increases the adhesion of cardiomyocytes (Braam et al., 2008; Michel, 2003). The resulting samples were covered with aluminum foil and left overnight on a shaker plate at 4°C prior to seeding cells.
2.5 Bioreactor culture
iPSC-CMs were cultured on Matrigel for 30 days after cardiac induction and seeded onto PDMS devices for 24 hours to allow for cell attachment. Cell-seeded PDMS stretching devices were mounted on the custom-built cyclic bioreactor and placed in an incubator at 37°C and 5% CO2. Samples were cyclically stretched at 1 Hz, 5% length based strain for 48 hours(Clements et al., 1997; Govoni et al., 2013a; Hoit, 2011; Nguyen et al., 2013a; Ruan et al., 2015; Shimko and Claycomb, 2008b; Yamamoto et al., 2001). Static controls were set up in a similar fashion and subject to 5% strain. No-strain controls were placed in the incubator and not subjected to any strain (Chien et al., 2008; Gwak et al., 2008). Samples were removed after 48 hours and prepared for specified endpoint assays.
2.6 Characterization of iPSC-CM viability and morphology
Cells on PDMS devices were stained with a live/dead mammalian assay (Life-Tech) according to the manufacturer’s protocol. A Nikon Eclipse Ti inverted fluorescence microscope was used to image cells on the PDMS devices. For cell morphological analysis, images were imported into ImageJ (NIH, Bethesda, MA) and analyzed for cell area and density.
2.7 Analysis of gene expression by qRT-PCR
RNA was first extracted using Trizol (Invitrogen) and the RNeasy Mini Kit. cDNA was synthesized using the iScipt cDNA synthesis kit (Bio-Rad) and run for qRT-PCR using a thermocycler (Bio-Rad S1000™) with Platinum® Supermix (Life Technologies). The temperature cycle used for PCR went as follows: hot start for 3 minutes at 94°C; denaturation for 30 seconds at 94°C; annealing for 30 seconds; and extension for 1 minute at 72 °C. GAPDH was used as a house keeping gene.
2.8 Analysis of Immunofluorescence staining
Fixed cells were permeabilized in PBS with 0.2% Triton X-100 and blocked overnight in PBS, followed by treatment with either primary donkey anti-cTNT antibodies or primary mouse anti-MLC-2v antibodies (both diluted to 1:200 in PBS). Samples were then incubated with either secondary donkey anti-goat Cy3-conjugated antibodies or secondary goat anti-mouse FITC conjugated antibodies (Abcam) (both diluted to 1:200 in PBS), followed by counterstaining of nuclei with Hoechst. Images of the cells were taken with a Nikon Eclipse Ti inverted fluorescence microscope, and all images were imported into ImageJ (NIH) for measurements of average fluorescence intensity per cell.
2.9 Statistical Analysis
Prism 5 Software was used for data analysis. All results are reported as the mean ± SEM. Unless otherwise noted, N=4 for biological replicates where the data was analyzed by two-way analysis of variance (ANOVA). The Bonferroni post hoc test was used for comparison between groups, and one-way ANOVA was used for analysis of unrelated groups. A value of p<0.05 was considered to be statistically significant.
3. Results
3.1 Bioreactor device characterization
A bioreactor was designed to produce anisotropic strain on iPSC-CMs in a controlled manner by operating two computer-programmable, compact micro-positioning stage linear actuators that can provide static or cyclic strains (See Fig. 1A, Videos S1, and the method section for details). In order to verify that the PDMS device used with the bioreactor system accurately mimicked the cardiac environment, the stiffness (Young’s modulus) of the device was measured. The ultimate loading program (5% strain, 1 Hz, 48 hour culture) was determined based on the constraint that the force and cycle experienced by the cells stay within physiological cardiac range (150 kPa, 1 Hz). This modulus was measured to be approximately 150 kPa, which compares well with that of the adult human myocardium (Bhana et al., 2010; Engelmayr et al., 2008b; Forte et al., 2008; Jawad et al., 2008) (Fig. 1B).
3.2 Confirmation of cardiac induction of iPSC derived from healthy and DCM donors
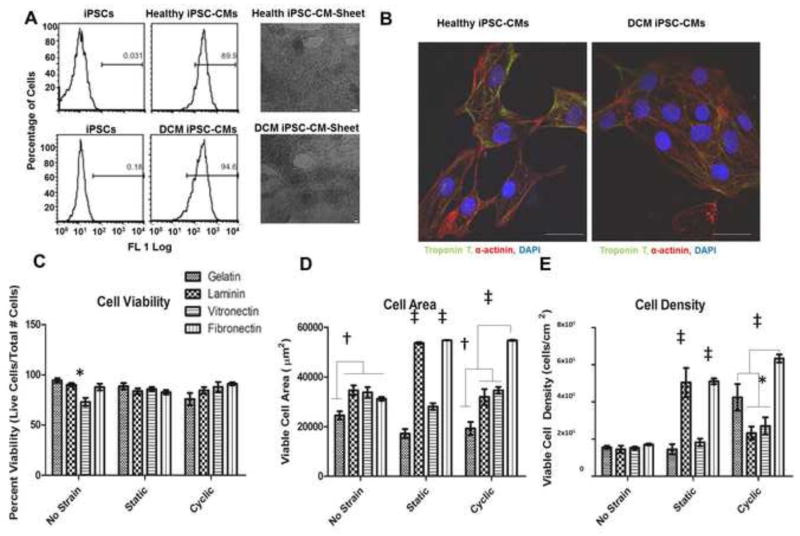
Directed differentiation of iPSCs derived from both healthy and DCM donors, outlined in Figs. 2A and 2B, successfully yielded iPSC-CMs. Visual inspection of the iPSC-CMs 10–13 days after differentiation showed both iPSC-CMs formed beating sheets of cells. Flow cytometry analysis demonstrated 90–95% of both cell types were positive for troponin T at 15 days post differentiation (Fig. 2A), which was confirmed by clear visualization of cardiac α-actinin and troponin T expression in both cell types using immunostaining (Fig. 2B).
Figure 2.
Characterization of healthy and DCM iPSC-CMs by (A) flow cytometry for expression of cardiac Troponin T with corresponding health and DCM iPSC-CM sheets (scale bars are 100 μm) and (B) immunostaining for expression of Troponin T and α-actinin. Evaluation of ECM coating materials under strain by (C) cell viability, (D) cell area, and (E) cell density of healthy iPSC-CMs. * P<0.05, † P<0.01, ‡ P<0.001, scale bars = 20μm
3.3 Effects of ECM Coating and Varying Strain Conditions on Cell Viability, Area, and Density
To determine an optimal extracellular matrix type for coating of the stretcher, PDMS devices were coated with gelatin, laminin, vitronectin, or fibronectin. Healthy iPSC-CMs were seeded on the PDMS devices that were either unstrained or exposed to 5% static strain, and 5% cyclic strain. Cell viability (Fig. 3C), viable cell area (Fig. 2D), and cell density (Fig. 2E) results show significant differences between the types of coating. There was a significant decrease (p<0.05) in cell viability of unstrained iPSC-CMs on a vitronectin coat. No other significant differences were observed between the groups. Under all three strain conditions, cells placed on fibronectin presented the largest amounts of viable cell area. Cyclic strain data showed that while there was a significant increase (P<0.05) in viable cell density of cells on gelatin compared to laminin and vitronectin, those plated on fibronectin showed the largest amounts of viable cell density (P<0.001) compared to the other three groups. These results suggest fibronectin as the best coating material among the tested ones for further experiments.
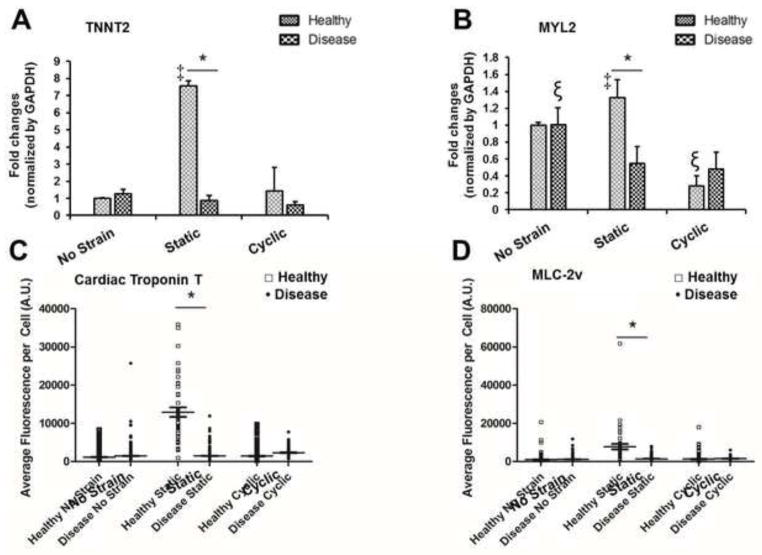
Figure 3.
Expression of cardiac markers in healthy and DCM iPSC-CMs under different mechanical stimulation conditions. (A) TNNT2 gene, (B) MYL2 gene, (C) Cardiac Troponin T protein and (D) MLC-2v protein. Expression of these markers was highest in healthy iPSC-CMs under 5% static at the levels of both gene and protein. * P<0.05 vs. the line paired group; ‡ P<0.05 vs. the other test groups; P<0.05 vs. the test groups of the same cell type.
3.4 Expression of Cardiac Markers
TNNT2 is the gene that codes a sarcomeric cardiac protein, cardiac troponin T (cTNT), and Myosin Light Chain 2 Gene (MYL2) encodes MLC-2v protein. PCR analysis of TNNT2 and MYL2 expression (Fig. 3A–B) showed that under static strain, the two genes were expressed significantly more (P<0.05) in healthy CMs compared to diseased CMs. The expression levels of these genes were highest under static strain among all the test conditions. There were no significant differences in the expression of these two genes between healthy and diseased CMs when unstrained and exposed to cyclic strain. There were also no significant differences in the express of TNN2 between diseased CMs subject to different strain types, but in diseased CMs, static and cyclic strain degreased MYL2 expression significantly (P<0.05) compared to no strain, indicating that DCM iPSC-CMs may not be healthy enough to exert positive responses to the strain conditions.
Protein expression of cTNT and MLC-2v (Fig. 3 C–D) indicate that under static strain, these two proteins were expressed significantly more in healthy CMs compared to diseased CMs (P<0.05), which are consistent with the results of gene expression (Fig. 3A–B). There were no significant differences in protein expression of cTNT and MLC-2v between healthy and DCM iPSC-CMs when exposed to cyclic and no strain.
3.5 Cell-Cell and Cell-Matrix Interaction
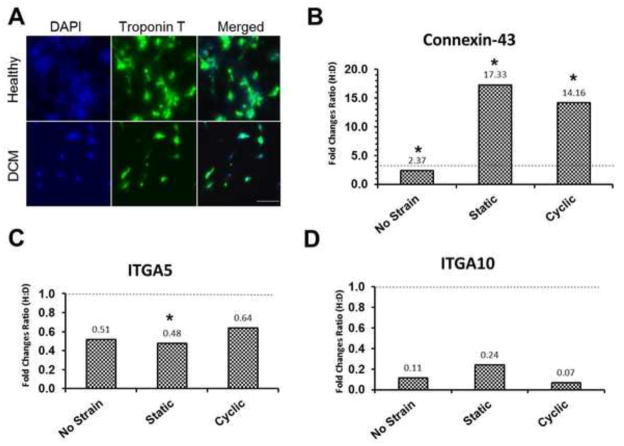
The difference in protein expression of cTNT between healthy and DCM iPSC-CMs under the static strain condition was confirmed visually by immunostaining in Fig. 4A. Moreover, healthy iPSC-CMs showed aggregated cell clusters over the culture area, which was not seen in DCM iPSC-CMs. This difference indicates that altered cell-cell and cell-matrix interactions of healthy versus diseased iPSC-CMs might play a role in regulating their cardiac structure and function. Therefore, gene expression levels of connexin-43 (cell-cell interaction) and integrins α5 and α10 (cell-matrix interaction) were examined by RT-PCR (Maitra et al., 2000; Palatinus et al., 2012). The three strain conditions showed 2.4–17.3 ratio of healthy to disease (H:D) connexin-43 expression (>1) (Fig. 4B), indicating an enhanced cell-cell interaction in healthy over DCM iPSC-CMs. On the other hand, expression of integrins α5 and α10 showed 0.07–0.64 H:D ratio (< 1), indicating an enhanced cell-matrix interaction in the diseased over healthy cells under the three strain conditions (Fig. 4C–D). While all strain groups show a substantial H:D ratio change between the expression of connexin-43 and both integrin subunits, the most notable changes are observed in the static condition. Cells exposed to cyclic strain also showed a relatively large change in H:D value between Cx43 and the integrin subunits, while a much smaller difference was observed in those that experienced no strain. Considering the fact that the H:D ratios of integrins α5 and α10 expression are similar for the three strain conditions, these results suggest the enhanced cell-cell interaction under the static and cyclic strain conditions as a critical aspect in maintaining cardiac structure and function of healthy iPSC-CMs over diseased cells.
Figure 4.
Cell-cell interaction of healthy iPSC-CMs was increased by mechanical stimulation. (A) Immunofluorescence images of cardiac Troponin T expression in healthy and DCM iPSC-CMs under 5% static condition. Gene expression ratios (Healthy: Disease=H:D) of (B) connexin 43, (C) integrin alpha 5 (ITGA5), and integrin alpha 10 (ITGA10) in iPSC-CMs under each condition. Fold changes normalized by GAPDH. * p<0.05, comparison between healthy iPSC-CMs and DCM iPSC-CMs under the same mechanical stimulation. Scale bar = 200 μm. Dashed line at a fold change ratio of 1 in all plots.
4. Discussion
The goal of this study is to determine effects of genetic backgrounds carried from healthy and DCM patients on iPSC responses to anisotropic mechanical strain. In particular, the causative roles of DCM and mechanostimulation in expression of sarcomere structure and cell-cell/cell-matrix interaction markers were investigated. Either healthy or DCM iPSC-CMs we used in this study were isolated on day 30 after cardiac differentiation in order to keep identical maturity for all experiments. Additionally, the metabolic selection method was used for purifying CMs in the iPSC-derived cell population. It was expected that these minimized the variations resulted from each derivative.
Our study indicates that healthy and DCM iPSC-CMs show selective responses to different types of strain. It was found that local increases in stationary mechanical strain (~ 150 kPa) might help maintenance of cardiac structure and function of healthy iPSC-CMs because this values is similar to the range of the adult human myocardium (Engelmayr et al., 2008b; Forte et al., 2008; Jawad et al., 2008). However, this effect was not the same for DCM iPSC-CMs, most likely due to an altered genetic background. Results from this study suggest the dominant role of cell-cell interaction in driving the healthy iPSC-CM phenotype. In the analysis of matrix coatings, fibronectin proved to be the most suitable for this experiment in terms of viable cell area and cell density.
The measured Young’s modulus value of PDMS device (~150 kPa) matches the stiffness values in the previously studied cardiac tissue models, and native rat myocardium has a Young’s modulus in the range of 10 to 150 kPa (Bhana et al., 2010; Engelmayr et al., 2008a; Jawad et al., 2007). This suggests that the fabricated PDMS devices are able to create a mechanical environment similar to that of heart tissue. Static strain seemed to promote the healthy phenotype of iPSC-CMs, as evidenced by increased expression of genes and proteins associated with cardiac structure and function. Studies with mouse models have demonstrated that cTNT deficient CMs failed to assemble sarcomere structures from thick and thin filaments and were not able to produce heartbeats (Ameen et al., 2008). Higher expression of this protein in healthy iPSC-CMs under static conditions indicates a preferential differentiation toward a healthier CM phenotype with an overall increase in contractile function of the heart. DCM iPSC-CMs, on the other hand, did not respond to static strain in the same way that healthy iPSC-CMs did. MLC2 expression is also related to contractility of the heart, as well as thick-filament stabilization. The increased expression of MLC2 gene and protein under healthy, static conditions can explain a critical symptom of DCM, most notably a diminished systolic function accompanied by an inability to generate the necessary contractile force. Therefore, this result supports the idea that CM structure and function are affected by a pathological status (altering cellular mechanism) related to DCM (Marian and Roberts, 1994; Morita et al., 2005; Willott et al., 2010). A previous study reported reduced beating rates and contractility with altered sarcomere structures in iPSC-CMs derived from DCM patients (Sun et al., 2012). Our study provides additional insight into differences in cardiac-specific structural organization and cell-cell/cell-matrix interactions between normal and DCM iPSC CMs in response to different strain types.
Next, the effects of DCM on cell-matrix and cell-cell interactions under the three types of strain were studied. Connexin-43 expression, the principle protein in gap junction interactions, was much higher in healthy iPSC-CMs than in DCM iPSC-CMs in all three stretching conditions. Reduction in Cx43 expression accompanies human ventricular disease and correlates with arrhythmic changes and contractile dysfunction in animal models (Severs et al., 2008). Cx43 expression is expected to decrease as lowered cell-cell interaction results in diminished electrical and mechanical coupling and thus a less cohesive and contractile tissue. DCM cells, suffering from the inability to generate an adequate contractile and uniform force, show a greatly reduced expression of this vital protein. In contrast, expression of specific cell-matrix promoting proteins (integrins α5 and α10) was significantly higher in iPSC-CMs derived from DCM patients. In fetal CMs, integrins α5 expression increases to improve cell attachment to ECM components such as collagen type I and fibronectin, whereas in adult CMs, its decreased expression results in reduced production of fibronectin receptors (Maitra et al., 2000). This result indicates that DCM-derived CMs may have more cell-matrix interactive, fetal phenotypes as evidenced by the increased expression of ITGA5.
Future studies will aim to further elucidate the effects of static strain on cardiac phenotype and cell-cell communication following damage from dilated cardiomyopathy using electrophysiological tools. Understanding the maturation mechanism of DCM iPSC-CMs at molecular, cellular, and physiological levels will ultimately lead to future development of DCM patient-specific cell therapy.
Supplementary Material
Acknowledgments
This research work was funded and supported by NIH R01HL104040, NIH UH2 TR000491, NIH R01ES010563, NIH R01ES016931, 1R01 NS078289, VA Merit 101BX000771, NSF CAREER CBET 1056046, the National Center for Research Resources Grant UL1 RR024975 and the National Center for Advancing Translational Sciences Grant UL1 TR000445. Polymer characterization was conducted through the use of the core facilities of the Vanderbilt Institute of Nanoscale Sciences and Engineering (VINSE), which was supported by NSF EPS 1004083.
Footnotes
The conflict of interest statement
The authors declare no conflicts of interest, financial or otherwise.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aboud AA, Tidball AM, Kumar KK, Diana Neely M, Han B, Ess KC, Hong CC, Erikson KM, Hedera P, Bowman AB. PARK2 patient neuroprogenitors show increased mitochondrial sensitivity to copper. Neurobiol Dis. 2014a doi: 10.1016/j.nbd.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aboud AA, Tidball AM, Kumar KK, Neely MD, Ess KC, Erikson KM, Bowman AB. Genetic risk for Parkinson’s disease correlates with alterations in neuronal manganese sensitivity between two human subjects. Neurotoxicology. 2012;33:1443–1449. doi: 10.1016/j.neuro.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aboud AA, Tidball AM, Kumar KK, Neely MD, Han B, Ess KC, Hong CC, Erikson KM, Hedera P, Bowman AB. PARK2 patient neuroprogenitors show increased mitochondrial sensitivity to copper. Neurobiology of disease. 2014b;73c:204–212. doi: 10.1016/j.nbd.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameen C, Strehl R, Bjorquist P, Lindahl A, Hyllner J, Sartipy P. Human embryonic stem cells: current technologies and emerging industrial applications. Critical reviews in oncology/hematology. 2008;65:54–80. doi: 10.1016/j.critrevonc.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Bhana B, Iyer RK, Chen WL, Zhao R, Sider KL, Likhitpanichkul M, Simmons CA, Radisic M. Influence of substrate stiffness on the phenotype of heart cells. Biotechnology and bioengineering. 2010;105:1148–1160. doi: 10.1002/bit.22647. [DOI] [PubMed] [Google Scholar]
- Braam SR, Zeinstra L, Litjens S, Ward-van Oostwaard D, van den Brink S, van Laake L, Lebrin F, Kats P, Hochstenbach R, Passier R, Sonnenberg A, Mummery CL. Recombinant vitronectin is a functionally defined substrate that supports human embryonic stem cell self-renewal via alphavbeta5 integrin. Stem Cells. 2008;26:2257–2265. doi: 10.1634/stemcells.2008-0291. [DOI] [PubMed] [Google Scholar]
- Burridge PW, Matsa E, Shukla P, Lin ZC, Churko JM, Ebert AD, Lan F, Diecke S, Huber B, Mordwinkin NM, Plews JR, Abilez OJ, Cui B, Gold JD, Wu JC. Chemically defined generation of human cardiomyocytes. Nat Methods. 2014;11:855–860. doi: 10.1038/nmeth.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien KR, Domian IJ, Parker KK. Cardiogenesis and the Complex Biology of Regenerative Cardiovascular Medicine. Science. 2008;322:1494–1497. doi: 10.1126/science.1163267. [DOI] [PubMed] [Google Scholar]
- Christoforou N, Liau B, Chakraborty S, Chellapan M, Bursac N, Leong KW. Induced pluripotent stem cell-derived cardiac progenitors differentiate to cardiomyocytes and form biosynthetic tissues. PloS one. 2013;8:e65963. doi: 10.1371/journal.pone.0065963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements ML, Banes AJ, Faber JE. Effect of Mechanical Loading on Vascular α1D- and α1B-Adrenergic Receptor Expression. Hypertension. 1997;29:1156–1164. doi: 10.1161/01.hyp.29.5.1156. [DOI] [PubMed] [Google Scholar]
- Engelmayr GC, Cheng M, Bettinger CJ, Borenstein JT, Langer R, Freed LE. Accordion-Like Honeycombs for Tissue Engineering of Cardiac Anisotropy. Nat Mater. 2008a;7:1003–1010. doi: 10.1038/nmat2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmayr GC, Jr, Cheng M, Bettinger CJ, Borenstein JT, Langer R, Freed LE. Accordion-like honeycombs for tissue engineering of cardiac anisotropy. Nat Mater. 2008b;7:1003–1010. doi: 10.1038/nmat2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forte G, Carotenuto F, Pagliari F, Pagliari S, Cossa P, Fiaccavento R, Ahluwalia A, Vozzi G, Vinci B, Serafino A, Rinaldi A, Traversa E, Carosella L, Minieri M, Di Nardo P. Criticality of the biological and physical stimuli array inducing resident cardiac stem cell determination. Stem Cells. 2008;26:2093–2103. doi: 10.1634/stemcells.2008-0061. [DOI] [PubMed] [Google Scholar]
- Gandaglia A, Huerta-Cantillo R, Comisso M, Danesin R, Ghezzo F, Naso F, Gastaldello A, Schittullo E, Buratto E, Spina M, Gerosa G, Dettin M. Cardiomyocytes in vitro adhesion is actively influenced by biomimetic synthetic peptides for cardiac tissue engineering. Tissue Eng Part A. 2012;18:725–736. doi: 10.1089/ten.tea.2011.0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govoni M, Muscari C, Guarnieri C, Giordano E. Mechanostimulation protocols for cardiac tissue engineering. BioMed research international. 2013a;2013:918640. doi: 10.1155/2013/918640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govoni M, Muscari C, Guarnieri C, Giordano E. Mechanostimulation Protocols for Cardiac Tissue Engineering. BioMed Research International. 2013b;2013:918640. doi: 10.1155/2013/918640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwak SJ, Bhang SH, Kim IK, Kim SS, Cho SW, Jeon O, Yoo KJ, Putnam AJ, Kim BS. The effect of cyclic strain on embryonic stem cell-derived cardiomyocytes. Biomaterials. 2008;29:844–856. doi: 10.1016/j.biomaterials.2007.10.050. [DOI] [PubMed] [Google Scholar]
- Herman DS, Lam L, Taylor MRG, Wang L, Teekakirikul P, Christodoulou D, Conner L, DePalma SR, McDonough B, Sparks E, Teodorescu DL, Cirino AL, Banner NR, Pennell DJ, Graw S, Merlo M, Di Lenarda A, Sinagra G, Bos JM, Ackerman MJ, Mitchell RN, Murry CE, Lakdawala NK, Ho CY, Barton PJR, Cook SA, Mestroni L, Seidman JG, Seidman CE. Truncations of Titin Causing Dilated Cardiomyopathy. New England Journal of Medicine. 2012;366:619–628. doi: 10.1056/NEJMoa1110186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoit BD. Strain and Strain Rate Echocardiography and Coronary Artery Disease. Circulation: Cardiovascular Imaging. 2011;4:179–190. doi: 10.1161/CIRCIMAGING.110.959817. [DOI] [PubMed] [Google Scholar]
- Jawad H, Ali NN, Lyon AR, Chen QZ, Harding SE, Boccaccini AR. Myocardial tissue engineering: a review. Journal of tissue engineering and regenerative medicine. 2007;1:327–342. doi: 10.1002/term.46. [DOI] [PubMed] [Google Scholar]
- Jawad H, Lyon AR, Harding SE, Ali NN, Boccaccini AR. Myocardial tissue engineering. Br Med Bull. 2008;87:31–47. doi: 10.1093/bmb/ldn026. [DOI] [PubMed] [Google Scholar]
- Kitamura H, Ohnishi Y, Yoshida A, Okajima K, Azumi H, Ishida A, Galeano EJ, Kubo S, Hayashi Y, Itoh H, Yokoyama M. Heterogeneous Loss of Connexin43 Protein in Nonischemic Dilated Cardiomyopathy with Ventricular Tachycardia. Journal of Cardiovascular Electrophysiology. 2002;13:865–870. doi: 10.1046/j.1540-8167.2002.00865.x. [DOI] [PubMed] [Google Scholar]
- Koch-Schneidemann S, Gehr P, Rutishauser B, Eppenberger HM. Attachment of adult rat cardiomyocytes (ARC) on laminin and two laminin fragments. J Struct Biol. 1994;113:107–116. doi: 10.1006/jsbi.1994.1037. [DOI] [PubMed] [Google Scholar]
- Maitra N, Flink IL, Bahl JJ, Morkin E. Expression of alpha and beta integrins during terminal differentiation of cardiomyocytes. Cardiovascular research. 2000;47:715–725. doi: 10.1016/s0008-6363(00)00140-1. [DOI] [PubMed] [Google Scholar]
- Marian AJ, Roberts R. Molecular basis of hypertrophic and dilated cardiomyopathy. Texas Heart Institute Journal. 1994;21:6–15. [PMC free article] [PubMed] [Google Scholar]
- Michel JB. Anoikis in the cardiovascular system: known and unknown extracellular mediators. Arterioscler Thromb Vasc Biol. 2003;23:2146–2154. doi: 10.1161/01.ATV.0000099882.52647.E4. [DOI] [PubMed] [Google Scholar]
- Miskon A, Ehashi T, Mahara A, Uyama H, Yamaoka T. Beating behavior of primary neonatal cardiomyocytes and cardiac-differentiated P19.CL6 cells on different extracellular matrix components. J Artif Organs. 2009;12:111–117. doi: 10.1007/s10047-009-0449-4. [DOI] [PubMed] [Google Scholar]
- Morita H, Seidman J, Seidman CE. Genetic causes of human heart failure. The Journal of clinical investigation. 2005;115:518–526. doi: 10.1172/JCI200524351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MD, Tinney JP, Yuan F, Roussel TJ, El-Baz A, Giridharan G, Keller BB, Sethu P. Cardiac Cell Culture Model (CCCM) as a Left Ventricle Mimic for Cardiac Tissue Generation. Analytical chemistry. 2013a;85 doi: 10.1021/ac401910d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MD, Tinney JP, Yuan F, Roussel TJ, El-Baz A, Giridharan G, Keller BB, Sethu P. Cardiac Cell Culture Model As a Left Ventricle Mimic for Cardiac Tissue Generation. Analytical Chemistry. 2013b;85:8773–8779. doi: 10.1021/ac401910d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita K, Matsumura Y, Sato Y, Okada A, Morizane A, Okamoto S, Hong H, Nakagawa M, Tanabe K, Tezuka K, Shibata T, Kunisada T, Takahashi M, Takahashi J, Saji H, Yamanaka S. A more efficient method to generate integration-free human iPS cells. Nature methods. 2011;8:409–412. doi: 10.1038/nmeth.1591. [DOI] [PubMed] [Google Scholar]
- Palatinus JA, Rhett JM, Gourdie RG. The connexin43 carboxyl terminus and cardiac gap junction organization. Biochimica et biophysica acta. 2012;1818:1831–1843. doi: 10.1016/j.bbamem.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajala K, Pekkanen-Mattila M, Aalto-Set #228, #228 K. Cardiac Differentiation of Pluripotent Stem Cells. Stem Cells International 2011. 2011 doi: 10.4061/2011/383709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RS, Borg TK. Integrins and the myocardium. Circ Res. 2001;88:1112–1119. doi: 10.1161/hh1101.091862. [DOI] [PubMed] [Google Scholar]
- Ruan JL, Tulloch NL, Saiget M, Paige SL, Razumova MV, Regnier M, Tung KC, Keller G, Pabon L, Reinecke H, Murry CE. Mechanical Stress Promotes Maturation of Human Myocardium From Pluripotent Stem Cell-Derived Progenitors. Stem cells (Dayton, Ohio) 2015;33:2148–2157. doi: 10.1002/stem.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz K, Carrier L, Guicheney P, Komajda M. Molecular Basis of Familial Cardiomyopathies. Circulation. 1995;91:532–540. doi: 10.1161/01.cir.91.2.532. [DOI] [PubMed] [Google Scholar]
- Sehnert AJ, Huq A, Weinstein BM, Walker C, Fishman M, Stainier DY. Cardiac troponin T is essential in sarcomere assembly and cardiac contractility. Nature genetics. 2002;31:106–110. doi: 10.1038/ng875. [DOI] [PubMed] [Google Scholar]
- Severs NJ, Bruce AF, Dupont E, Rothery S. Remodelling of gap junctions and connexin expression in diseased myocardium. Cardiovascular research. 2008;80:9–19. doi: 10.1093/cvr/cvn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimko VF, Claycomb WC. Effect of Mechanical Loading on Three-Dimensional Cultures of Embryonic Stem Cell-Derived Cardiomyocytes. Tissue engineering Part A. 2008a;14:49–58. doi: 10.1089/ten.2007.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimko VF, Claycomb WC. Effect of mechanical loading on three-dimensional cultures of embryonic stem cell-derived cardiomyocytes. Tissue engineering Part A. 2008b;14:49–58. doi: 10.1089/ten.2007.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon AM, Goodenough DA, Paul DL. Mice lacking connexin40 have cardiac conduction abnormalities characteristic of atrioventricular block and bundle branch block. Current biology: CB. 1998;8:295–298. doi: 10.1016/s0960-9822(98)70113-7. [DOI] [PubMed] [Google Scholar]
- Sun N, Yazawa M, Liu J, Han L, Sanchez-Freire V, Abilez OJ, Navarrete EG, Hu S, Wang L, Lee A, Pavlovic A, Lin S, Chen R, Hajjar RJ, Snyder MP, Dolmetsch RE, Butte MJ, Ashley EA, Longaker MT, Robbins RC, Wu JC. Patient-specific induced pluripotent stem cells as a model for familial dilated cardiomyopathy. Science translational medicine. 2012;4:130ra147. doi: 10.1126/scitranslmed.3003552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terracio L, Rubin K, Gullberg D, Balog E, Carver W, Jyring R, Borg TK. Expression of collagen binding integrins during cardiac development and hypertrophy. Circulation research. 1991;68:734–744. doi: 10.1161/01.res.68.3.734. [DOI] [PubMed] [Google Scholar]
- Wang X, Chun YW, Zhong L, Chiusa M, Balikov DA, Frist AY, Lim CC, Maltais S, Bellan L, Hong CC, Sung HJ. A temperature-sensitive, self-adhesive hydrogel to deliver iPSC-derived cardiomyocytes for heart repair. International Journal of Cardiology. 2015;190:177–180. doi: 10.1016/j.ijcard.2015.04.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willecke K, Eiberger J, Degen J, Eckardt D, Romualdi A, Guldenagel M, Deutsch U, Sohl G. Structural and functional diversity of connexin genes in the mouse and human genome. Biological chemistry. 2002;383:725–737. doi: 10.1515/BC.2002.076. [DOI] [PubMed] [Google Scholar]
- Willott RH, Gomes AV, Chang AN, Parvatiyar MS, Pinto JR, Potter JD. Mutations in Troponin that cause HCM, DCM AND RCM: what can we learn about thin filament function? Journal of molecular and cellular cardiology. 2010;48:882–892. doi: 10.1016/j.yjmcc.2009.10.031. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Dang QN, Maeda Y, Huang H, Kelly RA, Lee RT. Regulation of Cardiomyocyte Mechanotransduction by the Cardiac Cycle. Circulation. 2001;103:1459–1464. doi: 10.1161/01.cir.103.10.1459. [DOI] [PubMed] [Google Scholar]
- Yang T, Chun YW, Stroud DM, Mosley JD, Knollmann BC, Hong C, Roden DM. Screening for Acute IKr Block Is Insufficient to Detect Torsades de Pointes Liability: Role of Late Sodium Current. Circulation. 2014;130:224–234. doi: 10.1161/CIRCULATIONAHA.113.007765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Klos M, Wilson GF, Herman AM, Lian X, Raval KK, Barron MR, Hou L, Soerens AG, Yu J, Palecek SP, Lyons GE, Thomson JA, Herron TJ, Jalife J, Kamp TJ. Extracellular matrix promotes highly efficient cardiac differentiation of human pluripotent stem cells: the matrix sandwich method. Circ Res. 2012;111:1125–1136. doi: 10.1161/CIRCRESAHA.112.273144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.