Summary
Functional PNS regeneration requires injured axons to return to their original synaptic targets, yet the mechanisms underlying target-selective regeneration have remained elusive. Using live-cell imaging in zebrafish we find that regenerating motor axons exhibit a strong preference for their original muscle territory, and that axons probe both correct and incorrect trajectories extensively before selecting their original path. We show that this process requires the glycosyltransferase lh3 and that post-injury expression of lh3 in Schwann cells is sufficient to restore target-selective regeneration. Moreover, we demonstrate that Schwann cells neighboring the transection site express the lh3 substrate collagen4a5 and that during regeneration collagen4a5 destabilizes axons probing inappropriate trajectories to ensure target-selective regeneration, possibly through the axonal repellant slit1a. Our results demonstrate that selective ECM components match subpopulations of regenerating axons with their original targets, and reveal a previously unappreciated mechanism that conveys synaptic target selection to regenerating axons in vivo.
Keywords: zebrafish, peripheral nerve, motor axon, regeneration, Schwann cell, lh3, collagen4a5
Introduction
Axons of the peripheral nervous system have the remarkable ability to regenerate following injury and to form functional connections with their original targets. Damage to peripheral nerves such as trauma, disease or chemical insult triggers the well-characterized program of Wallerian degeneration that results in axonal fragmentation and debris clearance involving immune and Schwann cells (Vargas and Barres, 2007; Waller, 1849). Concomitantly, denervated Schwann cells de-differentiate to support axonal regrowth from the proximal nerve stump (Rosenberg et al., 2014; Zochodne, 2008). There, intrinsic and extrinsic factors promote sprouting of axonal growth cones, which then begin to reestablish functional connections with their original synaptic targets (Brushart, 2011; reviewed in Zochodne, 2008).
Not surprisingly, the degree of functional regeneration depends largely on the type of injury (Kruspe et al., 2014). For example, crush injuries leave the nerve-ensheathing basal lamina intact, providing an uninterrupted tube-like substrate leading regenerating axons back to their appropriate targets (Haftek and Thomas, 1968; Scherer and Easter, 1984; Sketelj et al., 1989; Westerfield and Powell, 1983). In contrast, nerve transections disrupt the continuity of the nerve and nerve basal lamina, forcing regenerating axons to navigate across the injury gap through an acellular environment (Forman and Berenberg, 1978; Forman et al., 1979). This challenge is even greater in cases when regenerating axons encounter a nerve branch choice point distal to the injury site. Axons that fail to select appropriate branch-specific trajectories frequently miss their original targets, thereby decreasing the degree of functional regeneration (reviewed in Brushart, 2011). Moreover, misguided axons can innervate inappropriate targets, leading to involuntary muscle contractions such as those observed in facial palsy (Kimura et al., 1975; Spector et al., 1991). Several studies argue that this sparse and/or ectopic axonal reinnervation is the result of regenerating axons selecting their path at branch points in a stochastic manner (English, 2005; Scherer, 1986; Westerfield, 1987; Westerfield and Powell, 1983), while others conclude that regenerating axons somehow ‘recognize’ their original trajectory (Brushart, 1988; Grimm, 1971; Kuffler, 1986a; Lee and Farel, 1988; Mark, 1965; Sperry and Arora, 1965; Stephenson, 1979). However, the mechanisms and molecules that enable regenerating axons to select their original trajectory at branch choice points in vivo have remained elusive.
Extracellular matrix (ECM) components and their modifying enzymes are known to provide critical guidance to developing axons, and while several ECM components are transcriptionally upregulated following peripheral nerve injury, their roles in axonal regeneration have not been well defined in vivo (Chen et al., 2011; Kubo et al., 2002; Nix et al., 2014). In regenerating peripheral nerves, ECM components are the second most upregulated class of genes, and though regenerating axons associate with the ECM as they to return to their targets (Chen et al., 2011; Chernousov and Carey, 2000; Kubo et al., 2002; Nix et al., 2014), the role of ECM components and their modifying enzymes has not been fully elucidated in genetic loss of function studies due to their frequent essential requirement during development (George et al., 1993; Guo et al., 1991; Löhler et al., 1984; Myllyharju, 2004; Poschl, 2004; Ruotsalainen et al., 2006; Smyth et al., 1999). Here, we take advantage of the optical transparency and stereotyped peripheral motor nerve architecture in larval zebrafish to determine the role of ECM components in target specific regeneration of spinal motor axons. We find that the collagen-modifying glycosyltransferase lysyl hydroxylase 3 (lh3) is critical for regenerative growth and guidance of axons of the dorsal but not ventral nerve branch, and that lh3 expression during regeneration and in Schwann cells is sufficient to restore dorsal regeneration. Furthermore we show that in vivo lh3 exerts its function at least in part through its well-established substrate collagen4a5 (Ruotsalainen et al., 2006; Wang et al., 2000), and that following nerve transection collagen4a5 mRNA is selectively upregulated in Schwann cells at the lesion site. Combined our results revise the widely-held assumption that during regeneration ECM components serve primarily as permissive substrates, and reveal an underappreciated yet specific role in directing regenerating axons towards their original targets.
RESULTS
Regenerating motor axons select their original trajectory with high fidelity
Following complete nerve transection, peripheral axons can successfully traverse a short, acellular injury gap, yet whether axons randomly extend towards their original targets when confronted with a path choice or whether mechanisms for target-selective innervation exist has long been a point of contention (Brushart, 1993; English, 2005; Kuffler, 1986a; Scherer, 1986; Westerfield and Powell, 1983). As a first step to distinguish between these possibilities in a live vertebrate system, we took advantage of the simple architecture of larval zebrafish peripheral motor nerves. Each motor nerve consists of approximately 100 fasciculated axons, which separate into two main nerve branches shortly after exiting from the spinal cord: a ventral nerve branch consisting of 60–80 axons with synaptic targets in the ventral myotome, and a dorsal nerve branch consisting of 20–30 axons innervating the dorsal myotome (Myers et al., 1986; Westerfield, 1987; Westerfield et al., 1986 and Figure 1A).
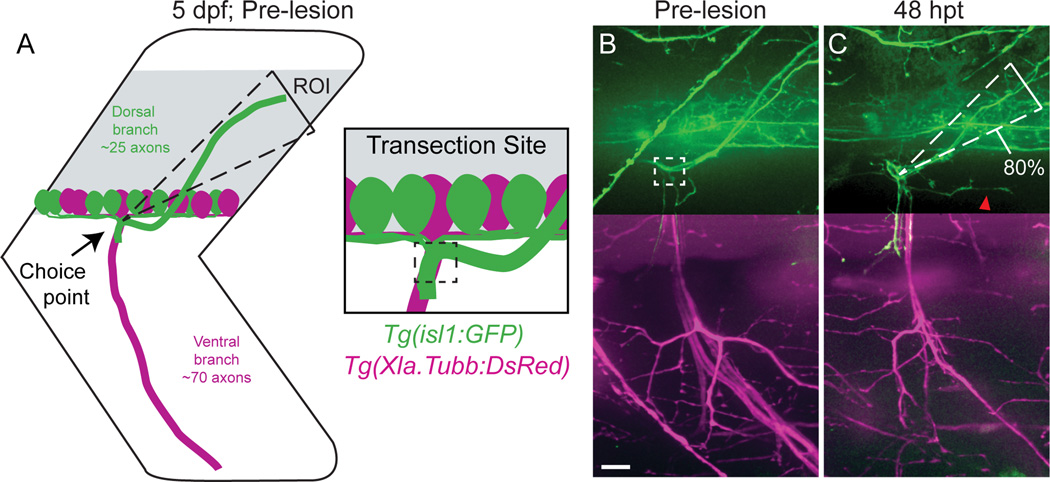
Figure 1. Regenerating motor axons select their original trajectory with high fidelity. (A,B).
Zebrafish peripheral motor axons traverse a common path then diverge to innervate functionally distinct myotomal regions (dashed triangle, dorsal ROI; dashed boxes, nerve transection site; scale bar = 10µm). (C) By 48 hpt, dorsal axons regrow with great fidelity to the original trajectory (80% of fascicles that developed in the dorsal ROI regrew on the dorsal path, n = 14 larvae, 26 nerves; red arrowhead, misguided regrowth). In B and C, we omitted Tg(Xla.Tubb:DsRed) signal from the dorsal panel as expression in the spinal cord overwhelms the max projection image. The ventral panel shows expression from both transgenes.
To test whether regenerating motor axons preferentially select their original branch-specific nerve path, we laser-transected the entire motor nerve proximal to where the trajectories of the dorsal and ventral branches diverge, creating a ~9µm gap between the proximal and distal nerve stumps (Binari et al., 2013; Lewis and Kucenas, 2014; Rosenberg et al., 2014). We labeled both ventral and dorsal nerve axons using the Tg(Xla.Tubb:DsRed) transgene (Peri and Nüsslein-Volhard, 2008), and selectively labeled the dorsal branch with the Tg(isl1:GFP) transgene (Uemura et al., 2005), thereby enabling us to monitor target-selective regeneration in vivo (Figure 1A,B). Prior to nerve transection (pre-lesion), the majority of Tg(isl1:GFP) labeled fascicles extend within a very narrow area that spans 20° of the dorsal myotome (Figure 1A, B). Forty-eight hours post nerve transection, 80% of these fascicles regenerated to this original area (Figure 1C; see Experimental Procedures for more details on quantification). This is a significantly higher fraction than the 50% expected for a ‘random’ mechanism given a binary choice between the 20° dorsal target area and regions outside, demonstrating that following complete nerve transection, regenerating axons of the dorsal nerve branch retain the ability to select their original branch-specific trajectory. Furthermore, transection of only the dorsal nerve branch resulted in the same degree of branch-specific regrowth of Tg(isl1:GFP) positive fascicles (data not shown), indicating that target-selective regeneration of dorsal nerve axons occurs independently of injury to ventral nerve axons. Together these results reveal that when confronted with a choice point, regenerating zebrafish motor axons select their original path with high fidelity, consistent with the existence of non-random genetic mechanisms that promote target-selective regeneration.
Regenerating axons probe the transection gap extensively before selecting their original path
We next used live-cell imaging to examine the behavior of regenerating axons as they encounter a branch choice point. One possibility is that regenerating axons exclusively rely on a predetermined intrinsic program that instructs growth cones to extend rapidly onto the appropriate path without probing the environment at choice points. Alternatively, regenerating growth cones might integrate extrinsic cues to select their appropriate path. A prediction for this latter scenario is that as regenerating growth cones cross the injury gap and encounter a choice point, they would extensively probe their environment for instructive cues. To determine the extent to which regenerating growth cones probe their environment, we transected the dorsal nerve branch and monitored in vivo growth cone dynamics of pioneering axons as they cross the injury gap and encounter the branch choice point (Figure 2A–F; Movie S1). Following transection, axons proximal to the injury site retracted, while distal axons underwent Wallerian degeneration, which is conserved in zebrafish (Cajal, 1928; Gaudet et al., 2011; Lewis and Kucenas, 2014; Martin et al., 2010; Rosenberg et al., 2012; 2014; Vargas and Barres, 2007; Waller, 1849). Between 7 and 11 hours post-transection (hpt) we observed the first axons sprouting growth cones into the injury gap, where they probed the environment with short bursts of extension and retraction. These bursts of extension and retraction occurred at almost equal frequencies towards the correct dorsal path and towards the incorrect ventral path (Figure 2B,C; Movie S1; dorsal: 17.1±1.46 bursts; ventral: 16.9±1.68 bursts; n=20 nerves; See Experimental Procedures for quantification). Over the next 2–4 hours, ventrally directed axons extended and collapsed frequently, while growth cones extending along the correct dorsal path stabilized (Figure 2D,E; n = 15/16 nerves; Movie S1). These stabilized, dorsally directed axons then extended rapidly, eventually reaching their original synaptic target regions in the dorsal myotome (Figure 2F; Movie S1). Thus, as regenerating axons of the dorsal nerve branch encounter the nerve branch point, they explore both the correct dorsal and incorrect ventral path before ultimately selecting the path to their original synaptic targets. This extensive probing behavior strongly supports the idea that regenerating growth cones rely on extrinsic cues to navigate their branch choice point.
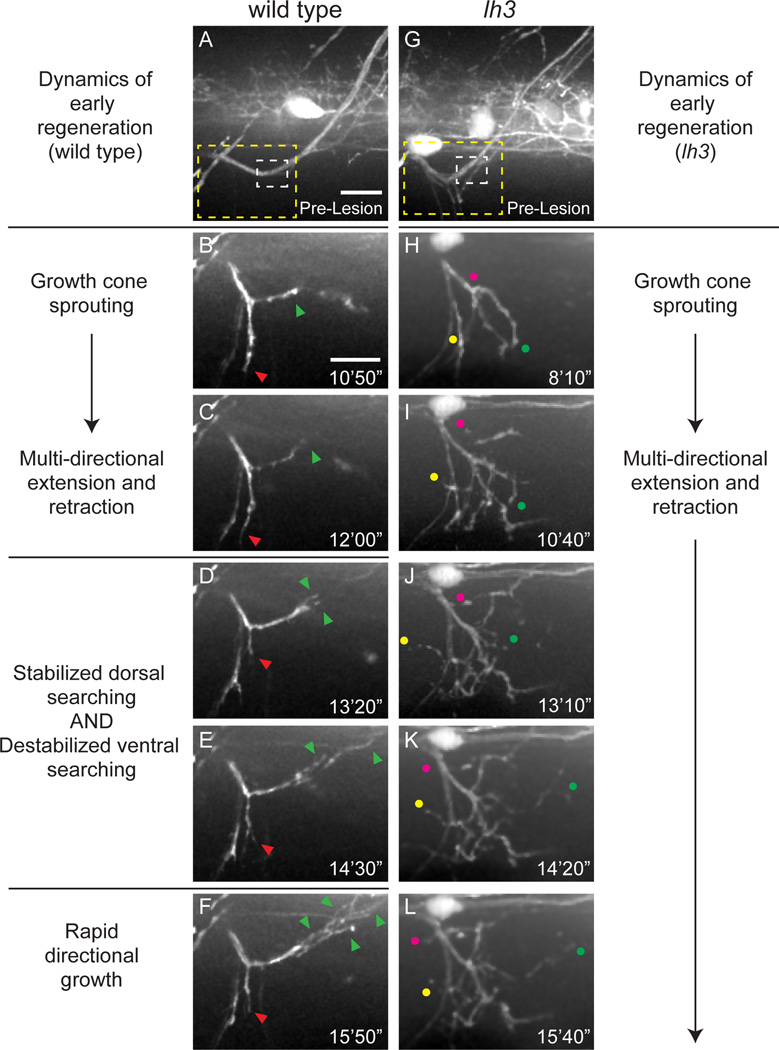
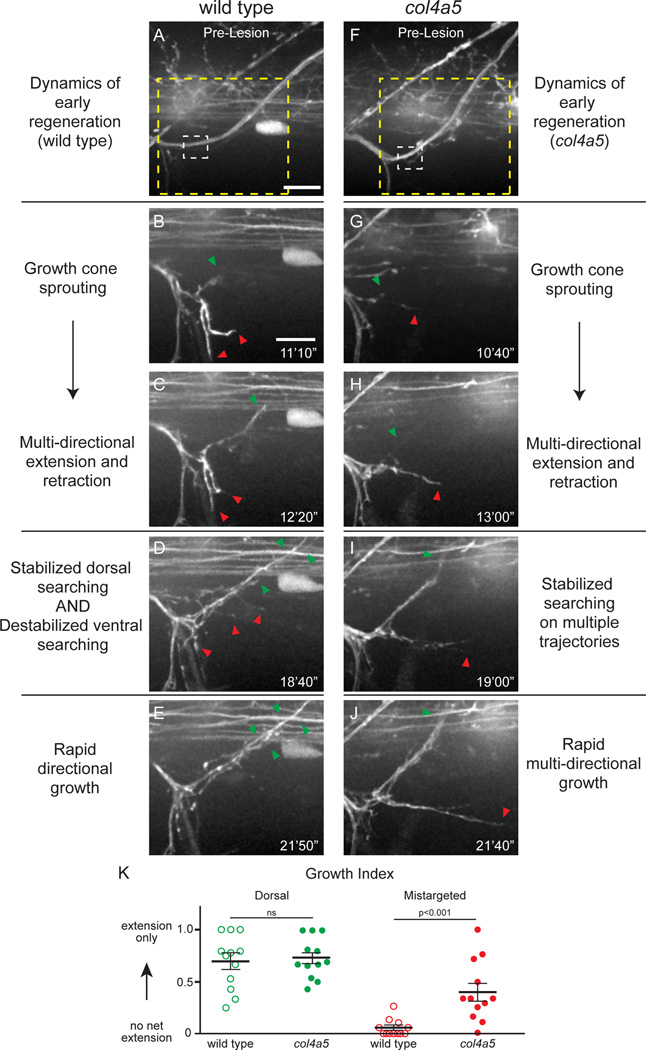
Figure 2. lh3 is required for pathway stabilization in early regeneration.
(A) Wild type dorsal nerve prior to nerve transection (white dashed box, transection site; yellow dashed box, region magnified in B–F; scale bar = 10µm). (B,C) Regenerating wild type dorsal axons sprout growth cones and probe the injury gap through multi-directional extension and retraction (red arrowhead, ventral probing; green arrowhead, dorsal probing; scale bar, 10µm). (D–F) Axons then destabilize non-dorsal searching and stabilize dorsal searching leading to rapid directional growth (n=8 larvae, 15/16 nerves). (G) Conditional lh3 mutant dorsal nerves develop indistinguishably from wild type (white dashed box, transection site; yellow dashed box, region magnified in H–L). (H–L), In the absence of lh3, axons sprout growth cones after transection. Axons probe the myotome multidirectionally (magenta, yellow and green dots track individual fascicles), but fail to stabilize dorsal searching and destabilize ventral searching (n = 11 larvae; 10/26 nerves). When lh3 mutant axons stabilized growth, these axons often grew on non-dorsal paths (n = 8/26 nerves, p<0.001).
lh3 is required for growth of regenerating axons and target-selective regeneration
Regenerating axons exhibit highly dynamic behaviors as they probe the transection gap, suggesting that cues in the extracellular environment might lead them back to their original trajectory. Therefore, we chose to test components of the extracellular matrix (ECM) for specific roles in this process. Collagens are abundant in the ECM, and it has long been noted that axons regenerate along basal laminae rich in Collagens (Carey et al., 1983; Chernousov and Carey, 2000; Martin and Timpl, 1987). Given that vertebrate genomes express a large number of Collagen-encoding genes (28 in mammals, 42 in zebrafish), we decided to test the role of Collagens in nerve regeneration by analyzing a single gene whose function is critical for post-translational Collagen modifications. Collagens are modified by ~20 isoenzymes, including glycosyltransferases whose functions are critical for collagen assembly, secretion and function (Myllyharju, 2004). Of these, lysyl hydroxylase 3 (lh3) is a well-characterized glycosyltransferase that modifies a known set of Collagens for proper secretion and deposition in the ECM (Norman and Moerman, 2000 and Figure S3; Ruotsalainen et al., 2006; Sipilä et al., 2007).
To bypass the requirement of lh3 during development (Schneider and Granato, 2006; Zeller and Granato, 1999), we generated a conditional, heat-inducible Tg(hsp70l:lh3myc) transgene to restore early motor nerve development in lh3 mutants, and then examined dorsal nerve regeneration in animals lacking lh3 during regeneration (hereafter ‘conditional lh3 mutants’ see Supplemental Experimental Procedures, and Figure S1). At 5 days post fertilization (dpf), peripheral motor nerves in these conditional lh3 mutants were indistinguishable from those of wild type siblings, including the presence of closely associated Schwann cells (compare Figure 2A, G, and data not shown). Following dorsal nerve transection in conditional lh3 mutants, we observed distal motor axon fragmentation and axonal debris removal, followed by proximal growth cones sprouting with kinetics comparable to those observed in wild type siblings (Figure 2H–I; Movie S2 and data not shown). Like in wild type, regenerating lh3 mutant growth cones probed the transection gap through multi-directional extension and retraction (Figure 2I–L). However, in contrast to wild type growth cones, many lh3 mutant axons failed to consolidate onto their original dorsally directed path, and instead repeatedly exhibited short bursts of extension and retraction which lasted for the duration of recording (10 hours; n=10/26 nerves; Movie S2). In cases where lh3 mutant axons stabilized growth, these axons often grew into aberrant regions of the myotome (n=8/26 nerves; data not shown). To quantify the role of lh3 beyond the early stages of axonal regrowth, we first analyzed dorsal axon regrowth extent at 48 hpt, when wild type peripheral motor axons have regrown sufficiently to restore neuromuscular function (Rosenberg et al., 2012). In wild type siblings, over 80% of dorsal nerves regrew axons into the dorsal myotome. In contrast, in lh3 mutants only 60% of dorsal nerves regrew axons into the dorsal myotome, demonstrating that lh3 is required to support growth of regenerating dorsal nerves in vivo (Figure 3AD, quantified in G “dorsal” using categories described in G inset and Supplementary Experimental Procedures; regrowth = extent categories 3–5; p<0.001).
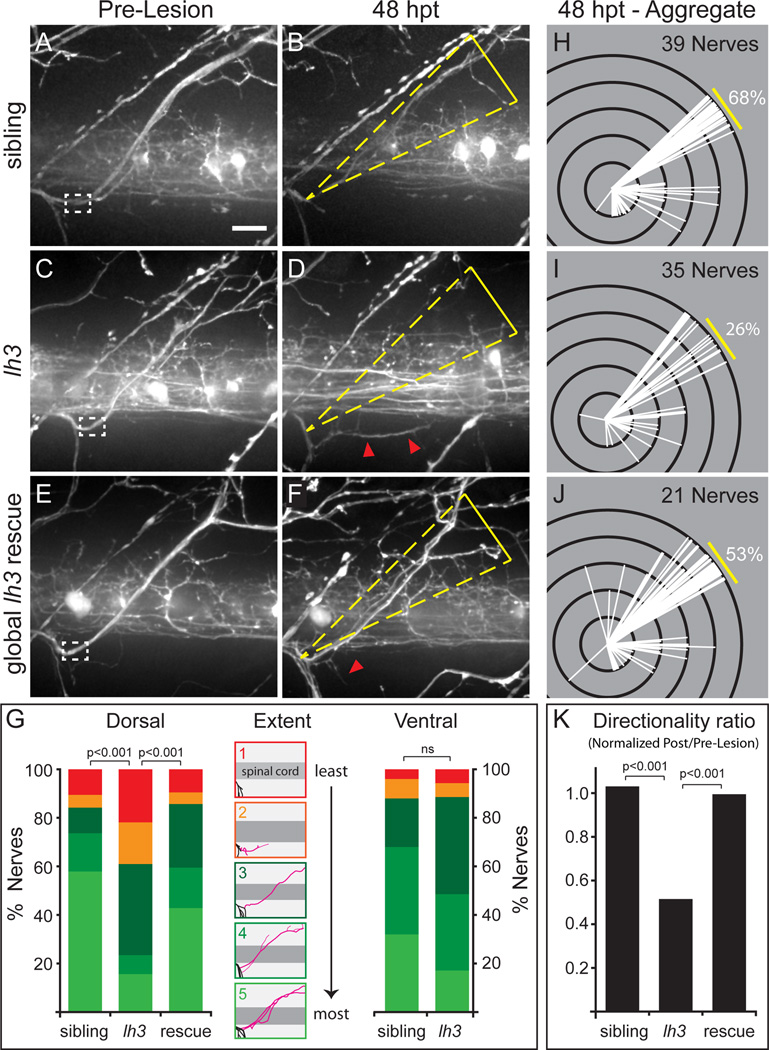
Figure 3. lh3 is required for regenerative axonal growth and target-selective regeneration.
(A–D) Compared to sibling nerves (A,B) conditional lh3 mutant motor nerves (C,D) regrew fewer fascicles, and regrowth often targeted non-dorsal regions (white dashed box, transection site, yellow triangle, dorsal ROI; red arrowheads, misguided fascicles; scale bar=10µm). (E,F) lh3 expression after transection rescued this defect. (G) lh3 is required for regenerative growth across populations (wild type sibling, n=13 larvae, 39 nerves; lh3, n=13 larvae, 35 nerves; Global lh3 rescue, n=8 larvae, 21 nerves). Camera lucida tracings of regrowth “extent” categories described in Supplementary Experimental Procedures (black = uninjured axons, pink = regenerated axons). Regenerating ventral axons do not require lh3 function (sibling, n = 9 larvae, 25 nerves; lh3, n = 16 larvae, 35 nerves). (H–K) Modified Sholl analysis reveals that in comparison to siblings (H), fewer lh3 fascicles (I) regrew to the dorsal myotome. This defect was partially rescued by ubiquitous lh3 transgene expression during regeneration (J). (K) These differences were statistically significant after adjusting for developmental dorsal axon patterning in the directionality ratio.
We noticed that instead of returning to their original dorsal muscle targets regenerating dorsal nerve axons in lh3 mutants frequently invaded lateral as well as ventral regions of the myotome (Figure 3D). To quantify the precision with which dorsal nerve axons regrew to their original dorsal targets, we applied a modified Sholl analysis (Li and Hoffman-Kim, 2008; Sholl, 1953). In wild type siblings, 68% of regenerating fascicles from the dorsal nerve regrew and formed synapses on muscle fibers within a 20° region of their original synaptic target area while in lh3 mutants regrowth to this area was reduced to 26%, resulting in increased ectopic regrowth either adjacent to their original target area or into the ventral myotome (Figure 3H,I, Figure S2). To also take into account the number of fascicles present prior to nerve transection, we introduced a directionality ratio (% of fascicles in target area pre transection ÷ % of fascicles in target area post transection normalized to wild type; Experimental Procedures). This confirmed that in lh3 mutants the proportion of regenerating axons that extend along their original trajectory is significantly decreased (Figure 3K). We noted that in lh3 mutants some fascicles regrew just outside the 20° region. Including these fascicles in our analysis did not change the statistical significance between mutants and wild type (p<0.001). Thus, lh3 plays dual roles in regeneration by promoting the overall growth of regenerating dorsal nerve axons, and by directing their growth to their original target area.
We next asked whether lh3 is required for regeneration of all motor axons, or whether lh3 function is selective for dorsal nerve regeneration. For this, we transected ventral nerves in conditional lh3 mutant larvae. We detected no significant difference in the extent or fidelity of lh3 ventral nerve regeneration when compared to wild type siblings (Figure 3G “ventral” and data not shown). Combined, these data demonstrate that lh3 selectively promotes regeneration and target-selectivity of regenerating dorsal nerve axons.
Finally, we tested if lh3 functions during the process of regeneration. To address this, we induced lh3 expression from Tg(hsp70l:lh3myc) ~6hr after dorsal nerve transection, just before the first regenerating growth cones emerge. This almost completely restored the extent of dorsal nerve outgrowth at 48 hours post transection in lh3 mutants (Figure 3E–G), and significantly increased the ability of regenerating axons to return to their original target area (Figure 3J,K, p<0.001). Combined, these data provide compelling evidence that lh3 functions to promote regrowth and target-selective regeneration of dorsal nerve axons during regeneration.
The lh3 substrate collagen4a5 (col4a5) directs regenerating dorsal nerve axons
To identify the relevant in-vivo lh3 substrates for dorsal nerve regeneration, we took a candidate approach. lh3 predominantly glycosylates fibrillar collagens and basal laminar collagens (Anttinen et al., 1978; Sipilä et al., 2007; Wang et al., 2000), and we therefore examined mutants for three lh3 basal laminar collagen substrates. We focused on collagen4a5 (col4a5) and collagen18a1 (col18a1) because their expression is upregulated following peripheral nerve injury (Arthur-Farraj et al., 2012; Kubo et al., 2002; Siironen et al., 1992), and also included collagen19a1 (col19a1) because of its role in motor axon guidance during zebrafish development (Beattie et al., 2000; Hilario et al., 2010). Each of these collagens contains several predicted lh3 glycosylation sites required for proper secretion and deposition into the ECM (Figure S3 and Hautala et al., 1992; Ruotsalainen et al., 2006; Wang et al., 2000). Consistent with this we find that transgenic Col4a5 expression in mutants lacking lh3 activity, but not in wild type embryos, caused aberrant Col4a5 protein localization, providing direct evidence that in zebrafish lh3 is required for Col4a5 localization (Figure S3). To determine the in-vivo roles of the three collagens in peripheral nerve regeneration, we obtained existing zebrafish col4a5 and col19a1 mutants (Hilario et al., 2010; Xiao and Baier, 2007), and generated several TALEN induced col18a1 mutations predicted to abolish col18a1 function (see Supplemental Experimental Procedures).
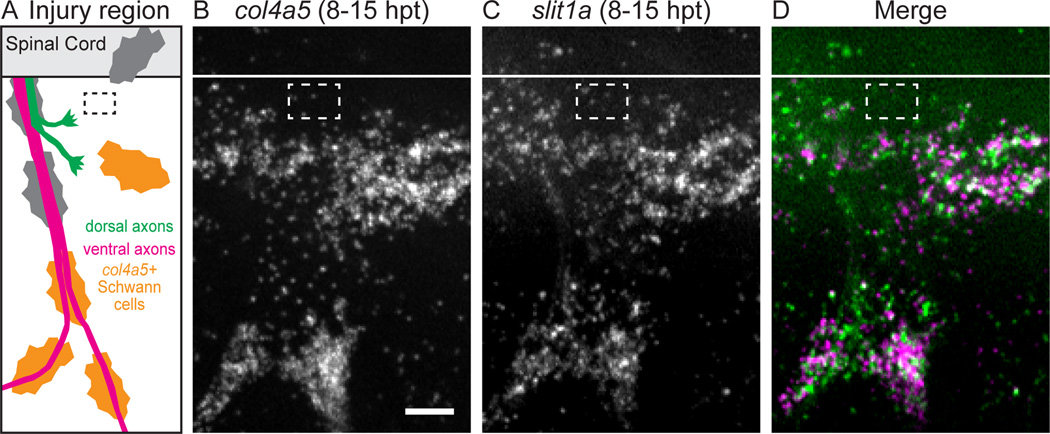
Like lh3 mutants, none of these mutants exhibited defects in ventral axon regeneration (data not shown). Importantly, dorsal axon regeneration was unaffected in col18a1 and col19a1 mutants, demonstrating that the mere removal of a basement membrane collagen is not sufficient to disrupt regeneration (Figure S3). Furthermore, dorsal nerve development in col4a5 mutants was indistinguishable from that in wild type siblings (compare Figure 4A,D). However, similar to lh3 mutant fascicles, 57% of col4a5 mutant fascicles regenerated into aberrant lateral and ventral regions of the myotome, demonstrating that col4a5 is critical for dorsal axon regeneration in vivo, and that lh3 operates — at least partially — through col4a5 to mediate peripheral nerve regeneration (Figure 4E,F,G). Finally, we asked when and where col4a5 is expressed during nerve regeneration (Figure 4H–K). In the absence of nerve transection, col4a5 mRNA is detectable in spinal cord cells adjacent to the central canal (data not shown), but was undetectable along the ventral or dorsal nerve path (Figure 4I, data not shown). In contrast, between 8 and 15 hpt, col4a5 mRNA was upregulated in Schwann cells just ventral and ventrolateral to the transection site (Figure 4H,J,K). Thus, col4a5 mRNA is upregulated precisely when and where regenerating dorsal nerve axons select their original trajectory.
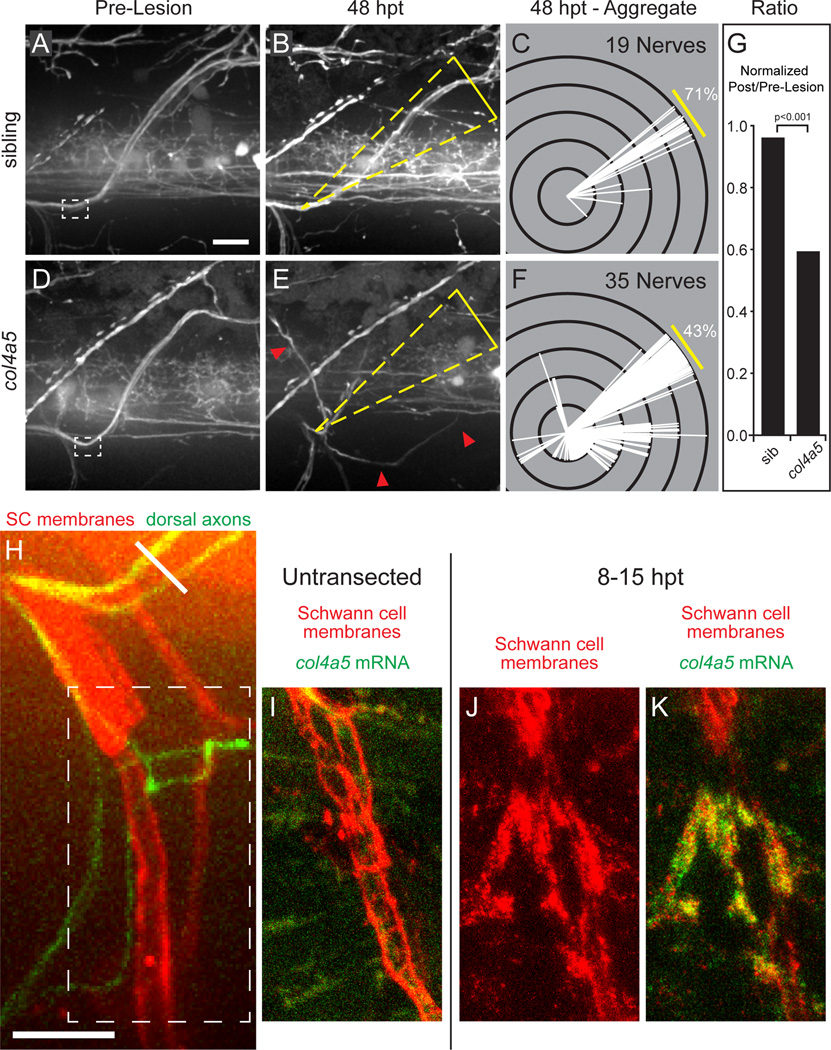
Figure 4. The lh3 substrate collagen4a5 (col4a5) is upregulated after nerve transection and directs regenerating dorsal nerve axons.
(A–F) After nerve transection, sibling nerves (A,B) regenerate to the original outgrowth pathway whereas col4a5 nerves regrow into aberrant regions of the myotome (D,E; yellow triangles, dorsal ROI; red arrowheads, misguided fascicle). (C,F) Modified Sholl analysis reveals that in comparison to siblings (n = 7 larvae, 19 nerves), fewer col4a5 fascicles regrew to the dorsal ROI (n = 12 larvae, 35 nerves). (G) These differences were statistically significant after adjusting for developmental dorsal axon patterning in the directionality ratio. (H) Region of transected nerves showing col4a5 mRNA signal in J–K (oblique white line, transection site; white dashed box, region of nerve shown in I–K). (I) col4a5 in situ hybridization in untransected hemisegments revealed sparse signal (n = 12 larvae, 36/54 nerves). (J,K) 8–15 hours post-transection, col4a5 mRNA was upregulated in Schwann cells (shown in isolation in J) ventral and ventrolateral to the transection site (n = same 12 larvae; 52/60 nerves; p < 0.001). All scale bars = 10µm.
Col4a5 promotes target specific regeneration by destabilizing misdirected axons
We next wanted to understand how col4a5 directs dorsal nerve regrowth. Given that col4a5 is a constituent of the basement membrane, we first examined basement membrane integrity in col4a5 mutants. Immunohistochemistry and electron microscopy revealed no difference between wild-type siblings and col4a5 mutants in the basal lamina directly at the dorsal nerve choice point or at the neuromuscular junction, arguing against a significant defect in basal lamina integrity causing the observed regeneration phenotype (Figure S4). Regenerating axons extend in close association with the remaining the basal lamina of the injured nerve, and we therefore asked if and to what extent regenerating axons in col4a5 mutants retained their ability to grow. For this we quantified average and maximum forward axonal growth rates in col4a5 mutants following nerve transection. In col4a5 mutants regenerative growth rates were indistinguishable from those in wild type animals (wild type: average = 0.15mm/day; maximum = 0.51mm/day; n = 11 fascicles in 11 nerves; col4a5: average = 0.14mm/day; maximum = 0.48mm/day; n = 17 fascicles in 17 nerves; Experimental Procedures).
We therefore considered that rather than regulating growth rates col4a5 might promote target specific regeneration by destabilizing axons probing incorrect trajectories. For this we analyzed growth cone behaviors of regenerating col4a5 dorsal nerve axons in vivo. Similar to wild type axons, between 7 and 11 hpt regenerating col4a5 axons sprout growth cones into the injury gap, and like wild type axons, they immediately and extensively probe their environment (compare Figure 5A–C to Figure 5F–H; Movie S3). As they explored their environment col4a5 mutant axons extended and retracted onto the dorsal path with the same frequency when compared to wild type axons (Figure 5K; See Experimental Procedures for growth index quantification). In contrast, unlike in wild type animals regenerating axons in col4a5 mutants that probed ventral or ventrolateral territories frequently stabilized and grew (Figure 5D,E; 5I,J; Movie S3; quantified in Figure 5K). Importantly, a significant fraction of these aberrantly projecting axons were the first to enter the transection gap (38%, 5/13 nerves). While we cannot formally exclude a potential role for col4a5 in axonal fasciculation, our data is consistent with the idea that rather than defasciculating from axons directed towards the correct dorsal targets, these axons lacked proper guidance early in regeneration. Thus, during regeneration col4a5 does not control axonal growth rates but instead axonal directionality. These data provide compelling evidence that as dorsal axons navigate their branch choice point, col4a5 destabilizes misdirected dorsal axons to promote regeneration towards the original trajectory.
Figure 5. col4a5 destabilizes aberrant growth early in regeneration.
(A) Wild type dorsal nerve prior to nerve transection (white dashed box, transection site; yellow dashed box, region magnified in B–F; scale bar = 10µm). (B,C) Wild type axons sprout growth cones and probe all regions of the injury gap. (D,E) Over time, axons destabilize searching on non-dorsal paths and stabilize searching on the dorsal path leading to rapid directional growth (n = 8 larvae, 15/16 nerves). (F) col4a5 dorsal nerves develop indistinguishably from wild type siblings (white dashed box, transection site; yellow dashed box, region magnified in H–L). (G,H) Regenerating col4a5 axons sprout growth cones and search all regions of the injury gap (I) Over time, axons stabilize searching on the dorsal path but fail to destabilize searching on non-dorsal paths. (J) This leads to invasion of non dorsal regions of the myotome (n = 8 larvae, 17/25 nerves; p<0.0001). Red arrowheads, ventral searching; green arrowhead, dorsal searching; scale bar = 10µm. (K) The net proportion of extension and retraction movements was similar between wild type and col4a5 fascicles on the dorsal path. In contrast, while wild type fascicles extended and retracted with equal frequency, mistargeted col4a5 fascicles extended more frequently and stabilized to grow on aberrant trajectories.
Finally, we asked whether destabilization of misdirected axons correlates with the expression of repulsive guidance cues. Given their well established roles in growth cone repulsion and their ability to bind to col4a5 (Xiao et al., 2011; Yebra et al., 2003), we focused on Netrin and Slit. We have previously shown that both of the zebrafish netrin homologues are expressed in motor neurons and Schwann cells in 5dpf larvae, and that the netrin receptor dcc is required for ventral nerve regeneration (Rosenberg et al., 2014). To test whether Netrin-DCC signaling is required for dorsal nerve regeneration we transected dorsal nerves in dcc mutant larvae. We did not observe any defects in dcc mutant nerve regeneration (data not shown). We therefore examined the expression of the four slit homologues in zebrafish - slit1a, slit1b, slit2 and slit3. Prior to and following dorsal nerve transection we observed slit1b, slit2, and slit3 mRNAs in the spinal cord, but failed to detect expression in transected dorsal nerves (data not shown). In contrast, 8–15 hpt we observed robust upregulation of slit1a mRNA expression ventral and ventrolateral to the lesion site, in the same regions we observed col4a5 mRNA (Figure 6A–C). Double in-situ hybridization confirmed that col4a5 and slit1a mRNA expression strongly co-localizes to a small group of Schwann cells (Figure 6D and Figure 4H–K). Thus, col4a5 is required to direct dorsal nerve regeneration, and together with the guidance repellent slit1a is upregulated in a small group of Schwann cells located ventral and ventrolateral to the transection gap, suggesting a pivotal role for Schwann cells in col4a5-dependent regeneration.
Figure 6. Slit1a is upregulated with collagen4a5 after nerve transection.
(A) Schematic showing approximate region imaged for in situ hybridization (black dashed box, transection site) (B–D) col4a5 mRNA (B) and slit1a mRNA (C) are co-expressed (D) ventral to the spinal after nerve transection (n = 6 larvae, 27/30 nerves). White line, dorsal aspect of spinal cord; white dashed box, approximate transection site; scale bar = 10 µm.
lh3 function in peripheral glia directs regenerating axons
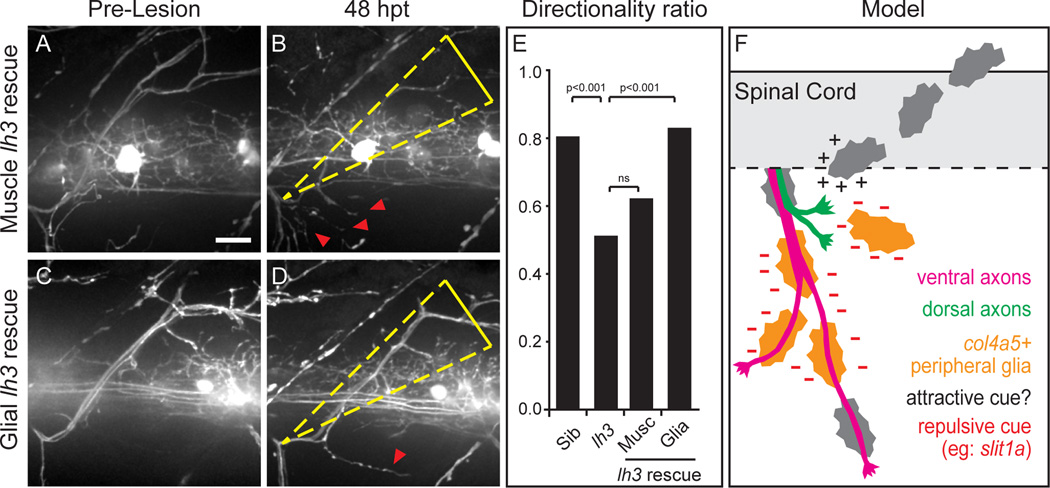
To identify the cell types relevant for lh3 and col4a5 in axonal regeneration, we employed a transgenic rescue strategy. Like in mammals, zebrafish peripheral motor nerves consist of several cell types, most prominently neurons, perineural glia, and Schwann cells (Brushart, 2011; Kucenas et al., 2008; Lyons and Talbot, 2014; Zochodne, 2008). In addition, peripheral nerves are in close contact with muscle fibers. Given that during regeneration col4a5 mRNA expression localizes to Schwann cells (Figure 4J,K), we tested whether transgenic expression of the col4a5 glycosyltransferase lh3 in Schwann cells is sufficient to restore dorsal nerve regeneration in lh3 mutants. As a control we also generated transgenic lines expressing lh3 in somitic muscle directly adjacent to the path of regenerating axons. Though lh3 expression from the Tg(aActin:lh3-mkate) transgene was detectable in muscle cells adjacent to the nerve path, this was insufficient to restore lh3 axon regeneration (Figure 7A,B,E). In contrast, Schwann cell-specific expression of Tg(sox10:lh3-mkate) in lh3 mutants restored axon regeneration (Figure 7C–E). Importantly, in lh3 mutants, the number and position of Sox10+ Schwann cells along the dorsal motor nerve were indistinguishable from that in wild type siblings (lh3=6.49 ± 0.16, n=81 nerves; wild type=6.46 ± 0.14, n=90 nerves). Thus, lh3 function in Schwann cells — but not in muscle — is sufficient to direct dorsal nerve axons during regeneration. Combined, our data suggest a model in which lh3 functions in a small group of Schwann cells to ensure proper secretion and/localization of Col4a5; col4a5 de-stabilizes incorrectly projecting axons – possibly through slit1a – thereby promoting target specific regeneration (Figure 7F).
Figure 7. lh3 in peripheral glia rescues lh3 dorsal axon regeneration defects.
(A–E) Expression of lh3 in all muscles Tg(aActin:lh3mkate) fails to rescue lh3 axon regeneration (A,B). Transgenic expression of lh3 in peripheral glia Tg(sox10:lh3mkate) (C,D) significantly rescued these guidance defects (E). (F) After nerve transection, regenerating motor axons cross the injury gap to return to distal Schwann cells and reinnervate dorsal muscle targets. lh3 glycosylation of ECM components and the lh3 substrate col4a5 are required for targeting dorsal, but not ventral motor axon regeneration. Col4a5 is upregulated in ventral and ventrolateral Schwann cells where it may act to present canonical guidance cues – such as slit1a – to destabilize regenerating axons. Dashed white boxes outline the nerve transection site; dashed yellow triangle, dorsal ROI; red arrowheads, aberrant regrowth; scale bar = 10µm.
DISCUSSION
Non-neuronal cells, including fibroblasts and Schwann cells, are known to generate an extrinsic milieu that promotes axon regeneration (Paíno et al., 1994; Parrinello et al., 2010; Richardson et al., 1980; Schröder et al., 1993; Xu et al., 1997) but whether this environment also provides regenerating axons with target-specificity has been controversial. Here, using live-imaging of regenerating vertebrate axons, we demonstrate that following nerve transection, axons confronted with a trajectory choice select the appropriate path back to their original targets, and this process depends on extrinsic cues. We identify a molecular pathway by which Schwann cell expression of the glycosyltransferase lh3 and expression of lh3 post-transection are required to convey target specificity. We show that one lh3 substrate, col4a5, is upregulated in a defined subset of Schwann cells when regenerating axons select their original trajectory, and that col4a5 destabilizes mistargeted axons to provide target specificity to a subset of regenerating axons in vivo. Finally, we find that nerve transection induces upregulation of the canonical axon guidance repellent slit1a in cells expressing col4a5, providing a potential mechanism by which col4a5 promotes target-selective regeneration (Figure 7F). Together, our results provide compelling evidence that regenerating axons targeted to different synaptic sites utilize specific ECM components that direct them back onto their original trajectories.
Zebrafish spinal motor axons regenerate to their original developmental targets
Since Ramon y Cajal’s original experiments demonstrating axonal misdirection during PNS regeneration (Cajal, 1928), it has become clear that the degree of target-selective reinnervation varies. For example, fully transected sciatic nerve axons of the peroneal and tibial branches regenerating through a 5mm Y-shaped tube displayed no preferential regeneration towards the appropriate distal nerve stump (Abernethy et al., 1992), while transection and surgical apposition of transected mouse sciatic nerves resulted in ~85% of the common fibular branch axons re-innervating their original muscle targets (English, 2005). In contrast, crushing the motor nerve such that the perineurium and the distal Schwann cell tubes remained intact resulted in over 90% of regenerating motor axons innervating their original muscle fibers (Nguyen et al., 2002). Thus, depending on the location and severity of the injury, regenerating axons display varying degrees of target-selective reinnervation. However, the in vivo behaviors of regenerating axons as they negotiate branch points have remained elusive.
In this study we fully transected motor nerves and generated a ~9µm injury gap, which destroys Schwann cells in the injury gap and induces characteristic regeneration-associated morphological changes in Schwann cells neighboring the lesion site (Lewis and Kucenas, 2014; Rosenberg et al., 2012). We find that under these conditions axons retain a high degree of target specificity (80%), indicating a non-random mechanism of reinnervation, consistent with previous reports (Brushart, 1988; Grimm, 1971; Kuffler, 1986a; Lee and Farel, 1988; Mark, 1965; Sperry and Arora, 1965; Stephenson, 1979). We observed that regenerating axons initially extend highly dynamic growth cones randomly towards both correct and incorrect targets before selecting their appropriate path. Moreover, our in vivo studies revealed that misprojecting growth cones collapse in one of the first morphological steps towards target-selectivity, consistent with repulsive forces playing a role in this process. Thus, live-cell imaging reveals that target-specific innervation is a multistep process that includes extensive interactions of regenerating axons with their environment. Indeed, endpoint analysis of transected mouse sciatic nerve axons (Witzel et al., 2005) revealed similar pathway sampling, suggesting that this is an evolutionarily conserved mechanism.
lh3 reveals a novel role for collagens in target-selective peripheral nerve regeneration
Components of the extracellular matrix including heparan sulfate proteoglycans, collagens, and enzymes that modify them post-translationally have well-documented roles in developmental axon guidance (Ackley et al., 2001; Bülow and Hobert, 2006; Poulain and Chien, 2013; Xiao et al., 2011). With a few exceptions (Chen, 2003; Edwards and Hammarlund, 2014), the in vivo roles of ECM components and their modifying enzymes in axonal regeneration are less established. This is in part because genetic knockouts of ECM components often have developmental phenotypes that preclude the analysis of nerve regeneration at later stages (George et al., 1993; Guo et al., 1991; Löhler et al., 1984; Myllyharju, 2004; Poschl, 2004; Ruotsalainen et al., 2006; Smyth et al., 1999). In vitro, there is compelling evidence that ECM molecules of the nerve basal lamina facilitate regrowth (Forman and Berenberg, 1978; Kuffler, 1986b; Martini, 1994; Nathaniel and Pease, 1963; Pollard and Fitzpatrick, 1973; Scherer and Easter, 1984). For example, axons from an excised mouse sciatic nerve grow on acellular Schwann cell basal lamina, suggesting that ECM components are sufficient to support axonal regrowth (Ide et al., 1983). These and other ex vivo experiments have contributed to the notion that during regeneration components of the ECM serve as permissive substrates (Uziyel et al., 2000; Wang et al., 1992a; 1992b; Werner et al., 2000). However, our genetic and live-cell imaging data indicate a much more directive role for the ECM during in vivo regeneration.
Using an inducible transgene, we demonstrate that lh3 is required during nerve regeneration independent of its role during development. During development, lh3 is required in a subset of muscle cells to guide motor axons from the spinal cord to their targets, independently of col4a5 (Zeller and Granato, 1999). Following nerve transection, we find that lh3 expression in Schwann cells is required for target-selectivity of the dorsal but not ventral nerve axons, and that this process also requires the lh3 substrate col4a5 (Figures 3–5,7). Importantly, col4a5 does not regulate axonal growth rates but instead directs regenerating axons towards their original targets by destabilizing mistargeted axons (Figure 5, Movie S3). Thus, independent of their developmental roles, lh3 and col4a5 provide regenerating axons with target specificity, demonstrating that in vivo ECM collagens provide more than a permissive substrate for axon regeneration.
lh3 and col4a5 reveal a Schwann cell dependent repair mechanism that ensures target-selectivity
Our data provide compelling evidence that lh3 and col4a5 specifically direct regenerating axons of the dorsal nerve branch, matching these axons with their original targets and thereby achieving target-selective regeneration. While lh3 promotes growth and directionality of dorsal nerve axons, col4a5 appears critical only for axonal directionality, consistent with the idea that lh3 exerts its various functions through different substrates, including col4a5. Given the large number of collagens in the vertebrate genome, it is unclear precisely which other collagen or group of collagens play critical roles in peripheral nerve regeneration. Furthermore, although the exact contribution of individual glycosylation sites on collagens are not well established, collagens are glycosylated by additional glycosyltransferases such as GLT25D1 and GLT25D2 (Schegg et al., 2009), increasing the complexity of this system.
How do lh3 and col4a5 selectively direct dorsal motor axons? While expression of lh3 in all Schwann cells restores target-selectivity, the relevant substrates, including col4a5, might be expressed only in a relevant subset of these cells. In fact, we find that col4a5 is upregulated in Schwann cells ventral and ventrolateral to the injury gap (Figure 4,6). These data are consistent with rodents studies demonstrating that following peripheral nerve transection collagen4 is upregulated in Schwann cells, and that Schwann cells respond to injury with independent expression phenotypes depending on the nerve they associate with and their proximity to the injury site (Brushart et al., 2013; Höke et al., 2006; Siironen et al., 1992). The spatially restricted expression of col4a5 also suggests a local mechanism by which col4a5 might either directly or indirectly guide regenerating axons. For example, Collagen4 subunits can bind Integrin receptors and Discoidin Domain Receptors (Leitinger and Hohenester, 2007), and regenerating axons express Integrins (Lefcort et al., 1992; Vogelezang et al., 2001), providing a compelling scenario by which Schwann cells expressing Collagen4a5 might selectively guide dorsal nerve axons through Integrin receptors expressed on these but not on ventral nerve axons. Alternatively, Collagen4a5 might bind and concentrate axonal guidance ligands to direct regenerating growth cones expressing the cognate guidance receptor. In fact, Col4a5 can bind Netrin and Slit, which are both upregulated after peripheral nerve transection in rodents (Xiao et al., 2011; Yebra et al., 2003). We find that Netrin-DCC signaling is dispensable for dorsal nerve regeneration, but that slit1a is upregulated with col4a5 in Schwann cells ventral and ventrolateral to the transection site. Thus, one possible scenario is that in response to injury Schwann cells ventral to the transection site secrete Collagen4a5, which binds and accumulates Slit, thereby forming a repulsive barrier to direct dorsal axons onto their original, dorsal path (Figure 7F). Although future studies are required to determine whether these mechanisms operate in isolation or in combination, our data reveal for the first time that in vivo, distinct ECM components serve to selectively direct a subpopulation of regenerating axons towards their original targets. Moreover, our results provide a compelling mechanistic framework underlying target-selective regeneration.
Experimental Procedures
Zebrafish genetics and transgenes
Transgenic lines were generated in the Tübingen or Tupfel longfin (TLF) genetic background (see Supplemental Experimental Procedures) and maintained as previously described (Mullins et al., 1994). The following mutant strains were used: lh3TV2O5 (Schneider and Granato, 2006), col4a5s510 (Xiao and Baier, 2007), col19a1b393 (Hilario et al., 2010); col18a1 mutants were generated via TALEN injection and multiple alleles were identified as described (Dahlem et al., 2012). All zebrafish work was conducted in accordance with Institutional Animal Care and IACUC regulatory standards.
Whole-mount fluorescent in situ hybridization and Immunohistochemistry
Nerve transections were performed in 5dpf Tg(isl1:GFP); Tg(nkx2.2a:GFP) larvae to fluorescently label the dorsal nerve branch and surrounding peripheral glia, respectively (Kucenas et al., 2008; Uemura et al., 2005). Larvae were fixed between 8–15 hpt for 2hrs in 4% PFA in PBS, and in situ hybridization was performed using RNAscope (ACDbio; Gross-Thebing et al., 2014). Nerves were imaged in 1µm sections on a 60× immersion lens on an Olympus Spinning disk confocal microscope using Slidebook Software, and were processed for analysis as described below. Anti-Sox10 (1:2000, gift from S. Kucenas) and anti-Laminin (1:100, Sigma) were used to stain 5dpf larvae as described (Rosenberg et al., 2014; Wolman et al., 2015). Nerves were imaged in 0.5–1µm sections with a 40X water immersion lens on a Zeiss LSC 710 confocal scanning microscope.
Nerve transection
Transection of both ventral and dorsal peripheral motor nerves was performed as previously described, resulting in a ~9µm injury gap measured between proximal and distal nerve endings immediately following transection (Rosenberg et al., 2012). For Figure 1, dorsal and ventral nerves were transected on the common path, ~5µm from the spinal cord exit point. For Figures 2–7, only the dorsal nerves were transected ~10µm from the spinal cord exit point.
Live-cell imaging
Anesthetization, mounting and imaging of embryos was carried out as previously described (Rosenberg et al., 2012).
Image processing
For live imaging (Figures 1–5, 7), image stacks were compressed into maximum intensity projections (MIPs), and processed using ImageJ and Adobe Photoshop to normalize brightness and contrast. For fixed imaging (Figures 4, 6, S1–S4) MIPs were adjusted to equivalent brightness and contrast in ImageJ for comparison.
Axon Regeneration Quantification
Axon growth extent was quantified as described in Supplemental Experimental Procedures. Axon growth directionality was quantified 48 hours post transection using a modified Sholl analysis (Li and Hoffman-Kim, 2008; Sholl, 1953) as illustrated in Figure S2. Line thickness was selected proportional to the number of fascicles that crossed at a given intersection point. The proportion (p) of fascicles (F) within the ROI (25°–45° from the horizontal) at 48hpt was divided by the proportion that developed in this ROI pre-lesion. In wild type (wt) animals, ~70% of fascicles regrew into the dorsal ROI at 48 hpt, and the Directionality ratio for a given genotype “X” was defined in relation to this as:
Axon extension and retraction bursts were defined as growth or retraction of >1µm between timelapse frames (10min) and the frequency was defined as the cumulative number of these bursts counted until an axon remained on the same trajectory >1hr. Axon growth rates were calculated as previously described (Rosenberg et al., 2014). To define a Growth Index, axons were monitored for direction and were labeled “dorsal” if they extended to dorsal regions or “mistargeted” if they extended to nondorsal regions. Axons were scored “1” if they extended >1µm or “-1” if they retracted >1µm between frames (extension/retraction rarely exceeded 1.5–2µm between frames) and were scored “0” if they moved <1µm. We defined the Growth index for a given fascicle as:
In this case, extension only has a growth index of 1 and no net growth has a growth index of 0.
Statistical Analysis
Fisher’s exact and Student’s t tests were performed on all applicable datasets.
Supplementary Material
Acknowledgments
We would like to thank Dr. Chandresekhar (UMo), Dr. Baier (MPI, Munich), Dr. Beattie (OSHU), and Dr. Kucenas (UVA) for providing reagents. We would like to thank Dr. Stout from the CDB imaging core for technical assistance and members of the Granato lab for comments and discussion. This work was supported by grants from the NIH to J.I-B. (NS076197), and to M.G. (HD37975, EY024861).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors Contributions
J.I.-B. and M.G. designed research; J.I.-B., C.F.-A. and V.S. performed research; J.I.-B. and C.F.-A. analyzed data; J.I.-B. and M.G. wrote the paper.
The authors declare no competing interests.
REFERENCES
- Abernethy DA, Rud A, Thomas PK. Neurotropic influence of the distal stump of transected peripheral nerve on axonal regeneration: absence of topographic specificity in adult nerve. J. Anat. 1992;180(Pt 3):395–400. [PMC free article] [PubMed] [Google Scholar]
- Ackley BD, Crew JR, Elamaa H, Pihlajaniemi T, Kuo CJ, Kramer JM. The NC1/endostatin domain of Caenorhabditis elegans type XVIII collagen affects cell migration and axon guidance. The Journal of Cell Biology. 2001;152:1219–1232. doi: 10.1083/jcb.152.6.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anttinen H, Myllylä R, Kivirikko KI. Further characterization of galactosylhydroxylysyl glucosyltransferase from chick embryos. Amino acid composition and acceptor specificity. Biochem. J. 1978;175:737–742. doi: 10.1042/bj1750737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur-Farraj PJ, Latouche M, Wilton DK, Quintes S, Chabrol E, Banerjee A, Woodhoo A, Jenkins B, Rahman M, Turmaine M, et al. c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron. 2012;75:633–647. doi: 10.1016/j.neuron.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie CE, Melancon E, Eisen JS. Mutations in the stumpy gene reveal intermediate targets for zebrafish motor axons. Development. 2000;127:2653–2662. doi: 10.1242/dev.127.12.2653. [DOI] [PubMed] [Google Scholar]
- Binari LA, Lewis GM, Kucenas S. Perineurial Glia Require Notch Signaling during Motor Nerve Development but Not Regeneration. Journal of Neuroscience. 2013;33:4241–4252. doi: 10.1523/JNEUROSCI.4893-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brushart TM. Preferential reinnervation of motor nerves by regenerating motor axons. J. Neurosci. 1988;8:1026–1031. doi: 10.1523/JNEUROSCI.08-03-01026.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brushart TM. Motor axons preferentially reinnervate motor pathways. J. Neurosci. 1993;13:2730–2738. doi: 10.1523/JNEUROSCI.13-06-02730.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brushart TM. Nerve Repair. New York: Oxford University Press; 2011. [Google Scholar]
- Brushart TM, Aspalter M, Griffin JW, Redett R, Hameed H, Zhou C, Wright M, Vyas A, Höke A. Schwann cell phenotype is regulated by axon modality and central-peripheral location, and persists in vitro. Experimental Neurology. 2013;247:272–281. doi: 10.1016/j.expneurol.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülow HE, Hobert O. The molecular diversity of glycosaminoglycans shapes animal development. Annu. Rev. Cell Dev. Biol. 2006;22:375–407. doi: 10.1146/annurev.cellbio.22.010605.093433. [DOI] [PubMed] [Google Scholar]
- Cajal SRY. Cajal's Degeneration and Regeneration of the Nervous System. Oxford, UK: Oxford University Press; 1928. [Google Scholar]
- Carey DJ, Eldridge CF, Cornbrooks CJ, Timpl R, Bunge RP. Biosynthesis of type IV collagen by cultured rat Schwann cells. The Journal of Cell Biology. 1983;97:473–479. doi: 10.1083/jcb.97.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Wang Z, Ghosh-Roy A, Hubert T, Yan D, O'Rourke S, Bowerman B, Wu Z, Jin Y, Chisholm AD. Axon Regeneration Pathways Identified by Systematic Genetic Screening in C. elegans. Neuron. 2011;71:1043–1057. doi: 10.1016/j.neuron.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZL. Laminin 1 is critical for Schwann cell differentiation, axon myelination, and regeneration in the peripheral nerve. The Journal of Cell Biology. 2003;163:889–899. doi: 10.1083/jcb.200307068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernousov MA, Carey DJ. Schwann cell extracellular matrix molecules and their receptors. Histol. Histopathol. 2000;15:593–601. doi: 10.14670/HH-15.593. [DOI] [PubMed] [Google Scholar]
- Dahlem TJ, Hoshijima K, Jurynec MJ, Gunther D, Starker CG, Locke AS, Weis AM, Voytas DF, Grunwald DJ. Simple methods for generating and detecting locus-specific mutations induced with TALENs in the zebrafish genome. PLoS Genet. 2012;8:e1002861. doi: 10.1371/journal.pgen.1002861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards TJ, Hammarlund M. Syndecan Promotes Axon Regeneration by Stabilizing Growth Cone Migration. CellReports. 2014;8:272–283. doi: 10.1016/j.celrep.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English AW. Enhancing axon regeneration in peripheral nerves also increases functionally inappropriate reinnervation of targets. J. Comp. Neurol. 2005;490:427–441. doi: 10.1002/cne.20678. [DOI] [PubMed] [Google Scholar]
- Forman DS, Berenberg RA. Regeneration of motor axons in the rat sciatic nerve studied by labeling with axonally transported radioactive proteins. Brain Res. 1978;156:213–225. doi: 10.1016/0006-8993(78)90504-8. [DOI] [PubMed] [Google Scholar]
- Forman DS, Wood DK, DeSilva S. Rate of regeneration of sensory axons in transected rat sciatic nerve repaired with epineurial sutures. J. Neurol. Sci. 1979;44:55–59. doi: 10.1016/0022-510x(79)90222-3. [DOI] [PubMed] [Google Scholar]
- Gaudet AD, Popovich PG, Ramer MS. Wallerian degeneration: gaining perspective on inflammatory events after peripheral nerve injury. J Neuroinflammation. 2011;8:110. doi: 10.1186/1742-2094-8-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- Grimm LM. An evaluation of myotypic respecification in axolotls. J. Exp. Zool. 1971;178:479–496. doi: 10.1002/jez.1401780406. [DOI] [PubMed] [Google Scholar]
- Gross-Thebing T, Paksa A, Raz E. Simultaneous high-resolution detection of multiple transcripts combined with localization of proteins in whole-mount embryos. BMC Biol. 2014;12:266. doi: 10.1186/s12915-014-0055-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo XD, Johnson JJ, Kramer JM. Embryonic lethality caused by mutations in basement membrane collagen of C. elegans. Nature. 1991;349:707–709. doi: 10.1038/349707a0. [DOI] [PubMed] [Google Scholar]
- Haftek J, Thomas PK. Electron-microscope observations on the effects of localized crush injuries on the connective tissues of peripheral nerve. J. Anat. 1968;103:233–243. [PMC free article] [PubMed] [Google Scholar]
- Hautala T, Byers MG, Eddy RL, Shows TB, Kivirikko KI, Myllylä R. Cloning of human lysyl hydroxylase: complete cDNA-derived amino acid sequence and assignment of the gene (PLOD) to chromosome 1p36.3----p36.2. Genomics. 1992;13:62–69. doi: 10.1016/0888-7543(92)90202-4. [DOI] [PubMed] [Google Scholar]
- Hilario JD, Wang C, Beattie CE. Collagen XIXa1 is crucial for motor axon navigation at intermediate targets. Development. 2010;137:4261–4269. doi: 10.1242/dev.051730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höke A, Redett R, Hameed H, Jari R, Zhou C, Li ZB, Griffin JW, Brushart TM. Schwann cells express motor and sensory phenotypes that regulate axon regeneration. Journal of Neuroscience. 2006;26:9646–9655. doi: 10.1523/JNEUROSCI.1620-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide C, Tohyama K, Yokota R, Nitatori T, Onodera S. Schwann cell basal lamina and nerve regeneration. Brain Res. 1983;288:61–75. doi: 10.1016/0006-8993(83)90081-1. [DOI] [PubMed] [Google Scholar]
- Jing L, Gordon LR, Shtibin E, Granato M. Temporal and spatial requirements of unplugged/MuSK function during zebrafish neuromuscular development. PLoS ONE. 2010;5:e8843. doi: 10.1371/journal.pone.0008843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura J, Rodnitzky RL, Okawara SH. Electrophysiologic analysis of aberrant regeneration after facial nerve paralysis. Neurology. 1975;25:989–993. doi: 10.1212/wnl.25.10.989. [DOI] [PubMed] [Google Scholar]
- Kruspe M, Thieme H, Guntinas-Lichius O, Irintchev A. Motoneuron regeneration accuracy and recovery of gait after femoral nerve injuries in rats. Neuroscience. 2014;280:73–87. doi: 10.1016/j.neuroscience.2014.08.051. [DOI] [PubMed] [Google Scholar]
- Kubo T, Yamashita T, Yamaguchi A, Hosokawa K, Tohyama M. Analysis of genes induced in peripheral nerve after axotomy using cDNA microarrays. J. Neurochem. 2002;82:1129–1136. doi: 10.1046/j.1471-4159.2002.01060.x. [DOI] [PubMed] [Google Scholar]
- Kucenas S, Takada N, Park H-C, Woodruff E, Broadie K, Appel B. CNS-derived glia ensheath peripheral nerves and mediate motor root development. Nat Neurosci. 2008;11:143–151. doi: 10.1038/nn2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffler DP. Accurate reinnervation of motor end plates after disruption of sheath cells and muscle fibers. J. Comp. Neurol. 1986a;250:228–235. doi: 10.1002/cne.902500209. [DOI] [PubMed] [Google Scholar]
- Kuffler DP. Isolated satellite cells of a peripheral nerve direct the growth of regenerating frog axons. J. Comp. Neurol. 1986b;249:57–64. doi: 10.1002/cne.902490106. [DOI] [PubMed] [Google Scholar]
- Lee MT, Farel PB. Guidance of regenerating motor axons in larval and juvenile bullfrogs. J. Neurosci. 1988;8:2430–2437. doi: 10.1523/JNEUROSCI.08-07-02430.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefcort F, Venstrom K, McDonald JA, Reichardt LF. Regulation of expression of fibronectin and its receptor, alpha 5 beta 1, during development and regeneration of peripheral nerve. Development. 1992;116:767–782. doi: 10.1242/dev.116.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitinger B, Hohenester E. Mammalian collagen receptors. Matrix Biology. 2007;26:146–155. doi: 10.1016/j.matbio.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Lewis GM, Kucenas S. Perineurial Glia Are Essential for Motor Axon Regrowth following Nerve Injury. Journal of Neuroscience. 2014;34:12762–12777. doi: 10.1523/JNEUROSCI.1906-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GN, Hoffman-Kim D. Evaluation of neurite outgrowth anisotropy using a novel application of circular analysis. J. Neurosci. Methods. 2008;174:202–214. doi: 10.1016/j.jneumeth.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löhler J, Timpl R, Jaenisch R. Embryonic lethal mutation in mouse collagen I gene causes rupture of blood vessels and is associated with erythropoietic and mesenchymal cell death. Cell. 1984;38:597–607. doi: 10.1016/0092-8674(84)90514-2. [DOI] [PubMed] [Google Scholar]
- Lyons DA, Talbot WS. Glial Cell Development and Function in Zebrafish. Cold Spring Harb Perspect Biol. 2014 doi: 10.1101/cshperspect.a020586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark RF. Fin movement after regeneration of neuromuscular connections: An investigation of myotypic specificity. Experimental Neurology. 1965;12:292–302. [Google Scholar]
- Martin GR, Timpl R. Laminin and other basement membrane components. Annu. Rev. Cell Biol. 1987;3:57–85. doi: 10.1146/annurev.cb.03.110187.000421. [DOI] [PubMed] [Google Scholar]
- Martin SM, O'Brien GS, Portera-Cailliau C, Sagasti A. Wallerian degeneration of zebrafish trigeminal axons in the skin is required for regeneration and developmental pruning. Development. 2010;137:3985–3994. doi: 10.1242/dev.053611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini R. Expression and functional roles of neural cell surface molecules and extracellular matrix components during development and regeneration of peripheral nerves. J. Neurocytol. 1994;23:1–28. doi: 10.1007/BF01189813. [DOI] [PubMed] [Google Scholar]
- Mullins MC, Hammerschmidt M, Haffter P, Nusslein-Volhard C. Large-scale mutagenesis in the zebrafish: in search of genes controlling development in a vertebrate. Current Biology. 1994;4:189–202. doi: 10.1016/s0960-9822(00)00048-8. [DOI] [PubMed] [Google Scholar]
- Myers PZ, Eisen JS, Westerfield M. Development and axonal outgrowth of identified motoneurons in the zebrafish. J. Neurosci. 1986;6:2278–2289. doi: 10.1523/JNEUROSCI.06-08-02278.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myllyharju J. Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends in Genetics. 2004;20:33–43. doi: 10.1016/j.tig.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Nathaniel EJ, Pease DC. Collagen and basement membrane formation by Schwann cells during nerve regeneration. J. Ultrastruct. Res. 1963;52:550–560. doi: 10.1016/s0022-5320(63)80084-2. [DOI] [PubMed] [Google Scholar]
- Nguyen QT, Sanes JR, Lichtman JW. Pre-existing pathways promote precise projection patterns. Nat Neurosci. 2002;5:861–867. doi: 10.1038/nn905. [DOI] [PubMed] [Google Scholar]
- Nix P, Hammarlund M, Hauth L, Lachnit M, Jorgensen EM, Bastiani M. Axon Regeneration Genes Identified by RNAi Screening in C. elegans. Journal of Neuroscience. 2014;34:629–645. doi: 10.1523/JNEUROSCI.3859-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman KR, Moerman DG. The let-268 locus of Caenorhabditis elegans encodes a procollagen lysyl hydroxylase that is essential for type IV collagen secretion. Developmental Biology. 2000;227:690–705. doi: 10.1006/dbio.2000.9897. [DOI] [PubMed] [Google Scholar]
- Paíno CL, Fernandez-Valle C, Bates ML, Bunge MB. Regrowth of axons in lesioned adult rat spinal cord: promotion by implants of cultured Schwann cells. J. Neurocytol. 1994;23:433–452. doi: 10.1007/BF01207115. [DOI] [PubMed] [Google Scholar]
- Parrinello S, Napoli I, Ribeiro S, Digby PW, Fedorova M, Parkinson DB, Doddrell RDS, Nakayama M, Adams RH, Lloyd AC. EphB Signaling Directs Peripheral Nerve Regeneration through Sox2-Dependent Schwann Cell Sorting. Cell. 2010;143:145–155. doi: 10.1016/j.cell.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peri F, Nüsslein-Volhard C. Live imaging of neuronal degradation by microglia reveals a role for v0-ATPase a1 in phagosomal fusion in vivo. Cell. 2008;133:916–927. doi: 10.1016/j.cell.2008.04.037. [DOI] [PubMed] [Google Scholar]
- Pollard JD, Fitzpatrick L. A comparison of the effects of irradiation and immunosuppressive agents on regeneration through peripheral nerve allografts: an ultrastructural study. Acta Neuropathol. 1973;23:166–180. doi: 10.1007/BF00685770. [DOI] [PubMed] [Google Scholar]
- Poschl E. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development. 2004;131:1619–1628. doi: 10.1242/dev.01037. [DOI] [PubMed] [Google Scholar]
- Poulain FE, Chien C-B. Proteoglycan-Mediated Axon Degeneration Corrects Pretarget Topographic Sorting Errors. Neuron. 2013;78:49–56. doi: 10.1016/j.neuron.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson PM, McGuinness UM, Aguayo AJ. Axons from CNS neurons regenerate into PNS grafts. Nature. 1980;284:264–265. doi: 10.1038/284264a0. [DOI] [PubMed] [Google Scholar]
- Rosenberg AF, Isaacman-Beck J, Franzini-Armstrong C, Granato M. Schwann Cells and Deleted in Colorectal Carcinoma Direct Regenerating Motor Axons Towards Their Original Path. Journal of Neuroscience. 2014;34:14668–14681. doi: 10.1523/JNEUROSCI.2007-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg AF, Wolman MA, Franzini-Armstrong C, Granato M. In Vivo Nerve-Macrophage Interactions Following Peripheral Nerve Injury. Journal of Neuroscience. 2012;32:3898–3909. doi: 10.1523/JNEUROSCI.5225-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruotsalainen H, Sipilä L, Vapola M, Sormunen R, Salo AM, Uitto L, Mercer DK, Robins SP, Risteli M, Aszodi A, et al. Glycosylation catalyzed by lysyl hydroxylase 3 is essential for basement membranes. Journal of Cell Science. 2006;119:625–635. doi: 10.1242/jcs.02780. [DOI] [PubMed] [Google Scholar]
- Schegg B, Hülsmeier AJ, Rutschmann C, Maag C, Hennet T. Core glycosylation of collagen is initiated by two beta(1-O)galactosyltransferases. Mol. Cell. Biol. 2009;29:943–952. doi: 10.1128/MCB.02085-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer SS. Reinnervation of the extraocular muscles in goldfish is nonselective. J. Neurosci. 1986;6:764–773. doi: 10.1523/JNEUROSCI.06-03-00764.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer SS, Easter SS. Degenerative and regenerative changes in the trochlear nerve of goldfish. J. Neurocytol. 1984;13:519–565. doi: 10.1007/BF01148079. [DOI] [PubMed] [Google Scholar]
- Schneider VA, Granato M. The Myotomal diwanka (lh3) Glycosyltransferase and Type XVIII Collagen Are Critical for Motor Growth Cone Migration. Neuron. 2006;50:683–695. doi: 10.1016/j.neuron.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Schröder JM, May R, Weis J. Perineurial cells are the first to traverse gaps of peripheral nerves in silicone tubes. Clin Neurol Neurosurg. 1993;95(Suppl):S78–S83. doi: 10.1016/0303-8467(93)90040-n. [DOI] [PubMed] [Google Scholar]
- Sholl DA. Dendritic organization in the neurons of the visual and motor cortices of the cat. J. Anat. 1953;87:387–406. [PMC free article] [PubMed] [Google Scholar]
- Siironen J, Sandberg M, Vuorinen V, Röyttä M. Laminin B1 and collagen type IV gene expression in transected peripheral nerve: reinnervation compared to denervation. J. Neurochem. 1992;59:2184–2192. doi: 10.1111/j.1471-4159.1992.tb10110.x. [DOI] [PubMed] [Google Scholar]
- Sipilä L, Ruotsalainen H, Sormunen R, Baker NL, Lamandé SR, Vapola M, Wang C, Sado Y, Aszodi A, Myllylä R. Secretion and assembly of type IV and VI collagens depend on glycosylation of hydroxylysines. J. Biol. Chem. 2007;282:33381–33388. doi: 10.1074/jbc.M704198200. [DOI] [PubMed] [Google Scholar]
- Sketelj J, Bresjanac M, Popović M. Rapid growth of regenerating axons across the segments of sciatic nerve devoid of Schwann cells. J. Neurosci. Res. 1989;24:153–162. doi: 10.1002/jnr.490240205. [DOI] [PubMed] [Google Scholar]
- Smyth N, Vatansever HS, Murray P, Meyer M, Frie C, Paulsson M, Edgar D. Absence of basement membranes after targeting the LAMC1 gene results in embryonic lethality due to failure of endoderm differentiation. The Journal of Cell Biology. 1999;144:151–160. doi: 10.1083/jcb.144.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector JG, Lee P, Peterein J, Roufa D. Facial nerve regeneration through autologous nerve grafts: a clinical and experimental study. Laryngoscope. 1991;101:537–554. doi: 10.1288/00005537-199105000-00017. [DOI] [PubMed] [Google Scholar]
- Sperry RW, Arora HL. Selectivity in regeneration of the oculomotor nerve in the cichlid fish, Astronotus ocellatus. J Embryol Exp Morphol. 1965;14:307–317. [PubMed] [Google Scholar]
- Stephenson RS. Axon reflexes in axolotl limbs: evidence that branched motor axons reinnervate muscles selectively. Experimental Neurology. 1979;64:174–189. doi: 10.1016/0014-4886(79)90013-x. [DOI] [PubMed] [Google Scholar]
- Uemura O, Okada Y, Ando H, Guedj M, Higashijima S-I, Shimazaki T, Chino N, Okano H, Okamoto H. Comparative functional genomics revealed conservation and diversification of three enhancers of the isl1 gene for motor and sensory neuron-specific expression. Developmental Biology. 2005;278:587–606. doi: 10.1016/j.ydbio.2004.11.031. [DOI] [PubMed] [Google Scholar]
- Uziyel Y, Hall S, Cohen J. Influence of laminin-2 on Schwann cell-axon interactions. Glia. 2000;32:109–121. doi: 10.1002/1098-1136(200011)32:2<109::aid-glia10>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Vargas ME, Barres BA. Why is Wallerian degeneration in the CNS so slow? Annu. Rev. Neurosci. 2007;30:153–179. doi: 10.1146/annurev.neuro.30.051606.094354. [DOI] [PubMed] [Google Scholar]
- Vogelezang MG, Liu Z, Relvas JB, Raivich G, Scherer SS, ffrench-Constant C. Alpha4 integrin is expressed during peripheral nerve regeneration and enhances neurite outgrowth. Journal of Neuroscience. 2001;21:6732–6744. doi: 10.1523/JNEUROSCI.21-17-06732.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller A. Experiments on the section of the glossopharyngeal and hypoglossal nerves of the frog, and observations of the alterations produced thereby in the structure of their primitive fibres. Philos. Trans. R. Soc. Lond. 1849;140:423–429. [Google Scholar]
- Wang C, Valtavaara M, Myllylä R. Lack of collagen type specificity for lysyl hydroxylase isoforms. DNA Cell Biol. 2000;19:71–77. doi: 10.1089/104454900314582. [DOI] [PubMed] [Google Scholar]
- Wang GY, Hirai K, Shimada H. The role of laminin, a component of Schwann cell basal lamina, in rat sciatic nerve regeneration within antiserum-treated nerve grafts. Brain Res. 1992a;570:116–125. doi: 10.1016/0006-8993(92)90571-p. [DOI] [PubMed] [Google Scholar]
- Wang GY, Hirai K, Shimada H, Taji S, Zhong S-Z. Behavior of axons, Schwann cells and perineurial cells in nerve regeneration within transplanted nerve grafts: effects of anti-laminin and anti-fibronectin antisera. Brain Res. 1992b;583:216–226. doi: 10.1016/s0006-8993(10)80027-7. [DOI] [PubMed] [Google Scholar]
- Werner A, Willem M, Jones LL, Kreutzberg GW, Mayer U, Raivich G. Impaired axonal regeneration in alpha7 integrin-deficient mice. Journal of Neuroscience. 2000;20:1822–1830. doi: 10.1523/JNEUROSCI.20-05-01822.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. Substrate interactions affecting motor growth cone guidance during development and regeneration. J. Exp. Biol. 1987;132:161–175. doi: 10.1242/jeb.132.1.161. [DOI] [PubMed] [Google Scholar]
- Westerfield M, Powell SL. Selective reinnervation of limb muscles by regenerating frog motor axons. Brain Res. 1983;312:301–304. doi: 10.1016/0165-3806(83)90146-3. [DOI] [PubMed] [Google Scholar]
- Westerfield M, McMurray JV, Eisen JS. Identified motoneurons and their innervation of axial muscles in the zebrafish. J. Neurosci. 1986;6:2267–2277. doi: 10.1523/JNEUROSCI.06-08-02267.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witzel C, Rohde C, Brushart TM. Pathway sampling by regenerating peripheral axons. J. Comp. Neurol. 2005;485:183–190. doi: 10.1002/cne.20436. [DOI] [PubMed] [Google Scholar]
- Wolman MA, Jain RA, Marsden KC, Bell H, Skinner J, Hayer KE, Hogenesch JB, Granato M. A Genome-wide Screen Identifies PAPP-AA- Mediated IGFR Signaling as a Novel Regulator of Habituation Learning. Neuron. 2015;85:1200–1211. doi: 10.1016/j.neuron.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao T, Baier H. Lamina-specific axonal projections in the zebrafish tectum require the type IV collagen Dragnet. Nat Neurosci. 2007;10:1529–1537. doi: 10.1038/nn2002. [DOI] [PubMed] [Google Scholar]
- Xiao T, Staub W, Robles E, Gosse NJ, Cole GJ, Baier H. Assembly of lamina-specific neuronal connections by slit bound to type IV collagen. Cell. 2011;146:164–176. doi: 10.1016/j.cell.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XM, Chen A, Guénard V, Kleitman N, Bunge MB. Bridging Schwann cell transplants promote axonal regeneration from both the rostral and caudal stumps of transected adult rat spinal cord. J. Neurocytol. 1997;26:1–16. doi: 10.1023/a:1018557923309. [DOI] [PubMed] [Google Scholar]
- Yebra M, Montgomery AMP, Diaferia GR, Kaido T, Silletti S, Perez B, Just ML, Hildbrand S, Hurford R, Florkiewicz E, et al. Recognition of the Neural Chemoattractant Netrin-1 by Integrins alpha6beta4 and alpha3beta1 Regulates Epithelial Cell Adhesion and Migration. Dev. Cell. 2003;5:695–707. doi: 10.1016/s1534-5807(03)00330-7. [DOI] [PubMed] [Google Scholar]
- Zeller J, Granato M. The zebrafish diwanka gene controls an early step of motor growth cone migration. Development. 1999;126:3461–3472. doi: 10.1242/dev.126.15.3461. [DOI] [PubMed] [Google Scholar]
- Zochodne DW. Neurobiology of Peripheral Nerve Regeneration. 2008:1–318. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.