Abstract
Introduction
Chronic treatment with nicotine is known to increase the α4β2-nAChR sites in brain, to decrease α6β2-nAChR sites and to have minimal effect on α3β4- and α7-nAChR populations. Varenicline is now used as a smoking cessation treatment, with and without continued smoking or nicotine replacement therapy. Varenicline, like nicotine, upregulates the α4β2-nAChR sites; however, it is not known whether varenicline treatment changes expression of the other nAChR subtypes.
Methods
Using a mouse model, chronic treatments (10 days) with varenicline (0.12mg/kg/hr) and/or nicotine (1 mg/kg/hr), alone or in combination, were compared for plasma and brain levels of drugs, tolerance to subsequent acute nicotine and expression of four subtypes of nAChR using autoradiography.
Results
The upregulation of α4β2-nAChR sites elicited by chronic varenicline was very similar to that elicited by chronic nicotine. Treatment with both drugs somewhat increased up-regulation, indicating that these doses were not quite at maximum effect. Similar down-regulation was seen for α6β2-nAChR sites. Varenicline significantly increased both α3β4- and α7-nAChR sites while nicotine had less effect on these sites. The drug combination was similar to varenicline alone for α3β4-nAChR sites, while for α7 sites the drug combination was less effective than varenicline alone. Varenicline had small but significant effects on tolerance to acute nicotine.
Conclusions
Effects of varenicline in vivo may not be limited to the α4β2*-nAChR subtype. In addition, smoking cessation treatment with varenicline may not allow receptor numbers to be restored to baseline and may, in addition, change expression of other receptor subtypes.
Keywords: Varenicline, Nicotine, Chronic treatment, Nicotinic receptors
1. Introduction
Although the incidence of tobacco use in many parts of the world has declined in recent years, many people continue to use tobacco, primarily through cigarette smoking. The health consequences of cigarette smoking are widely recognized and a significant number of current smokers express a desire to quit. However, the success rate for quitting is quite low.
It is well established that exposure to nicotine elicits changes in the expression of the neuronal nicotinic cholinergic receptors (nAChR). Increases in the most widely expressed nAChR subtype (α4β2*-nAChR) occurs in mouse, rat and human brain following exposure to nicotine (Marks, Burch et al. 1983, Schwartz and Kellar 1983, Benwell, Balfour et al. 1988, Perry, Davila-Garcia et al. 1999, Marks, McClure-Begley et al. 2011). The time-course of reversal of this upregulation after cessation of treatment in mice is approximately a week in mice (Marks, Stitzel et al. 1985, Turner, Castellano et al. 2011, Yohn, Turner et al. 2014). In addition, nicotine-induced decreases in the expression of the α6β2*-nAChR subtype have been observed in rodent and monkey brain following chronic nicotine treatment (Perez, Bordia et al. 2008, Perez, Ly et al. 2012, Marks, Grady et al. 2014). While α4β2*- and α6β2*-nAChRs have been implicated in some smoking behaviors, other subtypes including α3, β4 and α5 subunits have been shown to be important for appetite, aversion and withdrawal (Salas, Pieri et al. 2004, Frahm, Slimak et al. 2011, George, Lloyd et al. 2011, Jackson, Sanjakdar et al. 2013, Picciotto and Kenny 2013, Stoker and Markou 2013).
Several forms of nicotine replacement (NRT), including nicotine in gum, patches and lozenges, are in clinical use to reduce tobacco withdrawal symptoms and replacement provides significant harm reduction. Although NRT has been somewhat successful, it has not generally achieved the desired level of long-term quit rates. Subsequently, varenicline (Chantix), which has activity at nAChR somewhat different from nicotine including partial agonist activity at the α4β2*-nAChR subtype, was developed and is now widely used as a smoking cessation aid. While varenicline treatment has been somewhat more helpful than NRT for some smokers, long-term quit-rates are somewhat disappointing (Stapleton, Watson et al. 2008, Kralikova, Kmetova et al. 2013). Combination treatment strategies including co-treatment with nicotine and varenicline (Ebbert, Burke et al. 2009, Hajek, McRobbie et al. 2011, Hajek, Smith et al. 2013) have been employed in attempts to improve the quit-rate.
Varenicline exhibits higher affinity than nicotine for the α4β2*-nAChR, and was initially considered selective for this subtype (Coe, Brooks et al. 2005). However, varenicline also exhibits activity at additional nAChR subtypes, including α3β4*-nAChR and α7-nAChR (Grady, Drenan et al. 2010, Papke, Wecker et al. 2010, Campling, Kuryatov et al. 2013). Studies using rodent and cell culture models to evaluate the effects of chronic varenicline exposure have demonstrated that, similar to nicotine, these treatments elicit an up-regulation of α4β2*-nAChR binding sites (Turner, Castellano et al. 2011, Hussmann, Turner et al. 2012, Hussmann, DeDominicis et al. 2014). In humans, at therapeutic doses, varenicline may have effects on nAChRs other than β2* (Campling, Kuryatov et al. 2013). Effects of chronic varenicline on receptor expression of subtypes other than β2*-nAChR have not been reported.
Nicotine-induced changes in expression are indicative of receptor interaction. It is currently unknown whether changes in nAChR expression are maintained by all smoking cessation treatments, although some compounds under investigation appear to allow return to baseline for α4β2*-nAChR (Turner, Castellano et al. 2010, Hussmann, Turner et al. 2012, Hussmann, DeDominicis et al. 2014, Yohn, Turner et al. 2014). Whether reversal of these changes is an important aspect of a successful quit attempt is also not known.
We compared chronic varenicline to nicotine, as well as co-treatment with both compounds in a mouse model to investigate selectivity of upregulation as a marker for drug effects at four different subtypes of nAChR. These four nAChR binding sites were quantitated in multiple brain regions. The results show that nicotine or varenicline treatment alone elicits very similar effects on ligand binding to α4β2*-nAChR and α6β2*-nAChR sites, but that varenicline treatment elicits significantly greater changes in populations of α3β4*nAChR and α7-nAChR sites than does nicotine treatment. Co-treatment effects were increased for some sites, but not all.
2. Methods
2.1 Materials
[125I]-Epibatidine (2200 Ci/mmol) and [125I]-α-bungarotoxin (110 Ci/mmol) were obtained from Perkin-Elmer NEN, Boston, MA. [125I]-α-conotoxin MII (2200 Ci/mmol) was synthesized as previously described (Whiteaker et al, 2000). NaCl, KCl, MgSO4, CaCl2, Na2HPO4, NaH2PO4, bovine serum albumin, polyethyleneglycol, polyethylenimine, nicotine, and cytisine were obtained from Sigma Chemical Co., St. Louis, MO. Ketamine, xylazine, acepromazine and buphrenophine were obtained from MWI Veterinary Supply. Sucrose was obtained from Roche Diagnostics, Indianapolis, IN. HEPES and NaHEPES were products of Amresco, Solon, OH. Silastic tubing a product of Dow Chemical were obtained through VWR International. Glass filters Type B were products of MicroFiltration Systems, Dublin, CA and glass fiber filters Type A/E were products of Pall Life Sciences, Port Washington, NY. Nylon mesh and 22 gauge stainless steel tubing were obtained from Small Parts, Inc. 5I-Epibatidine was a generous gift of Dr. Kenneth Kellar, Georgetown University. Varenicline tartrate was synthesized and kindly supplied by Targacept, Inc. (Winston-Salem, NC). Varenicline internal standard (PF-00142282) was kindly supplied by Pfizer (Groton, CT). Deuterated nicotine standard (d4 ± nicotine), ammonium hydroxide and ammonium acetate were purchased from Sigma-Aldrich (St Louis, MO). UPLC-grade acetonitrile, formic acid and methanol were purchased from VWR (Radnor, PA).
2.2 Mice
C57Bl/6J mice were bred and maintained at the Institute for Behavioral Genetics. After weaning, mice housed with same sex littermates had free access to food and water on a 12-hr light/dark cycle at 22°C. All care and treatment protocols were approved by the Animal Care and Utilization Committee of the University of Colorado and followed the guidelines for the care and use of mice by the National Institutes of Health. All efforts were made to minimize the number of animals treated by using a preliminary dosing study and by analyzing all mice treated in the two compound study for tolerance as well as all four autoradiography binding protocols.
2.3 Chronic treatment
Methods previously described for continuous infusion (Marks, Burch et al. 1983, Marks, McClure-Begley et al. 2011) were followed with minor modifications. Mice were anesthetized by intraperitoneal injection of a ketamine (100 mg/kg)/xylazine (10 mg/kg). A cannula constructed of silastic tubing (0.30 mm inner diameter, 0.64 mm outer diameter) was inserted 8 mm into the vein and anchored to the underlying tissue with surgical silk thread. The silastic tubing was connected to 22 gauge stainless steel tubing attached to a nylon circle (1 cm diameter) which was affixed to the back of the mouse between the scapulae. Following surgery each mouse was injected with 0.1 mg/kg buprenorphine and placed in a freshly bedded cage. The mouse was warmed and monitored repeatedly until awakening.
The day after surgery the cannula was checked for free flow. The mouse was weighed and transferred to an infusion chamber (15 cm × 15 cm × 30 cm, l × w × h). The stainless steel tubing was connected to polyethylene tubing attached to a 1ml syringe mounted on a Harvard Infusion pump that delivered isotonic sterile saline at a rate of 35μl/hr. Saline infusion was maintained for two days before beginning drug treatment. Four treatment groups were used: saline-infused (controls), 1.0 mg/kg/hr nicotine, 0.12 mg/kg/hr varenicline and 1.0 mg/kg/hr nicotine plus 0.12 mg/kg/hr varenicline. In addition, a preliminary experiment in which mice were treated with saline, 0.12 or 0.6 mg/kg/hr varenicline was performed to establish the appropriate varenicline dose for the larger study. All drug doses are free base.
Mice for analytical studies of blood/brain levels were treated as above with either1.0 mg/kg/hr nicotine or 0.12 mg/kg/hr varenicline for 10 days.
2.4 Testing for tolerance
Mice chronically-treated for 10 days with saline, nicotine, varenicline or the combination of nicotine plus varenicline, were withdrawn from treatment for 24 hours. Mice from each treatment group were randomly assigned to groups for acute ip nicotine treatment followed by a test battery (Tritto, McCallum et al. 2004). Two hours after the first tolerance test, a second dose of nicotine was administered followed by a second test battery. The nicotine doses for the first acute treatment groups were given either saline (no nicotine) or 0.5 mg/kg nicotine; the second groups were given either 1 mg/kg or 1.5 mg/kg. Injection volume was 0.01 ml/g body weight. The test battery was run as follows: 2 min after injection the mouse was placed in the center of a red plastic Y-maze and allowed to explore for 3 min; crosses and rears were measured by infrared beam breaks. The mouse was then placed in the middle of circular open field arena under white light and allowed to explore for 5 min; activity was measured by infrared beam breaks. After that test the mouse was singly housed and body temperature was measured 15 min after the injection. Preliminary experiments established that the responses to acute nicotine challenge for mice receiving two injections spaced at least two hours apart were comparable to those for animals receiving a single acute challenge dose.
2.5 Membrane binding
Mice that had been treated with saline, 0.12 or 0.6 mg/kg/hr varenicline were withdrawn from treatment for 24 hr. Following cervical dislocation and decapitation, cortex, striatum, hippocampus and thalamus were dissected from each mouse. The binding of [125I]-epibatidine was measured essentially as described previously (Whiteaker, Jimenez et al. 2000). Radioactivity was determined using a Perkin-Elmer Wallac Tri-Lux Microbeta plate counter following addition of 150 μL of National Diagnostics Ecoscint XR scintillation fluid (National Diagnostics, Atlanta, GA). Cytisine-sensitive [125I]-epibatidine binding sites (primarily α4β2*-nAChR sites) were determined as the difference in binding between incubations without and with 50 nM cytisine included in the incubations. Blanks were determined in samples containing 10 μM nicotine.
2.6 Determination of blood and brain levels after chronic drug treatment
The methods of Obach et al (2006) and Vieira-Brock et al. (2011) were used with some modifications, including deproteination, as described below.
2.6.1 Sample collection
After 10 days of treatment with nicotine or varenicline, mice were removed from the infusion system and blood and brain collected within 5 min. Whole blood was drawn into syringes containing EDTA (0.5 ml, 500 mM) by cardiac puncture and kept ice-cold until centrifugation (1000×g for 10 min within 30 min of collection). Plasma was removed and frozen (−80°C) until time of assay. Whole brains were quickly dissected and flash frozen in isopentane on dry ice, and stored at −80°C until assay.
2.6.2 Sample preparation
Nicotine and varenicline were extracted from plasma samples according to methods of Obach et al (2006) with some modifications. Standards and samples were extracted and analyzed in an identical fashion. All standards were diluted into blank matrix (eg. plasma or brain) before extractions. Briefly, internal standards (PF-00142282 or d4 ± nicotine) were added to plasma samples (200 μl) and standards ;samples were deproteinated by the addition of 0.1% formic acid in acetonitrile. Samples were then extracted with methyl t-butyl ether (3×3 ml), dried under a stream of nitrogen and reconstituted in HPLC mobile phase initial conditions (100 μl) for injection.
For brain tissue (~200-300 g), internal standards (as above) were added and then samples were homogenized in phosphate buffered saline (pH 7.2) using a Precellys®24 with Cryolys (5,000 RPM × 15 sec) (Precellys, Rockville MD). The samples were deproteinated by the addition of acetonitrile/ 0.1% formic acid (v/v). Supernatants were collected and separated on an Oasis®MCX (60 mg, 3 ml) solid phase exchange column (Waters, Milford, MA). Columns were conditioned with HPLC grade methanol (2 ml) followed by 2% aqueous formic acid (2 ml). After samples were loaded, the columns were washed with 2% formic acid (1 ml) followed by methanol (1 ml). Analytes were eluted with 5% (v/v) ammoniated methanol (1.5 ml) then methylene chloride: isopropyl alcohol: ammonium hydroxide (78:20:2 v/v; 1.5 ml). The samples were acidified with 2M HCL (100 μl) before evaporation under nitrogen and reconstituted in HPLC mobile phase initial conditions (100 μl) for injection.
2.6.2 Quantitation by UPLC-MS/MS
The LC system consisted of a Waters Acquity UPLC instrument equipped with a binary pump and a 96-vial autosampler (Waters, Milford, MA, USA). Chromatographic separations were accomplished using a Kinetex C18 1.7 μm, 100 × 2.1mm column (Phenomenex, Torrence, CA). Column temperature was set at 30 °C and the autosampler was kept at 8 °C. Reverse phase separation from plasma and brain samples was run using a gradient elution with mobile phase A (10 mM ammonium acetate in water) and mobile phase B (10mM ammonium acetate in methanol) at 0.3 mL/min. The gradient was started at 5% B and held for 1.8 min. The conditions were decreased to 70% B at 2 min and held until 5 min, followed by column reconditioning for 5 min.
The chromatographic system was coupled to a 4000 QTRAP ® MS/MS Triple quadrupole (Sciex, Framingham, MA) equipped with an ESI interface operated in positive ionization mode. Data acquisition handling and instrument control were performed by the Analyst software version V6.1 (Sciex, Framingham, MA). Quantification was achieved using multiple reaction monitoring (MRM). Standard curves were generated using the reference standards obtained in ratio to the internal standards (above). Transitions are as follows: m/z 163→132 and (nicotine), 167→136 and ([d4]-nicotine), 177→98 (cotinine), 180→80 ([d3]-cotinine), 212→169 (varenicline) and 215→170 (PF-00142282). Each standard was infused for optimization of the mass spectrometer conditions. Nitrogen was used as desolvation gas at a flow rate of 800 L/h and a temperature of 400 °C, and argon as collision gas at a flow rate of 0.2 mL/min. Source temperature was set at 200 °C and capillary voltage at 4.5 kV. Dwell time for each ion was 200 ms. Other parameters included a declustering potential of 50 V, collision exit potential of 5 V, entrance potential of 12 V, and collision energy of 25 V.
2.7 Autoradiography
2.7.1 Tissue Preparation
Mice that had been treated with saline, 0.12 or 0.6 mg/kg/hr varenicline were withdrawn from treatment for 24 hr and tested for tolerance to nicotine as described (section 2.4). Two hours after completion of the second tolerance test each mouse was euthanized by cervical dislocation, its brain was rapidly (<1 min) removed from the skull and quickly frozen by immersion in isopentane (−35°C) for 10 s. The frozen brain was wrapped in aluminum foil and stored at −70°C until sectioning.
For sectioning, frozen brains were mounted with M-1 Embedding Matrix (Anatomical Pathology, Pittsburgh, PA). Subsequently, 14 μm coronal sections were cut using either a Leica CM 1850 cryostat/microtome (Leica, Nussloch, Germany) or an IEC Minotome (Damon Corp., Needham, MA) at −14°Cand thaw mounted on Fisher Suprafrost/Plus microscope slides. A series of ten sets of slides were prepared from each brain to allow comparison of results for several different experiments on adjacent or near-adjacent sections. Slides containing the brain sections were stored, desiccated at −70°C, until use.
2.7.2 [125I]-Epibatidine Autoradiography
Slides with tissue sections prepared were warmed to room temperature in a desiccator, transferred to Bel-Art (Wayne, NJ) slide racks and rehydrated by incubation at 22°C for 15 min in isotonic buffer (NaCl, 144 mM; KCl, 2.2 mM, CaCl2, 2.0 mM, MgSO4, 1.0 mM; HEPES, 25 mM; pH = 7.5). Rehydrated slides were subsequently transferred to isotonic buffer containing 200 pM [125I]-epibatidine (specific activity 2200 Ci/mmol mixed with unlabeled 5I-epibatidine to yield a final specific activity of 110 Ci/mmol, a 20-fold dilution). Samples were incubated for 2 h at 22°C. Samples were washed as follows (all solutions at 4°C): Twice for 30 s in isotonic buffer, twice for 5 s in hypotonic buffer (0.1 ×) and twice for 5 s in 10 mM HEPES, pH = 7.5. Parallel series of sections were used to determine [125I]-epibatidine (200 pM) binding levels in the presence of 50 nM cytisine (sufficient to block most binding to α4β2-nAChR sites, while leaving other subtypes relatively unaffected (Whiteaker, McIntosh et al. 2000, Whiteaker, Peterson et al. 2002)). Tissue sections from β2 null mutant mice or samples incubated in the presence of 10 μM nicotine were used to establish blanks; these blanks did not differ from film background (Whiteaker, Cooper et al. 2006).
2.7.3 [125I]-α-conotoxin MII autoradiography
[125I]-α-conotoxin MII [125I]-α-CtxMII binding was performed as previously described (Whiteaker, McIntosh et al. 2000). Sections were incubated for 10 min in binding buffer containing 1 mM phenylmethylsulfonyl fluoride (PMSF)). Sections were then incubated with 0.5 nM [125I]-α-conotoxin MII in binding buffer with the addition of the protease inhibitors leupeptin, pepstatin, and aprotinin (10 μg/ml each) and bovine serum albumin (0.1% w/v), for 2 h at 22° C. Samples were washed as follows: once for 30s in isotonic buffer containing 0.1% BSA at 22°C, twice for 10s in ice-cold 10 mM HEPES, pH 7.5. Tissue sections from β2 null mutant mice were again used to establish blanks.
2.7.4 [125I]-α-Bungarotoxin autoradiography
[125I]-α-Bungarotoxin binding was performed using a modification of a previously described method (Pauly, Marks et al. 1991). Sections were incubated for 10 min in binding buffer containing 1 mM phenylmethylsulfonyl fluoride (PMSF)). Sections were then incubated with 1 nM [125I]-α-bungarotoxin in binding buffer with the addition bovine serum albumin (0.1% w/v), for 3 h at 22° C. Slides were subsequently incubated for 10 min at 22° C in binding buffer containing 0.1% bovine serum albumin followed by a 5-min incubation at 22° C in protein-free binding buffer. Slides were subsequently washed in ice-cold protein free binding buffer twice for 30 s followed by two 5 s washes in 0.1× protein free binding buffer. Final rinses (2 × 5 s each) were conducted in ice-cold 5 mM HEPES, pH 7.5. Blanks were established by including 1 mM nicotine in the incubation buffer.
2.7.5 Image Development and Quantitation
After samples were washed, slides were air dried and stored overnight under vacuum in a dessicator. Slides were initially exposed to Packard Super Resolution Phosphor screens, Type XR (Perkin Elmer, Shelton, CT). After exposure images were captured with a Packard Cyclone PhosphorImager. Subsequently, slides were exposed to Kodak MR autoradiography film (Eastman-Kodak, Rochester, NY) to provide higher resolution images. Tissue paste samples prepared from whole brain homogenates containing measured amounts of 125I were used to construct standard curves. The Phosphor screens produce a linear relationship between signal intensity and tissue radioactivity content over several orders of magnitude and all ligand binding values fell within this linear range. The regression line calculated for the standard curve was used to convert the measured value of pixels/mm2 to the cpm/mg wet weight. Signal intensity in fmol/mg wet weight was subsequently calculated from the specific activity of each ligand. Brain regions were identified using a mouse brain atlas (Paxinos and Franklin 2004) as a guide. Multiple (4-6) measurements were made in each brain region of each mouse and the average of these measurements defined the signal intensity for each region.
2.7.6 Statistical Analysis
ED50 values for producing hypolocomotion were calculated by non-linear curve fitting to the equation: (response after acute nicotine) = (response after saline)/[1+(nicotine dose/ED50)N] where ED50 is the dose producing 50% of the maximal effect and N is a slope factor (Hill coefficient). ED50 values for hypothermia were also calculated by non-linear curve fitting with a modified equation: (body temperature after nicotine) = (maximal temperature decrease)/[1+(nicotine dose/ED50)N] + (maximal body temperature decrease).
IBM SPSS Statistical Package, v 21, was used for statistical analyses. The effect of chronic nicotine and/or varenicline treatment on binding site densities was initially analyzed using a three-way ANOVA (independent variables: nicotine dose, varenicline dose and brain region) and then using a two-way ANOVA for each brain region (nicotine dose and varenicline dose as the independent variables). Group means were compared with Duncan’s post hoc test.
3. Results
3.1. Preliminary experiment to choose dose of chronic varenicline
The aim of this initial test was to choose a dose of varenicline that would elicit up-regulation of α4β2-nAChR sites approximately equivalent to that elicited by treatment with 1.0 mg/kg/hr nicotine. In order to estimate a varenicline dose to be used in the chronic treatments, equivalent to 1.0 mg/kg/hr nicotine, we determined Ki values by inhibition of [125I]-epibatidine binding to be 1.6 ± 0.08 nM for nicotine and 0.11 ± 0.01 nM for varenicline using membranes prepared from cortex of C57Bl/6J mice. Therefore, in order to choose a dose of varenicline that would produce significant up-regulation, but also to not be quite at maximum effect in order to see any additive effects, mice were treated with saline, and free base doses of 0.12 and 0.60 mg/kg/hr varenicline. In light of the slower metabolism of varencline compared to that of nicotine (Obach, Reed-Hagen et al. 2006, Matta, Balfour et al. 2007), a withdrawal of 24 hours from varenicline treatment was used before the preparation of samples for membrane binding in contrast to the two-hour withdrawal used for nicotine (t1/2 of 6-7 min for nicotine in mouse vs. 1.4 hrs for varenicline).
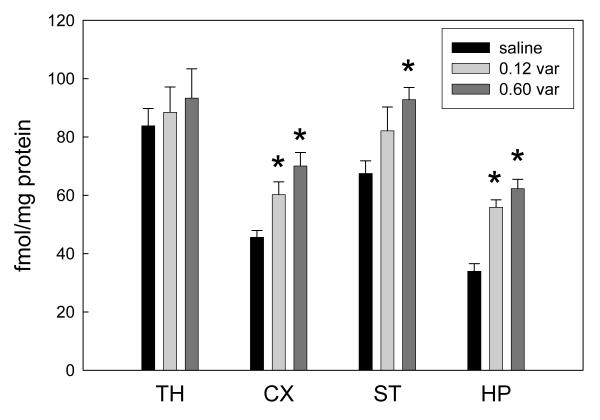
α4β2-nAChR binding sites from four brain regions were assayed by membrane binding for the cytisine-sensitive component of [125I]-epibatidine binding. Thalamus (TH), cortex (CX), striatum (ST) and hippocampus (HP) were chosen as representative brain regions that exhibit different extents of upregulation following chronic nicotine treatment (Marks, Pauly et al. 1992, Marks, McClure-Begley et al. 2011). Significant upregulation was seen with both doses for CX and HP, while only the higher dose produced significant upregulation in ST (Figure 1). Neither dose upregulated α4β2-nAChR binding sites in TH. The 0.12 mg/kg/hr dose of varenicline was chosen for further study because it produced measurable upregulation, but was not quite at the maximal effective dose. This dose appears elicit changes about equivalent to 1 mg/kg/hr nicotine (McCallum et al, 2006). The ratio of drug dosing used here (0.12 mg varenicline vs 1 mg nicotine) is similar to the ratio of usual doses for smoking cessation in humans of ~2 mg/day varenicline vs 14-21 mg/day NRT.
Figure 1.
Upregulation of α4β2*-nAChR by chronic treatment with varenicline. Mice were treated with the indicated doses of varenicline as mg/kg/hr by constant infusion for 10 days. After 24 hr of withdrawal, brains were dissected and cytisine-sensitive 125I-epibatidine binding to membrane preparations of the indicated regions were determined. * indicates significantly different from saline control by one-way ANOVA. Region code: TH, thalamus; CX, cortex; ST, striatum; HP, hippocampus.
3.2 Tolerance to acute dose of nicotine after chronic treatments
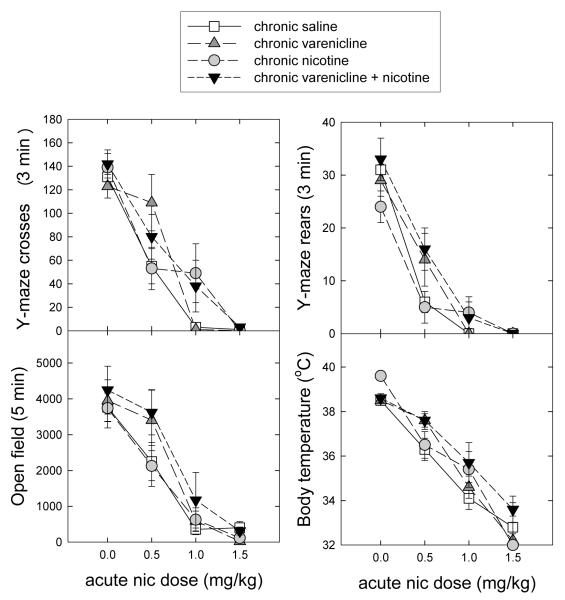
After chronic treatment for 10 days and withdrawal for 24 hours and an acute injection of saline or various doses of nicotine, four responses were measured, Y-maze crosses, Y-maze rears, open field activity and body temperature. Results are shown in Figure 2. Analysis by two-way ANOVA for effects of nicotine and/or varenicline indicated a significant main effect of chronic varenicline for Y-maze crosses and rears (crosses F(1,8)=13.342, P=0.006; rears F(1,8)=19.360, P=0.002) but no effect of chronic nicotine and no interaction. There was no significant effect of any treatment on distance traveled in the open field. For body temperature there was a significant effect of nicotine (F(1,8)=11.314, P=0.010) as well as for varenicline treatment (F(1,8)=16.480, P=0.004), with no interaction between the drugs.
Figure 2.
Tolerance tests after various chronic treatments. Mice were treated with 0.12 mg/kg/hr varenicline, 1 mg/kg/hr nicotine or the combination by constant infusion for 10 days followed by 24 hr withdrawal before tolerance to the indicated doses of nicotine was measured.
In addition to the ANOVA, ED50 values for nicotine dose-response curves were calculated and are presented in Table 1. By one-way ANOVA followed by Dunnett’s test for difference from the saline group, significant differences were seen for the varenicline treatment group for Y-maze crosses and rears, as well as for the varenicline plus nicotine group for Y-maze rears and for body temperature. While the varenicline and varenicline plus nicotine groups tended to higher ED50 values for the other measures, these changes were not significant by ANOVA. All shifts were to higher ED50 values, indicating that chronic varenicline or varenicline plus nicotine treatment, elicited some tolerance to the acute effects of nicotine. In contrast, no significant tolerance was detected following treatment with the 1.0 mg/kg/hr dose of nicotine, as has been previously reported for this dose (McCallum et al, 2006).
Table 1.
ED50 values for effect of acute nicotine following 24 hr withdrawal
| Chronic treatment | Y-maze cross | Y-maze rear | Open field | Body temperature |
|---|---|---|---|---|
| Saline | 0.49 ± 0.04 | 0.38 ± 0.03 | 0.60 ± 0.06 | 0.80 ± 0.05 |
| 1.0 mg/kg/hr Nicotine | 0.47 ± 0.06 | 0.38 ± 0.05 | 0.55 ± 0.06 | 0.88 ± 0.09 |
| 0.12 mg/kg/hr Varenicline | 0.65 ± 0.08* | 0.49 ± 0.05* | 0.70 ± 0.10 | 0.91 ± 0.06 |
| Nicotine + Varenicline | 0.57 ± 0.06 | 0.49 ± 0.04* | 0.78 ± 0.40 | 1.12 ± 0.09* |
ED50 values (as mg/kg) for acute nicotine-induced hypolocomotion and hypothermia by ip injection of nicotine determined by curve-fit. The mean of all groups was fixed as the starting point for each test and the curve-fit hill coefficient of saline-treated mice was used for analysis of all groups.
indicates significant difference (P<0.05) from chronic saline treatment group by one-way ANOVA with Dunnett’s test.
3.3 Determination of brain and plasma levels of drugs after chronic treatment
Levels of nicotine and varenicline after 10 days of treatment with either 1.0 mg/kg/hr nicotine or 0.12 mg/kg/hr varenicline were determined from plasma and whole brain samples as described in Methods. Quantitation by UPLC-MS/MS resulted in the following levels: plasma nicotine: 225.1 ± 34.4 ng/ml (N=8); brain nicotine: 379.6 ± 75.4 ng/g wet wt (N=7), ratio 1.7; plasma varenicline: 57.9 ± 9.9 ng/ml; brain varenicline: 262.5 ± 37.6 ng/g wet wt (N=9), ratio 4.5.
3.4 Autoradiographic analysis of nAChR binding sites
3.4.1 α4β2-nAChR binding sites were measured as cytisine-sensitive [125I]-epibatidine binding; data is compiled for 43 brain regions for 4 treatments in Table 2.
Table 2.
Cytisine-sensitive [125I]-Epibatidine Binding (fmol/mg wet weight)
| Plot code |
Brain Region | Saline N=6 mice |
1.0 mg/kg/hr Nicotine N=6 mice |
0.12 mg/kg/hr Varenicline N=5 mice |
Nicotine plus Varenicline N=6 mice |
Main effect of Nicotine |
Main effect of Varenicline |
Nicotine x Varenicline interaction |
|---|---|---|---|---|---|---|---|---|
| A | Frontal Cortex | 3.10±0.13 | *4.50±0.37 | 4.13±0.12 | *4.76±0.36 | F1,20=10.61 | F1,20=4.49 | F1,20=1.63 |
| B | Orbital Cortex | 5.26±0.51 | 7.19±0.62 | 6.70±0.34 | 7.87±0.45 | F1,20=9.25 | F1,20=4.35 | F1,20=0.55 |
| C | Cingulate Cortex | 7.57±0.79 | 9.48±1.00 | 9.42±0.72 | 10.48±1.17 | F1,20=2.24 | F1,20=2.05 | F1,20=0.18 |
| D | Cortex, Outer | 3.43±0.17 | *5.01±0.48 | *5.45±0.27 | *5.31±0.26 | F1,20=5.01 | F1,20=12.94 | F1,20=7.20 |
| E | Cortex, Inner | 6.02±0.33 | 7.89±0.58 | *9.28±0.42 | *9.35±0.50 | F1,20=4.01 | F1,20=24.03 | F1,20=3.50 |
| F | Retrosplenial Cortex | 10.23±0.69 | 12.24±0.79 | *14.30±1.19 | 13.60±1.19 | F1,20=2.55 | F1,20=7.20 | F1,20=1.79 |
| G | Hippocampus | 3.38±0.20 | 4.36±0.30 | *4.98±0.17 | *5.55±0.22 | F1,20=11.03 | F1,20=35.70 | F1,20=0.71 |
| H | Subiculum | 12.03±0.41 | *17.37±1.64 | *16.79±1.00 | *18.44±0.96 | F1,20=6.57 | F1,20=6.03 | F1,20=1.61 |
| I | Septum | 6.23±0.30 | 7.04±0.36 | 7.63±0.44 | *8.74±0.72 | F1,20=3.39 | F1,20=8.73 | F1,20=0.83 |
| J | Olfactory Tubercle | 4.28±0.28 | *6.04±0.43 | *6.86±0.66 | *7.02±0.45 | F1,20=3.61 | F1,20=17.28 | F1,20=3.31 |
| K | Nucleus Accumbens | 4.86±0.26 | *6.40±0.35 | *6.37±0.55 | *7.23±0.41 | F1,20=12.72 | F1,20=14.88 | F1,20=0.32 |
| L | Striatum | 6.71±0.41 | 8.15±0.60 | 7.93±0.70 | 9.32±0.55 | F1,20=6.71 | F1,20=5.91 | F1,20=0.04 |
| M | Substantia nigra pars compacta |
11.30±1.19 | 14.42±2.45 | 14.41±1.28 | 16.32±1.04 | F1,20=2.52 | F1,20=2.04 | F1,20=0.19 |
| N | Substantia nigra, pars reticulate |
10.24±1.74 | 13.75±2.24 | 10.89±1.76 | 14.12±1.31 | F1,20=2.27 | F1,20=0.28 | F1,20=0.04 |
| O | Ventral Tegmental Area |
15.74±1.75 | 20.75±3.99 | 19.51±1.87 | 23.06±0.76 | F1,20=3.24 | F1,20=1.45 | F1,20=0.12 |
| P | Anterodorsal thalamic nucleus |
28.42±1.56 | 27.55±1.94 | 28.00±2.55 | 33.08±2.21. | F1,20=1.00 | F1,20=1.48 | F1,20=2.00 |
| Q | Laterodorsal thalamic nucleus |
21.98±1.24 | 23.73±1.19 | 24.14±1.82 | 26.34±1.15 | F1,20=2.18 | F1,20=2.19 | F1,20=0.03 |
| R | Mediodorsal thalamic nucleus |
22.63±1.42 | 24.23±1.39 | 23.47±1.79 | 26.46±1.27 | F1,20=2.43 | F1,20=1.10 | F1,20=0.22 |
| S | Ventrolateral thalamic nucleus |
17.53±0.94 | 20.00±3.71 | 22.07±2.00 | 22.62±1.11 | F1,20=1.19 | F1,20=6.68 | F1,20=0.48 |
| T | Hypothalamus | 5.70±0.48 | 7.53±0.44 | *8.78±0.80 | *10.32±0.30 | F1,20=11.34 | F1,20=34.41 | F1,20=0.08 |
| U | Medial Habenula | 63.62±11.60 | 60.77±13.20 | 38.89±14.24 | 70.73±15.22 | F1,20=3.24 | F1,20=0.23 | F1,20=2.62 |
| V | Fasiculus Retroflexus | 22.86±1.70 | 29.64±6.79 | 26.48±6.82 | 33.30±4.44 | F1,20=1.20 | F1,20=1.09 | F1,20=0.17 |
| W | Interpeduncular nucleus |
49.45±13.65 | 68.91±19.16 | 30.23±19.84 | 62.01±12.20 | F1,20=2.17 | F1,20=0.37 | F1,20=0.01 |
| X | Optic tracts | 4.21±0.43 | 4.42±0.70 | 5.32±1.41 | 5.49±0.44 | F1,20=0.48 | F1,20=6.21 | F1,20=0.05 |
| Y | Dorsolateral geniculate nucleus |
21.83±2.83 | 24.77±3.32 | 22.50±2.47 | 24.98±1.52 | F1,20=1.74 | F1,20=0.00 | F1,20=0.07 |
| Z | Pregeniculate nucleus | 14.26±1.34 | 14.90±2.50 | 14.04±1.74 | 16.77±1.35 | F1,20=0.58 | F1,20=0.11 | F1,20=0.10 |
| a | Zona Incerta | 10.37±0.30 | 13.37±1.15 | *15.63±1.35 | *16.18±0.96 | F1,20=3.14 | F1,20=16.30 | F1,20=1.49 |
| b | Olivary Pretectal Nucleus |
10.53±1.06 | *13.64±2.85 | *14.78±1.70 | *19.82±1.06 | F1,20=4.73 | F1,20=3.85 | F1,20=1.90 |
| c | Superior Colliculus, superficial gray |
11.99±1.19 | 12.65±2.09 | 11.56±2.45 | 15.36±1.39 | F1,20=2.19 | F1,20=2.85 | F1,20=0.20 |
| d | Superior Colliculus, optic nerve layer |
17.65±1.00 | 20.83±1.93 | 21.07±2.02 | 23.43±1.62 | F1,20=3.10 | F1,20=3.63 | F1,20=0.05 |
| e | Medial Geniculate Nucleus |
16.21±1.08 | 18.60±2.53 | 18.91±1.61 | 20.91±0.96 | F1,20=1.64 | F1,20=1.99 | F1,20=0.02 |
| f | Inferior Colliculus, Dorsal Cortex Anterior |
7.30±0.50 | *9.65±0.57 | *11.42±0.78 | *12.75±0.40 | F1,17=10.20 | F1,17=39.06 | F1,17=0.78 |
| g | Inferior Colliculus, Central Nucleus |
4.09±0.39 | *6.09±0.67 | *5.83±0.36 | *6.51±0.28 | F1,19=7.78 | F1,19=5.05 | F1,19=1.89 |
| h | Inferior Colliculus, External Cortex |
3.36±0.47 | 4.82±1.00 | *5.02±0.55 | *6.82±1.07 | F1,15=3.23 | F1,15=9.47 | F1,15=0.32 |
| i | Inferior Colliculus, Dorsal Cortex Posterior |
4.48±0.72 | *9.04±1.76 | *10.63±2.04 | *8.10±1.41 | F1,13=0.67 | F1,13=7.61 | F1,13=5.50 |
| j | Anterior Olfactory Area |
0.98±0.13 | 0.84±0.16 | 1.88±0.76 | 1.11±0.18 | F1,20=0.03 | F1,20=4.56 | F1,20=1.46 |
| k | Accessory Olfactory Bulb |
0.83±2.10 | 3.48±2.08 | 1.98±1.45 | 4.47±2.21 | F1,20=3.26 | F1,20=0.13 | F1,20=0.02 |
| l | Periaquiductal Gray | 8.72±0.51 | *12.37±1.64 | *12.08±1.04 | *13.21±0.85 | F1,19=4.50 | F1,19=3.46 | F1,19=1.24 |
| m | Deep Mesencephalic Area |
9.72±0.61 | *13.42±1.66 | *13.46±1.01 | *15.78±0.96 | F1,19=6.37 | F1,19=6.53 | F1,19=0.34 |
| n | Pontine Nucleus | 10.78±1.19 | 12.44±1.44 | 14.33±2.37 | 16.69±2.31 | F1,18=0.42 | F1,12=2.81 | F1,12=0.07 |
| o | Dorsal Tegmental Nucleus |
9.13±1.42 | 11.39±0.77 | *14.10±2.02 | *14.80±1.58 | F1,12=0.50 | F1,12=10.67 | F1,12=1.63 |
| p | Hindbrain | 6.29±1.32 | 8.27±0.72 | 11.04±2.02 | 10.03±1.13 | F1,12=3.07 | F1,12=3.47 | F1,12=0.92 |
| q | Cerebellum | 1.05±0.07 | 1.80±0.29 | 1.49±0.30 | 1.80±0.33 | F1,12=2.64 | F1,12=0.44 | F1,12=0.46 |
Results were initially analyzed using three-way ANOVA with chronic nicotine dose, chronic varenicline dose and brain region as the independent variables. The highly significant effect of brain region (F43,824=303.72) was anticipated owing to the large variation in α4β2*-nAChR sites across the brain. The significant effects of chronic nicotine (F1,824 = 28.52) and chronic varenicline (F1,824 = 27.13) treatments demonstrated an overall effect of drug treatment on these binding sites. In order to reduce the large initial differences in the density of binding sites among the regions, data were normalized to site densities in each region of saline-treated mice and also analyzed by three-way ANOVA. The main effect of brain regions was significantly reduced, as expected (F42,766 = 5.19), but regional differences remained significant indicating regional differences in response to chronic drug treatment. Analysis following normalization revealed even larger effects of both nicotine (F1,766 = 91.88) and varenicline (F1,766 = 182.33) treatments.. This analysis also detected significant nicotine by varenicline (F1,766 = 19.71) and varenicline by brain region (F42,766 = 1.86) interactions.
Results for the effects of chronic nicotine or varenicline treatments were also examined by two-way ANOVAs for each brain region. As shown in Table 2, significant effects of either nicotine or varenicline treatment were most apparent in forebrain regions such as cerebral cortical areas as well as hippocampus, hypothalamus and some midbrain regions. Cytisine-sensitive [125I]-epibatidine binding in thalamic nuclei was relatively resistant to treatment with either drug.
3.4.2 Cytisine-resistant [125I]-epibatidine binding measures several non-α4β2-nAChR sites. In the presence of cytisine and at the concentration of [125I]-epibatidine used for these experiments, only high affinity cytisine-resistant sites are measured; these include α3β4*-, α6β2*-, α2β2*-, α3β2-nAChR (Baddick and Marks 2011) Data from the 4 treatment groups for 17 brain regions that contain sufficient numbers of these sites to permit quantitation, are presented in Table 3.
Table 3.
Cytisine-Resistant [125I]-Epibatidine Binding (fmol/mg wet weight)
| Plot code |
Brain Region | Saline N=6 mice |
1.0 mg/kg/hr Nicotine N=6 mice |
0.12 mg/kg/hr Varenicline N=5 mice |
Nicotine plus Varenicline N=6 mice |
Main effect of Nicotine |
Main Effect of Varenicline |
Nicotine x Varenicline interaction |
|---|---|---|---|---|---|---|---|---|
| A | Olfactory Bulbs, Glomerular Cell Layer |
0.514±0.050 | 0.756±.058 | 0.824±0.072 | 0.920±0.055 | F(1,18)=8.14 | F(1,18)=15.90 | F(1,18)=1.53 |
| B | Olfactory Bulbs, Inner Plexiform Layer |
0.604±0.080 | 0.920±0.040 | 0.942±0.071 | 1.163±0.083 | F(1,18)=14.60 | F(1,18)=17.09 | F(1,18)=0.45 |
| C | Accessory Olfactory Bulb | 13.91±1.12 | 14.92±0.96 | 13.73±0.94 | 14.53±0.58 | F(1,18)=0.98 | F(1,18)=0.10 | F(1,18)=0.01 |
| D | Olfactory Tubercles | 0.78±.013 | 0.715±0.055 | 0.933±0.140 | 0.807±0.007 | F(1,20)=0.89 | F(1,20)=1.52 | F(1,20)=0.10 |
| E | Nucleus Accumbens | 0.860±0.111 | 0.935±0.047 | 1.092±.109 | 1.024±.061 | F(1,20)=0.00 | F(1,20)=3.65 | F(1,20)=0.72 |
| F | Striatum | 1.058±.100 | 1.203±.024 | 1.330±.182 | 1.280±.074 | F(1,20)=.019 | F(1,20)=2.49 | F(1,20)=0.78 |
| G | Optic tracts | 2.262±0.098 | 2.300±0.210 | 2.340±0.309 | 2.620±0.238 | F(1,20)=0.44 | F(1,20)=0.69 | F(1,20)=0.26 |
| H | Dorsolateral Geniculate Nucleus |
5.726±.211 | 5.568±.220 | 5.633±.715 | 5.468±.276 | F(1,20)=0.15 | F(1,20)=0.05 | F(1,20)=0.00 |
| I | Pregeniculate Nucleus | 5.648±.164 | 5.228±.0.109 | 5.308±.535 | 5.327±.294 | F(1,20)=0.36 | F(1,20)=0.13 | F(1,20)=0.43 |
| J | Olivary Pretectal Nucleus | 4.412±0.355 | 5.740±0.705 | 5.246±.587 | 4.421±0.419 | F(1,20)=0.21 | F(1,20)=0.20 | F(1,20)=3.89 |
| K | Superior Colliculus, Superficial Gray |
7.46±0.17 | 8.18±0.38 | 11.49±1.45 | 9.41±0.52 | F(1,20)=0.70 | F(1,20)=10.35 | F(1,20)=2.92 |
| L | Medial Habenula | 56.79±9.65 | 61.62±9.01 | 65.75±8.79 | 71.28±10.20 | F(1,20)=0.29 | F(1,20)=0.93 | F(1,20)=0.00 |
| M | Fasiculus retroflexus | 25.16±0.80 | 25.82±1.70 | 28.72±2.99 | 25.30±1.18 | F(1,20)=0.53 | F(1,20)=0.65 | F(1,20)=1.15 |
| N | Interpeduncular nucleus | 81.86±6.18 | 80.21±4.96 | 107.87±10.27 | 97.95±9.81 | F(1,20)=0.45 | F(1,20)=6.50 | F(1,20)=0.23 |
| O | Inferior Colliculus, Dorsal Cortex |
4.60±0.59 | 5.48±0.80 | 7.10±0.96 | 7.14±1.10 | F(1,14)=0.22 | F(1,14)=4.49 | F(1,14)=0.19 |
| P | Inferior Colliculus, External Cortex |
1.62±0.25 | 2.23±0.30 | 3.16±0.0.26 | 2.43±0.46 | F(1,11)=0.03 | F(1,14)=6.59 | F(1,14)=3.88 |
| Q | Laterodorsal tegmental nucleus |
2.19±0.53 | 2.73±0.10 | 3.63±0.91 | 2.13±0.50 | F(1,11)=0.61 | F(1,11)=0.46 | F(1,11)=2.80 |
Results were initially analyzed using three-way ANOVA with chronic nicotine dose, chronic varenicline dose and brain region (omitting mHab, fr and IPN that exhibit the highest expression of these sites) as the independent variables. As expected owing to the large differences in binding site densities among the brain regions, a highly significant effect of brain region was found (F16,312 = 228.56, P<0.001)). A significant effect of chronic varenicline treatment was also detected (F1,312 = 7.27, P =0.007), but the response to chronic nicotine treatment was not significant (F1,312 = 0.013). The significant varenicline by brain region interaction (F16,332 = 2.75, P<0.001) indicated that response to varenicline varied among the brain regions. In order to reduce the large initial differences in the density of binding sites among the regions, data were normalized to site densities in each region of saline-treated mice and also analyzed by three-way ANOVA. Values for mHab, fr and IPN were included in this analysis. Although the main effect of brain region was significantly reduced (F19.375 = 7.98, P<0.001)), this difference remained statistically significant. The effect of chronic varenicline treatment was retained (F1,375,=49.43, P<0.001) and a modest effect of chronic nicotine treatment was indicated (F1,375 = 4.89, P=0.028).
Results for the effects of chronic nicotine or varenicline treatments were also examined by two-way ANOVAs for each brain region. As shown in Table 3, significant effects of either treatment were noted only in the olfactory bulbs, while chronic varenicline treatment elicited increases in areas of the superior and inferior colliculi in addition to the olfactory bulbs.
3.4.3 [125I]-α-bungarotoxin binding, a measure of α7 nAChR sites
[125I]-α-bungarotoxin binding values are presented in Table 4. Results were initially analyzed using three-way ANOVA with chronic nicotine dose, chronic varenicline dose and brain region as the independent variables. As expected owing to the large differences in binding site densities among the brain regions, a highly significant effect of brain region was found (F36,714 = 151.37). In addition, significant effects of both chronic nicotine (F1,714 = 15.40, P< 0.001) and varenicline (F1,714 = 37.48, P <0.001) were obtained. However, the overall pattern of response to treatment with the two drugs was different. An increase in the density of [125I]-α-bungarotoxin binding sites was noted following chronic varenicline treatment, while a decrease in the density of [125I]-α-bungarotoxin binding sites was noted following chronic nicotine treatment. When the results were normalized to the density of [125I]-α-bungarotoxin binding sites in saline-treated mice, the effect of brain region was no longer significant (F35,696 = 1.26, P = 0.15), while the main effects of nicotine (F1,696 = 14.45, P<0.001) and varenicline (F1,696 = 48.17) treatment and the direction of the effects (decrease and increase, respectively) were retained.
Table 4.
[125I]-α-Bungarotoxin binding following chronic nicotine and varenicline treatments.
| Plot code |
Brain Region | Saline N=6 mice |
1.0 mg/kg/hr Nicotine N=6 mice |
0.12 mg/kg/hr Varenicline N=5 mice |
Nicotine Plus Varenicline N=6 mice |
Main Effect of Nicotine |
Main Effect of Varenicline |
Nicotine x Varenicline Interaction |
|---|---|---|---|---|---|---|---|---|
| A | Olfactory bulb, glomerular layer | 1.000.04 | 0.94±0.06 | 1.00±0.12 | 0.93±0.08 | F1,21=0.48 | F1,21=0.00 | F1,21=0.01 |
| B | Accessory olfactory nucleus | 2.25±0.23 | 2.20±0.23 | 2.14±0.21 | 2.31±0.27 | F1,21=0.06 | F1,21=0.00 | F1,21=0.21 |
| C | Cortex, outer | 1.11±0.04 | 1.0408.23 | 1.28±0.11 | 1.15±0.10 | F1,21=1.25 | F1,21=2.46 | F1,21=0.13 |
| D | Cortex, middle | 1.76±0.07 | 1.70±0.13 | 2.18±0.20 | 1.99±0.18 | F1,21=0.64 | F1,21=5.29 | F1,21=0.19 |
| E | Cortex, inner | 1.25±0.06 | 1.29±0.08 | 1.60±0.13 | 1.48±0.17 | F1,21=0.11 | F1,21=4.83 | F1,21=0.42 |
| F | Cingulate cortex | 1.25±0.06 | 1.46±0.07 | 1.46±0.18 | 1.34±0.13 | F1,21=0.62 | F1,21=2.30 | F1,21=0.04 |
| G | Retrosplenial cortex | 1.12±0.03 | 1.01±0.04 | 1.25±0.13 | 1.19±0.14 | F1,21=0.67 | F1,21=2.34 | F1,21=0.05 |
| H | Amygdala | 2.56±0.12 | 2.48±0.15 | 3.08±0.24 | 2.80±0.31 | F1,21=0.66 | F1,21=3.45 | F1,21=0.19 |
| I | Hippocampus, stratum oriens | 2.24±0.07 | 2.21±0.12 | 2.71±0.34 | 2.48±0.39 | F1,21=0.21 | F1,21=1.66 | F1,21=0.13 |
| J | Hippocampus, stratum radiatum | 2.24±0.07 | 2.30±0.14 | 2.75±0.33 | 2.44±0.38 | F1,21=0.21 | F1,21=1.34 | F1,21=0.45 |
| K | Hippocampus, molecular layer | 2.82±0.05 | 2.82±0.16 | 3.54±0.41 | 3.19±0.36 | F1,21=0.35 | F1,21=3.39 | F1,21=0.34 |
| L | Hippocampus, pyramidal cell layer | 6.55±0.28 | 6.15≈0.47 | 6.73±0.65 | 6.58±0.74 | F1,18=0.24 | F1,18=0.29 | F1,18=0.05 |
| M | Septum | 0.52±0.05 | 0.44±0.04 | 0.78±0.09 | 0.56±0.07 | F1,20=4.83 | F1,20=7.80 | F1,20=0.96 |
| N | Caudate Putamen | 2.30±0.07 | 2.23±0.11 | 2.80±0.29 | 2.40±0.13 | F1,21=1.96 | F1,21=4.00 | F1,21=0.96 |
| O | Mediodorsal thalamic nucleus | 1.98±0.14 | 2.02±0.12 | 2.53±0.28 | 2.40±0.31 | F1,21=0.04 | F1,21=3.99 | F1,21=0.13 |
| P | Subthalamic nucleus | 4.66±0.33 | 4.76±0.34 | 4.89±0.63 | 5.64±0.65 | F1,20=0.66 | F1,20=1.12 | F1,20=0.38 |
| Q | Field of Forel | 3.41±0.33 | 3.47±0.26 | 3.98±0.40 | 3.90±0.37 | F1,21=0.00 | F1,21=2.08 | F1,21=0.04 |
| R | Prerubral field | 9.51±0.39 | 9.46±0.61 | 10.09±1.37 | 9.81±0.65 | F1,19=0.05 | F1,19=0.39 | F1,19=0.02 |
| S | Zona incerta | 1.83±0.08 | 1.73±0.10 | 1.89±0.21 | 2.07±0.33 | F1,20=0.40 | F1,20=0.85 | F1,20=0.43 |
| T | Red nucleus | 5.38±0.25 | 5.61±0.54 | 6.47±1.17 | 5.53±0.46 | F1,18=0.35 | F1,18=0.73 | F1,18=0.99 |
| U | Hypothalamus | 1.62±0.05 | 1.51±0.08 | 1.96±0.22 | 1.73±0.19 | F1,21=1.25 | F1,21=3.15 | F1,21=0.14 |
| V | Posterior hypothalamic area | 2.55±0.18 | 2.41±0.14 | 2.58±0.25 | 2.66±0.23 | F1,20=0.03 | F1,20=0.50 | F1,20=0.28 |
| W | Medial tuberal nucleus | 3.07±0.23 | 3.09±0.38 | 3.13±0.30 | 3.64±0.42 | F1,20=0.53 | F1,20=0.73 | F1,20=0.46 |
| X | Medial mammilary nucleus | 3.37±0.30 | 3.03±0.69 | 4.15±0.80 | 3.27±0.34 | F1,19=1.30 | F1,20=0.90 | F1,20=0.25 |
| Y | Interpeduncular nucleus, medial | 3.15±0.31 | 2.56±0.19 | 2.46±0.24 | 3.01±0.25 | F1,18=0.01 | F1,18=0.19 | F1,18=4.48 |
| Z | Interpeduncular nucleus, lateral | 3.45±0.17 | 3.15±0.31 | 3.21±0.28 | 3.55±.028 | F1,18=0.00 | F1,18=0.09 | F1,18=1.37 |
| a | Medial preoptic area | 1.25±0.19 | 0.88±0.06 | 1.88±0.38 | 1.12±0.23 | F1,21=5.38 | F1,21=3.29 | F1,21=0.64 |
| b | Anterior pretectal nucleus | 1.81±0.13 | 1.98±0.13 | 2.30±0.14 | 2.25±0.22 | F1,20=0.13 | F1,20=4.96 | F1,20=0.42 |
| c | Pretectal nucleus | 2.80±0.12 | 2.84±0.19 | 3.49±0.57 | 3.31±0.42 | F1,19=0.04 | F1,19=2.74 | F1,19=0.09 |
| d | Pregeniculate nucleus | 3.08±0.15 | 2.91±0.16 | 3.38±0.33 | 3.32±0.32 | F1,20=0.20 | F1,20=1.94 | F1,20=0.04 |
| e | Superior colliculus, superficial gray | 5.17±0.27 | 4.63±0.30 | 5.51±0.74 | 4.99±0.32 | F1,18=1.88 | F1,18=0.81 | F1,18=0.00 |
| f | Superior colliculus, optic nerve layer |
2.39±0.13 | 2.33±0.19 | 2.56±0.28 | 2.53±0.17 | F1,18=0.06 | F1,18=0.95 | F1,18=0.00 |
| g | Inferior colliculus | 9.64±0.33 | 9.05±1.03 | 9.67±0.71 | 7.98±0.40 | F1,16=3.02 | F1,16=0.62 | F1,16=0.71 |
| h | Dorsal tegmental nucleus | 16.13±0.81 | 13.97±1.57 | 16.86±1.98 | 12.47±0.80 | F1,15=6.81 | F1,15=0.10 | F1,15=0.80 |
| i | Pontine central gray | 3.30±0.21 | 3.17±0.45 | 3.22±0.20 | 3.04±0.33 | F1,14=0.20 | F1,14=0.10 | F1,14=0.01 |
| j | Pons | 1.18±0.06 | 1.25±0.19 | 1.21±0.03 | 1.22±0.13 | F1,16=0.09 | F1,16=0.00 | F1,16=0.07 |
| k | Cerebellum | 0.51±0.04 | 0.53±0.04 | 0.51±0.10 | 0.66±0.18 | F1,16=0.69 | F1,16=0.46 | F1,16=0.39 |
The effects of chronic nicotine and or varenicline treatment in each of the 37 brain regions quantitated were subsequently examined using two-way ANOVAs. In general the effects of drug treatment were relatively modest. Although [125I]-α-bungarotoxin binding tended to increase following varenicline treatment and decrease or remain unchanged following chronic nicotine treatment, significant main effects of varenicline treatment were noted in only four brain regions and for nicotine treatment in only two brain regions.
3.4.4 [125I]-α-conotoxin MII binding, a measure of α6β2- and α3β2-nAChR sites.
[125I]-α-conotoxin MII binding results are complied in Table 5 for the 8 regions with significant expression of these binding sites. In the mouse most of this binding in the dopaminergic regions is to α6β2*nAChR sites; for the optic regions much is α6β2*nAChR with some smaller amount of α3β2*-nAChR, especially in superior colliculus.
Table 5.
[125I]-α-Conotoxin Binding (fmol/mg wet weight)
| Plot code |
Brain Region | Saline N=6 mice |
1.0 mg/kg/hr Nicotine N=6 mice |
0.12 mg/kg/hr Varenicline N=5 mice |
Nicotine plus Varenicline N=6 mice |
Main effect of Nicotine |
Main effect of Varenicline |
Nicotine x Varenicline Interaction |
|---|---|---|---|---|---|---|---|---|
| A | Olfactory Tubercles | 0.111±0.028 | 0.063±0.025 | 0.058±0.015 | *0.041±0.008 | F(1,16)=4.17 | F(1,16)=5.15 | F(1,16)=0.15 |
| B | Nucleus Accumbens | 0.137±0.018 | 0.113±0.027 | 0.096±0.022 | *0.073±o.005 | F(1,16)=1.71 | F(1,16)=4.88 | F(1,16)=0.00 |
| C | Striatum | 0.105±0.011 | 0.102±0.020 | 0.112±0.019 | 0.088±0.007 | F(1,16)=0.88 | F(1,16)=0.06 | F(1,16)=0.57 |
| D | Optic Tracts | 0.163±0.023 | 0.136±0.015 | 0.169±0.032 | 0.126±0.009 | F(1,16)=2.62 | F(1,16)=0.01 | F(1,16)=0.14 |
| E | Dorsolateral Geniculate Nucleus | 0.434±0.036 | 0.356±0.029 | 0.334±0.046 | 0.321±0.025 | F(1,16)=1.65 | F(1,16)=3.72 | F(1,16)=0.84 |
| F | Pregeniculate Nucleus | 0.489±0.033 | 0.392±0.022 | 0.362±0.061 | 0.362±0.029 | F(1,16)=1.44 | F(1,16)=3.77 | F(1,16)=1.44 |
| G | Olivary Pretectal Nucleus | 0.371±0.016 | 0.290±0.032 | 0.299±0.032 | *0.278±0.040 | F(1,16)=4.26 | F(1,16)=2.97 | F(1,16)=1.51 |
| H | Superior Colliculus, Superficial Gray |
0.378±0.015 | 0.328±0.034 | 0.340±0.041 | 0.398±0.017 | F(1,16)=0.02 | F(1,16)=0.32 | F(1,16)=3.79 |
The initial three-way ANOVA examining the effect of brain region as well as chronic nicotine and varenicline treatments on [125I]-α-conotoxin MII binding yielded results similar to those obtained for the other ligands: an expected significant effect of brain region (F7,128 = 109.95, P<0.001) as well as main effects of both chronic nicotine (F1,128 = 11.34, P = 0.001) and varenicline (F1,128 = 12.26, P =0.001). When the results were normalized to the binding in saline-treated mice, the main effect of brain region was decreased, but remained significant (F7,128 = 4.45, P<0.001), while the effects of chronic nicotine (F1,128 = 13.42, P<0.001) and varenicline (F1,128 = 11.70, P = 0.001) treatments remained relatively stable. It should be noted that for both drug treatments, an overall decrease in [125I]-α-conotoxin MII binding was observed. The largest decreases tended to be found in the group treated with both drugs.
3.5 Graphical representation of the effects of chronic nicotine and/or varenicline treatment on nAChR binding sites.
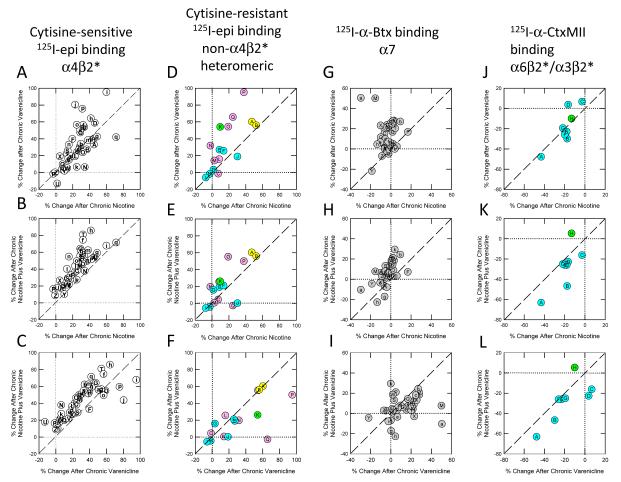
The statistical analyses presented above indicate that nicotine and/or varenicline treatment elicited differential effects on the four classes of nAChR binding sites. In order to examine these results further and provide a visual representation of the effects of the drug treatments on the ligand binding, the graphical approach shown in Figure 3 was used. The ligand binding data shown for chronic drug treatments in Tables 2, 3, 4 and 5 were normalized to the binding measured for saline-infused mice. Subsequently scattergrams were constructed comparing the effects of each set of treatments [first row: nicotine (X-axis) to varenicline (Y-axis), second row: nicotine (X-axis) to nicotine plus varenicline (Y-axis) and third row: varenicline (X-axis) to nicotine plus varenicline (Y-axis)]. The letter codes used to identify each brain region are provided in the appropriate tables. Color-coding indicates major types of binding sites measured: α4β2, white, using cytisine-sensitive epibatidine; α3β4, pink, using cytisine-resistant epibatidine in regions known to express significant amounts of these sites; α6β2, blue, using cytisine-resistant epibatidine or α-conotoxin MII in regions known to contain significant α6; α3β2 + α6β2, green, using cytisine-resistant epibatidine or α-conotoxin MII in regions known to contain significant α6 as well as α3; α2, yellow, using cytisine-resistant epibatidine in regions known to express significant amounts of α2; and α7, gray, using α-bungarotoxin. Lines of unit slope are included in each figure. Thus each point that falls below the line indicates that the treatment depicted on the X-axis elicited a greater response and conversely each point that falls above the line indicates that the treatment depicted on the Y-axis elicited a greater effect. A value deviating by at least 5% from the line of unit slope was considered to indicate a difference between the groups.
Figure 3.
Comparison of effects of treatments on various subtypes of nAChR. N=6 mice/group. Panels A, B, C compare cytisine-sensitive [125I]-epibatidine binding, representing α4β2*-nAChR (white symbols) as % change from saline-treated mice. Panels D, E, F compare cytisine-resistant [125I]-epibatidine binding, representing a mixture of non- α4β2* heteromeric subtypes that differ among regions. These subtypes are color-coded as follows: α4β2 white, α3β4 pink, α6β2 blue, α3β2 + α6β2 green, α2 yellow, α7 gray. Panels G, H, I compare [125I]-α-bungarotoxin binding, representing α7-nAChR (gray symbols), and panels J, K, L compare [125I]-α-conotoxin MII binding, color-coded as above. Treatment comparisons are the same across rows; A, D, G, J compare treatments with nicotine alone and varenicline alone; B, E, H, K compare nicotine alone to nicotine + varenicline; and C, F, I, L compare varenicline alone to nicotine + varenicline. For all plots dotted lines indicate no change from saline, and dashed line indicates equivalent changes by the two treatments being compared.
3.5.1 Cytisine-sensitive [125I]-epibatidine binding sites, primarily α4β2-nAChR sites.
Visual inspection of Figure 3a, the scattergram comparing the effects of chronic nicotine to chronic varenicline treatment, suggests that treatment with either of these drugs has a similar effect on cytisine-sensitive [125I]-epibatidine binding sites. The fact that 16 of the regions display higher binding after varenicline treatment, 10 of the regions display higher binding after nicotine treatment and 16 of the regions display the same binding is consistent with the visual inspection. Overall, binding measured after chronic varenicline treatment was modestly higher than that after nicotine treatment (6.2±2.7%, t42=2.27, P<0.05).
Visual inspection of Figures 3b and 3c suggest that the treatment with nicotine plus varenicline elicits larger increases in cytisine-sensitive [125I]-epibatidine binding sites than treatment with either drug alone. Indeed, treatment with both drugs elicited a larger increase in binding in 39 brain regions than treatment with nicotine alone (Fig 3b). Binding in four of the regions did not differ between the groups. Overall, binding was 17.9±1.8% higher in mice treated with both drugs (t42=9.93, P<0.001). Similarly, treatment with both drugs elicited a larger increase in binding in 34 brain regions, while binding following treatment with varenicline alone was higher in 5 regions Fig 3c). Binding in 4 brain regions did not differ between the treatments. Overall, binding was 11.7±2.2% higher in mice treated with both drugs (t42 = 5.36, P<0.001).
The observation that treatment with both nicotine and varenicline elicited a larger increase in binding sites than treatment with either drug alone is likely to have occurred because the doses chosen for nicotine and varenicline treatment do not by themselves elicit maximal up-regulation.
3.5.2 Cytisine-resistant [125I]-epibatidine binding sites, heteromeric nAChR sites, excluding α4β2-nAChR sites.
Visual inspection of Figure 3d, the scattergram comparing the effects of chronic nicotine to chronic varenicline treatment, suggests that treatment with varenicline has a larger effect on cytisine-resistant [125I]-epibatidine binding sites than does treatment with nicotine. Consistent with this impression is the fact that 11 of the brain regions displayed higher binding following varenicline treatment, while only 2 regions displayed higher binding following nicotine treatment. Binding in 4 regions did not differ between the treatments. Overall, binding in the varenicline treated mice was 17.1±4.8% greater than binding in nicotine treated mice (t16 = 3.57, P<0.005).
Visual inspection of Figure 3e, the scattergram comparing the effects of chronic nicotine to chronic nicotine plus varenicline treatment, also suggests that co-treatment with nicotine and varenicline has a larger effect on cytisine-resistant [125I]-epibatidine binding sites than does treatment with nicotine alone. As was the case for varenicline treatment, 11 of the brain regions displayed higher binding following treatment with both drugs, while only 2 regions displayed higher binding following nicotine treatment. Binding in 4 regions did not differ between the treatments. However, the difference in binding site densities between these two treatment groups (9.2±4.6%) was smaller than that between nicotine and varenicline treatments and was not statistically significant (t16 = 2.00, P>0.05).
Visual inspection of Figure 3f suggests that treatment with varenicline alone elicits similar changes in cytisine-resistant [125I]-epibatidine binding sites as does treatment with both drugs. Consistent with this impression is the observation that binding site densities were higher in 8 regions, lower in 5 regions and the same in 4 regions when the binding following varenicline treatment is compared to that following treatment with both drugs. Binding in regions from mice treated with varenicline alone tended to be higher (7.8±5.9%), but this difference was not statistically significant (t16 = 1.32, P>0.05).
This analysis indicates that significantly greater and more widely distributed increases in cytisine-resistant [125I]-epibatidine binding sites occurs following chronic varenicline treatment than following chronic nicotine treatment. However, treatment with both drugs appears to elicit changes intermediate between those of either drug alone.
3.5.3 [125I]-α-bungarotoxin binding sites, α7-nAChR sites
Visual inspection of Figure 3g conveys two major points: chronic nicotine treatment had little effect on the density of [125I]-α-bungarotoxin binding sites (illustrated by clustering of the points around zero at the X-axis) and chronic varenicline treatment elicited increases in the density of [125I]-α-bungarotoxin binding sites. This impression is reinforced by the observation that 28 of the regions in mice treated with varenicline displayed higher binding than that in nicotine treated mice, while no regions displayed higher binding following nicotine treatment. Binding in nine regions did not differ between the two treatment groups. Overall, [125I]-α-bungarotoxin binding in varenicline treated mice was 16.6±2.8% higher than in nicotine treated mice (t33 = 5.83, P<0.001).
Visual inspection of Figure 3h conveys a similar impression to that of Figure 3g in for the comparison of effects of nicotine compared to nicotine plus varenicline treatments. [125I]-α-Bungarotoxin binding site density was greater in 28 regions following treatment with both drugs and higher in 3 regions following nicotine treatment. No differences were noted in 6 regions. Overall, [125I]-α-bungarotoxin binding was significantly higher in mice treated with both drugs (9.6±1.5%, t33 = 6.46, P<0.001), although the magnitude of the difference was somewhat smaller than that between nicotine and varenicline treated mice.
Visual inspection of Figure 3I suggests that [125I]-α-bungarotoxin binding following treatment with both drugs is similar to that following treatment with varenicline alone. However, the observation that a significant number of points that fall below the line (20) indicate that treatment with varenicline alone elicits larger increases in [125I]-α-bungarotoxin binding sites than treatment with both drugs (6 regions have higher binding following co-treatment and 8 regions do not differ). Indeed, the overall 6.9±2.7% higher binding noted for mice treated with varenicline alone is significant (t33 = 2.58, P<0.02).
This analysis indicates that significantly greater and more widely distributed increases occurs in [125I]-α-bungarotoxin binding sites following chronic varenicline treatment than following chronic nicotine treatment. However, treatment with both drugs appears to elicit changes intermediate between those of either drug alone. This pattern is similar to that observed for the cytisine resistant [125I]-epibatidine binding sites.
3.5.4 [125I]-α-Conotoxin MII binding sites, primarily α6β2*nAChR sites with some α3β2*-nAChR sites.
Visual inspection of Figure 3j conveys a significantly different pattern for the effects of chronic nicotine or varenicline treatment on [125I]-α-conotoxin MII binding sites than those for the other ligands. Chronic drug treatment tends to decrease the density of these binding sites. In addition, the effects of chronic nicotine and varenicline appear to be very similar with 4 regions showing greater response to nicotine treatment and 4 regions showing greater response to varenicline treatment. Overall, the 0.9±3.7% difference between the two treatment groups is not significant (t7 = 0.24, P>0.05).
As suggested by the visual inspection of Figures 3k and 3l, treatment with both nicotine and varenicline has a modestly larger effect on [125I]-α-conotoxin MII binding sites than treatment with either drug alone. Following treatment with both drugs a larger decrease in [125I]-α-conotoxin MII binding sites binding sites was noted in 7 regions compared to mice treated with nicotine alone and in 6 regions compared to mice treated with varenicline alone. However, the 8.4±4.9% (t7=1.72, P>0.05) and 9.3±4.8% (t7 = 1.93, P>0.05) differences are not statistically significant.
This analysis suggests that chronic nicotine and varenicline treatment have quite similar effects on the regulation of [125I]-α-conotoxin MII binding sites.
4. Discussion
4.1 Upregulation of α4β2*-nAChR following varenicline treatment
Two chronic treatment doses of varenicline (0.12 and 1.0 mg/kg/hr) were used to determine an appropriate dose for more detailed comparison to the effects of chronic treatment with nicotine, varenicline or both drugs. Analysis of the effects of treatment using ligand binding to tissue homogenates with these two doses of varenicline established that both doses elicited up-regulation of cytisine-sensitive [125I]-epibatidine binding (primarily α4β2*-nAChR sites). The extent of up-regulation achieved following treatment with the lower dose was not maximal, subsequently this dose was chosen for the additional experiments. The results obtained confirm previous reports (Turner, Castellano et al, 2011) indicating that chronic varenicline treatment (0.075 mg/kg/hr) in mice (F1 hybrid 129svJ:C57/6J) resulted in significant upregulation of α4β2*-nAChR sites in cortex, striatum and hippocampus with no change in thalamus after a 24 hour withdrawal. We observed the same pattern of upregulation using C57Bl/6J mice and 0.12 or 1.0 mg/kg/hr varenicline with a 24 hr withdrawal (Fig 1). The effects of sub-maximal varenicline (0.12 mg/kg/hr) treatment on the α4β2*-nAChR sites are very similar to those observed following a sub-maximal dose of chronic nicotine (1.0 mg/kg/hr) as measured here by quantitative autoradiography of 43 brain regions (Table 3 and Fig 3A).
4.2. Plasma and brain levels of nicotine and varenicline
In the current study, we compared nicotine and varenicline in chronic treatment protocols in C57Bl/6J mice that elicited similar extents of upregulation for the α4β2-nAChR subtype in order to compare effects of these equipotent doses. The doses chosen (1.0 mg/kg/hr nicotine and 0.12 mg/kg/hr varenicline) are considerably higher than doses that would be used for humans for reasons discussed below. Steady-state plasma levels of nicotine (225±34.4 ng/ml) were approximately 4-fold higher than those of varenicline (57.9±9.9 ng/ml) consistent with the lower chronic treatment dose but slower metabolism of varenicline relative to nicotine. Both drugs had higher levels in brain than plasma (ratio of 1.7 for nicotine and 4.5 for varenicline), in agreement with previous studies in rats reporting nicotine brain:plasma ratios of 2.5 – 5.0 (Rowell and Li 1997; Vieira-Brock et al, 2011; Doura et al, 2008; Hussman, Turner et al, 2012; Ghosheh et al., 2001) and varenicline brain:plasma ratio of 1.5 (Hussmann, Turner et al, 2012). Our results for plasma nicotine are somewhat higher (225 ng/ml) than a previous report (40-80 ng/ml) after similar chronic treatment protocols (Marks, Rowell et al., 2004). Plasma levels measured after this treatment are about 5-fold higher than found in humans on smoking cessation treatments (Dobrinas et al, 2011).
The plasma and brain levels of drugs in various mammalian species are affected by numerous factors, such as route of administration and metabolism rates. Humans require much lower doses than rodents to achieve similar plasma levels. For example, plasma level of nicotine measured 2 hr after application of a Nicoderm® (21mg/24hr) patch (average weight 70 kg or ~0.3 mg/kg/day) was 44 ng/ml in humans (Shakleya and Huestis, 2009), while a rat plasma level of 43 ng/ml was measured after sc nicotine infusion of 2.4 mg/kg/day (Rowell and Li, 1997). Other comparable studies have reported plasma levels of nicotine in rats of 146-313 ng/ml after treatment with 6.0 mg/kg/day (Hussman, Turner et al, 2012; Doura et al, 2008) and 6.5 ng/ml after 0.8 mg/kg/day ((Ghosheh et al, 2001). Mice require even higher doses of nicotine (12-24 mg/kg/day) to achieve similar plasma levels (40-80 ng/ml) than do rats or humans (Marks, Rowell et al., 2004; Matta et al, 2007). Another study reported nicotine plasma levels of 160 ng/ml in C57Bl/6 mice after a single sc dose of 1 mg/kg (Siu and Tyndale, 2007). In a smoking cessation trial with human smokers, pre-quit plasma levels of up to 52 ng/ml were measured (Dobrinas et al, 2011), indicating that plasma levels in the same range as measured in a number of rodent studies are relevant.
Among species, varenicline metabolism rates differ. As for nicotine, mice have much higher rates of clearance of varenicline than rats and humans (t ½ of 1.4, 4, and 17.6 hr respectively) (Obach et al, 2006). In humans, a single oral dose (1 mg or ~0.014 mg/kg assuming 70 kg as average weight) of varenicline results in a plasma level of 4-6 ng/ml (Obach et al, 2006; Al-Haj et al, 2013). In a smoking cessation study of humans taking varenicline, plasma levels of up to 20 ng/ml were reported (Dobrinas et al, 2011). In mice, a single oral dose of 3 mg (or ~120 mg/kg assuming an average mouse weight of 25 g), resulted in a peak plasma level of 293 ng/ml (Obach et al, 2006), possibly indicating that a pharmacologically equivalent dose for a mouse is many times higher than a human dose. In rats treated with varenicline (1.2 mg/kg/day), plasma levels were 98 ng/ml and brain levels, 143 ng/ml (Hussmann, Turner et al, 2012).
4.3. Regulation of α4β2*-nAChR
Autoradiographic analyses demonstrated that the regional pattern and the extent of up-regulation of cytisine-sensitive [125I]-epibatidine binding (primarily α4β2*-nAChR sites) following chronic treatment with nicotine and varenicline were very similar. In addition, treatment with both drugs resulted in an additional, but non-additive, increase in binding site density. The relative resistance of thalamic nuclei to up-regulation in contrast to the substantial increases noted for cortical and hippocampal sites determined autoradiographically are consistent with the results obtained with tissue homogenates. The similarity of results obtained following nicotine and varenicline treatment suggest that similar mechanisms underlie the changes in α4β2*nAChR expression following these chronic treatments. This assertion is supported by the observation that changes in receptor expression following treatment with both drugs are not additive, but are similar to the maximum increase noted for nicotine infusion (Marks, McClure-Begley et al. 2011).
4.4. Regulation of non-α4β2*-nAChR
The effects of chronic varenicline treatment on the expression of nAChR sites other than α4β2*-nAChR have not been previously reported and these effects are significant new findings.
[125I]-α-conotoxin MII binding sites (measuring primarily α6β2β3*-nAChR sites plus some α3β2*-nAChR sites) were found to be similarly affected by chronic varenicline or nicotine treatments. However, as reported previously for chronic nicotine treatment (Perez, Bordia et al. 2008, Perez, Ly et al. 2012, Marks, Grady et al. 2014), chronic nicotine as well as chronic varenicline treatment differentially decreases the expression of [125I]-α-conotoxin MII binding sites. The magnitude of the changes elicited by these two drugs was similar and co-treatment resulted in modestly larger decreases. These results also suggest similar mechanisms of action.
Chronic varenicline treatment elicited significantly more changes in cytisine-resistant [125I]-epibatidine binding than did chronic nicotine treatment. It should be noted that receptors measured as cytisine-resistant [125I]-epibatidine binding sites include several nAChR subtypes such as α2*-nAChR, α3β2*-nAChR, α3β4*-nAChR, α6β2β3*nAChR so that care must be taken in evaluating the effects of the drug treatments. Areas have been color-coded for the predominant subtype in Figure 3.
Neither nicotine nor varenicline treatment elicited significant changes in cytisine-resistant [125I]-epibatidine binding in brain regions containing α6*-nAChR sites, including limbic and visual areas.
Significant differences were noted between the effects of nicotine and varenicline in regions known to contain α3β4*-nAChR such as interpeduncular nucleus and the dorsal cortex of the inferior colliculus. Chronic varenicline treatment elicited increases in several of these brain areas know to express α3β4*-nAChR, while nicotine treatment did not. This subtype is rarely affected by nicotine treatment in vivo (Peng, Gerzanich et al. 1997, Nguyen, Rasmussen et al. 2003). Thus it appears that varenicline has a larger effect on expression of α3β4*-nAChR than does nicotine.
Chronic treatment with either nicotine or varenicline elicited similar, significant increases cytisine-resistant [125I]-epibatidine binding in both the glomerular cell layer and the internal plexiform layer of the olfactory bulbs. These regions as well as IPN contain significant α2 mRNA expression (Whiteaker, Wilking et al. 2009). α2*-nAChR have been determined to be mostly α2β4* in olfactory bulb and α2β2* in IPN (Whiteaker, Wilking et al. 2009). Although these specific changes in olfactory bulb (possibly α2*-nAChR) sites may not be directly relevant in modulating nicotine dependence and withdrawal, there is evidence that the α2 subunit expressed in the IPN modulates somatic signs of nicotine withdrawal (Salas, Sturm et al. 2009). However, unlike the relatively simple receptor composition in olfactory bulbs, IPN contains a wide diversity of nAChR subtypes, including a dense expression of α3β4*-nAChR. Consequently, evaluation of the effects of chronic treatment on α2*-nAChR in IPN with the methods used in this study is not possible.
Chronic nicotine treatment by intravenous infusion has previously been shown to have little effect on [125I]-α-bungarotoxin binding in C57BL/6 mice (Pauly, Marks et al. 1991). That observation is confirmed in the present study. In contrast, chronic varenicline treatment elicited an overall increase in [125I]-α-bungarotoxin binding; the magnitude of the increase varied among brain regions. As was the case with cytisine resistant [125I]-epibatidine binding, co-treatment with nicotine and varenicline tended to reduce the up-regulation of [125I]-α-bungarotoxin binding elicited by varenicline treatment alone.
Varenicline is an effective agonist at heterologously expressed α3β4-nAChR and α7-nAChR (Campling, Kuryatov et al. 2013) so it may not be surprising that chronic varenicline treatment alters the expression of these receptors. This modulation indicates that under the conditions of the current experiment the concentrations of varenicline are adequate to elicit regulatory changes in these receptor subtypes. Experiments with cells expressing α3β4-nAChR or α7-nAChR have demonstrated that nicotine-induced increases in expression can occur, but drug concentrations attained in vivo may not be sufficiently high to trigger the responses (Peng, Gerzanich et al. 1997, Nguyen, Rasmussen et al. 2003, Moretti, Mugnaini et al. 2010).
The differential effect of chronic varenicline treatment, especially the increased expression of α3β4*-nAChR sites, may partially explain the difference in tolerance development observed between nicotine-treated and varenicline-treated mice. Although mice do become tolerant to the effects of nicotine following chronic treatment with the drug, tolerance to the tests employed in the current study is not usually observed for C57BL/6J mice at the 1.0 mg/kg/hr dose. The tolerance to acutely administered nicotine following chronic varenicline treatment is relatively modest, but the fact that this differs from nicotine-treated mice suggests that the varenicline-induced changes in α3β4*-nAChR may contribute to the reduced responsiveness.
If varenicline treatment elicits similar selective changes in distinct receptor subtypes in humans as it does in mice, these differential changes may contribute to the somewhat different pharmacology and efficacy as a smoking cessation aide between nicotine and varenicline. Furthermore, differing receptor interactions may suggest reasons for the improved efficacy seen with some nicotine/varenicline co-treatment approaches. It is unknown whether the level of upregulation of various subtypes of nAChR supported by chronic nicotine and/or varenicline maintains craving or causes any of the various reported side effects of varenicline. However, given the lowered upregulation tendency of the combination treatment in mice for non-α4β2 and α7 sites, using co-treatment may decrease some side effects.
Highlights.
Regulation of nAChR binding sites by chronic treatment with varenicline ± nicotine
Varenicline promotes up-regulation of α4β2-nAChR sites, similar to nicotine
Varenicline promotes more up-regulation of non-α4β2-nAChR sites than nicotine
Varenicline promotes more up-regulation of α7-nAChR sites than nicotine
Varenicline promotes down-regulation of α6β2 sites, similar to nicotine
Acknowledgements
This project was supported by Grants DA003194, DA012242, DA015663 and DA019375 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Chemical Compounds:
Nicotine (PubChem CID: 942)
Varenicline (PubChen CID: 5310966)
Epibatidine (PubChem CID: 3073763)
Cytisine (PubChem CID: 10235)
References
- Al-Haj A, Alawi M, Arafat T, Hourani MK. Method Development, validation and bioequivalence in human plasma by liquid chromatography tandem mass spectrometry. J. Chromatogr B Analyt. Technol. Biomed. Life Sci. 2013;931:134–139. doi: 10.1016/j.jchromb.2013.05.019. [DOI] [PubMed] [Google Scholar]
- Baddick CG, Marks MJ. An autoradiographic survey of mouse brain nicotinic acetylcholine receptors defined by null mutants. Biochem Pharmacol. 2011;82(8):828–841. doi: 10.1016/j.bcp.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benwell ME, Balfour DJ, Anderson JM. Evidence that tobacco smoking increases the density of (−)-[3H]nicotine binding sites in human brain. J Neurochem. 1988;50(4):1243–1247. doi: 10.1111/j.1471-4159.1988.tb10600.x. [DOI] [PubMed] [Google Scholar]
- Campling BG, Kuryatov A, Lindstrom J. Acute activation, desensitization and smoldering activation of human acetylcholine receptors. PLoS One. 2013;8(11):e79653. doi: 10.1371/journal.pone.0079653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, Sands SB, Davis TI, Lebel LA, Fox CB, Shrikhande A, Heym JH, Schaeffer E, Rollema H, Lu Y, Mansbach RS, Chambers LK, Rovetti CC, Schulz DW, Tingley FD, 3rd, O’Neill BT. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005;48(10):3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- Dobrinas M, Choong E, Noetzli M, Cornuz J, Ansermot N, Eap CB. Quantification of nicotine, cotinine, trans-3°-hydroxycotinine and varenicline in human plasma by a sensitive and specific UPLC-tandem mass-spectrometry procedure for a clinical study on smoking cessation. J. Chromatogr B Analyt. Technol. Biomed. Life Sci. 2011;879(30):3574–3582. doi: 10.1016/j.jchromb.2011.09.046. [DOI] [PubMed] [Google Scholar]
- Doura MB, Gold AB, Keller AB, Perry DC. Adult and periadolescent rats differ in expression of nicotinic cholinergic receptor subtypes and in the response of these subtypes to chronic nicotine exposure. Brain Research. 2008;1215:40–52. doi: 10.1016/j.brainres.2008.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbert JO, Burke MV, Hays JT, Hurt RD. Combination treatment with varenicline and nicotine replacement therapy. Nicotine Tob Res. 2009;11(5):572–576. doi: 10.1093/ntr/ntp042. [DOI] [PubMed] [Google Scholar]
- Frahm S, Slimak MA, Ferrarese L, Santos-Torres J, Antolin-Fontes B, Auer S, Filkin S, Pons S, Fontaine JF, Tsetlin V, Maskos U, Ibanez-Tallon I. Aversion to nicotine is regulated by the balanced activity of beta4 and alpha5 nicotinic receptor subunits in the medial habenula. Neuron. 2011;70(3):522–535. doi: 10.1016/j.neuron.2011.04.013. [DOI] [PubMed] [Google Scholar]
- George O, Lloyd A, Carroll FI, Damaj MI, Koob GF. Varenicline blocks nicotine intake in rats with extended access to nicotine self-administration. Psychopharmacology (Berl) 2011;213(4):715–722. doi: 10.1007/s00213-010-2024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosheh OA, Dwoskin LP, Miller DK, Crooks PA. Accumulation of nicotine and its metabolites in rat brain after intermittent or continuous peripheral administration of [2′-14C]nicotine. Drug Metabolism and Disposition. 2001;29:645–651. [PubMed] [Google Scholar]
- Grady SR, Drenan RM, Breining SR, Yohannes D, Wageman CR, Fedorov NB, McKinney S, Whiteaker P, Bencherif M, Lester HA, Marks MJ. Structural differences determine the relative selectivity of nicotinic compounds for native alpha 4 beta 2*-, alpha 6 beta 2*-, alpha 3 beta 4*- and alpha 7-nicotine acetylcholine receptors. Neuropharmacology. 2010;58(7):1054–1066. doi: 10.1016/j.neuropharm.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek P, McRobbie HJ, Myers KE, Stapleton J, Dhanji AR. Use of varenicline for 4 weeks before quitting smoking: decrease in ad lib smoking and increase in smoking cessation rates. Arch Intern Med. 2011;171(8):770–777. doi: 10.1001/archinternmed.2011.138. [DOI] [PubMed] [Google Scholar]
- Hajek P, Smith KM, Dhanji AR, McRobbie H. Is a combination of varenicline and nicotine patch more effective in helping smokers quit than varenicline alone? A randomised controlled trial. BMC Med. 2013;11:140. doi: 10.1186/1741-7015-11-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussmann GP, DeDominicis KE, Turner JR, Yasuda RP, Klehm J, Forcelli PA, Xiao Y, Richardson JR, Sahibzada N, Wolfe BB, Lindstrom J, Blendy JA, Kellar KJ. Chronic sazetidine-A maintains anxiolytic effects and slower weight gain following chronic nicotine without maintaining increased density of nicotinic receptors in rodent brain. J Neurochem. 2014;129(4):721–731. doi: 10.1111/jnc.12653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussmann GP, Turner JR, Lomazzo E, Venkatesh R, Cousins V, Xiao Y, Yasuda RP, Wolfe BB, Perry DC, Rezvani AH, Levin ED, Blendy JA, Kellar KJ. Chronic sazetidine-A at behaviorally active doses does not increase nicotinic cholinergic receptors in rodent brain. J Pharmacol Exp Ther. 2012;343(2):441–450. doi: 10.1124/jpet.112.198085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Sanjakdar SS, Muldoon PP, McIntosh JM, Damaj MI. The alpha3beta4* nicotinic acetylcholine receptor subtype mediates nicotine reward and physical nicotine withdrawal signs independently of the alpha5 subunit in the mouse. Neuropharmacology. 2013;70C:228–235. doi: 10.1016/j.neuropharm.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kralikova E, Kmetova A, Stepankova L, Zvolska K, Davis R, West R. Fifty-two-week continuous abstinence rates of smokers being treated with varenicline versus nicotine replacement therapy. Addiction. 2013;108(8):1497–1502. doi: 10.1111/add.12219. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Burch JB, Collins AC. Effects of chronic nicotine infusion on tolerance development and nicotinic receptors. J Pharmacol Exp Ther. 1983;226(3):817–825. [PubMed] [Google Scholar]
- Marks MJ, Grady SR, Salminen O, Paley MA, Wageman CR, McIntosh JM, Whiteaker P. alpha6beta2*-subtype nicotinic acetylcholine receptors are more sensitive than alpha4beta2*-subtype receptors to regulation by chronic nicotine administration. J Neurochem. 2014 doi: 10.1111/jnc.12721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MJ, McClure-Begley TD, Whiteaker P, Salminen O, Brown RW, Cooper J, Collins AC, Lindstrom JM. Increased nicotinic acetylcholine receptor protein underlies chronic nicotine-induced up-regulation of nicotinic agonist binding sites in mouse brain. J Pharmacol Exp Ther. 2011;337(1):187–200. doi: 10.1124/jpet.110.178236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MJ, Pauly JR, Gross SD, Deneris ES, Hermans-Borgmeyer I, Heinemann SF, Collins AC. Nicotine binding and nicotinic receptor subunit RNA after chronic nicotine treatment. J Neurosci. 1992;12(7):2765–2784. doi: 10.1523/JNEUROSCI.12-07-02765.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MJ, Rowell PP, Cao J-Z, Grady SR, McCallum SE, Collins AC. Subsets of acetylcholine-stimulated 86Rb+ efflux and [125I]-epibatidine binding sites in C57BL/6 mouse brain are differentially affected by chronic nicotine treatment. Neuropharmacology. 2004;46:1141–1157. doi: 10.1016/j.neuropharm.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Stitzel JA, Collins AC. Time course study of the effects of chronic nicotine infusion on drug response and brain receptors. J Pharmacol Exp Ther. 1985;235(3):619–628. [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, McCallum SE, Parameswaran N, Perkins KA, Picciotto MR, Quik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, Zirger JM. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl) 2007;190(3):269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- McCallum SE, Collins AC, Paylor R, Marks MJ. Deletion of the beta 2 nicotinic acetylcholine receptor subunit alters development of tolerance to nicotine and eliminates receptor upregulation. Psychopharmacology. 2006;184:314–327. doi: 10.1007/s00213-005-0076-6. [DOI] [PubMed] [Google Scholar]
- Moretti M, Mugnaini M, Tessari M, Zoli M, Gaimarri A, Manfredi I, Pistillo F, Clementi F, Gotti C. A comparative study of the effects of the intravenous self-administration or subcutaneous minipump infusion of nicotine on the expression of brain neuronal nicotinic receptor subtypes. Mol Pharmacol. 2010;78(2):287–296. doi: 10.1124/mol.110.064071. [DOI] [PubMed] [Google Scholar]
- Nguyen HN, Rasmussen BA, Perry DC. Subtype-selective up-regulation by chronic nicotine of high-affinity nicotinic receptors in rat brain demonstrated by receptor autoradiography. J Pharmacol Exp Ther. 2003;307(3):1090–1097. doi: 10.1124/jpet.103.056408. [DOI] [PubMed] [Google Scholar]
- Obach RS, Reed-Hagen AE, Krueger SS, Obach BJ, O’Connell TN, Zandi KS, Miller S, Coe JW. Metabolism and disposition of varenicline, a selective alpha4beta2 acetylcholine receptor partial agonist, in vivo and in vitro. Drug Metab Dispos. 2006;34(1):121–130. doi: 10.1124/dmd.105.006767. [DOI] [PubMed] [Google Scholar]
- Papke RL, Wecker L, Stitzel JA. Activation and inhibition of mouse muscle and neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther. 2010;333(2):501–518. doi: 10.1124/jpet.109.164566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauly JR, Marks MJ, Gross SD, Collins AC. An autoradiographic analysis of cholinergic receptors in mouse brain after chronic nicotine treatment. J Pharmacol Exp Ther. 1991;258(3):1127–1136. [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. Elsevier Academic Press; 2004. [Google Scholar]
- Peng X, Gerzanich V, Anand R, Wang F, Lindstrom J. Chronic nicotine treatment up-regulates alpha3 and alpha7 acetylcholine receptor subtypes expressed by the human neuroblastoma cell line SH-SY5Y. Mol Pharmacol. 1997;51(5):776–784. doi: 10.1124/mol.51.5.776. [DOI] [PubMed] [Google Scholar]
- Perez XA, Bordia T, McIntosh JM, Grady SR, Quik M. Long-term nicotine treatment differentially regulates striatal alpha6alpha4beta2* and alpha6(nonalpha4)beta2* nAChR expression and function. Mol Pharmacol. 2008;74(3):844–853. doi: 10.1124/mol.108.048843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez XA, Ly J, McIntosh JM, Quik M. Long-term nicotine exposure depresses dopamine release in nonhuman primate nucleus accumbens. J Pharmacol Exp Ther. 2012;342(2):335–344. doi: 10.1124/jpet.112.194084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry DC, Davila-Garcia MI, Stockmeier CA, Kellar KJ. Increased nicotinic receptors in brains from smokers: membrane binding and autoradiography studies. J Pharmacol Exp Ther. 1999;289(3):1545–1552. [PubMed] [Google Scholar]
- Picciotto MR, Kenny PJ. Molecular mechanisms underlying behaviors related to nicotine addiction. Cold Spring Harb Perspect Med. 2013;3(1):a012112. doi: 10.1101/cshperspect.a012112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell PP, Li M. Dose-response relationship for nicotine-induced up-regulation of rat brain nicotinic receptors. J. Neurochem. 1997;68:1982–1989. doi: 10.1046/j.1471-4159.1997.68051982.x. [DOI] [PubMed] [Google Scholar]
- Salas R, Pieri F, De Biasi M. Decreased signs of nicotine withdrawal in mice null for the beta4 nicotinic acetylcholine receptor subunit. J Neurosci. 2004;24(45):10035–10039. doi: 10.1523/JNEUROSCI.1939-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Sturm R, Boulter J, De Biasi M. Nicotinic receptors in the habenulo-interpeduncular system are necessary for nicotine withdrawal in mice. J Neurosci. 2009;29(10):3014–3018. doi: 10.1523/JNEUROSCI.4934-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RD, Kellar KJ. Nicotinic cholinergic receptor binding sites in the brain: regulation in vivo. Science. 1983;220(4593):214–216. doi: 10.1126/science.6828889. [DOI] [PubMed] [Google Scholar]
- Shakleya DM, Huestis MA. Simultaneous and sensitive measurement of nicotine, cotinine, trans-30-hydroxycotinine and nornicotine in human plasma by liquid chromatography-tandem mass spectrometry. J. Chromatogr B Analyt. Technol. Biomed. Life Sci. 2009;877:3537–3542. doi: 10.1016/j.jchromb.2009.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton JA, Watson L, Spirling LI, Smith R, Milbrandt A, Ratcliffe M, Sutherland G. Varenicline in the routine treatment of tobacco dependence: a pre-post comparison with nicotine replacement therapy and an evaluation in those with mental illness. Addiction. 2008;103(1):146–154. doi: 10.1111/j.1360-0443.2007.02083.x. [DOI] [PubMed] [Google Scholar]
- Stoker AK, Markou A. Unraveling the neurobiology of nicotine dependence using genetically engineered mice. Curr Opin Neurobiol. 2013;23(4):493–499. doi: 10.1016/j.conb.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu ECK, Tyndale RF. Characterization and comparison of nicotine and cotinine metabolism in vitro and in vivo in DBA/2 and C57BL/6 mice. Mol. Pharmacol. 2007;71:826–834. doi: 10.1124/mol.106.032086. [DOI] [PubMed] [Google Scholar]
- Tritto T, McCallum SE, Waddle SA, Hutton SR, Paylor R, Collins AC, Marks MJ. Null mutant analysis of responses to nicotine: deletion of beta2 nicotinic acetylcholine receptor subunit but not alpha7 subunit reduces sensitivity to nicotine-induced locomotor depression and hypothermia. Nicotine Tob Res. 2004;6(1):145–158. doi: 10.1080/14622200310001656966. [DOI] [PubMed] [Google Scholar]
- Turner JR, Castellano LM, Blendy JA. Nicotinic partial agonists varenicline and sazetidine-A have differential effects on affective behavior. J Pharmacol Exp Ther. 2010;334(2):665–672. doi: 10.1124/jpet.110.166280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JR, Castellano LM, Blendy JA. Parallel anxiolytic-like effects and upregulation of neuronal nicotinic acetylcholine receptors following chronic nicotine and varenicline. Nicotine Tob Res. 2011;13(1):41–46. doi: 10.1093/ntr/ntq206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira-Brock PL, Miller EI, Nielsen SM, Fleckenstein AE, Wilkins DG. Simultaneous quantification of nicotine metabolites in rat brain by liquid-chromatography-tandem mass spectrometry. J. Chromatogr B Analyt. Technol. Biomed. Life Sci. 2011;879:3465–3474. doi: 10.1016/j.jchromb.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteaker P, Cooper JF, Salminen O, Marks MJ, McClure-Begley TD, Brown RW, Collins AC, Lindstrom JM. Immunolabeling demonstrates the interdependence of mouse brain alpha4 and beta2 nicotinic acetylcholine receptor subunit expression. J Comp Neurol. 2006;499(6):1016–1038. doi: 10.1002/cne.21181. [DOI] [PubMed] [Google Scholar]
- Whiteaker P, Jimenez M, McIntosh JM, Collins AC, Marks MJ. Identification of a novel nicotinic binding site in mouse brain using [(125)I]-epibatidine. Br J Pharmacol. 2000;131(4):729–739. doi: 10.1038/sj.bjp.0703616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteaker P, McIntosh JM, Luo S, Collins AC, Marks MJ. 125I-alpha-conotoxin MII identifies a novel nicotinic acetylcholine receptor population in mouse brain. Mol Pharmacol. 2000;57(5):913–925. [PubMed] [Google Scholar]
- Whiteaker P, Peterson CG, Xu W, McIntosh JM, Paylor R, Beaudet AL, Collins AC, Marks MJ. Involvement of the alpha 3 subunit in central nicotinic binding populations. Journal of Neuroscience. 2002;22(7):2522–2529. doi: 10.1523/JNEUROSCI.22-07-02522.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteaker P, Wilking JA, Brown RW, Brennan RJ, Collins AC, Lindstrom JM, Boulter J. Pharmacological and immunochemical characterization of alpha2* nicotinic acetylcholine receptors (nAChRs) in mouse brain. Acta Pharmacol Sin. 2009;30(6):795–804. doi: 10.1038/aps.2009.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yohn NL, Turner JR, Blendy JA. Activation of alpha4beta2*/alpha6beta2* nicotinic receptors alleviates anxiety during nicotine withdrawal without upregulating nicotinic receptors. J Pharmacol Exp Ther. 2014;349(2):348–354. doi: 10.1124/jpet.113.211706. [DOI] [PMC free article] [PubMed] [Google Scholar]