Abstract
Background
Selective serotonin reuptake inhibitors (SSRIs) are widely prescribed for mood and other disorders. However, their neural effects are difficult to study due to patient compliance and drug history variability, and rarely studied in those prescribed SSRIs for non-mood disorders. Here we evaluated SSRI effects on neural volumetrics in depressed and nondepressed monkeys.
Methods
42 socially-housed cynomolgus monkeys were randomized to treatment balanced on pretreatment depressive behavior and body weight. Monkeys were trained for oral administration of placebo or 20mg/kg sertraline HCl daily for 18 months and depressive and anxious behavior recorded. Volumes of neural regions of interest in depression were measured in magnetic resonance images and analyzed by 2 (depressed, nondepressed) × 2 (placebo, sertraline) ANOVA.
Results
Sertraline reduced anxiety (p=0.04) but not depressive behavior (p=0.43). Left Brodmann’s Area (BA)32 was smaller in depressed than nondepressed monkeys (main effect of depression: p<0.05). Sertraline and depression status interacted to affect volumes of left anterior cingulate cortex (ACC), left BA24, right hippocampus (HC), and right anterior HC (sertraline X depression interactions: all p’s < 0.05). In the Placebo group, depressed monkeys had smaller right anterior HC and left ACC than nondepressed monkeys. In nondepressed monkeys, sertraline reduced right HC volume, especially right anterior HC volume. In depressed monkeys sertraline increased left ACC volume. In nondepressed monkeys, sertraline reduced left BA24 volumes resulting in smaller BA24 volumes in nondepressed than sertraline-treated depressed monkeys.
Conclusions
These observations suggest that SSRIs may differentially affect neural structures in depressed and nondepressed individuals.
Keywords: Depression, SSRI, Nonhuman Primate, Anterior Cingulate Cortex, Hippocampus, MRI
1. Introduction
Depression is prevalent, debilitating, and nearly twice as common in women as men (Kessler et al., 2003). Volumetric differences in neural structures between depressed and nondepressed individuals, as measured via magnetic resonance imaging (MRI), are widely reported. Among the most commonly reported differences are reduced volumes of hippocampus (HC), amygdala, and cingulate cortex in depressed individuals, although there is some variability in these observations (Arnone, McIntosh, Ebmeier, Munafo, & Anderson, 2012; Grieve, Korgaonkar, Koslow, Gordon, & Williams, 2013; Koolschijn, van Haren, Lensvelt-Mulders, Hulshoff Pol, & Kahn, 2009; McKinnon, Yucel, Nazarov, & MacQueen, 2009; Neumeister et al., 2005; Y. I. Sheline, 1996; Y. I. Sheline, Sanghavi, Mintun, & Gado, 1999; Tang et al., 2007). Factors such as age, sex, life stress history, number of bouts of depression, and medication history may contribute to this variability.
One potential mechanism through which antidepressant therapies promote remission is increased neurogenesis and synaptic connectivity through synaptogenesis and reorganization or reintegration of new neurons into depression neurocircuitry (Duman & Li, 2012; Mahar, Bambico, Mechawar, & Nobrega, 2014). A few studies suggest that neural volumes of major depressive disorder (MDD) patients that are medicated may differ from those who are unmedicated. In longitudinal studies following medication both no change and an increase in hippocampal volume have been reported (Frodl et al., 2008; Vythilingam et al., 2004); and an increase in dorsolateral prefrontal cortex has been reported (Smith, Chen, Baxter, Fort, & Lane, 2013). In case-control studies increased volumes of neural regions associated with medication have been reported for the body of the hippocampus (Malykhin, Carter, Seres, & Coupland, 2010), and dentate gyrus (Huang et al., 2013), but an apparent decrease in white matter volume in the left dorsolateral prefrontal cortex and left putamen (Zeng et al., 2012).
Selective serotonin reuptake inhibitors (SSRIs), including sertraline HCl (Zoloft®), are the third most prescribed drug in the United States (Pratt, Brody, & Gu, 2011). Use of these medications has increased 400% over the past 15 years, in part because antidepressants are prescribed for a number of disorders other than depression (Pratt et al., 2011). It has been estimated that 11% of Americans over 12 years of age take antidepressant medication. Antidepressant usage is most common in middle-aged women (40–59 years of age) (Pratt et al., 2011). Over 60% of those taking antidepressants have taken it for two years or more (Mojtabai & Olfson, 2011; Pratt et al., 2011). In addition to depression, SSRIs are prescribed for bulimia (McElroy, Guerdjikova, Mori, & O'Melia, 2012), hot flashes (Shams et al., 2014), obsessive compulsive disorder (Chouinard, 2006), stroke recovery (Mead et al., 2012) and sexual dysfunction (Moreland & Makela, 2005). Although widely prescribed for disorders other than anxiety and depression, there are no studies of the effects of SSRI on brain volumes in individuals without psychiatric diagnoses. Thus, antidepressant effects on neural volumes in nonpsychiatric populations may have important implications for public health.
Studies evaluating neural changes following prolonged antidepressant use are difficult to do under controlled experimental conditions in human subjects because of difficulties with compliance to treatment, and complex drug histories. Here we report the evaluation of the effects of long term SSRI treatment on volumes of specific brain regions in adult female cynomolgus monkeys (Macaca fascicularis), a well-established nonhuman primate (NHP) model of depression (Shively & Willard, 2012; Willard & Shively, 2012). Briefly, depressive behavior in socially housed female cynomolgus monkeys occurs in captivity without experimental manipulation. Socially subordinate females are more likely than dominants to display depressive behavior; however not all subordinates display depressive behavior and some socially dominant animals do also (Shively & Willard, 2012; Willard & Shively, 2012). Behavioral depression in adult female cynomolgus macaques appears similar to human depression in physiological, neurobiological, and behavioral characteristics, including reduced body mass, hypothalamic-pituitary-adrenal axis perturbations, autonomic dysfunction, increased cardiovascular disease risk, reduced hippocampal volume, altered serotonergic function, decreased activity levels, and increased mortality (Shively & Willard, 2012; Willard & Shively, 2012). The menstrual cycles of cynomolgus macaques are similar to those of women in terms of length and hormonal fluctuations. Behaviorally depressed monkeys have low concentrations of ovarian steroids, with preserved menses (Shively & Willard, 2012; Willard & Shively, 2012). The macaque hippocampus (HC) more closely parallels the cellular organization and connectivity patterns of the human hippocampus than does that of the rat (Amaral & Lavenex, 2007), and macaques have complex and differentiated cortical areas, similar to those of human beings, that are important in human depression (Carmichael, Clugnet, & Price, 1994; Machado, Snyder, Cherry, Lavenex, & Amaral, 2008). Our group has previously reported reduced anterior hippocampal volume in untreated, behaviorally depressed female cynomolgus macaques. Postmortem in vitro analysis (Willard, Friedman, Henkel, & Shively, 2009) and pre-mortem in vivo MRI measures (Willard, Daunais, Cline, & Shively, 2011) demonstrated region-specific reductions in hippocampal volume in depressed versus nondepressed females. The goal of the present study was to determine the effects of long-term treatment using a commonly prescribed antidepressant, sertraline HCl (Zoloft®), on the volume of depression–related neural regions in depressed and nondepressed NHPs. Examining the effects of sertraline in both depressed and nondepressed monkeys allowed us to test the hypothesis that sertraline treatment has differential effects on brain volumes depending on depression status.
2. Materials and Methods
2.1 Subjects
Forty-five adult, reproductive-aged female cynomolgus macaques were imported directly from Indonesia (Institut Pertanian Bogor, Bogor, Indonesia) and quarantined in single cages for one month. Following quarantine, the monkeys were housed in social groups of n = 4–5, in indoor pens (3.05m × 3.05m × 3.05m), in a climate-controlled building with outdoor exposure, 12/12 light/dark, and water ad libitum. All monkeys were fed a Western-like diet, designed to be similar to that consumed by some Americans, with 44% of calories from fat, and 0.29 mg/Cal cholesterol which is approximately equal to a human consumption of 500 mg cholesterol/2000 calories (Groban, Kitzman, Register, & Shively, 2014; Shively, Register, Higley, & Willard, 2014). The monkeys were all of reproductive age, approximately 15.7 ± 0.3 years of age, estimated from dentition, which approximates a human age of about 45 years. Over the course of the 3.5 year study, three animals died of causes unrelated to the experiment resulting in a sample size of 42. MRI images were not available for one animal, leaving a final sample size of 41. All procedures involving primates were conducted using protocols approved by the Institutional Animal Care and Use Committee of Wake Forest University Health Sciences and were in compliance with all institutional, state, and federal laws for the usage of primates in laboratory settings.
2.2. Experimental Design
The monkeys lived in these stable social groups for 18 months, during which depressive behavior was recorded. At the end of the 18 months, the monkeys were assigned by social group to either placebo (n=20) or sertraline (n=21) treatment balanced on body weight (BW), body mass index (BMI) and the rate of depressive behavior during the pretreatment period using stratified randomization (Table 1). This approach provides balance on variables that were measured as well as those that were not measured, because these variables are correlated with many other physiological characteristics (Kernan, Viscoli, Makuch, Brass, & Horwitz, 1999; Lachin, Matts, & Wei, 1988). There was also no difference in the groups in total plasma cholesterol, high density lipoprotein cholesterol, quality of ovarian function, and age, all of which could affect brain morphometry (Shively, Register, Appt, & Clarkson, 2015). The monkeys were treated with sertraline for 18 months.
Table 1.
Characteristics of the Study Population
| Variables | Placebo Non Depressedb n=12 |
Placebo Depressedb n=8 |
Sertraline Non Depressedb n=12 |
Sertraline Depressedb n=9 |
Sertraline | Depression |
|---|---|---|---|---|---|---|
| Characteristics of Study Population Prior to Treatmenta | ||||||
| Depressive Behavior freq/hr | 0.29 (0.51) |
1.95 (0.62) |
0.02 (0.51) |
1.51 (0.59) |
F[1,38]=0.40 p=0.53 |
F[1,38]=7.93 P=0.008 |
| BW kg | 3.86 (0.29) |
2.96 (0.22) |
3.92 (0.26) |
3.12 (0.07) |
F[1,38]=0.22 p=0.64 |
F[1,38]=12.13 P<0.001 |
| BMI kg/m2 | 50.8 (2.75) |
41.7 (2.54) |
44.1 (2.30) |
40.0 (1.31) |
F[1,38]=3.12 p=0.09 |
F[1,38]=7.54 p=0.01 |
| Characteristics of Study Population During the Treatment Phasea | ||||||
| Depressive Behavior freq/hr | 0.28 (0.84) |
5.11 (1.03) |
0.11 (0.84) |
6.44 (0.97) |
F[1,38]=0.40 p=0.53 |
F[1,38]=36.4 p<0.0001 |
| BW kg | 4.18 (0.35) |
3.06 (0.24) |
3.89 (0.28) |
3.03 (0.08) |
F=[1,38]=0.34 p≤0.56 |
F[1,38]=13.0 p≤0.01 |
| BMI kg/m2 | 52.8 (3.53) |
42.3 (2.62) |
44.6 (2.39) |
39.7 (1.32) |
F=[1,38]=3.9 p≤0.06 |
F=[1,38]=8.1 p=0.01 |
Determined by analysis of variance
Data depicted as mean (SEM)
BW:Body Weight; BMI: Body Mass Index
2.3. Oral Dosing
All monkeys were trained to comply with an oral dosing regimen. The monkeys were trained to exit their social group pens and enter a dosing cage where they were individually offered vehicle (vanilla pudding) orally from a metal dosing syringe. After consuming the vehicle, the monkeys were immediately released into their home cage. This initial dose training was completed in about two weeks. Following training, either 20 mg/kg sertraline HCl (Zoloft®) in vehicle, or vehicle alone (placebo) was administered orally daily at about 08:00 am for 18 months. Sertraline was chosen as it has little effect on body weight, unlike some commonly prescribed SSRIs, which is important since dose was based on body weight (Beyazyuz, Albayrak, Egilmez, Albayrak, & Beyazyuz, 2013). The dose was recalculated for current body weight 10 times during the 18 month period. Dose compliance was tracked daily as per cent of dose accepted. Monkeys in the placebo group were 99.6% with 10,107 doses; those in the sertraline group were 97.8% compliant with 11,262 doses.
2.4. Behavioral Observations
Behaviors indicative of depression and anxiety were recorded twice a week during 10-minute focal observations for 12 months prior to treatment, and during the last 12 months of the 18 month treatment phase (~33.3 hours/monkey). As in previous studies, depressive behavior was defined as a slumped body posture (head lower than shoulders), with open eyes (to distinguish this behavior from rest), accompanied by a lack of responsiveness to environmental stimuli (Shively, Laber-Laird, & Anton, 1997). This behavior was easily recognizable (Figure 1A) and inter-rater reliability, determined biannually, was ≥ 0.92 throughout the experiment. The average frequency/hour of depression during each phase was calculated from these observations. Examination of the distribution of rate of depression during the treatment phase revealed a bimodal distribution. Thus, we compared monkeys that exhibited depressive behavior an average of once or less per hour (n=24, 59%), with those that exhibited depressive behavior an average of more than once per hour (n=17, 41%). In two previous studies rates of depression were 38% (Shively et al., 1997) and 42% (Shively et al., 2005); thus the distribution in the present study was consistent with previous work. Previous studies carried out over a 25 year period have demonstrated that monkeys classified using this definition of behavioral depression also have reduced body mass, hypothalamic-pituitary-adrenal axis perturbations, autonomic dysfunction, increased coronary artery disease, dyslipidemia, poor ovarian function, reduced hippocampal volume, altered central serotonergic function, decreased activity levels, and increased mortality compared to their nondepressed counterparts (Shively & Willard, 2012; Willard & Shively, 2012).
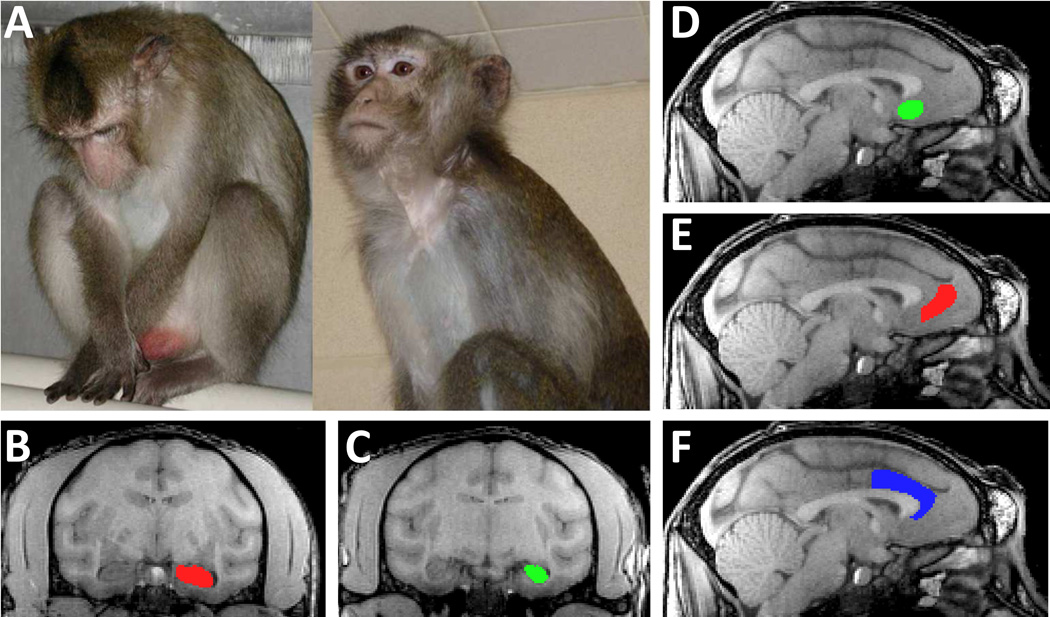
Figure 1.
A. Monkey exhibiting depressive like behavior (left) and alert behavior (right). B–C. A structural MRI image of a monkey brain in the coronal plane, depicting the anterior hippocampus (HC) (B) in which the hippocampal head is present, and the posterior HC (C). D–F. Sagittal images of a monkey brain depicting the anterior cingulate cortical regions measured, including Brodmann’s area (BA)25 (D), BA32 (E), and BA24 (F). See text for detailed description of anatomical delineations.
The rate of self-directed behaviors including scratching and self-grooming was recorded as a behavioral indicator of anxiety (Coleman, Robertson, & Bethea, 2011; Maestripieri, Schino, Aureli, & Troisi, 1992; Schino, Perretta, Taglioni, Monaco, & Troisi, 1996; Shively et al., 2015; Troisi, 2002; Troisi et al., 2000). Scratching was operationally defined as moving the fingertips repeatedly across the same skin area for a duration longer than one second, and self-grooming was defined as combing skin, hair, teeth or nails with hands.
2.5. Anthropometrics
BW and trunk length were measured at the end of the pretreatment phase, and again after 16 months of treatment. Body mass index (BMI) was estimated as the ratio of BW to the square of trunk length measured from the suprasternal notch to the pubic symphysis (kg/m2) (Shively, Register, & Clarkson, 2009).
2.6. Plasma and CSF Assays
Within a month of the treatment phase MRIs, four hours after their morning dose, the monkeys were sedated with 10–15 mg/kg ketamine HCl and blood samples were collected by femoral venipuncture for plasma sertraline and desmethylsertraline determinations. At the same time, and just prior to the onset of treatment, cerebrospinal fluid (CSF) samples were collected for determination of levels of the serotonin metabolite 5-hydroxyindole acetic acid (5-HIAA). CSF samples were collected by inserting a 22-gauge needle percutaneously into the cisterna magna space while the animal was sedated and restrained in lateral recumbency. Approximately 1.0–1.5 cc of spinal fluid was obtained and frozen at −70°C until metabolite determinations were made. These samples were collected less than 10 minutes following sedation.
Plasma levels of sertraline and desmethylsertraline were analyzed by gas chromatography as previously described (NMS Labs, Willow Grove, PA) (26). CSF levels of 5-HIAA were analyzed using high-pressure liquid chromatography (HPLC) with electrochemical detection as previously described (Shively et al., 2007). All intra- and inter-assay coefficients of variation were <10%.
2.7. MRI Image Acquisition and Analysis
At the end of the treatment phase, and while daily dosing continued, T1-weighed (T1w) images were acquired in the monkeys while under isoflurane anesthesia in head restraint to minimize motion artifacts. Images were acquired on a 3.0T GE Scanner (General Electric; Milwaukee, Wisconsin) equipped with a Twin Speed Gradient Coil in Zoom Mode (maximum gradient strength 4G/cm and a slew rate of 150 mT/m/msec) using an 8 channel NHP receive only RF coil with a form factor specifically designed for NHP imaging. Axial high-resolution T1w structural scans with isotropic voxels were acquired with a 3D spoiled gradient echo (3DSPGR) inversion recovery sequence (inversion time (TI): 600 ms; echo time (TE): 2.9 ms; repetition time (TR): 13.6 ms; flip angle: 15 degrees; receiver bandwidth: 31.25 kHz; in-plane matrix size: 256 × 256; field of view: 12.8 cm; in-plane resolution: 0.5 mm; slice thickness: 0.5 mm; number of slices: 128; acquisition time: about 14 min). Proton density (PDw) and T2 weighted (T2w) images were acquired separately with a fast-spin echo-pulse sequence (TE: 15 ms/80 ms respectively; TR: 8.5 sec; echo-train length: 8; receiver bandwidth: 15.63 kHz; voxel size: 0.5 × 0.5 × 1.0 mm; field of view: 12.8 × 12.8 × 6 cm; acquisition time for PDw and T2w images: about 16 min). T1w, T2w and PDw images were converted from DICOM to NIFTI format, and the T2w and PDw images were co-registered to the T1w image (Friston et al., 1995) allowing the images to be analyzed in a common native space.
2.8. Regions of Interest (ROI)
The volumes of 8 ROIs representing key components of depression neurocircuitry (Figure 1B–F) were measured bilaterally (16 ROIs per monkey) for a total of 656 ROIs overall. All ROIs were measured on T1w images, and coregistered T2w and PDw images were viewed when delineating difficult boundaries. ROI anatomical boundaries were defined based on macaque neuroanatomy (Carmichael et al., 1994; Machado et al., 2008) with Brodmann’s areas (BA) in parentheses: whole HC, anterior and posterior HC, whole ACC, subgenual ventral ACC (BA25), dorsal ACC (BA24), rostral ACC (BA32), and amygdala. Whole, anterior and posterior HC ROIs were measured with boundaries defined as follows (Machado et al., 2008): Ventral/medial: white matter superior to parahippocampal cortex; Dorsal/lateral: temporal horn of the lateral ventricle; Anterior: 3rd slice posterior to optic chiasm; Posterior: fornix emerging from HC. The anterior HC was delineated from the posterior HC by the presence of the uncus, as in previous studies (Willard et al., 2011; Willard et al., 2009). Four ROIs were measured in the ACC representing the whole ACC, BA24 (dorsal ACC), BA25 (ventral subgenual ACC), and BA32 (rostral ACC) according to the following boundaries (Carmichael et al., 1994; Machado et al., 2008): Anterior: slice posterior to anterior-most slice in which the cingulate and rostral sulci are both visible; Posterior: area 25 - slice anterior to posterior-most image with rostral sulcus; area 24 - midpoint slice between anterior and posterior commissures; Ventral: fundus of rostral sulcus or fundus of corpus callosum sulcus; Dorsal: fundus of cingulate sulcus. The border between ROIs of BA24 and BA32 was delineated by a horizontal line drawn at the anterior-most slice with the genu of the corpus callosum present, whereas the border between ROIs of BA32 and BA25 was delineated by a vertical line drawn midway through the genu of the corpus callosum. Amygdala ROI boundaries were defined as follows: Anterior: slice where optic chiasm begins. Posterior: HC starts to emerge and lateral ventricle begins to take a more vertical position. Medial: set as the entire area of gray matter proximal to the optic tract. Lateral: white matter of temporal lobe located laterally to the amygdala Dorsal: unspecified area horizontally in line with the superior temporal sulcus. Ventral: extend to the bottom of the brain image, excluding when the parahippocampal gyrus emerges (Carmichael et al., 1994).
Regions of interest (ROIs) were identified and manually segmented using contouring tools in MRIcro software (Rorden & Brett, 2000). ROI volumes were computed by adding voxels within the ROI from each slice and multiplying by the voxel volume (0.125 mm3). All regions were segmented in the coronal plane and volumes calculated using MRIcro software (Rorden & Brett, 2000). All subsequent brain region volumes were normalized whole brain volume. Intraobserver and interobserver reliability were ≥ 98% and those who measured ROIs were blinded to treatment.
2.9. Statistical Analysis
The levels of CSF 5-HIAA were compared between the pretreatment and treatment phase using paired t-test. Anxious and depressive behavior was analyzed using a 2 (pretreatment, treatment phase) × 2 (placebo, sertraline group) mixed-models analysis of variance. The remaining dependent variables were analyzed by a 2 (nondepressed, depressed) × 2 (placebo, sertraline) ANOVA. 2 × 2 interactions were further analyzed with Duncan’s post hoc tests. All statistics were two-sided tests, with the alpha level set at p ≤ 0.05.
3. Results
3.1. Characteristics of the Study Population at the Time of Randomization to Treatment, and During Treatment (Table 1)
Monkeys assigned to the placebo and sertraline treatment groups were similar in BW, BMI, and depressive behavior rate at the time of randomization and during the treatment phase. Monkeys that were classified as depressed during the pretreatment phase had significantly lower BW and BMI compared to non-depressed animals, as well as higher rates of depressive behavior at the time of randomization and during the treatment phase. There were no significant depression X sertraline interactions at baseline or during treatment (all p’s > 0.10).
3.2. Circulating Levels of Sertraline and Desmethylsertraline and CSF Levels of 5-HIAA (Table 2A)
Table 2.
| A. Circulating Sertraline and Desmethylsertraline and CSF 5-hydroxyindole acetic acid (5-HIAA)a | ||||
|---|---|---|---|---|
| Placebo | Sertraline | |||
| Baseline | Treatment | Baseline | Treatment | |
| Plasma Sertraline | ----- | ----- | ------ | 132.0 (19.8) |
| Plasma Desmethylsertraline | ----- | ----- | ------ | 182.0 (30.0) |
| CSF 5-HIAA | 44.9 (3.5) | 44.8 (3.1) | 46.2 (3.7)b | 26.6 (2.38)b |
| B. Effects of Sertraline on Behavioral Indicators of Depression and Anxietyc | |||||
|---|---|---|---|---|---|
| Placebo | Sertraline | ||||
| Baseline | Treatment | Baseline | Treatment | Phase X Treatment | |
| Depressive Behavior | 1.06 (0.37) | 2.58 (0.69) | 0.69 (0.37) | 2.96 (0.69) | F[1,40]=0.64, p=0.43 |
| Anxious Behavior | 42.5 (4.10) | 41.8 (2.83) | 43.2 (4.06) | 35.4 (2.80) | F[1,40]=4.40, p=0.04 |
Mean (SEM) in ng/ml; CSF: Cerebrospinal fluid
t(20) = 9.97, p < 0.000001
Mean (SEM) in frequency/hour
Circulating concentrations of sertraline and desmethylsertraline indicate that the drug was successfully delivered and metabolized. CSF 5-HIAA decreased significantly (p<0.000001) during sertraline treatment indicating that the drug crossed the blood brain barrier and affected central serotonergic function. The magnitude of the decrease in CSF 5-HIAA in the sertraline treated group were similar to those observed in patients treated with sertraline (Y. Sheline, Bardgett, & Csernansky, 1997).
3.3. Effects of Sertraline on Behavioral Indicators of Depression and Anxiety (Table 2B)
Sertraline had no effect on rates of depressive behavior (p=0.43), although it significantly reduced behavioral indicators of anxiety (p=0.04) as recently described (Shively et al., 2015).
3.4. Regions of Interest Volumes
A complete list of means, SEMs, and statistical outcomes are presented in Table 3, and significant effects are graphed in Figures 2 and 3. There was no significant difference between depressed and non-depressed monkeys and no effect of sertraline treatment on whole brain volume.
Table 3.
Volumes of regions of interest in depressed and non-depressed monkeys following Sertraline HCl or placebo treatment.
| Brain Regiona |
Placebo | Sertraline | Depression | Sertraline | Depression X Sertraline Interaction |
|||
|---|---|---|---|---|---|---|---|---|
| Non- Depressed (n=12)b |
Depressed (n=8)b |
Non- Depressed (n=12)b |
Depressed (n=9)b |
|||||
| Whole Brain | 63,500 (1,438) | 61,657 (1,761) | 66,185 (1,438) | 64,420 (1,660) | F[1,37]=1.3 p=0.26 | F[1,37]=3.0 p=0.09 | F[1,37]=0.0 p=0.98 | |
| Left ACC | 0.54 (0.03)d | 0.48 (0.02)c | 0.51 (0.01) | 0.53 (0.02)d | F[1,37]=1.9 p=0.18 | F[1,37]=0.8 p=0.37 | F[1,37]=7.6 p=0.007 | |
| BA25 | 0.11 (0.01) | 0.11 (0.01) | 0.1(0.01) | 0.12 (0.01) | F[1,37]=2.2 p=0.15 | F[1,37]=0.0 p=0.91 | F[1,37]=1.9 p=0.17 | |
| BA32 | 0.24 (0.02) | 0.19 (0.02) | 0.25 (0.01) | 0.22 (0.01) | F[1,37]=5.2 p=0.03 | F[1,37]=1.5 p=0.23 | F[1,37]=0.6 p=0.46 | |
| BA24 | 0.18 (0.01)d | 0.17 (0.01) | 0.16 (0.01)c | 0.18 (0.01)d | F[1,37]=0.2 p=0.69 | F[1,37]=0.2 p=0.63 | F[1,37]=8.0 p=0.009 | |
| Right ACC | 0.54 (0.01) | 0.52 (0.02) | 0.50 (0.01) | 0.51 (0.02) | F[1,37]=0.2 p=0.65 | F[1,37]=3.4 p=0.07 | F[1,37]=0.5 p=0.48 | |
| BA25 | 0.12 (0.01) | 0.11 (0.01) | 0.11 (0.01) | 0.12 (0.01) | F[1,37]=0.0 p=0.98 | F[1,37]=0.4 p=0.53 | F[1,37]=1.4 p=0.24 | |
| BA32 | 0.23 (0.01) | 0.24 (0.02) | 0.23 (0.01) | 0.21 (0.02) | F[1,37]=0.1 p=0.74 | F[1,37]=0.8 p=0.39 | F[1,37]=0.4 p=0.50 | |
| BA24 | 0.19 (0.01) | 0.17 (0.01) | 0.17 (0.01) | 0.18 (0.01) | F[1,37]=0.4 p=0.52 | F[1,37]=1.1 p=0.30 | F[1,37]=2.9 p=0.10 | |
| Left HC | 0.54 (0.02) | 0.51 (0.02) | 0.50 (0.02) | 0.50 (0.02) | F[1,37]=0.6 p=0.44 | F[1,37]=2.1 p=0.15 | F[1,37]=0.38 p=0.54 | |
| Anterior | 0.29 (0.01) | 0.26 (0.02) | 0.26 (0.01) | 0.26 (0.02) | F[1,37]=0.9 p=0.36 | F[1,37]=0.5 p=0.49 | F[1,37]=0.7 p=0.42 | |
| Posterior | 0.26 (0.01) | 0.26 (0.01) | 0.24 (0.01) | 0.24 (0.01) | F[1,37]=0.2 p=0.89 | F[1,37]=2.9 p=0.10 | F[1,37]=0.0 p=0.99 | |
| Right HC | 0.56 (0.02)c | 0.52 (0.02) | 0.50 (0.02)d | 0.53 (0.02) | F[1,37]=0.2 p=0.89 | F[1,37]=3.4 p=0.07 | F[1,37]=4.9 p=0.03 | |
| Anterior | 0.33 (0.01)c | 0.28 (0.01)d | 0.26 (0.01)d | 0.30 (0.01) | F[1,37]=0.6 p=0.46 | F[1,37]=3.5 p=0.07 | F[1,37]=11.2 p=0.001 | |
| Posterior | 0.24 (0.01) | 0.25 (0.01) | 0.24 (0.01) | 0.23 (0.01) | F[1,37]=0.0 p=0.99 | F[1,37]=0.6 p=0.45 | F[1,37]=0.2 p=0.69 | |
| Left Amy | 0.33 (0.02) | 0.3 (0.02) | 0.3 (0.02) | 0.28 (0.02) | F[1,37]=1.2 p=0.28 | F[1,37]=1.0 p=0.32 | F[1,37]=0.26 p=0.61 | |
| Right Amy | 0.31 (0.01) | 0.29 (0.02) | 0.28 (0.0) | 0.27 (0.02) | F[1,37]=0.6 p=0.44 | F[1,37]=2.2 p=0.15 | F[1,37]=0.06 p=0.81 | |
Whole brain volume is depicted as mm3; regions of interest measures are expressed as average proportion of whole brain volume.
Data depicted as mean (SEM)
cell means marked by c are significantly different than those marked d using Duncan’s post hoc test.
ACC: Anterior Cingulate Cortex; BA: Brodmann’s Area; HC: Hippocampus; Amy: Amygdala
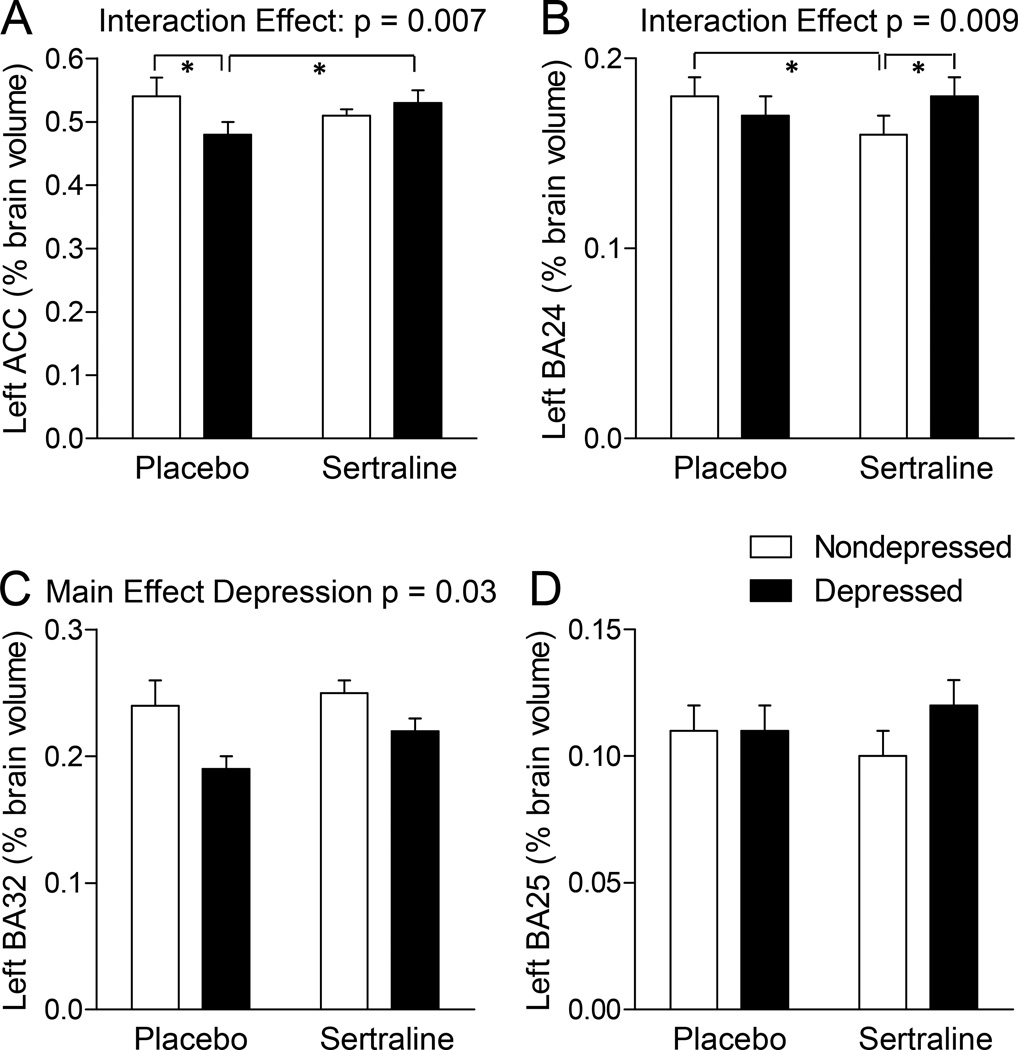
Figure 2. The Effects of Sertraline in the Anterior Cingulate Cortex (ACC) of Depressed and Nondepressed Monkeys.
A–B. There were significant sertraline X depression interactions in (A) the left ACC, and (B) Brodmann’s area (BA)24 of the left ACC. In these regions, sertraline increased volumes in depressed monkeys and decreased volumes in nondepressed monkeys. C. There was a significant main effect of depression in BA32 of the left ACC; depressed monkeys had smaller BA32 volumes than nondepressed monkeys. D. No effects of depression or sertraline were observed in BA25. Data are presented as average percent of total brain volume ± SEM. * p<0.05, Duncan’s post hoc test.
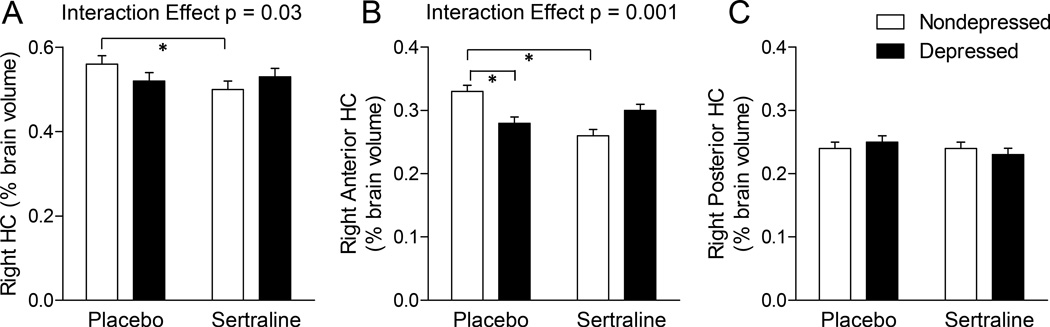
Figure 3. The Effects of Sertraline in the Hippocampus (HC) of Depressed and Nondepressed Monkeys.
A–B. There was a significant sertraline X depression interaction in the right HC (A), which appears to be driven by the same interaction in the anterior region of the right HC (B). Specifically, sertraline increased volume in depressed monkeys whereas in nondepressed monkeys sertraline decreased volume. C. No effects of depression or sertraline were observed in the right posterior HC. Data are presented as average percent of total brain volume ± SEM. * p<0.05, Duncan’s post hoc test.
Anterior Cingulate Cortex
In the left ACC, there was a significant sertraline X depression interaction (Figure 2A, p = 0.007). Post hoc tests suggested that in the placebo group, depressed monkeys had a smaller left ACC volume than nondepressed monkeys (post hoc p<0.05), and that in depressed monkeys, sertraline treatment resulted in larger left ACC volumes (post hoc p<0.05). The overall interaction in the left ACC appeared to be due in large part to effects on BA24 (Figure 2B, sertraline X depression interaction p= 0.009) but not on BA25 or BA32. In BA24 post hoc tests suggested that nondepressed monkeys treated with sertraline had smaller volumes than sertraline-treated depressed monkeys and placebo-treated nondepressed monkeys (both post hoc p’s<0.05). In addition, the left BA32 was smaller in depressed than nondepressed monkeys (Figure 2C, main effect of depression p = 0.03). There were no significant main or interaction effects of sertraline or depression observed in the right ACC.
Hippocampus
There were no significant main or interaction effects of sertraline or depression observed in the left HC. In the right HC there was a significant sertraline X depression interaction (Figure 3A, p = 0.03). Post hoc tests suggested that among nondepressed monkeys, those treated with sertraline had smaller right HC volumes (p<0.05). This effect appeared to be largely due to a significant sertraline X depression interaction in the anterior HC (Figure 3B, p = 0.002). Post hoc tests suggested that placebo-treated nondepressed monkeys had larger right anterior HC volumes than placebo-treated depressed monkeys, and nondepressed monkeys treated with sertraline (both p’s<0.05). In the whole, left, and right amygdala there were no significant main or interaction effects of sertraline or depression.
4. Discussion and Conclusions
This is the first published study examining the effects of long term SSRI treatment on neuroanatomical structures associated with depression in a placebo-controlled randomized preclinical trial. An important characteristic of the trial was randomization, which controls for pretreatment individual differences. Another important strength of the trial design was the stratified assignment to treatment groups balanced on pretreatment characteristics including BW, BMI, and rates of depressive behavior. This design feature allowed the analysis of the effects of treatment on individuals that did and did not display depressive behavior. Since sertraline and other SSRIs are widely prescribed for disorders other than depression, the effects of sertraline on neural structures in nondepressed individuals may be as important as the effects in depressed individuals. Another key feature of this study was the use of subjects that were naïve to antidepressant treatment prior to the onset of the study. A number of other subject characteristics or parameters of the study that might affect brain volumetrics were also tightly controlled including sex, diet, other drug exposures, light/day cycles, housing, and treatment compliance. Plasma concentrations of sertraline and desmethylsertraline demonstrated that the drug was successfully delivered via oral dosing and metabolized, and CSF monoamine metabolite concentrations evidenced effects on the CNS similar to those observed in human beings (Y. Sheline et al., 1997).
This preclinical trial was further strengthened by the use of a well-established NHP model of depression. A series of 19 papers over 15 years from our laboratory has established many similarities between the behavioral biology of depression in this NHP model and that of depressed human beings, and have been reviewed (Shively & Willard, 2012; Willard & Shively, 2012). More recently, the model has begun to be studied in other laboratories (Camus et al., 2014; Hennessy, McCowan, Jiang, & Capitanio, 2014; Xu et al., 2015). Shared characteristics of depression in human beings and macaques include risk factors such as low social status (Shively et al., 1997), loss due to separation from family (Suomi, Eisele, Grady, & Harlow, 1975), temperament (Hennessy et al., 2014), and adverse early experiences (Seay, Hansen, & Harlow, 1962). Similar to human beings, depressed monkeys have low levels of activity (Camus et al., 2014; Shively et al., 2008; Xu et al., 2015), low levels of social interaction, and anhedonia (Shively et al., 2005). We have described temporal patterns of depressive behavior (Shively et al., 2005). Over the course of a year we observed 3 general patterns of depressive behavior: One subgroup of monkeys rarely or never displayed depression (25% displayed depression in less than 25% of the months), one subgroup displayed depression intermittently (33% displayed depression in 25–74% of the months), and one subgroup displayed depression nearly all the time (42% displayed depression in 75% or more of the months). The number of months a monkey displays the depressed posture is highly correlated with the average time spent in, or frequency/hour of, the depressed posture throughout the phase (r = 0.84, p < 0.001) (Shively et al., 2005).
Depressive behavior in this NHP model is accompanied by many physiological characteristics which are shared with depressed human beings. These include perturbed HPA axis (Chilton et al., 2011; Shively, Musselman, & Willard, 2009; Shively, Williams, Laber-Laird, & Anton, 2002), increased cardiovascular disease (Shively & Clarkson, 2009), perturbed bone metabolism (Shively et al., 2005), high mortality (Shively et al., 2005), perturbed serotonin neurotransmission (Shively et al., 2006), and hippocampal atrophy (Willard et al., 2011; Willard et al., 2009) due to alterations in cell number and neuropil (Willard, Riddle, Forbes, & Shively, 2013), accompanied by altered expression of glial and synaptic markers (Willard, Hemby, Register, McIntosh, & Shively, 2014). But, for the purposes of this study, perhaps the most important trait shared by human and nonhuman primates, is the elaboration and differentiation of cortical areas important to human depression, such as Brodmann’s areas 24, 25, and 32, which are not well-represented in rodents or other animal models (Insel, 2007; Vogt et al., 2013). The use of this primate model allows a placebo-controlled long term randomized trial design in a preclinical model closely related to human beings, facilitating basic research to clinical translation.
Sertraline treatment resulted in a significant decrease in behavioral indices of anxiety between the pretreatment and treatment phases, analyzed using repeated measures (Shively et al., 2015). Sertraline is widely prescribed and has been shown to be efficacious for the treatment of anxiety disorders in people (Baldwin, Woods, Lawson, & Taylor, 2011; Sheehan & Kamijima, 2009). Sertraline also significantly decreased aggression and increased affiliative behaviors including grooming and sitting in physical contact in these monkeys, which were previously reported (Shively et al., 2014). Thus, sertraline treatment affected brain function, social, and mood-related behavior. The efficacy of sertraline and other SSRIs for depression has been questioned in part due to publication bias (Naudet et al., 2013; Turner, Matthews, Linardatos, Tell, & Rosenthal, 2008). Reported remission rates vary from 28 to <50%, and it is thought that there is little difference in efficacy between second-generation antidepressants (Gartlehner et al., 2011; Trivedi et al., 2006). Due to the small reported effect sizes of SSRIs for depression, this study could not be powered to detect a decrease in depressive behavior.
Depression was also associated with differences in neural volumes. Depressed animals had smaller BA32 than nondepressed monkeys. This is a new observation from preclinical models as Brodmann’s areas have not previously been studied in a nonhuman primate model of depression and are not well differentiated in the rodent brain. In the placebo group, depressed monkeys had smaller left ACC volumes than nondepressed monkeys, which is also novel observation in this model that reflects observations made in depressed patients(Arnone et al., 2012). Right anterior HC volumes were also reduced in depressed versus nondepressed monkeys in the placebo group. One of the most robust observations in major depressive disorder patients is reduced anterior hippocampal volume (Goodkind et al., 2015).
Most importantly, the effects of sertraline treatment were different in depressed versus nondepressed individuals. In nondepressed monkeys, sertraline reduced right HC volume, mostly the right anterior HC which was reduced by 21%. In depressed monkeys sertraline increased left ACC volume. However, in nondepressed monkeys, sertraline reduced left BA24 volumes. This resulted in nondepressed monkeys having smaller BA24 volumes than depressed monkeys in the sertraline treated group. A large literature documents myriad differences in the anatomy and physiology of depressed versus nondepressed brains, thus perhaps it is not surprising that they respond differently to medications. This observation is important in light of the growing proportion of antidepressant prescriptions without a psychiatric diagnosis (Chouinard, 2006; McElroy et al., 2012; Mead et al., 2012; Mojtabai & Olfson, 2011; Moreland & Makela, 2005; Pratt et al., 2011; Shams et al., 2014).
These observations are made even more intriguing in light of sertraline effects on the cardiovascular system in depressed and nondepressed individuals in this same study. We measured the extent of atherosclerosis, a principal cause of myocardial infarction, in the coronary arteries of these NHPs. Coronary artery atherosclerosis was more extensive in depressed than nondepressed monkeys, and more extensive in those treated with sertraline than placebo. These effects were additive; thus coronary artery atherosclerosis was most extensive in sertraline-treated depressed animals (Shively et al., 2015).
Depression is twice as common in women than men suggesting a role for ovarian function. Previously, using histomorphometry, we observed that depressed females have smaller anterior HC (Willard et al., 2009). However, in a previous MRI study of ovariectomized monkeys, both the anterior and posterior HC were smaller in depressed versus nondepressed individuals suggesting that the presence of ovaries protected the posterior HC from atrophy (Willard et al., 2011). In that study there were no laterality effects of depression such as those observed in the study of intact females reported here (Willard et al., 2011). Thus, the presence of ovaries appears to contribute to bilateral asymmetry in depression in females. This observation adds to a growing body of evidence that the presence of ovaries may modify the neural characteristics of depression.
There were no effects of chronic sertraline treatment on amygdala volumes. However, this may be due to assessment of the amygdala as a whole rather than dividing the amygdala into subnuclei, which was beyond the limits of the resolution of manual segmentation. Alternatively, some amygdala differences may be functional but not morphologic; thus, they may not be apparent in structural analyses. The literature is mixed as to whether amygdala volumes are associated with depression in human studies (Keller et al., 2008; Mervaala et al., 2000; Tamburo et al., 2009; von Gunten, Fox, Cipolotti, & Ron, 2000; Xia et al., 2004).
Limitations in this study may include those imposed by the use of manual segmentation to delineate neural regions. Techniques such as automated segmentation allow for much finer discrimination of gray versus white matter and anatomic bias. Using a combination of both manual and automated segmentation increases precision when measuring neural regions. Unfortunately, advancements in automated segmentation have been a challenge in animal models, due to a relative lack of MRI templates and digital atlases. As the quality and availability of templates and atlases increases, advanced analyses of the depression and drug effects on brain structure in primate models will be possible. In addition to advancements in segmenting techniques, these observations would be strengthened by pretreatment (baseline) MRIs. Randomization of subjects is used to control for preexperimental subject variation, thus mitigating the need for pretreatment values. Nonetheless, the availability of baseline volumes may increase power and precision. Finally, while the translational value of this model is compelling due to similarity to human beings in central nervous system structure and function, and characteristics of depression, the observations of different effects in nondepressed versus depressed brains needs to be confirmed in clinical studies.
In summary, a series of papers have described an extensive list of consequences of sertraline treatment, in an established NHP model, with physiologically relevant doses on anxiety, aggression, affiliation, the cardiovascular system, and serotonin neurotransmission, as well as the volumetric changes in neural areas critical to mood-related behavior and depressive disorders reported here. The data presented here suggest that the neural effects of SSRIs may be different in depressed subjects than in individuals prescribed these medications for disorders other than depression. Given the number of different disorders for which SSRIs are prescribed, these observations may have important implications for human health. Although these observations in NHPs are compelling, the clinical significance of differential effects of SSRIs on regional volumes needs to be further investigated in studies of patient populations. Volumetric differences in depressed versus nondepressed monkeys in HC and especially the ACC suggest this NHP model may be useful to assess efficacy of novel depression treatments.
Highlights.
SSRI effects on neuroanatomy were evaluated in depressed and nondepressed monkeys.
SSRIs reduced anxiety but did not affect depression.
Neural areas associated with human depression were smaller in depressed monkeys.
SSRIs differentially affected neural volumes in depressed vs. nondepressed monkeys.
Anterior cingulate & hippocampal areas were smallest in SSRI-treated nondepressed.
Acknowledgments
This research was funded in part by NIH HL087103, the Roena Kulynych Center for Memory and Cognition Research, and the Translational Science Institute of Wake Forest School of Medicine. We thank Haiying Chen MD, PhD for consultation on design and analysis matters.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
There are no financial interests or potential conflicts of interest.
Contributor Information
Stephanie L. Willard, Center for Neurobiology and Behavior, University of Pennsylvania
Beth Uberseder, Department of Pathology, Wake Forest School of Medicine.
Ashlee Clark, Neuroscience Graduate Program, Wake Forest School of Medicine.
James B. Daunais, Department of Physiology & Pharmacology, Wake Forest School of Medicine.
Warwick D. Johnston, Integrated Physiology and Pharmacology Program, Wake Forest School of Medicine
David Neely, Department of Pathology, Wake Forest School of Medicine.
Adreanna Massey, Department of Pathology, Wake Forest School of Medicine.
Jeff D. Williamson, Department of Internal Medicine, Wake Forest School of Medicine.
Robert A. Kraft, Department of Biomedical Engineering, Wake Forest School of Medicine
J. Daniel Bourland, Department of Radiation Oncology, Wake Forest School of Medicine
Sara R. Jones, Department of Physiology & Pharmacology, Wake Forest School of Medicine.
Carol A. Shively, Department of Pathology, Wake Forest School of Medicine.
References
- Amaral D, Lavenex P. Hippocampal neuroanatomy. In: Anderson P, Morris R, Amaral D, Bliss T, O’Keefe J, editors. The Hippocampus Book. New York: Oxford University Press; 2007. pp. 37–114. [Google Scholar]
- Arnone D, McIntosh AM, Ebmeier KP, Munafo MR, Anderson IM. Magnetic resonance imaging studies in unipolar depression: systematic review and meta-regression analyses. Eur Neuropsychopharmacol. 2012;22(1):1–16. doi: 10.1016/j.euroneuro.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Baldwin D, Woods R, Lawson R, Taylor D. Efficacy of drug treatments for generalised anxiety disorder: systematic review and meta-analysis. BMJ. 2011;342:d1199. doi: 10.1136/bmj.d1199. [DOI] [PubMed] [Google Scholar]
- Beyazyuz M, Albayrak Y, Egilmez OB, Albayrak N, Beyazyuz E. Relationship between SSRIs and Metabolic Syndrome Abnormalities in Patients with Generalized Anxiety Disorder: A Prospective Study. Psychiatry Investig. 2013;10(2):148–154. doi: 10.4306/pi.2013.10.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camus SM, Rochais C, Blois-Heulin C, Li Q, Hausberger M, Bezard E. Depressive-like behavioral profiles in captive-bred single- and socially-housed rhesus and cynomolgus macaques: a species comparison. Front Behav Neurosci. 2014;8:47. doi: 10.3389/fnbeh.2014.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael ST, Clugnet MC, Price JL. Central olfactory connections in the macaque monkey. J Comp Neurol. 1994;346(3):403–434. doi: 10.1002/cne.903460306. [DOI] [PubMed] [Google Scholar]
- Chilton FH, Lee TC, Willard SL, Ivester P, Sergeant S, Register TC, Shively CA. Depression and altered serum lipids in cynomolgus monkeys consuming a Western diet. Physiol Behav. 2011;104(2):222–227. doi: 10.1016/j.physbeh.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouinard G. The search for new off-label indications for antidepressant, antianxiety, antipsychotic and anticonvulsant drugs. J Psychiatry Neurosci. 2006;31(3):168–176. [PMC free article] [PubMed] [Google Scholar]
- Coleman K, Robertson ND, Bethea CL. Long-term ovariectomy alters social and anxious behaviors in semifree ranging Japanese macaques. Behav Brain Res. 2011;225(1):317–327. doi: 10.1016/j.bbr.2011.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Li N. A neurotrophic hypothesis of depression: role of synaptogenesis in the actions of NMDA receptor antagonists. Philos Trans R Soc Lond B Biol Sci. 2012;367(1601):2475–2484. doi: 10.1098/rstb.2011.0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiak RS, Turner R. Analysis of fMRI time-series revisited. Neuroimage. 1995;2(1):45–53. doi: 10.1006/nimg.1995.1007. [DOI] [PubMed] [Google Scholar]
- Frodl T, Jager M, Smajstrlova I, Born C, Bottlender R, Palladino T, Meisenzahl EM. Effect of hippocampal and amygdala volumes on clinical outcomes in major depression: a 3-year prospective magnetic resonance imaging study. J Psychiatry Neurosci. 2008;33(5):423–430. [PMC free article] [PubMed] [Google Scholar]
- Gartlehner G, Hansen RA, Morgan LC, Thaler K, Lux LJ, Van Noord M, Lohr KN. Second-Generation Antidepressants in the Pharmacologic Treatment of Adult Depression: An Update of the 2007 Comparative Effectiveness Review. Rockville (MD): Agency for Healthcare Research and Quality (US); 2011. AHRQ Comparative Effectiveness Reviews. [PubMed] [Google Scholar]
- Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, Jones-Hagata LB, Etkin A. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72(4):305–315. doi: 10.1001/jamapsychiatry.2014.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve SM, Korgaonkar MS, Koslow SH, Gordon E, Williams LM. Widespread reductions in gray matter volume in depression. Neuroimage Clin. 2013;3:332–339. doi: 10.1016/j.nicl.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groban L, Kitzman DW, Register TC, Shively CA. Effect of depression and sertraline treatment on cardiac function in female nonhuman primates. Psychosom Med. 2014;76(2):137–146. doi: 10.1097/PSY.0000000000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy MB, McCowan B, Jiang J, Capitanio JP. Depressive-like behavioral response of adult male rhesus monkeys during routine animal husbandry procedure. Front Behav Neurosci. 2014;8:309. doi: 10.3389/fnbeh.2014.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Coupland NJ, Lebel RM, Carter R, Seres P, Wilman AH, Malykhin NV. Structural changes in hippocampal subfields in major depressive disorder: a high-field magnetic resonance imaging study. Biol Psychiatry. 2013;74(1):62–68. doi: 10.1016/j.biopsych.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Insel TR. Neuroscience. Shining light on depression. Science. 2007;317(5839):757–758. doi: 10.1126/science.1147565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller J, Shen L, Gomez RG, Garrett A, Solvason HB, Reiss A, Schatzberg AF. Hippocampal and amygdalar volumes in psychotic and nonpsychotic unipolar depression. Am J Psychiatry. 2008;165(7):872–880. doi: 10.1176/appi.ajp.2008.07081257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernan WN, Viscoli CM, Makuch RW, Brass LM, Horwitz RI. Stratified randomization for clinical trials. J Clin Epidemiol. 1999;52(1):19–26. doi: 10.1016/s0895-4356(98)00138-3. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Wang PS. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289(23):3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Koolschijn PC, van Haren NE, Lensvelt-Mulders GJ, Hulshoff Pol HE, Kahn RS. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Hum Brain Mapp. 2009;30(11):3719–3735. doi: 10.1002/hbm.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachin JM, Matts JP, Wei LJ. Randomization in clinical trials: conclusions and recommendations. Control Clin Trials. 1988;9(4):365–374. doi: 10.1016/0197-2456(88)90049-9. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Snyder AZ, Cherry SR, Lavenex P, Amaral DG. Effects of neonatal amygdala or hippocampus lesions on resting brain metabolism in the macaque monkey: a microPET imaging study. Neuroimage. 2008;39(2):832–846. doi: 10.1016/j.neuroimage.2007.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestripieri D, Schino G, Aureli F, Troisi A. A Modest Proposal - Displacement Activities as an Indicator of Emotions in Primates. Animal Behaviour. 1992;44(5):967–979. doi: [Google Scholar]
- Mahar I, Bambico FR, Mechawar N, Nobrega JN. Stress, serotonin, and hippocampal neurogenesis in relation to depression and antidepressant effects. Neurosci Biobehav Rev. 2014;38:173–192. doi: 10.1016/j.neubiorev.2013.11.009. [DOI] [PubMed] [Google Scholar]
- Malykhin NV, Carter R, Seres P, Coupland NJ. Structural changes in the hippocampus in major depressive disorder: contributions of disease and treatment. J Psychiatry Neurosci. 2010;35(5):337–343. doi: 10.1503/jpn.100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy SL, Guerdjikova AI, Mori N, O'Melia AM. Current pharmacotherapy options for bulimia nervosa and binge eating disorder. Expert Opin Pharmacother. 2012;13(14):2015–2026. doi: 10.1517/14656566.2012.721781. [DOI] [PubMed] [Google Scholar]
- McKinnon MC, Yucel K, Nazarov A, MacQueen GM. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J Psychiatry Neurosci. 2009;34(1):41–54. [PMC free article] [PubMed] [Google Scholar]
- Mead GE, Hsieh CF, Lee R, Kutlubaev MA, Claxton A, Hankey GJ, Hackett ML. Selective serotonin reuptake inhibitors (SSRIs) for stroke recovery. Cochrane Database Syst Rev. 2012;11:Cd009286. doi: 10.1002/14651858.CD009286.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mervaala E, Fohr J, Kononen M, Valkonen-Korhonen M, Vainio P, Partanen K, Lehtonen J. Quantitative MRI of the hippocampus and amygdala in severe depression. Psychol Med. 2000;30(1):117–125. doi: 10.1017/s0033291799001567. [DOI] [PubMed] [Google Scholar]
- Mojtabai R, Olfson M. Proportion of antidepressants prescribed without a psychiatric diagnosis is growing. Health Aff (Millwood) 2011;30(8):1434–1442. doi: 10.1377/hlthaff.2010.1024. [DOI] [PubMed] [Google Scholar]
- Moreland AJ, Makela EH. Selective serotonin-reuptake inhibitors in the treatment of premature ejaculation. Ann Pharmacother. 2005;39(7–8):1296–1301. doi: 10.1345/aph.1E069. [DOI] [PubMed] [Google Scholar]
- Naudet F, Millet B, Charlier P, Reymann JM, Maria AS, Falissard B. Which placebo to cure depression? A thought-provoking network meta-analysis. BMC Med. 2013;11:230. doi: 10.1186/1741-7015-11-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumeister A, Wood S, Bonne O, Nugent AC, Luckenbaugh DA, Young T, Drevets WC. Reduced hippocampal volume in unmedicated, remitted patients with major depression versus control subjects. Biol Psychiatry. 2005;57(8):935–937. doi: 10.1016/j.biopsych.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Pratt LA, Brody DJ, Gu Q. Antidepressant use in persons aged 12 and over: United States, 2005–2008. NCHS Data Brief. 2011;(76):1–8. [PubMed] [Google Scholar]
- Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol. 2000;12(4):191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- Schino G, Perretta G, Taglioni AM, Monaco V, Troisi A. Primate displacement activities as an ethopharmacological model of anxiety. Anxiety. 1996;2(4):186–191. doi: 10.1002/(SICI)1522-7154(1996)2:4<186::AID-ANXI5>3.0.CO;2-M. doi: 10.1002/(sici)1522-7154(1996)2:4<186::aidanxi5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Seay B, Hansen E, Harlow HF. Mother-infant separation in monkeys. J Child Psychol Psychiatry. 1962;3:123–132. doi: 10.1111/j.1469-7610.1962.tb02047.x. [DOI] [PubMed] [Google Scholar]
- Shams T, Firwana B, Habib F, Alshahrani A, Alnouh B, Murad MH, Ferwana M. SSRIs for hot flashes: a systematic review and meta-analysis of randomized trials. J Gen Intern Med. 2014;29(1):204–213. doi: 10.1007/s11606-013-2535-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Kamijima K. An evidence-based review of the clinical use of sertraline in mood and anxiety disorders. Int Clin Psychopharmacol. 2009;24(2):43–60. doi: 10.1097/yic.0b013e3282f4b616. [DOI] [PubMed] [Google Scholar]
- Sheline Y, Bardgett ME, Csernansky JG. Correlated reductions in cerebrospinal fluid 5-HIAA and MHPG concentrations after treatment with selective serotonin reuptake inhibitors. J Clin Psychopharmacol. 1997;17(1):11–14. doi: 10.1097/00004714-199702000-00003. [DOI] [PubMed] [Google Scholar]
- Sheline YI. Hippocampal atrophy in major depression: a result of depression-induced neurotoxicity? Mol Psychiatry. 1996;1(4):298–299. [PubMed] [Google Scholar]
- Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci. 1999;19(12):5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively CA, Clarkson TB. The unique value of primate models in translational research. Nonhuman primate models of women's health: introduction and overview. Am J Primatol. 2009;71(9):715–721. doi: 10.1002/ajp.20720. [DOI] [PubMed] [Google Scholar]
- Shively CA, Friedman DP, Gage HD, Bounds MC, Brown-Proctor C, Blair JB, Buchheimer N. Behavioral depression and positron emission tomography-determined serotonin 1A receptor binding potential in cynomolgus monkeys. Arch Gen Psychiatry. 2006;63(4):396–403. doi: 10.1001/archpsyc.63.4.396. [DOI] [PubMed] [Google Scholar]
- Shively CA, Laber-Laird K, Anton RF. Behavior and physiology of social stress and depression in female cynomolgus monkeys. Biol Psychiatry. 1997;41(8):871–882. doi: 10.1016/S0006-3223(96)00185-0. [DOI] [PubMed] [Google Scholar]
- Shively CA, Musselman DL, Willard SL. Stress, depression, and coronary artery disease: modeling comorbidity in female primates. Neurosci Biobehav Rev. 2009;33(2):133–144. doi: 10.1016/j.neubiorev.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively CA, Register TC, Adams MR, Golden DL, Willard SL, Clarkson TB. Depressive behavior and coronary artery atherogenesis in adult female cynomolgus monkeys. Psychosom Med. 2008;70(6):637–645. doi: 10.1097/PSY.0b013e31817eaf0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively CA, Register TC, Appt SE, Clarkson TB. Effects of Long-Term Sertraline Treatment and Depression on Coronary Artery Atherosclerosis in Premenopausal Female Primates. Psychosom Med. 2015 doi: 10.1097/PSY.0000000000000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively CA, Register TC, Clarkson TB. Social stress, visceral obesity, and coronary artery atherosclerosis in female primates. Obesity (Silver Spring) 2009;17(8):1513–1520. doi: 10.1038/oby.2009.74. [DOI] [PubMed] [Google Scholar]
- Shively CA, Register TC, Friedman DP, Morgan TM, Thompson J, Lanier T. Social stress-associated depression in adult female cynomolgus monkeys (Macaca fascicularis) Biol Psychol. 2005;69(1):67–84. doi: 10.1016/j.biopsycho.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Shively CA, Register TC, Higley JD, Willard SL. Sertraline effects on cerebrospinal fluid monoamines and species-typical socioemotional behavior of female cynomolgus monkeys. Psychopharmacology (Berl) 2014;231(7):1409–1416. doi: 10.1007/s00213-013-3329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively CA, Willard SL. Behavioral and neurobiological characteristics of social stress versus depression in nonhuman primates. Exp Neurol. 2012;233(1):87–94. doi: 10.1016/j.expneurol.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively CA, Williams JK, Laber-Laird K, Anton RF. Depression and coronary artery atherosclerosis and reactivity in female cynomolgus monkeys. Psychosom Med. 2002;64(5):699–706. doi: 10.1097/01.psy.0000021951.59258.c7. [DOI] [PubMed] [Google Scholar]
- Shively CA, Wood CE, Register TC, Willard SL, Lees CJ, Chen H, Cline JM. Hormone therapy effects on social behavior and activity levels of surgically postmenopausal cynomolgus monkeys. Psychoneuroendocrinology. 2007;32(8–10):981–990. doi: 10.1016/j.psyneuen.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Smith R, Chen K, Baxter L, Fort C, Lane RD. Antidepressant effects of sertraline associated with volume increases in dorsolateral prefrontal cortex. J Affect Disord. 2013;146(3):414–419. doi: 10.1016/j.jad.2012.07.029. [DOI] [PubMed] [Google Scholar]
- Suomi SJ, Eisele CD, Grady SA, Harlow HF. Depressive behavior in adult monkeys following separation from family environment. J Abnorm Psychol. 1975;84(5):576–578. doi: 10.1037/h0077066. [DOI] [PubMed] [Google Scholar]
- Tamburo RJ, Siegle GJ, Stetten GD, Cois CA, Butters MA, Reynolds CF, 3rd, Aizenstein HJ. Amygdalae morphometry in late-life depression. Int J Geriatr Psychiatry. 2009;24(8):837–846. doi: 10.1002/gps.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Wang F, Xie G, Liu J, Li L, Su L, Blumberg HP. Reduced ventral anterior cingulate and amygdala volumes in medication-naive females with major depressive disorder: A voxel-based morphometric magnetic resonance imaging study. Psychiatry Res. 2007;156(1):83–86. doi: 10.1016/j.pscychresns.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Fava M. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- Troisi A. Displacement activities as a behavioral measure of stress in nonhuman primates and human subjects. Stress. 2002;5(1):47–54. doi: 10.1080/102538902900012378. [DOI] [PubMed] [Google Scholar]
- Troisi A, Belsanti S, Bucci AR, Mosco C, Sinti F, Verucci M. Affect regulation in alexithymia: an ethological study of displacement behavior during psychiatric interviews. J Nerv Ment Dis. 2000;188(1):13–18. doi: 10.1097/00005053-200001000-00003. [DOI] [PubMed] [Google Scholar]
- Turner EH, Matthews AM, Linardatos E, Tell RA, Rosenthal R. Selective publication of antidepressant trials and its influence on apparent efficacy. N Engl J Med. 2008;358(3):252–260. doi: 10.1056/NEJMsa065779. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Hof PR, Zilles K, Vogt LJ, Herold C, Palomero-Gallagher N. Cingulate area 32 homologies in mouse, rat, macaque and human: cytoarchitecture and receptor architecture. J Comp Neurol. 2013;521(18):4189–4204. doi: 10.1002/cne.23409. [DOI] [PubMed] [Google Scholar]
- von Gunten A, Fox NC, Cipolotti L, Ron MA. A volumetric study of hippocampus and amygdala in depressed patients with subjective memory problems. J Neuropsychiatry Clin Neurosci. 2000;12(4):493–498. doi: 10.1176/jnp.12.4.493. [DOI] [PubMed] [Google Scholar]
- Vythilingam M, Vermetten E, Anderson GM, Luckenbaugh D, Anderson ER, Snow J, Bremner JD. Hippocampal volume, memory, and cortisol status in major depressive disorder: effects of treatment. Biol Psychiatry. 2004;56(2):101–112. doi: 10.1016/j.biopsych.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Willard SL, Daunais JB, Cline JM, Shively CA. Hippocampal volume in postmenopausal cynomolgus macaques with behavioral depression. Menopause. 2011;18(5):582–586. doi: 10.1097/gme.0b013e3181fcb47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard SL, Friedman DP, Henkel CK, Shively CA. Anterior hippocampal volume is reduced in behaviorally depressed female cynomolgus macaques. Psychoneuroendocrinology. 2009;34(10):1469–1475. doi: 10.1016/j.psyneuen.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard SL, Hemby SE, Register TC, McIntosh S, Shively CA. Altered expression of glial and synaptic markers in the anterior hippocampus of behaviorally depressed female monkeys. Neurosci Lett. 2014;563:1–5. doi: 10.1016/j.neulet.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard SL, Riddle DR, Forbes ME, Shively CA. Cell number and neuropil alterations in subregions of the anterior hippocampus in a female monkey model of depression. Biol Psychiatry. 2013;74(12):890–897. doi: 10.1016/j.biopsych.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard SL, Shively CA. Modeling depression in adult female cynomolgus monkeys (Macaca fascicularis) Am J Primatol. 2012;74(6):528–542. doi: 10.1002/ajp.21013. [DOI] [PubMed] [Google Scholar]
- Xia J, Chen J, Zhou Y, Zhang J, Yang B, Xia L, Wang C. Volumetric MRI analysis of the amygdala and hippocampus in subjects with major depression. J Huazhong Univ Sci Technolog Med Sci. 2004;24(5):500–502. 506. doi: 10.1007/BF02831120. [DOI] [PubMed] [Google Scholar]
- Xu F, Wu Q, Xie L, Gong W, Zhang J, Zheng P, Xie P. Macaques exhibit a naturally-occurring depression similar to humans. Sci Rep. 2015;5:9220. doi: 10.1038/srep09220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng LL, Liu L, Liu Y, Shen H, Li Y, Hu D. Antidepressant treatment normalizes white matter volume in patients with major depression. PLoS One. 2012;7(8):e44248. doi: 10.1371/journal.pone.0044248. [DOI] [PMC free article] [PubMed] [Google Scholar]