Abstract
The purpose of this study was to determine whether scores on two temperament dimensions (fearlessness and disinhibition) correlated differentially with gray matter volumes in two limbic regions (amygdala and hippocampus). It was predicted that the fearlessness dimension would correlate with low gray matter volumes in the amygdala and the disinhibition dimension would correlate with low gray matter volumes in the hippocampus after controlling for age, IQ, regular substance use, and total brain volume. Participants were 191 male adolescents (age range = 13–19 years) incarcerated in a maximum-security juvenile facility. Structural magnetic resonance imaging (MRI) analysis of the limbic and paralimbic regions of the brain was conducted. The temperament dimensions were estimated with items from the Psychopathy Checklist: Youth Version (PCL: YV: Forth et al., 2003). Analyses showed that the fearlessness dimension correlated negatively with gray matter volumes in the amygdala and the disinhibition dimension correlated negatively with gray matter volumes in the hippocampus but not vice versa. These findings provide preliminary support for the construct validity of the fearlessness and disinhibition temperament dimensions and offer confirmatory evidence for involvement of the amygdala and hippocampus in fear conditioning and behavioral inhibition, respectively.
Keywords: fearlessness, disinhibition, amygdala, hippocampus
1. Introduction
The limbic system or paleopallium is one of the most important subcortical regions of the brain for understanding and predicting human behavior. When limbic structures and connections fail to function properly behavioral abnormalities often result (Anderson, Bechara, Damasio, Tranel, & Damasio, 1999) . The specific abnormality, of course, depends on a number of factors, including the specific limbic structures involved. Several major reviews have concluded that the concept of a single limbic system is outmoded and that there are actually (at least) two separate limbic systems: an amygdalar limbic system centered around emotion (amygdala, orbitofrontal cortex, and anterior cingulate cortex) and a hippocampal limbic system centered around memory (hippocampus, posterior cingulate cortex, and fornix – mammillary body – anterior thalamus – posterior cingulate circuit: Bannerman et al., 2004; Rolls, 2015). The amygdala—one of two almond-shaped structures on either side of the thalamus—plays a major role in fear conditioning. Lesions and hypofunction in this area of the brain have been found to correspond with a diminished fear response and weakened fear conditioning in humans (Phelps et al., 2001; Sah et al., 2003). Dysfunction in the hippocampal region of the human brain, on the other hand, more often leads to memory problems and poor behavioral control (Cherbuin et al., 2012; Shechner et al., 2014). These relatively specific behavioral effects occur even though the hippocampus and amygdala are connected by a series of neural pathways. The fact that different structures in the limbic system have both shared and unique effects on behavior make them invaluable for testing certain brain-behavior relationships.
Creating a fearlessness dimension from Lykken’s (1957) low-fear hypothesis and a disinhibition dimension from Krueger et al.’s (2007) externalizing spectrum, Walters (2008) constructed a two-dimensional model of temperament designed to explain crime-related constructs like psychopathy and antisocial personality. This model, which shares features in common with Fowles and Dindo’s (2009) dual-process model of psychopathy, was designed to identify the roots of proactive and reactive criminal thinking and behavior (Walters, 2012). In an effort to create as pure a measure of these two dimensions as possible, Walters (2015) took items from the Psychopathy Checklist-Revised (PCL-R: Hare, 2003) and created a fearlessness factor by merging Facets 1 (interpersonal) and 2 (affective), after one item from Facet 1 (i.e., grandiosity) and one item from Facet 2 (i.e., failure to accept responsibility) that seemed to bear little resemblance to fearlessness were removed, and a disinhibition factor from Facet 3 (lifestyle), after one item with little apparent relevance to disinhibition (i.e., parasitic orientation) was removed. Two of the three omitted items were the weakest loading items on their respective facets in large-scale confirmatory factor analyses (CFA) conducted by Cooke and Michie (2001) and Hare and Neumann (2006). A CFA of the Walters (2015) data revealed that the six Facet 1 and 2 items and four Facet 3 items achieved good overall fit with their respective latent factors and slightly better fit than the traditional two-, three-, and four-factor models of psychopathy.
The purpose of the current study was to evaluate the construct validity of the two PCL-R factors used to measure fearlessness and disinhibition in the previous Walters (2015) investigation. This was accomplished by pairing the fearlessness and disinhibition temperament dimensions, measured by means of the PCL: YV, with MRI derived gray matter volume (GMV) measures taken from the amygdala and hippocampus regions of male adolescent offenders’ brains. It was hypothesized that fearlessness would achieve a significant negative correlation with amygdalar GMV levels but not hippocampal GMV levels and disinhibition would achieve a significant negative correlation with hippocampal GMV levels but not amygdalar GMV levels. Most of the research on the role of the limbic system in fear conditioning and behavioral inhibition comes from animal studies, although there are a few human studies on which to base a rationale for this study. The rationale for the first part of the hypothesis tested in this study comes from human research showing that the amygdala plays a greater role in fear conditioning than the hippocampus (Shechner et al., 2014). In fact, individual differences in fear conditionability have been found to be stable, heritable, and specific to neural areas in and around the amygdala (MacNamara et al., 2015). The rational for the second part of the hypothesis comes from human research showing that the hippocampus is more consistently associated with behavioral inhibition than the amygdala (Cherbuin et al., 2008). Recent research conducted on hyperactivity in medication-naïve children (Posner et al., 2014) and impulsivity in abstinent heroin addicts (Zhai et al., 2014) indicate that hippocampus is heavily involved in behavioral inhibition.
2. Method
2.1 Participants
The sample used in this study consisted of 191 male adolescents housed in a maximum security youth detention facility in New Mexico drawn from the Southwest Advanced Neuroimaging Cohort–Youth study (SWANC-Y; NIMH R01 MH071896-01; PI: Kiehl). The rationale for using juveniles instead of adults as participants in this study was research showing that temperament or its expression is shaped by experience (Kagan, 2010). Given that experience increases with age we sought a sample of seriously offending individuals with the least amount of life experience. There were originally 218 male juvenile offenders considered for inclusion in this study but 9 met one or more of the exclusionary criteria (history of psychosis, seizures, traumatic brain injury, major medical problems, or fluency in English below a fourth grade reading level) and 18 were removed for excessive motion during the MRI procedure. The ethnic breakdown for the sample of 191 male adolescents was 56.6% Hispanic, 14.8% white, 11.7% Native American, and 16.9% other/unspecified. The average participant was 17.32 years of age (SD = 1.18, range = 13–19), with 9.36 years of education (SD = 1.36, range = 6–13), and an estimated Wechsler IQ of 92.80 (SD = 12.06, range = 63–140).
2.2 Measures
2.2.1 Independent Variables
Gray matter volumes (GMVs) from the amygdala and hippocampus served as the independent variables in this study. GMVs were estimated using voxel-based morphometry (VBM) derived from high-resolution T1-weighted structural magnetic resonance imaging (MRI) scans. A multi-echo MPRAGE pulse sequence (repetition time = 2530-ms, echo times = 1.64-ms, 3.50-ms, 5.36-ms, 7.22-ms, inversion time = 1100-ms, flipangle = 7″, slice thickness = 1.3-mm, matrix size = 256 × 256) was used to create 128 sagittal slices with an in-plane resolution of 1.0 mm × 1.0 mm. Four measures of GMV were sampled by fitting a 10-mm sphere centered on the left amygdala (MNI coordinates: x = −12, y = −40, z = 8), right amygdala (x = 24, y = −38, z = 2), left hippocampus (x = −16, y = 2, z = −18), and right hippocampus (x = 30, y = 10, z = −32). The values obtained for the left and right amygdala were then averaged and multiplied by 100. The reason for multiplying by 100 was that otherwise the scores were too small to permit analysis using all four control variables. The values for the left and right hippocampus were also averaged and multiplied by 100. Each region of interest was selected a priori. For a complete description of the scanning parameters and analytic procedures the reader is referred to Ermer, Cope, Nyalakanti, Calhoun, and Kiehl (2013).
2.2.2 Dependent Variables
The fearlessness and disinhibition dimensions were derived from the Psychopathy Checklist: Youth Version (PCL: YV: Forth et al., 2003), a 20-item rating scale designed to measure psychopathy in youth. As outlined in Walters (2015), the fearlessness score is based on six items from Facets 1 and 2 of the PCL-R/YV (superficial charm, pathological lying, conning/manipulative, lack of remorse or guilt, shallow affect, and callousness) and the disinhibition score is based on four items from Facet 3 of the PCL-R/YV (need for stimulation, lack of realistic long-term goals, impulsivity, and irresponsibility). Each PCL: YV item is rated on a three-point scale: 0 = does not apply, 1 = applies somewhat, 2 = definitely applies. Approximately 12% of the PCL: YV protocols were double-rated, with good agreement between raters for the total PCL: YV score: Intraclass Correlation Coefficient (ICC: 1, 1) = .90.
2.2.3 Control Variables
Four control variables were included in this study: age in years, IQ, regular substance use, and total brain volume. A Wechsler full-scale IQ was estimated from the Vocabulary and Matrix Reasoning subtests of the Wechsler Adult Intelligence Scale (participants 16 years of age and older: Wechsler, 1997) or Wechsler Intelligence Scale for Children (participants under 16 years of age: Wechsler, 2003). A modified version of the Addiction Severity Index (McLellan et al., 1992) was used to assess regular substance use. Regular substance use was calculated by summing the number of years a participant regularly used various substances (alcohol and illegal drugs, three or more times for at least one month), dividing this number by the participant’s age, multiplying the result by 100, and using a square root transformation of the product to correct for skew (Ermer et al., 2013). The fourth and final control variable was total brain volume (white + gray matter), which is commonly included in studies using the VBM methodology.
2.3 Procedure
Participants for this study were recruited from a maximum-security youth detention facility in New Mexico. All testing took place between June 2007 and March 2011. Individuals who were 18 years of age or older at the time they were approached about the study provided their informed consent to participate and individuals under the age of 18 gave their informed assent to participate in conjunction with written parental/guardian approval. All participants were compensated for the time they spent in the study at a rate commensurate with the standard institutional pay rate. The study and procedure were approved by the Institutional Review Boards at the University of New Mexico Health Sciences Center and Kutztown University.
2.4 Analytic Strategy
Two regression equations were simultaneously computed using the structural equation modeling (SEM) program MPlus 5.2 (Muthén and Muthén, 1998–2007). Each dependent variable (fearlessness and disinhibition) was regressed onto the independent variables (GMV amygdala and GMV hippocampus) and four control measures (age, IQ, regular substance use, and total brain volume). Results were then evaluated against the prediction that amygdalar GMV levels would correlate negatively with fearlessness but not with disinhibition and hippocampal GMV levels would correlate negatively with disinhibition but not with fearlessness.
There were no missing data for any of the independent or dependent variables included in this study. Two of the control variables, however, were affected by missing data. Four percent of the participants were missing data on substance use and 7% of the sample had missing IQ scores. Missing data were handled with full information maximum likelihood (FIML). This approach to missing data analysis estimates a likelihood function for each participant based on observed relationships between non-missing data and then uses them to construct model parameters and standard errors for all participants.
3. Results
Descriptive statistics for the independent, dependent, and control variables can be found in Table 1. Although only the hippocampus–disinhibition correlation achieved Bonferroni-corrected significance, both the hippocampus–disinhibition (p < .01) and amgydala–fearlessness (p < .01) correlations achieved univariate significance and both the hippocampus–fearlessness (p = .09) and amygdala–disinhibition (p > .10) correlations failed to achieve univariate significance. Although the hippocampus correlated significantly higher than the amygdala with disinhibition, Steiger’s (1980) Z = 1.71, p < .05 (one-tailed test), the amygdala and hippocampus did not differ significantly in their correlations with fearlessness, Steiger’s Z = 0.86, p > .10. There was no evidence of multicollinearity between the seven predictor variables used in the two regressions (Tolerance = .767–.959; Variance Inflation Factor = 1.043–1.303).
Table 1.
Descriptive Statistics and Correlations for the 8 Variables Included in the Current Study
| Variable | n | M | SD | Range | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age | 191 | 17.32 | 1.18 | 13–19 | 0.02 | −0.09 | −0.12 | 0.15 | 0.13 | −0.09 | −0.17 |
| 2. IQ | 178 | 92.80 | 12.06 | 63–140 | 0.11 | 0.19 | 0.02 | −0.01 | 0.05 | −0.15 | |
| 3. Substance Use | 184 | 5.52 | 2.70 | 0–11.40 | −0.01 | −0.06 | −0.11 | 0.27* | 0.28* | ||
| 4. Brain Volume | 191 | 1266.47 | 99.55 | 1031–1533 | −0.25* | −0.34* | −0.03 | 0.02 | |||
| 5. GMV Amygdala | 191 | 15.71 | 0.82 | 13.32–17.94 | 0.16 | −0.20 | −0.07 | ||||
| 6. GMV Hippocampus | 191 | 7.60 | 0.76 | 5.53–9.73 | −0.12 | −0.23* | |||||
| 7. Fearlessness | 191 | 5.14 | 2.55 | 0–11 | 0.48* | ||||||
| 8. Disinhibition | 191 | 5.41 | 1.68 | 0–8 |
Note. Age = chronological age in years; IQ = estimated full scale Wechsler IQ; Substance Use = regular substance use; Brain Volume = total brain volume (white + gray matter); GMV Amygdala = average gray matter volume for left and right amygdala x 100; GMV Hippocampus = average gray matter volume for left and right hippocampus ×100; Fearlessness = fearlessness temperament dimension; Disinhibition = disinhibition temperament dimension; n = number of non-missing cases; M = mean, SD = standard deviation; Range = range of scores in current sample.
p < .0018 (Bonferroni-corrected alpha: .05 / 28 correlations).
The right and left amygdalae each correlated significantly with the fearlessness temperament dimension (r = −.18, p < .05) and failed to correlate with the disinhibition temperament dimension (r = −08 to −.05, p > .10). The right and left hippocampi likewise correlated significantly with disinhibition (r = −.20, p < .01) but failed to correlate with fearlessness (r = −.13 to −.08, p > .07). Based on these results the GMV values for the left and right amygdalae were averaged and the GMV values for the left and right hippocampi were averaged prior to conducting the regression analysis.
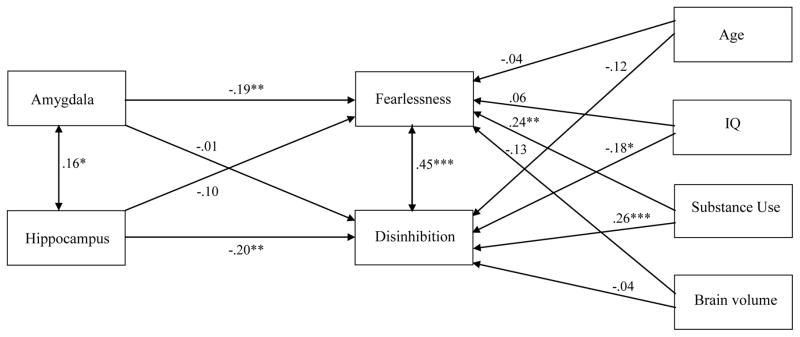
A regression analysis composed of two regression equations, one for each dependent variable, was computed using SEM. The results, as summarized in Table 2, denote that measured GMV levels in the amygdala correlated significantly with fearlessness but not with disinhibition whereas measured GMV levels in the hippocampus correlated significantly with disinhibition but not with fearlessness net the effects of age, IQ, regular substance use, and total brain volume. The standardized coefficients for all of the correlations in the model are reproduced in Figure 1.
Table 2.
Structural Equation Modeling Regression Analysis
| Variable | b(95% CI) | β | t | p |
|---|---|---|---|---|
| Fearlessness | ||||
| Age | −0.093(−0.386, 0.201) | −0.043 | −0.62 | 0.536 |
| IQ | 0.013(−0.018, 0.043) | 0.059 | 0.80 | 0.426 |
| Substance Use | 0.222(0.090, 0.354) | 0.235 | 3.29 | 0.001 |
| Brain Volume | −0.003(−0.007, 0.001) | −0.128 | −1.67 | 0.091 |
| GMV Amygdala | −0.603(−1.036, −0.169) | −0.193 | −2.72 | 0.006 |
| GMV Hippocampus | −0.346(−0.830, 0.138) | −0.103 | −1.40 | 0.161 |
| Disinhibition | ||||
| Age | −0.174(−0.363, 0.014) | −0.123 | −1.82 | 0.069 |
| IQ | −0.024(−0.043, −0.005) | −0.175 | −2.50 | 0.012 |
| Substance Use | 0.163(0.079, 0.248) | 0.264 | 3.81 | <0.001 |
| Brain Volume | −0.001(−0.003, 0.002) | −0.036 | −0.48 | 0.629 |
| GMV Amygdala | −0.019(−0.298, 0.259) | −0.010 | −0.14 | 0.891 |
| GMV Hippocampus | −0.436(−0.746, −0.125) | −0.197 | −2.75 | 0.006 |
| Fearlessness with Disinhibition | 1.621(1.053, 2.189) | 0.446 | 5.59 | <0.001 |
| Amygdala with Hippocampus | 0.102(0.014, 0.190) | 0.164 | 2.26 | 0.024 |
Note. Fearlessness = regression equation with the fearlessness temperament dimension as the outcome; Disinhibition = regression equation with the disinhibition temperament dimension as the outcome; Age = chronological age in years; IQ = estimated full scale Wechsler IQ; Substance Use = regular substance use; Brain Volume = total brain volume (white + gray matter); GMV Amygdala = average gray matter volume for left and right amygdala × 100; GMV Hippocampus = average gray matter volume for left and right hippocampus ×100; Fearlessness = fearlessness temperament dimension; Disinhibition = disinhibition temperament dimension; Fearlessness with Disinhibition = correlation between the fearlessness and disinhibition temperament dimensions; Amygdala with Hippocampus = correlation between the amygdala and hippocampus average gray matter volumes; N = 191.
Figure 1.
Cross-sectional regressions of fearlessness and disinhibition on the two limbic structures (amygdala, hippocampus) and four control variables (age, IQ, substance use, and brain volume)
Note. Standardized beta coefficients are reported; N = 191.
*p <..05; **p < .01; ***p < .001.
A confirmatory factor analysis (CFA) of the two-dimensional model performed with an asymptotic distribution free (ADF) estimator (WLSMV) achieved poor absolute fit in the current study (comparative fit index (CFI) = .87, Tucker-Lewis Index (TLI) = .87, root mean square error of approximation (RMSEA) = .120) but achieved significantly better relative fit than a one-factor model in which all 10 PCL: YV items were loaded onto a single factor (DIFF TEST (1) = 4.88, p < .05). When latent factor scores from the CFA replaced the raw summed scores in the SEM analysis, there was little change in the results: i.e., the amygdala–fearlessness (t = −2.56, p < .05) and hippocampus–disinhibition (t = −2.79, p < .01) effects remained significant and the hippocampus–fearlessness (t = −1.93, p = .053) and amygdala–disinhibition (t = −1.64, p = .100) effects remained non-significant.
4. Discussion
A two-dimensional model of temperament designed to explain, in part, the origins of psychopathy, antisocial personality, and other crime-related constructs was tested using data from MRI scans and youth measures of psychopathy (PCL: YV) in an ultra-high risk sample. As predicted, GMV levels in the amygdala and hippocampus correlated differentially with the two temperament dimensions designed to explain psychopathy, antisocial personality, and criminal lifestyle—namely, fearlessness and disinhibition. Previous research had shown that the amygdala is more intimately involved in fear conditioning than the hippocampus (Shechner et al., 2014) and that the hippocampus is more closely tied to behavioral inhibition than the amygdala (Cherbuin et al.. 2008). When tested in a group of serious juvenile offenders, lower amygdalar GMV levels correlated with PCL: YV items thought to measure weak fear conditioning (fearlessness) and lower hippocampal GMV levels correlated with PCL: YV items thought to measure weak behavioral control (disinhibition). Just as the two sets of PCL-R items achieved high loadings on their respective latent traits and predicted recidivism beyond the antisocial facet in the Walters (2015) investigation, scores on the youth version of this instrument (PCL-YV)—whether configured as raw or latent (factor) scores—correlated differentially with GMV levels in the amygdala and hippocampus in the current study.
Whereas low hippocampal GMVs were significantly more diagnostic of disinhibition than low amygdala GMVs, there was not a significant difference in the correlations between fearlessness and gray matter volumes in the amygdala and hippocampus, even though only the amygadala–fearlessness correlation was significant. This suggests that the amygdala and hippocampus may both be involved in fear conditioning (see also Baeuchl, Meyer, Hoostädter, Diener, & Flor, 2015) and that relationships between limbic structures and temperament dimensions are more complex and inter-dependent than indicated by the simple models tested in the current study.
By way of review, fearlessness is a core feature of what has traditionally been referred to as primary psychopathy (Karpman, 1941; Lykken, 1957). It involves a characteristically weak response to social disapproval and punishment and reflects such classic psychopathic traits as interpersonal deception and manipulation, lack of guilt and remorse, and a general sense of callousness and weak empathy. Cognitively, it is associated with proactive or predatory criminal thinking. Fearlessness should not be confused with Zuckerman’s (1979) thrill-seeking subscale of the Sensation Seeking Scale (SSS) which features fearlessness in response to physical challenge or danger. Disinhibition, on the other hand, is characterized by weak impulse control, intolerance of boredom, and general externalizing tendencies (Krueger et al., 2007; Patrick et al., 2009). Cognitively, it corresponds with reactive or impulsive criminal thinking and shares some but not all of the features of secondary psychopathy (Karpman, 1941). The disinhibition temperament dimension should not be confused with Zukerman’s (1979) Disinhibition subscale, which focuses on drug use and criminal behavior in general. Parenthetically, Levenson et al. (1995) found that Zuckerman’s Disinhibition subscale correlated comparably with measures of primary and secondary psychopathy.
It has been proposed that both the amygdala and hippocampus are involved in Gray and McNaughton’s (2003) behavioral inhibition system (BIS). Although there is research supporting this position (Barrós-Loscertales et al., 2006), a more recent study on this issue found GMV levels in the hippocampus correlated with BIS sensitivity but GMV levels in the amygdala did not (Cherbuin et al.. 2008). Differences in sampling or BIS measures may account for these differences in outcome but one thing is clear — the results of the current study are more consistent with Cherbuin et al.’s (2008) finding that BIS sensitivity is restricted to the hippocampus than with Barrós-Loscertales et al. (2006) finding that BIS sensitivity is equivalent in the hippocampus and amygdala. Additional research employing a definition of behavioral inhibition that does not include any remnants of fear conditioning should help resolve this issue. In the meantime the conclusion from the current study is that low levels of GMV in the amygdala result in a moral-emotive deficit, whereas low levels of GMV in the hippocampus are more apt to culminate in a behavioral control deficit. This could very well be reflected in the types of crimes these individuals commit later in life, with preliminary research suggesting that fearlessness/proactive tendencies more often lead to instrumental crimes (e.g., burglary, robbery) and disinhibition/reactive tendencies more often lead to impulsive crimes (e.g., assault, domestic violence: Walters et al., 2007).
Understanding the relationship between fearlessness and disinhibition can go a long way toward explaining their impact on psychopathy, antisocial personality, and other crime-related constructs. In the current study the fearlessness and disinhibition dimensions correlated three times higher than GMV levels in the amygdala and hippocampus. This replicates previous research on the two-dimensional model which has consistently shown that the two dimensions are highly correlated regardless of whether they are measured as fearlessness and disinhibition (Walters, 2015) or as proactive and reactive criminal thinking (Walters, 2008). Whereas these dimensions have traditionally been treated as categories or types, starting with Karpman’s (1941) primary–secondary psychopathy breakdown and continuing up through the callous/unemotional specifier for conduct disorder in DSM-5 (APA, 2013), a strong correlation between fearlessness and disinhibition suggests that these are overlapping dimensions. The dimensional nature of psychopathy and other crime-related constructs (Haslam et al., 2012), not to mention the dimensionality of the proactive/fearlessness and reactive/disinhibition divisions themselves (Walters, 2009), has been fairly well established. What this means is that while some offenders may be high on one dimension but not on the other, most offenders are high on both dimensions or low on both dimensions.
Despite recent efforts to highlight the role of temperament in future criminal offending (DeLisi and Vaughn, 2014), early temperament is not a particularly strong or consistent predictor of delinquency and crime (van der Voort et al., 2013). We would do well to note, however, that temperament, or at least its expression, changes over time as a result of a child’s ongoing interactions with the environment (Kagan, 2010). This was the primary reason why an adolescent sample was employed in the current study. Moreover, there is evidence that early temperament may be connected to later behavior by means of a chain of proximal intervening variables. Walters (2014), for example, determined that a difficult temperament at age one correlated minimally with delinquency at age nine but that an indirect chain running from temperament at age one to delinquency at age nine via age five externalizing behavior was significant. Although this particular chaining effect involved a disinhibited temperament, chains related to fearlessness are also likely. Clinicians providing assessment and intervention services to at-risk youth should accordingly be sensitive to chaining effects and take pains to assess and break the chain when possible given that ignoring it will likely keep an individual locked in an escalating pattern of ongoing criminality.
One limitation of this study is that it was cross-sectional rather than prospective in nature. In conducting this research we assumed that although the variables were collected concurrently the GMV levels preceded the behaviors measured with the PCL: YV. This, however, may not always have been the case. Behaviors assessed with the PCL: YV could have occurred years earlier and perhaps in some way influenced the concurrently measured GMV. This was one reason why regular substance use was employed as a control variable. A second limitation of this study is that participants were 13 years of age and older. A great deal can happen during the first 13 years of life to alter GMV levels. An adolescent sample was selected over an adult sample based on the fact that less life experience translates into a lower chance of a significant alteration occurring in early temperament. Based on this logic, an even younger sample would have been preferable. A third limitation of this study is that it was conducted on incarcerated male offenders. There is no way to know what effect, if any, incarceration or gender had on the GMV levels obtained in this study although it is possible that the current results have limited generalizability to non-incarcerated and female populations.
Some of the aforementioned problems with the current study could be rectified by conducting a prospective investigation on a younger sample of non-incarcerated youth. The study might begin by measuring GMV limbic levels in 8 to 9 year old boys and girls thought to be at-risk for future externalizing behavior. Once these individuals reach the age at which the PCL: YV can be administered (i.e., 12 years of age; Forth et al., 2003) a PCL: YV evaluation could then be conducted. Several years after this, both official and self-reported delinquency and parental and teacher ratings of antisocial behavior could be collected on participants. This way, we could more thoroughly investigate the effects of putative amygdalar and hippocampal anomalies on early adolescent measures of psychopathy and mid- to late-adolescent measures of antisocial behavior, delinquency, and criminality.
Highlights.
Structural magnetic resonance imaging analysis of the limbic regions of the brain.
PCL: YV used to measure fearlessness and disinhibition temperament dimensions.
Fearlessness correlated negatively with gray matter volumes in the amygdala.
Disinhibition correlated negatively with gray matter volumes in the hippocampus.
Study provides support for the construct validity of the two dimensions.
Acknowledgments
Funding
This research was supported by NIMH R01 MH071896-01 & NICHD R01 HD082257-01 (KAK). We are grateful to the staff and inmates of the New Mexico Children, Youth and Families Department for their support and assistance in making this research possible and the Kiehl lab for assistance with data collection and preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5. Author; Washington, DC: 2013. [Google Scholar]
- Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nature Neuroscience. 1999;2:1032–1037. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- Baeuchl C, Meyer P, Hoppstädter M, Diener C, Flor H. Contextual fear conditioning in humans using feature-identical contexts. Neurobiology of Learning and Memory. 2015;121:1–11. doi: 10.1016/j.nlm.2015.03.001. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JNP, McHugh SE, Deacon RMJ, Yeeb BK, et al. Regional dissociations within the hippocampus—memory and anxiety. Neuroscience and Biobehavioral Reviews. 2004;28:273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Barrós-Loscertales A, Meseguer V, Sanjuan A, Belloch V, Parcet MA, et al. Behavioral inhibition system activity is associated with increased amygdala and hippocampal gray matter volume: A voxel-based morphometry study. Neuroimage. 2006;33:1011–1015. doi: 10.1016/j.neuroimage.2006.07.025. [DOI] [PubMed] [Google Scholar]
- Cherbuin N, Windsor TD, Anstey KJ, Maller JJ, Meslin C, Sachdeve PS. Hippocampal volume is positively associated with behavioural inhibition (BIS) in a large community-based sample of mid-life adults: The PATH through life study. Social Cognitive and Affective Neuroscience. 2008;3:262–269. doi: 10.1093/scan/nsn018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke DJ, Michie C. Refining the construct of psychopathy: Towards a hierarchical model. Psychological Assessment. 2001;13:171–188. [PubMed] [Google Scholar]
- DeLisi M, Vaughn MG. Foundation for a temperament-based theory of antisocial behavior and criminal justice system involvement. Journal of Criminal Justice. 2014;42:0–25. [Google Scholar]
- Ermer E, Cope LM, Nyalakanti PK, Calhoun VD, Kiehl KA. Aberrant paralimbic gray matter in incarcerated male adolescents with psychopathic traits. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52:94–103. doi: 10.1016/j.jaac.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forth AE, Kosson D, Hare RD. Psychopathy Checklist: Youth Version Technical Manual. Multi-Health Systems; Toronto: 2003. [Google Scholar]
- Fowles DC, Dindo L. Temperament and psychopathy: A dual-process model. Current Directions in Psychological Science. 2009;18:179–183. [Google Scholar]
- Gray JA, McNaughton N. The Neuropsychology of Anxiety: An Enquiry into the Function of the Septo-Hippocampal System. 2. Oxford University Press; Oxford, England: 2003. [Google Scholar]
- Hare RD. The Hare Psychopathy Checklist–Revised Manual. 2. Multi-Health Systems; Toronto: 2003. [Google Scholar]
- Hare RD, Neumann CS. The PCL-R assessment of psychopathy: Development, structural properties, and new directions. In: Patrick CJ, editor. Handbook of Psychopathy. Guilford; New York: 2006. pp. 58–88. [Google Scholar]
- Haslam N, Holland E, Kuppens P. Categories versus dimensions in personality and psychopathology: A quantitative review of taxometric research. Psychological Medicine. 2012;42:903–920. doi: 10.1017/S0033291711001966. [DOI] [PubMed] [Google Scholar]
- Kagan J. Emotions and temperament. In: Burnstein MH, editor. Handbook of Cultural Developmental Science. Psychology Press; New York: 2010. pp. 175–194. [Google Scholar]
- Karpman B. On the need for separating psychopathy into two distinct clinical types: Symptomatic and idiopathic. Journal of Criminology and Psychopathology. 1941;3:112–137. [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, Benning SD, Kramer MD. Linking antisocial behavior, substance use, and personality: An integrative quantitative model of the adult externalizing spectrum. Journal of Abnormal Psychology. 2007;116:645–666. doi: 10.1037/0021-843X.116.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson MR, Kiehl KA, Fitzpatrick CM. Assessing psychopathic attributes in a noninstitutionalized population. Journal of Personality and Social Psychology. 1995;68:151–158. doi: 10.1037//0022-3514.68.1.151. [DOI] [PubMed] [Google Scholar]
- Lykken DT. A study of anxiety in the sociopathic personality. Journal of Abnormal and Social Psychology. 1957;55:6–10. doi: 10.1037/h0047232. [DOI] [PubMed] [Google Scholar]
- MacNamara A, Rabinak CA, Fitzgerald DA, Zhou XJ, Shankman SA, et al. Neural correlates of individual differences in fear learning. Behavioural Brain Research. 2015;287:34–41. doi: 10.1016/j.bbr.2015.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G. The fifth edition of the Addiction Severity Index. Journal of Substance Abuse Treatment. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Muthén B, Muthén L. Mplus User’s Guide. 5. Muthen and Muthen; Los Angeles: 1998–2007. [Google Scholar]
- Patrick CJ, Fowles DC, Krueger RF. Triarchic conceptualization of psychopathy: Developmental origins of disinhibition, boldness, and meanness. Development and Psychopathology. 2009;21:913–938. doi: 10.1017/S0954579409000492. [DOI] [PubMed] [Google Scholar]
- Phelps EA, O’Connor KJ, Gatenby C, Gore JC, Grillon C, Davis M. Activation of the left amygdala to a cognitive representation of fear. Nature Neuroscience. 2001;4:437–441. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- Posner J, Siciliano F, Wang Z, Liu J, Sonuga-Barke E, Greenhill L. A multimodal MRI study of the hippocampus in medication-naïve children with ADHD: What connects ADHD and depression? Psychiatry Research: Neuroimaging. 2014;224:112–118. doi: 10.1016/j.pscychresns.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET. Review: Limbic systems for emotion and for memory, but no single limbic system. Cortex. 2015;62:119–157. doi: 10.1016/j.cortex.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ESL, Lopez de Armentia M, Power J. The amygdaloid complex: Anatomy and physiology. Physiology Review. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Shechner T, Hong M, Britton JC, Pine DS, Fox NA. Fear conditioning and extinction across development: Evidence from human studies and animal models. Biological Psychology. 2014;100:1–12. doi: 10.1016/j.biopsycho.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger JH. Tests for comparing elements of a correlation matrix. Psychological Bulletin. 1980;87:245–252. [Google Scholar]
- van der Voort A, Linting M, Juffer F, Bakersman-Kranenburg MJ, van IJzendoorn MH. Delinquent and aggressive behaviors in early-adopted adolescents: Longitudinal predictions from child temperament and maternal sensitivity. Children and Youth Services Review. 2013;35:439–446. [Google Scholar]
- Walters GD. Self-report measures of psychopathy, antisocial personality, and criminal lifestyle: Testing and validating a two-dimensional model. Criminal Justice and Behavior. 2008;35:1459–1483. [Google Scholar]
- Walters GD. Latent structure of a two-dimensional model of antisocial personality disorder: Construct validation and taxometric analysis. Journal of Personality Disorders. 2009;23:647–660. doi: 10.1521/pedi.2009.23.6.647. [DOI] [PubMed] [Google Scholar]
- Walters GD. Crime in a Psychological Context: From Career Criminals to Criminal Careers. Sage; Thousand Oaks, CA: 2012. [Google Scholar]
- Walters GD. Pathways to early delinquency: Exploring the individual and collective contributions of difficult temperament, low maternal involvement, and externalizing behavior. Journal of Criminal Justice. 2014;42:321–326. [Google Scholar]
- Walters GD. A two-dimensional model of psychopathy and antisocial behavior: A multi-sample investigation using items from the Psychopathy Checklist-Revised. Personality and Individual Differences. 2015;78:88–93. [Google Scholar]
- Walters GD, Frederick AA, Schlauch C. Postdicting arrests for proactive and reactive aggression with the PICTS proactive and reactive composite scales. Journal of Interpersonal Violence. 2007;22:1415–1430. doi: 10.1177/0886260507305556. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3. Psychological Corporation; New York: 1997. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. 4. Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- Zhai TY, Shao YC, Xie CM, Ye EM, Zou F, et al. Altered intrinsic hippocampus declarative memory network and its association with impulsivity in abstinent heroin dependent subjects. Behavioural Brain Research. 2014;272:209–217. doi: 10.1016/j.bbr.2014.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M. Sensation Seeking: Beyond the Optimal Level of Arousal. Erlbaum; Hillsdale, NJ: 1979. [Google Scholar]