Abstract
B-lymphocytes play an essential regulatory role in the adaptive immune response through antibody production during infection. A less known function of B-lymphocytes is their ability to respond directly to infectious antigens through stimulation of pattern recognition receptors expressed on their surfaces. β-glucans are carbohydrates present in the cell wall of many pathogenic fungi that can be detected in the peripheral blood of patients during infection. They have been shown to participate in the innate inflammatory response as they can directly activate peripheral macrophages and dendritic cells. However, their effect as direct stimulators of B-lymphocytes has not been yet fully elucidated. The aim of this study was to examine the molecular mechanisms and cytokine profiles generated following β-glucan stimulation of B-lymphocytes, compared with the well-established TLR-9 agonist CpG-oligodeoxynucleotide (CpG) and study the participation of β-glucan stimulated B-cells in the innate immune response. Herein, we demonstrate that β-glucan activated B-lymphocytes upregulate pro-inflammatory cytokines (TNFα, IL-6 and IL-8). Interestingly, β-glucan, unlike CpG, had no effect on B-lymphocyte proliferation or IgM production. When compared with CpG (TLR9 agonist), β-glucan-activated cells secreted significantly higher levels of IL-8. Furthermore, IL-8 secretion was partially mediated by Dectin-1 and required SYK, MAPKs and the transcription factors NF-κB and AP-1. Moreover, we observed that conditioned media from β-glucan stimulated B-lymphocytes elicited neutrophil chemotaxis. These studies suggest that β-glucan activated B-lymphocytes have an important and novel role in fungal innate immune responses.
Keywords: Pneumocystis, beta-glucan, B-Lymphocytes, Neutrophils chemotaxis, Innate immunity
INTRODUCTION
B-lymphocytes orchestrate the adaptive immune response by producing antibodies upon direct contact with activated T-cells. A lesser-known function of B-cells is their T-cell independent participation in stimulating the innate immune system by releasing chemokines and pro-inflammatory cytokines following challenge with pathogen-associated molecular patterns (PAMs). PAMs are highly conserved molecules shared across multiple microorganisms, including fungi that are recognized by pattern recognition receptors (PRRs). Among the different PRRs, toll like receptors (TLRs) are one of the best-studied family. In B-lymphocytes, engagement of TLRs by specific ligands triggers the up-regulation of activation markers, proliferation, cytokine secretion and antibody production (1–3). In particular, the activation of TLR-9 by CpG-containing oligodeoxynucleotides stimulates activation markers such as CD80 and CD86, induces B-cell proliferation and secretion of IL-6 and IL-10, and triggers production of natural antibodies, mostly of the IgM isotype.(4–9).
CpG motifs are found in bacterial and viral DNA, and while in fungi there is not a clear CpG-equivalent, β-glucan carbohydrates found in the cell wall act as potent immune modulators and also use PRRs to stimulate host cells. During fungal infections β-glucan levels are elevated in peripheral blood of patients and these cell wall components strongly contribute to the anti-fungal inflammatory response (10, 11). Interestingly, patients with Pneumocystis pneumonia (PcP) tend to have very high levels of circulating β-glucans compared with other fungal infections (10, 12).
β-glucans mainly consist of 1,3-linked monomers of D-glucose, with variable amounts of 1,6-linked side chains. The ratio of 1,3 to 1,6 linkages in addition to the rest of the architectural cell wall differs among various fungal organisms and, therefore, differentially influences host immunological responses. β-glucan particles mainly signal through the Dectin-1 receptor, a PRR belonging to the C-lectin receptor family (13). β-glucans are known to activate macrophages and dendritic cells (DCs). Upon activation, DCs generate a cytokine milieu that prompts T-cells to polarize into robust T helper-1 (Th1) and T helper-17 (Th17) responses important for fungal host defense (14–17). Limited data in mice suggest that β-glucans can also directly activate B-lymphocytes and generate a T-cell independent antibody response (18). However, their role in human B-lymphocyte activation has not been fully elucidated. This is important as most opportunistic fungal infections occur in CD4-depleted individuals and a better understanding of the T-cell independent mechanisms against fungal disease are crucial to generate tools that would enhance non-CD4 immune responses to fight these severe and frequently fatal infections.
Herein, we investigated the molecular mechanisms leading to activation of peripheral human B-lymphocytes by β-glucans and we delineated their participation in innate immune responses. Our results demonstrate that β-glucan activated B-lymphocytes secrete pro-inflammatory cytokines, particularly IL-8. Secretion of IL-8 involves Dectin-1 receptors, MAPKs and the transcription factors NF-κB and AP-1. Moreover, our data show that activated B-lymphocytes contribute to neutrophil chemotaxis and confirm the participation of B-lymphocytes in the innate immune response against fungal infections.
MATERIALS AND METHODS
Reagents and antibodies
Endotoxin-free buffers and reagents were scrupulously used in all experiments. Curdlan and Zymosan were purchased from Sigma Chemical Co. (St Louis, MO), Zymosan A Texas Red conjugate was from Life Technologies (Carsbad, CA), and Pustulan from Elicityl SA (Crolles, France). Pneumocystis and Aspergillus β-glucan preparations were isolated as previously described (19, 20). MAPK inhibitors, PD98059 and JNK inhibitor II, the SYK inhibitor, Piceatannol, and the NF-κB inhibitor, Bay11-7085 were all obtained from Calbiochem, Inc. (San Diego, CA). AP-1 inhibitor, SR 11302, was from R&D Systems, Inc. (Minneapolis, MN). Phosphorothioate-protected CpG oligonucleotide ODN 2006 (5′-TCGTCGTTTTGTCGTTTTGTCGTT-3′) was commercially synthesized by Integrated DNA Technologies, Inc. The fMLF peptide was purchased from Sigma and recombinant human IL-8 was from R&D Systems, Inc. (Minneapolis, MN). Antibodies recognizing the cell signaling components SYK, p-SYK, ERK1/2, p-ERK1/2, JNK, p-JNK were from Cell Signaling Technology, Inc. (Danvers, MA). The neutralizing antibody for Dectin-1 was purchased from AbD Serotec (Raleigh, North Carolina) and for IL-8 was purchased from Abcam (Cambridge, MA). Goat anti-human Dectin-1 antibody from R&D Systems, Inc. (Minneapolis, MN) was used for immunoprecipitation and a Rabbit anti-human Dectin-1 antibody from LifeSpan BioSciences, Inc. (Seattle WA) for immunoblot. Isotype control antibody was from R&D Systems, Inc. Soluble glucan phosphate was a gift from Dr. Williams, East Tennessee State University, Johnson City. Murine RAW 264.7 macrophages were purchased from ATCC and cultured in Dulbecco modified Eagle medium containing 10% fetal bovine serum, 2 mM L-glutamine, penicillin (10,000 U/liter), and streptomycin (1 mg/liter). All the other reagents were obtained from Sigma-Aldrich (St. Louis, MO) unless specified otherwise.
Leucocyte Isolation and Culture
B-Lymphocytes were isolated from acid citrate dextrose anticoagulated blood obtained from healthy volunteer blood donors using RossetteSep B-cell enrichment cocktail according to the manufacturer’s protocol (StemCell Technologies, Vancouver, Canada). The enriched B-lymphocyte population was repeatedly observed to contain greater than 90% CD19+ B-lymphocytes. Cells were maintained in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum and 1% Antibiotic-Antimycotic solution (Life Technologies, Grand Island, NY). PMNs were isolated from heparinized whole blood (100 ml) that was collected from healthy human donors. The blood was layered over a Polymorphprep resolving media (Accurate Chemical, Westbury, NY) and spun at 500 × g for 35 min. The PMN rich fraction was collected and the RBCs lysed with Red Blood Cell Lysis Buffer. The PMNs were washed twice with phosphate buffered saline (PBS), and then resuspended in RPMI-1640 supplemented with 10% heat-treated fetal bovine serum and 1% Antibiotic-Antimycotic.
Cytokine and IgM detection
B-lymphocytes (4 × 105 cells/well in 96-well plates) were cultured with 100 μg/ml of the various β-glucan preparations or with 2.5 μg/ml of CpG, unless otherwise indicated, in a total of 0.2 ml culture medium for 24 hours or 5 days unless otherwise indicated. Cell supernatants from cells stimulated for 24 hours were then analyzed for IL-8 and IL-10 production or after 5 days stimulation for IgM production using Ready-SET-Go Elisa kits (eBioscience, Inc., San Diego, CA) according to the manufacturer’s instructions. Inhibitors and neutralizing antibodies were incubated with B-lymphocytes for 1 hour prior to addition the various β-glucan preparations.
Phagocytosis assay
Zymosan A Texas Red particles were rinsed with PBS, resuspended in culture medium, sonicated and mixed for different periods of time (0, 1, 4 and 8 hours) with Raw cells cultured on coverslips or B-lymphocytes that were transferred to a Poly-L lysine (Sigma-Aldrich, St. Louis, MO) coated coverslips. Cells were rinsed with PBS, fixed with 4% formaldehyde and mounted on slides. Cells were viewed with a Zeiss 510 Confocal Laser Scanning Microscope and images were captured using Zen2010 software.
Neutrophil chemotaxis
Chemotaxis of primary human PMNs was assessed in 24-well plates using a 3 μm-pore size Transwell filter system (Costar, Cambridge, MA). Migration buffer (400 μl) containing the indicated chemoattractants or conditioned media was added to the lower chamber and PMNs were loaded onto the inserts at a density of 1 × 106 cells/100 μl. The cells were then allowed to migrate for 2 hours at 37°C. Following incubations, 40 μl of 0.5 M EDTA was added to the lower chamber and the plate was incubated for 15 min at 4°C. The number of cells in the lower chamber was counted using a hemocytometer and the percentage of migrating cells was determined.
Proliferation assay
B-lymphocyte proliferation was determined by 3H-labeled thymidine incorporation during DNA synthesis. Briefly, B-lymphocytes were seeded at a density of 1.5 × 105 cells/well in 96-well plates. Cells were cultured with the indicated stimulants for 96 hours at 37°C in the presence of 5% CO2. The cells were pulsed with 3H-thymidine (8 μCi/ml) (Amersham, Buckinghamshire, England) during the last 18 hours of the 96-hour culture. Cells were harvested using a FilterMate Harvester (PerkinElmer), and uptake of 3H-thymidine was measured with a TopCount NXT Microplate Scintillation and a Luminescence Counter (PerkinElmer).
RNA isolation and real-time qPCR analysis
Total cellular RNA was isolated from B-lymphocytes using Tri-Reagent (Molecular Research Center, Inc, Cincinnati, OH) according to the manufacturer’s instructions. For cDNA synthesis, total RNA concentrations and purity was determined using a Nanodrop ND-1000 spectrophotometer, and 1 μg RNA was employed in a 20 μl reaction mixture using a Verso cDNA Synthesis Kit (Thermo scientific). Quantitative real-time PCR was performed in 10 μl reaction volumes in a 96-well plate using 2 μl of diluted cDNA with SYBR Premix Ex Taq (Clontech Laboratories, Inc.) on a CFX96 Real-Time PCR Detection System (Bio-Rad, Hercules, CA). The data were analyzed with CFX manager software, version 3.1 (Bio-Rad). Relative transcript expression of each target gene was determined using the comparative Ct method, and the amounts of various cytokines and receptor transcripts were normalized to GAPDH transcripts in the same cDNA samples. The results were expressed as % of the target gene relative to that of GAPDH (100%) and plotted as the mean ± standard error of the mean (SEM). The primer pairs used in these assays are listed in Supplement Table 1.
Cellular viability
Cell viability was assessed using the XTT Cell Proliferation Kit II (Roche Molecular Biochemicals, Indianapolis, IN) according to the manufacturer’s protocol. This assay measures the conversion of sodium-3′-[1-(phenylaminocarbonyl)-3,4-tetrazolium]-bis(4-methoxy-6-nitro) benzenesulfonic acid hydrate (XTT) to a formazan dye through electron coupling in metabolically active mitochondria using the coupling reagent N-methyldibenzopyrazine methyl sulfate. Only metabolically active cells are capable of mediating this reaction, which is detected by absorbance of the dye at 450–500 nm. Briefly, 50 μl of the XTT labeling mixture was added to the 100 μl of growth medium containing B-lymphocytes and different concentrations of the inhibitors. The XTT labeling mixture was added in parallel samples 24 hours after the addition of the various inhibitors. A set of blanks were also included that did not contain cells and were treated identically as the normal samples. In addition, a set of solvent controls was also included for each inhibitor. Absorbance was measured at 6 hours after XTT addition. All treatments were performed in multiple replicates. Only inhibitor concentrations that elicited less than 20% net toxicity were used in these assays.
Preparation of Cell Lysates, Immunoprecipitation and Western Blotting
Total cellular proteins were obtained from B-lymphocytes following the described culture conditions. Briefly, the cells were washed with cold PBS twice and lysed in RIPA buffer (50 mM Tris-HCl pH 7.4, 15 mM NaCl, 0.25% deoxycholic acid, 1% NP-40, 1 mM EDTA) freshly supplemented with 1 mM phenylmethylsulfonyl fluoride (PMSF), mammalian protease inhibitor mixture, 10 mM Na Fluoride and 1 mM Na Orthovanadate. Cells were kept on ice for 15 minutes and then the lysates were centrifuged at 12,000 × g for 10 min at 4°C. The resultant soluble supernatant contained total cellular protein. Protein concentrations were determined with the Bio-Rad protein assay (Hercules, CA) using BSA as the standard. For immunoprecipitation cell lysates were collected from multiple donors, pooled and 0.5 mg of total protein used for each analysis. Pierce Classic IP Kit (Pierce Biotech, Rockford, IL) was used according to manufacturer’s instructions. Equal amounts of total cellular proteins were separated on SDS-10% polyacrylamide gels with Precision Plus Protein Dual Color Standards (Bio-Rad; Hercules, CA) being used as the molecular weight standards. Proteins were then transferred to Immobilon-P membranes (Millipore, Bedford, MA). Membranes were blocked at room temperature for 1 hour with 2% BSA/PBST (PBS, pH 7.4, 2% BSA, 0.1% Tween 20) and incubated overnight at 4°C in a blocking solution containing primary antibodies at the appropriate dilutions. After washing with PBST, the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies for 1hour. Immunoreactive bands were detected with the ECL enhanced chemiluminescence Western blotting detection kit (Amersham, Buckinghamshire, England).
Statistical analyses
All data are presented as the mean ± SEM, from multiple experimental runs, unless otherwise stated. The data were first analyzed using one-way ANOVA and posttest Sidak’s comparisons unless otherwise indicated. Statistical analysis was performed using GraphPad Prism Version 6 (GraphPad Software, La Jolla, CA).
RESULTS
β-Glucan induces the secretion of pro-inflammatory cytokines by B-lymphocytes in the absence of T-lymphocytes
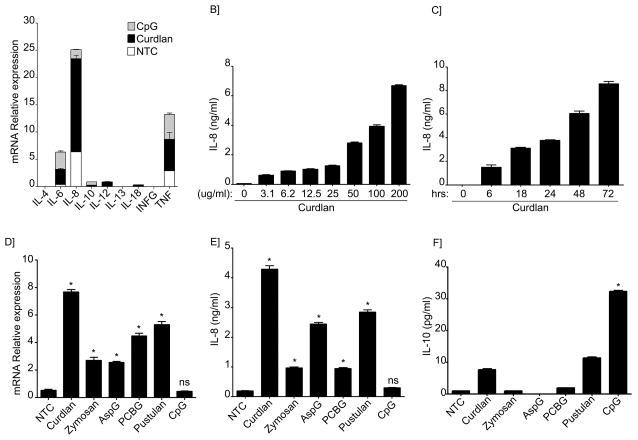
T-cell independent mechanisms of B-lymphocyte activation have been long recognized. For instance, bacterial components such as lipoproteins and CpG are well known to induce cytokine and antibody production upon TLR activation in B-lymphocytes (4, 5, 21, 22). More recently, Curdlan, a particulate product containing largely β-1,3-glucan, has also been shown to activate murine B-lymphocytes and induce IgM production similarly to CpG (18). To investigate whether human B-lymphocytes also responded to β-glucans, peripheral blood B-lymphocytes were isolated from healthy donors as described above and cultured in the presence of Curdlan. CpG was used as a positive control. As shown in Figure 1A, Curdlan-stimulated cells expressed a variety of pro-inflammatory cytokines including TNFα IL-6 and IL-8. Surprisingly, CpG-treated cells while they had similar amounts of IL-6 and TNFα they expressed very low levels of IL-8 (Figure 1A). In contrast, IL-8 mRNA expression levels from Curdlan-stimulated cells were significantly higher than the other cytokines tested. To further explore this observation, we determined the levels of IL-8 protein in the cell culture supernatants. As shown in Figure 1B and 1C, our results confirmed that cells released significant quantities of IL-8 following Curdlan stimulation.
Figure 1. Cytokine profile from β-glucan stimulated human peripheral B-lymphocytes differs from CpG stimulated cells.
(A) Quantitative real-time PCR for indicated mRNAs in non-treated cells (NTC) or B-cells stimulated with CpG or Curdlan. Stacked bars represent the relative expression of the cytokines tested after specific stimuli of B-lymphocytes as indicated. Segments of each bar are not additive. Expression was normalized to GAPDH. (B) IL-8 was measured by ELISA in the cell supernatant after Curdlan stimulation at the indicated concentrations or (C) after stimulation with Curdlan for the indicated period of time. (D) Quantitative real-time PCR for IL-8 mRNAs in NTC or B-cells stimulated with the different β-glucan preparations or CpG. Expression was normalized to GAPDH. (E) IL-8 and (F) IL-10 were measured by ELISA in the supernatants of NTC, after stimulation with different β-glucan preparations or CpG as indicated. Shown are data from a representative experimental run, typical of at least three separate experimental preparations, performed with different B-cell preparations. *p<0.0001 when compared with the NTC.
Next, since β-glucan preparations can be very diverse (soluble versus particulate, 1,3 β-glucan versus 1,6 β-glucan predominant linkages), we further investigated the stimulatory effect of various β-glucan preparations on IL-8 production. As shown in Figure 1D and E, all of the β-glucan preparations tested which included preparations with enriched particulate β-1,3-glucan contents (Zymosan, Aspergillus β-glucan and Pneumocystis β-glucan) and with soluble 1,6 β-glucan (Pustulan) induced significant amounts of IL-8. In addition, our results demonstrate that the relative expression of IL-8 mRNA correlated well with the levels of secreted protein (Figure 1D and E). Interestingly, compared with Curdlan-stimulated cells, CPG-treated cells induced higher amounts of IL-10 expression although the relative mRNA expression and protein secretion were overall in the low range (Figure 1A and F).
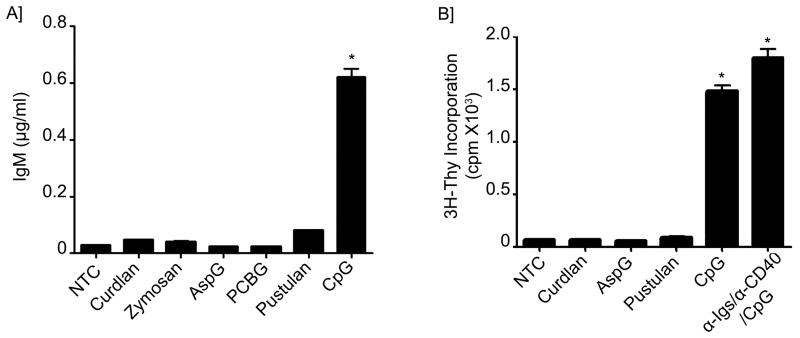
Subsequently, we investigated the ability of β-glucan to induce antibody production by determining the levels of IgM released into the cell supernatant after either β-glucan or CpG stimulation. Surprisingly, while CpG was able to induce large amounts of IgM, none of the β-glucan preparations tested induced significant IgM release (Figure 2A and Supplement Figure 1A). We further postulated that even though β-glucan did not itself induce IgM secretion it may still exert an additive stimulatory effect to CpG-mediated IgM secretion. Therefore, to test this hypothesis, B-lymphocytes were stimulated with Curdlan in combination with different concentrations of CpG. Interestingly, the addition of Curdlan to CpG failed to show any additive effect on CpG-mediated IgM production (Supplement Figure 1B).
Figure 2. Lack of IgM production and B-cell proliferation after β-glucan stimulation.
(A) IgM was measured by ELISA in the supernatants of B-lymphocytes from NTC, treated with different β-glucan preparations or CpG as indicated for a total of 5 days. (B) 3H-Thymidine incorporation was assessed in NTC, stimulated cells with different β-glucan preparations, CpG or Immunoglobulin cocktail control. Shown are data from a representative experimental run, typical of at least three separate experimental preparations, performed with different B-cell preparations. *p<0.0001 when compared with the NTC.
To further investigate whether the lack of IgM production was secondary to an inability of β-glucans to induce B-lymphocyte proliferation, 3H-Thymidine incorporation by the B-cells was assessed following β-glucan stimulation. Compared with CpG and the Immunoglobulin cocktail control, none of the β-glucan preparations induced significant B-cell proliferation, suggesting that β-glucans are not mitogens for human B-lymphocytes (Figure 2B).
Since fungal organisms express multiple antigenic determinants, we next investigated if Curdlan in combination with other potential receptors ligands would result in an enhanced B-lymphocyte response. Therefore, Curdlan was used in combination with BCR ligand (BCRL), TLR2 ligand (TLR2L) or different cytokines known to activate B-lymphocytes (23). Our results showed that with the exception of the combination of TLR2L and Curdlan, which clearly showed an additive effect on the production of IL-8, none of the other combinations resulted in a significant increase of IL-8 secretion, IgM production or B-lymphocyte proliferation (Supplement Figure 1C–H). Taken together, this data indicates that β-glucan-stimulated B-lymphocytes elicit different cytokine and antibody profiles compared with CpG-stimulated B-lymphocytes and suggests that they activate B-lymphocytes using different receptors and exert different functions in innate host responses to microorganisms.
Dectin-1 and SYK-mediated IL-8 secretion by β-glucan stimulated B-lymphocytes
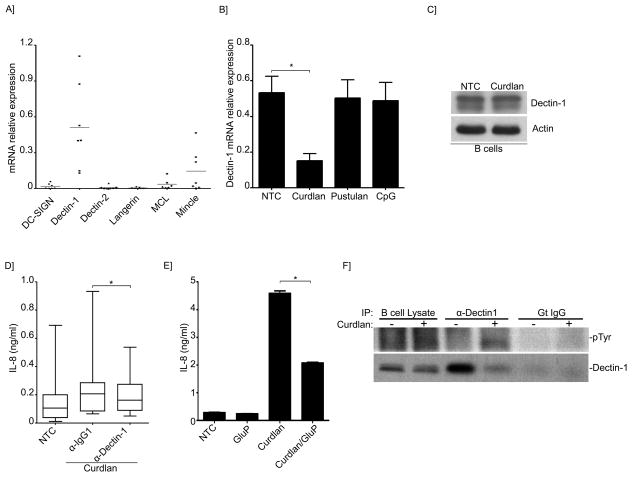
C-type lectins are important host receptors that recognize fungal organisms (24). In particular, Dectin-1 has been well characterized as the prominent β-1,3-glucan receptor in macrophages and dendritic cells (13, 15, 25, 26). C-lectin receptors including Dectin-1 have also been shown to be expressed by B-lymphocytes (27, 28). However, the role of these receptors in human B-lymphocytes remains poorly elucidated. Therefore, we further investigated the basal mRNA expression of the major C-lectin receptors in our human peripheral B-lymphocytes. As shown in Figure 3A and consistent with previous observations, Dectin-1 was expressed in all the donors tested, however the relative expression of this receptor varied considerably among the individual donors. In addition to Dectin-1, Mincle and to a lesser degree MCL were also expressed at baseline in some, but not all, of the individuals tested (Figure 3A). Other receptors including Dectin-2, DC-Sign and Langerin were not significantly expressed at baseline (Figure 3A). Remarkably, the relative expression of Dectin-1 decreased following β-glucan stimulation (Figure 3B). Next, Dectin-1 expression was confirmed at the protein level (Figure 3C). Interestingly, Dectin-1 was expressed slightly more in memory cells (CD27+) than in naïve (CD27−) cells, but the differences were not statistically significant (Supplement Figure 2). To further determine the participation of Dectin-1 in the IL-8 pathway, neutralizing antibodies to Dectin-1 or glucan phosphate, a soluble form of beta-glucan that acts as a competitive carbohydrate antagonist for Dectin-1(13, 29), were used prior to stimulation with Curdlan. IL-8 secretion following Curdlan stimulation was significantly decreased, although only partially, in the presence of the neutralizing antibodies and glucan phosphate (Figure 3D and E). While these results strongly infer the role of Dectin-1 in β-glucan signaling of B-lymphocytes, the partial inhibition of IL-8 indicate the potential involvement of other receptors. Further evidence confirming the participation of Dectin-1 in β-glucan signaling, were demonstrated by tyrosine phosphorylation at the immunoreceptor tyrosine-based activation motif (ITAM) being evident only after Curdlan stimulation compared with controls (Figure 3F). Activation of Dectin-1 upon ligand binding requires phosphorylation of this ITAM-motif, which is located in its cytoplasmic tail and mediates its intracellular signaling functions (30).
Figure 3. Dectin-1 participates in IL-8 secretion induced by β-glucans.
(A) Quantitative real-time PCR for the indicated C-lectin receptors mRNAs in B-lymphocytes. Expression was normalized to GAPDH. Shown are the data from 7 different B-cell preparations. (B) Quantitative real-time PCR for Dectin-1 mRNAs in B-lymphocytes of NTC, or after stimulation with Curdlan, pustulan or CpG. Expression was normalized to GAPDH. Shown are the data from 3 different B-cell preparations. *p< 0.05 (C) Immunoblot analysis of Dectin-1 in unstimulated or Curdlan-stimulated B-lymphocytes. (D)IL-8 was measured by ELISA in the supernatant of NTC and after Curdlan stimulation. Cells were pretreated with Dectin-1 neutralizing antibodies or isotype control as indicated. Data were analyzed by the non-parametric Wilcoxon matched-pairs signed rank test to compare the medians of 5 different B-cell preparations. (E) Secretion of IL-8 into the cell supernatant was measured by ELISA of NTC or Curdlan-stimulated cells. Cells were pretreated with glucan phosphate as indicated. Shown is a representative run of 3 independent experiments performed with 3 different B-cell preparations. *p< 0.01. (F) Endogenous Dectin-1 was immunoprecipitated from unstimulated and Curdlan-stimulated cells phospho-tyrosine antibody was used to detect Dectin-1 phosphorylation in the immunoprecipitates. Total Dectin-1 was used as a control.
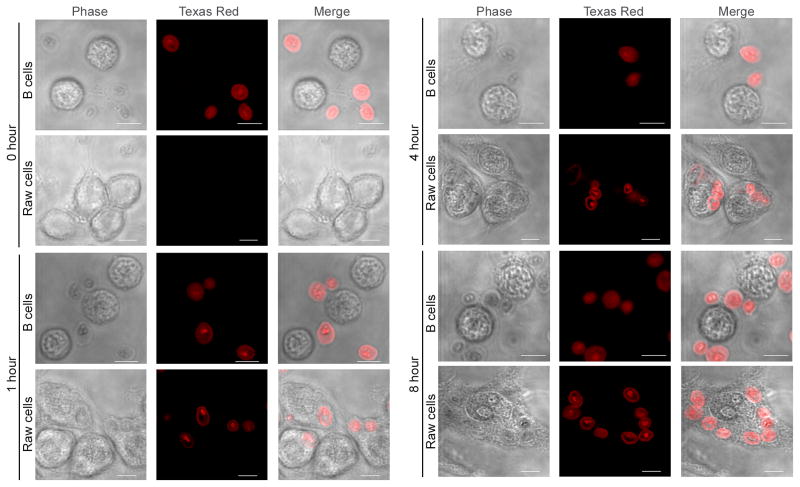
We next sought to investigate whether internalization of β-glucan particles was needed for IL-8 secretion. Fluorescently-labelled zymosan particles where incubated with B-lymphocytes for different periods of time and internalization was assessed by confocal fluorescence microscopy. RAW cells, a mouse macrophage cell line known to internalize β-glucan particles, were used as a positive control (31). Interestingly, after 1 hour incubation zymosan particles started to show association with the plasma membrane of the B-lymphocytes but there was no evidence of internalization of the particles even after incubation for 8 hours (Figure 4). To the contrary, RAW cells did show internalization of zymosan after 1 hour incubation, which was still evident at 4 and at 8 hours (Figure 4).
Figure 4. IL-8 secretion does not require internalization of β-glucan particles in B-lymphocytes.
Fluorescently labeled zymosan particles were incubated for the indicated periods of time with B-lymphocytes or RAW cells. Internalization was assessed by confocal fluorescence microscopy. Bar = 5 μm.
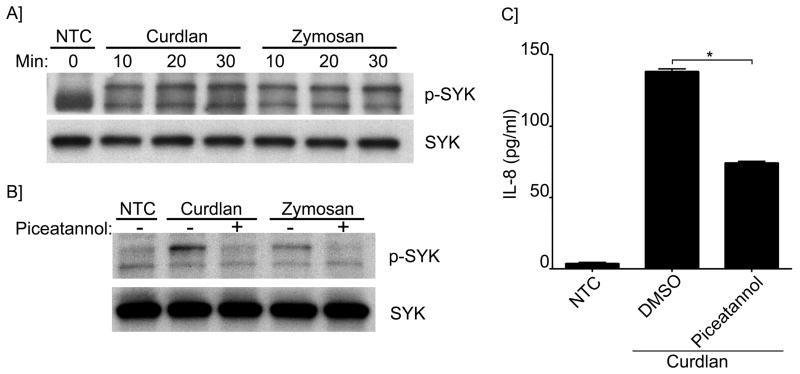
Dectin-1 activation usually involves SYK-mediated signaling pathways (30). We therefore next investigated the role of SYK after β-glucan stimulation. As shown in Figures 5A and B, SYK phosphorylation was induced in the presence of both Curdlan and Zymosan, and was inhibited by the SYK inhibitor, piceatannol. Moreover, IL-8 secretion was also found to be significantly decreased in the presence of piceatannol (Figure 5C). These results strongly support that Dectin-1 and SYK are involved in β-glucan mediated stimulation of B-cells leading to secretion of IL-8.
Figure 5. Syk activation and participation in IL-8 secretion induced by β-glucans.
(A) Immunoblot analysis of phospho- and total-Syk in unstimulated and stimulated cells with Curdlan or Zymosan as indicated; (B) for some conditions cells were pretreated with piceatannol as indicated. (C) IL-8 was measured by ELISA in the cell supernatant of NTC and after Curdlan stimulation. Cells were pretreated with piceatannol or vehicle control as indicated. *p<0.0001. Shown are data from a representative experimental run, typical of at least three separate experimental preparations, performed with different B-cell preparations.
ERK, JNK and the transcription factors NF-κB and AP-1 participate in IL-8 signaling triggered by β-glucan
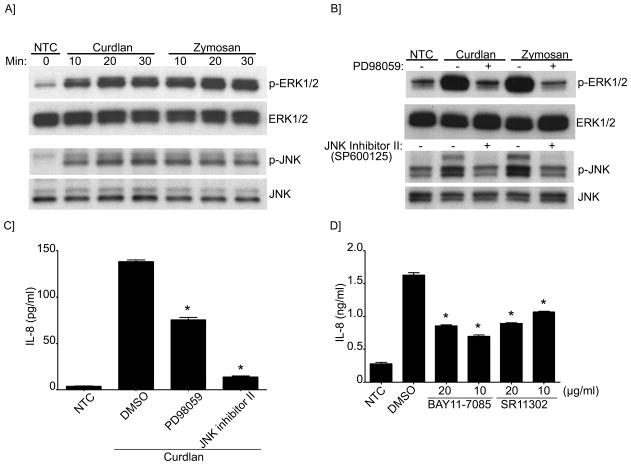
IL-8 secretion is mediated by MAPKs and the transcription factors NF-κB and AP-1 (32, 33). Accordingly, we next investigated the activation of ERK and JNK by β-glucans. As shown in Figure 6A and B, ERK and JNK were phosphorylated following β-glucan stimulation. Furthermore, the use of selective inhibitors for ERK and JNK abrogated β-glucan induced phosphorylation (Figure 6B) and impaired IL-8 secretion (Figure 6C). These effects were enhanced when the SYK inhibitor was used in combination with either ERK or JNK inhibitors, indicative of complementary pathways (Supplement Figure 3A). Next, we investigated the participation of the transcription factors NF-κB and AP-1 in the regulation of IL-8 using specific blocking reagents. Our data demonstrated that in the presence of the NF-κB and AP-1 inhibitors, IL-8 secretion was significantly impaired (Figure 6D), confirming their participation of these transcription factors in IL-8 activation following stimulation of B-cells with β-glucans.
Figure 6. MAPKs (ERK and JNK) and the transcription factors AP-1 and NF-κB participate in IL-8 secretion.
(A) Immunoblot analysis of ERK and JNK (phosphorylated and total) in NTC and stimulated cells with Curdlan or Zymosan as indicated; (B) for some conditions cells were pretreated with PD98059 (ERK inhibitor) or JNK inhibitor II as indicated. (C) IL-8 concentration was measured by ELISA in the cell supernatant of NTC and after Curdlan stimulation. Cells were pretreated with PD98059 and JNK inhibitor II or (D) BAY11 (NF-κB inhibitor), SR11 (AP-1 inhibitor) as indicated. DMSO was used as the vehicle control. Shown are data from a representative experimental run, typical of at least three separate experimental preparations, performed with different B-cell preparations. *p<0.0001 when compared with DMSO control.
B-lymphocyte activation by β-glucans promotes neutrophil chemotaxis
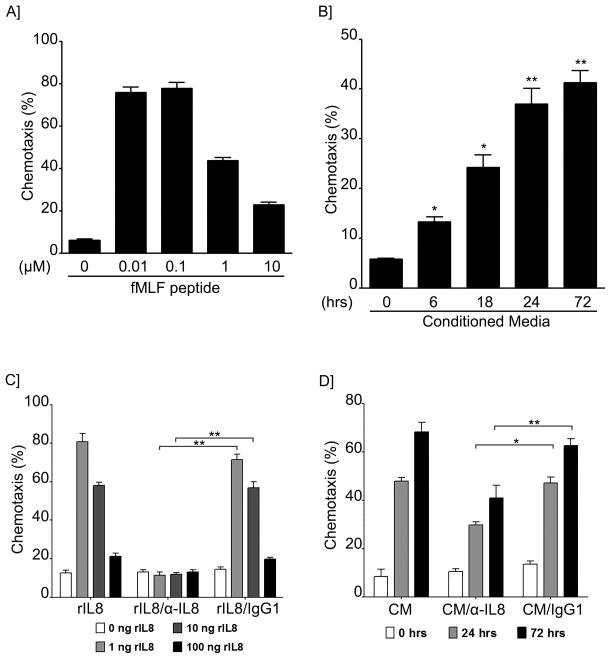
To further explore the role of β-glucan activated B-lymphocytes in innate immunity, and since IL-8 is an important chemoattractant for neutrophils, we next studied the chemotaxis response of freshly isolated neutrophils to the media derived from β-glucan activated B-cells. Our data had previously shown that the levels of IL-8 in the cell supernatant increased progressively within 72 hours of β-glucan stimulation (Figure 1C). Consistent with this, conditioned media from Curdlan β-glucan-stimulated cells containing increased levels of IL-8 were tested for their ability to stimulate chemotaxis of freshly isolated neutrophils. As anticipated, neutrophils exhibited a significant chemotactic response to the conditioned media from the β-glucan activated B-lymphocytes (Figure 7A and B). Furthermore, neutrophil chemotaxis increased in parallel to the concentration of IL-8 in the conditioned media (Figure 7B). The fMLF peptide, a well-known neutrophil chemoattractant, served as a positive control. To further demonstrate the participation of the secreted IL-8 from the activated B-lymphocytes in neutrophil recruitment, the conditioned media were pre-incubated with recombinant anti-IL-8 prior to addition to the neutrophil culture. As shown in Figure 7C and D, recombinant anti- IL-8 antibody significantly reduced neutrophil chemotaxis compared with isotype control. In addition to IL-8, increased mRNA expression of other chemokines such as CXCL-1, CXCL-2 and CXCL-3 was also observed (Supplement Figure 3B). These data indicate that β-glucan activated B-lymphocytes participate in the innate immune response by releasing pro-inflammatory cytokines and by inducing neutrophil chemotaxis. Moreover, β-glucan stimulated human B-lymphocytes are an important source of IL-8, which, among other activities, participate in neutrophil chemotaxis.
Figure 7. Conditioned media from β-Glucan-activated B-lymphocytes induced neutrophil chemotaxis and is partially mediated by IL-8.
Transwell chemotaxis assay of peripheral neutrophils towards different concentrations of (A) fMLF peptide (positive control) or (B) towards conditioned media form β-glucan treated B-lymphocytes for the indicated periods of time. Shown are data from a representative experimental run, typical of at least three separate experimental preparations, performed with different B-cell preparations. *p<0.05 and **p<0.01 when compared with NTC. Transwell chemotaxis assay of peripheral neutrophils towards different concentrations of recombinant IL-8 (C) or conditioned media (D) from β-glucan treated B cells in the presence or absence of anti-IL-8 or isotype control. *p<0.001 and **p<0.0001. Shown are data from a representative experimental run, typical of at least three separate experiments performed with different B cell preparations.
DISCUSSION
In this study we demonstrated that β-glucan can directly activate human B-lymphocytes leading to the generation of a cytokine response important for neutrophil recruitment. In particular, we have shown that β-glucan activated B-lymphocytes generate a cytokine signature that differs from the CpG-generated cytokine profile. While β-glucan activated cells induced higher levels of IL-8, CpG-stimulated cells secreted more IL-10. In addition, while CpG was able to induce B-cell proliferation and a secreted antibody response, β-glucan stimulated B-lymphocytes lacked the ability to do so even in the presence of different co-stimulatory signals (CPG, BCRL, TLR2L and cytokines). The lack of antibody production after β-glucan stimulation differs from the mouse model where Curdlan did seem to stimulate IgM secretion and B-lymphocyte proliferation (18). While the exact mechanisms that may explain the different response between mice and human are not known and could just be explained by the differences in the experimental methodology; there are clear evidences that mice and human immunology are in many aspects very diverse (34). One of the main obvious differences resides in their peripheral blood differential cell count. While human blood is rich in neutrophils (50–70%) the mouse blood has a predominance of lymphocytes (75–90%). These differences could potentially alter B-lymphocyte responses to infectious agents particularly since they have less circulating neutrophils. Class switching in response to IL-13 is also different between mice and humans as IL-13 has no effect in mice B-lymphocytes yet induces IgE class switching in humans (35). Moreover, molecules like BLNK, involved in Dectin-1 signaling, also seem to play different roles, as they can block B-lymphocyte development more severely in humans than in mice (34). While these are just a few examples, the different responses in proliferation and antibody production following β-glucan activation of mice and human B-lymphocytes may well add to the immunological diversity among different species. Herein, we also present evidence that human B-lymphocytes responses to two different antigens (DNA vs carbohydrate) are very dissimilar indicative that B-cell immunological responses are antigen-dependent. Our results further emphasize the important role of B-lymphocytes in the interface between the innate and the adaptive immune responses.
β-1,3-glucans can be elevated in the bronchoalveolar lavage and peripheral blood of patients with invasive fungal infections (10, 11). Interestingly, the levels of β-1,3-glucans are substantially higher in patients with Pneumocystis pneumonia compared with other fungal infections (10, 12, 36, 37). β-glucans, once thought to be merely involved in fungal cell wall rigidity, are now known to be potent immune regulators. In the particular case of Pneumocystis, we have previously shown that Pneumocystis derived β-glucans drive human dendritic cell activation and modulation of Th1 and Th17 responses important for fungal defense (14, 15). β-glucans utilize PRR expressed on the host cell surface, particularly Dectin-1, to activate antigen-presenting cells. (26) The data shown here is consistent with prior knowledge that Dectin-1 is present on human B-lymphocytes (28). Our data shows similar expression between memory and naïve B-lymphocytes. Interestingly, we noticed individual variations of Dectin-1 basal expression among donors, and expression of Dectin-1 did not increase following Curdlan stimulation. Furthermore, our data strongly indicate that Dectin-1 mediates β-glucan signaling since β-glucan stimulation resulted in Dectin-1 phosphorylation and both neutralizing Dectin-1 antibodies and glucan phosphate significantly decreased β-glucan mediated IL-8 secretion of B-lymphocytes. Our data also showed the participation of SYK, the MAPKs ERK and JNK, and the transcription factors AP-1 and NF-κB. Interestingly, β-glucan activation of IL-8 did not required β-glucan internalization. This is consistent with our prior published data in murine macrophages where β-glucan internalization was not required for activation of the downstream inflammatory pathways (31). Taken together these observations indicate that while Dectin-1 participates in β-glucan mediated B-lymphocyte activation and IL-8 secretion, alternative and parallel activation pathways beyond Dectin-1 alone, must be present, particularly in those patients with low levels of Dectin-1 expression. For instance, it is known that B-cells also express complement and scavenger receptors known to participate in β-glucan signaling (38, 39).
Immunity to opportunistic fungal infections requires the presence of functional CD4+ T-cell-mediated immune responses. This was widely recognized during the HIV/AIDS pandemic where patients suffered a wide range of opportunistic diseases in the setting of very severe CD4+ T-cell immunosuppression. In the case of Pneumocystis pneumonia, CD4+ cell levels less than 200 cells/μL place patients at significant risk for infection. However, patients with impaired B-lymphocyte responses alone as a consequence of B-cell depletion therapies such as Rituximab (a monoclonal antibody that binds to the CD20 antigen on B lymphocytes) have also been found to be susceptible to Pneumocystis pneumonia (40–42). While the exact mechanism through which B-cell deficient patients are susceptible to Pneumocystis pneumonia has not been fully elucidated, animal models of pneumonia suggest that B-lymphocytes play an important role modulating the CD4+ T-cell response. In particular, Lund et al (43) showed in their murine model of Pneumocystis pneumonia in which B-lymphocytes lacked MHC-II but had normal CD4+ T-cells, that the mice failed to clear the infection due to an inability of the CD4+ to mount a sustained or memory response. These data suggest that B-lymphocytes are important not only through antibody production, but also through their ability to regulate the quality of the CD4+ T-cell mediated immune response (43).
Moreover, B-lymphocytes are also being increasingly recognized for T-cell independent functions. In this regard, a recent study by Hoyt et al. (44) demonstrated that mice with lymphocyte impairment who also lacked type I interferon signaling developed progressive bone marrow failure (decreased total bone marrow cell count and neutrophils) following Pneumocystis infection. However, when these mice were reconstituted only with B-lymphocytes, they were protected from bone marrow failure, indicating that B-cells participated in the regulation of the hematopoiesis. The authors further verify that these activities were independent of CD4+ T cells and did not require migration of the B-lymphocytes to the bone marrow (44). Our data also provides evidence for a novel, and potentially important, role of B-lymphocytes as helpers of neutrophil recruitment. We hypothesize that the ability of B-lymphocytes to respond to circulating β-glucans allows them to initiate an inflammatory cascade involving recruitment of neutrophils, even in the absence of T-cells.
Neutrophils are an important first step in control of infection. Activated neutrophils migrate to the sites of inflammation where they participate in the clearance of microorganisms through phagocytosis and by release of antimicrobial molecules, as well as neutrophil extracellular traps (NETs). It is also now recognized that activated neutrophils provide signals that are important for the maturation of other innate immune cells (macrophage and dendritic cells) as well as for the regulation of T and B-cells. In particular, it is now better understood that neutrophils can contribute to B-cell survival and differentiation by releasing cytokines such as BAFF, APRIL and IL-21(45). Furthermore, recent investigations have shown that neutrophils accumulate in the splenic marginal zone where they activate B-lymphocytes to secrete antibodies (45). Our data indicates that β-glucan activated B-lymphocytes release factors such as IL-8 that promote the recruitment of neutrophils. In addition to helping with clearance the infection, neutrophil recruitment also likely contributes to further B-cell activation, which in turn subsequently induces even greater neutrophil recruitment further amplifying the host inflammatory response.
In an intact immune system the regulation of the inflammatory and anti-inflammatory pathways are tightly controlled resulting in a balanced immune response that is ultimately needed to successfully clear the infection. However, in immunocompromised patients where these pathways are altered, the consequences of an exaggerated immune response may be more detrimental than the infection itself. This is well documented in patients who suffer from Pneumocystis pneumonia as a result of hematological malignancies or other immunosuppressive disorders instead of AIDS (46). For instance, patients without AIDS who have Pneumocystis pneumonia exhibit lower number of organisms in the BAL while having increased neutrophilic infiltration in the lung and worsen oxygenation compared to patients with AIDS. These observations indicate that increased lung inflammation is associated with worsening respiratory failure and this is not directly related to the burden of infection itself (46). The data in our current study provides further evidence for the role of B-lymphocytes in the innate immune response and how these cells can further contribute to inflammation. These observations could potentially provide insights to modulate the immune response and minimize tissue damage from exuberant immune reactions to opportunistic fungal diseases such as Pneumocystis pneumonia.
In conclusion, we have demonstrated that human circulating B-lymphocytes can be activated by β-glucans, independently of T cells. β-glucan activated B-lymphocytes express a unique cytokine profile that differs from CpG stimulated B-Cells. While β-glucan is not able to induce the production of natural antibodies from the B-cells, these fungal components were able induce the release of pro-inflammatory cytokines important for neutrophil recruitment. These observations provide further evidence supporting the importance of B-lymphocytes in fungal innate immune responses.
Supplementary Material
Acknowledgments
Funding source: Funded by NIH grants K08 (HL112849) to EMC and R01 (HL62150) to AHL, and funds from the Annenberg Foundation to EMC and AHL.
We thank Drs. Diane Jelinek, Hirohito Kita, Anne J. Novak, the members of Dr. Limper’s laboratory and the Mayo Clinic Thoracic Diseases Research Unit for their many helpful discussions.
BIBLIOGRAPHY
- 1.Gerondakis S, Grumont RJ, Banerjee A. Regulating B-cell activation and survival in response to TLR signals. Immunol Cell Biol. 2007;85:471–475. doi: 10.1038/sj.icb.7100097. [DOI] [PubMed] [Google Scholar]
- 2.Meyer-Bahlburg A, Rawlings DJ. Differential impact of Toll-like receptor signaling on distinct B cell subpopulations. Front Biosci (Landmark Ed) 2012;17:1499–1516. doi: 10.2741/4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fillatreau S. Cytokine-producing B cells as regulators of pathogenic and protective immune responses. Ann Rheum Dis. 2013;72:ii80–84. doi: 10.1136/annrheumdis-2012-202253. [DOI] [PubMed] [Google Scholar]
- 4.Yehudai D, Snir A, Peri R, Halasz K, Haj T, Odeh M, Kessel A. B cell-activating factor enhances interleukin-6 and interleukin-10 production by ODN-activated human B cells. Scand J Immunol. 2012;76:371–377. doi: 10.1111/j.1365-3083.2012.02752.x. [DOI] [PubMed] [Google Scholar]
- 5.Klinman DM, Yi AK, Beaucage SL, Conover J, Krieg AM. CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc Natl Acad Sci U S A. 1996;93:2879–2883. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartmann G, Krieg AM. Mechanism and function of a newly identified CpG DNA motif in human primary B cells. J Immunol. 2000;164:944–953. doi: 10.4049/jimmunol.164.2.944. [DOI] [PubMed] [Google Scholar]
- 7.Wagner M, Poeck H, Jahrsdoerfer B, Rothenfusser S, Prell D, Bohle B, Tuma E, Giese T, Ellwart JW, Endres S, Hartmann G. IL-12p70-dependent Th1 induction by human B cells requires combined activation with CD40 ligand and CpG DNA. J Immunol. 2004;172:954–963. doi: 10.4049/jimmunol.172.2.954. [DOI] [PubMed] [Google Scholar]
- 8.Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, Koretzky GA, Klinman DM. Cpg Motifs in Bacterial-DNA Trigger Direct B-Cell Activation. Nature. 1995;374:546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 9.Krieg AM. CpG motifs: the active ingredient in bacterial extracts? Nat Med. 2003;9:831–835. doi: 10.1038/nm0703-831. [DOI] [PubMed] [Google Scholar]
- 10.Karageorgopoulos DE, Qu JM, Korbila IP, Zhu YG, Vasileiou VA, Falagas ME. Accuracy of beta-D-glucan for the diagnosis of Pneumocystis jirovecii pneumonia: a meta-analysis. Clin Microbiol Infect. 2013;19:39–49. doi: 10.1111/j.1469-0691.2011.03760.x. [DOI] [PubMed] [Google Scholar]
- 11.Odabasi Z, Mattiuzzi G, Estey E, Kantarjian H, Saeki F, Ridge RJ, Ketchum PA, Finkelman MA, Rex JH, Ostrosky-Zeichner L. Beta-D-glucan as a diagnostic adjunct for invasive fungal infections: validation, cutoff development, and performance in patients with acute myelogenous leukemia and myelodysplastic syndrome. Clin Infect Dis. 2004;39:199–205. doi: 10.1086/421944. [DOI] [PubMed] [Google Scholar]
- 12.Theel ES, Jespersen DJ, Iqbal S, Bestrom JE, Rollins LO, Misner LJ, Markley BJ, Mandrekar J, Baddour LM, Limper AH, Wengenack NL, Binnicker MJ. Detection of (1, 3)-beta-D-glucan in bronchoalveolar lavage and serum samples collected from immunocompromised hosts. Mycopathologia. 2013;175:33–41. doi: 10.1007/s11046-012-9579-y. [DOI] [PubMed] [Google Scholar]
- 13.Brown GD, Gordon S. Immune recognition. A new receptor for beta-glucans. Nature. 2001;413:36–37. doi: 10.1038/35092620. [DOI] [PubMed] [Google Scholar]
- 14.Carmona EM, Kottom TJ, Hebrink DM, Moua T, Singh RD, Pagano RE, Limper AH. Glycosphingolipids Mediate Pneumocystis Cell Wall beta-Glucan Activation of the IL-23/IL-17 Axis in Human Dendritic Cells. Am J Respir Cell Mol Biol. 2012 doi: 10.1165/rcmb.2011-0159OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carmona EM, Vassallo R, Vuk-Pavlovic Z, Standing JE, Kottom TJ, Limper AH. Pneumocystis cell wall beta-glucans induce dendritic cell costimulatory molecule expression and inflammatory activation through a Fas-Fas ligand mechanism. J Immunol. 2006;177:459–467. doi: 10.4049/jimmunol.177.1.459. [DOI] [PubMed] [Google Scholar]
- 16.LeibundGut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, Schweighoffer E, Tybulewicz V, Brown GD, Ruland J, Reis e Sousa C. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8:630–638. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- 17.Qi H, Denning TL, Soong L. Differential induction of interleukin-10 and interleukin-12 in dendritic cells by microbial toll-like receptor activators and skewing of T-cell cytokine profiles. Infect Immun. 2003;71:3337–3342. doi: 10.1128/IAI.71.6.3337-3342.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar H, Kumagai Y, Tsuchida T, Koenig PA, Satoh T, Guo Z, Jang MH, Saitoh T, Akira S, Kawai T. Involvement of the NLRP3 inflammasome in innate and humoral adaptive immune responses to fungal beta-glucan. J Immunol. 2009;183:8061–8067. doi: 10.4049/jimmunol.0902477. [DOI] [PubMed] [Google Scholar]
- 19.Vassallo R, Standing JE, Limper AH. Isolated Pneumocystis carinii cell wall glucan provokes lower respiratory tract inflammatory responses. J Immunol. 2000;164:3755–3763. doi: 10.4049/jimmunol.164.7.3755. [DOI] [PubMed] [Google Scholar]
- 20.Manners DJ, Masson AJ, Patterson JC. The heterogeneity of glucan preparations from the walls of various yeasts. J Gen Microbiol. 1974;80:411–417. doi: 10.1099/00221287-80-2-411. [DOI] [PubMed] [Google Scholar]
- 21.Agrawal S, Gupta S. TLR1/2, TLR7, and TLR9 signals directly activate human peripheral blood naive and memory B cell subsets to produce cytokines, chemokines, and hematopoietic growth factors. J Clin Immunol. 2011;31:89–98. doi: 10.1007/s10875-010-9456-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–760. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 23.Darce JR, Arendt BK, Wu X, Jelinek DF. Regulated expression of BAFF-binding receptors during human B cell differentiation. J Immunol. 2007;179:7276–7286. doi: 10.4049/jimmunol.179.11.7276. [DOI] [PubMed] [Google Scholar]
- 24.Hardison SE, Brown GD. C-type lectin receptors orchestrate antifungal immunity. Nat Immunol. 2012;13:817–822. doi: 10.1038/ni.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ariizumi K, Shen GL, Shikano S, Xu S, Ritter R, 3rd, Kumamoto T, Edelbaum D, Morita A, Bergstresser PR, Takashima A. Identification of a novel, dendritic cell-associated molecule, dectin-1, by subtractive cDNA cloning. J Biol Chem. 2000;275:20157–20167. doi: 10.1074/jbc.M909512199. [DOI] [PubMed] [Google Scholar]
- 26.Brown GD, Taylor PR, Reid DM, Willment JA, Williams DL, Martinez-Pomares L, Wong SY, Gordon S. Dectin-1 is a major beta-glucan receptor on macrophages. J Exp Med. 2002;196:407–412. doi: 10.1084/jem.20020470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawata K, Illarionov P, Yang GX, Kenny TP, Zhang W, Tsuda M, Ando Y, Leung PS, Ansari AA, Eric Gershwin M. Mincle and human B cell function. J Autoimmun. 2012;39:315–322. doi: 10.1016/j.jaut.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willment JA, Marshall AS, Reid DM, Williams DL, Wong SY, Gordon S, Brown GD. The human beta-glucan receptor is widely expressed and functionally equivalent to murine Dectin-1 on primary cells. Eur J Immunol. 2005;35:1539–1547. doi: 10.1002/eji.200425725. [DOI] [PubMed] [Google Scholar]
- 29.Graham LM, Tsoni SV, Willment JA, Williams DL, Taylor PR, Gordon S, Dennehy K, Brown GD. Soluble Dectin-1 as a tool to detect beta-glucans. J Immunol Methods. 2006;314:164–169. doi: 10.1016/j.jim.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 30.Brown GD. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nature reviews Immunology. 2006;6:33–43. doi: 10.1038/nri1745. [DOI] [PubMed] [Google Scholar]
- 31.McCann F, Carmona E, Puri V, Pagano RE, Limper AH. Macrophage internalization of fungal beta-glucans is not necessary for initiation of related inflammatory responses. Infect Immun. 2005;73:6340–6349. doi: 10.1128/IAI.73.10.6340-6349.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stein B, Baldwin AS., Jr Distinct mechanisms for regulation of the interleukin-8 gene involve synergism and cooperativity between C/EBP and NF-kappa B. Molecular and cellular biology. 1993;13:7191–7198. doi: 10.1128/mcb.13.11.7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kunsch C, Lang RK, Rosen CA, Shannon MF. Synergistic transcriptional activation of the IL-8 gene by NF-kappa B p65 (RelA) and NF-IL-6. J Immunol. 1994;153:153–164. [PubMed] [Google Scholar]
- 34.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 35.Snapper CM, Marcu KB, Zelazowski P. The immunoglobulin class switch: beyond “accessibility”. Immunity. 1997;6:217–223. doi: 10.1016/s1074-7613(00)80324-6. [DOI] [PubMed] [Google Scholar]
- 36.Marty FM, Koo S, Bryar J, Baden LR. (1->3)beta-D-glucan assay positivity in patients with Pneumocystis (carinii) jiroveci pneumonia. Ann Intern Med. 2007;147:70–72. doi: 10.7326/0003-4819-147-1-200707030-00018. [DOI] [PubMed] [Google Scholar]
- 37.Sax PE, Komarow L, Finkelman MA, Grant PM, Andersen J, Scully E, Powderly WG, Zolopa AR. Blood (1->3)-beta-D-glucan as a diagnostic test for HIV-related Pneumocystis jirovecii pneumonia. Clin Infect Dis. 2011;53:197–202. doi: 10.1093/cid/cir335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jozefowski S, Yang Z, Marcinkiewicz J, Kobzik L. Scavenger receptors and beta-glucan receptors participate in the recognition of yeasts by murine macrophages. Inflamm Res. 2012;61:113–126. doi: 10.1007/s00011-011-0395-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Bruggen R, Drewniak A, Jansen M, van Houdt M, Roos D, Chapel H, Verhoeven AJ, Kuijpers TW. Complement receptor 3, not Dectin-1, is the major receptor on human neutrophils for beta-glucan-bearing particles. Mol Immunol. 2009;47:575–581. doi: 10.1016/j.molimm.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 40.Martin-Garrido I, Carmona EM, Specks U, Limper AH. Pneumocystis pneumonia in patients treated with rituximab. Chest. 2013;144:258–265. doi: 10.1378/chest.12-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bonilla-Abadia F, Betancurt JF, Pineda JC, Velez JD, Tobon GJ, Canas CA. Pneumocystis jirovecii pneumonia in two patients with systemic lupus erythematosus after rituximab therapy. Clin Rheumatol. 2014;33:415–418. doi: 10.1007/s10067-013-2475-0. [DOI] [PubMed] [Google Scholar]
- 42.Chang H, Yeh HC, Su YC, Lee MH. Pneumocystis jiroveci pneumonia in patients with non-Hodgkin’s lymphoma receiving chemotherapy containing rituximab. J Chin Med Assoc. 2008;71:579–582. doi: 10.1016/S1726-4901(08)70173-4. [DOI] [PubMed] [Google Scholar]
- 43.Lund FE, Hollifield M, Schuer K, Lines JL, Randall TD, Garvy BA. B cells are required for generation of protective effector and memory CD4 cells in response to Pneumocystis lung infection. J Immunol. 2006;176:6147–6154. doi: 10.4049/jimmunol.176.10.6147. [DOI] [PubMed] [Google Scholar]
- 44.Hoyt TR, Dobrinen E, Kochetkova I, Meissner N. B Cells Modulate Systemic Responses to Pneumocystis murina Lung Infection and Protect On-Demand Hematopoiesis via T Cell-Independent Innate Mechanisms when Type I Interferon Signaling Is Absent. Infect Immun. 2015;83:743–758. doi: 10.1128/IAI.02639-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Puga I, Cols M, Barra CM, He B, Cassis L, Gentile M, Comerma L, Chorny A, Shan M, Xu W, Magri G, Knowles DM, Tam W, Chiu A, Bussel JB, Serrano S, Lorente JA, Bellosillo B, Lloreta J, Juanpere N, Alameda F, Baro T, de Heredia CD, Toran N, Catala A, Torrebadell M, Fortuny C, Cusi V, Carreras C, Diaz GA, Blander JM, Farber CM, Silvestri G, Cunningham-Rundles C, Calvillo M, Dufour C, Notarangelo LD, Lougaris V, Plebani A, Casanova JL, Ganal SC, Diefenbach A, Arostegui JI, Juan M, Yague J, Mahlaoui N, Donadieu J, Chen K, Cerutti A. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat Immunol. 2012;13:170–180. doi: 10.1038/ni.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Limper AH, Offord KP, Smith TF, Martin WJ., 2nd Pneumocystis carinii pneumonia. Differences in lung parasite number and inflammation in patients with and without AIDS. Am Rev Respir Dis. 1989;140:1204–1209. doi: 10.1164/ajrccm/140.5.1204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.