Abstract
Src and Fyn are two Src family kinase (SFK) members that are expressed in mammalian brains and play important roles in the regulation of a variety of neuronal and synaptic substrates. Here we investigated the responsiveness of these SFKs to changing dopamine receptor signals in dopamine responsive regions of adult rat brains in vivo. Pharmacological activation of dopamine D1 receptors (D1Rs) by a systemic injection of the selective agonist SKF81297 increased phosphorylation of SFKs at a conserved and activation-associated autophosphorylation site (Y416) in the striatum, indicating activation of SFKs following SKF81297 injection. The dopamine D2 receptor (D2R) agonist quinpirole had no effect. Blockade of D1Rs with an antagonist SCH23390 did not alter striatal Y416 phosphorylation, while the D2R antagonist eticlopride elevated it. Between Src and Fyn, SKF81297 seemed to preferentially facilitate Fyn phosphorylation. Activation of muscarinic acetylcholine M4 receptors (M4Rs) with a positive allosteric modulator VU0152100 suppressed SFK Y416 responses to SKF81297. Additionally, SKF81297 induced a correlated increase in phosphorylation of N-methyl-D-aspartate (NMDA) receptor GluN2B subunits at a Fyn site (Y1472), which was attenuated by VU0152100. SKF81297 also enhanced synaptic recruitments of active Fyn and GluN1/GluN2B-containing NMDA receptors. These data demonstrate that D1Rs regulate Fyn and downstream NMDA receptors in striatal neurons in vivo. Acetylcholine through activating M4Rs inhibits Fyn and NMDA receptors in their sensitivity to D1R signaling.
Keywords: Acetylcholine, glutamate, Fyn, D1 receptor, D2 receptor, muscarinic M4 receptor, NMDA, tyrosine phosphorylation
1. Introduction
A large number of non-receptor tyrosine kinases are classified into several subfamilies (Neet and Hunter, 1996). Among these subfamilies is the Src family kinase (SFK) which has nine known members, including Src, Fyn (isoform 1; also known as isoform a or FynB), Yes, Lyn, and Lck. These five SFKs are expressed in the mammalian brain (Omri et al., 1996; Kalia et al., 2004; Bongiorno-Borbone et al., 2005). In particular, Src and Fyn are enriched at synaptic sites and have been most thoroughly investigated for their vital roles in modulating excitatory synaptic transmission (Kalia et al., 2004; Ohnishi et al., 2011; Schenone et al., 2011). Available data show that Src and Fyn target the synaptic N-methyl-D-aspartate (NMDA) receptor and directly tyrosine-phosphorylate these receptors to modulate the strength and efficacy of synaptic transmission (Groveman et al., 2012; Trepanier et al., 2012). Of note, SFK activity is also regulated by a phosphorylation-dependent mechanism. Phosphorylation of a conserved residue, tyrosine 416 (Y416), in the activation loop by an autophosphorylation mechanism enables activation of the enzyme (Roskoski, 2005; Okada, 2012).
The striatum is a key structure in the basal ganglia and is linked to the pathogenesis of various neurodegenerative and neuropsychiatric disorders. This structure is characterized by abundant expression of dopamine D1 receptors (D1Rs) and D2 receptors (D2Rs), which are generally segregated into two types of medium spiny neurons (MSNs): D1Rs in striatonigral output neurons and D2Rs in striatopallidal output neurons (Gerfen et al., 1990; Aubert et al., 2000; Bertran-Gonzalez et al., 2010). Given that Src and Fyn are enriched in both output neurons (Pascoli et al., 2011), dopamine via activating D1Rs and D2Rs may actively modulate these SFKs. An early study showed that the D2R antagonist haloperidol increased striatal Fyn although not Src phosphorylation at Y416 in wild type but not Fyn knock out mice (Hattori et al., 2006). However, at present, detailed D1R and D2R linkage to Src and Fyn SFKs has not been fully studied in the striatum as well as other dopamine responsive brain regions.
In addition to 90-95% of MSNs, the striatum contains a few groups of interneurons. Among these interneurons are large aspiny cholinergic interneurons, which make up 1-2% of the total cell population and provide intrinsic acetylcholine (ACh) to the entire striatum via their extremely dense and branched axonal arbors (Bolam et al., 1984; Phelps et al., 1985). The principal receptor that ACh interacts with in the striatum is muscarinic ACh receptors (mAChRs), through which ACh acts as an inhibitory drive to balance tonic and phasic dopamine input and maintain MSN homeostasis. The supportive evidence is that mAChR blockade usually augmented dopamine-stimulated motor activity and striatal gene expression (Chou et al., 1992; Bernard et al., 1993; Morelli et al., 1993; Wang and McGinty, 1996a; 1996b; 1997).
Here we carried out a series of neuropharmacological experiments to investigate the distinct role of D1Rs and D2Rs in regulating SFK phosphorylation in vivo. The effect of D1R and D2R agonists and antagonists following a systemic injection on SFK Y416 phosphorylation was examined in the two key dopamine responsive regions: the striatum and medial prefrontal cortex (mPFC). In addition, the mAChR regulation of the dopamine-SFK system was investigated by assessing the effect of a positive allosteric modulator (PAM) selective for M4 mAChRs (M4Rs) on dopamine-induced SFK phosphorylation in both brain regions.
2. Methods
2.1. Animals
We used adult male Wistar rats (210-300 g; Charles River, New York, NY) that were housed in pairs. The animal room was on a 12-h/12-h light/dark cycle and was controlled at a constant temperature of 23°C and humidity of 50 ± 10% with food and water available ad libitum. All animal use and procedures were in strict accordance with the US National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Missouri-Kansas City.
2.2. Drug treatment
An intraperitoneal (i.p.) injection of systemically active agents was given to rats in a volume of 1 ml/kg. The dose of agents was calculated as the salt. Most pharmacological agents have been used in our previous work where their effective doses were chosen from (Wang and McGinty, 1995). Age-matched rats received an acute injection of saline (1 ml/kg) and served as controls.
2.3. Synaptic protein fractionation
Rats were deeply anesthetized with sodium pentobarbital (65 mg/kg, i.p.) and decapitated. Rat brains were removed immediately. We then cut brains into coronal slices. From these slices, the entire striatum, including the caudate putamen and nucleus accumbens, and the mPFC, including the anterior cingulate, prelimbic cortex and infralimbic cortex, were dissected at 4°C. The dissected brain tissue was homogenized in isotonic sucrose homogenization buffer (SHB) containing 0.32 M sucrose, 10 mM HEPES, pH 7.4, 2 mM EDTA, a protease inhibitor cocktail, and a phosphatase inhibitor cocktail (Thermo Scientific, Rochester, NY). After centrifugation at 800 g (10 min), the supernatant was collected and centrifuged again at 10,000 g (30 min) to obtain P2 pellets containing crude synaptosomal plasma membranes. To prepare postsynaptic density (PSD) fractions, the P2 pellet was washed once with 1 volume of SHB and centrifuged (10,000 g, 15 min, 4°C). We then resuspended the pellet in the SHB containing 0.5% Triton X-100 (v/v). After slow rotation for 20 min (4°C), the suspension was centrifuged (32,000 g, 20 min, 4°C) to obtain the supernatant (Triton X-100-soluble non-PSD membranes, i.e., peri/extrasynaptic and presynaptic membranes) and the PSD-enriched pellet (Triton X-100-insoluble postsynaptic membranes, also known as PSDI). All pellets (P2 and PSD) were resuspended and solubilized in SHB containing 0.5% Triton X-100, 1% sodium dodecyl sulfate (SDS), 1% deoxycholic acid, and 1 mM dithiothreitol with gentle rotation (1 h, 4°C). Protein concentrations were determined. Samples were stored at −80°C until use.
2.4. Western blot
Immunoblot was performed as described previously (Jin et al., 2013). Briefly, proteins were loaded and separated on SDS NuPAGE Novex 4-12% gels (Invitrogen, Carlsbad, CA). They were then transferred to the polyvinylidene fluoride membrane (Millipore, Bedford, MA). The membrane was blocked in a blocking buffer (5% nonfat dry milk in phosphate-buffered saline and 0.1% Tween 20) for 1 h, washed and incubated in the blocking buffer containing a primary rabbit or mouse antibody overnight at 4°C. The membrane was washed and incubated for 1 h in a horseradish peroxidase-linked secondary antibody against rabbit or mouse (Jackson Immunoresearch Laboratory, West Grove, PA). Immunoblots were visualized by the enhanced chemiluminescence reagent (GE Healthcare Life Sciences, Piscataway, NJ) with a MagicMark XP Western protein standard (Invitrogen) served for protein size determination. Optical density of immunoblots was measured using NIH gel analysis software. Values of optical density were normalized to actin or tubulin.
2.5. Immunoprecipitation
P2 pellets (synaptosomal fraction) were prepared as described above and were solubilized in SHB containing Triton X-100 (0.5%, v/v) and 1% sodium deoxycholate for 1 h at 4°C. Solubilized proteins were incubated with a mouse antibody against Src or Fyn. The complex was precipitated with 50% protein A or G agarose/sepharose bead slurry (GE Healthcare). Proteins were separated on Novex 4-12% gels and probed with a rabbit antibody against Src, Fyn, or phospho-Src family at Y416 (pan pY416). HRP-conjugated secondary antibodies and enhanced chemiluminescence were used to visualize proteins.
2.6. Antibodies and pharmacological agents
Primary antibodies used in this study include rabbit polyclonal antibodies against Src (Cell Signaling Technology, Danvers, MA), Fyn (Santa Cruz Biotechnology, Santa Cruz, CA), GluN1 (Millipore), GluN2A (Millipore), GluN2B (Millipore), phosphorylated GluN2B at tyrosine 1472 (pY1472; PhosphoSolutions), or actin (Sigma-Aldrich, St. Louis, MO), or mouse antibodies against Src (Cell Signaling), Fyn (Santa Cruz), or tubulin (Millipore). The rabbit antibody against pan pY416 was purchased from Cell Signaling. This antibody reacts with the Src family members when phosphorylated at the activation residue: Y416 (chicken Src), Y419 (rat Src), and Y420 (rat Fyn). Pharmacological agents, including (±)-6-chloro-PB hydrobromide (SKF81297), (-)-quinpirole hydrochloride, R(+)-SCH23390 hydrochloride, and S-(-)-eticlopride hydrochloride, were purchased from Sigma. VU0152100 [3-amino-N-(4-methoxybenzyl)-4,6-dimethylthieno[2,3-b]pyridine carboxamide] was purchased from Axon Medchem (Reston, VA). All agents were freshly prepared at the day of experiments. VU0152100 was dissolved in 10% Tween 80 and dH2O with the pH adjusted to approximately 7.0 using 1 N NaOH. Other agents were dissolved in physiological saline.
2.7. Statistics
The results are presented as means ± SEM and were statistically analyzed. In details, these results were analyzed using one-way analysis of variance (ANOVA) followed by Kruskal-Wallis comparison of groups or a Mann-Whitney test. Probability levels of < 0.05 were considered statistically significant.
3. Results
3.1. D1R agonist activates SFKs
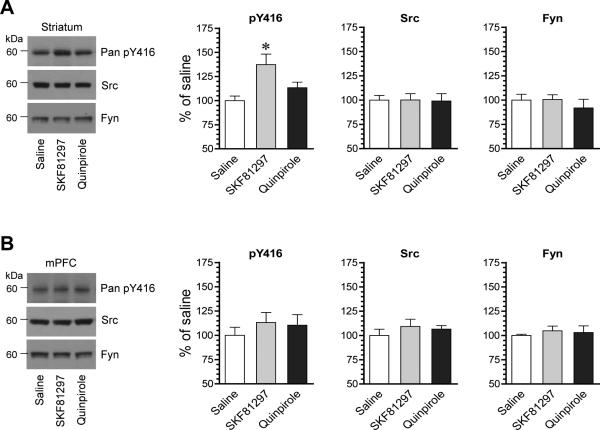
We first examined the responsiveness of SFKs in phosphorylation to D1R or D2R activation. To this end, we subjected rats to a single dose of the D1R agonist SKF81297 (3 mg/kg, i.p.) or the D2R agonist quinpirole (3 mg/kg, i.p.). We then sacrificed rats 20 min after drug injection to collect brain tissue for immunoblot analysis of changes in SFK phosphorylation. Using a pan Src pY416 antibody that detects phosphorylation of both rat Src and Fyn at a conserved common activation site (Src: Y419; Fyn: Y420), we found that SKF81297 induced a moderate increase in Y416 phosphorylation in the striatum, while quinpirole did not (Fig. 1A). In the mPFC, no change was observed in Y416 phosphorylation following administration of either agonist (Fig. 1B). In both brain regions, the total amount of Src and Fyn proteins showed a minimal change in SKF81297- or quinpirole-treated rats as compared to saline-treated control rats. These results indicate that selective stimulation of D1Rs activates SFKs as manifested by increased phosphorylation in an activation site in the striatum although not in the mPFC in vivo.
Figure 1. Effects of dopamine receptor agonists on Y416 phosphorylation in the rat striatum and mPFC.
(A) Effects of SKF81297 and quinpirole on Y416 phosphorylation and Src and Fyn expression in the striatum. Note that SKF81297 induced a significant increase in Y416 phosphorylation. (B) Effects of SKF81297 and quinpirole on Y416 phosphorylation and Src and Fyn expression in the mPFC. Representative immunoblots are shown left to the quantified data. Rats were given a single dose of SKF81297 (3 mg/kg, i.p.) or quinpirole (3 mg/kg, i.p.) and sacrificed 20 min after drug injection for immunoblot analysis. Data are presented as means ± SEM (n = 4 per group). *P < .05 versus saline (one-way ANOVA).
3.2. Effects of D1R and D2R antagonists on SFK phosphorylation
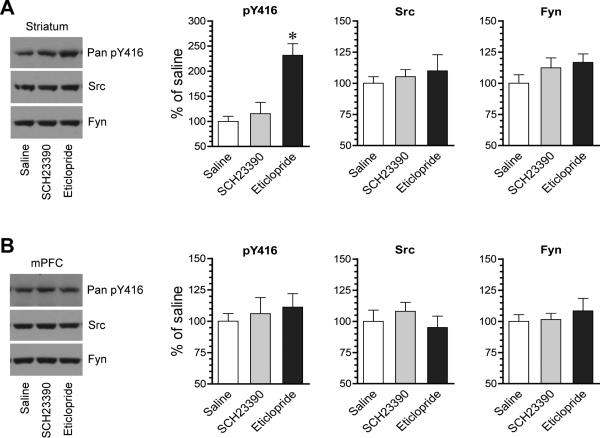
We next determined the role of two dopamine receptors in maintaining basal SFK phosphorylation by testing the impact of pharmacological blockade of D1Rs or D2Rs on SFK phosphorylation. The D1R antagonist SCH23390 (0.1-0.5 mg/kg, i.p.) or the D2R antagonist eticlopride (0.5 mg/kg, i.p.) was injected. Rats were sacrificed 20 min after drug injection for immunoblot analysis. In the striatum, SCH23390 did not significantly alter pY416 levels (Fig. 2A). Interestingly, in contrast to SCH23390, eticlopride markedly elevated Y416 phosphorylation. In the mPFC, neither SCH23390 nor eticlopride affected basal levels of pY416 proteins (Fig. 2B). The two antagonists had no significant effect on the total Src and Fyn abundance in the striatum and mPFC. These data reveal a minimal D1R drive in controlling basal SFK activity and an existence of an inhibitory tone of D2Rs on basal SFK Y416 phosphorylation in striatal neurons under normal conditions. As a result, blocking D2Rs releases this tonic inhibition, leading to a higher level of Y416 phosphorylation.
Figure 2. Effects of dopamine receptor antagonists on Y416 phosphorylation in the rat striatum and mPFC.
(A) Effects of SCH23390 and eticlopride on Y416 phosphorylation and Src and Fyn expression in the striatum. (B) Effects of SCH23390 and eticlopride on Y416 phosphorylation and Src and Fyn expression in the mPFC. Representative immunoblots are shown left to the quantified data. Rats were given a single dose of SCH23390 (0.1-0.5 mg/kg, i.p.) or eticlopride (0.5 mg/kg, i.p.) and sacrificed 20 min after drug injection for immunoblot analysis. Data are presented as means ± SEM (n = 4 per group). *P < .05 versus saline (one-way ANOVA).
3.3. M4Rs antagonize D1Rs in regulating SFK phosphorylation
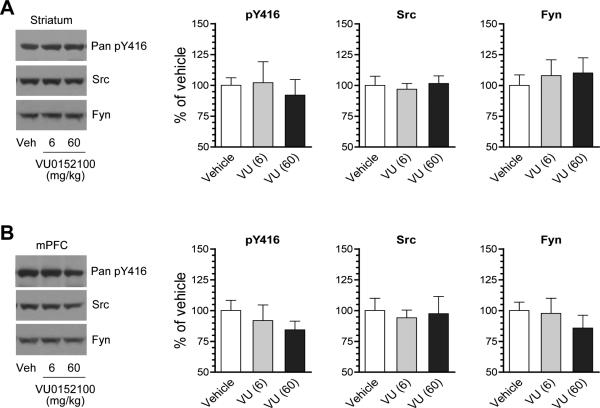
M4Rs are a principal subtype of mAChRs in the striatum and are found to be predominantly co-expressed with D1Rs in postsynaptic striatonigral neurons (Ince et al., 1997; Santiago and Potter, 2001). As Gαi/o-coupled receptors, M4Rs inhibit adenylyl cyclase and thus cAMP-dependent protein kinase A (PKA) (Wess, 1996). To determine the role of M4Rs in SFK phosphorylation, we investigated the effect of selective activation of M4Rs on SFK phosphorylation. VU0152100 is a systemically active PAM selective for M4Rs (Brady et al., 2008). We thus used this PAM to directly activate M4Rs. A single injection of VU0152100 at 6 mg/kg (i.p.) did not alter pY416 levels in the striatum as compared to vehicle control (Fig. 3A). At 60 mg/kg, VU0152100 induced a slight decrease in pY416 levels, although it did not reach a statistically significant difference (P > 0.05). In the mPFC, the PAM at both doses did not alter pY416 levels (Fig. 3B). The Src and Fyn abundance in the two areas remained unchanged following VU0152100 administration. The subtype selectivity of VU0152100 to M4Rs has been recently demonstrated in vivo: the PAM at 56.6 mg/kg (i.p.) largely blocked motor responses to the dopamine indirect agonist amphetamine in wild type rats and mice but not in M4R knockout mice (Brady et al., 2008; Byun et al., 2014). The inability of VU0152100 to alter pY416 levels suggests that M4R activation has a minimal impact on basal SFK phosphorylation in striatal and mPFC neurons.
Figure 3. Effects of the M4R PAM on Y416 phosphorylation in the rat striatum and mPFC.
(A) Effects of VU0152100 (VU) on Y416 phosphorylation and Src and Fyn expression in the striatum. (B) Effects of VU0152100 on Y416 phosphorylation and Src and Fyn expression in the mPFC. Representative immunoblots are shown left to the quantified data. Rats were given a single injection of vehicle (Veh) or VU0152100 (6 or 60 mg/kg, i.p.) sacrificed 20 min after drug injection for immunoblot analysis. Data are presented as means ± SEM (n = 4 per group).
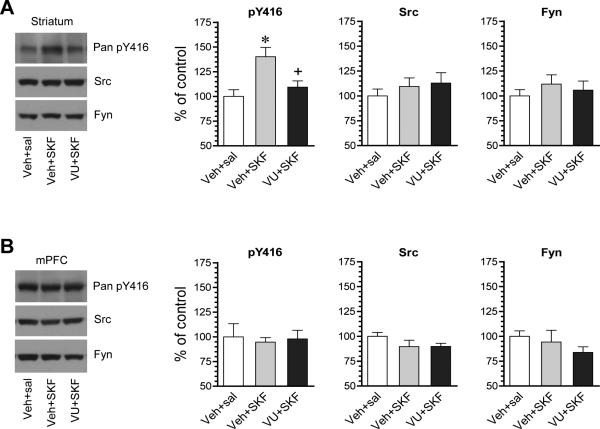
To determine the putative regulatory role of M4Rs in the D1R-mediated SFK phosphorylation, we tested the effect of the M4R PAM on the D1R agonist-stimulated SFK phosphorylation. In rats pretreated with VU0152100 (60 mg/kg, i.p.; 20 min prior to SKF81297), SKF81297 (3 mg/kg, i.p.; 20 min prior to tissue collection) no longer produced a significant increase in pY416 protein levels in the striatum (Fig. 4A). No change was observed in pY416 levels in the mPFC after all drug treatments (Fig. 4B). Src and Fyn in their abundances remained insensitive to SKF81297 or VU0152100 injected alone or jointly. These data demonstrate that activation of VU0152100-sensitive M4Rs suppresses the D1R agonist-induced SFK Y416 phosphorylation in striatal neurons.
Figure 4. Effects of the M4R PAM on SKF81297-stimulated Y416 phosphorylation in the rat striatum.
(A) Effects of VU0152100 (VU) on SKF81297 (SKF)-stimulated Y416 phosphorylation in the striatum. Note that VU0152100 significantly reduced the SKF81297-stimulated Y416 phosphorylation. (B) Effects of VU0152100 and SKF81297 coadministration on Y416 phosphorylation in the mPFC. Representative immunoblots are shown left to the quantified data. Rats were given vehicle (Veh) or VU0152100 (60 mg/kg, i.p.) 20 min prior to saline (sal) or SKF81297 (3 mg/kg, i.p.) and sacrificed 20 min after SKF81297 injection for immunoblot analysis. Data are presented as means ± SEM (n = 4 per group). *P < .05 versus vehicle + saline. +P < .05 versus vehicle + SKF81297 (one-way ANOVA).
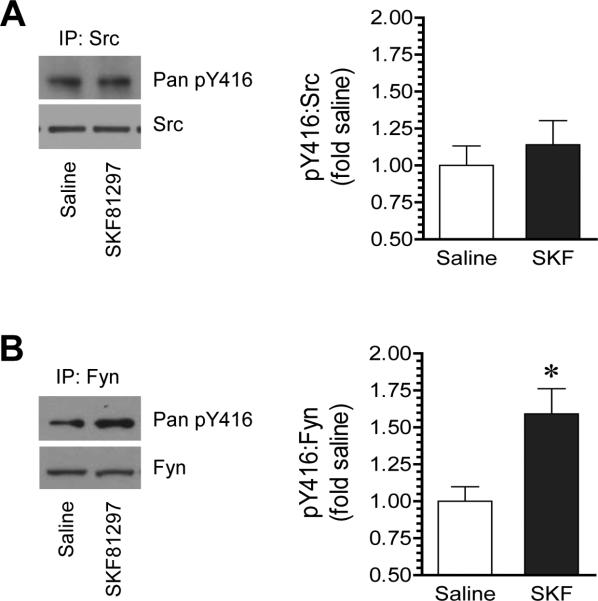
3.4. SKF81297 selectively activates Fyn
To determine the specific SFK family member(s) that were responsive to D1R signals, we tested the effect of SKF81297 on Y416 phosphorylation in immunopurified Src and Fyn proteins. Following SKF81297 injection (3 mg/kg, i.p., 20 min), rats were sacrificed. Striatal tissue was collected for immunoprecipitation with an anti-Src or anti-Fyn antibody. Changes in Y416 phosphorylation were then detected in Src and Fyn immunoprecipitates. We did not observe a significant change in pY416 signals in Src precipitates in response to SKF81297 (Fig. 5A). Remarkably, pY416 protein levels in Fyn precipitates were markedly enhanced in SKF81297-treated rats relative to saline-treated rats (Fig. 5B). These data indicate that D1R activation preferentially activates Fyn in the striatum. A much higher level of Fyn than Src abundance in striatal neurons is noteworthy (Pascoli et al., 2011).
Figure 5. Effects of SKF81297 on phosphorylation of Src and Fyn in the rat striatum.
(A) Effects of SKF81297 on phosphorylation of immunopurified Src poteins. (B) Effects of SKF81297 on phosphorylation of immunopurified Fyn proteins. Note that SKF81297 selectively increased Fyn phosphorylation. Representative immunoblots are shown left to the quantified data. Rats were given a single dose of SKF81297 (3 mg/kg, i.p.) and sacrificed 20 min after drug injection for immunoprecipitation (IP) of Src and Fyn. Data are presented as means ± SEM (n = 4-5 per group). *P < .05 versus saline (Mann-Whitney test).
3.5. D1Rs and M4Rs regulate tyrosine phosphorylation of GluN2B
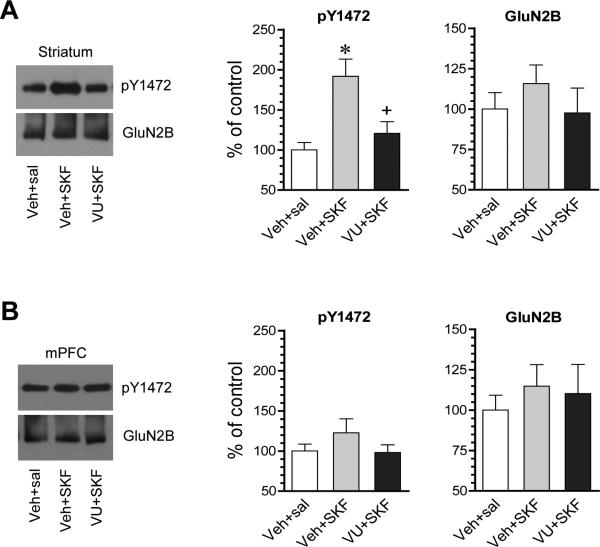
Fyn phosphorylated NMDA glutamate receptors in HEK 293T cells co-expressed NMDA receptors and Fyn (Nakazawa et al., 2001). A principal phosphorylation site is Y1472 in C-terminal tails of GluN2B subunits (Cheung and Gurd, 2001; Nakazawa et al., 2001). Fyn is also believed to phosphorylate GluN2B Y1472 in the telencephalon since Y1472 phosphorylation was much less in Fyn knockout mice than wild-type mice (Nakazawa et al., 2001). We thus evaluated whether the D1R agonist that activated Fyn in above studies alters GluN2B Y1472 phosphorylation in striatal neurons. SKF81297 (3 mg/kg, i.p.; 20 min) increased phosphorylation of GluN2B at Y1472 in the striatum as detected by a phospho- and site-specific antibody (Fig. 6A). In the mPFC, SKF81297 did not significantly alter Y1472 phosphorylation (Fig. 6B). Thus, D1R activation was effective to induce concurrent phosphorylation of GluN2B Y1472 in striatal neurons. We next tested whether the D1R agonist-stimulated GluN2B phosphorylation is sensitive to the M4R PAM which was demonstrated to block the D1R-triggered Fyn activation. We found that the M4R PAM VU0152100 (60 mg/kg, i.p.; 20 min prior to SKF81297) largely reduced the Y1472 phosphorylation induced by SKF81297 (Fig. 6A). This indicates that the D1R-regulated GluN2B phosphorylation, similar to the D1R-triggered Fyn activation, is subject to the inhibitory modulation by M4Rs.
Figure 6. Effects of the M4R PAM on SKF81297-stimulated Y1472 phosphorylation in the rat striatum and mPFC.
(A) Effects of VU0152100 (VU) on SKF81297 (SKF)-stimulated Y1472 phosphorylation in the striatum. Note that VU0152100 reduced the SKF81297-stimulated Y1472 phosphorylation. (B) Effects of VU0152100 and SKF81297 coadministration on Y1472 phosphorylation in the mPFC. Representative immunoblots are shown left to the quantified data. Rats were given vehicle (Veh) or VU0152100 (60 mg/kg, i.p.) 20 min prior to saline (sal) or SKF81297 (3 mg/kg, i.p.) and sacrificed 20 min after SKF81297 injection for immunoblot analysis. Data are presented as means ± SEM (n = 4 per group). *P < .05 versus vehicle + saline. +P < .05 versus vehicle + SKF81297 (one-way ANOVA).
3.6. SKF81297 enhances synaptic delivery of Fyn and NMDA receptors
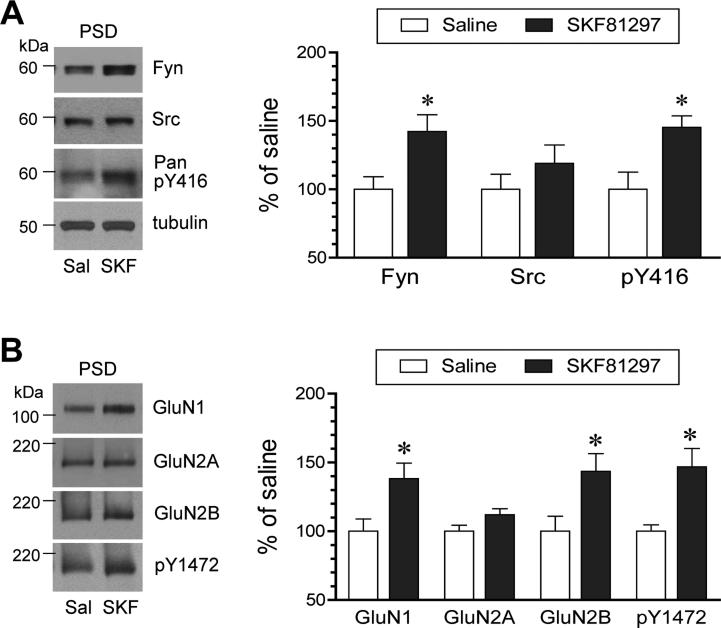
To determine whether SKF81297 impacts synaptic trafficking of SFKs and NMDA receptors, we monitored changes in the abundance of these proteins in the PSD compartment. As shown in Fig. 7A, SKF81297 (3 mg/kg, i.p.; 45 min) increased an amount of Fyn proteins in PSD samples extracted from rat striatal tissue. The pY416 protein level was also enhanced in parallel, although the Src protein level was not significantly affected by the agonist. Apparently, D1R stimulation leads to concurrent increases in Fyn and phosphorylated Fyn proteins in the PSD. As to synaptic NMDA receptor expression, we found that SKF81297 induced a moderate increase in the abundance of GluN1 and GluN2B proteins in striatal PSD fractions (Fig. 7B). The agonist also showed a tendency to elevate GluN2A proteins, although it did not reach a significantly statistical level. A higher level of pY1472 protein signals was detected in the same samples. These results suggest that D1R stimulation enhances the synaptic recruitment of NMDA receptors in striatal neurons, simultaneously along with increased synaptic delivery of Fyn.
Figure 7. Effects of SKF81297 on SFK and NMDA receptor expression and phosphorylation in the PSD of rat striatal neurons.
(A) Effects of SKF81297 (SKF) on Fyn and Src expression and Y416 phosphorylation in the PSD. Note that SKF81297 enhanced Fyn expression and Y416 phosphorylation. (B) Effects of SKF81297 on NMDA receptor subunit expression and Y1472 phosphorylation in the PSD. Note that SKF81297 enhanced GluN1 and GluN2B expression and Y1472 phosphorylation. Representative immunoblots are shown left to the quantified data. Rats were given an injection of SKF81297 (3 mg/kg, i.p.) and sacrificed 45 min after SKF81297 injection for biochemical fractionation of PSD proteins. Data are presented as means ± SEM (n = 4 per group). *P < .05 versus saline (Mann-Whitney test).
4. Discussion
This study investigated the role of dopamine and ACh in regulating SFK phosphorylation. We found that D1R activation enhanced SFK Y416 phosphorylation in the striatum although not the mPFC. M4R activation suppressed Y416 responses to the D1R agonist. Between Src and Fyn, the D1R agonist seemed to selectively target Fyn. Finally, the D1R agonist induced parallel phosphorylation of NMDA receptor GluN2B subunits at a Fyn site (Y1472) and synaptic trafficking of Fyn and GluN1/2B. These data demonstrate that SFKs (primarily Fyn) in striatal neurons are subject to the modulation by D1Rs and M4Rs and are likely engaged in linking dopamine and Ach signaling to NMDA receptors.
Due to the homology in amino acid sequences and structures, SFKs share a common step for activation (Thomas and Brugge, 1997). Both Src and Fyn have an N-terminal region containing regulatory Src homology 2 and 3 (SH2 and SH3) domains and a large C-terminal catalytic domain with active intramolecular interactions. In an inactive state, the SH2 domain binds to the catalytic domain. Activation signals release SH2 and transform the kinase into an active state. Active SFKs then autophosphorylate Y416 and render high enzymatic activity. As such, Y416 phosphorylation has been widely used as a marker of SFK activation. By measuring Y416 phosphorylation, we found that dopamine activates SFKs in a receptor subtype-, kinase isoform-, and brain region-specific manner.
The direct effect of dopamine receptor stimulation on SFK phosphorylation in the striatum has been less studied. Given a well-defined role of D1Rs in upregulating tyrosine phosphorylation and activity of NMDA receptors in striatal neurons via SFKs (see below), D1Rs are believed to be positively coupled to SFKs. Indeed, we found that the D1R agonist SKF81297 enhanced striatal SFK phosphorylation. Similarly, D1R agonists increased Fyn but not Src phosphorylation in mouse cultured striatal neurons (Pascoli et al., 2011) and rat hippocampal slices (Yang et al., 2012). In contrast to D1Rs, the D2R agonist quinpirole unaffected SFK phosphorylation in the striatum, while the D2R antagonist eticlopride (this study) or haloperidol (Hattori et al., 2006) elevated it. Thus, D2Rs, as opposed to D1Rs, are negatively coupled to SFKs. D1Rs and D2Rs are believed to target SFKs in the D1R-bearing striatonigral and D2R-bearing striatopallidal MSNs, respectively, although direct evidence for this cell type-specific event needs to be provided in the future. The D1R- and D2R-associated cAMP-PKA pathway is assumed to link these receptors to SFKs. In heterologous cells in vitro, PKA phosphorylated a specific residue (Fyn S21/Src S17) to allow Y416 autophosphorylation and activation of SFKs (Schmitt and Stork, 2002; Yeo et al., 2011). The PKA activator forskolin also activated Fyn although not Src in mouse spinal dorsal horn neurons (Yang et al., 2011). However, the D1R agonist SKF38393 activated Fyn in mouse cultured striatal neurons via the β subunit of G proteins but not the Gα-coupled cAMP-PKA pathway (Pascoli et al., 2011). Future studies need to clarify whether this is the case in vivo and whether the signaling pathway linking dopamine receptors to SFKs is cell type-specific. Of note, adenosine A2a receptors form heteromeric complexes with D2Rs in striatopallidal neurons (Ferraro et al., 2012). Thus, it is intriguing to investigate whether the A2a-D2R interplay plays a role in the regulation of SFKs in the indirect pathway.
Both Src and Fyn are associated with and regulate NMDA receptors (Kalia et al., 2004; Ohnishi et al., 2011; Groveman et al., 2012; Trepanier 2012). A general model is that SFKs phosphorylate Y1472 in GluN2B, a predominant phosphotyrosine protein in the PSD (Kennedy, 1997), to increase synaptic delivery of NMDA receptors and enhance channel activity. Src and Fyn seem to exert a region-specific role. In the striatum, Fyn is noticeably expressed to a much greater degree than Src (Pascoli et al., 2011) and is a preferred SFK member linking D1Rs to NMDA receptors. In details, the D1R agonist SKF82958 activated Fyn to tyrosine-phosphorylate and synaptically deliver NMDA receptor subunits including GluN2B in rat striatal but not cortical and cerebellar slices in vitro (Dunah and Standaert, 2001; Dunah et al., 2004). SKF82958 but not quinpirole increased GluN2B Y1472 phosphorylation and selectively triggered insertion of GluN2B but not GluN2A into the plasma membrane in cultured striatal neurons, which was blocked by the tyrosine kinase inhibitor genistein (Hallett et al., 2006). SKF38393 activated Fyn rather than Src to phosphorylate GluN2B Y1472 to augment NMDA receptor-mediated Ca2+ influx in mouse cultured striatal neurons (Pascoli et al., 2011). The Fyn-GluN2B coupling in the dorsal striatum was critical for alcohol drinking behavior (Wang et al., 2007). Thus, even though there was a Src-GluN2A system in the striatum (Taniguchi et al., 2009), Fyn is a prominent kinase linking D1Rs to GluN2B. Consistent with this, we found that D1R stimulation preferentially activated Fyn and led to a concurrent increase in GluN2B Y1472 phosphorylation and synaptic delivery of GluN2B in rat striatal neurons in vivo. On the contrary, D1R signals did not significantly affect Fyn and GluN2B phosphorylation in the mPFC. This indicates that the D1R regulation of the two proteins is region-specific. While mechanisms underlying this region difference are unclear at present, it may be derived from the difference of the two regions in cytostructural, neurochemical, and physiological properties (Tritsch and Sabatini, 2012; Volman et al., 2013).
Although the non-selective mAChR agonist carbachol increased Src Y416 phosphorylation in a human-derived cell line (Watcharasit et al., 2001), the M4R-mediated subtype-specific regulation of SFKs is unclear. The striatum is among brain areas with the highest level of M4Rs. Specifically, M4Rs are colocalized with D1Rs in striatonigral MSNs (Ince et al., 1997; Santiago and Potter, 2001). With a recently available M4R selective and systemically active PAM (Brady et al., 2008), one could investigate the M4R-SFK connection in adult animal brains in vivo. In fact, we found that activation of M4Rs with the M4R PAM VU0152100 did not alter constitutive SFK phosphorylation. Interestingly, VU0152100 activity-dependently inhibited SFK responses to the D1R agonist. Gαi protein-coupled M4Rs, as opposed to Gαs protein-coupled D1Rs, are negatively coupled to the cAMP-PKA pathway (Wess, 1996). Thus, activation of M4Rs may inhibit the response of the cAMP-PKA pathway to D1R signals and thereby suppress the D1R/PKA-dependent activation of Fyn. Our results support a model in which the two receptors form a delicate balance to control SFK activity, which contributes to maintaining the homeostasis of MSNs and basal ganglia outflow related to behavior (Jeon et al., 2010).
A dopamine D1 receptor (D1R) agonist increased Fyn phosphorylation in the striatum.
A positive modulator of muscarinic M4 receptors (M4R) inhibited this increase.
D1Rs increased tyrosine phosphorylation of GluN2B, while M4Rs decreased it.
D1Rs and M4Rs form a balance regulating Fyn and NMDA receptors in striatal neurons.
Acknowledgements
This work was supported by NIH grants R01DA10355 (JQW) and R01MH61469 (JQW). Authors wish to thank Drs. Bing Xue and Elton Chen for their technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Chemical compounds studied in this article
SKF81297 (PubChem CID: 1218); (-)-quinpirole hydrochloride (PubChem CID: 55397);
SCH23390 (PubChem CID: 5018); S-(-)-eticlopride hydrochloride (PubChem CID: 11973707);
VU0152100 (PubChem CID: 864492)
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Aubert I, Ghorayeb I, Normand E, Bloch B. Phenotypical characterization of the neurons expressing the D1 and D2 dopamine receptors in the monkey striatum. J. Comp. Neurol. 2000;418:22–32. [PubMed] [Google Scholar]
- Bernard V, Dumartin B, Lamy E, Bloch B. Fos immunoreactivity after stimulation or inhibition of muscarinic receptors indicates anatomical specificity for cholinergic control of striatal efferent neurons and cortical neurons in the rat. Eur. J. Neurosci. 1993;5:1218–1225. doi: 10.1111/j.1460-9568.1993.tb00976.x. [DOI] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Herve D, Girault JA, Valjent E. What is the degree of segregation between striatonigral and striatopallidal projections? Front Neuroanat. 2010;4:136. doi: 10.3389/fnana.2010.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolam JP, Wainer BH, Smith AD. Characterization of cholinergic neurons in the rat neostriatum. A combination of choline acetyltransferase immunocytochemistry, Golgi-impregnation and electron microscopy. Neuroscience. 1984;12:711–718. doi: 10.1016/0306-4522(84)90165-9. [DOI] [PubMed] [Google Scholar]
- Bongiorno-Borbone L, Kadare G, Benfenati F, Girault JA. FAK and PYK2 interact with SAP90/PSD-95-associated protein-3. Biochem. Biophys. Res. Commun. 2005;337:641–646. doi: 10.1016/j.bbrc.2005.09.099. [DOI] [PubMed] [Google Scholar]
- Brady AE, Jones CK, Bridges TM, Kennedy JP, Thompson AD, Heiman JU, Breininger ML, Gentry PR, Yin H, Jadhav SB, Shirey JK, Conn PJ, Lindsley CW. Centrally active allosteric potentiators of the M4 muscarnic acetylcholine receptor reverse amphetamine-induced hyperlocomotor activity in rats. J. Pharmacol. Exp. Ther. 2008;327:941–953. doi: 10.1124/jpet.108.140350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun NE, Grannan M, Bubser M, Barry RL, Thompson A, Rosanelli J, Gowrishankar R, Kelm ND, Damon S, Bridges TM, Melancon BJ, Tarr JC, Brogan JT, Avison MJ, Deutch AY, Wess J, Wood MR, Lindsley CW, Gore JC, Conn PJ, Jones CK. Antipsychotic drug-like effects of the selective M4 muscarinic acetylcholine receptor positive allosteric modulator VU0152100. Neuropsychopharmacology. 2014;39:1578–1593. doi: 10.1038/npp.2014.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung HH, Gurd JW. Tyrosine phosphorylation of the N-methyl-D-aspartate receptor by exogenous and postsynaptic density-associated Src-family kinases. J. Neurochem. 2001;78:524–534. doi: 10.1046/j.1471-4159.2001.00433.x. [DOI] [PubMed] [Google Scholar]
- Chou H, Ogawa N, Asanuma M, Hirata H, Mori A. Muscarinic cholinergic receptor-mediated modulation on striatal c-fos mRNA expression induced by levodopa in rat brain. J. Neural. Transm. 1992;90:171–181. doi: 10.1007/BF01250959. [DOI] [PubMed] [Google Scholar]
- Dunah AW, Sirianni AC, Fienberg AA, Bastia E, Schwarzschild MA, Standaert DG. Dopamine D1-dependent trafficking of striatal N-methyl-D-aspartate glutamate receptors requires Fyn protein tyrosine kinase but not DARPP-32. Mol. Pharmacol. 2004;65:121–129. doi: 10.1124/mol.65.1.121. [DOI] [PubMed] [Google Scholar]
- Dunah AW, Standaert DG. Dopamine D1 receptor-dependent trafficking of striatal NMDA glutamate receptors to the postsynaptic membrane. J. Neurosci. 2001;21:5546–5558. doi: 10.1523/JNEUROSCI.21-15-05546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro L, Beggiato S, Tomasini MC, Fuxe K, Antonelli T, Tanganelli S. A(2)/D(2) receptor heteromerization in a model of Parkinson's disease. Focus on striatal aminoacidergic signaling. Brain Res. 2012;1476:96–107. doi: 10.1016/j.brainres.2012.01.032. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr., Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Groveman BR, Feng S, Fang XQ, Plueger M, Lin SX, Bienkiewicz EA, Yu X. The regulation of N-methyl-D-aspartate receptors by Src kinase. FEBS J. 2012;279:20–28. doi: 10.1111/j.1742-4658.2011.08413.x. [DOI] [PubMed] [Google Scholar]
- Hallett PJ, Spoelgen R, Hyman BT, Standaert DG, Dunah AW. Dopamine D1 activation potentiates striatal NMDA receptors by tyrosine phosphorylation-dependent subunit trafficking. J. Neurosci. 2006;26:4690–4700. doi: 10.1523/JNEUROSCI.0792-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori K, Uchino S, Isosaka T, Maekawa M, Iyo M, Sato T, Kohsaka S, Yagi T, Yuasa S. Fyn is required for haloperiodol-induced catalepsy in mice. J. Biol. Chem. 2006;281:7129–7135. doi: 10.1074/jbc.M511608200. [DOI] [PubMed] [Google Scholar]
- Ince E, Ciliax BJ, Levey AI. Differential expression of D1 and D2 dopamine and m4 muscarinic acetylcholine receptor proteins in identified striatonigral neurons. Synapse. 1997;27:357–366. doi: 10.1002/(SICI)1098-2396(199712)27:4<357::AID-SYN9>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Jeon J, Dencker D, Wörtwein G, Woldbye DP, Cui Y, Davis AA, Levey AI, Schütz G, Sager TN, Mork A, Li C, Deng CX, Fink-Jensen A, Wess J. A subpopulation of neuronal M4 muscarinic acetylcholine receptors plays a critical role in modulating dopamine-dependent behaviors. J. Neurosci. 2010;30:2396–2405. doi: 10.1523/JNEUROSCI.3843-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin DZ, Guo ML, Xue B, Fibuch EE, Choe ES, Mao LM, Wang JQ. Phosphorylation and feedback regulation of metabotropic glutamate receptor 1 by calcium/calmodulin-dependent protein kinase II. J. Neurosci. 2013;33:3402–3412. doi: 10.1523/JNEUROSCI.3192-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia LV, Gingrich JR, Salter MW. Src in synaptic transmission and plasticity. Oncogene. 2004;23:8007–8016. doi: 10.1038/sj.onc.1208158. [DOI] [PubMed] [Google Scholar]
- Kennedy MB. The postsynaptic density at glutamatergic synapses. Trends Neurosci. 1997;20:264–268. doi: 10.1016/s0166-2236(96)01033-8. [DOI] [PubMed] [Google Scholar]
- Morelli M, Fenu S, Cozzolino A, Pinna A, Carta A, Di Chiara G. Blockade of muscarinic receptors potentiates D1 dependent turning behavior and c-fos expression in 6-hydroxydopamine-lesioned rats but does not influence D2 mediated response. Neuroscience. 1993;53:673–678. doi: 10.1016/0306-4522(93)90615-m. [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Komai S, Tezuka T, Hisatsune C, Umemori H, Semba K, Mishina M, Manabe T, Yamamoto T. Characterization of Fyn-mediated tyrosine phosphorylation sites on GluRε2 (NR2B) subunit of the N-methyl-D-aspartate receptor. J. Biol. Chem. 2001;276:693–699. doi: 10.1074/jbc.M008085200. [DOI] [PubMed] [Google Scholar]
- Neet K, Hunter T. Vertebrate non-receptor protein-tyrosine kinase families. Genes Cell. 1996;1:147–169. doi: 10.1046/j.1365-2443.1996.d01-234.x. [DOI] [PubMed] [Google Scholar]
- Ohnishi H, Murata Y, Okazawa H, Matozaki T. Src family kinases: modulators of neurotransmitter receptor function and behavior. Trends Neurosci. 2011;34:629–637. doi: 10.1016/j.tins.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Okada M. Regulation of the Src family kinase by Csk. Int. J. Biol. Sci. 2012;8:1385–1397. doi: 10.7150/ijbs.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omri B, Crisanti P, Marty MC, Alliot F, Fagard R, Molina T, Pessac B. The Lck tyrosine kinase is expressed in brain neurons. J. Neurochem. 1996;67:1360–1364. doi: 10.1046/j.1471-4159.1996.67041360.x. [DOI] [PubMed] [Google Scholar]
- Pascoli V, Besnard A, Herve D, Pages C, Heck N, Girault JA, Caboche J, Vanhoutte P. Cyclic adenosine monophosphate-independent tyrosine phosphorylation of NR2B mediates cocaine-induced extracellular signal-regulated kinase activation. Biol. Psychiatry. 2011;69:218–227. doi: 10.1016/j.biopsych.2010.08.031. [DOI] [PubMed] [Google Scholar]
- Phelps PE, Houser CR, Vaughn JE. Immunocytochemical localization of choline acetyltransferase within the rat neostriatum: a correlated light and electron microscopic study of cholinergic neurons and synapses. J. Comp. Neurol. 1985;238:286–307. doi: 10.1002/cne.902380305. [DOI] [PubMed] [Google Scholar]
- Roskoski R., Jr. Src kinase regulation by phosphorylation and dephosphorylation. Biochem. Biophys. Res. Commun. 2005;331:1–14. doi: 10.1016/j.bbrc.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Santiago MP, Potter LT. Biotinylated m4-toxin demonstrates more M4 muscarinic receptor protein on direct than indirect striatal projection neurons. Brain Res. 2001;894:12–20. doi: 10.1016/s0006-8993(00)03170-x. [DOI] [PubMed] [Google Scholar]
- Schenone S, Brullo C, Musumeci F, Biava M, Falchi F, Botta M. Fyn kinase in brain diseases and cancer: the search for inhibitors. Curr. Med. Chem. 2011;18:2921–2942. doi: 10.2174/092986711796150531. [DOI] [PubMed] [Google Scholar]
- Schmitt JM, Stork PJ. PKA phosphorylation of Src mediates cAMP's inhibition of cell growth via Rap1. Mol. Cell. 2002;9:85–94. doi: 10.1016/s1097-2765(01)00432-4. [DOI] [PubMed] [Google Scholar]
- Taniguchi S, Nakazawa T, Tanimura A, Kiyama Y, Tezuka T, Watabe AM, Katayama N, Yokoyama K, Inoue T, Izumi-Nakaseko H, Kakuta S, Sudo K, Iwakura Y, Umemori H, Inoue T, Murphy NP, Hashimoto K, Kano M, Manabe T, Yamamoto T. Involvement of NMDAR2A tyrosine phosphorylation in depression-related behavior. EMBO J. 2009;28:3717–3729. doi: 10.1038/emboj.2009.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu. Rev. Cell. Dev. Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- Trepanier CH, Jackson MF, MacDonald JF. Regulation of NMDA receptors by the tyrosine kinase Fyn. FEBS J. 2012;279:12–19. doi: 10.1111/j.1742-4658.2011.08391.x. [DOI] [PubMed] [Google Scholar]
- Tritsch NX, Sabatini BL. Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron. 2012;76:33–50. doi: 10.1016/j.neuron.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volman SF, Lammel S, Margolis EB, Kim Y, Richard JM, Roitman MF, Lobo MK. New insights into the specificity and plasticity of reward and aversion encoding in the mesolimbic system. J. Neurosci. 2013;33:17569–17576. doi: 10.1523/JNEUROSCI.3250-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Carnicella S, Phamluong K, Jeanblanc J, Ronesi JA, Chaudhri N, Janak PH, Lovinger DM, Ron D. Ethanol induces long-term facilitation of NR2B-NMDA receptor activity in the dorsal striatum: implications for alcohol drinking behavior. J. Neurosci. 2007;27:3593–9602. doi: 10.1523/JNEUROSCI.4749-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JQ, McGinty JF. Differential effects of D1 and D2 dopamine receptor antagonists on acute amphetamine- or methamphetamine-induced up-regulation of zif/268 mRNA expression in rat forebrain. J. Neurochem. 1995;65:2706–2715. doi: 10.1046/j.1471-4159.1995.65062706.x. [DOI] [PubMed] [Google Scholar]
- Wang JQ, McGinty JF. Scopolamine augments c-fos and zif/268 messenger RNA expression induced by the full D1 dopamine receptor agonist SKF-82958 in the intact rat striatum. Neuroscience. 1996a;72:601–616. doi: 10.1016/0306-4522(95)00597-8. [DOI] [PubMed] [Google Scholar]
- Wang JQ, McGinty JF. Muscarinic receptors regulate striatal neuropeptide gene expression in normal and amphetamine-treated rats. Neuroscience. 1996b;75:43–56. doi: 10.1016/0306-4522(96)00277-1. [DOI] [PubMed] [Google Scholar]
- Wang JQ, McGinty JF. Intrastriatal injection of a muscarinic receptor agonist and antagonist regulates striatal neuropeptide mRNA expression in normal and amphetamine-treated rats. Brain Res. 1997;748:62–70. doi: 10.1016/s0006-8993(96)01244-9. [DOI] [PubMed] [Google Scholar]
- Watcharasit P, Tucholski J, Jope RS. Src family kinase involvement in muscarinic receptor-induced tyrosine phosphorylation in differentiated SH-SY5Y cells. Neurochem Res. 2001;26:809–816. doi: 10.1023/a:1011612118779. [DOI] [PubMed] [Google Scholar]
- Wess J. Molecular biology of muscarinic acetylcholine receptors. Crit. Rev. Neurobiol. 1996;10:69–99. doi: 10.1615/critrevneurobiol.v10.i1.40. [DOI] [PubMed] [Google Scholar]
- Yang HB, Yang X, Cao J, Li S, Liu YN, Suo ZW, Cui HB, Guo Z, Hu XD. cAMP-dependent protein kinase activated Fyn in spinal dorsal horn to regulate NMDA receptor function during inflammatory pain. J. Neurochem. 2011;116:93–104. doi: 10.1111/j.1471-4159.2010.07088.x. [DOI] [PubMed] [Google Scholar]
- Yang K, Trepanier C, Sidhu B, Xie YF, Li H, Lei G, Salter MW, Orser BA, Nakazawa T, Yamamoto T, Jackson MF, MacDonald JF. Metaplasticity gated through differential regulation of GluN2A versus GluN2B receptors by Src family kinases. EMBO J. 2012;31:805–816. doi: 10.1038/emboj.2011.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo MG, Oh HJ, Cho HS, Chun JS, Marcantonio EE, Song WK. Phosphorylation of Ser 21 in Fyn regulates its kinase activity, focal adhesion targeting, and is required for cell migration. J. Cell. Physiol. 2011;226:236–247. doi: 10.1002/jcp.22335. [DOI] [PubMed] [Google Scholar]