Abstract
The α7nAChR agonist, PNU-282987, has previously been shown to have a neuroprotective effect against loss of retinal ganglion cells (RGCs) in an in vivo glaucoma model when the agent was injected into the vitreous chamber of adult Long Evans rat eyes. Here, we characterized the neuroprotective effect of PNU-282987 at the nerve fiber and retinal ganglion cell layer, determined that neuroprotection occurred when the agonist was applied as eye drops and verified detection of the agonist in the retina, using LC/MS/MS. To induce glaucoma-like conditions in adult Long Evans rats, hypertonic saline was injected into the episcleral veins to induce scar tissue and increase intraocular pressure. Within one month, this procedure produced significant loss of RGCs compared to untreated conditions. RGCs were quantified after immunostaining with an antibody against Thy 1.1 and imaged using a confocal microscope. In dose-response studies, concentrations of PNU-282987 were applied to the animal’s right eye two times each day, while the left eye acted as an internal control. Eye drops of PNU-282987 resulted in neuroprotection against RGC loss in a dose-dependent manner using concentrations between 100 µM and 2 mM PNU-282987. LC/MS/MS results demonstrated that PNU-282987 was detected in the retina when applied as eye drops, relatively small amounts of PNU-282987 were measured in blood plasma and no PNU-282987 was detected in cardiac tissue. These results support the hypothesis that eye drop application of PNU-282987 can prevent loss of RGCs associated with glaucoma, which can lead to neuroprotective treatments for diseases that involve α7nAChRs.
Keywords: neuroprotection, acetylcholine receptors, glaucoma, retinal ganglion cells, nerve fiber layer, adult rats
1. Introduction
Glaucoma is characterized as a neuropathic disease that causes damage to the optic nerve and progressive degeneration of retinal ganglion cells (RGCs) in the retina, resulting in irreversible loss of vision (Foster et al., 2002; Guo et al., 2005). All current treatments for glaucoma are focused on reducing an increase of intraocular pressure (IOP), the primary risk factor associated with glaucoma (Chauhan et al., 2002; Levkovitch-Verbin et al., 2002; Damji et al., 2003). Eye drop medications for glaucoma treatments decrease the production of aqueous humor or affect drainage of fluid through the trabecular meshwork. Surgical procedures cut small holes in the eye to drain the aqueous humor or lasers are used to produce holes in the trabecular meshwork to increase outflow of aqueous humor (Cairns, 1968). Eye drop or surgery treatment is also used in normotensive glaucoma patients, even when IOP measurements never exceed 22 mmHg (Mi et al., 2014; Jeong et al. 2014). However, these treatments alone are insufficient to halt the progression of blindness associated with glaucoma (Lickter et al., 2001; Heijl et al., 2002; Kass et al., 2002; Beidoe and Mousa, 2012; Jeong et al., 2014). As a result, new treatment options focused on preventing the loss of neurons from the retina are required.
In previous studies from Iwamoto et al. (2014), the loss of retinal ganglion cells (RGCs) was prevented in an in-vivo rat model of glaucoma when intravitreal injections of a specific α7 nicotinic acetylcholine receptor agonist (α7nAChR), N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl]-4-chlorobenzamide hydrochloride (PNU-282987) (Bodnar et al., 2005; Hajos et al., 2005), were delivered into the vitreal chamber of the adult Long Evans rat eyes before a procedure to induce glaucoma-like conditions. RGCs contain acetylcholine (ACh) receptors (Keyser et al., 1988; Whiting et al., 1991; Keyser et al., 1993; Kanada et al., 1995) and receive cholinergic input from a well-described population of starburst amacrine cells common to a majority of vertebrate retina (Massey and Redburn, 1987). They are the only source of ACh in the vertebrate retina. A growing body of evidence indicates that nAChRs play a key role in neuroprotection within the brain against several neurodegenerative diseases. Specifically, activation of α7 nAChRs in the brain have been linked to neuroprotection against several neurodegenerative diseases (Conejero-Goldberg et al., 2008; Liu et al., 2012). There is strong evidence that α7 nAChRs are neuroprotective, reducing β-amyloid induced toxicity in Alzheimer’s disease (Kawamata and Shimohama 2011; Oz et al., 2013) and that the α7 nAChRs plays a role in the pathophysiology of schizophrenia (Winterer et al., 2013; Young and Geyer, 2013). In the retina, pig and rat in vitro studies have demonstrated that the loss of RGCs normally associated with glutamate-induced excitotoxicity involves activation of α7 nAChRs (Wehrwein et al., 2004; Thompson et al., 2006; Iwamoto et al., 2013). In addition, intravitreal injections of the α7 nAChR agonist, PNU-282987, in adult Long Evans rats in vivo prevented the loss of RGCs normally associated with a procedure to induce glaucoma-like conditions (Iwamoto et al., 2014). However, the use of intravitreal injections is invasive and reduces the appeal of developing an α7 nAChR agonist for potential glaucoma treatment. To address this issue, eye drops of PNU-282987 were applied to adult Long Evans rats before and after the procedure to induce glaucoma-like conditions according to the method described by Morrison et al., (1997) to test the hypothesis that eye drop application of a specific α7 nicotinic acetylcholine receptor agonist can prevent loss of RGCs in an in-vivo adult rat glaucoma model. The effect of eye drop application on RGCs in the retinal ganglion cell layer was addressed and its advantages over systemic applications discussed.
2. Materials and Methods
2.1 Animals
Adult Long Evans rats (males and females 3 months of age) were used for all in vivo studies. Rats were kept at Western Michigan University’s animal facility until needed and were cared for in accordance with the approved guidelines of the Institutional Animal Care and Use Committee (IACUC).
2.2 Inducing Glaucoma-Like Conditions
The procedure to induce glaucoma-like conditions in rats was modified from the procedure initially described by Morrison et al., (1997). These modified procedures were fully described in Iwamoto et al., (2014). Briefly, Long Evans rats were anesthetized with 0.1ml/100gm KAX via intraperitoneal injections until no reflexes were observed. KAX is a combination of 5 ml of ketamine (100 mg/ml), 2.5 ml xylazine (20 mg/ml), 1 ml acepromazine (10 mg/ml), and 0.5 ml sterile water. A topical anesthetic of 0.5% procaine hydrochloride was applied to the eye before the procedure to induce glaucoma. Once the animal exhibited no reflexes, the episcleral vein of the right eye in each experimental animal was injected with 50 µl of 2 M NaCl using a glass microinjection needle (Iwamoto et al., 2014). Injection of 2 M hypertonic saline caused blanching of the episcleral veins and subsequent scarring of the vascular system (Morrison et al., 1997). Visualization of blanching in the episcleral veins correlated directly with significant loss of RGCs after one month and an increase of intraocular pressure (IOP) (Morrison et al., 1997; Johnson and Tomarev, 2011; Iwamoto et al., 2014). Following the procedure to induce glaucoma-like conditions, animals were closely watched to ensure full recovery before being returned to the animal facility.
2.3 Eye Drop Application
Previous in vivo studies on adult Long Evans rats injected the α7 nAChR agonist, PNU-282987, directly into the vitreal chamber of adult Long Evans rats to gain access to the retina. In this study, the α7 nAChR specific agonist was introduced as eye drops to Long Evans rats prior to the procedure that induced glaucoma-like conditions. Four different concentrations of PNU-282987 were used in a dose-dependent study and included 100 µM, 500 µM, 1 mM, and 2 mM. These concentrations were 10 times the concentration used in studies where PNU-282987 was injected intravitreally (Iwamoto, 2014), as only a small percentage of any eye drop reaches the retina through the back of the eye. The ACh agonist was dissolved in dimethyl sulfoxide (DMSO) to make a stock solution and then diluted in PBS. The eye drops were applied for three days before the injection of hypertonic solution, as previous studies demonstrated that the neuroprotective agent needed to be applied 3 days before an insult for neuroprotection to occur (Wehrwein et al., 2004; Iwamoto et al., 2013). Following surgery, eye drops were applied twice a day for one month based on previous LC/MS/MS studies that demonstrated evidence of PNU-282987 in the retina up to 12 hours after intravitreal injections (Iwamoto et al., 2014). Animals were sacrificed after one month. Previous studies have demonstrated that significant loss of RGCs in the periphery (4 mM from the optic nerve head) occurred one month following the procedure to induce glaucoma (Iwamoto et al., 2014). Eye drops were delivered to the bulbar conjunctiva, which provided the best access for the eye drops to seep into the eye socket while also retaining consistency in the administration of PNU-282987. In vehicle control experiments, eye drops of sterile PBS containing up to 1% DMSO were delivered to the right eye of experimental rats 3 days before the procedure to induce glaucoma-like conditions and for 1 month following the procedure.
2.4 Labeling RGCs
After rats were exposed to PNU-282987 for a month, they were sacrificed and retinas were removed from the eyes. Rats were euthanized in a carbon dioxide chamber. Both left and right retinas were peeled off the back of eyecups after removal of the cornea, lens and vitreous humor. Care was taken to peel the retina off the back of the remaining eyecup in one piece to maintain anatomical and geographical orientation (Iwamoto et al., 2014). Whole retinas were then flat-mounted, pinned out in a sylgard dish with the RGC layer facing upward using cactus needles, and fixed in 10% formalin overnight at 4° C. After the samples were fixed, the tissue was rinsed with PBS three times. To block nonspecific binding, the tissue was incubated in 2% BSA in PBS containing 0.02% saponin for 30 minutes at room temperature before applying the primary antibody (anti-Thy 1.1) to label RGCs. Anti-Thy 1.1 (mouse anti-rat, BD Biosciences) is a monoclonal antibody against glycoproteins found exclusively on the plasma membrane of RGCs in the retina (Barnstable and Drager, 1984). Preliminary serial dilution studies determined that optimal results were obtained when the primary antibody was diluted 1:300 in 0.02% saponin in PBS with 2% BSA. Fixed flat-mounted retinas were kept at 4° C in a humidified chamber for 5 days. After 5 days, the retina was rinsed 3 times using PBS and incubated in fluorescent secondary antibody (goat anti-mouse IgG), Alexa Fluor 595 (Invitrogen/Molecular Probes), for visualization (1:300) for another 5 days. The retina tissue was then rinsed 3 times with PBS and mounted on glass slides using 50% PBS and 50% glycerol. Preliminary time and dose-dependent studies using the antibodies was performed to select the concentration and incubation period that produced optimal results.
In control studies, experiments were conducted to determine the specificity of the antibodies used. In some experiments, retinal flat-mounts were processed with the primary antibody omitted, while other experiments substituted non-immune mouse immunoglobulin (dilution: 0.1 – 1.0 µg/ml) for the monoclonal antibody. In other experiments, preabsorption controls were performed where the primary antibody and Thy 1.1 antigen were added together before applying to tissue. No significant epifluorescence was observed under any of these conditions.
2.5 Quantification of RGC survival
Retinal tissue was visualized using a Zeiss confocal microscope. Using the Z stack function of the confocal microscope, images were obtained from the periphery of the retina, 4 mm from the optic nerve head. This distance from the ONH was based on previous studies showing that the procedure to induce glaucoma-like conditions produced the greatest amount of damage to RGCs in the periphery (Iwamoto, 2014). Images were obtained throughout the entire nerve fiber and RGC layer in 1 µm increments to analyze nerve fiber thickness and RGC density. As the distribution of RGCs is uneven in different regions of the rat retina (Dreher et al., 1985), images were obtained from four 200 µm2 regions of each retina, 4 mm away from the center of the optic nerve head. The total number of Thy 1.1 labeled RGCs in each section were counted, averaged and compared in experimental and control retinas as in previous studies (Iwamoto et al., 2014). RGC counts, anatomical characteristics and axon fascicles were analyzed using Metamorph and ImageJ software. Results were graphed using GraphPad software.
2.6 LC/MS/MS Analysis of Rat Retina, Blood Plasma and Heart Tissue
LC/MS/MS (liquid chromatography: mass spectroscopy with triple quad capabilities) was performed according to standard procedures on retina, blood plasma and heart samples removed from sacrificed Long Evans rats at various time points following eye drop applications. Retinas, blood plasma and heart samples (atria and ventricle) were removed from asphyxiated rats after different concentrations of PNU-282987 were applied to rat eyes as eye drops for various amounts of time. Three different concentrations of PNU-282987 was applied to rat eyes (500 µM, 1 mM, 2 mM) for five different time periods (1, 2, 4, 8 and 12 hours) before the animals were sacrificed. Collected samples were removed from experimental animal, rinsed and weighed. 2 retinas from each animal were used for each time point and for each concentration. The pooling of 2 retinas for each time and concentration point was required to deliver enough tissue for HPLC analysis. All experiments were repeated in triplicate. Tissues were placed on ice and sent immediately to the Michigan Innovation Center of Kalamazoo, MI for LC/MS/MS analysis and quantification of PNU-282987. At the Innovation Center, each of the samples were placed in −80°C until processed. LC/MS/MS was performed on a Waters Quatro Micro triple quadruple mass spectrometer using positive ion electrospray ionization at the Michigan Innovation Center of Kalamazoo. A Waters CapLC capillary HPLC was configured for on-line SPE.
2.7 Statistical Analysis
All cell counts were compared to the internal control counts for each trial. Statistical analysis was performed on all normalized data using Kruskal-Wallis non-parametric analysis of variance (ANOVA) with post hoc multiple comparisons (Dunn’s test) for studies. For multiple comparison data that was not normalized, statistical analysis was performed using a two-way ANOVA with correction for multiple comparisons. P<0.05 was considered statistically significant for all tests.
3. Results
3.1 Glaucoma Effects on RGCs
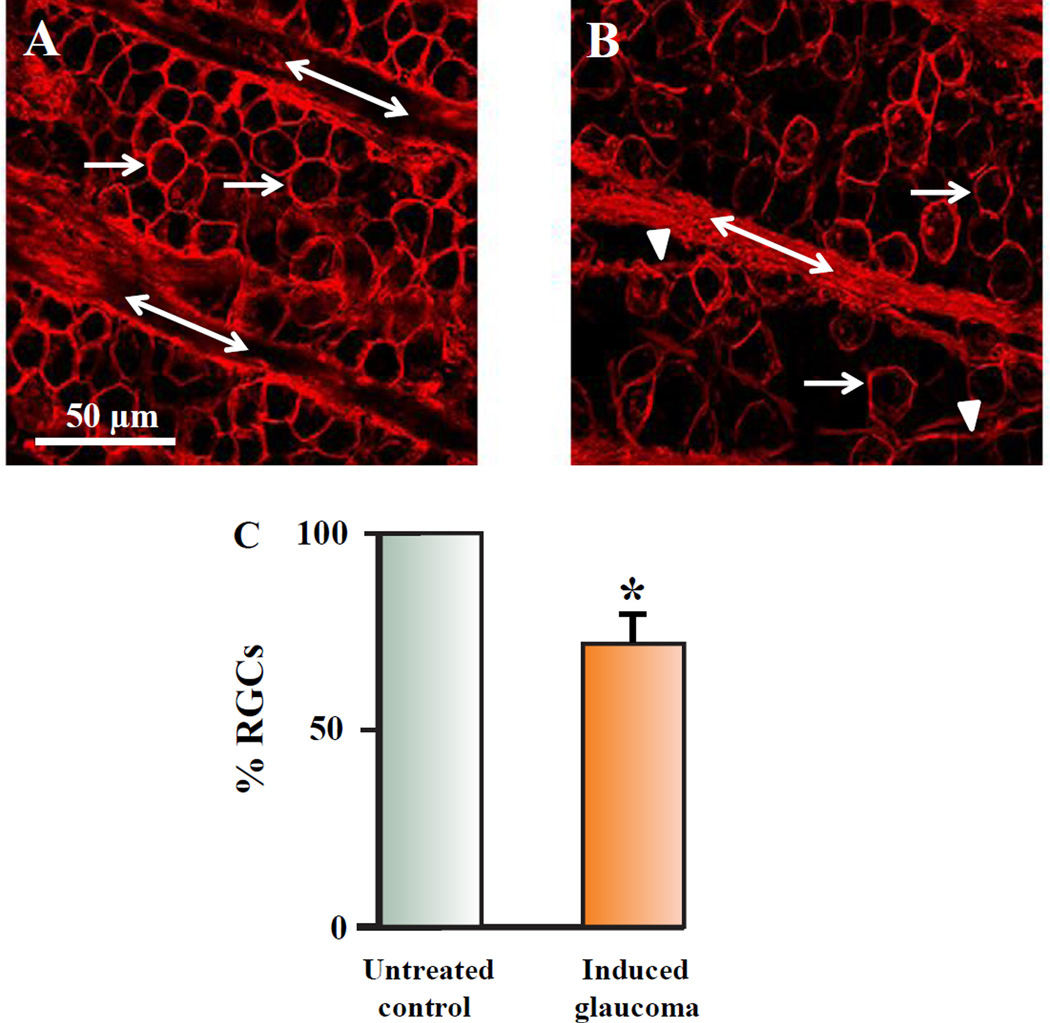
Figure 1 demonstrates the effect of the procedure to induce glaucoma-like conditions in the retinal nerve fiber and ganglion cell layer. Figure 1A illustrates an example of a typical confocal image of labeled RGCs and labeled RGC axon fascicles that was obtained from the control untreated eye of a Long Evans rat 4mm from the nasal side of the optic nerve head (ONH). Figure 1B illustrates an image from the same retinal location (Iwamoto et al., 2014), from the same rat, but in the right eye, which underwent the hypertonic injection to induce glaucoma-like conditions one month previously. Rats injected with hypertonic saline to induce glaucoma-like conditions experienced a noticeable RGC loss compared to the internal control (arrows) and a change in RGC axon fasciculation compared to the internal control image. The axons of RGCs combine as fascicles on their way to exit the retina through the ONH (double arrows). After the procedure to induce glaucoma-like conditions, defasciculation of axon strands off the main axon fascicles was observed (arrowheads) (Fig 1B). Defasciculation was characterized as strands of RGC axons and connective tissue, between 2 and 10 µms in diameter, that clearly disengaged from the main axon fascicle. Although the number of axons in each strand was not quantified, each strand clearly contained RGC axons that labeled with anti-Thy 1.1 antibody. Figure 1C summarizes the results of RGC loss one month after hypertonic injection into the episcleral veins to induce glaucoma-like conditions. One month following the procedure to induce glaucoma-like conditions, there was an average decrease in the percent of RGCs by 27.4% (+/− 2.9; N=20) when cell counts were normalized and compared to the internal control left eyes. As such, each “N” represents the number of rats used unless otherwise stated. The results of 20 experimentally treated retinas from 20 different rats were compared to their internal control retinas. The loss of RGCs one month after hypertonic saline injections correlated with a significant increase in IOP in all experimental animals (Table 1). IOP measurements were obtained using a Tonopen tonometer according to the procedure outlined by Iwamoto et al. (2013).
Figure 1.
Effects of glaucoma in the nerve fiber layer and RGC layer. 1A represents a confocal image obtained from an adult Long Evans rat retina that was labeled with an antibody against Thy 1.1 4 mm from the ONH in control untreated conditions. The arrows point to RGC soma, while the double arrows demonstrate the axon fascicles in the nerve fiber layer. 1B illustrates an image obtained from the same rat, from the same retinal geographic location in an eye injected with 2 M NaCl in the episcleral veins. The image shown was obtained one month after the procedure to induce glaucoma-like conditions. The arrow heads point to axon strands defasciculating from the main axon bundles. Figure 1C demonstrate bar graphs that plot the average loss of RGCs associated with the procedure to induce glaucoma-like conditions after one month. The star represents significant differences from the control untreated condition. Error bars represent S.E.
Table 1.
IOP measurements obtained under different experimental conditions. IOP measurements were obtained using a Tonopen according to the procedure outlined by Iwamoto et al. (2013).
| Experimental Conditions: |
Mean IOP measurement from each animal (mmHg) |
Average IOP for each experimental condition |
|---|---|---|
| Control untreated (N=20) |
13.2; 11.8; 12.7; 13.5; 11.6; 12.8; 12.9; 13.3; 12.8; 14.1; 12.8; 12.7; 13.6; 15.1; 14.2; 13.7; 14.3; 12.8; 11.7; 11.9 |
13.08 +/− 0.20 mmHg |
| 4 weeks after hypertonic saline injection (N=20) |
19.5; 22.5; 23.6; 18.9; 23.5; 25.2; 23.4; 22.5; 26.2; 18.7; 19.8; 18.7; 18.5; 22.9; 21.3; 20.5; 21.3; 24.5; 25.6; 24.2 |
22.06 +/− 0.70 mm Hg |
| 4 weeks after hypertonic saline injection and 2 mM PNU (N=12) |
21.3; 22.5; 20.7; 21.6; 19.8; 18.6; 18.9; 22.8; 23.3; 21.5; 20.9; 22.5 |
21.2 +/− 0.42 mm Hg |
| 4 weeks after PNU only 2 mM PNU (N=4) |
14.8; 13.9; 14.2; 12.8 | 13.93 +/− 0.36 mm Hg |
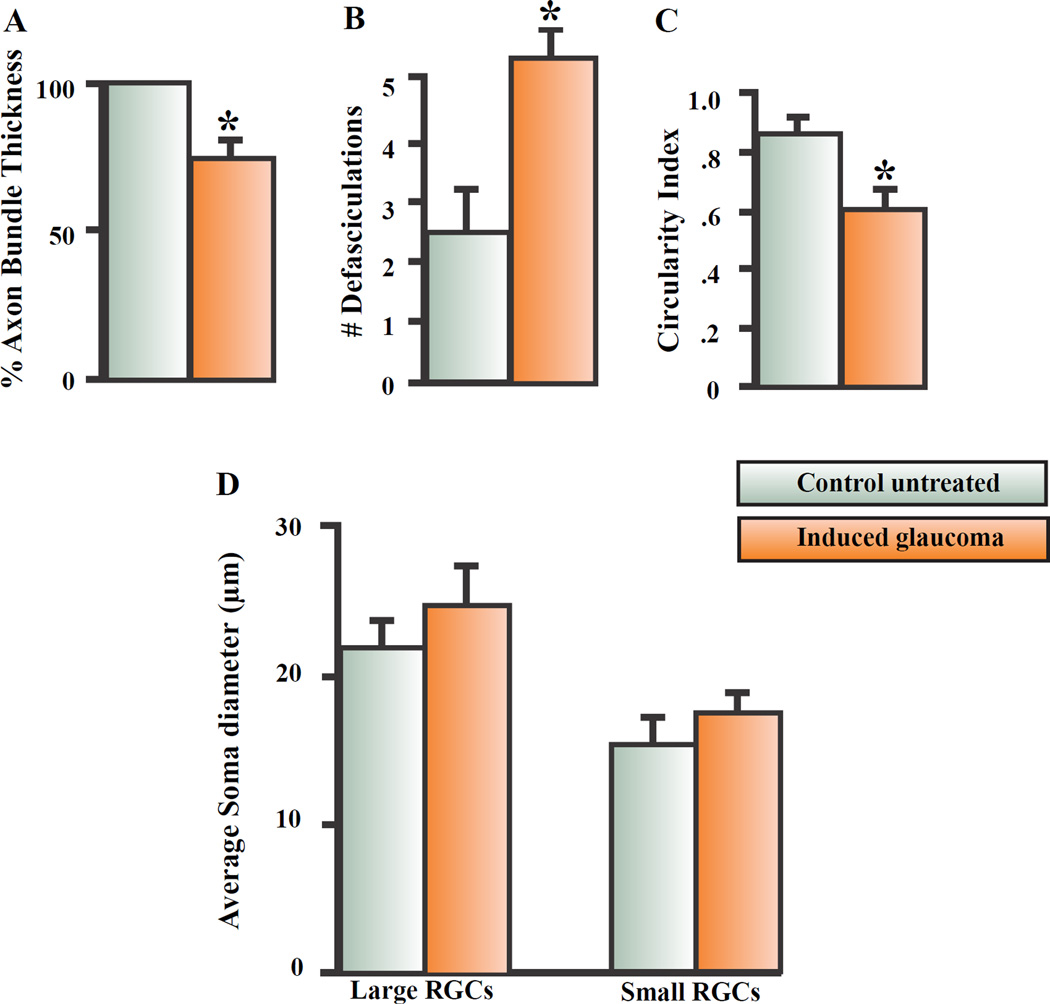
Figure 2 summarizes the effect of hypertonic injections on RGC soma morphology in the RGC layer and RGC axon fasciculation in the nerve fiber layer. Specifically, the thickness of the large axon fascicles, defasciculation of axon strands off the main axon bundles, circularity of the RGC soma and size of the large and small RGCs were quantified in control untreated and experimentally manipulated retinas and compared. As seen in figure 2A, the thickness of the axon fascicles traveling towards the ONH significantly decreased by an average of 25.6% (+/− 2.9; N=9) one month following the procedure to induce glaucoma compared to the axon bundle thickness measured from corresponding internal control retinas. Axon bundle thickness was measured from the same retinal location in all animals to ensure direct comparisons from 200 µm2 confocal images taken 4 mm from the ONH. The decreased thickness of the main axon fascicles corresponded with an increased number of defasciculating axon strands off the main axon bundle after the procedure to induce glaucoma (fig. 2B). As shown in figure 2B, under control conditions, an average of 2.5 (+/−0.7; N=9) RGC axon strands emerged off the main axon fascicles in 200 µm2 confocal images obtained from the peripheral retina. One month after the procedure to induce glaucoma, the number of defasciculating axon strands off the main axon bundle significantly increased to an average of 5.3 (+/−0.5; N=9).
Figure 2.
Summary of effects on nerve fiber layer and RGC morphology. 2A represents the average change in axon bundle thickness that occurred one month after inducing glaucomalike conditions in adult Long Evan rats. 2B summarizes the average effect of defasciculating axon strands off the main axon one month after inducing glaucoma-like conditions. 2C summarizes the average change in RGC soma circularity and 2D represents the average change in large and small RGC soma diameter that occurred one month after inducing glaucoma-like conditions. The star represents significant differences from the control untreated condition. Error bars represent S.E.
Besides changes to the RGC axon fascicles in the nerve fiber layer, there were also morphological changes that occurred in the RGC somas after the procedure to induce glaucoma. Labeling the glycoproteins in the plasma membrane of RGCs with anti-Thy 1.1 allowed us to directly measure the diameter of soma size as well as soma circularity (roundness of the soma). Figure 2C summarizes the RGC circularity analysis using ImageJ software. Under control untreated conditions, RGCs 4 mm from the ONH had an average circularity index of 0.92 +/− .04 (N=9). In this case, each “N” represented the average RGC circularity index obtained after averaging all RGCs from confocal images of 9 different retinas. ImageJ software defines a perfect circle as 1.00. Any object less circular is issued a lower score. One month after the procedure to induce glaucoma, the circularity index of the remaining RGCs significantly decreased to 0.61 +/− .06 (N=9) and indicated increased blebbing in the RGC membranes.
In the rat retina, RGCs are subdivided into large and small RGCs based on their morphology diameter and sensitivity to certain stressors (Thanos, 1988; Glovinsky et al., 1991, 1993; Calkins et al., 2007). Large RGCs have soma diameters between 20–35µm, while small RGCs have soma diameters between 7–12 µm (Glovinsky et al., 1991; 1993). In figure 2D, after labeling the plasma membranes of fixed RGCs with anti-Thy 1.1 antibody, the effect of inducing glaucoma-like conditions on the diameter of RGC somas in large and small RGCs was analyzed and summarized. The effect of the procedure had no significant effect on the average soma diameter in large RGCs. The average diameter of large RGCs measured 22 µm (+/− 2.0; N=100) under control conditions and 24 µm (+/−2.5; N=100) one month after inducing glaucoma. In untreated controls, the diameter of small RGCs averaged 11 µm (+/− 2.2; N=100) compared to an average of 10 (+/− 2.4; N=100) one month after the procedure to induce glaucoma-like conditions.
3.2 Neuroprotection with an a7nAChR agonist
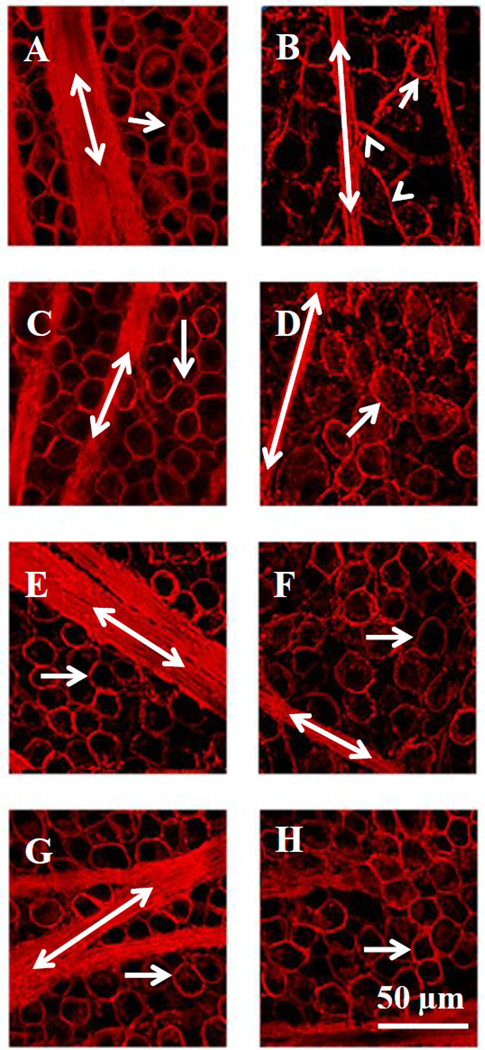
The confocal images in figure 3 illustrate the neuroprotective effect of the a7nAChR agonist, PNU-282987, on RGC survival after inducing glaucoma-like conditions. The left images in figure 3 (A, C, E and G) represent internal control untreated confocal images while the right images (figs. 3B, D, F, H) represent images obtained one month after the procedure to induce glaucoma-like conditions. Each right and left image in the same row was obtained from the same animal and from the same temporal region of the retina, 4 mm from the ONH. The effect of inducing glaucoma is illustrated in figure 3B. One month after inducing glaucoma-like conditions, there was a loss of RGCs (arrows), the main axon fascicle thickness decreased (double arrows), and defasciculation of axon strands off the main axon bundles increased (arrowheads) compared to the internal control image (fig. 3A). In figure 3D, the animal was treated with 100 µM PNU-282987 as eye drops for 3 days before the procedure to induce glaucoma and for one month following the procedure, when the animals were euthanized and retinas were processed for analysis. As shown in figure 3D, RGC soma and axon morphologies are significantly different from the internal control images (fig. 3C) as 100 µM PNU-282987 treatment failed to provide neuroprotection when applied as eye drops. However, less disruption of the RGC’s soma and axon occurred when 500 µM PNU-282987 was applied as eye drops (fig. 3F compared to Fig. 3E) and normal RGC soma and axon characteristics were illustrated if eye drops of 2 mM PNU-282987 were applied (Fig. 3H compared to fig. 3G).
Figure 3.
Confocal images illustrating the neuroprotective effect of PNU-282987. The left column represents control untreated images obtained from different rats (A, C, E and G). RGCs (arrow) and axon fascicles (double labeled arrows) were labeled with an antibody against Thy 1.1. The corresponding confocal images in the right column were obtained from the same animal and from the same retinal geographic location as shown in the right column, but the episcleral veins were injected with 2 M NaCl to induce glaucoma-like conditions (B) or treated with 100 µM (D), 500 µM (F) or 2 mM PNU-282987 (H) eye drops before and after the procedure to induce glaucoma-like conditions. The arrowhead points to defasciculating axon strands.
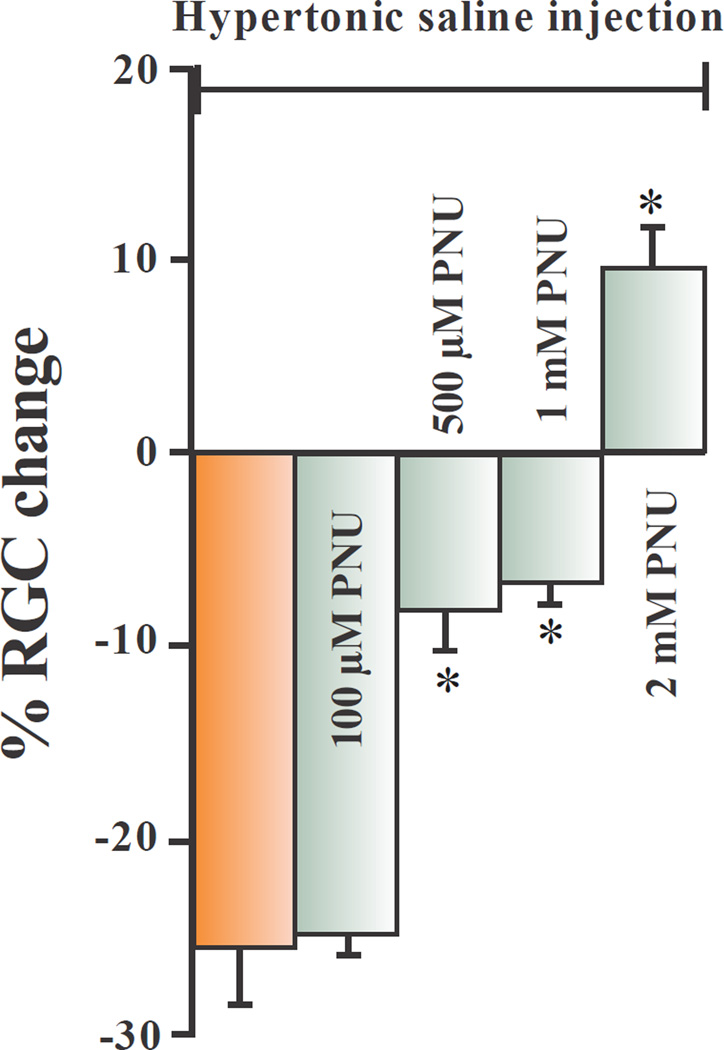
Figure 4 summarizes the dose-response effect of the α7nAChR agonist, PNU-282987, on RGC survival when the α7nAChR agonist was applied as eye drops. The left bar graph in figure 4 represents the average normalized percent loss of RGCs one month after hypertonic saline injections into the episcleral veins of adult Long Evans rats. The other bars graphs represent the normalized percent change of RGCs when treated with different concentrations of PNU-282987 before and after the procedure to induce glaucoma. Without PNU-282987 eye drops, the average loss of RGCs was 27.4% (+/− 2.9; N=20) compared to internal controls. 100 µM PNU-282987 eye drop application had no significant neuroprotective effect on RGC survival (N=9). However, if adult Long Evans rat eyes were treated with 500 µM PNU-282987 before and after the procedure to induce glaucoma, RGC survival significantly increased. With 500 µM PNU-282987 treatment, the procedure to induce glaucoma led to an average decrease in RGCs by 7.5% (+/− 2.5; N=9) compared to the typical 27% decrease that occurred in the absence of PNU-282987. If 1 mM PNU-282987 was applied as eye drops to experimentally manipulated rat eyes, there was an average decrease in RGCs of 6.0% (+/− 1.5; N=9) compared to internal controls and if 2 mM PNU-282987 was applied before and after the procedure to induce glaucoma, there was an average increase in RGC of 9.5% (+/− 2.5; N=12) compared to internal controls. Eye drop treatments of 500 µM, 1 mM and 2 mM PNU-282987 significantly affected the loss of RGCs normally associated with the procedure to induce glaucoma-like conditions. In vehicle control experiments, sterile PBS containing up to 1% DMSO was applied to experimental eyes instead of PNU-282987 for 3 days before the procedure to induce glaucoma-like conditions and for a month following the procedure. After one month, retinas were processed to view RGCs. After quantification of RGC survival, there was no significant difference in RGC counts between experimental retinas and internal control retinas (N=6). In addition, PNU-282987, by itself, had no significant effect on IOP measurement (Table 1, N=4). However, application of PNU-282987, by itself, did affect RGC counts in a dose-dependent manner (Webster et al., 2015), although significantly different from results obtained in this study when PNU-282987 was applied in conjunction with the procedure to induce glaucoma-like conditions.
Figure 4.
Dose response neuroprotection with PNU-282987. This bar graph summarizes the dose-dependent effect of PNU-282987 on RGC survival after the procedure to induce glaucoma-like conditions. The brown bar graph represents the average percent loss of RGCs that occurred one month after hypertonic injections into the episcleral veins. Each green bar graph represents the average effect of experimental retinas treated with different concentrations of PNU-282987 as eye drops before and after the procedure to induce glaucoma-like conditions. The * represents significant neuroprotection from RGC loss associated with inducing glaucoma-like conditions. Error bars represent S.E.
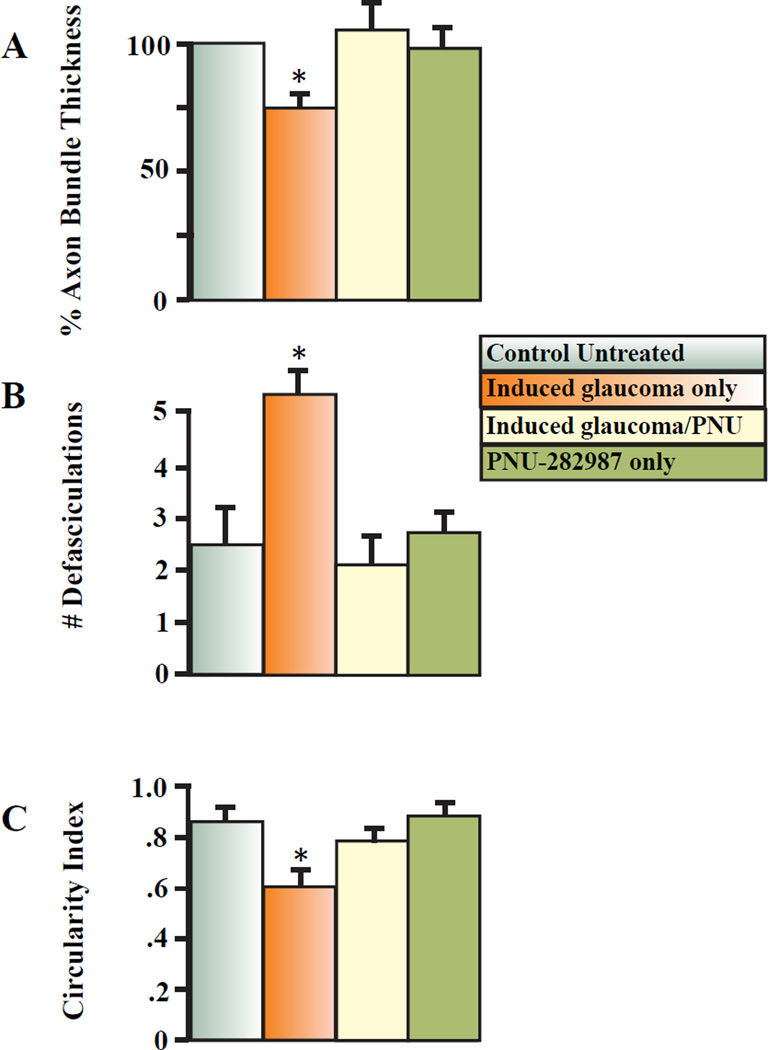
The bar graphs shown in figure 5 summarize the effect of the α7nAChR agonist, PNU-282987, on axon bundle thickness, defasciculating axon strands off the main axon bundle and circularity of the RGC somas. The top bar graphs represent an averaged normalized percent change in the thickness of the axon fascicles that are found 4 mm from the ONH under control conditions, after the procedure to induce glaucoma-like conditions, with the procedure and eye drop treatment with the α7nAChR agonist and after treatment with 2 mM PNU-282987 alone (bars from left to right respectively) (fig. 5A). As seen by these results, eye drop treatment with the α7nAChR agonist significantly prevented the change in axon bundle thickness normally associated with the procedure to induce glaucoma. Without the α7nAChR agonist treatment, axon fascicle thickness decreased by an average of 25.6% (+/− 2.9; N=9). However, with eye drop treatment of α7nAChR agonist, axon fascicle thickness increased by an average of 5.1% (+/− 10.5; N=9). PNU-282987 by itself had no significant effect on axon bundle thickness compared to the axon bundle thickness analyzed in control untreated retinas.
Figure 5.
PNU-2828987’s effect on the nerve fiber layer and on RGC morphology. 5A illustrates normalized bar graphs under 4 different conditions. Each bar graph represents an averaged normalized value obtained for axon bundle thickness. 5B summarizes the change in the number of defasciculating axons that occurred under the 4 different conditions, while 5C summarizes the change in circularity index that occurs to the RGC soma under each of the 4 conditions. All measurements were obtained from 200 µm2 confocal images and treatment with PNU-282987 was as eye drops. The * represents significant change from control untreated conditions (left bar in each panel). Error bars represent S.E.
In figure 5B, the number of defasciculations off the main axon bundles were summarized under control untreated conditions, one month after induction of glaucoma, after eye drop treatment with 2 mM of the α7nAChR agonist in combination with the procedure to induce glaucoma, and after eye drop treatment with PNU-282987 alone (bar graphs from left to right respectively). As seen by these results, eye drop treatment with the α7nAChR agonist significantly prevented axon strand separation from the main axon fascicles. Without the α7nAChR agonist treatment, the number of axon strands defasciculating from the main axon bundles averaged 5.3 (+/− 0.5 N=9) one month after the procedure to induce glaucoma. However, with eye drop treatment of the α7nAChR agonist, the number of defasciculating axon strands off the main axon bundles were statistically the same as control levels (2.1 +/− 0.6: N=9). If the α7nAChR agonist was applied alone, it had no significant effect on defasciculation of axon strands off the main axon fascicles.
In figure 5C, the circularity index of the RGC somas was summarized under control untreated conditions, one month after induction of glaucoma, after eye drop treatment with 2 mM of the α7nAChR agonist in combination with the procedure to induce glaucoma, and after eye drop treatment with PNU-282987 alone (bar graphs from left to right respectively). Again, eye drop application of 2 mM PNU-282987 prevented the change in RGC circularity index associated with the procedure to induce glaucoma-like conditions. With the procedure to induce glaucoma alone, the average circularity index of RGCs was 0.61 (+/− .06; N=9). However, if the procedure to induce glaucoma was performed in conjunction with 2 mM α7nAChR agonist treatment, the circularity index for RGCs increased significantly to 0.79 (+/− 0.03; N=9). Again, application of PNU-282987 by itself had no significant effect on the circularity index of RGCs compared to internal control conditions.
3.3 Detection of PNU-282987 in rat tissue
The next experiment was designed to determine if PNU-282987 could be detected and quantified in the retina, plasma or heart tissue of adult Long Evans rats when applied as eye drops. LC/MS/MS was performed on retina, plasma and heart samples removed from sacrificed Long Evans rats at various time points following eye drop applications using three different concentrations of PNU-282987. Specifically, retinas were removed from euthanized Long Evans rats 1, 2, 4, 8 and 12 hours after applying 100 µM, 500 µM or 2 mM PNU-282987 directly to the rat eyes. Removed tissue was rinsed and sent to the Michigan Innovation Center of Kalamazoo for LC/MS/MS detection of PNU-282987.
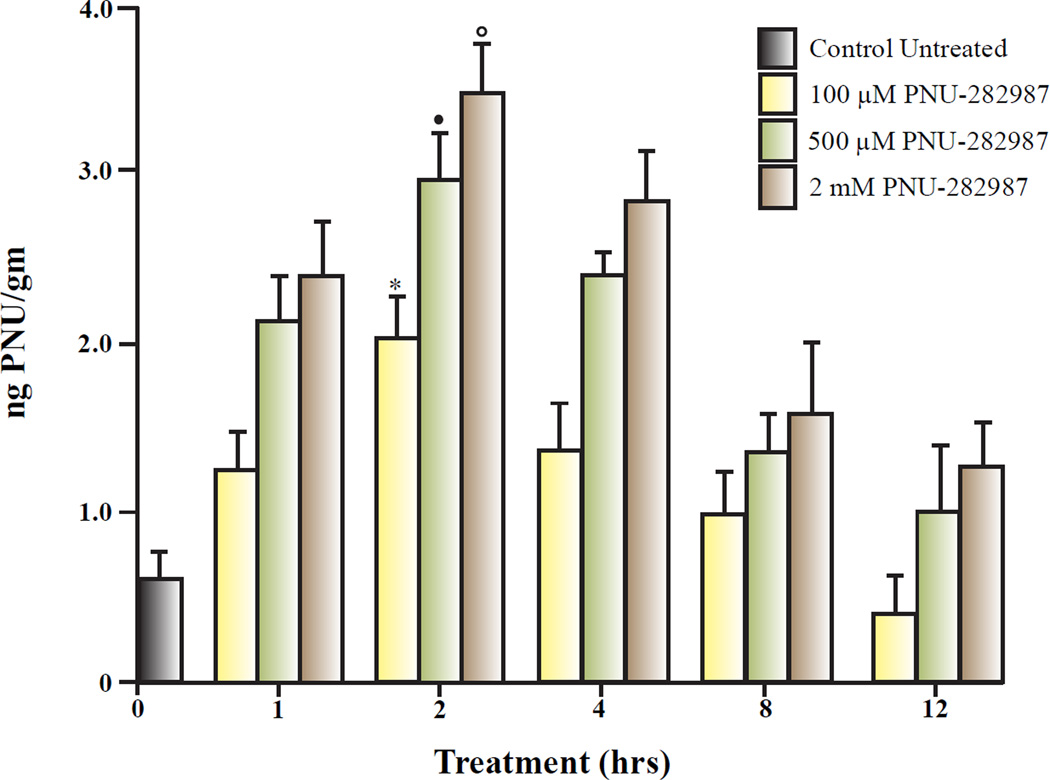
Results from LC/MS/MS studies demonstrated that all three doses of PNU-282987 could be detected in the retina (figure 6). Under control untreated conditions, 0.6 ngPNU/gm (± 0.15) was detected and represents the background level of compounds in the retina that had a similar chemical structure to PNU-282987. The highest amount of PNU-282987 detected in the retina for any time interval was recorded 2 hours after the α7nAChR was applied. Two hours after application, LC/MS/MS detected 2.1 ngPNU/gm (± 0.35) in the retina after 100 µM eye drops were used, 2.85 ngPNU/gm (± 0.45) after 500 µM eye drops were applied and 3.40 ngPNU/gm (± 0.48) after 2 mM PNU eye drops were applied. Each of the values obtained 2 hour after application of PNU-282987 eye drops calculations were significantly different from all other time intervals for each particular concentration. PNU-282987 detection was the lowest for all three concentrations after animals were sacrificed 12 hours after eye drop application and were statistically the same as control untreated conditions at this time point.
Figure 6.
LC/MS/MS analysis of PNU-282987 applied as eye drops. Each bar graph represents the average ng PNU-282987 per gram of animal weight measured from isolated retinas treated with various concentrations of PNU-282987. Different concentrations of PNU-282987 were applied directly to rat eyes and then sacrificed from 0 – 12 hours. At different time points, the treated retinas were removed and processed for PNU-282987 detection using LC/MS/MS analysis at the Western Michigan Innovation Center. The * represents significant difference from all other time points when 100 µM PNU-282987 was applied as eye drops. The closed circle represents significant difference from all other time points when 500 µM PNU-282987 was applied as eye drops and the open circle represents significant difference from all other time points when 2 mM PNU-282987 was applied as eye drops. Error bars represent S.E.
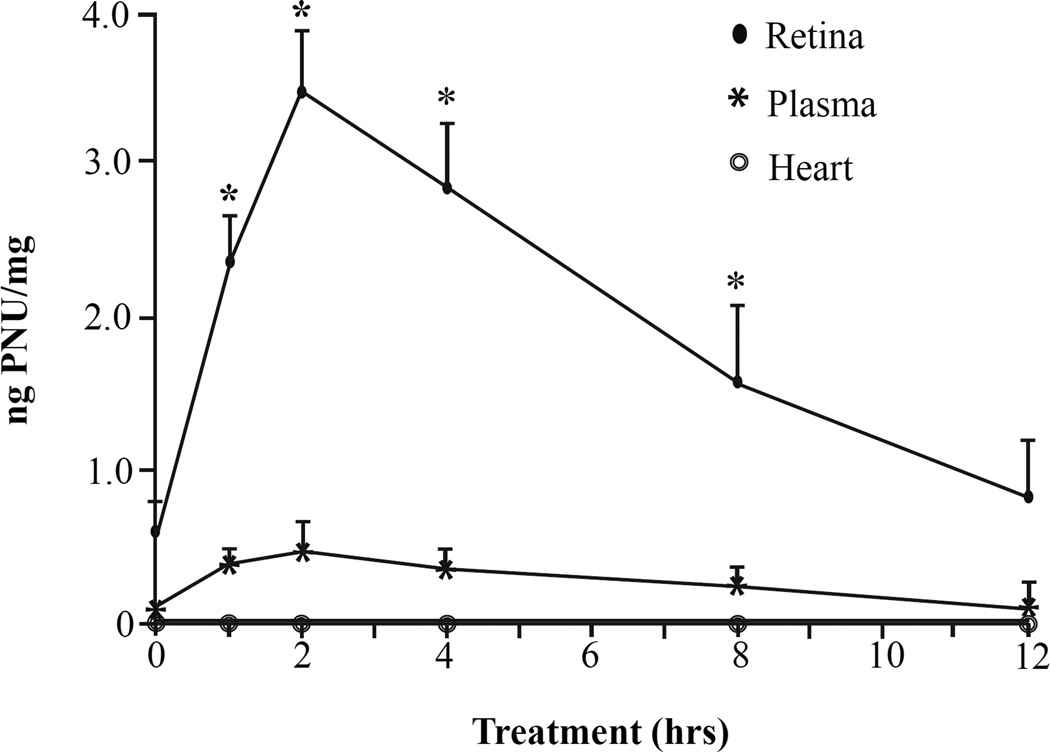
LC/MS/MS experiments were also conducted to determine if traces of PNU-282987 could be detected in blood plasma and heart tissues after eye drop application. Samples of retina, blood plasma, and heart tissue, (separated into atria and ventricle) were collected in lab, rinsed and weighed before sending to the Kalamazoo Innovation Center for analysis after 2 mM PNU-282987 was applied as eye drops for various amounts of time. The results of these studies are shown in figure 7. The highest levels of PNU-282987 were measured in the retina when 2 mM PNU-282987 was applied as eye drops and animals were sacrificed 2 hours later. There was a steady decrease in detection of PNU in the retina as more time was added before the animals were sacrificed. PNU-282987 detection occurred in the retina when animals were sacrificed between 1 and 8 hours compared to control untreated conditions. When animals were sacrificed 12 hours after eye drop application of PNU-282987, the level of detection was comparable to the levels measured in control untreated retina. As can be seen from figure 7, significantly lower levels of PNU-282987 were detected in blood plasma, but levels in blood plasma peaked at 2 hours (0.42 ng PNU/mg +/−0.02; N=3), similar to the peak measured in the retina. However, PNU-282987 was not detected in the atrial or ventricular heart tissue at any time point. All experiments were repeated in replicates (N=3) and averaged together.
Figure 7.
Detection of PNU-282987 in retina, plasma and heart tissue. To generate this line graph, 2 mM PNU-282987 was applied in eye drops directly to the rat eyes for various amounts of time before the animals were sacrificed. Once sacrificed, retina, blood plasma, atria and ventricular heart tissue were removed and analyzed using LC/MS/MS analysis. The closed circles represent the average amount of PNU-282987 detected in the retina at the various time points. The stars represent the average amount of PNU-282987 detected in blood plasma, while the open circles represent the average amount of PNU-2828987 detected in heart tissue at the various time points. The * represents significant change from control untreated conditions. Error bars represent S.E.
4. Discussion
4.1 Neuroprotection
The results from this study demonstrated that eye drop application of the α7 nAChR agonist, PNU-282987, prevented the loss of RGCs that is normally associated with the procedure to induce glaucoma-like conditions in a dose-dependent manner. The main pathway for absorption of eye drops is through the cornea. However, even with these barriers, approximately 1% of agents applied directly to the cornea reach the retina depending on the agent’s vehicle (Ahmed and Patton, 1985; Loftsson and Brewster, 2008), either working its way from the anterior of the eye to the retina, or by drops that are absorbed through the back of the eye as eye drops follow the curvature of the eye structure. Treating the retina directly with neuroprotective eye drops has recently been used to analyze N-methyl-N-nitrosourea-induced photoreceptor cell death (Lin et al., 2014), to study glaucomatous optic neuropathy (Roberti et al., 2014) and to preserve visual function at the retinal ganglion cell layer (Prokai-Tatrai et al., 2013).
However, to verify that PNU-282987 could be detected in the retina when applied as eye drops, LC/MS/MS analysis was performed. Detection of the α7 nAChR agonist was detected in a time and dose-dependent manner, supporting the hypothesis that eye drop application of PNU-282987 reached the retina to provide neuroprotection. However, twelve hours following application, the levels of detectable PNU-282987 decreased to control untreated retinal levels. As a result, eye drops were applied twice each day; 12 hours apart, to keep detectable levels of PNU-282987 present in the retina.
The amount of PNU-282987 that reaches the retina after long term topical administration led to neuroprotection if eye drops between 500 µM and 2 mM PNU-282987 was used. How do these concentrations relate to previously reported concentrations that provide neuroprotection in adult RGCs? In previous studies using intravitreal injections of PNU-282987, 5 µl of 100 µM PNU-282987 provided complete neuroprotection against loss of RGCs (Iwamoto et al., 2014). When injected into the vitreal chamber, 100 µM PNU-282987 would dissolve in 50 µl of vitreous humor (Sha and Kwong, 2006), to make a final concentration of 10 µM. Although only a small amount of PNU-282987 would reach the retina with one eye drop application, it is likely that repeated application of 500 µM and 2 mM PNU-282987 eye drops result in a final concentration near 10 µM PNU-282987 in the retina after 1 month to provide neuroprotection.
Initial studies using PNU-282987 were designed to investigate its neuroprotective effects in Alzheimer’s disease and schizophrenia (Hajos et al, 2005; DelBarrio et al., 2011; McLean et al., 2011). Although successful in animal models, PNU-282987 proved to be unsuitable for human use when applied systemically because it inhibited hERG potassium channels in the heart (Walker et al., 2006). However, confining the α7 nAChR agonist to the eye could eliminate this harmful side effect. To address this issue, 2 mM PNU-282987 eye drops were applied to adult Long Evans rat eyes for various amounts of time before tissues from the animals were removed and analyzed with LC/MS/MS. Although PNU-282987 could be detected in the retina, relatively small amounts could be detected in the plasma and no PNU-282987 was detected in any heart tissue. This supports the development of this α7 nAChR agonist as a potential therapeutic agent for treating glaucoma that doesn’t solely address intraocular pressure.
4.2 Glaucoma induction
In this paper, we have characterized the morphological changes that occurred after inducing glaucoma-like conditions in RGCs and axon fascicles as the RGC axons gather. In the nerve fiber layer, the thickness of the axon fascicles was significantly reduced one month following the procedure to induce glaucoma-like conditions. This was directly correlated with a loss of RGC. As more RGCs are lost, fewer axons reduced the thickness of the axon fascicles. This was also supported by evidence of defasciculation, where strands of connective tissue and axons disconnected from the main axon bundles. Different studies have observed defasciculation in the optic nerve during glaucoma-like conditions that also occurs with RGC loss and degeneration of optic nerve myelin (Fu and Sretavan, 2010; Soto et al., 2011). Onset of axon degeneration may be an early event during the manifestation of glaucoma and could be associated with degeneration of supporting proteins that bundle axon fascicles (Fu and Sretevan, 2010).
Besides an effect on axon fascicles, the procedure to induce glaucoma also had a direct effect on RGC morphology. In particular, the plasma membrane circularity index significantly decreased from control untreated conditions. The change in circularity is likely an effect of plasma membrane blebbing in compromised RGCs that are later lost due to apoptosis. However, if the eyes are treated with the α7 nAChR agonist, PNU-282987, the cell bodies of RGCs retained their circular shape and the blebbing of the plasma membrane was absent.
Different studies have shown a discrepancy in the characterization of RGC somata size under experimental glaucoma models. Some studies have shown an increase in RGC somata size (Ahmed et al., 2001) while others have shown a decrease in RGC somata size (Jacobs et al., 2005; Kalesnykas et al., 2012) in glaucoma models. However, in this study, it was shown that the diameter of the remaining RGC’s soma did not significantly change one month following the procedure to induce glaucoma. This is likely due to RGCs that were already phagocytized after apoptosis. However, all changes to the morphology of the RGCs and to axon fascicles were eliminated if eyes were treated with the α7 nAChR agonist, PNU-282987. Specifically, in the presence of PNU-282987, the circularity index of the RGCs, the thinning of the main axon bundles and the amount of defasciculation of axon strands returned to control untreated conditions.
4.3 Physiological implications
ACh has been found to have a neuroprotective effect against loss of RGCs in an in vitro model of excitotoxicity (Wehrwein et al., 2004) as well as in an in vivo rat model of glaucoma (Iwamoto et al., 2014). If ACh has a neuroprotective role under physiological conditions, it should help to prevent cells from dying under glaucoma-like conditions. In the vertebrate retina, the only source of ACh is from starburst amacrine cells. Starburst amacrine cells are a subtype of retinal amacrine cell that release ACh under physiological conditions onto RGCs (Famiglietti, 1983; Masland, 1988; Grimes, 2011). In the retina, starburst amacrine cells are known to play an important function in the development of the retina and play a role in directional-selectivity (Wassle, 2001; Taylor and Vaney, 2003; Masland, 2005; Enciso et al., 2010). Although recent studies have demonstrated that induced hypertension does not reduce the number of displaced amacrine cells in the ganglion cell layer (Agudo-Barriuso et al., 2013; Ortin-Martinez et al., 2015), it is possible that ACh release is compromised under a glaucoma-like environment. If ACh release is diminished or sensitivity of post synaptic receptors is reduced under glaucomatous conditions, the RGCs could lose their neuroprotection, leading to apoptosis. Further studies are needed to elucidate this possible scenario and to determine if ACh has another function in the retina.
4.4 Applications
In this study, the neuroprotective effect of the α7 nAChR agonist, PNU-282987, has been shown to prevent the loss of RGCs in glaucoma-like conditions in a dose-dependent manner when applied as eye drops. These results have clinical significance as they suggest a potential alternative treatment for treating glaucoma that directly treats the retina in a non-invasive manner instead of focusing exclusively on IOP measurements. Alternatively a potential treatment could be devised to protect RGCs directly with an α7nAChR agonist eye drop in conjunction with current treatments designed to lower IOPs in glaucoma patients.
4.5 Conclusions
In conclusion, the data presented in this study demonstrated that an α7 nAChR agonist, PNU-282987, significantly prevented the loss of RGCs associated with experimental glaucoma when applied as eye drops. PNU-282987 prevented all morphological changes to the nerve fiber layer and to the RGCs that normally occurred after the procedure to induce glaucoma-like conditions. In addition, the α7 nAChR agonist was detected and quantified in retinal tissue when applied as eye drops but could not be detected in the heart, where it has previously been shown to have adverse side effects. Results from this study support the potential of nAChR agonists to be used as a potential treatment for glaucoma.
Highlights.
The α7 nAChR agonist, PNU-282987, prevents loss of RGCs in a rat glaucoma model.
An α7 nAChR agonist prevents loss of RGCs after eye drop application.
An α7 nAChR agonist protects against changes in the nerve fiber layer and RGC layer
PNU-282987 is detected in retina after eye drop application, but not in heart tissue.
Acknowledgements
This work was supported by an NIH NEI grant (EY 022795) issued to Dr. C. Linn. Special thanks to Dr. Rob Eversole for his confocal microscopy expertise.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agudo-Barriuso M, Villegas-Perez MP, de Imperial JM, Vidal-Sanz M. Anatomical and functional damage in experimental glaucoma. Curr. Opin. Pharmacol. 2013;13(1):5–11. doi: 10.1016/j.coph.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Ahmed FA, Chaudhary P, Sharma SC. Effects of increased intraocular pressure on rat retinal ganglion cells. Int. J. Dev. Neurosci. 2011;19:209–218. doi: 10.1016/s0736-5748(00)00073-3. [DOI] [PubMed] [Google Scholar]
- Ahmed I, Patton TF. Importance of the noncorneal absorption route in topical ophthalmic drug delivery. Invest. Ophthalmol. Vis. Sci. 1985;26(4):584–587. [PubMed] [Google Scholar]
- Barnstable CJ, Drager UC. Thy-1 antigen: a ganglion cell specific marker in rodent retina. Neurosci. 1984;11:847–855. doi: 10.1016/0306-4522(84)90195-7. [DOI] [PubMed] [Google Scholar]
- Beidoe G, Mousa SA. Current primary open-angle glaucoma treatments and future directions. Clin. Ophthalmol. 2012;6:1699–1707. doi: 10.2147/OPTH.S32933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar A, Cortes-Burgos L, Cook K, Dinh D, Groppi V, Hajos M, Higdon N, Hoffmnn W. Discovery and structure-activity relationship of quinuclidine benzamides as agonists of alpha7 nicotinic acetylcholine receptors. J. Med. Chem. 2005;48(4):905–908. doi: 10.1021/jm049363q. [DOI] [PubMed] [Google Scholar]
- Cairns JE. Trabeculectomy. preliminary report of a new method. Amer. J. Ophthalmol. 1968;66(4):673–679. [PubMed] [Google Scholar]
- Calkins DJ, Sterling P. Microcircuitry for two types of achromatic ganglion cell in primate fovea. J. Neurosci. 2007;27(10):2646–2653. doi: 10.1523/JNEUROSCI.4739-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan BC, Pan J, Archibald ML, LeVatte TL, Kelly ME, Tremblay F. Effect of intraocular pressure on optic disc topography, electroretinography, and axonal loss in a chronic pressure-induced rat model of optic nerve damage. Invest. Ophthalmol. Vis. Sci. 2002;43:2969–2976. [PubMed] [Google Scholar]
- Conejero-Goldberg C, Davies P, Ulloa L. Alpha7 nicotinic acetylcholine receptor: A link between inflammation and neurodegeneration. Neurosci. Biobehav. Rev. 2008;32(4):693–706. doi: 10.1016/j.neubiorev.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damji KF, Behki R, Wang L. Canadian perspectives in glaucoma management: Setting target intraocular pressure range. Can. J. Ophthalmol. 2003;38(3):189–197. doi: 10.1016/s0008-4182(03)80060-1. [DOI] [PubMed] [Google Scholar]
- Del Barrio L, Martín-de-Saavedra MD, Romero A, Parada E, Egea J, Avila J, McIntosh JM, Wonnacott S, López MG. Neurotoxicity induced by okadaic acid in the human neuroblastoma SH-SY5Y line can be differentially prevented by α7 and β2 nicotinic stimulation. Toxicol. Sci. 2011;123(1):193–205. doi: 10.1093/toxsci/kfr163. [DOI] [PubMed] [Google Scholar]
- Dreher B, Sefton AJ, Ni SY, Nisbett G. The morphology, number, distribution and central projections of Class I retinal ganglion cells in albino and hooded rats. Brain Behav. Evol. 1985;26(1):10–48. doi: 10.1159/000118764. [DOI] [PubMed] [Google Scholar]
- Enciso GA, Rempe M, Dmitriev AV, Gavrikov KE, Terman D, Mangel SC. A model of direction selectivity in the starburst amacrine cell network. J. Comput. Neurosci. 2010;28(3):567–578. doi: 10.1007/s10827-010-0238-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famiglietti EV. Starburst amacrine cells and cholinergic neurons: mirror-symmetric on and off amacrine cells of rabbit retina. Brain Res. 1983;261:138–144. doi: 10.1016/0006-8993(83)91293-3. [DOI] [PubMed] [Google Scholar]
- Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. Brit. J. Ophthalmol. 2002;86:238–242. doi: 10.1136/bjo.86.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu CT, Sretevan D. Laser-induced ocular hypertension in albino CD-1 mice. Invest. Ophthalmol. Vis. Sci. 2010;51(2):980–990. doi: 10.1167/iovs.09-4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glovinsky Y, Quigley HA, Dunkelberger GR. Retinal ganglion cell loss is size dependent in experimental glaucoma. Invest. Ophthalmol. Vis. Sci. 1991;32(3):484–491. [PubMed] [Google Scholar]
- Glovinsky Y, Quigley HA, Pease ME. Foveal ganglion cell loss is size dependent in experimental glaucoma. Invest. Ophthalmol. Vis. Sci. 1993;34(2):395–400. [PubMed] [Google Scholar]
- Grimes W. Amacrine cell-mediated input to bipolar cells: Variations on a common mechanistic theme. Vis. Neurosci. 2011;1(29):41–49. doi: 10.1017/S0952523811000241. [DOI] [PubMed] [Google Scholar]
- Guo L, Moss SE, Alexander RA, Ali RR, Fitzke FW, Cordeiro MF. Retinal ganglion cell apoptosis in glaucoma is related to intraocular pressure and IOP-induced effects on extracellular matrix. Invest. Ophthalmol. Vis. Sci. 2005;46(1):175–185. doi: 10.1167/iovs.04-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajós M, Hurst R, Hoffmann W, Krause M, Wall T, Higdon N, Groppi V. The selective alpha7 nicotinic acetylcholine receptor agonist PNU-282987 N-(3R)-1-Azabicyclo2.2.2oct-3-yl-4-chlorobenzamide hydrochloride enhances GABAergic synaptic activity in brain slices and restores auditory gating deficits in anesthetized rats. J. Pharm. Exp. Ther. 2005;312(3):1213–1222. doi: 10.1124/jpet.104.076968. [DOI] [PubMed] [Google Scholar]
- Heijl A, Leske MC, Bengtsson B, Hyman L. Hussein M. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch. Ophthalmol. 2002;120(10):1268–1279. doi: 10.1001/archopht.120.10.1268. [DOI] [PubMed] [Google Scholar]
- Iwamoto K, Mata D, Linn DM, Linn CL. Neuroprotection of rat retinal ganglion cells mediated through alpha7 nicotinic acetylcholine receptors. Neurosci. 2013;237:184–198. doi: 10.1016/j.neuroscience.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto K, Birkholtz P, Schipper A, Mata D, Linn DM, Linn CL. A nicotinic acetylcholine receptor agonist prevents loss of retinal ganglion cells in a glaucoma model. Invest. Ophthalmol. Vis. Sci. 2014;55(2):1078–1087. doi: 10.1167/iovs.13-12688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobs TC, Libby RT, Ben Y, John SW, Masland RH. Retinal ganglion cell degeneration is topological but not cell type specific in DBA/2J mice. J. Cell Biol. 2005;171:313–325. doi: 10.1083/jcb.200506099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JH, Park KH, Jeoung JW, Kim DM. Preperimetric normal tension glaucoma study: long-term clinical course and affect of therapeutic lowering of intraocular pressure. Acta Ophthalmol. 2014;92(3):e185–e193. doi: 10.1111/aos.12277. [DOI] [PubMed] [Google Scholar]
- Johnson TV, Tomarev SI. Rodent models of glaucoma. Brain Res. Bull. 2011;81(2–3):349–358. doi: 10.1016/j.brainresbull.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalesnykas G, Oglesby EN, Zack DJ, Cone FE, Steinbart MR, Tian J, Pease ME, Quigley HA. Retinal ganglion cell morphology after optic nerve crush and death in experimental glaucoma and experimental glaucoma. Invest. Ophthalmol. Vis. Sci. 2012;53(7):3847–3857. doi: 10.1167/iovs.12-9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda M, Hashimoto M, Kaneko A. Neuronal nicotinic acetylcholine receptors of ganglion cells in the cat retina. Jpn. J. Physiol. 1995;45:491–508. doi: 10.2170/jjphysiol.45.491. [DOI] [PubMed] [Google Scholar]
- Kass MA, Heuer DK, Higginbotham EJ. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch. Ophthalmol. 2002;120(6):701–713. doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]
- Kawamata J, Shimohama S. Stimulating nicotinic receptors trigger multiple pathways attenuating cytotoxicity in models of Alzheimer’s and Parkinson’s diseases. J. Alzheimer’s Dis. 2011;24(2):95–109. doi: 10.3233/JAD-2011-110173. [DOI] [PubMed] [Google Scholar]
- Keyser KT, Hughes TE, Whiting PJ, Lindstrom JM, Karten HJ. Cholinoceptive neurons in the retina of the chick: an immunohistochemical study of the nicotinic acetylcholine receptors. Vis. Neurosci. 1988;1:349–366. doi: 10.1017/s0952523800004120. [DOI] [PubMed] [Google Scholar]
- Keyser KT, Britto LGR, Schoepfer R, Whiting P, Cooper J, Conroy W, Brozozowska-Prechtl A, Karten HJ, Lindstrom J. Three subtypes of α-Bgt-sensitive nicotinic ACh receptors are expressed in chick retina. J. Neurosci. 1993;13:442–454. doi: 10.1523/JNEUROSCI.13-02-00442.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levkovitch-Verbin H, Quigley HA, Martin KR, Valenta D, Baumrind LA, Pease ME. Translimbal laser photocoagulation to the trabecular meshwork as a model of glaucoma in rats. Invest. Ophthalmol. Vis. Sci. 2002;43:402–410. [PubMed] [Google Scholar]
- Lichter PR, Musch DC, Gillespie BW, Guire KE, Janz NK. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmol. 2001;108(11):1943–1953. doi: 10.1016/s0161-6420(01)00873-9. [DOI] [PubMed] [Google Scholar]
- Lin JL, Wang YD, Ma Y, Zhong CM, Zhu MR, Chen WP, Lin BQ. Protective effects of naringenin eye drops on N-methyl-N-nitrosourea-induced photoreceptor cell death in rats. Int. J. Ophthalmol. 2014;7(3):391–396. doi: 10.3980/j.issn.2222-3959.2014.03.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Cai H, Zhang P, Li H, Liu H, Li Z. Activation of ERK1/2 and PI3K/Akt by IGF-1 on GAP-43 expression in DRG neurons with excitotoxicity induced by glutamate in vitro. Cell Mol. Neurobiol. 2012;32:191–200. doi: 10.1007/s10571-011-9746-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftsson T, Brewster ME. Physicochemical properties of water and its effect on drug delivery. Int. J. Pharm. 2008;354(1–2):248–254. doi: 10.1016/j.ijpharm.2007.08.049. [DOI] [PubMed] [Google Scholar]
- Masland RH. Amacrine cells. Trends Neurosci. 1988;11:405–411. doi: 10.1016/0166-2236(88)90078-1. [DOI] [PubMed] [Google Scholar]
- Masland RH. The many roles of starburst amacrine cells. Trends Neurosci. 2005;28(8):395–396. doi: 10.1016/j.tins.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Massey S, Redburn D. Transmitter circuits in the vertebrate retina. Prog. Neurobiol. 1987;28:55–96. doi: 10.1016/0301-0082(87)90005-0. [DOI] [PubMed] [Google Scholar]
- McLean SL, Grayson B, Idris NF, Lesage AS, Pemberton DJ, Mackie C, Neill JC. Activation of α7 nicotinic receptors improves phencyclidine-induced deficits in cognitive tasks in rats: implications for therapy of cognitive dysfunction in schizophrenia. Eur. Neuropsychopharmacol. 2011;21(4):333–343. doi: 10.1016/j.euroneuro.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Mi XS, Yuang TF, So KF. The current research status of normal tension glaucoma. Clin. Interv. Aging. 2014;9:1563–1571. doi: 10.2147/CIA.S67263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JC, Moore CG, Deppmeier LM, Gold BG, Meshul CK, Johnson EC. A rat model of chronic pressure-induced optic nerve damage. Exp. Eye Res. 1997;64:85–96. doi: 10.1006/exer.1996.0184. [DOI] [PubMed] [Google Scholar]
- Ortín-Martínez A, Salinas-Navarro M, Nadal-Nicolás FM, Jiménez-López M, Valiente-Soriano FJ, García-Ayuso D, Bernal-Garro JM, Avilés-Trigueros M, Agudo-Barriuso M, Villegas-Pérez MP, Vidal-Sanz M. Laser-induced ocular hypertension in adult rats does not affect non-RGC neurons in the ganglion cell layer but results in protracted severe loss of cone-photoreceptors. Exp. Eye. Res. 2015;132:17–33. doi: 10.1016/j.exer.2015.01.006. [DOI] [PubMed] [Google Scholar]
- Oz M, Lorke DE, Yang KH, Petroianu G. On the interaction of β-amyloid peptides and α7-nicotinic acetylcholine receptors in Alzheimer’s disease. Curr. Alzheimer Res. 2013;10(6):618–630. doi: 10.2174/15672050113109990132. [DOI] [PubMed] [Google Scholar]
- Prokai-Tatrai K, Xin H, Nguyen V, Szarka S, Blazics B, Prokai L, Koulen P. 17β-estradiol eye drops protect the retinal ganglion cell layer and preserve visual function in an in vivo model of glaucoma. Mol. Pharm. 2013;10(8):3253–3261. doi: 10.1021/mp400313u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberti G, Tanga L, Parisi V, Sampalmieri M, Centofanti M, Manni G. A preliminary study of the neuroprotective role of citicoline eye drops in glaucomatous optic neuropathy. Ind. J. Ophthalmol. 2014;62(5):549–553. doi: 10.4103/0301-4738.133484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha O, Kwong WH. Postnatal developmental changes of vitreous and lens volumes in Sprague-Dawley rats. Neuroembryol. Aging. 2006;4(4):183–188. [Google Scholar]
- Soto I, Pease ME, Son JL, Shi X, Quigley HA, Marsh-Armstrong N. Retinal ganglion cell loss in a rat ocular hypertension model is sectorial and involves early optic nerve axon loss. Invest. Ophthalmol. Vis. Sci. 2011;52(1):434–441. doi: 10.1167/iovs.10-5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WR, Vaney DI. New directions in retinal research. Trends Neurosci. 2003;26(7):379–385. doi: 10.1016/S0166-2236(03)00167-X. [DOI] [PubMed] [Google Scholar]
- Thanos S. Alterations in the morphology of ganglion cell dendrites in the adult rat retina after optic nerve transection and grafting of peripheral nerve segments. Cell Tiss. Res. 1988;254(3):599–609. doi: 10.1007/BF00226510. [DOI] [PubMed] [Google Scholar]
- Thompson SA, Smith O, Linn DM, Linn CL. Acetylcholine neuroprotection against glutamate-induced excitotoxicity in adult pig retinal ganglion cells is partially mediated through alpha4 nAChRs. Exp. Eye Res. 2006;83(5):1135–1145. doi: 10.1016/j.exer.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Walker DP, Wishka DG, Piotrowski DW. Design, synthesis, structure-activity relationship, and in vivo activity of azabicyclic aryl amides as alpha7 nicotinic acetylcholine receptor agonists. Bioorg. Med. Chem. 2006;14(24):8219–8248. doi: 10.1016/j.bmc.2006.09.019. [DOI] [PubMed] [Google Scholar]
- Wässle H. Knock out of direction selectivity in the retina. Neuron. 2001;30(3):644–646. doi: 10.1016/s0896-6273(01)00335-x. [DOI] [PubMed] [Google Scholar]
- Webster M, Gossman C, Linn CL. Proliferation of retinal ganglion cells in a mammalian model. ARVO abst. 2015:2173435. [Google Scholar]
- Wehrwein E, Thompson SA, Coulibaly SF, Linn DM, Linn CL. Acetylcholine protection of adult pig retinal ganglion cells from glutamate-induced excitotoxicity. Invest. Ophthalmol. Vis. Sci. 2004;45(5):1531–1543. doi: 10.1167/iovs.03-0406. [DOI] [PubMed] [Google Scholar]
- Whiting PJ, Schoepfer R, Conroy WG, Gore MJ, Keyser KT, Shimasaki S, Esch F, Lindstrom JM. Expression of nicotinic acetylcholine receptor subtypes in brain and retina. Mol. Brain Res. 1991;10:61–70. doi: 10.1016/0169-328x(91)90057-5. [DOI] [PubMed] [Google Scholar]
- Winterer G, Gallinat J, Brinkmeyer J, Musso F, Kornhuber J, Thuerauf N, Rujescu D, Favis R, Sun Y, Franc MA, Ouwerkerk-Mahadevan S, Janssens L, Timmers M, Streffer JR. Allosteric alpha-7 nicotinic receptor modulation and P50 sensory gating in schizophrenia: a proof-of-mechanism study. Neuropharm. 2013;64:197–204. doi: 10.1016/j.neuropharm.2012.06.040. [DOI] [PubMed] [Google Scholar]
- Young JW, Geyer MA. Evaluating the role of the alpha-7 nicotinic acetylcholine receptor in the pathophysiology and treatment of schizophrenia. Biochem. Pharmacol. 2013;86(8):1122–1132. doi: 10.1016/j.bcp.2013.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]