Abstract
Class switch recombination (CSR) generates isotype-switched antibodies with distinct effector functions. B cells express phosphatase and tensin homolog (PTEN) and multiple isoforms of class IA phosphoinositide 3-kinase (PI3K) catalytic subunits, including p110α and p110δ, whose roles in CSR remain unknown or controversial. Here, we demonstrate a direct effect of PTEN on CSR signaling by acute deletion of Pten specifically in mature B cells, thereby excluding the developmental impact of Pten deletion. We show that mature B cell-specific PTEN overexpression enhances CSR. More importantly, we establish a critical role of p110α in CSR. Furthermore, we identify a cooperative role of p110α and p110δ in suppressing CSR. Mechanistically, dysregulation of p110α or PTEN reversely affects activation-induced deaminase expression via modulating AKT activity. Thus, our study reveals that a signaling balance between PTEN and PI3K isoforms is essential to maintain normal CSR.
Keywords: class switch recombination, activation induced deaminase, Phosphoinositide 3-kinase signaling, PTEN, antibody deficiency
Introduction
Class switch recombination (CSR) is a region-specific DNA recombination process that enables the switch of the constant (C) regions of immunoglobulin heavy chain (IgH) molecules to generate isotype-switched antibodies (1-3). In mice, there are 8 sets of CH exons organized as 5’--VDJ--Cμ--Cδ--Cγ3--Cγ1--Cγ2b--Cγ2a--Cε--Cα--3’ at the Igh locus (1). Naïve B cells initially express surface IgM encoded by Cμ. Upon CSR, the assembled V(D)J exon is juxtaposed next to one of the sets of downstream CH exons, allowing B cells to produce different IgH classes (e.g. IgG, IgE, and IgA) with distinct effector functions that are encoded by different CH genes (e.g. Cγ, Cε, and Cα), respectively (1). The essential molecular components of CSR include: (1) active germline transcription of CH genes that renders a given C region accessible for recombination (1, 3, 4); (2) switch (S) regions that are highly repetitive and specific DNA sequences, located 5’ of each set of CH exons except Cδ (5); (3) activation induced deaminase (AID) that deaminates cytosine (C) and converts it into uracil (U), thereby resulting in U:G mismatch; (4) subsequent recognition and processing of the AID-initiated U:G mismatch by mismatch repair (MMR) and base excision repair (BER) pathways that generate DNA double strand breaks (DSBs) in the upstream donor Sμ and a downstream acceptor S region (6, 7); (5) repair of the AID-initiated DSBs via non-homologous end-joining (NHEJ) that eventually completes CSR via re-joining the two broken S regions (8, 9). Both classical and alternative NHEJ contribute to the repair of S region DSBs (8, 9).
While AID-mediated molecular mechanisms of CSR are well characterized, control of CSR by upstream signaling is less well understood. Previous studies suggest that phosphoinositide 3-kinase (PI3K) and its antagonizing lipid phosphatase PTEN play a critical role in regulating CSR (10, 11). PI3K catalyzes the phosphorylation of PI(4,5)P2 and converts it into PI(3,4,5)P3, whereas PTEN effects the reverse reaction and converts PI(3,4,5)P3 back to PI(4,5)P2. Thus, PI3K and PTEN act antagonistically to maintain the proper cellular level of PI(3,4,5)P3, which promotes activation of downstream kinases including AKT and 3-phosphoinositide dependent protein kinase 1(PDK1) by PH domain-mediated localization at the plasma membrane. Prior studies showed that CD19Cre-mediated Pten deficiency in B cells results in a reduced level of CSR (12, 13). However, since CD19Cre mediates efficient deletion at pre-B cell developmental stage (14), it remains formally possible that CD19Cre-mediated deletion of Pten may affect B cell development that subsequently impairs CSR. Furthermore, the effects of Pten deletion on IgE CSR have not been directly evaluated.
The role of PI3Ks in CSR remains less well understood and appears to be much more complicated, probably due to the fact that there are multiple isoforms of PI3K expressed in B cells. B cells express three isoforms of class I PI3K catalytic subunits, p110α, p110δ, and p110γ (10). To date, only a role for p110δ in CSR has been suggested. It was shown that germline p110δ deletion in B cells does not affect CSR to IgG1, using an in vitro CSR culture assay that can reveal the B cell intrinsic role of any given factor in CSR (15). B cell-specific deletion of p110δ (CD19cre) has no effect on T-dependent antibody or germinal center (GC) responses except that it strongly promotes antigen-specific IgE production, implicating specific dysregulation in IgE CSR (16). Overall, genetic deletion of p110δ has no significant effect on IgG1 CSR but strongly promotes IgE CSR. On the other hand, pharmacologic inhibition of p110δ in wt B cells potently enhances the percentage of IgG1+ and IgE+ B cells (17). The discrepancy regarding IgG1 CSR probably results from compensatory effects of other PI3K isoforms in the p110δ-deleted B cells. To avoid the complication that deleting one subunit can affect the expression of the others, a knock-in allele was generated that carried an inactive point mutation of p110δ (D910A) (18). p110δD910A (inactive) mutant mice had reduced in vivo Ab responses to T-dependent and T-independent antigens (18). In contrast, p110δD910A mutant B cells exhibit an increased level of CSR to IgG1 and IgE in an in vitro CSR assay (17), suggesting that p110δ normally suppresses CSR. Taken together, these studies suggest that a B cell intrinsic role of p110δ in CSR has not been clearly elucidated. Thus, further studies are needed to address the potential underlying mechanisms whereby the components of PI3K pathway modulate CSR. It remains unknown whether other PI3K isoforms of catalytic subunit (p110α, β or γ) have any effects on CSR. Previous data showed that p110α compensates for p110δ in B cell development (19). Thus, we reason that other PI3K isoforms may also play a critical role in CSR that may explain the inconsistent phenotypes caused by p110δ deletion versus point mutation.
Defects in CSR can lead to Hyper-IgM syndrome (HIGM) and common variable immunodeficiency (CVID) in human patients, characterized by increased serum IgM and reduced IgG levels, and susceptibility to recurrent infections (20). Genetic defects in CD40/CD40L and AID account for many cases of HIGM (20, 21). It has been suggested that dysregulated PI3K or PTEN activity may also contribute to the development of HIGM or CVID, especially for the cases without genetic classification that might carry mutations in genes regulating PI3K or PTEN activity (22). Consistent with these notions, emerging studies have reported that mutations of PI3K components, e.g. p110δ and p85α, have important implications in human immunodeficiency (23-27). For instance, a recent study described activated PI3K-δ syndrome (APDS), a primary immunodeficiency associated with a dominant gain-of-function mutation in the p110δ protein, which occurs at residue 1021 with glutamic acid replaced by lysine (E1021K) (23). APDS patients have increased serum level of IgM but reduced IgG2 and impaired vaccine responses (23). Furthermore, mutations of p110α are frequently observed in cancer patients (28) and its constitutively active mutant cooperates with c-myc to cause Burkitt lymphomagenesis in mouse (29). Therefore, it would be of great interest to further elucidate the B cell intrinsic roles of PI3K isoforms in regulating CSR and the relevant downstream signaling components.
In the current study, we employed various genetic models and pharmacological inhibitors to investigate how different PI3K isoforms coordinate with PTEN to regulate CSR. Using deletion or constitutive activation, our results reveal that a delicate signaling balance between PI3K and PTEN is essential for a normal level of CSR. We further discuss the implications of our study in the context of antibody-mediated immune diseases.
Materials and Methods
Generation of genetically modified mouse models
To delete the Pten gene specifically in mature B cells in an inducible manner, Ptenflox/flox mice (a kind gift from Dr. Robert Rickert) (30) were bred with hCD20TamCre (a kind gift from Dr. Mark Shlomchik) (31) and Rosa26-flox-stop-YFP mice (32) to generate the compound mutant mice termed PTEN-KO-YFP mice. hCD20TamCre mice contain a human CD20 promoter-driven transgenic fusion gene composed of Cre recombinase and estrogen receptor (ER) nuclear localization domain (Cre-ER), which can be activated by tamoxifen injection to induce the translocation of the Cre-ER fusion protein into the nucleus, thereby mediating deletion of the Pten floxed allele acutely. Thus, our lineage-specific inducible system allows the deletion of Pten in mature B cells without the complication of impaired B cell development. In addition, these PTEN-KO-YFP mutant B cells express YFP as a surrogate marker for Pten deletion. To generate the control mice, hCD20TamCre mice were intercrossed with Rosa26-flox-stop-YFP mice, in which YFP was expressed in B cells upon Cre-ER activation by tamoxifen (termed YFP-KI mice). To achieve the overexpression of PTEN specifically in mature B cells, Rosa26-flox-stop-Pten-2A-YFP mice were generated (Getahun A and Cambier JC, manuscript in preparation), which contain a silenced Pten cDNA preceded by a STOP cassette and linked to a YFP cDNA by a 2A peptide encoding sequence, targeted into Rosa26 locus. Rosa26-flox-stop-Pten-2A-YFP mice were intercrossed with hCD20TamCre mice, and the compound mutant mice were termed PTEN-Tg-YFP or PTEN-Tg mice.
To generate mice in which mature B cells can be induced to express a constitutively active form of p110α, Rosa26-flox-stop-P110α*-IRES-GFP (p110α*-GFP) mice (a kind gift of Dr. Klaus Rajewsky) (33) were bred with hCD20TamCre mice to generate p110α*-GFP TamCre mice. These mice contain a cDNA encoding a constitutively active form of P110α (designated as p110α*) (34) that is preceded by a loxP flanked STOP cassette and followed by an IRES and GFP cDNA in Rosa26 locus (33). Transcription of the KI p110α* allele can be induced by tamoxifen injection as described above, thereby allowing the expression of constitutive activated p110α* in mature B cells without affecting B cell development. In addition, the p110α*-GFP-TamCre mice express GFP as a surrogate marker for p110α* activation. Moreover, Rosa26-flox-stop-P110α*-IRES-GFP (p110α*-GFP) mice (33) were bred with Cγ1Cre (35) mice to generate p110α*-GFP-Cγ1Cre mice which were used for proliferation assay (Figure 4C), PI3K inhibitor rescue experiments (Figure 5B), and RT-PCR (Figure 7F).
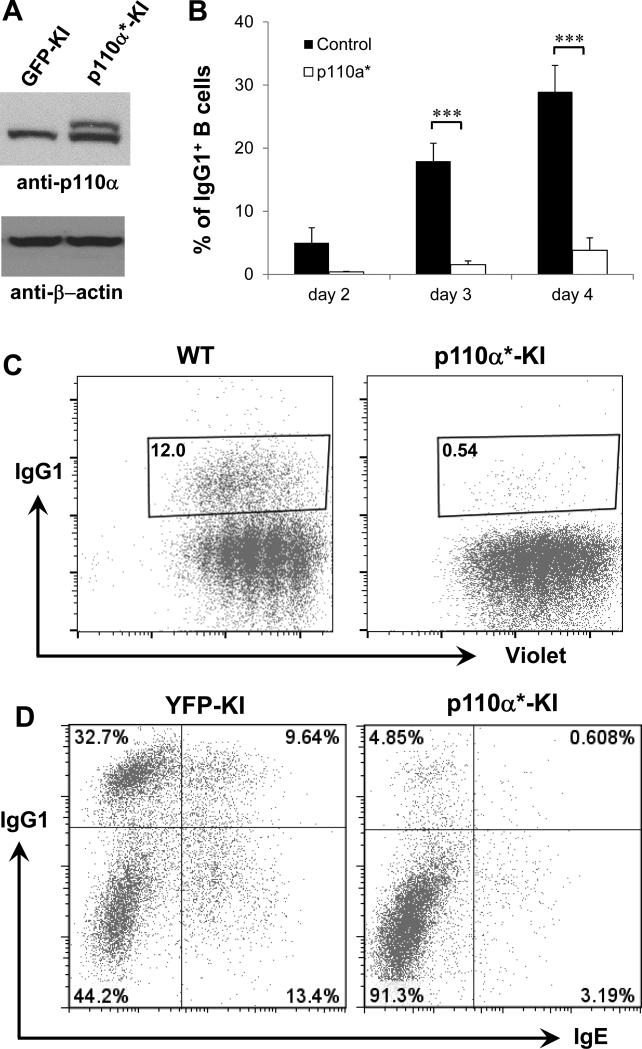
Figure 4. Hyperactivation of p110α inhibits IgG1 and IgE CSR.
(A) Western blotting analysis of p110α protein. Day 3 activated B cells from WT or p110α* KI mice were employed for western blot with anti-p110α antibody. p110α* mutant B cells expressed both endogenous and KI pl10α protein. (B) Kinetics of IgG1 CSR level at various time points in anti-CD40/IL4-activated B cells. The percentage of IgG1+ B cells was shown for p110α* mutant or control samples. Statistical analyses were calculated by a Student's t-Test with two-tailed distribution and two-sample equal variance. p=0.090 (day2, control vs KI, n=5), ***p=3.427×10−5 (day3, control vs KI, n=9), ***p=5.514×10−5 (day4, control vs KI, n=9). Data are presented as mean±s.e.m. (C) Proliferation assay for p110α* mutant and control B cells activated with anti-CD40/IL4 for 3 days. Representative FACS plots were shown from three independent experiments. Numbers in the gate are percentages of IgG1+ B cells. (D) Purified B cells from indicated genotypes (n=4 per group) were cultured for 4 days, and stained with anti-IgG1 and anti-IgE intracellularly and analyzed by flow cytometry. p110α*: constitutively active form of p110α.
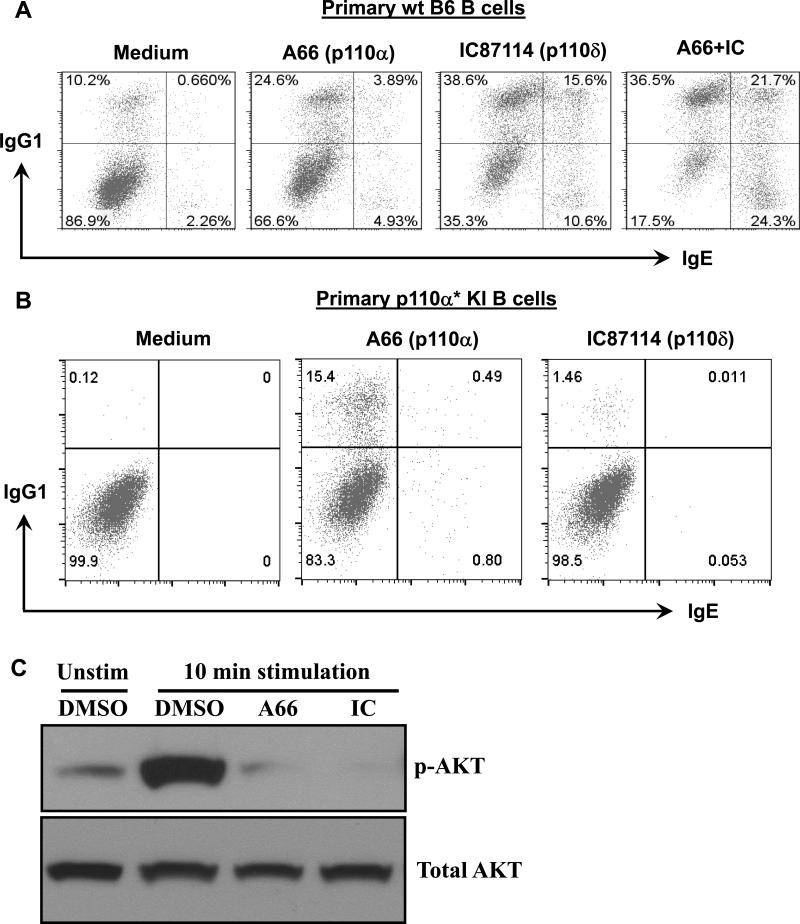
Figure 5. The cooperative roles of p110α and p110δ in suppressing CSR.
(A) WT B6 B cells were stimulated with anti-CD40 and IL4 (medium) for 3 days in the presence of p110α-specific (A66, 5μM), p110δ-specific (IC87114, 5μM), or both inhibitors (A66+IC), and analyzed by flow cytometry for IgG1 and IgE CSR. (B) Primary p110α* KI B cells were activated with anti-CD40 and IL4 (medium) for 3 days in the presence of p110α-specific (A66) or p110δ-specific (IC87114) inhibitor. Representative FACS plots were shown from three independent experiments for panel A and B. (C) Western blotting analysis of AKT phosphorylation. WT B6 B cells were stimulated with anti-CD40/IL4 in the presence of p110α-specific (A66) or p110δ-specific (IC) inhibitor for 10 mins (10’ stimulation). Different groups of B cells were employed for western blot analysis with anti-phospho-AKT or anti-AKT antibody. Representative western plots were shown from three independent experiments for panel C.
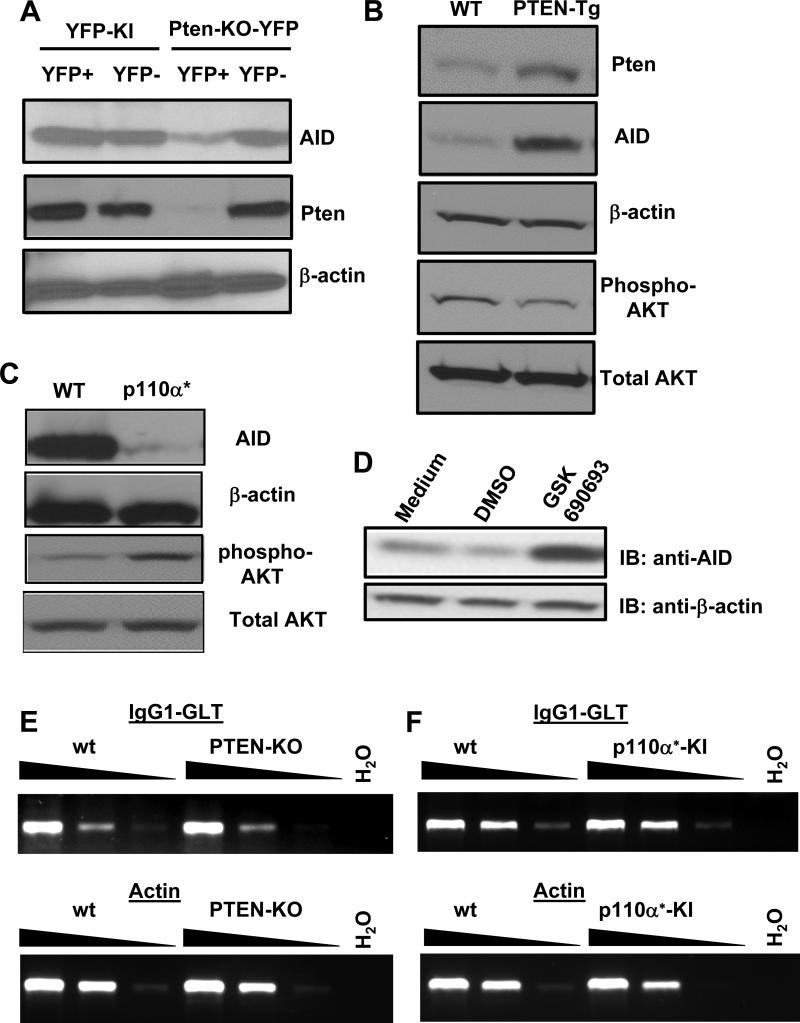
Figure 7. AID expression is reduced in p110α hyperactive or Pten deficient B cells and correlates to the level of phosphor-AKT reversely.
(A) B cells from indicated genotypes were stimulated with anti-CD40 plus IL4 for 3 days, and sorted as YFP positive or negative populations, respectively. Cell lysates were resolved by SDS-PAGE and sequentially immuno-blotted with anti-AID, anti-Pten and anti-β-actin specific antibodies. (B) WT or PTEN-Tg B cells were activated as described above and employed for western blot analysis using the indicated antibodies. (C) Activated WT or p110α* mutant B cells lysates were resolved by SDS-PAGE and sequentially immuno-blotted with indicated antibodies. (D) WT B cells were stimulated for 2 days under indicated conditions (Medium: anti-CD40/IL4; DMSO: solvent for GSK690693). Lysates from different groups were resolved by SDS-PAGE and sequentially immuno-blotted (IB) with anti-AID and anti-β-actin specific antibodies. Representative western plots were shown from three independent experiments for panel A-D. (E and F) Semi-quantitative RT-PCR analysis of germline transcripts (GLT) of IgG1 in day 4 activated B cells from wt, PTEN-KO, or p110α* KI mice. cDNA samples were diluted in 1:5 serials for actin (1:5, 1:25 and 1:125) and 1:3 serials for IgG1 GLT (no dilution, 1:3 and 1:9). Representative data were shown from three independent experiments.
To activate Cre-ER fusion protein, mice were injected intraperitoneally twice on consecutive days with tamoxifen (2 mg in 100μl corn oil per mouse each time). 7 or 10 days after tamoxifen injection, mice were utilized for experiments and B cells expressed YFP or GFP upon the successful activation of Cre-ER fusion protein. In general, 6 to 16 week old mice were used for experiments. WT B6 and Balb/c mice were purchased from Jackson Laboratory. Animal work was approved by the Institutional Animal Care and Use Committee of University of Colorado Anschutz Medical Campus (Aurora, CO) and National Jewish Health (Denver, CO).
Cell culture, flow cytometry and cell sorting
Spleens were harvested from mice of various genotypes following treatment with tamoxifen or control mice, and naïve B cells were isolated (StemCell mouse B cell isolation EASY kit) according to the manufacturer's instructions. Purified B cells (purity>95%, data not shown) were stimulated in vitro with anti-CD40 (0.5μg/ml, eBioscience, clone HM40-3) plus interleukin-4 (IL4) (10ng/ml, Fisher Scientific) in 10% FBS RPMI lymphocyte medium for various days. Activated B cells were examined with flow cytometry to detect the percentage of IgG1+ or IgE+ class-switched B cells. Intracellular IgE was detected using methods based on previous studies (36-38). Briefly, single cell suspension was treated for 1 min with acid buffer (0.085M NaCl, 0.005M KCl, 0.01M EDTA, and 0.05M NaAcetate [pH 4]) to remove cytophilic IgE (defined as extrinsic, CD23-bound) (39, 40), then the samples were neutralized with 3 ml of cell culture medium and washed with 2% FBS 1×PBS buffer sequentially. Cells were fixed and permeabilized with BD Cytofix/Cytoperm kit (BD Biosciences, San Jose, CA). Intracellular staining was performed with APC-anti-IgG1 and PE-anti-IgE (Biolegend, San Diego, CA) using a BD Biosciences intracellular staining kit (BD Biosciences, San Jose, CA) according to manufacturer's instructions. All analyses were performed with a FACSCalibur (BD Biosciences), and results were analyzed with FlowJo software. In addition, YFP or GFP positive B cells were sorted with Beckman Coulter MoFlo XDP Sorter and employed for subsequent biochemical analysis. Proliferation assay was performed with the CellTrace™ Violet Cell Proliferation Kit for flow cytometry (Life Technologies, Thermo Fisher Scientific) according to manufacturer's instructions. Analysis was performed on BD LSRII or BD LSRFortessa platform. CH12F3 cells were cultured in CH12 medium and activated with transforming growth factor-β (TGFβ), anti-CD40 and IL4 to induce IgA CSR as described previously (41).
Chemical inhibitors, Western blotting and antibodies
p110α specific inhibitor (A66), p110δ specific inhibitor (IC87114) and AKT inhibitor (GSK690693) were purchased from SelleckChem (Houston, TX) and used at the concentration of 5μM, 5μM and 1μM, respectively. Western blot antibodies included: anti-PTEN, anti-pan-AKT, anti-phospho-AKTT308 and AKTS473, and anti-p110α (Cell Signaling Technology, Beverly, MA), anti-p110δ (Abcam, Cambridge, MA), anti-β-actin (Santa Cruz Biotechnology, Santa Cruz, CA), and HRP-conjugated anti-mouse IgG and anti-rabbit IgG (Jackson Immunoresearch Laboratory, West Grove, PA). ECL western blot detection reagents were purchased from GE Healthcare (Piscatoway, NJ). Flow cytometry antibodies included: PE-conjugated anti-mouse IgE (Biolegend, San Diego, CA), FITC or APC-conjugated anti-mouse IgG1, FITC, PercP or PE-conjugated anti-mouse B220 (BD Biosciences, San Jose, CA).
RT-PCR analysis for germline transcripts
Semi-quantitative RT-PCR was performed as described previously (42). Total RNA was purified with TriPure (Roche) and used for RT reaction according to manufacturer's instructions (Promega). Forward primer for Actin: 5'-TGGAATCCTGTGGCATCCATGAAAC-3'; Reverse primer for actin: 5'-TAAAACGCAGCTCAGTAACAGTCCG-3'. Forward primers for IgG1 germline transcript (GLT) (Iγ1): 5'-GGCCCTTCCAGATCTTTGAG-3'; Reverse primer for IgG1 GLT (Cγ1 exon1): 5'-CAGGGTCACCATGGAGTTAGTT-3'. PCR reaction conditions: 94°C 3min, 94°C 1 min, 60°C 45s, 72°C 45s, 30 cycles, 72°C 10 min.
CRISPR/Cas9 targeting
We employed a new strategy of gene-targeting based on CRISPRs (Clustered Regularly Interspaced Short Palindromic Repeats) (43-45) to delete p110α or p110δ gene. We followed a detailed protocol as described previously (46). p110α or p110δ-specific guide oligos were cloned into pX330 plasmids containing a Cas9 expression cassette and the cloning site for guide RNA (Broad Institute, MIT, Cambridge, MA), and the resultant plasmids were used to transfect CH12F3 mouse B cells with the NucleofectorTM kit (Lonza, Walkersville, MD). In general, the transfection efficiency was higher than 90%. We have successfully identified pooled clones that carry non-homologous end-joining (NHEJ)-mediated insertions and deletions (indels) by Cel-I assay (data not shown). Subsequently, a limiting dilution assay was employed to screen for single clones carrying NHEJ events on both alleles. Genomic DNA was isolated from single clones and employed for PCR screening with locus-specific primers. PCR products were cut with MboII for p110α targeting which resulted in three fragments for wt allele (77bp, 106bp, 244bp) and two fragments for NHEJ allele (77bp, ~349bp) or with BbvI for p110δ targeting which resulted into two fragments for wt allele (179bp, 208bp) and one fragment for NHEJ allele (~387bp). Sanger sequencing was employed to validate the indels in multiple mutant clones. Sequences of oligos and primers:
p110α guide oligo-Forward: 5’- CACCGAGTTCACCCGAAGATGGTCG-3’
p110α guide oligo-Reverse: 5’- AAACCGACCATCTTCGGGTGAACTC-3’
p110α PCR screening-Forward: 5’-AACAGGTGGTTTTCTTCTTTTGTTCA-3’
p110α PCR screening-Reverse: 5’-CTTCACGGTTGCCTACTGGTTCA-3’
p110δ guide oligo-Forward: 5’- CACCGAGAAGTCAACAACCACGCTC-3’
p110δ guide oligo-Reverse: 5’- AAACGAGCGTGGTTGTTGACTTCTC-3’
p110δ PCR screening-Forward: 5’-GGTGGCTTAAATTGGTGGGAGTCA-3’
p110δ PCR screening-Reverse: 5’ GGGAGGGGGAAAGGAAAATAAGGT-3’
Results
Deletion of Pten results in a significant reduction of IgG1 CSR
Previous studies showed that CD19cre-mediated deletion of Pten resulted in a significant reduction in CSR (12, 13). However, CD19cre exhibits a deletion efficiency of 75-80% in pre-B cells (14), thus, it remains possible that CD19cre-mediated Pten deletion might affect pre-B cell development, impairing CSR indirectly. To absolutely exclude the potential impact of Pten deletion on B cell development, we employed an inducible experimental system that allows the acute deletion of the Pten gene in mature B cells via tamoxifen injection (see details in Material and Methods). In addition, we crossed an inducible YFP reporter cassette into Pten conditional knock-out (KO) background (30), whose expression can also be induced acutely via tamoxifen injection. Thus, in the compound mutant PTEN-KO-YFP mice, YFP expression serves as a surrogate marker for deletion of the floxed Pten allele. Tamoxifen was injected into Pten-KOYFP and control YFP-KI mice that carry the YFP reporter cassette only. Moreover, we included wild type (wt) C57BL/6 (B6) mice treated with or without tamoxifen as controls.
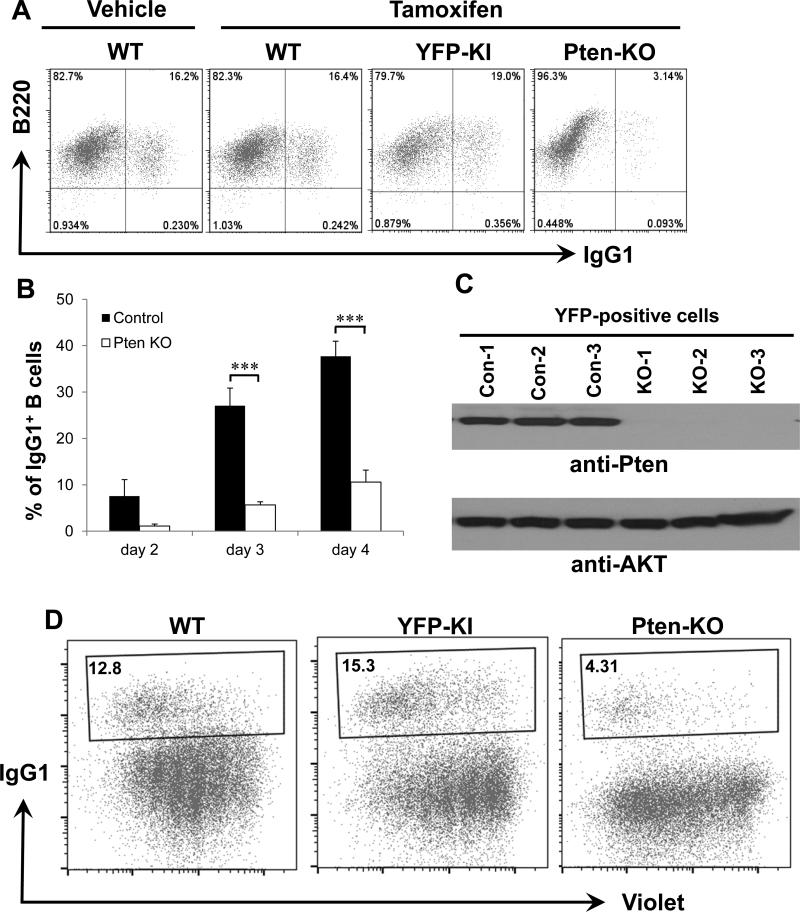
CSR can be induced in vitro by activating B cells with anti-CD40 in the presence of cytokines such as IL-4. We have employed this in vitro CSR assay to identify the B cell intrinsic defects in CSR previously (42, 47). Splenic B cells were isolated from mice of various genotypes and stimulated with anti-CD40 and IL-4 for the indicated number of days. Activated B cells were gated on YFP positive populations if applicable and analyzed by flow cytometry for IgG1 CSR. Our data showed that the percentage of IgG1+ switched B cells was comparable among wt B6 (WT) treated with or without tamoxifen and control YFP-KI B cells after 3 days of stimulation (Figure 1A). In contrast, Pten-KO-YFP mutant B cells exhibited a striking reduction in IgG1 CSR (Figure 1A). Consistently, kinetic studies showed that the percentage of IgG1+ B cells was significantly reduced in Pten deficient mutant B cells (PTEN-KO) as compared to control B cells at day 3 and 4 after stimulation (Figure 1B). We confirmed that, compared to the sorted YFP positive B cells from YFP-KI mice, the sorted B cells from PTEN-KO-YFP mice did not express PTEN protein but expressed equal amount of AKT shown by western blot with corresponding specific antibodies (Figure 1C). Since CSR level can be affected by the proliferation status of activated B cells, we next investigated whether Pten deficiency altered the proliferation of mutant B cells. Our results showed that PTEN KO cells did not exhibit any proliferation defects as compared to their wt counterparts (Figure 1D). Thus, we conclude that the acute deletion of Pten significantly reduces IgG1 CSR in a mature B cell autonomous manner.
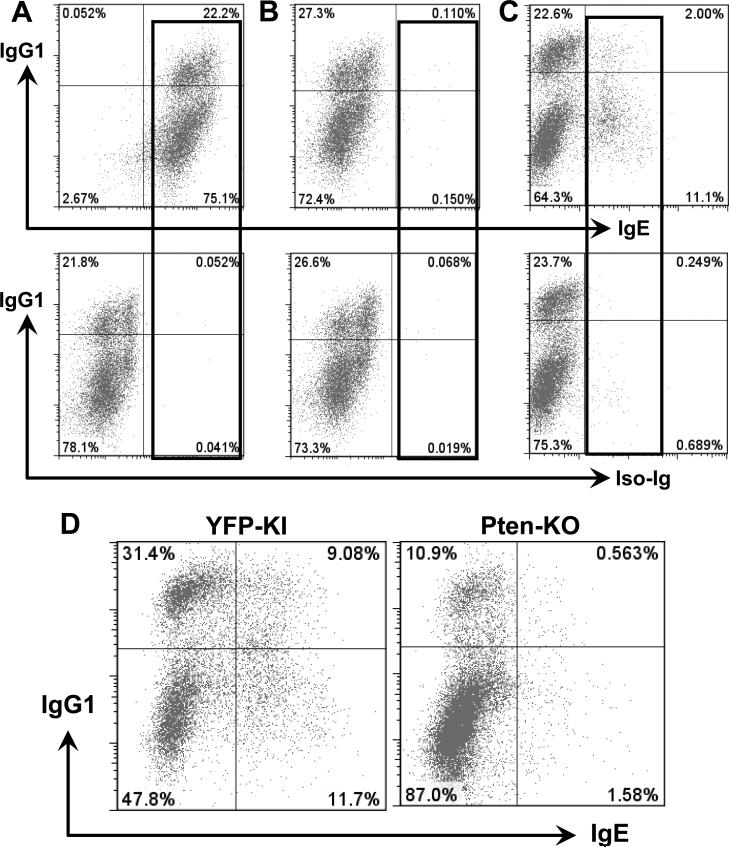
Figure 1. Pten deficiency in mature B cells inhibits IgG1 CSR.
(A) Representative FACS plots of IgG1 CSR in day 3 activated B cells from indicated genotypes (n=5 per group) treated with vehicle or tamoxifen. Numbers in quadrates indicate the percentages of IgG1+ B cells. YFP+ cells were gated for the YFP-KI and PTEN KO but not WT mice. (B) Kinetics of IgG1 CSR level at various time points in anti-CD40/IL4-activated B cells. The percentage of IgG1+ B cells was shown for PTEN-KO or control samples. Statistical analyses were calculated by a Student's t-Test with two-tailed distribution and two-sample equal variance. p=0.107 (day2, control vs KO, n=5), ***p=8.785×10−5 (day3, control vs KO, n=6), ***p=3.754×10−5 (day4, control vs KO, n=6). Data are presented as mean±s.e.m. (C) Western blotting analysis of PTEN protein. Day 3 activated B cells were sorted for YFP positive population from either YFP-KI control (Con) or PTEN-KO-YFP (KO) mice that were subjected to western blot with anti-PTEN antibody. WT: wild type; YFP: yellow fluorescence protein; KI: knock-in; KO: knock-out. (D) Proliferation assay for PTEN-KO and control B cells activated with anti-CD40/IL4 for 3 days. Numbers in the gate are percentages of IgG1+ B cells. Populations with less Violet staining indicate more cell divisions. All experiments were repeated at least three times independently and representative plots were shown.
Anti-CD40/IL4 induces production of secreted but not membrane-bound IgE and Ptendeficiency dramatically reduces IgE CSR
Since it was reported that CSR to IgG1 and IgE was differentially regulated by p110δ deficiency (16), we attempted to investigate how acute deletion of Pten affects IgE CSR. Surprisingly, flow cytometry analysis showed that 100% of anti-CD40/IL4-stimulated WT B cells were IgE+ when examined with anti-IgE antibody for routine surface staining, whereas only about 20% of stimulated B cells were IgG1+ (Figure 2A, top). Such IgE positive staining appeared to be specific since isotype Ig control did not generate any positive signals (Figure 2A, bottom). We postulated that this puzzling phenomenon might be caused by the low affinity IgE receptor, FcεRII (CD23), expressed on the surface of anti-CD40/IL4-activated B cells (data not shown), which captured the secreted IgE, thereby resulting in 100% of B cells positive for IgE. To test this possibility, we pretreated the cultured B cells with acid buffer (see details in Material and Methods). Unexpectedly, this acidic treatment removed all of the IgE staining, in contrast, it did not affect IgG1 surface staining (Figure 2B). These results suggested that the positive IgE surface staining is probably caused by the weak association between secreted IgE and its low affinity FcεRII. Moreover, anti-CD40/IL-4 stimulation appears to only induce secreted but not membrane bound IgE, due to the complete absence of positive IgE staining upon acid treatment (Figure 2B). In addition, lipopolysaccharide/IL-4 stimulation only promoted secreted IgE production (data not shown).
Figure 2. Pten deficiency in mature B cells causes IgE CSR defects.
Purified WT B cells were cultured with anti-CD40 and IL4 for 3 days and analyzed by flow cytometry. (A) The activated B cells were stained with anti-IgG1 and anti-IgE or isotype control (Iso-Ig) with routine surface staining method. (B) The cells were pretreated with acidic buffer, and stained as described in (A). (C) The cells were pretreated with acidic buffer, and stained with anti-IgG1 and anti-IgE or isotype control intracellularly. (D) Intracellular staining showed that Pten deficiency resulted in a dramatic reduction in IgE CSR in day 4 activated B cells (n=4 per group). Numbers in the quadrates are percentages of different populations. All experiments were repeated at least three times independently and representative plots were shown.
Since surface staining cannot reliably evaluate the level of IgE CSR induced by anti-CD40/IL-4, we adopted a method by pre-treating cells with acidic buffer then employing an intracellular staining approach to detect IgE CSR (36-38). After 3 days of stimulation, about 11% of wt B6 B cells displayed positive staining for intracellular IgE whereas isotype control-Ig staining was negligible (Figure 2C). Thus, intracellular IgE staining is a reliable method to evaluate IgE CSR level. Next, we employed this method to evaluate the previously untested effects of Pten deficiency on IgE CSR. We found that Pten deficient B cells exhibited a markedly reduced level of IgE CSR as compared to control YFP-KI B cells (Figure 2D). Given that our mouse models exclude the possible effects of Pten deletion on B cell development, we thus definitively demonstrate that Pten deficiency significantly reduces the percentage of IgG1+ and IgE+ switched B cells via impairing CSR process per se.
Overexpression of PTEN in mature B cells promotes CSR
Since Pten deficiency significantly inhibits CSR, we hypothesized that overexpressing PTEN in B cells may promote CSR. To test this hypothesis, we employed a newly generated Pten transgenic mouse model, in which the overexpression of PTEN was induced with a similar strategy as described above via human CD20 promoter-driven Tam-Cre, termed PTEN-Tg-YFP-TamCre mice (PTEN-Tg) (Figure 3A). Upon tamoxifen injection, Pten transgene is constitutively expressed from the Rosa26 locus in mature B cells while the YFP reporter expression is a surrogate marker for PTEN overexpression (Figure 3A). Indeed, we detected a higher level of PTEN protein in the Tg B cells (Figure 3B). Control YFP-KI and PTEN-Tg B cells were purified, stimulated with anti-CD40/IL4 and assayed for IgG1 and IgE CSR level. Our results showed that the percentage of IgG1+ or IgE+ switched B cells was significantly increased in PTEN-Tg B cells compared to control YFP-KI B cells at different time points (Figure 3C). Furthermore, PTEN-Tg B cells did not exhibit any proliferation defects as compared to their wt counterparts (Figure 3D). Thus, we conclude that a higher level of PTEN expression in mature B cells promotes CSR.
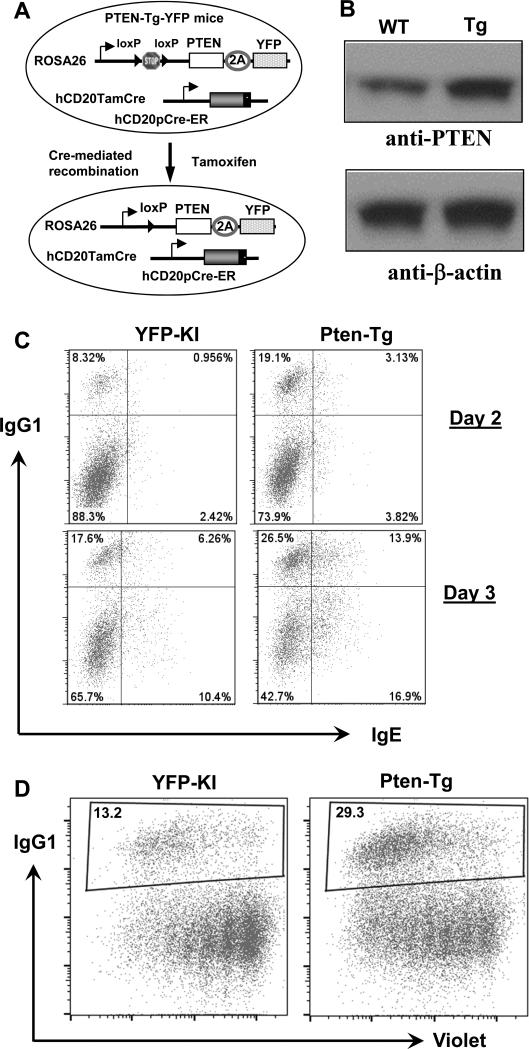
Figure 3. Overexpression of PTEN promotes IgG1 and IgE CSR.
(A) Schematics of PTENTg mice generation. Tamoxifen injection induces the Cre-mediated deletion of STOP cassette, thereby permitting the constitutive expression of PTEN in mature B cells. (B) Western blottinganalysis of PTEN protein. PTEN-Tg B cells expressed a higher level of PTEN protein. (C) B cells from indicated genotypes were stimulated for 2 or 3 days, respectively, stained with anti-IgG1 and anti-IgE intracellularly and analyzed by flow cytometry. Representative FACS plots were shown from three independent experiments. Numbers in the quadrates are percentages of different populations. (D) Proliferation assay for PTEN-Tg and control B cells activated with anti-CD40/IL4 for 3 days. Representative FACS plots were shown from three independent experiments. Numbers in the gate are percentages of IgG1+ B cells.
Constitutive activation of p110α dramatically reduces IgG1 and IgE CSR
B cells express an appreciable amount of class IA PI3K isoforms of catalytic subunit, p110α and p110δ. Previous studies of genetic deletion or pharmacological inhibition revealed a controversial role of p110δ in regulating CSR (15-18), which prompted us to investigate how p110α regulates CSR. To test whether constitutive activation of p110α modulates CSR in a mature B cell intrinsic manner, we generated a complex mouse model using a similar approach as described above (see details in Material and Methods). We employed a previously established KI model termed p110α*-GFP mice (33), which were crossed with hCD20TamCre mice (31) to generate p110α*-GFP-hCD20TamCre mice (termed p110α* KI mice). In the compound mutant mice, tamoxifen injection induces the expression of constitutive activated p110α* in mature B cells without compromising B cell development. In addition, GFP expression serves as a surrogate marker for the activation of p110α* allele. Our data demonstrated the successful induction of p110α* allele in KI B cells which encoded a protein with a slightly higher molecular weight than wt counterpart (Figure 4A), due to the modification of wt p110α (34). To test the effects of constitutively active p110α* allele on CSR, we isolated splenic B cells from various genotypes of mice and activated them in culture with anti-CD40 and IL4. Kinetic studies demonstrated a drastic reduction of IgG1 CSR in p110α* KI B cells compared to control B cells at different time points (Figure 4B). Furthermore, p110α* KI B cells did not exhibit any proliferation defects as compared to their wt counterparts (Figure 4C). Thus, we conclude that constitutive activation of p110α results in a dramatic reduction of IgG1 CSR.
Since PI3K pathway may play a critical role in regulating IgE CSR (16, 17, 19, 38, 48), we employed the intracellular IgE staining to investigate whether constitutive activation of p110α affected IgE CSR. Our data demonstrated that hyperactivation of p110α significantly inhibited the level of IgE CSR evidenced by the reduction of IgE+ and IgG1+IgE+ switched B cells (Figure 4D). The IgG1+IgE+ double producers likely represent the sequentially switched B cells that initially switch to IgG1 and subsequently switch to IgE. Taken together, we conclude that constitutive activation of p110α also drastically inhibits IgE CSR.
Both p110α and p110δ participate in regulating the normal level of CSR and inhibition of p110α* hyperactivity rescues the defective CSR
Given the striking inhibitory effect of p110α* on CSR, we next tested whether endogenous p110α plays a role in regulating CSR. To do so, we took advantage of a pharmacological inhibitor of p110α (A66) that specifically blocks the activity of p110α. Our results showed that pharmacological inhibition of p110α enhanced the level of CSR in WT B cells (Figure 5A), suggesting that p110α normally suppresses CSR. Moreover, we found that a p110δ specific inhibitor (IC87114) increased the percentage of IgG1+ and IgE+ switched B cells, to an even greater extent (Figure 5A). More importantly, inhibition of both p110α and p110δ additively promotes CSR, especially for IgE+ and IgG1+IgE+ double producers (Figure 5A). Taken together, these data demonstrate an inhibitory role of p110α and p110δ in regulating CSR that appear to function cooperatively.
To further confirm the role of p110α* per se in reducing CSR, we performed CSR rescue assays of p110α* KI B cells using p110α (A66) or p110δ (IC87114) specific inhibitors. Our results demonstrate that only the p110α inhibitor rescued the CSR defects in p110α* mutant KI B cells whereas the p110δ specific inhibitor did not (Figure 5B). Taken together, our study suggests that p110α* independently regulates CSR and does not act through p110δ. To further elucidate the mechanisms mediating the effects of PI3K inhibitors, we tested whether inhibiting p110α or p110δ affected the phosphorylation level of AKT which is an immediate downstream element of PI3K signaling pathways. Phosphorylation of AKT Ser473 and Thr308 is required for the full activation of AKT and often used as readouts of PI3K activity. We found that both inhibitors suppressed the phosphorylation of AKT at Ser473 compared to the control group after 10 minutes of treatment (Figure 5C). These data indicate that PI3K isoforms including p110α and p110δ might function through AKT to suppress CSR.
AKT inhibition rescues the CSR defects in p110α* KI or Pten deficient B cells
AKT acts as a downstream effector of the PI3K pathway, and its activation has been suggested to downregulate AID expression and reduce CSR (12, 13). To test this relationship in our model, we investigated how AKT inhibition affected CSR in WT B6 B cells by employing a pan-AKT inhibitor, GSK690693. After two days of activation, the AKT inhibitor promoted IgG1 CSR remarkably, leading to an almost 10 fold increase of IgG1+ B cells (Figure 6A). While WT B6 B cells produced a minimal level of IgE after two days of activation, AKT inhibition resulted in an average 5-6 fold increase of IgE+ B cells (Figure 6A). In addition, we found that AKT inhibition promoted IgG1 and IgE CSR in WT B cells from different genetic backgrounds such as Balb/c mice (data not shown). Thus, we conclude that AKT activation normally inhibits CSR.
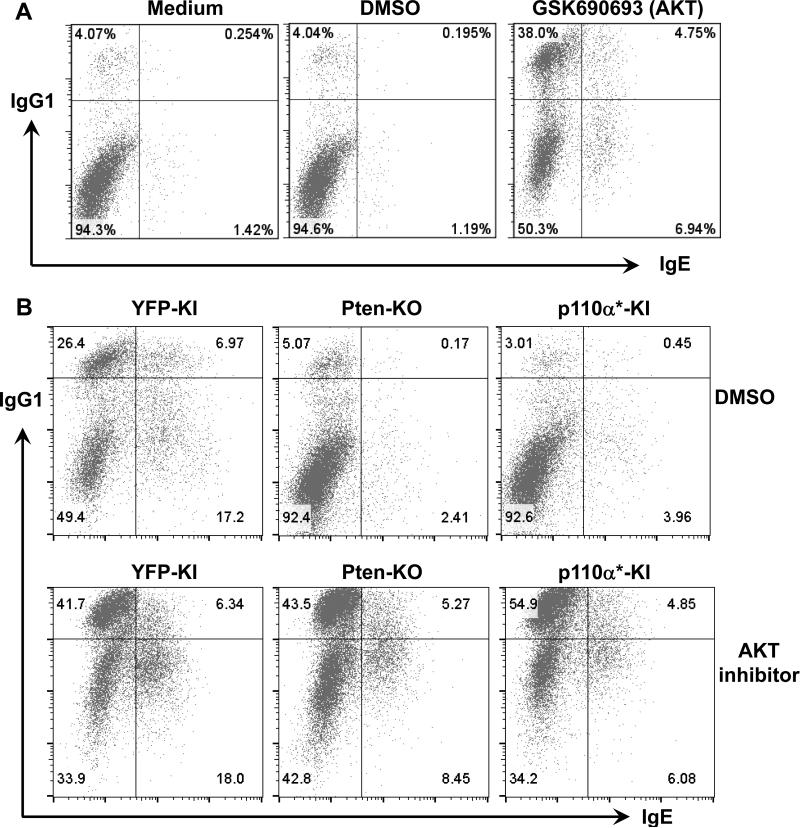
Figure 6. Inhibition of AKT promotes IgG1 and IgE CSR in WT B cells and rescues CSR defects in p110α* KI or PTEN-KO B cells.
(A) WT B cells were stimulated for 2 days under indicated conditions. GSK690693: pan-AKT specific inhibitor (1μM); DMSO: solvent for GSK690693. (B) Genetically modified B cells from indicated genotypes were stimulated for 4 days under indicated conditions. Cells were then stained with anti-IgG1 and anti-IgE intracellularly and analyzed by flow cytometry. Numbers in the quadrates are percentages of different populations. Representative FACS plots were shown from at least five independent experiments for panel A and B.
We predict that both Pten deficiency and constitutive activation of p110α* may activate AKT. We thus tested whether AKT inhibition can rescue the CSR defects in Pten deficient and p110α* KI B cells by treating these B cells with the pan-AKT inhibitor GSK690693 or DMSO control. Our data demonstrated that AKT inhibition completely rescued the defects of IgG1 CSR in both Pten deficient and p110α* KI B cells, to a level comparable to control YFP-KI B cells (Figure 6B). Thus, these results suggest that Pten deficiency or p110α activation results in IgG1 CSR defects mainly via activating AKT. Interestingly, inhibition of AKT only partially rescued IgE CSR in both Pten deficient and p110α* KI B cells, indicating that PTEN and p110α employ an additional AKT-independent mechanism(s) to regulate IgE CSR.
PTEN and PI3K isoforms regulate AID expression via modulating AKT activity
To address how Pten deficiency and p110α hyperactivation inhibit CSR at molecular level, we examined the level of AID expression in various genotypes of B cells. Our results showed that the expression of AID protein was significantly reduced in anti-CD40/IL4 stimulated YFP+ Pten deficient B cells as compared to YFP− control B cells (Figure 7A). In contrast, control YFP-KI B cells expressed AID protein robustly upon anti-CD40/IL4 stimulation (Figure 7A). Thus, we conclude that Pten deficiency significantly inhibits AID expression that contributes to the impaired CSR level, which is consistent with previous findings that CD19cre-mediated Pten deletion reduced AID transcript expression (13). Next, we investigated whether overexpressing PTEN affected the expression of AID protein. Using western blot analysis, we confirmed that activated PTEN-Tg B cells expressed a higher level of PTEN protein (Figure 7B). More importantly, the expression of AID protein was also significantly increased in PTEN-Tg B cells, to a greater extent than the increase of PTEN protein (Figure 7B). These data suggest that the fine-tuning of PTEN protein concentration might perturb the signaling balance between PTEN and PI3Ks, thereby producing a more notable effect on AID expression. Since PI3Ks counteract PTEN, we predicted that hyperactivation of p110α would decrease the expression of AID. Indeed, p110α* KI B cells exhibited a remarkably reduced level of AID protein expression (Figure 7C). Overall, we conclude that the signaling balance between PTEN and PI3Ks is critical for regulating the expression of AID protein; both PTEN deficiency and PI3K hyperactivation suppress AID expression, thereby resulting in defective CSR.
Our data suggested that CSR defects caused by Pten deficiency or p110α activation mainly act through the AKT pathway (Figure 6B). Thus, we next examined how dysregulation of Pten and p110α affected the phosphorylation of AKT at Ser473 that is required for complete activation of AKT. We found that overexpressing PTEN reduced the phosphorylation of AKT (Figure 7B) whereas p110α* activation enhanced the level of AKT phosphorylation (Figure 7C). Taken together, these data reveal a reverse correlation between the level of AID expression and AKT activation. Consistently, we found that AKT inhibition strongly promoted AID protein expression in WT B cells stimulated with anti-CD40/IL4 (Figure 7D). In addition, we examined the Igh germline transcription in Pten deficient or p110* mutant B cells. Our data showed that neither Pten deficiency nor p110α hyperactivation had an obvious effect on the level of IgG1 germline transcripts (Figure 7E and F) or IgE germline transcripts (data not shown). Thus, we conclude that dysregulation of PTEN and p110α regulates CSR via modulating AKT activity, which in turn affects AID protein expression.
Depletion of p110α or p110δ in B cells promotes IgA CSR
In order to investigate the effects of endogenous PI3K components on CSR, we employed a genome-editing approach based on CRISPRs (clustered regularly interspaced short palindromic repeats) using CH12F3 mouse B cell line. CH12F3 cell line is a well-established model for studying CSR, which expresses IgM on surface initially and switches to become IgA+ upon stimulation with anti-CD40, IL4 and TGFβ (41). The advantage of CRISPRs is that they can be used to quickly achieve homozygous deletion of a given gene (43-45). By using the CRISPR-based genome-editing approach, we targeted the p110α gene in the CH12 cell line and showed that NHEJ-mediated indels resulted in the deletion of start codon or frame-shift of p110α (Figure 8A). Western blot analysis confirmed the absence of p110α protein whereas the expression of p110δ and p110γ was not affected (Figure 8B), indicating a high specificity of CRISPR targeting in our system. In addition, we employed a similar approach to generate the p110δ deficient CH12 cell lines (data not shown). Our results showed that p110α deficiency promoted IgA CSR, as evidenced by the increased percentage of IgA+ cells (Figure 8C). Similarly, p110δ deficient CH12 cells underwent IgA CSR efficiently at early time points (day1 and day 1.5), with a CSR level significantly higher than control CH12 cells (Figure 8D). However, the level of IgA CSR became comparable between WT and p110δ deficient CH12 cells at later time point (day 3) (Figure 8D). Overall, these genetic studies further demonstrate that both p110α and p110δ normally inhibit CSR and play a cooperative role in regulating CSR, consistent with the pharmacologic blockade experiments shown above (Figure 5A).
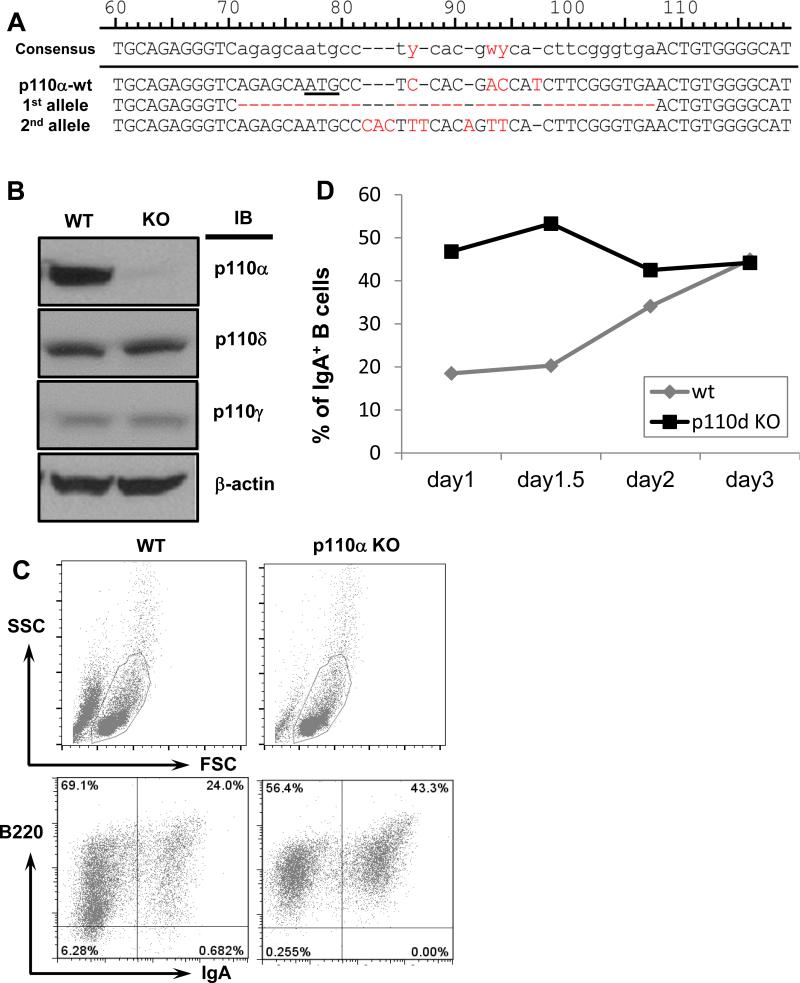
Figure 8. Genome-editing of p110α or p110δ in CH12F3 cells via CRISPR/Cas9 approach.
(A) Genetic deletion of the p110α gene in CH12F3 cells. Sanger sequencing results of p110α targeted alleles: Top: wt allele, ATG start codon is underlined; Middle: 1st targeted allele contains 32bp deletion; Bottom: 2nd targeted allele contains multiple insertions and deletions (Indels). Mismatched nucleotides are in red fonts. Indels result in the deletion of start codon or frame-shift of p110α, which also eliminate the MboII site. (B) Western blotting analysis of p110 catalytic subunits. WT and p110α KO clones were employed for western blot with anti-p110α, p110δ, p110γ or anti-β-actin antibody. (C) FACS plots of IgA CSR. WT or p110α KO clones were stimulated for 1.5 days in the presence of anti-CD40/IL4/TGFβ, and analyzed by flow cytometry for IgA+ CH12 cells. Top panel: FSC and SSC plots; bottom panel: B220 and IgA plots (gated in the FSC/SSC plot of the top panel). Representative FACS plots were shown from three independent experiments. (D) Kinetics of IgA CSR in p110δ KO CH12F3 cells. WT or p110δ KO clones were stimulated and analyzed as described in (C) for various time points. Representative data from one experiment was shown out of three independent experiments.
Discussion
CSR is required for generation of isotype-switched antibodies that mediate effective humoral immunity, and also contribute to allergic and autoimmune diseases. In this study, we employed various genetic models and pharmacological inhibitors to reveal the effects of imbalanced signaling between B cell intrinsic PTEN and PI3K isoforms on CSR. Compared to previous studies, our analytic system eliminates the confounding effects of Pten deletion on B cell development that may influence CSR indirectly, thus, we demonstrate definitively that Pten deletion dramatically reduces CSR in a mature B cell autonomous manner. In addition, our data reveal that modest overexpression of PTEN significantly promotes CSR. More importantly, we uncover a previously unrecognized role of p110α in CSR, and find that p110α and p110δ act coordinately to antagonize PTEN, suppressing CSR in a physiological context. Taken together, our studies reveal a delicate signaling balance between PTEN and PI3K isoforms that is essential to maintain a normal level of CSR.
Prior studies addressing this question employed CD19Cre to delete the Pten gene, and demonstrated reduced CSR of IgG isotypes (12, 13). However, CD19Cre leads to a deletion efficiency of 75–80% in BM-derived pre-B cells and 90–95% in splenic B cells (14); thus, it remains possible that CD19Cre-mediated Pten deletion might perturb pre-B cell development, which in turn affects CSR indirectly. Moreover, CD19Cre-mediated Pten deletion results in the expansion of B1 and marginal zone B cells (13, 30), which may also affect the ability of B cells to undergo CSR. Addressing the unresolved question of PTEN's effect on CSR will require a better controlled model system. In the current study, we take an advantage of a newly developed genetic model in which the Pten gene is deleted in mature B cells acutely, with a narrow time window from genetic deletion to CSR assay, thereby unequivocally excluding the indirect effects of Pten deletion. Consistent with previous studies using CD19Cre (12, 13), our results show that acute deletion of Pten dramatically reduces IgG1 CSR in a mature B cell autonomous manner. Thus, we conclude that PTEN directly regulates CSR signaling, instead of influencing CSR via modulating B cell development or differentiation of B cell subsets.
Previous studies have not evaluated the effects of Pten deletion on IgE CSR, which requires a much more complicated procedure for detection. Based on a previous study (36), we developed a reliable method to readily monitor the percentage of IgE switched B cells. Our data clearly demonstrate that PTEN is required for a normal level of IgE CSR. Intriguingly, the defects in IgG1 CSR were completely rescued by AKT inhibition in PTEN-KO B cells whereas the IgE CSR defects were only partially rescued, suggesting that Pten deficiency might have differential effects on the switching of different isotypes. In addition, we developed a novel transgenic model in which PTEN protein is subtly overexpressed in a mature B cell autonomous manner. Our results show that even such a modest upregulation of PTEN protein can enhance the level of CSR significantly. Consistent with our findings, PTEN deficiency-induced defective CSR was partially rescued by attenuating the activity of p110δ (12, 15). Thus, we propose that fine-tuning PTEN protein concentration probably perturbs the signaling balance between PTEN and PI3K, which results in the significant alteration of CSR level.
PI3K kinase is composed of catalytic and regulatory subunits. B lymphocytes express three isoforms of catalytic subunits, p110α, p110δ and p110γ (10). Deletion of both p110α and p110δ dramatically impairs B cell development whereas single deletion of either isoform does so, albeit to a much less extent, demonstrating a compensatory role of p110α and p110δ in B cell development (19). Thus, we reason that other PI3K isoforms might compensate p110δ to regulate CSR, which may explain the inconsistent phenotypes caused by p110δ deletion (15) versus point mutation (17). Consistent with our hypothesis, we found that p110α hyperactivation strongly inhibits CSR and, in contrast, its genetic deletion or pharmacological blockade promotes CSR. Thus, our studies reveal an important role of p110α in CSR, which has not been previously recognized. Furthermore, we show that p110α- or p110δ-specific inhibitor enhances CSR individually, and combined treatment of both inhibitors promotes CSR additively, especially for IgE CSR, consistent with the dramatically increased serum IgE level in p110α/p110δ double KO mice (19). Thus, we demonstrate that p110α and p110δ act coordinately to suppress CSR per se. Our data is also in line with previous reports showing that p110δ pharmacological blockade or p110δD910A point mutation resulted in a higher level of CSR (17). Therefore, we suggest that the lack of CSR defects in p110δ KO B cells is probably due to the compensatory effects of p110α. Of note, our kinetic studies also show that the deletion of p110δ gene in CH12F3 cells indeed promotes CSR at early time points; however, such promoting effects diminish at later time points, resulting in a comparable CSR efficiency between wt and p110δ deficient cells. These results are consistent with the possibility that p110α might exert its compensatory effects in p110δ deficient B cells, especially at later time points, to suppress CSR. In addition, our data suggest the importance of kinetic analysis of CSR efficiency. Taken together, our study may explain the lack of CSR defects in p110δ deletion mutant and clarify the discrepancy between genetic and pharmacological blockade data. We conclude that p110α and p110δ act coordinately to antagonize PTEN's activity to control the efficiency and kinetics of CSR.
AKT is a downstream effector of PI3Ks and has three isoforms AKT1, 2, and 3 (49). Since AKT inhibition fully rescued the IgG1 CSR defects in Pten-KO and p110α* KI B cells, we suggest that PTEN/PI3Ks regulate IgG1 CSR via acting through AKT. Deletion or pharmacological inhibition of p110δ promotes IgE CSR; however, it was previously reported that the effects of p110δ on IgE CSR were independent of AKT since an AKT1/2 specific inhibitor, AKT I, did not affect IgE CSR in this setting (38). In contrast, we found that a pan-AKT inhibitor, GSK690693 and another AKT1/2 specific inhibitor, AKT VIII, promote IgE CSR in WT B cells (Figure 6A and data not shown). Moreover, the pan-AKT inhibitor rescued the IgE CSR defects in p110α* KI and PTEN-KO B cells significantly. We thus conclude that PTEN/PI3Ks regulate IgE CSR by acting through AKT, at least, partially. The phenotypic discrepancy might be caused by the utilization of different AKT inhibitors, which may be clarified using AKT1/2/3 deficient B cells.
Recent next generation sequencing (NGS) studies report that the components of PI3K pathway, e.g. p110δ and p85α (a regulatory subunit), harbor mutations that predispose to human immunodeficiency (23-27). These data further support our hypothesis that the signaling balance between PTEN and PI3K pathway plays a critical role in regulating antibody deficiency. Two independent studies reported a mutation in p110δ (E1021K) in human patients of PI3K-δ syndrome (23, 26), which was also previously detected in a pediatric patient with primary B cell immunodeficiency (50). Both studies showed that the E1021K mutation was a gain-of-function mutation, causing hyperactivation of p110δ (23, 26), while one of them also reported two additional gain-of-function mutations (N334K and E525K) and focused on uncovering the abnormal functions of patient T cells (26). We suggest that certain gain-of-function mutations in p110δ may lead to intrinsic CSR defects, similar to what we discovered in p110α* KI B cells. Overall, our data are consistent with the mutational studies of human patients in that they support our findings that hyperactivation of PI3K pathway drastically inhibits CSR. Furthermore, we predict that gain-of-function mutations of p110α may reduce CSR in human patients, thereby predisposing to antibody deficiency. Thus, it will be of great interest to determine if cases of antibody deficiency that currently have no genetic classification carry mutations in genes that regulate PI3K or PTEN activity. However, it remains unknown whether these patient-derived PI3K mutations affect the CSR process in a B cell intrinsic manner. A recent report also identified two mutations of p110δ in primary immunodeficient patients including the E1021K mutation and a previously unreported C416R mutation; furthermore, it suggested that the C416R mutation had no effects on CSR process (24). However, there remain multiple caveats in this case report study (24), thereby limiting its conclusions, for instance, it was unclear whether the naïve mutant B cells indeed had CSR defects and whether other cell types also influenced the activation of these B cells. Moreover, it has not been directly tested whether this C416R mutation of p110δ regulates CSR in a well-controlled system. We suggest that a new genome-editing approach based on CRISPR/Cas9 would offer an ideal experimental system to test such questions. CRISPR system is advantageous to other gene-targeting approaches because it is extremely efficient and allows generating deletions or mutations on both alleles simultaneously (43-45), as shown by our current study. We envision that such an experimental system would facilitate rapid validation of the functional significance of newly identified mutations, thereby greatly accelerating the conversion of genomic studies into translational applications.
Acknowledgements
We thank Dr. Robert Rickert for Ptenflox/flox mice, Dr. Mark Shlomchik for hCD20TamCre mice, and Dr. Klaus Rajewsky for Rosa26-flox-stop-P110α*-IRES-GFP (p110α*-GFP) mice and Cγ1Cre mice. PTEN-Tg mice were generated by mouse genetic core at National Jewish Health. We thank Tanya Kadoishi, Michael Rice and Stephanie Cung for technical help. We apologize to those whose work was not cited due to length restrictions.
Footnotes
This work was supported by University of Colorado School of Medicine and Cancer Center startup funds, a Boettcher Foundation Webb-Waring Biomedical Research Award, an American Society of Hematology Scholar Award, a fund from Cancer League of Colorado, R21CA184707, R21AI110777 and R01CA166325 to J.H.W, and by P01 AI022295 and R01 AI077597 to J.C.C. and A.G. X.C. is supported by NIH Training grant T32 AI074491. We apologize to those whose work was not cited due to length restrictions. The authors disclose no potential conflicts of interest.
Reference
- 1.Chaudhuri J, Basu U, Zarrin A, Yan C, Franco S, Perlot T, Vuong B, Wang J, Phan RT, Datta A, Manis J, Alt FW. Evolution of the immunoglobulin heavy chain class switch recombination mechanism. Adv Immunol. 2007;94:157–214. doi: 10.1016/S0065-2776(06)94006-1. [DOI] [PubMed] [Google Scholar]
- 2.Stavnezer J, Guikema JE, Schrader CE. Mechanism and regulation of class switch recombination. Annu Rev Immunol. 2008;26:261–292. doi: 10.1146/annurev.immunol.26.021607.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang JH. The role of activation-induced deaminase in antibody diversification and genomic instability. Immunol Res. 2013;55:287–297. doi: 10.1007/s12026-012-8369-4. [DOI] [PubMed] [Google Scholar]
- 4.Manis JP, Tian M, Alt FW. Mechanism and control of class-switch recombination. Trends Immunol. 2002;23:31–39. doi: 10.1016/s1471-4906(01)02111-1. [DOI] [PubMed] [Google Scholar]
- 5.Hackney JA, Misaghi S, Senger K, Garris C, Sun Y, Lorenzo MN, Zarrin AA. DNA targets of AID evolutionary link between antibody somatic hypermutation and class switch recombination. Adv Immunol. 2009;101:163–189. doi: 10.1016/S0065-2776(08)01005-5. [DOI] [PubMed] [Google Scholar]
- 6.Chahwan R, Edelmann W, Scharff MD, Roa S. AIDing antibody diversity by error-prone mismatch repair. Seminars in immunology. 2012;24:293–300. doi: 10.1016/j.smim.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Noia JM, Neuberger MS. Molecular mechanisms of antibody somatic hypermutation. Annu Rev Biochem. 2007;76:1–22. doi: 10.1146/annurev.biochem.76.061705.090740. [DOI] [PubMed] [Google Scholar]
- 8.Boboila C, Alt FW, Schwer B. Classical and alternative end-joining pathways for repair of lymphocyte-specific and general DNA double-strand breaks. Adv Immunol. 2012;116:1–49. doi: 10.1016/B978-0-12-394300-2.00001-6. [DOI] [PubMed] [Google Scholar]
- 9.Chen Z, Wang JH. Generation and repair of AID-initiated DNA lesions in B lymphocytes. Front Med. 2014;8:201–216. doi: 10.1007/s11684-014-0324-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baracho GV, Miletic AV, Omori SA, Cato MH, Rickert RC. Emergence of the PI3-kinase pathway as a central modulator of normal and aberrant B cell differentiation. Curr Opin Immunol. 2011;23:178–183. doi: 10.1016/j.coi.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pauls SD, Lafarge ST, Landego I, Zhang T, Marshall AJ. The phosphoinositide 3-kinase signaling pathway in normal and malignant B cells: activation mechanisms, regulation and impact on cellular functions. Frontiers in immunology. 2012;3:224. doi: 10.3389/fimmu.2012.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Omori SA, Cato MH, Anzelon-Mills A, Puri KD, Shapiro-Shelef M, Calame K, Rickert RC. Regulation of class-switch recombination and plasma cell differentiation by phosphatidylinositol 3-kinase signaling. Immunity. 2006;25:545–557. doi: 10.1016/j.immuni.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki A, Kaisho T, Ohishi M, Tsukio-Yamaguchi M, Tsubata T, Koni PA, Sasaki T, Mak TW, Nakano T. Critical roles of Pten in B cell homeostasis and immunoglobulin class switch recombination. J Exp Med. 2003;197:657–667. doi: 10.1084/jem.20021101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rickert RC, Roes J, Rajewsky K. B lymphocyte-specific, Cre-mediated mutagenesis in mice. Nucleic Acids Res. 1997;25:1317–1318. doi: 10.1093/nar/25.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janas ML, Hodson D, Stamataki Z, Hill S, Welch K, Gambardella L, Trotman LC, Pandolfi PP, Vigorito E, Turner M. The effect of deleting p110delta on the phenotype and function of PTEN-deficient B cells. J Immunol. 2008;180:739–746. doi: 10.4049/jimmunol.180.2.739. [DOI] [PubMed] [Google Scholar]
- 16.Rolf J, Bell SE, Kovesdi D, Janas ML, Soond DR, Webb LM, Santinelli S, Saunders T, Hebeis B, Killeen N, Okkenhaug K, Turner M. Phosphoinositide 3-kinase activity in T cells regulates the magnitude of the germinal center reaction. J Immunol. 2010;185:4042–4052. doi: 10.4049/jimmunol.1001730. [DOI] [PubMed] [Google Scholar]
- 17.Zhang TT, Okkenhaug K, Nashed BF, Puri KD, Knight ZA, Shokat KM, Vanhaesebroeck B, Marshall AJ. Genetic or pharmaceutical blockade of p110delta phosphoinositide 3-kinase enhances IgE production. The Journal of allergy and clinical immunology. 2008;122:811–819 e812. doi: 10.1016/j.jaci.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Okkenhaug K, Bilancio A, Farjot G, Priddle H, Sancho S, Peskett E, Pearce W, Meek SE, Salpekar A, Waterfield MD, Smith AJ, Vanhaesebroeck B. Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science. 2002;297:1031–1034. doi: 10.1126/science.1073560. [DOI] [PubMed] [Google Scholar]
- 19.Ramadani F, Bolland DJ, Garcon F, Emery JL, Vanhaesebroeck B, Corcoran AE, Okkenhaug K. The PI3K isoforms p110alpha and p110delta are essential for pre-B cell receptor signaling and B cell development. Science signaling. 2010;3:ra60. doi: 10.1126/scisignal.2001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.International Union of Immunological Societies Expert Committee on Primary, I. Notarangelo LD, Fischer A, Geha RS, Casanova JL, Chapel H, Conley ME, Cunningham-Rundles C, Etzioni A, Hammartrom L, Nonoyama S, Ochs HD, Puck J, Roifman C, Seger R, Wedgwood J. Primary immunodeficiencies: 2009 update. The Journal of allergy and clinical immunology. 2009;124:1161–1178. doi: 10.1016/j.jaci.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liadaki K, Sun J, Hammarstrom L, Pan-Hammarstrom Q. New facets of antibody deficiencies. Curr Opin Immunol. 2013;25:629–638. doi: 10.1016/j.coi.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Omori SA, Rickert RC. Phosphatidylinositol 3-kinase (PI3K) signaling and regulation of the antibody response. Cell Cycle. 2007;6:397–402. doi: 10.4161/cc.6.4.3837. [DOI] [PubMed] [Google Scholar]
- 23.Angulo I, Vadas O, Garcon F, Banham-Hall E, Plagnol V, Leahy TR, Baxendale H, Coulter T, Curtis J, Wu C, Blake-Palmer K, Perisic O, Smyth D, Maes M, Fiddler C, Juss J, Cilliers D, Markelj G, Chandra A, Farmer G, Kielkowska A, Clark J, Kracker S, Debre M, Picard C, Pellier I, Jabado N, Morris JA, Barcenas-Morales G, Fischer A, Stephens L, Hawkins P, Barrett JC, Abinun M, Clatworthy M, Durandy A, Doffinger R, Chilvers ER, Cant AJ, Kumararatne D, Okkenhaug K, Williams RL, Condliffe A, Nejentsev S. Phosphoinositide 3-kinase delta gene mutation predisposes to respiratory infection and airway damage. Science. 2013;342:866–871. doi: 10.1126/science.1243292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crank MC, Grossman JK, Moir S, Pittaluga S, Buckner CM, Kardava L, Agharahimi A, Meuwissen H, Stoddard J, Niemela J, Kuehn H, Rosenzweig SD. Mutations in PIK3CD can cause hyper IgM syndrome (HIGM) associated with increased cancer susceptibility. Journal of clinical immunology. 2014;34:272–276. doi: 10.1007/s10875-014-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deau MC, Heurtier L, Frange P, Suarez F, Bole-Feysot C, Nitschke P, Cavazzana M, Picard C, Durandy A, Fischer A, Kracker S. A human immunodeficiency caused by mutations in the PIK3R1 gene. J Clin Invest. 2014;124:3923–3928. doi: 10.1172/JCI75746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lucas CL, Kuehn HS, Zhao F, Niemela JE, Deenick EK, Palendira U, Avery DT, Moens L, Cannons JL, Biancalana M, Stoddard J, Ouyang W, Frucht DM, Rao VK, Atkinson TP, Agharahimi A, Hussey AA, Folio LR, Olivier KN, Fleisher TA, Pittaluga S, Holland SM, Cohen JI, Oliveira JB, Tangye SG, Schwartzberg PL, Lenardo MJ, Uzel G. Dominant-activating germline mutations in the gene encoding the PI(3)K catalytic subunit p110delta result in T cell senescence and human immunodeficiency. Nat Immunol. 2014;15:88–97. doi: 10.1038/ni.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lucas CL, Zhang Y, Venida A, Wang Y, Hughes J, McElwee J, Butrick M, Matthews H, Price S, Biancalana M, Wang X, Richards M, Pozos T, Barlan I, Ozen A, Rao VK, Su HC, Lenardo MJ. Heterozygous splice mutation in PIK3R1 causes human immunodeficiency with lymphoproliferation due to dominant activation of PI3K. J Exp Med. 2014;211:2537–2547. doi: 10.1084/jem.20141759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Jr., Kinzler KW. Cancer genome landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sander S, Calado DP, Srinivasan L, Kochert K, Zhang B, Rosolowski M, Rodig SJ, Holzmann K, Stilgenbauer S, Siebert R, Bullinger L, Rajewsky K. Synergy between PI3K signaling and MYC in Burkitt lymphomagenesis. Cancer Cell. 2012;22:167–179. doi: 10.1016/j.ccr.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anzelon AN, Wu H, Rickert RC. Pten inactivation alters peripheral B lymphocyte fate and reconstitutes CD19 function. Nat Immunol. 2003;4:287–294. doi: 10.1038/ni892. [DOI] [PubMed] [Google Scholar]
- 31.Khalil AM, Cambier JC, Shlomchik MJ. B cell receptor signal transduction in the GC is short-circuited by high phosphatase activity. Science. 2012;336:1178–1181. doi: 10.1126/science.1213368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC developmental biology. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Srinivasan L, Sasaki Y, Calado DP, Zhang B, Paik JH, DePinho RA, Kutok JL, Kearney JF, Otipoby KL, Rajewsky K. PI3 kinase signals BCR-dependent mature B cell survival. Cell. 2009;139:573–586. doi: 10.1016/j.cell.2009.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klippel A, Reinhard C, Kavanaugh WM, Apell G, Escobedo MA, Williams LT. Membrane localization of phosphatidylinositol 3-kinase is sufficient to activate multiple signal-transducing kinase pathways. Mol Cell Biol. 1996;16:4117–4127. doi: 10.1128/mcb.16.8.4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Casola S, Cattoretti G, Uyttersprot N, Koralov SB, Seagal J, Hao Z, Waisman A, Egert A, Ghitza D, Rajewsky K. Tracking germinal center B cells expressing germ-line immunoglobulin gamma1 transcripts by conditional gene targeting. Proc Natl Acad Sci U S A. 2006;103:7396–7401. doi: 10.1073/pnas.0602353103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Erazo A, Kutchukhidze N, Leung M, Christ AP, Urban JF, Jr., Curotto de Lafaille MA, Lafaille JJ. Unique maturation program of the IgE response in vivo. Immunity. 2007;26:191–203. doi: 10.1016/j.immuni.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiong H, Dolpady J, Wabl M, Curotto de Lafaille MA, Lafaille JJ. Sequential class switching is required for the generation of high affinity IgE antibodies. J Exp Med. 2012;209:353–364. doi: 10.1084/jem.20111941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang TT, Makondo KJ, Marshall AJ. p110delta phosphoinositide 3-kinase represses IgE switch by potentiating BCL6 expression. J Immunol. 2012;188:3700–3708. doi: 10.4049/jimmunol.1103302. [DOI] [PubMed] [Google Scholar]
- 39.Katona IM, Urban JF, Jr., Finkelman FD. B cells that simultaneously express surface IgM and IgE in Nippostrongylus brasiliensis-infected SJA/9 mice do not provide evidence for isotype switching without gene deletion. Proc Natl Acad Sci U S A. 1985;82:511–515. doi: 10.1073/pnas.82.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katona IM, Urban JF, Jr., Scher I, Kanellopoulos-Langevin C, Finkelman FD. Induction of an IgE response in mice by Nippostrongylus brasiliensis: characterization of lymphoid cells with intracytoplasmic or surface IgE. J Immunol. 1983;130:350–356. [PubMed] [Google Scholar]
- 41.Nakamura M, Kondo S, Sugai M, Nazarea M, Imamura S, Honjo T. High frequency class switching of an IgM+ B lymphoma clone CH12F3 to IgA+ cells. Int Immunol. 1996;8:193–201. doi: 10.1093/intimm/8.2.193. [DOI] [PubMed] [Google Scholar]
- 42.Chen Z, Ranganath S, Viboolsittiseri SS, Eder MD, Chen X, Elos MT, Yuan S, Hansen E, Wang JH. AID-initiated DNA lesions are differentially processed in distinct B cell populations. J Immunol. 2014;193:5545–5556. doi: 10.4049/jimmunol.1401549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jinek M, East A, Cheng A, Lin S, Ma E, Doudna J. RNA-programmed genome editing in human cells. eLife. 2013;2:e00471. doi: 10.7554/eLife.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, Norville JE, Church GM. RNA-guided human genome engineering via Cas9. Science. 2013;339:823–826. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. Genome engineering using the CRISPR-Cas9 system. Nature protocols. 2013;8:2281–2308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang JH, Gostissa M, Yan CT, Goff P, Hickernell T, Hansen E, Difilippantonio S, Wesemann DR, Zarrin AA, Rajewsky K, Nussenzweig A, Alt FW. Mechanisms promoting translocations in editing and switching peripheral B cells. Nature. 2009;460:231–236. doi: 10.1038/nature08159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Doi T, Obayashi K, Kadowaki T, Fujii H, Koyasu S. PI3K is a negative regulator of IgE production. Int Immunol. 2008;20:499–508. doi: 10.1093/intimm/dxn009. [DOI] [PubMed] [Google Scholar]
- 49.Xu N, Lao Y, Zhang Y, Gillespie DA. Akt: a double-edged sword in cell proliferation and genome stability. Journal of oncology. 2012;2012:951724. doi: 10.1155/2012/951724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jou ST, Chien YH, Yang YH, Wang TC, Shyur SD, Chou CC, Chang ML, Lin DT, Lin KH, Chiang BL. Identification of variations in the human phosphoinositide 3-kinase p110delta gene in children with primary B-cell immunodeficiency of unknown aetiology. International journal of immunogenetics. 2006;33:361–369. doi: 10.1111/j.1744-313X.2006.00627.x. [DOI] [PubMed] [Google Scholar]