Abstract
Maintenance of cerebral viability and function is an important goal of critical care in victims of injury due to ischemia and hypovolemia. As part of the multiple organ dysfunction syndrome, the brain function after trauma is influenced by systemic inflammatory response. We investigated the effect of EF24, an anti-inflammatory bis-chalcone, on cerebral bioenergetics in a rat model of 45% hemorrhagic shock. The rats were treated with EF24 (0.4 mg/kg) or EF24 with an artificial oxygen carrier liposome-encapsulated hemoglobin (LEH). The volume of LEH administered was equal to the shed blood. The brain was collected after 6 h of shock for biochemical assays. EF24 treatment showed significant recovery of ATP, phosphocreatine, and NAD/NADH ratio. It also increased citrate synthase activity and cytochrome c oxidase subunit IV expression which were reduced in shock brain. Furthermore, it reduced the shock-induced accumulation of pyruvate and pyruvate dehydrogenase kinase-1 expression, suggesting that EF24 treatment improves cerebral energetics by restoring perturbed pyruvate metabolism in the mitochondria. These effects of EF24 were associated with reduced poly(ADP-ribose) polymerase cleavage and a significant improvement in the levels of nerve growth factor and brain-derived neurotrophic factor in shock brain. Co-administration of LEH with EF24 was only marginally more effective as compared to the treatment with EF24 alone. These results show that EF24 treatment sets up a pro-survival phenotype in shock by resurrecting cerebral bioenergetics. Since EF24 was effective in the absence of accompanying fluid resuscitation, it has potential utility as a pre-hospital pharmacotherapy in shock due to accidental blood loss.
Keywords: Brain, cerebral metabolism, Hypovolemic shock, Trauma, Resuscitation
BACKGROUND
Prognosis of critical care in victims of severe blood loss is primarily dependent on the success of interventions targeting hypovolemia and tissue hypoxia. The brain is a unique organ because the host physiological response strives to protect it from hypoxic and mal-perfusion insults by increasing cerebral oxygen extraction ratio and diverting cardiac output from splanchnic organs (Kovach & Sandor 1976, Awasthi et al. 2007a). However, these compensatory mechanisms begin to fail when a large amount of blood is lost, or when the shock is prolonged without adequate resuscitation, leading to perturbed cellular metabolism, energy depletion, and neuronal cell death. Intensivists correct inadequate tissue perfusion and cellular hypoxia by resuscitation with fluids ranging from crystalloids to colloids and by transfusion with whole blood and packed red blood cells (pRBCs). However, in addition to the volume and oxygen deficits, the pathology of shock is also characterized by an early onset of systemic inflammation which affects organs in a global and interdependent fashion within a phenomenon called multiple organ dysfunction syndrome (MODS) which is the leading cause of death among intensive care unit (ICU) patients (Brattstrom et al. 2010).
The neurologic component of MODS in the victims of hemorrhagic shock is primarily a consequence of systemic inflammatory response syndrome (SIRS) and reduced cerebral perfusion pressure. Whereas perfusion pressure could be managed by resuscitation fluids, therapeutic treatment of SIRS remains a major challenge in ICU. The major trigger for SIRS in hemorrhagic shock is the dysfunction of intestinal barrier, secondary to the ischemic injury to the intestinal epithelium (Moore et al. 2004, Grenz et al. 2012, Rhodes et al. 1973). The causal role of inflammatory processes initiated by intestinal injury in brain dysfunction has been reported (Hsieh et al. 2011, Zhou et al. 2012). Therefore, pharmacologic intervention to correct intestinal injury in hemorrhagic shock is expected to show improvement in brain metabolism as well.
Recently, we have shown that treatment of severely hypovolemic rats with EF24, 3,5-bis(2-fluorobenzylidene)piperidin-4-one, protected gut barrier function and reduced systemic inflammatory response (Yadav et al. 2014a). The primary goal of this study was to evaluate the status of brain energetics in response to a treatment with EF24 in shock model of 45% hemorrhage in rats. EF24 suppresses inflammation by interfering with nuclear factor-kB (NF-kB) pathway, putatively by inhibiting the β isoform of inhibitor of kB kinase (IKKβ) (Vilekar et al. 2012, Kasinski et al. 2008). NF-kB is a convergence point for the signaling networks from various pro-inflammatory pattern recognition receptors (PRRs), such as interleukin-1 receptor type I. Canonical NF-kB pathway is induced by hypoxia (Oliver et al. 2009, Fitzpatrick et al. 2011).
Considering that the neurological dysfunction in hemorrhagic shock is also influenced by cerebral perfusion pressure and hypoxia, the secondary goal of this study was to investigate if the co-resuscitation with EF24 and liposome-encapsulated hemoglobin (LEH), an artificial oxygen carrier, will have additive effect on brain metabolic activity. Previously we have shown that LEH infusion to correct oxygen and volume deficit has salutary effects on brain metabolism in a rat model of hemorrhagic shock (Awasthi et al. 2010, Awasthi et al. 2007b). From the results of this study we conclude that EF24 treatment alone is effective in improving the degraded cerebral bioenergetics, and that LEH co-administration has no significant additional benefits in hemorrhagic shock.
METHODS
Unless otherwise mentioned, all chemicals were obtained from Sigma-Aldrich (St. Louis, MO) and/or various suppliers represented by VWR Scientific (West Chester, PA). For in vivo work, the rats were purchased from Harlan (Indianapolis, IN, USA). EF24 was synthesized in-house by the procedures published elsewhere (Vilekar et al. 2012, Vilekar et al. 2014). The LEH was prepared by encapsulating hemoglobin inside the liposomes composed of dipalmitoylphosphatidylcholine, cholesterol, hexadecylcarbamoylmethylhexadecanoate (HDAS) and HDAS-poly(ethylene glycol)-2000. The methods of preparation and characteristics of LEH are described in the supplemental material; they are also a part of previously published work (Nag et al. 2013, Agashe et al. 2010, Yadav et al. 2014b).
Rat model of hypovolemic shock
The animal experiments were performed according to the NIH Animal Use and Care Guidelines and were approved by the Institutional Animal Care and Use Committee of the University Of Oklahoma Health Sciences Center. Male Sprague Dawley rats (250–300 g, 9–10 months of age) were purchased from Harlan (Indianapolis, IN, USA), housed in regular light/dark cycles of 12/12 and allowed to acclimatize for at least 5 days. The method of femoral artery catheterization in rats has been described elsewhere (Awasthi et al. 2007b). Briefly, the left femoral artery was cannulated with a Teflon-tipped catheter and the catheter was subcutaneously tunneled and secured to the nape; the rats were allowed at least 2 days to recover from surgery. On the day of the experiment, the rats were handled under isoflurane (2–3%) anesthesia in medical air stream (2 l per min) containing 21% oxygen and 78% nitrogen. The rats were heparinized with 100 units of heparin to prevent catheter blockade. Hypovolemic shock was induced by withdrawing approximately 45% of circulating blood at the rate of 1.5 ml/min. The total volume of blood was estimated approximately 6% of the total body weight. The rats were treated (described below), while allowing them to wake up and freely compensate. After 6 h of hemorrhage, the surviving rats were euthanized with an overdose of SOMNASOL, Euthanasia-III Solution (Butler Schein Animal Health, Dublin, OH). The brain was isolated, cleaned with ice-cold saline, and preserved in liquid nitrogen. We employed 6 h as the endpoint for this study because in our previous study we found a significant difference in 6 h-survival of rats between treatments (Yadav et al. 2013).
EF24 treatment and resuscitation
The cannulated rats were clustered a priori in four groups (n = 6/group): control (CTRL), hemorrhagic shock+vehicle (VEH), HS+EF24 (EF), and HS+EF24+LEH (EFLEH). No blinding of the investigators was done. Isovolemic resuscitation with pre-warmed LEH (37°C) was intravenously given at 1.5 ml/min after 15 min of the completion of blood withdrawal. EF24 was administered intraperitoneally after the resuscitation was complete. A sterile solution of EF24 was prepared in normal saline using poly(ethylene glycol)-400 as a co-solvent (3 parts saline + 1 part PEG400). The drug treatment consisted of approximately 0.4 mg/Kg bodyweight in 100 µl volume. The VEH group received equivalent amounts of vehicle (25% PEG400 in saline) in an identical fashion.
Hemodynamics
Blood pressure was digitally monitored by instrumenting the rats to a data acquisition system consisting of IX118 and ETH-255 amplifier (iWorx Systems, Dover, NH). The femoral artery catheter was connected to a BP-102 transducer system via a 23G×12” blunt-ended butterfly infusion set. For blood gas monitoring, approximately 200 µl whole blood sample was collected and introduced into a disposable blood gas cartridge placed in a VetStat Electrolyte and Blood Gas Analyzer (IDDEX Laboratories, Westbrook, Maine).
Preparation of tissue homogenate
Brain tissue (100 mg) was rinsed in ice-cold PBS to remove remnant of blood and transferred in buffer containing 1% Igepal, 2 mM EDTA, 150 mM NaCl, and 25 mM Tris-HCL, pH 7.5, supplemented with protease and phosphatase inhibitors. The protein was extracted by homogenization using a dounce homogenizer, followed by centrifugation at 20,000 rcf for 15 min. The supernatants were transferred into fresh tubes and stored at −80°C.
Adenosine triphosphate (ATP) and phosphocreatine
We determined ATP using an assay kit based on luciferin-luciferase system (Sigma-Aldrich, St. Louis, MO). The light output was converted into nM ATP per mg of protein by comparing with ATP standards. To determine phosphocreatine, first the endogenous ATP levels were destroyed in the tissue extract, and the phosphocreatine is allowed to generate ATP de novo from exogenously added ADP (Ronner et al. 1999). Briefly, the extracted tissue sample (5 µl) was allowed to react with 5 µl of an ATP→ADP reagent containing 390 mM 2-amino-2-methyl-a-propanol (pH 8.8), 20 mM glucose, 4 mM MgCl2, 40 µM ATP-free ADP, 0.04% BSA, 24 mM NaF, and 7 U/ml hexokinase. After allowing 15 min at room temperature, 5 µl of 5 mM EDTA was added and the reaction mixture was immersed in boiling water path for 2 min. To the cooled reaction mixture, 5 µl of the phosphocreatine→ATP reagent (containing 715 mM imidazole (pH 6.5), 15 mM MgCl2, 0.07% BSA, 18 mM NaF, and 500 U/ml creatine kinase) was added. A series of control reactions and standard ATP were also performed simultaneously. The control reagent solutions were prepared without hexokinase or creatine kinase enzymes. The reaction was allowed to proceed for 50 min, before addition of 100 µl of stopping solution containing 0.2 M NaOH, 1.2 mM EDTA, followed by immersion in boiling water bath for 2 min. Finally, 20 µl of reaction mixture was used for the determination of ATP as described above.
Nicotinamide adenine dinucleotide (NAD) and NADH
The brain tissue nicotinamides NAD and NADH were determined in equal amounts of homogenate proteins by using a kit from Abcam (Cambridge, MA). The values were expressed as a ratio of NAD and NADH calculated from a standard curve generated from absorbance values at 450 nm.
Lactate and pyruvate
The lactate and pyruvate content in brain tissue (nM/mg) was estimated by using a commercially available kit from Cayman Chemical (Ann Arbor, Michigan).
Citrate synthase assay
We measured citrate synthase activity in brain tissue homogenates by using a commercially available 96-well kit from Cayman Chemical (Ann Arbor, Michigan). The enzyme activity was normalized to protein concentration and expressed as nmol/mg protein/min.
Immunoblotting
The isolated frozen tissues were minced and incubated on ice for 30 min in ice-cold buffer consisting of 10% NP-40, 5 M NaCl, 1 M HEPES, 0.1 M ethyleneglycoltetraacetic acid, 0.5 M ethylenediaminetetraacetic acid, 0.1 M phenylmethylsulfonyl fluoride, 0.2 M sodium orthovanadate, 1 M NaF, 2 µg/mL aprotinin, and 2 µg/mL leupeptin. The protein was extracted by homogenization using a dounce homogenizer, followed by centrifugation at 20,000 rcf for 10 min. The proteins were separated by sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE), electro-transferred to nitrocellulose membranes, blocked (5% milk in TBST) and incubated with primary and secondary antibodies in a standard manner. The primary antibody were: rabbit polyclonal poly-ADP ribose polymerase-1 (PARP), goat polyclonal pyruvate dehydrogenase kinase-1 (PDK1), rabbit monoclonal cytochrome c oxidase subunit 4 isoform I (COX4), rabbit affinity-isolated polyclonal β-actin (actin). They were obtained from Cell Signaling Technology (Danvers, MA), Santa Cruz Biotech (SCBT, Dallas, TX), and Sigma-Aldrich (St. Louis, MO), respectively, and were used at dilutions 1:1,000, 1:200, 1:1,000, and 1:5,000, respectively. The horse radish peroxidase (HRP)-conjugated goat anti-rabbit IgG (1:10,000) was from Sigma-Aldrich and HRP-conjugated donkey anti-goat IgG (1:10,000) was from Santa Cruz Biotech. The immunoreactive bands were detected by SuperSignal West Femto detection reagent (Thermo Fischer Scientific, Rockford, IL). The blots were imaged using Ultraquant image acquisition machine (Claremont, CA) and the densitometric readings of immunoreactive bands were normalized with those of actin using Image J 1.46r freeware (National Institutes of Health, USA) in at least three replicates (Fig. S1, supplemental material).
RNA Extraction and Real time-PCR
The total RNA was extracted from brain tissue using Trizol reagent (Invitrogen, CA) and quantified by absorbance values at 260 nm. The reverse transcriptase reaction was performed for 1 h at 42°C using 2 µg of total RNA, 1 µg of oligo(dT), 200 U of M-MLV reverse transcriptase enzyme, 500 µM dNTP mix, and 25 U of RNase inhibitor (Promega, Madison, WI). The resultant cDNA was used to carry out 40 PCR cycles consisting of 15 s at 95°C, 30 s at 58°C, and 30 s at 72°C on an ABIPrism 7000 sequence detection system (Applied biosytems, Foster City, CA). The reactions were performed using SybrGreen II (Qiagen, Valencia, CA) and Go Taq Colorless master mix (Promega, Madison, WI). Each PCR reaction was set up in triplicate wells in a total volume of 25 µl. The reaction mixture contained the cDNA equivalent of 20 ng total RNA. The quantitative values of the genes of interest were normalized using β-actin as the endogenous reference, and fold-increase over control values was calculated using the relative quantification method of 2-ΔΔ Ct. The primer sequences were: δ-aminolevulinate synthase-1 (ALAS1) forward-TCTATGGGGCTTCAGGTGGA and reverse-ACGGTGTCGATCAGCAAACT, mitochondrial transcription factor A (TFAM) forward-TTCCAGGGGGCTAAGGATGA reverse-CACACTGCGACGGATGAGAT, cytochrome c oxidase subunit IV isoform 1 (COX4) forward-CAGCAGTGGCAGAATGTTGG and reverse-CGAAGGCACACCGAAGTAGA, peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1α) forward-TGGAGTGACATAGAGTGTGCTG reverse-TATGTTCGCGGGCTCATTGT, and PDK1 forward-CGGCATAGAGCGGCAGGTT and reverse-CCTTGCCAGCCTCATACCGA.
Data analysis
The results were analyzed by one-way analysis of variance (ANOVA) applying the Bonferroni post-test using Prism 6 software (GraphPad, San Diego, CA, USA). A p < 0.05 was considered statistically significant. No attempt was made to statistically analyze the densitometric values because of the semi-quantitative nature of data and pitfalls associated with quantitative analyses of Western blot density.
RESULTS AND DISCUSSION
There is growing realization that the inflammatory response is the main factor which determines the clinical outcome in hemorrhagic shock (Angele et al. 2008, Moore et al. 2004). Therefore, therapies targeting the causes of shock-associated SIRS are needed to minimize tissue damage in multiple organs. The leading cause of SIRS is the loss of gut barrier function because of ischemic injury to the gut epithelium (Moore et al. 2004, Grenz et al. 2012, Rhodes et al. 1973) which has also been implicated in brain dysfunction (Hsieh et al. 2011, Zhou et al. 2012). In previous reports, we described that an anti-inflammatory molecule EF24 exerts a pro-survival effect in a rat model of 50% hemorrhagic shock (Yadav et al. 2014a, Yadav et al. 2013) by preventing gut injury. In this work, we investigated the effect of EF24 on brain energy crisis caused by hemorrhagic shock in the same model. We also supplemented EF24-treatment with LEH infusion to study whether co-administration of LEH will augment the efficacy of EF24. Recently, we have shown that LEH preparation used in this work protects brain metabolic activity after hemorrhagic shock (Rao et al. 2015). The data discussed below indicate that EF24 administration alone considerably improves the bioenergetics of brain, and the additional benefits of LEH infusion were only incremental.
The effect of EF24 treatment on hemodynamic responses to hemorrhagic shock
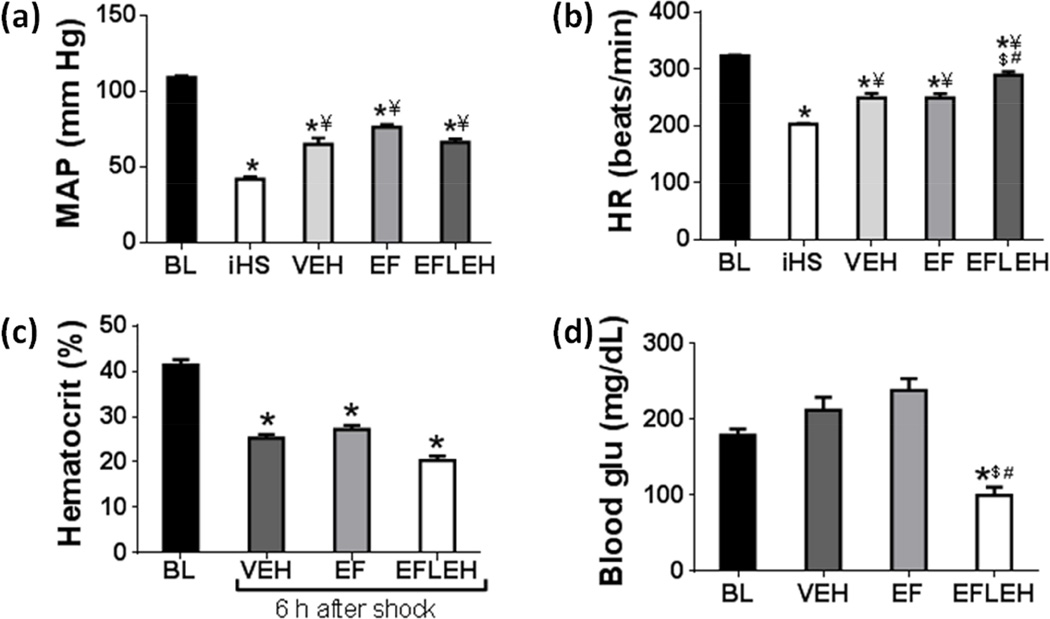
The mean arterial pressure (MAP) and heart rate (HR) recorded at baseline (BL), immediately after shock (iHS), and 6 h after treatments with vehicle (VEH), EF24 (EF), and EF24+LEH (EFLEH) are provided in Fig. 1. Commensurate to the blood loss, the MAP immediately after hemorrhage reduced from 109.3 mmHg to 42 mmHg (Fig. 1a). After 6 h of compensation, the MAP increased to 64.8, 76.2, 66.1 mmHg in VEH, EF, and EFLEH groups, respectively; these values were significantly different from the MAP recorded immediately after shock (p < 0.05 vs. iHS). However, the 6 h MAP values in VEH, EF, and EFLEH groups remained significantly lower as compared to the baseline MAP (p < 0.05). The difference in MAP between EF and EFLEH groups was not significant. The changes in HR in various groups (Fig. 1b) followed the same pattern as that of MAP. Like MAP, the HR immediately after hemorrhage also dropped from the baseline levels of 324 to 203 bpm. Both VEH and EF groups showed significant recovery to approximately 250 bpm after 6 h of compensation. Although LEH co-resuscitation further improved the HR in EFLEH group (290 bpm), it was still significantly lower than the baseline HR.
Figure 1.
Hemodynamics in rats hemorrhaged by 45% of blood. (a) Mean arterial pressure (MAP), (b) Heart rate, (c) Hematocrit, and (d) Blood glucose at baseline (BL), immediately after hemorrhage (iHS), and after 6 h of shock, treated with vehicle (VEH), EF24 (EF), or EF24+LEH (EFLEH). *P < 0.05 vs. BL, ¥P < 0.05 vs. iHS, $P < 0.05 vs. VEH, and #P < 0.05 vs. EF (ANOVA + Bonferroni test).
The hematocrit values essentially reflected the loss of 45–50% blood from circulation (Fig. 1c). Hematocrit in EFLEH group (20%) was noticeably lower than the hematocrit in VEH and EF groups (25% and 27%, respectively), perhaps due to the dilution effect of infused fluid. The blood glucose (glu) concentration was higher in VEH and EF group (212 and 238 mg/dL, respectively), but was lower in EFLEH group (99.6 mg/dL) as compared to the CTRL group (179 mg/dL, Fig. 1d). The difference in glu concentration between EFLEH and all other groups was statistically significant (p < 0.05).
The blood gas parameters, measured at baseline and after 6 h of treatments are provided in Table 1. Like hematocrit, the total hemoglobin (Hb) concentration decreased from baseline levels of 14.8 g/dL to 7.3 g/dL after hemorrhage. The Hb concentration in EF and EFLEH groups were 7.7 g/dL and 6.7 g/dL, respectively. The differences in Hb content among VEH, EF, and EFLEH groups were not statistically significant. Cations (Na+ and K+), pH, pCO2, pO2, and SO2 levels were not also different among various groups, but Cl− was significantly reduced in EF group. LEH co-administration brought EF24-induced reduction in Cl− levels to normal levels. Total CO2, HCO3−, and base excess in VEH and EF groups was not significantly different from baseline levels, but these parameters in EFLEH group were significantly reduced as compared to all other groups. Correspondingly, the anion gap in EFLEH group was significantly increased as compared to the baseline levels, suggesting the presence of anion gap acidosis. Similar effect was observed when the hemorrhaged rats were infused with LEH alone (Rao et al. 2015). Although the precise reason for this phenomenon is unclear, we suspect albumin in our LEH preparation as a possible factor. Hyperalbuminemia is known to cause high anion gap because it remains as an unmeasured anion under physiological conditions, but consumes bicarbonate buffer (Feldman et al. 2005).
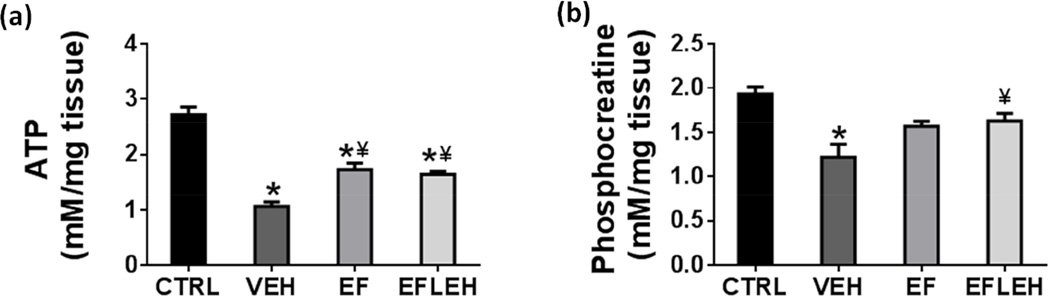
EF24 treatment increases the cerebral ATP and phosphocreatine reduced by hemorrhagic shock
ATP is the energy currency of all eukaryotic cells, but the brain cells also store energy in the form phosphocreatine (Kekelidze et al. 2001, Patra et al. 2008). As needed, phosphocreatine is consumed in a continuous and efficient manner to replenish ATP by a reaction catalyzed by creatine kinase. Hemorrhagic shock caused a significant reduction in ATP concentration (Fig. 2a), from 2.72 to 1.1 mM/mg (60% reduction, p < 0.05). EF24 alone and EF24+LEH were equally effective in partially restoring the ATP cellular content to 1.73 and 1.65 mM/mg, respectively (p < 0.05 vs. VEH). Like in the case of ATP, hemorrhagic shock also decreased phosphocreatine content from 1.9 nM to 1.2 nM (37% reduction). EF24 as well as EF24+LEH treatments increased phosphocreatine concentration to approximately 1.6 nM (84% of CTRL level), but only EF24+LEH group showed significant recovery as compared to the VEH group. The differences in phosphocreatine concentration in CTRL, EF, and EFLEH groups were not statistically significant. Since LEH co-resuscitation did not show additive effect, the partial recovery of depleted levels of ATP and phosphocreatine after EF24 treatment appears to be independent of volume resuscitation.
Figure 2.
Effect of EF24 on the energy metabolites in brain tissue of hypovolemic rats. (a) ATP content (n = 4/group) and (b) Phosphocreatine concentration (n = 4/group). The rats were treated with EF24 (EF), corresponding vehicle (VEH), or EF24+LEH (EFLEH). *P < 0.05 vs. CTRL, ¥P < 0.05 vs. VEH (ANOVA + Bonferroni test).
Our observations of the effect of shock on energy metabolites are different from those made by Okuda et al. and Mongan et al (Okuda et al. 1989, Mongan et al. 2001). Both these studies reported ATP remaining unchanged, but the measurements were limited to the acute phase (< 2 h) of shock. In contrast, the shock duration in our model is significantly prolonged, lasting for 6 h and possibly entering the decompensatory stage when energy metabolites start to deplete (Sun et al. 2014). It is possible that during the acute phase of shock, the compensatory mechanisms are able to preserve cerebral metabolism.
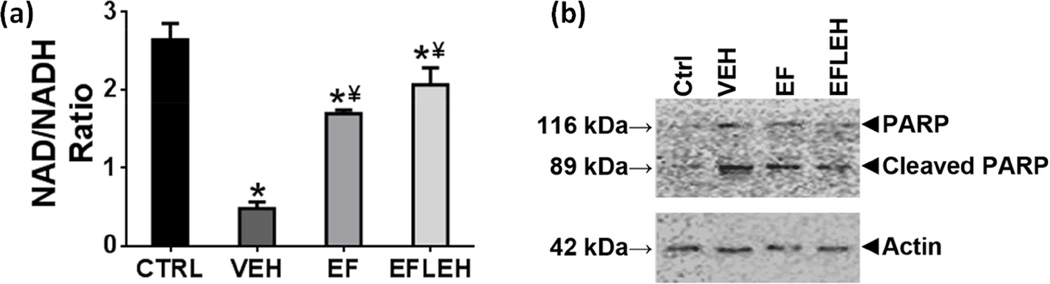
EF24 treatment recovers NAD/NADH balance in brain tissue
The cellular ATP is synthesized from glucose in a pathway where NAD and NADH act as electron acceptor and donor, respectively. Hemorrhagic shock caused an approximately 80% reduction in NAD/NADH ratio from 2.6 to 0.5 (Fig. 3a). In EF24 and EF24+LEH groups the ratio was significantly recovered to 1.7 and 2.1, respectively (65% and 81% of the NAD/NADH ratio in CTRL group, respectively). Although major consumption of cellular NAD occurs during glycolysis, rapid activation of PARP has also been implicated in the depletion of cytosolic NAD in hemorrhagic shock (Mongan et al. 2001). PARP is a key mediator of cell death in excitotoxicity, ischemia, and oxidative stress in neurons (Alano et al.). We found that hemorrhagic shock significantly increased the cleavage of PARP in brain tissue (Fig. 3b). Treatment with EF24 and EF24+LEH reduced PARP cleavage, but EF24 was more effective in suppressing the PARP cleavage.
Figure 3.
Effect of EF24 on (a) NAD/NADH ratio (n = 4/group) and (b) A representative immunoblot showing cleavage of PARP (n=3). The rats were treated with EF24 (EF), corresponding vehicle (VEH), or EF24+LEH (EFLEH). *P < 0.05 vs. CTRL, ¥P < 0.05 vs. VEH (ANOVA + Bonferroni test).
The primary suspect for the depletion of NAD in hemorrhaged brain was the stress-induced glycolysis because under acute stress conditions, the cellular metabolism shifts from oxidative phosphorylation to glycolysis (Pfeiffer et al. 2001). The glycolytic phenotype provides a state of apoptotic resistance in stress conditions by maintaining high levels of intermediates to support anabolic reactions in cells (Lunt & Vander Heiden 2011). Although stress-induced glycolysis is a cellular survival mechanism as it does not require mitochondrial function and is independent of oxygen availability, it cannot go on forever because it continuously consumes NAD in the process. Without NAD regeneration from oxidative phosphorylation, the exhaustion of cellular NAD signals a point of no return in hemorrhagic shock when irreversible decoupling of various metabolic steps occurs. The brain is especially sensitive to stress-induced glycolytic consumption of NAD because even under normal circumstances neuronal cells continuously invoke glycolysis for the ATP needed to maintain ionic homeostasis. Given that EF24 helps to restore NAD/NADH balance, it is possible that EF24 reinstates oxidative phosphorylation in the mitochondria. In addition, since the administration of EF24 reduced the activation of PARP, EF24 treatment also contributes to the preservation of NAD pools in brain of hemorrhaged rats by inhibiting this mechanism.
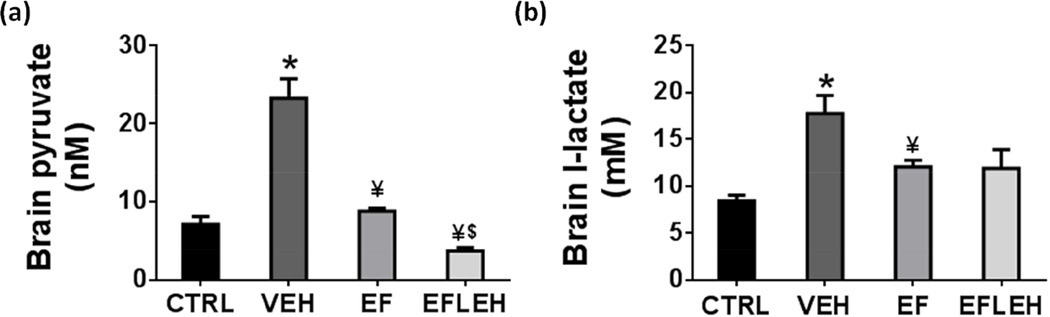
EF24 decreases hemorrhagic shock-induced accumulation of pyruvate in the brain
The mitochondrial oxidative phosphorylation regenerates NAD by consuming glycolytic product pyruvate in the TCA cycle. Therefore, in order to explain the recovery of energy metabolites, we determined the concentration of pyruvate in brain tissue of rats treated with EF24 (Fig. 4a). Pyruvate is not only present at the intersection of glycolysis and the oxidative phosphorylation, it also juxtaposes between the cellular anabolic and catabolic pathways by participating in analprotic pathways (Hassel 2000). Therefore, failure to correctly utilize pyruvate contributes to many pathologies (Gray et al. 2014), including the deranged energy metabolism in hemorrhagic shock (Hwabejire et al. 2013, Lin et al. 2005, Mongan et al. 2001). We found that hemorrhagic shock significantly increases the pyruvate concentration (p < 0.001, Fig. 4a). EF24 treatment reduced pyruvate to 8.8 nM (p < 0.001 vs. VEH) which was not significantly different from that in CTRL group. In EF24+LEH group, the pyruvate concentration was further decreased to 3.7 nM which was significantly lower than that in the EF24 alone group (p < 0.05).
Figure 4.
The concentration of monocarboxylic acids in brain tissue of hypovolemic rats treated with EF24 (EF), corresponding vehicle (VEH), or EF24+LEH (EFLEH). (a) Pyruvate concentration (n ≥ 4/group). (b) Lactate concentration (n = 5/group). *P < 0.05 vs. CTRL, ¥P < 0.05 vs. VEH, $P < 0.05 vs. EF (ANOVA + Bonferroni test).
Lactate is another monocarboxylic acid, the tissue concentration of which is affected by hypoxia. The effects of hemorrhagic shock and EF24 treatment on brain lactate concentration are shown in Fig. 4b. In VEH group, brain lactate increased from control levels of 8.4 mM to 17.7 mM (p < 0.05). EF24 treatment resulted in a significant reduction of lactate accumulation to 12.1 mM as compared to the VEH group (p <0.05). There was no additional effect of LEH infusion on EF24-treated rats, but the difference in lactate levels of EFLEH and VEH groups was not statistically significant. The effect of EF24 on tissue lactate concentration is interesting, because lactate is also a source of energy in brain (Dienel 2012). Under hypoxic stress, pyruvate can be reductively metabolized to lactate to primarily maintain high levels of glycolytic intermediates to support anabolic reactions in cells (Lunt & Vander Heiden 2011). In the process, aerobic glycolysis also produces ATP, albeit in a very inefficient manner (Pfeiffer et al. 2001). Brain has the special ability to utilize blood and tissue lactate to produce ATP by converting it back into pyruvate in the mitochondria.
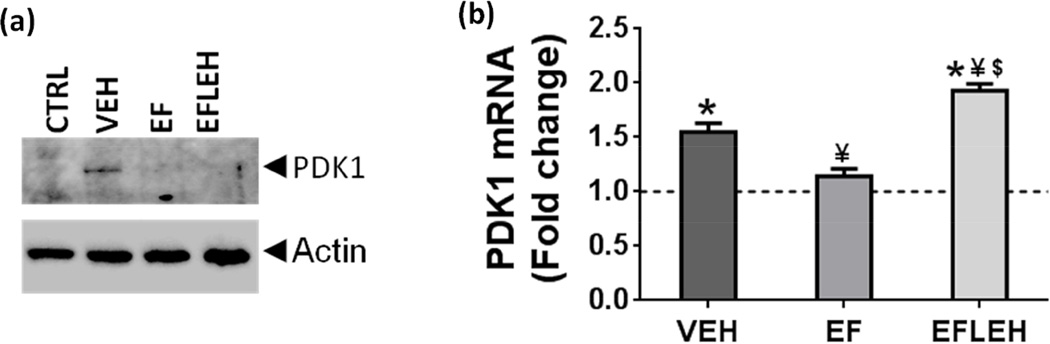
As far as pyruvate metabolism is concerned, we hypothesized that EF24 affects intracellular pyruvate accumulation by increasing the influx of pyruvate in the TCA cycle to produce acetyl-CoA, a step catalyzed by pyruvate dehydrogenase (PDH) complex. The activity of PDH is negatively regulated by its phosphorylation by PDKs at multiple sites (Zhang et al. 2014). We determined the expression of PDK1, and found that in VEH group, the expression of PDK1 was significantly increased as compared to that in CTRL group (Fig. 5a). EF24 and EF24+LEH treatments reduced the hemorrhagic shock-induced expression of PDK1. Recently Jian et al also reported that trauma and hemorrhage increases the expression of PDK1, suggesting that a possible shift in cellular energetics from mitochondria to glycolysis occurs after trauma and hemorrhage (Jian et al. 2014). Since the expression of PDK1 is transcriptionally regulated by hypoxia-inducible factor-1α (HIF-1α) (Kim et al. 2006), and considering that the transcriptional activity of HIF-1α is upregulated in hypoxic brain, we examined the expression of PDK1 transcripts in brain tissue (Fig. 5b). Hemorrhagic shock resulted in 55% increase in PDK1 mRNA in VEH group as compared to the CTRL group. EF24 significantly reduced the shock-induced increase in PDK1 mRNA, but the samples from EFLEH group showed significantly high levels of PDK1 mRNA (Fig. 5b). These results suggest that EF24 is able to improve pyruvate metabolism in hemorrhagic shock by reducing the expression of PDK1 at transcriptional level. The effect of EF24 on PDK expression would be manifested in an increase in PDH activity associated with mitochondrial fraction of the cells. However, more work is required to explain the suppressive effect of LEH co-administration on PDK1 protein expression even when it increased the PDK1 mRNA levels.
Figure 5.
Expression of PDK1 in brain tissue of hypovolemic rats treated with EF24 (EF), corresponding vehicle (VEH), or EF24+LEH (EFLEH). (a) A representative immunoblot showing PDK1 protein expression (n = 3/group). (b) PDK1 mRNA (n ≥ 4/group). *P < 0.05 vs. CTRL, ¥P < 0.05 vs. VEH, $P < 0.05 vs. EF (ANOVA + Bonferroni test).
EF24 recovers citrate synthase activity and increases COX4 expression in hemorrhaged rats
For EF24 treatment to reduce hemorrhagic shock-induced intracellular pyruvate accumulation, improvement in mitochondrial metabolism is the primary expectation. We studied mitochondrial function by first determining the activity of citrate synthase which catalyzes the condensation of oxaloacetate with acetyl-CoA in the first reaction of the TCA cycle. The assay revealed that hemorrhagic shock caused a significant reduction in the activity of citrate synthase (Table 2). The activity decreased from 5.5 to 4.2 nmol/min (a 34% reduction). EF24 treatment brought the citrate synthase activity in hemorrhaged rats to 5.04 nmol/min (92% of CTRL level). In EF24+LEH group, the citrate synthase activity was 5.3 nmol/min (approximately 98% of the CTRL level).
Table 2.
Citrate synthase activity in brain tissue homogenates of rats (n = 4/group). The rats were hemorrhaged by 45% blood followed by treatment with EF24 (EF), corresponding vehicle (VEH), or EF24+LEH (EFLEH).
| Group | Cit synthase (nmol/mg/min) |
|---|---|
| Control | 5.48 ± 0.18 |
| HS→VEH | 4.20 ± 0.26* |
| HS→EF24 | 5.03 ± 0.11¥ |
| HS→EF24+LEH | 5.33 ± 0.20¥ |
P < 0.05 vs. CTRL and
P < 0.05 vs. VEH (ANOVA + Bonferroni test).
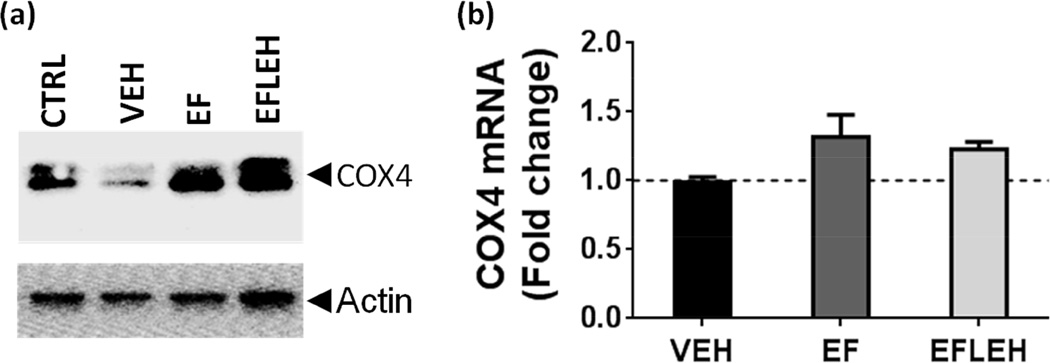
Next, we assessed the effect of hemorrhagic shock and the treatments on the expression of mitochondria-specific COX4 subunit of cytochrome oxidase c (Fig. 6). COX4 is one of the 13 subunits in the hetero-oligomeric enzyme cytochrome c oxidase localized to the inner mitochondrial membrane and serves as a terminal enzyme complex in the respiratory chain (Li et al. 2006). In our model, COX4 protein showed approximately 34% reduction in VEH group (Fig. 6a). In EF and EFLEH groups, COX4 expression was almost similar to that in CTRL group. High COX4 expression has been shown to increase the cellular oxygen consumption and optimizes the electron transfer chain activity in the mitochondria (Fukuda et al. 2007). Like PDK1, the expression of cytochrome c oxidase subunits is also regulated by HIF-1α. However unlike PDK1, the expression of COX4 mRNA was not altered in VEH group as compared to CTRL group, and there was insignificant increase in COX4 mRNA in EF and EFLEH groups (Fig. 6b, p > 0.05). Thus, the expression of COX4 in hemorrhagic shock is reduced primarily at protein level, possibly by enhanced degradation. Incidentally, the induction of HIF-1α has been shown to increase the degradation of COX4 subunit from cytochrome c oxidase isoform I by regulating a mitochondrial protease LON (Fukuda et al. 2007).
Figure 6.
COX4 expression in brain tissue after hemorrhagic shock and treatment with EF24 (EF), corresponding vehicle (VEH), or EF24+LEH (EFLEH). (a) A representative immunoblot showing COX4 protein expression (n = 3/group). (b) COX4 mRNA (n=6/group). The dotted line represents the mRNA expression in control group of rats. *P < 0.05 vs. CTRL, ¥P < 0.05 vs. VEH, and $P < 0.05 vs. EF (ANOVA + Bonferroni test).
EF24 treatment does not affect mitochondrial biogenesis
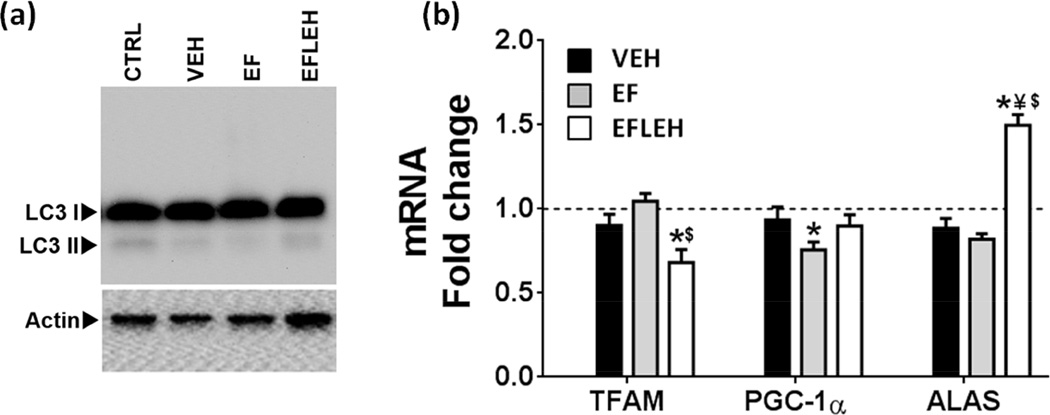
In addition to the mitochondrial function, the induction of mitochondrial turnover is yet another possible mechanism by which cellular metabolism can be influenced. For example, ischemic injury can result into autophagic destruction of damaged mitochondria (Kozlov et al.). We examined the expression of LC3BII as a marker of autophagy (Fig. 7a). Autophagy is a homeostatic mechanism by which a cell gets rid of unnecessary or dysfunctional organelles that may be essential for adaptation to a stress condition. Alternatively, the autophagy process also recycles the mitochondrial mass and is associated with mitochondrial biogenesis (Kubli & Gustafsson 2012, Yuan et al. 2009). The immunoblot data for LC3B expression suggest that basal level of autophagy is not substantially altered in brain tissue by hemorrhagic shock or EF24 treatment (Fig. 7a).
Figure 7.
Mitochondrial biogenesis in brain tissue after hemorrhagic shock and treatment with EF24 (EF), corresponding vehicle (VEH), or EF24+LEH (EFLEH). (a) A representative immunoblot showing LC3BI and LC3BII protein expression (n = 3/group). (b) TFAM, ALAS, and PGC-1α mRNA (n=6/group). The dotted line represents the mRNA expression in control group of rats. *P < 0.05 vs. CTRL, ¥P < 0.05 vs. VEH, and $P < 0.05 vs. EF (ANOVA + Bonferroni test).
Trauma and hemorrhagic shock have also been reported to cause decreased mitochondrial DNA content and the expression of transcription factors regulating mitochondrial biogenesis in left ventricular tissue of a rat model (Jian et al.). We examined three factors, namely TFAM, PGC-1α, and ALAS, which are considered essential for mitochondrial biogenesis (Fig. 7b). As compared to the CTRL group, the TFAM and ALAS mRNAs were unchanged in VEH and EF groups, but PGC-1α mRNA was significantly reduced in EF group. In efleh group, we obtained mixed results- the PGC-1α mRNA remained unchanged, the TFAM mRNA was significantly reduced and the ALAS mRNA was significantly increased as compared to the CTRL group.
Since hemorrhagic shock had no apparent influence on the levels of TFAM, PGC-1α, and ALAS transcripts, we infer that shock of 6 h duration due to 45% blood loss does not affect mitochondrial biogenesis in brain. Although EF24 was found to decrease PGC-1α mRNA expression, the reduction was only by approximately 25%. Thus, we conclude that EF24 has no effect on mitochondrial biogenesis in cerebral tissue of hemorrhaged rats. On the other hand, LEH co-administration resulted in a significant increase in ALAS mRNA. We speculate that the volume correction by LEH infusion induces ALAS expression to enable house-keeping functions of isoform 1 of ALAS.
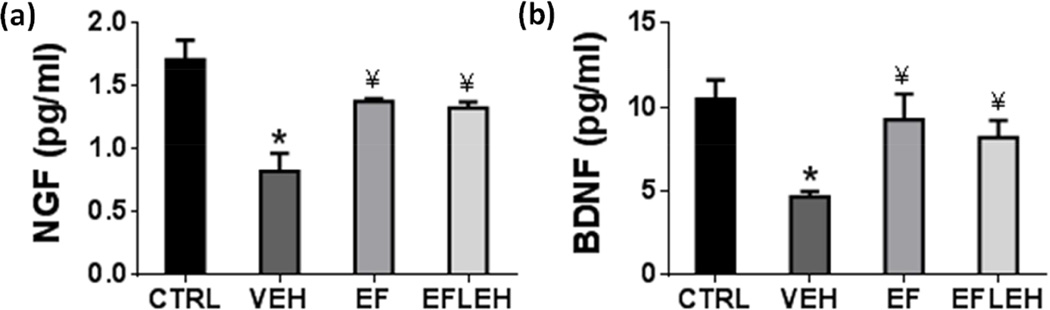
EF24 treatment restores NGF and BDNF in brain
Another symptom of deranged energy metabolism in hypovolemic shock was the reduced levels of neurotrophins, NGF and BDNF. NGF signaling is neuroprotective and regulates repair functions in brain (Sofroniew et al. 2001), whereas BDNF signaling is important for synaptic plasticity, and its circulating levels correlate with mood, cognition, and motor function (Lu et al. 2014). ATP binding to NGF has been shown to be a prerequisite for its neuroprotective bioactivity (Ferenz et al. 2012). Similarly, BDNF can stimulate its receptors only if it is bound to ATP (Ferenz et al. 2012). In order to assess the effect of improved energy metabolism by EF24 treatment on neuronal health and growth, we determined the concentration of neurotrophins NGF and BDNF in brain tissue (Fig. 8). Hemorrhagic shock reduced NGF from 1.7 pg/ml to 0.8 pg/ml (p < 0.05), whereas treatments with EF24 and EF24+LEH significantly increased it to approximately 1.4 pg/ml (Fig. 8a). The brain concentration of BDNF was also reduced by hemorrhagic shock (Fig. 8b). Hemorrhagic shock decreased BDNF from 10.5 to 4.7 pg/ml (p < 0.05). EF24 and EF24+LEH treatments increased BDNF concentration to 9.3 pg/ml and 8.2 pg/ml, respectively. The NGF and BDNF levels in the EF and EFLEH groups were comparable to those in CTRL group. These results show that by increasing the ATP pools in brain of hemorrhaged rats, EF24 was able to beneficially influence the neurotrophic factors responsible for neuronal growth and function.
Figure 8.
Effect of EF24 treatment on the expression of neurotrophic factors in brain tissue of hemorrhaged rats. (a) Nerve growth factor (NGF, n = 4/group) and (b) Brain-derived neurotrophic factor (BDNF, n = 4/group). The rats were treated with EF24 (EF), corresponding vehicle (VEH), or EF24+LEH (EFLEH). *P < 0.05 vs. CTRL, ¥P < 0.05 vs. VEH (ANOVA + Bonferroni test).
Summary
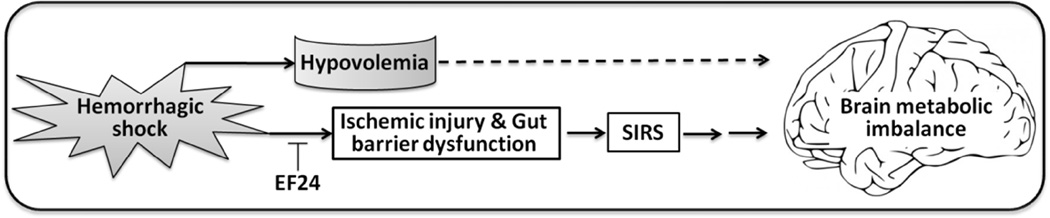
We show for the first time that EF24 administration in hemorrhagic shock resurrects cerebral energy metabolism by correcting the accumulation of pyruvate and improving mitochondrial function. Given the marginal additional benefits of LEH co-administration, we hypothesize that cerebral metabolic alterations in hemorrhagic shock are mainly a result of SIRS (Fig. 9) which is primarily caused by ischemic injury to the peripheral tissues, especially the gut. Gut barrier dysfunction is known to trigger SIRS and cerebral damage (Grenz et al. 2012, Hsieh et al. 2011, Moore et al. 2004, Rhodes et al. 1973, Zhou et al. 2012). This hypothesis is also supported by our recent report where we showed that EF24 prevents gut barrier dysfunction and this effect was associated with rapid accumulation of EF24 in the intestinal tissue (Yadav et al. 2014a). Interestingly, brain tissue accumulates negligible amounts of administered EF24 (Lagisetty et al. 2012). Overall, we conclude that while correction of volume and oxygen deficits is of paramount importance in overall well-being of an organism in hemorrhagic shock, pre-hospital administration of EF24 may be helpful in preserving brain metabolism before hospital-based intensive care is made available. Such a scenario is especially applicable in field conditions or whenever there are limited medical resources or prolonged transport times (Crookes et al. 2004). At the same time, the data in Fig. 3, Fig. 4a, and Fig. 6a noticeably indicates that EF24 treatment in LEH administered rats has additive salutary effect, suggesting that EF24 is effective after volume replacement resuscitation as well. However, further studies are required to conclusively address the post-resuscitation efficacy of EF24 in the context of resuscitation-induced ischemia-reperfusion injury.
Figure 9.
A hypothetical model to explain the salutary effects of EF24 on the brain in hemorrhagic shock. EF24 in small volume was as effective in restoring cerebral energy stores as was a combination of EF24 and LEH. Given our previous observations that the majority of administered EF24 localizes in intestinal tissue (Lagisetty et al. 2012, Yadav et al. 2014a) and negligible amounts accumulated in the brain, it appears that the cerebral metabolic derangement after blood loss is a combined consequence of hypovolemia-induced perfusion/oxygen deficit and inflammatory processes triggered by access of gut contents to systemic space, secondary to the dysfunction of intestinal barrier.
Supplementary Material
Table 1.
Arterial blood gases in the rat model of hemorrhagic shock treated with EF24 and EF24+LEH.
| Baseline | VEH 6 h | EF 6 h | EFLEH 6 h | |
|---|---|---|---|---|
| Total Hb (g/dL) | 14.80 ± 0.30 | 7.34 ± 0.32* | 7.71 ± 0.34* | 6.70 ± 0.27* |
| pH | 7.50 ± 0.01 | 7.43 ± 0.06 | 7.49 ± 0.01 | 7.33 ± 0.11 |
| pCO2 | 39.36 ± 1.15 | 41.38 ± 3.26 | 36.89 ± 1.93 | 32.80 ± 5.10 |
| pO2 (mm Hg) | 88.27 ± 2.42 | 109.00 ± 5.37 | 98.67 ± 3.65 | 104.20 ± 16.99 |
| SO2 (%) | 95.73 ± 0.56 | 95.25 ± 1.84 | 95.67 ± 0.41 | 96.00 ± 0.91 |
| HCO3 (mM) | 29.45 ± 0.91 | 25.20 ± 1.88 | 26.46 ± 1.63 | 15.92 ± 1.15*$ƺ |
| Total CO2 (mM) | 29.75 ± 0.47 | 26.76 ± 1.84 | 27.60 ± 1.68 | 16.74 ± 2.05*$ƺ |
| Base excess (mM) | 5.61 ± 0.37 | 1.44 ± 2.77 | 3.68 ± 1.52 | −8.88 ± 3.82*$ƺ |
| Anion gap | 9.43 ± 0.97 | 12.28 ± 3.03 | 14.50 ± 3.92 | 23.635 ± 0.98* |
| Na+ (mM) | 140.82 ± 0.42 | 138.63 ± 0.94 | 140.11 ± 1.38 | 141.80 ± 1.93 |
| K+ (mM) | 4.27 ± 0.06 | 4.78 ± 0.75 | 4.21 ± 0.20 | 5.58 ± 1.12 |
| Cl − (mM) | 107.09 ± 0.61 | 105.13 ± 1.01 | 103.11 ± 1.41* | 108.20 ± 1.02ƺ |
The data are provided as mean ± sem. The blood gas was measured at baseline (before the induction of hemorrhagic shock) and after 6 h of treatment with vehicle (VEH), EF24 (EF), and EF24+LEH (EFLEH).
P value < 0.05 vs Baseline*, HS$, and EFƺ by ANOVA and Tukey’s multiple comparison test.
Highlights.
EF24 treatment recovers brain energy metabolites and reduced PARP cleavage in 50% hemorrhaged rats.
EF24 improves cerebral energetics by restoring perturbed mitochondrial pyruvate metabolism.
EF24 treatment increases the levels of NGF and BDNF in shock brain.
Co-administration of artificial oxygen-carrier LEH with EF24 is marginally more effective than EF24 alone.
ACKNOWLEDGEMENTS
The work was supported by a grant from National Heart, Lung & Blood Institute [R01HL104286]. The authors thank Dr. Alamdar Hussain, Assistant Professor of Pharmaceutical Sciences at the University of Oklahoma Health Sciences Center, for providing assistance in animal experiments. The authors also acknowledge the effort of Dr. Vivek Yadav (postdoctoral fellow) in preparation and characterization of LEH used in this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST DISCLOSURE
The authors do not have any conflict of interest to disclose.
REFERENCES
- Agashe H, Lagisetty P, Awasthi S, Awasthi V. Improved formulation of liposome-encapsulated hemoglobin with an anionic non-phospholipid. Colloids Surf B Biointerfaces. 2010;75:573–583. doi: 10.1016/j.colsurfb.2009.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alano CC, Garnier P, Ying W, Higashi Y, Kauppinen TM, Swanson RA. NAD+ depletion is necessary and sufficient for poly(ADP-ribose) polymerase-1-mediated neuronal death. J Neurosci. 2010;30:2967–2978. doi: 10.1523/JNEUROSCI.5552-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angele MK, Schneider CP, Chaudry IH. Bench-to-bedside review: latest results in hemorrhagic shock. Crit Care. 2008;12:218. doi: 10.1186/cc6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasthi V, Agashe H, Doblas S, Towner R. Magnetic resonance spectroscopy for evaluation of liposome-encapsulated hemoglobin as a resuscitation fluid. Artif Cells Blood Substit Immobil Biotechnol. 2010;38:69–78. doi: 10.3109/10731191003634638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasthi V, Yee SH, Jerabek P, Goins B, Phillips WT. Cerebral oxygen delivery by liposome-encapsulated hemoglobin: a positron-emission tomographic evaluation in a rat model of hemorrhagic shock. J Appl Physiol (1985) 2007a;103:28–38. doi: 10.1152/japplphysiol.00136.2006. [DOI] [PubMed] [Google Scholar]
- Awasthi V, Yee SH, Jerabek P, Goins B, Phillips WT. Cerebral oxygen delivery by liposome-encapsulated hemoglobin: a positron-emission tomographic evaluation in a rat model of hemorrhagic shock. J Appl Physiol. 2007b;103:28–38. doi: 10.1152/japplphysiol.00136.2006. [DOI] [PubMed] [Google Scholar]
- Brattstrom O, Granath F, Rossi P, Oldner A. Early predictors of morbidity and mortality in trauma patients treated in the intensive care unit. Acta Anaesthesiol Scand. 2010;54:1007–1017. doi: 10.1111/j.1399-6576.2010.02266.x. [DOI] [PubMed] [Google Scholar]
- Crookes BA, Cohn SM, Bonet H, Burton EA, Nelson J, Majetschak M, Varon AJ, Linden JM, Proctor KG. Building a better fluid for emergency resuscitation of traumatic brain injury. J Trauma. 2004;57:547–554. doi: 10.1097/01.ta.0000135162.85859.4c. [DOI] [PubMed] [Google Scholar]
- Dienel GA. Brain lactate metabolism: the discoveries and the controversies. J Cereb Blood Flow Metab. 2012;32:1107–1138. doi: 10.1038/jcbfm.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman M, Soni N, Dickson B. Influence of hypoalbuminemia or hyperalbuminemia on the serum anion gap. J Lab Clin Med. 2005;146:317–320. doi: 10.1016/j.lab.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Ferenz KB, Gast RE, Rose K, Finger IE, Hasche A, Krieglstein J. Nerve growth factor and brain-derived neurotrophic factor but not granulocyte colony-stimulating factor, nimodipine and dizocilpine, require ATP for neuroprotective activity after oxygen-glucose deprivation of primary neurons. Brain Res. 2012;1448:20–26. doi: 10.1016/j.brainres.2012.02.016. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick SF, Tambuwala MM, Bruning U, et al. An intact canonical NF-kappaB pathway is required for inflammatory gene expression in response to hypoxia. J Immunol. 2011;186:1091–1096. doi: 10.4049/jimmunol.1002256. [DOI] [PubMed] [Google Scholar]
- Fukuda R, Zhang H, Kim JW, Shimoda L, Dang CV, Semenza GL. HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell. 2007;129:111–122. doi: 10.1016/j.cell.2007.01.047. [DOI] [PubMed] [Google Scholar]
- Gray LR, Tompkins SC, Taylor EB. Regulation of pyruvate metabolism and human disease. Cell Mol Life Sci. 2014;71:2577–2604. doi: 10.1007/s00018-013-1539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenz A, Clambey E, Eltzschig HK. Hypoxia signaling during intestinal ischemia and inflammation. Curr Opin Crit Care. 2012;18:178–185. doi: 10.1097/MCC.0b013e3283514bd0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassel B. Carboxylation and anaplerosis in neurons and glia. Mol Neurobiol. 2000;22:21–40. doi: 10.1385/MN:22:1-3:021. [DOI] [PubMed] [Google Scholar]
- Hsieh YH, McCartney K, Moore TA, Thundyil J, Gelderblom M, Manzanero S, Arumugam TV. Intestinal ischemia-reperfusion injury leads to inflammatory changes in the brain. Shock. 2011;36:424–430. doi: 10.1097/SHK.0b013e3182295f91. [DOI] [PubMed] [Google Scholar]
- Hwabejire JO, Jin G, Imam AM, et al. Pharmacologic modulation of cerebral metabolic derangement and excitotoxicity in a porcine model of traumatic brain injury and hemorrhagic shock. Surgery. 2013;154:234–243. doi: 10.1016/j.surg.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Jian B, Yang S, Chaudry IH, Raju R. Resveratrol restores sirtuin 1 (SIRT1) activity and pyruvate dehydrogenase kinase 1 (PDK1) expression after hemorrhagic injury in a rat model. Mol Med. 2014;20:10–16. doi: 10.2119/molmed.2013.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian B, Yang S, Chen D, Chaudry I, Raju R. Influence of aging and hemorrhage injury on Sirt1 expression: possible role of myc-Sirt1 regulation in mitochondrial function. Biochim Biophys Acta. 2011;1812:1446–1451. doi: 10.1016/j.bbadis.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasinski AL, Du Y, Thomas SL, et al. Inhibition of IkappaB kinase-nuclear factor-kappaB signaling pathway by 3,5-bis(2-flurobenzylidene)piperidin-4-one (EF24), a novel monoketone analog of curcumin. Mol Pharmacol. 2008;74:654–661. doi: 10.1124/mol.108.046201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kekelidze T, Khait I, Togliatti A, Benzecry JM, Wieringa B, Holtzman D. Altered brain phosphocreatine and ATP regulation when mitochondrial creatine kinase is absent. J Neurosci Res. 2001;66:866–872. doi: 10.1002/jnr.10060. [DOI] [PubMed] [Google Scholar]
- Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Kovach AG, Sandor P. Cerebral blood flow and brain function during hypotension and shock. Annu Rev Physiol. 1976;38:571–596. doi: 10.1146/annurev.ph.38.030176.003035. [DOI] [PubMed] [Google Scholar]
- Kozlov AV, Bahrami S, Calzia E, Dungel P, Gille L, Kuznetsov AV, Troppmair J. Mitochondrial dysfunction and biogenesis: do ICU patients die from mitochondrial failure? Ann Intensive Care. 2011;1:41. doi: 10.1186/2110-5820-1-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubli DA, Gustafsson AB. Mitochondria and mitophagy: the yin and yang of cell death control. Circ Res. 2012;111:1208–1221. doi: 10.1161/CIRCRESAHA.112.265819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagisetty P, Subramaniam D, Sahoo K, Anant S, Awasthi V. Anticancer activity of an imageable curcuminoid 1-[2-aminoethyl-(6-hydrazinopyridine-3-carbamidyl)-3,5-bis-(2-fluorobenzylidene)-4-piperidone (EFAH) Chem Biol Drug Des. 2012;79:194–201. doi: 10.1111/j.1747-0285.2011.01271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Park JS, Deng JH, Bai Y. Cytochrome c oxidase subunit IV is essential for assembly and respiratory function of the enzyme complex. J Bioenerg Biomembr. 2006;38:283–291. doi: 10.1007/s10863-006-9052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T, Koustova E, Chen H, Rhee PM, Kirkpatrick J, Alam HB. Energy substrate-supplemented resuscitation affects brain monocarboxylate transporter levels and gliosis in a rat model of hemorrhagic shock. J Trauma. 2005;59:1191–1202. doi: 10.1097/01.ta.0000188646.86995.9d. discussion 1202. [DOI] [PubMed] [Google Scholar]
- Lu B, Nagappan G, Lu Y. BDNF and synaptic plasticity, cognitive function, and dysfunction. Handb Exp Pharmacol. 2014;220:223–250. doi: 10.1007/978-3-642-45106-5_9. [DOI] [PubMed] [Google Scholar]
- Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 2011;27:441–464. doi: 10.1146/annurev-cellbio-092910-154237. [DOI] [PubMed] [Google Scholar]
- Mongan PD, Capacchione J, Fontana JL, West S, Bunger R. Pyruvate improves cerebral metabolism during hemorrhagic shock. Am J Physiol Heart Circ Physiol. 2001;281:H854–H864. doi: 10.1152/ajpheart.2001.281.2.H854. [DOI] [PubMed] [Google Scholar]
- Moore FA, McKinley BA, Moore EE. The next generation in shock resuscitation. Lancet. 2004;363:1988–1996. doi: 10.1016/S0140-6736(04)16415-5. [DOI] [PubMed] [Google Scholar]
- Nag OK, Yadav VR, Hedrick A, Awasthi V. Post-modification of preformed liposomes with novel non-phospholipid poly(ethylene glycol)-conjugated hexadecylcarbamoylmethyl hexadecanoic acid for enhanced circulation persistence in vivo. Int J Pharm. 2013;446:119–129. doi: 10.1016/j.ijpharm.2013.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda M, Muneyuki M, Sogabe T, Miura I. Effects of various catecholamines on highenergy phosphates of rat liver and brain during hemorrhagic shock measured by 31P-NMR spectroscopy. J Anesth. 1989;3:200–209. doi: 10.1007/s0054090030200. [DOI] [PubMed] [Google Scholar]
- Oliver KM, Garvey JF, Ng CT, Veale DJ, Fearon U, Cummins EP, Taylor CT. Hypoxia activates NF-kappaB-dependent gene expression through the canonical signaling pathway. Antioxid Redox Signal. 2009;11:2057–2064. doi: 10.1089/ars.2008.2400. [DOI] [PubMed] [Google Scholar]
- Patra S, Bera S, SinhaRoy S, Ghoshal S, Ray S, Basu A, Schlattner U, Wallimann T, Ray M. Progressive decrease of phosphocreatine, creatine and creatine kinase in skeletal muscle upon transformation to sarcoma. FEBS J. 2008;275:3236–3247. doi: 10.1111/j.1742-4658.2008.06475.x. [DOI] [PubMed] [Google Scholar]
- Pfeiffer T, Schuster S, Bonhoeffer S. Cooperation and competition in the evolution of ATP-producing pathways. Science. 2001;292:504–507. doi: 10.1126/science.1058079. [DOI] [PubMed] [Google Scholar]
- Rao G, Hedrick A, Yadav V, Xie J, Hussain A, Awasthi V. The Brain metabolic activity after resuscitation with liposome-encapsulated hemoglobin (LEH) in a rat model of hypovolemic shock. J Cerebral Blood Flow Metab. 2015 doi: 10.1038/jcbfm.2015.82. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes RS, Depalma RG, Robinson AV. Intestinal barrier function in hemorrhagic shock. J Surg Res. 1973;14:305–312. doi: 10.1016/0022-4804(73)90032-2. [DOI] [PubMed] [Google Scholar]
- Ronner P, Friel E, Czerniawski K, Frankle S. Luminometric assays of ATP, phosphocreatine, and creatine for estimation of free ADP and free AMP. Anal Biochem. 1999;275:208–216. doi: 10.1006/abio.1999.4317. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Howe CL, Mobley WC. Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci. 2001;24:1217–1281. doi: 10.1146/annurev.neuro.24.1.1217. [DOI] [PubMed] [Google Scholar]
- Sun N, Li LZ, Luo W, Luo Q. Cerebral hemodynamic change and metabolic alteration in severe hemorrhagic shock. Adv Exp Med Biol. 2014;812:217–223. doi: 10.1007/978-1-4939-0620-8_29. [DOI] [PubMed] [Google Scholar]
- Vilekar P, Awasthi S, Natarajan A, Anant S, Awasthi V. EF24 suppresses maturation and inflammatory response in dendritic cells. Int Immunol. 2012;24:455–464. doi: 10.1093/intimm/dxr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilekar P, King C, Lagisetty P, Awasthi V, Awasthi S. Antibacterial activity of synthetic curcumin-derivatives: 3,5-bis(benzylidene)-4-piperidone (EF24) and EF24-dimer linked via diethylenetriaminepentacetic acid (EF2DTPA) Applied Biochem Biotechnol. 2014 doi: 10.1007/s12010-014-0741-5. In press. [DOI] [PubMed] [Google Scholar]
- Yadav VR, Hussain A, Sahoo K, Awasthi V. Remediation of Hemorrhagic Shock-Induced Intestinal Barrier Dysfunction by Treatment with Diphenyldihaloketones EF24 and CLEFMA. J Pharmacol Exp Ther. 2014a;351:413–422. doi: 10.1124/jpet.114.217331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav VR, Nag O, Awasthi V. Biological evaluation of liposome-encapsulated hemoglobin surface-modified with a novel PEGylated nonphospholipid amphiphile. Artif Organs. 2014b;38:625–633. doi: 10.1111/aor.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav VR, Sahoo K, Roberts PR, Awasthi V. Pharmacologic Suppression of Inflammation by a Diphenyldifluoroketone, EF24, in a Rat Model of Fixed-Volume Hemorrhage Improves Survival. J Pharmacol Exp Ther. 2013;347:346–356. doi: 10.1124/jpet.113.208009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H, Perry CN, Huang C, Iwai-Kanai E, Carreira RS, Glembotski CC, Gottlieb RA. LPS-induced autophagy is mediated by oxidative signaling in cardiomyocytes and is associated with cytoprotection. Am J Physiol Heart Circ Physiol. 2009;296:H470–H479. doi: 10.1152/ajpheart.01051.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Hulver MW, McMillan RP, Cline MA, Gilbert ER. The pivotal role of pyruvate dehydrogenase kinases in metabolic flexibility. Nutr Metab (Lond) 2014;11:10. doi: 10.1186/1743-7075-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Huang WQ, Li C, Wu GY, Li YS, Wen SH, Lei WL, Liu KX. Intestinal ischemia/reperfusion enhances microglial activation and induces cerebral injury and memory dysfunction in rats. Crit Care Med. 2012;40:2438–2448. doi: 10.1097/CCM.0b013e3182546855. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.