Abstract
Opioid and α2-adrenoceptor (AR) agonists are analgesic when administered in the spinal cord and show a clinically beneficial synergistic interaction when co-administered. However, α2-AR antagonists can also inhibit opioid antinociception, suggesting a complex interaction between the two systems. The α2A-AR subtype is necessary for spinal adrenergic analgesia and synergy with opioids for most agonist combinations. Therefore, we investigated whether spinal opioid antinociception and opioid-adrenergic synergy were under allosteric control of the α2A-AR. Drugs were administered intrathecally in wild type (WT) and α2A-knock-out (KO) mice and antinociception was measured using hot water tail immersion or substance P behavioral assays. The α2A-AR agonist clonidine was less effective in α2A-KO mice in both assays. The absence of the α2A-AR resulted in 10–70-fold increases in the antinociceptive potency of the opioid agonists morphine and DeltII. In contrast, neither morphine nor DeltII synergized with clonidine in α2AKO mice, indicating that the α2AAR has both positive and negative modulatory effects on opioid antinociception. Depletion of descending adrenergic terminals with 6-OHDA resulted in a significant decrease in morphine efficacy in WT but not in α2A-KO mice, suggesting that endogenous norepinephrine acts through the α2A-AR to facilitate morphine antinociception. Based on these findings, we propose a model whereby ligand-occupied versus ligand-free α2A-AR produce distinct patterns of modulation of opioid receptor activation. In this model, agonist-occupied α2A-ARs potentiate opioid analgesia, while non-occupied α2A-ARs inhibit opioid analgesia. Exploiting such interactions between the two receptors could lead to the development of better pharmacological treatments for pain management.
Keywords: α2-adrenoceptor, opioid receptor, norepinephrine, spinal cord, morphine, analgesia
1. Introduction
1.1 Adrenergic modulation of nociception
The adrenergic system is a critical modulator of nociception, including at the spinal level where nociceptive information is relayed from the periphery to the central nervous system. The activation of spinal adrenoceptors by norepinephrine (NE) released from descending noradrenergic fibers or by exogenously administered α2-adrenoceptor (α2-AR) agonists generates an antinociceptive effect (Eisenach et al., 1996). These receptors are located both pre- and post-synaptically on sensory neurons and in the spinal cord where they inhibit neurotransmitter release and hyperpolarize secondary dorsal horn neurons (Fairbanks et al., 2009). The α-adrenergic receptor family is divided into α1- and α2-adrenoceptors, and there are three α2 receptor subtypes: α2A, α2B and α2C. Poor subtype selectivity among α2-AR ligands makes it difficult to distinguish the functions of each subtype; genetically modified mice therefore play a critical role in elucidating the relative roles of each subtype.
The α2A-AR is the predominant mediator of the antinociceptive effects of α2-adrenoceptor agonists at the spinal level, although the α2C-AR is also involved depending on the ligand tested (Fairbanks et al., 2002; Stone et al., 1997). Elsewhere in the central nervous system, the α2A-AR also mediates the anti-hypertensive and sedative effects of α2-AR-selective agonists such as clonidine and dexmedetomidine, limiting their use as analgesic agents (Lakhlani et al., 1997; MacMillan et al., 1996). Thus, spinal administration of α2-AR agonists is a useful strategy to minimize such unwanted side effects.
1.2 Opioid-adrenergic interactions in pain and antinociception
Activation of spinal µ- and δ-opioid receptors, MOPr and DOPr, respectively, have the potential to mediate analgesia. Interactions between opioid and adrenergic systems in the spinal cord are well documented. Of clinical importance is the interaction between opioid and α2-AR agonists such as morphine and clonidine: while co-administration results in analgesic synergy, the interaction remains additive for sedative and cardiovascular side effects, thus widening the therapeutic window between desired and undesired effects (Stone et al., 2014). The identification of receptor subtypes mediating such synergistic interactions is critical for understanding these mechanisms and for development of novel synergy-based therapies. MOPr, DOPr, α2A- and α2C-AR have been shown to mediate opioid-adrenergic synergy depending on the opioid-α2-AR agonist combination used (Chabot-Dore et al., 2014). Morphine/clonidine synergy - one of the most clinically-relevant and widely studied combinations – requires the activation of MOPr (Chabot-Doré et al., 2013; Guo et al., 2003), but the α2-AR subtype necessary for this interaction has not been clearly identified. Morphine also interacts synergistically with spinally administered NE (Roerig et al., 1992), suggesting that the endogenous adrenergic system regulates opioid-mediated antinociception, but again the α2-AR subtype(s) involved remain(s) unknown. Inhibition of morphine antinociception by α2-AR antagonists has also been observed; the regulation of opioid antinociception by adrenoceptors is therefore bidirectional and complex (Browning et al., 1982; Iglesias et al., 1992; Morales et al., 2001; Stone et al., 1997).
1.3 Intra-receptor allostery potentially regulates opioid-adrenergic interactions
Opioid receptors and adrenoceptors belong to the G protein-coupled receptor (GPCR) superfamily of transmembrane receptors and both preferentially couple to Gαi/o. GPCRs can exist as homo- and hetero-oligomers in cells and oligomerization can affect many aspects of GPCR function, including their maturation during biosynthesis, surface expression, ligand binding, signal transduction and internalization, all of which may have important pharmacological implications (Terrillon and Bouvier, 2004). These functional and pharmacological effects can result from allosteric interactions between receptors in an oligomer and, in some cases, only require the mere presence of the allosteric partner. For example, activation of the dopamine receptor D2 (DRD2) produces an anorexigenic effect that requires the presence, but not the activation of the ghrelin receptor GHSR1a (Kern et al., 2012).
In order for allosteric interactions to be relevant in vivo, receptors of interest must be co-expressed and physically interact in the tissue or cell of interest (Kenakin et al., 2010). An extensive body of functional and anatomical evidence points towards the co-localization of α2A-AR and MOPr or DOPr receptors in the spinal cord (see Chabot-Doré et al. (2014) for a detailed review) and MOPr and α2A-AR have been shown to heterodimerize in brainstem nuclei (Sun et al., 2015). DOPr and α2A-AR co-localize in the substance P (SP)-expressing DRG neuron subpopulation and interact both physically and functionally when co-expressed in vitro (Riedl et al., 2009; Rios et al., 2004). Similarly, MOPr and α2A-AR are expressed in DRG neurons (Riedl et al., 2009; Stone et al., 1998; Wang et al., 2010) and co-localize extensively in cultured DRG neurons (Tan et al., 2009). Furthermore, these receptors form dimers in heterologous expression systems as detected using bioluminescence resonance energy transfer (BRET; (Jordan et al., 2003), fluorescence resonance energy transfer (FRET; (Vilardaga et al., 2008), or co-immunoprecipitation (Jordan et al., 2003; Zhang and Limbird, 2004). Cross-desensitization between morphine and clonidine and cross-internalization of MOPr and α2A-AR following agonist treatment in cultured DRG neurons further support the capacity of these receptors to interact (Tan et al., 2009). Moreover, it was shown in expression systems that the morphine-bound MOPr allosterically inhibits the α2A-AR (Vilardaga et al., 2008). Therefore, α2A-AR/MOPr and α2A-AR/DOPr hetero-oligomers likely form in the spinal cord, making this neuronal population a good substrate to test whether the α2A-AR allosterically regulate opioid antinociception.
1.4 Study aims
The objective of this study was to determine if the α2A-adrenoceptor allosterically regulates spinal opioid antinociception and opioid-adrenergic synergy. The antinociceptive efficacy of spinal morphine, the opioid subtype-selective agonists [d-Ala2]-deltorphin II (DeltII) and [dAla2, NMe-Phe4, Gly-ol5]-enkephalin (DAMGO), and the interaction between morphine/clonidine and DeltII/clonidine were therefore determined in the presence and absence of the α2A-AR in mice in vivo. We further investigated the role of α2A-AR in the endogenous regulation of morphine antinociception by NE. Our findings lead us to propose a mechanism for the distinct patterns of allosteric regulation of opioid receptor-mediated antinociception by ligand-occupied versus ligand-free α2A-adrenoceptors.
2. Materials and Methods
2.1 Animals
All procedures were approved by the Animal Care Committee at McGill University and conformed to the ethical guidelines of the Canadian Council on Animal Care. All efforts were made to minimize animal suffering, to reduce the number of animals used, and to utilize alternatives to in vivo techniques, if available. We used C57BL/6 mice (Charles River, Quebec, Canada) as wild-type (WT) controls. Mice with a targeted gene deletion introducing a premature stop codon in the Adra2a gene were developed on a mixed C57BL/6 × FVB/129 background (Altman et al., 1999). Congenic α2A-KO mice backcrossed to a standard C57BL/6 background were obtained from Jackson Laboratory (Bar Harbor, Maine, stock #004367). Mice were bred in house and genotyping was performed to assign Adra2a genotypes in WT and α2A-KO mice as described (Peterhoff et al., 2003). Mice were housed in groups of 2–5 and maintained in ventilated cages on a regular 12 hour light/dark cycle and given ad libitum access to food and filtered water. Aged-matched 3–6 month old males were used in all studies and experimenters were blind to both genotype and treatment. Animals were re-randomized to control for the effect of previous drug exposure and re-tested up to 3 times with a minimum drug washout period of 4 days between experiments. The re-randomization across groups minimizes the potential that previous drug exposure confounds subsequent results. The potential effects of repeated handling were analyzed in the vehicle-treated mice and no effects of previous handling were observed in either the SP or the tail immersion assays.
Experiments with α2C-KO mice and their controls were approved by the Institutional Animal Care and Use Committee of the University of Minnesota. α2C-KO mice were developed at Stanford University, Palo Alto, California (Link et al., 1995) and purchased from Jackson Labs (Bar Harbor, Maine, stock # 002512) after 17 generations of backcrossing to C57BL/6 background. α2C-KO and C57BL/6 mice WT controls were bred and housed in University of Minnesota, Minneapolis where experiments were performed.
Some of the experiments performed in WT mice have been previously published as part of another study (Chabot-Doré et al., 2013). Three stains of mice were tested in parallel – WT, DOPr-KO and α2A-KO mice – so that they could share a WT control group, thus reducing animal use and maximizing resources. As a result, the WT data from Figures 2A–D (2nd row), Figure 3D,E and Figure 4A,B and the α2A-KO clonidine dose-response curve in the substance P assay in Figure 2A were previously published (Chabot-Doré et al., 2013). These data are included here to provide the necessary WT control data needed to put the current α2A-KO experiments in context.
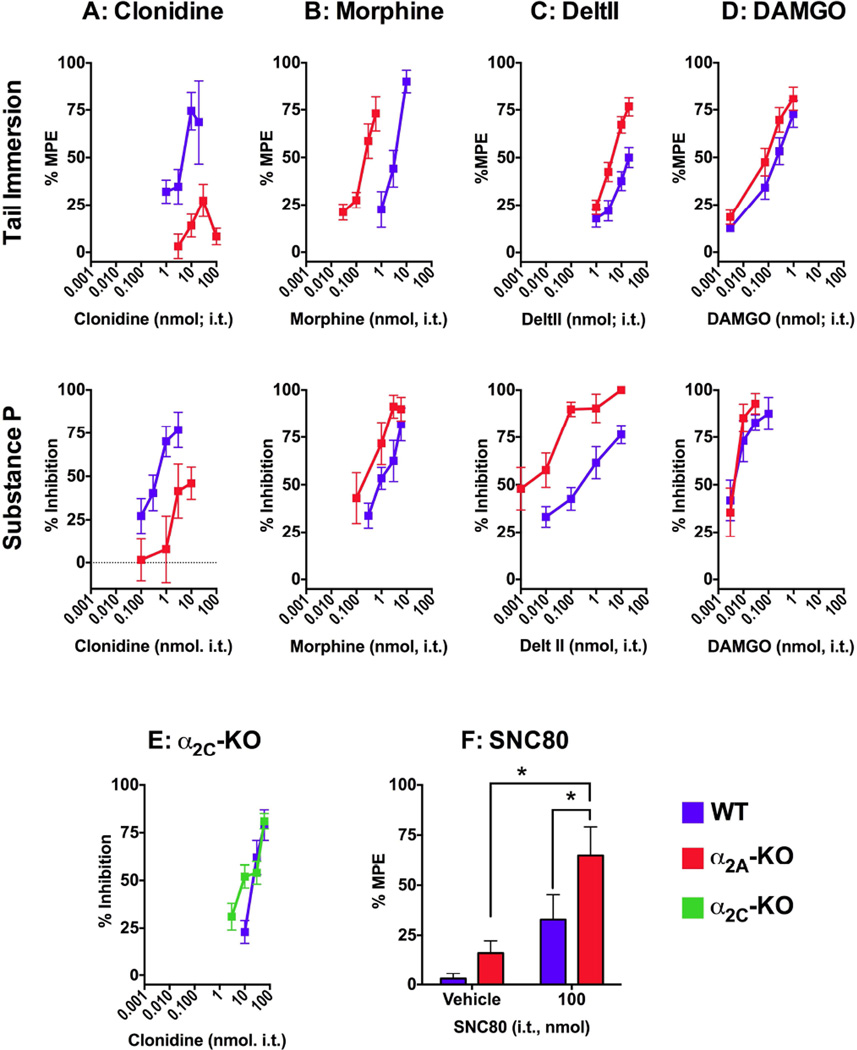
Figure 2. Dose-response analysis of spinal clonidine-, morphine-, DeltII- and DAMGO-mediated antinociception in WT and α2A-KO mice.
Dose-response curves were constructed for each drug administered i.t. in the hot water tail immersion assay (upper row) and the SP behavioral assay (middle row). A) Clonidine was efficacious in the tail immersion assay and the SP behavioral assay in WT mice but clonidine potency and efficacy were reduced in α2A-KO mice in both assays. B) Morphine was more potent in α2A-KO mice than in WT mice in both assays. C) DeltII was more potent in α2A-KO mice than in WT mice in both assays D) DAMGO was more potent in α2A-KO mice in the tail immersion assay but not in the SP behavioral assay. E) Clonidine was equally efficacious and potent in WT and α2C-KO mice. F) 100 nmol of SNC 80 was more effective in α2A-KO mice in the tail immersion assay compared to WT or vehiclecontrols. A–D) Dose-response curves generated with the SP behavioral assay in WT mice and the clonidine dose response curve in α2A-KO mice were published in Chabot-Doré et al. (2013) as part of a study conducted in parallel with this one. DeltII and DAMGO antinociception were measured with a cumulative dosing protocol in the tail immersion ED50 values are reported in Table 2. *p ≤ 0.01.
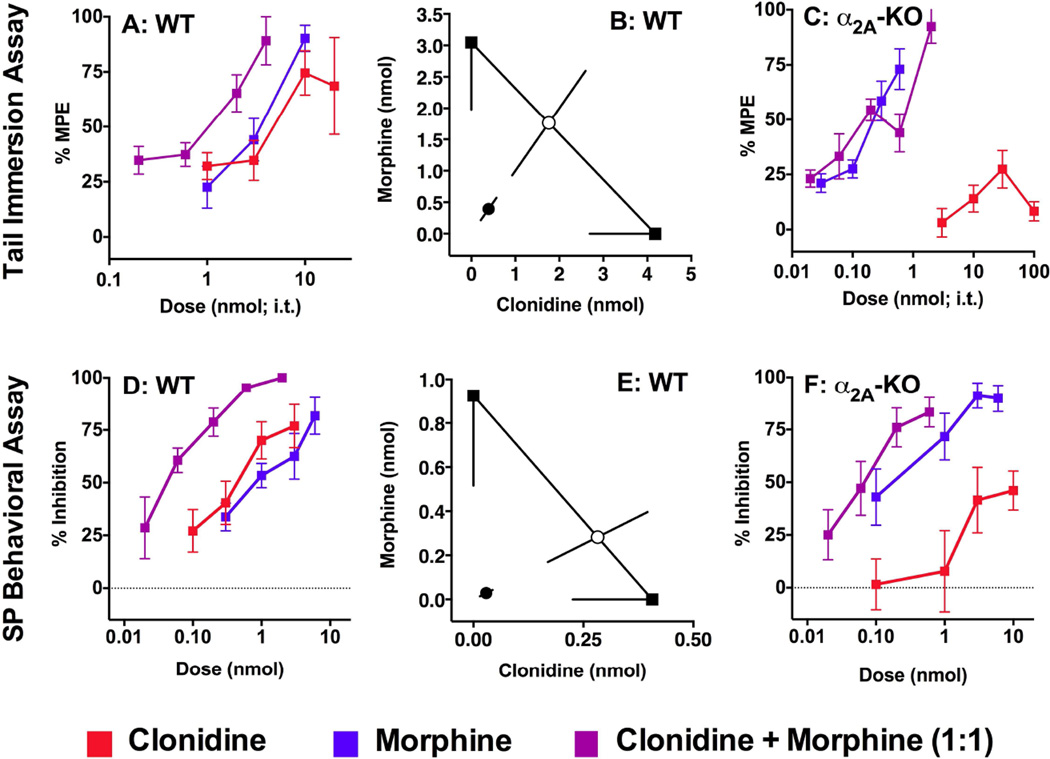
Figure 3. Synergistic interaction between clonidine and morphine in WT but not α2A-KO mice.
Dose-response curves for spinal morphine, clonidine and their combination in WT mice in the hot water tail immersion assay (A) and the SP behavioral assay (D). B, E) Isobolographic analysis of the morphine and clonidine interaction in WT mice: the morphine ED50 value with lower CI is plotted on the y axis, and the clonidine ED50 value with lower CI is on the x axis. The measured experimental ED50 value (●) for the drug combination was lower than the theoretical additive ED50 value (○), indicating that morphine and clonidine interacted in a synergistic manner (p < 0.05). In α2A-KO mice, since clonidine failed to reach 50% efficacy in the hot water tail immersion assay (C) or in the SP behavioral assay (F), isobolographic analysis was not performed. However, when clonidine was added to morphine in a 1:1 ratio, the ED50 values for morphine and clonidine were not significantly different than morphine alone (p < 0.05), showing that adding clonidine to morphine did not change its potency (F). The calculated experimental and theoretical ED50 values for the morphine and clonidine combinations are reported in Table 3. D, E were published in Chabot-Doré et al. (2013) as part of a study conducted in parallel with this one.
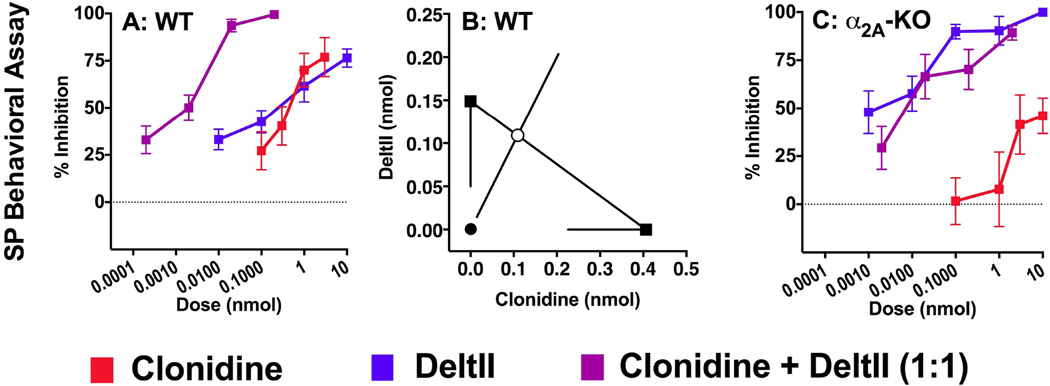
Figure 4. Synergistic interaction between clonidine and DeltII in WT but not α2A-KO mice.
(A) Dose-response curves of spinal DeltII, clonidine and their combination in WT mice in the SP behavioral assay. (B) Isobolographic analysis of the DeltII and clonidine interaction in WT mice: the morphine ED50 value with lower CI is plotted along the y axis, and the clonidine ED50 value with lower CI along the x axis. The measured experimental ED50 value (●) for the drug combination was lower than the theoretical additive ED50 value (○), indicating that DeltII and clonidine interact in a synergistic manner (p < 0.05). (C) In α2A-KO mice, since clonidine reached less than 50% efficacy isobolographic analysis was not performed. However, the doseresponse curves of DeltII and of clonidine+DeltII overlapped and ED50 values were not significantly different (p < 0.05), showing that adding clonidine to DeltII did not change its potency. The calculated experimental and theoretical ED50 values for the DeltII+clonidine combinations are reported in Table 3. A, B were published in Chabot-Doré et al. (2013) as part of a study conducted in parallel with this one.
2.2 Drug preparation
Morphine sulphate was purchased from Medisca Pharmaceutica (St-Laurent, QC, Canada). Clonidine HCl and [d-Ala2, NMe-Phe4, Gly-ol5]-enkephalin (DAMGO) were purchased from R&D Systems (MN, USA) and were dissolved in saline. [d-Ala2]-deltorphin II (DeltII; R&D Systems) was dissolved in acidified saline (0.9% NaCl, 0.05 M acetic acid). For cumulative dose-response curve experiments evaluating DAMGO and DeltII, artificial cerebrospinal fluid (ACSF, Harvard Apparatus, MA, USA) with 0.05 M acetic acid was used. (+)-4-[(αR)-α-((2S,5R)-4-Allyl-2,5-di-methyl-1-piperazinyl)-3-methoxybenzyl]-N,N-diethyl-benzamide (SNC 80; R&D Systems) stock and working solutions were dissolved in saline with 0.3% tartaric acid. Substance P (SP) was purchased from AnaSpec (CA, USA) and concentrated stocks were dissolved in acidified saline. Working SP solutions were dissolved in saline at a total dose of 15 ng/5µl alone or mixed with other drugs. 6-hydroxydopamine (6-OHDA, Sigma) was dissolved in saline with 0.2 mg/ml ascorbic acid and administered i.t. at 20 µg/5 µl. Vehicle solutions consisted of the diluents used for the respective drug tested. Drug doses administered intrathecally are expressed as total nmol or pmol in 5µl and drug combination doses are graphed as total drug dose (i.e. the sum of each drug).
2.3 Drug administration
Intrathecal (i.t.) drug administration was done by direct lumbar puncture in a volume of 5 µl adapted from the method of Hylden and Wilcox (1980) in conscious mice. Briefly, mice were immobilized in a cotton cloth and held by the pelvic girdle while a 30 gauge 1/2 inch needle mounted on a 50 µl Luer lip syringe (Hamilton, Reno, NV) was inserted in the intervertebral space and directed forward inside the vertebral column to deliver 5 µl of drug.
2.4 Behavioral assays
Animals were acclimatized to the testing room in their home cage for 45–60 minutes and to the testing chambers for 60 minutes before testing, if applicable.
2.4.1 Tail immersion assay
Mice were immobilized in a cotton cloth and the bottom 2/3 of the tail was immersed in a 49°C hot water bath. The latency for animals to withdraw their tails from the water was recorded with a hand-held stopwatch and cutoff time was set to 12 seconds to avoid tissue damage. To obtain reliable and stable measures, mice were given at least two training sessions on separate days during which baseline measurements were taken three times. Measurements were taken before (baseline latency) and after drug administration (experimental latency) and expressed as the percent of the maximal possible effect (MPE): % MPE = (Experimental Latency – Baseline Latency × 100) / (12 sec – Baseline Latency).
2.4.2 Radiant heat paw withdrawal assay
Thermal heat threshold was measured by focusing a radiant heat beam (IITC Life Science Inc, Woodland Hills, CA) onto the center of the plantar surface of the hind paw and measuring the latency to withdraw the hind paw (Hargreaves et al., 1988). Three measurements at 60 minute intervals were used to calculate an average. A 17 second cutoff was set to prevent tissue damage.
2.4.3 Mechanical sensitivity
Calibrated von Frey filaments (Stoelting Co., IL, USA) were applied on the plantar surface of the hind paw for 4 seconds or until paw withdrawal, and the 50% threshold to withdraw (grams) was calculated according to the up-down method (Chaplan et al., 1994) and as described by Millecamps et al. (2011).
2.4.4 Substance P behavioral assay
The antinociceptive action of single drugs and their combinations was tested in the SP behavioral assay developed by Hylden and Wilcox (1981). Briefly, 15 ng of SP was administered i.t. alone (control) or co-administered with a single drug or a drug combination (experimental) in a volume of 5µl. The number of caudally directed biting, licking and scratching behaviors were counted for 1 min and results are expressed as the percent inhibition of SP-induced behaviors: % Inhibition = (Control – Experimental) × 100/Control.
Control values represent the average number of SP-induced behaviors elicited by 15 ng of SP collected from 2–3 mice of each strain on the different experimental days (WT: n = 13; α2A-KO: n =16).
2.4.5 Analysis of baseline sensory thresholds
Comparison of raw baseline values (means ± SEM) obtained in behavioral assays between WT and α2A-KO were compared with a two-sided unpaired parametric T-test with a 95% confidence level (GraphPad Software, Inc.).
2.5 In vivo pharmacology
2.5.1 Dose-response curves of morphine, clonidine and their combinations
Mice were treated with single drug doses administered i.t. and measurements were taken with the SP behavioral assay or 10 minutes post injection with the tail immersion assay. Morphine or DeltII were combined with clonidine in 5 µl at an equi-effective 1:1 ratio and are expressed as total drug dose. Each dose represents a group of 4–12 animals.
2.5.2 Cumulative dose-response curves of DAMGO and DeltII
A total of four consecutive injections were performed with increasing doses of the drug tested. For DAMGO, we injected 0.003, 0.07, 0.3 and 1 nmol i.t. and for DeltII, we injected 1, 3, 10 and 20 nmol i.t. Mice were tested 3 min after i.t. injection and re-injected 2 minutes later, allowing for a total of 5 minutes between each injection. Vehicle-treated mice were included to confirm that the repeated injections did not change the tail flick latencies (data not shown). Initial experiments indicated that these peptide agonists rapidly lose their efficacy following i.t. injection with a return to baseline at 30 minutes. The same group of WT (n = 12) and α2A-KO (n = 12) mice was used to test both DAMGO and DeltII by allowing a washout period of one week between experiments.
2.5.3 Morphine antinociception in 6-OHDA-lesioned mice
Pre-lesion baseline tail flick latencies were measured in groups of 7–10 WT or α2A-KO mice before injecting them i.t. with 20 µg of 6-OHDA or vehicle solution. 3 days post-6-OHDA administration, baseline tail flick latencies were measured and full morphine dose-response curves were obtained using a cumulative drug administration protocol with a maximum of five consecutive injections of increasing morphine doses. Morphine doses were injected at 5 minutes intervals and tail flick latencies were measured at 4 minutes post injection. Because of the potency difference between WT and α2A-KO mice, we used a different range of morphine doses to construct the cumulative dose-response curve in each strain: in WT mice, we used 1, 3, 6, 10 and 30 nmol i.t.; in α2A-KO mice, we used 0.1, 0.3, 1, 3 and 6 nmol i.t.
2.5.4 Dose-response and isobolographic analysis
Dose-response curve graphs were generated with GraphPad Prism 6.0. A minimum of 4 animals were used per dose and each dose point is expressed as the mean % MPE or % inhibition ± S.E.M. ED50 values and 95% confidence interval (CI) were calculated using a minimum of three doses in the linear portion of each dose-response curve following the method of Tallarida and Murray (1987) with the FlashCalc 4.5.3 pharmacological statistics software package generously supplied by Dr. Michael Ossipov. Statistical comparisons of potencies are based on the confidence limits of the ED50 values.
Isobolographic analysis is the method of choice for evaluating drug interactions (Tallarida, 2006). As a pre-requisite for this analysis, each drug alone or in combination must be at least 50% efficacious. Drug combination ratios were chosen according to the relative potency of each drug by determining an approximately equally effective potency ratio between the agonists based on their respective ED50 values. To test for interactions between agonists, the ED50 values and S.E.M. of all dose-response curves were arithmetically arranged around the ED50 value using equation (ln(10) × ED50) × (S.E. of log ED50) (Tallarida, 1992). This manipulation is required to perform an isobolographic analysis to evaluate if an interaction is synergistic, additive or sub-additive (Tallarida, 1992). When testing an interaction between two drugs, a theoretical additive ED50 value is calculated for the combination based on the dose-response curves of each drug administered separately. This theoretical value is then compared by t test with the observed experimental ED50 value of the combination. An interaction is considered synergistic if the experimental ED50 is significantly less (P < 0.05) than the calculated theoretical additive ED50.
Visualization of drug interactions can be facilitated by the use of an isobologram, i.e. a graphical representation of isobolographic analysis. The isobologram depicts the ED50 value of each drug as the x-y-intercept. The line connecting these two points depicts the dose combination expected to yield 50% efficacy if the interaction is purely additive and is called the theoretical additive line. The theoretical additive ED50 is determined mathematically and plotted on this line with its CI spanning perpendicularly from the line. The experimental ED50 for the combination is plotted at the corresponding x,y co-ordinates along with its 95% confidence interval for comparison with the theoretical additive ED50 value. All dose-response and isobolographic analyses were performed with FlashCalc 4.5.3.
2.5.4 SNC80 antinociception
WT and α2A-KO mice were tested in the hot water tail flick assay (n=4–7). We measured the effect of 100 nmol of SNC80 or vehicle (i.t.) at 3 minutes post-injection since our pilot experiments determined that SNC80 effect is maximal at 3 minutes and returned to baseline values at 10 minutes (data not shown). Comparison between SNC80 and vehicle treatment in WT and α2A-KO was done with a 2-way ANOVA followed by a Bonferroni multiple comparison T test with GraphPad Prism 6.0.
2.6 Tissue collection
Mice were deeply anesthetized with isofluorane and decapitated. Spinal cords were ejected through the rostral end of the vertebral canal by injecting PBS from the caudal end. DRGs were dissected out under a stereomicroscope. Tissues were frozen on dry ice and stored at −80°C.
2.7 Quantitative gene expression analysis of opioid receptors
Total RNA from spinal cords and DRGs was extracted and DNase digested (RNeasy Lipid Tissue Mini Kit, Quiagen). Conversion of mRNA to cDNA and subsequent quantification of DOPr and MOPr RNA by real time PCR using SYBR-Green was performed at the Genome Québec RNomic Center (Sherbrooke, QC, Canada). Primer sequences for opioid receptor genes (Oprm1, Oprd1) and housekeeping genes (Gapdh, Hptr1, Ubc) are shown in Table 1. Four mice of each strain were used and amplification reactions were performed in triplicate for each tissue sample. Relative expression and quality control evaluation was computed using the qBase method (Hellemans et al., 2007) which used three reference housekeeping genes (Gapdh, Hptr1, Ubc) to normalize expression values for each sample. Results were then normalized to the WT condition, giving it an arbitrary relative expression value of 1. Comparisons of relative gene expression between WT and α2A-KO mice were done in GraphPad Prism 6.0 with a two-sided unpaired Mann-Whitney U test.
Table 1.
Sequence of forward (F) and reverse (R) primers used for quantitative PCR analysis of gene expression in WT and α2A-KO mouse SC and DRG.
| Gene | Primer | Sequences (5’ → 3’) |
|---|---|---|
| Oprm1 | F R |
TGGTCACAGCCATCACCATCATG CATCAGGTAGTTAACACTCTGAAAGGGCA |
| Oprd1 | F R |
GTCCCTCGCCCTAGCCATCG GCCAGATTGAAGATGTAGATGTTGGTGG |
| Gapdh | F R |
TGACGTGCCGCCTGGAGAAA AGTGTAGCCCAAGATGCCCTTCAG |
| Hptr1 | F R |
GCTTGCTGGTGAAAAGGACCTCTCGAAG CCCTGAAGTACTCATTATAGTCAAGGGCAT |
| Ubc | F R |
CGTCGAGCCCAGTGTTACCACCAAGAAGG CCCCCATCACACCCAAGAACAAGCACAAG |
2.8 Western blot analysis
Spinal cords were collected from age-matched WT and α2A-KO mice (n = 3 per strain) and homogenized with an automated tissue homogenizer (Precellys 24, Bertin Technologies) with 1.4 mm ceramic beads (Mo Bio Laboratories) in homogenizing buffer (25 mM Tris HCl pH 7.4, 1 mM EDTA, 2 mM MgCl2) supplemented with proteinase inhibitor cocktail (Roche). The homogenate was centrifuged 4 min at 1000g and the supernatant containing the total protein fraction was denatured by boiling in sample buffer (TrisHCl 50 mM, 2% sodium dodecyl sulfate (SDS), 10% glycerol, 2.5% β-mercaptoethanol, bromophenol blue, pH 6.8). 40 µg of each sample was run in a 10% acrylamide SDS-PAGE and proteins were transferred onto a nitrocellulose membrane. Immunoblotting of the membranes was performed with 5% non-fat dry milk in TBST (0.1M Tris-HCl, 0.9% NaCl, 0.1% Tween 20, pH 7.4) as a blocking and antibody diluent solution. MOPr-immunoreactive (ir) bands were detected with a rabbit polyclonal antisera raised against amino acids 384–398 of the predicted MOR1 sequence (NHQLENLEAETAPLP; (Arvidsson et al., 1995)) diluted 1:2500 followed by an HRP-coupled mouse-anti-rabbit IgG (1:10 000; Jackson ImmunoResearch). Membranes were stripped and reprobed to detect glyceraldehyde-3-phosphate dehydrogenase (GAPDH)-ir band with a mouse monoclonal antisera (1:10 000; MAB374, Millipore) and an HRP-coupled goat-anti-mouse IgG (1:10 000; Jackson ImmunoResearch). Immunoreactivity was revealed by enhanced chemiluminescence and visualized by exposing membranes to a light sensitive film. Densitometry was performed using ImageJ64 analysis software (NIH) where the 67 kDa MOPr band from each mouse sample was normalized to GAPDH levels. Comparison of protein expression levels was done by comparing average normalized densitometry values between WT and α2A-KO mice in GraphPad Prism 6.0 with a two-sided unpaired Mann-Whitney U test.
2.9 [3H]-DeltII saturation and competition binding assay
WT and α2A-KO mouse spinal cords were homogenized with a handheld rotor stator homogenizer (TissueRuptor, Quiagen) in cold homogenization buffer (50 mM TrisHCl, 2.5 mM EDTA, pH 7.0) supplemented with protease inhibitors (Roche). The homogenate was centrifuged 10 minutes at 1000 g at 4°C and the supernatant was centrifuged at 48 000 g for 20 minutes at 4°C. The pellet was resuspended in binding buffer (50 mM TrisHCl, 3 mM MgCl2, pH 7.4 with protease inhibitor) and incubated at 37°C for 15 min to dissociate receptor-bound endogenous opioid peptides and degrade monoamines. The membranes were re-centrifuged at 48 000 g and resuspended in binding buffer.
Binding experiments were performed with the DOP-selective radioligand deltorphinII (d-Ala2), [Tyrosyl-3,5-3H] ([3H]-DeltII, PerkinElmer, MA, USA). In saturation binding experiments, 200 µg of spinal cord membrane protein from WT or α2A-KO mice was combined with five concentrations of [3H]-DeltII (0.3–10 nM) in a final sample volume of 300 µl. Nonspecific binding was determined by the addition of the nonselective opioid receptor antagonist naloxone (10 µM, R&D systems). In competition binding experiments, 200 µg of spinal cord membrane protein was combined with 1 nM of 3H-DeltII and the DOR-selective antagonist naltrindole (R&D Systems) at concentrations ranging from 1 µM to 100 fM. Samples were prepared in triplicate and incubated for 60 min in a 37°C water bath. Spinal cord membranes were collected by filtration on a grade GF/C glass microfiber filter (Whatman) that was previously soaked overnight in binding buffer with 0.5% polyethyleneimine and washed three times with ice-cold washing buffer (25 mM TrisHCl, pH 7.4). Filters were transferred into 5 ml polypropylene tube and filled with 3 ml of Ecolume liquid scintillation cocktail (MP Biomedicals, OH, USA). Tubes were quantified with a Wallace Winspectral 1414 liquid scintillation counter for saturation binding experiments and a Beckman Coulter LS 6500 for competition binding experiments. All ligand binding analyses were done in GraphPad Prism 6.0. For saturation binding, total [3H]-DeltII binding and non-specific binding data were fitted using a global nonlinear regression model and non-specific binding was subtracted from total binding to obtain a specific binding curve. Bmax and Kd with 95% CI values were obtained by nonlinear regression of specific binding. Competition binding data were fitted to a one-site non-linear regression model to determine naltrindole Ki and IC50 values with 95% CI. Strain differences were evaluated with an extra sum-of-squares F test to compare the best fit values of Bmax, Kd and Ki.
2.10 HPLC analysis of catecholamines
Spinal cords from WT and α2A-KO mice treated with 6-OHDA or vehicle solution were collected and processed for HPLC analysis (n = 3 per experimental group). Spinal cord samples were weighed and then sonicated in 150 µl of solution containing six parts of 0.2 M perchloric acid and one part of antioxidant solution (Kankaanpaa et al., 2001). The homogenates were centrifuged at 17,000 g for 35 min at 4 °C, and the supernatant was filtered using Millex-HV syringe filters (PVDF-membrane, 0.45 µm pores; Millipore). The concentrations of norepinephrine (NE), dopamine (DA) and epinephrine were determined with a UHPLC (Dionex UltiMate3000 RS, Thermo Scientific) combined with a coulometric array detector (Coularray 5600A, Thermo Scientific). Mobile phase consisted of 10 % acetonitrile in a 0.1 M phosphate solution (pH 3.0), supplemented with 1 mM EDTA and 1.5 mM 1-octanesulfonic acid, and the analytes were separated in a column (Acclaim™ 120, C18, 3 um, 2.1 × 100 mm, Thermo Scientific) that was kept at 30 °C. Eight coulometric cells of the detector were set to 50–450 mV. The data was analyzed with the CoulArray software using peak area as a measure for the amount of analyte. Each value was normalized against the weight of the sample to get final values as ng/mg tissue. Comparison between vehicle and 6-OHDA treatment in WT and α2A-KO spinal cords was done with a 2-way ANOVA followed by a Tukey multiple comparison T test with GraphPad Prism 6.0.
3. Results
3.1 α2A-KO mice are hypersensitive to noxious heat
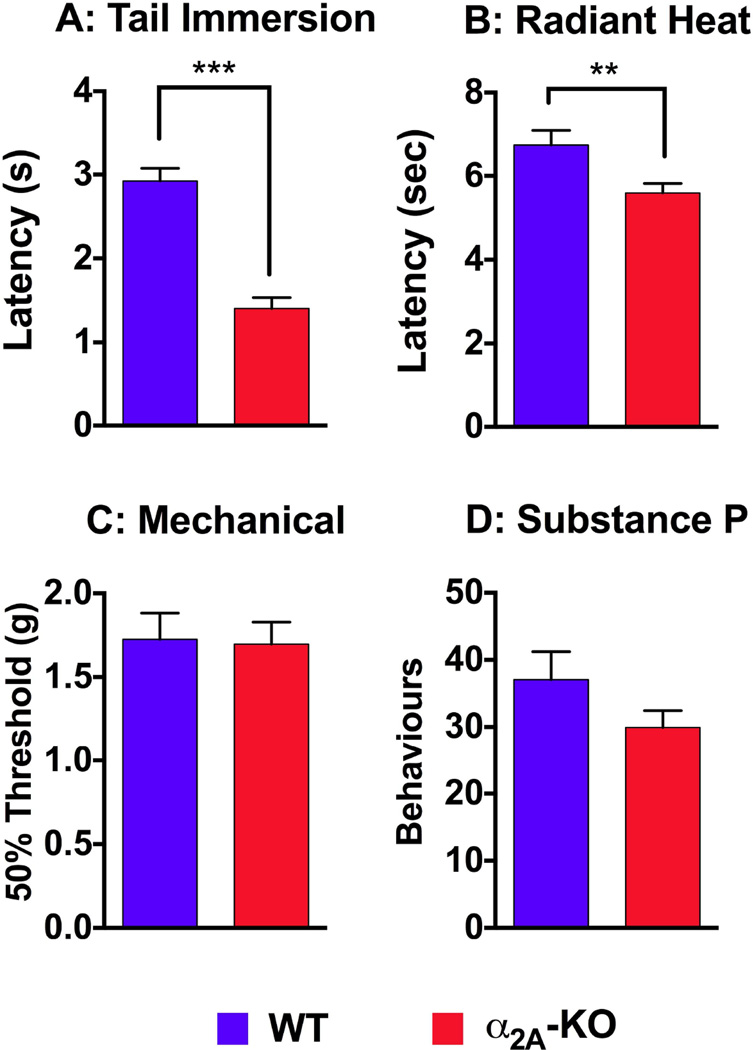
The baseline sensitivity of α2A-KO mice compared to WT mice to assess the role of the α2A-AR in normal thermal and mechanical nociception. Compared to WT mice, α2A-KO mice were more sensitive to heat stimuli in the tail immersion assay (Figure 1A; WT: 2.9 ± 0.2 sec. (n=22); α2AKO: 1.4 ± 0.1 sec. (n=16); p<0.0001). Similarly, α2A-KO mice were more sensitive than WT mice to heat from a radiant light beam focused on the hind paw (Figure 1B; WT: 6.8 ± 0.4 sec. (n=15); α2A-KO: 5.6 ± 0.2 sec. (n=23); p = 0.007). There were no differences between WT and α2A-KO mice in 50% mechanical withdrawal thresholds (Figure 1C; WT: 1.8 ± 0.2 g (n=17); α2A-KO: 1.7 ± 0.1 g (n=16)) or in the number of nocifensive behaviors induced by intrathecally administered SP (Figure 1D; WT: 37 ± 4 counts (n=13); α2A-KO: 30 ± 3 counts (n=16)). Together, these data indicate that α2A-KO mice are more sensitive to heat stimuli than WT mice, but that mechanical sensitivity and responsively to intrathecal SP were not affected.
Figure 1. Comparison of thermal, mechanical and SP-induced nociceptive thresholds between WT and α 2A-KO mice.
Tail flick latencies measured in the hot water (49°C) tail immersion assay (A) and paw withdrawal latencies measured with the radiant heat assay (B) were more rapid in α2A-KO mice. C) 50% mechanical threshold measured with von Frey filaments were similar on the hind paws of WT and α2A-KO mice. D) The number of nocifensive behaviors induced by administration of 15 ng of SP i.t. were not significantly different between WT and α2A-KO mice. Two-tailed unpaired t-test, ** p < 0.01, *** p < 0.0001.
3.2 Spinal adrenergic and opioid antinociception in α2A-KO and WT mice
The role of α2A-AR in spinal antinociception mediated by clonidine, morphine, DeltII and DAMGO were evaluated in the hot water tail immersion assay (Figure 2, top row) and in the SP behavioral assay (Figure 2, second row) by comparing the ED50 values for each drug in WT and α2A-KO mice (Table 2). All drugs dose-dependently increased tail flick latencies and reduced SP-induced behaviors in WT and α2A-KO mice (Figure 2) with the exception of clonidine, which had reduced potency and efficacy in the α2A-KO mice (Figure 2A). In contrast, clonidine potency and efficacy in the SP assay was not different in α2C-KO mice compared to WT controls (Figure 2E). These data implicate the α2A-AR in clonidine antinociception, although we cannot exclude a role for the α2B-AR subtype.
Table 2.
Comparison of ED50 values (nmol (95% CI)) for spinal morphine, DeltII and DAMGO in WT and α2A-KO mice in the tail immersion and SP behavioral assays.
| Assay | Strain | Clonidine | Morphine | DeltII | DAMGO |
|---|---|---|---|---|---|
| Tail immersion assay | WT | 4.2 (2.0–4.7) |
3.1 (2.0–4.7) |
21.2 (11.4–39.2) |
0.17 (0.09–0.32) |
| α2A-KO | N/A§ | 0.26 (0.18–0.38) |
4.3 (3.4–5.5) |
0.06 (0.03–0.10) |
|
| Potency ratio | N/A | 12:1* | 5:1* | 3:1* | |
| SP behavioral assay | WT# | 0.4 (0.2–0.7) |
0.93 (0.52–1.66) |
0.15 (0.05–0.44) |
0.0034 (0.0017–0.007) |
| α2A-KO | 7.0§ (2.9–16.6) |
0.12 (0.03–0.46) |
0.0021 (0.0006–0.008) |
0.0041 (0.0026–0.0065) |
|
| Potency ratio | 1:18* | 8:1* | 70:1* | 1:1 | |
| α2C-WT | 21.4 (9–51) |
||||
| α2C-WT | 12.5 (4.5–35) |
ED50 significantly different, WT vs. α2A-KO, t-test (p < 0.05)
Efficacy < 50%.
Values reported in Chabot-Doré et al. (2013)
Morphine had similar efficacy, but was more potent in α2A-KO mice than in WT mice in both assays (Figure 2B). The potency of the DOPr-selective peptide agonist DeltII was also increased in α2A-KO mice compared to WT mice (Figure 2C). The limited solubility of DeltII prevented us from testing it at higher doses to assess maximal efficacy. We also tested the antinociceptive response to the non-peptide DOPr-selective agonist SNC80 (Figure 2F). A high dose of SNC80 administered i.t. (100 nmol) elicited antinociception in both strains compared to vehicle but with greater efficacy in α2A-KO compared to WT mice (2 way ANOVA; strain: F(1, 37) = 4.48, p = 0.04, dose: F(1, 37) = 13.6, p = 0.0007, interaction: F(1, 37) = 0.78, p = 0.38). The antinociceptive response of the MOPr-selective peptide agonist DAMGO was more potent in α2A-KO mice than in WT mice in the tail flick assay, but no difference in potency was observed in the SP behavioral assay (Figure 2D). Overall, these experiments indicate that in the absence of the α2AAR, opioid spinal antinociception is potentiated.
3.3 Morphine-clonidine spinal antinociceptive synergy requires α2A-AR
We tested if the synergistic interaction between the MOPr-preferring morphine and clonidine requires the α2A-AR by evaluating this combination in WT and α2A-KO mice in the hot water tail immersion and SP behavioral assays. In WT mice, the calculated ED50 values for spinal morphine and clonidine were within one order of magnitude in both assays; we therefore combined them at a 1:1 ratio to test their interaction (Figure 3A, D). The potency of the combination was increased compared to each drug administered alone in the tail immersion assay (Figure 3A) and the SP behavioral assay (Figure 3D). Isobolographic analysis demonstrated that morphine combined with clonidine interacted synergistically in both assays (Figure 3B, E, Table 3). The interaction between morphine and clonidine was also assessed α2AKO mice. Since a minimum of 50% efficacy is required for isobolographic analysis and clonidine did not reach this threshold in either assay, isobolographic analysis was not performed. As an alternative, we combined morphine and clonidine in the α2A-KO mice in the same ratio (1:1) used in WT mice to test whether the addition of clonidine would alter the morphine doseresponse. The ED50 values for the morphine + clonidine combinations were not significantly different from morphine alone in either assay (Figures 3C, F, Table 3), suggesting that the α2AAR is required for the synergistic interaction between morphine and clonidine.
Table 3.
Experimental and Theoretical ED50 values (nmol (± 95% SEM)) for drug combinations in WT and α2A-KO mice.
| Drug | Assay | WT # Experimental |
Theoretical | Interaction | α2A-KO Experimental |
Theoretical | Interaction |
|---|---|---|---|---|---|---|---|
| Morphine + Clonidine | TI | 0.79 (±0.35) |
3.52 (±1.67) |
Synergistic* | 0.29 (±0.13) |
N/A | No interaction§ |
| SP | 0.058 (±0.029) |
0.56 (±0.27) |
Synergistic* | 0.071 (±0.044) |
N/A | No interaction§ |
|
| DeltII + Clonidine | SP | 0.0010 (±0.0005) |
0.22 (±0.19) |
Synergistic* | 0.009 (±0.015) |
N/A | No interaction§ |
Indicates that the experimental ED50 value < theoretical ED50 value (t-test, p < 0.05)
Since clonidine did not reach 50% efficacy in α2A-KO mice, isobolographic analysis was not performed (N/A). We therefore compared the ED50 value for the drug combinations to the ED50 values of DeltII or morphine alone (t test, p > 0.05).
SP behavioral assay values reported in Chabot-Doré et al. (2013).
3.4 Deltorphin II-clonidine spinal antinociceptive synergy requires α2A-AR
To evaluate the role of the α2A-AR in interactions with DOPr agonists, the DOPr agonist DeltII was tested in combination with clonidine in WT and α2A-KO mice. In WT mice, DeltII and clonidine inhibited SP-induced behaviors with similar potency. We therefore co-administered these agonists at a 1:1 mixture reflecting their equi-effective ratio. The potency of the combination was increased compared to either drug administered alone (Figure 4A). Isobolographic analysis revealed that the experimental ED50 value of the drug combination was significantly lower than the theoretical additive ED50 value; the drug interaction is therefore synergistic (Figure 4B, Table 3). In α2A-KO mice, as clonidine did not inhibit more than 50% of SP-induced behaviors, isobolographic analysis was not performed. However, using the same 1:1 ratio to combine DeltII and clonidine as in WT mice, we tested if the addition of clonidine to DeltII modulated its potency in α2A-KO mice. Clonidine did not shift the DeltII dose-response curve, suggesting that there is no interaction between DeltII and clonidine in α2A-KO mice (Figure 4C, Table 3).
3.5 MOPr expression level is unchanged in α2A-KO mice
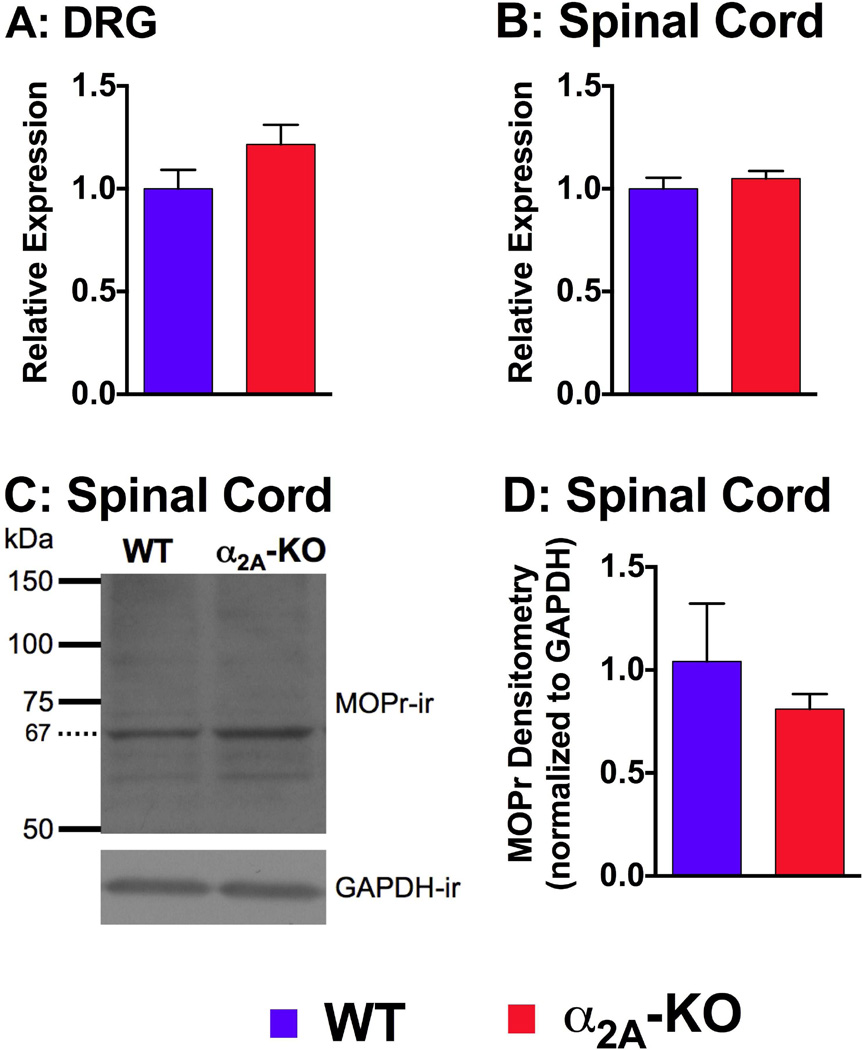
MOPr is the primary receptor mediating morphine antinociceptive effects and an increase in its expression could render morphine more potent. MOPr mRNA and protein expression in WT and α2A-KO were therefore examined. Quantitative PCR analysis of the cDNA corresponding to MOPr mRNA was compared in DRG and spinal cord extracts from WT and α2A-KO mice. No significant differences in MOPr expression between WT and α2A-KO mice were observed in either DRG (WT = 1.0 ± 0.1, α2A-KO = 1.2 ± 0.1, p = 0.15; Figure 5A) or spinal cord (1.0 ± 0.05, α2A-KO = 1.1 ± 0.04, p = 0.47; Figure 5B). We further analyzed the expression of MOPr by western blot analysis of purified membrane extracts from WT and α2A-KO spinal cords (Figure 5C). The relative MOPr density, normalized to GAPDH, was not different between WT and α2A-KO mice (nonparametric t test, p = 0.7; Figure 5D). We conclude that neither MOPr mRNA nor protein expression were altered in α2A-KO. Thus changes in MOPr expression do not explain the increased potency of morphine in α2A-KO mice compared to WT mice.
Figure 5. Analysis of MOPr mRNA and protein expression in dorsal root ganglia (DRG) and spinal cords (SC) from WT and α2A-KO mice.
Quantitative PCR analysis of the Oprm1 gene transcript from WT and α2A-KO DRG (A) and SC (B) showed no significant strain differences in MOPr mRNA levels. Relative expression was obtained by normalizing expression values to three internal housekeeping reference genes (Gapdh, Hptr1, Ubc) and then normalized with the WT value of that tissue as a reference. C) Representative western blot showing the 67 kDa MOPr-immunoreactive (ir) bands that were used to quantify MOPr levels in the spinal cord of WT and α2A-KO mice. D) Densitometry analysis of western blot MOPr-immunoreactive bands relative to GAPDH showed no strain difference between WT and α2A-KO.
3.6 DOPr expression level is unchanged and DeltII binding affinity is unaffected in α2A-KO mice
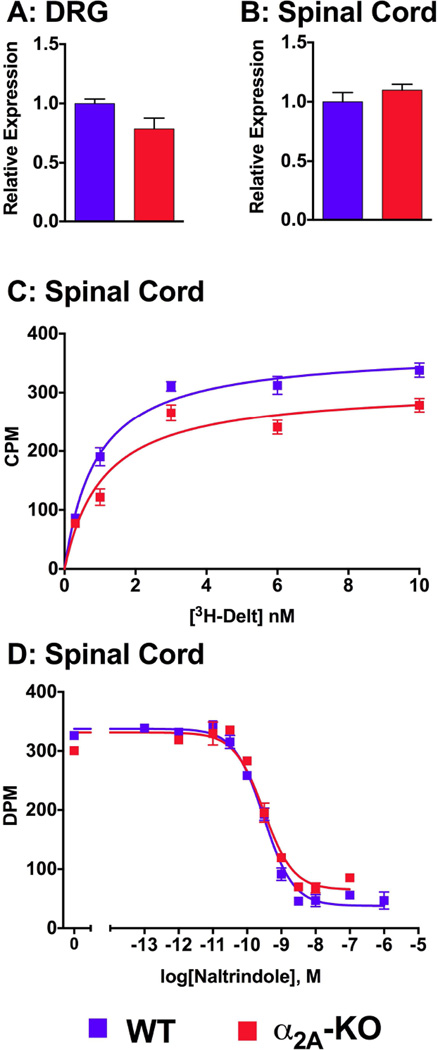
As the antinociceptive responses to DeltII and SNC80 were enhanced in α2A-KO mice, we tested the hypothesis that DOPr expression levels were upregulated in these mice. We analyzed DOPr mRNA expression in spinal cord and DRG levels in WT and α2A-KO mice by quantative PCR. No significant differences were found in the relative expression of DOPr receptors in DRGs between WT (1.00 ± 0.04) and α2A-KO mice (0.79 ± 0.09; p = 0.07; Figure 6A). Similarly, relative DOPr expression in spinal cord tissues from WT mice (1.00 ± 0.08) was not different from that of α2A-KO mice (1.10 ± 0.05; P = 0.33; Figure 6B).
Figure 6. Analysis of DOPr mRNA and [3H]-DeltII binding in WT and α2A-KO mice.
Quantitative PCR analysis of the Oprd1 gene transcript from WT and α2A-KO DRG (A) and spinal cord (B) mRNA extracts showed no strain differences in DOPr mRNA expression. Relative expression was obtained by normalizing expression values to three internal house keeping reference genes (Gapdh, Hptr1, Ubc) and then normalized with the WT value of that tissue as a reference. C) Saturation ligand binding performed on spinal cord membrane protein extracts from WT and α2A-KO mice showing specific binding obtained by subtracting nonspecific from total [3H]-DeltII binding. The binding was concentration-dependent and no significant strain difference was detected. D) Competition of 1 nM [3H]-DeltII binding to spinal cord membranes by naltrindole was similar in WT and α2A-KO mice. CPM = counts per minute. DPM = disintregrations per minute.
Saturation binding assays were conducted to measure the number of binding sites and the affinity of [3H]-DeltII in SC membrane preparations from WT and α2A-KO mice. This method was selected as an alternative to western blot analysis since the selectivity of DOPr antibodies is controversial (Scherrer et al., 2009; Wang et al., 2010). [3H]-DeltII bound to membrane preparations in a concentration-dependent manner (Figure 6C) and the total number of specific binding sites was similar in both strains (WT: Bmax = 373 (319–426) CPM, α2A-KO: Bmax = 309 (201–417) CPM; nonlinear regression, p = 0.14) and apparent dissociation constant (WT: KD = 0.9 (0.4–1.4) nM, α2A-KO: KD = 1.1 (−0.4–2.6) nM; nonlinear regression, p = 0.68) were not significantly different. We further evaluated [3H]-DeltII affinity using competition binding assays. The affinity of naltrindole, a DOP-selective antagonist, determined by competition with [3H]-DeltII in WT and α2A-KO was similar in both strains (p = 0.29; Figure 6D). Naltrindole competitively inhibited [3H]-DeltII binding with a KI value of 0.17 (0.12–0.25) nM in WT membrane preparations and a KI value of 0.14 (0.11–0.18) nM in α2A-KO membrane preparations. Thus, the absence of the α2A-AR did not alter DeltII affinity or DOPr expression levels in a manner that would explain the increased DOPr-mediated antinociceptive potency or efficacy observed in α2A-AR mice.
3.7 Endogenous norepinephrine is not necessary for the potentiation of morphine antinociception in α2A-KO mice
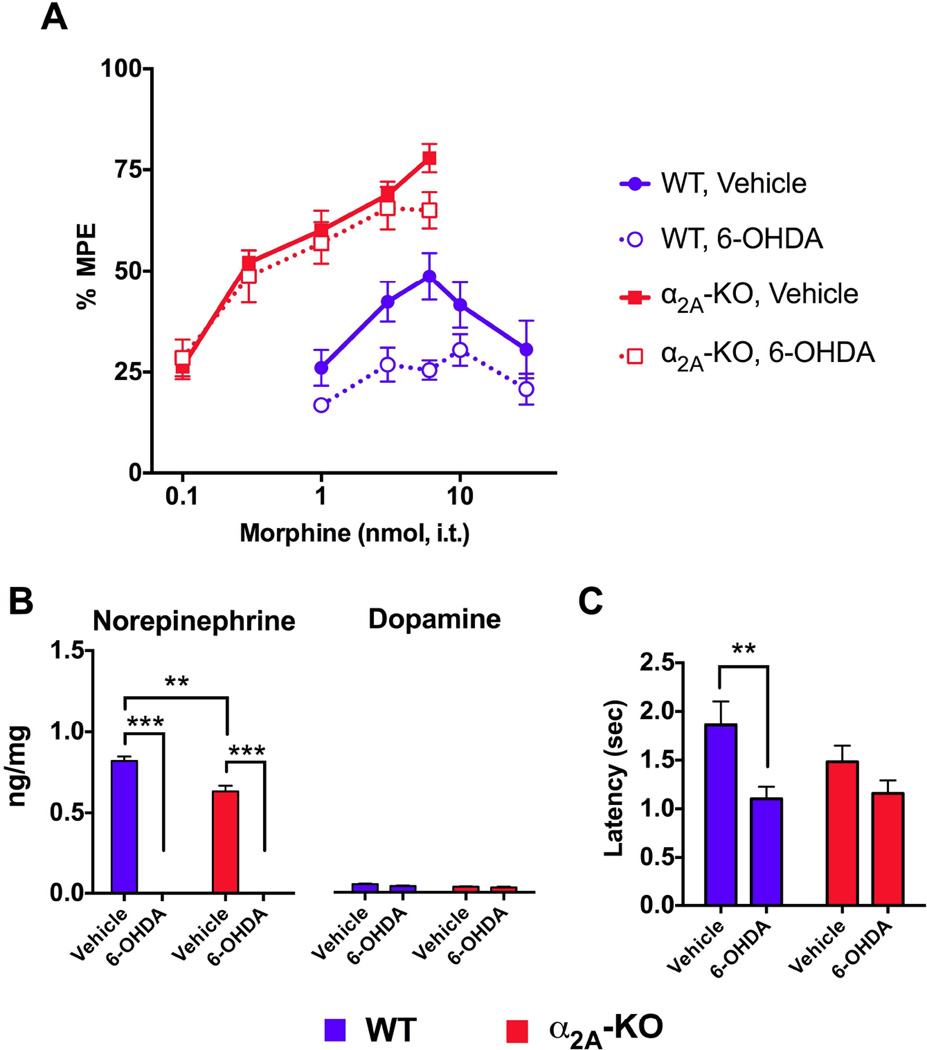
Disruption in α2A-AR function can result in elevated NE levels in the central nervous system (Lakhlani et al., 1997). This is due to the loss of negative feedback by presynaptic α2A-AR autoreceptors, which normally attenuate NE release. Since co-administration of morphine with NE at the spinal cord results in analgesic synergy (Roerig et al., 1992), we hypothesized that elevated NE levels, due to the loss of feedback inhibition in α2A-KO, could contribute to the increased spinal morphine efficacy observed in these mice. To test this hypothesis, the toxin 6-OHDA was injected intrathecally to eliminate spinal catecholaminergic fibers that release NE in the spinal cord (Fasmer et al., 1986). In WT mice, morphine efficacy was reduced in mice treated with 6-OHDA compared to mice treated with vehicle, confirming the role of endogenous NE in the antinociceptive effect of morphine (Figure 7A). In α2A-KO mice, increased morphine potency was observed as before (WT vs. α2A-KO; vehicle-treated) and 6-OHDA treatment had no effect on morphine potency or efficacy in these animals (Figure 7A, Table 5).
Figure 7. Effect of selective elimination of spinal catecholaminergic nerve terminals on morphine antinociception and heat nociception.
WT and α2A-KO mice were injected i.t. with either 6-OHDA or vehicle to eliminate catecholaminergic nerve fibers containing NE. A) Morphine dose-response curves were constructed using a cumulative dosage protocol. 6-OHDA reduced morphine response in WT mice, but not in α2A-KO mice. ED50 values are reported in Table 5. B) HPLC analysis of spinal content in norepinephrine (NE) and dopamine (DA) in ng/mg of spinal cord tissue. Vehicle-treated α2A-KO mice have lower NE and DA levels than WT mice. 6-OHDA treatment completely eliminated NE in both strains. C) Tail flick latency values were lower in 6-OHDAtreated WT mice compared to vehicle-treated mice. The 6-OHDA treatment had no effect on baseline latencies of α2A-KO mice. *** p < 0.001 ** p < 0.01.
Table 5.
ED50 values for morphine in WT and α2A-KO mice treated with 6-OHDA (i.t.).
| Strain | Treatment | ED50 (95% CI) |
|---|---|---|
| WT | Vehicle | 6.2 nmol (3.9 – 9.9) |
| 6-OHDA | No efficacy | |
| α2A-KO | Vehicle | 0.48 nmol (0.36 – 0.64) |
| 6-OHDA | 0.55 nmol (0.33 – 0.91) |
The elimination of catecholaminergic fibers may also affect levels of neurotransmitters other than NE. We therefore performed HPLC analysis to evaluate the effect of 6-OHDA treatment on levels of NE, epinephrine and dopamine (DA) in spinal cords of WT and α2A-KO mice (Figure 7B). Levels of NE recovered from spinal cord supernatant were lower in vehicle-treated α2A-KO mice compared to vehicle-treated WT mice. However, in both strains, NE was undetectable in 6-OHDA-treated mice (2 way ANOVA; strain: F(1, 8) = 18.0, p = 0.003, treatment: F(1, 8) = 1083, p = 0.0001, interaction: F(1, 8) = 18.0, p = 0.003). The amount of DA in the spinal cord was much lower compared to NE in both strains and α2A-KO mice and 6-OHDA treatment did not significantly alter DA levels in either mouse strain (2 way ANOVA; strain: F(1, 8) = 10, p = 0.01, treatment: F(1, 8) = 5, p = 0.06, interaction: F(1, 8) = 1.2, p = 0.3). Epinephrine was undetectable in the spinal cord of all experimental groups. Thus, α2A-KO mice have lower catecholamine levels in their spinal cord, but 6-OHDA treatment selectively eliminated NE, which was in both cases, the most abundant catecholamine neurotransmitter.
Together, these results show that increased morphine potency was maintained in α2A-KO mice despite the selective lesion of noradrenergic fibers, suggesting that the loss of negative feedback inhibition of NE in the α2A-KO mice is not responsible for the effects on morphine antinociception.
3.8 Descending noradrenergic inhibition of noxious heat sensitivity is mediated through the α2A-AR
Descending noradrenergic fibers modulate endogenous inhibition of nociceptive stimuli at the spinal cord level by activating α2-ARs (Gilsbach et al., 2009). We hypothesized that the increased sensitivity to heat observed in α2A-KO mice (Figure 1) was due to a loss of sensitivity to descending endogenous NE inhibition in these mice. To test this, tail flick latencies from WT and α2A-KO mice in which descending NE fibers were eliminated were compared (Figure 7B). 6-OHDA-treated WT mice were more sensitivity to heat compared to vehicle-treated mice (WT vehicle = 1.9 ± 0.2 sec., WT 6-OHDA = 1.1 ± 0.1 sec., p = 0.01), confirming the role of descending noradrenergic fibers in heat nociception. In α2A-KO mice, no such difference was observed in tail flick latencies between 6-OHDA-and vehicle-treated mice (α2A-KO vehicle = 1.5 ± 0.2, α2A-KO 6-OHDA = 1.2 ± 0.1, p = 0.14), suggesting that the α2A-AR subtype mediates endogenous NE modulation of heat nociception.
4. Discussion
4.1 The α2A-adrenoceptor modulates heat nociception
The hypersensitivity to heat observed in α2A-KO mice compared to WT mice in the tail flick and radiant heat assays suggests that heat nociception is endogenously modulated by the α2A-AR under normal conditions. These data also demonstrate that the hypersensitivity is not site-specific, since α2A-KO mice were more sensitive to heat applied on either the tail or the plantar surface of the hind paw. Changes in thermal thresholds were not observed in previous studies using α2A-D79N mice where a point mutation rendered the α2A-AR dysfunctional and decreased its expression levels (Lakhlani et al., 1997; MacMillan et al., 1996; Malmberg et al., 2001; Surprenant et al., 1992). Thus endogenous inhibition of sensitivity to noxious heat could require the physical presence but not necessarily the normal signaling properties of the α2A-AR. Differences in heat nociception were also not previously reported using α2A-KO mice in the radiant heat assay (Lähdesmäki et al., 2003). It is possible that heat hyperalgesia in α2A-KO mice depends on the experimental paradigm which could differentially activate C and Aδ fibers (Yeomans and Proudfit, 1996). Consistent with previous reports, a strain difference in tactile threshold was not observed (Lähdesmäki et al., 2003; Malmberg et al., 2001). These results suggest a modality-specific role for the α2A-AR in the endogenous modulation of nociception. Descending noradrenergic pathways contribute to endogenous inhibition of pain through activation of α2-ARs. α2-AR antagonists decrease tail flick and hot plate latencies in rats (Sagen and Proudfit, 1984), suggesting that adrenergic receptors help set the threshold for thermal nociception. A decreased sensitivity to heat but not to mechanical stimuli was reported in rats and mice following the lesioning of catecholaminergic fibers with 6-OHDA (Fasmer et al., 1986; Kuraishi et al., 1983). Furthermore, Jasmin et al. (2002) observed a similar thermal, but not mechanical, hypersensitivity in dopamine β-hydroxylase-KO mice, which lack the enzyme that converts dopamine to NE. Similarly, increased sensitivity to heat was noted in mice with elevated extracellular NE levels resulting from a genetic deletion of the NE transporter (Bohn et al., 2000). These studies all suggest that endogenous NE modulates heat nociception. Here, we confirm the increase in heat sensitivity in WT mice following an i.t. 6-OHDA treatment and show that similar changes are not matched in 6-OHDA-treated vs. vehicle-treated α2A-KO mice. Thus, the α2A-AR is involved in the inhibition of noxious heat via descending noradrenergic fibers in the spinal cord in naïve animals.
4.2 Unifying model: The α2A-adrenoceptor may allosterically modulate spinal opioid antinociception
In this study, a set of seemingly contradictory observations regarding regulation of opioid antinociception by the α2A-AR are presented. These observations are summarized in Figure 8A using morphine as an example, although similar observations were made with DeltII. First, morphine and DeltII potency were enhanced in the absence of the α2A-AR. Second, we report that morphine and DeltII synergy with clonidine is mediated by the α2A-AR, illustrating that opioid antinociception is enhanced when the α2A-AR is activated by clonidine. Third, morphine antinociception was diminished following the depletion of spinal NE in WT, but not in α2A-KO mice.
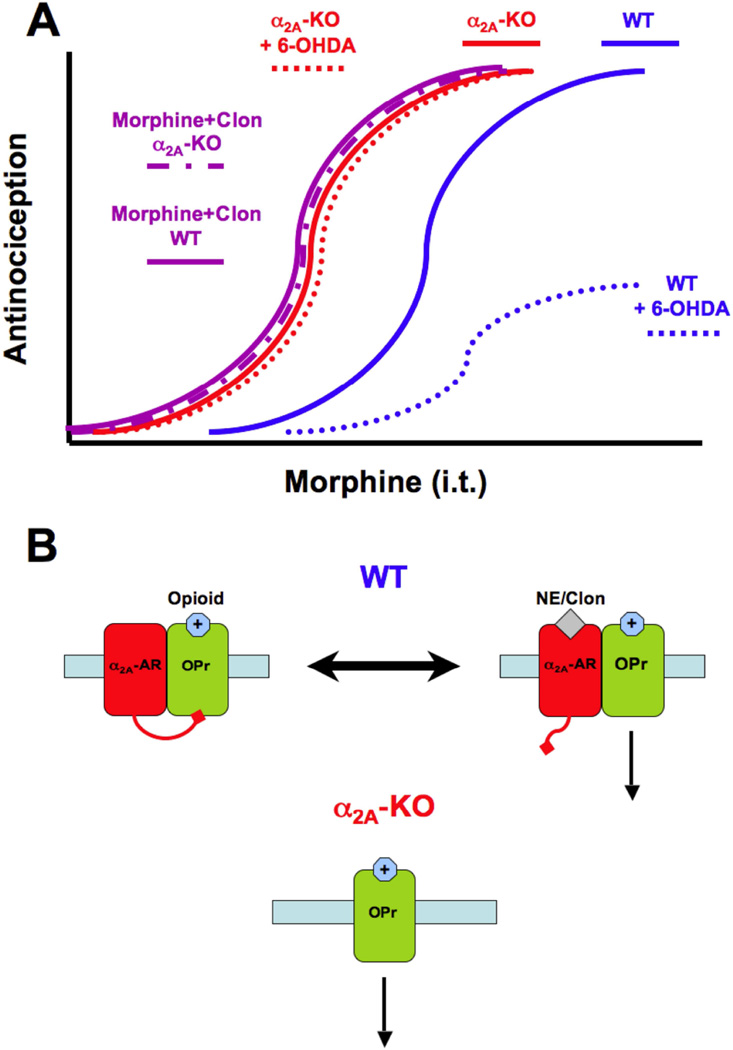
Figure 8. Proposed model of allosteric regulation of opioid receptors by the α 2A-adrenoceptor.
A) Schematic showing dose-response curves obtained for spinal morphine under different experimental conditions. In WT mice, morphine produces a dose-dependent antinociceptive effect (blue solid line), which is reduced in mice treated with 6-OHDA (blue dashed line) and potentiated by the addition of clonidine (purple solid line). In α2A-KO mice, morphine is more potent than in WT mice (red solid line) and neither the 6-OHDA treatment (red dashed line) nor the addition of clonidine (purple dashed line) shifted the morphine dose-response curve. B) In the proposed model, in WT mice, opioid receptors (OPr, green) are inhibited by the α2A-AR (red) in its ligand-free inactive state, which results in decreased opioid receptor antinociceptive output. When an agonist like clonidine (Clon) or norepinephrine (NE) activates α2A-AR, this inhibitory action on opioid receptors is removed, resulting in the synergism between the two agonistoccupied receptors. Under normal conditions, tonic NE release from descending noradrenergic fibers maintains an equilibrium state between ligand-free and agonist-occupied α2A-AR. When NE is depleted, the equilibrium is shifted towards the inactive and inhibitory α2A-AR, and when exogenous clonidine is administered, the equilibrium is shifted towards an active α2A-AR state, which disinhibits the opioid system. In α2A-KO mice, opioid receptors are not subjected to inhibition by the α2A-AR regardless of the presence or absence of α2A-AR ligands and are thus always fully activated.
The above findings lead us to propose allosteric regulation as the basis by which the α2A-AR modulates spinal opioid receptor function in an activation state-dependent manner (Figure 8B). This model predicts that ligand-free α2A-ARs inhibit opioid receptor function, as was observed after depleting spinal NE. The model also predicts that this inhibitory action is removed when the α2A-AR is occupied by agonists, thereby potentiating opioid analgesia, such as when clonidine is given in combination with morphine or DeltII. Finally, it predicts that loss of the α2A-AR in the KO animals removes this constitutive inhibitory action on opioid receptors, leaving the opioid receptors fully functional and suggesting at least two types of modulation – positive and negative - by the α2A-AR. According to this model, the tonic release of NE in the spinal cord creates an equilibrium between the ligand-occupied and ligand-free α2A-ARs that give rise to an intermediate response to opioid agonists in WT mice under normal conditions. Removing NE will favor the constitutive inhibition while the addition of α2A-AR agonists, such as clonidine, shifts the equilibrium towards the occupied receptor, which potentiates opioid responses.
4.3 Functional consequences of allosteric interactions within GPCR oligomers
G protein-coupled receptors are in direct physical contact within hetero-oligomers and the binding of a ligand on one receptor can cause functional changes at the other receptor through allosteric interactions (Kenakin et al., 2010). State-dependent α2A-AR regulation of opioid effects such as reported here are consistent with previously reported allosteric interactions. Vilardaga et al. (2008) showed that MOPr allosterically modulates α2A-AR upon morphine binding by reducing conformational changes in the α2A-AR and Gi activation by NE. The reduction in Gi signaling was accompanied by inhibition of the ERK1/2 signaling pathway. The ability of α2A-AR to allosterically modulate MOPr was not investigated in that study; however, if the allosteric interaction is symmetric, our model would predict that the α2A-AR has similar allsoteric effects on the MOPr. Jordan et al. (2003) observed a decrease in GTPγS binding and MAPK phosphorylation when MOPr/α2A-AR-expressing cells were treated with morphine/clonidine, which could result from decreased Gi signaling output (Jordan et al., 2003). When activated individually, MOPr and α2A-AR normally activate MAPK signaling pathways. In the presence of agonists for both receptors, the MOPr/α2A-AR may switch to different signaling pathways as indicated by the suppression of Gi-protein coupling and downstream signaling. Protein kinase C (PKC) is a likely downstream target of the new signaling pathway engaged by the co-activation of MOPr and α2A-AR since inhibiting PKC blocks the analgesic synergy between morphine and clonidine, but not the antinociceptive effect of each drug alone (Wei and Roerig, 1998). In our model, we postulate that the un-liganded α2A-AR constitutively inhibits the opioid receptor and that activating the α2A-AR would relieve this constitutive inhibition and engage alternative signaling pathways.
Allosteric interactions between GPCR heterodimers may change ligand binding properties, leading to negative or positive cooperativity that can be detected in binding experiments. For example, changes in the shape and slope of saturation and competition binding curves could reflect alterations in receptor density or ligand affinity (Albizu et al., 2006; Durroux, 2005). Our behavioral results and allosteric interaction model predict that, in the absence of the α2A-AR, the number of binding sites or binding affinity would increase. Using [3H]-DeltII binding to spinal cord membrane preparations from WT and α2A-KO mice, we saw a small decrease in maximal binding sites (Bmax) in α2A-KO mice, but ligand affinity (KD and Ki) was not different from WT mice. These results suggest that the presence of an un-liganded α2A-AR does not affect [3H]DeltII binding properties. However, allosteric interactions usually occur when the orthosteric binding site is occupied by an agonist or an antagonist. For example, Browning et al. (1982) reported a displacement of [3H]DADLE, a DOR agonist, by α2AR ligands in rat brain membranes (Browning et al., 1982). Competition binding experiments with [3H]DeltII in the presence of α2A-AR ligands would provide further insights into the hypothesized allosteric interactions.
Although we propose a model based on allosteric interactions, it is possible that functional interactions could be mediated in the absence of direct physical interactions between the receptors (e.g. via intracellular crosstalk). We cannot exclude other possibilities such as molecular crosstalk between the two receptors which might be mediated by constitutive activity of the α2A-AR. That said, there is significant precedent in the literature for allosteric interactions between antagonist binding (which does not activate signaling) on one receptor partner modulating signaling via the other (Goupil et al., 2015; Wrzal et al., 2012). Such experiments could be conducted on the putative α2A-AR/OPr dimers in future studies. Results from pharmacological studies using α2-AR antagonists to investigate opioid-adrenergic interactions are not consistent with our observations in α2A-KO mice, indicating that blocking the α2A-AR is different from removing it. Combining morphine with the α2-AR antagonists yohimbine or idazoxan has been reported to decrease morphine efficacy (Browning et al., 1982; Iglesias et al., 1992; Morales et al., 2001; Stone et al., 1997). Interestingly, this interaction was paralleled in binding studies on CNS membranes whereby α2-AR ligands could displace radiolabeled µ or δ opioid ligands (Browning et al., 1982). These two classes of ligands do not bind the same receptor sites, again suggesting that an allosteric interaction rather than a direct competition affected opioid binding properties. Further, since yohimbine and idazoxan are antagonists for the α2-AR rather than agonists, they would not be expected to activate cellular signaling and thus their effects would not simply be due to molecular crosstalk between the two receptor pathways. In our model, α2-AR antagonists would counteract opioid effects by displacing NE bound to the α2A-AR, thus stabilizing the receptor complex in an inactive conformation. This model, however, does not explain how ultra low doses of α2A-AR antagonists have the opposite effect on morphine antinociception, i.e. an increase in morphine potency and prolonged antinociceptive effect (Milne et al., 2014). Finally, when measuring the effect of drug interactions at the behavioral level, one cannot exclude the possibility that the interaction involves distinct cells in the system under study.
4.4 Morphine is more potent in absence of the α2A-AR
Other studies have examined the role of α2A-AR in the antinociceptive action of morphine using genetically modified mice. In α2A-D79N mice, spinal morphine potency in the warm water tail immersion assay was not different from WT mice, but a decreased potency in the SP assay was reported (Stone et al., 1997). In another study, no difference in systemic morphine antinociceptive effect was observed between WT and α2A-D79N mice in the hot plate assay (Lakhlani et al., 1997). Our data report the opposite: the potency of morphine is augmented in α2A-KO mice with both the tail immersion and the SP behavioral assay. This potentiation is not due to an upregulation of MOPr mRNA or protein. The divergent results between α2A-D79N and α2A-KO mice raise two possibilities: 1) morphine potentiation requires the complete absence of α2A-AR and/or 2) the dysfunctional α2A-D79N is capable of preventing morphine potentiation. A similar observation was reported in which the D2 dopamine receptor (DRD2) is allosterically regulated by a co-localized and un-liganded ghrelin receptor GHSR1a (Kern et al., 2012). The mere presence of GHSR1a, in the absence of its activation (since this brain region never sees ghrelin), was sufficient to modulate DRD2 signaling output and to produce an anorexigenic response to dopamine. In absence of GHSR1a, dopamine did not affect food intake in GHSR1a-KO mice. This supports our assertion that the opioid response observed in α2A-KO mice is due to the loss of the negative allosteric influence of the unliganded α2A-AR.
Using α2A-KO mice, Ozdogan et al. (2006) observed an enhanced response to morphine (i.p.) and other partial opioid agonists with the tail flick assay, but not for the full agonist fentanyl or with the hot plate assay. This is consistent with our observation of greater potentiation of spinal morphine efficacy compared to the small potentiation of the full MOP agonist, DAMGO (i.t.) in α2A-KO mice. In contrast, using a single morphine test dose (i.p.), the same group previously reported no strain difference in morphine effect (Ozdogan et al., 2004). The use of different doses, administration routes or behavioral assays could be a source of discrepancy between studies.
4.5 DOP agonists are more potent and effective in α2A-KO mice
In both the tail flick and the SP behavioral assays, DeltII antinociception was enhanced in α2A-KO mice. This is in contrast with previous work showing that DeltII-mediated antinociception was unchanged in α2A-D79N mice in the SP behavioral assay (Fairbanks et al., 2002; Stone et al., 2007; Stone et al., 1997). Since we have demonstrated that DOPr mediates DeltII efficacy in the SP behavioral assay (Chabot-Doré et al., 2013), we are confident that DOPr also mediates DeltII antinociception in α2A-KO mice. However, because the antinociceptive effect of DeltII in the tail immersion assay may not be DOPr-mediated (Scherrer et al., 2009; van Rijn et al., 2012), we cannot conclude with certainty that the enhanced DeltII efficacy in α2A-KO mice is mediated by DOPr in this assay. Nevertheless, the enhancement was observed with another DOPr-selective agonist, SNC80, demonstrating that the effect extends to another agonist. Upregulation of functional DOPr has been reported following inflammation, nerve injury, morphine treatment and prolonged exposure to ethanol (Cahill et al., 2003; Cahill et al., 2001; Gendron et al., 2006; Lucido et al., 2005; van Rijn et al., 2012). It is therefore possible that the absence of the α2A-AR triggers a similar upregulation. Our results show that neither Oprd1 transcript levels nor the number of [3H]-DeltII binding sites were augmented in α2A-KO mice compared to WT mice. Other mechanisms leading to DOPr upregulation – increased surface expression or change in signaling output – have not been investigated and therefore are not excluded.
4.6 The α2A-AR is necessary for clonidine-mediated synergistic interactions with DeltII and morphine
The spinal analgesic effect of clonidine is attributed to the α2A-AR (Fairbanks and Wilcox, 1999), an observation we confirmed with α2A-KO mice in both the SP behavioral assay (Chabot- Doré et al., 2013) and the tail immersion assay. Other α2-AR agonists such as dexmetomidine, UK 14,304 and ST-91 also mediate their antinociceptive effects at least in part through the α2AAR (Lakhlani et al., 1997; Stone et al., 2007; Stone et al., 1997). While the synergistic interaction between DeltII and UK 14,304 was lost in α2A-D79N mice (Stone et al., 1997), synergy is maintained when DeltII is combined with either ST91 (Stone et al., 2007) or moxonidine (Fairbanks et al., 2002). Therefore, the role of the α2A-AR in synergy between α2-AR agonists and DeltII depends on the adrenergic ligand tested, with the α2C-AR contributing to this interaction in some cases (Fairbanks et al., 2002). In the current study, clonidine synergy with DeltII was absent in α2A-KO mice, suggesting that the α2A-AR is necessary for clonidine’s synergistic interaction with DeltII. In a nerve injury model, α2-AR analgesic efficacy was bi-directionally modulated by activating or inhibiting DOPr, suggesting that this receptor pair is a valid therapeutic target for neuropathic pain (Aira et al., 2015).
Consistent with the DeltII results, the synergistic interaction between morphine and clonidine was also absent in the α2A-KO mice. To our knowledge, this is the first report demonstrating that the α2A-AR is necessary in this widely studied and clinically relevant drug interaction.
4.7 Endogenous norepinephrine modulates opioid antinociception
A subset of α2A-ARs in the spinal cord are expressed on descending noradrenergic nerve fibers, where they act as autoreceptors regulating the release of NE via negative feedback inhibition (Gilsbach et al., 2009). The absence of α2A-AR could disrupt this negative feedback inhibition, resulting in increased NE release which could potentiate the effects of spinally administered morphine. If elevated spinal NE levels mediate the increased opioid response in α2A-KO, the 6-OHDA treatment would have decreased morphine potency and efficacy to the same level in both strains. Instead, the chemical lesion did not affect the potentiated morphine response in α2A-KO mice. Therefore, the enhanced potency of morphine in α2A-KO mice is not due to an excess of NE in α2A-KO mice and the mechanism must be independent of endogenous NE levels. Since we demonstrated that morphine efficacy decreased in 6-OHDA-treated WT mice, but not in α2A-KO mice, we also conclude that, under normal conditions, spinal morphine efficacy requires the action of endogenous NE on α2A-AR.
The link between the endogenous noradrenergic system and opioid responses has been studied by manipulating spinal NE levels, but the adrenergic receptor requirement has not been adressed. Depleting spinal levels of NE by different methods has been shown to reduce the antinociceptive effect of morphine in rodents (Berge and Ogren, 1984; Jasmin et al., 2003; Zhong et al., 1985). Others have found that these treatments had no effect on morphine antinociception (Milne et al., 2008; Pappas et al., 1982; Sawynok et al., 1991). These discrepancies may be attributed to differences in experimental design such as the choice of behavioral assay and time point. Morphine efficacy is also decreased in mice lacking dopamine β-hydroxylase (Dbh), the enzyme that converts dopamine to NE (Jasmin et al., 2002). Interestingly, the reverse is also true: when NE levels are upregulated such as in NE transporter (NET)-KO mice, morphine efficacy is enhanced (Bohn et al., 2000). These studies point at a role for the noradrenergic system in modulating morphine antinociception. Our study further identifies the α2A-AR as the receptor mediating this effect by showing that α2A-KO mice are not responsive to a decrease in spinal NE levels.
4.8 Use of genetically modified animals
The interpretation of the results presented here has certain limitations. Our experimental approach relies primarily on the comparison of WT mice with a mouse strain carrying a genetic deletion in the Adra2A gene. This approach provides the opportunity to assess α2A-AR function. For example, while clonidine is a non-selective α2-AR agonist with affinity for all three α2-AR subtypes (α2A-AR, α2B-AR, α2C-AR), our results show that α2A-AR is the principal mediator of clonidine analgesia and synergistic interaction with opioids in the spinal cord. The use of transgenic KO mice presents certain caveats that must be considered. For example, global KO suppresses the expression of α2A-AR not only in DRGs and the spinal cord, but also in all tissues. Deletion of these receptors could therefore lead to compensatory mechanisms or dysfunctions in the expression of other genes or in the regulation of other physiological systems, introducing confounding factors in the interpretation of our results. The pattern of autoradiographic labeling of a non-selective α2-AR ligand in the brain of α2A-KO mice was similar to the distribution of α2C-AR in WT mice, suggesting that there are no profound changes in other α2-AR binding sites due to the deletion (Lahdesmaki et al., 2002). We also confirmed that the expression of MOPr and DOPr were unchanged in α2A-KO mice, but the possibility that other genes are dysregulated cannot be ruled out. Given that no changes were observed between α2A-KO and WT mice in a) mechanical nociceptive thresholds, b) sensitivity to intrathecal SP and c) the antinociceptive responses to DAMGO, some components of nociception and antinociception are normal in these mice.
4.9 Clinical implications
Uncovering the role of the α2A-AR in the modulation of pain and opioid sensitivity by NE raises the possibility that an imbalance in adrenergic signaling in the spinal cord may be related to some chronic pain conditions. For example, the etiology of fibromyalgia syndrome (FMS) is still largely unknown. Cerebrospinal fluid (CSF) levels of SP are higher in FMS patients than in normal individuals (Russell, 1998), but the CSF levels of methoxyhydroxyphenylglycol, a metabolite of NE, are lower (Russell et al., 1992). This suggests that FMS patients have reduced noradrenergic tone, which reduces the inhibition of SP release from nociceptors and sensitizes the nociceptive circuit in the spinal cord. Our results suggest that low levels of NE in the spinal cord would also decrease the effect of endogenous or exogenous opioids in FMS patients and restoring NE levels could attenuate pain symptoms associated with FMS. Tramadol is a noradrenergic reuptake inhibitor and a weak MOP agonist that is efficacious for the treatment of FMS (Russell et al., 2000). According to our proposed model, the success of this treatment could be attributed to its dual action: 1) the reuptake inhibitor restores spinal NE levels, which favors the agonist-occupied form of the α2AAR and releases the inhibition on MOPr so that 2) the action of tramadol on MOPr can be fully effective.
4.10 Conclusion
Here we propose a model of activation state-dependent allosteric modulation of opioid receptor function by α2A-ARs. According to the model, activation of α2A-ARs facilitates and the inactive form inhibits opioid antinociception. This model is supported by previous studies showing coexpression in vivo and physically interactions in vitro, and accounts for many of the seemingly contradictory opioid-adrenergic interactions observed in pre-clinical and clinical settings. Finally, this model has implications for understanding functional interactions between other coexpressed G protein-coupled receptor pairs in vivo.
Highlights.
The role of α2A-adrenoceptors (ARs) in spinal opioid antinociception was investigated.
The α2A-AR is necessary for analgesic synergy between clonidine and opioid agonists.
Opioid antinociception is enhanced in α2A-AR-KO mice.
A model of activation state-dependent allosteric modulation is proposed
The model has implications for allosteric modulation of other G protein-coupled receptor pairs.
Acknowledgements
We would like to thank Sarah Amrani, Serge Zaretsky and Tharsika Sinnathamby for participation in preliminary experiments and method optimization and Dr Michael Ossipov for generously supplying FlashCalc 4.5.3 software.
This work was supported by a Canadian Institutes for Health Research (CIHR) operating grants (MOP-86691 to LSS and MOP-130309 to TEH), National Institutes of Health, National Institute on Drug Abuse Grants #R01 DA015438 to GLW and #K01 DA000509 to CAF, funds from the Canadian Foundation for Innovation (CFI 13691) to LSS, a Chercheur-Boursier award from Fonds de Recherche en Santé du Québec (FRSQ) to LSS and Réseau de recherche en santé buccodentainre et osseuse (RSBO) infrastructure support to LSS. AJCD received studentship support from CIHR, the McGill University Integrated Program in Neuroscience (IPN) and the Louise and Alan Edwards Foundation and travel support from the Quebec Pain Research Network and the IPN. LD is the Canada Excellence Research Chair in Personalized Pain Medicine (CERC 08) and received funds from the0020Canada Foundation for Innovation (CFI 32151). Funding sources had no role in the planning, execution, analysis and interpretation of experiments, or in the manuscript preparation.
Abbreviations
- α2-AR
α2-adrenoceptor
- α2A-AR
α2A-adrenoceptor
- DeltII
[d-Ala2]-deltorphin II
- DAMGO
[d-Ala2, NMe-Phe4, Gly-ol5]-enkephalin
- DOPr
delta opioid receptor
- MOPr
mu opioid receptor
- i.t.
intrathecal
- Oprd1
mouse delta opioid receptor gene
- Oprm1
mouse mu opioid receptor gene
- DA
dopamine
- DRG
dorsal root ganglia
- SC
spinal cord
- MPE
maximal possible effect
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- NE
norepinephrine
- SP
substance P
- 6-OHDA
6-hydroxydopamine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aira Z, Barrenetxea T, Buesa I, Azkue JJ. Plasticity of α2-adrenergic spinal antinociception following nerve injury_ Selective, bidirectional interaction with the delta opioid receptor. Brain research. 2015;1594:190–203. doi: 10.1016/j.brainres.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Albizu L, Balestre MN, Breton C, Pin J-P, Manning M, Mouillac B, Barberis C, Durroux T. Probing the Existence of G Protein-Coupled Receptor Dimers by Positive and Negative Ligand-Dependent Cooperative Binding. Molecular Pharmacology. 2006;70:1783–1791. doi: 10.1124/mol.106.025684. [DOI] [PubMed] [Google Scholar]
- Altman JD, Trendelenburg AU, MacMillan L, Bernstein D, Limbird L, Starke K, Kobilka BK, Hein L. Abnormal regulation of the sympathetic nervous system in alpha2A-adrenergic receptor knockout mice. Molecular Pharmacology. 1999;56:154–161. doi: 10.1124/mol.56.1.154. [DOI] [PubMed] [Google Scholar]
- Arvidsson U, Riedl M, Chakrabarti S, Lee JH, Nakano AH, Dado RJ, Loh HH, Law PY, Wessendorf MW, Elde R. Distribution and targeting of a mu-opioid receptor (MOR1) in brain and spinal cord. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1995;15:3328–3341. doi: 10.1523/JNEUROSCI.15-05-03328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berge OG, Ogren SO. Limited involvement of central noradrenergic pathways in morphine-induced antinociception. Neuropharmacology. 1984;23:1179–1185. doi: 10.1016/0028-3908(84)90236-3. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Xu F, Gainetdinov RR, Caron MG. Potentiated opioid analgesia in norepinephrine transporter knock-out mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:9040–9045. doi: 10.1523/JNEUROSCI.20-24-09040.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning S, Lawrence D, Livingston A, Morris B. Interactions of drugs active at opiate receptors and drugs active at alpha 2-receptors on various test systems. British Journal of Pharmacology. 1982;77:487–491. doi: 10.1111/j.1476-5381.1982.tb09322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill CM, Morinville A, Hoffert C, O'Donnell D, Beaudet A. Upregulation and trafficking of delta opioid receptor in a model of chronic inflammation: implications for pain control. Pain. 2003;101:199–208. doi: 10.1016/s0304-3959(02)00333-0. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Morinville A, Lee MC, Vincent JP, Collier B, Beaudet A. Prolonged morphine treatment targets delta opioid receptors to neuronal plasma membranes and enhances delta-mediated antinociception. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:7598–7607. doi: 10.1523/JNEUROSCI.21-19-07598.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabot-Doré A-J, Millecamps M, Stone LS. The delta-opioid receptor is sufficient, but not necessary, for spinal opioid-adrenergic synergy. Journal of Pharmacology and Experimental Therapeutics. 2013 doi: 10.1124/jpet.113.206581. [DOI] [PubMed] [Google Scholar]
- Chabot-Dore A-J, Schuster DJ, Stone LS, Wilcox GL. Analgesic synergy between opioid and α2 -adrenoceptors. British Journal of Pharmacology. 2014 doi: 10.1111/bph.12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. Journal of neuroscience methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Durroux T. Principles: a model for the allosteric interactions between ligand binding sites within a dimeric GPCR. Trends in pharmacological sciences. 2005;26:376–384. doi: 10.1016/j.tips.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Eisenach JC, De Kock M, Klimscha W. alpha(2)-adrenergic agonists for regional anesthesia. A clinical review of clonidine (1984–1995) Anesthesiology. 1996;85:655–674. doi: 10.1097/00000542-199609000-00026. [DOI] [PubMed] [Google Scholar]
- Fairbanks CA, Stone LS, Kitto KF, Nguyen HO, Posthumus IJ, Wilcox GL. alpha(2C)-Adrenergic receptors mediate spinal analgesia and adrenergic-opioid synergy. The Journal of Pharmacology and Experimental Therapeutics. 2002;300:282–290. doi: 10.1124/jpet.300.1.282. [DOI] [PubMed] [Google Scholar]
- Fairbanks CA, Stone LS, Wilcox GL. Pharmacological profiles of alpha 2 adrenergic receptor agonists identified using genetically altered mice and isobolographic analysis. Pharmacology & therapeutics. 2009;123:224–238. doi: 10.1016/j.pharmthera.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks CA, Wilcox GL. Moxonidine, a selective alpha2-adrenergic and imidazoline receptor agonist, produces spinal antinociception in mice. The Journal of Pharmacology and Experimental Therapeutics. 1999;290:403–412. [PubMed] [Google Scholar]
- Fasmer OB, Berge OG, Tveiten L, Hole K. Changes in nociception after 6- hydroxydopamine lesions of descending catecholaminergic pathways in mice. Pharmacology, biochemistry, and behavior. 1986;24:1441–1444. doi: 10.1016/0091-3057(86)90207-8. [DOI] [PubMed] [Google Scholar]
- Gendron L, Lucido AL, Mennicken F, O'Donnell D, Vincent J-P, Stroh T, Beaudet A. Morphine and pain-related stimuli enhance cell surface availability of somatic delta-opioid receptors in rat dorsal root ganglia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:953–962. doi: 10.1523/JNEUROSCI.3598-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilsbach R, Röser C, Beetz N, Brede M, Hadamek K, Haubold M, Leemhuis J, Philipp M, Schneider J, Urbanski M, Szabo B, Weinshenker D, Hein L. Genetic dissection of alpha2-adrenoceptor functions in adrenergic versus nonadrenergic cells. Molecular Pharmacology. 2009;75:1160–1170. doi: 10.1124/mol.109.054544. [DOI] [PubMed] [Google Scholar]
- Goupil E, Fillion D, Clement S, Luo X, Devost D, Sleno R, Petrin D, Saragovi HU, Thorin E, Laporte SA, Hebert TE. Angiotensin II type I and prostaglandin F2alpha receptors cooperatively modulate signaling in vascular smooth muscle cells. The Journal of biological chemistry. 2015;290:3137–3148. doi: 10.1074/jbc.M114.631119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X-h, Fairbanks CA, Stone LS, Loh HH. DPDPE-UK14,304 synergy is retained in mu opioid receptor knockout mice. Pain. 2003;104:209–217. doi: 10.1016/s0304-3959(03)00007-1. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome biology. 2007;8:R19. doi: 10.1186/gb-2007-8-2-r19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. European journal of pharmacology. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- Hylden JL, Wilcox GL. Intrathecal substance P elicits a caudally-directed biting and scratching behavior in mice. Brain research. 1981;217:212–215. doi: 10.1016/0006-8993(81)90203-1. [DOI] [PubMed] [Google Scholar]
- Iglesias V, Alguacil LF, Alamo C, Cuenca E. Effects of yohimbine on morphine analgesia and physical dependence in the rat. European journal of pharmacology. 1992;211:35–38. doi: 10.1016/0014-2999(92)90258-6. [DOI] [PubMed] [Google Scholar]
- Jasmin L, Boudah A, Ohara PT. Long-term effects of decreased noradrenergic central nervous system innervation on pain behavior and opioid antinociception. The Journal of comparative neurology. 2003;460:38–55. doi: 10.1002/cne.10633. [DOI] [PubMed] [Google Scholar]
- Jasmin L, Tien D, Weinshenker D, Palmiter RD, Green PG, Janni G, Ohara PT. The NK1 receptor mediates both the hyperalgesia and the resistance to morphine in mice lacking noradrenaline. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:1029–1034. doi: 10.1073/pnas.012598599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan BA, Gomes I, Rios C, Filipovska J, Devi LA. Functional interactions between mu opioid and alpha 2A-adrenergic receptors. Molecular Pharmacology. 2003;64:1317–1324. doi: 10.1124/mol.64.6.1317. [DOI] [PubMed] [Google Scholar]
- Kankaanpaa A, Meririnne E, Ariniemi K, Seppala T. Oxalic acid stabilizes dopamine, serotonin, and their metabolites in automated liquid chromatography with electrochemical detection. Journal of chromatography. B, Biomedical sciences and applications. 2001;753:413–419. doi: 10.1016/s0378-4347(00)00553-3. [DOI] [PubMed] [Google Scholar]
- Kenakin T, Agnati LF, Caron M, Fredholm B, Guidoli D, Kobilka B, Lefkowitz RW, Lohse M, Woods A, Fuxe K. International Workshop at the Nobel Forum, Karolinska Institutet on G protein-coupled receptors: finding the words to describe monomers, oligomers, and their molecular mechanisms and defining their meaning. Can a consensus be reached? Journal of receptor and signal transduction research. 2010;30:284–286. doi: 10.3109/10799893.2010.512438. [DOI] [PubMed] [Google Scholar]
- Kern A, Albarran-Zeckler R, Walsh HE, Smith RG. Apo-ghrelin receptor forms heteromers with DRD2 in hypothalamic neurons and is essential for anorexigenic effects of DRD2 agonism. Neuron. 2012;73:317–332. doi: 10.1016/j.neuron.2011.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuraishi Y, Harada Y, Aratani S, Satoh M, Takagi H. Separate involvement of the spinal noradrenergic and serotonergic systems in morphine analgesia: the differences in mechanical and thermal algesic tests. Brain research. 1983;273:245–252. doi: 10.1016/0006-8993(83)90849-1. [DOI] [PubMed] [Google Scholar]
- Lahdesmaki J, Sallinen J, MacDonald E, Kobilka BK, Fagerholm V, Scheinin M. Behavioral and neurochemical characterization of alpha(2A)-adrenergic receptor knockout mice. Neuroscience. 2002;113:289–299. doi: 10.1016/s0306-4522(02)00185-9. [DOI] [PubMed] [Google Scholar]
- Lähdesmäki J, Scheinin M, Pertovaara A, Mansikka H. The alpha2A-adrenoceptor subtype is not involved in inflammatory hyperalgesia or morphine-induced antinociception. European journal of pharmacology. 2003;468:183–189. doi: 10.1016/s0014-2999(03)01677-7. [DOI] [PubMed] [Google Scholar]
- Lakhlani PP, MacMillan LB, Guo TZ, McCool BA, Lovinger DM, Maze M, Limbird LE. Substitution of a mutant alpha2a-adrenergic receptor via "hit and run" gene targeting reveals the role of this subtype in sedative, analgesic, and anesthetic-sparing responses in vivo. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:9950–9955. doi: 10.1073/pnas.94.18.9950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link RE, Stevens MS, Kulatunga M, Scheinin M, Barsh GS, Kobilka BK. Targeted inactivation of the gene encoding the mouse alpha 2c-adrenoceptor homolog. Mol Pharmacol. 1995;48:48–55. [PubMed] [Google Scholar]
- Lucido AL, Morinville A, Gendron L, Stroh T, Beaudet A. Prolonged morphine treatment selectively increases membrane recruitment of delta-opioid receptors in mouse basal ganglia. Journal of molecular neuroscience : MN. 2005;25:207–214. doi: 10.1385/JMN:25:3:207. [DOI] [PubMed] [Google Scholar]
- MacMillan LB, Hein L, Smith MS, Piascik MT, Limbird LE. Central hypotensive effects of the alpha2a-adrenergic receptor subtype. Science (New York, NY) 1996;273:801–803. doi: 10.1126/science.273.5276.801. [DOI] [PubMed] [Google Scholar]
- Malmberg AB, Hedley LR, Jasper JR, Hunter JC, Basbaum AI. Contribution of alpha(2) receptor subtypes to nerve injury-induced pain and its regulation by dexmedetomidine. British Journal of Pharmacology. 2001;132:1827–1836. doi: 10.1038/sj.bjp.0704032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millecamps M, Tajerian M, Sage EH, Stone LS. Behavioral signs of chronic back pain in the SPARC-null mouse. Spine. 2011;36:95–102. doi: 10.1097/BRS.0b013e3181cd9d75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne B, Jhamandas K, Sutak M, Grenier P, Cahill CM. Analgesia, enhancement of spinal morphine antinociception, and inhibition of tolerance by ultra-low dose of the α2A-adrenoceptor selective antagonist BRL44408. European journal of pharmacology. 2014;743:89–97. doi: 10.1016/j.ejphar.2014.08.040. [DOI] [PubMed] [Google Scholar]
- Milne B, Sutak M, Cahill CM, Jhamandas K. Low doses of alpha 2-adrenoceptor antagonists augment spinal morphine analgesia and inhibit development of acute and chronic tolerance. British Journal of Pharmacology. 2008;155:1264–1278. doi: 10.1038/bjp.2008.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales L, Perez-Garcia C, Alguacil LF. Effects of yohimbine on the antinociceptive and place conditioning effects of opioid agonists in rodents. British Journal of Pharmacology. 2001;133:172–178. doi: 10.1038/sj.bjp.0704057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdogan UK, Lähdesmäki J, Hakala K, Scheinin M. The involvement of alpha 2A-adrenoceptors in morphine analgesia, tolerance and withdrawal in mice. European journal of pharmacology. 2004;497:161–171. doi: 10.1016/j.ejphar.2004.06.051. [DOI] [PubMed] [Google Scholar]
- Ozdogan UK, Lähdesmäki J, Scheinin M. The analgesic efficacy of partial opioid agonists is increased in mice with targeted inactivation of the alpha2A-adrenoceptor gene. European journal of pharmacology. 2006;529:105–113. doi: 10.1016/j.ejphar.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Pappas B, Ings R, Roberts D. Neonatal intraspinal 6-hydroxydopamine, 5,7-dihydroxytryptamine or their combination: effects on nociception and morphine analgesia. European journal of pharmacology. 1982;86:157–166. doi: 10.1016/0014-2999(82)90313-2. [DOI] [PubMed] [Google Scholar]
- Peterhoff M, Sieg A, Brede M, Chao C-M, Hein L, Ullrich S. Inhibition of insulin secretion via distinct signaling pathways in alpha2-adrenoceptor knockout mice. European journal of endocrinology / European Federation of Endocrine Societies. 2003;149:343–350. doi: 10.1530/eje.0.1490343. [DOI] [PubMed] [Google Scholar]
- Riedl MS, Schnell SA, Overland AC, Chabot-Doré A-J, Taylor AM, Ribeiro-da- Silva A, Elde RP, Wilcox GL, Stone LS. Coexpression of alpha 2A-adrenergic and delta-opioid receptors in substance P-containing terminals in rat dorsal horn. The Journal of comparative neurology. 2009;513:385–398. doi: 10.1002/cne.21982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios C, Gomes I, Devi LA. Interactions between delta opioid receptors and alpha-adrenoceptors. Clinical and experimental pharmacology & physiology. 2004;31:833–836. doi: 10.1111/j.1440-1681.2004.04076.x. [DOI] [PubMed] [Google Scholar]
- Roerig SC, Lei S, Kitto K, Hylden JK, Wilcox GL. Spinal interactions between opioid and noradrenergic agonists in mice: multiplicativity involves delta and alpha-2 receptors. The Journal of Pharmacology and Experimental Therapeutics. 1992;262:365–374. [PubMed] [Google Scholar]
- Russell IJ. Advances in fibromyalgia: possible role for central neurochemicals. The American journal of the medical sciences. 1998;315:377–384. doi: 10.1097/00000441-199806000-00006. [DOI] [PubMed] [Google Scholar]
- Russell IJ, Kamin M, Bennett RM, Schnitzer TJ, Green JA, Katz WA. Efficacy of tramadol in treatment of pain in fibromyalgia. Journal of clinical rheumatology : practical reports on rheumatic & musculoskeletal diseases. 2000;6:250–257. doi: 10.1097/00124743-200010000-00004. [DOI] [PubMed] [Google Scholar]
- Russell IJ, Vaeroy H, Javors M, Nyberg F. Cerebrospinal fluid biogenic amine metabolites in fibromyalgia/fibrositis syndrome and rheumatoid arthritis. Arthritis and rheumatism. 1992;35:550–556. doi: 10.1002/art.1780350509. [DOI] [PubMed] [Google Scholar]
- Sagen J, Proudfit HK. Effect of intrathecally administered noradrenergic antagonists on nociception in the rat. Brain research. 1984;310:295–301. doi: 10.1016/0006-8993(84)90152-5. [DOI] [PubMed] [Google Scholar]
- Sawynok J, Reid A, Nance D. Spinal antinociception by adenosine analogs and morphine after intrathecal administration of the neurotoxins capsaicin, 6-hydroxydopamine and 5,7-dihydroxytryptamine. The Journal of Pharmacology and Experimental Therapeutics. 1991;258:370–380. [PubMed] [Google Scholar]
- Scherrer G, Imamachi N, Cao YQ, Contet C, Mennicken F, O'Donnell D, Kieffer BL, Basbaum AI. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell. 2009;137:1148–1159. doi: 10.1016/j.cell.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone LS, Broberger C, Vulchanova L, Wilcox GL, Hökfelt T, Riedl MS, Elde R. Differential distribution of alpha2A and alpha2C adrenergic receptor immunoreactivity in the rat spinal cord. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:5928–5937. doi: 10.1523/JNEUROSCI.18-15-05928.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone LS, German JP, Kitto KF, Fairbanks CA, Wilcox GL. Morphine and Clonidine Combination Therapy Improves Therapeutic Window in Mice: Synergy in Antinociceptive but Not in Sedative or Cardiovascular Effects. PLoS ONE. 2014;9:e109903. doi: 10.1371/journal.pone.0109903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone LS, Kitto KF, Eisenach JC, Fairbanks CA, Wilcox GL. ST91 [2-(2,6-diethylphenylamino)-2-imidazoline hydrochloride]-mediated spinal antinociception and synergy with opioids persists in the absence of functional alpha-2A- or alpha-2C-adrenergic receptors. The Journal of Pharmacology and Experimental Therapeutics. 2007;323:899–906. doi: 10.1124/jpet.107.125526. [DOI] [PubMed] [Google Scholar]
- Stone LS, MacMillan LB, Kitto KF, Limbird LE, Wilcox GL. The alpha2a adrenergic receptor subtype mediates spinal analgesia evoked by alpha2 agonists and is necessary for spinal adrenergic-opioid synergy. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17:7157–7165. doi: 10.1523/JNEUROSCI.17-18-07157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G-C, Ho W-Y, Chen B-R, Cheng P-W, Cheng W-H, Hsu M-C, Yeh T-C, Hsiao M, Lu P-J, Tseng C-J. GPCR dimerization in brainstem nuclei contributes to the development of hypertension. British Journal of Pharmacology. 2015;172:2507–2518. doi: 10.1111/bph.13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surprenant A, Horstman DA, Akbarali H, Limbird LE. A point mutation of the alpha 2-adrenoceptor that blocks coupling to potassium but not calcium currents. Science (New York, NY) 1992;257:977–980. doi: 10.1126/science.1354394. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ. Statistical analysis of drug combinations for synergism. Pain. 1992;49:93–97. doi: 10.1016/0304-3959(92)90193-F. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ. An overview of drug combination analysis with isobolograms. The Journal of Pharmacology and Experimental Therapeutics. 2006;319:1–7. doi: 10.1124/jpet.106.104117. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ, Murray RB. Tallarida and Murray (1987) Manual of pharmacologic calculations with computer programs. 1987 [Google Scholar]
- Tan M, Walwyn WM, Evans CJ, Xie C-W. p38 MAPK and beta-arrestin 2 mediate functional interactions between endogenous micro-opioid and alpha2A-adrenergic receptors in neurons. The Journal of biological chemistry. 2009;284:6270–6281. doi: 10.1074/jbc.M806742200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrillon S, Bouvier M. Roles of G-protein-coupled receptor dimerization. EMBO reports. 2004;5:30–34. doi: 10.1038/sj.embor.7400052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn RM, Brissett DI, Whistler JL. Emergence of Functional Spinal Delta Opioid Receptors After Chronic Ethanol Exposure. BPS. 2012;71:232–238. doi: 10.1016/j.biopsych.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilardaga J-P, Nikolaev VO, Lorenz K, Ferrandon S, Zhuang Z, Lohse MJ. Conformational cross-talk between alpha2A-adrenergic and mu-opioid receptors controls cell signaling. Nature chemical biology. 2008;4:126–131. doi: 10.1038/nchembio.64. [DOI] [PubMed] [Google Scholar]
- Wang HB, Zhao B, Zhong YQ, Li KC, Li ZY, Wang Q, Lu YJ, Zhang ZN, He SQ, Zheng HC, Wu SX, Hokfelt TG, Bao L, Zhang X. Coexpression of delta-and mu-opioid receptors in nociceptive sensory neurons. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:13117–13122. doi: 10.1073/pnas.1008382107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei ZY, Roerig SC. Spinal morphine/clonidine antinociceptive synergism is regulated by protein kinase C, but not protein kinase A activity. The Journal of Pharmacology and Experimental Therapeutics. 1998;287:937–943. [PubMed] [Google Scholar]
- Wrzal PK, Devost D, Petrin D, Goupil E, Iorio-Morin C, Laporte SA, Zingg HH, Hebert TE. Allosteric interactions between the oxytocin receptor and the beta2-adrenergic receptor in the modulation of ERK1/2 activation are mediated by heterodimerization. Cellular signalling. 2012;24:342–350. doi: 10.1016/j.cellsig.2011.09.020. [DOI] [PubMed] [Google Scholar]
- Yeomans DC, Proudfit HK. Nociceptive responses to high and low rates of noxious cutaneous heating are mediated by different nociceptors in the rat: electrophysiological evidence. Pain. 1996;68:141–150. doi: 10.1016/S0304-3959(96)03177-6. [DOI] [PubMed] [Google Scholar]
- Zhang YQ, Limbird LE. Hetero-oligomers of alpha2A-adrenergic and mu-opioid receptors do not lead to transactivation of G-proteins or altered endocytosis profiles. Biochemical Society transactions. 2004;32:856–860. doi: 10.1042/BST0320856. [DOI] [PubMed] [Google Scholar]
- Zhong FX, Ji XQ, Tsou K. Intrathecal DSP4 selectively depletes spinal noradrenaline and attenuates morphine analgesia. European journal of pharmacology. 1985;116:327–330. doi: 10.1016/0014-2999(85)90171-2. [DOI] [PubMed] [Google Scholar]