Abstract
Our objective was to determine whether the frequencies of non-ovarian cancers (NOC) within families in a large Familial Ovarian Cancer Registry (FOCR) are significantly different from the frequencies listed in the SEER database. The FOCR was established in 1981. Registry members are families with two or more first degree relatives who have a diagnosis of ovarian cancer, three or more cases of cancer on one side of the family with at least one being ovarian, at least one female with two or more primary cancers in which one is ovary, or a history of two or more cancers in the family with at least one being ovarian cancer diagnosed before the age of 45. The data was analyzed to find relative rates of 10 of the most common cancers found within the database, with the exception of ovarian and breast. These include bladder, CNS, cervical, colorectal, liver, lung, pancreas, prostate, stomach, and uterine. Cancers were further stratified by age at diagnosis, and compared to information in the SEER database. There are 2,671 pedigrees and a total of 50,454 individuals within the FOCR. There are 1,938 families with two or more relatives with ovarian cancer, accounting for 4,816 individuals with ovarian cancer. The total number of individuals with ovarian cancer is 5,421. Of these individuals with ovarian cancer, 2,249 have been verified with testing or physician correspondence. The frequencies of the NOCs within the registry were higher than that of the general population as described in the SEER database. In particular, the overall frequencies of cancers of the bladder, cervix, prostate, and uterus were higher within the FOCR at 2.3, 7.4, 25.2, and 11.9 per 1,000 respectively. Furthermore, diagnoses of both cervical and uterine cancers tended to occur at an earlier age within the FOCR. The overall frequencies of cancers of the bladder, cervix, prostate, and uterus are higher in the FOCR compared with a general population database. Future studies on segregation analysis and genome-wide linkage studies are warranted on families with NOC within the Familial Ovarian Cancer Registry.
Keywords: NOC, SEER, cervical, uterine, bladder, prostate
Introduction
A disease affecting 1/70 women over their lifetime, ovarian cancer accounted for an estimated 14,030 deaths in the US in 2013, with 22,240 new cases diagnosed [1]. Hereditary cancer syndromes account for up to 15% of ovarian and breast cancers. Though there are many established genetic susceptibilities for ovarian cancer, including but not limited to BRCA1, BRCA2, MSH2, and MLH1, there are still many families that carry a strong family history of ovarian and other cancers without having any of these inheritable mutations. After identifying two families with multiple affected individuals over the course of two years, the familial ovarian cancer registry (FOCR) was established in 1981 at Roswell Park Cancer Institute (RPCI) [3]. The registry collects and records any and all factors that may be contributing to familial ovarian cancer, including but not limited to environmental, genetic, and social risk factors. The registry is the only established research resource in the United States with continuously recorded familial ovarian cancer lineage and is therefore an invaluable resource for research endeavors associated with cancer genetics [4]. Consequently, given the FOCR’s vast content, including non-ovarian cancers (NOC), we aim to utilize the registry to better understand the incidence of other cancers within families that have already shown a high predisposition to ovarian cancer. In a 20-year analysis of the registry, greater than two-thirds of the enrolled families had diagnoses of ovarian cancer without evidence of breast cancer, suggesting another genetic component of hereditary ovarian cancer outside of the widely accepted hereditary breast/ovarian cancer syndrome [2]. Whole family pedigrees are constructed from member data, including men and women with non-ovarian cancers (NOC). While we have looked in depth at the ovarian and breast cancers within the registry, we had not yet delved into the frequencies of NOCs within the database.
We hypothesize that the incidence of NOCs within the pedigrees contained in the FOCR may also be higher than that of the general population, as found in the SEER database, due to yet unknown genetic predispositions. This can have far-reaching implication for the future of cancer care.
Materials and methods
Ethics statement
The Familial Ovarian Cancer Registry: Family and Medical History and Biosample Resource (CIC 95–27) protocol has been reviewed and approved the Roswell Park Cancer Institute IRB Board.
Study subjects and characteristics of family members
Subjects for this study were derived from the Familial Ovarian Cancer Registry (FOCR - formerly referred to as the Gilda Radner Familial Ovarian Cancer Registry). The Familial Ovarian Cancer Registry (FOCR) is a self-referred registry of families with two or more ovarian cancer cases in genetic relatives. Since its inception in 1981, its primary function is to receive family cancer information voluntarily contributed from around the United States by ovarian cancer patients, referring physicians, self-referred women, and patients of the Roswell Park Cancer Institute (RPCI) Gynecologic Oncology Department. The FOCR objectives include (i) obtaining detailed family histories from individuals who are from families with two or more cases of ovarian cancer or a syndrome possibly related to ovarian cancer; (ii) documenting through medical records and through pathologist review of tissues the occurrence of cancer; (iii) collecting, processing and storing biological samples, when possible, from Registry participants; and (iv) making the information and biological samples available for research under Institutional Review Board approved research protocols.
Recruitment of subjects
Registry participants are recruited through pro-bands that meet at least one of the following criteria: (i) family history of two or more cases of ovarian cancer; (ii) family history of one case of ovarian cancer and two cases of cancer at any other site; (iii) family history of at least one female with two or more primary tumors with one of the primaries being ovarian cancer; (iv) family history of two or more cases of cancer with at least one case being ovarian cancer, and the other cancer considered to be of early onset (≤ 45 years old). In addition to meeting at least one of the preceding criteria, each participant is required to sign a consent form for potential collection of biospecimens. Individuals unable to give consent as a result of mental, intellectual, or cognitive deficits are excluded, however, such could appear in the Registry through collection of family history data but without collection of bio-samples from them. Subjects for this study consisted of 50,454 adult members from 2,671 different pedigrees from the FOCR. Of these, there were a total of 1,938 families with two or more cases of ovarian cancer. The remaining pedigrees met criteria ii–iv.
Data collection
Data were collected via forms filled out by subjects upon entry into the registry. All subjects gave authorization to release medical records and archival tissues (when available). Both the completed Family History Form and retrieved medical records were reviewed to ascertain eligibility before subjects are entered into the Registry. Information collected on individuals who do not meet inclusion criteria at the time of collection are not entered into the Registry, and were either destroyed, placed in an inactive locked file, or returned to the individuals upon request. Once an individual has been identified to meet eligibility criteria, a second survey is filled out by the individual, detailing epidemiologic data. Furthermore, they receive a blood sampling kit for biosample collection. Further permission is ascertained from the individual to invite other members of their pedigree to participate. Those invited individuals that consent to participate also fill out the required forms and donate a biospecimen if able and agreeable.
Construction of pedigrees
Family history forms detailing patient-family relationships enable the construction of detailed pedigrees. Pedigrees used in this study were constructed from relatives ascertained through probands and if necessary, dummy individuals were added to families for the purpose of connecting relatives within pedigrees, with affected status set to “unknown” preventing this data from being used in the registry.
SEER database
Several SEER databases were utilized to analyze the FOCR data. In order to obtain data estimates of cancer cases over a 30 year period, SEER 9 was utilized, because it spanned the time period of 1973–2011, most closely matching the time of existence of the FOCR. The SEER prevalence was related in an estimate of the number of individuals with a given cancer in 2009, and was thus divided by the total United States population (as estimated by the United States Census Bureau). For example, the prevalence of prostate cancer in the SEER 9 database is 2,496,784. If this is applied to the latest US Census Bureau population estimate of 151,781,326 males living in the US, the rate is 1.6% [5]. However, prostate cancer was reported in 580 individuals within the database, a frequency of approximately 2.5%. This methodology was used for all NOCs analyzed in the study. Furthermore, when stratifying our affected pedigree members by cancer type and age, we needed a comparator. As this is not easily observable and available information in SEER 9, SEER 18 was utilized because it provided population estimates of the percent of cases by age group for each type of cancer for a period of 2007–2011.
Statistical analysis
The familial ovarian cancer registry at Roswell Park Cancer Institute was reviewed to identify all NOCs listed throughout the pedigrees. This data was analyzed using Statistical Analysis System (SAS) to identify the relative rates of the 10 most common cancers found within the database, with the exception of ovarian and breast. These included bladder, CNS, cervical, colorectal, liver, lung, pancreas, prostate, stomach, and uterine cancers. This data was used to compare frequencies of the cancers within the database and was further stratified into age-specific frequencies of each cancer as they are seen in the SEER 9 database. The SEER 9 database was referenced due to its longevity, with data dating back to 1973, giving the best comparison to FOCR data. Frequencies were determined by using prevalence rates in the SEER 9 database and dividing by the population as determined by the 2010 US Census Bureau [5].
Results
Of the 50,454 individuals identified from the database for this study, 23,041 (45.67%) were male and 25,917 (51.37%) were female. 1496 (2.97%) were listed ambiguously as “cousin” within the family pedigrees, accounting for the discrepancy. As expected in a familial ovarian cancer database, the majority of affected individuals were ovarian and breast cancer patients at 5,421 and 2,017 respectively. These numbers reflect patients with pathologically confirmed cases or as by correspondence with their treating physicians.
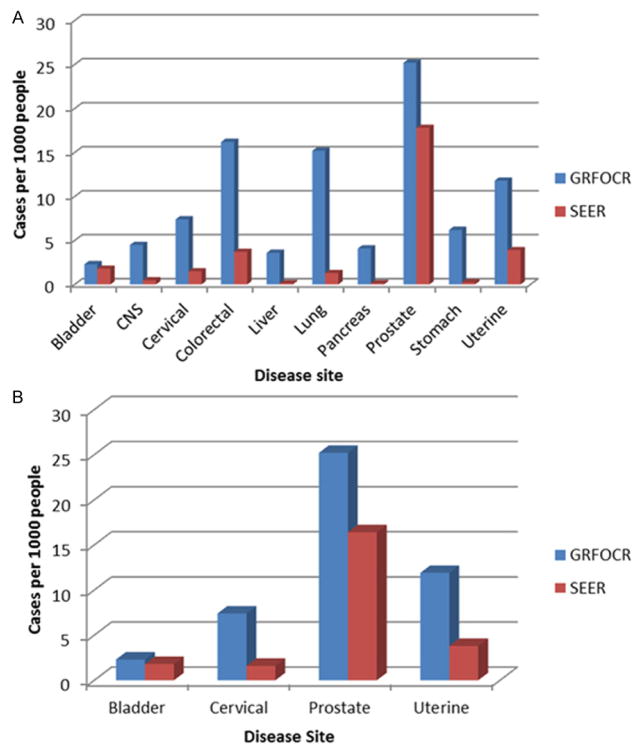
After excluding ovarian and breast, the 10 most frequent cancers listed within pedigrees are shown in Table 1. These include colorectal (819), lung (768), prostate (580), stomach (311), uterine (308), central nervous system (226), pancreas (206), cervical (191), liver (184), and bladder (115). The frequencies of each of these NOCs within the registry were significantly higher than that of the general population as described in the SEER database (Table 1, Figure 1A). The prevalence of each of these ten cancers within the “SEER stat fact sheets” are as follows: Colorectal (1,162,4–26), lung (402326), prostate (2,707,821), stomach (74035), uterine (610804), central nervous system (144463), pancreas (435-38), cervical (249632), liver (45942), and bladder (571-518) [1] (Table 1). These values were divided by the total number of applicable individuals in the population, as outlined by the 2010 US Census. Therefore, sex-specific cancers had a denominator of 151,781,326 for males and 156,964,212 for females, with a total population of 308,745,538 applicable to calculations of cancers that affect both sexes.
Table 1.
Number of cases per 1000, as calculated using the number of affected individuals in FOCR and estimates of affected individuals from SEER and the population estimates from the 2010 U.S. Census
| Disease Site | FOCR | SEER | P-value | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| # Affected | Database Population | Cases per 1000 | # Affected | USCB Population | Cases per 1000 | ||
| Bladder | 115 | 50454 | 2.29 | 571518 | 308745538 | 1.85 | < 0.01 |
| CNS | 226 | 50454 | 4.5 | 144463 | 308745538 | .47 | < 0.01 |
| Cervical | 191 | 25917 | 7.37 | 249632 | 156964212 | 1.6 | < 0.01 |
| Colorectal | 819 | 50454 | 16.2 | 1162426 | 308745538 | 3.78 | < 0.01 |
| Liver | 184 | 50454 | 3.65 | 45942 | 308745538 | 0.15 | < 0.01 |
| Lung | 768 | 50454 | 15.2 | 402326 | 308745538 | 1.3 | < 0.01 |
| Pancreas | 206 | 50454 | 4.1 | 43538 | 308745538 | 0.14 | < 0.01 |
| Prostate | 580 | 23041 | 25.2 | 2707821 | 151781326 | 17.8 | < 0.01 |
| Stomach | 311 | 50454 | 6.2 | 74035 | 308745538 | 0.24 | < 0.01 |
| Uterine | 308 | 25917 | 11.9 | 610804 | 156964212 | 3.9 | < 0.01 |
| Ovary | 5421 | 25917 | 209.2 | 188867 | 156964212 | 1.2 | * |
| Breast | 2017 | 25917 | 77.8 | 2899726 | 156964212 | 18.4 | * |
P-values not calculated for these statistics because of bias apparent secondary to the fact that the index person for each pedigree qualifies by meeting criteria of familial ovarian cancer syndrome, and therefore a high proportion of these will also have breast cancer secondary to BRCA mutations.
Figure 1.
A. Side-by-side comparison of SEER and FOCR prevalence (per 1000) in the ten most prevalent non-ovarian and non-breast cancers within the FOCR. B. Cancers less often associated with sites of metastatic spread, and thus most likely to be primary tumor sites (P < 0.01 by Chi Square Test).
To avoid possible bias generated by the fact that the NOCs within the database are reported by the index pedigree patient, that may or may not understand the difference between multiple synchronous primaries and one primary with metastatic disease, we then focused on four cancers of which it was more likely to be a primary tumor site rather than a sight of metastasis. This led us to focus specifically on bladder, cervix, prostate, and uterine cancers. In particular, the overall frequencies of cancers of the bladder, cervix, prostate, and uterus were higher within the FOCR at 2.3 (115/50,454), 7.4 (191/25,917), 25.2 (580/23,041), and 11.9 (308/25,917) per 1,000 respectively (Figure 1B). Furthermore, diagnoses of cervical, uterine and CNS cancers tended to occur at an earlier age within the FOCR (P < .01 by ChiSquare Test) (Table 2).
Table 2.
Frequencies (%) of non-ovarian cancers within the GRFOCR and SEER databases by age at diagnosis (P < .01 by Chi Square Test)
| GRFOCR
| ||||||||
|---|---|---|---|---|---|---|---|---|
| ≤ 19 | 20–34 | 35–44 | 45–54 | 55–64 | 65–74 | > 75 | n = | |
| Bladder | 2.6 | 1.7 | 6 | 9.5 | 13 | 15.6 | 51.3 | 115 |
| Cervical | 1.5 | 31.4 | 12.5 | 8.9 | 4.1 | 6.2 | 35 | 191 |
| Prostate | 0 | 0 | 0.34 | 2.7 | 10.8 | 20.6 | 65.3 | 580 |
| Uterine | 0.65 | 7.1 | 9.7 | 17.5 | 9.7 | 7.4 | 47.7 | 308 |
| CNS | 2.2 | 3.1 | 6.2 | 10.6 | 10.1 | 9.7 | 57.9 | 226 |
| Colorectal | 0.12 | 1.5 | 3.3 | 8.1 | 11.7 | 12.8 | 62.5 | 819 |
| Liver | 0 | 1.1 | 4.3 | 5.4 | 13.5 | 16.8 | 58.6 | 184 |
| Lung | 0.13 | 0.52 | 2.1 | 6.6 | 12.2 | 14.1 | 64.3 | 768 |
| Pancreas | 0 | 0 | 1.5 | 8.3 | 12.1 | 15.5 | 62.6 | 206 |
| Stomach | 0.32 | 0.96 | 2.6 | 5.5 | 4.8 | 9.0 | 76.8 | 311 |
|
| ||||||||
| SEER
| ||||||||
| Bladder | 0.1 | 0.4 | 1.6 | 7.4 | 18.4 | 27.4 | 44.7 | 83,931 |
| Cervical | 0.2 | 14 | 25.9 | 23.8 | 16.7 | 10.7 | 8.7 | 17,257 |
| Prostate | 0 | 0 | 0.6 | 9.5 | 31.6 | 35.6 | 22.7 | 287,904 |
| Uterine | 0 | 1.5 | 6 | 19.1 | 32.9 | 22.8 | 17.7 | 53,468 |
| CNS | 13.1 | 8.8 | 8.8 | 14.9 | 19.5 | 16.7 | 18.2 | 26,898 |
| Colorectal | 0.1 | 1.2 | 4.0 | 13.8 | 20.8 | 24 | 36.1 | 190,152 |
| Liver | 1.0 | 0.8 | 2.6 | 17.8 | 32.1 | 22.4 | 23.4 | 31,640 |
| Lung | 0 | 0.3 | 1.4 | 8.8 | 21.3 | 31.4 | 36.8 | 253,634 |
| Pancreas | 0.1 | 0.4 | 2.1 | 9.7 | 20.8 | 26.0 | 40.9 | 49,621 |
| Stomach | 0.1 | 1.6 | 4.6 | 12.2 | 19.7 | 24.7 | 37.2 | 30,963 |
Discussion
This study demonstrates that the ten most frequent NOCs in the database all occurred at a frequency higher than is seen in the general population. This finding raises a number of questions. First, although only a few genetic susceptibilities to cancer have been identified, this data suggests there may be additional genes within individuals contained in the pedigrees of the FOCR that predispose them to cancer at other sites. Further investigation is needed into the genomes of patients and families demonstrating hereditary cancer syndromes to uncover more genes for targeting and surveillance. This is not merely supported by the increase in observed cases, but by the earlier occurrence of these cases as compared to the general population as represented by SEER. These two factors taken together would suggest the existence of yet unidentified genetic susceptibilities within these families.
Conversely, a limitation of the database lies in the fact that the pedigree information is largely patient reported. For example, the recruited individuals are those that meet the criteria listed in the introduction. Those individuals are ideally confirmed with physician correspondence and/or pathology reports. However, the patient creates the pedigree information for their family, reporting on cancers and diseases of each individual, affected and unaffected. Pathologic verification is not always possible and this may further confound our results. Despite this confounder, the results still appear robust because of the demonstrated results in the cancers that are unlikely to be sites of metastasis. Because in some individuals synchronous primaries versus secondary sites of metastasis can be confirmed nor disputed, we ventured to focus on cancers that were listed individually, and/or are not frequent sites of metastatic spread. Therefore, we chose to focus on bladder, cervical, prostate, and uterine cancer.
It became very evident in analyzing our data that not only do these four cancers occur more frequently within the registry population, but the age at diagnosis was significantly earlier than in the SEER population (Table 2). So, despite the ambiguity of some of the non-verified data, and though it is clear that the data is probably biased in that in order to qualify for this database, one must already have multiple cases of cancer within his or her family, it sheds light on the fact that further genetic links exist that have yet to be explored, outside of the oft analyzed BRCA, lynch syndromes, etc. Furthermore, the younger age at diagnosis for many of our cancers is corroborated by the existing data on the hereditary ovarian cancer cases seen of the first 1000 families that were enrolled, which was 53.5 years versus the 60.8 years that was reported in SEER at the time [6].
The FOCR is a powerful, versatile platform that has proven useful for studying ovarian and NOC. In order to further corroborate our findings, the next step is to conduct segregation analysis as well as genome-wide linkage studies on families with NOC. Ideally we will further enhance our collection of information on NOCs within the FOCR.
Acknowledgments
We acknowledge the patients and their families who voluntarily contributed to this database. Additionally, research reported in this publication was supported by the National Institutes of Health Award Number 5T32 CA 108456 and the Roswell Park Cancer Institute-University of Pittsburg Cancer Institute Ovarian Cancer Specialized Program of Research Excellence National Institutes of Health P50CA159981-01A1. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosure of conflict of interest
None.
References
- 1.Surveillance, Epidemiology, and End Results Program. National Cancer Institute; Apr Web 14, 2014. Surveillance, Epidemiology, and End Results Program Turning Cancer Data Into Discovery. n.d. http://www.seer.cancer.gov/ [Google Scholar]
- 2.Piver MS. Hereditary Ovarian Cancer: Lessons from the First Twenty Years of the Gilda Radner Familial Ovarian Cancer Registry. Gynecol Oncol. 2002;85:9–17. doi: 10.1006/gyno.2001.6465. [DOI] [PubMed] [Google Scholar]
- 3.Lurain JR, Piver MS. Familial ovarian cancer. Gynecol Oncol. 1979;8:185–92. doi: 10.1016/0090-8258(79)90024-6. [DOI] [PubMed] [Google Scholar]
- 4.Ovarian Cancer. Familial Ovarian Cancer Registry. Roswell Park Cancer Institute; Apr Web 14, 2014. n.d. http://www.ovariancancer.com/ [Google Scholar]
- 5.Howden L, Meyer J. 2010 Census Briefs: Age and Sex Composition 2010. United States Census Bureau. U.S. Dept of Commerce; Apr-May. 2011. Web. Jan.–Feb. 2014 http://www.census.gov/prod/cen2010/briefs/c2010br-03.pdf. [Google Scholar]
- 6.Piver MS, Goldberg JM, Tsukada Y, Mettlin CJ, Jishi MF, Natarajan N. Characteristics of familial ovarian cancer: a report of the first 1,000 families in the Gilda Radner Familial Ovarian Cancer Registry. Eur J Gynecol Oncol. 1996;17:169–76. [PubMed] [Google Scholar]