Abstract
Epilepsy is a highly prevalent seizure disorder which tends to progress in severity and become refractory to treatment. Yet no therapy is proven to halt disease progression or to prevent the development of epilepsy. Because a high fat low carbohydrate ketogenic diet (KD) augments adenosine signaling in the brain and because adenosine not only suppresses seizures but also affects epileptogenesis, we hypothesized that a ketogenic diet might prevent epileptogenesis through similar mechanisms. Here, we tested this hypothesis in two independent rodent models of epileptogenesis. Using a pentylenetetrazole kindling paradigm in mice, we first show that a KD, but not a conventional antiepileptic drug (valproic acid), suppressed kindling-epileptogenesis. Importantly, after treatment reversal, increased seizure thresholds were maintained in those animals kindled in the presence of a KD, but not in those kindled in the presence of valproic acid. Next, we tested whether a KD can halt disease progression in a clinically relevant model of progressive epilepsy. Epileptic rats that developed spontaneous recurrent seizures after a pilocarpine-induced status epilepticus were treated with a KD or control diet (CD). Whereas seizures progressed in severity and frequency in the CD-fed animals, KD-fed animals showed a prolonged reduction of seizures, which persisted after diet reversal. KD-treatment was associated with increased adenosine and decreased DNA methylation, the latter being maintained after diet discontinuation. Our findings demonstrate that a KD prevented disease progression in two mechanistically different models of epilepsy, and suggest an epigenetic mechanism underlying the therapeutic effects.
Keywords: TLE, acquired epilepsy, chemoconvulsant, adenosine, epigenetics, DNA methylation
Introduction
Epilepsy affects about 50 million persons worldwide; it is estimated that up to 30–35% of all cases are pharmacoresistant. In particular, seizures originating from the temporal lobe tend to progress in severity and frequency and may become refractory to treatment. Epileptogenesis refers to a complex process, involving epigenetic changes, inflammatory mechanisms, glial activation, and reorganization of neuronal circuitry, during which a normal brain becomes epileptic (Pitkanen and Lukasiuk, 2011; Vezzani et al., 2011). Antiepileptogenic and disease-modifying therapies that halt disease progression are therefore urgently needed. A ketogenic diet (KD) is a high-fat, low-carbohydrate and restricted-protein diet that is often a last resort to treat epilepsy in children who are resistant to antiepileptic drugs (Kossoff and Rho, 2009). Beyond its anticonvulsant effects, clinical evidence suggests that a KD might also have antiepileptogenic effects. Follow-up studies of epileptic children treated with a KD suggest that the diet may afford long-lasting seizure protection even after its discontinuation (Caraballo et al., 2011). Whether this stems from an antiepileptogenic effect of the diet or simply reflects spontaneous remission of seizures cannot be decided. Unlike the clear anticonvulsant success of the KD, which has been related to metabolic changes induced by the diet (Bough, 2008; Yellen, 2008), the potential antiepileptogenic activity of a KD remains poorly documented and evaluated.
We recently provided direct evidence that changes in DNA methylation patterns are a key determinant of the progression of epilepsy and that treatment with anticonvulsants that boost adenosine may reverse DNA hypermethylation and break the cycle of increasing seizure severity. Accordingly, adenosine has been identified as an endogenous agent of the brain with potent and long-lasting antiepileptogenic properties (Williams-Karnesky et al., 2013). Because a KD was shown to suppress seizures by augmenting adenosine signaling in the brain (Masino et al., 2011) and because adenosine has not only anticonvulsive, but also antiepileptogenic properties (Williams-Karnesky et al., 2013), we hypothesized that a KD might prevent epileptogenesis or halt disease progression via augmentation of adenosine signaling. We tested the antiepileptogenic effect of a KD in two different rodent models of epileptogenesis and show consistent increases in seizure thresholds and decreases in spontaneous seizure activity, which were maintained even after reversal to a normal diet. Seizure suppression in KD-fed epileptic animals was in line with a rise in brain adenosine and a sustained decrease in DNA methylation status.
2. Materials & Methods
2.1. Animals, drug, and diet treatment
All animal procedures were conducted in accordance with protocols approved by the Legacy Institutional Animal Care and Use Committee (IACUC) and the principles outlined in the NIH Guide for the Care and Use of Laboratory Animals. All studies were performed in adult male CD-1 mice or Wistar rats (Charles River Laboratories, Wilmington, MA, USA). Pentylenetetrazole, pilocarpine, scopolamine, and valproic acid (all from Sigma Aldrich, St. Louis, MO, USA) were dissolved in 0.9% w/v saline to achieve the desired dosages via the intraperitoneal (i.p.) or subcutaneous (s.c.) routes. Ketogenic diet (KD: 8.6% protein w/w, 75.1% fat w/w, 3.2% carbohydrates w/w) for rodents was obtained from Bio-Serv (#F3666, Bio-Serv, Frenchtown, NJ, USA) and supplied ad libitum. Caloric composition of the KD is: 93.4% fat, 4.7% protein, and 1.8% carbohydrates. Standard chow (control diet, CD) with a caloric composition of 13.5% fat, 28.5% protein, and 58.0% carbohydrates was used in control animals or after diet reversal.
2.2. Pentylenetetrazole (PTZ) kindling
Kindling is a process whereby repeated stimulation of the brain initiates permanent alterations in neural circuitry, resulting in increased convulsive response to the same stimulus. Kindling paradigms are therefore widely used to assess the efficacy of potential antiepileptogenic treatments (Loscher, 2002). We used a chemical kindling paradigm, based on the repeated administration of subconvulsant doses of PTZ (El Yacoubi et al., 2008). Prior to the induction of kindling, 4 month old mice were randomly assigned to one of three groups: CD, KD-I, or KD-II. Mice were fed either a KD or CD for 8 weeks, and KD treatment was maintained throughout the 29 day kindling period. During chemical kindling, sub-convulsive doses of PTZ were injected i.p. every other day (including weekends) adhering to the following schedule: 25 mg/kg from study day 1–15 and 30 mg/kg from day 17–29. After each PTZ injection, mice were monitored 30 min for incidence and severity of seizures using a modified Racine scale (0 = no response, 1 = mouth and facial jerks, 2 = nodding or myoclonic body jerks, 3 = forelimb clonus, 4 = rearing, falling down, hindlimb and forelimb clonus). For diet reversal, half of the animals on the KD received a glucose injection (30% w/v; 2 g/kg) on study day 29 after the last kindled seizure, and those mice were then reverted to CD feeding for the remainder of the experiment. Five days later, all animals were subjected to a single 30 mg/kg, i.p. dose of PTZ to assess the maintenance of previous seizure thresholds. Identical protocols for kindling and seizure threshold studies were followed when kindling a different set of animals in the presence or absence of the antiepileptic drug valproic acid (VPA, 200 mg/kg, i.p.). In those studies VPA was injected 30 min prior to each PTZ injection.
2.3. Pilocarpine model of epileptogenesis
Temporal lobe epilepsy (TLE) is characterized by repeated spontaneous seizures that are initiated in the hippocampal formation. A period of prolonged status epilepticus (SE) initiates epileptogenic processes leading to spontaneous convulsive seizures and histologic changes associated with TLE (Dudek et al., 2006). SE was induced in adult male Wistar rats (250–275 g) with pilocarpine (280 mg/kg, i.p.) (Klitgaard et al., 1998). Scopolamine (1 mg/kg, s.c.) was injected 30 min before pilocarpine to reduce peripheral cholinergic effects. Animals that experienced no SE during 60 min after the first pilocarpine administration were treated a second time with half the initial dose. Animals that did not develop SE after the second dose were excluded from further experimentation. SE was terminated 2 hours after its onset with diazepam (5 mg/kg, s.c.) to reduce post-ictal mortality. Only rats that exhibited at least 2 hours of convulsive Racine stage-4 seizures were used. Control rats were treated with saline (0.9 % w/v NaCl, i.p.) instead of pilocarpine, but likewise received scopolamine and diazepam. Starting 3 weeks after the initial SE, all animals were intermittently (3–4 week bins) video monitored (24 hours/day, 7 days/week) to quantify the number of convulsive stage 4–5 seizures. All rats were video monitored at the same time, and all captured videos were included in the analysis. A custom “software robot” was utilized to create sequential 8 hour video files. To account for occasional video loss due to technical difficulties, seizures per week calculations were normalized to the number of hours analyzed. Diet treatment was initiated after the conclusion of week 6 after the SE. At this time point all animals had a seizure rate of at least 3 seizures per week. All animals were monitored for up to 23 additional weeks by intermittent video monitoring and retrospective scoring and analyses performed by investigators blinded to the experimental conditions. Behavioral seizures were confirmed by EEG analysis in selected animals, with bilateral recording screw electrodes implanted at stereotaxic coordinates: AP, −4.5 mm; ML, ±4.0 mm. Electrical brain activity was amplified (Grass Technologies) and digitized (PowerLab; AD Instruments). EEG seizure activity was defined as high-amplitude rhythmic discharges that clearly represented a new pattern of tracing that lasted at least 5 seconds.
2.4. Quantification of β-hydroxybutyrate
Blood levels of β -hydroxybutyrate (BHB) were measured by Precision Xtra ketone test strips (Abbott Laboratories). Blood was collected from the lateral tail vein on study day 18. Increased blood concentration of BHB was taken as evidence of ketonemia in animals fed with the KD.
2.5. Adenosine Quantification
After decapitation, the brain was removed and hippocampal tissue dissected out, frozen in liquid nitrogen, and stored at −80 °C. Adenosine analyses were performed on a high performance liquid chromatography (HPLC) system coupled to a multiple wavelength detector (Agilent 1100 series). Samples were eluted on a C18 column with a particle size of 5 µm and a flow rate of 0.8 mL/min using a mobile phase containing water, acetonitrile and methanol (88:7:5, %v/v). The retention time of adenosine was around 6 min at a detection wavelength of 258 nm. All peak areas were within the linear range of the standard curves. Adenosine values were extrapolated from the linear regression curve calculated on the basis of standard solutions. Extracellular adenosine levels are presented as the mean of four samples.
2.6. DNA methylation assay
Total genomic DNA was isolated from fresh frozen tissues using a DNeasy Blood and Tissue Kit (Qiagen). Global DNA methylation status was assessed using commercial 5-mC DNA ELISA kits (Epigentech or ZymoResearch) as per the manufacturer’s instructions and as described previously (Williams-Karnesky et al., 2013). Samples shown in each figure were run in a single assay, and are normalized to the control CD group.
2.7. Statistics
Quantitative data were analyzed using GraphPad Prism software (Version 6.02). Data sampled at continuous intervals was analyzed by repeated measures ANOVA (RM-ANOVA), with post hoc Fisher’s least significant differences (LSD) comparisons and presented as mean ± SEM in a line graph. Data sampled at non-continuous intervals was analyzed by t-Test at representative time points and presented in a bar graph, unless otherwise noted. Changes in mouse ketone levels, and rat average seizure durations were analyzed by one-way ANOVA, with post hoc Fisher’s LSD comparisons. Rat seizure rates were compared at each time point examined by t-Test. Rat adenosine and 5-mC levels were analyzed using two-way ANOVA, with post hoc Fisher’s LSD comparisons. Significance indicated in figures is by post hoc test, unless noted.
3. Results
3.1. A ketogenic diet attenuates kindling epileptogenesis in mice
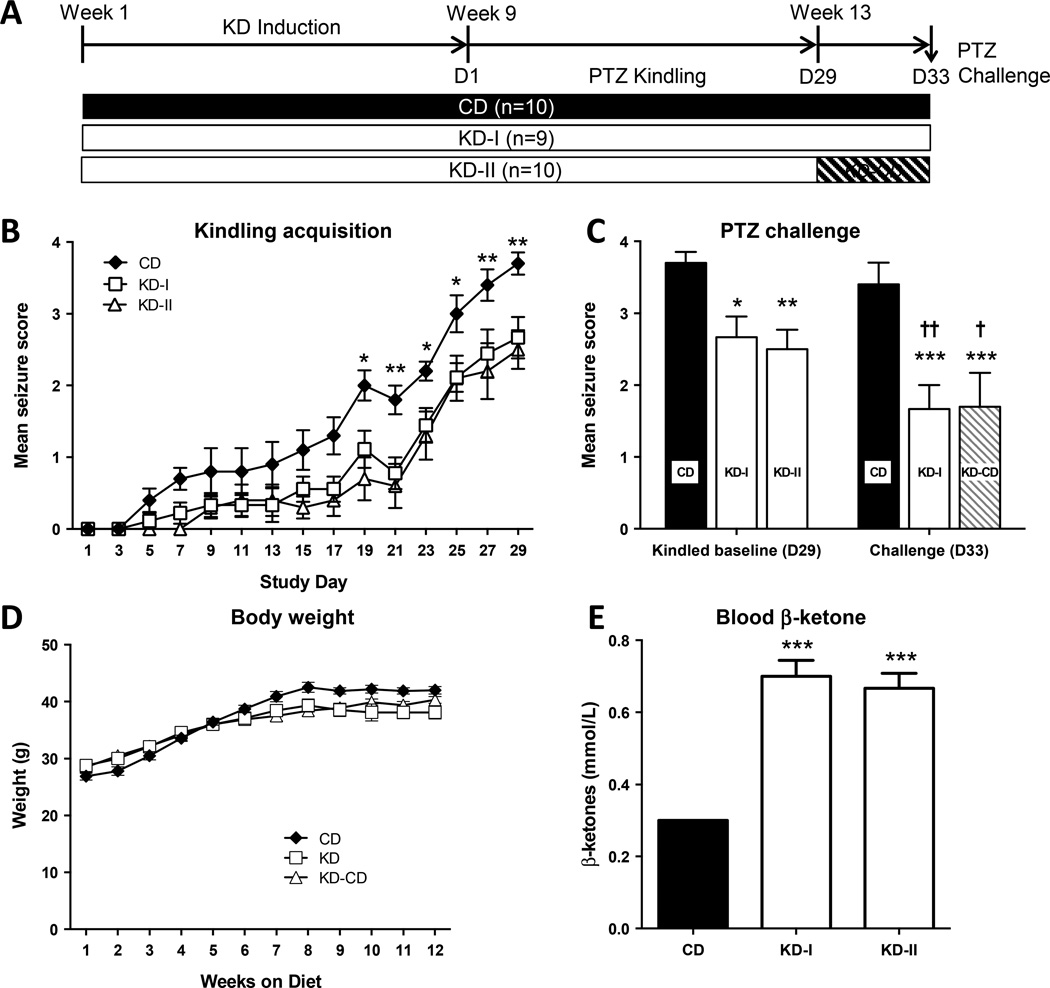
To test whether KD therapy can attenuate the development of kindling-epileptogenesis in adult animals, we fed 4 month old male CD-1 mice KD chow (two separate groups KD-I and KD-II, n=9–10 each). Age-matched controls (n=10) were maintained on a CD. PTZ kindling was initiated after 8 weeks of KD induction (Fig. 1A). Seizures gradually became more severe towards the end of the PTZ kindling period, and maintenance of the kindled state in CD-fed animals after a challenge 4 days later suggested acquisition of a kindled state. Overall, the average seizure scores in the KD-fed animals were substantially lower during the later stages of kindling when compared with CD-fed animals (RM-ANOVA, F2,26 = 13.25, P = 0.0001) (Fig. 1B). Post hoc comparison of the KD-I and KD-II groups demonstrated no difference between the two groups at any time point prior to the diet switch (study day 1 – 29). Seizures became more severe towards the end of the PTZ kindling period, regardless of diet. After 15 stimulations, 100% of all CD-fed animals displayed stage-3 seizures or higher, whereas in KD-fed animals only 37% of all animals reached comparable seizure stages. Overall, more PTZ injections were necessary to induce seizures of similar severity in animals maintained on a KD compared to animals fed with a CD (right-shift of kindling curve), indicating that the KD delayed kindling epileptogenesis. This finding is in contrast to an acute seizure test, in which a single ictogenic dose of PTZ (50 mg/kg) was delivered to an independent set of seizure naïve animals after 8 weeks of KD or CD feeding. In this experiment, KD treatment did not significantly modify seizure severity (CD: 3.0 ± 0.4, n=10; KD: 3.8 ± 0.1, n=10, p = 0.068), a finding consistent with previous experiments showing no effect of a KD on acute PTZ-induced seizures after 2–4 weeks of KD feeding (Samala et al., 2008; Uhlemann and Neims, 1972). Thus, the effects of a KD on PTZ seizures are restricted to a chronic PTZ administration protocol. Together, these results corroborate earlier studies (Hansen et al., 2009) demonstrating that a KD exhibits antiepileptogenic effects in the mouse PTZ kindling model. Regardless of diet, animals continuously gained weight until week 8, after which bodyweight stabilized and remained constant until the end of experimental testing (Fig. 1D). KD-induced ketonemia was confirmed by a significant increase in blood β-ketone levels in animals fed with a KD (one-way ANOVA, F2,15=39.12, P=0.0001) (Fig. 1E). These findings validate the effectiveness of the KD in elevating blood ketone levels.
Figure 1. Suppression of PTZ kindling epileptogenesis by a KD in adult mice.
(A) Experimental design and timeline. Animals were maintained on a control diet (CD) or on a ketogenic diet (two independent groups, KD-I and KD-II). Kindling started at D1 in all groups. (B) Suppression of kindling epileptogenesis by a KD is suggested by a right-shift of the kindling curve. Starting at day 19, the average seizure score was significantly reduced in the KD-fed groups compared to control (** P < 0.01; * P < 0.05). (C) Following the kindling stimulus on day 29, mice in the KD-II group were returned to a control diet for 4 days, when they received a PTZ challenge. Kindling suppression is observed on D29 in both KD groups, and maintained on D33, even after diet reversal (*P < 0.05, **P < 0.01, ***P < 0.005 with respect to the date matched CD fed group). Seizures in the CD fed mice were consistent between D29 and D33, and reduced in both KD-fed groups on D33 († P < 0.05, †† P < 0.01 with respect to the diet matched D29 group). (D) Weight increased during the first 6 weeks on the diet, and was stable during kindling (weeks 8–12). (E) Blood ketone levels as determined at study day 18 in a subset of CD and KD treated animals (n = 6 each); *** P < 0.0001 with respect to CD.
3.2. Antiepileptogenic effect of the KD is maintained after diet reversal
A single injection of glucose is known to terminate the acute effects of KD therapy immediately (Kawamura et al., 2014; Masino et al., 2011). Therefore, glucose injection of KD-fed animals followed by reversal to a CD is an experimental strategy to distinguish antiictogenic effects of KD therapy during kindling from lasting antiepileptogenic effect after diet reversal. To distinguish between both possibilities we used a diet reversal design whereby the KD-II group was injected with glucose (30% w/v; 2 g/kg) and reverted to CD (KD-CD, n=10) after completion of PTZ kindling (day 29) (Fig. 1A). Four days following the diet switch, all three groups of mice (CD, KD-I, and KD-CD) were challenged with a single injection of PTZ (30 mg/kg) to quantify seizure susceptibility (D33) (Fig. 1C). We found a significant main effect of the challenge (RM-ANOVA, F1,26 = 12.41, P = 0.0016), and of the diet (RM-ANOVA, F2,26 = 9.469, P = 0.0008). After 4 days of diet reversal (D33), the mean seizure scores in the KD-I and KD-CD groups were significantly lower compared to the CD group (***P < 0.005), whereas the KD-I and KD-CD groups did not differ from each other (P = 0.942), indicating a lasting antiepileptogenic effect of the KD. Furthermore, while mean seizure scores in the CD subjects were similar at D29 and D33 as would be expected in successfully kindled animals, KD-I and KD-CD subjects showed lower seizures scores on D33 compared to D29 (†P < 0.05, ††P < 0.01). Together, these results indicate that a KD exerts long-lasting protective effects against seizures induced by PTZ even after discontinuation of diet therapy, consistent with an antiepileptogenic effect.
3.3. Attenuation of epileptogenesis is independent of seizure suppression during kindling
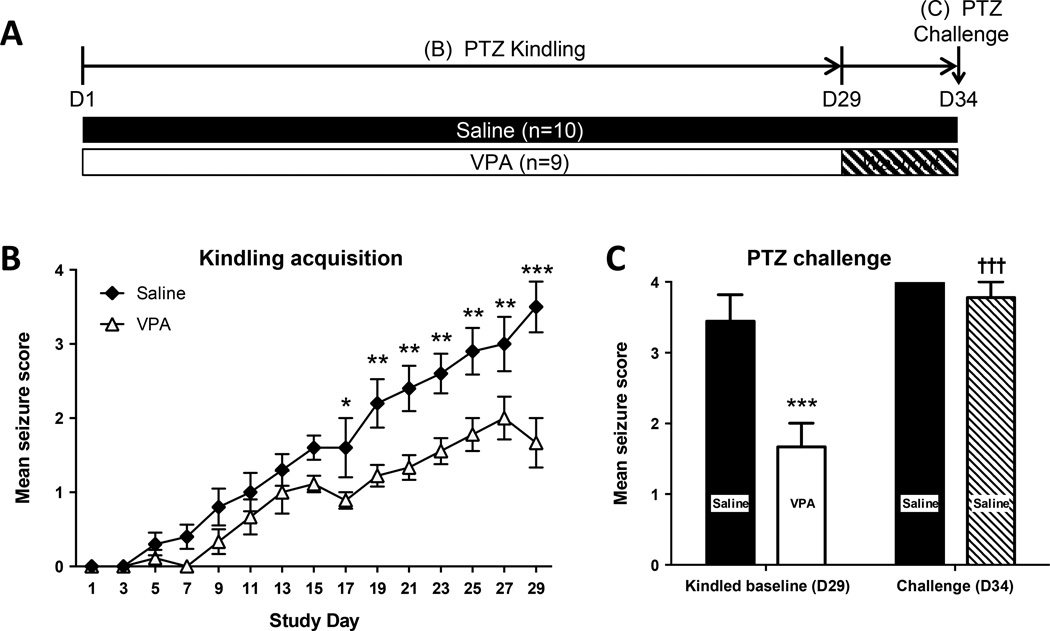
To investigate whether the antiepileptogenic effect in the PTZ kindling model can be attributed to seizure suppression, two additional groups of mice were kindled in the presence or absence of the antiepileptic drug valproic acid (VPA, 200 mg/kg, i.p.) (Fig. 2A). Overall, kindling proceeded more slowly in the VPA-treated mice (F1,17=22.64, P = 0.0002, Fig. 2B). After 5 days of VPA washout (D34), the mean seizure score increased significantly in both the saline treated group (†P < 0.05) and in the VPA group (†††P < 0.005) as compared to the kindled baseline of saline- or VPA-injected animals at D29 (Fig. 2C). Importantly, and in contrast to the KD experiment in Fig. 1 both groups reacted similarly to the challenge at D34 (P>0.05, Fig. 2C). The similarity of the challenge response at D34 and the lack of a lasting effect of VPA after the washout period is in marked contrast to a lasting effect of KD treatment (Fig. 1) and indicates that KD treatment, but not seizure suppression, is mechanistically linked to the enduring antiepileptogenic effects of a KD.
Figure 2. Effects of valproate (VPA) on PTZ kindling.
(A) Mice were kindled with PTZ in the presence of VPA or saline. After VPA washout, the seizure thresholds to PTZ (at D34) were assessed. (B) VPA attenuated kindled seizures (* P < 0.05, ** P < 0.01, *** P < 0.005 vs saline). (C) Effects of a PTZ challenge before (D29) and after (D34) the VPA washout period. At D29 the combination of VPA and PTZ resulted in a significant attenuation of the mean seizure score compared to the saline treated mice (*** P < 0.0001). Mean seizure score in the VPA washout group (D34) was the same as the saline control, but significantly higher than the D29 VPA time point (††† P < 0.0001).
3.4. KD prevents disease progression in a rat model of chronic epilepsy
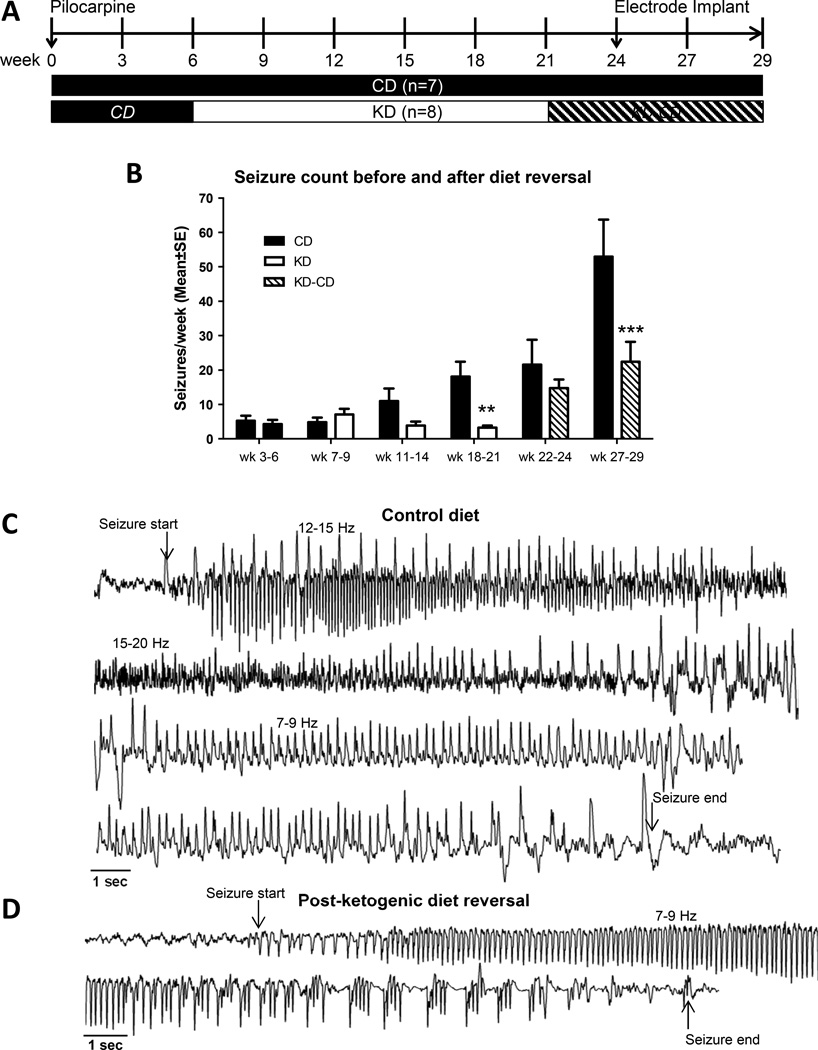
While epilepsy can result from a variety of underlying triggers (e.g. febrile status epilepticus or traumatic brain injury) the goal of this study was to target common mechanisms of disease progression that become amenable to treatment after disease onset. We followed here a unique approach by initiating antiepileptogenic treatment after disease onset, thereby setting ourselves apart from most research efforts that initiate ‘antiepileptogenic’ treatments before, during, or shortly after a precipitating event. To ascertain whether a KD can prevent seizures and disease progression in a model of chronic epilepsy, we evaluated its effects in the rat pilocarpine model, which is characterized by an initial precipitating injury, a latent period, hippocampal sclerosis and reorganization of neuronal networks leading to spontaneous epileptogenicity in the limbic system (Curia et al., 2008). Spontaneous convulsive seizures in this model increase in severity and frequency over time and reflect ongoing disease progression and continuous epileptogenesis. A group of epileptic rats (n = 15) was prepared by pilocarpine-induced SE and subjected to CD or KD treatment followed by diet reversal to allow a critical assessment of lasting antiepileptogenic effects (Fig. 3A). Epilepsy progression was intermittently video monitored in 3 to 4 week bins starting 3 weeks after the systemic administration of pilocarpine (Fig. 3A). Continuous epileptogenesis over 29 weeks in animals on the CD was evidenced by a progressive increase in the number of seizures after the initial SE (Fig. 3B). KD treatment was initiated 6 weeks after the SE in half of the animals and maintained for 15 weeks (Fig. 3A). At this time (week 21) all animals received an intraperitoneal injection of glucose (30% w/v; 2 g/kg) to terminate the effects of the KD, and the KD animals were switched to a CD (KD-CD), which was maintained until termination of the experiment 8 weeks later (Fig. 3A). Seizure frequency was assessed by video scoring in 3–4 week time bins. As expected, KD treatment had a delayed onset, with no influence on seizure frequency during weeks 7–9 and 11–14 (P = 0.7161 and 0.2511, respectively). During weeks 18–21 after the SE, there was a significant reduction of spontaneous recurrent seizures in the KD fed rats (P = 0.0184) compared to spontaneous recurrent seizures in the CD fed rats during the same time frame. Seizure rate and severity in the CD-fed animals increased throughout the 29 week study, reflecting ongoing epileptogenesis and disease progression (Fig. 3B). During the first 3 weeks after diet reversal (weeks 22–24), rats that had been on the KD had a slightly attenuated seizure phenotype compared to CD-fed rats (P = 0.2744). The lack of statistical significance might be due to an initial rebound effect after diet reversal in the KD-CD group. Importantly, 6–8 weeks after diet reversal (wk 27–29, Fig. 3B) the KD-CD group had significantly fewer seizures than the CD group (P < 0.0001), indicating a lasting protective effect of the KD. To confirm and validate the epileptic phenotype, four animals from each group received cortical electrodes and seizures scored on video were validated in the EEGs of those animals from week 27–29 after pilocarpine. Animals on the CD displayed robust prolonged seizures showing typical evolution of seizure activity with different patterns and frequencies (Fig. 3C), while seizure activity was strikingly attenuated (Fig. 3C) in KD-fed rats after diet reversal (Fig. 3D). After completion of the seizure recording in week 29 all brains were harvested for adenosine and 5-methylcytosine (5-mC) analysis.
Figure 3. Antiepileptogenic effects of a KD in the rat pilocarpine model of chronic epilepsy.
(A) Experimental design. 6 weeks after pilocarpine induced SE, animals were randomly assigned to ketogenic diet (KD) therapy or control diet (CD). Diet reversal to control diet (KD-CD) was implemented in week 21. Spontaneous convulsive seizures were assessed by intermittent video monitoring, with additional EEG recordings in weeks 28 and 29. (B) Number of seizures per week as determined by video monitoring and analysis during indicated weekly (wk) bins. Seizures in the KD-fed group were significantly reduced compared to control in weeks 18–21 while still on KD (*P < 0.05), and in weeks 27–29, after diet reversal (***P < 0.005). (C) Sample EEG recorded from an animal fed control diet in week 29. A single representative seizure is shown and split across 4 lines. (D) Sample EEG recorded from KD-fed rat after diet reversal in week 29. A single representative seizure is shown and split across 2 lines.
3.5. KD increases adenosine in the epileptic brain
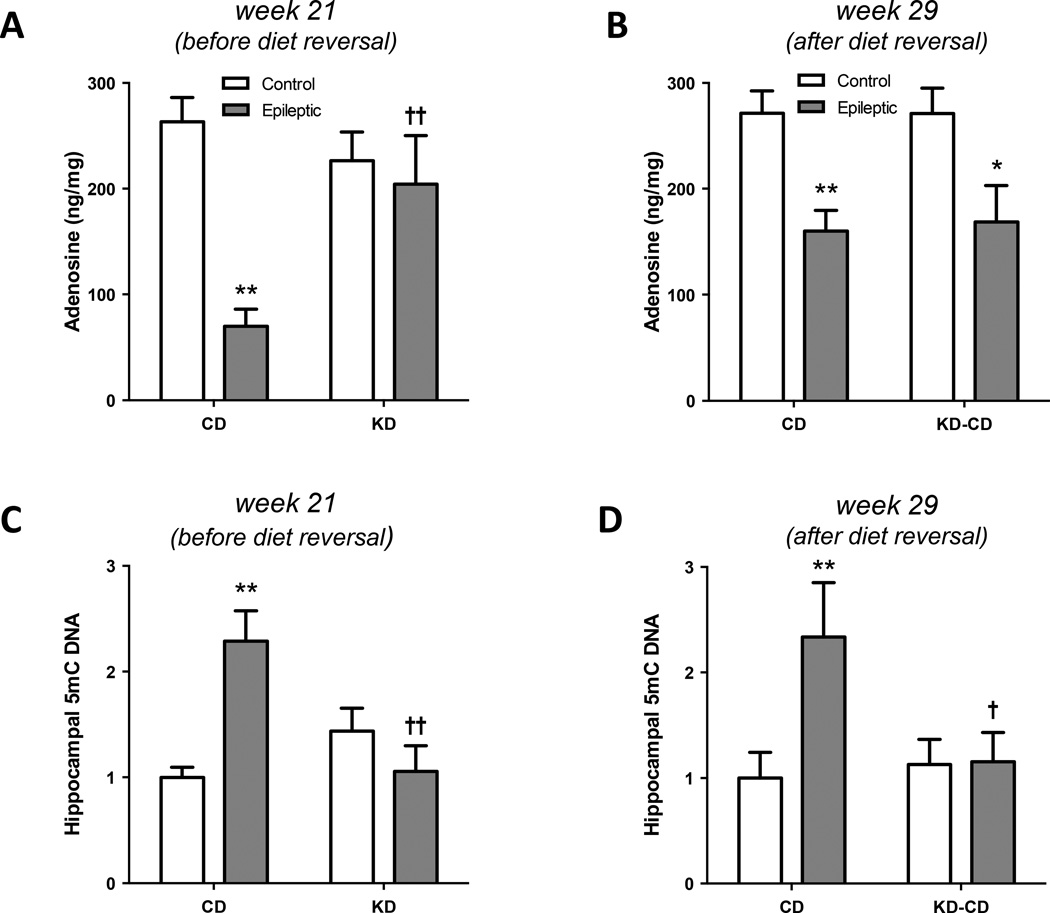
To provide mechanistic insight into the antiepileptic effects of the KD in this model, an additional set of CD and KD fed epileptic animals was generated and sacrificed in week 21, while still on the diet regimen. We found a significant interaction between epilepsy and diet on hippocampal adenosine levels (F1,12 = 6.004, P = 0.0306). Epileptic animals on the CD showed a significant reduction in hippocampal adenosine levels (**P < 0.01) compared to non-epileptic controls, in line with adenosine deficiency being a pathological hallmark of the epileptic brain (Aronica et al., 2013; Boison, 2012). Importantly, a KD restored adenosine levels in epileptic animals to those commonly found in healthy control animals (P = 0.2561), indicating that a KD restores adenosine homeostasis in epileptic animals (Fig. 4A). This finding is in line with our previous demonstration that a KD suppressed seizures in transgenic mice by augmentation of adenosine signaling in the brain (Masino et al., 2011). In contrast to brains that were harvested during KD treatment, an elevation of adenosine in the hippocampus of epileptic animals treated with a KD was no longer present 8 weeks after diet reversal, indicating that the increase of hippocampal adenosine observed earlier is a direct effect of the KD (Fig. 4B). We found a significant main effect of epilepsy (F1,19 = 15.17, P = 0.0010), but no remaining influence of diet. Adenosine levels in the epileptic animals were significantly reduced compared to non-epileptic control animals (**P < 0.01 for the CD, *P < 0.05 for the KD-CD) regardless of prior exposure to the KD and consistent with an increased incidence of seizures in the KD-CD group at week 27–29 (adenosine deficient) compared to week 18–21 (adenosine augmentation by KD).
Figure 4. Hippocampal adenosine and 5-methylcytosine content.
(A) Hippocampal adenosine levels were measured from control or epileptic animals fed a CD or KD. Adenosine deficiencies in the epileptic CD-fed rats were reversed in the KD-fed rats (**P < 0.01 vs Control-CD, †† P < 0.01 vs Epileptic-CD). (B) Hippocampal adenosine levels from control or epileptic animals fed a CD or reversed to CD following KD treatment. (**P < 0.01, *P < 0.05 vs diet-matched Control). (C) Hippocampal 5-mC DNA from control or epileptic animals fed a CD or KD. DNA hypermethylation observed in the epileptic CD fed rats was reversed in the epileptic KD-fed rats (**P < 0.01 vs Control-CD, †† P < 0.01 vs Epileptic-CD). (D) Hippocampal 5-mC DNA from control or epileptic animals fed a CD or diet reversed following KD treatment (KD-CD). DNA 5-mC levels were maintained at control levels in the diet reversed (KD-CD) group (**P < 0.01 vs Control-CD, † P < 0.05 vs Epileptic-CD).
3.6. Ketogenic diet therapy reverses DNA hypermethylation long-term
We have previously shown that an adenosine deficit causes DNA hypermethylation through receptor-independent interference with the transmethylation pathway (Williams-Karnesky et al., 2013). Therefore, we reasoned that a KD might affect DNA methylation in the epileptic brain. To test this hypothesis, we quantified the hippocampal 5-methylcytosine (5-mC) content with an ELISA based assay. In line with general hypermethylation of hippocampal DNA in the epileptic brain (Kobow and Blumcke, 2011; Qureshi and Mehler, 2010), the global DNA methylation status in whole hippocampal isolates was increased in epileptic animals maintained on a CD compared with the hippocampal DNA methylation status from non-epileptic control animals maintained on the CD (**P < 0.01). In contrast, the DNA methylation status in KD-fed epileptic animals was reduced to levels similar to those found in either CD- or KD-fed non-epileptic controls (Fig. 4C). In contrast to the acute diet-dependent changes in adenosine levels, the reduction of the global hippocampal 5-mC status in the KD-CD group was maintained over at least 8 weeks after diet reversal, indicating long-lasting epigenetic changes induced by the KD treatment (Fig. 4C). The maintenance of DNA methylation changes even after diet reversal supports the hypothesis that the reduced seizure incidence in weeks 27–29 in the KD-CD group compared to the CD group (Fig. 3B) is due to an antiepileptogenic effect of the KD, linked to a long-lasting epigenetic mechanism, but independent of an adenosine-dependent antiictogenic effect.
4. Discussion
Here, we provide direct evidence in two mechanistically different models of epilepsy (i) that a KD increases hippocampal adenosine, an effect limited to the duration of the KD treatment, (ii) that a KD effectively suppresses seizures in adult mice and rats, and (iii) that a KD attenuates disease progression and epileptogenesis long-term. We further show that this antiepileptogenic disease-modifying effect was specific to KD therapy and independent of seizure suppression. Because KD therapy increases adenosine signaling in the brain and because adenosine prevents epileptogenesis through an epigenetic mechanism (Williams-Karnesky et al., 2013), we suggest that the profound antiepileptogenic activity of KD therapy demonstrated here is directly dependent on the adenosine augmenting properties of KD therapy.
4.1. Evidence for antiepileptogenic effects of KD therapy
Although limited clinical studies suggest remission of epilepsy following KD therapy in at least a subset of pediatric patients (Caraballo et al., 2011), controlled clinical studies to assess antiepileptogenic effects of KD therapy have not been undertaken. Clinical antiepileptogenesis studies are limited by the fact that KD therapy is often used as a ‘last resort’ in highly refractory patients. A valid clinical study would need to initiate KD therapy immediately after the initial diagnosis of epilepsy. This is a challenging approach in terms of clinical study design and regulatory requirements. In addition, the underlying mechanisms of long-lasting effects of KD therapy are poorly understood and rodent studies have yielded conflicting results. Importantly, our studies were performed in adult mice and rats to avoid developmental confounds associated with KD therapy in developing rodents. Immature weanling rats subjected to lithium/pilocarpine induced SE and then treated with KD therapy developed significantly less spontaneous seizures when reaching adulthood, but showed impaired brain growth and deficits in visual-spatial memory, suggesting that KD therapy might interfere with normal brain development in immature rodents (Zhao et al., 2004). In contrast, adult rats that were subjected to lithium/pilocarpine induced SE three weeks after the onset of KD treatment showed neuroprotection in the hippocampus (Linard et al., 2010), but antiepileptogenic effects in this study could not be determined because epileptogenesis might have been confounded by onset of diet treatment prior to triggering epileptogenesis and because of the high experimental variability within the diet treatment groups. Although not statistically significant, in this study 3 out of 24 KD treated animals did not develop epilepsy within 70 days after the SE, whereas all of 15 control diet fed animals developed epilepsy during the same time frame (Linard et al., 2010). A more recent study demonstrated robust attenuation of pilocarpine-induced SE in rats that were pretreated with a triheptanoin supplemented KD (Gama et al., 2015). Similarly, rats electrically kindled in the amygdala in the presence of a KD showed attenuation of kindling epileptogenesis, however a diet reversal paradigm to study lasting antiepileptogenic effects has not been performed (Jiang et al., 2012). Although the latter three studies show beneficial effects of KD therapy, disease progression after onset of epilepsy has not previously been investigated. The studies above including our own are limited to chemoconvulsants and electrical kindling-based epilepsy models. It would be worthwhile to investigate whether KD therapy exerts similar antiepileptogenic effects in pediatric and symptomatic generalized epilepsies.
4.2. Novel study design to assess antiepileptogenesis
A recent comprehensive review on antiepileptogenic strategies (Loscher and Brandt, 2010) suggests that the majority of published ‘antiepileptogenesis’ studies are ‘prophylactic’. In most cases the ‘antiepileptogenic’ treatment was initiated during or shortly after an epilepsy precipitating event (e.g. SE). This common approach is problematic for two reasons: (i) The proposed treatment may directly interfere with the quality and or quantity of the epilepsy initiating trigger. Unless this potential confound is carefully controlled for, it may be impossible to distinguish true antiepileptogenic effects from factors that modulate the precipitating injury. For example an anticonvulsant or neuroprotective drug may reduce the epilepsy precipitating seizure severity or neuronal cell loss, respectively. (ii) From a translational standpoint, prophylactic therapies would require the identification of subjects that develop epilepsy after a precipitating event from those that don’t. Currently, a reliable biomarker to identify persons at risk for developing epilepsy is not yet available. Therefore, implementation of antiepileptogenic treatments immediately after the diagnosis of epilepsy appears to be more pragmatic.
In many forms of epilepsy, including TLE, epileptogenesis is a continuous process leading to a progressive course of epilepsy including an increased seizure load, an increase in comorbid symptoms, and the development of pharmacoresistance (Kobow et al., 2012; Kobow and Blumcke, 2011; Pitkanen and Engel, 2014; Pitkanen et al., 2007; Pitkanen and Lukasiuk, 2009; Qureshi and Mehler, 2010; Stables et al., 2002; van Vliet et al., 2014). Therefore, antiepileptogenic treatments initiated after disease onset, i.e. ideally immediately after diagnosis would still have the potential to halt further disease progression, and to avoid the development of pharmacoresistance and major comorbidities. With this rationale in mind we designed a here a novel treatment paradigm tailored to (i) avoid interference with the epileptogenesis triggering SE and (ii) to adhere to a clinically translatable scenario. Using the rat pilocarpine model of progressive TLE we initiated KD treatment after the diagnosis of epilepsy, i.e. at a time point when all animals had developed a seizure rate of several spontaneous seizures per week. This novel procedure to assess antiepileptogenesis also allowed us to reduce variation within groups and to monitor epilepsy progression long-term.
4.3. Distinction between anticonvulsant and antiepileptogenic effects
A conceptual challenge in the development of antiepileptogenic strategies is the distinction between anticonvulsant effects that suppress seizures (‘antiictogenic’) from those that interfere with development and/or progression of epilepsy (‘antiepileptogenic’). This challenge has elegantly been solved in the rat kindling model, where antiepileptogenic properties of conventional antiepileptic drugs have been studied by first kindling the animals in the presence of the drug, followed by a washout period, and continued kindling in the absence of the drug. Reduced kindling stages after drug washout were interpreted as antiepileptogenic (Silver et al., 1991). Using a similar experimental approach we previously demonstrated antiepileptogenic effects of adenosine in the rat kindling model (Szybala et al., 2009). Here we used an innovative diet reversal paradigm to distinguish antiictogenic from antiepileptogenic effects in our two rodent models of epileptogenesis. KD therapy attenuated seizure activity during PTZ kindling, an effect that could be attributed to the well characterized anticonvulsant properties of KD therapy; however, following diet reversal to a control diet significantly lower seizure scores were maintained, suggesting a prominent antiepileptogenic effect (Fig. 1 B, C). In contrast, valproic acid did not have any effect on seizure reduction following a 5 day drug washout period, suggesting that valproic acid in this model is anticonvulsant, but not antiepileptogenic (Fig. 2). Likewise, in the rat pilocarpine model we observed robust suppression of seizure activity during active KD treatment, which might be due to seizure suppression and/or antiepileptogenic effects (Fig. 3B). Maintenance of seizure reduction compared to control animals 6 to 9 weeks after diet reversal again suggests lasting antiepileptogenic effects of KD therapy several weeks beyond the discontinuation of KD treatment. Future longer term studies over several months will be needed to assess whether these antiepileptogenic effects lead to a permanent modification of the epilepsy phenotype. In two different experimental paradigms we demonstrated at least partial antiepileptogenic effects of KD therapy. Since the therapeutic effects of KD therapy require strict adherence to severe (and non-palatable) dietary restrictions and are not apparent until the diet has been in effect for several weeks, it might not be a practical approach for epilepsy prevention during the latent period of epileptogenesis, when preventive therapies might be most effective. Therefore, mechanistic understanding of the epileptogenic properties of KD therapy is essential to translate the KD into a more widely accessible antiepileptogenic therapy.
4.4. Mechanisms of KD therapy
Ketogenic diets have been used to treat epileptic seizures for over 90 years, yet until recently the underlying mechanisms of KD therapy have remained enigmatic. The exact combination of acute vs. chronic mechanisms and their relative importance are still under investigation, but it is likely that several combine to suppress seizures. Metabolic therapy with the KD reduces available glucose and forces the brain to use ketones as a primary energy source (Kawamura et al., 2014). Metabolic changes leading to disruption of glutamatergic synaptic transmission, inhibition of glycolysis, increase in adenosine, and activation of ATP-sensitive potassium channels are thought to underlie the therapeutic effects of this type of dietary intervention (Bough, 2008; Lutas and Yellen, 2013; Masino et al., 2011; Yellen, 2008). In addition, KD-therapy was shown to exert epigenetic effects on the DNA methylome, however the underlying mechanism has not been identified (Kobow et al., 2013). A growing body of evidence supports the idea that a ketogenic diet leads to increased adenosine signaling in the brain most likely via reduced expression of adenosine kinase (ADK) (Masino et al., 2011). Our findings of increased hippocampal adenosine in epileptic animals fed with a KD, alongside with reduced seizures in those animals (Fig. 3B and 4A) provide the first direct evidence to support previous work indicating that adenosine augmentation is one of the anticonvulsant mechanisms of the diet. This effect was specific, since after diet reversal, the adenosine concentrations of the previously KD-fed animals were equal to the control group (Fig. 4B). Metabolic therapies can also affect gene expression, providing a possible explanation for antiepileptogenic effects of the diet. It was shown that the glycolytic inhibitor 2-deoxy-d-glucose potently reduced the progression of kindling epileptogenesis and blocked seizure-induced increases in brain-derived neurotrophic factor (BDNF) and its receptor, TrkB. This effect was dependent on neuron-restrictive silencer factor, a transcription factor which recruits the NADH-binding C-terminal binding protein, a co-repressor which generates a repressive chromatin environment around the BDNF promoter (Garriga-Canut et al., 2006). However, recent work suggests neuron-restrictive silencer factor may not be required for the effects of a KD (Hu et al., 2011) suggesting there may be both common and disparate mechanisms among metabolic therapies. Together, these findings suggest that metabolic therapies can affect epilepsy on multiple levels by a combination of receptor mediated and epigenetic mechanisms, which may lead to long-term alterations of neuronal circuitry. Although not investigated here, several of the mechanisms of KD therapy mentioned here might contribute to the antiepileptogenic effects of the diet. For example, KD therapy increases β-hydroxybutyrate, an endogenous histone deacetylase inhibitor, which could be a mechanistic basis linking KD therapy with epigenetic modification of gene expression (Shimazu et al., 2013). Because KD therapy augments adenosine (Masino et al., 2011) and because adenosine prevents epileptogenesis (Williams-Karnesky et al., 2013) we focused here on the adenosine-related effects of KD therapy, which however does not exclude the possible contribution of additional mechanisms to the antiepileptogenic effects of metabolic therapies.
4.5. Adenosine – an epigenetic mediator of KD therapy
The purine ribonucleoside adenosine is a well-recognized endogenous anticonvulsant and seizure terminator (Dunwiddie, 1980). Its anticonvulsant effects are largely based on activation of presynaptic G-protein coupled adenosine A1 receptors (A1R), which provide presynaptic inhibition of glutamate release, and by activation of postsynaptic A1Rs, which stabilize the postsynaptic membrane potential by promoting G-protein coupled inwardly rectifying potassium currents (Chen et al., 2013). Our data showing increased hippocampal adenosine (Fig. 4A) and seizure suppression during KD treatment (Fig. 3B) are consistent with the A1R-dependent mechanism of seizure suppression. Accordingly, adenosine augmentation therapy is highly effective in the suppression of kindled seizures (Huber et al., 2001). Recently, an additional, adenosine receptor-independent function of adenosine as regulator of the DNA-methylome has been identified (Williams-Karnesky et al., 2013). Adenosine is an obligatory end product of biochemical transmethylation reactions, which transfer methyl groups from S-adenosylmethionine (SAM) to an acceptor, such as DNA and result in the formation of S-adenosylhomocysteine (SAH), which subsequently is cleaved into adenosine and homocysteine. If adenosine is not constantly removed by ADK, the levels of SAH rise and inhibit DNA methyltransferases (Boison et al., 2002). We further provided the first direct evidence that DNA hypermethylation contributed to the progression of epilepsy, since the reversal of the hypermethylated state of hippocampal DNA to normal levels induced by a transient therapeutic dose of adenosine halted ongoing epileptogenesis long-term (Williams-Karnesky et al., 2013).
Since our current data show that a KD increases brain adenosine (Fig. 4A) and because adenosine prevents epileptogenesis through an epigenetic mechanism (Williams-Karnesky et al., 2013), we conclude by association that the antiepileptogenic effects of KD-therapy described here can at least partly be attributed to an adenosine-associated epigenetic mechanism. This conclusion is further supported by recent findings showing that KD-therapy reduced DNA methylation in the hypermethylated epileptic brain (Kobow et al., 2013). Accordingly, in epileptic animals we demonstrate an association of decreased hippocampal adenosine with increased hippocampal 5-mC (Fig. 4A,C) suggesting that the adenosine deficiency – a characteristic of the epileptic brain (Aronica et al., 2011) – contributes to a state of DNA hypermethylation in epilepsy. Increased cytosine methylation (5-mC) is indeed a characteristic finding in chronic epilepsy and thought to be involved in the progression and maintenance of the epileptic state (Kobow and Blumcke, 2011). Likewise, our current findings show a prolonged normalization of the global DNA-methylation status – even after diet reversal – in conjunction with the antiepileptogenic effects induced by a KD (Fig. 3B & 4D). Additional studies are warranted to provide direct causal links between KD therapy, adenosine augmentation, DNA methylation changes and antiepileptogenic effects, for example by combining KD therapy with agents that interfere with the transmethylation pathway.
5. Conclusions
Our present findings show that a KD exerts significant antiepileptogenic effects in two etiologically different rodent models in conjunction with augmentation of adenosine and restoration of global DNA methylation levels. The increase in adenosine is likely to exert epigenetic effects on the landscape of the DNA-methylome, which has independently been shown to be influenced by KD therapy (Kobow et al., 2013). Therefore, adenosine-related epigenetic effects might contribute to the antiepileptogenic effects of the KD demonstrated here. Thus, even transient KD therapy might be uniquely suited to reset the ‘epileptogenic clock’ and to provide long-lasting relief from seizures even after discontinuation of the diet. Clinical studies showing seizure freedom after diet discontinuation support this view (Caraballo et al., 2011; Dressler et al., 2010). At this time, carefully controlled clinical studies are urgently needed to critically assess specific opportunities whereby a KD could lead to persistent seizure remission.
Highlights.
Ketogenic diet therapy attenuates epileptogenesis and disease progression after epilepsy onset in adult rodents.
Ketogenic diet therapy increases adenosine in the brain.
Ketogenic diet therapy restores DNA methylation status in epileptic hippocampus, which is maintained after return to normal diet.
Acknowledgments
This work was supported through grants from the NINDS (R01 NS065957) and the US Department of the Army (W81XWH-12-1-0283). We would like to thank Ariel Weingarten and Ellena Basada for their contributions to seizure analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
D.B. and S.A.M. designed the study and wrote the manuscript. T.A.L and D.R. analyzed data. K.K.A. and S.Q.C. performed experiments.
Competing financial interests
The authors declare no competing financial interests.
References
- Aronica E, Sandau US, Iyer A, Boison D. Glial adenosine kinase - A neuropathological marker of the epileptic brain. Neurochem Int. 2013;63:688–695. doi: 10.1016/j.neuint.2013.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronica E, Zurolo E, Iyer A, de Groot M, Anink J, Carbonell C, van Vliet EA, Baayen JC, Boison D, Gorter JA. Upregulation of adenosine kinase in astrocytes in experimental and human temporal lobe epilepsy. Epilepsia. 2011;52:1645–1655. doi: 10.1111/j.1528-1167.2011.03115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D. Adenosine dysfunction in epilepsy. Glia. 2012;60:1234–1243. doi: 10.1002/glia.22285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D, Scheurer L, Zumsteg V, Rülicke T, Litynski P, Fowler B, Brandner S, Mohler H. Neonatal hepatic steatosis by disruption of the adenosine kinase gene. Proc Natl Acad Sci USA. 2002;99:6985–6990. doi: 10.1073/pnas.092642899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bough K. Energy metabolism as part of the anticonvulsant mechanism of the ketogenic diet. Epilepsia. 2008;49(Suppl 8):91–93. doi: 10.1111/j.1528-1167.2008.01846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraballo R, Vaccarezza M, Cersosimo R, Rios V, Soraru A, Arroyo H, Agosta G, Escobal N, Demartini M, Maxit C, Cresta A, Marchione D, Carniello M, Panico L. Long-term follow-up of the ketogenic diet for refractory epilepsy: multicenter Argentinean experience in 216 pediatric patients. Seizure. 2011;20:640–645. doi: 10.1016/j.seizure.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Chen JF, Eltzschig HK, Fredholm BB. Adenosine receptors as drug targets--what are the challenges? Nat Rev Drug Discov. 2013;12:265–286. doi: 10.1038/nrd3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curia G, Longo D, Biagini G, Jones RS, Avoli M. The pilocarpine model of temporal lobe epilepsy. J Neurosci Methods. 2008;172:143–157. doi: 10.1016/j.jneumeth.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler A, Stocklin B, Reithofer E, Benninger F, Freilinger M, Hauser E, Reiter-Fink E, Seidl R, Trimmel-Schwahofer P, Feucht M. Long-term outcome and tolerability of the ketogenic diet in drug-resistant childhood epilepsy--the Austrian experience. Seizure. 2010;19:404–408. doi: 10.1016/j.seizure.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Dudek FE, Clark S, Williams PA, Grabenstatter HL. Kainate-Induced Status Epilepticus: A Chronic Model of Acquired Epilepsy. In: Pitkanen A, Schwartzkroin PA, Moshe SL, editors. Models of Seizures and Epilepsy. Burlington, MA: Elsevier Academic Press; 2006. pp. 415–432. [Google Scholar]
- Dunwiddie TV. Endogenously released adenosine regulates excitability in the in vitro hippocampus. Epilepsia. 1980;21:541–548. doi: 10.1111/j.1528-1157.1980.tb04305.x. [DOI] [PubMed] [Google Scholar]
- El Yacoubi M, Ledent C, Parmentier M, Costentin J, Vaugeois JM. Evidence for the involvement of the adenosine A(2A) receptor in the lowered susceptibility to pentylenetetrazol-induced seizures produced in mice by long-term treatment with caffeine. Neuropharmacology. 2008;55:35–40. doi: 10.1016/j.neuropharm.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Gama IR, Trindade-Filho EM, Oliveira SL, Bueno NB, Melo IT, Cabral-Junior CR, Barros EM, Galvao JA, Pereira WS, Ferreira RC, Domingos BR, da Rocha Ataide T. Effects of ketogenic diets on the occurrence of pilocarpine-induced status epilepticus of rats. Metab Brain Dis. 2015;30:93–98. doi: 10.1007/s11011-014-9586-4. [DOI] [PubMed] [Google Scholar]
- Garriga-Canut M, Schoenike B, Qazi R, Bergendahl K, Daley TJ, Pfender RM, Morrison JF, Ockuly J, Stafstrom C, Sutula T, Roopra A. 2-Deoxy-D-glucose reduces epilepsy progression by NRSF-CtBP-dependent metabolic regulation of chromatin structure. Nat Neurosci. 2006;9:1382–1387. doi: 10.1038/nn1791. [DOI] [PubMed] [Google Scholar]
- Hansen SL, Nielsen AH, Knudsen KE, Artmann A, Petersen G, Kristiansen U, Hansen SH, Hansen HS. Ketogenic diet is antiepileptogenic in pentylenetetrazole kindled mice and decrease levels of N-acylethanolamines in hippocampus. Neurochem Int. 2009;54:199–204. doi: 10.1016/j.neuint.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Hu XL, Cheng X, Fei J, Xiong ZQ. Neuron-restrictive silencer factor is not required for the antiepileptic effect of the ketogenic diet. Epilepsia. 2011;52:1609–1616. doi: 10.1111/j.1528-1167.2011.03171.x. [DOI] [PubMed] [Google Scholar]
- Huber A, Padrun V, Deglon N, Aebischer P, Mohler H, Boison D. Grafts of adenosine-releasing cells suppress seizures in kindling epilepsy. Proc. Natl. Acad. Sci. USA. 2001;98:7611–7616. doi: 10.1073/pnas.131102898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Yang Y, Wang S, Ding Y, Guo Y, Zhang MM, Wen SQ, Ding MP. Ketogenic diet protects against epileptogenesis as well as neuronal loss in amygdaloid-kindling seizures. Neurosci Lett. 2012;508:22–26. doi: 10.1016/j.neulet.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Kawamura M, Jr, Ruskin DN, Geiger JD, Boison D, Masino SA. Ketogenic diet sensitizes glucose control of hippocampal excitability. J Lipid Res. 2014;55:2254–2260. doi: 10.1194/jlr.M046755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klitgaard H, Matagne A, Gobert J, Wulfert E. Evidence for a unique profile of levetiracetam in rodent models of seizures and epilepsy. Eur J Pharmacol. 1998;353:191–206. doi: 10.1016/s0014-2999(98)00410-5. [DOI] [PubMed] [Google Scholar]
- Kobow K, Auvin S, Jensen F, Loscher W, Mody I, Potschka H, Prince D, Sierra A, Simonato M, Pitkanen A, Nehlig A, Rho JM. Finding a better drug for epilepsy: antiepileptogenesis targets. Epilepsia. 2012;53:1868–1876. doi: 10.1111/j.1528-1167.2012.03716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobow K, Blumcke I. The methylation hypothesis: do epigenetic chromatin modifications play a role in epileptogenesis? Epilepsia. 2011;52(Suppl 4):15–19. doi: 10.1111/j.1528-1167.2011.03145.x. [DOI] [PubMed] [Google Scholar]
- Kobow K, Kaspi A, Harikrishnan KN, Kiese K, Ziemann M, Khurana I, Fritzsche I, Hauke J, Hahnen E, Coras R, Muhlebner A, El-Osta A, Blumcke I. Deep sequencing reveals increased DNA methylation in chronic rat epilepsy. Acta Neuropathol. 2013;126:741–756. doi: 10.1007/s00401-013-1168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossoff EH, Rho JM. Ketogenic diets: evidence for short- and long-term efficacy. Neurotherapeutics. 2009;6:406–414. doi: 10.1016/j.nurt.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linard B, Ferrandon A, Koning E, Nehlig A, Raffo E. Ketogenic diet exhibits neuroprotective effects in hippocampus but fails to prevent epileptogenesis in the lithium-pilocarpine model of mesial temporal lobe epilepsy in adult rats. Epilepsia. 2010;51:1829–1836. doi: 10.1111/j.1528-1167.2010.02667.x. [DOI] [PubMed] [Google Scholar]
- Loscher W. Animal models of epilepsy for the development of antiepileptogenic and disease-modifying drugs. A comparison of the pharmacology of kindling and post-status epilepticus models of temporal lobe epilepsy. Epilepsy Res. 2002;50:105–123. doi: 10.1016/s0920-1211(02)00073-6. [DOI] [PubMed] [Google Scholar]
- Loscher W, Brandt C. Prevention or modification of epileptogenesis after brain insults: experimental approaches and translational research. Pharmacol Rev. 2010;62:668–700. doi: 10.1124/pr.110.003046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutas A, Yellen G. The ketogenic diet: metabolic influences on brain excitability and epilepsy. Trends Neurosci. 2013;36:32–40. doi: 10.1016/j.tins.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masino SA, Li T, Theofilas P, Sandau US, Ruskin DN, Fredholm BB, Geiger JD, Aronica E, Boison D. A ketogenic diet suppresses seizures in mice through adenosine A1 receptors. J Clin Inv. 2011;121:2679–2683. doi: 10.1172/JCI57813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkanen A, Engel J., Jr Past and present definitions of epileptogenesis and its biomarkers. Neurotherapeutics. 2014;11:231–241. doi: 10.1007/s13311-014-0257-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkanen A, Kharatishvili I, Karhunen H, Lukasiuk K, Immonen R, Nairismagi J, Grohn O, Nissinen J. Epileptogenesis in experimental models. Epilepsia. 2007;48(Suppl 2):13–20. doi: 10.1111/j.1528-1167.2007.01063.x. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Lukasiuk K. Molecular and cellular basis of epileptogenesis in symptomatic epilepsy. Epilepsy Behav. 2009;14(Suppl 1):16–25. doi: 10.1016/j.yebeh.2008.09.023. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Lukasiuk K. Mechanisms of epileptogenesis and potential treatment targets. Lancet Neurol. 2011;10:173–186. doi: 10.1016/S1474-4422(10)70310-0. [DOI] [PubMed] [Google Scholar]
- Qureshi IA, Mehler MF. Epigenetic mechanisms underlying human epileptic disorders and the process of epileptogenesis. Neurobiol Dis. 2010;39:53–60. doi: 10.1016/j.nbd.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samala R, Willis S, Borges K. Anticonvulsant profile of a balanced ketogenic diet in acute mouse seizure models. Epilepsy Res. 2008;81:119–127. doi: 10.1016/j.eplepsyres.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, Le Moan N, Grueter CA, Lim H, Saunders LR, Stevens RD, Newgard CB, Farese RV, Jr, de Cabo R, Ulrich S, Akassoglou K, Verdin E. Suppression of oxidative stress by beta-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2013;339:211–214. doi: 10.1126/science.1227166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver JM, Shin C, McNamara JO. Antiepileptogenic effects of conventional anticonvulsants in the kindling model of epilespy. Ann Neurol. 1991;29:356–363. doi: 10.1002/ana.410290404. [DOI] [PubMed] [Google Scholar]
- Stables JP, Bertram EH, White HS, Coulter DA, Dichter MA, Jacobs MP, Loscher W, Lowenstein DH, Moshe SL, Noebels JL, Davis M. Models for epilepsy and epileptogenesis: report from the NIH workshop, Bethesda, Maryland. Epilepsia. 2002;43:1410–1420. doi: 10.1046/j.1528-1157.2002.06702.x. [DOI] [PubMed] [Google Scholar]
- Szybala C, Pritchard EM, Wilz A, Kaplan DL, Boison D. Antiepileptic effects of silk-polymer based adenosine release in kindled rats. Exp Neurol. 2009;219:126–135. doi: 10.1016/j.expneurol.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlemann ER, Neims AH. Anticonvulsant properties of the ketogenic diet in mice. J Pharmacol Exp Ther. 1972;180:231–238. [PubMed] [Google Scholar]
- van Vliet EA, Otte WM, Gorter JA, Dijkhuizen RM, Wadman WJ. Longitudinal assessment of blood-brain barrier leakage during epileptogenesis in rats. A quantitative MRI study. Neurobiol Dis. 2014;63:74–84. doi: 10.1016/j.nbd.2013.11.019. [DOI] [PubMed] [Google Scholar]
- Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nat Rev Neurol. 2011;7:31–40. doi: 10.1038/nrneurol.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams-Karnesky RL, Sandau US, Lusardi TA, Lytle NK, Farrell JM, Pritchard EM, Kaplan DL, Boison D. Epigenetic changes induced by adenosine augmentation therapy prevent epileptogenesis. J Clin Inv. 2013;123:3552–3563. doi: 10.1172/JCI65636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen G. Ketone bodies, glycolysis, and KATP channels in the mechanism of the ketogenic diet. Epilepsia. 2008;49(Suppl 8):80–82. doi: 10.1111/j.1528-1167.2008.01843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Stafstrom CE, Fu DD, Hu Y, Holmes GL. Detrimental effects of the ketogenic diet on cognitive function in rats. Pediatr Res. 2004;55:498–506. doi: 10.1203/01.PDR.0000112032.47575.D1. [DOI] [PubMed] [Google Scholar]