Abstract
Background
Germinal matrix intraventricular hemorrhage (IVH) is the most common type of intracranial hemorrhage observed in preterm neonates. It is a precursor of poor neurocognitive development, cerebral palsy and death. The pathophysiology is not well defined, but damage to the fragile germinal matrix vasculature may be due to free radicals generated during inflammation and as a consequence of ischemia followed by reperfusion. Assessment of the oxidative stress status in these infants is therefore important. Urinary allantoin concentration was measured in preterm neonates as a marker of oxidative stress associated with IVH.
Study design
Urine was collected from 44 preterm neonates at four time points between 24 and 72 hours of life (HOL) and the allantoin content was determined by gas chromatography mass spectrometry (GCMS). Records were retrospectively reviewed and the incidence and severity of IVH was categorized as follows: no IVH (n=24), mild (grade 1-2) IVH (n=13) and severe (grade 3-4) IVH (n=7).
Results
Neonates with severe IVH showed significantly elevated allantoin levels vs subjects with no IVH from 36 HOL (0.098±0.013µmol and 0.043±0.007µmol, respectively, p=.002). The allantoin concentration remained elevated even at 72 HOL (0.079±0.014µmol and 0.033±0.008µmol, respectively, p=.021). There were no significant differences in allantoin levels in the no IVH and mild IVH groups. IVH was diagnosed by head imaging on average at about 11th post-natal day.
Conclusion
Urinary allantoin levels were significantly elevated during the first 3 days of life in the neonates subsequently diagnosed with severe IVH, suggesting that oxidative stress might be a crucial factor in IVH pathogenesis. Further studies are needed to assess the usefulness of urinary allantoin in early identification of preterm infants at risk for or with severe IVH, and monitoring of the response to interventions designed to prevent or treat it.
INTRODUCTION
About 12% of live births in the United States in 2008 were classified as premature and 20-25% of premature babies develop intraventricular hemorrhage (IVH) each year [1]. IVH is the most common type of intracranial hemorrhage that occurs within the ventricular cistern in premature babies ≤ 32 weeks gestation weighing < 1500g [2]. It usually develops during the first week of life, with about 75% of the cases occurring within the first 72 hours and 90% being diagnosed by the end of the first postnatal week [3, 4]. Predisposing risk factors include early gestational age, low birth weight, infection, inflammation and respiratory distress [5, 6]. IVH has neurological and developmental sequelae that include injuries to the white matter, hydrocephalus, poor motor and cognitive development, cerebral palsy and poor executive function [7, 8]. This makes IVH a major pediatric public health problem [9].
The mechanism and pathophysiology of IVH is not well understood. However, three major factors may be involved in the development of this condition: 1) very fragile germinal matrix microvasculature, 2) poorly regulated cerebral blood flow and 3) low response capacity of the hemostatic system characterized by relatively low platelets and coagulation factors. Hemorrhage of immature, fragile germinal matrix microvasculature is thought to occur in part due to fluctuations in cerebral blood flow. Cerebral blood flow is known to increase in response to several conditions including hypotension, acidosis, hypoxemia or hypercapnia [1, 10].
In spite of marked improvements in the quality of perinatal care, the incidence of IVH has remained undiminished over the past several decades [7]. While this may be partially due to the multifactorial nature of the disorder, attempted interventions and study are further complicated by the fragile nature of this patient population. Moreover, the availability of blood samples from this cohort for research purposes is necessarily very limited and generally reserved for already well established and clinically essential assays. Consequently, previous studies investigating oxidative status in premature infants employed single point assays using cord blood [11, 12]. As a result information is lacking about the changes in oxidative stress markers over time. Furthermore, there are currently no appropriate biochemical markers that could be used to identify infants with elevated risk of IVH, with IVH or that would be helpful in monitoring the effectiveness of preventive or therapeutic interventions. For this reason, attempts are being made to develop minimally invasive, potentially useful diagnostic tools that do not rely on further blood sampling, hence the use of urine in this study.
Allantoin is usually produced in humans by the non-enzymatic oxidation of uric acid by free radicals [13, 14] following oxidative stress [15], and by other agents associated with inflammation [16, 17]. It was identified as a marker of oxidative stress in adults and validated by other known markers of oxidative stress [18-21, 14, 22, 23]. It was shown to be elevated in ischemic stroke [24], smoking [25], during intense exercise [26], ozone challenge [15], and injection of doxorubicin to breast cancer patients [25]. In neonates, elevated allantoin concentrations were linked to increased ATP utilization [27] and necrotizing enterocolitis [28]. Allantoin concentration is not affected by storage at −80°C or by the freeze/thaw cycle [29, 30, 13]. The reported detection limit for allantoin is about 2 pmol [31, 15].
Our hypothesis is that oxidative and inflammatory reactions occurring prior to or during IVH will lead to the increased production of allantoin that will be excreted in the urine over time. Therefore, urinary allantoin was measured in preterm newborns at 12 hour intervals from 24 to 72 hours of life (HOL) to determine whether it might be a non-invasive marker for impending or ongoing IVH.
METHODS
Subject enrolment and sample collection
The Loma Linda University Institutional Review Board approved the study protocol and informed consent documents. Infants of less than 34 weeks gestational age, who were admitted to the Loma Linda University Children’s Hospital Neonatal Intensive Care Unit (NICU) between October 2008 and December 2011, were identified as study candidates. Infants with congenital anomalies were excluded from the study.
After informed consent was obtained from a legal guardian, the investigators collaborated with the clinical staff to obtain urine samples from the subjects. Samples were collected by placing cotton balls over the urethral meatus. Urine-soaked cotton balls were removed from the diaper with every change of diaper and stored at 4oC. Samples that were free of stool were combined into 12-hour aliquots from 24 – 72 HOL. Urine was extracted from the cotton ball by applying pressure, filtered through a syringe filter and stored at − 80°C.
Screening for IVH and classification of subjects
The medical records were reviewed to identify subjects in whom IVH was diagnosed and to determine the grade of the hemorrhage. IVH was diagnosed by head ultrasound and/or magnetic resonance imaging (MRI). The infants were grouped according to Papile’s classification of IVH [32]. Cases in which imaging showed no signs of bleeding were classified in the no IVH group. The infants with grades 1 and 2 IVH, who had hemorrhage that was still contained within the germinal matrix and ventricles without dilatations of the ventricles, were grouped as mild IVH. The severe IVH group included those infants with hemorrhage within or beyond the ventricles with ventriculomegaly and/or parenchymal involvement.
Measurement of allantoin
Allantoin was measured in the urine by the adaptation of the method developed by Gruber [15, 13]. In summary, 25 µL of urine was spiked with 400 µL of 10 µM internal standard (DL-allantoin- 5-13C;1-15N). The spiked samples were simultaneously deproteinized and extracted with 100 µL acetonitrile, vortexed and centrifuged at 20 000 g, 4oC, for 5 minutes. The supernatant was dried using the speed vacuum drier and derivatized with 50 µL of MTBSTFA (i.e. N-methyl-N-tert-butyl-dimethyl-silyltrifluoroacetamide) in pyridine (1:1 vol/vol). The derivatization process was facilitated by incubation at 50oC for 2 hours. Analysis was performed on Agilent 6890N Network GC System connected to an Agilent 5973 Inert Mass Selective Detector (both Agilent Technologies, Inc, Santa Clara, California). Separation was performed using an Agilent 122-5532G capillary column (25.7 m length, 0.25 mm internal diameter). Allantoin was quantified using selected ion monitoring mode with the 398.00 m/z ion being monitored for allantoin and the 400.00 m/z for DL-allantoin- 5-13C;1-15N. The ion abundance ratios (398.00/ 400.00) were converted to micromolar concentrations by use of a standard curve.
Statistical Analyses
Demographic data for categorical variables were analyzed using one-way ANOVA analysis, Chi-square and Mann-Whitney U tests. Repeated measures ANOVA was used to compare the urinary allantoin concentration between the groups and over time. All statistical analyses were performed using IBM SPSS for windows version 22. Differences were considered significant at P < 0.05.
RESULTS
Forty-four infants were enrolled and categorized based upon the presence and extent of IVH. The 1- and 5-minute Apgar scores were not obtained for four of the infants that were transported shortly after home delivery to the Loma Linda University Children’s Hospital NICU. The subjects were homogenous for estimated gestational age (EGA), birth weight and 1-minute Apgar score. There were no significant differences in the incidences of respiratory distress syndrome (RDS) and necrotizing enterocolitis (NEC) and, requirement for mechanical ventilation support. IVH was diagnosed after about 17 and 11 postnatal days in neonates with mild and severe IVH, respectively. The 5-minute Apgar score for the severe IVH group (3.86±2.54; p=0.002) was significantly lower than the scores in the no IVH (6.82±1.59) and mild IVH (6.27±1.56) groups (Table 1).
Table 1.
Subject Demographics
| No IVH (n=24) | Mild IVH (n=13) | Severe IVH (n=7) | P-value | |
|---|---|---|---|---|
| EGA(weeks) | 27.5±2.9 | 26.2±1.3 | 25.6±2.3 | .112† |
| Birth Weight (g) | 1043±414 | 932±264 | 894±288 | .550† |
| 1-Minute APGAR | 4.64±2.38 | 4.09±2.91 | 2.43±2.30 | .145† |
| 5-Minute APGAR | 6.82±1.59 | 6.27±1.56 | 3.86±2.54 | .002a† |
| IVH Diagnosis, DOL Mean ± St.Dev. Median (Range) |
NA |
45.9±52.0 17 (2-158) |
11.1±6.5 11 (3-21) |
0.099†† 0.341* |
| NEC | 3 (12.5%) | 2 (15.4%) | 1 (14.3%) | .855†† |
| RDS | 19 (79.2%) | 12 (92.3%) | 6 (85.7%) | .136†† |
| Mechanical Ventilation | 21 (87.5%) | 13 (100%) | 7 (100%) | .146†† |
| Incomplete Data | 2 | 2 | 0 |
Abbreviations: EGA, estimated gestational age; DOL, day of life; NA, not applicable; NEC, necrotizing enterocolitis; RDS, respiratory distress syndrome. Data are mean ± standard deviation.
Severe IVH significantly different from mild and no IVH
ANOVA test
Chi-Square test
Mann-Whitney U test.
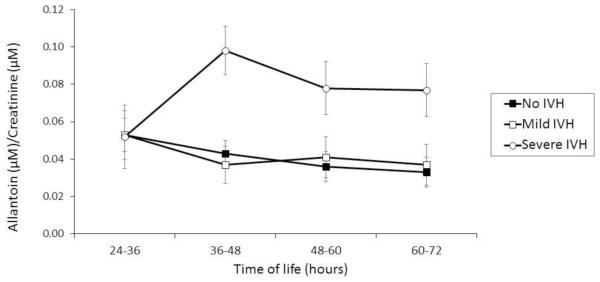
The urinary allantoin concentration was similar in the three groups at 24-36 HOL. Between 36 and 72 HOL the concentration of allantoin in the no IVH and mild IVH groups remained relatively stable at 0.043±0.007 and 0.037±0.01µmol, respectively. A significant elevation in allantoin (0.098±0.013µmol) was observed between 36 – 48 HOL in neonates with severe IVH (p = .002) compared to those with mild IVH or to controls. Allantoin remained significantly elevated (0.079±0.14µmol; p= .021) in this group even at 72 HOL (Figure 1).
Figure 1.
Urinary allantoin concentration between 24 and 72 hours of life in infants with no IVH (n=22), mild IVH (n=11) and severe IVH (n=7). The allantoin concentration in infants with severs IVH was significantly elevated at 36-48 hours (p=0.002), 48-60 hours (p=0.039) and 60-72 hours (p=0.021).
DISCUSSION
The objective of this study was to evaluate changes in urinary allantoin, a marker for oxidative stress, in premature infants who developed IVH. We found that allantoin was significantly elevated in infants with severe IVH compared to those with no IVH or with mild IVH. This elevation was observed at 36 HOL and it persisted up to 72 HOL. This is consistent with prior observations that neonates with poor outcomes experience increased oxidative stress shortly after birth [33, 12, 11].
Allantoin, a non-enzymatic product of purine and marker of oxidative stress [15, 22], is produced when uric acid is oxidized by reactive oxygen species [17]. The mechanism of allantoin production from uric acid by the action of free radicals is well documented [34, 16, 35]. Hellsten and coworkers observed a more than two-fold increase in plasma allantoin during recovery in adults who exercised [26]. This suggests that oxygen radicals generated during hyperoxia may be responsible for the production of allantoin from urate in humans [13]. Apart from this, oxidative stress induced by inflammation and toxicity has been shown to increase allantoin levels, making it a potential marker of oxidative stress [36, 14]. Based on these reports we can infer that oxidative stress is the likely cause of the elevated allantoin we observed in the neonates with severe IVH.
Our study documented urinary allantoin content over time in preterm neonates. The greatest increase in content was found at 36-48 HOL in neonates with severe IVH. Prior studies measured oxidative stress markers only at a single point in time in cord blood [11, 12]. This may be partially due to the difficulty associated with repeated blood sampling in this fragile population. We chose urinary allantoin because it can be repeatedly measured over time and could permit tracking of the progression of oxidative stress in infants with IVH. In addition, it may be helpful in following the effectiveness of any therapeutic interventions they may be receiving.
Most of the neonates (>79%) experienced some degree of respiratory distress shortly after birth and were placed on mechanical ventilation at some point during hospitalization (Table 1), however, the group that developed severe IVH appeared to have had a more difficult transition to extrauterine life and were possibly poorly perfused as indicated by the significantly lower Apgar score at 5 minutes. Severe IVH has been correlated to low 5-minute Apgar scores [37-39]. The incidence and severity of IVH has been partially linked to the timing, severity and intensity of hypoxia-ischemia, as well as the method of reperfusion employed to correct this [40]. Basu et al., demonstrated a correlation between hyperoxemia and increased cerebral blood flow in neonates who developed IVH between 24 −48 HOL [41]. We observed a similar time course of the elevation of allantoin in the infants with severe IVH, whereby allantoin levels significantly increased at 36 HOL in this group. This timing further suggests that the rise in allantoin may be linked to perinatal events such as maternal/placental inflammation and ischemia-reperfusion. Therefore, urinary allantoin may serve in identifying which neonates are experiencing oxidative stress and need further monitoring for morbidities associated with prematurity.
Severe IVH is known to have poor neurological outcome. If urinary allantoin indicates increased free radical production and possible tissue injury, then the significantly higher allantoin levels presented here are consistent with reports associating severe IVH (grade 3-4) with more adverse neurological outcomes [3]. IVH as well as retinopathy of prematurity (ROP), necrotizing enterocolitis (NEC) and bronchopulmonary dysplasia (BPD) have been termed oxygen-radical diseases (ORD) of neonatology [42, 33]. Free radicals generated during hypoxia-ischemia and reperfusion are suspected to play a role in their pathogenesis in the low-birth weight preterm newborn [43], but the resulting lesions present differently in different tissues [44]. Allantoin levels were found to be elevated in NEC [28] and BPD [45, 21]. Further studies are needed to better characterize such associations.
The exact timing of IVH development is not clear, but it is suspected to occur sometime during the first week of life [4]. The 5-minute Apgar indicated that the neonates who developed severe IVH were sicker and perhaps more poorly perfused. But IVH was not diagnosed in this group by the conventional head imaging until about the 11th post-natal day, on average. Preterm newborns do not usually undergo head imaging when they are unstable and possibly intubated. The observed elevated urinary allantoin from 36-72 HOL was associated with severe IVH. The timing of this elevated urinary allantoin may not give an indication of exact timing of IVH development, but it may indicate ongoing physiological changes prior to or during IVH. Urinary allantoin, probably in combination with low Apgar scores, may be helpful to the clinician toward earlier consideration of IVH risks.
In conclusion, elevated allantoin level at 36 HOL which persisted up to 72 HOL was associated with severe IVH, which was diagnosed on average at about the 11th day of life. These findings support previous work suggesting that oxidative stress plays an important role in the pathophysiology of IVH [11, 46, 47]. Further studies are needed to establish the possible usefulness of urinary allantoin, during the first 2-3 days of life, in identifying neonates at risk for severe IVH. It may also be helpful in monitoring of the effectiveness of therapeutic interventions designed to limit further progression of IVH and reduce the neurological sequelae.
Acknowledgments
Supported in part by Department of Basic Sciences, Department of Earth and Biological Sciences and by NIH Grant NR011209
Footnotes
Conflict of Interest
Ijeoma Esiaba, Danilyn M. Angeles, Megan S. Holden, John B.C. Tan, Yayesh Asmerom, Gerald Gollin and Danilo S. Boskovic declare that they have no conflict of interest.
Compliance with Ethics Requirements
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). Informed consent was obtained from the parents of all patients for their inclusion in the study.
REFERENCES
- 1.Ballabh P. Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatr Res. 2010;67(1):1–8. doi: 10.1203/PDR.0b013e3181c1b176. doi:10.1203/PDR.0b013e3181c1b176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi IR, Lee JH, Park MS, Kim JY, Park KH, Kim G, et al. Early neurodevelopment in very low birth weight infants with mild intraventricular hemorrhage or those without intraventricular hemorrhage. Korean J Pediatr. 2012;55(11):414–9. doi: 10.3345/kjp.2012.55.11.414. doi:10.3345/kjp.2012.55.11.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCrea HJ, Ment LR. The Diagnosis, Management and Postnatal Prevention of Intraventricular Hemorrhage in the Preterm Neonate. Clinics in perinatology. 2008;35(4):1–17. doi: 10.1016/j.clp.2008.07.014. doi:10.1016/j.clp.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang BY, Castillo M. Hypoxic-Ischemic Brain Injury: Imaging Findings from Birth to Adulthood. Radiographics. 2008;28(2):417–39. doi: 10.1148/rg.282075066. doi:10.1148/rg.282075066. [DOI] [PubMed] [Google Scholar]
- 5.Poralla C, Hertfelder HJ, Oldenburg J, Mueller A, Bartmann P, Heep A. Elevated Interleukin-6 Concentration and Alterations of the Coagulation System Are Associated with the Development of Intraventricular Hemorrhage in Extremely Preterm Infants. Neonatology. 2012;102(4):270–5. doi: 10.1159/000341266. doi:10.1159/000341266. [DOI] [PubMed] [Google Scholar]
- 6.Bhandari V, Bizzarro MJ, Shetty A, Zhong X, Page GP, Zhang H, et al. Familial and genetic susceptibility to major neonatal morbidities in preterm twins. Pediatrics. 2006;117(6):1901–6. doi: 10.1542/peds.2005-1414. doi:10.1542/peds.2005-1414. [DOI] [PubMed] [Google Scholar]
- 7.Ballabh P. Pathogenesis and Prevention of Intraventricular Hemorrhage. Clinics in perinatology. 2014;41(1):47. doi: 10.1016/j.clp.2013.09.007. doi:10.1016/j.c1p.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graham EM, Holcroft CI, Rai KK, Donohue PK, Allen MC. Neonatal cerebral white matter injury in preterm infants is associated with culture positive infections and only rarely with metabolic acidosis. American journal of obstetrics and gynecology. 2004;191(4):1305–10. doi: 10.1016/j.ajog.2004.06.058. doi:10.1016/j.ajog.2004.06.058. [DOI] [PubMed] [Google Scholar]
- 9.Ment LR, Aden U, Lin AP, Kwon SH, Choi M, Hallman M, et al. Gene-environment interactions in severe intraventricular hemorrhage of preterm neonates. Pediatr Res. 2014;75(1):241–50. doi: 10.1038/pr.2013.195. doi:10.1038/pr.2013.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strahle J, Garton HJ, Maher CO, Muraszko KM, Keep RF, Xi G. Mechanisms of hydrocephalus after neonatal and adult intraventricular hemorrhage. Translational stroke research. 2012;3(Suppl 1):25–38. doi: 10.1007/s12975-012-0182-9. doi:10.1007/s12975-012-0182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perrone S, Tataranno ML, Negro S, Longini M, Marzocchi B, Proietti F, et al. Early identification of the risk for free radical-related diseases in preterm newborns. Early human development. 2010;86(4):241–4. doi: 10.1016/j.earlhumdev.2010.03.008. doi:10.1016/j.earlhumdev.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Weinberger B, Nisar S, Anwar M, Ostfeld B, Hegyi T. Lipid peroxidation in cord blood and neonatal outcome. Pediatr Int. 2006;48(5):479–83. doi: 10.1111/j.1442-200X.2006.02257.x. doi:10.1111/j.1442-200X.2006.02257.x. [DOI] [PubMed] [Google Scholar]
- 13.Plank MS, Calderon TC, Asmerom Y, Boskovic DS, Angeles DM. Biochemical measurement of neonatal hypoxia. Journal of visualized experiments : JoVE. 2011;(54) doi: 10.3791/2948. doi:10.3791/2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Il'yasova D, Scarbrough P, Spasojevic I. Urinary biomarkers of oxidative status. Clinica chimica acta; international journal of clinical chemistry. 2012;413(19-20):1446–53. doi: 10.1016/j.cca.2012.06.012. doi:10.1016/j.cca.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gruber J, Tang SY, Jenner AM, Mudway I, Blomberg A, Behndig A, et al. Allantoin in human plasma, serum, and nasal-lining fluids as a biomarker of oxidative stress: avoiding artifacts and establishing real in vivo concentrations. Antioxidants & redox signaling. 2009;11(8):1767–76. doi: 10.1089/ars.2008.2364. doi:10.1089/ars.2008.2364. [DOI] [PubMed] [Google Scholar]
- 16.Kaur H, Halliwell B. Action of biologically-relevant oxidizing species upon uric acid. Identification of uric acid oxidation products. Chemico-biological interactions. 1990;73(2-3):235–47. doi: 10.1016/0009-2797(90)90006-9. [DOI] [PubMed] [Google Scholar]
- 17.Becker BF, Kastenbauer S, Kodel U, Kiesl D, Pfister HW. Urate oxidation in CSF and blood of patients with inflammatory disorders of the nervous system. Nucleosides, nucleotides & nucleic acids. 2004;23(8-9):1201–4. doi: 10.1081/NCN-200027469. doi:10.1081/ncn-200027469. [DOI] [PubMed] [Google Scholar]
- 18.Causse E, Pradelles A, Dirat B, Negre-Salvayre A, Salvayre R, Couderc F. Simultaneous determination of allantoin, hypoxanthine, xanthine, and uric acid in serum/plasma by CE. Electrophoresis. 2007;28(3):381–7. doi: 10.1002/elps.200600205. doi:10.1002/elps.200600205. [DOI] [PubMed] [Google Scholar]
- 19.Kand'ar R, Zakova P. Allantoin as a marker of oxidative stress in human erythrocytes. Clin Chem Lab Med. 2008;46(9):1270–4. doi: 10.1515/CCLM.2008.244. doi:10.1515/cclm.2008.244. [DOI] [PubMed] [Google Scholar]
- 20.Skalicky J, Muzakova V, Kandar R, Meloun M, Rousar T. Oxidative stress and metabolic syndrome in obese adults with and without controlled diet restriction. Bratislavske lekarske listy. 2009;110(3):152–7. [PubMed] [Google Scholar]
- 21.Ogihara T, Okamoto R, Kim HS, Nagai A, Morinobu T, Moji H, et al. New evidence for the involvement of oxygen radicals in triggering neonatal chronic lung disease. Pediatr Res. 1996;39(1):117–9. doi: 10.1203/00006450-199601000-00017. doi:10.1203/00006450-199601000-00017. [DOI] [PubMed] [Google Scholar]
- 22.Seet RC, Lee CY, Chan BP, Sharma VK, Teoh HL, Venketasubramanian N, et al. Oxidative damage in ischemic stroke revealed using multiple biomarkers. Stroke; a journal of cerebral circulation. 2011;42(8):2326–9. doi: 10.1161/STROKEAHA.111.618835. doi:10.1161/strokeaha.111.618835. [DOI] [PubMed] [Google Scholar]
- 23.Zinellu A, Sotgia S, Loriga G, Deiana L, Satta AE, Carru C. Oxidative stress improvement is associated with increased levels of taurine in CKD patients undergoing lipid-lowering therapy. Amino acids. 2012;43(4):1499–507. doi: 10.1007/s00726-012-1223-0. doi:10.1007/s00726-012-1223-0. [DOI] [PubMed] [Google Scholar]
- 24.Deulofeut R, Critz A, Adams-Chapman I, Sola A. Avoiding hyperoxia in infants <= 1250 g is associated with improved short- and long-term outcomes. J Perinatol. 2006;26(11):700–5. doi: 10.1038/sj.jp.7211608. doi:10.1038/sj.jp.7211608. [DOI] [PubMed] [Google Scholar]
- 25.Il'yasova D, Spasojevic I, Wang F, Tolun AA, Base K, Young SP, et al. Urinary biomarkers of oxidative status in a clinical model of oxidative assault. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2010;19(6):1506–10. doi: 10.1158/1055-9965.EPI-10-0211. doi:10.1158/1055-9965.epi-10-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hellsten Y, Tullson PC, Richter EA, Bangsbo J. Oxidation of urate in human skeletal muscle during exercise. Free Radic Biol Med. 1997;22(1-2):169–74. doi: 10.1016/s0891-5849(96)00286-9. doi:10.1016/s0891-5849(96)00286-9. [DOI] [PubMed] [Google Scholar]
- 27.Asmerom Y, Slater L, Boskovic DS, Bahjri K, Holden MS, Phillips R, et al. Oral sucrose for heel lance increases adenosine triphosphate use and oxidative stress in preterm neonates. The Journal of pediatrics. 2013;163(1):29–35. doi: 10.1016/j.jpeds.2012.12.088. e1. doi:10.1016/j.jpeds.2012.12.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plank M, Slater L, Asmerom Y, Angeles KR, Mannoia K, Boskovic D, et al. Altered urinary excretion of allantoin and purine catabolites in neonates with necrotizing enterocolitis. Faseb J. 2012:26. [Google Scholar]
- 29.Tolun AA, Zhang HY, Il'yasova D, Sztaray J, Young SP, Millington DS. Allantoin in human urine quantified by ultra-performance liquid chromatography-tandem mass spectrometry. Anal Biochem. 2010;402(2):191–3. doi: 10.1016/j.ab.2010.03.033. doi:10.1016/j.ab.2010.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berthemy A, Newton J, Wu DL, Buhrman D. Quantitative determination of an extremely polar compound allantoin in human urine by LC-MS/MS based on the separation on a polymeric amino column. J Pharm Biomed Anal. 1999;19(3-4):429–34. doi: 10.1016/s0731-7085(98)00200-3. doi:10.1016/s0731-7085(98)00200-3. [DOI] [PubMed] [Google Scholar]
- 31.Chung WY, Benzie IFF. Plasma allantoin measurement by isocratic liquid chromatography with tandem mass spectrometry: Method evaluation and application in oxidative stress biomonitoring. Clin Chim Acta. 2013;424:237–44. doi: 10.1016/j.cca.2013.06.015. doi:10.1016/j.cca.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 32.Papile L, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92(4):529–34. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 33.Saugstad OD. Update on oxygen radical disease in neonatology. Curr Opin Obstet Gynecol. 2001;13(2):147–53. doi: 10.1097/00001703-200104000-00009. doi:10.1097/00001703-200104000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Grootveld M, Halliwell B. Measurement of allantoin and uric acid in human body fluids. A potential index of free-radical reactions in vivo? The Biochemical journal. 1987;243(3):803–8. doi: 10.1042/bj2430803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marklund N, Ostman B, Nalmo L, Persson L, Hillered L. Hypoxanthine, uric acid and allantoin as indicators of in vivo free radical reactions. Description of a HPLC method and human brain microdialysis data. Acta Neurochir. 2000;142(10):1135–42. doi: 10.1007/s007010070042. doi:10.1007/s007010070042. [DOI] [PubMed] [Google Scholar]
- 36.Il'yasova D, Kennedy K, Spasojevic I, Wang F, Tolun AA, Base K, et al. Individual responses to chemotherapy-induced oxidative stress. Breast cancer research and treatment. 2011;125(2):583–9. doi: 10.1007/s10549-010-1158-7. doi:10.1007/s10549-010-1158-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lie KK, Groholt EK, Eskild A. Association of cerebral palsy with Apgar score in low and normal birthweight infants: population based cohort study. BMJ (Clinical research ed) 2010;341:c4990. doi: 10.1136/bmj.c4990. doi:10.1136/bmj.c4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shankaran S, Lin AP, Maller-Kesselman J, Zhang HP, O'Shea TM, Bada HS, et al. Maternal Race, Demography, and Health Care Disparities Impact Risk for Intraventricular Hemorrhage in Preterm Neonates. J Pediatr. 2014;164(5):1005. doi: 10.1016/j.jpeds.2014.01.036. doi:10.1016/j.jpeds.2014.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaudier FL, Goldenberg RL, Nelson KG, PeraltaCarcelen M, DuBard MB, Hauth JC. Influence of acid-base status at birth and Apgar scores on survival in 500-1000-g infants. Obstetrics and gynecology. 1996;87(2):175–80. doi: 10.1016/0029-7844(95)00407-6. doi:10.1016/0029-7844(95)00407-6. [DOI] [PubMed] [Google Scholar]
- 40.Alvarez-Diaz A, Hilario E, de Cerio FG, Valls-i-Soler A, Alvarez-Diaz FJ. Hypoxic-ischemic injury in the immature brain - Key vascular and cellular players. Neonatology. 2007;92(4):227–35. doi: 10.1159/000103741. doi:10.1159/000103741. [DOI] [PubMed] [Google Scholar]
- 41.Basu S, Barman S, Shukla R, Kumar A. Effect of oxygen inhalation on cerebral blood flow velocity in premature neonates. Pediatr Res. 2014;75(2):328–35. doi: 10.1038/pr.2013.219. doi:10.1038/pr.2013.219. [DOI] [PubMed] [Google Scholar]
- 42.Saugstad OD. HYPOXANTHINE AS AN INDICATOR OF HYPOXIA - ITS ROLE IN HEALTH AND DISEASE THROUGH FREE-RADICAL PRODUCTION. Pediatr Res. 1988;23(2):143–50. doi: 10.1203/00006450-198802000-00001. doi:10.1203/00006450-198802000-00001. [DOI] [PubMed] [Google Scholar]
- 43.Rogers S, Witz G, Anwar M, Hiatt M, Hegyi T. Antioxidant capacity and oxygen radical diseases in the preterm newborn. Arch Pediatr Adolesc Med. 2000;154(6):544–8. doi: 10.1001/archpedi.154.6.544. [DOI] [PubMed] [Google Scholar]
- 44.Gitto E, Pellegrino S, D'Arrigo S, Barberi I, Reiter RJ. Oxidative stress in resuscitation and in ventilation of newborns. Eur Resp J. 2009;34(6):1461–9. doi: 10.1183/09031936.00032809. doi:10.1183/09031936.00032809. [DOI] [PubMed] [Google Scholar]
- 45.Saugstad OD. Bronchopulmonary dysplasia and oxidative stress: are we closer to an understanding of the pathogenesis of BPD? Acta Paediatr. 1997;86(12):1277–82. doi: 10.1111/j.1651-2227.1997.tb14897.x. doi:10.1111/j.1651-2227.1997.tb14897.x. [DOI] [PubMed] [Google Scholar]
- 46.Dani C, Cecci A, Bertini G. Role of Oxidative Stress as Physiopathologic Factor in the Preterm Infant. Minerva Pediatr. 2004;56(4):381–94. [PubMed] [Google Scholar]
- 47.Takashima S, Itoh M, Oka A. A History of Our Understanding of Cerebral Vascular Development and Pathogenesis of Perinatal Brain Damage Over the Past 30 Years. Semin Pediatr Neurol. 2009;16(4):226–36. doi: 10.1016/j.spen.2009.09.004. doi:10.1016/j.spen.2009.09.004. [DOI] [PubMed] [Google Scholar]