Abstract
Bipolar Disorder (BD) is associated with impairment in a number of areas including poor work functioning, often despite the remission of mood symptoms. The present study aimed to examine the role of sleep disturbance and cognitive functioning in occupational impairment in BD. Twenty-four euthymic BD participants and 24 healthy control participants completed a week of prospective assessment of sleep disruption via self-report and actigraphy, a battery of neuropsychological tests of executive functioning, working memory, and verbal learning, and assessments of work functioning. BD participants experienced significantly poorer cognitive functioning as well as greater months of unemployment and greater incidence of being fired than controls. Moderation analyses revealed that both poor sleep and cognitive functioning were associated with poor work performance in BD participants, but not control participants. Sleep and cognitive functioning may be impaired in euthymic BD and are associated with poor work functioning in this population. More research should be conducted to better understand how sleep and cognitive functioning may interact in BD.
Keywords: ipolar disorder, sleep, cognitive functioning, functional outcomes
1. Introduction
Bipolar Disorder (BD) is a chronic, highly recurrent and debilitating condition affecting roughly 5.7 million adults in the United States (National Institute of Mental Health, 2011). Indeed, the World Health Organization ranks BD as the sixth leading cause of disability in the world (World Health Organization; 2006). Reports of poor work performance and high rates of utilization of disability income are frequent in the literature (Judd et al., 2005; 2008). However, research is mixed regarding the underpinnings of this sustained dysfunction (see Sanchez-Moreno et al., 2009 for a thorough review), thus necessitating further research into which variables might be the best predictors of functioning in BD.
The most robust clinical predictor of long-term functional impairment is subsyndromal depressive symptoms (Altshuler et al., 2002; Fagiolini et al., 2005; Judd et al., 2005; Bonnin et al., 2010), although studies also have shown associations between older age, presence of mixed episodes, and increased number of previous hospitalizations and poor functioning (Rosa et al., 2009). Comparatively understudied are the contributions of cognitive functioning and sleep to sustained work disability, as both are often found to be impaired despite remission of mood symptoms and may in fact be inter-related (see Boland and Alloy, 2013 for a review of the theoretical relationship between sleep and neurocognition in BD). Numerous reports point to widespread cognitive deficits in the euthymic phase of BD, including deficits in verbal memory, verbal learning, attention, and executive functioning (Robinson et al., 2006; Torres et al., 2007; Arts et al., 2008, Bora et al., 2009; Kurtz et al., 2009; Mann-Wrobel et al., 2011; Peters et al., 2014). Studies including indices of work performance in their analyses have consistently reported associations between work disability and verbal learning and memory (Dickerson et al., 2004; Martinez-Àran et al., 2004). Indeed, in a cross-sectional analysis, Martinez-Àran and colleagues (2004) found that in euthymic BD individuals, verbal learning deficits were associated with poor functional outcome, whereas clinical variables were not.
Research also points to persistent sleep disruption in the euthymic phase (Millar et al., 2004; Harvey et al., 2005). Sleep disturbance, including reduced need for sleep, increased sleep (hypersomnia), sleep fragmentation, and increased sleep onset latency, has been associated with poor functional outcomes in their broader sense, including poor quality of life, lower Global Assessment of Functioning (GAF) scores, and self-reported symptoms of daytime dysfunction as a consequence of sleep disturbance (Harvey et al., 2005; Giglio et al., 2008; Gruber et al., 2009). Only one study to date has specifically examined the role of sleep disturbance in work functioning in BD; however, this study only assessed subjective duration of sleep and found that both short and long duration of sleep was associated with poor work functioning in BD (Gruber et al., 2009).
The present study provides a focused examination of work disability by examining the relative contributions of sleep disturbance and cognitive impairment. We hypothesized that participants with BD would experience poorer cognitive and work functioning relative to healthy controls. BD participants in this study are compared to healthy sleepers, and although we expect BD participants to demonstrate poorer sleep relative to controls due to the study design, we hypothesize that scores on self-report measures of sleep disturbance and actigraphic assessments of sleep parameters for BD participants will reflect sleep disturbance based on known cutoff scores and values. Given the established chronicity of sleep and cognitive functioning impairments in BD, as well as frequent reports of overall poor functional outcomes in this population, we also hypothesized that sleep and cognitive functioning deficits would be associated with poor work performance among BD individuals more strongly than among healthy controls.
2. Method
2.1 Participants
Participants were 48 adults, aged 18 to 65 recruited from the Temple University campus and the greater Philadelphia community. Participants carried a diagnosis of bipolar I or II disorder in the euthymic phase or had no history of mood or sleep disorders, were willing to commit to a week of actigraphic assessment, and were able to read, write, and speak English. Exclusion criteria included active suicidal or homicidal ideation, current psychotic symptoms, baseline IQ below 70, and an uncontrolled severe medical condition.
2.2 Procedure
All procedures were approved by the Institutional Review Board of Temple University. In Phase I, 1,954 individuals aged 18 to 65 completed screening questionnaires consisting of the General Behavior Inventory (GBI; Depue et al. 1989), a questionnaire assessing the severity and duration of affective symptoms, and the Insomnia Severity Index (ISI; Morin et al., 2011), a questionnaire assessing severity of insomnia symptoms. Eligible participants completed a psychiatric diagnostic interview (Schedule for Affective Disorders and Schizophrenia – Lifetime Version (SADS-L; Endicott and Spitzer, 1978), an interview of current insomnia symptoms, and self-reports of mood symptoms (Beck Depression Inventory [BDI]; Beck et al., 1996; Altman Self-Rating Mania Scale [ASRM]; Altman et al., 1997). Seventy-four participants were administered the diagnostic interview, from which 26 were disqualified for the following reasons: diagnosis of BD-NOS (n = 8), diagnosis of cyclothymia (n = 4), diagnosis of major depression (n = 8), bipolar but symptomatic (n = 6). Participants then completed a brief assessment of intellectual functioning (Kaufman Brief Intelligence Test; Kaufman and Kaufman, 1990).
Bipolar participants had to score > 13 on the depression subscale and > 11 on the hypomania/biphasic subscale of the GBI (Depue et al., 1989). Control participants had to score < 7 on both the depression and hypomania subscales of the GBI and < 7 on the ISI. Scores of 7 and below represent the lower quartile of the ISI, and would thus provide a good demonstration of satisfactory sleep. Individuals who endorsed any current or past mood disorder diagnosis or any current sleep disruption were excluded from the control group, as were individuals who reported satisfactory sleep via the use of sleeping medications. BD participants were included only if they scored < 5 on the ASRM and < 13 on the BDI.
Full study participants completed a Pittsburgh Sleep Quality Index (PSQI; see Measures) and were fitted with an actigraph. Research has shown that the use of actigraphy is useful in the assessment of sleep/wake activity and that data collected via actigraphy is more accurate than data collected through standard sleep/wake diaries (Webster et al., 1982; Brooks et al., 1993; Sadeh, 1995; Ancoli-Israel, et al., 2003). Actigraphy took place for one week prior to cognitive testing. Cognitive testing and work functioning assessment took place at Temple University at the culmination of the one-week actigraphy period. Cognitive and work functioning assessments were conducted by assistants who were blind to participants’ group assignments as well as to actigraphy results.
2.3 Measures
2.3.1 Sleep measures
Insomnia Severity Index (ISI). The ISI (Morin et al., 2011) is a 7-item questionnaire used to assess the severity of insomnia symptoms utilizing a 5-point Likert-type scale. Internal consistency for this measure is excellent (α = 0.90) with good convergent validity (Morin et al., 2011). A study of the psychometrics of the ISI revealed a cutoff score of 10 to be adequate in community samples for the diagnosis of insomnia (Morin et al., 2011).
Unstructured Clinical Interview for Insomnia. An unstructured clinical interview was utilized to diagnose primary insomnia. Interviewers collected data on sleep onset latency, total sleep time, number of mid-sleep and early-morning awakenings, symptoms of obstructive sleep apnea, nightmares, and sleep-related behaviors. Information on medical conditions was collected to determine if medical exclusion was warranted.
The Pittsburgh Sleep Quality Index (PSQI). The PSQI (Buysse, 1989) is a self-report measure that assesses sleep quality and disturbance over a one-month time interval. The measure generates component scores of subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction. This measure has good internal consistency (α = 0.83) and test-retest reliability (r = 0.85).
Actigraphy. All participants were equipped with wristwatch actigraphy for 7 days and nights. The device used was an Actiwatch AW-64 (Mini Mitter, Philips Respironics Inc., Bend, OR, USA). This device features a sensitivity of 0.05 g and a bandwidth between 3 and 11 Hz, with a sampling frequency of 32 Hz. Actigraphy data were stored in 15-second epochs for a continuous period of 7 days. Sleep onset data were analyzed using the Immobile Minutes algorithm in Actiware 5.57 (Mini Mitter Philips Respironics Inc.), which generated wake, rest, and sleep intervals for each participant for each night of actigraphy. Participants completed subjective sleep-wake diaries in addition to actigraphy. Sleep diaries required participants to log the time they got into bed, time they attempted to sleep, estimate of time to fall asleep, number of awakenings, total length of awakenings, final wake time, and time out of bed. Sleep diaries were compared to actigraphy data to broadly define the sleep period. Data were inspected by the PI, who was not blind to participant diagnosis. Given this potential confound and to reduce the potential for researcher bias, analyses of sleep data relied heavily on software identification of sleep periods in the actigraphy data.
2.3.2. Cognitive functioning measures
Kauffman Brief Intelligence Test Second Edition (KBIT-II). The KBIT-II (Kaufman and Kaufman, 1990) was used to obtain a baseline assessment of intelligence. Correlations between the KBIT and the Wechsler Adult Intelligence Scale - Revised (WAIS-R; Wechsler, 1976), a longer and more comprehensive intelligence test, were 0.83, 0.77, and 0.88 for Verbal, Nonverbal, and Composite scales, respectively (Naugle et al., 1993). The KBIT-II has been shown to be a valid measure for verbal, performance, and general intellectual functioning in psychiatric samples (Hays et al., 2002) and has been utilized in other examinations of cognitive functioning in bipolar samples (Nadkami et al., 2010, Torres et al., 2011, Kozicky et al., 2012).
Stroop subtest of the Delis-Kaplan Executive Functioning System (DKEFS). The Stroop subtest of the DKEFS (Delis et al., 2004) is a test of cognitive inhibition and set shifting abilities. Participants are required to alternate between reading the name of a color and the color of the ink with which the word is printed. Shifting costs are computed as the difference in task completion time between the shifting trial and their own baseline trials.
Tower of London. The Tower of London (Shallice, 1982) task assesses executive planning. The specific version utilized in this study consisted of the Tower of London subtest of the DKEFS (Delis et al., 2004). The test consists of two boards with pegs and several discs of varying diameters, the goal being to move the discs from one peg to another without violating a set of rules given to the participant by the test administrator.
California Verbal Learning Test (CVLT-II). The CVLT-II (Woods et al., 2006) is a learning test that has been used extensively in neuropsychology to assess auditory/verbal learning and memory. The CVLT-II measures recall, recognition, encoding strategies, learning rates, and error types. Short and long-term free and cued recall trials are administered, as are forced choice and recognition discrimination trials.
Digit Span (subtest of the Wechsler Memory Scale – Fourth Edition). In the digit span (Wechsler, 1997), a test of working memory, participants were presented with a string of numbers and asked to repeat them verbatim. Length of strings increased with each subsequent set. Participants were then asked to repeat a list of numbers in reverse order.
2.3.3. Work Functioning Measure
Work Performance Questionnaire. This is an original questionnaire created for this study to obtain reports of vocational data. The questionnaire asked participants to report total months of employment, number of firings, and number of incidents of self-terminated employment in his or her lifetime.
2.3.4 Diagnostic and mood symptom measures
Schedule for Affective Disorders and Schizophrenia – Lifetime. The Schedule for Affective Disorders and Schizophrenia – Lifetime (SADS-L; Endicott and Spitzer, 1978) is a semi-structured diagnostic interview used to assess current and lifetime history of Axis I disorders. The mood disorders and psychosis sections of an expanded SADS-L (exp-SADS-L; see Nusslock et al., 2007; Alloy et al., 2008) was administered to assess the presence of current or past major depressive disorder, bipolar spectrum disorder, or psychosis.
The General Behavior Inventory (GBI). The GBI (Depue et al., 1989) was used as a screening questionnaire to select individuals likely to carry a diagnosis of BD. The GBI is a psychometrically strong measure, with internal consistency α’s of 0.90 to 0.96, test-retest reliability r’s of 0.71 – 0.74, satisfactory sensitivity (0.78) and superior specificity (0.99) for bipolar spectrum disorders (Depue et al., 1989).
Beck Depression Inventory (BDI-II). The BDI-II (Beck et al., 1996) is a 21-item self-report measure of symptoms of depression, and is the gold standard of self-report measures of the severity of depression. It has been shown to have good internal consistency (α’s = 0.81–0.86) and retest reliability (r’s = 0.48–0.86) as well as validity in nonclinical samples (Beck et al., 1996).
Altman Self-Rating Mania Scale (ASRM). The ASRM (Altman et al., 1997) is a 5-item self-report scale designed to assess both presence and severity of manic symptoms. The ASRM correlates significantly with the Clinician Administered Rating Scale for Mania (CARS-M) and the Young Mania Rating Scale (YMRS) and has high sensitivity and specificity (Altman et al., 1997). Internal consistency of the ASRM is good (α = 0.79; Altman et al., 1997).
2.4 A priori variable selection and statistical plan
To reduce the number of statistical tests and to increase generalizability of findings, the target variables for analysis were selected a priori from a large number of potential outcome variables. Sleep variables chosen for analysis were ISI score, PSQI total and sub-scores, and actigraph variables of sleep onset latency, wake time after sleep onset, sleep duration, and efficiency. Utilizing results from meta-analyses of cognitive functioning in BD as a guide (e.g., Bora et al., 2009), specific variables were selected to match earlier reports of deficits in cognitive functioning. This procedure yielded the following cognitive variables: Short-and long-term cued and free recall and recognition from the CVLT, Digit Span forward and backward, the Stroop Inhibition/Switching condition, and the total score of the Tower test.
Given the presence of multiple dependent variables, and because tests of neuropsychological functioning and assessments of sleep quality are expectedly correlated within each domain, a protective multivariate analysis of covariance analysis (MANCOVA) was performed to assess differences in sleep, cognitive, and work functioning across groups. Significant MANCOVA findings were followed up with sanctioned ANCOVA analyses to examine variables driving the group differences. Age and baseline IQ were correlated with several cognitive measures in this sample, and thus, were entered as covariates for analyses of cognitive variables. BDI scores differed between groups, and thus, BDI was included as a covariate in all analyses.
Following analyses of group differences, moderation analyses were performed to assess whether BD diagnosis moderated relationships between sleep and work functioning, and cognitive performance and work functioning. Given the large number of possible combinations of variables for moderation analyses, correlations between sleep/cognitive and work functioning were conducted as a screening procedure to reduce the number of tests performed. Only those variables that were significantly associated with the outcome variables were included in moderation analyses.
3. Results
3.1 Sample description and associations among study variables
Table 1 presents demographic and clinical characteristics of the study sample. BD participants did not differ in terms of hypomanic symptoms, but differed significantly from control participants on depression symptoms (F(1, 46) = 21.4, p < 0.001). BD and control participants did not significantly differ on gender, ethnicity, age, or years of education. Although insomnia and sleep disturbance may differ by gender, gender was not associated with any sleep index in this sample.
Table 1.
Demographic and clinical variables in bipolar and control groups.
| Bipolar Mean (SD) |
Control Mean (SD) |
|
|---|---|---|
| Age | 32.63 (11.61) | 30.96 (12.9) |
| Years of Education | 13.96 (1.85) | 14.50 (1.96) |
| Baseline IQ | 101.08 (13.99) | 102.33 (10.96) |
| BDI | 5.08 (4.20)* | 0.92 (1.35) |
| ASRM | 2.75 (2.47) | 2.38 (3.47) |
| Bipolar n (%) |
Control n (%) |
|
| Gender (Female) | 15 (62.5%) | 14 (58.3%) |
| Race | ||
| Caucasian | 19 (79.2%) | 14 (58.3%) |
| African-American | 2 (8.3%) | 6 (25%) |
| Asian | 1 (4.2%) | 1 (4.2%) |
| Hispanic | 1 (4.2%) | 0 (0%) |
| Other | 1 (4.2%) | 3 (12.5%) |
BDI = Beck Depression Inventory; ASRM = Altman Self Rating Mania Scale.
=significant group difference; p <.001
3.2 Group Differences in Sleep, Cognitive Functioning, and Work Functioning
MANCOVA indicated significant group differences on sleep variables (Pillai’s Trace F = 2.57, p = 0.02). As expected due to the study design, BD participants exhibited worse sleep than controls on five of the 12 items, specifically on self-reported symptoms of sleep disturbance (ISI, PSQI Daytime Dysfunction, PSQI Sleep Onset Latency, PSQI Sleep Quality, and Total PSQI score).
MANCOVA analyses of cognitive variables, controlling for age and IQ, yielded Pillai’s Trace F = 2.66, p = 0.02 for the main effect. BD participants performed worse than controls on CVLT short delay cued recall, CVLT Recognition, Digit Span Forward, and the Stroop task.
MANCOVA analyses of work functioning variables, controlling for participant age, yielded Pillai’s Trace F = 6.75, p = 0.001. BD participants had significantly more lifetime firings than controls and greater months of unemployment in their lifetimes. Groups did not significantly differ in terms of number of incidents of lifetime job quitting. Complete analyses are presented in Table 2.
Table 2.
Cognitive, Sleep, and Work Performance in Euthymic Bipolar Participants and Healthy Controls.
| Cognitive Functioning Variables | Bipolar | Control | ANCOVA | p |
|---|---|---|---|---|
| CVLT Short Delay Cued Recall | 11.04 (3.01) | 12.58 (2.38) | 4.32 | 0.04 |
| CVLT Long Delay Cued Recall | 11.08 (2.75) | 12.37 (2.85) | 2.94 | 0.09 |
| CVLT Short Delay Free Recall | 10.54 (2.52) | 11.79 (3.02) | 1.57 | 0.22 |
| CVLT Long Delay Free Recall | 10.92 (2.46) | 11.67 (3.35) | 0.73 | 0.40 |
| CVLT Recognition | 14.08 (1.84) | 15.08 (1.25) | 5.24 | 0.03 |
| Digit Span Forward | 9.71 (1.39) | 11.33 (1.69) | 8.60 | 0.01 |
| Digit Span Backward | 8.58 (2.06) | 9.08 (1.74) | 0.12 | 0.73 |
| Stroop Inhibition/Switching | 55.82 (12.81) | 50.85 (7.96) | 4.76 | 0.04 |
| Tower Test | 15.91 (4.77) | 17.04 (2.91) | 1.81 | 0.19 |
| Sleep Functioning Variables | ||||
| Insomnia Severity Index | 10.87 (5.10) | 2.58 (2.75) | 11.46 | 0.002 |
| PSQI Onset Latency | 1.29 (0.85) | 0.50 (0.59) | 6.36 | 0.02 |
| PSQI Duration | 0.46 (0.51) | 0.17 (0.38) | 0.24 | 0.63 |
| PSQI Disturbance | 1.29 (0.86) | 0.88 (0.34) | 0.60 | 0.44 |
| PSQI Daytime Dysfunction | 1.26 (0.75) | 0.38 (0.495) | 8.69 | 0.01 |
| PSQI Efficiency | 0.42 (0.50) | 0.21 (0.41) | 0.05 | 0.82 |
| PSQI Sleep Quality | 1.21 (0.66) | 0.29 (0.46) | 9.73 | 0.004 |
| PSQI Total | 7.32 (3.23) | 2.46 (1.38) | 15.32 | <0.001 |
| Actigraphy Onset Latency | 23.25 (14.11) | 22.87 (15.51) | 1.27 | 0.27 |
| Actigraphy Wake After Onset | 34.14 (15.23) | 34.69 (13.29) | 0.01 | 0.94 |
| Actigraphy Duration | 458.43 (69.12) | 462.26 (53.10) | 0.89 | 0.35 |
| Actigraphy Efficiency | 83.95 (5.23) | 84.88 (4.37) | 2.14 | 0.15 |
| Work Functioning Variables | ||||
| Lifetime Firings | 1.13 (1.54) | 0.08 (0.28) | 17.02 | <0.001 |
| Lifetime Unemployment | 31.50 (48.66) | 7.71 (7.36) | 4.70 | 0.04 |
Results are presented as means (SD)
3.3 Moderation analyses of associations between cognitive and work functioning
Across all participants, Digit Span Forward, Stroop Inhibition/Switching, and the Tower of London test were significantly associated with number of lifetime firings, and CVLT short delay cued recall, CVLT long delay cued recall, CVLT recognition, and the Tower of London test were significantly associated with lifetime unemployment, so these cognitive variables were utilized for tests of moderation. Correlations are presented in Table 3.
Table 3.
Correlations between sleep, cognitive, and work disability variables.
| Lifetime Firing | Lifetime Quit | Lifetime Unemployment | ||
|---|---|---|---|---|
| Cognitive Variables | ||||
| CVLT Short Delay Free Recall | −0.28 | −0.07 | −0.18 | |
| CVLT Short Delay Cued Recall | −0.27 | −0.10 | −0.35* | |
| CVLT Long Delay Free Recall | −0.21 | −0.09 | −0.14 | |
| CVLT Long Delay Cued Recall | −0.22 | −0.11 | −0.34* | |
| CVLT Recognition | −0.28 | −0.23 | −0.35* | |
| Digit Span Forward | −0.42* | −0.09 | −0.27 | |
| Digit Span Backward | 0.03 | −0.02 | 0.09 | |
| Stroop Inhibition/Switching | 0.58** | −0.04 | 0.20 | |
| Tower of London | −0.47** | −0.19 | −0.42** | |
| Sleep Variables | ||||
| Insomnia Severity Index | 0.30* | 0.36* | 0.07 | |
| PSQI Day Dysfunction | −0.05 | 0.15 | −0.01 | |
| PSQI Efficiency | 0.41** | 0.14 | 0.37** | |
| PSQI Sleep Duration | 0.15 | 0.20 | 0.23 | |
| PSQI Sleep Disturbance | 0.23 | 0.36* | 0.22 | |
| PSQI Onset Latency | 0.04 | 0.27 | 0.29* | |
| PSQI Sleep Quality | 0.13 | 0.29* | 0.30* | |
| PSQI Total | 0.30* | 0.33* | 0.34* | |
| Actigraphy Sleep Duration | −0.07 | 0.03 | 0.04 | |
| Actigraphy Onset Latency | −0.07 | −0.17 | −0.18 | |
| Actigraphy Efficiency | 0.05 | −0.10 | 0.08 | |
| Actigraphy Disturbance | −0.04 | 0.04 | 0.19 | |
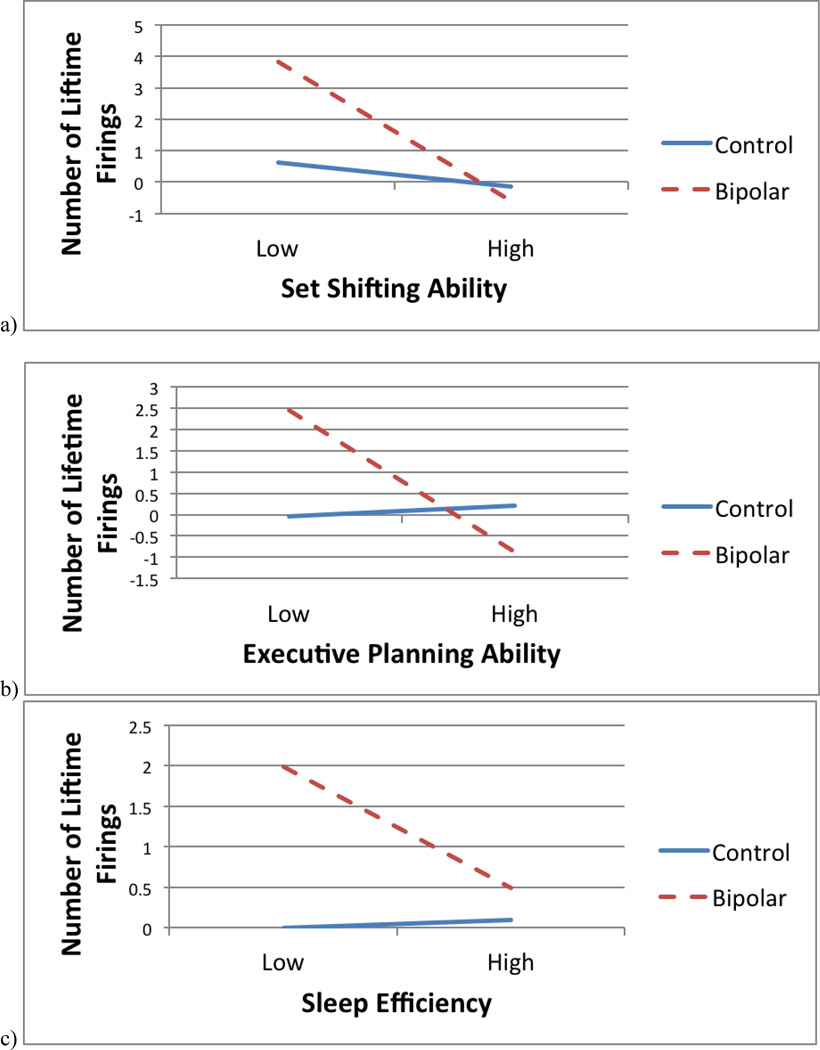
There was a significant interaction between diagnostic status and Stroop Inhibition/Switching and lifetime firings (t(42) = 2.00, p = 0.05), such that poorer set shifting ability was associated with increased lifetime firings for BD participants (t = 4.44, p < 0.001), but not for controls (t = 0.46, p = 0.65) (see Figure 1a). There was also a significant interaction between diagnostic status and performance on the Tower test (t(42) = −2.47, p = 0.02), such that deficits in executive planning were associated with greater lifetime firings for BD participants (t = −4.06, p < 0.001), but not for controls (t = 0.33, p = 0.74) (see Figure 1b). The interaction between diagnostic status and digit span forward was not significantly associated with lifetime firings.
Figure 1.
Interactions between diagnostic status and (a) set-shifting ability, (b) executive planning ability, and (c) sleep efficiency predicting number of lifetime firings.
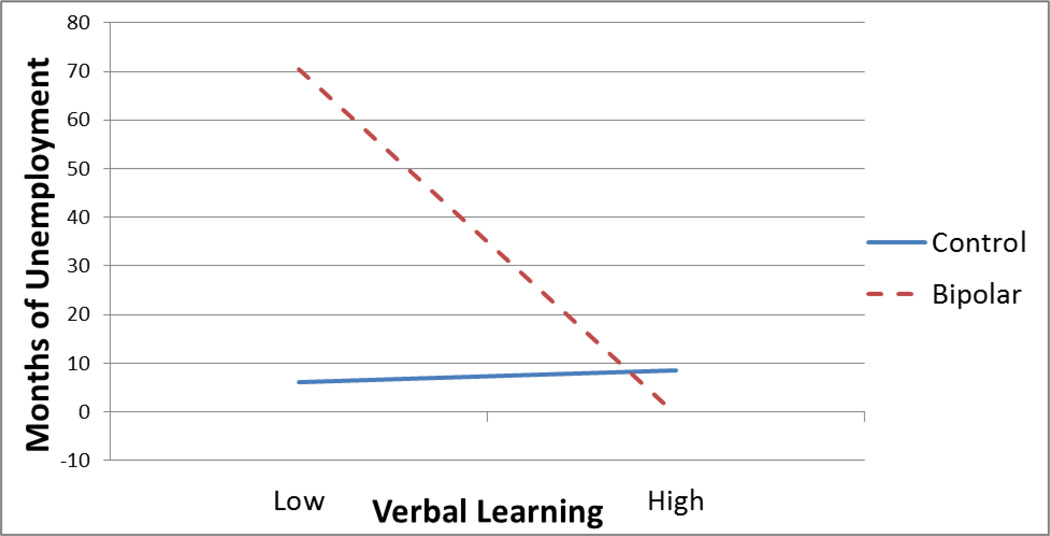
There was a significant interaction between CVLT Long Delay Cued Recall and diagnostic group associated with lifetime unemployment (t(43) = −2.31, p = 0.03), such that worse performance on long delay cued recall was associated with greater months of lifetime unemployment for BD participants (t = −3.07, p < 0.01), but not for controls (t = 0.13, p = 0.90) (see Figure 2). All other analyses were non-significant.
Figure 2.
Interaction between diagnostic status and verbal learning predicting months of lifetime unemployment.
3.4 Moderation analyses of associations between sleep and work functioning
PSQI Sleep Efficiency, PSQI Total Score, and ISI were significantly correlated with lifetime firings; PSQI Total Score, Sleep Quality, Sleep Disturbance, and Insomnia Severity Index score were all significantly correlated with number of lifetime incidents of quitting one’s job; and PSQI Sleep Efficiency, Latency, Quality, and total score were significantly correlated with lifetime unemployment.
The interaction between bipolar diagnosis and PSQI sleep efficiency was significant, t(43) = 2.21, p = 0.03, such that poorer sleep efficiency (indicated by a higher PSQI score) was significantly associated with greater number of lifetime firings for BD participants (t = 3.73, p < 0.001), whereas this relationship was not significant for control participants (t = 0.17, p = 0.87) (see Figure 1c). Diagnostic category did not moderate the relationship between PSQI Total Score or ISI and number of lifetime firings.
The interaction between diagnostic status and PSQI Sleep Disturbance reached trend level significance (t(41) = 1.84, p = 0.07), such that increased sleep disturbance was associated with marginally increased lifetime occurrences of quitting one’s job among BD participants (t = 2.31, p = 0.03), but not controls (t = −0.71, p = 0.48). The interaction between diagnostic status and PSQI Sleep Efficiency reached trend level significance (t(41) = 1.76, p = 0.09), such that increased sleep disturbance was associated with marginally increased lifetime unemployment among BD participants (t = 2.93, p = 0.005), but not controls (t = 0.11, p = 0.91). Diagnostic category did not significantly moderate the relationships between any other sleep variable and lifetime unemployment or of quitting one’s job.
3.5 Exploratory analyses of relationship between sleep and cognitive functioning
Given that sleep disturbance and cognitive functioning were both related to work disability in the BD sample, we conducted post-hoc correlational analyses to explore any possible relationships between sleep disturbance and cognitive functioning. All relationships were nonsignificant except for PSQI Daytime Dysfunction, which was significantly negatively correlated with backward digit span (r = −0.57, p = 0.03).
4. Discussion
Overall, findings from this study were generally supportive of study hypotheses. Sleep disturbance among BD participants was overwhelmingly greater when measured by self-report, although this is due in part to the design of the study, which included a comparison group of healthy sleepers. What is perhaps more important is that the mean score on the ISI was 10.9 for participants with BD in the euthymic phase, which is above the recommended cutoff score of 10 for detecting insomnia in community samples (Morin et al., 2011) and is suggestive of persistent sleep disturbance despite remission of affective symptoms. However, no objective differences in sleep parameters were observed on actigraphy. Although on the surface this may appear to be a confusing result, discrepancies in objective versus subjective assessments of sleep disruption are not uncommon in analyses of sleep disruption in BD (Harvey et al., 2005). Indeed, this also is a common finding in the insomnia literature, as individuals with insomnia have been found to overestimate sleep latency and wake time after sleep onset as well as to underestimate sleep duration (Mercier et al., 2002). There may be other explanations as well. It is possible that medications may be objectively ameliorating some sleep symptoms, but leaving lingering sedative effects that individuals attribute to poor sleep. Another possibility is that lingering cognitive distortions about sleep developed throughout the course of affective episodes where sleep disturbance is much more pronounced. Sleep may still be mildly disturbed during the interepisode period, but negative beliefs about sleep and ingrained sensitivity to disturbance may magnify subjective report. Finally, as mentioned, objective sleep disturbance may exist but only mildly, and this sample was likely underpowered to detect such small effect sizes.
It is important to note that a limitation of actigraphy is that it is not equipped to measure sleep architecture. A polysomnography study could potentially uncover changes in sleep architecture during periods of euthymia in the absence of objective behavioral markers of sleep disturbance. Moreover, anomalies identified via polysomnography could potentially be related to work performance. Utilizing polysomnographic techniques in future studies of work disability in BD is an important future direction, as findings could potentially inform the development of pharmacologic agents aimed at improving work functioning.
Cognitive performance was likewise poorer among BD participants than controls. Results from this study are consistent with previous examinations of cognitive functioning in BD (Robinson et al., 2006; Bora et al., 2009; Peters et al., 2014), demonstrating comparatively worse performance in the domains of verbal learning, working memory, and executive function. Importantly, these deficits remained after controlling for the contribution of sub-threshold depressive symptoms.
Analyses of work functioning indicated that BD participants had greater lifetime histories of unemployment, but also greater incidence of being fired from their jobs. Being terminated from a job can stem from a number of pathways including patterns of work inappropriate behavior (e.g., persistent absence from work, failure to meet job expectations), as well as more acute events (e.g., insubordination, losing one’s temper at work, behaving inappropriately). These pathways are speculative; the current study did not assess participants’ specific grounds for termination. However, the overall pattern of results would suggest that individuals with BD play some role in their own job loss. It is also important to note that the base rate of firings in the control group was low (0.08), which is potentially affected by the presence of college students in the sample. Thus, whereas our findings are consistent with the literature on occupational functioning among individuals with BD, more focused examinations utilizing a control group with a broader range of employment histories are needed.
Although the present study was unable to examine the temporal associations of sleep, cognitive functioning, and work disability, it generated some important hypotheses about how individuals with BD become unemployed. For example, the associations between executive functioning deficits and increased number of instances of being fired from one’s job could suggest that problems with response inhibition, difficulty switching between ways of thinking to meet situational demands, lack of planning ability, or increased impulsivity may lead to particularly egregious errors in the workplace that would result in termination. On the other hand, deficits in verbal learning may bring with them separate work-related challenges. Poor retention of verbal information could impact one’s ability to navigate job interviews or possibly lead to securing only lower-level positions with frequent turnover rates. In a similar vein, individuals with poor sleep efficiency may arrive late to work, miss shifts, or generally underperform as a result of lack of sleep. These explanations are naturally speculative, but further research into the differential effects of sleep disturbance and cognitive functioning on employment variables would be beneficial and could inform vocational rehabilitation programs for individuals with BD.
BD participants exhibited only subjective, not objective, deficits in sleep efficiency relative to controls. Thus, when interpreting the associations between sleep efficiency and incidence of job firings, it is important to note that it is ultimately the perception of reduced sleep efficiency that is linked to poor work performance. We can speculate that the perception of poor sleep quality results from carry-over of cognitive distortions about sleep that may have developed during acute episodes where objective sleep disturbance may have been more pronounced (Harvey et al., 2005; Carney et al., 2011). This has potentially important implications for the treatment of sleep disturbance in BD, as addressing sleep-related cognitive distortions may be as important as improving sleep behaviors in bringing about improvements in functional outcomes (Morin et al., 2002). Both the cognitive and behavioral aspects of sleep disturbance are equally addressed in Cognitive Behavioral Treatment for Insomnia (CBT-I), thus making this a potentially useful treatment for individuals with BD. The role of negative beliefs about sleep should be explored in future studies of factors influencing work disability in BD.
Given that sleep efficiency and frontal executive deficits were both significantly associated with work disability, it is possible that sleep disturbance and cognitive functioning may interact to increase the likelihood of workplace dysfunction. This line of inquiry is relatively new (see McKenna and Eyeler, 2012 and Boland and Alloy, 2013 for extensive reviews); however, evidence of sleep-mediated cognitive deficits in BD has been uncovered (Volkert et al., 2014). Similarly, we demonstrated a correlational relationship between daytime dysfunction (as a consequence of sleep disturbance) and working memory. Thus, it is possible that sleep disturbance may lead to certain cognitive deficits, which then produce deficits in work performance. Exploration of the independent relationships between endogenous and sleep-mediated cognitive deficits to work disability in BD is a worthwhile direction for future research.
Strengths of this study include careful measurement of sleep disruption via both subjective and objective methods, the use of well-validated measures of cognitive functioning, and a specific focus on employment-related disability. There are several limitations that should also be noted. First, although null actigraph findings have been observed in other studies of euthymic BD, we cannot rule out that our lack of group differences are attributable to a lack of greater data scrutiny by an independent technician blind to diagnosis and level of sleep disturbance. Second, the overall sample size was small, precluding analyses that may have answered important questions about the relationship between sleep and cognitive functioning in BD. Similarly, our sample was likely underpowered to detect what are likely small effect sizes in actigraphy variables and for moderation. Results should thus be interpreted cautiously. Although our study demonstrated sleep disturbance in BD that is consistent with the literature on euthymic BD, the presence of anxiety disorders or ADHD and their potential contributions to sleep disturbance was not assessed in this sample. Furthermore, analyses were cross-sectional and retrospective in nature, thus limiting interpretations about causality. For example, we were unable to distinguish whether job loss or unemployment contributed to stress that impacted sleep continuity. Likewise, the inclusion of college students hampered our ability to assess concurrent relationships between sleep/cognitive functioning and current employment, due to problems in operationalizing employment (i.e., whether to potentially inflate the number of “employed” by designating full-time student status as employment, or vice versa) as well as quality of current work functioning between college students and the rest of the population. Future studies should incorporate longitudinal designs that may better assess these potentially bidirectional relationships between sleep, cognitive impairments and work disability. College students are also a population known to be associated with sleep disturbance (Lund et al., 2009; Bulboltz et al., 2010). Although college students were distributed equally across groups, their inclusion in the study may limit generalizability to other (e.g., treatment-seeking) populations. Finally, the use of medications must be addressed. Many of the medications prescribed for the treatment of BD impact sleep functioning, including alterations in sleep architecture and duration; however, limiting analyses to an unmedicated BD sample would not have been feasible as pharmacotherapy for BD is the gold standard for treatment. Future studies should examine how the effects of medications impact sleep, cognitive, and work functioning.
Nevertheless, there are important clinical implications that stem from this study. First, sleep may not need to be objectively disturbed in order for individuals with BD to report sleep-related impairment. Thus, as mentioned earlier, specific focus on sleep-relevant cognitive distortions and/or expectations about sleep would thus be advantageous for this population. Additionally, individuals with BD may benefit from targeted vocational rehabilitation programs that take into account their history, if any, of being fired from previous jobs in an effort to help them avoid similar dismissals in the future. Given this study’s associations between cognitive functioning and work disability, cognitive remediation may be a worthy addition to treatment, especially if tailored to fit the context of vocational rehabilitation. Future research distinguishing between sleep-mediating and exogenous cognitive deficits also would help inform treatment decisions, as sleep treatment may ameliorate cognitive deficits in the former scenario.
Highlights.
We examine the role of cognitive deficits and sleep disturbance in work disability among individuals with bipolar disorder.
Cognitive functioning is disturbed among individuals with bipolar disorder compared to healthy controls.
Self-reported insomnia severity among individuals with bipolar disorder in the euthymic phase exceeds the recommended cutoff score for detecting insomnia in community samples.
Cognitive deficits and poor sleep efficiency are associated with poor work performance for individuals with bipolar disorder but not for healthy controls.
Sleep is an important target for treatment for bipolar disorder and may have important implications for reducing functional disability.
Acknowledgements
This research was supported by a 2013 award from the Civic Foundation to Elaine M. Boland. Lauren B. Alloy was supported by National Institute of Mental Health Grants MH 77908 and MH102310. Jonathan P. Stange, Jessica L. Hamilton, and Samantha L. Connolly were supported by National Research Service Awards F31MH099761, F31MH106184 and F31MH106181, respectively, from NIMH. Writing of this manuscript was supported by the Office of Academic Affiliations, Advanced Fellowship Program in Mental Illness Research and Treatment, Department of Veterans Affairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Elaine M. Boland developed and organized the study, collected and analyzed data, and composed the manuscript. Jonathan P. Stange provided statistical consultation and composed and edited the manuscript. Ashleigh Molz Adams, Denise R. LaBelle, Mian L. Ong, Jessica L. Hamilton, Samantha L. Connolly, Angelo B. Cedeño collected data and provided manuscript edits. Lauren B. Alloy provided diagnostic consultation, supervised the study procedures, and edited the manuscript.
References
- Alloy LB, Abramson LY, Walshaw PD, Cogswell A, Grandin LD, Hughes ME, Iacoviello BM, Whitehouse WG, Urosevic S, Nusslock R, Hogan ME. Behavioral approach system (BAS) and behavioral inhibition system (BIS) sensitivities and bipolar spectrum disorders: Prospective prediction of bipolar mood episodes. Bipolar Disorders. 2008;10:310–322. doi: 10.1111/j.1399-5618.2007.00547.x. [DOI] [PubMed] [Google Scholar]
- Altshuler LL, Gitlin MJ, Mintz J, Leight KL, Frye MA. Subsyndromal depression is associated with functional impairment in patients with bipolar disorder. Journal of Clinical Psychiatry. 2002;63:807–811. doi: 10.4088/jcp.v63n0910. [DOI] [PubMed] [Google Scholar]
- Altman EG, Hedeker D, Peterson JL, Davis JM. The Altman Self-Rating Mania Scale. Biological Psychiatry. 1997;42:948–955. doi: 10.1016/S0006-3223(96)00548-3. [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S, Roth T. Characteristics of insomnia in the United States: results of the 1991 National Sleep Foundation Survey. Sleep 22 Supplement. 1997;2:S347–S353. [PubMed] [Google Scholar]
- Arts B, Jabben N, Krabbendam L, van Ox J. Meta-analyses of cognitive functioning in euthymic bipolar patients and their first-degree relatives. Psychological Medicine. 2008;38:771–785. doi: 10.1017/S0033291707001675. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Boland EM, Alloy LB. Sleep disturbance and cognitive deficits in bipolar disorder: Toward an integrated examination of disorder maintenance and functional impairment. Clinical Psychology Review. 2013;33:33–44. doi: 10.1016/j.cpr.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnín CM, Martinez-Arán A, Torrent C, Pacchiarotti I, Rosa AR, Franco C, Murru A, Sanchez-Moreno J, Vieta E. Clinical and neurocognitive predictors of functional outcome in bipolar euthymic patients: A long-term, follow-up study. Journal of Affective Disorders. 2010;121:156–160. doi: 10.1016/j.jad.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Bora E, Yucel M, Pantelis C. Cognitive endophenotypes of bipolar disorder: A meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. Journal of Affective Disorders. 2009;113:1–20. doi: 10.1016/j.jad.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Brooks JO, III, Friedman L, Bliwise DL, Yesavage JA. Use of the wrist actigraph to study insomnia in older adults. Sleep. 1993;16:151–155. doi: 10.1093/sleep/16.2.151. [DOI] [PubMed] [Google Scholar]
- Buboltz WC, Brown F, Soper B. Sleep habits and patterns of college students: A preliminary study. Journal of American College Health. 2010;50:131–135. doi: 10.1080/07448480109596017. [DOI] [PubMed] [Google Scholar]
- Buysse D, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Carney CE, Harris AL, Friedman J, Segal ZV. Residual sleep beliefs and sleep disturbance following cognitive behavioral therapy for major depression. Depression and Anxiety. 2011;28:464–470. doi: 10.1002/da.20811. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Holdnack J. Reliability and validity of the Delis-Kaplan Executive Function System: An update. Journal of the International Neuropsychological Society. 2004;10:301–303. doi: 10.1017/S1355617704102191. [DOI] [PubMed] [Google Scholar]
- Depue RA, Krauss S, Spoont MR, Arbisi P. General Behavior Inventory identification of unipolar and bipolar affective conditions in a nonclinical university population. Journal of Abnormal Psychology. 1989;98:117–126. doi: 10.1037//0021-843x.98.2.117. [DOI] [PubMed] [Google Scholar]
- Dickerson FB, Boronow JJ, Stallings CR, Origioni AE, Cole S, Yolken RH. Association between cognitive functioning and employment status of persons with bipolar disorder. Psychiatric Services. 2004;55:54–58. doi: 10.1176/appi.ps.55.1.54. [DOI] [PubMed] [Google Scholar]
- Endicott J, Spitzer RL. A diagnostic interview: The schedule for affective disorders and schizophrenia. Archives of General Psychiatry. 1978;35(7):837–844. doi: 10.1001/archpsyc.1978.01770310043002. [DOI] [PubMed] [Google Scholar]
- Fagiolini A, Kupfer DJ, Masalehdan A, Scott J, Houck PR, Frank E. Functional impairment in the remission phase of bipolar disorder. Bipolar Disorders. 2005;7:281–285. doi: 10.1111/j.1399-5618.2005.00207.x. [DOI] [PubMed] [Google Scholar]
- Giglio LMF, Andreazza AC, Andersen M, Cereser KM, Walz JC, Sterz L, Kapczinski F. Sleep in bipolar patients. Sleep Breath. 2009;13:169–173. doi: 10.1007/s11325-008-0215-5. [DOI] [PubMed] [Google Scholar]
- Gruber J, Harvey AG, Wang PW, Brooks JO, III, Thase ME, Sachs GS, Ketter TA. Sleep functioning in relation to mood, function, and quality of life at entry to the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) Journal of Affective Disorders. 2009;114:41–49. doi: 10.1016/j.jad.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AG, Schmidt DA, Scarna A, Semler CN, Goodwin GM. Sleep-related functioning in euthymic patients with bipolar disorder, patients with insomnia, and subjects without sleep problems. American Journal of Psychiatry. 2005;162:50–57. doi: 10.1176/appi.ajp.162.1.50. [DOI] [PubMed] [Google Scholar]
- Hays JR, Reas DL, Shaw JB. Concurrent validity of the Wechsler Abbreviated Scale of Intelligence and the Kaufman Brief Intelligence Test among psychiatric inpatients. Psychological Reports. 2002;90:355–359. doi: 10.2466/pr0.2002.90.2.355. [DOI] [PubMed] [Google Scholar]
- Judd LL, Akiskal HS, Schettler PJ, Endicott J, Leon AC, Solomon DA, Coryell W, Maser JD, Keller MB. Psychosocial disability in the course of bipolar I and II disorders: A prospective, comparative, longitudinal study. Archives of General Psychiatry. 2005;62(12):1322–1330. doi: 10.1001/archpsyc.62.12.1322. [DOI] [PubMed] [Google Scholar]
- Judd LL, Schettler PJ, Solomon DA, Maser JD, Coryell W, Endicott J, Akiskal HS. Psychosocial disability and work role function compared across the long-term course of bipolar I, bipolar II, and unipolar major depressive disorders. Journal of Affective Disorders. 2008;108:49–58. doi: 10.1016/j.jad.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test. Circle Pines, MN: American Guidance Service; 1990. [Google Scholar]
- Kozicky J, Torres IJ, Bond DJ, Lam RW, Yatham LN. Comparison of neuropsychologcal effects of adjunctive risperidone or quetiapine in euthymic patients with bipolar I disorder. International Clinical Psychopharmacology. 2012;27:91–99. doi: 10.1097/YIC.0b013e32834e3bea. [DOI] [PubMed] [Google Scholar]
- Kurtz NM, Gerraty RT. A meta-analytic investigation of neurocognitive deficits in bipolar illness: Profile and effects of clinical state. Neuropsychology. 2009;23:551–562. doi: 10.1037/a0016277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund HG, Reider BD, Whiting AB, Prichard JR. Sleep patterns and predictors of disturbed sleep in a large population of college students. Journal of Adolescent Health. 2009;46:124–132. doi: 10.1016/j.jadohealth.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Mann-Wrobel MC, Carreno JT, Dickinson D. Meta-analysis of neurpsychological function in euthymic bipolar disorder: an update and investigation of moderator variables. Bipolar Disorders. 2011;13:334–342. doi: 10.1111/j.1399-5618.2011.00935.x. [DOI] [PubMed] [Google Scholar]
- Martinez-Àran A, Vieta E, Reinares M, Colom F, Torrent C, Sanchez-Moreno J, Benabarre A, Goikolea JM, Comes M, Salamero M. Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. American Journal of Psychiatry. 2004;161:262–270. doi: 10.1176/appi.ajp.161.2.262. [DOI] [PubMed] [Google Scholar]
- McKenna BS, Eyler LT. Overlapping prefrontal systems involved in cognitive and emotional processing in euthymic bipolar disorder and following sleep deprivation: A review of functional neuroimaging studies. Clinical Psychology Review. 2012;32:650–663. doi: 10.1016/j.cpr.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar A, Espie CA, Scott J. The sleep of remitted bipolar outpatients: A controlled naturalistic study using actigraphy. Journal of Affective Disorders. 2004;80:145–153. doi: 10.1016/S0165-0327(03)00055-7. [DOI] [PubMed] [Google Scholar]
- Morin CM, Belleville G, Belanger L, Ivers H. The Insomnia Severity Index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34:601–608. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin CM, Blais F, Savard J. Are changes in beliefs and attitudes about sleep related to sleep improvements in the treatment of insomnia? Behaviour Research and Therapy. 2002;40:741–752. doi: 10.1016/s0005-7967(01)00055-9. [DOI] [PubMed] [Google Scholar]
- Nadkami RB, Fristad MA. Clinical course of children with a depressive spectrum disorder and transient manic symptoms. Bipolar Disorders. 2010;12:494–503. doi: 10.1111/j.1399-5618.2010.00847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naugle RI, Gordon CJ, Tucker GD. Validity of the Kaufman Brief Intelligence Test. Psychological Assessment. 1993;5:182–186. [Google Scholar]
- National Institute of Mental Health Fact Sheet on Bipolar Disorder. http://www.nimh.nih.gov/health/publications/bipolar-disorder/index.shtml.
- Nusslock R, Abramson LY, Harmon-Jones E, Alloy LB, Hogan ME. A goal-striving life event and the onset of bipolar episodes: Perspective from the Behavioral Approach System (BAS) dysregulation theory. Journal of Abnormal Psychology. 2007;116:105–115. doi: 10.1037/0021-843X.116.1.105. [DOI] [PubMed] [Google Scholar]
- Peters AT, Peckham AD, Stange JP, Sylvia LG, Hansen NS, Salcedo S, Rauch SL, Nierenberg AA, Dougherty DD, Deckersbach T. Correlates of real world executive dysfunction in bipolar I disorder. Journal of Psychiatry Research. 2014;53:87–93. doi: 10.1016/j.jpsychires.2014.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson LJ, Thompson JM, Gallagher P, Goswami U, Young AH, Ferrier N, Moor PB. A meta-analysis of cognitive deficits in euthymic patients with bipolar disorder. Journal of Affective Disorders. 2006;93:105–115. doi: 10.1016/j.jad.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Rosa AR, Reinares M, Franco C, Comes M, Torrent C, Sanchez-Moreno J, Martinez-Aran A, Salamero M, Kapczinski Vieta E. Clinical predictors of functional outcome of bipolar patients in remission. Bipolar Disorders. 2009;11:401–409. doi: 10.1111/j.1399-5618.2009.00698.x. [DOI] [PubMed] [Google Scholar]
- Sadeh A. The role of actigraphy in sleep medicine. Sleep Medicine Reviews. 1995;6:113–124. doi: 10.1053/smrv.2001.0182. [DOI] [PubMed] [Google Scholar]
- Sanchez-Moreno J, Martinez-Àran A, Tabares-Seisdedos R, Torrent C, Vieta E, Ayuso-Mateos JL. Functioning and Disability in Bipolar Disorder: An extensive review. Psychotherapy and Psychosomatics. 2009;78:285–297. doi: 10.1159/000228249. [DOI] [PubMed] [Google Scholar]
- Shallice T. Specific impairments of planning. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 1982;298:199–209. doi: 10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- Torres IJ, Boudreau VG, Yatham LN. Neuropsychological functioning in euthymic bipolar disorder: a meta-analysis. Acta Psychiatrica Scandinavica. 2007;116:17–26. doi: 10.1111/j.1600-0447.2007.01055.x. [DOI] [PubMed] [Google Scholar]
- Torres IJ, DeFreitas CM, DeFreitas VG, Bond DJ, Kunz M, Honer WG, Lam RW, Yatham LN. Relationship between cognitive functioning and 6-month clinical and functional outcome in patients with first manic episode bipolar I disorder. Psychological Medicine. 2011;41:971–982. doi: 10.1017/S0033291710001613. [DOI] [PubMed] [Google Scholar]
- Volkert J, Kopf J, Kazmaier J, Glaser F, Zierhut KC, Schiele MA, Kittel-Schneider S, Reif A. Evidence for cognitive subgroups in bipolar disorder and the influence of subclinical depression and sleep disturbances. European Neuropsychopharmacology. 2014;25:192–202. doi: 10.1016/j.euroneuro.2014.07.017. [DOI] [PubMed] [Google Scholar]
- Webster JB, Kripke SM, Mullaney DJ, Wyborney G. An activity-based sleep monitor system for ambulatory use. Journal of Sleep Research and Sleep Medicine. 1982;5:389–399. doi: 10.1093/sleep/5.4.389. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale – Third Edition. Texas: The Psychological Corporation; 1997. [Google Scholar]
- Woods SP, Delis DC, Scott JC, Kramer JH, Holdnack JA. The California Verbal Learning Test - second edition: test-retest reliability, practice effects, and reliable change indices for the standard and alternate forms. Archives of Clinical Neuropsychology. 2006;21:413–20. doi: 10.1016/j.acn.2006.06.002. [DOI] [PubMed] [Google Scholar]
- World Health Organization [WHO] The World Health Report 2006: Working Together for Health. Geneva: WHO; 2006. [Google Scholar]