Abstract
Reproductive lives of men and women may provide significant insight into later-life morbidity and mortality. Sociological, biological, and evolutionary theories predict a relationship between reproductive history and later-life health; however, current research is lacking consensus on the direction of the relationship. Parity, early age at first birth and last birth, birth weight of offspring, having a child die as an infant, and having a preterm birth may have long-term effects on health for both men and women. In this study, the relationship between these measures of reproductive history and later-life health is examined using the Utah Population Database (a rich source of longitudinal data), and Medicare claims data from 1992–2009. Later-life health is measured using annual Charlson comorbidity index scores, a construct that summarizes most serious illnesses afflicting older individuals. Group-based trajectory modeling that accounts for nonrandom attrition due to death is used to identify the number and types of morbidity trajectories by sex and age for 52,924 individuals aged 65–84 in 1992. For females, early age at first birth, high parity, and having a preterm or high-birth-weight baby are associated with increased risks of comorbidity; later age at last birth is associated with a decreased risk of comorbidity. For males, early age at first birth and having a child with an abnormal birth weight leads to increased risk of comorbidity. The results suggest that both biological and social factors play important roles in the relationships between fertility and morbidity profiles at older ages.
Keywords: Fertility, Health trajectories, Life course, Morbidity, Biodemography
Introduction
Variations in the frequency, severity, and sequences of illness among older adults suggest that morbidity is not an inevitable consequence of aging. To examine the sources of this variation, we adopt a life course approach, which advocates the idea of periods of plasticity wherein individuals may experience physiological or social change that alters their future health trajectories. The question of variability and patterning of illness profiles are examined herein by using data for 1992–2009 from the Centers for Medicare & Medicaid Services (CMS) linked to the Utah Population Database (UPDB), a rich source of longitudinal data. Fertility information is derived from a combination of sources within UPDB including genealogical records from two sources—the Genealogical Society of Utah and linked Utah vital records—including birth certificate data. Fertility characteristics we investigate include parity, early age at first birth and last birth, birth weight of offspring, having a child die as an infant, and having a preterm birth. The goals of this analysis are twofold: (1) to identify distinct trajectories of comorbidity from 1992 to 2009 for individuals aged 66–84 in 1992 by sex and birth cohort; and (2) to estimate the association between measures of fertility and these later-life comorbidity trajectories while controlling for early-life conditions (ELCs).
Background
Considerable research investigating the relationship between reproductive history and health has focused on adult and older-age mortality. An important but smaller set of studies has also demonstrated the relationship between reproductive history and a variety of later-life health measures (Davey Smith et al. 2004; Grundy and Tomassini 2005; Henretta 2008; Kravdal 1995; Lawlor et al. 2003; Myklestad et al. 2012; Spence 2008; Yi and Vaupel 2004). It is common for studies of reproductive history and health to focus on females; however, it is also true that reproductive history affects males through mechanisms that may be more social in origin. In addition, including males in the analyses serves to sharpen the possible mechanisms underlying the association between fertility and later morbidity risk.
To date, no studies have investigated the relationship between reproductive history and comorbidity trajectories during the post-reproductive period. This is an important area for research because studies of all-cause mortality or single diseases do not allow for more comprehensive analyses of sets of diseases that are the hallmark of aging, a feature that is addressed here by the use of morbidity trajectories.
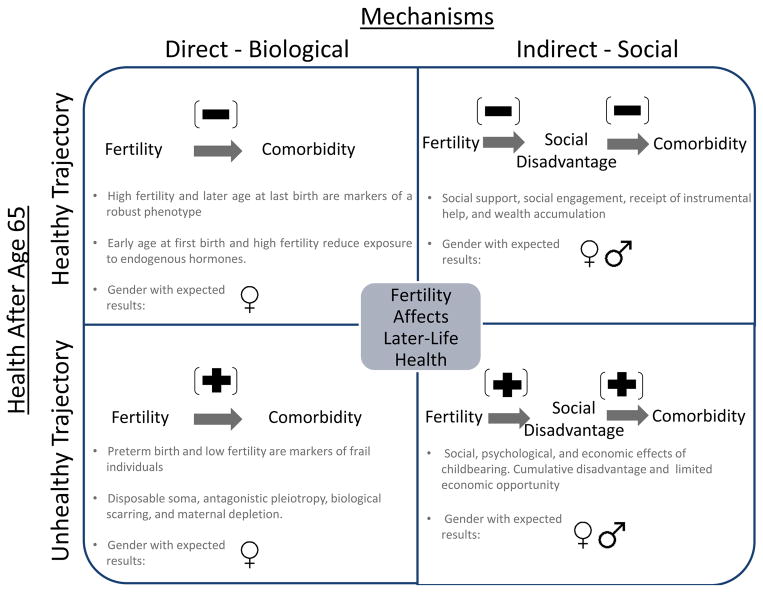
Evolutionary, biological, and social theories predict that reproductive costs represented by parity, age at first birth, age at last birth, interbirth intervals, twinning, birth weight of offspring, and giving birth prematurely may all be associated with later-life morbidity and mortality for both males and females. Ignoring these potential developmental origins of adult disease may lead to confounded estimates linking fertility behavior to later morbidity patterns. Figure 1 summarizes the direct and indirect mechanisms that may affect health trajectories after age 65. The past literature that has examined reproductive history includes both clinical and populations’ samples—methodological distinctions that may influence generalizability of findings. Although clinical samples are biased, they often have better measures and provide important information about mechanisms linking reproductive outcomes to later-life health. Therefore, both types of studies are discussed herein.
Fig. 1.
Expected relationship between fertility and later-life comorbidity trajectories
Direct Biological Evolutionary and Genetic Theories Linking Reproductive Health to Aging
Evolutionary theories of disposable soma and antagonistic pleiotropy help to articulate the relationship between fertility and mortality. For females, these theories predict that young age at first birth and high parity are associated with increased comorbidity later in life (Gagnon et al. 2009; Smith et al. 2002). High parity, late age at last birth, multiple births, and short birth intervals may also be associated with decreased comorbidity later in life. For example, it has been suggested that genetic variants influence both late female fertility and slowed rates of somatic aging (Smith et al. 2009a). Fertility may also be an indication of health status. Women with higher fertility, with shorter birth intervals, who have had twins, and with later ages at last birth may have increased longevity because elevated fertility is a marker of a robust phenotype (Hawkes 2010; Robson and Smith 2011, 2012).
Male fertility may also be a biomarker for overall male somatic health (Eisenberg 2012; Eisenberg et al. 2011). Male subfertility has been linked to increased risk for cancer (testicular, prostate, and colon), reduced lifespan, and possibly cardiovascular disease and metabolic syndrome (Walsh et al. 2009a, 2009b, 2010).
Direct Biological Effects of Reproductive Health and Biological Indicators of Later-Life Health
From a life course epidemiology perspective, physiological scarring has been used to define an event that permanently alters the physiological functioning of an organism. Pregnancy may trigger physiological changes that favorably or adversely affect later-life health among women. Increased parity and early age at first birth have been shown to lower risk of postmenopausal reproductive cancers. One of the well-characterized pathways relates to pregnancy, which affects lifetime exposure to endogenous hormones (progesterone and estrogen), which in turn are associated with cancer risk later in life (Kelsey et al. 1993; Kobayashi et al. 2012; Lukanova and Kaaks 2005). There is an inverse association between parity and cancer incidence in tissues sensitive to hormone levels, such as breast, endometrial, and ovarian (Kelsey et al. 1993; Kobayashi et al. 2012; Kvale et al. 1994; Permuth-Wey and Sellers 2009). Age at first birth is also a well-established risk factor for breast cancer, with younger age at first birth being protective (MacMahon et al. 1973).
Reproductive behaviors may lead to additional physiological changes that adversely affect a woman’s health. Pregnancy-related biological responses lead to increased risks for coronary heart disease and obesity later in life (Bastian et al. 2005; Lawlor et al. 2003). Short birth spacing and multiple births (twins) may leave a physiological imprint on the mother. Specifically, the maternal depletion hypothesis argues that physiological demands of pregnancy diminish physical resources and that short birth intervals do not give the mother ample time to recover from the stresses of the previous pregnancy (Jelliffe and Jelliffe 1978; Kirkwood and Rose 1991). These theories predict increased comorbidities with high parity, short birth intervals, and multiple births.
Characteristics of a mother’s offspring at time of birth can be used to gauge the woman’s health during her reproductive period and may predict health status as she ages. Examples of these markers include birth weight of her child and whether her child was born prematurely (Bellamy et al. 2009; Carr et al. 2006; Smith et al. 1997, 2000). There are at least two reasons for these associations. First, they may be indicators of the social and physical environment of the mother, with conditions affecting the development and health of both mother and fetus. Second, they may be indicative of genetic variants carried by the mother or father that predispose them to cardiovascular disease (Myklestad et al. 2012). These studies suggest that high- and low-birth-weight and preterm babies are associated with adverse health outcomes later in life of the parents.
Indirect Social Mechanisms Linking Reproductive History to Later-Life Health
Social theories predict both positive and negative relationships between parity and later-life morbidity. The social benefits of adult children may negate adverse physiological effects of having children because they may provide social support, social engagement, and instrumental help to their parents. Strong social support may reduce stress, minimize risky behavior, and foster feelings of meaning. Individuals with more social support and intimate ties have better health and lower mortality (Berkman and Syme 1979; House et al. 1988). Children may provide a support network later in life, and more children may increase the chance of parents having regular contact (Uhlenberg and Cooney 1990) and receipt of help from their children (Grundy and Read 2012). Later ages at first birth may also allow for accumulation of wealth and resources that may be associated with fewer adverse health outcomes later in life.
Psychological, social, and economic impacts of having children may also lead to a positive relationship between parity and later-life morbidity. Increasing parity is associated with obesity and coronary heart disease for both men and women, whereby lifestyle factors associated with high parity may lead to increased risks of morbidity (Lawlor et al. 2003). Increased parity may not necessarily translate to increased social support. Smith et al. (2002) suggested that high-parity children may have fewer resources to devote to parents; and because of the intergenerational transmission of fertility, high parity may actually lead to decreased social support.
Early parenthood may limit opportunities for education and employment (Ross and Huber 1985; Waldron et al. 1998), which leads to adverse health consequences later in life (Mirowsky 2005; Phelan et al. 2010). Mirowsky (2005) suggested that the optimal period for childbirth in relation to health is from the late-20s to mid-30s and that the social and economic benefits of delayed childbearing far outweigh presumed biological costs. The current findings in the literature present conflicting findings related to the benefits of age at last birth after 39, with some studies suggesting that late ages at last birth are protective (Smith et al. 2002, 2009b) and others suggesting they are damaging (Mirowsky 2005).
Finally, infant mortality may be a marker for maternal health, limited resources, and adverse environments surrounding childbearing (Geronimus 1992; McCormick et al. 1984). Environments that lead to adverse health outcomes of the infant may also be risky for the parents. Therefore, the death of a child during infancy may be associated with poor health later in life.
Events Throughout the Life Course That Affect Fertility and Morbidity
Examining the relationship between fertility and morbidity without controlling for early-life circumstances can lead to a bias assessment of their association. Early-life factors affect both reproductive health and behaviors of men and women (Doblhammer and Oeppen 2003; Rich-Edwards 2002); therefore, part of the observed association between fertility and later-life morbidity is possibly a reflection of genetic makeup at birth or physiological changes during childhood. For example, adverse childhood and adolescent circumstances are related to early motherhood (Geronimus and Korenman 1992) and later-life health (Galobardes et al. 2008; Preston et al. 1998; Smith et al. 2009b). These are important confounders that must be controlled for when studying effects of fertility on later-life morbidity. The current study includes measures of early-life circumstances that may affect fertility and later-life health outcomes.
This study aims to identify distinct comorbidity trajectories from 1992–2009 and to identify whether and how measures of fertility and reproductive health are associated with morbidity profiles later in life for both males and females. Health trajectories at younger ages are not considered because Medicare data are not available prior to age 65. From a life course epidemiology perspective, this is a valid age range to study because early stressors affect later-life health, and a study of those after age 65 is apropos. Much of the world has fertility today that is not so different from the elderly cohorts that we study in Utah; thus, our results may speak to other populations. Understanding the relationship between multiple measures of fertility in both sexes allows us to increase our understanding of the long-term implications of reproduction.
Data
The majority of life-span epidemiological studies examine health influences of conditions early in life and in adulthood with relatively modest sample sizes. This study addresses this limitation by using data drawn from the Utah Population Database (UPDB). UPDB has supported numerous biodemographic, epidemiological, and genetic studies largely because of its sample size, pedigree complexity, and linkages across data sources. Because of longstanding and ongoing efforts to add new sources of data and update records as they become available, the full UPDB contains data on more than 8 million individuals, including all statewide death certificate records (1904–present) and all Medicare claims (1992–2009).
Given cohort differences in mortality and quickly changing morbidity risks by age and sex, we conduct all analyses by sex and two age categories (66–74 and 75–84); the first age category begins at 66 to eliminate problems of prorating partial year coverage of individuals who become Medicare eligible partway into a year when turning 65. Ages are considered in 1992, the first year in which UPDB has been linked to Medicare data. Separating samples by age effectively controls for broad cohort differences and allows us to analyze the cohort-specific trends for an 18-year period. Individuals age 66–74 and 75–84 in 1992 are considered members of the young-old cohort (born 1918–1926) and middle-old cohort (born 1908–1917), respectively.
We select once-married parous individuals to limit complications related to fertility spanning more than one marriage partner. We also exclude individuals with a spouse deceased before the individual reached age 50 because they may not have completed childbearing (Gagnon et al. 2009). The Centers for Medicare & Medicaid Services (CMS) data pertain to individuals who survive to age 65, thus including individuals who have by definition completed childbearing. Selecting parous individuals helps in identification of multivariate effects of both intensity and timing of fertility on comorbidity trajectories. It is also a necessary restriction because identification of nulliparous women is not as comprehensive in the UPDB (Moorad et al. 2011). Note that only a small fraction of individuals are excluded for this reason and therefore represents a very small source of bias. However, this analysis is not intended to account for the impact of childlessness (Gagnon et al. 2009; Smith et al. 2002). Individuals are also required to have sufficient information about parents in the database, which allows for the inclusion of early-life circumstances in the model, most of which naturally relate to the parents.
CMS data allow us to assess whether an individual is sufficiently represented in the Medicare claims data so that they can contribute to the construction of morbidity trajectories. Our goal is to avoid characterizing someone as being disease-free when their health events are simply not well represented in the Medicare data. CMS provides a monthly indicator variable that describes when a beneficiary was enrolled in a managed care plan. As expected, few Medicare claims exist in the file for individuals during the time they are enrolled in a managed care plan (managed care organization (MCO) or Medicare Advantage). For the purposes of this analysis, we exclude persons who have at least one month of enrollment in a managed care plan. We also require individuals to have at least one full year of data—a requirement to model the outcome. Accordingly, those deceased in 1992 are excluded. Individuals were then followed for a maximum of 18 years (to 2009), our last year of Medicare data, or until death. The total sample size is N = 41,158 (Female66–74 = 12,190, Female75–79 = 10,099, Male66–74 = 11,349, and Male66–74 = 7,520). Sample exclusion criteria are shown in Table S1 in Online Resource 1.
An ancillary analysis of individuals aged 66 to 74 in 1992 was conducted based on those for whom we could obtain information from birth certificates. Birth certificate information in UPDB is available from 1915–1921 and 1938 to the present; however, birth weight was not recorded until 1947. Because we are interested in the effects of birth weight and prematurity on parental morbidity trajectories, individuals used in this analysis are required to have their first birth in 1947 or later with all births occurring in Utah (providing complete fertility information). Approximately 40 % (n = 4,142) of women and 55 % (n = 6,192) of men in this young-old cohort have birth certificate records for all births. Individuals age 66 and 74 in 1992 would have had their first birth at age 21 if they were the youngest members of the cohort, and at age 29 if they were the oldest. For females, the average age at first birth in the birth certificate sample is 2.5 years greater than among those excluded (25.8 vs. 23.3, p < .01); for males, it is 1.4 years greater (26.9 vs. 25.5, p < .01). Despite these sample selection restrictions, the benefits of linking birth outcomes to later-life health trajectories are justifiable.
Key Measures
Comorbidity
We observe morbidity episodes from Medicare claims collected over time for each individual. Health status is measured by the Charlson comorbidity index (CCI) (Charlson et al. 1987). In the present study, we adopt the Klabunde et al. (2000) variant of the CCI based on the SEER-Medicare Comorbidity macro (Healthcare Delivery Research 2010). The SEER-Medicare macro calculates the CCI with respect to cancer. Given that cancer originally was the index disease, it was not included as a comorbid condition in this SEER-Medicare program. Accordingly, we add cancer as comorbid disease. We identify specific episodes of the following 17 major morbidity conditions occurring during the interval 1992–2009 on a per annum basis that form the basis of the CCI. Items are coded as 0 if they do not occur at any time during the year, and as a weighted score based on ability to predict mortality if they do occur:
| 1. Myocardial Infarction |
| 2. Congestive Heart Failure |
| 3. Peripheral Vascular Disease |
| 4. Cerebrovascular disease |
| 5. Dementia |
| 6. Chronic pulmonary disease |
| 7. Rheumatologic disease |
| 8. Peptic Ulcer Disease |
| 9. Mild Liver Disease |
| 10. Diabetes (mild to moderate) |
| 11. Diabetes with chronic complications |
| 12. Hemiplegia or paraplegia |
| 13. Renal (kidney) disease |
| 14. Any malignancy |
| 15. Moderate or severe liver disease |
| 16. Metastatic Solid Tumor |
| 17. AIDS |
Demographic Characteristics
All models control for age in 1992, mean-centered within sex and age groups. Widowhood is a frequent occurrence among individuals in this age range and may be linked to changes in health status (Williams and Umberson 2004). Time-varying covariates are used to allow for altered shapes of the trajectories due to the death of a spouse. An indicator variable was created for each year of observation, defined as equal to 0 during all periods where the spouse was alive and equal to 1 during periods where the spouse was deceased.
Measures of Early-Life Conditions
Measures of age at parental death, childhood socioeconomic status (SES), familial excess longevity (FEL; described later in this section), and religious participation are generated from the data held within the UPDB. Death of a parent during childhood may have adverse effects on health later in life (Andersson et al. 1996; Norton et al. 2011; Umberson and Chen 1994), and disruption of the family may affect transition into adulthood, including timing of childbirth. Birth, marriage, and death dates are recorded comprehensively in the UPDB and are used to construct categories of parental death. Sex of the deceased parent may have different social and economic implications; therefore, two categories are created (one mother and one father) for each of the following circumstances related to parental death: (1) mother/father died when child was under age 18, (2) and parent deceased after child was age 18 (reference category). As well, a category is included for both parents deceased when child was under 18 (orphan).
Childhood SES may directly and indirectly influence marriage and reproductive success, timing of childbirth, and later-life comorbidity (Doblhammer and Oeppen 2003; Geronimus and Korenman 1992; Kuh and Ben-Shlomo 2004). Childhood SES is measured using usual occupation and industry information reported on their father’s death certificate for fathers who died in Utah and for whom we have a death certificate (deaths occurring from 1904 forward). Occupational descriptions are converted to Nam-Powers socioeconomic (NP SES) scores, a measure of income and education based on occupational categories that range from 1 to 99, with higher scores being associated with higher SES (Nam and Powers 1983). NP SES scores are not available for approximately 20 % of the sample, and values for these individual are imputed by substituting the mean plus a random number multiplied by the distribution of nonmissing values based on the standard deviation. An additional variable indicating missing values is also included (Paul et al. 2008). Approximately 30 % of fathers from this era were farmers. Farming may confer a survival advantage related to lifestyle factors (Gavrilov and Gavrilova 2012). As a result, a separate dummy variable is created for farming.
To control for unobservable genetic and shared environmental effects, we use a measure of family history of longevity: FEL (Kerber et al. 2001). This measure has been applied in a similar fashion in other life-span studies using UPDB (Garibotti et al. 2006; Kerber et al. 2012; Smith et al. 2009b). To construct FEL, we first measure individual-level excess longevity, defined as the difference between an individual’s attained age and the age to which that individual was expected to live according to a model that incorporates two basic life-span predictors (sex and birth year). Excess longevity is then extended to blood relatives who reached the age of 65 for each individual, a restriction to focus on years less affected by external causes of death. The kinship coefficient—the probability that an individual shares a particular allele with another individual—is used as a weight in calculating FEL. Averaging excess longevities of all blood kin over 65 for each individual, weighted by their kinship coefficients, generates a point estimate of FEL. We have found that individuals with high FEL live longer and experience more healthful disease trajectories as they age (Smith et al. 2009b, 2012a).
Active affiliation with the Church of Jesus Christ of Latter-day Saints (LDS) church is associated with increased life expectancy (Enstrom and Breslow 2008) and high fertility rates (Arland 1979). Individuals actively affiliated with the LDS church are more likely to abstain from alcohol and tobacco use, fast once per month, and participate in church-related social activities (Mineau et al. 2002). The UPDB contains information on baptism and endowment dates from family history records and are used to classify individuals as active followers, inactive, or nonmembers. Individuals with an endowment date have agreed to live their lives following the doctrine of the LDS church and are considered active church followers if endowed before age 40. Individuals with a baptism date but no endowment date are considered inactive, and individuals with no baptism or endowment date are considered nonmembers of the LDS church (reference category).
Measures of Fertility
Fertility information is derived from a combination of sources collected from genealogical records obtained from two sources: the Genealogical Society of Utah and linked vital records, including birth certificate data from 1915–1921 and 1938 to the present. All eligible women in the sample have completed their fertility by definition because they are required to survive to at least age 65 to be visible in the Medicare Claims data.
Parity is measured with a set of dummy variables indicating whether a woman had 1–2, 3–5, 6–8, or 9 or more children. On average, women had four children, and the category for 3–5 was used as a reference. To measure the effects of early and late childbirth, dummy variables are created for the following categories: age at first birth before the age of 18, 18–24 (reference category), and after age 25. Because very few men in the sample had a first birth before age 18, this category is combined with the 18–24 for men. For age at last birth, three categories are created: under 35 (reference group), 35–39, and 40 or older. A dummy variable is used to identify parents of twins. Short birth intervals are defined as interbirth intervals less than 18 months, and long intervals are defined as intervals >60 months (Conde-Agudelo et al. 2007). Separate variables are created to identify individuals with at least one short or long interbirth interval.
Infant mortality may be a marker for maternal health and adverse environments (McCormick et al. 1984). Individuals losing one or more children during the first year of life are identified with a dummy variable.
Birth certificates contain information on mother’s marital status, prematurity, and birth weight (starting in 1947). Using the information from the birth certificates, individuals are categorized as ever having a high-birth-weight (>4,000 grams) or low-birth-weight baby (<2,500 grams and carried 37+ weeks), which reflects Women, Infants, and Children (WIC) Nutrition Risk Criteria (Porter 1996). Preterm birth is defined as the birth of an infant before 37 weeks of gestation. Table 1 presents descriptive statistics of all the measures by sex and age group.
Table 1.
Descriptive statistics by gender and age group: Samples size (with percentages of total sample in parentheses) or, where indicated, means (with standard deviations in parentheses)
| Female 66–74 in 1992 (N = 12,190) | Female 75–84 in 1992 (N = 10,099) | Male 66–74 in 1992 (N = 11,349) | Male 75–84 in 1992 (N = 7,520) | |
|---|---|---|---|---|
| Early-Life Conditions | ||||
| Father’s Nam-Powers SES score (mean and SD) | 49.37 (19.54) | 47.4 (18.19) | 49.0 (19.14) | 47.09 (17.96) |
| Father farmer | 3,556 (29.2) | 3,699 (36.6) | 3,434 (30.3) | 2,786 (37.1) |
| Father missing SES | 2,982 (24.5) | 2,416 (23.9) | 2,620 (23.1) | 1,754 (23.3) |
| Active LDS | 6,452 (52.9) | 5,525 (54.7) | 5,876 (51.8) | 4,219 (56.1) |
| Inactive LDS | 2,886 (23.7) | 2,883 (28.6) | 2,643 (23.3) | 1,843 (24.5) |
| Non-LDS | 2,852 (23.4) | 1,691 (16.7) | 2,830 (24.9) | 1,458 (19.4) |
| FEL bottom 25 % | 2,841 (23.3) | 2,335 (23.1) | 2,693 (23.7) | 1,640 (21.8) |
| FEL middle 50 % | 5,321 (43.7) | 4,525 (44.8) | 4,869 (42.9) | 3,648 (48.5) |
| FEL top 25 % | 2,662 (21.8) | 2,653 (26.3) | 2,554 (22.5) | 1,757 (23.4) |
| FEL missing | 1,366 (11.2) | 586 (5.8) | 1,233 (10.9) | 475 (6.3) |
| Mother died when ego age <18 | 721 (5.9) | 746 (7.4) | 682 (6.0) | 555 (7.4) |
| Mother died when ego age 18+ | 11,379 (93.4) | 9,255 (91.6) | 10,583 (93.3) | 6,906 (91.8) |
| Father died when ego age <18 | 1,060 (8.7) | 943 (9.3) | 951 (8.4) | 714 (9.5) |
| Father died when ego age 18+ | 11,040 (90.6) | 9,058 (89.7) | 10,314 (90.9) | 6,747 (89.7) |
| Both parents died before age 18 | 90 (0.7) | 98 (1) | 84 (0.7) | 59 (0.8) |
| Fertility History | ||||
| Children 1–2 | 3,004 (24.6) | 3,138 (31.1) | 2,533 (22.3) | 2,104 (28.0) |
| Children 3–5 | 6,798 (55.8) | 5,287 (52.4) | 6,605 (58.2) | 4,087 (54.4) |
| Children 6–8 | 2,061 (16.9) | 1,410 (14.0) | 1,934 (17.0) | 1,135 (15.1) |
| Children 9+ | 327 (2.7) | 264 (2.6) | 277 (2.4) | 194 (2.6) |
| Infant death (Y/N) | 730 (6.0) | 757 (7.5) | 637 (5.6) | 527 (7.0) |
| Short birth interval (Y/N) | 3,380 (27.7) | 2,238 (22.2) | 3,489 (30.7) | 1,707 (22.7) |
| Long birth interval (Y/N) | 5,201 (42.7) | 4,684 (46.4) | 4,568 (40.3) | 3,513 (46.7) |
| Mother/Father of twin | 580 (4.8) | 489 (4.8) | 486 (4.3) | 370 (4.9) |
| Age at first birth <18 | 391 (3.2) | 316 (3.1) | n/a | n/a |
| Age at first birth 18–24 | 8,590 (70.5) | 5,871 (58.1) | 5,815 (51.2) | 2,687 (35.7) |
| Age at first birth 25+ | 3,209 (26.3) | 3,912 (38.7) | 5,534 (48.8) | 4,833 (34.3) |
| Age at last birth 35–39 | 1,848 (15.2) | 3,105 (30.8) | 2,864 (25.2) | 2,188 (29.1) |
| Age at last birth ≥40 | 3,827 (31.4) | 2,369 (23.5) | 2,864 (25.2) | 2,995 (39.8) |
| Age (mean and SD) | 70.09 (2.57) | 79.00 (4.76) | 70.0 (2.56) | 78.6 (2.78) |
| Information From Utah Birth Certificatesa | ||||
| At least 1 high–birth weight baby | 668 (16.1) | 1,126 (18.8) | ||
| At least 1 low–birth weight baby | 283 (6.8) | 434 (7.0) | ||
| At least one preterm birth | 572 (13.8) | 942 (15.2) | ||
| Adult Measures | ||||
| Spouse alive at baseline | 9,523 (78.1) | 5,023 (49.7) | 10,913 (96.2) | 6,662 (88.6) |
Age 66–74 in 1992 with birth certificate data on all births, making this a select sample. Females are younger (average age = 69, range 66–74) and had to have their first birth after age 20 (1947 is the first year birth certificates are available). Males are younger and had to have their first birth after age 20.
Constructing Morbidity Trajectories
We determine how reproductive history and health affect the likelihood of having a particular later-life comorbidity trajectory. Assessment of comorbidity trajectories are accomplished through the application of a finite mixture modeling approach available as a SAS procedure, called PROC TRAJ (Haviland et al. 2008; Jones and Nagin 2007; Nagin and Tremblay 2001). The group-based modeling approach allows for identification of distinct clusters of individual trajectories.
The response variable in this analysis is a weighted count of the number of comorbid conditions. Accordingly, a zero-inflated Poisson (ZIP) based distribution is used. Both Poisson and censored normal distributions were considered, but the ZIP provided the best fit for our data.
Trajectories were modeled so as to allow for two to six groups as a quadratic function of time with widowhood included as a time-varying covariate. Model fit is assessed using Bayesian information criterion (BIC). There are situations where BIC continues to increase as more groups are added, but these additional groups do not help to summarize the distinct features of the data parsimoniously (Nagin 2005). Therefore, average posterior probability of assignment, odds of correct classification, and estimated group probabilities versus the observed proportion of the sample assigned to the group (Nagin 2005) are also used to assess a model’s correspondence with the data.
Nonrandom attrition alters characteristics of the population over time and can lead to biased estimates. Because we exclude individuals enrolled in a managed care plan during any period of a given year, our only source of censorship is death. PROC TRAJ is used to simultaneously model comorbidity trajectories and probability of death, allowing the modeled probability of death to vary across trajectory groups. Individuals in the analysis are required to be alive in 1992, and therefore the probability of dropout during that year is zero. All models account for nonrandom attrition due to death using the PROC TRAJ extension created by Haviland (Haviland et al. 2011; Zimmer et al. 2012). This extension jointly models morbidity trajectories with the logit of the mortality probability by group that includes dependence on the prior period response until dropout and age.
Trajectory group membership probabilities can vary as a function of either time-invariant characteristics or characteristics established before the observation period (Jones and Nagin 2007). This component jointly estimates a multinomial logit model that captures the effects of stable characteristics on the probability of group membership. This makes it possible to test the effect of early-life conditions and fertility on the probability of membership in each group (Nagin and Odgers 2010).
For each group, the following analyses are conducted. First, we derive base trajectory groups in which comorbidity is a function of only time in order to select the best model. Second, we fit a model with the covariates from the demographic, early-life conditions (ELCs), and fertility domains. We examine the probability that trajectory membership associated with ELCs are mediated by timing of childbirth (age at first and last birth), preterm birth, and parity. All models account for nonrandom attrition due to death.
Addressing Sample Selection Bias
UPDB’s extensive data holdings and follow-up information allow us to test and correct for sample selection bias, which is often not feasible because of the lack of longitudinal population data. To correct for potential selection bias due to data restrictions, we use a Heckman two-stage modeling strategy (Heckman 1979) performed using Stata 11 (Gagnon et al. 2009). In the first stage, a probit model assesses factors leading to selection into the sample among all individuals (see Table S1, Online Resource 1). The dependent variable is a dichotomous indicator of selection into the sample. This equation generates the inverse Mills ratio (IMR) (Fu et al. 2004), which can be interpreted as the hazard of not being selected into the sample. The independent variables used in the selection equation are displayed in Table S2, Online Resource 1. Although other methods of sample selection correction exist, such as the Wooldridge method, we do not employ them because analyses have shown that other methods do not necessarily outperform the Heckman estimator, and both the Wooldridge and Heckman methods show satisfactory and comparable performance (Arulampalam and Stewart 2009).
Because our sample selection is largely based on having complete information in UPDB and non-enrollment in an MCO, we select variables that are closely related to enrollment and use them as independent variables in the selection equation s. The first variable is age in 1992, derived from CMS records. Second, the longitudinal information within the UPDB is more complete for individuals with a longer length of residence in the state of Utah. Because UPDB holds information on the birthplace of individuals, it is used to create an indicator variable to proxy for residential duration (born in Utah vs. outside Utah). Approximately 85 % of individuals selected into the sample were born in Utah, compared with 40 % of those not in the sample. Finally, area-level characteristics of an individual’s current place of residence may also predict selection into the sample. Information about an individual’s county of residence in 1992, based on CMS residential data, is used and is derived from the 1990 U.S. Census.
Table S2 shows large differences in county-level population and median family income between those selected and not selected. Of those selected into the final sample, 61 % reside in the Wasatch Front (the urban core of Utah that holds approximately 75 % of the population of Utah), compared with 48 % of the nonselected individuals. The UPDB has information on individuals who migrate out of state, and therefore we have scarce data on individuals who reside in other states. This contributes to the large difference in county-level population between the groups because a larger proportion of individuals in the nonselected group reside in populous counties in California (such as Los Angeles county, population of 8.8 million in 1992).
In the second stage of the selection modeling, trajectory models are estimated with the IMR from the first stage added as a covariate, with the goal being to account for possible sample selection bias in the final models. The far-right column in Table S2 shows that all variables included in the probit models significantly predict selection into the sample, with the exception of age in the equation for males aged 75–84. Increasing age and being born in Utah increase the probability of selection into the sample, while county median family income and population size are associated with a decreased probability of selection into the sample for all sexes and cohorts (with the aforementioned age exception).
Results
Trajectories of Comorbidity
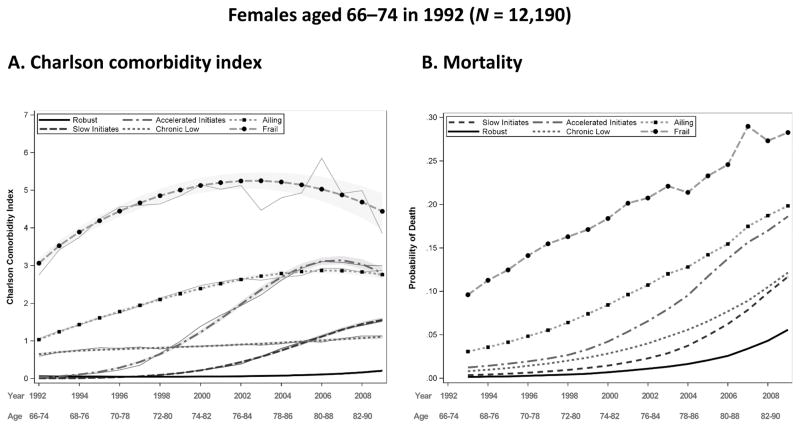
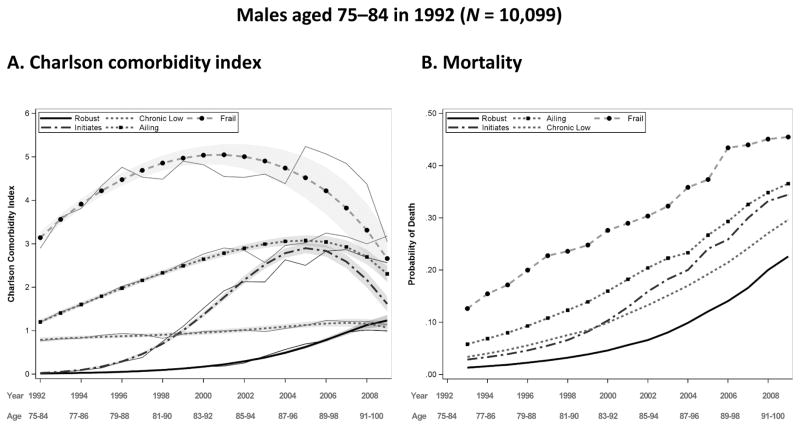
Trajectories were modeled for two to six groups as a quadratic function of time with widowhood included as a time-varying covariate. Model fit was assessed using the BIC and additional measures of model fit discussed in the Methods section. The best fitting models for both males and females ages 66–74 (the young-old) in 1992 reveal six distinct trajectory groups, while those for ages 75–84 (the middle-old) show five; one of the five categories of the middle-old (initiates) is further distinguished into two groups (slow initiates and accelerated initiates) for the young-old. Figures 2–5, panel A, show the predicted comorbidity trajectories by sex and age. The figures show the diversity of comorbidity experience over the 18-year period of follow-up. To aid in the interpretation of results, trajectory groups have been labeled as follows:
Fig. 2.
Predicted and observed Charlson comorbidity index (CCI) by year and group for females aged 66–74 in 1992, with 95 % confidence intervals displayed by gray shading (panel A), and predicted probability of death before the next year by group for females aged 66–74 in 1992 (panel B). Source: Utah Population Database and Centers for Medicare & Medicaid Services (CMS) data
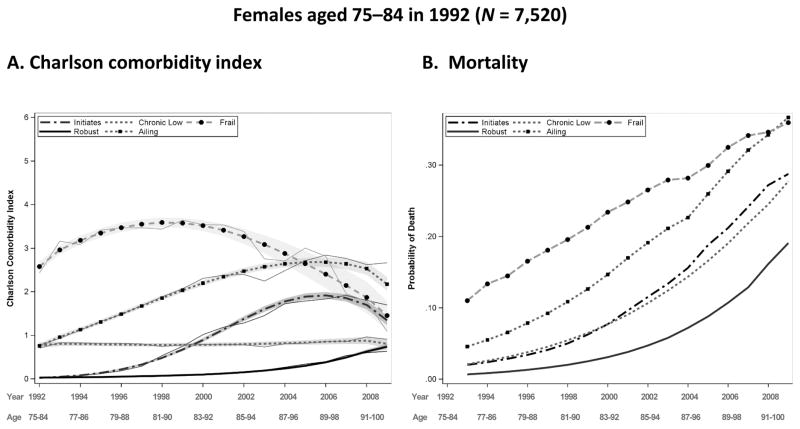
Fig. 5.
Predicted and observed Charlson comorbidity index (CCI) by year and group for males aged 75–84 in 1992, with a 95 % confidence intervals displayed by gray shading (panel A), and predicted probability of death before the next year by group for males aged 75–84 in 1992 (panel B). Source: Utah Population Database and Centers for Medicare & Medicaid Services (CMS) data
Robust: Absence of comorbid conditions
Initiates (for the middle-old group only): No comorbid conditions at baseline, with conditions increasing over time
Slow initiates (for the young-old group only): Comorbid conditions at the beginning, with gradually increasing numbers over time
Accelerated initiates (for the young-old group only): No comorbid conditions at the beginning, with conditions increasing quickly over time and then decelerating during the last two years of the 18-year period
Chronic low: Steady comorbidity over time
Ailing: Moderate levels of comorbidity at baseline that steadily increase over time
Frail: The highest level of comorbidity at baseline, remaining high over time.
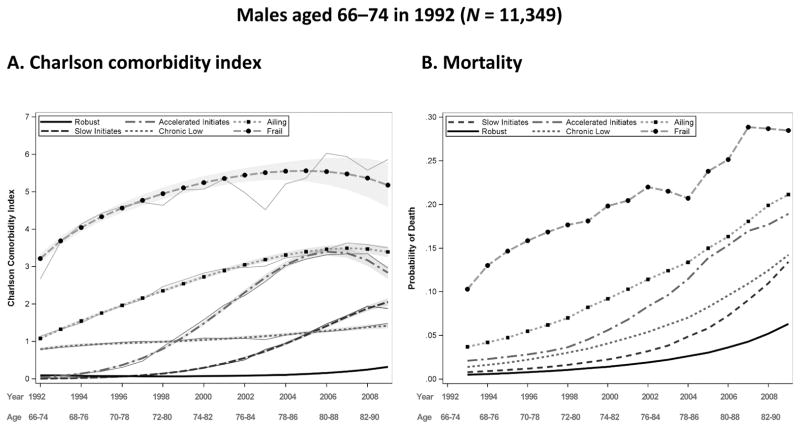
The shape of the trajectories is similar between males and females in their respective age groups; however, intercepts differ. For females in the young-old age category, trajectory membership is evenly distributed among the robust (19.1 %), slow initiates (18.8 %), chronic low (19.7 %), and ailing (21.8 %). Compared with females, males have lower percentages in the robust (15.7 %) and slow initiates (16.4 %) groups but higher percentages in the ailing (26.1 %), accelerated initiates (15.3 %), and frail groups (8 %). The frail category constitutes the smallest proportion of group membership for males and females, at 8 % and 7.3 %, respectively. These findings are somewhat unexpected given the health-survival paradox in which females have worse health and males have higher mortality. Nonetheless, recently reported prevalence estimates support our findings (Fried et al. 2012; Schiller et al. 2012).
Individuals in the middle-old cohort surviving the full 18-year period range in age from 92 to 101 in 2009. For both sexes, five distinct trajectory groups are identified. The ailing category, with a CCI of approximately 1 at baseline that gradually increases over time, has the highest trajectory membership for both sexes, with 29.7 % of females and 35.3 % of males in this category. Compared with the young-old, a smaller proportion of the middle-old fall into the robust category (19.1 % vs. 18.2 % for females and 15.7 % vs. 14.7 % for males). The robust in the middle-old cohort do not maintain a disease-free trajectory, with a predicted CCI of 0.74 for females and 1.5 for males by 2009. Although the pattern of this robust trajectory in the middle-old cohort is similar to the pattern of the slow initiates in the young-old cohort, individuals in the middle-old robust category have a slower rate of increase and end the period with a lower predicted CCI (the difference in CCI2009 is 0.82 for females and 0.44 for males). Compared with the young-old, is the middle-old experience a near doubling in the proportion of frail females (13.6 % vs. 7.3 %) and a 50 % increase for males (12.5 % vs. 8 %). Another notable difference between the young-old and middle-old is the maximum predicted CCI, which is higher in the young-old category for both sexes. Although the two cohorts are not directly comparable, the results suggest a possible decrease in the heterogeneity of morbidity (mortality selection) patterns with age, with fewer categories in the older age groups. The lower number of categories that fit the data may also be a function of the decreased sample size in the middle-old cohort.
We also find six distinct trajectory groups for the male and female samples when restricting the analysis to the birth certificate samples. The parameter estimates and estimated trajectory memberships are similar across samples (results not shown) with a slightly higher percentage in the robust group for both males and females compared with the full sample. This is not unexpected given the younger age distribution of these subsamples.
Panel B of Figs. 2–5 display the probability of dropout due to death for each sex and age group. This probability is modeled as a function of age and the comorbidity measurement in the previous year, and it is allowed to vary by trajectory group. Mortality trajectories follow a similar hierarchy as the comorbidity trajectories, with the robust group generally having the lowest levels of mortality and the frail group having the highest. Mortality in all groups naturally rise with time; the accelerated initiates have the greatest rate of increase in mortality for the young-old cohort, and the ailing and initiates have the greatest rate of increase for females and males, respectively, in the middle-old cohort. Females have lower probabilities of death than males in their respective cohorts and comorbidity groups, as expected, and the young-old have lower probabilities than the middle-old.
Widowhood alters the shape of the trajectory for some, but not all, trajectory groups. In general, death of a spouse between 1992 and 2009 leads to increases in the level of comorbidity within a trajectory. Individuals in the frail categories and males in the middle-old cohort are the least affected by widowhood, with few of the effects reaching significance (results not shown).
Fertility History and Later-Life Comorbidity
Tables 2–5 display the odds ratios for full models for the association among demographic, ELCs, and fertility measures and the probability of group membership. The reference group in all tables is the robust category. Thus, we are comparing the probability of membership in each trajectory groups relative to the probability of membership in the group with the healthiest trajectory. All results discussed here control for early-life events and demographic measures, and therefore results presented are fully adjusted. Unless otherwise noted, the results we highlight are significant at p < .05.
Table 2.
Effects of early-life conditions and fertility on comorbidity trajectory group membership versus robust group (19.1 %), Women age 66–74 in 1992: Odds ratios (95 % CI)
| Slow Initiates (18.8 %) | Accelerated Initiates (13.3 %) | Chronic Low (19.7 %) | Ailing (21.8 %) | Frail (7.3 %) | |
|---|---|---|---|---|---|
| Early-Life Conditions | |||||
| Age in 1992 | 2.47(1.88,3.26) | 2.54(1.89,3.41) | 3.52(2.71,4.58) | 3.01(2.34,3.87) | 3.16(2.24,4.45) |
| Active member of LDS church | 0.76(0.61,0.94) | 0.55(0.44,0.68) | 0.72(0.58,0.88) | 0.45(0.37,0.54) | 0.47(0.37,0.61) |
| Inactive member of LDS church | 0.84(0.66,1.06) | 0.57(0.45,0.73) | 0.78(0.62,0.98) | 0.59(0.48,0.73) | 0.56(0.42,0.73) |
| Nonmember (ref.) | 1 | 1 | 1 | 1 | 1 |
| Father’s Nam-Powers SES (unit = 10) | 1.02(0.98,1.05) | 0.98(0.94,1.02) | 1.01(0.97,1.04) | 0.99(0.96,1.03) | 0.96(0.91,1) |
| Father farmer | 1.01(0.85,1.2) | 0.8(0.67,0.96) | 1.1(0.93,1.29) | 0.79(0.68,0.92) | 0.74(0.6,0.93) |
| Missing SES | 0.91(0.76,1.09) | 0.91(0.76,1.1) | 1.08(0.91,1.28) | 0.85(0.73,1.01) | 1.08(0.87,1.34) |
| FEL in bottom quartile | 1.19(0.99,1.44) | 1.38(1.14,1.67) | 1.24(1.04,1.48) | 1.44(1.22,1.69) | 1.27(1.02,1.59) |
| FEL in mid 50 % (ref.) | 1 | 1 | 1 | 1 | 1 |
| FEL in top quartile | 0.79(0.67,0.94) | 0.75(0.62,0.91) | 0.71(0.61,0.84) | 0.58(0.49,0.68) | 0.7(0.56,0.87) |
| FEL missing | 0.97(0.74,1.27) | 0.75(0.57,1) | 0.84(0.64,1.09) | 0.56(0.44,0.72) | 0.45(0.32,0.63) |
| Orphaned before age 18 | 2.39(0.91,6.29) | 1.78(0.62,5.14) | 1.46(0.53,4.05) | 2.62(1.08,6.36) | 3.87(1.44,10.4) |
| Mother died before child age 18 | 1.1(0.82,1.49) | 1.28(0.94,1.73) | 1.11(0.83,1.48) | 1.24(0.95,1.63) | 1.48(1.05,2.1) |
| Father died before child age 18 | 1.12(0.87,1.45) | 1.38(1.07,1.78) | 1.12(0.88,1.43) | 1.24(0.99,1.56) | 1.32(0.98,1.78) |
| Both parents alive at age 18 (ref.) | 1 | 1 | 1 | 1 | 1 |
| Fertility | |||||
| 1–2 children | 0.87(0.72,1.06) | 1.07(0.88,1.31) | 0.89(0.74,1.06) | 0.95(0.8,1.13) | 1.13(0.9,1.42) |
| 3–5 children (ref.) | 1 | 1 | 1 | 1 | 1 |
| 6–8 children | 1.09(0.87,1.35) | 1.04(0.82,1.31) | 1.1(0.9,1.36) | 1.16(0.95,1.42) | 1.1(0.83,1.45) |
| 9+ children | 0.86(0.52,1.43) | 1.06(0.65,1.74) | 1.01(0.65,1.58) | 1.2(0.79,1.84) | 0.96(0.51,1.77) |
| Age at first birth <18 | 1.48(0.97,2.25) | 1.48(0.96,2.3) | 1.33(0.88,2.01) | 1.44(0.98,2.12) | 1.94(1.2,3.13) |
| Age at first birth 18–24 (ref.) | 1 | 1 | 1 | 1 | 1 |
| Age at first birth ≥25 | 1.04(0.88,1.24) | 0.86(0.71,1.03) | 0.93(0.79,1.1) | 0.86(0.74,1.02) | 1.06(0.86,1.31) |
| Age at last birth 35–39 | 0.85(0.71,1.02) | 0.83(0.68,1.01) | 0.81(0.68,0.97) | 0.82(0.69,0.97) | 0.76(0.61,0.96) |
| Age at last birth ≥40 | 0.65(0.5,0.84) | 0.8(0.61,1.05) | 0.82(0.65,1.04) | 0.66(0.52,0.83) | 0.75(0.54,1.03) |
| Mother of twins | 1.17(0.84,1.62) | 0.95(0.66,1.38) | 0.96(0.69,1.32) | 1.12(0.83,1.51) | 1.01(0.67,1.54) |
| One or more short birth intervals | 1.01(0.85,1.19) | 1.11(0.93,1.33) | 0.92(0.78,1.08) | 1.03(0.88,1.21) | 1.06(0.85,1.31) |
| One or more long birth intervals | 1.01(0.87,1.19) | 1.06(0.9,1.26) | 0.93(0.8,1.09) | 1.02(0.88,1.17) | 0.97(0.79,1.19) |
| One or more infant deaths | 0.93(0.69,1.24) | 0.67(0.47,0.95) | 0.83(0.62,1.1) | 1.02(0.79,1.32) | 1.13(0.8,1.6) |
| Sample Selection Bias | |||||
| IMR | 1.13(0.92,1.39) | 1.03(0.82,1.28) | 1.06(0.86,1.29) | 1.17(0.96,1.41) | 1.3(1.02,1.67) |
Note: Results shown in Table 2 are controlling for widowhood and jointly modeled with mortality trajectories.
Table 5.
Effects of early-life conditions and fertility on comorbidity trajectory group membership versus robust group (14.7%), Men age 75–84 in 1992: Odds ratios (95 % CI)
| Initiates (17.4 %) | Ailing (35.8 %) | Chronic Low (20.2 %) | Frail (12.5 %) | |
|---|---|---|---|---|
| Early-Life Conditions | ||||
| Age in 1992 | 1.60* | 2.24* | 2.12* | 2.82* |
| Active member of LDS church | 0.81 | 0.78† | 0.80 | 0.72* |
| Inactive member of LDS church | 1.01 | 0.98 | 0.97 | 0.97 |
| Nonmember (ref.) | 1.00 | 1.00 | 1.00 | 1.00 |
| Father’s Nam-Powers SES (unit = 10) | 1.00 | 1.00 | 1.01 | 0.96 |
| Father farmer | 0.95 | 0.94 | 0.95 | 1.09 |
| Missing SES | 1.06 | 0.85 | 0.90 | 0.98 |
| FEL in bottom quartile | 1.38* | 1.70* | 1.28† | 1.89* |
| FEL in mid 50 % (ref.) | 1.00 | 1.00 | 1.00 | 1.00 |
| FEL in top quartile | 0.80† | 0.77* | 0.91 | 0.67* |
| FEL missing | 0.71 | 0.76 | 0.99 | 0.84 |
| Orphaned before age 18 | 1.16 | 1.28 | 0.67 | 0.43 |
| Mother died before child age 18 | 1.14 | 1.36† | 1.11 | 1.00 |
| Father died before child age 18 | 1.01 | 0.97 | 0.79 | 1.00 |
| Both parents alive at age 18 (ref.) | 1.00 | 1.00 | 1.00 | 1.00 |
| Fertility | ||||
| 1–2 children | 1.01 | 0.95 | 1.07 | 0.97 |
| 3–5 children (ref.) | 1.00 | 1.00 | 1.00 | 1.00 |
| 6–8 children | 0.81 | 1.14 | 0.89 | 0.77 |
| 9+ children | 0.82 | 0.81 | 0.76 | 0.61 |
| Age at first birth <25 (ref.) | 1.00 | 1.00 | 1.00 | 1.00 |
| Age at first birth ≥25 | 0.97 | 0.82* | 0.86 | 0.71* |
| Age at last birth 35–39 | 0.96 | 0.85 | 0.79 | 0.73* |
| Age at last birth ≥40 | 0.94 | 0.74* | 0.94 | 0.81 |
| Father of twins | 0.86 | 0.71† | 1.25 | 1.12 |
| One or more short birth intervals | 1.16 | 0.92 | 0.98 | 1.15 |
| One or more long birth intervals | 1.11 | 1.05 | 1.12 | 1.10 |
| One or more infant deaths | 1.03 | 1.27 | 1.01 | 0.96 |
| Sample Selection Bias | ||||
| IMR | 1.21 | 1.38* | 1.15 | 0.98 |
p < .10;
p < .05
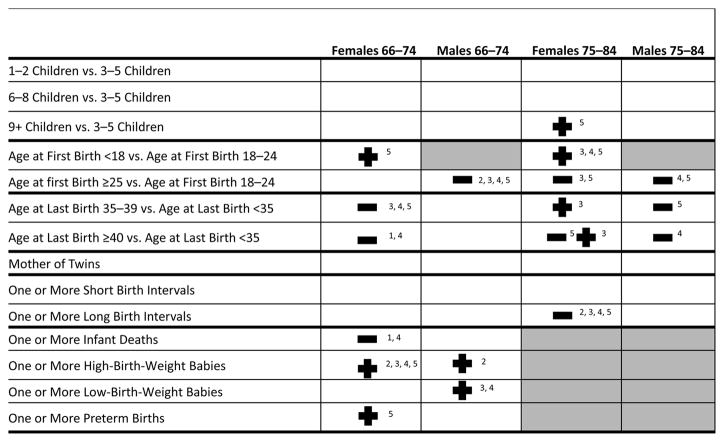
For females in the young cohort, we do not find a significant association between parity and trajectory membership. Significant relationships between age at first birth and membership in a trajectory group were detected. Table 2 shows that compared with women having their first birth between the ages of 19 and 24, young age at first birth (<18) nearly doubles the odds of being in the frail versus robust trajectory. The results also suggest that young age at first birth increases the odds of being in the other categories with increased comorbidity, but the differences do not reach significance. Older age at last birth confers a protective effect, with women who had their last birth at age 35 or later having decreased odds of being in a less-favorable trajectory. Women whose age at last birth was between the ages of 35 and 39 and after age 40 have a 24 % and a 25 % (p = .07), respectively, decrease in the odds of being in the frail versus robust group compared with females ending childbearing earlier. We see no evidence of an association among twinning, infant deaths, short birth intervals, long birth intervals, and later-life comorbidity trajectories for this group.
Table 3 shows the results for females in the middle-old cohort. We find little association between parity and trajectory membership, ceteris paribus, for females in the middle-old cohort with the exception of the frail group, where females having nine or more children are nearly twice as likely to be in the frail versus robust group. The relationship between age at first birth and trajectory membership persist and are similar to the patterns observed in the young-old cohort. Females having their first birth during the teenage years are more likely to be in the chronic low, ailing, and frail versus robust groups. Females in this cohort having their first birth after age 25 are less likely to be in the chronic low and frail categories. Compared with females having their last birth before the age of 35, females having their last child after the age of 35 are more likely to be in the chronic low group versus the robust group. However, last birth after the age of 40 have a 24 % decrease in the odds of being in the frail versus robust group. This inconsistency may be a reflection of dwindling sample size and mortality selection. As with the younger female cohort, we find no association among trajectory membership and twinning, infant death, and short birth intervals, ceteris paribus. Having one or more long birth interval reduces the odds of being in any of the groups with more comorbid conditions versus the robust, with a respective decrease of 24 %, 34 %, 18 %, and 27% in the risk of being in the initiates, chronic low, ailing, and frail versus robust group. (The difference in the magnitude of the effect across groups is not significant.)
Table 3.
Effects of early-life conditions and fertility on comorbidity trajectory group membership versus robust group (18.2%), Women age 75–84 in 1992: Odd ratios (95 % CI)
| Initiates (21.6 %) | Chronic Low (29.7 %) | Ailing (16.9 %) | Frail (13.6 %) | |
|---|---|---|---|---|
| Early-Life Conditions | ||||
| Age in 1992 | 1.84* | 3.89* | 2.77* | 2.95* |
| Active member of LDS church | 1.05 | 0.83 | 0.89 | 0.93 |
| Inactive member of LDS church | 1.12 | 0.79 | 0.97 | 1.05 |
| Nonmember (ref.) | 1.00 | 1.00 | 1.00 | 1.00 |
| Father’s Nam-Powers SES (unit = 10) | 1.02 | 1.02 | 1.02 | 1.00 |
| Father farmer | 0.93 | 1.07 | 1.14 | 0.93 |
| Missing SES | 1.01 | 1.07 | 1.11 | 1.08 |
| FEL in bottom quartile | 1.44* | 1.41* | 1.59* | 1.60* |
| FEL in mid 50 % (ref.) | 1.00 | 1.00 | 1.00 | 1.00 |
| FEL in top quartile | 0.77* | 0.83† | 0.65* | 0.61* |
| FEL missing | 0.91 | 1.11 | 0.81 | 0.78 |
| Orphaned before age 18 | 0.88 | 0.97 | 1.29 | 0.78 |
| Mother died before child age 18 | 1.16 | 1.31† | 0.99 | 1.35* |
| Father died before child age 18 | 1.19 | 1.15 | 1.27† | 1.24 |
| Both parents alive at age 18 (ref.) | 1.00 | 1.00 | 1.00 | 1.00 |
| Fertility | ||||
| 1–2 children | 0.98 | 0.96 | 0.90 | 0.89 |
| 3–5 children (ref.) | 1.00 | 1.00 | 1.00 | 1.00 |
| 6–8 children | 1.01 | 0.93 | 1.22† | 1.15 |
| 9+ children | 0.77 | 0.68 | 1.17 | 1.98* |
| Age at first birth <18 | 1.45 | 1.85* | 1.97* | 2.03* |
| Age at first birth 18–24 (ref.) | 1.00 | 1.00 | 1.00 | 1.00 |
| Age at first birth ≥ 25 | 0.86† | 0.77* | 0.90 | 0.78* |
| Age at last birth 35–39 | 1.07 | 1.38* | 1.01 | 1.11 |
| Age at last birth ≥ 40 | 1.13 | 1.34* | 0.89 | 0.76* |
| Mother of twins | 1.08 | 0.96 | 1.02 | 1.19 |
| One or more short birth intervals | 1.04 | 0.83 | 1.03 | 0.89 |
| One or more long birth intervals | 0.76* | 0.66* | 0.82* | 0.73* |
| One or more infant deaths | 0.78 | 0.84 | 1.00 | 1.07 |
| Sample Selection Bias | ||||
| IMR | 0.95 | 1.13 | 1.12 | 1.12 |
p < .10;
p < .05
We find no association between parity and trajectory membership for males in the young-old and middle-old cohorts, ceteris paribus. Having a later age at first birth (over the age of 25 vs. younger than 25) is protective for males in both cohorts. Table 4 shows that for males in the young-old cohort, having their first birth at the age of 25 or older reduces the odds of being in the accelerated initiates, chronic low, ailing, and frail groups by at least 20 % for each group compared with the robust. Table 5 shows that for males in the middle-old cohort, older age at first birth is associated with an 18 % and a 29 % reduction in the risk of being in the ailing and frail groups, respectively, compared with the robust. Males in the middle-old cohort having their last child after the age of 40 have a 26 % decrease in the odds of being in the ailing versus robust group. We do not find an association among twinning, age at last birth, short birth intervals, long birth intervals, and infant deaths and group membership for males in these cohorts.
Table 4.
Effects of early-life conditions and fertility on comorbidity trajectory group membership vs. robust group (15.7%), Men age 65–74 in 1992: Odds ratios (95 % CI)
| Slow Initiates (16.4 %) | Accelerated Initiates (15.3 %) | Chronic Low (18.5 %) | Ailing (26.1 %) | Frail (8 %) | |
|---|---|---|---|---|---|
| Early-Life Conditions | |||||
| Age in 1992 | 1.40* | 1.64* | 2.33* | 3.14* | 3.41* |
| Active member of LDS church | 0.76* | 0.55* | 0.82 | 0.48* | 0.40* |
| Inactive member of LDS church | 0.66* | 0.70* | 0.75* | 0.59* | 0.45* |
| Nonmember (ref.) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Father’s Nam-Powers SES (unit = 10) | 0.98 | 1.01 | 0.99 | 1.02 | 1.02 |
| Father farmer | 0.99 | 1.05 | 0.90 | 1.13 | 1.06 |
| Missing SES | 1.05 | 1.10 | 0.96 | 1.05 | 1.14 |
| FEL in bottom quartile | 1.20 | 1.19 | 1.28* | 1.37* | 1.30* |
| FEL in mid 50 % (ref.) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| FEL in top quartile | 0.73 | 0.71* | 0.68* | 0.66* | 0.60* |
| FEL missing | 0.86 | 0.88 | 0.87 | 0.55* | 0.50* |
| Orphaned before age 18 | 0.27* | 0.57 | 0.58 | 0.72 | 0.73 |
| Mother died before child age 18 | 1.21 | 1.18 | 1.14 | 1.21 | 1.12 |
| Father died before child age 18 | 1.14 | 1.17 | 1.08 | 1.25 | 1.07 |
| Both parents alive at age 18 (ref.) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Fertility | |||||
| 1–2 children | 1.04 | 1.17 | 1.14 | 1.01 | 1.25† |
| 3–5 children (ref.) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 6–8 children | 0.84 | 1.04 | 0.91 | 0.91 | 1.19 |
| 9+ children | 0.63 | 0.87 | 0.63 | 0.71 | 0.77 |
| Age at first birth <25 (ref.) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Age at first birth ≥25 | 0.88 | 0.79* | 0.78* | 0.74* | 0.78* |
| Age at last birth 35–39 | 0.99 | 1.15 | 0.98 | 1.02 | 1.12 |
| Age at last birth ≥40 | 1.04 | 1.21 | 0.88 | 1.09 | 0.94 |
| Father of twins | 0.94 | 0.95 | 1.20 | 1.01 | 1.13 |
| One or more short birth intervals | 1.11 | 0.94 | 1.17 | 1.01 | 1.13 |
| One or more long birth intervals | 1.08 | 0.98 | 1.17 | 0.97 | 1.06 |
| One or more infant deaths | 0.83 | 1.07 | 0.83 | 0.85 | 0.85 |
| Sample Selection Bias | |||||
| IMR | 0.89 | 0.90 | 0.91 | 1.06 | 0.98 |
p < .10;
p < .05
The IMR estimated from the probit model predicting selection into the sample was included in the analyses. Overall, the sample bias correction term, the IMR, has little effect on probability of group membership across all samples. We find that an increased hazard of nonselection is associated with an increase in likelihood of being in the frail group for females in the young-old cohort and the ailing group for males in the middle-old cohort. Individuals predicted not to be in the sample had a 30 % and a 38 % increase in the odds of being in the frail and ailing groups for the young-old females and middle-old males, respectively. An advantage of using the IMR term is that it simultaneously tests for and corrects selection bias.
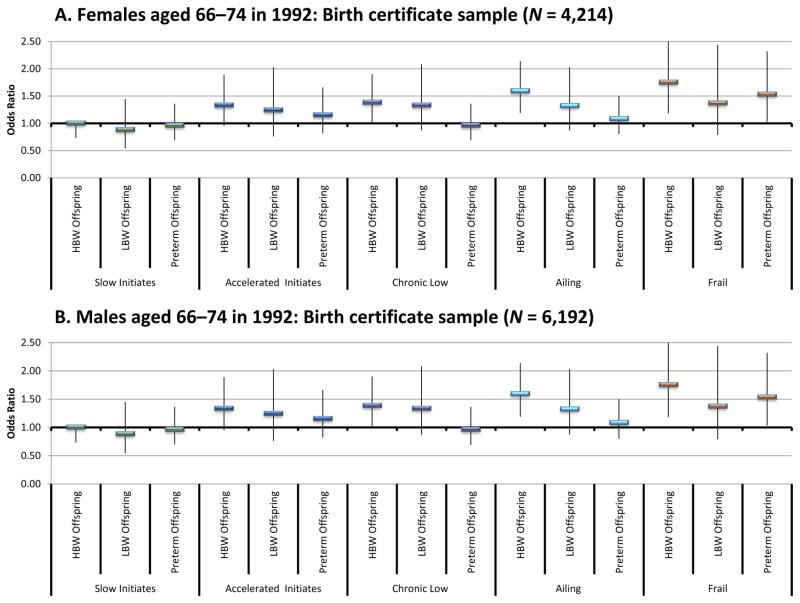
The results based on samples with birth certificate data are presented in Fig. 6. Individuals included in these models were required to have Utah birth certificate records in UPDB for all births. Thus, the results are based on a subsample in the young-old cohorts, with a higher percentage of the males from the full sample represented (NFemale = 4,124 and NMale = 6,192). This disparity arises because, on average, men have an older age at first birth and are therefore more likely to meet the selection criteria. All models control for early-life conditions, and the fertility covariates used in the earlier models and are otherwise constructed and estimated in the same manner.
Fig. 6.
Odds of comorbidity group membership by sex for the birth certificate sample based on multinomial logistic regression. Solid rectangles show the estimated odds ratio, and the thin vertical lines show the 95 % confidence intervals. Models control for early-life conditions, fertility variables, demographic characteristics, and jointly modeled with mortality trajectories. Source: Utah Birth Certificate data linked to the Utah Population Database. HBW = High birth weight; LBW = Low birth weight
We find that for females in the young-old cohort, having one or more high-birth-weight child increases odds of being in the chronic low, ailing, and frail versus the robust group by 39 %, 60 %, and 76 %, respectively. Females having one or more preterm births have a 54 % increase in the odds of being in the frail group versus the robust group. We do not find an association between ever having a low-birth-weight (carried to term) baby and later-life comorbidity trajectories for females. For males in the birth certificate analysis, we find no association between premature offspring and group membership. We do observe that males having one or more high-birth-weight child have a 30 % increase in the odds of being in the accelerated initiates versus the robust group. Fathers of low-birth-weight babies are also more likely to be in the chronic low and ailing groups compared with the robust group.
Discussion
In this study, we first sought to identify first distinct sex-specific trajectories of comorbidity for individuals in two age categories and then tested specific hypotheses relating measures of fertility histories to trajectory group membership. Our results show distinct heterogeneous patterns of comorbidity that range from a robust group whose members avoided major morbid conditions for the majority of the observation period, to a frail group characterized by high comorbidity throughout the entire period. Figure 7 summarizes the association between fertility history and comorbidity trajectories for both sexes and birth cohorts. The results show that fertility history is associated with comorbidity after the age of 65 for both females and males when controlling for early-life circumstances, although it is clear that fertility history has a greater impact on female health.
Fig. 7.
Summary of the multinomial logistic regression results for fertility related variables (Tables 5–8) by sex and age group. All models control for age, widowhood, church membership, childhood SES, familial excess longevity, and parental death during childhood. A plus sign (+) indicates increased risk of being in an unhealthy category versus robust; a minus sign (−) indicates decreased odds of being in an unhealthy category versus robust; 1 = Slow Initiates (age 65–74 only. Categories 1 and 2 were combined for the individuals age 75–79); 2 = Accelerated Initiates (age 65–69) or Initiates (age 75–79); 3 = Chronic low; 4 = Ailing; 5 = Frail. Source: Utah Birth Certificate data linked to the Utah Population Database
The observed relationships among parity, age at last birth, and comorbidity group membership present evidence consistent with a trade-off between fertility and aging for females in the young-old cohort. We found that adverse effects occur at nine or more births rather than the five or more births reported in other studies of contemporary populations (Doblhammer 2000; Grundy and Tomassini 2005), although these two studies top-coded fertility at five or more births, which is a likely consequence of lower fertility in their respective populations. Our findings support other studies showing high levels of fertility exact health costs later in life (Gagnon et al. 2009; Kitagawa and Hauser 1973). We also found a consistent protective relationship between age at last birth and comorbidity group membership in the young-old cohort. This is in line with the prediction that older ages of reproduction are a marker for slowed rates of aging (Perls et al. 1997; Smith et al. 2002). However, we did not find comparable strong protective effects for females in the middle-old cohort.
Related to the evolutionary theories discussed previously are the biological mechanisms through which fertility is linked to later-life comorbidity for women. We found some support of biological consequences to childbirth for women, although we did not find evidence supporting the maternal depletion hypothesis or the link between low birth weight and comorbidity for women (Davey Smith et al. 2004; Smith et al. 1997). Extremely high parity (nine or more births) has an adverse effect on later-life health for females but not males. Having at least one long birth interval has a protective effect for females in the middle-old cohort. Previous studies have linked long birth intervals to complications during reproductive years (Conde-Agudelo et al. 2007). However, this adverse relationship may be associated with a change of partner (Grundy and Kravdal 2014) and thus may not be seen in our sample of once-married individuals.
We found no evidence in favor of the robust phenotype hypothesis, which argues that fertility success is a marker for health and vitality. Other studies using these data have found evidence supporting the robust phenotype hypothesis (Robson and Smith 2011, 2012), but these studies used a historical cohort of women that survived to age 50. It is possible that twinning served as a selection filter, with only the most robust women giving birth to multiples during that historical period surviving to age 50. The same effect is not observed in this contemporary sample with women giving birth during a period when medical intervention could increase the rate of survival for mothers of twins.
This article provides evidence that social mechanisms explain a portion of the relationship between fertility and later-life health. We did not find strong evidence of decreased comorbidity for individuals with more children, and therefore found little indication that the social support hypothesis is consistent with the data. We did, however, find strong evidence supporting the association between age at first birth and comorbidity. Young motherhood is related to adverse health outcomes for both cohorts in this study, and later parenthood is protective for males and females when controlling for early-life circumstances, including childhood SES. The adverse effect of bearing children with low birth weight for fathers but not mothers and high birth weight of offspring for fathers and mothers suggests that adverse birth outcomes could be a marker of risky environments (Kramer et al. 2000). Sibling and spouse designs may improve understanding of the mechanisms linking reproductive history and health.
Fertility decisions and outcomes are heavily influenced by social and historical circumstances, making it important to consider the historical context of these individuals’ lives. The oldest members of the middle-old cohort would have been born in 1908 and entered childbearing age (assuming it is age 15) in 1923, with the childbearing years extending to 1965 for the youngest members of the cohort (assuming age at last birth is 50). The members of the young-old cohort would have initiated childbearing in 1933 and ended in 1974. Changes in managed childbirth, pregnancy, and cesarean deliveries changed rapidly during this period (Sewell 1998). Contraceptives and infertility drugs would have been available for some individuals in these cohorts, meaning that parity and late age at last birth may not be completely biologically determined. Individuals in both cohorts may have served during WWII, which would affect the timing of fertility. Individuals in both cohorts would have been parents during the Baby Boom, when total fertility rates (TFR) peaked at 3.8 (Westoff 1986). Members of the middle-old cohort would also have been children during the 1918 influenza pandemic, an exposure that may have left them physiologically scarred, affecting both fertility and mortality (Smith et al. 2012b).
This study has some limitations that should be addressed in future studies of the relationship between fertility and later-life health. First, results are based on once-married, parous individuals. Future studies should consider the relationship between nulliparity and later-life comorbidity for both men and women. Second, we were unable to consider the role of many early-life and reproductive outcomes (including the number of sibling, number of sons and daughters, proportion of offspring born prematurely, and proportion of offspring born high/low birth weight) and later-life health. Investigators may wish to consider the mediating role of fertility in the association between these early-life circumstances and later-life health. Third, our measures of SES are not exhaustive. Childhood SES measured from the father’s usual occupation may differ from the occupation during an individual’s childhood. In addition, measures of midlife SES or education are not available in the UPDB for the majority of individuals in the sample. Fourth, sample selection may affect the generalizability of our findings. The sample was limited to individuals linked to the UPDB, in a continuous first marriage until age 50, and enrolled in a Medicare managed care plan. We detected little evidence of sample selection bias, although this result is—as are all sample selection correction methods—a function of the selection correction methods used, including the covariates considered (Fu et al. 2004). Although other sample selection correction methods exist, we did not test any of them. We are currently in the process of linking 1930 and 1940 census records, which will allow for more information to be used to specify the section process, and future analysis will further investigate selection bias. In addition, the sample is representative of the Utah population, resulting in less racial heterogeneity, lower average ages at marriage and first birth, higher fertility, and a higher proportion of LDS population than found in the general U.S. population. Our innovative approach to studying the relationship between fertility and aging using a large, longitudinal population contributes important information to the study of aging and, certainly, replications of our approach are in order in other populations. Fifth, it is possible that the left-censoring at age 65, due to Medicare age restrictions, affects the generalizability of our findings because individuals were required to survive to age 65. Adverse health consequences associated with childrearing may lead to early mortality in the particularly vulnerable population.
Several key strengths of our analysis are noteworthy, including the use of massive amounts of data to portray a very dynamic outcome using comprehensive medical records. We also used an integrated strategy for examining both morbidity and mortality. The use of a longitudinal database with detailed demographic data allowed us to examine the role of fertility in later-life health while controlling for key early-life conditions for a large number of individuals. In addition, we were able to use birth certificates to link birth outcomes to later-life health trajectories for a subset of the sample.
Future investigations examining the relationship between fertility and later-life health would be beneficial for replicating our results, especially through the use of alternate modeling strategies as well as the inclusion of additional measures of later-life SES and social support. For example, investigations of the mediation effects of fertility on the pathway from childhood circumstances to later-life heath could be conducted using methods such as structural equation modeling. In addition, we did not control for SES during adulthood or current socioeconomic circumstance because these measures are sporadically available in UPDB for members of these birth cohorts. Fertility and later-life health are both affected by adult SES, and some of the observed relationship between fertility and health may be determined by SES. Additionally, final socioeconomic attainment may be altered because of fertility histories.
In conclusion, the arch that defines human aging is heterogeneous, and more research is needed to characterize this variation and the factors that influence it. While early-life conditions explain a portion of the heterogeneity in aging, midlife circumstances may also alter the trajectories of disease. We show that parity, timing of childbearing, and birth outcomes of offspring are significantly related to shape of later-life health patterns and trajectories. This study contributes to this literature because it assesses the associations of fertility history on a long-term process of chronic disease and how it unfolds during the later years of life. The differences in risk factors between men and women suggest that evolutionary, biological, and social mechanisms are all critical when studying aging.
Supplementary Material
Fig. 3.
Predicted and observed Charlson comorbidity index (CCI) by year and group for females aged 75–84 in 1992, with a 95 % confidence intervals displayed by gray shading (panel A), and predicted probability of death before the next year by group for females aged 75–84 in 1992 (panel B). Source: Utah Population Database and Centers for Medicare & Medicaid Services (CMS) data
Fig. 4.
Predicted and observed Charlson comorbidity index (CCI) by year and group for males aged 66–74 in 1992, with a 95 % confidence intervals displayed by gray shading (panel A), predicted probability of death before the next year by group for males aged 66–74 in 1992 (panel B). Source: Utah Population Database and Centers for Medicare & Medicaid Services (CMS) data
Acknowledgments
This work was supported by the National Institutes of Health – National Institute of Aging (Grant Nos. 1R21AG036938-01 and 2R01 AG022095). This work was performed under CMS DUA 14187. The authors wish to thank the Huntsman Cancer Foundation for database support provided to the Pedigree and Population Resource of the HCI, University of Utah. We also thank Alison Fraser and Diana Lane Reed for valuable assistance in managing the data. We are also grateful to Rebecca L. Utz, Antoinette Stroup, and Ming Wen for their invaluable feedback. Partial support for all datasets within the UPDB was provided by the HCI Cancer Center Support Grant (P30 CA42014) from National Cancer Institute.
References
- Andersson T, Hogberg U, Åkerman S. Survival of orphans in 19th century Sweden—The importance of remarriages. Acta Pædiatrica. 1996;85:981–985. doi: 10.1111/j.1651-2227.1996.tb14198.x. [DOI] [PubMed] [Google Scholar]
- Arland T. Religion and fertility: The case of Mormonism. Journal of Marriage and Family. 1979;41:131–142. [Google Scholar]
- Arulampalam W, Stewart MB. Simplified implementation of the Heckman estimator of the dynamic probit model and a comparison with alternative estimators. Oxford Bulletin of Economics and Statistics. 2009;71:659–681. [Google Scholar]
- Bastian LA, West NA, Corcoran C, Munger RG. Number of children and the risk of obesity in older women. Preventive Medicine. 2005;40:99–104. doi: 10.1016/j.ypmed.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. Lancet. 2009;373:1773–1779. doi: 10.1016/S0140-6736(09)60731-5. [DOI] [PubMed] [Google Scholar]
- Berkman LF, Syme SL. Social networks, host resistance, and mortality: A nine-year follow-up study of Alameda County residents. American Journal of Epidemiology. 1979;109:186–204. doi: 10.1093/oxfordjournals.aje.a112674. [DOI] [PubMed] [Google Scholar]
- Carr DB, Utzschneider KM, Hull RL, Tong J, Wallace TM, Kodama K American Diabetes Association GENNID Study Group. Gestational diabetes mellitus increases the risk of cardiovascular disease in women with a family history of type 2 diabetes. Diabetes Care. 2006;29:2078–2083. doi: 10.2337/dc05-2482. [DOI] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Chronic Diseases. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Conde-Agudelo A, Rosas-Bermudez A, Kafury-Goeta AC. Effects of birth spacing on maternal health: A systematic review. American Journal of Obstetrics & Gynecology. 2007;196:297–308. doi: 10.1016/j.ajog.2006.05.055. [DOI] [PubMed] [Google Scholar]
- Davey Smith G, Sterne JAC, Tynelius P, Rasmussen F. Birth characteristics of offspring and parental diabetes: Evidence for the fetal insulin hypothesis. Journal of Epidemiology and Community Health. 2004;58:126–128. doi: 10.1136/jech.58.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doblhammer G. Reproductive history and mortality later in life: A comparative study of England and Wales and Austria. Population Studies. 2000;54:169–176. doi: 10.1080/713779087. [DOI] [PubMed] [Google Scholar]
- Doblhammer G, Oeppen J. Reproduction and longevity among the British peerage: The effect of frailty and health selection. Proceedings of the Royal Society of London, Series B: Biological Sciences. 2003;270:1541–1547. doi: 10.1098/rspb.2003.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg ML. Parenthood, host resistance to the common cold, and impaired fertility [Letter to the editor] Psychosomatic Medicine. 2012;74:988. doi: 10.1097/PSY.0b013e318273880f. [DOI] [PubMed] [Google Scholar]
- Eisenberg ML, Park Y, Brinton LA, Hollenbeck AR, Schatzkin A. Fatherhood and incident prostate cancer in a prospective US cohort. International Journal of Epidemiology. 2011;40:480–487. doi: 10.1093/ije/dyq163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enstrom JE, Breslow L. Lifestyle and reduced mortality among active California Mormons, 1980–2004. Preventive Medicine. 2008;46:133–136. doi: 10.1016/j.ypmed.2007.07.030. [DOI] [PubMed] [Google Scholar]
- Fried V, Bernstein A, Bush M. Multiple chronic conditions among adults aged 45 and over: Trends over the past 10 years. Atlanta, GA: Centers for Disease Control and Prevention; 2012. NCHS Data Brief No. 100. [PubMed] [Google Scholar]
- Fu VK, Winship C, Mare RD. Sample selection bias models. In: Hardy M, Bryman A, editors. Handbook of data analysis. Thousand Oaks, CA: Sage Publications; 2004. pp. 409–430. [Google Scholar]
- Gagnon A, Smith KR, Tremblay M, Vézina H, Paré PP, Desjardins B. Is there a trade off between fertility and longevity? A comparative study of women from three large historical databases accounting for mortality selection. American Journal of Human Biology. 2009;21:533–540. doi: 10.1002/ajhb.20893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galobardes B, Lynch JW, Smith GD. Is the association between childhood socioeconomic circumstances and cause-specific mortality established? Update of a systematic review. Journal of Epidemiology and Community Health. 2008;62:387–390. doi: 10.1136/jech.2007.065508. [DOI] [PubMed] [Google Scholar]
- Garibotti G, Smith KR, Kerber RA, Boucher KM. Longevity and correlated frailty in multigenerational families. Journals of Gerontology, Series A: Biological Sciences and Medical Sciences. 2006;61:1253–1261. doi: 10.1093/gerona/61.12.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilov LA, Gavrilova NS. Biodemography of exceptional longevity: Early-life and mid-life predictors of human longevity. Biodemography and Social Biology. 2012;58:14–39. doi: 10.1080/19485565.2012.666121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus AT. The weathering hypothesis and the health of African-American women and infants: Evidence and speculations. Ethnicity & Disease. 1992;2:207–221. [PubMed] [Google Scholar]
- Geronimus AT, Korenman S. The socioeconomic consequences of teen childbearing reconsidered. Quarterly Journal of Economics. 1992;107:1187–1214. [Google Scholar]
- Grundy E, Kravdal Ø. Do short birth intervals have long-term implications for parental health? Results from analyses of complete cohort Norwegian Register data. Journal of Epidemiology and Community Health. 2014;68:958–964. doi: 10.1136/jech-2014-204191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy E, Read S. Social contacts and receipt of help among older people in England: Are there benefits of having more children? Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2012;67:742–754. doi: 10.1093/geronb/gbs082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy E, Tomassini C. Fertility history and health in later life: A record linkage study in England and Wales. Social Science & Medicine. 2005;61:217–228. doi: 10.1016/j.socscimed.2004.11.046. [DOI] [PubMed] [Google Scholar]
- Haviland AM, Jones BL, Nagin DS. Group-based trajectory modeling extended to account for nonrandom participant attrition. Sociological Methods & Research. 2011;40:367–390. [Google Scholar]
- Haviland A, Nagin DS, Rosenbaum PR, Tremblay RE. Combining group-based trajectory modeling and propensity score matching for causal inferences in nonexperimental longitudinal data. Developmental Psychology. 2008;44:422–436. doi: 10.1037/0012-1649.44.2.422. [DOI] [PubMed] [Google Scholar]
- Hawkes K. How grandmother effects plus individual variation in frailty shape fertility and mortality: Guidance from human–chimpanzee comparisons. Proceedings of the National Academy of Sciences. 2010;107(Suppl. 2):8977–8984. doi: 10.1073/pnas.0914627107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healthcare Delivery Research. SEER-Medicare: Calculation of comorbidity weights. Bethesda, MD: National Cancer Institute; 2010. Retrieved from http://healthcaredelivery.cancer.gov/seermedicare/program/comorbidity.html. [Google Scholar]
- Heckman JJ. Sample selection bias as a specification error. Econometrica. 1979;47:153–161. [Google Scholar]
- Henretta JC, Grundy EM, Okell LC, Wadsworth ME. Early motherhood and mental health in midlife: A study of British and American cohorts. Aging & Mental Health. 2008;12:605–614. doi: 10.1080/13607860802343084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House J, Landis K, Umberson D. Social relationships and health. Science. 1988;241:540–545. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- Jelliffe DB, Jelliffe EFP. Human milk in the modern world: Psychological, nutritional, and economic significance. New York, NY: Oxford University Press; 1978. [Google Scholar]
- Jones BL, Nagin DS. Advances in group-based trajectory modeling and an SAS procedure for estimating them. Sociological Methods & Research. 2007;35:542–571. [Google Scholar]
- Kelsey JL, Gammon MD, John EM. Reproductive factors and breast cancer. Epidemiologic Reviews. 1993;15:36–47. doi: 10.1093/oxfordjournals.epirev.a036115. [DOI] [PubMed] [Google Scholar]
- Kerber RA, O’Brien E, Boucher KM, Smith KR, Cawthon RM. A genome-wide study replicates linkage of 3p22-24 to extreme longevity in humans and identifies possible additional loci. PLoS ONE. 2012;7(4):e34746. doi: 10.1371/journal.pone.0034746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerber RA, O’Brien E, Smith KR, Cawthon RM. Familial excess longevity in Utah genealogies. Journals of Gerontology, Series A: Biological Sciences and Medical Sciences. 2001;56:B130–B139. doi: 10.1093/gerona/56.3.b130. [DOI] [PubMed] [Google Scholar]
- Kirkwood TBL, Rose MR. Evolution of senescence: Late survival sacrificed for reproduction. Philosophical Transactions of the Royal Society of London, Series B: Biological Sciences. 1991;332(1262):15–24. doi: 10.1098/rstb.1991.0028. [DOI] [PubMed] [Google Scholar]
- Kitagawa EM, Hauser PM. Differential mortality in the United States: A study in socioeconomic epidemiology. Cambridge, MA: Harvard University Press; 1973. [Google Scholar]
- Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. Journal of Clinical Epidemiology. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Sugiura H, Ando Y, Shiraki N, Yanagi T, Yamashita H, Toyama T. Reproductive history and breast cancer risk. Breast Cancer. 2012;19:302–308. doi: 10.1007/s12282-012-0384-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer MS, Séguin L, Lydon J, Goulet L. Socio-economic disparities in pregnancy outcome: Why do the poor fare so poorly? Paediatric and Perinatal Epidemiology. 2000;14:194–210. doi: 10.1046/j.1365-3016.2000.00266.x. [DOI] [PubMed] [Google Scholar]
- Kravdal Ø. Relationship between childbearing and cancer incidence due to biology or lifestyle? Examples of the importance of using data on men. International Journal of Epidemiology. 1995;24:477–484. doi: 10.1093/ije/24.3.477. [DOI] [PubMed] [Google Scholar]
- Kuh D, Ben-Shlomo Y. A life course approach to chronic disease epidemiology. New York, NY: Oxford University Press; 2004. [Google Scholar]
- Kvale G, Heuch I, Nilssen S. Parity in relation to mortality and cancer incidence: A prospective study of Norwegian women. International Journal of Epidemiology. 1994;23:691–699. doi: 10.1093/ije/23.4.691. [DOI] [PubMed] [Google Scholar]
- Lawlor DA, Emberson JR, Ebrahim S, Whincup PH, Wannamethee SG, Walker M, Smith GD. Is the association between parity and coronary heart disease due to biological effects of pregnancy or adverse lifestyle risk factors associated with child-rearing? Circulation. 2003;107:1260–1264. doi: 10.1161/01.cir.0000053441.43495.1a. [DOI] [PubMed] [Google Scholar]
- Lukanova A, Kaaks R. Endogenous hormones and ovarian cancer: Epidemiology and current hypotheses. Cancer Epidemiology Biomarkers & Prevention. 2005;14:98–107. [PubMed] [Google Scholar]
- MacMahon B, Cole P, Brown J. Etiology of human breast cancer: A review. Journal of the National Cancer Institute. 1973;50:21–42. doi: 10.1093/jnci/50.1.21. [DOI] [PubMed] [Google Scholar]
- McCormick MC, Shapiro S, Starfield B. High-risk young mothers: Infant mortality and morbidity in four areas in the United States, 1973–1978. American Journal of Public Health. 1984;74:18–23. doi: 10.2105/ajph.74.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineau GP, Smith KR, Bean LL. Historical trends of survival among widows and widowers. Social Science & Medicine. 2002;54:245–254. doi: 10.1016/s0277-9536(01)00024-7. [DOI] [PubMed] [Google Scholar]
- Mirowsky J. Age at first birth, health, and mortality. Journal of Health and Social Behavior. 2005;46:32–50. doi: 10.1177/002214650504600104. [DOI] [PubMed] [Google Scholar]
- Moorad JA, Promislow DEL, Smith KR, Wade MJ. Mating system change reduces the strength of sexual selection in an American frontier population of the 19th century. Evolution and Human Behavior. 2011;32:147–155. doi: 10.1016/j.evolhumbehav.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myklestad K, Vatten LJ, Magnussen EB, Salvesen KÅ, Smith GD, Romundstad PR. Offspring birth weight and cardiovascular risk in parents—A population-based Hunt 2 study. American Journal of Epidemiology. 2012;175:546–555. doi: 10.1093/aje/kwr347. [DOI] [PubMed] [Google Scholar]
- Nagin DS. Group-based modeling of development. Cambridge, MA: Harvard University Press; 2005. [Google Scholar]
- Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annual Review of Clinical Psychology. 2010;6:109–138. doi: 10.1146/annurev.clinpsy.121208.131413. [DOI] [PubMed] [Google Scholar]
- Nagin DS, Tremblay RE. Analyzing developmental trajectories of distinct but related behaviors: A group-based method. Psychological Methods. 2001;6:18–34. doi: 10.1037/1082-989x.6.1.18. [DOI] [PubMed] [Google Scholar]
- Nam CB, Powers MG. The socioeconomic approach to status measurement: With a guide to occupational and socioeconomic status scores. Houston, TX: Cap and Gown Press; 1983. [Google Scholar]
- Norton MC, Smith KR, Østbye T, Tschanz JT, Schwartz S, Corcoran C Cache County Investigators. Early parental death and remarriage of widowed parents as risk factors for alzheimer disease: The Cache County study. American Journal of Geriatric Psychiatry. 2011;19:814–824. doi: 10.1097/JGP.0b013e3182011b38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul C, Mason W, McCaffrey D, Fox S. A cautionary case study of approaches to the treatment of missing data. Statistical Methods and Applications. 2008;17:351–372. [Google Scholar]
- Perls TT, Alpert L, Fretts RC. Middle-aged mothers live longer. Nature. 1997;389:133. doi: 10.1038/38148. [DOI] [PubMed] [Google Scholar]
- Permuth-Wey J, Sellers T. Epidemiology of ovarian cancer. In: Verma M, editor. Cancer epidemiology. Totowa, NJ: Humana Press; 2009. pp. 413–437. [DOI] [PubMed] [Google Scholar]
- Phelan JC, Link BG, Tehranifar P. Social conditions as fundamental causes of health inequalities: Theory, evidence, and policy implications. Journal of Health and Social Behavior. 2010;51(Suppl. 1):S28–S40. doi: 10.1177/0022146510383498. [DOI] [PubMed] [Google Scholar]
- Porter DV. WIC nutrition risk criteria: A scientific assessment. Nutrition Today. 1996;31:216. [PubMed] [Google Scholar]
- Preston SH, Hill ME, Drevenstedt GL. Childhood conditions that predict survival to advanced ages among African-Americans. Social Science & Medicine. 1998;47:1231–1246. doi: 10.1016/s0277-9536(98)00180-4. [DOI] [PubMed] [Google Scholar]
- Rich-Edwards J. A life course approach to women’s reproductive health. In: Kuh D, Hardy R, editors. A life course approach to women’s health. Oxford, UK: Oxford University Press; 2002. pp. 23–43. [Google Scholar]
- Robson SL, Smith KR. Twinning in humans: Maternal heterogeneity in reproduction and survival. Proceedings of the Royal Society B: Biological Sciences. 2011;278:3755–3761. doi: 10.1098/rspb.2011.0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson SL, Smith KR. Parity progression ratios confirm higher lifetime fertility in women who bear twins. Proceedings of the Royal Society B: Biological Sciences. 2012;279:2512–2514. [Google Scholar]
- Ross CE, Huber J. Hardship and depression. Journal of Health and Social Behavior. 1985;26:312–327. [PubMed] [Google Scholar]
- Schiller J, Lucas J, Peregoy J. Summary health statistics for US adults: National Health Interview Survey, 2011. Hyattsville, MD: National Center for Health Statistics; 2012. Vital Halth Statistics Series 10, No. 256. [PubMed] [Google Scholar]
- Sewell JE. History of Medicine. Bethesda, MD: U.S. National Library of Medicine; 1998. Cesarean section—A brief history. Retrieved from https://www.nlm.nih.gov/exhibition/cesarean/ [Google Scholar]
- Smith KR, Gagnon A, Cawthon RM, Mineau GP, Mazan R, Desjardins B. Familial aggregation of survival and late female reproduction. Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2009a;64:740–744. doi: 10.1093/gerona/glp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KR, Hanson HA, Zimmer Z. Early life conditions and later life (65+) co-morbidity trajectories: The Utah Population Database linked to medicare claims data. Paper presented at the annual meeting of the Population Association of America; San Francisco, CA. 2012a. May, [Google Scholar]
- Smith GD, Hart C, Ferrell C, Upton M, Hole D, Hawthorne V, Watt G. Birth weight of offspring and mortality in the Renfrew and Paisley study: Prospective observational study. BMJ. 1997;315:1189–1193. doi: 10.1136/bmj.315.7117.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KR, Mineau GP, Bean LL. Fertility and post-reproductive longevity. Biodemography and Social Biology. 2002;49:185–205. [PubMed] [Google Scholar]
- Smith KR, Mineau GP, Garibotti G, Kerber R. Effects of childhood and middle-adulthood family conditions on later-life mortality: Evidence from the Utah Population Database, 1850–2002. Social Science & Medicine. 2009b;68:1649–1658. doi: 10.1016/j.socscimed.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KR, Reed DL, Hanson HA, Mineau GP, Fraser A. All in the family: Fertility and mortality consequences for child survivors of the 1918 Influenza Pandemic. Paper presented at the Social Science History Association 2012 Annual Meeting; Vancouver, British Columbia, Canada. 2012b. Nov, [Google Scholar]
- Smith GD, Whitley E, Gissler M, Hemminki E. Birth dimensions of offspring, premature birth, and the mortality of mothers. Lancet. 2000;356:2066–2067. doi: 10.1016/S0140-6736(00)03406-1. [DOI] [PubMed] [Google Scholar]
- Spence NJ. The long-term consequences of childbearing: Physical and psychological well-being of mothers in later life. Research on Aging. 2008;30:722–751. doi: 10.1177/0164027508322575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlenberg P, Cooney TM. Family size and mother-child relations in later life. Gerontologist. 1990;30:618–625. doi: 10.1093/geront/30.5.618. [DOI] [PubMed] [Google Scholar]
- Umberson D, Chen MD. Effects of a parent’s death on adult children: Relationship salience and reaction to loss. American Sociological Review. 1994;59:152–168. [Google Scholar]
- Waldron I, Weiss CC, Hughes ME. Interacting effects of multiple roles on women’s health. Journal of Health and Social Behavior. 1998;39:216–236. [PubMed] [Google Scholar]
- Walsh TJ, Croughan MS, Schembri M, Chan JM, Turek PJ. Increased risk of testicular germ cell cancer among infertile men. Archives of Internal Medicine. 2009a;169:351–356. doi: 10.1001/archinternmed.2008.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh TJ, Pera RR, Turek PJ. The genetics of male infertility. Seminars in Reproductive Medicine. 2009b;27:124–136. doi: 10.1055/s-0029-1202301. [DOI] [PubMed] [Google Scholar]
- Walsh TJ, Schembri M, Turek PJ, Chan JM, Carroll PR, Smith JF, Croughan MS. Increased risk of high-grade prostate cancer among infertile men. Cancer. 2010;116:2140–2147. doi: 10.1002/cncr.25075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westoff CF. Fertility in the United States. Science. 1986;234:554–559. doi: 10.1126/science.3532324. [DOI] [PubMed] [Google Scholar]
- Williams K, Umberson D. Marital status, marital transitions, and health: A gendered life course perspective. Journal of Health and Social Behavior. 2004;45:81–98. doi: 10.1177/002214650404500106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi Z, Vaupel JW. Association of late childbearing with healthy longevity among the oldest-old in China. Population Studies. 2004;58:37–53. doi: 10.1080/0032472032000175437. [DOI] [PubMed] [Google Scholar]
- Zimmer Z, Martin L, Nagin D, Jones B. Modeling disability trajectories and mortality of the oldest-old in China. Demography. 2012;49:291–314. doi: 10.1007/s13524-011-0075-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.