Abstract
Background
New approaches to ablation of atrial fibrillation (AF) include focal impulse and rotor modulation (FIRM) mapping, and initial results reported with this technique have been favorable. We sought to independently evaluate the approach by analyzing quantitative characteristics of atrial electrograms used to identify rotors and describe acute procedural outcomes of FIRM-guided ablation.
Methods and Results
All FIRM-guided ablation procedures (n=24; 50% paroxysmal) at University of California, Los Angeles Medical Center were included for analysis. During AF, unipolar atrial electrograms collected from a 64-pole basket catheter were used to construct phase maps and identify putative AF sources. These sites were targeted for ablation, in conjunction with pulmonary vein isolation in most patients (n=19; 79%). All patients had rotors identified (mean, 2.3±0.9 per patient; 72% in left atrium). Prespecified acute procedural end point was achieved in 12 of 24 (50%) patients: AF termination (n=1), organization (n=3), or >10% slowing of AF cycle length (n=8). Basket electrodes were within 1 cm of 54% of left atrial surface area, and a mean of 31 electrodes per patient showed interpretable atrial electrograms. Offline analysis revealed no differences between rotor and distant sites in dominant frequency or Shannon entropy. Electroanatomic mapping showed no rotational activation at FIRM-identified rotor sites in 23 of 24 patients (96%).
Conclusions
FIRM-identified rotor sites did not exhibit quantitative atrial electrogram characteristics expected from rotors and did not differ quantitatively from surrounding tissue. Catheter ablation at these sites, in conjunction with pulmonary vein isolation, resulted in AF termination or organization in a minority of patients (4/24; 17%). Further validation of this approach is necessary.
Keywords: atrial fibrillation, catheter ablation, electrophysiology
A trial fibrillation (AF) is the most common sustained cardiac arrhythmia in adults and is associated with thromboembolic complications, myocardial and neuronal remodeling, and increased mortality.1 Surgical and percutaneous treatments involve ablative therapy targeted at isolating or eliminating putative arrhythmia triggers and substrate that sustains AF. Although Haïssaguerre et al2 found pulmonary vein triggers in the majority of patients with paroxysmal AF, the mechanisms underlying AF maintenance remain elusive, leading to suboptimal outcomes for catheter ablation. Ablation results are particularly disappointing for longstanding persistent AF, in which complex substrate and patient-specific sustaining mechanisms have been reported.3,4 This has led to a wide range of procedural approaches to persistent AF ablation, with most operators targeting atrial substrate in addition to pulmonary vein isolation (PVI) and ablation of other triggers.5
More recently, physiological mapping of AF has been used to identify areas of the atrium that may be responsible for maintaining AF, in an attempt to identify patient-specific drivers to design tailored ablation strategies.6–9 One such method with promising results is focal impulse and rotor modulation (FIRM). This technique uses panoramic sampling of the atria and computational spatiotemporal mapping to identify putative regions of recurrent organized rotational activity, or rotors, as well as focal drivers.10 Narayan et al10–12 have reported the presence of stable rotors in humans and that ablation of FIRM-identified localized sources results in high rates of acute termination and long-term freedom from recurrent AF.
On the basis of previous mechanistic studies, rotor sites are expected to exhibit greater electrical periodicity, unique spectral characteristics, such as higher dominant frequency6 and higher Shannon entropy,9 distinguishing them from other atrial sites.13–15 Quantitative analysis of electrograms recorded at rotor sites identified by FIRM mapping has not yet been systematically validated and reported. Accordingly, we hypothesized that FIRM-identified localized sources would show distinctive characteristics in frequency domain and entropy analysis and evidence of rotational activity on electroanatomic mapping. In addition, we report the rates of termination, organization, or slowing of AF cycle length (AFCL) associated with rotor-directed ablation and compare patients with acute procedural success to those with acute failure.
Methods
Patient Population
The institutional review board at the University of California, Los Angeles approved retrospective review of this data. Consecutive patients undergoing FIRM-guided ablation for paroxysmal or persistent AF at University of California, Los Angeles Medical Center between January 2012 and July 2013 were included in the study. Patients included underwent only FIRM-guided ablation (FIRM-only) or FIRM-guided ablation followed by PVI (FIRM+PVI). Data from some of these patients have previously been reported in other publications (one patient in Shivkumar et al11, and 11 patients in Miller et al12).
Electrophysiology Study
Antiarrhythmic medications were discontinued in paroxysmal patients for 5 half-lives (60 days for amiodarone) and were left to operator discretion for persistent patients. All patients were anticoagulated for at least 30 days before ablation; warfarin was uninterrupted with a target international normalized ratio of 2 to 3 at ablation; novel oral anticoagulants were held for 24 hours and restarted the next day, with intravenous heparin overnight. All patients had transesophageal echocardiography to rule out left atrial (LA) thrombus immediately before the procedure. Catheters were advanced from the femoral and jugular veins to the right atrium, coronary sinus, and LA via transseptal puncture. A 64-pole basket catheter (Constellation; Boston Scientific, Natick, MA) was advanced through an 8.5F SL0 sheath (Daig Medical, Minnetonka, MN) to map electric activation in both atria. The basket catheter was manipulated to optimize electrode contact with the atrial surface and maximize coverage of the entire chamber. One of the electrodes from a quadripolar catheter situated in the superior vena cava was used as a unipolar reference.
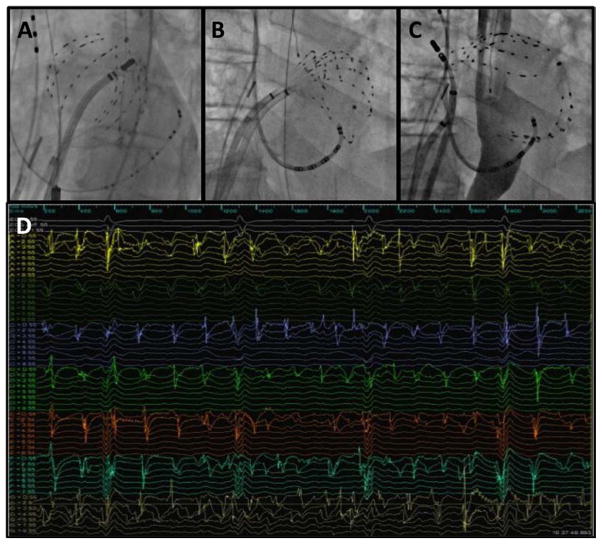
Three-dimensional (3D) electroanatomic mapping was performed using the Ensite Velocity system (NavX; St. Jude Medical, Minneapolis, MN). Intravenous heparin was infused before deployment of the basket catheter with a goal activated clotting time of >300 s. Figure 1 shows basket catheters in the LA via transseptal puncture (Figure 1A–1C) and a sample of the 64 unipolar electrograms recorded (Figure 1D). Unipolar electrograms were bandpass filtered between 0.05 and 500 Hz and recorded at 1-kHz sampling frequency (Prucka; GE Healthcare, Waukesha, WI).
Figure 1.
Left atrial basket catheter position and recordings. A, Fluoroscopic image of 64-pole Constellation basket catheter in left atrium (LA) with good chamber coverage and equidistant splines. B, Basket catheter with compressed splines, resulting in inadequate coverage of posterior and septal LA. C, Basket catheter with bunched splines, resulting in large areas of unsampled LA endocardium. D, Example of 64 unipolar electrograms recorded simultaneously during atrial fibrillation.
For patients presenting in sinus rhythm, AF was induced by atrial burst pacing, beginning at 500-ms cycle length and decreasing to 180 ms until AF was induced. If AF was not sustained for at least 5 minutes, isoproterenol was infused and pacing was repeated as necessary to induce sustained AF.
FIRM Mapping of AF Sources
The rationale, algorithms, and methods of identifying AF sources using FIRM mapping have been described previously.10,16 Briefly, basket catheter data are exported to a proprietary mapping system (RhythmView; Topera Medical, Palo Alto, CA), which filters out QRS complexes and T waves and analyzes the unipolar atrial electrograms, taking into account rate-dependent repolarization and conduction slowing to determine physiologically plausible activation paths. The resulting computational phase maps depict putative propagation of electric activity during AF, displayed as a gray-scale animation. Intraoperatively, 2 electrophysiologists examined the movies to qualitatively identify sites of recurrent clockwise or counterclockwise rotational activity or focal sources that initiated centrifugal atrial activation. Sources were targeted if both operators agreed on the location of a rotor or focal source. Rotor centers were localized on the basket catheter as either adjacent to a single electrode or a 1X1 electrode area bounded by adjacent electrodes on adjacent splines. Technical representatives from Topera Medical were present for all cases, and an experienced FIRM operator was on site for 18 of 24 (75%) cases, acting as a primary operator in 11 of 24 (46%) cases.
Catheter Ablation Procedure
FIRM sources in both the right atrium and LA were targeted using a 3.5-mm tip radiofrequency–irrigated ablation catheter (Thermocool; Biosense Webster, Diamond Bar, CA) at 25 to 35 Watts and at a maximum temperature of 42°C. The ablation catheter was directed to tissue adjacent to the basket electrode near the center of the identified rotor region, using fluoroscopy and electroanatomic mapping. Energy was applied until loss of local electrogram and drop in impedance at the site (20–40 s of radiofrequency energy). A lesion set was created, targeting the region within a radius of 1 to 2 cm of the rotor coordinate, until AF termination/organization or electrogram reduction of the rotor region was observed (5–10 minutes of radiofrequency energy). When AF organized into a regular atrial flutter or tachycardia, this was mapped and ablated with conventional approaches. When AF terminated, reinduction was attempted with the same pacing protocol and a new FIRM map was created. When AF did not terminate, the next FIRM-identified rotor site was targeted for ablation.
If the patient remained in AF after all FIRM sources were treated, direct-current cardioversion was performed. At that point, the procedure was terminated (FIRM-only group) or circumferential antral PVI was performed (FIRM+PVI) with a circular mapping catheter (Optima; St Jude Medical, St. Paul, MN) to verify LA–PV conduction block. No LA roof or mitral isthmus lines were created. Esophageal temperature was monitored, and energy delivery was interrupted for any temperature rise of >1°C.
Offline Data Analysis
Multipolar Basket Catheter Assessment
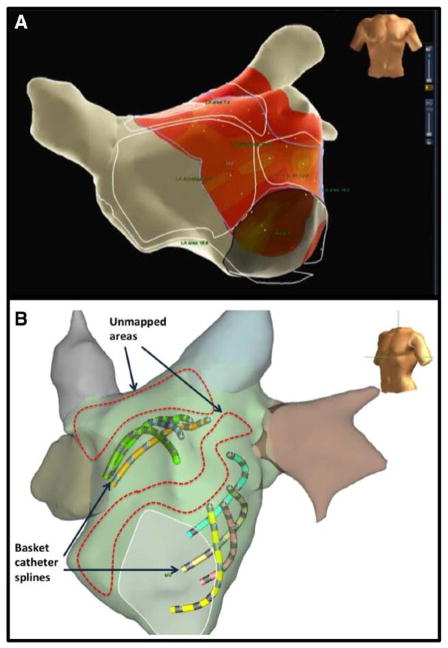
Atrial surface area coverage with the basket catheter was assessed by first calculating the endocardial LA surface area by segmentation of the electroanatomic 3D shell (Figure 2). We then determined the proportion of LA surface area within 10 mm of any basket catheter electrode, with the catheter deployed at the position leading to the first FIRM-identified LA source targeted for ablation.
Figure 2.
Analysis of basket catheter coverage. A, Electroanatomic map created with Ensite NAVX system. Left atrial (LA) surface was segmented to calculate total surface area outside pulmonary veins (outlined in white), and then 1-cm fill threshold was set to calculate the surface area near any basket electrode (colored area). B, Example of mapped and unmapped LA endocardial areas plotted on electroanatomic map.
Construction of Activation Maps
To assess adequacy of electric signals, basket catheter unipolar electrograms were studied to determine whether a voltage threshold could be used to exclude channels with only noise or indecipherable signals. Selection of a threshold value to allow automatic annotation with NavX was attempted. Two experienced electrophysiologists independently examined each electrogram and annotated timing of local atrial activation; an adequate electrogram was defined as one in which local activation time could be manually annotated at any gain setting. This was done at the peak deflection of each atrial electrogram in segments without a QRS complex to minimize distortion by ventricular activation. Electroanatomic mapping was used to create propagation and isochronal maps for 20 consecutive sequences, each 200 ms. Data were analyzed for rotational activity (defined as in other studies as ≥2 complete rotations of 360°) or focal sources with centrifugal atrial activation.
Frequency Domain Analysis and Entropy
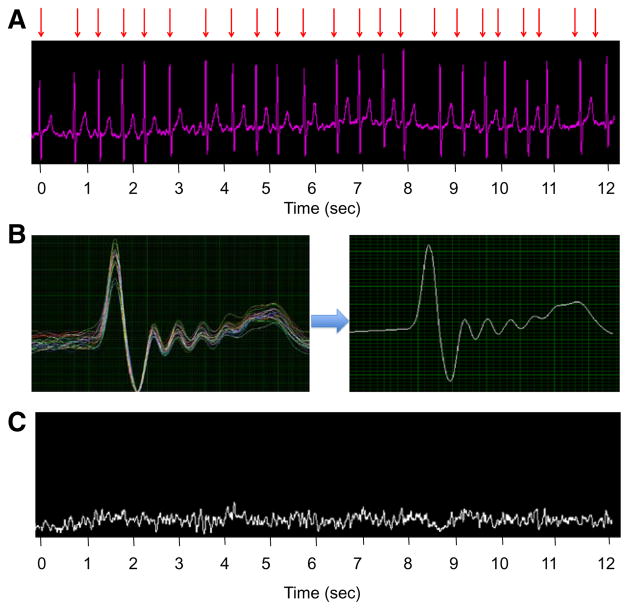
For quantitative analysis of atrial electric activity, far-field ventricular activity was subtracted from the raw unipolar signals by filtering to remove baseline drift and electric noise, locating the peak of each R wave, and selecting a surrounding segment that included the QRS complex and T wave (Figure 3). An aggregated QRS–T complex was subtracted from each segment, leaving atrial activity as the remainder signal for each of the 64 channels. The dominant frequency and Shannon entropy were then calculated from the remainder signal.
Figure 3.
Quantitative analysis of atrial unipolar electrograms. A, Raw unipolar signal from single electrode on a basket catheter, with red arrows indicating QRS complexes to be filtered out. B, QRS waveforms are plotted simultaneously to create an aggregated QRS–T complex. C, Remainder signal after QRS–T subtraction shows pure atrial electric activity.
Frequency domain and entropy analyses were performed using custom-made programs in LabVIEW 2013 software (National Instruments, Austin, TX). Briefly, a Fourier transform function was used to decompose each unipolar ECG into its component sinusoidal functions. The dominant frequency was defined as the frequency with the largest amplitude from 1 to 20 Hz, as calculated by the Fourier transform. Shannon entropy was calculated to measure the degree of disorganization for each waveform. This was calculated by the formula
where P(xn) is the probability of a given outcome and m is the number of possible outcomes. Applied to this analysis, each data point represents an instantaneous voltage measurement. For each channel, the range of voltage measurements was divided into 100 bins (m=100), and the proportion of all voltage measurements within each bin was given by P(xn).
Acute Ablation End points
Outcomes were assessed during the ablation procedure and in the immediate postprocedural period. For patients who began the procedure in sinus rhythm, the primary acute efficacy end point was defined as termination or organization of AF or slowing of AFCL by >10% during radiofrequency application and noninducibility after ablation. For patients who began the procedure in AF, acute efficacy was defined as termination of AF at any point during the procedure or slowing of AFCL by >10% from the beginning to the end of the procedure (including PVI). Safety end points included adverse events within 30 days of the index procedure. AFCL was determined for each patient at the start of the procedure, after each rotor ablation, and at the end of the procedure. A total of 30 cycle lengths were measured in the coronary sinus catheter and averaged for each cycle length measurement.
Statistical Analysis
Categorical variables were compared using the χ2 or Fisher exact tests, expressed as numbers and percentages. Continuous data are reported as mean±SD or median with 25% to 75% percentile range for small sample sizes. Patients were grouped based on acute procedural success and compared with those without acute success. Unpaired t test was used to estimate differences in continuous variables between groups. When comparing rotor sites to nonrotor sites, a linear mixed effects repeated measures model was used to account for within subject correlation among multiple observations from the same patient. We used the Z test of proportions to compare subgroups based on type of AF, previous ablation, and FIRM versus FIRM+PVI, with respect to acute outcomes. A 2-tailed P value of <0.05 was considered statistically significant. Stata statistical analysis software was used for all analyses.
Results
Patient Characteristics
During the study period, 24 FIRM-guided ablation procedures for AF were performed (Table 1). Patients were predominantly men (61%), half with paroxysmal AF, and 92% had failed at least 1 antiarrhythmic drug. Fifteen patients (63%) had previous PVI, of which 6 (25%) also had cavotricuspid isthmus ablation, and 6 (25%) had previous LA substrate modification. The mean duration of AF was 7.8±5.7 years, and mean LA volume by cardiac computed tomography or magnetic resonance imaging was 76±32 mL.
Table 1.
Baseline Characteristics
| All Patients, n=24 | Acute Procedural Success, n=12 | No Acute Procedural Success n=12 | P Value | |
|---|---|---|---|---|
| Age | 64±10 | 66±11 | 61±10 | 0.33 |
| Male, sex (%) | 14 (61) | 9 (75) | 5 (42) | 0.11 |
| Paroxysmal pattern of AF (%) | 12 (50) | 6 (50) | 6 (50) | 1.00 |
| AF duration (years from diagnosis) | 7.8±5.5 | 9.0±4.3 | 6.5±6.5 | 0.28 |
| Previous AF ablation (%) | 15 (63) | 8 (67) | 7 (58) | 0.69 |
| No. of previous AF ablations | 1.2±1.1 | 1.4±1.3 | 0.9±0.9 | 0.29 |
| No. of failed antiarrhythmic drugs | 1.7±1.0 | 1.5±0.9 | 1.8±1.0 | 0.41 |
| CHA2DS2-VaSC score | 1.7±1.0 | 1.8±1.1 | 1.6±1.0 | 0.57 |
| LVEF, % | 63±6.9 | 64±5.7 | 61±8.0 | 0.31 |
| LA volume on MRI, mL | 76±32 | 79±31 | 73±33 | 0.64 |
Values are n (%) or mean±SD. AF indicates atrial fibrillation; CHA2DS2-VASc, combined stroke risk score: congestive heart failure, hypertension, age ≥65 or 75 years, diabetes, prior stroke/transient ischemic attack, vascular disease, female sex; LA, left atrium; LVEF, left ventricular ejection fraction; and MRI, magnetic resonance imaging.
Quantitative Analysis of Rotor Sites
All patients had rotor sources identified by FIRM mapping (Table 2), with a mean of 2.3±0.9 total sources targeted per patient and more sites located in LA than right atrium (1.6±0.8 versus 0.6±0.8; P<0.01). On the basis of the segmented electroanatomic maps, only 54±15% of the total LA surface area outside the PVs was within 10 mm of any basket catheter electrode.
Table 2.
Rotor Mapping Results
| All Procedures, n=24 | Acute Procedural Success, n=12 | No Acute Procedural Success, n=12 | P Value | |
|---|---|---|---|---|
| No. of LA rotors identified | 1.6±0.8 | 1.7±0.9 | 1.5±0.7 | 0.61 |
| No. of RA rotors identified | 0.6±0.8 | 0.7±0.9 | 0.6±0.8 | 0.81 |
| Proportion of LA surface area mapped with basket catheter | 54±15% | 56±16% | 53±15% | 0.75 |
| No. of basket electrodes with adequate atrial electrogram | 31±17 | 27±18 | 35±16 | 0.28 |
| Dominant frequency at rotor sites. Hz | 4.8±1.3 | 4.8±1.4 | 5.0±1.3 | 0.62 |
| Dominant frequency at nonrotor sites, Hz | 4.7±1.6 | 4.8±1.6 | 4.6±1.7 | 0.06 |
| Shannon entropy at rotor sites | 4.8±0.5 | 4.8±0.3 | 4.9±0.7 | 0.35 |
| Shannon entropy at nonrotor sites | 4.9±0.6 | 4.8±0.5 | 5.0±0.6 | 0.08 |
| Patients with rotational activity or focal source at rotor site on electroanatomic mapping | 1 (4%) | 0 (0) | 1 (8%) | 0.34 |
Values are n (%), mean±SD. LA indicates left atrium; and RA, right atrium.
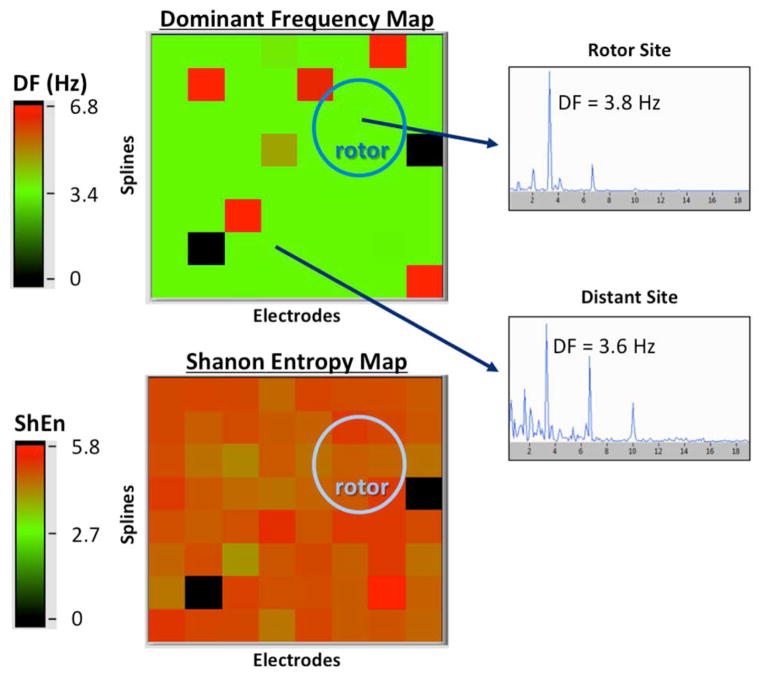
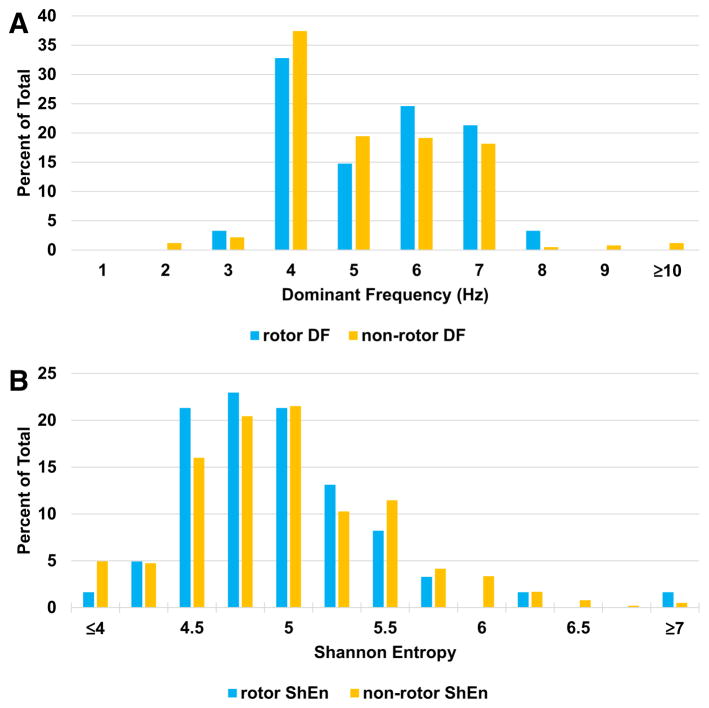
As shown in Table 2, the linear mixed model showed that electrograms recorded at FIRM-identified rotor sites when compared with the rest of the atrial sites were not different in dominant frequency (4.8±1.3 versus 4.7±1.6 Hz; P=0.84) or Shannon entropy (4.8±0.5 versus 4.9±0.6; P=0.31). This was true for patients with acute ablation success and those without acute success. Comparing patients who had undergone previous AF ablation (n=15) versus those without previous ablation (n=9), there was no difference in dominant frequency at rotor (4.8 versus 4.6 Hz; P=0.65) and nonrotor (4.7 versus 4.6 Hz; P=0.32) sites nor Shannon entropy at rotor (4.8 versus 4.7; P=0.65) and nonrotor (4.7 versus 4.8; P=0.07) sites. Figure 4 shows a representative checkerboard plot of the dominant frequency obtained at all 64 electrodes, with no difference at the rotor site. In Figure 5, histograms of dominant frequency and Shannon entropy recorded from all electrodes in all patients are shown, subdivided into rotor sites and nonrotor sites. Distribution was similar for both parameters.
Figure 4.
Example of frequency domain and entropy analysis. Both panels are 8×8 grids representing each electrode of the 64-channel basket catheter from a single patient. Top, Dominant frequency (DF) of each atrial unipolar signal is shown in a color map. Insets, Representative Fourier transforms illustrating similar DF at a focal impulse and rotor modulation (FIRM)–identified rotor site, and elsewhere in the atrium. Bottom, Similar Shannon entropy (ShEn) in a FIRM-identified rotor region and the rest of the left atrium.
Figure 5.
Detailed results of frequency domain and entropy analysis. A, Histogram of dominant frequency at all rotor and nonrotor sites shows similar frequency distribution. B, Histogram of Shannon entropy (ShEn) at all rotor and nonrotor sites shows similar frequency distribution.
Propagation Maps
As illustrated in Figure 6, the electroanatomic mapping system was not able to annotate activation times automatically because of poor signal quality. When an automatic minimum voltage threshold was set (0.15 mV in this example), no atrial electrograms were observed. Poor quality, difficult-to-interpret electrograms were observed in multiple electrodes from every case. Of the 64 basket catheter electrodes, a mean of 31.0±17.4 per patient had adequate electrograms to determine local activation time (range, 5–62) at the time when the first AF source in the LA was identified by FIRM mapping.
Figure 6.
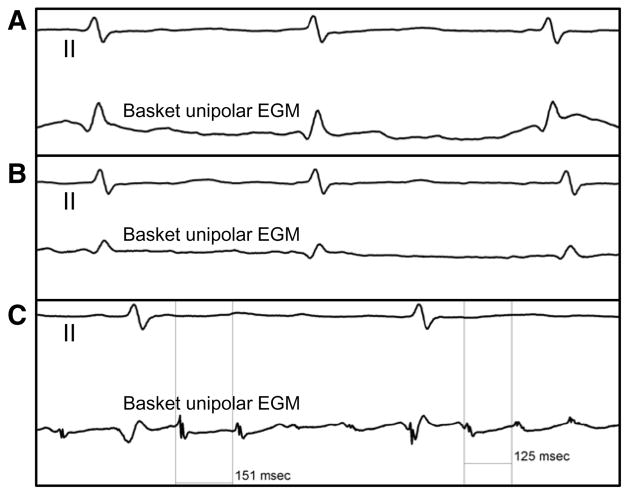
Atrial electrogram analysis: local activation time. A, Top, Surface ECG lead II. Bottom, Local electrogram (EGM) from a rotor site recorded by using a basket catheter. Only ventricular activation can be seen on the local tracing. B, On this unipolar recording with a voltage of 0.06 mV, low-amplitude atrial EGM can be seen, but activation time cannot be accurately annotated. C, On this unipolar recording with a voltage of 0.28 mV, atrial EGM is decipherable and shows variable cycle length.
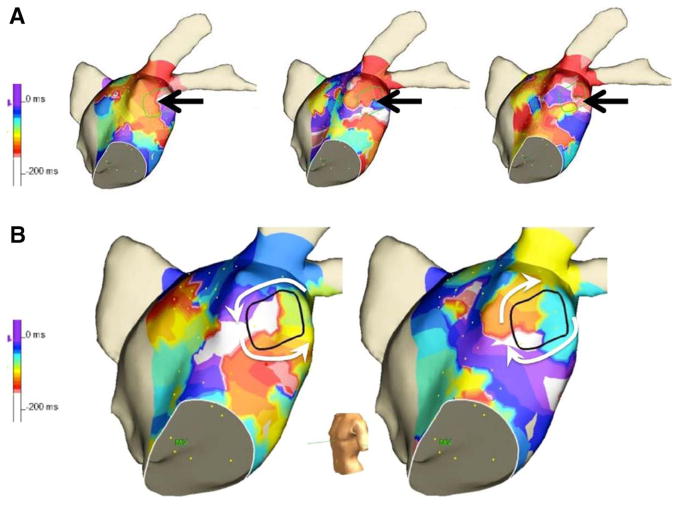
Figure 7A shows representative sequential LA activation maps, with a FIRM rotor site identified by black arrows. During most sequences, only disorganized activation patterns were observed. Interestingly, in the same patient, we also observed counterclockwise rotational activity for 1 cycle but opposite chirality (clockwise) in the next time sequence (Figure 7B). Rotational circuits (at least 2 consecutive rotations of the same chirality) were not identified in any of the 24 cases using electroanatomic activation maps. A focal source was observed in 1 case.
Figure 7.
Electroanatomic mapping. A, Sequential activation isochrone maps show disorganized activity with no rotation. B, Rotational activity seen, with change in chirality in sequential activation sequences.
Acute Results of FIRM-Guided Catheter Ablation
The majority (14/24; 58%) of patients were in sinus rhythm at the beginning of the procedure (4 persistent AF and 10 paroxysmal), and most of these (10/14; 71%) required isoproterenol and rapid atrial pacing to induce sustained AF for mapping. The remainder (10/24; 42%) were in AF at baseline and did not require pacing or isoproterenol for mapping. Pulmonary vein reconnection was identified in the majority of patients (10/14; 71%) who had undergone previous PVI. The average procedure length was 358 minutes, with 68 minutes of fluoroscopy time (Table 3).
Table 3.
Ablation Results
| All Procedures, n=24 | Acute Procedural Success, n=12 | No Acute Procedural Success, n=12 | P Value | |
|---|---|---|---|---|
| Procedure time, min | 358±72 | 351±70 | 365±77 | 0.63 |
| Fluoroscopy time, min | 68±24 | 71±24 | 65±24 | 0.55 |
| Total RF ablation time, min | 41±17 | 44±18 | 39±16 | 0.47 |
| Rotor RF ablation time, min | 20±11 | 20±12 | 19±11 | 0.99 |
| Nonrotor RF ablation time, min | 22±17 | 25±18 | 20±17 | 0.49 |
| AFCL preablation, ms | 189±36 | 183±25 | 196±46 | 0.40 |
| AFCL postablation, ms | 207±41 | 215±37 | 198±46 | 0.36 |
AFCL indicates atrial fibrillation cycle length; and RF, radiofrequency.
All patients underwent FIRM-guided ablation, and 19 of 24 (79%) also underwent PVI (entrance block documented in 13 and bidirectional block in 7). FIRM-guided and non–FIRM-guided radiofrequency ablation times were similar (19.8±11.0 and 22.4±16.8 minutes; P=0.55). There was no significant difference in radiofrequency time between paroxysmal and persistent patients (38.8 versus 44.1 minutes; P=0.44). Radiofrequency delivery was limited in 6 patients (FIRM or PVI) because of proximity of the phrenic nerve, atrioventricular node, or esophagus.
Overall, in 12 of 24 patients (50%), the acute end point was achieved: termination in 1, organization in 3, and AFCL slowing >10% in 8 (mean, 21.7±10.5%). There was no significant difference in the number of rotors (2.5 versus 2.2; P=0.52) or radiofrequency ablation time (44 versus 39 minutes; P=0.47) between those with acute success and failure. The majority of patients with acute success (8/12; 67%) were in sinus rhythm at the start of the procedure. Direct termination to sinus rhythm was not observed in any of the 10 patients who presented in AF, and all required direct-current cardioversion at some point in the procedure. Comparing patients with acute success and those with acute failure, there was no significant difference in age, proportion with previous ablation, AF duration, or LA size.
Periprocedurally, 4 patients (16.7%) had adverse events, with 2 heart failure exacerbations and 1 pericardial effusion requiring drainage. One patient, who underwent ablation of 2 rotors in the right atrium and 1 at the LA roof, as well as wide-area circumferential PVI, had sudden death 22 days after the procedure. Autopsy showed esophageal-left inferior PV fistula, near the PVI ablation line, distant from the rotor site at anterior LA roof.
Discussion
Major Findings
The major findings of this study are as follows: (1) the multipolar basket catheter provides inadequate coverage of the LA, with half the surface area unsampled, and decipherable atrial electrograms from only 48% of electrodes; (2) FIRM-identified rotor sites do not exhibit distinctive electrophysiological characteristics with regard to dominant frequency or Shannon entropy; (3) rotational activation (>1 rotation) on electroanatomic mapping was not observed at FIRM-identified rotor sites; and (4) ablation of rotor sites, even when accompanied by PVI, did not result in AF termination in the majority (20/24; 83%) of patients.
To the best of our knowledge, this is the first reported systematic quantitative study of electrograms recorded at putative rotor sites identified by FIRM mapping.
Feasibility of Panoramic Atrial Mapping With the Basket Catheter
We found the Constellation basket catheter left large areas of the atrial surface unsampled, usually because of anatomic variation leading to poor contact or clustering of splines. Even within regions that had adequate physical sampling (55% of LA surface area), the majority of atrial electrograms were of such poor quality that activation time could not be manually identified by 2 experienced electrophysiologists, and thus, they were indistinguishable from noise. The resulting activation maps were of such low resolution that it was difficult to precisely identify the site of any localized sources for ablation. Newer catheter designs might improve atrial coverage and signal quality (eg, FIRMap catheter, Topera Medical, Menlo Park, CA). Alternatively, other approaches, such as body surface mapping or high-resolution epicardial mapping, might provide better coverage and higher signal-to-noise ratio.
Quantitative Characteristics of FIRM-Identified Rotor Sites
In this study, electrograms recorded from sites identified as rotors did not differ quantitatively from other atrial sites in dominant frequency or Shannon entropy. In animal models, rotors characterized by a series of stable high-frequency electrograms around the repetitive circular path have been described.15 We did not see regular, monomorphic electric activity in any of the FIRM-identified rotor areas. Consistent with previous reports on human AF failing to demonstrate stable re-entrant rotors,17,18 we could not find evidence of sustained rotational activity on isochronal activation maps. Narayan et al19 have previously suggested that expectation of regular signals near a rotor core or antiphase signals at opposite equatorial points of a circular trajectory is a gross oversimplification of AF dynamics, in which rotors precess to produce variable electrograms.20 However, even if this is true, regular monomorphic electrograms should be observed close to the rotor core.
In summary, rotor sites were not mathematically distinct from background noise during AF using multiple established analytic methods and FIRM-identified rotor sites did not show quantitative characteristics that would allow for prediction of ablation success. Although the possibility that the proprietary FIRM algorithm processes electrophysiological information in a unique method beyond traditional mapping techniques cannot be excluded, objective corroborating analytical methods in this study could not confirm the presence and location of these purportedly stable localized sources.
Acute Results of Catheter Ablation at Rotor Sites
Catheter ablation of all FIRM-identified rotor sites did not lead to AF termination or organization in 83% of procedures, and only half could be considered successful with the less rigorous acute end point of AFCL slowing. These results were strikingly different from those reported in the CONFIRM study,21 in which 86% of patients achieved the primary acute end point, mostly by termination of AF rather than AFCL slowing. Possible explanations include statistical variation inherent to small sample size, adverse patient selection because of referral bias of particularly challenging patients with AF, and training effect of a new technology.
Targeting Rotors in AF Catheter Ablation
Rotor ablation did not result in AF termination in this study. One potential explanation is that the mechanism of AF in these patients was not dependent on the rotors identified and targeted for ablation. Even if the FIRM approach does indeed detect rotors, we did not observe regular periodic activation or mathematically distinct electric activity at these sites, perhaps because of rotor drift or temporal instability, and therefore, it is difficult to advocate ablation at these sites.
Theoretical concerns about rotor ablation in the treatment of AF persist. If FIRM-identified rotors are functional, focal ablation at the center should simply convert functional block to structural block and create fixed anatomic re-entry. To effectively treat rotor-driven fibrillation with catheter ablation, a line of conduction block should be created from the rotor core to an anatomic barrier. One mechanism by which focal ablation might be helpful is to increase the range over which the rotor core meanders, which could result in rotor collision with a tissue boundary, and hypothetically terminates spiral re-entry.22 However, even this explanation does not hold when the rotor meanders at baseline.
Study Limitations
This study is limited by a relatively small sample size, resulting in limited power to detect small differences. However, this is the largest independent detailed quantitative analysis to date of FIRM-identified rotor sites, and there was no trend toward unique rotor site characteristics that might be confirmed in a larger study. These were the initial cases performed using the FIRM system at our institution. Although it is possible that more experience would have yielded better mapping and ablation results, some of these procedures were performed by a developer of the FIRM technology, and most had an experienced FIRM operator onsite as an advisor.
One limitation inherent in approaches requiring mapping during AF is that many paroxysmal patients require induction of AF, which may spontaneously terminate even in the absence of ablation. Importantly, because most patients had PVI and FIRM-guided ablation, this study did not examine the effects of rotor ablation alone. Finally, this study examines only quantitative characteristics of rotor sites and acute ablation outcomes. Prospective studies of long-term outcomes are required to fully evaluate this technology.
Conclusions
Systematic quantitative analysis of FIRM-identified rotor sites did not reveal distinctive electrophysiological features or rotational activity. Catheter ablation of these sites resulted in AF termination or organization in a small minority of patients (17%). FIRM mapping and ablation require further validation in larger prospective randomized trials to compare acute procedural outcomes and long-term clinical efficacy of this approach with conventional ablation.
WHAT IS KNOWN
Ablation results are disappointing for longstanding persistent atrial fibrillation, in which complex substrate and patient-specific sustaining mechanisms have been implicated.
In an attempt to develop patient-specific ablation strategies, focal impulse and rotor modulation mapping has reportedly identified stable rotors, and ablation at these sites resulted in high rates of acute termination and long-term freedom from recurrent atrial fibrillation.
WHAT THE STUDY ADDS
We hypothesized that focal impulse and rotor modulation mapping–identified localized sources would exhibit distinctive characteristics in the frequency domain and entropy analysis and evidence of rotational activity on electroanatomic mapping.
In this first quantitative study of the atrial electrograms at focal impulse and rotor modulation mapping–identified rotor sites, we demonstrate that basket catheters insufficiently sample the left atrium and that the putative rotor sites are not distinct from the remainder of the atrium in frequency and entropy domains.
We observed atrial fibrillation termination or organization in only a small minority of patients (17%) undergoing catheter ablation with this technique.
Footnotes
Disclosures
None.
References
- 1.Piccini JP, Hammill BG, Sinner MF, Jensen PN, Hernandez AF, Heckbert SR, Benjamin EJ, Curtis LH. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993–2007. Circ Cardiovasc Qual Outcomes. 2012;5:85–93. doi: 10.1161/CIRCOUTCOMES.111.962688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 3.Brooks AG, Stiles MK, Laborderie J, Lau DH, Kuklik P, Shipp NJ, Hsu LF, Sanders P. Outcomes of long-standing persistent atrial fibrillation ablation: a systematic review. Heart Rhythm. 2010;7:835–846. doi: 10.1016/j.hrthm.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 4.Tilz RR, Rillig A, Thum AM, Arya A, Wohlmuth P, Metzner A, Mathew S, Yoshiga Y, Wissner E, Kuck KH, Ouyang F. Catheter ablation of longstanding persistent atrial fibrillation: 5-year outcomes of the Hamburg Sequential Ablation Strategy. J Am Coll Cardiol. 2012;60:1921–1929. doi: 10.1016/j.jacc.2012.04.060. [DOI] [PubMed] [Google Scholar]
- 5.Calkins H. Catheter ablation to maintain sinus rhythm. Circulation. 2012;125:1439–1445. doi: 10.1161/CIRCULATIONAHA.111.019943. [DOI] [PubMed] [Google Scholar]
- 6.Atienza F, Almendral J, Jalife J, Zlochiver S, Ploutz-Snyder R, Torrecilla EG, Arenal A, Kalifa J, Fernández-Avilés F, Berenfeld O. Real-time dominant frequency mapping and ablation of dominant frequency sites in atrial fibrillation with left-to-right frequency gradients predicts long-term maintenance of sinus rhythm. Heart Rhythm. 2009;6:33–40. doi: 10.1016/j.hrthm.2008.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cuculich PS, Wang Y, Lindsay BD, Faddis MN, Schuessler RB, Damiano RJ, Jr, Li L, Rudy Y. Noninvasive characterization of epicardial activation in humans with diverse atrial fibrillation patterns. Circulation. 2010;122:1364–1372. doi: 10.1161/CIRCULATIONAHA.110.945709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haissaguerre M, Hocini M, Denis A, Shah AJ, Komatsu Y, Yamashita S, Daly M, Amraoui S, Zellerhoff S, Picat MQ, Quotb A, Jesel L, Lim H, Ploux S, Bordachar P, Attuel G, Meillet V, Ritter P, Derval N, Sacher F, Bernus O, Cochet H, Jais P, Dubois R. Driver domains in persistent atrial fibrillation. Circulation. 2014;130:530–538. doi: 10.1161/CIRCULATIONAHA.113.005421. [DOI] [PubMed] [Google Scholar]
- 9.Ganesan AN, Kuklik P, Lau DH, Brooks AG, Baumert M, Lim WW, Thanigaimani S, Nayyar S, Mahajan R, Kalman JM, Roberts-Thomson KC, Sanders P. Bipolar electrogram shannon entropy at sites of rotational activation: implications for ablation of atrial fibrillation. Circ Arrhythm Electrophysiol. 2013;6:48–57. doi: 10.1161/CIRCEP.112.976654. [DOI] [PubMed] [Google Scholar]
- 10.Narayan SM, Krummen DE, Enyeart MW, Rappel WJ. Computational mapping identifies localized mechanisms for ablation of atrial fibrillation. PLoS One. 2012;7:e46034. doi: 10.1371/journal.pone.0046034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shivkumar K, Ellenbogen KA, Hummel JD, Miller JM, Steinberg JS. Acute termination of human atrial fibrillation by identification and catheter ablation of localized rotors and sources: first multicenter experience of focal impulse and rotor modulation (FIRM) ablation. J Cardiovasc Electrophysiol. 2012;23:1277–1285. doi: 10.1111/jce.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller JM, Kowal RC, Swarup V, Daubert JP, Daoud EG, Day JD, Ellenbogen KA, Hummel JD, Baykaner T, Krummen DE, Narayan SM, Reddy VY, Shivkumar K, Steinberg JS, Wheelan KR. Initial independent outcomes from focal impulse and rotor modulation ablation for atrial fibrillation: multicenter FIRM registry. J Cardiovasc Electrophysiol. 2014;25:921–929. doi: 10.1111/jce.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gray RA, Pertsov AM, Jalife J. Spatial and temporal organization during cardiac fibrillation. Nature. 1998;392:75–78. doi: 10.1038/32164. [DOI] [PubMed] [Google Scholar]
- 14.Skanes AC, Mandapati R, Berenfeld O, Davidenko JM, Jalife J. Spatiotemporal periodicity during atrial fibrillation in the isolated sheep heart. Circulation. 1998;98:1236–1248. doi: 10.1161/01.cir.98.12.1236. [DOI] [PubMed] [Google Scholar]
- 15.Mandapati R, Skanes A, Chen J, Berenfeld O, Jalife J. Stable microreentrant sources as a mechanism of atrial fibrillation in the isolated sheep heart. Circulation. 2000;101:194–199. doi: 10.1161/01.cir.101.2.194. [DOI] [PubMed] [Google Scholar]
- 16.Narayan SM, Krummen DE, Rappel WJ. Clinical mapping approach to diagnose electrical rotors and focal impulse sources for human atrial fibrillation. J Cardiovasc Electrophysiol. 2012;23:447–454. doi: 10.1111/j.1540-8167.2012.02332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee G, Kumar S, Teh A, Madry A, Spence S, Larobina M, Goldblatt J, Brown R, Atkinson V, Moten S, Morton JB, Sanders P, Kistler PM, Kalman JM. Epicardial wave mapping in human long-lasting persistent atrial fibrillation: transient rotational circuits, complex wavefronts, and disorganized activity. Eur Heart J. 2014;35:86–97. doi: 10.1093/eurheartj/eht267. [DOI] [PubMed] [Google Scholar]
- 18.de Groot NM, Houben RP, Smeets JL, Boersma E, Schotten U, Schalij MJ, Crijns H, Allessie MA. Electropathological substrate of longstanding persistent atrial fibrillation in patients with structural heart disease: epicardial breakthrough. Circulation. 2010;122:1674–1682. doi: 10.1161/CIRCULATIONAHA.109.910901. [DOI] [PubMed] [Google Scholar]
- 19.Narayan SM, Jalife J. CrossTalk proposal: Rotors have been demonstrated to drive human atrial fibrillation. J Physiol. 2014;592(Pt 15):3163–3166. doi: 10.1113/jphysiol.2014.271031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zlochiver S, Yamazaki M, Kalifa J, Berenfeld O. Rotor meandering contributes to irregularity in electrograms during atrial fibrillation. Heart Rhythm. 2008;5:846–854. doi: 10.1016/j.hrthm.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Narayan SM, Krummen DE, Shivkumar K, Clopton P, Rappel WJ, Miller JM. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) trial. J Am Coll Cardiol. 2012;60:628–636. doi: 10.1016/j.jacc.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spector P. Principles of cardiac electric propagation and their implications for re-entrant arrhythmias. Circ Arrhythm Electrophysiol. 2013;6:655–661. doi: 10.1161/CIRCEP.113.000311. [DOI] [PubMed] [Google Scholar]