Abstract
Humanin is a peptide that is cytoprotective against stresses in many cell types. We investigated whether a potent humanin analogue S14G-humanin (HNG) would protect against chemotherapy-induced damage to normal cells without interfering with the chemotherapy-induced suppression of cancer cells. Young adult male mice were inoculated iv with murine melanoma cells. After 1 week, cancer-bearing mice were randomized to receive either: no treatment, daily ip injection of HNG, a single ip injection of cyclophosphamide (CP), or CP+HNG and killed at the end of 3 weeks. HNG rescued the CP-induced suppression of leucocytes and protected germ cell from CP-induced apoptosis. Lung metastases were suppressed by HNG or CP alone, and further suppressed by CP+HNG treatment. Plasma IGF-1 levels were suppressed by HNG with or without CP treatment. To investigate whether HNG maintains its protective effects on spermatogonial stem cells, sperm output, and peripheral leucocytes after repeated doses of CP, normal adult male mice received: no treatment, daily sc injection of HNG, 6 ip injections of CP at 5-day intervals, and the same regimens of CP+HNG and killed at the end of 4 weeks of treatment. Cauda epididymal sperm counts were elevated by HNG and suppressed by CP. HNG rescued the CP-induced suppression of spermatogonial stem cells, sperm count and peripheral leucocytes. We conclude that HNG 1) protects CP-induced loss of male germ cells and leucocytes, 2) enhances CP-induced suppression of cancer metastases, and 3) acts as a caloric-restriction mimetic by suppressing IGF-1 levels. Our findings suggest that humanin analogues may be promising adjuvants to chemotherapy.
Onco-infertility and leucopenia are common adverse events in cancer patients after chemotherapy (1). Although the development of targeted cancer therapy has in some instances decreased adverse effects on the host, chemotherapy remains as a backbone of combination therapy for many cancers. Unfortunately, cancer survivors may suffer medical chemotherapy induced chronic complications after cure of the cancer. Infertility may result after many chemotherapy drugs. Preservation of fertility after successful chemotherapy treatment is important for cancer survivors in the reproductive age group (2–4). Chemotherapy also frequently causes leucopenia during treatment that increases susceptibility to bacterial and fungal infections leading to potentially life-threatening sepsis and delays in completing courses of chemotherapy (5). Leucopenia compromises the administration of adequate chemotherapy to cure cancer or efficiently suppress tumor growth. Although current therapy for leucopenia includes precautions and empiric treatment with antibiotics and/or granulocyte (macrophage) colony stimulating factor (6), there are no protectants available for onco-infertility.
We have focused on strategies to preserve male germ cells and prevent irreversible infertility from chemotherapy-induce toxicity. In our search for cytoprotective agents for fertility preservation against chemotherapy, we reported that humanin was able to protect male germ cells from apoptosis in response to various stresses (7–9), including chemotherapeutic agents in healthy rodents (10, 11). Humanin is a 24-amino acid micropeptide encoded by an open-reading frame from the mitochondrial 16S rRNA region (12–14), and is a functional circulating molecule in the blood stream (15). The extracellular actions of humanin are largely mediated through its cell membrane receptors (16, 17). Humanin has potent neuroprotective (18), antiapoptotic (19), and IGF binding protein-3 (IGFBP-3)-binding (20) cytoprotective effects with broad-spectrum actions on the brain, pancreas, heart, and testis (21, 22). Eriksson et al (23) recently showed that cotreatment of a potent humanin analog S14G-humanin (HNG) with a proteasome inhibitor bortezomib was effective in preventing bortezomib-induced bone growth impairment without interfering with bortezomib's anticancer effects in immunodeficient nude mice. Thus, HNG has been proposed as a protective agent against chemotherapy-induced side effects (24).
To investigate the benefits of HNG against chemotherapy-induced toxicity on germ cells and leukocytes in normal or tumor-bearing immunocompetent mice, and address the critical question of whether HNG may prevent the killing of cancer cells by chemotherapy, we studied the effects of HNG with or without a chemotherapeutic agent cyclophosphamide (CP) on cancer in mice. In this study, the mouse metastatic lung melanoma model was used as a prototype of a metastatic cancer. Inoculation of mouse melanoma cells induced lung metastases that could be easily quantified by counting melanoma nodules in the lung. This model was available and established in our institution (25). Though melanoma occurs mainly in older adults, data from older adolescents and adults showed that melanoma is the most common cancer for adults between 25 and 29 years and second commonest cancer in adolescent and young adults between 15 and 29 years (26). Thus, this mouse model of melanoma is relevant to adolescents and younger men where preservation of fertility is particularly important. We recognized that melanoma is presently treated with targeted immunotherapy even for advanced disease. We selected CP as a chemotherapeutic agent in this study because 1) CP is common example of an alkylating chemotherapeutic agent used in mouse studies, 2) the dose and time course of CP on suppression of spermatogenesis is very well defined (27, 28), 3) CP decreases peripheral leukocytes (29), and 4) CP treatment suppresses metastatic lung melanomas (30).
Our study showed that 1) HNG was cytoprotective in male germ cells and leukocytes exposed to the toxic effects of chemotherapy; 2) HNG by itself modestly suppressed the number of lung metastases, and enhanced the CP-induced cancer suppression; 3) HNG may act as a caloric-restriction mimetic by suppressing IGF-1 and increasing IGFBP-1 levels thus inhibiting the cancer growth. Coupling the prevention of chemotherapy-induced germ cell loss and reducing leucopenia, whereas enhancing chemotherapy efficacy renders humanin and its analogues promising adjuvants to cancer treatment.
Materials and Methods
Animals and reagents
Young adult (10-wk-old; body weight [BW], 24–28 g) male mice (C57BL/6J), purchased from The Jackson Laboratory, were housed in a standard animal facility under controlled temperature (22°C) and photoperiod (12 h light, 12 h dark) with free access to water and mouse chow. Animal handling and experimentation were in accordance with the recommendation of the American Veterinary Medical Association and were approved by the Los Angeles Biomedical Research Institute at Harbor-University of California-Los Angeles Medical Center Animal Care and Use Review Committee. CP monohydrate was obtained from Sigma and dissolved in saline at concentration of 20 mg/mL before use. Humanin analog HNG was synthesized by CPC Scientific and dissolved in saline at concentration of 1 or 2 mg/mL before use.
B16 mouse melanoma cell preparation and tail vein injection.
The B16 murine melanoma cell transfected with Luciferase gene driven by a cytomegalovirus promoter was a gift from Dr N. Craft and Dr K. Bruhn (Los Angeles Biomedical Research Institute) (25). Briefly, B16 cells were revived from frozen, cultured in DMEM (30–2002; ATCC) with 10% fetal bovine serum (Gibco, Life Technology Co) and penicillin-streptomycin in the 75-cm flask, and maintained in incubator supplied with 5% CO2 at 37°C. The second passage of B16 cells were harvested using 0.25% Trypsin-EDTA (Gibco, Life Technology Co), washed 3 times with PBS, passed 40-μm nylon mesh (BD Falcon), and resuspended in PBS (pH 7.4) at concentration of 1 × 106/mL; 200 mcl (2 × 105 B16 cells/mouse for experiment 1) or 100 mcl (1 × 105 B16 cells/mouse for experiment 2) of single cell suspension was iv injected through tail vein using 300-mcl insulin syringe with 29-gauge needle (Becton Dickinson).
Experimental protocol
Experiment 1. Determine the effects of HNG on male germ cell apoptosis and CP-induced suppression of lung metastasis melanoma in tumor-bearing mice
Forty-five young adult (12-wk-old) male mice (C57BL/6J) were grouped as control (n = 5); and 40 mice of each received intravenous tail vein inoculation with 2 × 105 B16 murine melanoma cells. After 1 week, 40 tumor-bearing mice were randomly divided into 4 groups with 10 mice in each group: 1) nontreated tumor-bearing (NT) mice, or mice treated with 2) HNG (5 mg/kg BW) daily ip injection (HNG), 3) a single CP (200 mg/kg BW) ip injection (CP), and 4) a single CP injection with daily HNG injection for 2 weeks (CP+HNG). All mice were killed 3 weeks after tumor inoculation and 2 weeks after HNG and/or CP treatment.
Experiment 2. Determine the effects of HNG on peripheral blood leukocytes, plasma IGF-1, and IGFBP-1 levels while confirming its additive or synergistic effects on CP-induced suppression of lung melanoma metastases in tumor-bearing mice
In the initial study (experiment 1) we inoculated 2 × 105 B16 cells per mouse. We showed that pulmonary melanoma formation in NT mice were prominent that led to the poor health at 14 days after tumor loading. Advised by our veterinarian and in line with the policy of animal welfare, we decreased the tumor loading dose to 1 × 105 B16 cells per mouse in experiment 2, to allow us to examine the effects of HNG on peripheral blood leukocytes, plasma IGF-1 and IGFBP-1 levels while confirming its additive or synergistic effects on CP-induced suppression of lung melanoma metastases. Twenty-five young adult (12-wk-old) male mice (C57BL/6J) were used for this experiment in which 5 mice were used as control and 20 mice of each were iv inoculated via the tail vein with 1 × 105 murine B16 melanoma cells. Twenty tumor-bearing mice were randomly divided into 4 groups with 5 mice in each group received 1) no treatment, 2) HNG alone, 3) CP alone, and 4) their combination treatment with same doses and experimental duration as described in experiment 1.
Experiment 3. Determine the effects of HNG on spermatogonial stem cells (SSCs), sperm output, and peripheral blood leukocytes in response to the repeated doses of CP treatment in normal mice
Thirty young adult (12-wk-old) male mice (C57BL/6J) were randomized into 4 groups: 1) 5 as control, 2) 5 received daily sc injection of HNG (10 mg/kg BW), 3) 10 were given 6 doses of CP (150 mg/kg BW) ip at 5-day intervals, and 4) 10 received both HNG and CP. All mice were killed at the end of 4 weeks of treatment.
Blood collection and tissue preparation
Mice were injected with heparin (1300 IU/kg BW, ip) 15 minutes before being killed by a lethal injection of sodium pentobarbital (200 mg/kg BW ip). BW was recorded at autopsy. Blood samples were collected from the right ventricle of each mouse immediately after death and used for complete blood count using an automated cell counter (VetScanHM2; ABAXIS). Plasma was separated and stored at −20°C for subsequent HNG, IGF-1, and IGFBP-1 measurements by specific and sensitive ELISA assays as previously described (15, 31). The humanin ELISA was used to measure plasma HNG levels because the antibody used in the assay cross-reacted completely with HNG. One testis from each mouse was removed, weighed, and snap frozen into liquid nitrogen. The contralateral testes of 5 mice from each group in experiment 1 were then fixed by vascular perfusion 5% glutaraldehyde in 0.05M cacodylate buffer (pH 7.4) for 30 minutes, preceded by a brief saline wash (7). The 5 controls and the remaining 5 mice from each group in experiment 1 were perfused with 10% buffered formalin (Newcomer Supply). The contralateral testes from mice in experiment 2 and 3 were immersed fixed into Bouin's solution (Newcomer Supply). One slice from the middle region of fixed testis was processed for routine paraffin embedding for hematoxylin and eosin staining, immunohistochemistry and/or terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick-end labeling assay. In the experiment 1 and 2, the lungs from each mouse were dissected out and postfixed into either 5% glutaraldehyde or 10% buffered formalin (Newcomer Supply) and used for tumor quantification, imaging, and pathological examination. The quantification of B16 melanoma nodules was performed by counting the number of tumor nodules on the surface of full lungs under LEICA MZ6 stereomicroscope (Leica Microsystem). The tumor size was measured on the lung imaging using a computer software program (Image-pro Plus 6.0; Media Cybernetics, Inc). A slice of the lung from each mouse was paraffin embedded and processed for sections. The hematoxylin and eosin stained sections were used for pathologic examination by our experienced pathologist (S.F.). In the experiment 3, the testicular histology was examined and spermatogenesis was estimated by counting the number of cross-sections of seminiferous tubules with various numbers of spermatogonia, spermatocytes, round or elongated spermatids, and shown as the percentage of the cross-sections containing germ cells per total cross-sections of seminiferous tubules. The SSCs were identified by Forkhead box protein O1 (FoxO1) immunohistochemistry (32) and quantified by counting FoxO1-positive spermatogonia in about 100 (average 109) cross-sections of seminiferous tubules per mouse. The number of SSCs (FoxO1-positive spermatogonia) per 100 seminiferous tubules was compared among the treatment groups. The cauda epididymal sperm count from each mouse in experiment 3 was performed as described previously (33).
Assessment of apoptosis
In situ detection of cells with DNA strand breaks was performed in paraffin-embedded testicular sections by the terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick-end labeling technique using an Apop Tag-peroxidase kit (Millipore) as described earlier (33). The rate of germ cell apoptosis was quantified from 5 mice per groups and expressed as apoptotic index (AI), which is the number of apoptotic germ cells per 100 cross-sections of seminiferous tubules on testicular sections as previously described (34).
Immunohistochemical analysis
FoxO1 localization in mouse testes was assessed by immunohistochemistry as FoxO1 has been shown to be a SSC-specific marker in testis (32). Briefly, Bouin-fixed and paraffin-embedded testicular sections were deparaffinized and rehydrated, and antigen retrieval was performed in sodium citrate buffer (pH 6.0) under microwave. Testicular sections were first incubated with a rabbit monoclonal anti-FoxO1-specific antibody (Cell Signaling Technology) at a concentration of 1:50 dilution at 4°C overnight (see Table 1). Immunoreactivity was detected using biotinylated antirabbit IgG secondary antibody followed by avidin-biotinylated horseradish peroxidase complex visualized with diaminobenzidine tetrahydrochloride (Imm PACTTM DAB) per the manufacturer's instructions (Rabbit VECTASTAIN ABC kit). Slides were counterstained with hematoxylin and viewed with the Zeiss Axioskop 40 microscope (10).
Table 1.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used |
|---|---|---|---|---|---|
| FoxO1 | FoxO1 Rabbit mAb | Cell Signaling Technology, 2880 | Rabbit Mab | 1:50 |
Statistical analyses
The comparisons of parameters of interest determined a priori were focused on the effects of HNG+CP vs CP alone and HNG or CP vs NT mouse. Statistical analyses were carried out using the SigmaStat 12.0 Program (Systat Software, Inc). Results were tested for statistical significance using one-way ANOVA with post hoc Tukey test or Student's t test. Differences were considered significant if P < .05.
Results
Experiment 1. HNG protects male germ cell from apoptosis while enhancing CP-induced suppression of lung melanoma metastases
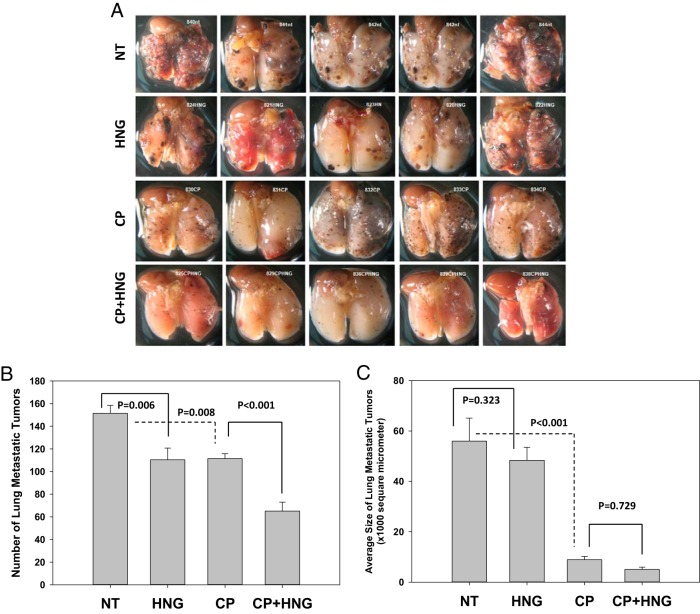
There were no changes in BW, but compared with NT (93.8 ± 4.28 mg) and HNG alone-treated (98.6 ± 0.93 mg) mice, testis weight was decreased by both CP (72.4 ± 2.99 mg) and HNG+CP (73.6 ± 2.73 mg) treatment. HNG by itself had no effect on the germ cell apoptosis (AI, 28.48 ± 4.31) as compared with NT mice (AI, 38.58 ± 3.25). As expected, CP treatment significantly (P < .043) increased germ cell apoptosis (AI, 61.79 ± 10.21) in comparison with NT and HNG-treated mice. HNG daily treatment for 2 weeks attenuated CP-induced germ cell apoptosis (AI, 24.92 ± 2.28) as compared with CP treatment alone (P < .001). HNG by itself significantly decreased the number of metastatic tumors in the lung (P = .006). CP treatment not only decreased number of tumors (P = .008) but also tumor size as compared with NT mice (Figure 1A). Importantly, combined HNG and CP treatment significantly (P < .001) decreased number of tumors compared with CP treatment alone (Figure 1B). Both CP alone, and CP+HNG treatment decreased tumor size as compared with NT and HNG-treated mice (P < .001) (Figure 1C). The pathologic examination reported that the lung metastatic melanoma appeared to be more differentiated after CP and HNG+CP treatment as compared with NT and HNG alone-treated mice. The differentiated melanoma cells in lungs were characterized as enlarged cells with heterochromatic nuclei and increased melanin granules in the cytoplasm as compared with a small undifferentiated melanoma cells with euchromatic nuclei and little cytoplasm.
Figure 1.
Representative photographs of glutaraldehyde-fixed lungs with metastatic melanomas from the NT, HNG-treated alone (HNG), CP alone (CP), and combined CP with HNG-treated (CP+HNG) mice inoculated with 2 × 105 B16 cells per mouse in experiment 1 (n = 10 mice per group) (A). The bar graph shows the number (B) and the size (C) of lung metastatic tumors. Values are the mean ± SEM. CP = cyclophosphamide; HNG = S14G-humanin
Experiment 2. HNG suppresses plasma IGF-1 and increases IGFBP-1 levels, and significantly rescues the CP-induced suppression of peripheral leukocytes while enhancing CP-induced tumor suppression
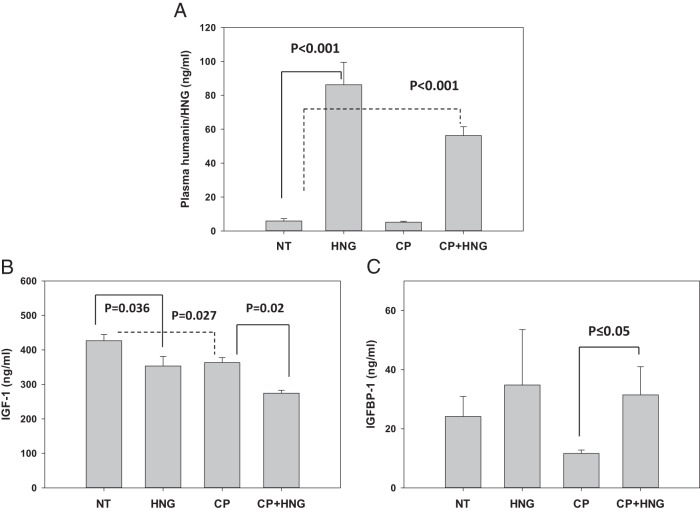
Administration of HNG increased plasma HNG levels (P < .001) in both groups treated with exogenous HNG (HNG and HNG+CP groups) as compared with NT and CP alone group (Figure 2A). HNG (P = .036) or CP (P = .027) treatment alone significantly suppressed plasma IGF-1 levels. Addition of HNG to CP further suppressed IGF-1 (P = .02) levels compared with CP alone (Figure 2B). Cotreatment of HNG with CP increases plasma IGFBP-1 levels when compared with CP alone (P ≤ .05) (Figure 2C).
Figure 2.
Plasma humanin/HNG (A), IGF-1 (B), and IGFBP-1 (C) levels in the NT, HNG-treated alone (HNG), CP alone (CP), and combined CP with HNG-treated (CP+HNG) mice in experiment 2 (n = 5 mice per group). Values are mean ± SEM.
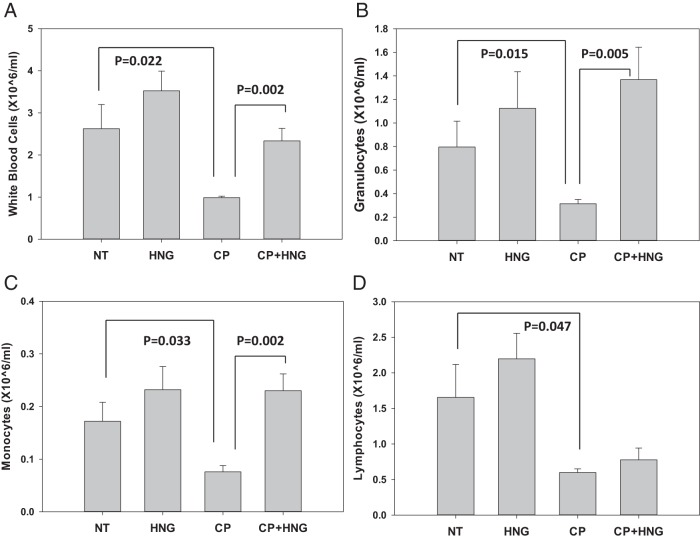
CP treatment significantly suppressed the number of white blood cells (WBCs) (P = .022) (Figure 3A), granulocytes (P = .015) (Figure 3B), monocytes (P = .033) (Figure 3C), and lymphocytes (P = .047) (Figure 3D) compared with NT mice. Importantly, addition of HNG to CP significantly increased WBCs (P = .002) (Figure 3A), granulocytes (P = .005) (Figure 3B), and monocytes (P = .002) (Figure 3C) compared with CP treatment alone, restoring these cell counts to the levels observed in NT mice. HNG+CP did not significantly rescue the decrease in lymphocytes induced by a single dose of CP (Figure 3D). There were no significant decreases in red blood cell or platelet counts in the groups treated with CP and HNG+CP groups (data not shown). Similar to experiment 1, treatment with HNG or CP significantly decreased (P < .001) the number of metastatic tumors in the lungs as compared with NT mice, addition of HNG to CP treatment further decreased number of tumors (P = .013) compared with CP treatment alone (Supplemental Figure 1). Even though the results are similar, the data from experiment 2 is confirmative and provided the experimental conditions to allow us to perform longer term survival studies without compromising of our study design when the mice are sick.
Figure 3.
Peripheral blood cell counts of WBC (A), granulocytes (B), monocytes (C), and lymphocytes (D) in the NT, HNG-treated alone (HNG), CP alone (CP), and combined CP with HNG-treated (CP+HNG) mice in experiment 2 (n = 5 mice per group). Values are mean ± SEM.
Experiment 3. HNG rescues CP-induced suppression of SSCs, sperm output, and peripheral leukocytes after repeated doses of CP in normal mice
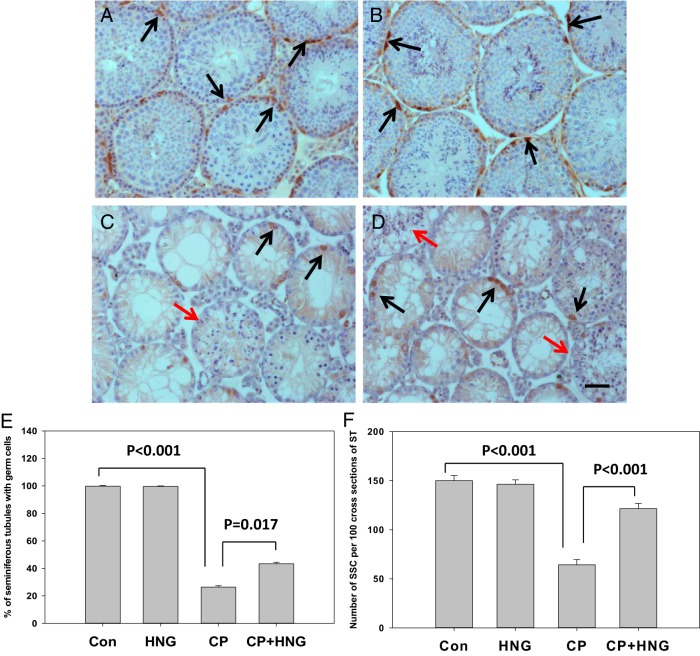
HNG was effective in counteracting the action of CP even after repeated doses of CP were administered. The results from experiment 3 showed that in comparison with control, plasma IGF-1 levels were significantly (P < .001) suppressed by HNG or CP, respectively, and further suppressed by CP+HNG treatment. HNG treatment alone had no effects on plasma IGFBP-1 levels. HNG significantly increased plasma IGFBP-1 levels suppressed by CP alone treatment (Supplemental Table 1). Repeated dose of CP treatment significantly (P < .001) decreased the number of leukocytes, granulocytes, monocytes, and lymphocytes as compared with control. Addition of HNG to CP significantly (P < .05) rescued the CP-induced suppression of WBC, granulocytes, monocytes, and lymphocytes (Supplemental Table 2). Repeated dose of CP treatment significantly (P < .001) decreased the number of seminiferous tubules containing germ cells (Figure 4C) as compared with either control or HNG treatment alone (Figure 4, A and B). Cotreatment of HNG with CP significantly (P = .017) increased the number of seminiferous tubule with germ cells as compared with CP alone treatment (Figure 4, D and E). The residual germ cells after repeated doses of CP treatment included the various numbers of spermatogonia, spermatocytes, round or elongated spermatids in different cross-sections of seminiferous tubules. Immunohistochemistry showed FoxO1-positive SSCs in testes from control, HNG alone, CP alone, and their combination (Figure 4, A–D). Quantification of SSCs (FoxO1-positive spermatogonia) showed that HNG treatment alone has no effect on the number of SSCs (Figure 4B); repeated CP treatment alone significantly (P < .001) decreased the number of SSC (Figure 4C) as compared with control; HNG significantly (P < .001) rescued CP-induced suppression of SSCs (Figure 4, D and F). Cauda epididymal sperm counts were significantly elevated by HNG (P = .039), and suppressed by CP (P < .001) as compared with control. HNG+CP significantly increased cauda epididymal sperm count (P = .02) as compared with repeated CP treatment in mice (Figure 5).
Figure 4.
Representative immunohistochemistry micrographs of mouse testis sections from control (n = 5) (A), HNG daily sc injection (n = 5) (B), 6 repeated dose of CP treatment (n = 10) (C), and CP+HNG-treated mice (n = 10) (D); scale bar, 0.05 mm. The cross-sections of seminiferous tubules containing elongated spermatids (red arrows) were noted both in CP (C) and CP+HNG (D)-treated mice. The FoxO1-positive SSCs (dark brown in color, black arrows) were observed in testes from all groups. The bar graph (E) shows the percentage of seminiferous tubules containing germ cells in control, HNG-treated alone, repeated CP alone, and combined CP with HNG-treated (CP+HNG, n = 10) mice. The bar graph (F) shows the number of SSCs (FoxO1-positive spermatogonia) per 100 cross-sections of seminiferous tubules from control (Con), HNG-treated alone, repeated CP alone, and combined CP with HNG-treated (CP+HNG) mice. Values are mean ± SEM.
Figure 5.
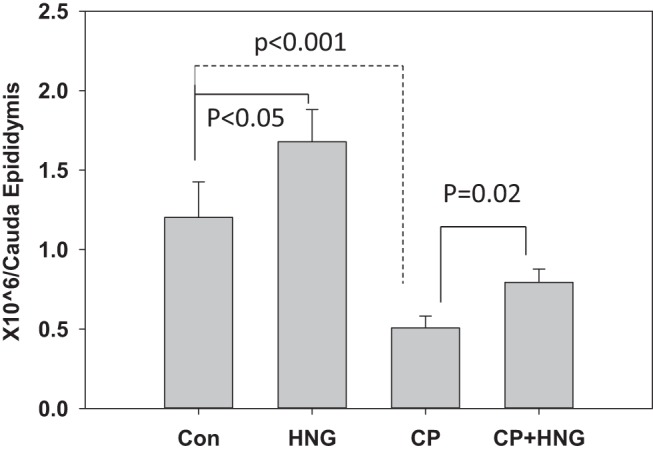
Cauda epididymal sperm count (million/cauda) in control (Con) (n = 5), HNG-treated alone (HNG) (n = 5), CP alone (CP) (n = 10), and combined CP with HNG-treated (CP+HNG) (n = 10) mice. Values are mean ± SEM.
Discussion
Recent advances in the understanding of cancer cell biology and multiple modality treatment have led to dramatically improved cancer survival (35). To improve the quality of life in cancer survivors, there is an increasing need for cytoprotective agents that reduce the treatment-related toxicity for normal tissues without affecting treatment efficacy on cancers. Most chemotherapeutic agents cause acute adverse effects on bone marrow suppression and chronic adverse effects, including subfertility/infertility, growth defects in children, and cognitive dysfunction in long term cancer survivors (36). Recent reports describe the new role of the mitochondrial peptide humanin in protecting against chemotherapy-induced adverse effects on bone growth and germ cell apoptosis (10, 11, 23). A recent report suggests that HNG by itself might have anticancer effects (23). We investigated in this study whether a potent humanin analog HNG will protect against chemotherapy-induced toxicity while exerting additive or synergistic effects on chemotherapy-induced metastatic tumor suppression.
We have previously demonstrated that a single ip injection of either humanin or its potent analog HNG attenuated CP-induced male germ cell apoptosis within 12 hours in healthy rats (10), and within 24 hours in healthy mice (11). HNG was able to protect against CP-induced spermatogonia, spermatocytes and round spermatids apoptosis in rodents (10). In this study, we showed that daily ip injection of HNG for 2 weeks significantly reduced CP-induced male germ cell apoptosis in metastatic tumor-bearing mice. Moreover, this effect is sustained with repeated dosing of CP, because we demonstrated that HNG daily sc injection for 4 weeks significantly increased the number of SSCs and cauda epididymal sperm count in mice with repeated doses of CP treatment. We provided the first evidence that coadministration of HNG with CP significantly attenuated the CP-induced suppression of the number of total WBCs, granulocytes and monocytes in healthy and metastatic tumor-bearing mice while enhancing the therapeutic effect of CP on tumor, respectively. The protective actions of humanin against stress-induced apoptosis of many cells initially led to the concerns that it might promote tumor growth. We found in this study that HNG by itself suppressed the number of metastatic lung melanoma. Moreover, we demonstrated that HNG enhanced the suppressive effects of CP on the number of metastatic lung melanomas as compared with CP treatment alone. Eriksson et al showed that HNG did not interfere with the ability of a proteasome inhibitor, bortesomib, to suppress neuroblastoma and medulloblastoma cell lines and xenografts in nude mice (23). Our results showed that HNG had synergistic/additive action with CP on metastatic melanoma. Therefore, our novel results in metastatic lung tumors as a model for cancer paves the way to the development of humanin analogues as an adjuvant therapy to enhance chemotherapy-induced tumor suppression while protecting against chemotherapy-induced adverse effects.
The mechanisms of action of humanin and its analogues on enhancing chemotherapy-induced tumor suppressive effects while simultaneously protecting normal cells and tissues against chemotherapy-induced adverse effects are divergent and suggest different signaling pathways. HNG is a 24-amino acid synthetic and potent analog of mitochondrial derived micropeptide humanin with the substitution of Gly for Ser14 in the humanin sequence (37). Humanin has cytoprotective, antiinflammatory, antioxidative properties, that may involve a systemic normalization of stress-response homeostasis in many cell types (38, 39). The cytoprotective effects of humanin on normal cells are mediated through at least 2 receptors, one of which is the immuno-modulating protein-coupled formyl peptide receptor-like 1 (16), and the other is the IL-like heterotrimeric cell membrane receptor composed of a CNTFRα/gp130/WSX-1 (17). Upon receptor binding, humanin activates Signal Transducer and Activator of Transcription 3 intracellular signaling pathways to promote cell survival (40). Humanin also binds IGFBP-3 and modulates IGF bioactivity to regulate cell growth, survival, and apoptosis (20). In addition, within cells humanin binds to Bcl-2-associated X protein and BH3 domain proteins preventing their entry to the mitochondria inhibiting initiation of apoptosis induced by stress (19, 41, 42). Our previous studies on humanin indicates that the protective effect of humanin against stress-induced (including chemotherapy) germ cell apoptosis is through interaction with the putative membrane receptors enhancing STAT-3 signaling and decrease p38 MAPK as well as through sequestration of Bcl-2-associated X protein in the cytoplasm and preventing its entry to the mitochondria to initiate the apoptosis cascade (9, 11).
The mechanisms of the novel finding of divergent but beneficial effects of HNG on cancer-bearing animals are intriguing. This phenomenon is reminiscent of the concept of differential protection, which was first proposed by Longo and coworkers using fasting and caloric-restriction regimens in rodent aging and cancer models (43). They demonstrated that fasting selectively protected normal cells and organs, but not cancer cells against oxidants and chemotherapeutic agents (44). There are many possible mechanisms of action of HNG on cancers. For example, binding of Insulin-like growth factor 1 (IGF-1) to its receptor (IGF1-R) activates the IGF-1 signaling pathway through Akt/mTOR promoting cancer growth (45, 46). Studies have shown that circulating humanin has an inverse relationship with IGF-1 and GH in mouse models and in men. Humanin levels are high in GH and IGF-1 liver-specific knockout mice and in GH receptor-deficient children. On the other hand humanin levels are suppressed in transgenic mice overexpressing GH and in children after GH treatment (47). We showed in this study that HNG not only inhibited plasma IGF-1 levels but also enhanced the CP-induced suppression of IGF-1 in tumor-bearing mice. The decrease of IGF-1 by HNG may inhibit IGF-1 bio-availability to its receptor leading to the suppression of melanoma growth (48). It is worth noting that strategies using blocking antibodies against IGF-1 receptor were not successful because of lack of efficacy and increased side effects; these were likely related to the increase in GH levels with IGF-1 receptor blockade which is not induced by fasting or humanin treatment (45, 49). Thus, the HNG-induced suppression of IGF-1 may play a role in the protection of normal cells and suppression of cancer cells.
Caloric restriction in animal models and men are associated with longevity and reduced inflammation, oxidative stress and risks of developing many types of cancer. High humanin levels are associated with longevity mimicking the effects of caloric restriction on lifespan (12, 47). Short term fasting in mice lowers IGF-1 and increases IGFBP-1 levels protecting against chemotherapy-induced cytotoxicity (50). HNG may influence tumor growth by altering nutrient levels, because humanin analogues have been proposed as a potential agent for treating patients with diabetes (38, 39). By regulating the metabolism of the host, HNG may sensitize the cancer cell making them more responsive to chemotherapy. HNG-induced suppression of IGF-1 mimicked the homeostatic conditions created by caloric restriction or fasting in the host suggesting that HNG may be a caloric-restriction mimetic with a promising therapeutic potential for patients with cancer. The laboratory is currently pursuing the studies of humanin action on body and tumor metabolism.
Because spermatogenesis and hematopoiesis are both active cell proliferating processes physiologically, we anticipate there would be some cytoprotective effects of HNG on leukocytes. We demonstrated that HNG rescued the CP-induced suppression of granulocytes and monocytes in both healthy and tumor-bearing mice. In tumor-bearing mice, HNG did not rescue lymphocytes suppressed by a single dose of CP injection. In contrast, HNG induced very small but significant protection on lymphocytes suppressed by the repeated dose of CP treatment in healthy mice. The differential cytoprotective effects of HNG on granulocytes and monocytes vs lymphocytes are intriguing. Myeloid progenitor cells produce granulocytes, monocytes, erythrocytes and megakaryocytes, and lymphoid produce NK cells, T and B lymphocytes in red bone marrow. The differential effect of HNG on protecting CP-induced loss of granulocytes and monocytes but less lymphocytes may be due to HNG having more cytoprotective action on myeloid progenitor than lymphoid lineage. The impact of CP on mature circulating lymphocytes that are not actively replicating is not well understood. CP may have direct toxic effect on the lymphocytes or it may affect the change in circulation of lymphocytes in lymph rather than blood. Because the action of CP on mature lymphocytes is not understood, the reason for the less cytoprotection of HNG on CP-induced decrease in lymphocyte count is not clear.
In summary, we demonstrated in this study that 1) HNG was cytoprotective in chemotherapy-induced SSCs, sperm output, and leukocytes in healthy and tumor-bearing mice; 2) HNG alone modestly suppressed the number of aggressive metastatic tumors, and additively/synergistically enhanced the chemotherapy-induced tumor suppression; and 3) HNG may act as a caloric-restriction mimetic by suppressing IGF-1 and increasing IGFBP-1 levels to protect normal cells against stress and suppress cancer. Thus, our findings suggest that humanin analogues are promising adjuvants to chemotherapy by reducing chemotherapy-induced leucopenia and low sperm count while enhancing CP-induced tumor suppression. In this study, our results lead us to speculate that the significant protection of HNG on SSCs against chemotherapy would result in the effective and efficient spermatogenesis recovery after cessation of chemotherapy. However, the protective effects of HNG on the genome stability of spermatozoa and the mutation rates in the offspring of CP-treated mice remain to be determined. We also speculate that humanin will protect other tissues (eg, heart and brain) subject to chemotherapy-induced damage.
Acknowledgments
Data from this manuscript was presented partially in abstract form at 16th International Congress of Endocrinology and 96th Annual Meeting of The Endocrine Society, Chicago, IL, 2014; 97th Annual Meeting of The Endocrine Society, San Diego, CA, 2015; and 40th American Society of Andrology, Salt Lake City, UT, 2015.
This work was supported by the UCLA Clinical and Translational Science Institute Grant UL1TR000124 (to Los Angeles Biomedical Research Institute and Harbor-UCLA Medical Center) and National Institutes of Health Grants R01AG034430, R01GM090311, and R01ES020812 (to P.C.).
Disclosure Summary: P.C. owns stock in CohBar, Inc. All other authors have nothing to disclose.
Footnotes
- AI
- apoptotic index
- BW
- body weight
- CP
- cyclophosphamide
- HNG
- S14G-humanin
- FoxO1
- Forkhead box protein O1
- IGFBP-3
- IGF binding protein-3
- NT
- nontreated tumor bearing
- SSC
- spermatogonial stem cell
- WBC
- white blood cell.
References
- 1. Armenian SH, Landier W, Hudson MM, Robison LL, Bhatia S. Children's Oncology Group's 2013 blueprint for research: survivorship and outcomes. Pediatr Blood Cancer. 2013;60(6):1063–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Loren AW, Mangu PB, Beck LN, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31(19):2500–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dohle GR. Male infertility in cancer patients: review of the literature. Int J Urol. 2010;17:327–331. [DOI] [PubMed] [Google Scholar]
- 4. Trost LW, Brannigan RE. Oncofertility and the male cancer patient. Curr Treat Options Oncol. 2012;13:146–160. [DOI] [PubMed] [Google Scholar]
- 5. Culakova E, Thota R, Poniewierski MS, et al. Patterns of chemotherapy-associated toxicity and supportive care in US oncology practice: a nationawide prospective cohort study. Cancer Med. 2014;3:434–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Crawford J, Ozer H, Stoller R, et al. Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. N Engl J Med. 1991;325:164–170. [DOI] [PubMed] [Google Scholar]
- 7. Lue Y, Swerdloff R, Liu Q, et al. Opposing roles of insulin-like growth factor binding protein 3 and humanin in the regulation of testicular germ cell apoptosis. Endocrinology. 2010;151:350–357. [DOI] [PubMed] [Google Scholar]
- 8. Jia Y, Lee KW, Swerdloff R, et al. Interaction of insulin-like growth factor-binding protein-3 and BAX in mitochondria promotes male germ cell apoptosis. J Biol Chem. 2010;285:1726–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jia Y, Lue YH, Swerdloff R, et al. The cytoprotective peptide humanin is induced and neutralizes Bax after pro-apoptotic stress in the rat testis. Andrology. 2013;1:651–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Surampudi P, Chang I, Lue Y, et al. The mitochondrial peptide humanin protects against chemotherapy-induced male germ cell apoptosis in rats. Andrology. 2015,3:582–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jia Y, Ohanyan A, Lue Y, et al. The Effects of Humanin and its analogues on male germ cell apoptosis induced by chemotherapeutic drugs. Apoptosis. 2015;20:551–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee C, Yen K, Cohen P. Humanin: a harbinger of mitochondrial-derived peptides? Trends Endocrinol Metab. 2013;24:222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yen K, Lee C, Mehta H, Cohen P. The emerging role of the mitochondrial-derived peptide humanin in stress resistance. J Mol Endocrinol. 2013;50:R11–R19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arakawa T, Hirano A, Shiraki K, Niikura T, Kita Y. Advances in characterization of neuroprotective peptide, humanin. Curr Med Chem. 2011;18:5554–5563. [DOI] [PubMed] [Google Scholar]
- 15. Chin YP, Keni J, Wan J, et al. Pharmacokinetics and tissue distribution of humanin and its analogues in male rodents. Endocrinology. 2013;154:3739–3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ying G, Iribarren P, Zhou Y, et al. Humanin, a newly identified neuroprotective factor, uses the G protein-coupled formylpeptide receptor-like-1 as a functional receptor. J Immunol. 2004;172:7078–7085. [DOI] [PubMed] [Google Scholar]
- 17. Hashimoto Y, Kurita M, Matsuoka M. Identification of soluble WSX-1 not as a dominant-negative but as an alternative functional subunit of a receptor for an anti-Alzheimer's disease rescue factor Humanin. Biochem Biophys Res Commun. 2009;389:95–99. [DOI] [PubMed] [Google Scholar]
- 18. Hashimoto Y, Niikura T, Tajima H, et al. A rescue factor abolishing neuronal cell death by a wide spectrum of familial Alzheimer's disease genes and Abeta. Proc Natl Acad Sci USA. 2001;98:6336–6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guo B, Zhai D, Cabezas E, et al. Humanin peptide suppresses apoptosis by interfering with Bax activation. Nature. 2003;423:456–461. [DOI] [PubMed] [Google Scholar]
- 20. Ikonen M, Liu B, Hashimoto Y, et al. Interaction between the Alzheimer's survival peptide humanin and insulin-like growth factor-binding protein 3 regulates cell survival and apoptosis. Proc Natl Acad Sci USA. 2003;100:13042–13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gong Z, Tas E, Muzumdar R. Humanin and age-related diseases: a new link? Front Endocrinol (Lausanne). 2014;5:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Colón E, Strand ML, Carlsson-Skwirut C, et al. Anti-apoptotic factor humanin is expressed in the testis and prevents cell-death in leydig cells during the first wave of spermatogenesis. J Cell Physiol. 2006;208:373–385. [DOI] [PubMed] [Google Scholar]
- 23. Eriksson E, Wickstrom M, Perup LS, et al. Protective role of humanin on bortezomib-induced bone growth impairment in anticancer treatment. J Natl Cancer Inst. 2014;106:djt459. [DOI] [PubMed] [Google Scholar]
- 24. Cohen P. New role for the mitochondrial peptide humanin: protective agent against chemotherapy-induced side effects. J Natl Cancer Inst. 2014;106(3):dju006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Craft N, Bruhn KW, Nguyen BD, et al. Bioluminescent imaging of melanoma in live mice. J Invest Dermatol. 2005;125:159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bleyer A, O'Leary M, Barr R, Ries LAG, eds. Cancer Epidemiology in Older Adolescents and Young Adults 15 to 29 Years of Age, Including SEER Incidence and Survival: 1975–2000. Bethesda, MD: National Cancer Institute, NIH Publication Number 06–5767; 2006. [Google Scholar]
- 27. Drumond AL, Weng CC, Wang G, Chiarini-Garcia H, Eras-Garcia L, Meistrich ML. Effects of multiple doses of cyclophosphamide on mouse testes: accessing the germ cells lost, and the functional damage of stem cells. Reprod Toxicol. 2011;32:395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meistrich ML, Finch M, da Cunha MF, Hacker U, Au WW. Damaging effects of fourteen chemotherapeutic drugs on mouse testis cells. Cancer Res. 1982;42:122–131. [PubMed] [Google Scholar]
- 29. Huyan XH, Lin YP, Gao T, Chen RY, Fan YM. Immunosuppressive effect of Cyclophosphamide on white blood cells and lymphocyte subpopulations from peripheral blood of Balb/c mice. Int Immunopharmacol. 2011;11:1293–1297. [DOI] [PubMed] [Google Scholar]
- 30. D'Agostini C, Pica F, Febbraro G, Grelli S, Chiavaroli C, Garaci E. Antitumour effect of OM-174 and cyclophosphamide on murine B16 melanoma in different experimental conditions. Int Immunopharmacol. 2005;5:1205–1212. [DOI] [PubMed] [Google Scholar]
- 31. Hwang DL, Lee PD, Cohen P. Quantitative ontogeny of murine insulin-like growth factor (IGF)-I, IGF-binding protein-3 and the IGF-related acid-labile subunit. Growth Horm IGF Res. 2008;18:65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goertz MJ, Wu Z, Gallardo TD, Hamra FK, Castrillon DH. Foxo1 is required in mouse spermatogonial stem cells for their maintenance and the initiation of spermatogenesis. J Clin Invest. 2011;121:3456–3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lue Y, Wang C, Lydon JP, Leung A, Li J, Swerdloff RS. Functional role of progestin and the progesterone receptor in the suppression of spermatogenesis in rodents. Andrology. 2013;1:308–317. [DOI] [PubMed] [Google Scholar]
- 34. Cai L, Hales BF, Robaire B. Induction of apoptosis in the germ cells of adult male rats after exposure to cyclophosphamide. Biol Reprod. 1997;56(6):1490–1497. [DOI] [PubMed] [Google Scholar]
- 35. Valdivieso M, Kujawa AM, Jones T, Baker LH. Cancer survivors in the United States: a review of the literature and a call to action. Int J Med Sci. 2012;9(2):163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kopp LM, Gupta P, Pelayo-Katsanis L, Wittman B, Katsanis E. Late effects in adult survivors of pediatric cancer: a guide for the primary care physician. Am J Med. 2012;125(7):636–641. [DOI] [PubMed] [Google Scholar]
- 37. Zapała B, Kaczyski Ł, Kie-Wilk B, et al. Humanins, the neuroprotective and cytoprotective peptides with antiapoptotic and anti-inflammatory properties. Pharmacol Rep. 2010;62(5):767–777. [DOI] [PubMed] [Google Scholar]
- 38. Muzumdar RH, Huffman DM, Atzmon G, et al. Humanin: a novel central regulator of peripheral insulin action. PLoS One. 2009;4:e6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hoang PT, Park P, Cobb LJ, et al. The neurosurvival factor Humanin inhibits β-cell apoptosis via signal transducer and activator of transcription 3 activation and delays and ameliorates diabetes in nonobese diabetic mice. Metabolism. 2010;59:343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hashimoto Y, Suzuki H, Aiso S, Niikura T, Nishimoto I, Matsuoka M. Involvement of tyrosine kinases and STAT3 in Humanin-mediated neuroprotection. Life Sci. 2005;77(24):3092–3104. [DOI] [PubMed] [Google Scholar]
- 41. Zhai D, Luciano F, Zhu X, Guo B, Satterthwait AC, Reed JC. Humanin binds and nullifies Bid activity by blocking its activation of Bax and Bak. J Biol Chem. 2005;280:15815–15824. [DOI] [PubMed] [Google Scholar]
- 42. Luciano F, Zhai D, Zhu X, et al. Cytoprotective peptide humanin binds and inhibits proapoptotic Bcl-2/Bax family protein BimEL. J Biol Chem. 2005;280:15825–15835. [DOI] [PubMed] [Google Scholar]
- 43. Raffaghello L, Lee C, Safdie FM, et al. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc Natl Acad Sci USA. 2008;105:8215–8220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lee C, Longo VD. Fasting vs dietary restriction in cellular protection and cancer treatment: from model organisms to patients. Oncogene. 2011;30:3305–3316. [DOI] [PubMed] [Google Scholar]
- 45. Heidegger I, Pircher A, Klocker H, Massoner P. Targeting the insulin-like growth factor network in cancer therapy. Cancer Biol Ther. 2011;11:701–707. [DOI] [PubMed] [Google Scholar]
- 46. Tognon CE, Sorensen PH. Targeting the insulin-like growth factor 1 receptor (IGF1R) signaling pathway for cancer therapy. Expert Opin Ther Targets. 2012;16:33–48. [DOI] [PubMed] [Google Scholar]
- 47. Lee C, Wan J, Miyazaki B, et al. IGF-I regulates the age-dependent signaling peptide humanin. Aging Cell. 2014;13(5):958–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Capoluongo E. Insulin-like growth factor system and sporadic malignant melanoma. Am J Pathol. 2011;178:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Arnaldez FI, Helman LJ. Targeting the insulin growth factor receptor 1. Hematol Oncol Clin North Am. 2012;26(3):527–542, vii–viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lee C, Safdie FM, Raffaghello L, et al. Reduced levels of IGF-I mediate differential protection of normal and cancer cells in response to fasting and improve chemotherapeutic index. Cancer Res. 2010;70:1564–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]