Abstract
There are clear epidemiological associations between circadian disruption, obesity, and pathogenesis of type 2 diabetes. The mechanisms driving these associations are unclear. In the current study, we hypothesized that continuous exposure to constant light (LL) compromises pancreatic β-cell functional and morphological adaption to diet-induced obesity leading to development of type 2 diabetes. To address this hypothesis, we studied wild type Sprague Dawley as well as Period-1 luciferase reporter transgenic rats (Per1-Luc) for 10 weeks under standard light-dark cycle (LD) or LL with concomitant ad libitum access to either standard chow or 60% high-fat diet (HFD). Exposure to HFD led to a comparable increase in food intake, body weight, and adiposity in both LD- and LL-treated rats. However, LL rats displayed profound loss of behavioral circadian rhythms as well as disrupted pancreatic islet clock function characterized by the impairment in the amplitude and the phase islet clock oscillations. Under LD cycle, HFD did not adversely alter diurnal glycemia, diurnal insulinemia, β-cell secretory function as well as β-cell survival, indicating successful adaptation to increased metabolic demand. In contrast, concomitant exposure to LL and HFD resulted in development of hyperglycemia characterized by loss of diurnal changes in insulin secretion, compromised β-cell function, and induction of β-cell apoptosis. This study suggests that circadian disruption and diet-induced obesity synergize to promote development of β-cell failure, likely mediated as a consequence of impaired islet clock function.
The incidence of type 2 diabetes (T2DM) has reached epidemic proportions raising the significance of understanding pathophysiology of this common disease. Pancreatic β-cell failure is a key feature of T2DM, characterized by compromised β-cell insulin secretory function and decreased survival (1, 2). Mechanisms underlying β-cell dysfunction are complex but are largely attributed to a decline in glucose-stimulated insulin secretion (3) as well as a loss of circadian control of insulin release (4, 5). Compromised β-cell survival in T2DM is associated with increased frequency of β-cell apoptosis attributed in part to induction of endoplasmic reticulum and oxidative stress (6).
Obesity is an important contributory factor to induction and progression of T2DM (7). This is largely attributed to obesity-mediated insulin resistance which places an increased metabolic demand on the β-cell to produce and secrete appropriate amounts of insulin to maintain normoglycemia (8, 9). Indeed, nondiabetic obese insulin resistant individuals remain normoglycemic due to compensatory increase in β-cell function (and mass) while retaining appropriate β-cell survival (10, 11). In contrast, β-cell failure and subsequent hyperglycemia occurs in a subset of individuals who fail to maintain adequate β-cell function and survival in lieu of increased metabolic demand associated with obesity (2, 12). Thus, understanding the molecular and physiological underpinnings of failed β-cell adaptation to obesity is critical for prevention and treatment of T2DM.
Circadian system plays an essential role in regulation of glucose homeostasis (13). Circadian control of physiological functions is a fundamental property of nearly all living organisms and provides physiological advantage by adapting internal metabolism to changes in the light-dark cycle (LD). The “master circadian clock” in mammals is localized in the suprachiasmatic nucleus (SCN) of the hypothalamus governed by a highly conserved set of core “clock” genes (molecular oscillators) regulated by a transcriptional-translational feedback loops (14). The SCN clock synchronizes molecular oscillators in peripheral tissues accomplished through combination of neuronal, behavioral and endocrine mechanisms (15). Robust circadian organization is particularly essential for pancreatic β-cells, given critical importance to restrain insulin production and secretion during the sleep phase (to prevent hypoglycemia), and activate insulin production and secretion during the active feeding phase (to avoid hyperglycemia) (16). Accordingly, β-cell molecular clocks have been shown to be essential for transcriptional regulation of insulin release, production, as well as control of β-cell survival and intracellular stress response (17–19). Consistent with these observations, disruption of circadian organization in humans promotes development of metabolic dysfunction and increases the propensity for development of T2DM (20–22). However, the mechanisms underlying increased susceptibility to T2DM in individuals exposed to circadian disruption remain to be elucidated.
Thus, in the current study, we hypothesized that circadian disruption accelerates development of T2DM by compromising normal β-cell functional and morphological adaptation to obesity-induced insulin resistance, mediated in part through loss of islet cell clock function. To address this hypothesis, we examined effects of biological interactions between circadian disruption (accomplished by prolonged exposure to constant light [LL]) and diet-induced obesity in vivo in rats by detailed assessment of 1) behavioral circadian rhythms, 2) islet circadian clock function, 3) glucose homeostasis, 4) islet function, and 5) islet turnover and survival.
Research Design and Methods
Study design
A total of 46 male Sprague Dawley rats and 19 male Wistar rats transgenic for the mouse Period-1 promoter linked to a luciferase reporter (Per1-LUC) were used in the current study. The generation and validation of Per1-LUC rats for the study of clock function have been previously described (19, 23). All rats were bred and housed individually before the study at the University of California Los Angeles animal housing facility and subjected to standard 12-hour light, 12-hour dark cycle (lights on at 6 am, lights of 6 pm, local time). Two weeks before initiation of study protocols, all rats were synchronized to standard living conditions with lights on at 6 am and lights of at 6 pm in a custom-made environmentally controlled soundproof chambers outfitted with the optical beam sensor system to monitor circadian behavioral rhythms in locomotor activity (Respironics). At 3 months of age, rats were randomly assigned into 4 experimental protocols for 10 weeks (Figure 1A): 1) LD-Chow; 10 weeks of standard LD cycle (lights on at 6 AM, lights off at 6 PM) on standard laboratory chow diet (14% fat, 32% protein, and 54% carbohydrates; Harlan Laboratories), 2) LD-HFD; 10 weeks of standard LD cycle on high fat diet (HFD) (60% fat, 20% protein, and 20% carbohydrates; Research Diets Inc), 3) LL-Chow; 10 weeks of 24-hour constant light exposure (25 watt fluorescent tubes 12″ above the cage at >100 Lux light intensity) on standard laboratory chow diet, and 4) LL-HFD; 10 weeks of 24-hour constant light exposure on HFD. The University of California Los Angeles Institutional Animal Care and Use Committee approved all experimental procedures described in the study.
Figure 1.
Induction of diet-induced obesity in rats exposed to LD or LL. A, A diagram of experimental design and timeline. Mean body mass gain (B), daily caloric intake (C), daily fat intake (D), and mass of epididymal fat depots (E) in rats exposed for 10 weeks to standard LD cycle on chow diet (LD-Chow, open bars; n = 11), 2) LD cycle on 60% HFD (LD-HFD, black bars; n = 15), 3) LL cycle on chow (LL-Chow, open striped bars; n = 8), and 4) LL cycle on HFD (LL-HFD, shaded striped bars; n = 15). Bar graphs represent mean ± SEM. *, P < .05 vs LD-Chow and LL-Chow; †, P < .05 vs LD-Chow and LL-Chow.
Circadian behavioral activity analyses
Rats exposed to the 4 experimental protocols (LD-Chow, LD-HFD, LL-Chow, and LL-HFD) were housed individually in standard rat cages equipped with infrared detectors, and 24-hour locomotor activity was recorded in 3-minute intervals. Circadian activity data were analyzed and plotted by using the normalized format in ClockLab software (Actimetrics, Inc). Data from last 14 days of each experimental protocol were used for final data analysis. To quantitatively assess circadian rhythmicity in locomotor activity, we used 2 independent methods (χ2 periodogram algorithm analysis and fast Fourier transform [FFT] analysis) (ClockLab, Actimetrics, Inc). In short, χ2 periodogram method estimates circadian periodicity by examining the data at different period lengths and provides quantification of variance of data at each time period relative to the defined confidence interval (99%) (24). FFT is an independent method that relies on fitting the activity data with multiple cosine functions with varying periods and amplitudes across the specified time series. The most significant (or dominant) periodicity in the data set is fit with the cosine function and recorded amplitude is used as a coefficient of “relative power” of circadian rhythmicity (25).
Islet Per1-LUC bioluminescence recording and data analysis
Per1-LUC rats were exposed to LD-Chow, LD-HFD, LL-Chow, and LL-HFD conditions for 10 weeks and subsequently euthanized. After euthanasia, pancreatic islets were isolated using standard collagenase method (performed always at 9 am local time which corresponds to Zeitgeber Time 3 in LD rats and projected Zeitgeber Time 3 in LL rats). Subsequently, 50 islets per animal were handpicked carefully matched by size and diameter (using an ocular measuring device) and plated onto polytetrafluoroethylene (PTFE) membranes (Millipore) in 35-mm culture dishes containing culture medium (serum-free, no sodium bicarbonate, no phenol red, DMEM [D5030-10L, Sigma-Aldrich]) supplemented with 10mM HEPES (pH 7.2), 2mM glutamine, B27 (2%; GIBCO), and 0.1mM luciferin. Dishes were sealed and placed into a LumiCycle luminometer (Actimetrics, Inc), which was kept inside a standard light-resistant tissue culture incubator at 37°C. Bioluminescence was measured by photomultiplier tube for 1 minute at intervals of 10 minutes and continuously recorded for at least 7 days. For analysis of rhythm parameters, original data were detrended by subtraction of the 24-hour running average from the raw data, and then smoothed with a 3-hour running average (LumiCycle Data Analysis; Actimetrics, Inc). Peak was defined as the highest point of smoothed data and the first peak after 24 hours in vitro was used as a phase marker. Amplitude was calculated as the difference between the highest and lowest 12-hour means for each circadian cycle. Period is calculated as the average time difference between adjacent peaks as previously described (19).
Assessment of plasma metabolic parameters
Body mass and food intake were measured at the start of baseline and final week of the study. During the final week (wk 10) of LD-Chow, LD-HFD, LL-Chow, or LL-HFD protocols, diurnal blood samples were collected at circadian times 4 hours (10 am), 10 hours (4 pm), 16 hours (10 pm), and 22 hours (4 am) with at least 12-hour gaps between individual blood draws on the same rat to avoid confounding effects of stress and blood loss. Also, the order of blood sampling from individual rats was changed during each blood collection to avoid confounding effects of stress and potential sampling bias. Blood was collected via lateral saphenous venipuncture using sterile 21-gauge, 1-inch syringe needle into chilled, EDTA-treated microcentrifuge tubes, and immediately centrifuged at 4°C for subsequent collection of plasma. Plasma glucose was measured immediately using the YSI 2300 STAT Plus Glucose & Lactate Analyzer (YSI Life Sciences) and plasma aliquots were frozen at −80°C for future analyses for insulin (Alpco Diagnostics) and triglycerides (WAKO).
Assessment of β-cell function in vitro
Pancreatic islets were isolated using standard collagenase procedure from rats exposed to either 10 weeks of LD-HFD or LL-HFD. To assess basal and glucose-stimulated insulin secretion islet perifusion experiments were performed (ACUSYST-S; Cellex Biosciences, Inc) as previously validated for the study of insulin secretion (26). Batches of 20 islets were first exposed to 40 minutes of 4mM glucose in Krebs Ringer bicarbonate buffer supplemented with 0.2% serum albumin, preheated to 37°C, and oxygenized with 95% O2 and 5% CO2 followed by 40 minutes of hyperglycemic perfusate (16mM glucose). Effluent was collected in 1-minute intervals and assayed for insulin by ELISA (Alpco Diagnostics) for subsequent determination of basal and glucose-stimulated insulin release.
Assessment of β-cell turnover by immunohistochemistry and immunofluorescence
Rats exposed to the 4 experimental protocols for 10 weeks were euthanized and pancreas was quickly excised and weighed, then fixed in 4% paraformaldehyde overnight at 4°C and subsequently embedded in paraffin and sectioned through the maximal length. Paraffin-embedded pancreatic sections (3 sections per animal spaced through the width of the pancreas) were stained first for hematoxylin/eosin, and insulin (guinea pig antiinsulin; Abcam) for determination of insulin positive area, and β-cell mass was measured by first quantifying the pancreatic cross-sectional area positive for insulin and multiplying this by the pancreatic weight. In addition, sections were costained by immunofluorescence for insulin (guinea pig antiinsulin; Abcam) and terminal deoxynucleotidyl transferase biotin-dUTP nick end-labeling (TUNEL) method (Roche Diagnostics) for quantification of β-cell apoptosis, and insulin and Ki-67 (mouse anti-Ki-67; BD Pharmingen) for determination of β-cell replication (for antibodies, please see Table 1). All islets per pancreatic section (∼200 cells per section) were examined in detail and counted at ×200 magnification (×20 objectives, ×10 ocular) for the total number of apoptotic and proliferating β-cells. Slides were visualized using Leica DM600 microscope (Leica Microsystems) and images acquired using OpenLab software (Improvision) and analyzed using ImagePro Plus software.
Table 1.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (if known) | Name of Antibody | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used |
|---|---|---|---|---|---|
| Insulin | Antiinsulin | Abcam, ab7842 | Guinea pig; polyclonal | 1:100 | |
| Glucagon | Antiglucagon | Sigma, g2654 | Mouse; monoclonal | 1:1000 | |
| Ki-67 | Anti-Ki67 | BD Pharmingen, 550609 | Mouse; monoclonal | 1:50 |
Statistical analysis and calculations
Diurnal changes in β-cell function and insulin sensitivity were estimated by using homeostasis model assessment indices (HOMAs) of insulin sensitivity (HOMAir = [glucose] × [insulin]/405; insulin in mU/L and glucose in mg/dL) and β-cell function (HOMAβ = [20 × insulin]/[glucose-3.5]; insulin in mU/L and glucose in mmol/L) (27). Statistical analysis was performed using t test when comparing 2 independent groups and ANOVA analysis when comparing multiple groups (with post hoc and repeated measures wherever appropriate) (GraphPad Prism v.6.0). Data in graphs are presented as mean ± SEM and assumed statistically significant at P < .05.
Results
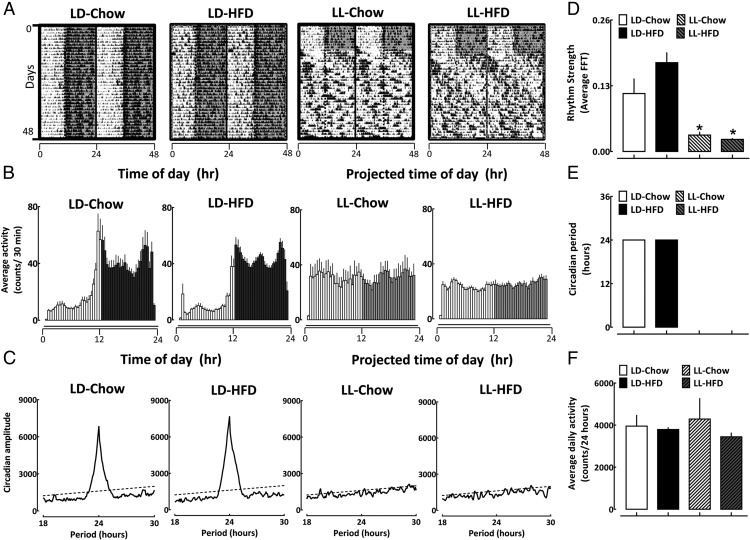
Body mass, total daily calorie, and fat-derived daily calorie intake increased comparably in LD and LL housed rats in response to 10 weeks of HFD (P > .05 for LD-HFD vs LL-HFD) (Figure 1, B–D). In addition, HFD led to a comparable epididymal fat accumulation (Figure 1E) and elevations in diurnal triglycerides (Supplemental Figure 1) in LD and LL, suggesting effective and equivalent induction of diet-induced obesity in both light treatment groups. When housed under LD conditions, both chow and HFD rats displayed robust 24-hour circadian locomotor activity rhythms (Figure 2, A and B) with no differences in the power of the circadian rhythm denoted by FFT values, circadian period, or average daily locomotor activity (P > .05) (Figure 2). In contrast, exposure to LL (in both LL-Chow and LL-HFD) led to profound loss of circadian rhythmicity characterized by loss of circadian period, amplitude and an approximately 90% decline in average FFT values compared with both LD-Chow and LD-HFD groups (P < .05 vs LD-Chow and LD-HFD) (Figure 2 and Supplemental Figure 2). Importantly, no significant difference between all 4 groups was observed in respect to average daily activity (Figure 2F).
Figure 2.
Induction of circadian disruption in rats exposed to LD or LL fed either regular chow or HFD. A, Representative behavioral profiles of gross motor activity in rats (double plotted) monitored for 1 week at baseline under standard LD cycle fed regular chow diet followed by 6 weeks of recordings in LD-Chow, LD-HFD, LL-Chow, or LL-HFD. Shaded areas represent periods of dark. Temporal profiles are presented corresponding to “time of day (h)” and “projected time of day (h)” for activity data in LD- and LL-treated rats, respectively. B, Mean circadian activity (expressed as counts/30 min) shown across the 24-hour day in rats exposed for 10 weeks to LD-Chow, LD-HFD, LL-Chow, or LL-HFD. Each bar represents mean ± SEM (n = 4–7 per group, per time point). Shaded areas represent periods of darkness under LD, and projected periods corresponding to darkness under LL. Temporal profiles are presented corresponding to time of day (h) and projected time of day (h) for activity data in LD- and LL-treated rats, respectively. C, Representative χ2 periodograms of activity recordings in LD-Chow, LD-HFD, LL-Chow, or LL-HFD rats. Broken horizontal lines represents statistical significant threshold in determination of dominant circadian period. Note absence of circadian period in LL-Chow and LL-HFD groups. A mean measure of circadian rhythm strength denoted by FFT values (D), mean χ2 periodogram derived circadian period (E), and average daily (24 hours) activity of respective groups (F). Bar graphs represent mean ± SEM (n = 4–7 per group). *, P < .05 vs LD-Chow and LD-HFD.
We next employed continuous tracking of islet cell bioluminescence (with Per1 luciferase fusion construct) to examine effects of biological interactions between light and diet-induced obesity on the islet circadian clock (Figure 3). Islets isolated from LD-Chow and LD-HFD displayed comparable sustained circadian rhythms in Per1 bioluminescence with robust amplitude, near 24 hours of oscillatory period, and the phase of circadian oscillations not significantly impacted by 10 weeks of HFD (Figure 3). In contrast, exposure of rats to LL (independent of diet) led to the dampening (∼50%) in the amplitude of Per1-driven luciferase oscillations evident in both LL-Chow and LL-HFD rats (P < .05, vs LD-Chow and LD-HFD) (Figure 3). Interestingly, LL-HFD animals demonstrated a significantly altered phase (peak, 13.17 ± 0.29 vs 9.56 ± 4.02 hours for LD-HFD vs LL-HFD; P < .05) (Figure 3C) and increased variance in the phase of Per1-driven luciferase oscillations. All 4 groups exhibited detectable near 24-hour period of islet clock oscillations.
Figure 3.
Real-time bioluminescence monitoring of islets isolated from Per1-LUC transgenic rats exposed to LD or LL fed either regular chow or HFD. A, Representative examples of 5 days of continuous recordings of Per-driven bioluminescence rhythms in islets isolated from Per1-LUC transgenic rats exposed for 10 weeks to LD-Chow, LD-HFD, LL-Chow, or LL-HFD. Mean amplitude (B), phase (C), and period of circadian clock oscillations (D) in islets isolated from Per1-LUC transgenic rats exposed for 10 weeks to LD-Chow, LD-HFD, LL-Chow, or LL-HFD conditions. Bar graphs represent mean ± SEM (n = 4–6 per group). Temporal profiles are presented corresponding to “time of day (h)” and “projected time of day (h)” in LD- and LL-treated rats, respectively. *, P < .05 vs LD-Chow and LD-HFD.
To assess regulation of glucose homeostasis, we next examined diurnal 24 hours profiles in plasma glucose, insulin, as well as calculated index of β-cell function (HOMAβ) and insulin resistance (HOMAir) (Figure 4). Under LD, HFD did not augment diurnal glycemia (P > .05, LD-Chow vs LD-HFD) (Figure 4A), suggestive of successful metabolic adaption to diet-induced obesity. In contrast, LL-HFD led to induction of hyperglycemia evident across the 24-hour day compared with all experimental groups (P < .05 for LL-HFD vs LD-Chow, LD-HFD, and LL-Chow) (Figure 4A). Furthermore, although there was no relationship between weight gain and the plasma glucose concentration under LD, increase in body weight during the 10 week study predicted the rise in plasma glucose concentrations under LL conditions, which was particularly evident at projected time of the day 16- and 22-hour time points (P < .05, r = 0.7) (Supplemental Figure 3).
Figure 4.
Regulation of diurnal glucose homeostasis in rats exposed to LD or LL fed either regular chow or HFD. A, Diurnal profiles in plasma glucose (A), insulin (B), and calculated index of β-cell function HOMAβ (C) in rats fed either chow or HFD and exposed to 10 weeks of either standard LD (left) or LL (middle) light conditions. Bar graphs (right) display mean ± SEM of AUC for measures of plasma glucose (A), insulin (B), and HOMAβ (C) across the 24-hour day in rats exposed for 10 weeks to LD-Chow, LD-HFD, LL-Chow, or LL-HFD. Plasma samples were obtained at 6-hour intervals and P values in each graph represent statistical effects of “time” and “diet” interaction for each variable under LD and LL conditions derived from repeated measures two-way ANOVA analysis (GraphPad Prism v.6.0). *, P < .05 vs LD-Chow, LL-Chow, and LD-HFD; †, P < .05 vs LD-Chow; ‡, P < .05 vs LD-Chow and LL-HFD.
LD animals exhibited statistically significant diurnal variations in plasma insulin concentrations with peak values restricted to the dark/night (feeding) phase of the circadian cycle (P < .05) (Figure 4B). In contrast, diurnal variations in insulin concentrations were absent under LL, thus insulin levels were constitutively elevated throughout the 24-hour period particularly evident under LL-HFD conditions (Figure 4B). The constitutively elevated insulin concentrations in LL-HFD were likely attributed in part to loss of diurnal variations in insulin sensitivity (Supplemental Figure 4). Although HFD led to a comparable increase in total insulin secretory output (measured as area under the curve [AUC] for insulin concentrations across the 24-h day) under LD and LL (Figure 4B), prevailing insulin levels in LL-HFD animals were not appropriate for the level of hyperglycemia indicating onset of β-cell dysfunction. Consistent with this, HFD resulted in a robust increase in a measure of β-cell function (derived from homeostasis model assessment, HOMAβ) under LD, but not under LL (P < .05 for LD-HFD vs LL-HFD) (Figure 4C). To confirm deleterious effects of LL-HFD on β-cell secretory capacity, we performed isolated islet perifusion experiments in islets from LD-HFD vs LL-HFD rats (Figure 5). LL-HFD islets demonstrated blunted glucose-stimulated insulin secretion (P < .05 for LD-HFD vs LL-HFD) (Figure 5) attributed to a decline in the amplitude of insulin secretory pulses in response to hyperglycemia (Figure 5), thus confirming presence of β-cell dysfunction.
Figure 5.
Measurements of glucose-stimulated insulin secretion in vitro by islet perifusion in isolated islets from rats exposed to LD or LL with concomitant exposure to HFD. A, Mean insulin concentration profiles during islet perifusion at low basal glucose 4mM (0–40 min) and hyperglycemic 16mM glucose (40–80 min) in isolated islets obtained from rats exposed to 10 weeks of either LD-HFD (black circles, n = 5) or LL-HFD (gray triangles, n = 5). B, Example of a representative pulsatile insulin secretion profile sampled at 1min intervals at 4mM (0–40 min) and 16mM glucose (40–80 min) in isolated islets from rats exposed to 10 weeks of either LD-HFD (black lines) or LL-HFD (gray lines). C, Mean rates of insulin secretion calculated as AUC from insulin concentrations obtained during islet perifusion at 4mM (0–40 min) and 16mM glucose (40–80 min) in isolated islets from rats exposed to 10 weeks of either LD-HFD (black bars, n = 5) or LL-HFD (gray bars, n = 5). *, P < .05 vs LD-HFD 4mM glucose, LL-HFD 4mM glucose and LL-HFD 16mM glucose.
We next examined effects of light and diet interactions on β-cell turnover (Figures 6 and 7). As expected, HFD provoked a 46% increase in β-cell mass in LD rats (13.6 ± 3 vs 20 ± 2 mg, P < .05 for LD-Chow vs LD-HFD), which corresponded to β-cell hyperplasia characterized by 4.2-fold increase in the frequency of β-cell proliferation (P < .05 for LD-Chow vs LD-HFD) (Figures 6 and 7). HFD induced similar effects on β-cell expansion under LL, evident by 58% increase in β-cell mass and a 4.4-fold increase in the frequency of β-cell proliferation (P < .05 for LL-Chow vs LL-HFD) (Figures 6 and 7). However, although there was no significant increase (P = .46) in β-cell apoptosis in LD rats on a HFD, the frequency of β-cell apoptosis increased 2.8-fold in LL rats exposed to HFD, indicating compromised β-cell survival (P < .05 for LL-HFD vs all groups) (Figures 6 and 7).
Figure 6.
Islet morphology in rats exposed to LD or LL fed either regular chow or HFD. A, Representative examples of pancreatic sections imaged at ×4 stained for insulin (brown) and hematoxylin (blue) in rats after 10 weeks of LD-Chow, LD-HFD, LL-Chow, or LL-HFD. B–D, Representative examples of islets stained by immunofluorescence for insulin (green) and counterstained with either glucagon (B), replication marker Ki-67 (C), or apoptosis marker TUNEL (D) and nuclear stain Dapi (blue) imaged at ×20 in rats after 10 weeks of LD-Chow, LD-HFD, LL-Chow, or LL-HFD conditions. White arrowheads and corresponding high magnification insets highlight examples of Ki-67 and TUNEL-positive β-cells.
Figure 7.
Quantification of β-cell turnover in rats exposed to LD or LL fed either regular chow or HFD. Mean pancreatic mass (upper left), β-cell mass (upper right), frequency of β-cell proliferation (lower left), and frequency of β-cell apoptosis (lower right) in rats exposed to 10 weeks of LD-Chow, LD-HFD, LL-Chow, or LL-HFD. Bar graphs represent mean ± SEM (n = 5–6 per group). *, P < .05 vs LD-Chow; †, P < .05 vs LD-Chow and LL-Chow; ‡, P < .05 vs LD-Chow, LL-Chow, and LD-HFD.
Discussion
Obesity and corresponding insulin resistance are known to increase the risk of T2DM (28). However, nondiabetic obese individuals compensate for onset of insulin resistance by elevating insulin production and secretion and thus remain normoglycemic (8, 29). In those vulnerable to T2DM, this compensation fails due to β-cell failure characterized by β-cell secretory dysfunction and increased β-cell apoptosis (2, 12). In the current study, we investigated whether disruption of circadian rhythms (achieved by continuous exposure to LL) promotes development of T2DM by compromising normal β-cell functional and morphological adaptation to diet-induced obesity. We also addressed whether maladaptive response to obesity is mediated through loss of islet clock function? We report that concomitant exposure to LL and HFD leads to synergistic disruption of pancreatic islet clock characterized by the impaired amplitude and the phase islet clock transcriptional oscillations. Consequently, although exposure to HFD under LD leads to appropriate adaptive β-cell response and preservation of normal glucose homeostasis, HFD under LL results in development of diabetes characterized by loss of diurnal control of insulin secretion, impaired β-cell function, and increased rate of β-cell apoptosis.
Light is the strongest entrainment signal for the mammalian circadian system. Thus, inappropriate exposure to nighttime light (typical of shift work and other conditions common to modern lifestyle) is associated with the disruption of circadian rhythms and deleterious consequences for human health (30). Importantly, accumulating evidence from epidemiological, clinical, as well as animal studies consistently show that disruption of circadian rhythms negatively impacts metabolic health and augments susceptibility for T2DM (13, 31, 32). For example, series of studies by Fonken et al (33, 34) have eloquently demonstrated that exposure of mice to dim light (∼5 lux) at night (designed to recapitulate light pollution levels in urban areas) results in disruption of circadian rhythms in feeding and locomotor activity and coincides with induction of glucose intolerance and exaggerated inflammatory response. Interestingly, dim light at night is also associated with increased body mass and fat accumulation, which is attributed to the alter timing of feeding (with no change in total daily caloric intake) (34). Consistent with these observations, our results also show no difference in total daily caloric intake in rats between LD and LL and clear disruption in behavioral rhythms after exposure to LL. However, rats exposed to LL alone did not display increased body mass or epididymal fat accumulation on either chow or HFD. This discrepancy may be due multiple factors such as species differences, lighting condition as well as duration of treatment. Indeed, previous studies in rats have documented absence of weight gain after exposure to continuous bright light illumination (35). Nonetheless, absence of exaggerated weight gain in LL groups in our study have excluded a potential confounding factor in evaluating metabolic and islet effects of HFD (which in itself is associated with increased body mass).
LL impairs central clock function by inducing disynchronization of circadian oscillators in the SCN thus resulting in the loss (or dampening) of behavioral and endocrine circadian outputs (36). This observation is consistent with our results. Also, consistent with previous studies, LL led to dampening of the amplitude of circadian clock oscillators in pancreatic islets (19). In our study, islets isolated from rats exposed to LD-HFD did not exhibit significant alterations in the amplitude or phase of molecular oscillators in islets, consistent with some previous studies (37). In contrast, others have reported that exposure to HFD significantly alters clock gene expression in peripheral tissues (eg, liver and adipose tissue) (38) and recently has been shown to augment islet Per1 mRNA expression in mice (39). In our study, the most profound effect on islet clock was evident after combination of LL and HFD conditions, which synergized to impair cyclical expression and also altered the phase of circadian clock oscillations in islets. We have recently shown that feeding activity couples molecular oscillators in the islet to the SCN (40). Thus, behavioral arrhythmicity in feeding and locomotor activity induced by LL likely contributed to impaired islet clock functionality. Moreover, LL-induced loss of endocrine circadian outputs (eg, melatonin and corticosterone) (34, 35, 41, 42) might have also contributed to loss of islet circadian rhythms, because melatonin and corticosterone has been shown to modulate entrainment of peripheral oscillators (including pancreatic β-cells) (43, 44).
The use of Per1 bioluminescence recordings from pancreatic islets presents some limitations. Although rodent islets are largely comprised of β-cells, we cannot exclude potential contribution of other endocrine islet cell types (ie, α-cells) to measures of islet Per1 bioluminescence reported in our study. Thus, further studies will be needed to determine effects of circadian disruption and/or diet-induced obesity on clock function in individual islet endocrine cell types. In addition, the time of tissue isolation as well as procedures associated with tissue harvesting from Per1-Luc transgenic rats has been previously shown, for some tissues, to reset the phase of the circadian clock (45). However, our previous work suggests that pancreatic islet isolation and culture procedure does not appear to reset the phase of islet molecular clock. Firstly, we previously demonstrated that the phase of islet Per1-Luc bioluminescence reflected the temporal profile of in vivo Per-1 mRNA expression determined by qPCR (19). Furthermore, inversion of the phase of the LD cycle in vivo in Per1-Luc transgenic rats by 12 hours resulted in a near 12-hour phase reversal of the islet Per1-Luc bioluminescence signal, suggesting that changes in the phase of islet Per1 expression can be readily detected using in vitro islet Per1 bioluminescence recordings (19).
One of the key observations in our study is that concomitant exposure to LL and HFD recapitulates key features of pancreatic β-cell failure characteristic of T2DM. In particular, LL-HFD islets exhibit loss of diurnal variability in insulin concentrations, impaired glucose-stimulated insulin release, disrupted islet architecture and increased frequency of β-cell apoptosis. Of note, methodological limitations due to blood sampling limits precluded us from definitively assessing circadian rhythms in insulin secretion in vivo. Thus, the apparent lack of diurnal variability in insulin concentrations under LL does not necessarily reflect loss of endogenous circadian rhythmicity in vivo in islets. Indeed, persistent circadian oscillations with a distinct circadian period were still detected (albeit at suppressed amplitude) in islets of LL-exposed rats, which is consistent with previous studies in SCN-lesioned PER2::LUC mice (46).
Interestingly, β-cell mass in LL-HFD rats remained comparable with LD-HFD counterparts, suggesting that compensatory increase in β-cell proliferation/neogenesis is still able to account for increased β-cell turnover observed in LL-HFD rats in the 10 weeks of the present study. As in humans with evolving T2DM, persistent increase in β-cell apoptosis in LL-HFD will likely result in eventual decline in β-cell mass leading to further loss of metabolic control and exaggeration of diabetic phenotype (47). It is however important to note that at least in the short term, β-cell failure and consequent induction of hyperglycemia observed in our study in LL-HFD group is largely attributed to β-cell dysfunction associated with loss of glucose-stimulated insulin secretion.
These observations raise the question, what mechanism accounts for induction of β-cell failure after exposure to LL-HFD? This is likely attributed to LL-mediated loss of islet clock function resulting in compromised β-cell adaptation to oxidative stress associated with increased metabolic demand placed on the β-cell as a consequence of HFD. The support for this postulate comes from studies in which deletion of key components of the β-cell circadian clock has been shown to diminish glucose-stimulated insulin release, impair mitochondrial function and, importantly, compromise cellular response to oxidative stress (achieved via control of nuclear factor erythroid 2-related factor 2) (17, 18). Indeed, the molecular clock in β-cells appears to be essential for orchestrating cellular response to oxidative stress by regulating nuclear factor erythroid 2-related factor 2 levels, which subsequently regulates a myriad of downstream antioxidant enzymes (17). This is particularly important for the β-cell, which notoriously exhibits low antioxidant potential in the face of significant reliance on mitochondrial metabolism for regulation of insulin release and production (48). Circadian disruption can thus increase susceptibility to oxidative stress and consequent β-cell failure, particularly in context of gluco-/lipotoxicity (the known inducers of β-cell oxidative stress in T2DM) (49). Subsequently, clock-coordinated response to oxidative stress is critical for β-cells of rats exposed to LL-HFD, because in this group, insulin secretion is constitutively elevated throughout the 24-hour cycle driven by loss of circadian variations in fast/feeding cycles and insulin sensitivity. Furthermore, chronic exposure to LL-HFD has been shown to disrupt circadian rhythms in insulin sensitivity and exacerbate insulin resistance in mice (50), an observation consistent with our data. Thus, combination of constitutively elevated β-cell metabolic demand and loss of β-cell clock function makes LL-HFD animals particularly vulnerable to β-cell failure consistent with observed increase in the frequency of β-cell apoptosis and dysfunction.
Loss of melatonin secretion, consequent of exposure to LL (41), may be another contributory mechanism by which LL compromises normal adaption to HFD. Melatonin secretion and subsequent receptor signaling is important in the regulation of organisms' circadian rhythms and entrainment of circadian clocks, including β-cells (44). Because pineal production and secretion of melatonin is primarily influenced by the duration and intensity of exposure to light (51), exposure to artificial lighting at night results in profound suppression of nighttime melatonin production and secretion (52). Although, melatonin's involvement in regulation of glucose homeostasis and β-cell health has been proposed decades ago (53), more recently activation of melatonin receptor signaling has been demonstrated to play an important role in β-cell protection against oxidative stress in human islets (54). Moreover, in humans, loss of nocturnal melatonin secretion or presence of melatonin receptor genetic variant is associated with higher risk of developing β-cell failure and T2DM (55, 56). Together, these findings emphasize the potential importance of melatonin signaling in regulation of β-cell function and survival in T2DM.
In conclusion, lifestyle factors associated with disruption of circadian rhythms and promotion of obesity are becoming increasingly commonplace in today's societies. Additionally, there is also increased understanding of a causative interrelationship between circadian disruption and development of obesity and vice versa in humans (31). The results of our study suggest that combination of circadian disruption and diet-induced obesity synergize to promote development of pancreatic islet failure recapitulating many features of islet pathology present in T2DM. This data provides a potential mechanism underlying common epidemiological association between circadian disruption, obesity, and T2DM.
Acknowledgments
This work was supported by the National Institutes of Health Grant DK098468 (to A.V.M.), the O'Keefe Foundation (CSC and AVM), and the Center for Regenerative Medicine (Mayo Clinic).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AUC
- area under the curve
- FFT
- fast Fourier transform
- HFD
- high-fat diet
- HOMA
- homeostasis model assessment index
- LD
- standard light-dark cycle
- LD-Chow
- 10 weeks of standard LD cycle on regular chow diet
- LL-Chow
- 10 weeks of 24 hour LL exposure on regular chow diet
- LD-HFD
- 10 weeks of standard LD cycle exposure on HFD
- LL
- constant light
- LL-HFD
- 10 weeks of 24-hour LL exposure on HFD
- Per1-LUC
- Period-1 promoter linked to a luciferase reporter
- SCN
- suprachiasmatic nucleus
- T2DM
- type 2 diabetes
- TUNEL
- terminal deoxynucleotidyl transferase biotin-dUTP nick end-labeling.
References
- 1. Seltzer HS, Allen EW, Herron AL, Jr, Brennan MT. Insulin secretion in response to glycemic stimulus: relation of delayed initial release to carbohydrate intolerance in mild diabetes mellitus. J Clin Invest. 1967;46:323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. β-Cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. [DOI] [PubMed] [Google Scholar]
- 3. Brunzell JD, Robertson RP, Lerner RL, et al. Relationships between fasting plasma glucose levels and insulin secretion during intravenous glucose tolerance tests. J Clin Endocrinol Metab. 1976;42:222–229. [DOI] [PubMed] [Google Scholar]
- 4. Boden G, Chen X, Polansky M. Disruption of circadian insulin secretion is associated with reduced glucose uptake in first-degree relatives of patients with type 2 diabetes. Diabetes. 1999;48:2182–2188. [DOI] [PubMed] [Google Scholar]
- 5. Lee A, Ader M, Bray GA, Bergman RN. Diurnal variation in glucose tolerance. Cyclic suppression of insulin action and insulin secretion in normal-weight, but not obese, subjects. Diabetes. 1992;41:750–759. [DOI] [PubMed] [Google Scholar]
- 6. Papa FR. Endoplasmic reticulum stress, pancreatic β-cell degeneration, and diabetes. Spring Harb Perspect Med. 2012;2:a007666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chan JM, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care. 1994;17:961–969. [DOI] [PubMed] [Google Scholar]
- 8. Kahn SE. The relative contributions of insulin resistance and β-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia. 2003;46:3–19. [DOI] [PubMed] [Google Scholar]
- 9. Bergman RN, Phillips LS, Cobelli C. Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and β-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest. 1981;68:1456–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kahn SE, Prigeon RL, McCulloch DK, et al. Quantification of the relationship between insulin sensitivity and β-cell function in human subjects. Evidence for a hyperbolic function. Diabetes. 1993;42:1663–1672. [DOI] [PubMed] [Google Scholar]
- 11. Saisho Y, Butler AE, Manesso E, Elashoff D, Rizza RA, Butler PC. β-Cell mass and turnover in humans: effects of obesity and aging. Diabetes Care. 2013;36:111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kahn SE. Clinical review 135: the importance of β-cell failure in the development and progression of type 2 diabetes. J Clin Endocrinol Metab. 2001;86:4047–4058. [DOI] [PubMed] [Google Scholar]
- 13. Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9:764–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Buijs RM, Kalsbeek A. Hypothalamic integration of central and peripheral clocks. Nat Rev Neurosci. 2001;2:521–526. [DOI] [PubMed] [Google Scholar]
- 16. Boden G, Ruiz J, Urbain JL, Chen X. Evidence for a circadian rhythm of insulin secretion. Am J Physiol. 1996;271:E246–E252. [DOI] [PubMed] [Google Scholar]
- 17. Lee J, Moulik M, Fang Z, et al. Bmal1 and β-cell clock are required for adaptation to circadian disruption, and their loss of function leads to oxidative stress-induced β-cell failure in mice. Mol Cell Biol. 2013;33:2327–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marcheva B, Ramsey KM, Buhr ED, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Qian J, Block GD, Colwell CS, Matveyenko AV. Consequences of exposure to light at night on the pancreatic islet circadian clock and function in rats. Diabetes. 2013;62:3469–3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lin YC, Hsiao TJ, Chen PC. Persistent rotating shift-work exposure accelerates development of metabolic syndrome among middle-aged female employees: a five-year follow-up. Chronobiol Int. 2009;26:740–755. [DOI] [PubMed] [Google Scholar]
- 21. Pan A, Schernhammer ES, Sun Q, Hu FB. Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women. PLoS Med. 2011;8:e1001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Suwazono Y, Dochi M, Oishi M, Tanaka K, Kobayashi E, Sakata K. Shiftwork and impaired glucose metabolism: a 14-year cohort study on 7104 male workers. Chronobiol Int. 2009;26:926–941. [DOI] [PubMed] [Google Scholar]
- 23. Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291:490–493. [DOI] [PubMed] [Google Scholar]
- 24. Sokolove PG, Bushell WN. The chi square periodogram: its utility for analysis of circadian rhythms. J Theor Biol. 1978;72:131–160. [DOI] [PubMed] [Google Scholar]
- 25. Herzog ED, Kiss IZ, Mazuski C. Measuring synchrony in the mammalian central circadian circuit. Methods Enzymol. 2015;552:3–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Song SH, Kjems L, Ritzel R, et al. Pulsatile insulin secretion by human pancreatic islets. J Clin Endocrinol Metab. 2002;87:213–221. [DOI] [PubMed] [Google Scholar]
- 27. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- 28. Carey DG, Jenkins AB, Campbell LV, Freund J, Chisholm DJ. Abdominal fat and insulin resistance in normal and overweight women: direct measurements reveal a strong relationship in subjects at both low and high risk of NIDDM. Diabetes. 1996;45:633–638. [DOI] [PubMed] [Google Scholar]
- 29. Polonsky KS, Given BD, Van Cauter E. Twenty-four-hour profiles and pulsatile patterns of insulin secretion in normal and obese subjects. J Clin Invest. 1988;81:442–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Reddy AB, O'Neill JS. Healthy clocks, healthy body, healthy mind. Trends Cell Biol. 2010;20:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fonken LK, Nelson RJ. The effects of light at night on circadian clocks and metabolism. Endocr Rev. 2014;35:648–670. [DOI] [PubMed] [Google Scholar]
- 32. Rakshit K, Thomas AP, Matveyenko AV. Does disruption of circadian rhythms contribute to β-cell failure in type 2 diabetes? Curr Diab Rep. 2014;14:474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fonken LK, Lieberman RA, Weil ZM, Nelson RJ. Dim light at night exaggerates weight gain and inflammation associated with a high-fat diet in male mice. Endocrinology. 2013;154:3817–3825. [DOI] [PubMed] [Google Scholar]
- 34. Fonken LK, Workman JL, Walton JC, et al. Light at night increases body mass by shifting the time of food intake. Proc Natl Acad Sci USA. 2010;107:18664–18669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tapia-Osorio A, Salgado-Delgado R, Angeles-Castellanos M, Escobar C. Disruption of circadian rhythms due to chronic constant light leads to depressive and anxiety-like behaviors in the rat. Behav Brain Res. 2013;252:1–9. [DOI] [PubMed] [Google Scholar]
- 36. Ohta H, Yamazaki S, McMahon DG. Constant light desynchronizes mammalian clock neurons. Nat Neurosci. 2005;8:267–269. [DOI] [PubMed] [Google Scholar]
- 37. Yanagihara H, Ando H, Hayashi Y, Obi Y, Fujimura A. High-fat feeding exerts minimal effects on rhythmic mRNA expression of clock genes in mouse peripheral tissues. Chronobiol Int. 2006;23:905–914. [DOI] [PubMed] [Google Scholar]
- 38. Kohsaka A, Laposky AD, Ramsey KM, et al. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6:414–421. [DOI] [PubMed] [Google Scholar]
- 39. Vieira E, Marroquí L, Batista TM, et al. The clock gene Rev-erbα regulates pancreatic β-cell function: modulation by leptin and high-fat diet. Endocrinology. 2012;153:592–601. [DOI] [PubMed] [Google Scholar]
- 40. Rakshit K, Qian J, Colwell CS, Matveyenko AV. The islet circadian clock: entrainment mechanisms, function and role in glucose homeostasis. Diabetes Obes Metab. 2015;17(suppl 1):115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Honma S, Kanematsu N, Katsuno Y, Honma K. Persistence of circadian oscillation while locomotor activity and plasma melatonin levels became aperiodic under prolonged continuous light in the rat. Neurosci Lett. 1996;216:49–52. [DOI] [PubMed] [Google Scholar]
- 42. Jung CM, Khalsa SB, Scheer FA, et al. Acute effects of bright light exposure on cortisol levels. J Biol Rhythms. 2010;25:208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Balsalobre A, Brown SA, Marcacci L, et al. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289:2344–2347. [DOI] [PubMed] [Google Scholar]
- 44. Nishiyama K, Hirai K. The melatonin agonist ramelteon induces duration-dependent clock gene expression through cAMP signaling in pancreatic INS-1 β-cells. PLoS One. 2014;9:e102073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yoshikawa T, Yamazaki S, Menaker M. Effects of preparation time on phase of cultured tissues reveal complexity of circadian organization. J Biol Rhythms. 2005;20:500–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yoo SH, Yamazaki S, Lowrey PL, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA. 2004;101:5339–5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rhodes CJ. Type 2 diabetes-a matter of β-cell life and death? Science. 2005;307:380–384. [DOI] [PubMed] [Google Scholar]
- 48. Tiedge M, Lortz S, Drinkgern J, Lenzen S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes. 1997;46:1733–1742. [DOI] [PubMed] [Google Scholar]
- 49. Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and β-cell dysfunction. Endocr Rev. 2008;29:351–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Coomans CP, van den Berg SA, Houben T, et al. Detrimental effects of constant light exposure and high-fat diet on circadian energy metabolism and insulin sensitivity. FASEB J. 2013;27:1721–1732. [DOI] [PubMed] [Google Scholar]
- 51. Snyder SH, Axelrod J, Zweig M. Circadian rhythm in the serotonin content of the rat pineal gland: regulating factors. J Pharmacol Exp Ther. 1967;158:206–213. [PubMed] [Google Scholar]
- 52. Graham C, Cook MR, Gerkovich MM, Sastre A. Examination of the melatonin hypothesis in women exposed at night to EMF or bright light. Environ Health Perspect. 2001;109:501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Milcou SM, Vrejoin G, Marcean R, Nanu L. [Effect of a hypoglycemic pineal hormone on the endocrine pancreas in alloxanized animals; morphological study.] Ann Endocrinol (Paris). 1957;18:621–627. [PubMed] [Google Scholar]
- 54. Costes S, Boss M, Thomas AP, Matveyenko AV. Activation of melatonin signaling promotes β-cell survival and function. Mol Endocrinol. 2015:29:682–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lyssenko V, Nagorny CL, Erdos MR, et al. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet. 2009;41:82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. McMullan CJ, Schernhammer ES, Rimm EB, Hu FB, Forman JP. Melatonin secretion and the incidence of type 2 diabetes. JAMA. 2013;309:1388–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]