Abstract
While the predominant function of all tendons is to transfer force from muscle to bone and position the limbs, some tendons additionally function as energy stores, reducing the cost of locomotion. Energy storing tendons experience extremely high strains and need to be able to recoil efficiently for maximum energy storage and return. In the equine forelimb, the energy storing superficial digital flexor tendon (SDFT) has much higher failure strains than the positional common digital extensor tendon (CDET). However, we have previously shown that this is not due to differences in the properties of the SDFT and CDET fascicles (the largest tendon subunits). Instead, there is a greater capacity for interfascicular sliding in the SDFT which facilitates the greater extensions in this particular tendon (Thorpe et al., 2012). In the current study, we exposed fascicles and interfascicular matrix (IFM) from the SDFT and CDET to cyclic loading followed by a test to failure. The results show that IFM mechanical behaviour is not a result of irreversible deformation, but the IFM is able to withstand cyclic loading, and is more elastic in the SDFT than in the CDET. We also assessed the effect of ageing on IFM properties, demonstrating that the IFM is less able to resist repetitive loading as it ages, becoming stiffer with increasing age in the SDFT. These results provide further indications that the IFM is important for efficient function in energy storing tendons, and age-related alterations to the IFM may compromise function and predispose older tendons to injury.
Abbreviations: CDET, common digital extensor tendon; CSA, cross sectional area; IFM, interfascicular matrix; PBS, phosphate buffered saline; SDFT, superficial digital flexor tendon.
Keywords: Viscoelastic, Fatigue, Mechanics, Structure-function, Fascicle, Endotenon
Highlights
-
•
Fascicle sliding enables high levels of extension in energy storing tendons.
-
•
Sliding mechanics are governed by the interfascicular matrix (IFM).
-
•
We assessed IFM extension and recovery.
-
•
IFM elasticity and recovery are greater in energy storing tendons.
-
•
The IFM plays an important role in the function of energy storing tendons.
1. Introduction
Tendon is a hierarchical fibre-composite material, in which collagen molecules aggregate to form sub-units of increasing diameter, the largest of which is the fascicle. At the larger hierarchical levels, the collagenous subunits are interspersed with a non-fibrous matrix (for a detailed review of tendon structure, please refer to Thorpe et al. (2013a) and references therein). Tendons can be separated into two groups according to their function, positional or energy storing. Positional tendons transfer force from muscle to bone to facilitate locomotion, whereas energy storing tendons have an additional function, stretching and recoiling to store and release energy, increasing the efficiency of locomotion (Alexander, 1991, Biewener, 1998). While it is well established that energy storing tendons have specific mechanical properties for optimal energy storage, the functional specialisations that result in these distinct properties are not well understood.
Previous work has shown that energy storing tendons experience much higher strains in vivo than their positional counterparts. For example, strains of up to 11% and 16% have been recorded in the human Achilles (Lichtwark and Wilson, 2005) and equine superficial digital flexor tendons (SDFT) (Stephens et al., 1989) respectively. In contrast, maximum strains in the human anterior tibialis tendon and equine Common digital extensor tendon (CDET), both of which are purely positional in function, range from 2 to 3% (Maganaris and Paul, 1999, Birch et al., 2008). Correspondingly, in vitro testing to failure has demonstrated that energy storing tendons have a consistently higher failure strain than positional tendons (Batson et al., 2003, Thorpe et al., 2012). In addition, our previous work has shown that fascicles from the energy storing SDFT have a specialised helical structure to enable more efficient recovery from loading than their counterparts in the CDET, as befits their energy storing function (Thorpe et al., 2013b).
However, we have also shown that, despite the energy storing SDFT failing at higher strains than the positional CDET, fascicles from the SDFT actually have a lower failure strain than their counterparts from CDET (Thorpe et al., 2012). Taken together, these data suggest that, while the fascicles in the SDFT are specialised to maximise energy storage and return, mechanisms other than greater fascicle extensions facilitate the high strain characteristics of energy storing tendons.
Fascicles are bound together by the interfascicular matrix (IFM, also referred to as the endotenon), and we previously hypothesised that energy storing tendons have a specialised IFM which allows sliding between adjacent fascicles, resulting in the greater capacity for tendon extension. To investigate this, we developed a shear model, which allows the mechanical properties of the IFM to be determined (Thorpe et al., 2012). The results of this study showed that, while there were no differences in failure properties between the IFM in the SDFT and CDET, there were significant differences in the shape of the force extension curve between tendon types, such that at low forces (up to 60% failure force) there was greater extension in the SDFT IFM than in the CDET IFM (Thorpe et al., 2012). This greater extensibility in the SDFT IFM allows greater sliding between fascicles, thus enabling the large extensions that occur in this tendon during exercise. However, it has not been established if this sliding behaviour is elastic and reversible, which would be required for the energy storing function of the SDFT.
We have also previously assessed the effect of increasing age on the properties of the IFM, demonstrating that the low stiffness behaviour of the SDFT IFM is lost with ageing (Thorpe et al., 2013c). This is important, as the risk of injury to energy storing tendons increases with ageing (Kasashima et al., 2004, Perkins et al., 2005, Knobloch et al., 2008, Hess, 2010), and the stiffening of the SDFT IFM may contribute to this, as fascicles are being loaded at an earlier point during tendon extension, increasing the risk of fascicle damage and subsequent tendon injury.
In addition to allowing sliding between fascicles, effective energy storing behaviour requires the IFM to behave elastically and recover from loading. While the recovery capacity of fascicles and tendons has been studied previously, showing more elastic behaviour in energy storing tendons (Wang et al., 1995, Vereecke and Channon, 2013, Thorpe et al., 2014b), no previous work has attempted to establish if the IFM is able to recover from loading. The aim of this study was therefore to assess the recovery capacity and failure properties of fascicles and IFM in the SDFT and CDET from young and old horses. We have used the accepted equine model as it is well established that the equine SDFT and human Achilles are analogous in terms of function and age-related injury risk (Innes and Clegg, 2010, Lui et al., 2010). It was hypothesised that the IFM has the ability to recover after cyclic loading, and that elasticity is greater in the energy storing SDFT than in the positional CDET. Further, we hypothesised that the ability of the IFM to recover decreases with ageing and the stiffness of the IFM increases, specifically in the SDFT.
2. Materials and methods
Forelimbs distal to the carpus were collected from horses aged 3 to 7 years (young: n=5) and those aged 17 to 20 years (old: n=5) euthanized at a commercial equine abattoir. The SDFT and CDET were dissected free at the level of the metacarpophalangeal joint and wrapped in tissue paper dampened in phosphate buffered saline (PBS) and then in tin foil and frozen at −80 °C. Previous work has established that one freeze-thaw cycle does not affect tendon viscoelastic or failure properties (Huang et al., 2011). On the day of testing, tendons were allowed to thaw at room temperature and samples for fascicle and IFM testing were dissected as described in 2.1, 2.2. Sample hydration was maintained during dissection and testing by storing the samples on tissue paper dampened with PBS. All testing was performed at room temperature.
2.1. Determination of fascicle mechanical properties
Fascicles were isolated from the SDFT and CDET as previously described (Thorpe et al., 2014c). Approximately 10 fascicles, 40 mm in length, were dissected from each tendon. Following our previously validated methods, the diameter of each fascicle was measured in a single plane along a 10 mm section in the middle of the fascicle, and the smallest diameter recorded and used to calculate cross sectional area (CSA), assuming a circular shape (Thorpe et al., 2012).
An electrodynamic testing machine (Instron ElectroPuls 1000) equipped with a 250 N load cell was used to determine fascicle mechanical properties. Fascicles were gripped using pneumatic grips (gripping pressure of 4 bar) with a layer of rubber (0.3 mm thick) and sandpaper (0.1 mm thick) over each grip surface. The distance between the grips was set to 20 mm. Fascicles were pre-loaded to 0.1 N, which represents a load of approximately 2% of fascicle failure load, to remove any slack in the sample. After the pre-load, the value of grip to grip distance was recorded as the gauge length. Fascicles were then preconditioned with 10 loading cycles between 0 and 3% strain (approx. 25% of failure strain) using a sine wave at frequency of 1 Hz. Immediately after preconditioning, fascicles were pulled to failure at a strain rate of 5% per second. Force and extension data were continuously recorded at 100 Hz during preconditioning and the failure test. For each test, the location of sample failure was also recorded, and any samples that did not break in the middle were excluded from the analysis.
From preconditioning data, the percentage hysteresis and percentage stress relaxation were calculated between the first and last preconditioning cycles. To calculate sample failure properties from the quasi-static test to failure, the displacement at which the initial pre-load was reached, prior to preconditioning, was taken as the start point for the test to failure in all specimens, and engineering stress and strain were calculated using the CSA and effective gauge length for each sample. A continuous modulus was calculated across every 10 data points of each stress strain curve, from which the maximum modulus value was determined.
2.2. Determination of IFM mechanical properties
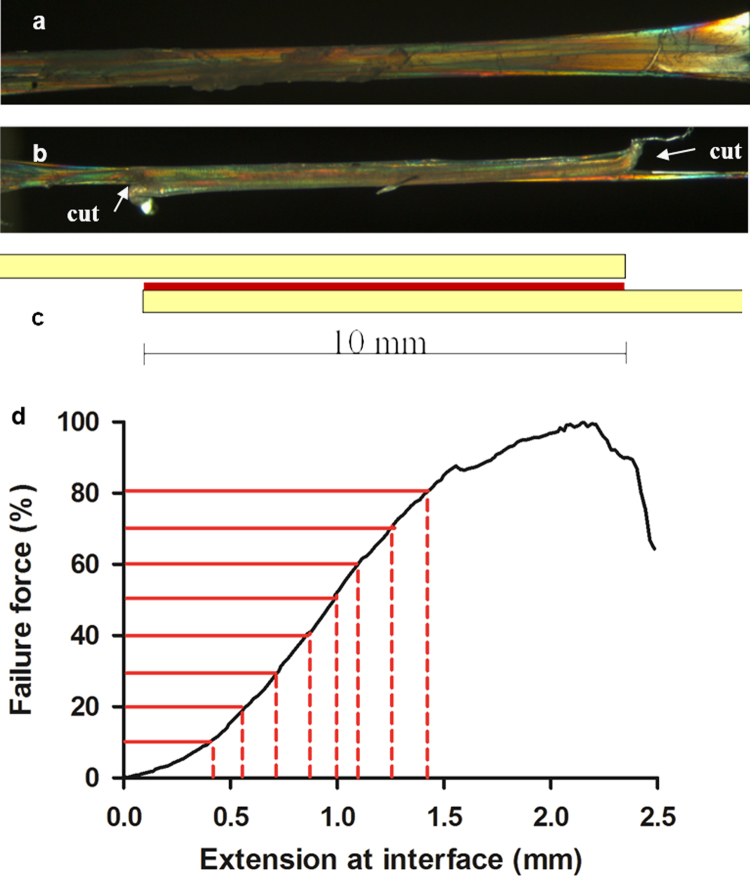
Approximately 12 groups of two intact fascicles (bound by interfascicular matrix), were dissected from each tendon as described previously (Thorpe et al., 2012). The fascicles were secured into a custom-made dissection rig, which was placed under a stereomicroscope fitted with an analyser and rotatable polarising lens (Leica). This generates elliptically polarised light, which enables clear visualisation of the individual collagen fascicles (Fig. 1a). The opposing end of each fascicle was cut transversely, leaving a consistent 10 mm length of intact IFM (Fig. 1b and c).
Fig. 1.
Images illustrating dissection for mechanical testing of the IFM. Two fascicles, bound by IFM, were dissected from the SDFT and CDET (a). The opposing end of each fascicle was cut transversely, leaving a 10 mm length of intact IFM connecting the fascicles (b and c). The fascicles were then pulled apart to failure, and the amount of extension at different percentages of failure force was calculated (d). Adapted with kind permission from eCM journal (Thorpe et al., 2013c).
After removal from the dissection rig, the intact end of each fascicle was secured in an electrodynamic testing machine (Instron ElectroPuls 1000) using pneumatic grips, with a grip to grip distance of 20 mm. A preload of 0.02 N was applied (equivalent to approximately 1% of failure load) and the distance between the grips, after pre-load, was recorded as the effective gauge length. The samples were then pre-conditioned with 10 loading cycles between 0 and 0.5 mm of extension (approx. 25% of failure extension) using a sine wave at frequency of 1 Hz. The samples were then pulled apart to failure at a speed of 1 mm/s. Force and extension data were continuously recorded at 100 Hz during preconditioning. For each test, the location of sample failure was also recorded, and any fascicles that broke, rather than pulling apart were excluded from further analysis.
The per cent hysteresis and stress relaxation that occurred during preconditioning was calculated for each sample as described in Section 2.1. The displacement at which the initial pre-load was reached was taken as the start point for the test to failure and the extension was measured as grip-to-grip displacement. A force-extension curve was derived for each sample, from which the amount of IFM extension was calculated at different percentages of IFM failure load as described previously (Fig. 1d) (Thorpe et al., 2012). The stiffness was calculated across every 10 data points of each force extension curve. The point at which the maximum stiffness was reached was taken as the yield point, and force and extension at the yield point were established.
2.3. Direct determination of the effect of preconditioning on IFM properties
To directly determine the effect of preconditioning on the properties of the IFM, and allow comparison of the current data with our previous study in which IFM samples were not preconditioned, the SDFT and CDET were harvested from an additional 2 horses (aged 4 and 5 years), enabling a paired analysis of IFM response before and after preconditioning. Samples (n=20) were dissected from each tendon, half of which were preconditioned and tested to failure (as described in Section 2.2) while the remaining samples were immediately tested to failure (as described in our previous methodology (Thorpe et al., 2012)). The amount of IFM extension was calculated at different percentages of failure load for both test groups, as described in Section 2.2.
2.4. Statistical analysis
Statistical differences between tendon types or age groups were determined using Analysis of Variance (Minitab 17). A general linear model was fitted to the data, with tendon type, age group and horse number included as factors. Data were tested for normality using the Anderson–Darling test. Data that did not follow a normal distribution were transformed using a Box-Cox transformation. Data are displayed as mean±SD.
3. Results
3.1. Fascicle material properties
Fascicle material properties are shown in Table 1. Considering viscoelastic properties of the fascicles determined during preconditioning, total hysteresis was significantly greater in CDET fascicles than in those from the SDFT (p<0.0001) and did not alter with ageing. The percentage stress relaxation did not differ significantly between SDFT and CDET fascicles from young horses, but increased significantly with ageing in the CDET (p<0.0001), resulting in significantly greater stress relaxation in CDET fascicles than in SDFT fascicles from old horses (p<0.0001, Table 1).
Table 1.
Material properties of fascicles from the SDFT and CDET from horses aged 3 to 7 years (young age group) and 17 to 20 years (old age group). Data are displayed as mean±SD.
| SDFT fascicle |
CDET fascicle |
|||
|---|---|---|---|---|
| Young | Old | Young | Old | |
| CSA (mm2) | 0.06±0.02 | 0.06±0.03 | 0.07±0.03 | 0.08±0.03 |
| Hysteresis (%) | 35.95±5.90 | 36.01±6.20 | 42.78±6.77b | 47.02±6.26b |
| Stress relaxation (%) | 12.09±2.22 | 12.04±3.99 | 14.13±3.29 | 19.45±5.28b,d |
| Failure stress (MPa) | 44.46±13.68 | 50.82±15.34c | 48.20±13.84 | 41.358±11.28a |
| Failure strain (%) | 11.63±2.09 | 11.61±2.26 | 14.69±2.54b | 15.45±2.28b |
| Elastic modulus (MPa) | 589.94±149.52 | 676.84±177.16c | 528.81±175.57 | 417.20±121.08b,c |
Significant difference between tendon types: ap<0.01, bp<0.001.
Significant difference between age groups: cp<0.05, dp<0.001.
In agreement with our previous studies of fascicle failure properties (Thorpe et al., 2012), there was no significant difference in failure stress or modulus between fascicles from the young SDFT and CDET, with values in the current study similar to those reported previously (Thorpe et al., 2013c). Further supporting previous findings, failure strain was significantly higher in fascicles from the CDET than those from the SDFT (p<0.0001). Ageing resulted in a significant increase in failure stress and modulus in SDFT fascicles (p≤0.043), and a decrease in modulus in fascicles from the CDET (p=0.032, Table 1).
3.2. IFM mechanical properties
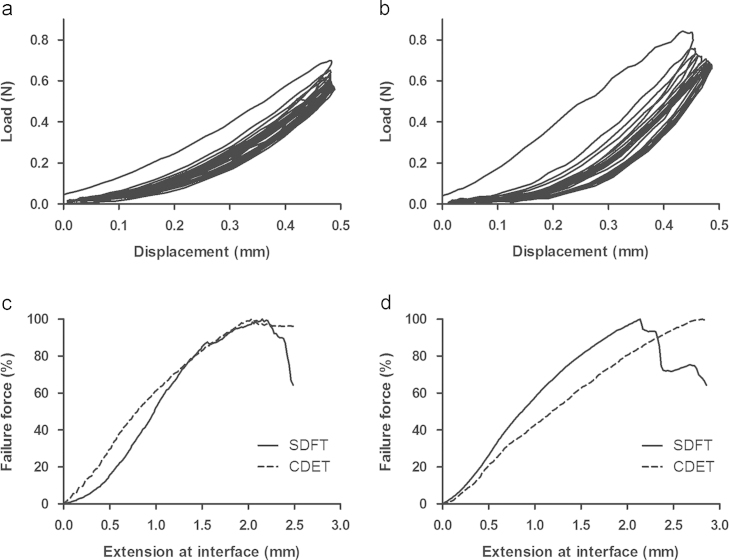
Typical preconditioning response and force extension curves for the SDFT and CDET IFM are shown in Fig. 2, and IFM mechanical properties are shown in Table 2.
Fig. 2.
Typical response of the IFM to 10 preconditioning cycles, in the young SDFT (a) and CDET (b). After preconditioning, IFM samples were pulled to failure. Typical IFM force-extension curves to failure are shown for the SDFT and CDET of young (c) and old (d) horses.
Table 2.
Mechanical properties of the IFM in the SDFT and CDET from young horses (aged 3 to 7 years) and old horses (aged 17 to 20 years). Data are displayed as Mean±SD.
| SDFT IFM |
CDET IFM |
|||
|---|---|---|---|---|
| Young | Old | Young | Old | |
| Hysteresis (%) | 44.72±7.70 | 44.79±6.60 | 55.99±7.57b | 57.95±4.73b |
| Stress relaxation (%) | 14.48±6.67 | 17.42±4.45c | 20.44±6.03a | 28.45±6.20b,e |
| Extension at failure (mm) | 1.96±0.45 | 1.84±0.51 | 2.26±0.70 | 3.02±0.70b,d |
| Force at failure (N) | 1.91±0.66 | 2.30±0.74 | 1.94±0.88 | 2.38±0.91 |
| Maximum stiffness (N/mm) | 1.54±0.43 | 2.14±0.44e | 1.67±0.49 | 1.45±0.48b |
| Extension at yield point (mm) | 0.92±0.28 | 0.59±0.20e | 0.53±0.19b | 0.63±0.28 |
| Force at yield point (N) | 0.88±0.41 | 0.83±0.42 | 0.52±0.34b | 0.56±0.38a |
Significant difference between tendon types: ap<0.01, bp<0.001.
Significant difference between age groups: cp<0.05, dp<0.01, ep<0.001.
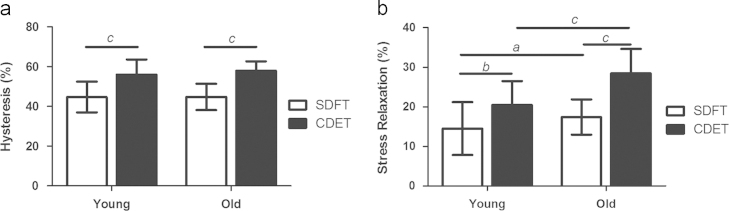
Assessment of viscoelastic properties during preconditioning cycles demonstrated greater hysteresis in the IFM of the CDET than the SDFT in samples from both young and old horses (p≤0.001, Fig. 3a). Hysteresis did not alter with ageing in either tendon type. The percentage of stress relaxation was significantly greater in CDET than SDFT IFM samples, in both young and old horses (p≤0.009. Fig. 3b). Stress relaxation also increased with ageing in both tendon types (p<0.03, Fig. 3b).
Fig. 3.
Total hysteresis (a) and stress relaxation (b) in the IFM of the SDFT and CDET during preconditioning, showing the response from young and old horses. Significant differences: a, p<0.05; b, p<0.01; c, p<0.001. Data are displayed as mean±SD.
Concerning the quasi-static test to failure that followed preconditioning, there were no significant differences in IFM failure load or failure extension between tendon types in young horses. However, failure extension increased significantly with ageing in CDET samples (p<0.0001), leading to a significantly greater failure extension in CDET samples than in SDFT samples in aged horses (p=0.005, Table 2).
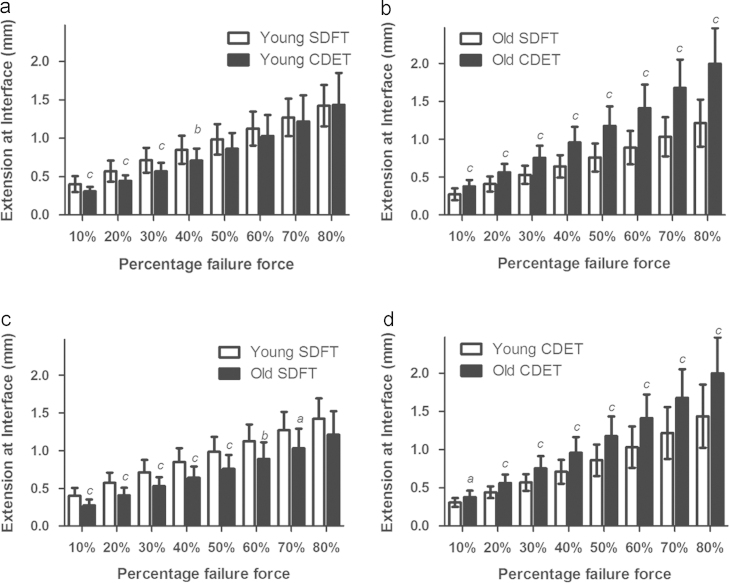
While there were no significant differences in IFM maximum stiffness or failure properties between samples from the SDFT and CDET from young horses, the shape of the force extension curve differed significantly between tendon types (Fig. 2c and Fig. 4a). At and below 40% of failure force, there was significantly greater extension within the SDFT IFM than the CDET IFM (Fig. 4a). This resulted in the amount of extension at the yield point, defined as the point at which the maximum stiffness was reached, being significantly greater in the SDFT (p<0.001, Table 2). Further, the amount of force at the yield point was significantly greater.
Fig. 4.
Amount of IFM extension at different percentages of failure load, showing IFM extension in SDFT and CDET samples from young (a) and old horses (b), and how IFM extension is altered with ageing in the SDFT (c) and CDET (d). Significant differences: a, p<0.05; b, p<0.01; c, p<0.001. Data are displayed as mean±SD.
With ageing, the shape of the IFM force-extension curve changed significantly in both tendon types (Fig. 2d and Fig. 4c and d). In the SDFT, while there were no changes in the failure properties of the IFM with ageing, maximum stiffness increased, and the amount of extension at the yield point decreased (p<0.001, Table 2). This resulted in significantly less extension at the interface through the early stages of the curve, up to 70% of failure load (p≤0.04. Fig. 4c) in aged SDFT samples. In the CDET, ageing resulted in a significant increase in failure extension (p=0.005) and a corresponding increase in IFM extension at all percentages of failure load (p≤0.01). However, there were no alterations in maximum stiffness or yield point with ageing in the CDET.
3.3. Effect of preconditioning on IFM mechanical properties
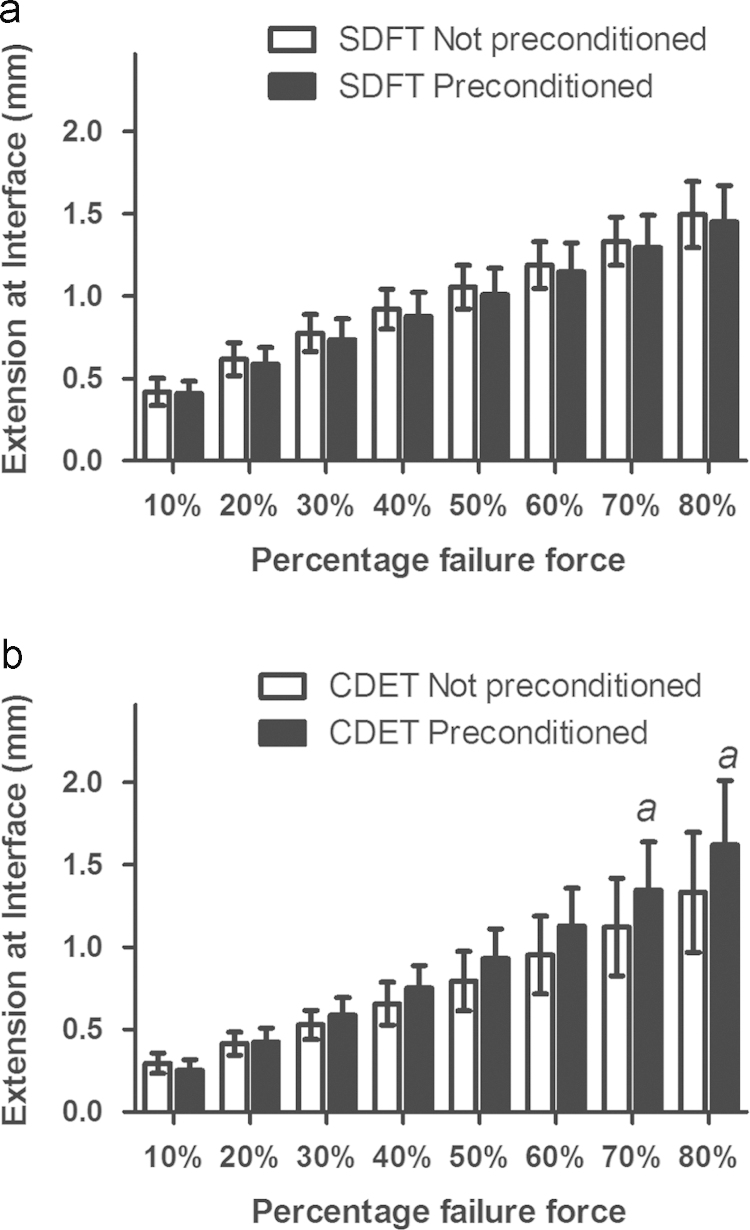
Direct comparison of the failure properties of preconditioned and non-preconditioned IFM samples demonstrated that preconditioning did not have a significant effect on the failure load of samples from either tendon type. Failure extension was not affected by preconditioning in SDFT samples, but there was a trend towards increased failure extension in samples from the CDET that had been preconditioned (p=0.051).
There was a consistent trend towards decreased extension at all percentages of failure load in preconditioned samples from the SDFT, (Fig. 5a) but this was not significant. In the CDET, preconditioning resulted in a significant increase in extension at 70 and 80% of failure load (p<0.048, Fig. 5b).
Fig. 5.
Effect of preconditioning on IFM mechanical properties in the SDFT (a) and CDET (b). Significant differences: a, p<0.05. Data are displayed as mean±SD.
4. Discussion
In this study, we demonstrate for the first time that the low stiffness mechanical behaviour seen in tendon IFM during loading is not simply due to irreversible deformation. Instead, our data show that the IFM is able to resist and recover from cyclic loading. Furthermore, in support of our hypothesis, the ability of the IFM to recover was greater in the energy storing SDFT (lower stress relaxation) than in the positional CDET indicating more elastic behaviour in the SDFT IFM. However, we also demonstrated increased stress relaxation in the IFM with ageing in both tendon types, and alterations in the shape of the force extension curve with increasing age in the SDFT specifically, indicating that the IFM becomes less elastic with ageing, and is also less able to perform its mechanical function in the aged SDFT.
It is well established that extension at the lower levels of the tendon hierarchy occurs due to sliding between adjacent fibres and fibrils, rather than due to fibril or fibre extension (Cheng and Screen, 2007, Thorpe et al., 2013b, Szczesny and Elliott, 2014). Further, more recent work has shown that this response differs between tendon types, with greater levels of inter-fibre sliding measured in the positional CDET than in the energy storing SDFT (Thorpe et al., 2013b). However, with the exception of our previous work (Thorpe et al., 2012), very few studies have assessed if this sliding behaviour occurs at the fascicular level. It has been reported that there is little force transmission between fascicles from the human Achilles tendon (Haraldsson et al., 2008) or within the bovine Achilles and digital flexor tendons (Purslow, 2009). However, these experiments were carried out at very low forces and therefore support our hypothesis that small forces are sufficient to facilitate inter-fascicle sliding, particularly in energy storing tendons. The current study and our previous results (Thorpe et al., 2012, Thorpe et al., 2013c), all demonstrate greater capacity for interfascicular sliding at low forces in the energy storing SDFT than in the positional CDET. Further, in the current study we have shown that greater forces and extensions are required to plastically deform the IFM in the SDFT, building further evidence that the IFM is specialised in energy storing tendons to allow fascicle sliding, providing an important extension mechanism in energy storing tendons, and enabling the large extensions required by this tendon type.
The current study adopted 10 preconditioning cycles to investigate fascicle and IFM response to cyclic loading, followed by a quasi-static test to failure, providing data to compare with our original study of fascicle and IFM quasi-static properties with no preconditioning. Many previous studies have investigated how tendons and fascicles respond to cyclic loading, although none have investigated the IFM. These previous studies have shown that energy storing tendons and their constituent fascicles are more fatigue resistant than positional tendons (Ker et al., 2000, Thorpe et al., 2015). Hysteresis has also previously been shown to be greater in positional tendons and their constituent fascicles than in energy storing tendons (Thorpe et al., 2013b, Vereecke and Channon, 2013), a finding supported by our current study results. However, previous studies have also shown greater stress relaxation in fascicles from positional tendons (Screen et al., 2012, Shepherd et al., 2013). Whilst perhaps surprising that the current study did not show this response, it is important to recall that only 10 cycles of preconditioning have been characterised in this work. This is far fewer than monitored in a typical cyclic stress relaxation test, and only tracks the initial stages of the stress relaxation response, which may provide insufficient time for significant differences to become apparent. Indeed, 1800 cycles of loading were required before a significant difference in the stress relaxation response of bovine flexor and extensor tendon fascicles was seen (Shepherd et al., 2013).
While tendon and fascicle viscoelastic responses to loading have been well characterised, to the author׳s knowledge this is the first study to assess the viscoelastic response of the IFM. Comparing fascicles and IFM from each tendon type, hysteresis was larger in the IFM than fascicles in both tendon types (8% greater in the SDFT and 10–13% greater in the CDET). Stress relaxation was 2–5% higher in the IFM than fascicles in the SDFT but 6–10% higher in the CDET. When comparing IFM mechanics between the CDET and SDFT directly, both hysteresis and stress relaxation were significantly higher in the CDET than the SDFT IFM, all indicating a more elastic IFM in the SDFT with a greater ability to recover from cyclic loading.
The quasi-static tests to failure can be usefully considered in light of our previous work (Thorpe et al., 2012), facilitating an analysis of how preconditioning influences IFM mechanics. However, to support this analysis further, we have also directly compared IFM response before and after preconditioning. The current quasi-static failure tests of the SDFT and CDET IFM still showed greater extensibility at low forces in the SDFT than CDET, even after preconditioning. However, in the current study, the difference post preconditioning was only significant up to 40% failure force, compared to 60% of failure force in samples tested without the preconditioning step as reported previously (Thorpe et al., 2013c). Preconditioning protocols are used to provide a consistent loading history in a sample prior to testing, and it is thought that the viscoelastic response of fascicles during cyclic testing occurs as a result of both water movement through the tissue and structural reorganisation through the tissue hierarchy, fully aligning the tissue in direction of loading (Screen et al., 2011). The structural changes occurring during pre-conditioning of the IFM remain unclear, but it is interesting to note that after preconditioning, the low stiffness behaviour in the SDFT IFM is still evident, providing further support that IFM extension does not result in immediate damage, but the IFM is more elastic in energy storing tendons.
Having previously shown changes to the quasi-static properties of the IFM with ageing (Thorpe et al., 2013c), in the current study, we also investigated how ageing affects the viscoelastic behaviour of fascicles and the IFM, and if the IFM in aged samples was less able to tolerate load after preconditioning.
Regarding age-related changes in fascicle properties, the viscoelastic properties of SDFT fascicles were not altered with ageing, but stress relaxation increased with ageing in CDET fascicles. We have previously considered only viscoelastic behaviour in SDFT fascicles, showing an increase in hysteresis with ageing in the SDFT (Thorpe et al., 2013b). However, in our previous study, fascicles were exposed to strains higher than those used in the current study, and held at these strains for 1 min before returning, so the results of this and the current study are not directly comparable. The results of the current study suggest that fascicles are equally well able to recover from low levels of cyclic loading, regardless of age.
Fascicle quasi-static tests to failure showed that failure stress and elastic modulus increased with age in SDFT fascicles, whereas elastic modulus decreased with ageing in the CDET. Whilst the CDET data agrees with previous findings (Thorpe et al., 2013c), we previously demonstrated no alterations in fascicle failure properties in the SDFT with increasing age. It is possible that the increases in SDFT fascicle failure stress and modulus are due to the age-related formation of advanced glycation end-product crosslinks, which have been shown to accumulate with age in the SDFT (Thorpe et al., 2010), and can result in increased tissue stiffness (Reddy, 2004). It has also recently been demonstrated that protein profile alters with ageing in tendon fascicles (Peffers et al., 2014), which could additionally affect mechanical properties.
While some age-related alterations in fascicle mechanical properties were identified, the majority of ageing changes occurred within the IFM, with alterations in both viscoelastic and quasi-static properties. The amount of stress relaxation in the IFM increased with ageing in both the SDFT and CDET, indicating that the ability of the IFM to recover from applied load decreases with age. Further, whilst the quasi-static failure properties of the IFM were unaltered with ageing in the SDFT, the failure extension of the IFM increased with age in the CDET. This increase in CDET IFM extension with ageing is likely due to a degree of sample elongation associated with preconditioning; stress relaxation was greatest in the old CDET, and almost double that measured in the SDFT IFM, suggesting that the greatest amount of structural realignment occurred in these samples (Screen et al., 2011).
There were also significant alterations in IFM stiffness and the shape of the IFM force extension curves with increasing age. In the SDFT, there was an increase in IFM maximum stiffness, and a decrease in the extension at which this stiffness was reached, in old compared to young samples. There was a corresponding decrease in the amount of extension at the fascicular interface below 70% of failure load with ageing in the SDFT. This supports our previous findings in samples without preconditioning (Thorpe et al., 2013c) that the SDFT IFM becomes stiffer with ageing, such that there is less capacity for interfascicular sliding.
However, the CDET showed greater extension at the interface with increasing age. This does not match our previous findings, but is likely a direct result of the preconditioning cycles and the increased overall extensibility of the aged CDET IFM. If the extension at the interface is calculated as a percentage of the failure extension (as opposed to as an absolute value) for both the young and old CDET IFM samples, no significant differences are evident with ageing in CDET IFM samples (data not shown).
There are several limitations of this study that should be considered. The unbalanced shear model used to test the IFM may result in interface rotation and tension perpendicular to the loading axis, potentially generating errors. However, it is not possible to use a balanced test design without causing considerable damage to the samples during dissection. Further, a short length of IFM was tested in isolation, this is likely to be very different to the loading conditions the IFM experiences in situ. However, it is evident from the results that, even at this short length, the IFM is able to withstand cyclic loading. It is therefore likely that, in vivo, the IFM has an even greater ability to recover from loading. An additional factor that will affect fascicle and IFM viscoelastic properties is the strain rate used during the test procedure. Samples were tested at a rate of 5%/s, which is much lower than the in vivo strain rates; it has previously been estimated that the SDFT experiences strain rates of up to 200%/s during high speed exercise (Stephens et al., 1989). Future work should investigate the effect of different strain rates on tendon and IFM viscoelastic properties.
While it is evident that the mechanical properties of the IFM differ between functionally distinct tendons, the structural specialisations that result in these differences are yet to be determined. Indeed, very little is known about the general composition of the IFM, although a small number of studies have shown that this matrix contains collagen type III, proteoglycans, elastic fibres and lubricin (Ritty et al., 2003, Funakoshi et al., 2008, Smith et al., 2011, Sodersten et al., 2012, Grant et al., 2013). Further, a recent study has demonstrated that the turnover of this matrix is greater than the turnover of the fascicular matrix (Thorpe et al., 2014a), indicating that the IFM may be more prone to damage and therefore require a greater rate of repair than the fascicles. More work is required to characterise the IFM in tendons with different functions, in order to determine which proteins enable the sliding and recovery behaviour of this matrix, and how alterations to the composition and organisation of the IFM with ageing contribute to the alterations in IFM mechanical properties that we have observed.
5. Conclusions
There is now increasing evidence to explain how energy storing tendons are specialised to meet the mechanical demands placed upon them. Previous studies suggest that the greater energy storing capacity is provided by a helical structure at the fascicle level, allowing the fascicle to act as springs (Thorpe et al., 2013b). However, this does not provide the additional extensibility required by this tendon type. The data presented in the current, and previous studies (Thorpe et al., 2012, Thorpe et al., 2013c) strongly indicate that the high strain capacity of energy storing tendons is provided by interfascicular sliding, whereby the mechanical properties of the IFM allows the tendon to stretch and store energy when loaded in vivo. Indeed, this is the first study to show that, when tested in isolation, the IFM exhibits reversible extension behaviour, and has the ability to recover from a number of loading cycles, maintaining its mechanical properties. Further, the IFM in the energy storing SDFT has a greater ability to recover from loading than its counterpart in the positional CDET. The data in this study also indicate that the IFM is less able to resist repetitive loading as it ages, becoming stiffer with increasing age in the SDFT. Full understanding of fascicle and IFM specialisation in energy storing tendons and the age-related changes that result in loss of function is important for the development of effective preventative measures and treatments for age-related tendon injury.
Acknowledgements
This work was funded by a project grant from the Biotechnology and Biological Sciences Research Council, UK (BB/K008412). The authors would like to thank Dr Stephen Thorpe for his assistance with the statistical analysis.
References
- Alexander R.M. Energy-saving mechanisms in walking and running. J. Exp. Biol. 1991;160:55–69. doi: 10.1242/jeb.160.1.55. [DOI] [PubMed] [Google Scholar]
- Batson E.L., Paramour R.J., Smith T.J., Birch H.L., Patterson-Kane J.C., Goodship A.E. Are the material properties and matrix composition of equine flexor and extensor tendons determined by their functions? Equine Vet. J. 2003;35:314–318. doi: 10.2746/042516403776148327. [DOI] [PubMed] [Google Scholar]
- Biewener A.A. Muscle-tendon stresses and elastic energy storage during locomotion in the horse. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1998;120:73–87. doi: 10.1016/s0305-0491(98)00024-8. [DOI] [PubMed] [Google Scholar]
- Birch H.L., Worboys S., Eissa S., Jackson B., Strassburg S., Clegg P.D. Matrix metabolism rate differs in functionally distinct tendons. Matrix Biol. 2008;27:182–189. doi: 10.1016/j.matbio.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Cheng V.W.T., Screen H.R.C. The micro-structural strain response of tendon. J. Mater. Sci. 2007;42:8957–8965. [Google Scholar]
- Funakoshi T., Schmid T., Hsu H., Spector M. Lubricin distribution in the goat infraspinatus tendon: a basis for interfascicular lubrication. J. Bone Jt. Surg. Am. 2008;90:803–814. doi: 10.2106/JBJS.G.00627. [DOI] [PubMed] [Google Scholar]
- Grant T.M., Thompson M.S., Urban J., Yu J. Elastic fibres are broadly distributed in tendon and highly localized around tenocytes. J. Anat. 2013;222:573–579. doi: 10.1111/joa.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraldsson B.T., Aagaard P., Qvortrup K., Bojsen-Moller J., Krogsgaard M., Koskinen S., Kjaer M., Magnusson S.P. Lateral force transmission between human tendon fascicles. Matrix Biol. 2008;27:86–95. doi: 10.1016/j.matbio.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Hess G.W. Achilles tendon rupture: a review of etiology, population, anatomy, risk factors, and injury prevention. Foot Ankle Spec. 2010;3:29–32. doi: 10.1177/1938640009355191. [DOI] [PubMed] [Google Scholar]
- Huang H., Zhang J., Sun K., Zhang X., Tian S. Effects of repetitive multiple freeze-thaw cycles on the biomechanical properties of human flexor digitorum superficialis and flexor pollicis longus tendons. Clin. Biomech. 2011;26:419–423. doi: 10.1016/j.clinbiomech.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Innes J.F., Clegg P. Comparative rheumatology: what can be learnt from naturally occurring musculoskeletal disorders in domestic animals? Rheumatology. 2010;49:1030–1039. doi: 10.1093/rheumatology/kep465. [DOI] [PubMed] [Google Scholar]
- Kasashima Y., Takahashi T., Smith R.K., Goodship A.E., Kuwano A., Ueno T., Hirano S. Prevalence of superficial digital flexor tendonitis and suspensory desmitis in Japanese Thoroughbred flat racehorses in 1999. Equine Vet. J. 2004;36:346–350. doi: 10.2746/0425164044890580. [DOI] [PubMed] [Google Scholar]
- Ker R.F., Wang X.T., Pike A.V. Fatigue quality of mammalian tendons. J. Exp. Biol. 2000;203:1317–1327. doi: 10.1242/jeb.203.8.1317. [DOI] [PubMed] [Google Scholar]
- Knobloch K., Yoon U., Vogt P.M. Acute and overuse injuries correlated to hours of training in master running athletes. Foot Ankle Int. 2008;29:671–676. doi: 10.3113/FAI.2008.0671. [DOI] [PubMed] [Google Scholar]
- Lichtwark G.A., Wilson A.M. In vivo mechanical properties of the human Achilles tendon during one-legged hopping. J. Exp. Biol. 2005;208:4715–4725. doi: 10.1242/jeb.01950. [DOI] [PubMed] [Google Scholar]
- Lui P.P.Y., Maffulli N., Rolf C., Smith R.K.W. What are the validated animal models for tendinopathy? Scand. J. Med. Sci. Sports. 2010;21:3–17. doi: 10.1111/j.1600-0838.2010.01164.x. [DOI] [PubMed] [Google Scholar]
- Maganaris C.N., Paul J.P. In vivo human tendon mechanical properties. J. Physiol. 1999;521:307–313. doi: 10.1111/j.1469-7793.1999.00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peffers M.J., Thorpe C.T., Collins J.A., Eong R., Wei T.K.J., Screen H.R.C., Clegg P.D. Proteomic analysis reveals age-related changes in tendon matrix composition, with age- and injury-specific matrix fragmentation. J. Biol. Chem. 2014;289:25867–25878. doi: 10.1074/jbc.M114.566554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins N.R., Reid S.W.J., Morris R.S. Risk factors for injury to the superficial digital flexor tendon and suspensory apparatus in thoroughbred racehorses in New Zealand. N. Z. Vet. J. 2005;53:184–192. doi: 10.1080/00480169.2005.36503. [DOI] [PubMed] [Google Scholar]
- Purslow, P.P., 2009. The shear modulus of connections between tendon fascicles. In Proceedngs of the Science and Technology for Humanity (TIC-STH), 2009 IEEE Toronto International Conferenceed, pp. 134–136.
- Reddy G.K. Cross-linking in collagen by nonenzymatic glycation increases the matrix stiffness in rabbit achilles tendon. Exp. Diabesity Res. 2004;5:143–153. doi: 10.1080/15438600490277860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritty T.M., Roth R., Heuser J.E. Tendon cell array isolation reveals a previously unknown fibrillin-2-containing macromolecular assembly. Structure. 2003;11:1179–1188. doi: 10.1016/s0969-2126(03)00181-3. [DOI] [PubMed] [Google Scholar]
- Screen H.R.C., Seto J., Krauss S., Boesecke P., Gupta H.S. Extrafibrillar diffusion and intrafibrillar swelling at the nanoscale are associated with stress relaxation in the soft collagenous matrix tissue of tendons. Soft Matter. 2011;7:11243–11251. [Google Scholar]
- Screen H.R.C., Toorani S., Shelton J.C. Microstructural stress relaxation mechanics in functionally different tendons. Med. Eng.Phys. 2012;35:96–102. doi: 10.1016/j.medengphy.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Shepherd J.H., Legerlotz K., Demirci T., Klemt C., Riley G.P., Screen H.R. Functionally distinct tendon fascicles exhibit different creep and stress relaxation behaviour. Proc. Inst. Mech. Eng. H. 2013;228:49–59. doi: 10.1177/0954411913509977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K.D., Vaughan-Thomas A., Spiller D.G., Innes J.F., Clegg P.D., Comerford E.J. The organisation of elastin and fibrillins 1 and 2 in the cruciate ligament complex. J. Anat. 2011;218:600–607. doi: 10.1111/j.1469-7580.2011.01374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodersten F., Hultenby K., Heinegard D., Johnston C., Ekman S. Immunolocalization of collagens (I and III) and cartilage oligomeric matrix protein (COMP) in the normal and injured equine superficial digital flexor tendon. Connect. Tissue Res. 2012;54:62–69. doi: 10.3109/03008207.2012.734879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens P.R., Nunamaker D.M., Butterweck D.M. Application of a Hall-effect transducer for measurement of tendon strains in horses. Am. J. Vet. Res. 1989;50:1089–1095. [PubMed] [Google Scholar]
- Szczesny S.E., Elliott D.M. Interfibrillar shear stress is the loading mechanism of collagen fibrils in tendon. Acta Biomater. 2014 doi: 10.1016/j.actbio.2014.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe C.T., Birch H.L., Clegg P.D., Screen H.R. The role of the non-collagenous matrix in tendon function. Int. J. Exp. Pathol. 2013;94:248–259. doi: 10.1111/iep.12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe C.T., Chaudhry S., Lei I.I., Varone A., Riley G.P., Birch H.L., Clegg P.D., Screen H.R.C. Tendon overload results in alterations in cell shape and increased markers of inflammation and matrix degradation. Scand. J. Med. Sci. Sports. 2014 doi: 10.1111/sms.12333. http://dx.doi.org/10.1111/sms.12333, published online: 30 DEC 2014. [DOI] [PubMed] [Google Scholar]
- Thorpe C.T., Klemt C., Riley G.P., Birch H.L., Clegg P.D., Screen H.R. Helical sub-structures in energy-storing tendons provide a possible mechanism for efficient energy storage and return. Acta Biomater. 2013;9:7948–7956. doi: 10.1016/j.actbio.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Thorpe C.T., Riley G.P., Birch H.L., Clegg P.D., Screen H.R. Fascicles from energy-storing tendons show an age-specific response to cyclic fatigue loading. J. R. Soc. Interface. 2014;11:20131058. doi: 10.1098/rsif.2013.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe C.T., Riley G.P., Birch H.L., Clegg P.D., Screen H.R.C. Effect of fatigue loading on structure and functional behaviour of fascicles from energy-storing tendons. Acta Biomateri. 2014:S1742–S7061. doi: 10.1016/j.actbio.2014.04.008. [DOI] [PubMed] [Google Scholar]
- Thorpe C.T., Spiesz E.M., Chaudhry S., Screen H.R.C., Clegg P.D. Science in brief: recent advances into understanding tendon function and injury risk. Equine Vet. J. 2015;47:137–140. doi: 10.1111/evj.12346. [DOI] [PubMed] [Google Scholar]
- Thorpe C.T., Streeter I., Pinchbeck G.L., Goodship A.E., Clegg P.D., Birch H.L. Aspartic acid racemization and collagen degradation markers reveal an accumulation of damage in tendon collagen that is enhanced with aging. J. Biol. Chem. 2010;285:15674–15681. doi: 10.1074/jbc.M109.077503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe C.T., Udeze C.P., Birch H.L., Clegg P.D., Screen H.R.C. Specialization of tendon mechanical properties results from interfascicular differences. J. R. Soc. Interface. 2012;9:3108–3117. doi: 10.1098/rsif.2012.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe C.T., Udeze C.P., Birch H.L., Clegg P.D., Screen H.R.C. Capacity for sliding between tendon fascicles decreases with ageing in injury prone equine tendons: a possible mechanism for age-related tendinopathy? Eur. Cells Mater. 2013;25:48–60. doi: 10.22203/ecm.v025a04. [DOI] [PubMed] [Google Scholar]
- Vereecke E.E., Channon A.J. The role of hind limb tendons in gibbon locomotion: springs or strings? J. Exp. Biol. 2013;216:3971–3980. doi: 10.1242/jeb.083527. [DOI] [PubMed] [Google Scholar]
- Wang X.T., Ker R.F., Alexander R.M. Fatigue rupture of wallaby tail tendons. J. Exp. Biol. 1995;198:847–852. doi: 10.1242/jeb.198.3.847. [DOI] [PubMed] [Google Scholar]