Abstract
Recently, murine hematopoietic progenitor stem cells (HSCs) and very small embryonic-like stem cells (VSELs) were demonstrated to express receptors for sex hormones including follicle-stimulating hormone (FSH). This raised the question of whether FSH therapy at clinically applied doses can mobilize stem/progenitor cells in humans. Here we assessed frequencies of VSELs (referred to as Lin−CD235a−CD45−CD133+ cells), HSPCs (referred to as Lin−CD235a−CD45+CD133+ cells), and endothelial progenitor cells (EPCs, identified as CD34+CD144+, CD34+CD133+, and CD34+CD309+CD133+ cells) in fifteen female patients subjected to the FSH therapy. We demonstrated that FSH therapy resulted in statistically significant enhancement in peripheral blood (PB) number of both VSELs and HSPCs. In contrast, the pattern of responses of EPCs delineated by different cell phenotypes was not uniform and we did not observe any significant changes in EPC numbers following hormone therapy. Our data indicate that FSH therapy mobilizes VSELs and HSPCs into peripheral blood that on one hand supports their developmental origin from germ lineage, and on the other hand FSH can become a promising candidate tool for mobilizing HSCs and stem cells with VSEL phenotype in clinical settings.
1. Introduction
Maintenance of appropriate size and composition of both stem cell and progenitor cell pool is tightly regulated by continuous responding to surrounding and long-range orchestrating signals. Interestingly, sex hormones appeared lastly as important regulators of hematopoietic stem/progenitor cells (HSPCs) proliferation [1]. Recently, Nakada and colleagues revealed that hematopoietic stem cells (HSCs) expressed high levels of estrogen receptor and the administration of estradiol increased HSC cell division and self-renewal [2]. In support of this notion, murine HSPCs along with very small embryonic-like stem cells (VSELs) were also recently demonstrated to express receptors for pituitary-derived sex hormones, namely, follicle-stimulating hormone (FSH) and luteinizing hormone (LH) [3]. In concert with this finding, murine HSPCs and VSELs following either in vitro or in vivo FSH and LH stimulation presented with high proliferative response as evidenced by BrdU incorporation. In the light of above mentioned observations, it is tempting to hypothesize the existence of developmental link between HSCs and VSELs and primordial germ cells (PGCs) that are naturally responsive to sex hormones [4, 5].
To date, however, it remained unknown whether the fact that stem cells are susceptible to signaling mediated by sex hormones can be used for mobilization of these cells in clinical settings. Moreover, based on the currently available scarce data, it is difficult to speculate if therapies using sex hormones will affect only fate of primordial stem cells and HSCs or rather would exert their actions toward all progenitor cell populations. Therefore, in the current study, we wished to investigate the effects of FSH therapy at clinically applied doses on mobilization of HSCs and VSELs as well as populations of endothelial progenitor cells (EPCs). In this study, EPCs were chosen as an example of easily identifiable, highly differentiated, and relatively numerous progenitor cell populations that account for endothelial repair and thus largely contribute to maintenance of appropriate vasculature [6–8]. On the other hand, quantification of decreased numbers of EPCs was found to improve prognostication of cardiovascular diseases (CVD) [9–11]. Thus, the search for therapeutic approaches aimed at efficient mobilization of functional EPCs is continuously warranted.
Here we tested in human model the actions of widely accepted regimens of FSH treatment with regard to three stem/progenitor cell subsets at different developmental hierarchy and differentiation level, namely, VSELs, HSCs, and EPCs. Moreover, given the previous reports indicating the crucial role of stroma derived factor-1 (SDF-1) for mobilization of stem cells [12–14], we set out to analyze whether any actions of clinically applied gonadotropins could affect not only stem cells and progenitor cells but also mediators regulating their migratory pathways.
2. Material and Methods
2.1. Patients and FSH Stimulation
For the purpose of the study we recruited fifteen women aged 32.9 ± 3.9 years (range: 27–39 years) who were prepared for in vitro fertilization and underwent controlled FSH ovarian stimulation. FSH stimulation has been initiated on 3rd day of menstrual cycle and FSH dose was adjusted based on patient age, ovarian reserve, and previous response to FSH stimulation (if performed). Only two patients received stimulation based on combination of FSH and LH. EDTA-anticoagulated peripheral blood was collected twice: before FSH ovarian stimulation (or in five cases within first days of such stimulation) and at the end of FSH stimulation (days 7–11). Mean daily dose of FSH (either Gonal F, Merck Serono, or Puregon, Schering, or, in two patients, Menopur, Ferring) was 194.4 IU. Detailed characteristics of hormonal status of analyzed patients are presented in Table 1.
Table 1.
The clinical and hormonal characteristics of female patients (n = 15) subjected to FSH stimulation.
| Studied parameter | Mean | SD | Minimum | Maximum |
|---|---|---|---|---|
| Age (years) | 32.9 | 3.9 | 27 | 39 |
| Duration of stimulation (days) | 8.8 | 1.1 | 8 | 11 |
| Mean daily dose of FSH (IU) | 194.4 | 43.8 | 120 | 262.5 |
| Number of mature follicles after FSH stimulation | 10.9 | 3.4 | 5 | 16 |
| Estradiol at 7th day (pg/mL) | 1153 | 405 | 540 | 1684 |
| Progesterone at 7th day (ng/mL) | 0.5 | 0.15 | 0.25 | 0.67 |
| LH at 7th day (mIU/mL) | 1.1 | 0.67 | 0.31 | 2.33 |
| Estradiol at the last day (pg/mL) | 2314 | 1367 | 1367 | 3294 |
| Progesterone at the last day (ng/mL) | 0.8 | 0.33 | 0.32 | 1.52 |
| LH at the last day (mIU/mL) | 1.7 | 0.85 | 0.72 | 2.97 |
All patients' samples were collected upon the approval of Ethics Committee of the Medical University of Bialystok.
2.2. Extracellular Staining and Flow Cytometry
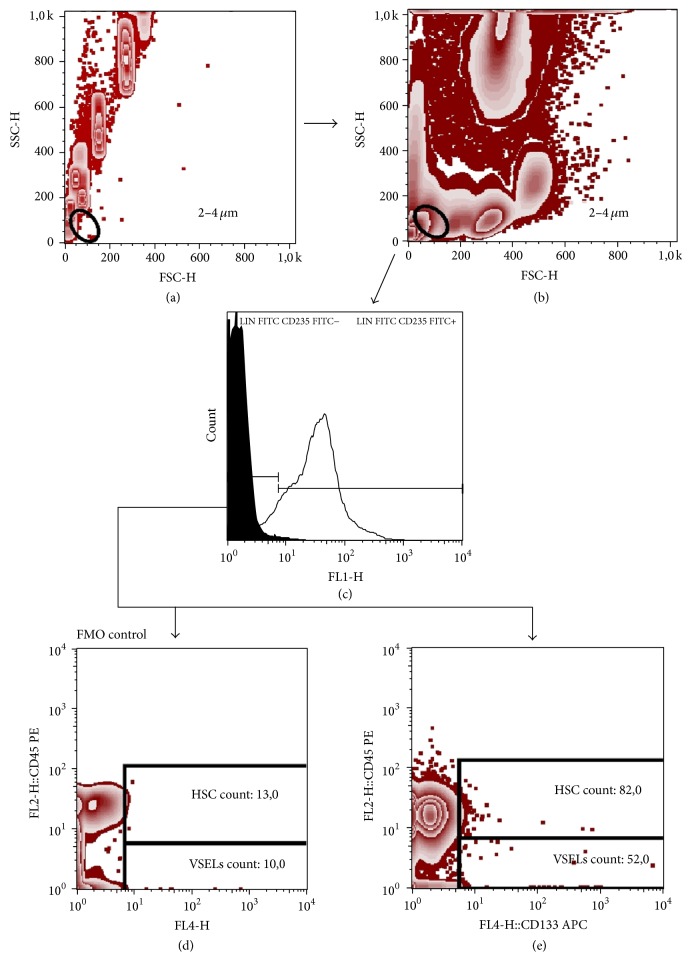
170 μL of fresh EDTA-anticoagulated whole blood was stained with the set of murine anti-human monoclonal antibodies described in detail in Table 2. Samples were incubated for 30 min at room temperature in the dark. Thereafter, 2 mL of FACS lysing solution (BD) was added, followed by 15 min incubation in the dark. Cells were washed twice with cold PBS (phosphate-buffered saline) and fixed with CellFix (BD Biosciences). Appropriate fluorescence-minus-one (FMO) controls were used for setting compensation and for assuring correct gating. The gating strategy for HSCs and VSELs is shown in Figure 1, while gating strategy for identifying CD34+ cells and EPCs is presented in Figure 2. Samples were acquired using FACSCalibur flow cytometer (BD Biosciences). Obtained data were analyzed using FlowJo version 7.6.5 software (Tree Star).
Table 2.
Detailed characteristics of monoclonal antibodies used in the study.
| Name | Clone | Isotype | Format | Additional information | Manufacturer |
|---|---|---|---|---|---|
| Mouse anti-human anti-CD34 | 8G12 | IgG1 | FITC | This antibody binds to 105–120 kDa single-chain transmembrane glycoprotein, IVD | Becton Dickinson |
| Anti-human lineage cocktail 2 (lin 2) | FITC | Becton Dickinson | |||
| Mouse anti-human anti-CD3 | SK7 | IgG1 | This antibody binds to epsilon chain of the CD3 antigen, IVD | ||
| Mouse anti-human anti-CD19 | SJ25C1 | IgG1 | This antibody recognizes a 90 kDa antigen, IVD | ||
| Mouse anti-human anti-CD20 | L27 | IgG1 | This antibody binds to phosphoprotein with a molecular weight of 35 or 37 kilodaltons (kDa), depending on the degree of phosphorylation, IVD | ||
| Mouse anti-human anti-CD14 | MφP9 | IgG2b | This antibody reacts with a 53–55 kDa glycosylphosphatidylinositol- (GPI-) anchored and single chain glycoprotein, IVD | ||
| Mouse anti-human anti-CD56 | NCAM16.2 | IgG2b | This antibody recognizes a heavily glycosylated 140 kDa isoform of NCAM, a member of the immunoglobulin (Ig) superfamily, IVD | ||
| Mouse anti-human anti-CD235a | GA-R2 | IgG2b | FITC | This antibody binds to glycophorin A, a sialoglycoprotein present on human red blood cells (RBC) and erythroid precursor cells | Becton Dickinson |
| Mouse anti-human anti-CD45 | HI30 | IgG1 | PE | This antibody binds to 190, 190, 205, and 220 kDa protein isoforms, RUO | Becton Dickinson |
| Mouse anti-human anti-CD144 | 55-7H1 | IgG1 | PE | This antibody reacts with calcium-independent epitope on cadherin 5, RUO | Becton Dickinson |
| Mouse anti-human anti-CD309 | 89106 | IgG1 | PE | This antibody reacts with CD309 (vascular endothelial growth factor receptor-2 (VEGFR-2)) | Becton Dickinson |
| Mouse anti-human anti-CD133 | AC133 | IgG1 | APC | This antibody reacts with epitope 1 of CD133, RUO | Miltenyi Biotec |
IVD: this clone is used for in vitro diagnostics; RUO: suitable for research use only.
Figure 1.
Representative FACS plots demonstrating gating strategy for HSC and VSELs. First, 2–4 μm size events were gated based on a forward and side scatter (FSC/SSC) dot plot (a). Then the 2–4 μm gate was visualized on sample data using a FSC/SSC dot plot (b). Next, 2–4 μm events were displayed on histogram plot (black peak, FMO control; grey peak identifies positive staining) and Lin−CD235a− events were gated (c). Finally, FMO control was used to set the HSC and VSELs gate and exclude the background noise (d). Next, HSCs were defined as Lin−CD235a−CD45+CD133+ cells (upper gate) and VSELs were referred to as Lin−CD235a−CD45−CD133+ cells (e).
Figure 2.
Representative FACS plots demonstrating the gating strategy for CD34+ cells and EPCs. PBMCs were gated based on forward and side scatter (FSC/SSC) plot (b). CD34+ cells were gated on CD34/SSC plot (c). CD34+CD144+ cells (upper right quadrant, Q2) were gated based on CD34/CD144 dot plot (d). FMO control was used to set the gates (a). In order to determine CD34+CD144+CD309+ cell numbers, the PBMC events were displayed on the basis of FMO control sample (e) and gates were set to exclude the random noise. Next, the gates were visualized on CD34/CD133 plot (f) and CD34+CD133+ cells were gated (upper right quadrant, Q2). Finally, CD34+CD133+CD309+ cells subsets were gated on histogram plot (g) (black peak, control; grey peak, positive staining).
2.3. Enzyme-Linked Immunosorbent Assays
SDF-1 plasma levels were quantified by means of commercially available enzyme-linked immunosorbent assays (ELISA, DuoSet, R&D). Samples were directly assayed according to manufacturer's instructions. The protein levels in the specimens were calculated from a reference curve generated by using reference standards. The detection range of used ELISA set was between 7,81 and 500 pg/mL. The samples were analyzed with automated light absorbance reader (LEDETEC 96 system). Results were calculated by MicroWin 2000 software.
2.4. Statistics
Statistical analysis was carried out using GraphPad Prism 6 (GraphPad software). Wilcoxon test was used. The differences were considered statistically significant at p < 0.05. The results are presented as medians (interquartile range).
3. Results
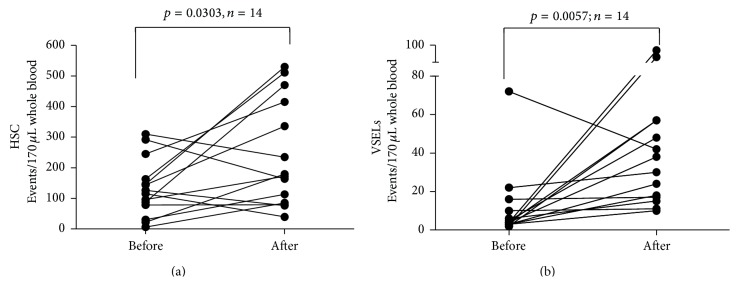
First, we analyzed the effects of FSH administration on the frequencies of circulating HSCs (Figure 3(a)). We found that FSH treatment resulted in statistically significant increase in Lin−CD133+CD45+ HSC numbers ((from 125.5 (66–133.5) to 175.5 (83.5–428.8) cells per 170 μL of whole blood), p = 0.0303). Similarly, FSH treatment led to significant enhancement of Lin−CD133+CD45− VSEL levels (Figure 3(b)). Following FSH therapy, VSEL numbers increased from 4 cells (3–11,5) to 34 cells (16,5–57) per 170 μL of whole blood (p = 0.0057).
Figure 3.
Summary of analyses of time-course changes of the numbers of HSCs (a) and VSELs (b) during FSH therapy.
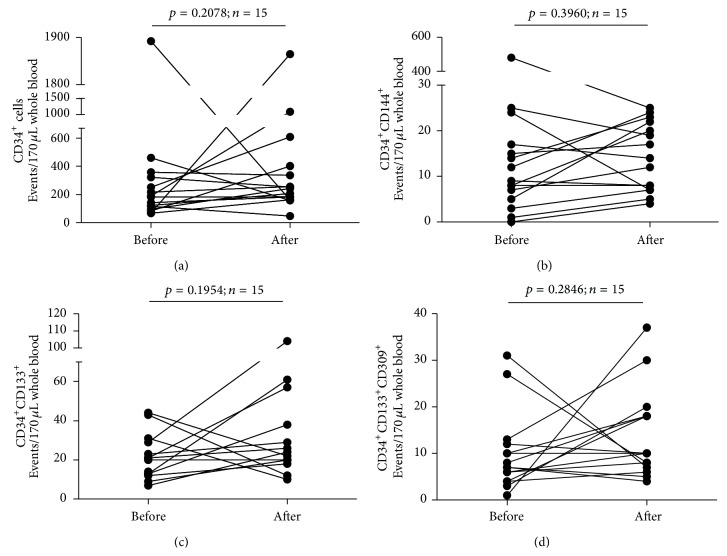
Next, we set out to investigate the influence of FSH administration on the frequencies of entire population of CD34+ cells and the population of EPCs delineated by such phenotypes as CD34+CD144+, CD34+CD133+, and CD34+CD133+CD309+ cells. Notably, we did not observe any significant changes in the numbers of single CD34+ cells following FSH administration (Figure 4(a)). Similarly, we did not demonstrate any significant changes in numbers of EPCs identified as CD34+CD144+ cells (Figure 4(b)), CD34+CD133+ cells (Figure 4(c)), and CD34+CD133+CD309+ cells (Figure 4(d)).
Figure 4.
Summary of analyses of time-course changes of the numbers of CD34+ cells (a), CD34+CD144+cells (b), CD34+CD133+ cells (c), and CD34+CD133+CD309+ cells (d) in the course of FSH therapy.
Finally, we wished to investigate whether FSH administration affected serum levels of SDF-1, one of crucial mobilizing factors for VSELs and HSCs (Figure 5). However, we did not find any significant change in SDF-1 concentrations upon completion of FSH administration (p > 0.05).
Figure 5.
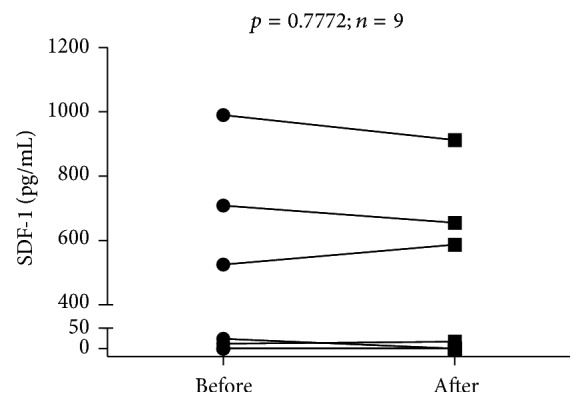
Time-course analysis of individual changes in serum levels of SDF-1 in the course of FSH therapy.
4. Discussion
Here we demonstrated that administration of pituitary-derived sex hormones at clinically applied doses allowed for efficient mobilization of frequencies of VSELs and HSCs but not endothelial progenitor cells. These findings build a platform for developing novel sex-hormone- based therapeutic strategies aimed at enhancement of either VSELs or HSCs numbers in conditions that would require efficient mobilization of these cells subsets.
Notably, it was shown that the links between gonadotropin hormones and stem cells may extend far beyond the effects exerted on VSELs and HSCs. Tadokoro and colleagues showed that FSH was one of crucial regulators of germinal stem cell (GSC) fate [15]. FSH regulated homeostatic control of glial cell line-derived neurotrophic factor (GDNF) which in turn accelerated proliferation of GSCs. These findings indicated that regulation of GCS population size is regulated by the GDNF/FSH pathway. It remains to be established whether effects of FSH treatment on VSELs and HSCs were related to actions mediated by GDNF. Recently, in concert with this notion, Tourkova and colleagues demonstrated that mesenchymal stem cells (MSC) expressing FSH-R responded with adhesion and proliferation following addition of FSH [16]. Moreover, short-term treatment by FSH at doses comparable to those observed in menopause augmented MSC proliferation by affecting signaling associated with Erk1/2 phosphorylation.
It remains elusive whether mobilizing effects of FSH/LH therapy on VSELs and HSCs in female patients were related to their direct effects on bone marrow-derived stem cells or rather on mobilization of stem cells localized in ovaries. On the other hand, both possibilities do not have to be mutually exclusive. Previously, it was demonstrated that stem cells found in ovaries have the phenotype of VSELs and they can give rise to more differentiated ovarian GCS [17, 18]. This was confirmed by later studies by Parte and colleagues who demonstrated that VSELs and GSCs were present in ovary surface epithelium (OSE) [19]. In this study, FSH treatment resulted in prominent proliferation of stem cell-harboring OSE and release of functional stem cells from ovaries. Moreover, Patel and colleagues found that FSH treatment resulted in increased clonal expansion of ovary-associated stem cells (both VSELs and ovarian GCSs), while FSH receptors were expressed only on ovarian stem cells but not on ovarian epithelial cells [20]. Similarly, Sriraman and colleagues showed that PMSG (FSH analog) increased VSELs numbers in chemoablated female mice [21]. Nevertheless, the question of how effects of gonadotropin hormones on stem cell population size are related to ovaries would require enrollment of male patients subjected to therapy with FSH and/or LH, the condition that due to ethical reasons certainly could not be achieved in the settings of the current study.
Notably, in our study, we did not demonstrate any significant effects of gonadotropin therapy on the levels of EPCs. In concert with this notion, in a representative group of patients with inflammatory bowel disease, Garolla and colleagues did not find relationships between levels of FSH or LH and numbers of EPCs [22]. In some contrast, however, reduced numbers of EPCs were found in hypogonadotropic hypogonadal male patients presenting with low levels of FSH and LH [23]. Interestingly, receptor for FSH is expressed by the endothelium of blood vessels in the majority of metastatic tumors [24]. Therefore, despite lack of significant effects of gonadotropin therapy on numbers of circulating EPCs analyzed in the current study, the subject of mutual relationships among EPCs, endothelial cells, and pituitary-derived sex hormones certainly deserves further investigation.
Our findings also bring about an interesting perspective for understanding relationships between stem and progenitor cells and elevated levels of FSH and LH that are detected at elderly age. In the light of our data, it is tempting to hypothesize that enhanced FSH and LH levels in elderly individuals could represent the mechanism of enhancing otherwise diminished hematopoiesis and decreased stem cell numbers. Similarly, this observation was previously documented in mouse model, wherein the numbers of VSELs were shown to be highest in young subjects and decreased with age [25].
Given these data, FSH and LH therapy could become an attractive and easily available tool enabling mobilization of stem cells in regenerative medicine. However, given the broad spectrum of the effects exerted by sex hormones, the safety of such approach would need to be examined in further clinical studies performed in different groups of patients including males. One has to keep in mind that FSH and LH were found to be involved in the growth of certain tumors. As an example, Ji and colleagues found significantly higher levels of mRNA encoding receptor for FSH (FSHR) in invasive ovarian tumors compared to low malignant tumors and normal OSE [26]. Similarly, it was reported that overexpression of FSHR in OSE cells led to an increase in expression of proteins involved in ovarian cancer development such as EGFR, c-myc, and HER2/neu. Thus any therapeutic strategies aimed at enhancement of the size of stem/progenitor cell pool by the use of gonadotropin-based therapies would need to be carefully investigated in terms of clinical safety.
Altogether, our data support recent findings on the role of FSH in the biology of VSELs and HSCs that were reported by our group in mouse model [4]. Thus, these data support a concept of a developmental link between germ line, VSELs, and hematopoiesis [4, 5]. Finally, we demonstrated here for the first time that mobilization of stem cells with VSELs phenotype and HSPCs can be achieved by the use of widely available therapeutic regimens based on pituitary-derived sex hormones.
Acknowledgments
The study was supported by funds of Leading National Research Center (KNOW) of Medical University of Bialystok. The authors wish to thank students of Scientific Society of Medical University of Bialystok for help in delivering blood samples.
Conflict of Interests
The University of Louisville is the owner of patents on VSELs and some areas of VSEL technology, which are licensed to Neostem Inc., New York. None of the authors have any stock in Neostem Inc., New York, or any other biotechnological stem cell company.
Authors' Contribution
Monika Zbucka-Kretowska, Andrzej Eljaszewicz, and Danuta Lipinska equally contributed to the work.
References
- 1.Gancz D., Gilboa L. Hormonal control of stem cell systems. Annual Review of Cell and Developmental Biology. 2013;29:137–162. doi: 10.1146/annurev-cellbio-101512-122331. [DOI] [PubMed] [Google Scholar]
- 2.Nakada D., Oguro H., Levi B. P., et al. Oestrogen increases haematopoietic stem-cell self-renewal in females and during pregnancy. Nature. 2014;505(7484):555–558. doi: 10.1038/nature12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mierzejewska K., Borkowska S., Suszynska E., et al. Hematopoietic stem/progenitor cells express several functional sex hormone receptors—novel evidence for a potential developmental link between hematopoiesis and primordial germ cells. Stem Cells and Development. 2015;24(8):927–937. doi: 10.1089/scd.2014.0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ratajczak M. Z., Marycz K., Poniewierska-Baran A., Fiedorowicz K., Zbucka-Kretowska M., Moniuszko M. Very small embryonic-like stem cells as a novel developmental concept and the hierarchy of the stem cell compartment. Advances in Medical Sciences. 2014;59(2):273–280. doi: 10.1016/j.advms.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Scaldaferri M. L., Klinger F. G., Farini D., et al. Hematopoietic activity in putative mouse primordial germ cell populations. Mechanisms of Development. 2015;136:53–63. doi: 10.1016/j.mod.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Rusak M., Radzikowska U., Glowinska-Olszewska B., et al. Endothelial progenitor cell levels in juvenile idiopathic arthritis patients; effects of anti-inflammatory therapies. Pediatric Rheumatology. 2015;13(1, article 6) doi: 10.1186/s12969-015-0001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khakoo A. Y., Finkel T. Endothelial progenitor cells. Annual Review of Medicine. 2005;56:79–101. doi: 10.1146/annurev.med.56.090203.104149. [DOI] [PubMed] [Google Scholar]
- 8.Goerke S. M., Obermeyer J., Plaha J., Stark G. B., Finkenzeller G. Endothelial progenitor cells from peripheral blood support bone regeneration by provoking an angiogenic response. Microvascular Research. 2015;98:40–47. doi: 10.1016/j.mvr.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Głowińska-Olszewska B., Moniuszko M., Hryniewicz A., et al. Relationship between circulating endothelial progenitor cells and endothelial dysfunction in children with type 1 diabetes: a novel paradigm of early atherosclerosis in high-risk young patients. European Journal of Endocrinology. 2013;168(2):153–161. doi: 10.1530/eje-12-0857. [DOI] [PubMed] [Google Scholar]
- 10.Lavoie J. R., Stewart D. J. Genetically modified endothelial progenitor cells in the therapy of cardiovascular disease and pulmonary hypertension. Current Vascular Pharmacology. 2012;10(3):289–299. doi: 10.2174/157016112799959413. [DOI] [PubMed] [Google Scholar]
- 11.Sen S., McDonald S. P., Coates P. T. H., Bonder C. S. Endothelial progenitor cells: novel biomarker and promising cell therapy for cardiovascular disease. Clinical Science. 2011;120(7):263–283. doi: 10.1042/cs20100429. [DOI] [PubMed] [Google Scholar]
- 12.Ratajczak M. Z., Kim C. H., Abdel-Latif A., et al. A novel perspective on stem cell homing and mobilization: review on bioactive lipids as potent chemoattractants and cationic peptides as underappreciated modulators of responsiveness to SDF-1 gradients. Leukemia. 2012;26(1):63–72. doi: 10.1038/leu.2011.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kucia M., Reca R., Miekus K., et al. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells. 2005;23(7):879–894. doi: 10.1634/stemcells.2004-0342. [DOI] [PubMed] [Google Scholar]
- 14.Kucia M., Ratajczak J., Reca R., Janowska-Wieczorek A., Ratajczak M. Z. Tissue-specific muscle, neural and liver stem/progenitor cells reside in the bone marrow, respond to an SDF-1 gradient and are mobilized into peripheral blood during stress and tissue injury. Blood Cells, Molecules, and Diseases. 2004;32(1):52–57. doi: 10.1016/j.bcmd.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 15.Tadokoro Y., Yomogida K., Ohta H., Tohda A., Nishimune Y. Homeostatic regulation of germinal stem cell proliferation by the GDNF/FSH pathway. Mechanisms of Development. 2002;113(1):29–39. doi: 10.1016/s0925-4773(02)00004-7. [DOI] [PubMed] [Google Scholar]
- 16.Tourkova I. L., Witt M. R., Li L., et al. Follicle stimulating hormone receptor in mesenchymal stem cells integrates effects of glycoprotein reproductive hormones. Annals of the New York Academy of Sciences. 2015;1335(1):100–109. doi: 10.1111/nyas.12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parte S., Bhartiya D., Telang J., et al. Detection, characterization, and spontaneous differentiation in vitro of very small embryonic-like putative stem cells in adult mammalian ovary. Stem Cells and Development. 2011;20(8):1451–1464. doi: 10.1089/scd.2010.0461. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Virant-Klun I., Zech N., Rozman P., et al. Putative stem cells with an embryonic character isolated from the ovarian surface epithelium of women with no naturally present follicles and oocytes. Differentiation. 2008;76(8):843–856. doi: 10.1111/j.1432-0436.2008.00268.x. [DOI] [PubMed] [Google Scholar]
- 19.Parte S., Bhartiya D., Manjramkar D. D., Chauhan A., Joshi A. Stimulation of ovarian stem cells by follicle stimulating hormone and basic fibroblast growth factor during cortical tissue culture. Journal of Ovarian Research. 2013;6, article 20 doi: 10.1186/1757-2215-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel H., Bhartiya D., Parte S., Gunjal P., Yedurkar S., Bhatt M. Follicle stimulating hormone modulates ovarian stem cells through alternately spliced receptor variant FSH-R3. Journal of Ovarian Research. 2013;6, article 52 doi: 10.1186/1757-2215-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Sriraman K., Bhartiya D., Anand S., Bhutda S. Mouse ovarian very small embryonic-like stem cells resist chemotherapy and retain ability to initiate oocyte-specific differentiation. Reproductive Sciences. 2015 doi: 10.1177/1933719115576727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garolla A., D’Incà R., Checchin D., et al. Reduced endothelial progenitor cell number and function in inflammatory bowel disease: a possible link to the pathogenesis. American Journal of Gastroenterology. 2009;104(10):2500–2507. doi: 10.1038/ajg.2009.332. [DOI] [PubMed] [Google Scholar]
- 23.Foresta C., de Toni L., Selice R., Garolla A., di Mambro A. Increased osteocalcin-positive endothelial progenitor cells in hypogonadal male patients. Journal of Endocrinological Investigation. 2010;33(7):439–442. doi: 10.1007/bf03346620. [DOI] [PubMed] [Google Scholar]
- 24.Siraj A., Desestret V., Antoine M., et al. Expression of follicle-stimulating hormone receptor by the vascular endothelium in tumor metastases. BMC Cancer. 2013;13, article 246 doi: 10.1186/1471-2407-13-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kucia M., Reca R., Campbell F. R., et al. A population of very small embryonic-like (VSEL) CXCR4+SSEA-1+Oct-4+ stem cells identified in adult bone marrow. Leukemia. 2006;20(5):857–869. doi: 10.1038/sj.leu.2404171. [DOI] [PubMed] [Google Scholar]
- 26.Ji Q., Liu P. I., Chen P. K., Aoyama C. Follicle stimulating hormone-induced growth promotion and gene expression profiles on ovarian surface epithelial cells. International Journal of Cancer. 2004;112(5):803–814. doi: 10.1002/ijc.20478. [DOI] [PubMed] [Google Scholar]