Abstract
Lead (Pb) is a well-studied environmental contaminant that has many negative health effects, especially for children. Both racial/ethnic and income disparities have been documented with respect to exposure to Pb in soils. The objectives of this study were to assess whether soil Pb concentrations in rural and urban areas of South Carolina USA, previously identified as having clusters of intellectual disabilities (ID) in children, were positively associated with populations of minority and low-income individuals and children (≤6 years of age). Surface soils from two rural and two urban areas with identified clusters of ID were analyzed for Pb and concentrations were spatially interpolated using inverse distance weighted analysis. Population race/ethnicity and income-to-poverty ratio (ITPR) from United States Census 2000 block group data were aerially interpolated by block group within each area. Urban areas had significantly higher concentrations of Pb than rural areas. Significant positive associations between black, non-Hispanic Latino, individuals and children ≤6 years of age and mean estimated Pb concentrations were observed in both urban (r = 0.38, p = 0.0007) and rural (r = 0.53, p = 0.04) areas. Significant positive associations also were observed between individuals and children with an ITPR < 1.00 and Pb concentrations, though primarily in urban areas. Racial/ethnic minorities and low ITPR individuals, including children, may be at elevated risk for exposure to Pb in soils.
Keywords: Lead, Residential soil, Inverse distance weighted analysis, GIS, Intellectual disabilities, Health disparities, Environmental justice, Low income
Introduction
Lead (Pb) is a ubiquitous metal in soils due to the use of leaded gasoline, as well as atmospheric fallout from incinerators and coal-fired power plants. Negative neurological effects from exposure to Pb have been documented in adults (Shih et al. 2006), and effects on infants and children may be more pronounced and severe given the rapid brain development in the early years of life (Bellinger 2008a, b; Calderón et al. 2003). In addition, soil ingestion is especially relevant for young children (≤6 years of age), due to their increased hand-to-mouth activity (ATSDR 2007).
Many studies have quantified metal concentrations in large, urban areas with potential non-point and point source contamination (Bityukova et al. 2000; Chen et al. 2010; Guan and Peart 2006; Odewande and Abimbola 2008; Tume et al. 2008; Turer et al. 2001; Wang 2008; Xia et al. 2011). Other published studies have focused on residential and rural areas suspected of contamination due to point sources and have quantified soil metal contamination from specific sources, such as industry (Hovmand et al. 2008; Stafilov et al. 2010) or pesticide application (Markovic et al. 2010; Sanghi and Sasi 2001). A few studies have measured soil metal concentrations in residential urban and rural locations without regard to specific contaminant sources (Birke and Rauch 2000; Langner et al. 2011; Yesilonis et al. 2008). We are one of the few groups to investigate whether soil metal concentrations in rural and urban residential areas, selected without regard to specific contaminant sources, may contribute to the negative health outcome of children (Aelion et al. 2008, 2009a, b; Davis et al. 2009).
The current study is part of a larger effort in which clusters of intellectual disabilities (ID) in children were identified statistically, without regard to environmental conditions or potential contaminant sources in South Carolina (SC), USA (Zhen et al. 2008, 2009). Thus, no sources of contamination were identified prior to the evaluation of the higher prevalence of ID. The population used was a low-income cohort of women and children on Medicaid assistance; thus, all were below the established US poverty limits.
The objectives of the current study were to compare two rural and two urban areas identified in our previous research as containing a cluster of ID in children (Cai et al. 2011; Kim et al. 2009, 2010; Zhen et al. 2008, 2009), and to determine, within these sampling areas, whether high populations of racial/ethnic minorities, and individuals with a low income-to-poverty ratio (ITPR), were positively associated with spatially interpolated Pb concentrations at the United States (US) Census 2000 block group level. These groups, both historically and currently, have been disproportionately exposed to environmental contaminants (Brown 1995). Data from the individuals from the previous study were not collected, but, instead, the cluster areas identified were used and Census data on race/ethnicity and ITPR for those areas were collected.
We hypothesize that both racial/ethnic minority and low-income individuals, and their children ≤6 years of age, will reside disproportionately in areas with higher estimated soil Pb concentrations when compared among the four sites. We also hypothesize that this will occur regardless of whether the location is urban or rural, because individuals are distributed similarly in both types of locations even though population numbers are different, and that environmental contamination may exist in both types of locations because SC is generally non-industrial.
Materials and methods
South Carolina is a small state (area of ∼ 83,000 km2) located in the southeastern United States (US) where approximately half of the population (2.1 million) lives outside of urban US Census 2000 areas (population of ≥50,000 people) (US ERS 2012) (Fig. 1). As part of the larger study in SC, Bayesian local likelihood cluster analysis was used to identify areas of increased prevalence of ID in children based on International Classification of Diseases and Related Health Problems Version 9 (ICD-9) codes (Cai et al. 2011; Kim et al. 2009, 2010; Zhen et al. 2008, 2009). Environmental conditions were unknown for this selection process, and there was no bias toward industrially contaminated or urban areas. Two locations (Areas 4 and 5) were classified as urban and two were classified as rural (Areas 6 and 27) (US Census 2009). The areas of sampling locations ranged from 65 to 140 km2. Areas 4 and 5 were located in the geological Blue Ridge/Piedmont area of SC and the majority surface soil type was Cecil sandy loam (USDA 2012). Area 6 was located in the Pliocene coastal plain and Area 27 is in the Pleistocene coastal plain of SC. The majority soil types in Areas 6 and 27 were Coxville sandy loam and Blanton loamy fine sand, respectively (USDA 2012). Areas 4, 5, and 27 had average soil pH values from 5.3 to 5.5, and Area 6 had an average pH of 4.7 (USDA 2012).
Fig. 1. Map of the contiguous United States and location of the state of South Carolina.
Soil sampling was carried out on a uniform grid of approximately 120 samples per area, and surface soil samples of ∼100 g were taken at a depth of up to 5 cm. Lead was analyzed using inductively coupled plasma optical emission spectroscopy (ICP-OES; Aelion and Davis 2007, Aelion et al. 2008, 2009a, b; Davis et al. 2009). Sampling locations, corresponding soil Pb concentrations and US Census 2000 block group polygons for SC were imported into ArcMap (ArcGIS version 10, ESRI, Red-lands, CA). The coordinate system used was NAD 1983 UTM Zone 17 N. Because the estimated Pb concentration of each cell is assumed to be homogenous for the entire cell area, we set the cell size to 30 × 30 m so that only one sampling location was located within a cell.
Inverse distance weighted (IDW) analysis was used to spatially interpolate measured Pb concentrations across each sampling area separately. IDW is a spatial interpolation method that assigns more weight in the interpolation process to measurements from closer points (Babak and Deutsch 2009); in other words, each raster cell value of the sampling area is a distance weighted average of nearby measured observations. Studies have shown results from IDW to be comparable to more complex spatial interpolation methods, like kriging (Kravchenko 2003; Mueller et al. 2004) and it has been used extensively to estimate different geochemical soil parameters, including soil pH (Mueller et al. 2004; Robinson and Metternicht 2006) and soil metal concentrations (Mueller et al. 2004; Xie et al. 2011). Because we sampled on a regular grid pattern, samples within each sampling area were approximately equidistant, which minimized error.
For each sampling area's IDW, the power (friction of distance) was optimized, and the fewest number of neighbors was chosen to minimize the root mean squared error (RMSE). The US Census 2000 block groups that intersected each sampling area were then identified. These selected block groups were used as separate geographical units. Using the zonal statistics function in ArcMap, both the mean estimated Pb soil concentration (the average of estimated Pb concentrations at all 30 × 30 m cells of the sampling area within each block group), and the total area (whole or portion) of the block group that was within a sampling area, were calculated. Therefore, for each block group that intersected a sampling area, there was a resulting mean estimated soil Pb concentration and an area (in m2) corresponding to the area of the block group that was located within that sampling area. By aggregating estimated Pb concentrations by block group, we also reduced the error associated with each individual cell's estimated Pb concentration (Epstein 1983).
US Census 2000 data by block group were downloaded from the Census 2000 data engine for SC for the total SC population. To estimate the racial/ethnic and income distributions for block groups in our sampling areas, we used areal interpolation. For this method, we assumed a homogeneous distribution of individuals within each block group; this was specifically for the estimation of Census data in block groups that were located only partially within sampling areas. This assumption of areal interpolation has been deemed appropriate for analyses on the aggregate level (Riebel and Agrawal 2007). We estimated the density of individuals (number of individuals m−2) for each block group by dividing the total population of the block group by the total area of the block group. This density was then multiplied by the fraction of the area of the block group that was located within each sampling area (either whole or partial) to obtain the total number of individuals estimated to be living within each area by block group. This was also carried out for children ≤6 years of age.
US Census race/ethnicity data were categorized into the following groups: white, non-Hispanic-Latino (non-HL); black, non-HL; HL; and other (comprised of Asian, Native American, Pacific Islanders, and individuals who consider themselves of other races). US Census economic level data for ITPR were categorized from poorest to less poor, as <1.00, 1.00–1.99, and ≥2.00. ITPR of 1 is equal to an annual income of $17,463 US dollars (USD) for a family of four (two adults and two children), and ITPR of 2 is equal to an annual income of $34,926 USD. The median 1999 household income for each block group and total numbers of individuals who were living both below and at or above the poverty level (ITPR equal to 1) also were obtained.
In the current study, both total numbers of individuals and children ≤6 years of age were estimated by race/ethnicity and by ITPR for each block group in each sampling area. These values were calculated by obtaining the proportion of individuals within each race/ethnicity and ITPR category, and multiplying this proportion by the total estimated number of individuals living in each sampling area by block group. As both race/ethnicity and ITPR data are estimated from a smaller regional sample by the US Census, there is variability in the total numbers estimated by race/ethnicity and ITPR for each area.
Block groups within each sampling area were categorized further by race/ethnicity, ITPR, and by soil Pb concentrations. Block groups where >50 % of the population were of racial/ethnic minority (considered to be any category other than white, non-HL), and, separately, where >50 % of the population had an ITPR < 1.00, were identified. Block groups were also identified where the mean estimated Pb concentration was greater than the US Environmental Protection Agency (EPA) residential soil limit (RSL) of 400 mg kg−1; these values are set by the EPA to be used for risk assessment, and locations with concentrations above the RSL may warrant further investigation (EPA 2010). Also, using the EPA Toxic Release Inventory (TRI) Explorer, all reporting industrial facilities and those facilities that emitted Pb, and coal-fired power plants located within each sampling area's block groups were identified for the years 2000-2010 (EPA 2012).
Pearson correlations between mean estimated soil Pb concentrations and estimated number of both total individuals and children ≤6 years of age by both race/ethnicity and ITPR were calculated for sampling areas separately, and for combined urban (Areas 4 and 5) and rural (Areas 6 and 27) locations. Mean estimated soil Pb concentrations, median 1999 household incomes, and estimated number of both total individuals and children ≤6 years of age by both race/ethnicity and ITPR also were compared between sampling areas, and between urban and rural locations, using an analysis of variance. An α level of 0.05 was used to determine statistical significance and SAS Statistical Software (Version 9.2, SAS Institute, Cary, NC) was used to perform all statistical analyses.
Results
Measured mean and median Pb concentrations, and concentration ranges, from each sampling area are listed in Table 1. Estimated soil Pb concentrations, with darker colors indicating increasing concentrations of Pb, are shown in Fig. 2a-d. The ranges of estimated soil Pb concentrations that correspond to color gradient changes are based on the quartiles of measured soil Pb concentrations in all four sampling areas (e.g., concentrations ≥43.7 mg kg−1 are greater than or equal to the 75th percentile based on measured soil Pb concentration in all sampling areas).
Table 1. Mean, median, and range of measured soil lead (Pb) concentrations in two urban (4 and 5) and two rural (6 and 27) sampling areas of South Carolina.
| Mean (mg kg−1) | Median (mg kg−1) | Range (mg kg−1) | |
|---|---|---|---|
| Area 4 | 44.8 | 28.8 | 2.4–288 |
| Area 5 | 69.5 | 38.3 | 0.9–1,800 |
| Area 6 | 25.6 | 14.8 | 1.7–314 |
| Area 27 | 24.3 | 7.7 | 0.81–239 |
Fig. 2. Estimated soil lead (Pb) concentrations and identified block groups within sampling areas where >50 % of the population was racial/ethnic minority, >50 % of the population has income-to-poverty ratio (ITPR) < 1.00, and where the mean estimated Pb concentration was >400 mg kg−1 (the EPA residential soil limit or RSL) for urban Area 4 (a) and Area 5 (b), and rural Area 6 (c) and Area 27 (d).
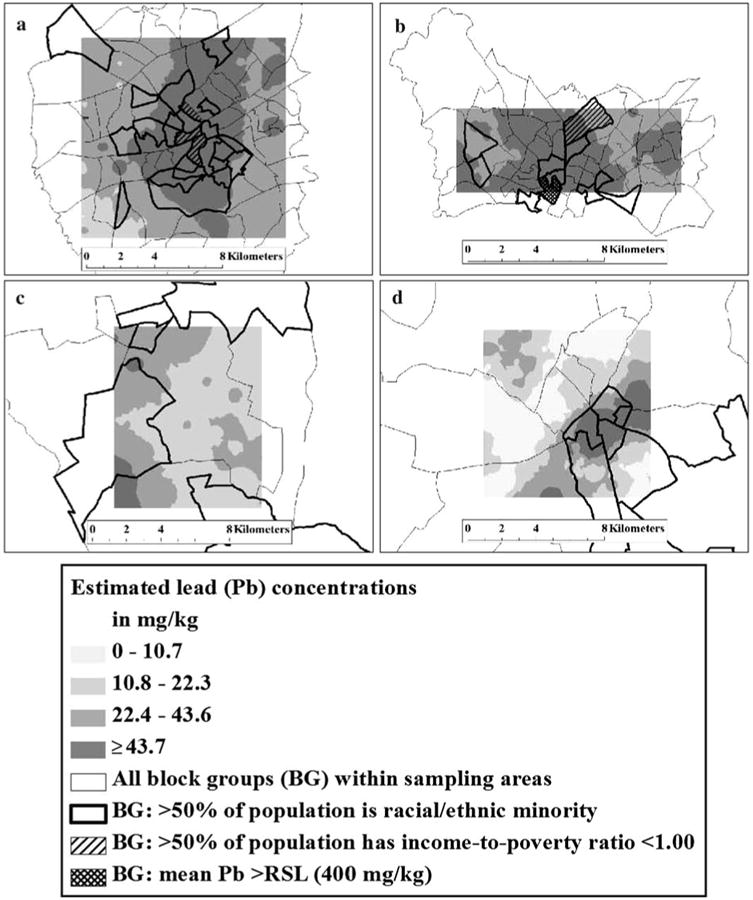
Overlain on the maps are all block groups located within each sampling area, block groups where >50 % of the population are of minority race/ethnicity (
 ), block groups where >50 % of the population had ITPR < 1.00 (
), block groups where >50 % of the population had ITPR < 1.00 (
 ), and block groups where the estimated mean Pb concentration is greater than the EPA RSL for Pb (
), and block groups where the estimated mean Pb concentration is greater than the EPA RSL for Pb (
 ). Urban Areas 4 and 5 contained 77 and 64 block groups, respectively, and rural Areas 6 and 27 contained 10 and 15 block groups, respectively.
). Urban Areas 4 and 5 contained 77 and 64 block groups, respectively, and rural Areas 6 and 27 contained 10 and 15 block groups, respectively.
The majority of Area 4 (Fig. 2a) had estimated Pb concentrations at or above the median measured Pb concentration of all sampling areas (22.4 mg kg−1). Estimated Pb concentrations at or above the 75th percentile (≥43.7 mg kg−1) overlapped block groups where >50 % of the population was of minority race/ethnicity. Three block groups in Area 4 had >50 % of the populations with an ITPR <1.00; these block groups also corresponded to both estimated Pb concentrations at or above the 75th percentile, and block groups where >50 % of the population was of minority race/ethnicity. Area 5 (Fig. 2b) had significantly higher estimated mean Pb concentrations than all other sampling locations (all p ≤ 0.04). All estimated Pb concentrations in Area 5 were at or above the median measured Pb concentration for all sampling areas, and two block groups had mean estimated Pb concentrations greater than the EPA RSL of 400 mg kg−1. There were fewer block groups where >50 % of the population were minority race/ethnicity in Area 5 compared to Area 4, and only one block group where >50 % of the population had ITPR <1.00.
Rural Areas 6 and 27 generally had lower estimated Pb concentrations (Fig. 2c, d, respectively) compared to the urban areas, and the majority of Areas 6 and 27 had estimated soil Pb concentrations at or below the 50th percentile of measured Pb concentrations in all sampling areas. Area 6 (Fig. 2c) had hot spots of high Pb concentrations (≥43.7 mg kg−1) in the western part of the sampling area, and there were four block groups where >50 % of the population was of minority race/ethnicity. In Area 27 (Fig. 2d), hot spots of Pb concentration were identified in the central and eastern parts of the sampling area (≥43.7 mg kg−1), and higher estimated Pb concentrations (≥43.7 mg kg−1) corresponded to block groups where >50 %of the population was of minority race/ethnicity in this section. Neither rural areas had block groups where >50 % of the population had ITPR <1.00.
In the block groups of urban Areas 4 and 5, there were seven and three EPA TRI facilities identified, respectively (data not shown). Of these, only one facility in each sampling area was an emitter of Pb. There were three TRI facilities located within block groups of Area 27; however, none were emitters of Pb. No TRI facilities were located within Area 6. Of all Pb-emitting TRI facilities identified in our study areas, none were coal-fired power plants.
In Area 4, the number of black, non-HL individuals was significantly positively associated with estimated mean Pb concentrations by block group (r = 0.38, p = 0.007; Table 2). In Area 5 and for the combined urban areas, both the numbers of white, non-HL (r = −0.29, p = 0.02) and other race/ethnicity (r = −0.27, p = 0.03) individuals were significantly negatively correlated with estimated mean Pb concentrations by block group. For Areas 4 and 5 and for the combined urban areas, the majority of significant associations for all individuals also were significant for children ≤6 years of age.
Table 2. Significant correlations and p-values for estimated mean lead (Pb) soil concentrations and estimated numbers of all individuals, and children ≤6 years of age, by race/ethnicity for block groups in urban Areas 4 and 5, rural Areas 6 and 27, and for urban and rural locations combined.
| All individuals | Children ≤6 years of age | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Black, non-HL | White, non-HL | HL | Othera | Black, non-HL | White, non-HL | HL | Other | |
| Area 4 | 0.38 | NA | NA | NA | 0.36 | NA | NA | NA |
| 0.0007 | 0.001 | |||||||
|
| ||||||||
| Area 5 | NA | −0.29 | NA | −0.27 | NA | −0.26 | NA | −0.25 |
| 0.02 | 0.03 | 0.04 | 0.04 | |||||
|
| ||||||||
| Area 6 | NA | NA | NA | NA | NA | NA | NA | NA |
|
| ||||||||
| Area 27 | 0.53 | NA | NA | NA | NA | NA | NA | NA |
| 0.04 | ||||||||
|
| ||||||||
| Urbanb | NA | −0.22 | NA | −0.17 | NA | −0.20 | NA | NA |
| 0.009 | 0.05 | 0.02 | ||||||
|
| ||||||||
| Ruralc | 0.45 | NA | NA | NA | 0.41 | NA | NA | NA |
| 0.03 | 0.04 | |||||||
HL hispanic latino, NA not applicable (correlation not significant at α = 0.05 level)
Other: includes Asian, Native American, and Pacific Islanders
Urban: Areas 4 and 5 combined
Rural: Areas 6 and 27 combined
There were no significant associations between estimated mean Pb concentrations and either all individuals, or children ≤6 years of age, by race/ethnicity in Area 6 (Table 2). In Area 27, there was a significant association between mean estimated Pb concentrations and numbers of black, non-HL individuals (r = 0.53, p = 0.04). When both rural areas were combined, there were significant positive associations between estimated Pb concentrations and numbers of both black, non-HL, individuals (r = 0.45, p = 0.03), and children ≤6 years of age (r = 0.41, p = 0.04).
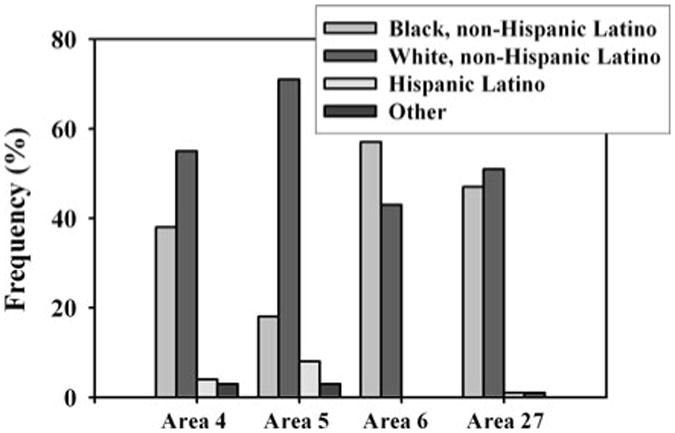
Urban Areas 4 and 5 had different racial distributions; in Area 4, the percentage of the population that was white and black, non-HL, was more similar (38 and 55 %, respectively) than in Area 5, where almost 75 % of the population was white, non-HL (Fig. 3). Rural Areas 6 and 27 had similar racial/ethnic distributions, although Area 6 was the only sampling location where the proportion of black, non-HL individuals was greater than the proportion of white, non-HL individuals. Area 6 also had the smallest total population (∼3,200) of all sampling locations; Area 27 had a total population of ∼10,000, and Areas 4 and 5 had total populations of ∼70,000 and ∼60,000, respectively. In all sampling areas, HL and other race/ethnicities made up a small percentage of the population (<10 %). The mean number of white, non-HL, individuals by block group in Area 5 was significantly greater than in Area 6 (p = 0.006), and the mean number of black, non-HL, individuals by block group in Area 5 was significantly lower than in Area 4 (p = 0.001); these significant relationships were also observed for children ≤6 years of age (data not shown).
Fig. 3. Population frequency distribution by race/ethnicity for urban Areas 4 and 5 and rural Areas 6 and 27.
Area 4 estimated Pb concentrations were significantly positively correlated with the poorest individuals, or those with ITPR <1.00 (r = 0.43, p = 0.0001; Table 3); the same was true for children ≤6 years of age. Individuals in Area 5 with ITPR ≥ 2.00 were significantly negatively correlated with estimated mean Pb concentrations (r = −0.29, p = 0.02), which was also true for children ≤6 years of age in that area. The combined urban areas had associations similar to those of Area 5. For the individual rural Areas 6 and 27, there were no significant associations between either all individuals or children ≤6 years of age by ITPR and estimated Pb concentrations (Table 3); however, for the combined rural areas, there was a marginally significant (r = 0.40, p = 0.05) positive association between individuals with ITPR <1.00 and estimated Pb concentrations.
Table 3. Significant correlations and p-values for estimated mean lead (Pb) soil concentrations and estimated numbers of all individuals, and children ≤6 years of age, by income-to-poverty (ITP) ratio for block groups in urban Areas 4 and 5, rural Areas 6 and 27, and for urban and rural locations.
| All individuals | Children ≤6 years of age | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| <1.00 | 1.00–1.99 | ≥2.00 | <1.00 | 1.00–1.99 | ≥2.00 | |
| Area 4 | 0.43 0.0001 |
NA | NA | 0.38 0.0006 |
NA | NA |
|
| ||||||
| Area 5 | NA | NA | −0.29 0.02 |
NA | NA | −0.26 0.04 |
|
| ||||||
| Area 6 | NA | NA | NA | NA | NA | NA |
|
| ||||||
| Area 27 | NA | NA | NA | NA | NA | NA |
|
| ||||||
| Urbana | NA | NA | −0.24 0.005 |
NA | NA | −0.21 0.01 |
|
| ||||||
| Ruralb | 0.40 0.05 |
NA | NA | NA | NA | NA |
NA not applicable (correlation not significant at α = 0.05 level)
Urban: Areas 4 and 5 combined
Rural: Areas 6 and 27 combined
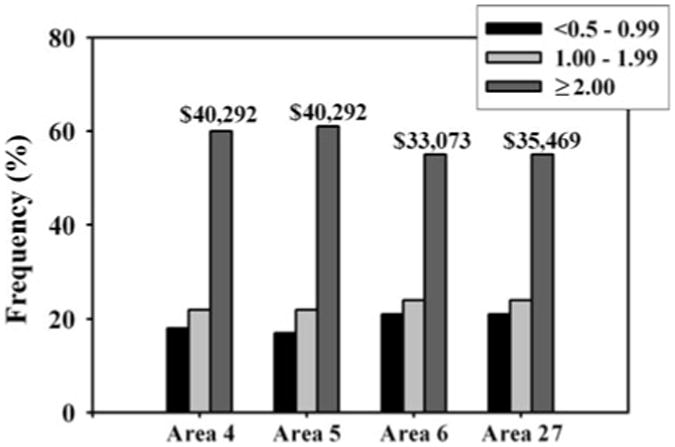
Average 1999 median household income by block group was not significantly different between sampling areas (Fig. 4). The majority (approximately 55–60 %) of the populations in each sampling area had ITPR ≥ 2.00, indicating an annual income of at least $34,926 USD (for a family of four). Approximately 20 % of the population in each sampling area had either ITPR < 1.00 or ITPR in the range of 1.00–1.99.
Fig. 4. Population frequency distribution by income-to-poverty ratio (ITPR) range (with average 1999 median income) for urban Areas 4 and 5 and rural Areas 6 and 27.
Discussion
Concentrations of Pb measured in our study are generally at or below those concentrations measured in other studies. Birke and Rauch (2000) measured median Pb concentrations of 50 and 109 mg kg−1 in low and high density residential areas of Berlin, Germany, respectively, and Yesilonis et al. (2008) reported a median soil Pb concentration of 89 mg kg−1 in Baltimore, Maryland, USA residential soils. Madrid et al. (2002) measured a mean total soil Pb concentration of 137 mg kg−1 in parks and gardens of Seville, Spain at 31 sites. These concentrations are approximately 2–13 times greater than our measured median (range, 8–38 mg kg−1) soil Pb concentrations and 2–6 times greater than our measured mean soil Pb concentrations (range, 24–70 mg kg−1). All of these study sites are more industrialized and populated than our sites. While our urban sites are classified as urban based on US Census data they are not large cities, nor are they heavily industrialized. Langner et al. (2011) measured Pb in suburban Iowa, USA, soils at 10 residential homes in Ankeny, Iowa (population 36,000), and their mean Pb concentration (55 mg kg−1) was greater than all of our mean Pb concentrations except Area 5 (70 mg kg−1), suggesting that our soil Pb concentrations are more representative of low-density residential areas.
Urban areas are expected to have greater metal concentrations than rural areas because they generally have more potential sources of Pb, including road networks (Aelion et al. 2012) and industries, which lead to higher soil Pb concentrations. We identified only one Pb-emitting TRI facility each in urban Areas 4 and 5, and no TRI facilities in rural Areas 6 and 27; overall, urban Areas 4 and 5 had higher measured and estimated Pb concentrations than the rural sampling Areas 6 and 27. Other studies have also reported higher background soil Pb concentrations in urban areas compared to rural areas. In the city of Wuhan, China (population of 7.5 million), a mean soil Pb concentration of 38 mg kg−1 was observed (with a range of 17–130 mg kg−1) by Gong et al. (2010) for samples taken in gardens or agricultural fields, most of which were outside the city limits. As in our study, they observed higher Pb concentrations in their urban, more populated sample locations compared to the non-urbanized and agricultural areas. In a comparison of urban (New Orleans, LA, USA) and rural (Lafourche Parrish, LA, USA) soil Pb concentrations, Mielke et al. (1997) found that all US Census tracts in the rural area had mean soil Pb concentrations <100 mg kg−1, and the majority (64.3 %) of US Census tracts in the urban area had mean soil Pb concentrations ≥100 mg kg−1.
Even though our measured and estimated soil Pb concentrations are generally low compared to large urban areas, they may still be an exposure risk for children living in these locations. We identified three block groups in Area 5 with a mean estimated soil Pb concentration greater than the PRG limit of 400 mg kg−1. Exposure of children to Pb in soils has been associated with negative neurological outcomes in many previous studies. Zahran et al. (2009) reported a significant dose–response relation between median measured blood Pb concentrations and school test scores in New Orleans, LA, USA. They found that for every 1 μg dL−1 increase in median blood Pb concentration, the school average standardized test score decreased 0.1 points. In a cohort study, Canfield et al. (2003) found that even low levels of Pb exposure (defined as blood Pb concentrations below 10 μg dL−1) in children were associated with lower intelligence quotient (IQ) test scores from ages 3 to 5. Other studies have also reported this association for other populations of children (Koller et al. 2004; Lanphear et al. 2005).
Racial disparities were observed in the current study with respect to block group race/ethnicity and soil Pb concentrations. In general, greater numbers of black, non-HL, individuals by block group were positively associated with mean estimated Pb concentrations in the urban Area 4, the rural Area 27, and rural areas combined; non-minority populations were negatively associated with Pb concentrations in urban Area 5. These finding were also true for children ≤6 years of age in all locations, which is important because children may not only be at higher risk for exposure to Pb, but also more biologically susceptible; the gastrointestinal bioaccessibility of Pb in soils for children has been estimated to be as high as 50 % (Bearer 1995). Other studies have concluded that minority populations often live in more contaminated environments. In a mining area in Pueblo, Colorado, USA, a significant positive association was observed between soil Pb concentrations and the proportion of HL individuals in the population (by Census tract) (Diawara et al. 2006). In New Orleans, LA, USA, Campanella and Mielke (2008) found that as soil Pb concentration increased, the percent of the population that was African-American also increased; African-Americans were also more than two times as likely as non-African-Americans to live where Pb soil concentrations were >100 mg kg−1. Zahran et al. (2009) found a significant positive association between percent African-American children at a school and median blood Pb concentrations.
The majority of populations in each sampling area had an ITPR ≥ 2.00. The number of individuals in the lowest income group (∼<$17,500 USD for a family of four) was positively correlated with soil Pb concentrations, and the number of individuals in the highest income group (>$35,000 USD for a family of four) was negatively correlated with soil Pb concentrations. Similarly, a general decrease in average median household income (from $45,500 to $24,000 USD) was observed by Campanella and Mielke (2008) as soil Pb concentrations increased from 2.5 to 20,000 mg kg−1 in New Orleans, LA, USA. In a rural area of northeastern Oklahoma, USA contaminated due to mining practices, Malcoe et al. (2002) found that children at ≤100 % of the federal poverty level were at 2.8 times the risk for having blood Pb levels higher than 10 μg dL−1 compared to children above the federal poverty level.
Regardless of urban or rural categorization, populations in our study sites were similar with respect to ITPR distributions, and dissimilar with respect to racial/ethnic population distributions. Also, associations between estimated Pb soil concentrations and both individuals and young children by race/ethnicity in all sampling areas were not only more numerous, but also more significant (e.g., lower p-values) compared to associations with individuals by ITPR. This may indicate that the potential for disparities in exposure to Pb in soils is more important for race/ethnicity than for income. This may be due in part to the racial segregation in neighborhoods that still exists in the US, including SC, and that many low-income individuals are also of minority race/ethnicity (specifically black, non-HL, in SC) (LaVeist et al. 2011; Williams and Collins 2001). Sampling areas with block groups where >50 % of the population had an ITPR < 1.00 overlapped almost entirely with block groups where >50 % of the population was of minority race/ethnicity.
Our study has some limitations. Because we used aggregate measures for soil Pb concentrations and both numbers of individuals by race/ethnicity and ITPR by block group in our study, our results are not necessarily transferable to the individual. Therefore, further research is needed to identify our reported associations at the individual level. There were also smaller sample sizes (i.e., fewer block groups) in the rural Areas 6 and 27 compared to the urban Areas 4 and 5. This may affect the power to observe statistically significant associations between estimated soil Pb concentrations and numbers of individuals and children ≤6 years of age by race/ethnicity and ITPR. However, we combined rural areas in the analyses to increase the sample size and significant associations were observed for all individuals.
Conclusions
Estimated soil Pb concentrations were higher in urban areas compared to rural areas in SC. Race/ethnicity of all individuals and children ≤6 years of age in both urban and rural areas were positively associated with estimated Pb soil concentrations. Higher numbers of black, non-HL individuals lived in areas with higher Pb concentrations compared to white non-HL individuals, suggesting a potential racial disparity for exposure to Pb in soils for all individuals and children. Higher numbers of individuals with ITPR < 1.00 (income < $17,463 USD) lived in areas with higher Pb concentrations than individuals with ITPR ≥ 2.00 (income > $34,926 USD). Associations between ITPR and estimated Pb soil concentrations in children were similar to those in adults but generally were limited to urban areas, potentially because of limited population numbers for children ≤6 years in rural areas. The disparity in potential for exposure to soil Pb may be more pronounced for race/ethnicity compared to income. However, the combined association of ethnicity and lower income with soil metal concentrations contributes to creating a population at higher risk for adverse health effects, which is of particular concern for infants and children living in both urban and rural areas.
Acknowledgments
Funding for this research was provided by a National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH) (grant number 5R01ES012895-01 and 5R01ES012895-06). We thank J. Davis, M. Engle, and S. Jayasinghe for their help with sampling.
Contributor Information
C. Marjorie Aelion, Email: maelion@schoolph.umass.edu, School of Public Health and Health Sciences, University of Massachusetts, 715 North Pleasant Street, Amherst, MA 01003, USA; Department of Environmental Health Sciences, University of South Carolina, 921 Assembly Street, Columbia, SC 29208, USA.
Harley T. Davis, Department of Environmental Health Sciences, University of South Carolina, 921 Assembly Street, Columbia, SC 29208, USA; Department of Epidemiology and Biostatistics, University of South Carolina, 800 Sumter Street, Columbia, SC 29028, USA
Andrew B. Lawson, Division of Biostatistics and Epidemiology, Medical University of South Carolina, 135 Cannon Street, Charleston, SC 29401, USA
Bo Cai, Department of Epidemiology and Biostatistics, University of South Carolina, 800 Sumter Street, Columbia, SC 29028, USA.
Suzanne McDermott, Department of Family and Preventive Medicine, University of South Carolina, 3209 Colonial Drive, Columbia, SC 29203, USA.
References
- Aelion CM, Davis HT. Use of a general toxicity test to predict heavy metal concentrations in residential soils. Chemosphere. 2007;67:1043–1049. doi: 10.1016/j.chemosphere.2006.10.042. [DOI] [PubMed] [Google Scholar]
- Aelion CM, Davis HT, Cai B, Lawson AB, McDermott S. Associations of estimated residential soil arsenic and lead concentrations and community-level environmental measures with mother-child health conditions in South Carolina. Health and Place. 2012;18:774–781. doi: 10.1016/j.healthplace.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aelion CM, Davis HT, McDermott S, Lawson AB. Metal concentrations in rural topsoil in South Carolina: Potential for human health impact. Science of the Total Environment. 2008;402:149–156. doi: 10.1016/j.scitotenv.2008.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aelion CM, Davis HT, Liu Y, Lawson AB, McDermott S. Validation of Bayesian kriging of arsenic, chromium, lead, and mercury in surface soils based on internode sampling. Environmental Science and Technology. 2009a;43:4432–4438. doi: 10.1021/es803322w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aelion CM, Davis HT, McDermott S, Lawson AB. Soil metal concentrations and toxicity: Associations with distances to industrial facilities and implications for human health. Science of the Total Environment. 2009b;407:2216–2223. doi: 10.1016/j.scitotenv.2008.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry) Lead ToxFAQ. [Accessed 1 March 2011];2007 Available: http://www.atsdr.cdc.gov/tfacts13.pdf.
- Babak O, Deutsch CV. Statistical approach to inverse distance interpolation. Stochastic Environmental Research and Risk Assessment. 2009;23:543–553. [Google Scholar]
- Bearer CF. Environmental health hazards—How children are different from adults. Future of Children. 1995;5:11–26. [PubMed] [Google Scholar]
- Bellinger DC. Lead neurotoxicity and socioeconomic status: Conceptual and analytical issues. Neurotoxicology. 2008a;29:828–832. doi: 10.1016/j.neuro.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger DC. Neurological and behavioral consequences of childhood lead exposure. PLoS Medicine. 2008b;5:690–692. doi: 10.1371/journal.pmed.0050115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birke M, Rauch U. Urban geochemistry: investigations in the Berlin metropolitan area. Environmental Geochemistry and Health. 2000;22:233–248. [Google Scholar]
- Bityukova L, Shogenova A, Birke M. Urban geochemistry: A study of element distributions in the soils of Tallinn (Estonia) Environmental Geochemistry and Health. 2000;22:173–193. [Google Scholar]
- Brown P. Race, class, and environmental health: a review and systematization of the literature. Environmental Research. 1995;69:15–30. doi: 10.1006/enrs.1995.1021. [DOI] [PubMed] [Google Scholar]
- Cai B, Lawson AB, McDermott S, Aelion CM. Variable selection for spatial predictors under Bayesian spatial model. Statistical Modelling. 2011;11:535–555. [Google Scholar]
- Calderón J, Ortiz-Pérez D, Yáñez L, Diaz-Barriga F. Human exposure to metals: Pathways of exposure, biomarkers of effect, and host factors. Ecotoxicology and Environmental Safety. 2003;56:93–103. doi: 10.1016/s0147-6513(03)00053-8. [DOI] [PubMed] [Google Scholar]
- Campanella R, Mielke HW. Human geography of New Orleans' high-lead geochemical setting. Environmental Geochemistry and Health. 2008;30:531–540. doi: 10.1007/s10653-008-9190-9. [DOI] [PubMed] [Google Scholar]
- Canfield RL, Henderson CR, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP. Intellectual impairment in children with blood lead concentrations below 10 μg per deciliter. The New England Journal of Medicine. 2003;348:1517–1526. doi: 10.1056/NEJMoa022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Xia X, Zhao Y, Zhang P. Heavy metal concentrations in roadside soils and correlation with urban traffic in Beijing, China. Journal of Hazardous Materials. 2010;181:640–646. doi: 10.1016/j.jhazmat.2010.05.060. [DOI] [PubMed] [Google Scholar]
- Davis HT, Aelion CM, McDermott S, Lawson AB. Identifying natural and anthropogenic sources of metals in urban and rural soils using GIS-based data, PCA, and spatial interpolation. Environmental Pollution. 2009;157:2378–2385. doi: 10.1016/j.envpol.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diawara MM, Litt JS, Unis D, Alfonso N, Martinez L, Crock JG, et al. Arsenic, cadmium, lead, and mercury in surface soils, Pueblo, Colorado: implications for population health risk. Environmental Geochemistry and Health. 2006;28:297–315. doi: 10.1007/s10653-005-9000-6. [DOI] [PubMed] [Google Scholar]
- EPA (Environmental Protection Agency) EPA Region 9 preliminary remediation goals. [Accessed 5 March 2011];2010 Available: http://www.epa.gov/reg3hwmd/risk/human/rb-concentration_table/Generic_Tables/pdf/ressoil_sl_table_run_NOVEMBER2010.pdf.
- Epstein S. Aggregation and beyond: some basic issues on the prediction of behavior. Journal of Personality. 1983;51:360–392. doi: 10.1111/j.1467-6494.1983.tb00338.x. [DOI] [PubMed] [Google Scholar]
- Gong M, Wu L, Bi X, Ren L, Wang L, Ma Z, et al. Assessing heavy-metal contamination and sources by GIS-based approach and multivariate analysis of urban-rural topsoils in Wuhan, central China. Environmental Geochemistry and Health. 2010;32:59–72. doi: 10.1007/s10653-009-9265-2. [DOI] [PubMed] [Google Scholar]
- Guan DS, Peart MR. Heavy metal concentrations in plants and soils at roadside locations and parks of urban Guangzhou. Journal of Environmental Sciences-China. 2006;16:495–502. [PubMed] [Google Scholar]
- Hovmand MF, Kemp K, Kystol J, Johnsen I, Riis-Nielsen T, Pacyna JM. Atmospheric heavy metal deposition accumulated in rural forest soils of southern Scandinavia. Environmental Pollution. 2008;155:537–541. doi: 10.1016/j.envpol.2008.01.047. [DOI] [PubMed] [Google Scholar]
- Kim JI, Lawson AB, McDermott S, Aelion CM. Variable selection for spatial random field predictors under a Bayesian mixed hierarchical spatial model. Spatial and Spatio-Temporal Epidemiology. 2009;1:95–102. doi: 10.1016/j.sste.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JI, Lawson AB, McDermott S, Aelion CM. Bayesian spatial modeling of disease risk in relation to multivariate environmental risk fields. Statistics in Medicine. 2010;29:142–157. doi: 10.1002/sim.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller K, Brown T, Spurgeon A, Levy L. Recent developments in low-level lead exposure and intellectual impairment in children. Environmental Health Perspectives. 2004;112:987–994. doi: 10.1289/ehp.6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravchenko AN. Influence of spatial structure on accuracy of interpolation methods. Soil Science Society of America Journal. 2003;67:1564–1571. [Google Scholar]
- Langner AN, Manu A, Tabatabai MA. Heavy metals distribution in an Iowa suburban landscape. Journal of Environmental Quality. 2011;40:83–89. doi: 10.2134/jeq2010.0229. [DOI] [PubMed] [Google Scholar]
- Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurstl P, Bellinger DC, et al. Low-level environmental lead exposure and children's intellectual function: an international pooled analysis. Environmental Health Perspectives. 2005;113:894–899. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVeist T, Pollack K, Thorpe R, Fesahazion R, Gaskin D. Place, not race: Disparities dissipate in southwest Baltimore when blacks and whites live under similar conditions. Health Affairs. 2011;30:1880–1887. doi: 10.1377/hlthaff.2011.0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrid L, Díaz-Barrientos E, Madrid F. Distribution of heavy metal contents of urban soils in parks of Seville. Chemosphere. 2002;49:1301–1308. doi: 10.1016/s0045-6535(02)00530-1. [DOI] [PubMed] [Google Scholar]
- Malcoe LH, Lynch RA, Kegler MC, Skaggs VJ. Lead sources, behaviors, and socioeconomic factors in relation to blood lead of Native American and white children: a community-based assessment of a former mining area. Environmental Health Perspectives. 2002;110:221–231. doi: 10.1289/ehp.02110s2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovic M, Cupac S, Durovic R, Millinovic J, Kljajic P. Assessment of heavy metal and pesticide levels in soil and plant products from agricultural area of Belgrade, Serbia. Archives of Environmental Contamination and Toxicology. 2010;58:341–351. doi: 10.1007/s00244-009-9359-y. [DOI] [PubMed] [Google Scholar]
- Mielke HW, Dugas D, Mielke PW, Smith KS, Smith SL, Gonzales CR. Associations between soil lead and childhood blood lead in urban New Orleans and rural Lafourche Parish of Louisiana. Environmental Health Perspectives. 1997;105:950–954. doi: 10.1289/ehp.97105950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller TG, Pusuluri NB, Mathias KK, Cornelius PL, Barnhise RI, Shearer SA. Map quality for ordinary kriging and inverse distance weighted interpolation. Soil Science Society of America Journal. 2004;68:2042–2047. [Google Scholar]
- Odewande AA, Abimbola AF. Contamination indices and heavy metal concentrations in urban soils of Ibadan metropolis, southwestern Nigeria. Environmental Geochemistry and Health. 2008;30:243–254. doi: 10.1007/s10653-007-9112-2. [DOI] [PubMed] [Google Scholar]
- Riebel M, Agrawal A. Areal interpolation of population counts using pre-classified land cover data. Population Research and Policy Review. 2007;26:619–633. [Google Scholar]
- Robinson TP, Metternicht G. Testing the performance of spatial interpolation techniques for mapping soil properties. Computers and Electronics in Agriculture. 2006;50:97–108. [Google Scholar]
- Sanghi R, Sasi KS. Pesticide and heavy metals in agricultural soil of Kanpur, India. Bulletin of Environmental Contamination and Toxicology. 2001;67:446–454. doi: 10.1007/s001280144. [DOI] [PubMed] [Google Scholar]
- Shih RA, Glass TA, Bandeen-Roche K, Carlson MC, Bolla KI, Todd AC, et al. Environmental lead exposure and cognitive function in community-dwelling older adults. Neurology. 2006;67:1556–1562. doi: 10.1212/01.wnl.0000239836.26142.c5. [DOI] [PubMed] [Google Scholar]
- Stafilov T, Šajn R, Pancevski Z, Boev B, Frontasyeva MV, Strelkova LP. Heavy metal contamination of topsoils around a lead and zinc smelter in the Republic of Macedonia. Journal of Hazardous Materials. 2010;175:896–914. doi: 10.1016/j.jhazmat.2009.10.094. [DOI] [PubMed] [Google Scholar]
- Tume P, Bech J, Sepulveda B, Tume L, Bech J. Concentrations of heavy metals in urban soils of Talcahuano (Chile): A preliminary study. Environmental Monitoring and Assessment. 2008;140:91–98. doi: 10.1007/s10661-007-9850-8. [DOI] [PubMed] [Google Scholar]
- Turer D, Maynard JB, Sansalone J. Heavy metal contamination in soils of urban highways: comparison between runoff and soil concentrations at Cincinnati, Ohio. Water, Air and Soil Pollution. 2001;132:293–314. [Google Scholar]
- United States (US) Census 2000. Census 2000 Urban and Rural Classification. [Accessed 5 March 2011];2009 Available: http://www.census.gov/geo/www/ua/ua_2k.html.
- United States (US) Economic Research Service (ERS) ERS/USDA rural definitions: State level maps. [Accessed 5 March 2011];2012 Available: http://www.ers.usda.gov/data/ruraldefinitions/SC.pdf.
- United States (US) Environmental Protection Agency (EPA) 2010 Toxic release inventory. [Accessed 12 April 2012];2012 Available: iaspub/epa.gov/triexplorer/tri_release.chemical.
- United States Department of Agriculture (USDA) Web soil survey. [Accessed 12 April 2012];2012 Available: websoilsurvey.nrcs.usda.gov/app/HomePage.htm.
- Wang XS. Heavy metals in urban soils of Xuzhou (China), Part I: Total concentration of heavy metals in soils. International Journal of Environment and Pollution. 2008;33:173–184. [Google Scholar]
- Williams DR, Collins C. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Reports. 2001;116:404–416. doi: 10.1093/phr/116.5.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X, Chen X, Liu R, Liu H. Heavy metals in urban soils with various types of land use in Beijing, China. Journal of Hazardous Materials. 2011;186:2043–2050. doi: 10.1016/j.jhazmat.2010.12.104. [DOI] [PubMed] [Google Scholar]
- Xie YF, Chen TB, Lei M, Yang J, Guo QJ, Song B, et al. Spatial distribution of soil heavy metal pollution estimated by different interpolation methods: Accuracy and uncertainty analysis. Chemosphere. 2011;82:468–476. doi: 10.1016/j.chemosphere.2010.09.053. [DOI] [PubMed] [Google Scholar]
- Yesilonis ID, Pouyat RV, Neerchal NK. Spatial distribution of metals in soils in Baltimore, Maryland: role of native parent material, proximity to major roads, housing age, and screening guidelines. Environmental Pollution. 2008;156:723–731. doi: 10.1016/j.envpol.2008.06.010. [DOI] [PubMed] [Google Scholar]
- Zahran S, Mielke HW, Weiler S, Berry KJ, Gonzales C. Children's blood lead and standardized test performance response as indicators of neurotoxicity in metropolitan New Orleans elementary schools. Neuro-Toxicology. 2009;30:888–897. doi: 10.1016/j.neuro.2009.07.017. [DOI] [PubMed] [Google Scholar]
- Zhen H, Lawson AB, McDermott S, Pande Lamichhane A, Aelion CM. A spatial analysis of mental retardation of unknown cause and maternal residence during pregnancy. Geospatial Health. 2008;2:173–182. doi: 10.4081/gh.2008.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen H, McDermott S, Lawson AB, Aelion CM. Are clusters of mental retardation correlated with clusters of developmental delay? Geospatial Health. 2009;4:17–26. doi: 10.4081/gh.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]


