Abstract
Tissue barriers that restrict passage of liquids, ions, and larger solutes are essential for the development of multicellular organisms. In simple organisms this allows distinct cell types to interface with the external environment. In more complex species, the diversity of cell types capable of forming barriers increases dramatically. Although the plasma membranes of these barrier-forming cells prevent flux of most hydrophilic solutes, the paracellular, or shunt, pathway between cells must also be sealed. This function is accomplished in vertebrates by the zonula occludens, or tight junction. The tight junction barrier is not absolute but is selectively permeable and is able to discriminate between solutes on the basis of size and charge. Many tight junction components have been identified over the past 20 years, and recent progress has provided new insights into the proteins and interactions that regulate structure and function. This review presents these data in a historical context and proposes an integrated model in which dynamic regulation of tight junction protein interactions determines barrier function.
Keywords: paracellular permeability, barrier function, claudin, occludin, ZO-1
THE TIGHT JUNCTION DEFINES EPITHELIAL BARRIER FUNCTION
The anatomic structure of the tight junction was first appreciated with the advent of transmission electron microscopy (1), which identified regions between adjacent epithelial cells where the intercellular space was virtually eliminated (Figure 1a). Subsequent studies using horseradish peroxidase or heavy metals as tracers demonstrated that the intercellular barrier to paracellular transport was specifically associated with the tight junction (2, 3). Moreover, the complete morphological occlusion of the intercellular space at this site led some investigators to conclude that the epithelial tight junction was a static, impermeant barrier.
Figure 1.
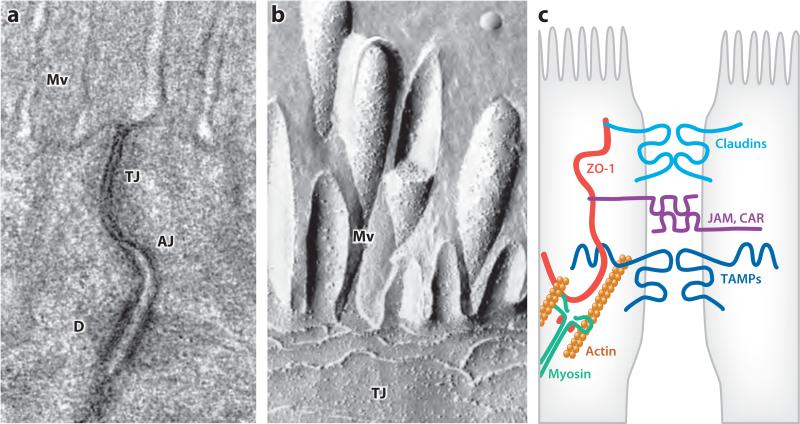
The apical junctional complex. (a) Transmission electron micrograph showing junctional complexes between two villous enterocytes. The tight junction (TJ) is just below the microvilli (Mv), followed by the adherens junction (AJ). The desmosomes (D) are located basolaterally. Panel a courtesy of Dr. Amanda Marchiando, The University of Chicago. (b) Freeze-fracture electron micrograph showing apical microvilli (Mv) and tight junction strands (TJ) in a cultured intestinal epithelial cell. Panel b courtesy of Dr. Eveline Schneeberger, Harvard Medical School. (c) Schematic showing interactions between F-actin, myosin II, zonula occludens-1 (ZO-1), claudins, tight junction–associated marvel proteins (TAMPs), and immunoglobulin superfamily members such as junctional adhesion molecules (JAM) and the coxsackie adenovirus receptor (CAR). Panel b reprinted from Reference 94 with permission.
Despite the ultrastructural data, physiological analyses suggested a role for bulk transmucosal transport that was not attributable to transcellular transport (4). Electrophysiological analyses using a vibrating probe suggested that this occurred across an ion-selective paracellular pathway (5). Ultrastructural analyses confirmed that such transport occurred across the tight junction rather than via gross epithelial defects, such as ulcers. Moreover, morphological examination showed that tight junction permeability to ionic lanthanum (La3+), an electron-dense paracellular tracer, varied significantly between tissues. For example, La3+ crossed tight junctions within rabbit ileum, but not those within rabbit gallbladder (3). Additionally, La3+ permeability correlated with previously observed differences in electrical conductance between tissues, suggesting that these two measures were assessing the same function and allowing for classification of epithelia as tight or leaky. However, this classification provided no clues as to the structural bases for these distinctions (3).
A potential explanation for differences between tight and leaky barriers was developed after freeze-fracture electron microscopy was used to assess the tight junction (2, 6). These images showed the tight junction to be a network of anastamosing strands composed of small punctae (Figure 1b). Strands could also be appreciated in transmission electron micrographs of negatively stained tight junctions isolated from subcellular fractions (2). Moreover, when tight and leaky epithelia were compared, it became apparent that, in most cases, the number of strands correlated positively with the tightness of the epithelium: “Tight” tight junctions typically had five or more strands, whereas “leaky” tight junctions often displayed only a single strand (7). Identification of tight junction components and their interactions has provided insight into the molecular determinants of barrier function (Figure 1c).
A SELECTIVELY PERMEABLE BARRIER
The relationship between tight junction strand number and barrier function made it possible to develop a mathematical model of trans–tight junction flux. Modeling each strand as an individual electrical resistor, one might consider the tight junction to be several resistors in series. This would result in a linear relationship between overall tight junction electrical resistance and strand number, as the overall resistance of any tight junction would be merely the sum of the resistances provided by each strand. However, the actual data did not conform to that model. Rather, the strand number was proportional to the log of overall tight junction resistance (8). This result can be explained by a model in which the strands are absolute barriers punctuated by pores that can be open or closed. In this model, the resistance of each strand is a function of the open probability of the pores. Because electrical resistance is measured instantaneously, this model predicts that tight junction resistance is proportional to the open probability raised to the negative power of the number of strands present (8).
The model above is satisfying in that it provides a unifying conceptual framework relating tight junction structure and function. However, it fails to account for several well-recognized tight junction characteristics. For example, paracellular charge selectivity varies across different epithelia. This would suggest the presence of different pores for anions and cations or, at least, means of modifying pore charge characteristics. Moreover, the existence of two strains of Madin-Darby canine kidney (MDCK) cells with similar tight junction ultrastructure and strand number, despite a greater-than-30-fold difference in electrical resistance, suggested that it might be possible to regulate ion conductance without dramatically impacting morphology.
In addition to charge selectivity, tight junctions across tissues, or even within a single epithelium, differ in size selectivity (Figure 2). The most striking example of this may be the mammalian small intestine, in which at least three differently sized routes for trans–tight junction macromolecular flux exist (9). Studies using radiolabeled probes of different sizes and autoradiography of rat jejunal tissue suggested that these distinct routes are spatially separated along the crypt-villus axis; the upper portion of the villus allows flux of solutes with radii up to ~6 Å, whereas the lower villus is permeable to a radius of ~10 Å (10). The crypt is far leakier and is permeable to molecules as large as ~60 Å (10). This scenario is consistent with freeze-fracture studies of crypt and villus epithelium, which have approximately four and approximately six strands per tight junction, respectively (11). The in vivo data are also consistent with more recent studies using high-resolution approaches to map size-selective characteristics of the paracellular barrier in cultured epithelial monolayers (12, 13). These in vitro analyses found that all cell lines have a pore pathway that is permeable to molecules with radii of ~4 Å or less and a second pathway, which we refer to as the leak pathway, for flux of larger noncharged solutes (12). Interestingly, the only cell line tested that exhibits villous differentiation, Caco-2, had a third pathway that allowed passage of molecules with radii of ~6.5 Å (12), consistent with the presence of three pathways in rat jejunum. Thus, a wealth of data spanning at least 25 years suggest that size selectivity is due to the presence of at least two classes of trans–tight junction flux pathways with differing size and charge selectivity. These pathways can also be distinguished on the basis of their capacity; the pore pathway is able to carry large quantities of small uncharged solutes and specific ions, whereas the leak pathway allows only small quantities of larger molecules to pass (12, 14–16). The identification of protein components of the tight junction and some of their functions has provided new understanding of the molecular characteristics of pore and leak pathways.
Figure 2.
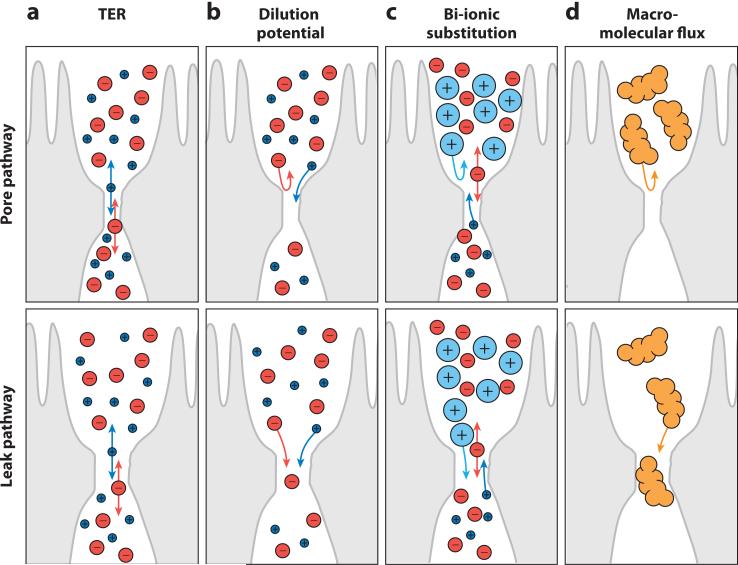
The tight junction is composed of at least two functionally distinct pathways: a high-capacity, charge-selective pore pathway that allows passage of small ions and uncharged molecules and a low-capacity leak pathway that allows flux of larger ions and molecules, regardless of charge. The permeability of these pathways can be measured using several complementary methods. However, these measure population averages; no tools are presently available to measure local tight junction permeability on a submicrometer scale. (a) Transepithelial electrical resistance (TER) measures the flux of all ions across the epithelium. This is typically done by applying a transepithelial current, by measuring the generated potential, and by using Ohm's law to calculate the resistance to current flow. The most common ions in physiological solutions, Na+ and Cl−, carry this current. These small ions do not discriminate between pore and leak pathways, and therefore TER cannot be used to measure tight junction size or charge selectivity. Increased permeability of either pathway reduces TER. Like all electrophysiological measures of tight junction function, TER reflects tight junction permeability best when ion conductance across the junction is far greater than across apical and basolateral membranes, such as in leaky epithelia. (b) Charge selectivity can be measured by the dilution potential technique. By inducing a transepithelial electrochemical gradient, e.g., by altering, iso-osmotically, apical or basolateral NaCl concentrations, one can establish a new equilibrium potential based on the relative paracellular permeabilities of Na+ and Cl−. For example, a permeability ratio close to 1.0 indicates no charge selectivity and does not alter the equilibrium potential across the monolayer. (Due to the higher mobility in free solution of Cl− relative to Na+, the dilution potential of a non-charge-selective pathway will be slightly negative. In practice, this makes a minimal contribution to measurements made across intact, charge-selective tight junctions.) (c) The bi-ionic substitution approach replaces Na+ on one side of the monolayer with organic cations of various sizes. The permeability of each organic cation can be determined and used to assess size selectivity of the pore pathway. (d ) Leak pathway permeability can be assessed by directly measuring macromolecular flux of tracers across the epithelium. Commonly used tracers include mannitol, sucrose, inulin, or polymers, such as polyethylene glycols or dextrans, of varying sizes. Because of their size, most of these molecules reflect the leak, but not the pore, pathway. However, some polyethylene glycols are small enough to allow analysis of pore pathway permeability.
MOLECULAR COMPONENTS OF THE TIGHT JUNCTION
The first identified protein specifically associated with the tight junction, zonula occludens-1 (ZO-1), was discovered in 1986 (17). Cingulin, a second tight junction–associated protein that, like ZO-1, lacks transmembrane domains was reported the following year (18). Subsequent studies identified additional peripheral membrane proteins related to ZO-1 and cingulin (19, 20).
Despite the discovery of ZO-1 and cingulin, it was clear that integral membrane proteins would be required for tight junction function and that these should have both adhesive and pore-forming properties. Thus, identification of the first tight junction–associated integral membrane protein was groundbreaking (21). This tetraspanning membrane protein, occludin, was determined to be a component of tight junction strands, to interact with ZO-1 and other tight junction–associated proteins (22, 23), and to possess adhesive properties (24). Nevertheless, tight junctions were present in occludin-deficient cells, the number of strands was identical to that of occludin-expressing cells, and barrier development was similar in the presence and absence of occludin (25). Although occludin knockout mice had a number of developmental and reproductive abnormalities, intestinal epithelial tight junctions were intact, and there was no obvious intestinal disease (26). However, both ZO-1 and occludin have been implicated in maintenance and regulation of the barrier to leak pathway flux (27–29).
The lack of an essential role for occludin in tight junction assembly or structure prompted a search for additional integral membrane tight junction proteins and led to the discovery of claudins 1 and 2 (30). Like occludin, claudins are tetraspanning membrane proteins that localize to tight junction strands and have two extracellular loops (31). A family of at least 24 claudin proteins was ultimately defined (14), and this family appears to regulate tight junction ion selectivity (32, 33), with individual family members forming cation- or anion-selective pores (16, 34–41). Site-directed mutagenesis of specific residues within the first extracellular loop of claudins can reverse the charge selectivity of the pore (35, 36), whereas inclusion of both acidic and basic residues in that region can reduce permeability to both cations and anions (42). These properties of claudins have been the topic of many reviews (43) and are not discussed in detail here.
A variety of other tight junction proteins, which may be classified as integral membrane, peripheral membrane, and signaling proteins, have also been identified (44). Several of these interact with the dense band of actin and myosin II that is present subjacent to the tight and adherens junctions. Because the roles of claudins, occludin, and ZO-1 have been studied most extensively, we focus on these as representative tight junction proteins.
PROTEINS AND LIPIDS CONTRIBUTE TO BARRIER FUNCTION
Despite the identification of a plethora of tight junction–associated proteins, the components that form the selectively permeable paracellular barrier are not well-defined. This may, in part, reflect our limited understanding of tight junction membrane structures. For example, some studies using a technique of direct, rapid freezing of newly assembled tight junctions identified cylinders within the junctions (45) that were interpreted as inverted phospholipid micelles formed from adjacent cell membranes. This suggested that the tight junction barrier might be the result of focal membrane fusion (45, 46). Although this model has fallen out of favor, we know that tight junction membranes are cholesterol-enriched, raft-like microdomains (47). Moreover, the observations that cholesterol depletion causes rapid barrier loss (48) and that occludin can interact with the raft component caveolin-1 (28, 29, 47) suggest that lipids are critical structural and functional components of the tight junction. However, advances in discovery of tight junction protein components have, in recent years, overshadowed the limited progress that has been made in understanding the roles of lipids.
Claudin proteins are strongly associated with tight junction barrier function (43). First, claudin proteins can form tight junction–like strands when expressed in fibroblasts, which do not otherwise form intercellular junctions and lack the majority of tight junction components (31). Second, consistent with an adhesive role of extracellular claudin domains, these strands form only at sites of cell-cell contact (31, 49), and several studies suggest that residues within the conserved second extracellular loop of claudins mediate interactions between proteins on adjacent cells (50–52). Finally, barrier function is reduced when some family members, including claudin-4 and claudin-5, are not expressed or are removed from the junction, whereas conversely their overexpression enhances barrier function (50, 53–55). Together with the finding that some claudins can enhance paracellular flux of specific ions, these data have led to the hypothesis that claudins can be divided into two broad categories: sealing and pore forming (56). However, the concept of naturally occurring sealing claudins is not universally accepted, as other models may also explain the data. For example, claudin-4 can be classified as either sealing or as anion-selective, pore forming (56). Evidence in favor of sealing includes the reduction in paracellular ion conductance when claudin-4 is overexpressed in MDCK cell monolayers (55) as well as the loss of barrier function induced by Clostridium perfringens enterotoxin, which binds to claudins 3 and 4 and induces their removal from the tight junction (54, 57–59). Nevertheless, it is also possible that claudin-4, and other claudins that increase transepithelial resistance, does not directly seal the paracellular space but displaces pore-forming claudins from tight junction strands. This model is supported by the observation that claudin-4 knockdown reduces paracellular Cl− permeability, as well as by data suggesting that claudin-8 increases barrier function by displacing claudin-2, which forms cation-selective pores, from the tight junction (60). Thus, although the specific role of claudins in forming paracellular pores is relatively well understood (16, 34, 37, 42, 61, 62), the manner in which claudins enhance barrier function is not.
TIGHT JUNCTION ORGANIZATION DEPENDS ON INTERPROTEIN INTERACTIONS
Until recently, the tight junction was considered to be a stable, heavily cross-linked protein complex (14, 43, 63–65). This model was supported by the numerous protein interactions present between tight junction proteins (Table 1). For example, claudin proteins can form tight junction–like strands, but not actual tight junctions, in fibroblasts (66). Importantly, these strands form only at sites of cell-cell contact, suggesting an important role of extracellular interactions between claudins in forming the strands (49, 52, 66). In epithelial cells, the site of claudin protein polymerization to form strands depends on ZO family protein expression (31), and cells lacking ZO-1 and ZO-2 fail to form tight junctions at all. This likely reflects a requirement for the PDZ (PSD95, Dlg, and ZO-1) domains of these proteins that bind to C-terminal YV motifs of most claudins (67) and are necessary for efficient junctional claudin delivery (65, 68, 69). Moreover, each of the tight junction–associated marvel proteins (TAMPs), occludin, tricellulin, and marvelD3, as well as other tight junction proteins can be incorporated into the tight junction–like strands formed by claudins (31, 70, 71). This may reflect the ability of occludin to interact directly with ZO-1 (23) and the ability of TAMPs to interact with one another (70), as no direct binding between cytoplasmic tails of claudins and TAMPs has been identified. Overall, it has been difficult to decipher the nature of specific protein interactions within tight junctions of living cells, and even in cell-free systems, very few have been characterized quantitatively (67). Thus, although the contribution of this plethora of protein interactions to barrier function is poorly understood, they are thought to be essential for maintenance of tight junction structure.
Table 1.
Interactions between tight junction–associated proteins
| Name | Structural signatures | Binding partners |
|---|---|---|
|
TRANSMEMBRANE PROTEINS
| ||
|
Claudin family
| ||
| Claudins | Four transmembrane domains, two cytoplasmic tails, two extracellular loops, one intracellular linker, one C-terminal PDZ-binding motif | Claudins (extracellular domains),c,e,f,h,i,k occludin (extracellular region),j marvelD3,h CD9,j CD81 (extracellular loop),g,i EpCAM,d,g ZO-1 (PDZ-binding motifs),a,b,e,f,h ZO-2,a,b,e,f ZO-3,a,b,e,f MUPP1 (PDZ-binding motifs),a,c PATJ (PDZ-binding motif ),b Clostridium perfringens enterotoxin (second extracellular loop)a |
|
Tight junction–associated MARVEL proteins (TAMPs) | ||
| Occludin | Four transmembrane domains (forming MARVEL domain), two cytoplasmic tails, two extracellular loops, one intracellular linker Occludin and tricellulin C-terminal tails contain an OCEL (coiled-coil) domain |
Occludin,b connexin26 (OCEL),b TGFβ RI and RII (second extracellular loop),d,j ZO-1 (OCEL),b,h ZO-2,a,b ZO-3,b cingulin (C-terminal cytoplasmic tail),b F-actin (C-terminal cytoplasmic tail),a VAP33 (OCEL),c,g,h c-Yes (OCEL),b,d,g PI3 kinase p85 (C-terminal coiled coil),b PKCζ (OCEL),b PKCη (C-terminal tail),a,e,h CK2 (C-terminal tail),j Itch (N-terminal cytoplasmic tail),c,d,e,h Nedd4-2 (C-terminal tail PY motif),e focal adhesion kinased |
| Tricellulin | MarvelD3,d ZO-1 (C-terminal cytoplasmic tail)a | |
| MarvelD3 | Occludin,d tricellulind | |
|
Junctional adhesion molecules (JAMs) | ||
| JAM-A/JAM-1 | Immunoglobulin (Ig) superfamily: one N-terminal extracellular region, one transmembrane region, one C-terminal cytosplasmic tail, C-terminal PDZ-binding motif; extracellular region contains two V-type Ig domains | JAM-A (extracellular region),b,j LFA-1/CD11a/CD18 (Ig domain 2),c,j αVβ3 integrin,d ZO-1 (PDZ-binding motif),a,b,d MUPP1 (PDZ-binding motif),a,b,c PATJ (PDZ-binding motif ),b Par3 (PDZ-binding motif ),a,d,h AF-6 (PDZ-binding motif),b,c,d,g cingulin (C-terminal cytoplasmic tail),b,d PDZ-GEF2,e,g reovirus sigma proteina,j |
| JAM-B/JAM-2 | JAM-C (extracellular region),a,h,j αVβ1 integrin,j ZO-1 (PDZ-binding motif),a,b,h Par3 (PDZ-binding motif)b,h | |
| JAM-C/JAM-3 | JAM-B (V domain),a,h,j JAM-C(V domain),b,j CD11b/CD18 (extracellular region),a,j ZO-1 (PDZ domain),a,b Par3 (PDZ-binding motif )b | |
| JAM-4/IGSF5 | JAM-4,h,j MAGI-1 (PDZ-binding motif)b,c,e,h | |
|
CAR-like CTX family proteins | ||
| CAR | CTX family of Ig superfamily proteins: extracellular loop contains one V-type and one C2-type Ig domain and C-terminal PDZ-binding motif | CAR (D1 domain),a ZO-1,d,f,g,h MUPP1 (PDZ-binding motif),a,c,d,e,g,h LNX (PDZ-binding motif),b,c,h F-actin (cytoplasmic tail),a,b,g,h microtubule (cytoplasmic tail),a,b,d,f,g Coxsackie virus,j adenovirus (D1 domain)a,b,j |
| ESAM | ESAM (extracellular region),j MAGI-1 (PDZ-binding motif)b,c,e | |
| CLMP | None determined | |
|
Popeye family proteins | ||
| Bves/popeye 1 | Three transmembrane domain, one N-terminal extracellular tail, one extracellular loop, one intracellular linker, one C-terminal cytoplasmic tail | Bves (extracellular region),j ZO-1 (C-terminal cytoplasmic tail),b,g VAMP3 (C-terminal cytoplasmic tail),b,c,e,g GEFT (C-terminal cytoplasmic tail)b,c,g |
|
PERIPHERAL MEMBRANE PROTEINS | ||
|
Zonula occludens (ZO) family
| ||
| ZO-1 | Three PDZ domains, one SH3 domain, one GuK domain, one actin-binding region; ZO-2 and ZO-3 have C-terminal PDZ-binding motifs | Claudins (PDZ1),a,b,e,f,h occludin (SH3-Guk domain),a,b JAM-A (PDZ3),a,b,d JAM-B (PDZ1-3),a,b,h JAM-C (PDZ1–3),a,b tricellulin (aa 1–888),a CAR,d,f,g,h Bves,b,g Neph1–3 (PDZ1),b,e,g connexins (PDZ domains),b,c,e,g ZO-2 (PDZ2),a,b ZO-3 (PDZ2),a,b AF6 (N-terminal to Guk domain),a,b,f,g α-catenin (SH3-Guk domain),a,b ARVCF (PDZ domains),a,b,e,h α-actinin-4 (PDZ1),b,d,e,g α-spectrin,d F-actin (actin-binding region),a,h,j ZONAB (SH3),b,e,j tuba,e,g,h Shroom2 (SH3-Guk),b,c,e,g,h Csk,b Gα(12) (SH3),e,h phosphoinositide (PDZ2)j |
| ZO-2 | Claudins (PDZ1)a,b,e,f,h, occludin (SH3-GuK)b,e, connexins (PDZ domains),b,e,h ZO-1 (PDZ2),a,b α-catenin (SH3-GuK),b,e ARVCF (PDZ domains),a,b,h F-actin,a sec6/8,d,j protein 4.1R (aa 1,054–1,118)b,c,d,e,g tuba,e,g Csk (aa 487–652),b,e Gα (12) (SH3),e,h SAF-B (PDZ1),b,c,e,h PKC,j Fos,b,d,g,j Jun,b,d,g,j C/EBP,b,d,g,j Scribble (C-terminal PDZ-binding motif),b,e,h phosphoinositide (PDZ2)j | |
| ZO-3 | Claudins (PDZ1),a,b,e,f,h occludin,b ZO-1 (PDZ2),d,e MUPP1 (C-terminal PDZ-binding motif),b PATJ (C-terminal PDZ-binding motif )b,e,h,g F-actin,a connexins (PDZ domains)b,e,h | |
|
Cingulin family | ||
| Cingulin | Globular N-terminal head and C-terminal tail domains and central α-helical rod domain | JAM-A,b,d ZO-1 (aa 1–378),b,e ZO-2 (aa 1–378),b,e ZO-3 (aa 1–378),b AF-6 (aa 1–378),b F-actin (aa 101–294),a,b myosin (aa 377–1,368),b GEF-H1 (aa 782–1,025)b,d,g,h |
| Cingulin-L1/JACOP | None determined | |
|
Angiomotin (Amot)/JEAP family | ||
| Amot | Coiled-coil domains and C-terminal PDZ-binding motifs; contains a lipid-binding domain | Angiostatin (BIG3 domain),b,c Rich1 (coiled-coil domain),e Amot-like 1,e MAGI-1,e,g MUPP1 (PDZ-binding motif),c,d,e PATJ (PDZ-binding motif),b,e Syx (PDZ-binding motif)b,e |
| Amotl1/JEAP | Amot (coiled coil),e MUPP1 (PDZ-binding motif),c,e PATJ (PDZ-binding motif),e Syx (PDZ-binding motif )b | |
| Amotl2/MASCOT | MAGI-1,e MUPP1 (PDZ-binding motif),c,d,e PATJ (PDZ-binding motif),e Syx (PDZ-binding motif )b | |
|
MAGUK with inverted domain structure (MAGI) family | ||
| MAGI-1 | Six PDZ domains, two WW domains, one Guk domain | JAM-4 (PDZ1 and PDZ4)b,c,e,h ESAM,b,c,e nephrin (PDZ2–3),a,b,c,d,e,f,g,h megalin (PDZ5),b,c Amot,e,g Amot-like 2,e β-catenin (PDZ5),c,d synaptopodin (WW2)b,e,h,j α-actinin-4 (PDZ5),b,e,h PDZ-GEF1 (PDZ1),d,e mNET1 (PDZ1),b,c,f PTEN (PDZ2),c,d,e,g,h TRIP6 (PDZ5)c,e |
|
MUPP/PATJ family | ||
| MUPP1/MPDZ | One L27 domain, 13 PDZ domains | Claudins (PDZ10),a,c CAR(PDZ13),a,c,d,e,g,h JAM-A (PDZ9),a,b,c nectin (PDZ5),b,h ZO-3 (PDZ7),b PALS (L27),a,b Amot (PDZ2–3),c,e Amot-like 1 (PDZ3),c,e Amot-like 2 (PDZ2–3),c,e Par6,b,e Syxb |
| PATJ | One L27 domain, ten PDZ domains | Claudin-1 (PDZ8),b JAM-A (PDZ3),b nectin (PDZ5),b,h ZO-3 (PDZ6)b,e,h,g PALS1 (L27),a,b Crumbs (N-terminal domain),e Par6,b,e Amot (PDZ3),c,e Amot-like 1 (PDZ1),c,e Amot-like 2 (PDZ2),c,e Syx (PDZ10)c |
|
Other peripheral membrane proteins | ||
| AF-6/l-afadin | Two Ras-binding domains, one forkhead-associated domain, one class V myosin homology region, DIL, one PDZ domain, and an actin-binding domain | Nectin,b,c,d,e,h JAM-A (PDZ),b,c,d,g ZO-1 (Ras-binding domain),a,b,f,g cingulin,b F-actin (aa 1,631–1,829),a profilin,b,c c-Src (PDZ domain),a,b,d,g ADIP (DIL domain),a,b,e Ras,b,c Rap1c,e,h |
| Pilt | hDlgb,c,g | |
| Barmotin/7H6 | None determined | |
| Symplekin | ZONABa,b,c,f,g | |
Parenthetical text indicates the domain used to bind identified partners. A fully referenced version of this table is available online (Supplemental Table 1; follow the Supplemental Material link from the Annual Reviews home page at http://www.annualreviews.org).
In vitro binding of bacterially expressed or purified proteins.
GST (glutathione S-transferase) fusion protein or peptide pulldown from eukaryotic cell lysates.
Yeast two-hybrid assay.
Coimmunoprecipitation of endogenous proteins.
Coimmunoprecipitation of overexpressed proteins.
Colocalization (by electron microscopy).
Colocalization (of endogenous proteins by fluorescence microscopy).
Colocalization (of overexpressed proteins by fluorescence microscopy).
Fluorescent resonant energy transfer.
Other methods.
TIGHT JUNCTION BARRIER FUNCTION IS PLASTIC
The discussion above implies that, although tight junction barrier properties may differ between tissues as a result of claudin expression, they are, for the most part, fixed within each cell type. This view is consistent with early morphological analyses of tight junction structure as well as ex vivo functional studies that showed preservation of paracellular barrier function until tissue death. However, a new model of the tight junction was needed when it was found that barrier function could be acutely regulated. The first study to demonstrate increased barrier function in response to an extracellular stimulus showed that kinetin and zeatin, both of which are plant hormones derived from adenine, were able to induce a marked increase in paracellular electrical resistance of Necterus gallbladder (72). Subsequent work found that the cell-permeant cAMP analog 8-Br-AMP had similar effects (73). In both of these cases, freeze-fracture electron microscopy identified a marked increase in tight junction strand number as well as extension of these strands into lateral cell membranes (72, 73). Similarly, transmucosal conductance of rabbit ileum was reduced by theophylline or cholera toxin, both of which increase intracellular cAMP (74). This was associated with decreased cation selectivity, i.e., a reduction in the relative permeability of Na+ to Cl− from 2.1 to 1.5 (74), consistent with a reduced abundance of paracellular Na+ channels as the cause of the observed decrease in conductance. Although the signaling pathways that increase barrier function in response to cAMP remain incompletely defined, similar functional and morphological changes could be induced by mucosal hyperosmotic stress in guinea pig jejunum (75). More recent data have linked hyperosmotic stress, protein kinase A, and rab13 to endocytic trafficking of tight junction proteins (76).
In contrast to the effects of cAMP, disruption of the cytoskeleton reduced epithelial barrier function of gastrointestinal mucosa and cultured epithelial monolayers (77–79). Cytochalasins, which cap and sever microfilaments, caused profound loss of electrical resistance and cation selectivity along with increased paracellular mannitol flux (79). This was associated with condensation of the dense perijunctional actomyosin ring that lies beneath the tight and adherens junctions (78, 79) as well as a marked decrease in the number of tight junction strands and interstrand junctions (79). Remarkably, these changes were energy dependent, suggesting that tight junction disruption following microfilament depolymerization was an active process. New insight into the mechanisms of this barrier loss has been provided by simultaneous, real-time analysis of tight junction structure and barrier function in epithelial monolayers treated with latrunculin A, which binds to and sequesters G-actin monomers. These studies assessed the organization of validated fluorescent fusion proteins during latrunculin A treatment (80). Remarkably, endocytosis of tagged occludin was temporally associated with barrier loss, whereas claudin-1 and ZO-1 redistribution occurred much later (80). Moreover, inhibition of occludin internalization by reduced temperature, hyperosmotic media, or extraction of membrane cholesterol also prevented latrunculin A–induced barrier loss. Thus, endocytosis is required for tight junction regulation following microfilament depolymerization. This may be of more general importance, as barrier loss associated with disassembly of both adherens and tight junctions following calcium chelation also depends on endocytosis (81).
PHYSIOLOGICAL BARRIER REGULATION OF THE TIGHT JUNCTION BARRIER
In the kidney, charge selectivity of the pore pathway varies along the length of the nephron, and such variance is critical to the physiology of tubular reabsorption (33, 82, 83). However, with the exception of stimuli that modify synthesis and turnover of tight junction proteins over extended intervals, paracellular permeability differences between nephron segments are relatively fixed. In contrast, the tight junction regulation induced by intestinal Na+ nutrient cotransport is an acute and reversible response to luminal nutrients (10, 84–86). The physiological import of this regulation has been the subject of controversy (87, 88). However, it is now generally accepted that increased tight junction permeability and activation of apical Na+-H+ exchange and Na+ absorption by Na+-glucose cotransport contribute significantly to intestinal water and solute absorption (16, 89–94). Electron microscopic examination demonstrated perijunctional actomyosin condensation during active Na+-glucose cotransport and provided initial insight into the mechanism of this process (85, 95). This was followed by biochemical analyses that demonstrated increased myosin II regulatory light-chain (MLC) phosphorylation during Na+-glucose cotransport–induced tight junction regulation, both in vitro (84) and in vivo (96). Although several kinases are capable of phosphorylating MLC (97) and regulating myosin phosphatase (98, 99), both in vitro and in vivo data demonstrate that myosin light-chain kinase (MLCK) is central to Na+-glucose cotransport–induced tight junction regulation (84).
PATHOPHYSIOLOGICAL BARRIER REGULATION OF THE TIGHT JUNCTION BARRIER
Interest in mechanisms of tight junction regulation was amplified by the recognition that intestinal permeability is increased in Crohn's and celiac disease patients (100, 101). Subsequent reports of increased intestinal permeability in healthy first-degree relatives of Crohn's disease patients (102, 103), as well as the association of barrier loss with disease reactivation (104), suggested that tight junction dysregulation might be of great clinical relevance. More recently, the association of tight junction barrier loss with intestinal disease has been explored in greater detail using mouse models (91, 105–112).
The clinical significance of intestinal barrier dysfunction has been the topic of numerous recent reviews and commentaries (14, 94, 113) and is not detailed here. However, as tumor necrosis factor (TNF) is central to intestinal barrier loss and overall pathogenesis of Crohn's disease (114, 115) and graft-versus-host disease (116, 117), it is a biologically important model stimulus. The effect of TNF on in vivo epithelial barrier function can be modeled in vitro using cultured monolayers (118, 119). Although both epithelial apoptosis and tight junction dysregulation have been suggested to contribute to permeability increases (120, 121), a variety of data indicate that MLCK is the primary intermediate through which TNF induces barrier loss, both in vitro (121–123) and in vivo (91, 107). Thus, TNF commandeers an existing physiological signaling pathway to dysregulate paracellular permeability. TNF also downregulates apical Na+-H+ exchange, thereby eliminating the transmucosal Na+ gradient that drives fluid absorption to allow reversal of net fluid transport and to cause diarrhea (91, 94). TNF-induced barrier loss is associated with increased paracellular flux of large, uncharged solutes (119, 123), consistent with regulation of the leak pathway (16).
Like that following actin depolymerization, barrier loss induced by inflammatory cytokines is associated with endocytosis of tight junction proteins (29, 91, 107, 123–126). Acute, in vivo studies suggest that TNF specifically induces occludin internalization (91, 107). Given the apparently normal intestinal barrier function of occludin knockout mice (26), this was an unexpected result. However, studies of occludin knockout mice exposed to stressors have not been reported, and activation of compensatory mechanisms has not been assessed in these animals. In contrast, although transgenic mice that overexpress enhanced green fluorescent protein (EGFP)-occludin within the intestinal epithelium are phenotypically normal under basal conditions, they are significantly protected from TNF-induced barrier loss and diarrhea (29). This may reflect the abundance of EGFP-occludin at intestinal epithelial tight junctions of TNF-treated transgenic, relative to wild-type, mice (29). TNF induces endocytic removal of occludin from intestinal epithelial tight junctions of wild-type mice by mechanisms that require MLCK; dynamin II; caveolin-1; and cholesterol-enriched, raft-like membrane domains (Figure 3) (29, 107). Prevention of occludin endocytosis by inhibition of MLCK or dynamin II, knockout of caveolin-1, or disruption of raft-like membrane domains prevents TNF-induced barrier loss and diarrhea (29). These data suggest that occludin contributes to tight junction organization and is essential for TNF-induced, MLCK-dependent tight junction regulation. Analyses in MDCK monolayers confirm the critical role of occludin in leak pathway maintenance (27). Moreover, knockdown of either occludin or caveolin-1, but not that of tricellulin, prevents TNF-induced leak pathway flux increases in MDCK monolayers (28). Thus, occludin is involved in multiple forms of cytoskeletally mediated leak pathway regulation. However, in vitro studies showed that occludin overexpression and knockdown increased and reduced sensitivity to cytokines, respectively (28), whereas the in vivo studies showed that occludin overexpression was protective (29). Further studies are needed to define the reasons for this difference, which may include the increased barrier function induced by occludin overexpression in vitro or activation of processes to compensate for occludin overexpression in vivo.
Figure 3.
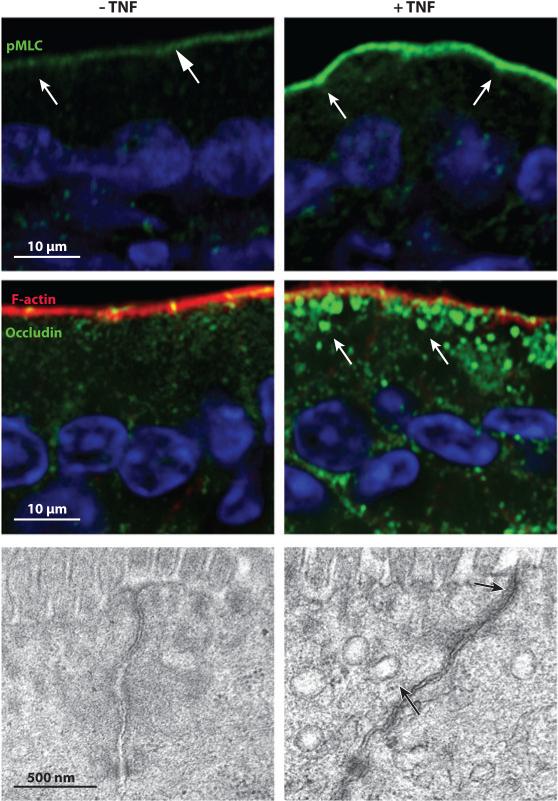
Tight junction regulation by tumor necrosis factor (TNF). Jejunal mucosa from control (left column) and TNF-treated (right column) mice were stained for phosphorylated MLC (pMLC; top row) or occludin and F-actin (middle row). Nuclei (blue) are shown for orientation. Arrows show enrichment of phosphorylated MLC at junctions (top row) and occludin-laden endocytic vesicles (middle row). Transmission electron microscopy (bottom row) shows perijunctional actomyosin condensation and endocytic vesicle accumulation (black arrows) after TNF treatment. Panels from Reference 29 with permission.
In contrast to TNF, interleukin (IL)-13, which is also implicated in inflammatory bowel disease, increases paracellular permeability to small, uncharged molecules and cations in vitro and in vivo (16, 62, 127). This barrier defect is not associated with MLCK activation or endocytosis but does require increased claudin-2 expression (16). Interestingly, clinical samples show that claudin-2 expression is augmented to a greater degree in ulcerative colitis relative to Crohn's disease (128) and correlates with the degree of IL-13 induction in patients with these diseases (62). Thus, IL-13 selectively activates the pore pathway by increasing claudin-2 expression. Although physiological regulation of the claudin-2-dependent pore pathway has not been described, claudin-2 expression is far greater in the developing neonatal gut (32), where it may compensate for the immaturity of other mechanisms of regulating paracellular transport. These data indicate that TNF and IL-13, cytokines that are central to intestinal barrier loss in disease, activate different signaling events to enhance trans–tight junction flux by distinct pathways. However, MLCK-dependent increases in intestinal epithelial leak pathway permeability are also able to induce mucosal IL-13 production, which, in turn, increases claudin-2 expression and pore pathway permeability (16). Thus, although pore and leak pathways are separable, they are likely to communicate with one another in vivo. This conclusion has implications for the durability of barrier regulation, as phosphorylation events, such as MLCK-dependent leak pathway regulation, can be activated and reversed rapidly, whereas synthesis of new proteins, such as claudin-2 to increase pore pathway cation flux, develops more slowly and is longer lasting.
TIGHT JUNCTION MOLECULAR STRUCTURE IS HIGHLY DYNAMIC
The data above demonstrate the remarkable plasticity of the tight junction with respect to barrier function. This is frequently, but not always, associated with modulation of tight junction ultrastructure or redistribution of tight junction–associated proteins. Although physiologically desirable, the ease with which the tight junction can be acutely regulated is difficult to reconcile with the model of the tight junction as a stable, heavily cross-linked structure. This raises the possibility that the molecular structure of the tight junction is more dynamic, even at steady state, than previously thought. This possibility would also be consistent with the mathematical models that predict tight junction flux pathways to have both open and closed states that can be defined by open probability (8). However, structural correlates of opening and closing have not been identified.
To better understand the dynamic behavior of the barrier, the technique of fluorescence recovery after photobleaching (FRAP), which allows analysis of fluorescent protein mobility in living cells and tissues, has been applied to tight junction proteins (Figure 4). FRAP analysis of non–tight junction strands formed by claudin-1-EGFP strands in fibroblasts showed that there was no fluorescent recovery within strands (49). Thus, although the strands were highly dynamic, the stability of claudin-1-EGFP within strands supported a view of the tight junction as a stable, heavily cross-linked, molecular complex. However, a detailed analysis of tight junction protein steady-state dynamics showed that, whereas EGFP-claudin-1 was relatively stable, other tight junction proteins have distinct exchange behaviors (65). These experiments were performed by systematically assessing the mobilities of different EGFP-tagged tight junction proteins expressed in MDCK monolayers by FRAP and fluorescence loss in photobleaching (FLIP) (65).
Figure 4.
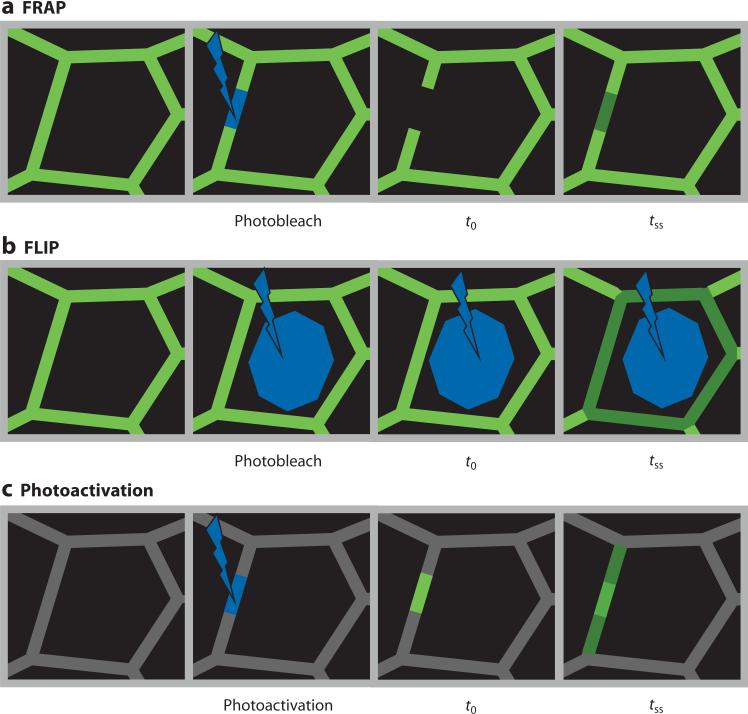
Analysis of protein interactions in living cells is greatly facilitated using fluorescent fusion proteins. Methods of measurement include fluorescence recovery after photobleaching (FRAP), fluorescence loss in photobleaching (FLIP), and time-lapse imaging of photoactivatable and photoswitchable fluoroproteins. (a) FRAP analysis of tight junction proteins is accomplished by photobleaching a defined segment of the junction followed by time-lapse imaging until a later time at which steady state is achieved (tss). Quantitative analysis of fluorescence recovery can be used to determine the size of the pool available for exchange, or mobile fraction, as well as the rate of exchange, as reflected by the time required for half-maximal recovery. (b) FLIP can be used to assess exchange between morphologically distinct protein pools. In the example shown, continuous photobleaching of the cytoplasm, sparing the tight junction, leads to reduced fluorescence at the tight junction. This indicates exchange between tight junction and cytoplasmic pools. Similarly, analysis of fluorescence in one area of the tight junction during continuous photobleaching of a separate area of the tight junction can be used to measure exchange between these areas. (c) Photoactivatable and photoswitchable fluoroproteins allow experiments that are essentially the reverse of FRAP. For example, targeted activation at one region of the tight junction allows measurement of the rate at which fluorescence moves away from the junction and simultaneous morphological and quantitative analysis of the sites to which the protein is trafficked.
FRAP analyses showed that EGFP-occludin is highly mobile at the tight junction (65). Given the critical role of occludin endocytosis in cytoskeletally mediated tight junction regulation (28, 29), this result suggested that vesicular traffic might be involved in occludin fluorescence recovery. However, neither ATP depletion nor brefeldin A, a guanine exchange factor inhibitor that interferes with membrane traffic, affected occludin FRAP kinetics (65). In contrast, occludin fluorescent recovery was markedly inhibited by disruption of raft-like membrane domains with methyl-β-cyclodextrin or global membrane stabilization by reduced temperature (65). A significant role for intracellular occludin pools in FRAP behavior was also excluded by FLIP analyses in which continuous photobleaching of the cytoplasm did not effect significant fluorescent loss at the tight junction (65). In contrast, FLIP studies in which the tight junction was photobleached continuously showed progressive loss of occludin fluorescence that extended from the bleached region, suggesting that occludin diffuses within the membrane (65). Consistent with these data, recovery of an elongated photobleached region began at the edges and moved progressively to the center. Moreover, occludin tagged with photoactivatable green fluorescent protein (GFP) diffused within the tight junction. Thus, diffusion within the membrane is the primary mechanism of occludin fluorescence recovery (65).
Like occludin, fluorescent-tagged ZO-1 is highly mobile at the tight junction (65, 129). One possible explanation of these data is that occludin and ZO-1 diffuse within the membrane bound to one another, as has been suggested for the E-cadherin-β-catenin complex (130). However, unlike occludin, ZO-1 exchange is energy dependent, is insensitive to methyl-β-cyclodextrin, and is only modestly affected by temperature (65). Moreover, FLIP studies in which the tight junction was photobleached continuously did not significantly reduce ZO-1 fluorescence in adjacent regions, and recovery was simultaneous at the center and edges of an elongated photobleached region (65). However, continuous photobleaching of the cytoplasm caused progressive loss of ZO-1 fluorescence at the tight junction, indicating communication between cytosolic and tight junction–associated ZO-1 pools (65). These data show that ZO-1 does not remain bound to occludin during exchange but exchanges with a cytosolic pool by an energy-dependent mechanism. Thus, two of three representative tight junction proteins are remarkably dynamic at the tight junction.
Exchange of claudin-1, occludin, and ZO-1; experimental data from FRAP and FLIP studies; and other features of protein trafficking were used to model the dynamic behavior of each protein (Figure 5a). This was straightforward in the case of claudin-1, which could be modeled as exclusively associated with the tight junction. However, to replicate the limited mobility of claudin-1, it was necessary to define tight junction–associated claudin-1 as existing in two pools: one that was able to diffuse and a second, which contained approximately 60% of total claudin-1, that was stably anchored. When a diffusion coefficient similar to previously reported values for transmembrane proteins was applied, this simple model accurately recapitulated EGFP-claudin-1 behavior in standard FRAP analyses as well as FLIP experiments in which either tight junction or cytoplasm was bleached continuously (65). Thus, in silico modeling supports the conclusion that tight junction–associated claudin-1 is present in two biophysically distinct pools. It will be important for future studies to define the respective functions of these pools and potential for communication between them.
Figure 5.
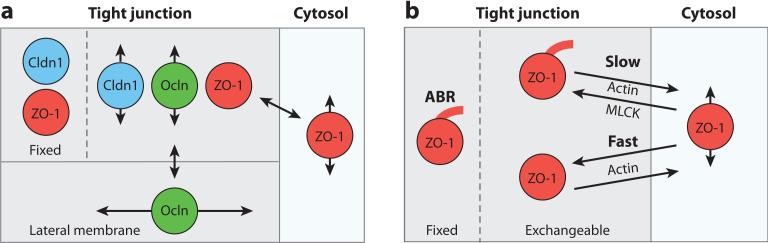
Models of tight junction protein behavior. (a) Computer simulations of tight junction FLIP (fluorescence loss in photobleaching) and FRAP (fluorescence recovery after photobleaching) behavior support the presence of different dynamic behaviors for claudin-1 (Cldn1), occludin (Ocln), and zonula occludens-1 (ZO-1). Cldn1 is localized at the tight junction, and fluorescence recovery occurs via diffusion of the mobile pool from adjacent unbleached areas of tight junction. The fixed pool does not exchange. Like Cldn1, Ocln diffuses within the tight junction, but exchange also occurs with a lateral membrane pool. However, unlike Cldn1, there is no fixed Ocln pool. Both fixed and exchangeable ZO-1 pools are present at the tight junction; the latter exchanges with a cytosolic ZO-1 pool. (b) The rate of ZO-1 exchange between the tight junction and cytosol is regulated through interactions with actin. ZO-1 dynamics are best modeled as containing two exchangeable pools at the tight junction. An actin-binding region (ABR)-anchored pool exchanges slowly between the cytosol and the tight junction and is sensitive to myosin light-chain kinase (MLCK) inhibition, whereas a MLCK-independent pool exchanges rapidly between the tight junction and the cytoplasm.
As detailed above, several pieces of data suggest that vesicular trafficking of cytoplasmic pools to and from the tight junction does not contribute to occludin FRAP behavior. However, even after adjusting pool sizes, EGFP-occludin behavior could not be reproduced using a model similar to that developed for claudin-1. Several models were tested, but only one in which occludin exchanged within and between tight junction and lateral membrane pools accurately recapitulated occludin behavior in FRAP and FLIP experiments (65). This is consistent with detection of both EGFP-occludin and endogenous occludin below the tight junction within lateral membranes (29, 131, 132). Interestingly, anchored pools were not required in this model, but it was necessary to assume that lateral membrane occludin diffuses more rapidly than that at the tight junction (65).
The FRAP and FLIP data indicate that ZO-1 does not diffuse at the membrane but exchanges with a large cytosolic pool. A model including a large cytosolic pool as well as exchangeable and anchored tight junction–associated ZO-1 pools was able to reproduce the results from the FRAP and FLIP studies (65). Consistent with the presence of numerous protein interaction domains within ZO-1, the in silico diffusion constant required to model cytosolic ZO-1 behavior was markedly less than that measured for free cytoplasmic EGFP. Thus, in silico modeling of actual FRAP and FLIP data can provide unique insight into the composition of intracellular pools and exchange reactions. In turn, this information can be used to probe the specific interactions involved in protein dynamic behavior.
MOLECULAR DETERMINANTS OF LEAK PATHWAY FLUX
As described above, MLCK is central to physiological and pathophysiological regulation of the leak pathway. To test the hypothesis that tight junction protein exchange contributes to barrier regulation, the FRAP behaviors of claudin-1, occludin, ZO-1, and actin were assessed in Caco-2 intestinal epithelial monolayers. Although claudin-1, occludin, and actin exchange was similar to that observed in MDCK monolayers, a slightly greater fraction of ZO-1 was anchored at the tight junction of Caco-2 cells (133). Pharmacological MLCK inhibition significantly elevated transepithelial electrical resistance (TER) of Caco-2 monolayers, but there was no change in localization or FRAP kinetics of claudin-1, occludin, or perijunctional actin. In contrast, ZO-1 fluorescence recovery was markedly reduced following MLCK inhibition (133). In vivo FRAP analyses of fluorescent-tagged ZO-1 expressed in jejunal epithelia of transgenic mice showed that, as was true in MDCK and Caco-2 monolayers, ZO-1 was highly dynamic. Moreover, pharmacological MLCK inhibition limited in vivo ZO-1 recovery (133). Thus, MLCK regulates both tight junction barrier function and exchange of ZO-1 between tight junction and cytosolic pools.
ZO-1 contains three PDZ domains, the most N-terminal of which binds to claudins (67), a centrally located SH3-guanylate kinase hinge region, the site of interaction with occludin, and an actin-binding region near the C terminus (134, 135). To better understand the mechanisms of MLCK-dependent ZO-1 exchange, FRAP behavior of a mutant ZO-1 lacking the actin-binding region (ABR), ZO-1ΔABR, was assessed (133). The fraction of ZO-1ΔABR stably associated with the tight junction was reduced, relative to full-length ZO-1. Moreover, MLCK inhibition did not affect exchange of ZO-1ΔABR. These data indicate that the ABR anchors ZO-1 at the tight junction, both at steady state and following MLCK inhibition (133). Consistent with this, expression of free ABR reduced full-length ZO-1 exchange to a level similar to that after MLCK inhibition (133). MLCK inhibition did not cause further reductions of full-length ZO-1 exchange in Caco-2 monolayers expressing free ABR. Thus, the ABR is a critical determinant of ZO-1 anchoring and acts as a dominant-negative inhibitor of ZO-1 exchange.
The central role of the ABR in ZO-1 anchoring and exchange raises the possibility that the ABR may also be involved in MLCK-dependent barrier regulation. The observation that ABR expression reduces TER supports this hypothesis. Importantly, ABR expression does not affect TER in ZO-1 knockdown cells, confirming that the TER loss induced by ABR expression in wild-type monolayers reflects a dominant-negative effect on ZO-1 and is not the result of global actin disruption. Finally, ABR expression prevents MLCK-dependent barrier regulation (133). Similarly, TER of Caco-2 monolayers in which ZO-1 expression was knocked down was unaffected by MLCK inhibition, either with or without ABR expression (133). As a whole, these data show that MLCK-dependent ZO-1 exchange and ABR-dependent ZO-1 anchoring are critical determinants of tight junction barrier function.
The conclusions above are consistent with a separate study showing increased leak pathway flux of MDCK cell monolayers after ZO-1 knockdown (15). MDCK cells lacking ZO-1 also demonstrated increased sensitivity to barrier loss induced by actin depolymerization, extracellular Ca2+ chelation, or myosin II motor inhibition (15). However, that report showed that ZO-1ΔABR was able to restore leak pathway barrier function in MDCK cells lacking full-length ZO-1 (15). The reasons for this difference are not entirely clear but may reflect greater ZO-1 anchoring in Caco-2 cells (133) or the failure of MLCK inhibition to raise TER in MDCK monolayers (L. Shen & J.R. Turner, unpublished observations). As noted above, occludin has also been linked to TNF-induced leak pathway regulation (28, 29). Moreover, ZO-1 and occludin, but not claudins, are redistributed following MLCK activation in cultured monolayers (136) as well as after immune activation in vivo (107). Thus, ZO-1 and occludin appear to be central to leak pathway function. It will be important for future analyses to further define the molecular roles of these proteins in leak pathway regulation as well as their contributions to signaling by distinct stimuli.
In addition to studies of deletion mutants and cells lacking ZO-1, mathematical modeling may provide further insight into and direct investigation of ZO-1 function. Such analyses show that the previous model of ZO-1 trafficking using a large cytosolic pool and both exchangeable and nonexchangeable (fixed) tight junction–associated ZO-1 pools (65), which was developed from data obtained using MDCK cells, is unable to explain the effects of MLCK inhibition on ZO-1 trafficking in Caco-2 cells as well as murine jejunal enterocytes (133). A model capable of explaining the Caco-2 data required division of the exchangeable tight junction–associated ZO-1 pool into two pools on the basis of sensitivity to MLCK inhibition (Figure 5b). Modeling of ZO-1 exchange before and after MLCK inhibition on this basis allowed definition of MLCK-dependent and MLCK-independent components of ZO-1 exchange (133). This model also required that the ABR be involved in anchoring the nonexchangeable ZO-1 pool as well as the pool that exchanges by a MLCK-dependent process (133). Although this model may require further refinement as new data are obtained, it does recapitulate the ZO-1 FRAP data obtained in vitro using both MDCK and Caco-2 monolayers as well as in vivo data from transgenic mice (133). Moreover, changes to a single constant (either exchange rate or size of one pool) are sufficient to reproduce the effects of MLCK inhibition or ABR deletion (133). This model will be useful as a guide to future studies of cytoskeletally mediated leak pathway regulation.
MOLECULAR DETERMINANTS OF PORE PATHWAY FLUX
As briefly described above and reviewed in detail elsewhere (43), the claudin residues responsible for ion selectivity and modulation of pore pathway permeability by changes in claudin protein expression have been well documented in cell culture (34, 37, 38, 40, 137–141), animal models (16, 32), and human disease (33, 62, 142). However, other than examples of acute claudin endocytosis, such as that following C. perfringens enterotoxin binding to claudins 3 and 4 (54, 57, 58), little information is available regarding posttranslational regulation of the pore pathway. However, palmitoylation can influence the trafficking and half-life of claudin proteins and, as a direct result, modify effects of claudins on paracellular permeability (141, 143). Additionally, interactions among claudin proteins to form pores (144) and to regulate pore opening and closing events are likely critical to barrier regulation. Finally, interactions between specific claudins and other tight junction proteins may regulate pore assembly and opening. For example, emerging data are consistent with a model in which phosphorylation of a specific site within the C-terminal occludin tail destabilizes occludin ZO-1 interactions. This enhances ZO-1-dependent claudin-2 stabilization at the tight junction, e.g., a reduced mobile fraction as assessed by FRAP, and enhances cation flux through claudin-2 pores (145). Conversely, the model suggests that occludin dephosphorylation facilitates assembly of a complex that includes occludin, ZO-1, and claudin-2; increases claudin-2 and ZO-1 exchange; and limits assembly or opening of claudin-2-based pores (145). Experiments that test this model and others are likely to yield a new molecular understanding of the pore and leak pathways of tight junction flux over the next decade.
CONCLUSIONS
Tight junctions form the major paracellular barrier to the flux of ions and molecules. The function of this barrier is complex, with at least two mechanisms of trans–tight junction flux: a pore pathway and a leak pathway. It is now well established that claudins are important in establishing the tight junction pore pathway, and recent findings have shown that ZO-1 and occludin are important in the leak pathway. However, regulation of these pathways can also be defined by interactions between multiple classes of tight junction proteins. Moreover, cross talk between the pore and leak pathways may occur via intracellular and extracellular signaling events that include kinase activation and cytokine release by nonepithelial cells. An integrated understanding of the mechanisms that determine barrier function and the interplay among these regulatory processes is being developed. Such an understanding will delineate multiple means of selectively modifying epithelial barrier function with respect to specific types of solutes and, ultimately, will provide new insight into the contributions of tight junction physiology and pathophysiology.
Tight junction: the most apical intercellular junction; limits paracellular flux
Paracellular transport: passive movement of water and solutes through the space between adjacent cells (paracellular space)
Transcellular transport: active or passive movement of water and solutes through cells by means of specific transmembrane transport proteins
Tight and leaky epithelia: refers to tissues that have paracellular (tight junction) electrical resistances that vary by several orders of magnitude
Strands: the anastamosing network of transmembrane particles, including claudins, that characterizes the freeze-fracture electron microscopy appearance of the tight junction
Barrier function: the ability of epithelium- and endothelium-lined surfaces to restrict free passage of water, ions, and larger solutes. This can be measured by a variety of techniques
Charge selectivity: the ability of the tight junction to differentially restrict paracellular flux of ions on the basis of charge
MDCK: Madin-Darby canine kidney
Size selectivity: the ability of the tight junction to differentially restrict paracellular flux of uncharged molecules on the basis of size
Pore pathway: a high-capacity, size- and charge-selective paracellular route that appears to be defined by the subset of claudins expressed
Leak pathway: a low-capacity, paracellular route that does not discriminate between solutes on the basis of charge and allows limited flux of large molecules; ZO-1 and occludin have been implicated in leak pathway regulation
Zonula occludens-1 (ZO-1): a tight junction–associated peripheral membrane protein with multiple sites for protein-protein interactions, often referred to as a scaffold or plaque protein, that organizes and regulates the tight junction. ZO-1 knockout results in embryonic lethality
Occludin: a tight junction–associated transmembrane protein that appears to play a regulatory role and organizes tight junction structure but is not essential for life
Claudins: a large family of tight junction–associated transmembrane proteins that define charge selectivity of the tight junction and are critical for barrier function. For example, claudin-2 forms a cation-specific pore that permits paracellular Na+ flux
PDZ (PSD95, Dlg, and ZO-1) domain: a protein domain that supports protein interactions. A YV motif at the C terminus of claudins binds to the first of three PDZ domains within ZO-1
TAMPs: tight junction–associated marvel proteins
MLC: myosin II regulatory light chain
Myosin light-chain kinase (MLCK): the Ca2+-calmodulin-dependent, serine-threonine kinase that phosphorylates the myosin II regulatory light chain. A single gene (MYLK) encodes smooth muscle and nonmuscle MLCK proteins, with two primary forms, long and short, expressed in various tissues
TNF: tumor necrosis factor
EGFP: enhanced green fluorescent protein
Fluorescence recovery after photobleaching (FRAP): one of several related techniques for measuring dynamic behavior of molecules in real time, often in live cells
Fluorescent protein: expressed by some jellyfish and coral; is commonly used as a fusion partner to track protein movement in live cells and tissues
FLIP: fluorescence loss in photobleaching
GFP: green fluorescent protein
ABR: actin-binding region
SUMMARY POINTS.
Tight junctions are the principal determinants of epithelial, and endothelial, paracellular barrier function.
The permeability of tight junctions varies significantly between tissues and can also be regulated in response to pharmacological, physiological, and pathophysiological stimuli.
There are at least two distinct pathways across the tight junction: a high-capacity pore pathway that allows small, uncharged solutes and specific ions to pass and a low-capacity leak pathway that is permeable to larger macromolecules but is not ion selective.
Charge selectivity and overall permeability of the pore pathway are defined primarily by the subset of claudins expressed but may also be regulated by protein interactions at the tight junction.
Permeability of the leak pathway can be acutely regulated by the cytoskeleton via mechanisms that involve ZO-1 and occludin.
Molecular interactions at the tight junction are dynamic at steady state. Differential modulation of the kinetics of these processes may underlie leak and pore pathway regulation.
Mathematical modeling of tight junction structure, permeability, and protein exchange dynamics can provide new insight into mechanisms of tight junction regulation.
FUTURE ISSUES.
The specific contributions of individual proteins to tight junction function are only beginning to be understood and are in need of further study.
The structure of claudin-based pores is unknown, and factors such as open probability and single-claudin pore conductance are presently only theoretical considerations. Improved understanding of these properties, both at steady state and during regulation, will require new technologies that measure local tight junction function.
An improved understanding of the biophysical characteristics of the leak pathway and the structures that allow large molecules to pass through the tight junction is required.
Mechanisms of protein delivery to and retrieval from the tight junction remain incompletely defined.
Improved in vivo characterization of protein interactions at the tight junction will inform future functional analyses. The effects of posttranslational protein modifications on these interactions, as well as on tight junction structure and function, deserve further analysis.
Application of sub-diffraction-limit optical imaging and other emerging imaging approaches to living cells may provide novel insight into the dynamics of tight junction strand organization.
The contribution of membrane lipids to tight junction structure and function at steady state and during barrier regulation requires further study.
Further molecular definition of signaling between transcellular transporters and paracellular leak and pore pathways is needed to understand the mechanisms that coordinate mucosal fluid and electrolyte transport in normal and pathological states.
ACKNOWLEDGMENTS
We are grateful to past and present members of our research group, our collaborators, and many others in the field for the insights they have provided into tight junction structure and function. We also thank Drs. James Madara and James Anderson for their critical reviews of the manuscript. We also apologize to colleagues whose outstanding work on tight junction physiology was not cited due to length restrictions. Our work is supported by the National Institutes of Health (R01DK61931, R01DK68271, and P01DK067887 to J.R.T.; F32DK082134 and K08DK088953 to C.R.W.), the Department of Defense (W81XWH-09-1-0341 to J.R.T.), the Broad Medical Research Foundation (IBD-022 to J.R.T.), the University of Chicago Digestive Disease Center (P30DK042086), the University of Chicago Cancer Center (P30CA14599), the University of Chicago Institute for Translational Medicine (UL1RR024999), the University of Chicago Medical Scientist Training Program (5T32GM007281), and a research fellowship award from the Crohn's and Colitis Foundation of America sponsored by Laura McAteer Hoffman (to L.S.).
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Farquhar M, Palade G. Junctional complexes in various epithelia. J. Cell Biol. 1963;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodenough DA, Revel JP. A fine structural analysis of intercellular junctions in the mouse liver. J. Cell Biol. 1970;45:272–90. doi: 10.1083/jcb.45.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Machen TE, Erlij D, Wooding FB. Permeable junctional complexes. The movement of lanthanum across rabbit gallbladder and intestine. J. Cell Biol. 1972;54:302–12. doi: 10.1083/jcb.54.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diamond JM, Bossert WH. Standing-gradient osmotic flow. A mechanism for coupling of water and solute transport in epithelia. J. Gen. Physiol. 1967;50:2061–83. doi: 10.1085/jgp.50.8.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foskett JK, Machen TE. Vibrating probe analysis of teleost opercular epithelium: correlation between active transport and leak pathways of individual chloride cells. J. Membr. Biol. 1985;85:25–35. doi: 10.1007/BF01872003. [DOI] [PubMed] [Google Scholar]
- 6.Staehelin LA, Mukherjee TM, Williams AW. Freeze-etch appearance of the tight junctions in the epithelium of small and large intestine of mice. Protoplasma. 1969;67:165–84. doi: 10.1007/BF01248737. [DOI] [PubMed] [Google Scholar]
- 7.Claude P, Goodenough DA. Fracture faces of zonulae occludentes from “tight” and “leaky” epithelia. J. Cell Biol. 1973;58:390–400. doi: 10.1083/jcb.58.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Claude P. Morphological factors influencing transepithelial permeability: a model for the resistance of the zonula occludens. J. Membr. Biol. 1978;39:219–32. doi: 10.1007/BF01870332. [DOI] [PubMed] [Google Scholar]
- 9.Naftalin RJ, Tripathi S. Passive water flows driven across the isolated rabbit ileum by osmotic, hydrostatic and electrical gradients. J. Physiol. 1985;360:27–50. doi: 10.1113/jphysiol.1985.sp015602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fihn BM, Sjoqvist A, Jodal M. Permeability of the rat small intestinal epithelium along the villus-crypt axis: effects of glucose transport. Gastroenterology. 2000;119:1029–36. doi: 10.1053/gast.2000.18148. [DOI] [PubMed] [Google Scholar]
- 11.Marcial MA, Carlson SL, Madara JL. Partitioning of paracellular conductance along the ileal crypt-villus axis: a hypothesis based on structural analysis with detailed consideration of tight junction structure-function relationships. J. Membr. Biol. 1984;80:59–70. doi: 10.1007/BF01868690. [DOI] [PubMed] [Google Scholar]
- 12.Van Itallie CM, Holmes J, Bridges A, Gookin JL, Coccaro MR, et al. The density of small tight junction pores varies among cell types and is increased by expression of claudin-2. J. Cell Sci. 2008;121:298–305. doi: 10.1242/jcs.021485. [DOI] [PubMed] [Google Scholar]
- 13.Watson CJ, Hoare CJ, Garrod DR, Carlson GL, Warhurst G. Interferon-gamma selectively increases epithelial permeability to large molecules by activating different populations of paracellular pores. J. Cell Sci. 2005;118:5221–30. doi: 10.1242/jcs.02630. [DOI] [PubMed] [Google Scholar]
- 14.Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harb. Perspect. Biol. 2009;1:a002584. doi: 10.1101/cshperspect.a002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Itallie CM, Fanning AS, Bridges A, Anderson JM. ZO-1 stabilizes the tight junction solute barrier through coupling to the perijunctional cytoskeleton. Mol. Biol. Cell. 2009;20:3930–40. doi: 10.1091/mbc.E09-04-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weber CR, Raleigh DR, Su L, Shen L, Sullivan EA, et al. Epithelial myosin light chain kinase activation induces mucosal interleukin-13 expression to alter tight junction ion selectivity. J. Biol. Chem. 2010;285:12037–46. doi: 10.1074/jbc.M109.064808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J. Cell Biol. 1986;103:755–66. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Citi S, Sabanay H, Jakes R, Geiger B, Kendrick-Jones J. Cingulin, a new peripheral component of tight junctions. Nature. 1988;333:272–75. doi: 10.1038/333272a0. [DOI] [PubMed] [Google Scholar]
- 19.Ohnishi H, Nakahara T, Furuse K, Sasaki H, Tsukita S, Furuse M. JACOP, a novel plaque protein localizing at the apical junctional complex with sequence similarity to cingulin. J. Biol. Chem. 2004;279:46014–22. doi: 10.1074/jbc.M402616200. [DOI] [PubMed] [Google Scholar]
- 20.Jesaitis LA, Goodenough DA. Molecular characterization and tissue distribution of ZO-2, a tight junction protein homologous to ZO-1 and the Drosophila discs-large tumor suppressor protein. J. Cell Biol. 1994;124:949–61. doi: 10.1083/jcb.124.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J. Cell Biol. 1993;123:1777–88. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nusrat A, Chen JA, Foley CS, Liang TW, Tom J, et al. The coiled-coil domain of occludin can act to organize structural and functional elements of the epithelial tight junction. J. Biol. Chem. 2000;275:29816–22. doi: 10.1074/jbc.M002450200. [DOI] [PubMed] [Google Scholar]
- 23.Furuse M, Itoh M, Hirase T, Nagafuchi A, Yonemura S, Tsukita S. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J. Cell Biol. 1994;127:1617–26. doi: 10.1083/jcb.127.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Itallie CM, Anderson JM. Occludin confers adhesiveness when expressed in fibroblasts. J. Cell Sci. 1997;110:1113–21. doi: 10.1242/jcs.110.9.1113. [DOI] [PubMed] [Google Scholar]
- 25.Saitou M, Fujimoto K, Doi Y, Itoh M, Fujimoto T, et al. Occludin-deficient embryonic stem cells can differentiate into polarized epithelial cells bearing tight junctions. J. Cell Biol. 1998;141:397–408. doi: 10.1083/jcb.141.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, et al. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol. Biol. Cell. 2000;11:4131–42. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu AS, McCarthy KM, Francis SA, McCormack JM, Lai J, et al. Knockdown of occludin expression leads to diverse phenotypic alterations in epithelial cells. Am. J. Physiol. Cell Physiol. 2005;288:1231–41. doi: 10.1152/ajpcell.00581.2004. [DOI] [PubMed] [Google Scholar]
- 28.Van Itallie CM, Fanning AS, Holmes J, Anderson JM. Occludin is required for cytokine-induced tight junction barrier regulation. J. Cell Sci. 2010;123:2844–52. doi: 10.1242/jcs.065581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marchiando AM, Shen L, Graham WV, Weber CR, Schwarz BT, et al. Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J. Cell Biol. 2010;189:111–26. doi: 10.1083/jcb.200902153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J. Cell Biol. 1998;141:1539–50. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furuse M, Sasaki H, Fujimoto K, Tsukita S. A single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J. Cell Biol. 1998;143:391–401. doi: 10.1083/jcb.143.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holmes JL, Van Itallie CM, Rasmussen JE, Anderson JM. Claudin profiling in the mouse during postnatal intestinal development and along the gastrointestinal tract reveals complex expression patterns. Gene Expr. Patterns. 2006;6:581–88. doi: 10.1016/j.modgep.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Simon DB, Lu Y, Choate KA, Velazquez H, Al-Sabban E, et al. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science. 1999;285:103–6. doi: 10.1126/science.285.5424.103. [DOI] [PubMed] [Google Scholar]
- 34.Amasheh S, Meiri N, Gitter AH, Schoneberg T, Mankertz J, et al. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J. Cell Sci. 2002;115:4969–76. doi: 10.1242/jcs.00165. [DOI] [PubMed] [Google Scholar]
- 35.Colegio OR, Van Itallie CM, McCrea HJ, Rahner C, Anderson JM. Claudins create charge-selective channels in the paracellular pathway between epithelial cells. Am. J. Physiol. Cell Physiol. 2002;283:142–47. doi: 10.1152/ajpcell.00038.2002. [DOI] [PubMed] [Google Scholar]
- 36.Colegio OR, Van Itallie C, Rahner C, Anderson JM. Claudin extracellular domains determine paracellular charge selectivity and resistance but not tight junction fibril architecture. Am. J. Physiol. Cell Physiol. 2003;284:1346–54. doi: 10.1152/ajpcell.00547.2002. [DOI] [PubMed] [Google Scholar]
- 37.Van Itallie CM, Fanning AS, Anderson JM. Reversal of charge selectivity in cation or anion-selective epithelial lines by expression of different claudins. Am. J. Physiol. Ren. Physiol. 2003;285:1078–84. doi: 10.1152/ajprenal.00116.2003. [DOI] [PubMed] [Google Scholar]
- 38.Yu AS, Enck AH, Lencer WI, Schneeberger EE. Claudin-8 expression in Madin-Darby canine kidney cells augments the paracellular barrier to cation permeation. J. Biol. Chem. 2003;278:17350–59. doi: 10.1074/jbc.M213286200. [DOI] [PubMed] [Google Scholar]
- 39.Van Itallie CM, Rogan S, Yu A, Vidal LS, Holmes J, Anderson JM. Two splice variants of claudin-10 in the kidney create paracellular pores with different ion selectivities. Am. J. Physiol. Ren. Physiol. 2006;291:1288–99. doi: 10.1152/ajprenal.00138.2006. [DOI] [PubMed] [Google Scholar]
- 40.Hou J, Renigunta A, Konrad M, Gomes AS, Schneeberger EE, et al. Claudin-16 and claudin-19 interact and form a cation-selective tight junction complex. J. Clin. Investig. 2008;118:619–28. doi: 10.1172/JCI33970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Günzel D, Amasheh S, Pfaffenbach S, Richter JF, Kausalya PJ, et al. bClaudin-16 affects transcellular Cl− secretion in MDCK cells. J. Physiol. 2009;587:3777–93. doi: 10.1113/jphysiol.2009.173401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu AS, Cheng MH, Angelow S, Günzel D, Kanzawa SA, et al. Molecular basis for cation selectivity in claudin-2-based paracellular pores: identification of an electrostatic interaction site. J. Gen. Physiol. 2009;133:111–27. doi: 10.1085/jgp.200810154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Annu. Rev. Physiol. 2006;68:403–29. doi: 10.1146/annurev.physiol.68.040104.131404. [DOI] [PubMed] [Google Scholar]
- 44.Matter K, Balda MS. Signalling to and from tight junctions. Nat. Rev. Mol. Cell Biol. 2003;4:225–36. doi: 10.1038/nrm1055. [DOI] [PubMed] [Google Scholar]
- 45.Kachar B, Reese TS. Evidence for the lipidic nature of tight junction strands. Nature. 1982;296:464–66. doi: 10.1038/296464a0. [DOI] [PubMed] [Google Scholar]
- 46.Pinto da Silva P, Kachar B. On tight-junction structure. Cell. 1982;28:441–50. doi: 10.1016/0092-8674(82)90198-2. [DOI] [PubMed] [Google Scholar]
- 47.Nusrat A, Parkos CA, Verkade P, Foley CS, Liang TW, et al. Tight junctions are membrane microdomains. J. Cell Sci. 2000;113:1771–81. doi: 10.1242/jcs.113.10.1771. [DOI] [PubMed] [Google Scholar]
- 48.Francis SA, Kelly JM, McCormack J, Rogers RA, Lai J, et al. Rapid reduction of MDCK cell cholesterol by methyl-beta-cyclodextrin alters steady state transepithelial electrical resistance. Eur. J. Cell Biol. 1999;78:473–84. doi: 10.1016/s0171-9335(99)80074-0. [DOI] [PubMed] [Google Scholar]
- 49.Sasaki H, Matsui C, Furuse K, Mimori-Kiyosue Y, Furuse M, Tsukita S. Dynamic behavior of paired claudin strands within apposing plasma membranes. Proc. Natl. Acad. Sci. USA. 2003;100:3971–76. doi: 10.1073/pnas.0630649100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Piehl C, Piontek J, Cording J, Wolburg H, Blasig IE. Participation of the second extracellular loop of claudin-5 in paracellular tightening against ions, small and large molecules. Cell Mol. Life Sci. 2010;7:2131–40. doi: 10.1007/s00018-010-0332-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Daugherty BL, Ward C, Smith T, Ritzenthaler JD, Koval M. Regulation of heterotypic claudin compatibility. J. Biol. Chem. 2007;282:30005–13. doi: 10.1074/jbc.M703547200. [DOI] [PubMed] [Google Scholar]
- 52.Piontek J, Winkler L, Wolburg H, Muller SL, Zuleger N, et al. Formation of tight junction: determinants of homophilic interaction between classic claudins. FASEB J. 2008;22:146–58. doi: 10.1096/fj.07-8319com. [DOI] [PubMed] [Google Scholar]
- 53.Amasheh S, Schmidt T, Mahn M, Florian P, Mankertz J, et al. Contribution of claudin-5 to barrier properties in tight junctions of epithelial cells. Cell Tissue Res. 2005;321:89–96. doi: 10.1007/s00441-005-1101-0. [DOI] [PubMed] [Google Scholar]
- 54.Sonoda N, Furuse M, Sasaki H, Yonemura S, Katahira J, et al. Clostridium perfringens enterotoxin fragment removes specific claudins from tight junction strands. Evidence for direct involvement of claudins in tight junction barrier. J. Cell Biol. 1999;147:195–204. doi: 10.1083/jcb.147.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van Itallie C, Rahner C, Anderson JM. Regulated expression of claudin-4 decreases paracellular conductance through a selective decrease in sodium permeability. J. Clin. Investig. 2001;107:1319–27. doi: 10.1172/JCI12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zeissig S, Bürgel N, Günzel D, Richter J, Mankertz J, et al. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn's disease. Gut. 2007;56:61–72. doi: 10.1136/gut.2006.094375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Van Itallie CM, Betts L, Smedley JG, 3rd, McClane BA, Anderson JM. Structure of the claudin-binding domain of Clostridium perfringens enterotoxin. J. Biol. Chem. 2008;283:268–74. doi: 10.1074/jbc.M708066200. [DOI] [PubMed] [Google Scholar]
- 58.Winkler L, Gehring C, Wenzel A, Muller SL, Piehl C, et al. Molecular determinants of the interaction between Clostridium perfringens enterotoxin fragments and claudin-3. J. Biol. Chem. 2009;284:18863–72. doi: 10.1074/jbc.M109.008623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robertson SL, Smedley JG, 3rd, McClane BA. Identification of a claudin-4 residue important for mediating the host cell binding and action of Clostridium perfringens enterotoxin. Infect. Immun. 2010;78:505–17. doi: 10.1128/IAI.00778-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Angelow S, Schneeberger EE, Yu AS. Claudin-8 expression in renal epithelial cells augments the paracellular barrier by replacing endogenous claudin-2. J. Membr. Biol. 2007;215:147–59. doi: 10.1007/s00232-007-9014-3. [DOI] [PubMed] [Google Scholar]
- 61.Angelow S, Yu AS. Structure-function studies of claudin extracellular domains by cysteine-scanning mutagenesis. J. Biol. Chem. 2009;284:29205–17. doi: 10.1074/jbc.M109.043752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heller F, Florian P, Bojarski C, Richter J, Christ M, et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550–64. doi: 10.1016/j.gastro.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 63.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat. Rev. Mol. Cell Biol. 2001;2:285–93. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- 64.Furuse M. Molecular basis of the core structure of tight junctions. Cold Spring Harb. Perspect. Biol. 2010;2:a002907. doi: 10.1101/cshperspect.a002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shen L, Weber CR, Turner JR. The tight junction protein complex undergoes rapid and continuous molecular remodeling at steady state. J. Cell Biol. 2008;181:683–95. doi: 10.1083/jcb.200711165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Furuse M, Sasaki H, Tsukita S. Manner of interaction of heterogeneous claudin species within and between tight junction strands. J. Cell Biol. 1999;147:891–903. doi: 10.1083/jcb.147.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J. Cell Biol. 1999;147:1351–63. doi: 10.1083/jcb.147.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Umeda K, Ikenouchi J, Katahira-Tayama S, Furuse K, Sasaki H, et al. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell. 2006;126:741–54. doi: 10.1016/j.cell.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 69.Muller D, Kausalya PJ, Claverie-Martin F, Meij IC, Eggert P, et al. A novel claudin 16 mutation associated with childhood hypercalciuria abolishes binding to ZO-1 and results in lysosomal mistargeting. Am. J. Hum. Genet. 2003;73:1293–301. doi: 10.1086/380418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Raleigh DR, Marchiando AM, Zhang Y, Shen L, Sasaki H, et al. Tight junction-associated MARVEL proteins marvelD3, tricellulin, and occludin have distinct but overlapping functions. Mol. Biol. Cell. 2010;21:1200–13. doi: 10.1091/mbc.E09-08-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ikenouchi J, Furuse M, Furuse K, Sasaki H, Tsukita S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J. Cell Biol. 2005;171:939–45. doi: 10.1083/jcb.200510043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bentzel CJ, Hainau B, Edelman A, Anagnostopoulos T, Benedetti EL. Effect of plant cytokinins on microfilaments and tight junction permeability. Nature. 1976;264:666–68. doi: 10.1038/264666a0. [DOI] [PubMed] [Google Scholar]
- 73.Duffey ME, Hainau B, Ho S, Bentzel CJ. Regulation of epithelial tight junction permeability by cyclic AMP. Nature. 1981;294:451–53. doi: 10.1038/294451a0. [DOI] [PubMed] [Google Scholar]
- 74.Powell DW. Intestinal conductance and permselectivity changes with theophylline and choleragen. Am. J. Physiol. 1974;227:1436–43. doi: 10.1152/ajplegacy.1974.227.6.1436. [DOI] [PubMed] [Google Scholar]
- 75.Madara JL. Increases in guinea pig small intestinal transepithelial resistance induced by osmotic loads are accompanied by rapid alterations in absorptive-cell tight-junction structure. J. Cell Biol. 1983;97:125–36. doi: 10.1083/jcb.97.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kohler K, Louvard D, Zahraoui A. Rab13 regulates PKA signaling during tight junction assembly. J. Cell Biol. 2004;165:175–80. doi: 10.1083/jcb.200312118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bentzel CJ, Hainau B, Ho S, Hui SW, Edelman A, et al. Cytoplasmic regulation of tight-junction permeability: effect of plant cytokinins. Am. J. Physiol. Cell Physiol. 1980;239:75–89. doi: 10.1152/ajpcell.1980.239.3.C75. [DOI] [PubMed] [Google Scholar]
- 78.Meza I, Ibarra G, Sabanero M, Martinez-Palomo A, Cereijido M. Occluding junctions and cytoskeletal components in a cultured transporting epithelium. J. Cell Biol. 1980;87:746–54. doi: 10.1083/jcb.87.3.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Madara JL, Barenberg D, Carlson S. Effects of cytochalasin D on occluding junctions of intestinal absorptive cells: further evidence that the cytoskeleton may influence paracellular permeability and junctional charge selectivity. J. Cell Biol. 1986;102:2125–36. doi: 10.1083/jcb.102.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shen L, Turner JR. Actin depolymerization disrupts tight junctions via caveolae-mediated endocytosis. Mol. Biol. Cell. 2005;16:3919–36. doi: 10.1091/mbc.E04-12-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ivanov AI, McCall IC, Parkos CA, Nusrat A. Role for actin filament turnover and a myosin II motor in cytoskeleton-driven disassembly of the epithelial apical junctional complex. Mol. Biol. Cell. 2004;15:2639–51. doi: 10.1091/mbc.E04-02-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kiuchi-Saishin Y, Gotoh S, Furuse M, Takasuga A, Tano Y, Tsukita S. Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J. Am. Soc. Nephrol. 2002;13:875–86. doi: 10.1681/ASN.V134875. [DOI] [PubMed] [Google Scholar]
- 83.Muto S, Hata M, Taniguchi J, Tsuruoka S, Moriwaki K, et al. Claudin-2-deficient mice are defective in the leaky and cation-selective paracellular permeability properties of renal proximal tubules. Proc. Natl. Acad. Sci. USA. 2010;107:8011–16. doi: 10.1073/pnas.0912901107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Turner JR, Rill BK, Carlson SL, Carnes D, Kerner R, et al. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am. J. Physiol. Cell Physiol. 1997;273:1378–85. doi: 10.1152/ajpcell.1997.273.4.C1378. [DOI] [PubMed] [Google Scholar]
- 85.Madara JL, Pappenheimer JR. Structural basis for physiological regulation of paracellular pathways in intestinal epithelia. J. Membr. Biol. 1987;100:149–64. doi: 10.1007/BF02209147. [DOI] [PubMed] [Google Scholar]
- 86.Sadowski DC, Meddings JB. Luminal nutrients alter tight-junction permeability in the rat jejunum: an in vivo perfusion model. Can. J. Physiol. Pharmacol. 1993;71:835–39. doi: 10.1139/y93-125. [DOI] [PubMed] [Google Scholar]
- 87.Turner JR, Madara JL. Physiological regulation of intestinal epithelial tight junctions as a consequence of Na+-coupled nutrient transport. Gastroenterology. 1995;109:1391–96. doi: 10.1016/0016-5085(95)90605-3. [DOI] [PubMed] [Google Scholar]
- 88.Lane JS, Whang EE, Rigberg DA, Hines OJ, Kwan D, et al. Paracellular glucose transport plays a minor role in the unanesthetized dog. Am. J. Physiol. Gastrointest. Liver Physiol. 1999;276:789–94. doi: 10.1152/ajpgi.1999.276.3.G789. [DOI] [PubMed] [Google Scholar]
- 89.Pappenheimer JR, Reiss KZ. Contribution of solvent drag through intercellular junctions to absorption of nutrients by the small intestine of the rat. J. Membr. Biol. 1987;100:123–36. doi: 10.1007/BF02209145. [DOI] [PubMed] [Google Scholar]
- 90.Meddings JB, Westergaard H. Intestinal glucose transport using perfused rat jejunum in vivo: model analysis and derivation of corrected kinetic constants. Clin. Sci. 1989;76:403–13. doi: 10.1042/cs0760403. [DOI] [PubMed] [Google Scholar]
- 91.Clayburgh DR, Musch MW, Leitges M, Fu YX, Turner JR. Coordinated epithelial NHE3 inhibition and barrier dysfunction are required for TNF-mediated diarrhea in vivo. J. Clin. Investig. 2006;116:2682–94. doi: 10.1172/JCI29218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Turner JR, Black ED. NHE3-dependent cytoplasmic alkalinization is triggered by Na+-glucose cotransport in intestinal epithelia. Am. J. Physiol. Cell Physiol. 2001;281:1533–41. doi: 10.1152/ajpcell.2001.281.5.C1533. [DOI] [PubMed] [Google Scholar]
- 93.Zhao H, Shiue H, Palkon S, Wang Y, Cullinan P, et al. Ezrin regulates NHE3 translocation and activation after Na+-glucose cotransport. Proc. Natl. Acad. Sci. USA. 2004;101:9485–90. doi: 10.1073/pnas.0308400101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Marchiando AM, Graham WV, Turner JR. Epithelial barriers in homeostasis and disease. Annu. Rev. Pathol. 2010;5:119–44. doi: 10.1146/annurev.pathol.4.110807.092135. [DOI] [PubMed] [Google Scholar]
- 95.Atisook K, Carlson S, Madara JL. Effects of phlorizin and sodium on glucose-elicited alterations of cell junctions in intestinal epithelia. Am. J. Physiol. Cell Physiol. 1990;258:77–85. doi: 10.1152/ajpcell.1990.258.1.C77. [DOI] [PubMed] [Google Scholar]
- 96.Berglund JJ, Riegler M, Zolotarevsky Y, Wenzl E, Turner JR. Regulation of human jejunal transmucosal resistance and MLC phosphorylation by Na+-glucose cotransport. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;281:1487–93. doi: 10.1152/ajpgi.2001.281.6.G1487. [DOI] [PubMed] [Google Scholar]
- 97.Yamashiro S, Totsukawa G, Yamakita Y, Sasaki Y, Madaule P, et al. Citron kinase, a Rho-dependent kinase, induces di-phosphorylation of regulatory light chain of myosin II. Mol. Biol. Cell. 2003;14:1745–56. doi: 10.1091/mbc.E02-07-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kimura K, Ito M, Amano M, Chihara K, Fukata Y, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science. 1996;273:245–48. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 99.Yamashiro S, Yamakita Y, Totsukawa G, Goto H, Kaibuchi K, et al. Myosin phosphatase-targeting subunit 1 regulates mitosis by antagonizing polo-like kinase 1. Dev. Cell. 2008;14:787–97. doi: 10.1016/j.devcel.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pearson AD, Eastham EJ, Laker MF, Craft AW, Nelson R. Intestinal permeability in children with Crohn's disease and celiac disease. Br. Med. J. (Clin. Res. Ed.) 1982;285:20–21. doi: 10.1136/bmj.285.6334.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ukabam SO, Clamp JR, Cooper BT. Abnormal small intestinal permeability to sugars in patients with Crohn's disease of the terminal ileum and colon. Digestion. 1983;27:70–74. doi: 10.1159/000198932. [DOI] [PubMed] [Google Scholar]
- 102.Hollander D, Vadheim CM, Brettholz E, Petersen GM, Delahunty T, Rotter JI. Increased intestinal permeability in patients with Crohn's disease and their relatives. A possible etiologic factor. Ann. Intern. Med. 1986;105:883–85. doi: 10.7326/0003-4819-105-6-883. [DOI] [PubMed] [Google Scholar]
- 103.Katz KD, Hollander D, Vadheim CM, McElree C, Delahunty T, et al. Intestinal permeability in patients with Crohn's disease and their healthy relatives. Gastroenterology. 1989;97:927–31. doi: 10.1016/0016-5085(89)91499-6. [DOI] [PubMed] [Google Scholar]
- 104.Wyatt J, Vogelsang H, Hubl W, Waldhoer T, Lochs H. Intestinal permeability and the prediction of relapse in Crohn's disease. Lancet. 1993;341:1437–39. doi: 10.1016/0140-6736(93)90882-h. [DOI] [PubMed] [Google Scholar]
- 105.Laukoetter MG, Nava P, Lee WY, Severson EA, Capaldo CT, et al. JAM-A regulates permeability and inflammation in the intestine in vivo. J. Exp. Med. 2007;204:3067–76. doi: 10.1084/jem.20071416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Su L, Shen L, Clayburgh DR, Nalle SC, Sullivan EA, et al. Targeted epithelial tight junction dysfunction causes immune activation and contributes to development of experimental colitis. Gastroenterology. 2009;136:551–63. doi: 10.1053/j.gastro.2008.10.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Clayburgh DR, Barrett TA, Tang Y, Meddings JB, Van Eldik LJ, et al. Epithelial myosin light chain kinase-dependent barrier dysfunction mediates T cell activation-induced diarrhea in vivo. J. Clin. Investig. 2005;115:2702–15. doi: 10.1172/JCI24970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Arrieta MC, Madsen K, Doyle J, Meddings J. Reducing small intestinal permeability attenuates colitis in the IL10 gene-deficient mouse. Gut. 2009;58:41–48. doi: 10.1136/gut.2008.150888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Olson TS, Reuter BK, Scott KG, Morris MA, Wang XM, et al. The primary defect in experimental ileitis originates from a nonhematopoietic source. J. Exp. Med. 2006;203:541–52. doi: 10.1084/jem.20050407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Madsen KL, Malfair D, Gray D, Doyle JS, Jewell LD, Fedorak RN. Interleukin-10 gene-deficient mice develop a primary intestinal permeability defect in response to enteric microflora. Inflamm. Bowel Dis. 1999;5:262–70. doi: 10.1097/00054725-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 111.Boirivant M, Amendola A, Butera A, Sanchez M, Xu L, et al. A transient breach in the epithelial barrier leads to regulatory T-cell generation and resistance to experimental colitis. Gastroenterology. 2008;135:1612–23. doi: 10.1053/j.gastro.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 112.Kaser A, Lee AH, Franke A, Glickman JN, Zeissig S, et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 2008;134:743–56. doi: 10.1016/j.cell.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Turner JR. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 114.Suenaert P, Bulteel V, Lemmens L, Noman M, Geypens B, et al. Anti-tumor necrosis factor treatment restores the gut barrier in Crohn's disease. Am. J. Gastroenterol. 2002;97:2000–4. doi: 10.1111/j.1572-0241.2002.05914.x. [DOI] [PubMed] [Google Scholar]
- 115.D'Haens G, Van Deventer S, Van Hogezand R, Chalmers D, Kothe C, et al. Endoscopic and histological healing with infliximab antitumor necrosis factor antibodies in Crohn's disease: a European multicenter trial. Gastroenterology. 1999;116:1029–34. doi: 10.1016/s0016-5085(99)70005-3. [DOI] [PubMed] [Google Scholar]
- 116.Cooke KR, Hill GR, Crawford JM, Bungard D, Brinson YS, et al. Tumor necrosis factor-α production to lipopolysaccharide stimulation by donor cells predicts the severity of experimental acute graft-versus-host disease. J. Clin. Investig. 1998;102:1882–91. doi: 10.1172/JCI4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Brown GR, Lindberg G, Meddings J, Silva M, Beutler B, Thiele D. Tumor necrosis factor inhibitor ameliorates murine intestinal graft-versus-host disease. Gastroenterology. 1999;116:593–601. doi: 10.1016/s0016-5085(99)70181-2. [DOI] [PubMed] [Google Scholar]
- 118.Mullin JM, Laughlin KV, Marano CW, Russo LM, Soler AP. Modulation of tumor necrosis factor-induced increase in renal (LLC-PK1) transepithelial permeability. Am. J. Physiol. Ren. Physiol. 1992;263:915–24. doi: 10.1152/ajprenal.1992.263.5.F915. [DOI] [PubMed] [Google Scholar]
- 119.Taylor CT, Dzus AL, Colgan SP. Autocrine regulation of epithelial permeability by hypoxia: role for polarized release of tumor necrosis factor α. Gastroenterology. 1998;114:657–68. doi: 10.1016/s0016-5085(98)70579-7. [DOI] [PubMed] [Google Scholar]
- 120.Gitter AH, Bendfeldt K, Schulzke JD, Fromm M. Leaks in the epithelial barrier caused by spontaneous and TNF-α-induced single-cell apoptosis. FASEB J. 2000;14:1749–53. doi: 10.1096/fj.99-0898com. [DOI] [PubMed] [Google Scholar]
- 121.Zolotarevsky Y, Hecht G, Koutsouris A, Gonzalez DE, Quan C, et al. A membrane-permeant peptide that inhibits MLC kinase restores barrier function in in vitro models of intestinal disease. Gastroenterology. 2002;123:163–72. doi: 10.1053/gast.2002.34235. [DOI] [PubMed] [Google Scholar]
- 122.Ma TY, Boivin MA, Ye D, Pedram A, Said HM. Mechanism of TNF-α modulation of Caco-2 intestinal epithelial tight junction barrier: role of myosin light-chain kinase protein expression. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;288:422–30. doi: 10.1152/ajpgi.00412.2004. [DOI] [PubMed] [Google Scholar]
- 123.Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR. Interferon-γand tumor necrosis factor-α synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am. J. Pathol. 2005;166:409–19. doi: 10.1016/s0002-9440(10)62264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bruewer M, Utech M, Ivanov AI, Hopkins AM, Parkos CA, Nusrat A. Interferon-γ induces internalization of epithelial tight junction proteins via a macropinocytosis-like process. FASEB J. 2005;19:923–33. doi: 10.1096/fj.04-3260com. [DOI] [PubMed] [Google Scholar]
- 125.Utech M, Ivanov AI, Samarin SN, Bruewer M, Turner JR, et al. Mechanism of IFN-γ-induced endocytosis of tight junction proteins: myosin II-dependent vacuolarization of the apical plasma membrane. Mol. Biol. Cell. 2005;16:5040–52. doi: 10.1091/mbc.E05-03-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schwarz BT, Wang F, Shen L, Clayburgh DR, Su L, et al. LIGHT signals directly to intestinal epithelia to cause barrier dysfunction via cytoskeletal and endocytic mechanisms. Gastroenterology. 2007;132:2383–94. doi: 10.1053/j.gastro.2007.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Heller F, Fuss IJ, Nieuwenhuis EE, Blumberg RS, Strober W. Oxazolone colitis, a Th2 colitis model resembling ulcerative colitis, is mediated by IL-13-producing NK-T cells. Immunity. 2002;17:629–38. doi: 10.1016/s1074-7613(02)00453-3. [DOI] [PubMed] [Google Scholar]
- 128.Prasad S, Mingrino R, Kaukinen K, Hayes KL, Powell RM, et al. Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells. Lab. Investig. 2005;85:1139–62. doi: 10.1038/labinvest.3700316. [DOI] [PubMed] [Google Scholar]
- 129.Capaldo CT, Macara IG. Depletion of E-cadherin disrupts establishment but not maintenance of cell junctions in Madin-Darby canine kidney epithelial cells. Mol. Biol. Cell. 2007;18:189–200. doi: 10.1091/mbc.E06-05-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherin-catenin-actin complex. Cell. 2005;123:889–901. doi: 10.1016/j.cell.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.McCarthy KM, Francis SA, McCormack JM, Lai J, Rogers RA, et al. Inducible expression of claudin-1-myc but not occludin-VSV-G results in aberrant tight junction strand formation in MDCK cells. J. Cell Sci. 2000;113(Pt. 19):3387–98. doi: 10.1242/jcs.113.19.3387. [DOI] [PubMed] [Google Scholar]
- 132.Muza-Moons MM, Schneeberger EE, Hecht GA. Enteropathogenic Escherichia coli infection leads to appearance of aberrant tight junctions strands in the lateral membrane of intestinal epithelial cells. Cell Microbiol. 2004;6:783–93. doi: 10.1111/j.1462-5822.2004.00404.x. [DOI] [PubMed] [Google Scholar]
- 133.Yu D, Marchiando AM, Weber CR, Raleigh DR, Wang Y, et al. MLCK-dependent exchange and actin binding region-dependent anchoring of ZO-1 regulate tight junction barrier function. Proc. Natl. Acad. Sci. USA. 2010;107:8237–41. doi: 10.1073/pnas.0908869107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fanning AS, Ma TY, Anderson JM. Isolation and functional characterization of the actin binding region in the tight junction protein ZO-1. FASEB J. 2002;16:1835–37. doi: 10.1096/fj.02-0121fje. [DOI] [PubMed] [Google Scholar]
- 135.Schmidt A, Utepbergenov DI, Mueller SL, Beyermann M, Schneider-Mergener J, et al. Occludin binds to the SH3-hinge-GuK unit of zonula occludens protein 1: potential mechanism of tight junction regulation. Cell Mol. Life Sci. 2004;61:1354–65. doi: 10.1007/s00018-004-4010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shen L, Black ED, Witkowski ED, Lencer WI, Guerriero V, et al. Myosin light chain phosphorylation regulates barrier function by remodeling tight junction structure. J. Cell Sci. 2006;119:2095–106. doi: 10.1242/jcs.02915. [DOI] [PubMed] [Google Scholar]
- 137.Furuse M, Furuse K, Sasaki H, Tsukita S. Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. J. Cell Biol. 2001;153:263–72. doi: 10.1083/jcb.153.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Alexandre MD, Lu Q, Chen YH. Overexpression of claudin-7 decreases the paracellular Cl− conductance and increases the paracellular Na+ conductance in LLC-PK1 cells. J. Cell Sci. 2005;118:2683–93. doi: 10.1242/jcs.02406. [DOI] [PubMed] [Google Scholar]
- 139.Angelow S, Kim KJ, Yu AS. Claudin-8 modulates paracellular permeability to acidic and basic ions in MDCK II cells. J. Physiol. 2006;571:15–26. doi: 10.1113/jphysiol.2005.099135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sas D, Hu M, Moe OW, Baum M. Effect of claudins 6 and 9 on paracellular permeability in MDCK II cells. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;295:1713–19. doi: 10.1152/ajpregu.90596.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Van Itallie CM, Gambling TM, Carson JL, Anderson JM. Palmitoylation of claudins is required for efficient tight-junction localization. J. Cell Sci. 2005;118:1427–36. doi: 10.1242/jcs.01735. [DOI] [PubMed] [Google Scholar]
- 142.Weber CR, Nalle SC, Tretiakova M, Rubin DT, Turner JR. Claudin-1 and claudin-2 expression is elevated in inflammatory bowel disease and may contribute to early neoplastic transformation. Lab. Investig. 2008;88:1110–20. doi: 10.1038/labinvest.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Van Itallie CM, Colegio OR, Anderson JM. The cytoplasmic tails of claudins can influence tight junction barrier properties through effects on protein stability. J. Membr. Biol. 2004;199:29–38. doi: 10.1007/s00232-004-0673-z. [DOI] [PubMed] [Google Scholar]
- 144.Mitic LL, Unger VM, Anderson JM. Expression, solubilization, and biochemical characterization of the tight junction transmembrane protein claudin-4. Protein Sci. 2003;12:218–27. doi: 10.1110/ps.0233903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Raleigh DR, Marchiando AM, Boe D, Wang Y, Shen L, Turner JR. Casein kinase 2 (CK2) phosphorylates occludin at Ser408 to increase intra-tight junction diffusion and reduce paracellular barrier function. FASEB J. 2010;24:1004.6. (Abstr.) [Google Scholar]