Abstract
Introduction
Social scientists have long recognized the important role that neighborhood crime can play in stress-related disease, but very little is known about potential biosocial mechanisms that may link the experience of living in high-crime neighborhoods with depression.
Objective
The current study introduces an integrated model that combines neighborhood, genetic, and epigenetic factors.
Methods
Hypotheses were tested with a sample of 99 African American women from the Family and Community Health Study (FACHS).
Results
Allele variants of the serotonin transporter gene (5-HTT) interact with neighborhood crime to predict depressive symptoms in a manner consonant with the differential susceptibility perspective. Furthermore, this association is mediated by DNA methylation of the promoter region of the serotonin transporter gene.
Conclusion
The findings provide support for an integrated model in which changes in DNA methylation, resulting from neighborhood crime, can result in an increase or decrease in gene activity which, in turn, influences depressive symptoms.
Keywords: neighborhood crime, depressive symptoms, 5-HTTLPR, DNA methylation, African American women
1. Introduction
Neighborhood studies have long recognized that individuals living in disordered or high crime neighborhoods are at greater risk for depression. Although the link between neighborhood crime and depression is robust, many individuals who reside in disordered neighborhoods do not become antisocial or depressed (Lei et al., 2014). The present study attempts to explain this heterogeneity of response by specifying moderating genetic polymorphisms and mediating epigenetic processes operating in the link between neighborhood conditions and depression.
First of all, past research has demonstrated that the serotonin system plays an important role in emotion regulation and the development of depression (Lesch et al., 1994). This has led to a series of studies examining the extent to which variation in the serotonin transporter gene (5-HTT) might increase risk for depression. While the findings are somewhat mixed, past research has provided evidence that possessing the short allele of 5-HTT increases the probability that stressful events will lead to depression (Karg et al., 2011). In particular, guided by the differential susceptibility perspective (Belsky & Pluess, 2009), recent studies revealed that the short allele of 5-HTT enhances a person’s sensitivity to environmental influence, whether that influence be adverse (i.e., “for worse effects”) or supportive (i.e., “for better effects”). The first goal of the present study is to investigate the extent to which variation in the 5-HTT gene moderates the association between neighborhood conditions and depressive symptoms in a “for better or for worse” manner.
The second goal of the present study is to examine the extent to which epigenetic regulation of the 5-HTT gene mediates the effect of neighborhood conditions on depressive symptoms. Although there are several mechanisms of epigenetic influence, the most pervasive and well-studied of these mechanisms is methylation (Beach et al., 2014). Importantly, epigenetic processes such as methylation are responsive to developmental and physiological cues, but they are also influenced by environmental conditions as well as genes. In the present study, we test the hypothesis that neighborhood context influences depression through methylation of 5-HTT. Further, we expect that carrying the short allele of 5-HTT amplifies this effect. In other words, we expect to find a pattern of conditional mediation. These arguments and hypotheses are elaborated in the sections that follow.
2. Neighborhood crime and depression
There is a long history of studies examining the link between neighborhood context and depression. Researchers (e.g. Browning et al., 2013) have concluded that residents in neighborhoods where crime and drug use are prevalent are likely to perceive that people in the neighborhood cannot be trusted, endorse feelings of powerlessness, believe that social controls no longer exist, and that life is essentially chaotic. Thus neighborhood crime and social disorder provide highly salient cues of threat and have significant potential to foster feelings of distress.
It is also the case, however, that there is much heterogeneity of response within neighborhoods. Given evidence that neighborhood effects on human behavior are often moderated by individual differences (Lei et al., 2014), understanding which individual characteristics influence the relationship between neighborhood contexts and mental health is crucial to the advancement of neighborhood studies. We expect that variability in the serotonin transporter gene may account, at least in part, for this heterogeneity of response.
3. Gene-neighborhood interaction
The neurotransmitter serotonin reduces depressed mood, anxiety, and irritability. It achieves this effect through a variety of avenues (Lesch et al., 1994). For example, it dampens reactivity of the amygdala, the limbic structure responsible for vigilance and emotional responsiveness to the environment. Further, it increases the linkage between the prefrontal cortex and the amygdala, thereby enhancing emotional regulation (Carver et al., 2008). The serotonin transporter gene (5-HTT) is a fundamental component of the serotonin system as it produces the protein that transports serotonin from the synaptic cleft to the presynaptic neuron. Given this crucial role, one would expect that variation in this gene that influences gene expression would be likely to affect emotion regulation and mood as well.
One variation linked to differential gene expression is the widely studied repeat element polymorphism in the promoter region of the gene. This polymorphic region (aka 5-HTTLPR) consists of two main variants, a short (12 repeats) and a long (14 repeats) allele. Among African Americans, a non-negligible portion of the population carries an extra-long variant that has 16 copies. A number of studies (e.g., Vijayendran et al., 2012) have indicated that the short allele is associated with reduced transcriptional efficiency of the protein produced by the serotonin transporter gene. The extra-long variant, however, is not associated with reduced expression, suggesting that contrasting the response of those carrying one or more short alleles to all others is appropriate in an African American Sample.
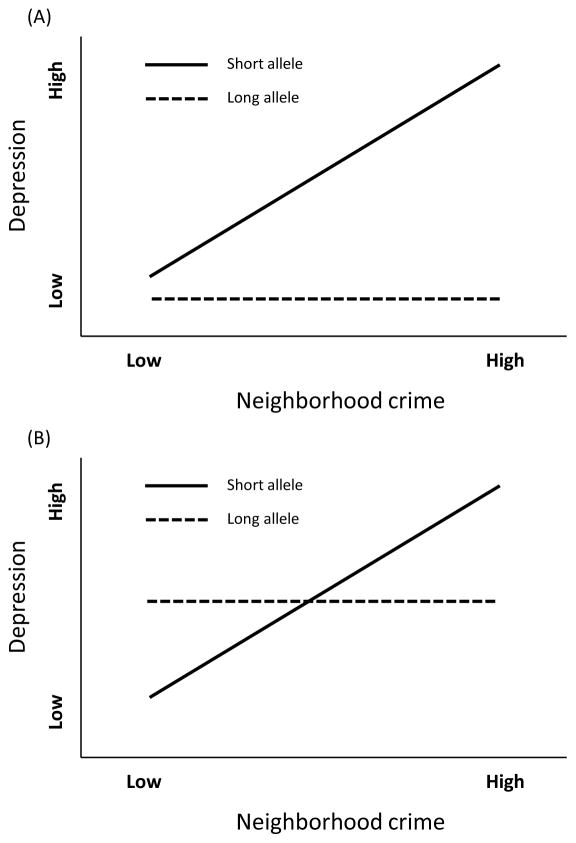
In the past decade, a number of social scientists have provided models of the manner in which genetic variations combine with environmental context to shape behavior (Shanahan & Hofer, 2005) and health outcomes (Seabrook & Avison, 2010). Indeed, research on this polymorphism indicates that the short allele does not directly affect depression. Rather, it exerts its influence by moderating the effect of the social environment on depression (Caspi et al., 2003). Most of this research has been guided by the stress-diathesis perspective. That is, the short allele amplifies the relationship between stress and depression. Support for the stress-diathesis perspective would be evident if a graph of the G×E effect showed a fan shape such that increases in adversity was associated with significantly increased depression among 5-HTTLPR short allele carriers, but greater adversity had no such effect for the long allele carriers, resulting in significantly different slopes in response to adversity for short vs. long allele carriers. This model is presented in Figure 1A.
Figure 1.
(A) The diathesis-stress hypothesis and (B) the differential susceptibility hypothesis.
In contrast to the stress-diathesis perspective, Belsky and his colleagues (Belsky & Pluess, 2009) have argued for another position. They have proposed the differential susceptibility perspective which suggests that genetic polymorphisms in genes such as the serotonin transporter amplify the impact of emotionally significant aspects of the environment, whether the environmental influence is adverse or supportive. Consistent with this viewpoint, findings across a number of laboratory studies indicate that carriers of the short allele of 5-HTTLPR do not just react more intensely to emotional stimuli. Rather, they show more vigilance, amygdala activity, and engagement in response to novel stimuli regardless of emotional valence. This suggests that the short allele increases general sensitivity and responsivity to emotionally significant aspects of the environment whether they are negative or positive. As shown in Figure 1B, support for the differential susceptibility perspective is evident when the slopes for the gene by environment interaction show a crossover pattern such that individuals with the short allele of 5-HTTLPR show more depressive symptoms than long allele carriers when the environment is adverse (i.e. “for worse effects”) but less depressive symptomatology than long allele carriers when the environment is supportive (i.e. “for better effects”).
Recently, there is mounting evidence that the differential susceptibility approach best describes the manner in which 5-HTTLPR interacts with the environment (e.g. Lei et al., 2014; Simons et al., 2011). Thus in the current study we expect to find that the 5-HTTLPR interacts with neighborhood conditions in a manner suggested by the differential susceptibility perspective: Individuals carrying the short allele of the 5-HTTLPR will evince higher risk of depression than the long allele carriers in response to high crime neighborhoods, whereas they will show lower risk of depression than the long allele carriers in response to a safe neighborhood.
4. DNA methylation as a link between gene-neighborhood interaction and depression
In recent years the life sciences have undergone a dramatic change in their understanding of genetic influences. The new paradigm places less emphasis upon DNA differences between people and more importance upon identifying the epigenetic factors that control or regulate DNA expression. Epigenetic regulation entails biochemical mechanisms that influence the genome to express (up regulate or down regulate) particular genes. One of the most pervasive and well-studied mechanisms is methylation (Carey, 2012). This occurs when a methyl group attaches to a segment of DNA. The effect of methylation, especially when it occurs in the promoter region of a gene, is typically to inhibit gene activity. Although methylation is responsive to developmental and physiological cues, it is also influenced by social environments. Indeed, from an evolutionary perspective, epigenetic changes such as methylation appear to be a fundamental process whereby a fixed genome can respond to changing environmental challenges and circumstances (Landecker & Panofsky, 2013).
Building upon this idea, adversity would be expected to foster increased methylation of the serotonin transporter gene which would have the result of increasing amygdala activity, depressed mood, vigilance, and negative scanning of the environment. From an evolutionary perspective, such a physiological and psychological response might be functional given a threatening environment (Andrews & Thomson, 2009), potentially increasing likelihood of survival albeit at the cost of other beneficial activities. On the other hand, a safe neighborhood would be expected to promote decreased methylation of the serotonin transporter gene and therefore less cortisol, amygdala activity and depression. This would be an adaptive response to a safe environmental context, facilitating less negative scanning and greater engagement with the environment.
Consistent with these arguments, studies have found that exposure to stressful events is associated with increased methylation of the serotonin transporter gene, which in turn increases the risk of depression (Philibert et al., 2008). Importantly for the present study, using genome-wide methylation analysis, Beach and colleagues (2014) provided evidence for the differential susceptibility approach, suggesting that the 5-HTTLPR amplifies the impact of the environment on DNA methylation in a “for better or for worse” manner. This study has identified numerous loci from 222 genes associated with depression and provides valuable insights regarding the G×E effect in explaining methylated pathways, but specific biological mechanisms are not entirely clear. Indeed, different CpG regions exert different effects on gene transcription and trigger different effects on neurotransmitter systems (Landecker & Panofsky, 2013). Although we cannot assume that candidate genes can carry the whole weight of genetic effects, they allow us to examine the specific biological function of genes. Further, the Beach et al (2014) study focused on amplification of the impact of SES related stress during childhood, thus examining a very different stressor and a different developmental context than the current investigation.
In line with previous studies, we focus on methylation of the serotonin transporter gene due to the central role of the serotonin system in neuropsychiatric disorders. We hypothesize that neighborhood crime will predict methylation of the serotonin transporter gene. Further, we expect that this association will be stronger for carriers of the short allele and that this interaction will conform to the cross-over pattern predicted by the differential susceptibility perspective. In the previous section, we developed the hypothesis that genetic variation in 5-HTTLPR will influence responses to neighborhood context in a “for better or for worse” manner. Here we hypothesize that methylation status of the serotonin transporter gene will mediate this G×E effect.
5. Methods
5.1. Participants
We tested our hypotheses using data from Waves 4 and 5 for 99 of the primary caregivers in the Family and Community Health Study (FACHS). FACHS is a longitudinal study of several hundred African American families that was initiated in 1997. Using a stratified random sampling procedure, the sampling strategy was intentionally designed to generate families representing a range of socioeconomic statuses and neighborhood settings. Details regarding recruitment are described by Simons and colleagues (2011).The first Wave of FACHS data was collected in 1997–1998 from 889 African-American children and their primary caregivers (PCs) (829 women and 60 men). The fourth and fifth Waves of data were collected in 2004–2005 and 2007–2008 to capture information when target youths were ages 17 to 18 and 20 to 21 years, respectively. Of the 889 PCs interviewed at Wave 1, 693 were interviewed again at Wave 5 (77.26% of the original sample).
At wave 5, 100 women were randomly selected from the roster of PCs to participate in an epigenetic assessment. Costs associated with the blood draws and epigenetic assays necessitated the drawing of a subsample. The current study is based upon the 99 women who participated in DNA methylation study, and were successfully genotyped for 5-HTTLPR gene. Mean age for this subsample was 48.33 years (SD = 9.30), 19.19% had less than a 12th grade education, 38.38% had an annual household income less than $25,000, 31% had lived in their neighborhoods for less than 2 years, and the mean of the proportion of households in a census tract below the federal poverty level was 20.88%. All 99 respondents are of African ancestry. Comparisons of this subsample of PCs with those who were not included in the methylation assessment did not reveal any significant differences with regard to the independent variables at the initial wave of the FACHS study.
The protocol and all study procedures were approved by the University institutional review board. Participants were asked to provide a blood sample at Wave 5. A certified phlebotomist drew four tubes of blood (30 ml) from each participant and shipped it the same day to a laboratory at the University of Iowa for preparation. Typical DNA yield for each pellet was between 10 and 15 mg of DNA.
5.2. Measures
Depressive symptoms
At Wave 5, the respondents completed the 20-item Center for Epidemiologic Studies Depression scale (CES-D; Radloff, 1977), a self-report measure of symptoms occurring during the previous week. The CES-D has been well validated and widely used to measure depressive symptoms in community samples. Cronbach’s alpha was .82. To control for previous depressive symptoms (Wave 4), we used the 5-item scale from the Mini-Mood and Anxiety Symptom Questionnaire (Mini-MASQ; Clark & Watson, 1995). Cronbach’s alpha was .82. Although the wording and exact content of these scales varies across the instruments, both scales are designed to measure depressive symptoms in the general population and are highly correlated (r = .47, p < .001).
Neighborhood crime
We measured neighborhood crime with an 11-item scale at Wave 4. Four of the eleven items were adapted from instruments developed for the Project on Human Development in Chicago Neighborhoods (see Sampson & Raudenbush, 2004). The items asked about the extent to which various criminal acts (drinking in public, people selling or using drugs, gang violence, and groups of teenagers or adults hanging out in the neighborhood and causing trouble) were a problem within the neighborhood. One item was used to assess how safe they felt in their neighborhoods. Respondents also completed a 6-item neighborhood deviance scale (Cutrona et al., 2000). The items focused on the extent to which people in their neighborhoods think it was okay if kids were drinking alcohol, having sex, smoking cigarettes, using drugs, and it was very easy for young people to get drugs or get alcohol. Scores were summed to form a measure of neighborhood crime. Higher scores indicate that respondents perceive their neighborhoods as high in crime and dangerous. Cronbach’s alpha was .83.
Genotyping
Genotype at the 5-HTTLPR located on chromosome 17q11.1-q12 has a functional polymorphism in the variable repeat sequence in the promoter region (Karg et al., 2011). The homozygous long allelic variant is related to higher concentrations of 5-HTT messenger RNA and a greater rate of reuptake than the homozygous short allelic variant. Among the 99 respondents, 4.1% were homozygous for the short allele (ss), 31.3% were heterozygous (sl), and 64.6% were homozygous for the long allele (ll). Using the Hardy-Weinberg equilibrium test, the observed distribution of 5-HTTLPR polymorphism did not differ significantly (chi-square = .01, df = 1, p = .92) from that predicted on the basis of simple Mendelian inheritance. Finally, consistent with previous studies (Beach et al., 2014), we treated 5-HTTLPR as dichotomous variables where individuals received a score of 1 if they were carrying at least one copy of the short allele and a score of 0 if they were homozygous for the long allele.
5-HTT methylation
DNA methylation status was determined using the Illumina Infinum (Sequenom, Inc., San Diego, CA) 450K HumanMethylation Beadchip. Details regarding processing and preparation of the methylation data are described by Dogan and Colleagues (2014). Methylation of CpG islands, which are cytosine-guanine dinucleotide-rich areas located mainly the promoter regions of genes near the transcription start site, are well known to contribute to gene silencing and are strongly related to transcriptional responses (Suzuki & Bird, 2008). Because of their greater potential regulatory significance, in the current study we focused on assessment of 5-HTT methylation occurring for CpG island associated CpG sites. Using the Illumina 450k array, 7 CpG sites (cg27569822, cg10901968, cg26741280, cg25725890, cg05016953, cg14692377, and cg03363743) were found to be within the CpG island associated with 5-HTT. As shown in Table 1, 5 of the 7 CpG residues are strongly associated with depressive symptoms, even after correcting for multiple testing using a false discovery rate (FDR) of less than .05. Confirmatory factor analysis used to assess the factor structure of the 7 CpG sites resulted in factor loadings that ranged from .233 to .761; items loaded as a single construct with excellent model fit (χ2 = 2.93, df = 14, p = .99). Finally, the composite index of 5-HTT methylation (5-HTTm) was calculated by averaging the standardized scores of these seven beta values.
Table 1.
Methylation at CpG sites in the promotor region of 5-HTT gene as predictors of depressive symptoms (N = 99).
| Depressive symptoms
|
||
|---|---|---|
| CpGs: Illumina ID
|
β
|
FDR p-value
|
| cg03363743 | 3.984 | <.001 |
| cg27569822 | 16.79 | <.001 |
| cg05016953 | 11.481 | <.001 |
| cg10901968 | 9.545 | .010 |
| cg14692377 | .389 | .612 |
| cg25725890 | 2.519 | .383 |
| cg26741280 | 8.411 | <.001 |
|
| ||
| SLC6A4 methylation index | .277 | |
FDR = false discovery rate to correct for the inflated probability of Type I error in multiple tests.
Control variables
To account for variables that could provide plausible rival explanations, our analyses included controls for several neighborhood and individual characteristics including age, relationship status (1 = single), education (1 = less than high school graduation), household income (1 = less than $25,000 per year), residential history (1 = living 2 years or less in a neighborhood), and neighborhood disadvantage. Cigarette consumption was measured with a single item: “On average, how many cigarettes do you usually smoke per day?” Neighborhood disadvantage was assessed by using the US Census Bureau’s 2010 American Community Survey (see Lei et al., 2014, for details).
6. Results
6.1. Initial findings
Descriptive statistics are presented in Table 2. The mean CES-D score was 14.64 (SD = 10.90). Of the 99 respondents, 25.3% were married and living with their husbands, and another 24.2% were cohabiting with a significant other. For the z-transformed neighborhood crime, the interquartile range was −.66 to .32 with a median score of −.41. Using independent t-tests, there were no mean differences between short (ss/sl) and long (ll) alleles of 5-HTTLPR on any of the variables. These findings are consistent with previous molecular genetic studies indicating 5-HTTLPR is not significantly related to either depressive symptoms or the environmental measures (Caspi et al., 2003).
Table 2.
Descriptive statistics for the total sample and by 5-HTTLPR.
|
5-HTTLPR
|
||||
|---|---|---|---|---|
| Characteristics a
|
Total Sample (n = 99)
|
ss/sl (n = 35)
|
ll (n = 64)
|
Statistical power
|
| Neighborhood crime (z-transformation) | 0.00 (1.00) | 0.03 (.96) | −0.02 (1.03) | |
| t(97) = .25 | 0.51 | |||
| SLC6A4 methylation index | 0.00 (.59) | −0.02 (.55) | 0.01 (.61) | |
| t(97) = −.26 | 0.51 | |||
| Depressive symptoms (mini-MASQ) (W4) | 6.55 (2.01) | 6.57 (2.05) | 6.53 (2.01) | |
| t(97) = .10 | 0.50 | |||
| Depressive symptoms (CES-D) (W5) | 14.64 (10.90) | 15.20 (11.58) | 14.33 (10.59) | |
| t(97) = .38 | 0.51 | |||
| Cigarette consumption | 5.24 (7.67) | 5.60 (7.62) | 5.05 (7.74) | |
| t(97) = .34 | 0.57 | |||
| Age (years) | 48.33 (9.30) | 46.17 (5.49) | 49.52 (10.69) | |
| t(97) = −1.73 | 0.76 | |||
| Single relationship | 33.33 (47.38) | 34.29 (48.16) | 32.81 (47.32) | |
| t(97) = .15 | 0.50 | |||
| Education (≤ high school) | 19.19 (39.58) | 11.43 (32.28) | 23.44 (42.70) | |
| t(97) = −1.45 | 0.69 | |||
| Income (≤ $25,000/yr) | 38.38 (48.88) | 34.29 (48.16) | 40.63(49.50) | |
| t(97) = −.62 | 0.54 | |||
| Living 2 years or less in a neighborhood | 31.00 (46.60) | 29.00 (45.80) | 33.00 (47.30) | |
| t(97) = −.43 | 0.52 | |||
| Neighborhood disadvantage | 0.00 (1.00) | 0.01 (1.13) | −0.01 (.93) | |
| t(97) = .08 | 0.50 | |||
Notes:
Characteristics of the total sample and by 5-HTTLPR are summarized as Mean/(SD) or %. t-test for differences by 5-HTTLPR.
p ≤ .10,
p ≤ .05,
p ≤ .01 (two-tailed tests).
Further, we tested for gene-environment correlation (rGE) as it is possible that genotype may influence selection into different types of neighborhoods, or exert a potentially confounding effect on outcomes (Shanahan & Hofer, 2005). In the current study, there was no significant zero-order correlation between neighborhood crime and 5-HTTLPR (r = .026, p = .802). In addition, there were no significant associations between 5-HTTLPR and either 5-HTTm (r = −.026, p = .798) or depressive symptoms (r = .038, p = .706). Thus, there was no evidence of an active rGE effect whereby people seek out or evoke environments that are compatible with their genetic predispositions. Nor was there evidence of a passive rGE whereby people find themselves in particular environments because they share genes with others who create or select their environments. Rather, our analyses indicated an absence of rGE effects in the current study.
6.2. The effect of G×E on depressive symptoms
We did not use multilevel modeling in this study because approximately 76.8% of census tracts had only one participant, and the results revealed that there was no significant intra-class correlation for depressive symptoms (ICC = .001, p > .50) or 5-HTTm (ICC = .01, p = .42) across our census tracts. To avoid overestimating our results, parameters in the regression models were examined using maximum likelihood estimate (MLE) with robust standard errors.
Poisson regression was used for the analyses reported in Table 3 given that depressive symptoms is a count variable. Before testing our hypotheses, we checked for potential multicollinearity among variables. The scores of VIF ranged between 1.07 for the 5-HTTLPR genotype and 1.41 for single relationship, and for all measures tolerance was above .70, indicating no evidence of multicollinearity. Model 1 shows that the main effect of neighborhood crime was significant (eβ = 1.165, p < .001), whereas the 5-HTTLPR was not. Model 2 added confounder variables. The result remains even after controlling for a variety of potential confounders. This finding is consistent with prior studies indicating that individuals living in high crime neighborhoods are at greater risk of depressive symptoms whereas the 5-HTTLPR gene generally has little main effect on depressive symptoms.
Table 3.
Poisson regression models depicting the effects of neighborhood crime and 5-HTTLPR on depressive symptoms (N = 99).
| Depressive symptoms
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Model 1
|
Model 2
|
Model 3
|
Model 4
|
|||||
| b | OR | b | OR | b | OR | b | OR | |
|
|
|
|
|
|
||||
| Neighborhood crime | .153 (.024) ** | 1.165 | .061 (.026) * | 1.063 | .002 (.032) | 1.002 | .020 (.031) | 1.020 |
| 5-HTTLPR (1 = ss/sl) | .053 (.055) | 1.054 | .104 (.057) † | 1.110 | .081 (.058) | 1.084 | .053 (.056) | 1.054 |
| Neighborhood crime × 5-HTTLPR | .195 (.054) ** | 1.215 | .096 (.054) † | 1.101 | ||||
| 5-HTT Methylation | .233 (.046) ** | 1.262 | ||||||
| Control variables | ||||||||
| Cigarette consumption | .010 (.003) ** | 1.010 | .009 (.003) ** | 1.009 | .005 (.004) | 1.005 | ||
| Age (mean centering) | .005 (.003) | 1.005 | .006 (.003) † | 1.006 | .004 (.003) | 1.004 | ||
| Single relationship | .133 (.065) * | 1.142 | .128 (.064) * | 1.137 | .158 (.065) * | 1.171 | ||
| Education (≤ high school) | .329 (.064) ** | 1.390 | .334 (.064) ** | 1.397 | .286 (.066) ** | 1.331 | ||
| Income (≤ $25,000/yr) | −.037 (.063) | .964 | −.040 (.063) | .961 | −.055 (.064) | 0.946 | ||
| Residence history | −.004 (.062) | .996 | −.024 (.062) | .976 | −.027 (.062) | 0.973 | ||
| Neighborhood disadvantage | .010 (.029) | 1.010 | .021 (.029) | 1.021 | .020 (.029) | 1.020 | ||
| Prior depressive symptoms | .119 (.012) ** | 1.1 | .129 (.012) ** | 1.138 | .131 (.012) ** | 1.140 | ||
| Constant | 2.652 (.033) ** | 14.182 | 1.663 (.096) ** | 5.275 | 1.605 (.097) ** | 4.978 | 1.621 (.098) ** | 5.058 |
| −2LL | 1161.99 | 996.73 | 984.20 | 974.20 | ||||
| Δ chi-square (df = 1) | 165.26** | 12.53 ** | 10.00 ** | |||||
| Statistical power for G × E | 96.70% | |||||||
Notes: Unstandardized (b) and OR = Odds Ratio shown with robust standard errors in parentheses; neighborhood crime is standardized by z-transformation (mean = 0 and SD =1).
p ≤ .10,
p ≤ .05,
p ≤ .01 (two-tailed tests).
Model 3 added the interaction of 5-HTTLPR with neighborhood crime to Model 2. The difference in deviance between Model 2 and Model 3 was significant (Δχ 2 = 12.53, df = 1, p < .001), indicating that the interaction effect improves the model fit. A standard deviation increase in neighborhood crime if respondents carried one or two copies of the short allele is related to a 21.77% increase in the odds of depressive symptoms. Thus, residing in a high crime neighborhood exerts a substantial mental health burden on residents, particularly those carrying a short allele of the 5-HTTLPR.
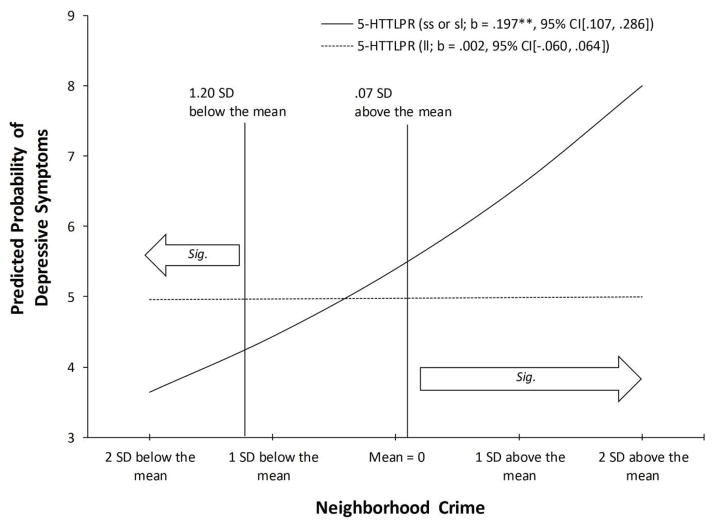
To further examine the interaction of neighborhood crime with the 5-HTTLPR, we plotted the effect in Figure 2 for levels of neighborhood crime ranging from 2 standard deviation (SD) below to 2 SD above from the sample mean. As expected, the graph showed that the association between neighborhood crime and depressive symptoms was significantly steeper for respondents who carried one or two copies of the short allele of 5-HTTLPR (b = .197, p < .001) than for those who carried only the long allele. This supports the hypothesis that individuals carrying the short allele are at increased risk for depressive symptoms in response to neighborhood crime compared to those with the long allele. Further, we followed the procedure developed by Roisman et al. (2012) to adjudicate between the diathesis-stress and differential susceptibility models of G×E. The proportion of interaction index was .38, close to the maximum potential value of 0.5, suggesting a relatively robust finding in favor of the susceptibility model. The results depicted in Figure 2 are consistent with the differential susceptibility model as there is a cross over pattern with a substantial region of significant difference in both the “for better” and “for worse” direction.
Figure 2.
Effect of neighborhood crime on depressive symptoms moderated by 5-HTTLPR. Numbers in parentheses refer to simple slopes with 95% confidence intervals. **p < 0.001.
To address the hypothesized indirect effects of 5-HTT methylation, Model 4 added 5-HTTm. As expected, inclusion of 5-HTTm reduces the interaction effect of neighborhood crime and 5-HTTLPR to nonsignificance. This result suggests that there is an indirect effect of the interaction terms on depressive symptoms operating through 5-HTTm.
6.3. The effect of G×E on 5-HTT methylation
Turning to the models that use 5-HTTm as the outcome, we first checked for potential multicollinearity among variables. VIF scores ranged between 1.065 and 1.406, and all measures of tolerance were above .70, indicating that multicollinearity is not a threat. As can be seen in Model 1 of Table 4, the main effect for neighborhood crime was marginal (b = .109, p = .060), and no significant main effect of 5-HTTLPR emerged. Model 2 added all control variables and presented that no other effects in the model other than age and cigarette consumption were significant. The results suggest that there is no evidence of an active rGE effect whereby people seek out environments that are compatible with their genetic predispositions. We then examined the model that included prior depressive symptoms. Model 3 shows that the effect of prior depressive symptoms was not significant. This pattern of results suggests that 5-HTTm is not directly affected by either neighborhood crime or 5-HTTLPR genotype. Finally, Model 4 added the multiplicative interaction term formed by multiplying neighborhood crime by 5-HTTLPR. As hypothesized, this interaction term was significant.
Table 4.
Regression models depicting the effects of neighborhood crime and 5-HTTLPR on 5-HTT Methylation (N = 99).
|
5-HTT Methylation
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Model 1
|
Model 2
|
Model 3
|
Model 4
|
|||||
| b | β | b | β | b | β | b | β | |
| Neighborhood crime | .109 (.058) † | .185 | .078 (.056) | .132 | .083 (.058) | .142 | −.007 (.068) | −.012 |
| 5-HTTLPR (1 = ss/sl) | −.038 (.121) | −.031 | .029 (.114) | .024 | .030 (.113) | .024 | .030 (.110) | .025 |
| Neighborhood crime × 5-HTTLPR | .266 (.113) * | .257 | ||||||
| Control variables | ||||||||
| Cigarette consumption | .026 (.007) ** | .338 | .026 (.007) ** | .344 | .026 (.007) ** | .336 | ||
| Age (mean centering) | .014 (.007) * | .223 | .014 (.007) * | .225 | .015 (.007) * | .246 | ||
| Single relationship | −.150 (.132) | −.121 | −.151 (.132) | −.122 | −.153 (.129) | −.123 | ||
| Education (≤ high school) | .205 (.142) | .139 | .209 (.143) | .141 | .220 (.139) | .149 | ||
| Income (≤ $25,000/yr) | .110 (.125) | .092 | .106 (.126) | .088 | .100 (.123) | .083 | ||
| Residence history | −.021 (.122) | −.017 | −.022 (.122) | −.017 | −.032 (.119) | −.025 | ||
| Neighborhood disadvantage | .020 (.055) | .035 | .017 (.055) | .029 | .033 (.054) | .056 | ||
| Prior depressive symptoms | −.009 (.028) | −.033 | .001 (.028) | .004 | ||||
| Constant | .013 (.072) | .023 | −.171 (.098) † | −.294 | −.110 (.206) | −.176 (.202) | −.301 | |
| R-square | .035 | .196 | .197 | .239 | ||||
| Statistical power for G × E | 82.40% | |||||||
Notes: Unstandardized (b) and standardized (β) coefficients shown with robust standard errors in parentheses; neighborhood crime is standardized by z-transformation (mean = 0 and SD =1).
p ≤ .10,
p ≤ .05,
p ≤ .01 (two-tailed tests).
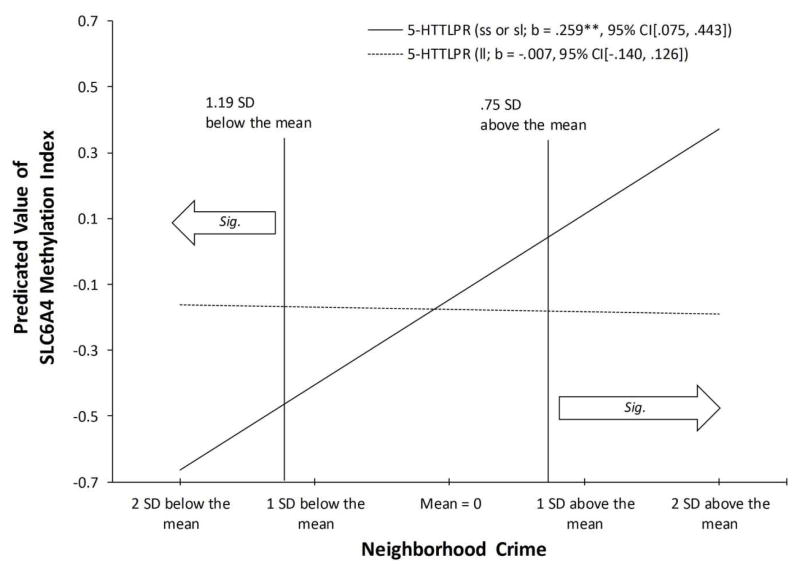
To interpret this finding, Figure 3 shows the graph of the interaction of neighborhood crime and 5-HTTLPR on methylation. Based on a post-hoc test of the interaction, the effect of neighborhood crime on 5-HTT methylation was significant for short allele carriers and was significantly steeper for respondents with at least one short allele at 5-HTTLPR (b = .259, p = .006) than for those with only long alleles. In addition, short allele carriers demonstrated significantly greater methylation than those with the long allele when the neighborhood context was more negative, whereas short allele carriers showed significantly lower methylation than those with the long allele when the neighborhood context was more positive. Thus the graph for this interaction indicated a pattern virtually identical to that depicted in Figure 2; the results present the expected cross over effect and provide strong support for the differential susceptibility perspective.
Figure 3.
Effect of neighborhood crime on 5-HTT methylation index moderated by 5-HTTLPR. Numbers in parentheses refer to simple slopes with 95% confidence intervals. **p < 0.001
6.4. Conditional indirect effect
As noted above, we found evidence for genetic moderation of neighborhood crime on depressive symptoms and on 5-HTTm. In addition, the G×E effect on depressive symptoms was no longer significant when 5-HTTm was included in the model predicting depressive symptoms. Further, using the indirect effect analysis option in Mplus7.2, the indirect effect of the G×E interaction on depressive symptoms through 5-HTTm was statistically significant (indirect effect = .062, p < .031) and accounted for 39.241% of the total variance. This pattern of findings suggests that the interaction of 5-HTTLPR × Neighborhood Crime on depressive symptoms is mediated by 5-HTT methylation. The final step in our analysis is to examine the conditional indirect effect of neighborhood crime on depressive symptoms through 5-HTTm by 5-HTTLPR genotype. Using the approach outlined by Preacher, Rucker, and Hayes (2007), the results show that there was no indirect effect of neighborhood crime on depressive symptoms through 5-HTTm when respondents carried the long allele. However, the indirect effect was significant, and significantly stronger than for long-allele homozygotes, when respondents carried at least one copy of the short 5-HTTLPR allele (indirect effect = .064, p = .015).
6.5. Power analyses
It is important to be cautious about effects observed with relatively small samples because lack of statistical power may increase the chances of false positive findings (Duncan & Keller, 2011). To examine the robustness of our results, we conducted Monte Carlo simulation in Mplus7.2 to estimate statistical power for n = 99. This method depends on 1,000 random sample replications generated from a population model with known parameters to determine statistical power for particular parameters (Thoemmes, MacKinnon, & Reiser, 2010). Thus, it is more flexibility in testing complex multivariate models. First, using the regression coefficients given in Model 3, Table 3 as population parameters, the statistical power of the interaction effect between neighborhood crime and 5-HTTLPR is .967. Second, the regression coefficients as effect sizes used in the simulation are given in Model 4, Table 4. The simulation analysis shows that statistical power for the G×E effect is .824. Taken together, both findings indicated that the G×E had large effect sizes in the prediction of depressive symptoms (β = .317) and 5-HTTm (β = .257). Our sample is of adequate size to test the theoretical models given observed effect sizes.
7. Discussion
Social scientists interested in health have long recognized the important role that neighborhoods can play in the development of mental and physical illness. Although progress has been made in identifying various biological processes (e.g., inflammation, allostatic load) that mediate the effect of neighborhood on physical health, there have been few attempts to specify the biological pathways that may link neighborhood conditions to mental illness through effects on gene methylation. The current study attempts to address this deficit by investigating various hypotheses regarding the extent to which the association between neighborhood context and depression is influenced by genetic variation and mediated by methylation of the serotonin transporter gene. Our results provide preliminary support for our hypotheses.
Consistent with previous studies, we confirmed that residing in a high crime neighborhood was associated with an increased risk of depressive symptoms. Further, consistent with prior research, we found that the 5-HTTLPR polymorphism had no direct effect on depressive symptoms. Rather, its influence was limited to its moderation of the association between neighborhood and depressive symptoms (Seabrook & Avison, 2010). In keeping with its hypothesized moderating effect, the short allele of 5-HTTLPR substantially (around 22%) increased the probability that neighborhood crime would predict depressive symptomatology, whereas there was no such effect among long allele carriers. Consistent with prior meta-analytic findings associating the G×E interaction at the 5-HTTLPR with depression (Karg et al., 2011), the 5-HTTLPR×neighborhood interaction explains substantial variation in depressive symptom. In addition, we demonstrate that this G×E effect was not confounded by an rGE correlation (Shanahan & Hofer, 2005) because there was no evidence that respondents’ genotype influenced their selection of neighborhood context.
Such findings are consistent with the idea that genes do not operate in isolation; rather, they interact with environmental conditions to influence depressive symptoms. This suggests that the 5-HTTLPR polymorphism involved in regulation of the serotonergic neurotransmitter systems is an individual difference that accounts, at least in part, for variations in the way that people respond to neighborhood influences. Thus, more detailed understanding of the mechanism accounting for the G×E effect is crucial for understanding heterogeneity in neighborhood effects on depressive symptoms.
Our results can also be seen as providing strong support for the differential susceptibility perspective relative to the stress-diathesis perspective as it pertains to the impact of neighborhood crime among those with short vs. long alleles of 5HTT. Individuals carrying the short allele evinced higher risk of depressive symptoms than the long allele carriers in response to high crime neighborhoods, whereas they showed lower risk of depressive symptoms than the long allele carriers in response to a safe neighborhood. This suggests that some individuals (carriers of the short allele) are more sensitive than others (carriers of two copies of the long allele) to the effects of neighborhood context. Whereas the stress-diathesis perspective suggests that individuals with the short allele have a genetic liability due to their tendency to be hyper-responsive to adversity, the differential susceptibility model suggests that that their enhanced environmental sensitivity offers enhanced opportunities for positive response to preventive intervention. Not only do environmental influences have the potential to produce illness, they also have the potential to foster greater positive outcomes. To the extent that this is true, preventive intervention may have different effects for some people, thereby expanding our conceptual models and increasing the range of outcomes we need to assess to fully appreciate the impact of environments on optimal health outcomes. In the current context, the results suggest that susceptible individuals may be particularly likely to thrive when they find themselves residing in low crime neighborhoods, but suffer mental health consequences when they reside in higher crime areas.
While the neighborhood×gene effects are interesting and potentially useful, they beg the question of whether there are biological mechanisms linking the observed interaction to outcomes. The current study fills an important research gap by investigating the potential role of DNA methylation of the serotonin transporter gene as a mediator of the impact of this G×E effect on depressive symptoms, examining differential methylation as a biological mechanism that can lead to lower gene expression and ultimately greater depressive symptoms.
To begin, we found a robust association between increased methylation (and hence down regulation) of the serotonin transporter gene and depressive symptoms. Consistent with previous studies (e.g. Beach et al., 2014), we did not find any main effect of 5-HTTLPR on depressive symptoms or DNA methylation. Rather, our analyses showed that neighborhood context interacted with the 5-HTTLPR polymorphism to influence methylation status. Importantly, as hypothesized, this interaction again conformed to the differential susceptibility model and so provided conceptual replication of the pattern reported by Beach et al (2014). Carriers of the short allele showed more methylation than those with the long allele in response to a high crime neighborhood whereas they demonstrated less methylation than those with the long allele in response to a neighborhood that is safe.
In addition, the results suggest that methylation status of the serotonin transporter gene mediated the interaction of 5-HTTLPR×neighborhood on depressive symptoms. Such results might be seen as consonant with an evolutionary perspective where adversity fosters increased methylation of the serotonin transporter gene which produces physiological and psychological consequences designed to deal with environmental threat (e.g., increases in cortisol, amygdala activity, and depressed mood); while conversely, benign circumstances promote decreased methylation of the serotonin transporter gene which promotes a physiological and psychological response appropriate for a safe environment.
Taken together, the patterns from this study suggest several important implications. While genotypes are fixed within an individual, DNA methylation varies depending on environmental factors. Thus, an integrated model that combines social factors, genotypes, and DNA methylation provides a more nuanced portrayal of the intersection of environment and genetic endowment that is particularly relevant to sociologists, psychologists, and social scientists. Although age and cigarette smoking were both related to methylation of loci within the CpG island for 5-HTTLPR, only smoking was associated with both methylation and depressive symptoms. Recent studies provide evidence that the 5-HTTLPR interacts with environmental context to influence smoking (Daw et al., 2013) in a “for better or for worse” manner. Further, 5-HTT methylation is associated with substance use (Philibert et al., 2008). Our data suggest that although self-reported cigarette consumption is significantly related to the composite index of 5-HTT methylation, it does not appear to be a confounding influence on the stress-depression relationship. Given the well documented association between depression and smoking (Kendler et al., 1993), this raises interesting questions for future research about whether smoking precedes or follows depression and whether methylation at some loci on 5-HTT is a consequence of smoking. This illustrates that specifying methylation mechanisms linking environmental factors to well-being has the potential to create interesting new sociological and psychological models that can point research in new directions.
8. Limitations
Although our study provides several important findings, it also suffered from various limitations. First of all, because our sample was limited to African American women it does not allow us to test for gender differences in effects on depression. Likewise, the current design did not allow us to test for differences across ethnic or racial groups. In some respects, however, this limitation might be seen as a strength. Myriad studies have indicated that African American women are at higher risk than other ethnic groups for exposure to high crime neighborhoods and for depression, suggesting this may be an ideal sample in which to examine the depressinogenic effect of high crime neighborhoods. In addition, there is also reason for concern that searching for genetic and epigenetic effects using multiple ethnic groups may increase the potential of finding spurious and misleading effects due to background variation in gene frequencies across ethnic groups. Nonetheless, it would be very useful for the current results to be replicated with samples that are more ethnically diverse and that include males.
Second, our assessment of methylation relied on whole blood. Some methylation patterns are tissue specific and vary depending on cell types, indeed this is a primary mechanism for cell type differentiation. As a result, we cannot assume that methylation patterns are similar between brain or other specific tissues and lymphocytes. Conversely, Thompson and colleagues (2013) found that DNA methylation patterns from peripheral blood and saliva had strong similarity, but differed from lymphoblastoid cell lines (LCLs) due to changes produced by the cell culturing processes. In general, it may be expected that methylation patterns will reflect similar effects among cell types that express the gene in question. Thus, methylation patterns may be more similar than different across many, but not all tissue types. This suggests the value of future research designed to better explicate associations of changes in methylation observed in peripheral samples with changes observed in specific brain regions.
Third, the present study was limited by its small sample size. Although the findings clearly need to be replicated with a larger sample to increase confidence in generalizability, our study had enough statistical power to test our hypotheses involving interaction effects. Finally, our study assessed methylation status at only one point in time. One might speculate that change in DNA methylation status over time can be explained by change in environmental stress over time. Future studies should focus on longitudinal changes in DNA methylation. Unfortunately, it is difficult to generate such data sets given the challenge of drawing blood from subjects scattered across neighborhoods and the costs of methylation assays.
9. Conclusion
Despite these limitations, our study provides insights for better understanding the link between neighborhood crime and depressive symptoms. The current study sheds light on the association between 5-HTTm and depressive symptoms, and on the manner in which such methylation varies depending on neighborhood context and genotype. As the editors of Nature (2012, p.143) recently noted “although our genes are fixed, their expression is highly dependent on what our environment throws at us. The current challenge is to work out precisely how environment affects our biological tissues and changes us.” The current investigation contributes to this important challenge.
Highlights.
The effects of neighborhood crime on depressive symptoms and methylation of the serotonin transporter gene are moderated by the 5-HTTLPR gene in a “for better” or “for worse” manner.
Methylation of the serotonin transporter gene mediates the interaction effect of neighborhood crime and the 5-HTTLPR gene on risk for depressive symptoms.
Neighborhoods serve as a critical context for epigenetic processes that are associated with depressive symptoms.
Acknowledgments
This research was funded by grants from the National Institute of Mental Health (R01 MH62699, R01MH080898, and R01MH60666) and the National Heart, Lung, Blood Institute (R01HL118045). Additional support for this study was derived from the Center for Translation and Prevention Science (Grant Number P30 DA027827) funded by the National Institute on Drug Abuse.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Declarations of Interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Man-Kit Lei, Center for Family Research, University of Georgia, USA.
Steven R. H. Beach, Department of Psychology, University of Georgia, USA
Ronald L. Simons, Department of Sociology, University of Georgia, USA
Robert A. Philibert, Department of Psychiatry, University of Iowa, USA
References
- Andrews PW, Thomson JA., Jr The bright side of being blue: depression as an adaptation for analyzing complex problems. Psychological Review. 2009;116:620–654. doi: 10.1037/a0016242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach SRH, Brody GH, Lei MK, Kim S, Cui J, Philibert RA. Is serotonin transporter genotype associated with epigenetic susceptibility or vulnerability? examination of the impact of socioeconomic status risk on African American youth. Development and Psychopathology. 2014;26:289–304. doi: 10.1017/S0954579413000990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Pluess M. The nature (and nurture?) of plasticity in early human development. Perspectives of Psychological Science. 2009;4:345–351. doi: 10.1111/j.1745-6924.2009.01136.x. [DOI] [PubMed] [Google Scholar]
- Browning CR, Soller B, Gardner M, Brooks-Gunn J. “Feeling Disorder” as a Comparative and Contingent Process Gender, Neighborhood Conditions, and Adolescent Mental Health. Journal of Health and Social Behavior. 2013;54:296–314. doi: 10.1177/0022146513498510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey N. The epigenetics revolution: how modern biology is rewriting our understanding of genetics, disease, and inheritance. New York: Columbia University Press; 2012. [Google Scholar]
- Carver CS, Johnson SL, Joormann J. Serotonergic function, two-mode models of self-regulation, and vulnerability to depression: What depression has in common with impulsive aggression. Psychological Bulletin. 2008;134:912–943. doi: 10.1037/a0013740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Clark L, Watson D. The mini mood and anxiety symptom questionnaire (Mini-MASQ) University of Iowa; 1995. Unpublished manuscript. [Google Scholar]
- Cutrona CE, Russell DW, Hessling RM, Brown PA, Murry V. Direct and moderating effects of community context on the psychological well-being of African American women. Journal of Personality and Social Psychology. 2000;79:1088–1101. doi: 10.1037//0022-3514.79.6.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw J, Shanahan M, Harris KM, Smolen A, Haberstick B, Boardman JD. Genetic Sensitivity to Peer Behaviors 5-HTTLPR, Smoking, and Alcohol Consumption. Journal of Health and Social Behavior. 2013;54:92–108. doi: 10.1177/0022146512468591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogan MV, Shields B, Cutrona C, Gao L, Gibbons FX, Simons R, Philibert RA. The effect of smoking on DNA methylation of peripheral blood mononuclear cells from African American women. BMC Genomics. 2014;15:151. doi: 10.1186/1471-2164-15-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan LE, Keller MC. A critical review of the first ten years of candidate gene-by-environment interaction research in psychiatry. American Journal of Psychiatry. 2011;168:1041–049. doi: 10.1176/appi.ajp.2011.11020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group NE. Life stress. Nature. 2012;490:143. [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Archives of General Psychiatry. 2011;68:444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS. Twin studies of psychiatric illness: Current status and future directions. Archives of General Psychiatry. 1993;50:905–915. doi: 10.1001/archpsyc.1993.01820230075007. [DOI] [PubMed] [Google Scholar]
- Landecker H, Panofsky A. From social structure to gene regulation, and back: A critical introduction to environmental epigenetics for sociology. Annual Review of Sociology. 2013;39:333–357. [Google Scholar]
- Lei MK, Simons RL, Edmond MB, Simons LG, Cutrona CE. The effect of neighborhood disadvantage, social ties, and genetic variation on the antisocial behavior of African American women: A multilevel analysis. Development and Psychopathology. 2014;26:1113–1128. doi: 10.1017/S0954579414000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Müller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Philibert RA, Gunter TD, Beach SR, Brody GH, Madan A. MAOA methylation is associated with nicotine and alcohol dependence in women. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2008;147:565–570. doi: 10.1002/ajmg.b.30778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Rucker DD, Hayes AF. Addressing moderated mediation hypotheses: Theory, methods, and prescriptions. Multivariate Behavioral Research. 2007;42:185–227. doi: 10.1080/00273170701341316. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Roisman GI, Newman DA, Fraley RC, Haltigan JD, Groh AM, Haydon KC. Distinguishing differential susceptibility from diathesis–stress: Recommendations for evaluating interaction effects. Development and Psychopathology. 2012;24:389–409. doi: 10.1017/S0954579412000065. [DOI] [PubMed] [Google Scholar]
- Sampson RJ, Raudenbush SW. Seeing disorder: Neighborhood stigma and the social construction of “broken windows”. Social Psychology Quarterly. 2004;67:319–342. [Google Scholar]
- Seabrook JA, Avison WR. Genotype-environment interaction and sociology: contributions and complexities. Social Science & Medicine. 2010;70:1277–84. doi: 10.1016/j.socscimed.2010.01.016. [DOI] [PubMed] [Google Scholar]
- Shanahan MJ, Hofer SM. Social context in gene–environment interactions: Retrospect and prospect. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2005;60:65–76. doi: 10.1093/geronb/60.special_issue_1.65. [DOI] [PubMed] [Google Scholar]
- Simons RL, Lei MK, Beach SR, Brody GH, Philibert RA, Gibbons FX. Social Environment, Genes, and Aggression Evidence Supporting the Differential Susceptibility Perspective. American Sociological Review. 2011;76:883–912. doi: 10.1177/0003122411427580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki MM, Bird A. DNA methylation landscapes: Provocative insights from epigenomics. Nature Reviews Genetics. 2008;9:465–476. doi: 10.1038/nrg2341. [DOI] [PubMed] [Google Scholar]
- Thoemmes F, MacKinnon DP, Reiser MR. Power analysis for complex mediational designs using Monte Carlo methods. Structural Equation Modeling. 2010;17:510–534. doi: 10.1080/10705511.2010.489379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson TM, Sharfi D, Lee M, Yrigollen CM, Naumova OY, Grigorenko EL. Comparison of whole-genome DNA methylation patterns in whole blood, saliva, and lymphoblastoid cell lines. Behavior Genetics. 2013;43(2):168–176. doi: 10.1007/s10519-012-9579-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayendran M, Cutrona C, Beach SRH, Brody GH, Russell D, Philibert RA. The relationship of the Serotonin Transporter (SLC6A4) extra long variant to gene expression in an African American Sample. American Journal of Medical Genetics: Part B, Neuropsychiatric Genetics. 2012;159B:611–612. doi: 10.1002/ajmg.b.32054. [DOI] [PMC free article] [PubMed] [Google Scholar]