Abstract
Neonatal period is the most vulnerable time for the occurrence of seizures, and neonatal seizures often pose a clinical challenge both for their acute management and frequency of associated long-term co-morbidities. Etiologies of neonatal seizures are known to play a primary role in the anti-epileptic drug responsiveness and the long-term sequelae. Recent studies have suggested that burden of acute recurrent seizures in neonates may also impact chronic outcomes independent of the etiology. However, not many studies, either clinical or pre-clinical, have addressed the long-term outcomes of neonatal seizures in an etiology-specific manner. In this review, we briefly review the available clinical and pre-clinical research for long-term outcomes following neonatal seizures. As the most frequent cause of acquired neonatal seizures, we focus on the studies evaluating long-term effects of HIE-seizures with the goal to evaluate (1) what parameters evaluated during acute stages of neonatal seizures can reliably be used to predict long-term outcomes? and (2) what available clinical and pre-clinical data are available help determine importance of etiology vs. seizure burdens in long-term sequelae.
Keywords: neonatal seizures, hypoxic–ischemic encephalopathy, neonatal brain injury, co-morbidities
Introduction
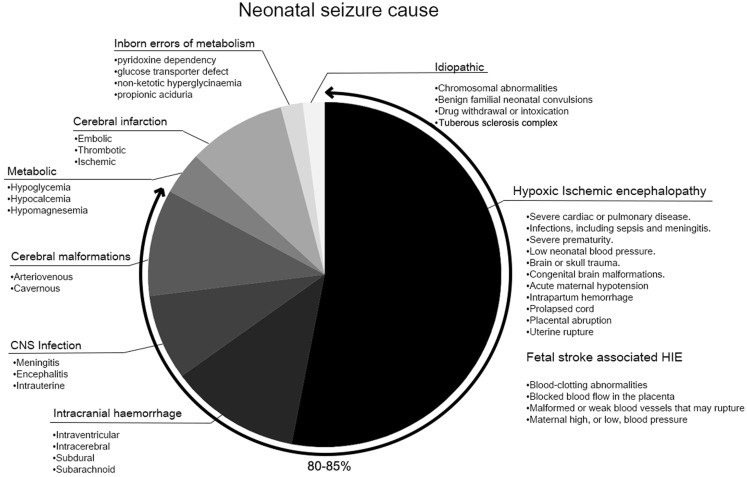
The incidence of seizures, 1.5–3/1,000 live births, is highest during the neonatal period (1, 2). Neonatal seizures remain a clinical challenge due to ambiguous presentations and, therefore, sometimes the failure of immediate detection. The lack of evidence-based management protocols, and poor outcomes add to that challenge (3). Most neonatal seizures are symptomatic rather than idiopathic (2) (Figure 1), and 80–85% are predominantly accounted for by hypoxic–ischemic encephalopathy (HIE), hemorrhage, metabolic disturbances, and infections (4, 5). Several mechanisms are known to play a role in seizure initiation in the immature brain.
Figure 1.
Neonatal seizure cause.
The immature brain has a higher seizure susceptibility due to multiple developmentally regulated features (1). One of which is the now established excitatory and trophic effect of the GABAergic system during cortical development (6, 7). Phenobarbital (PB) remains the first-line anti-epileptic drug (AED) for neonatal seizures (5, 8), however, with an efficacy of less than 50% (9). Consideration of other AEDs is (10) based on the underlying cause and characteristics of the seizures in neonates. Among many, the use of levetiracetam has increasingly become viable, as studies have reported its safety and efficacy on neonatal seizures associated with HIE and other etiologies (11). Increasing evidence suggests that neonatal seizures are associated with adverse neurodevelopmental outcomes, including epilepsy, cerebral palsy, developmental delay, and psychomotor deficits (12–14). However, whether neonatal seizures can independently impact long-term neurologic outcomes, or are a marker of the severity of underlying pathology, remains a topic of active debate (15, 16).
The lack of evidence-based treatments for neonatal seizures stems both from poor estimation of acute seizure burdens in the absence of continuous EEGs and the refractory nature of neonatal seizures. Without this evidence, the ability to effectively study and predict long-term neurodevelopmental effects of seizures remains a challenge. Clinical and pre-clinical studies on the long-term effects of seizures in neonates are needed to provide conclusive insights on (1) which acutely determined parameters predict the long-term sequelae of neonatal seizures, (2) how aggressively should the acute seizures be treated, and (3) are the current pro-active treatments like hypothermia and repeated doses of AEDs neuroprotective in the long-run?
Etiology
Acquired
Hypoxic–Ischemic Encephalopathy
Hypoxic–ischemic encephalopathy, reported in 1–2/1,000 live births, is the most prevalent underlying pathology for acquired neonatal seizures (17). HIE-seizures in neonates accompany high seizure burdens with frequent status epilepticus and electrographic seizures (18). HIE-seizures in neonates are known for their resistance to first-line AEDs like PB (19). The alternative treatment options for refractory seizures, such as levetiracetam and midazolam, have shown variable effects (20, 21).
Therapeutic hypothermia (TH) has become a standard practice for treating neonates with HIE, based on the evidence from pre-clinical and clinical studies that documented reduced brain injury in HIE-neonates that underwent TH (22–26). Clinical studies have documented that TH significantly reduced mortality and short-term morbidity, and improved AED efficacy in neonates with HIE (27–30). However, a recent study has reported no significant difference in survival or functional outcome by TH in neonates hospitalized for cardiac arrest (31). The long-term benefits of TH and its effect on chronic outcomes as related to neonatal seizures are awaiting further evaluation.
Pre-clinical modeling allows a thorough evaluation of both acute seizure burdens and the efficacy of treatment protocols with in vivo and in vitro experiments. Efficacies of AEDs are known to be model specific (32–34) and, therefore, caution must be exercised when making interpretations for translational purposes. Hypoxia is an important component of HIE, and its effect in a developing brain has been studied in a model of neonatal hypoxia (35). Global hypoxia (3–4% O2) in P10–12 rats induced acute seizure burden that was mild and age dependent, with no reported brain injury. A long-term study in this model further reported an increased seizure susceptibility to flurothyl-induced seizures but no significant association with neurobehavioral consequences (36). More recent study on this model reported an emergence of spontaneous seizures at juvenile period and significant prevalence of epilepsy at P180 evaluated by EEG (37). Ischemia represents another important cause of HIE. A newer model of neonatal ischemia-alone was characterized in P7, P10, and P12 mice. In contrast to the hypoxia model, ischemia-alone resulted in a status-like seizure burden and PB-resistance associated with neuronal injury (38). Another well-studied model for HIE is the combination of hypoxia and ischemia (HI; Rice–Vannucci model), which has widely been used to study neonatal HIE (39). In P7 rats, HI-induced seizures continued up to 48 h, with significantly decreased background EEG power (40). Lastly, chemoconvulsants have also been used to recapitulate the high-seizure load seen in neonatal HIE (41, 42). Seizures in brain slices, induced by kainic acid or Mg2+, displayed high seizure severity with status-like seizure activity and PB-resistance (42, 43). In vivo studies of chemoconvulsants, pilocarpine (44) and pentylenetetrazol (45), were also conducted in neonatal rats, but the seizure severity was not quantitated in either study. The characteristics of the seizures studied in these models differ by severity, response to AEDs, and the resultant neuronal injury (33). The long-term co-morbidities were evaluated in only a subset of these studies (Table 1), which is a drawback of some of the models being used and that needs further investigation.
Table 1.
Pre-clinical studies of neonatal seizures and their long-term parameters.
| Study | Model | Species | Age of insult | Chronic ages evaluated | Acute EEG seizure | Long-term EEG seizure | Injury | Long-term comorbidities |
|---|---|---|---|---|---|---|---|---|
| Stafstrom (6) | Kainic acid | Rat | PND 5, 10, 20, and 30 | 3 months | N.E | Evaluated | Evaluated CA3 cell loss in P20 and 30 | N.E |
| Jensen et al. (36) | Perinatal hypoxia | Rat | PND 5, 10, and 60 | 1–2 months | N.E | N.E | N.E | Water maze, open field, handling tests, susceptibility to flurothyl |
| Lee et al. (46) | Tetanus-toxin | Rat | PND 9–11 | Up to 6 months | N.E | N.E | Evaluated No injury | Chronic EEG abnormality |
| Huang et al. (47) | Flurothyl | Rat | PND 0–9 | 3 months | 50 seizures | N.E | N.E | Increased seizure susceptibility to flurothyl, impaired memory, change in HC morphology |
| Santos et al. (48) | Pilocarpine | Rat | PND 7–9 | 3 months | N.E | N.E | Evaluated No injury | Reduced exploratory skills CA1 hyperexcitability |
| Xiu-Yu et al. (44) | Pilocarpine | Rat | PND 1, 4, and 7 | P49 | N.E | N.E | Evaluated No injury | Altered neurogenesis |
| Kadam et al. (49) | Perinatal HI | Rat | PND 7 | 6 months | N.E | N.E | Evaluated Cortical lesions | Mossy fiber sprouting Cortical dysgenesis |
| Kadam et al. (49) | Perinatal HI | Mouse | PND12 | P33–39 | N.E | N.E | Evaluated Hemi: 34% HC: 61% | Rotarod, T-maze alteration, open field, cylinder test |
| Kadam et al. (49) | Perinatal HI | Rat | PND 7 | 2–12months | N.E | Evaluated | Evaluated Hemi: 30–78% | N.E |
| Rakhade et al. (37) | Perinatal hypoxia | Rat | PND 10 | 3–6 months | N.E | Evaluated | Evaluated No injury | Significant prevalence of epilepsy Increased mossy fiber sprouting in CA3 HC |
| Lugo et al. (50) | Flurothyl | Mouse | PND7–11 | P40 | N.E | N.E | N.E | Deficits in HC-dependent memory and social behavior |
| Kang et al. (38, 51) | Ischemia | Mouse | PND 7, 10, and 12 | N.E | Evaluated | N.E | Evaluated P18 | N.E |
| Bernard et al. (52) | Kainic acid | Rat | PND 7 | P6090 | N.E | N.E | N.E | Abnormal social interaction and restricted interests |
| Peng et al. (53) | Perinatal HI | Mouse | PND 7 | 11–12 months | N.E | Evaluated | Evaluated 11–12 month Hemi: 44–69% | N.E |
N.E, not evaluated; HC, hippocampus.
Acquired Non-HIE
CNS Infection (Neonatal Bacteremia and Meningitis)
Neonatal meningitis, occurring in every 0.25–1/1,000 live births, is a condition in which seizures are often detected (54) and long-term sequelae, such as hydrocephaly, brain edema, and subdural effusion, follow. Escherichia coli and group B Streptococcus are typical pathogens for bacterial meningitis and ~25% of neonates with meningitis suffer neurologic complications (55). Administration of dexamethasone, a steroid medication as an adjunct is included in current standard therapy, with minimal side effects reported clinically. (56).However, the data for seizure burdens, AEDs given, and evidence of injury are not readily available to allow evaluation of long-term neurologic outcomes for current management protocols. Additionally, dexamethasone has been shown to increase neuronal injury following asphyxia in preterm fetal sheep, despite later onset and shorter duration of acute seizures (57).
CNS inflammation is known to exacerbate seizure activity and the associated neuronal injury. The condition of prenatal intrauterine infection has been studied in a model of bacterial endotoxin lipopolysaccharide (LPS)-induced inflammation in perinatal rodents. Perinatal LPS exposure was reported to increase seizure susceptibility to chemoconvulsants in rats as adults (58). Similarly, it was also shown to have pro-convulsive and epileptogenic action in a rapid kindling model of neonatal seizures (59). In a long-term, in utero inflammation induced by a single-dose injection of LPS in CD1 pregnant mice resulted in behavioral abnormalities, chronic brain inflammation, neuronal loss, and impaired sleep structures in rodents (60, 61).
Hemorrhage/Trauma
Intracranial hemorrhage occurs in 3.8/10,000 live births and represents ~15% of seizures reported in the neonatal period (62). Infants with intracranial hemorrhage are at high risk for seizures, regardless of the etiology of hemorrhage. Parenchymal injury was independently predictive of acute seizures, and severity of acute seizures predicted later seizures (63).
Currently, no pre-clinical neonatal models are available to examine the association between intracranial hemorrhage and its acute seizure burdens and the long-term outcomes.
Metabolic Disorders
Neonatal seizures and epileptic encephalopathy, although rare, are associated with various inborn errors of metabolism (64) which include hypoglycemia and hypocalcemia. However, there is a lack of clinical or pre-clinical studies that provide insights about the acute quantifiable parameters associated with or co-morbidities caused by neonatal seizures due to metabolic causes.
Cortical Malformations
Developmental malformations of the brain are a cause of neonatal seizures with later development of refractory epilepsy (65). The majority of patients suffer developmental disabilities and epileptic seizures following cortical malformations at early ages (66). No clinical reports are available to evaluate acute parameters and long-term outcomes of seizures related to cortical malformations, possibly due to the variability of seizure locus and the seizure onset. Yet, epilepsy surgery has been reported to improve long-term seizure outcome in patients with focal cortical dysplasia (67).
The pathophysiology of cortical malformation has been characterized in neonatal freeze-lesion model in which microgyrus were surgically induced and hyperexcitability were observed (68). Long-term comorbidities associated with this model were reported in studies that evaluated long-term epileptogenesis (55, 69).
Neonatal Seizures – Genetic
Benign Familial Neonatal Seizures
Benign familial neonatal seizures constitute a small subset of neonatal seizures, often resulting in relatively favorable outcomes with spontaneous remission and normal psychomotor development (70, 71). The prevalent mutations identified include KCNQ2/3 and SCN2A, critical genes for ion channel subunits (72). Recent study on KCNQ2 mutation-positive families reported variable seizure onset and burden, although a higher seizure load at neonatal period suggested higher chance of developing seizures later in life (73). KCNQ2 mutations were also associated with epileptic encephalopathy, and KCNQ2 encephalopathy often manifests refractory seizures, cortical abnormalities, and severe neurodevelopmental delay (74–76).
Kcnq2 knock-out mice were lethal at perinatal stage, but conditional deletions of KCNQ2 channels induced neuronal hyperexcitability in cortical and CA1 pyramidal neurons with abnormal electrocorticogram activity and early death (77). Kcnq2 deficiency resulted in a significant downregulation of KCNQ3/5 protein expression levels, highlighting the critical function of KCNQ2 in maintaining normal neuronal excitability.
Tuberous Sclerosis Complex
Tuberous sclerosis complex (TSC), caused by mutations in TSC1 or TSC2, affects 1 in 6,000 live births (78). During early infancy, the majority of TSC patients manifested seizures that were refractory and recurring after remission (79). Brain MRIs of TSC patients have revealed focal cortical dysplasia (80), which often suggested worse neurodevelopmental outcomes such as epilepsy, cognitive impairment, and autism spectrum disorders (81, 82). TSC patients develop autism phenotypes, including cognitive deficits and anxiety (83). Longitudinal studies documented that the earlier and the more severe seizures predicted worse intellectual development (84, 85), this may be associated with long-term abnormal white matter development (86). Additionally, TSC tubers have been classified as sub-types based on their MRI properties. Type C cortical tubers are more likely to be associated with infantile spasms and epilepsy and associated with a worse phenotype (87). Therefore, both EEG and MRI may be good predictors for long-term prognosis for TSC.
Pre-clinical studies using conditional knock-out models of TSC1 or TSC2 have reported hyperactivation of mTORC1 signaling along with developmental abnormalities and lower seizure threshold (88, 89). However, very few of the rodent models elicit spontaneous seizures and, therefore, their impact on outcomes remains unknown.
Long-Term Co-Morbidities: Seizure Severity, and Injury
The long-term neurodevelopmental sequelae of neonatal seizures are prevalent (2). Nevertheless, very few clinical studies have evaluated the long-term outcomes of neonatal seizures by acute seizure burden and etiology (15). The severity of etiology, seizure burden, and brain injury are known to significantly affect the chronic outcomes, but distinguishing and understanding the role of each individual parameter on the long-term outcomes without standardized protocols across study centers are not feasible.
The underlying etiology has been determined to be one of the main prognostic factors for long-term sequelae in survivors of neonatal seizures (5, 90, 91). HIE, hemorrhage, CNS infection, and cerebral malformations are known to be associated with adverse outcomes compared to other etiologies of neonatal seizures (90) (Figure 1). Grades of neonatal encephalopathy assessed by encephalopathy scores or Sarnat staging are often used to predict neurodevelopmental outcome (92). The effect of hypothermia on improved AED efficacy was shown to depend on the severity of HIE, effective only in neonates with moderate, but not in severe HIE. (93). However, the standardized methodology for identifying the severity of HIE is not uniform. Additionally, severe HIE tends to associate with higher seizure burdens, as is the case in the study by Srinivasakumar et al. Therefore, it is difficult to conclude that etiology was the sole main factor and seizure burden did not exacerbate the encephalopathy.
Neonatal seizures are a significant risk factor for long-term sequelae, especially in the setting of HIE (17). The recurrent seizures themselves appear to cause additional neurodevelopmental consequences beyond that due to the underlying etiology (94). Prolonged seizures were shown to worsen brain damage in HIE brain (95, 96); indicating seizures themselves may have a harmful effect. HIE associated with status epilepticus frequently results in adverse neurodevelopmental outcomes (97, 98). The severity of clinical seizures comprehensively measured by seizure frequency, onset, EEG abnormalities, and number of AEDs used, was independently associated with the brain injury in HIE-neonates (95, 99). The temporal profile of electrographic seizure burdens in neonatal HIE has also been evaluated (18). Differential outcomes associated with the differential timing of onset of seizures, however, are not clear from these studies. Hence, increasing evidence suggests that neonatal seizures need to be controlled, to lessen the long-term co-morbidities above and beyond those associated with the underlying etiology alone (100, 101). Additionally, seizures in a developing brain can beget seizures (102, 103), and, therefore, it is difficult to delineate the role of the underlying etiology vs. prolonged repetitive seizures under these conditions.
Neonatal seizures, especially those that are PB resistant, significantly correlate to moderate–severe brain injury rather than mild or no injury (104). This study found that, the efficacy of a single dose of 20 mg/kg PB significantly differed by the severity of injury. Seizures were readily controlled in neonates with mild or no injury, whereas only 30% of neonates with moderate–severe injury responded to PB. Similarly, the severity of brain injury dictated the seizure burden recorded by video-EEG (93). The presence of brain injury and status epilepticus were highly predictive of the development of epilepsy later on in life (105). Neonatal MRI has demonstrated its possible clinical use for early identification of preterm babies at risk for later cognitive impairment (106). Similar protocols scanning neonates with seizures will help assess long-term outcomes more reliably.
The risk factors that can be used as parameters for predicting chronic outcomes of neonatal seizures remain unclear. A large cohort study at a tertiary center by Nunes et al. reported that the development of postnatal epilepsy and global developmental delay are common following neonatal seizures (107). For both co-morbidities, low birth weight, abnormal postnatal EEG and neuroimaging were also significant risk factors. Follow-up MRIs at 1 and 2 years of age with no evidence of lesion has been reported (108) to indicate better prognostication compared to those with detectable lesions. In a similar study, evaluating risk factors for the long-term sequelae following neonatal seizures, low Apgar score at 5 min, cesarean section, time of seizure onset, seizure type, and the abnormal background EEG were independently predictive of worse long-term outcome following neonatal seizures (90, 109). In line with this observation, lack of EEG recordings for seizure burden quantitation seems like a critical limitation for the interpretations made by studies where EEG seizure burden was not known (15). The identification and quantification of neonatal seizures are heavily dependent on quantitative EEG (15, 110), which remains the gold standard for determining seizure burdens. Additionally, other parameters such as initial injury severity, acute AED efficacy, and follow-up imaging can help provide important insights to help assess role of seizures in long-term outcomes. The severity of etiology, seizure burden, and brain injury can all affect the long-term outcomes of neonatal seizures. The grading of etiology at acute stages reflects the degree of brain injury and seizures are a significant risk factor for later brain injury as assessed by MRI (104, 111).
Using Pre-Clinical Models to Determine Long-Term Co-Morbidities Following Neonatal Seizures
In a hypoxia model of neonatal seizures, an increased seizure susceptibility was detected at 2 months post-hypoxia, but no neurobehavioral consequences or neuronal cell death (112) (Table 1). In another study using combined HI, the long-term effects of seizures were monitored with radio-telemetry for up to 12 months after seizure induction in P7 rats (49). This study reported that perinatal HI resulted in brain injury that ranged from 30 to 78% and temporally progressive epilepsy. However, the injury severity did not correlate to the severity of seizure rates of the chronic post-stroke epilepsy. But more importantly, the study showed that if the perinatal HI insult did not result in an infarct injury, no epilepsy was detected in such rats even with 1 year of continuous monitoring. One similar study using neonatal HI model has recently shown that brain injury can develop at later stages, 11 months post HI insult (53). Motor seizures were identified only in animals with cystic infarct, but none in the animals without infarct.
In pre-clinical models using chemoconvulsants (flurothyl and kainic acid), seizures in P7–11 rodents led to impaired social interaction and learning tested at P60 (50, 52), supporting the notion that the early life seizures may be associated with autism spectrum disorder and intellectual disability (113). By contrast, mTOR pathway was shown to be involved in the development of autistic-like behavior and chronic epilepsy in a model of neonatal hypoxia induced at P10 (114).
Conclusion
Lack of evidence-based or standardized clinical protocols for neonatal seizure management, poor efficacy of currently used AEDs, and dearth of clinical studies looking at long-term comorbidities, specifically by neonatal seizure severity and etiology, remain. Pre-clinical models have become the focus of research for investigating effects of neonatal seizures and novel therapeutics to subdue them efficaciously (51, 115, 116). The need for new pre-clinical models that are translationally viable is a critical need in the field (117). Since neonatal seizures are predominantly sub-clinical, EEG recording of electrographic seizures is crucial for estimating the true seizure burdens. Acute EEG seizure burdens are a good indicator of the severity of HIE. Additionally, evaluation of amplitude EEG (aEEG), with its potential benefit of easier application and interpretation, may enhance clinical management of neonatal seizures and prognosis of the outcomes (118). aEEG, a bedside neurophysiology tool that uses a limited number of channels to record raw EEG signal, is easy to record and interpret, without input from a neurologist. However, the limited sensitivity for seizure detection by aEEG makes conventional EEG the most reliable and globally used diagnostic and quantitative measure for neonatal seizures. Follow-up MRIs are a reliable indicator of the associated long-term brain injury. Diverse underlying etiologies of neonatal seizures may result in different types and severities of seizures, and, therefore, various long-term outcomes. Certain non-HIE related seizures may not result in severe long-term co-morbidities and, hence, etiology plays a critical role. However, lack of long-term data following rigorous acute standardized monitoring and treatment protocols hinders our ability to comprehensively understand these differences. Better pre-clinical modeling of neonatal pathologies that lead to neonatal seizures is already a benchmark set by the NIH (117, 119). As related to this review, the important guidelines highlighted for future pre-clinical studies are (1) whether the model recapitulates clinical comorbidities associated with neonatal seizures and (2) if available, whether certain treatments can prevent or reverse such consequences. The development of treatments to prevent long-term co-morbidities in patients at risk from a neonatal brain insult is a major unmet clinical need (100).
Author Contributions
SKK and SDK contributed equally to the writing of this review. SDK supervised and made final edits.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This publication was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R21HD073105 (SDK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Jensen FE. Neonatal seizures: an update on mechanisms and management. Clin Perinatol (2009) 36:881–900. 10.1016/j.clp.2009.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Volpe JJ. Neurology of the Newborn. Philadelphia, PA: W.B Saunders; (2008). [Google Scholar]
- 3.Zupanc ML. Neonatal seizures. Pediatr Clin North Am (2004) 51:961–78. 10.1016/j.pcl.2004.03.002 [DOI] [PubMed] [Google Scholar]
- 4.Shetty J. Neonatal seizures in hypoxic-ischaemic encephalopathy – risks and benefits of anticonvulsant therapy. Dev Med Child Neurol (2015) 57(Suppl 3):40–3. 10.1111/dmcn.12724 [DOI] [PubMed] [Google Scholar]
- 5.IRCCS ILAE, WHO. Guidelines on Neonatal Seizures (2011). [PubMed]
- 6.Stafstrom CE. Neurobiological mechanisms of developmental epilepsy: translating experimental findings into clinical application. Semin Pediatr Neurol (2007) 14:164–72. 10.1016/j.spen.2007.08.002 [DOI] [PubMed] [Google Scholar]
- 7.Holmes GL, Milh MD, Dulac O. Maturation of the human brain and epilepsy. Handb Clin Neurol (2012) 107:135–43. 10.1016/B978-0-444-52898-8.00007-0 [DOI] [PubMed] [Google Scholar]
- 8.Vento M, de Vries LS, Alberola A, Blennow M, Steggerda S, Greisen G, et al. Approach to seizures in the neonatal period: a European perspective. Acta Paediatr (2010) 99:497–501. 10.1111/j.1651-2227.2009.01659.x [DOI] [PubMed] [Google Scholar]
- 9.Painter MJ, Scher MS, Stein AD, Armatti S, Wang Z, Gardiner JC, et al. Phenobarbital compared with phenytoin for the treatment of neonatal seizures. N Engl J Med (1999) 341:485–9. 10.1056/NEJM199908123410704 [DOI] [PubMed] [Google Scholar]
- 10.Glauser T, Ben-Menachem E, Bourgeois B, Cnaan A, Guerreiro C, Kalviainen R, et al. Updated ILAE evidence review of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia (2013) 54:551–63. 10.1111/epi.12074 [DOI] [PubMed] [Google Scholar]
- 11.Mruk AL, Garlitz KL, Leung NR. Levetiracetam in neonatal seizures: a review. J Pediatr Pharmacol Ther (2015) 20:76–89. 10.5863/1551-6776-20.2.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Heide MJ, Roze E, van der Veere CN, Ter Horst HJ, Brouwer OF, Bos AF. Long-term neurological outcome of term-born children treated with two or more anti-epileptic drugs during the neonatal period. Early Hum Dev (2012) 88:33–8. 10.1016/j.earlhumdev.2011.06.012 [DOI] [PubMed] [Google Scholar]
- 13.McBride MC, Laroia N, Guillet R. Electrographic seizures in neonates correlate with poor neurodevelopmental outcome. Neurology (2000) 55:506–13. 10.1212/WNL.55.4.506 [DOI] [PubMed] [Google Scholar]
- 14.Bozzi Y, Casarosa S, Caleo M. Epilepsy as a neurodevelopmental disorder. Front Psychiatry (2012) 3:19. 10.3389/fpsyt.2012.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwon JM, Guillet R, Shankaran S, Laptook AR, McDonald SA, Ehrenkranz RA, et al. Clinical seizures in neonatal hypoxic-ischemic encephalopathy have no independent impact on neurodevelopmental outcome: secondary analyses of data from the neonatal research network hypothermia trial. J Child Neurol (2011) 26:322–8. 10.1177/0883073810380915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glass HC, Ferriero DM, Miller SP. Correspondence on “clinical seizures in neonatal hypoxic-ischemic encephalopathy have no independent impact on neurodevelopmental outcome: secondary analyses of data from the neonatal research network hypothermia trial”. J Child Neurol (2011) 26:532–4. 10.1177/0883073811399801 [DOI] [PubMed] [Google Scholar]
- 17.Tekgul H, Gauvreau K, Soul J, Murphy L, Robertson R, Stewart J, et al. The current etiologic profile and neurodevelopmental outcome of seizures in term newborn infants. Pediatrics (2006) 117:1270–80. 10.1542/peds.2005-1178 [DOI] [PubMed] [Google Scholar]
- 18.Lynch NE, Stevenson NJ, Livingstone V, Murphy BP, Rennie JM, Boylan GB. The temporal evolution of electrographic seizure burden in neonatal hypoxic ischemic encephalopathy. Epilepsia (2012) 53:549–57. 10.1111/j.1528-1167.2011.03401.x [DOI] [PubMed] [Google Scholar]
- 19.Kossoff E. Neonatal seizures due to hypoxic-ischemic encephalopathy: should we care? Epilepsy Curr (2011) 11:147–8. 10.5698/1535-7511-11.5.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furwentsches A, Bussmann C, Ramantani G, Ebinger F, Philippi H, Poschl J, et al. Levetiracetam in the treatment of neonatal seizures: a pilot study. Seizure (2010) 19:185–9. 10.1016/j.seizure.2010.01.003 [DOI] [PubMed] [Google Scholar]
- 21.Castro Conde JR, Hernandez Borges AA, Domenech ME, Gonzalez CC, Perera SR. Midazolam in neonatal seizures with no response to phenobarbital. Neurology (2005) 64:876–9. 10.1212/01.WNL.0000152891.58694.71 [DOI] [PubMed] [Google Scholar]
- 22.Burnsed JC, Chavez-Valdez R, Hossain MS, Kesavan K, Martin LJ, Zhang J, et al. Hypoxia-ischemia and therapeutic hypothermia in the neonatal mouse brain – a longitudinal study. PLoS One (2015) 10:e0118889. 10.1371/journal.pone.0118889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gano D, Orbach SA, Bonifacio SL, Glass HC. Neonatal seizures and therapeutic hypothermia for hypoxic-ischemic encephalopathy. Mol Cell Epilepsy (2014) 1(3):e88. 10.14800/mce.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmitt FC, Buchheim K, Meierkord H, Holtkamp M. Anticonvulsant properties of hypothermia in experimental status epilepticus. Neurobiol Dis (2006) 23:689–96. 10.1016/j.nbd.2006.05.008 [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Liu PP, Li LY, Zhang HM, Li T. Hypothermia reduces brain edema, spontaneous recurrent seizure attack, and learning memory deficits in the kainic acid treated rats. CNS Neurosci Ther (2011) 17:271–80. 10.1111/j.1755-5949.2010.00168.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Azzopardi D, Strohm B, Marlow N, Brocklehurst P, Deierl A, Eddama O, et al. Effects of hypothermia for perinatal asphyxia on childhood outcomes. N Engl J Med (2014) 371:140–9. 10.1056/NEJMoa1315788 [DOI] [PubMed] [Google Scholar]
- 27.Edwards AD, Brocklehurst P, Gunn AJ, Halliday H, Juszczak E, Levene M, et al. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ (2010) 340:c363. 10.1136/bmj.c363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shankaran S, Pappas A, McDonald SA, Vohr BR, Hintz SR, Yolton K, et al. Childhood outcomes after hypothermia for neonatal encephalopathy. N Engl J Med (2012) 366:2085–92. 10.1056/NEJMoa1112066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garfinkle J, Sant’Anna GM, Wintermark P, Ali N, Morneault L, Koclas L, et al. Cooling in the real world: therapeutic hypothermia in hypoxic-ischemic encephalopathy. Eur J Paediatr Neurol (2013) 17:492–7. 10.1016/j.ejpn.2013.03.006 [DOI] [PubMed] [Google Scholar]
- 30.Boylan GB, Kharoshankaya L, Wusthoff CJ. Seizures and hypothermia: importance of electroencephalographic monitoring and considerations for treatment. Semin Fetal Neonatal Med (2015) 20:103–8. 10.1016/j.siny.2015.01.001 [DOI] [PubMed] [Google Scholar]
- 31.Moler FW, Silverstein FS, Holubkov R, Slomine BS, Christensen JR, Nadkarni VM, et al. Therapeutic hypothermia after out-of-hospital cardiac arrest in children. N Engl J Med (2015) 372:1898–908. 10.1056/NEJMoa1411480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ben-Ari Y. Blocking seizures with the diuretic bumetanide: promises and pitfalls. Epilepsia (2012) 53:394–6. 10.1111/j.1528-1167.2011.03378.x [DOI] [PubMed] [Google Scholar]
- 33.Kang S, Kadam S. Pre-clinical models of acquired neonatal seizures: differential effects of injury on function of chloride co-transporters. Austin J Cerebrovasc Dis Stroke (2014) 1(6):1026. [PMC free article] [PubMed] [Google Scholar]
- 34.Vanhatalo S, Hellstrom-Westas L, de Vries LS. Bumetanide for neonatal seizures: based on evidence or enthusiasm? Epilepsia (2009) 50:1292–3. 10.1111/j.1528-1167.2008.01894.x [DOI] [PubMed] [Google Scholar]
- 35.Jensen FE. An animal model of hypoxia-induced perinatal seizures. Ital J Neurol Sci (1995) 16:59–68. 10.1007/BF02229075 [DOI] [PubMed] [Google Scholar]
- 36.Jensen FE, Holmes GL, Lombroso CT, Blume HK, Firkusny IR. Age-dependent changes in long-term seizure susceptibility and behavior after hypoxia in rats. Epilepsia (1992) 33:971–80. 10.1111/j.1528-1157.1992.tb01746.x [DOI] [PubMed] [Google Scholar]
- 37.Rakhade SN, Klein PM, Huynh T, Hilario-Gomez C, Kosaras B, Rotenberg A, et al. Development of later life spontaneous seizures in a rodent model of hypoxia-induced neonatal seizures. Epilepsia (2011) 52:753–65. 10.1111/j.1528-1167.2011.02992.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kang SK, Markowitz GJ, Kim ST, Johnston MV, Kadam SD. Age- and sex-dependent susceptibility to phenobarbital-resistant neonatal seizures: role of chloride co-transporters. Front Cell Neurosci (2015) 9:173. 10.3389/fncel.2015.00173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rice JE, III, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol (1981) 9:131–41. 10.1002/ana.410090206 [DOI] [PubMed] [Google Scholar]
- 40.Sampath D, White AM, Raol YH. Characterization of neonatal seizures in an animal model of hypoxic-ischemic encephalopathy. Epilepsia (2014) 55:985–93. 10.1111/epi.12646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boylan GB, Pressler RM. Neonatal seizures: the journey so far. Semin Fetal Neonatal Med (2013) 18:173–4. 10.1016/j.siny.2013.04.004 [DOI] [PubMed] [Google Scholar]
- 42.Dzhala VI, Talos DM, Sdrulla DA, Brumback AC, Mathews GC, Benke TA, et al. NKCC1 transporter facilitates seizures in the developing brain. Nat Med (2005) 11:1205–13. 10.1038/nm1301 [DOI] [PubMed] [Google Scholar]
- 43.Dzhala VI, Brumback AC, Staley KJ. Bumetanide enhances phenobarbital efficacy in a neonatal seizure model. Ann Neurol (2008) 63:222–35. 10.1002/ana.21229 [DOI] [PubMed] [Google Scholar]
- 44.Xiu-Yu S, Ruo-Peng S, Ji-Wen W. Consequences of pilocarpine-induced recurrent seizures in neonatal rats. Brain Dev (2007) 29:157–63. 10.1016/j.braindev.2006.08.009 [DOI] [PubMed] [Google Scholar]
- 45.Mares P. Age- and dose-specific anticonvulsant action of bumetanide in immature rats. Physiol Res (2009) 58:927–30. [DOI] [PubMed] [Google Scholar]
- 46.Lee CL, Hrachovy RA, Smith KL, Frost JD, Jr, Swann JW. Tetanus toxin-induced seizures in infant rats and their effects on hippocampal excitability in adulthood. Brain Res. (1995) 677(1):97–109. 10.1016/0006-8993(95)00127-C [DOI] [PubMed] [Google Scholar]
- 47.Huang L, Cilio MR, Silveira DC, McCabe BK, Sogawa Y, Stafstrom CE, et al. Long-term effects of neonatal seizures: a behavioral, electrophysiological, and histological study. Brain Res Dev. (1999) 118(1–2):99–107. 10.1016/S0165-3806(99)00135-2 [DOI] [PubMed] [Google Scholar]
- 48.Santos NF, Marques RH, Correia L, Sinigaglia-Coimbra R, Calderazzo L, Sanabria ER, et al. Multiple pilocarpine-induced status epilepticus in developing rats: a long-term behavioral and electrophysiological study. Epilepsia (2000) 41(Suppl 6):S57–63. [DOI] [PubMed] [Google Scholar]
- 49.Kadam SD, White AM, Staley KJ, Dudek FE. Continuous electroencephalographic monitoring with radio-telemetry in a rat model of perinatal hypoxia-ischemia reveals progressive post-stroke epilepsy. J Neurosci (2010) 30:404–15. 10.1523/JNEUROSCI.4093-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lugo JN, Swann JW, Anderson AE. Early-life seizures result in deficits in social behavior and learning. Exp Neurol (2014) 256:74–80. 10.1016/j.expneurol.2014.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kang SK, Johnston MV, Kadam SD. Acute TrkB-inhibition rescues phenobarbital-resistant seizures in a mouse model of neonatal ischemia. Eur J Neurosci (2015) 42:2792–804. 10.1111/ejn.13094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bernard PB, Castano AM, Beitzel CS, Carlson VB, Benke TA. Behavioral changes following a single episode of early-life seizures support the latent development of an autistic phenotype. Epilepsy Behav (2015) 44:78–85. 10.1016/j.yebeh.2015.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peng J, Li R, Arora N, Lau M, Lim S, Wu C, et al. Effects of neonatal hypoxic-ischemic episodes on late seizure outcomes in C57 black mice. Epilepsy Res (2015) 111:142–9. 10.1016/j.eplepsyres.2015.01.009 [DOI] [PubMed] [Google Scholar]
- 54.Khalessi N, Afsharkhas L. Neonatal meningitis: risk factors, causes, and neurologic complications. Iran J Child Neurol (2014) 8:46–50. [PMC free article] [PubMed] [Google Scholar]
- 55.Chu SM, Hsu JF, Lee CW, Lien R, Huang HR, Chiang MC, et al. Neurological complications after neonatal bacteremia: the clinical characteristics, risk factors, and outcomes. PLoS One (2014) 9:e105294. 10.1371/journal.pone.0105294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brouwer MC, Tunkel AR, van de Beek D. Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis. Clin Microbiol Rev (2010) 23:467–92. 10.1128/CMR.00070-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koome ME, Davidson JO, Drury PP, Mathai S, Booth LC, Gunn AJ, et al. Antenatal dexamethasone after asphyxia increases neural injury in preterm fetal sheep. PLoS One (2013) 8:e77480. 10.1371/journal.pone.0077480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Galic MA, Riazi K, Heida JG, Mouihate A, Fournier NM, Spencer SJ, et al. Postnatal inflammation increases seizure susceptibility in adult rats. J Neurosci (2008) 28:6904–13. 10.1523/JNEUROSCI.1901-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Auvin S, Shin D, Mazarati A, Sankar R. Inflammation induced by LPS enhances epileptogenesis in immature rat and may be partially reversed by IL1RA. Epilepsia (2010) 51(Suppl 3):34–8. 10.1111/j.1528-1167.2010.02606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dada T, Rosenzweig JM, Al SM, Firdaus W, Al RS, Borbiev T, et al. Mouse model of intrauterine inflammation: sex-specific differences in long-term neurologic and immune sequelae. Brain Behav Immun (2014) 38:142–50. 10.1016/j.bbi.2014.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Adler DA, Ammanuel S, Lei J, Dada T, Borbiev T, Johnston MV, et al. Circadian cycle-dependent EEG biomarkers of pathogenicity in adult mice following prenatal exposure to in utero inflammation. Neuroscience (2014) 275:305–13. 10.1016/j.neuroscience.2014.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gupta SN, Kechli AM, Kanamalla US. Intracranial hemorrhage in term newborns: management and outcomes. Pediatr Neurol (2009) 40:1–12. 10.1016/j.pediatrneurol.2008.09.019 [DOI] [PubMed] [Google Scholar]
- 63.Bansal S, Kebede T, Dean NP, Carpenter JL. Predictors of acute symptomatic seizures after intracranial hemorrhage in infants. Pediatr Crit Care Med (2014) 15:750–5. 10.1097/PCC.0000000000000221 [DOI] [PubMed] [Google Scholar]
- 64.Yu JY, Pearl PL. Metabolic causes of epileptic encephalopathy. Epilepsy Res Treat (2013) 2013:124934. 10.1155/2013/124934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takano T, Sokoda T, Akahori S, Sakaue Y, Sawai C, Takeuchi Y, et al. Enhanced capacity of epilepsy in brain malformation produced during early development. Pediatr Neurol (2006) 35:38–41. 10.1016/j.pediatrneurol.2005.11.007 [DOI] [PubMed] [Google Scholar]
- 66.Leventer RJ, Guerrini R, Dobyns WB. Malformations of cortical development and epilepsy. Dialogues Clin Neurosci (2008) 10:47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fauser S, Essang C, Altenmuller DM, Staack AM, Steinhoff BJ, Strobl K, et al. Long-term seizure outcome in 211 patients with focal cortical dysplasia. Epilepsia (2015) 56:66–76. 10.1111/epi.12876 [DOI] [PubMed] [Google Scholar]
- 68.Andresen L, Hampton D, Taylor-Weiner A, Morel L, Yang Y, Maguire J, et al. Gabapentin attenuates hyperexcitability in the freeze-lesion model of developmental cortical malformation. Neurobiol Dis (2014) 71:305–16. 10.1016/j.nbd.2014.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jacobs KM, Prince DA. Excitatory and inhibitory postsynaptic currents in a rat model of epileptogenic microgyria. J Neurophysiol (2005) 93(2):687–96. 10.1152/jn.00288.2004 [DOI] [PubMed] [Google Scholar]
- 70.Van Hove JL, Lohr NJ. Metabolic and monogenic causes of seizures in neonates and young infants. Mol Genet Metab (2011) 104:214–30. 10.1016/j.ymgme.2011.04.020 [DOI] [PubMed] [Google Scholar]
- 71.Steinlein OK, Conrad C, Weidner B. Benign familial neonatal convulsions: always benign? Epilepsy Res (2007) 73:245–9. 10.1016/j.eplepsyres.2006.10.010 [DOI] [PubMed] [Google Scholar]
- 72.Berkovic SF, Heron SE, Giordano L, Marini C, Guerrini R, Kaplan RE, et al. Benign familial neonatal-infantile seizures: characterization of a new sodium channelopathy. Ann Neurol (2004) 55:550–7. 10.1002/ana.20029 [DOI] [PubMed] [Google Scholar]
- 73.Grinton BE, Heron SE, Pelekanos JT, Zuberi SM, Kivity S, Afawi Z, et al. Familial neonatal seizures in 36 families: clinical and genetic features correlate with outcome. Epilepsia (2015) 56:1071–80. 10.1111/epi.13020 [DOI] [PubMed] [Google Scholar]
- 74.Pisano T, Numis AL, Heavin SB, Weckhuysen S, Angriman M, Suls A, et al. Early and effective treatment of KCNQ2 encephalopathy. Epilepsia (2015) 56:685–91. 10.1111/epi.12984 [DOI] [PubMed] [Google Scholar]
- 75.Weckhuysen S, Mandelstam S, Suls A, Audenaert D, Deconinck T, Claes LR, et al. KCNQ2 encephalopathy: emerging phenotype of a neonatal epileptic encephalopathy. Ann Neurol (2012) 71:15–25. 10.1002/ana.22644 [DOI] [PubMed] [Google Scholar]
- 76.Dalen Meurs-van der Schoor C, van Weissenbruch M, van Kempen M, Bugiani M, Aronica E, Ronner H, et al. Severe neonatal epileptic encephalopathy and KCNQ2 mutation: neuropathological substrate? Front Pediatr (2014) 2:136. 10.3389/fped.2014.00136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Soh H, Pant R, LoTurco JJ, Tzingounis AV. Conditional deletions of epilepsy-associated KCNQ2 and KCNQ3 channels from cerebral cortex cause differential effects on neuronal excitability. J Neurosci (2014) 34:5311–21. 10.1523/JNEUROSCI.3919-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Curatolo P, Bombardieri R, Jozwiak S. Tuberous sclerosis. Lancet (2008) 372:657–68. 10.1016/S0140-6736(08)61279-9 [DOI] [PubMed] [Google Scholar]
- 79.Vignoli A, La BF, Turner K, Scornavacca G, Chiesa V, Zambrelli E, et al. Epilepsy in TSC: certain etiology does not mean certain prognosis. Epilepsia (2013) 54:2134–42. 10.1111/epi.12430 [DOI] [PubMed] [Google Scholar]
- 80.Kotulska K, Jurkiewicz E, Domanska-Pakiela D, Grajkowska W, Mandera M, Borkowska J, et al. Epilepsy in newborns with tuberous sclerosis complex. Eur J Paediatr Neurol (2014) 18:714–21. 10.1016/j.ejpn.2014.06.009 [DOI] [PubMed] [Google Scholar]
- 81.Jozwiak S, Goodman M, Lamm SH. Poor mental development in patients with tuberous sclerosis complex: clinical risk factors. Arch Neurol (1998) 55:379–84. 10.1001/archneur.55.3.379 [DOI] [PubMed] [Google Scholar]
- 82.Chu-Shore CJ, Major P, Camposano S, Muzykewicz D, Thiele EA. The natural history of epilepsy in tuberous sclerosis complex. Epilepsia (2010) 51:1236–41. 10.1111/j.1528-1167.2009.02474.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gipson TT, Poretti A, Thomas EA, Jenkins KT, Desai S, Johnston MV. Autism phenotypes in tuberous sclerosis complex: diagnostic and treatment considerations. J Child Neurol (2015) 30(14):1871–6. 10.1177/0883073815600871 [DOI] [PubMed] [Google Scholar]
- 84.Overwater IE, Bindels-de HK, Rietman AB, Ten Hoopen LW, Vergouwe Y, Moll HA, et al. Epilepsy in children with tuberous sclerosis complex: chance of remission and response to antiepileptic drugs. Epilepsia (2015) 56:1239–45. 10.1111/epi.13050 [DOI] [PubMed] [Google Scholar]
- 85.Bolton PF, Clifford M, Tye C, Maclean C, Humphrey A, le MK, et al. Intellectual abilities in tuberous sclerosis complex: risk factors and correlates from the tuberous sclerosis 2000 Study. Psychol Med (2015) 45:2321–31. 10.1017/S0033291715000264 [DOI] [PubMed] [Google Scholar]
- 86.Baumer FM, Song JW, Mitchell PD, Pienaar R, Sahin M, Grant PE, et al. Longitudinal changes in diffusion properties in white matter pathways of children with tuberous sclerosis complex. Pediatr Neurol (2015) 52:615–23. 10.1016/j.pediatrneurol.2015.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gallagher A, Grant EP, Madan N, Jarrett DY, Lyczkowski DA, Thiele EA. MRI findings reveal three different types of tubers in patients with tuberous sclerosis complex. J Neurol (2010) 257:1373–81. 10.1007/s00415-010-5535-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Meikle L, Talos DM, Onda H, Pollizzi K, Rotenberg A, Sahin M, et al. A mouse model of tuberous sclerosis: neuronal loss of Tsc1 causes dysplastic and ectopic neurons, reduced myelination, seizure activity, and limited survival. J Neurosci (2007) 27:5546–58. 10.1523/JNEUROSCI.5540-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Feliciano DM, Su T, Lopez J, Platel JC, Bordey A. Single-cell Tsc1 knockout during corticogenesis generates tuber-like lesions and reduces seizure threshold in mice. J Clin Invest (2011) 121:1596–607. 10.1172/JCI44909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Anand V, Nair PM. Neonatal seizures: predictors of adverse outcome. J Pediatr Neurosci (2014) 9:97–9. 10.4103/1817-1745.139261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shellhass R. Etiology and Prognosis of Neonatal Seizures (2015).
- 92.van HM, Swaab H, de Vries LS, Jongmans MJ. Long-term cognitive and behavioral consequences of neonatal encephalopathy following perinatal asphyxia: a review. Eur J Pediatr (2007) 166:645–54. 10.1007/s00431-007-0437-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Srinivasakumar P, Zempel J, Wallendorf M, Lawrence R, Inder T, Mathur A. Therapeutic hypothermia in neonatal hypoxic ischemic encephalopathy: electrographic seizures and magnetic resonance imaging evidence of injury. J Pediatr (2013) 163:465–70. 10.1016/j.jpeds.2013.01.041 [DOI] [PubMed] [Google Scholar]
- 94.Chapman KE, Specchio N, Shinnar S, Holmes GL. Seizing control of epileptic activity can improve outcome. Epilepsia (2015) 56:1482–5. 10.1111/epi.13109 [DOI] [PubMed] [Google Scholar]
- 95.Miller SP, Weiss J, Barnwell A, Ferriero DM, Latal-Hajnal B, Ferrer-Rogers A, et al. Seizure-associated brain injury in term newborns with perinatal asphyxia. Neurology (2002) 58:542–8. 10.1212/WNL.58.4.542 [DOI] [PubMed] [Google Scholar]
- 96.Wirrell EC, Armstrong EA, Osman LD, Yager JY. Prolonged seizures exacerbate perinatal hypoxic-ischemic brain damage. Pediatr Res (2001) 50:445–54. 10.1203/00006450-200110000-00005 [DOI] [PubMed] [Google Scholar]
- 97.Van Rooij LG, de Vries LS, Handryastuti S, Hawani D, Groenendaal F, van Huffelen AC, et al. Neurodevelopmental outcome in term infants with status epilepticus detected with amplitude-integrated electroencephalography. Pediatrics (2007) 120:e354–63. 10.1542/peds.2006-3007 [DOI] [PubMed] [Google Scholar]
- 98.Dlugos DJ. The nature of neonatal status epilepticus – a clinician’s perspective. Epilepsy Behav (2015) 49:88–9. 10.1016/j.yebeh.2015.04.025 [DOI] [PubMed] [Google Scholar]
- 99.Younkin DP, Delivoria-Papadopoulos M, Maris J, Donlon E, Clancy R, Chance B. Cerebral metabolic effects of neonatal seizures measured with in vivo 31P NMR spectroscopy. Ann Neurol (1986) 20:513–9. 10.1002/ana.410200412 [DOI] [PubMed] [Google Scholar]
- 100.Jehi L, Wyllie E, Devinsky O. Epileptic encephalopathies: optimizing seizure control and developmental outcome. Epilepsia (2015) 56:1486–9. 10.1111/epi.13107 [DOI] [PubMed] [Google Scholar]
- 101.Berg AT, Zelko FA, Levy SR, Testa FM. Age at onset of epilepsy, pharmacoresistance, and cognitive outcomes: a prospective cohort study. Neurology (2012) 79:1384–91. 10.1212/WNL.0b013e31826c1b55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ben-Ari Y, Holmes GL. Effects of seizures on developmental processes in the immature brain. Lancet Neurol (2006) 5:1055–63. 10.1016/S1474-4422(06)70626-3 [DOI] [PubMed] [Google Scholar]
- 103.Holmes GL. Effects of seizures on brain development: lessons from the laboratory. Pediatr Neurol (2005) 33:1–11. 10.1016/j.pediatrneurol.2004.12.003 [DOI] [PubMed] [Google Scholar]
- 104.Glass HC, Nash KB, Bonifacio SL, Barkovich AJ, Ferriero DM, Sullivan JE, et al. Seizures and magnetic resonance imaging-detected brain injury in newborns cooled for hypoxic-ischemic encephalopathy. J Pediatr (2011) 159:731–5. 10.1016/j.jpeds.2011.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Glass HC, Hong KJ, Rogers EE, Jeremy RJ, Bonifacio SL, Sullivan JE, et al. Risk factors for epilepsy in children with neonatal encephalopathy. Pediatr Res (2011) 70:535–40. 10.1203/PDR.0b013e31822f24c7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ullman H, Spencer-smith S, Thompson D, Doyle L, Inder T, Anderson P, et al. Neonatal MRI is associated with future cognition and academic achievement in preterm children. Brain (2015) 138:3251–62. 10.1093/brain/awv244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nunes ML, Martins MP, Barea BM, Wainberg RC, Costa JC. Neurological outcome of newborns with neonatal seizures: a cohort study in a tertiary university hospital. Arq Neuropsiquiatr (2008) 66:168–74. 10.1590/S0004-282X2008000200005 [DOI] [PubMed] [Google Scholar]
- 108.Rutherford M, Pennock J, Schwieso J, Cowan F, Dubowitz L. Hypoxic-ischaemic encephalopathy: early and late magnetic resonance imaging findings in relation to outcome. Arch Dis Child Fetal Neonatal Ed (1996) 75:F145–51. 10.1136/fn.75.3.F145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Garfinkle J, Shevell MI. Prognostic factors and development of a scoring system for outcome of neonatal seizures in term infants. Eur J Paediatr Neurol (2011) 15:222–9. 10.1016/j.ejpn.2010.11.002 [DOI] [PubMed] [Google Scholar]
- 110.Abend NS, Wusthoff CJ, Goldberg EM, Dlugos DJ. Electrographic seizures and status epilepticus in critically ill children and neonates with encephalopathy. Lancet Neurol (2013) 12:1170–9. 10.1016/S1474-4422(13)70246-1 [DOI] [PubMed] [Google Scholar]
- 111.Shah DK, Wusthoff CJ, Clarke P, Wyatt JS, Ramaiah SM, Dias RJ, et al. Electrographic seizures are associated with brain injury in newborns undergoing therapeutic hypothermia. Arch Dis Child Fetal Neonatal Ed (2014) 99:F219–24. 10.1136/archdischild-2013-305206 [DOI] [PubMed] [Google Scholar]
- 112.Jensen FE, Applegate C, Burchfiel J, Lombroso CT. Differential effects of perinatal hypoxia and anoxia on long term seizure susceptibility in the rat. Life Sci (1991) 49:399–407. 10.1016/0024-3205(91)90448-K [DOI] [PubMed] [Google Scholar]
- 113.Bernard PB, Benke TA. Early life seizures: evidence for chronic deficits linked to autism and intellectual disability across species and models. Exp Neurol (2015) 263:72–8. 10.1016/j.expneurol.2014.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Talos DM, Sun H, Zhou X, Fitzgerald EC, Jackson MC, Klein PM, et al. The interaction between early life epilepsy and autistic-like behavioral consequences: a role for the mammalian target of rapamycin (mTOR) pathway. PLoS One (2012) 7:e35885. 10.1371/journal.pone.0035885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sampath D, Shmueli D, White AM, Raol YH. Flupirtine effectively prevents development of acute neonatal seizures in an animal model of global hypoxia. Neurosci Lett (2015) 607:46–51. 10.1016/j.neulet.2015.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cleary RT, Sun H, Huynh T, Manning SM, Li Y, Rotenberg A, et al. Bumetanide enhances phenobarbital efficacy in a rat model of hypoxic neonatal seizures. PLoS One (2013) 8:e57148. 10.1371/journal.pone.0057148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.NINDS/NIH guidelines. Curing the Epilepsies: The Promise of Research (2013).
- 118.Glass HC, Wusthoff CJ, Shellhaas RA. Amplitude-integrated electro-encephalography: the child neurologist’s perspective. J Child Neurol (2013) 28:1342–50. 10.1177/0883073813488663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.NINDS, NIH. Curing the Epilepsies: the Promise of Research. Bethesda, MD: NIH Publication; (2013). [Google Scholar]