Significance
Promyelocytic leukemia protein (PML) nuclear bodies (NBs) are subnuclear domains proposed to facilitate posttranslational modifications. Among NB partners, proteins are p53 and are most of the regulators. Overexpression of a single PML splice variant, PML IV, triggers p53-driven senescence. We demonstrate an interaction between PML IV C terminus and the ARF tumor suppressor. This interaction is required to promote p53 SUMOylation and subsequent stabilization. These results unexpectedly bridge three key tumor suppressors and stress the key role of PML NBs as SUMOylation factories and regulators of senescence.
Keywords: UBC9, posttranslational modification, nuclear matrix, splicing, tumor suppression
Abstract
Promyelocytic leukemia protein (PML) nuclear bodies (NBs) recruit multiple partners, including p53 and many of its regulators. NBs are believed to facilitate several posttranslational modifications and are key regulators of senescence. PML, the organizer of NBs, is expressed as a number of splice variants that all efficiently recruit p53 partners. However, overexpression of only one of them, PML IV, triggers p53-driven senescence. Here, we show that PML IV specifically binds ARF, a key p53 regulator. Similar to ARF, PML IV enhances global SUMO-1 conjugation, particularly that of p53, resulting in p53 stabilization and activation. ARF interacts with and stabilizes the NB-associated UBC9 SUMO-conjugating enzyme, possibly explaining PML IV-enhanced SUMOylation. These results unexpectedly link two key tumor suppressors, highlighting their convergence for global control of SUMO conjugation, p53 activation, and senescence induction.
Promyelocytic leukemia protein (PML) is the key organizer of PML nuclear bodies (NBs), organelles that recruit an ever-growing number of unrelated partner proteins (1). Initially discovered through its targeting by an oncogenic translocation (2), PML has drawn considerable attention through its complex link with SUMOylation, a posttranslational modification (PTM) by ubiquitin-like peptides (SUMO). Indeed, PML must be SUMOylated to recruit its partners within PML NBs, together with the universal SUMO-conjugating enzyme UBC9 then facilitating the partner’s modification (1, 3–5). Alternative splicing of human PML yields at least seven isoforms, and previous work has implicated these isoforms in specific interactions or functions (6–8). In particular, overexpression of a single isoform, PML IV, initiates p53 activation and drives senescence (8). Conversely, the requirement for PML in senescence induction was demonstrated by Ras’s inability to induce senescence in pml−/− cells (9, 10). In many other experimental systems, p53 activation and/or senescence induction can be antagonized by PML extinction (11, 12).
p53 is a multifunctional transcription factor, which is a key regulator of survival and proliferation (13). p53 activity is tightly regulated through multiple PTMs that control interactions with partner proteins or DNA. How exactly PML controls p53 is still debated. Numerous studies have pointed out the ability of PML to recruit p53 and many of its specific regulators (HIPK2, HAUSP, DAXX, CBP, HDM2, SIRT1, Chk2, MageA2, MOZ, etc.) into NBs (10, 14–20). Partner recruitment/retention within NBs was proposed to enhance various p53 PTMs, particularly acetylation of lysine K382. However, neither partner nor p53 recruitment is specific for PML IV (8), so the basis for the exquisite specificity of PML IV to control p53 signaling remains ill-understood. A study proposed that PML IV represses TBX2, a gene amplified in some cancers that controls the CDKN2A promoter (21). The CDKN2A locus encodes two related products, ARF and p16INK4A, both involved in senescence activation through p53 and pRB, respectively. Besides its control of p53 abundance, ARF also exerts p53-independent functions, notably on the regulation of cell growth (22). These functions were proposed to rely, at least in part, on ARF’s ability to enhance global SUMOylation (23–27).
Here, we demonstrate that PML IV specifically binds ARF and enhances p53 SUMO-1 conjugation, resulting in p53 stabilization and senescence. Our results unexpectedly bridge PML and ARF, two key senescence players, and highlight their roles in SUMOylation and control of p53-induced senescence.
Results
PML IV C Terminus Is Required to Trigger Senescence.
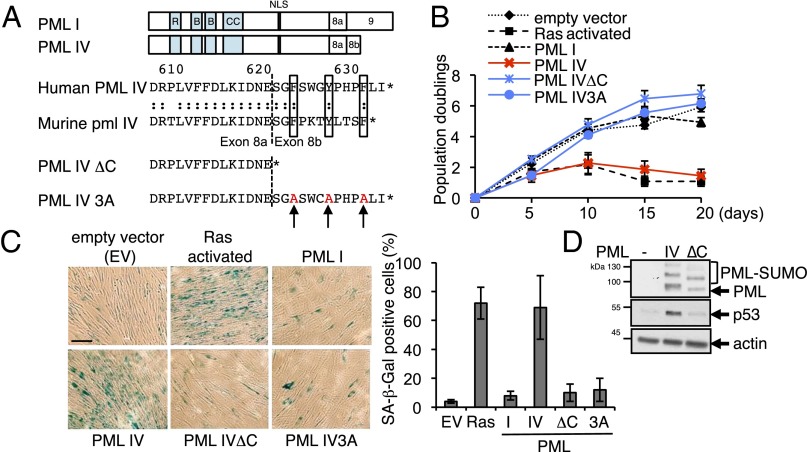
PML IV exon 8a is shared with PML I (Fig. 1A) and is thus unlikely to account on its own for senescence induction. Exon 8b, specific for PML IV, encodes a 13-amino-acid sequence conserved among species (Fig. 1A and Fig. S1). Remarkably, this short amino acid stretch contains seven cyclic amino acids with high steric hindrance. In particular, three bulky aromatic residues (human residues F623, Y627, and F631) are perfectly conserved between man and mouse (Fig. 1A) and in most mammals (Fig. S1).
Fig. 1.
PML IV C terminus is required for senescence induction. (A, Top) Comparison of human PML isoform I and IV. (Middle) Sequence alignment of human and murine PML IV C termini. The dotted line indicates the junction between exons 8a and 8b. The three conserved aromatic residues are boxed. (Bottom) PML IV∆C and PML IV3A mutants. (B) Proliferation of primary human WI38 fibroblasts infected by lentiviruses expressing the indicated constructs. (C) Quantification of SA-β-Gal staining of infected WI38 cells after 10 d (mean ± SD) from four independent experiments. (D) Western blot analysis of p53 and PML in WI38 cell extracts 3 d after transduction with indicated constructs.
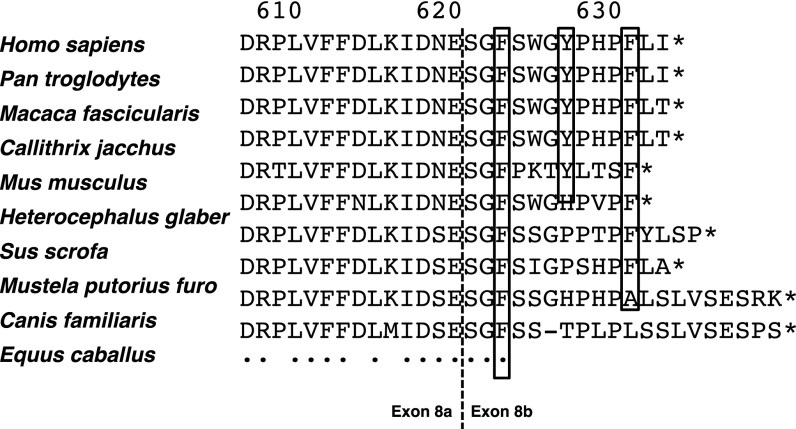
Fig. S1.
Partial PML IV alignment among species. Vertical dotted line indicates the exon 8a/8b boundary. Boxed residues are those mutated in PML IV3A. An asterisk represents end of coding sequence. A dot corresponds to a perfect conservation pattern among species.
To test whether these amino acids are involved in the specific function(s) of PML IV, we generated two mutants, either deleted for the PML IV-specific sequence (PML IVΔC) or with substitutions of aromatic residues for alanine (PML IV3A: F623A, Y627A, and F631A) (Fig. 1A). Overexpression of PML IVΔC, PML IV3A, or PML I in WI38 human primary fibroblasts failed to induce growth arrest, whereas PML IV or oncogenic Ras did (Fig. 1B) (8, 10). Moreover, Ras- and PML IV-transduced cells exhibited senescence-associated β-galactosidase activity (SA-β-Gal), whereas the two PML IV C-terminal mutants did not (Fig. 1C). In WI38 fibroblasts, PML I, IV, or IVΔC induced p53 recruitment within NBs, as previously shown (8). However, although PML IV stabilized p53 (8–10), the mutants defective for senescence induction did not (Fig. 1D). p53 silencing by shRNA reversed PML IV-induced growth arrest (8). Thus, PML IV stabilization of p53 and activation of senescence require the three bulky hydrophobic/cyclic residues within its C terminus.
In an attempt to identify p53 target genes specifically activated by PML IV, we compared the transcriptional changes elicited by PML IV or PML IV∆C. We performed microarray analysis in WI38 cells at early time points (12, 18, and 24 h) after infection with PML-expressing lentiviruses. A twofold induction of PML expression was demonstrated as early as 12 h postinfection, together with an unambiguous modulation of genes known to be regulated by PML [IFN targets (28, 29) and TBX2 (21)]. Importantly, no significant difference between PML IV and PML IV∆C transcriptional profiles was observed, suggesting that the primary changes elicited by PML IV are not transcriptional. Yet subsequent p53 activation should modulate gene expression at later time points, when the senescence program becomes activated.
PML IV Induces p53–SUMO-1 Conjugation.
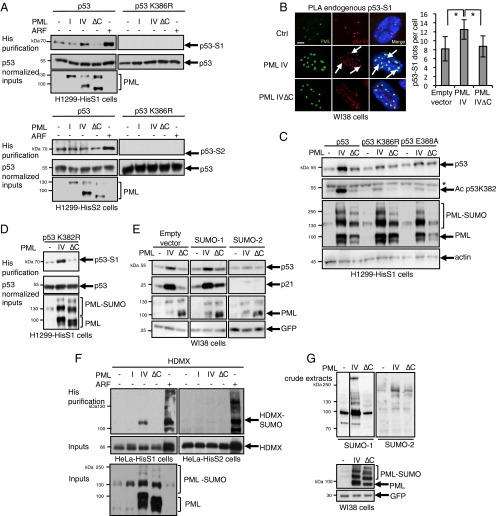
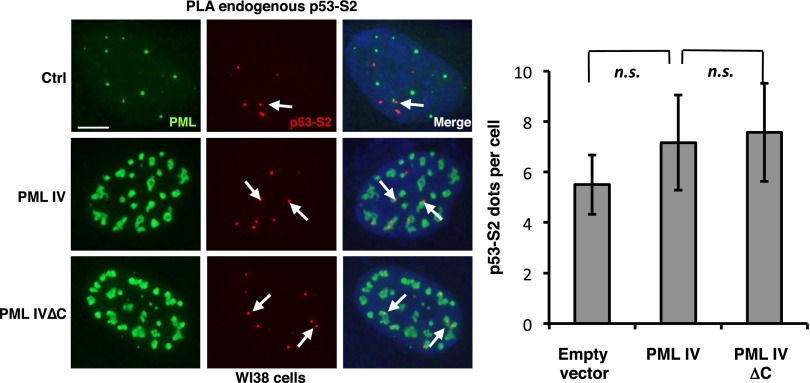
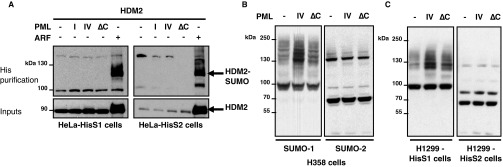
Upon stress, NBs facilitate partner SUMOylation through recruitment of UBC9 (1, 5). P53 can be SUMO-modified on a single consensus site K386 in its C-terminal regulatory region (30). To assess whether PML IV could facilitate p53 SUMO conjugation, we transiently transfected p53, or a p53 mutant defective for SUMOylation, in H1299 p53-null cells stably expressing His-tagged SUMO-1 or -2 (H1299-HisS1 or H1299-HisS2). His purification demonstrated a basal p53 modification on K386 by either SUMO-1 or SUMO-2 (Fig. 2A). Remarkably, PML IV enhanced p53 SUMOylation by SUMO-1, but not SUMO-2, whereas PML I or PML IV∆C did not (Fig. 2A). As expected, ARF expression also sharply enhanced p53 SUMO-1 conjugation (Fig. 2A) (23, 24, 26). Similar results were obtained in stable HeLa SUMO-1 transfectants. In PML IV-transduced WI38 cells, when analyzing p53/SUMO-1 physical vicinity using in situ Proximity Ligation Assay (PLA), we observed a significant increase in the interactions of endogenous SUMO-1 and p53 in PML IV- but not PML IV∆C-transduced cells (Fig. 2B). In contrast, PML IV did not enhance p53/SUMO-2 interactions (Fig. S2) (31).
Fig. 2.
PML IV increases p53 SUMOylation by SUMO-1. (A) H1299 cells stably expressing His–SUMO-1 or His–SUMO-2 were transfected with the indicated expression vectors 2 d before analysis. Purification of His–SUMO-1 (Top) and His–SUMO-2 (Right) conjugates followed by p53 Western blot. Samples were normalized for p53 expression to compare SUMOylation efficiency. (B) Conjugation of endogenous p53 by SUMO-1 was assessed by PLA in WI38 cells. (Left) Quantification of p53-S1 amplified dots (mean ± SD from three independent experiments). *P < 0.05. (Right) p53–SUMO-1 red dots; PML was detected by immunofluorescence (green). (Scale bar, 5 µm.) (C) H1299-HisS1 cells were transfected with p53, p53K386R, or p53E388A, in combination with empty vector, PML IV, or PML IV∆C. Protein extracts were immunoblotted with anti-PML, p53, acetyl K382 p53, and actin antibodies. Asterisk, nonspecific band. (D) H1299-HisS1 cells were transfected with p53K382R acetylation mutant, in combination with empty vector, PML IV, or PML IV∆C. Purification of His–SUMO-1 conjugates was followed by p53 Western blot. (E) Cotransduction of PML with SUMO paralogs in WI38 cells. Protein extracts were normalized for GFP level and immunoblotted with PML, p53, p21, and GFP antibodies. (F) His–SUMO-1 or His–SUMO-2 purification was performed in stably SUMO-expressing HeLa cells, transiently transfected by HDMX and PML or ARF constructs. Shown is the immunoblotting with HDMX or PML antibodies. (G) WI38 primary cells were transduced with PML expression vectors. After 3 d, global conjugation of endogenous SUMO-1 (Left) or SUMO-2 (Right) was analyzed by Western blot. Levels of GFP and PML are shown as controls.
Fig. S2.
PML IV does not increase p53 conjugation by SUMO-2 paralog. (Left) Conjugation of endogenous p53 by SUMO-2 was indirectly assessed by PLA (p53–SUMO-2 red dots) in WI38 cells. PML was detected by immunofluorescence (green). (Scale bar, 5 µm.) (Right) Quantification of p53-S2 PLA dots. n.s., nonsignificant.
We then questioned whether PML IV-enhanced p53 SUMOylation could be responsible for the p53 stabilization observed in WI38 cells. Cotransfection of p53 and PML IV in H1299-HisS1 cells sharply enhanced p53 expression (Fig. 2C). In contrast, point mutants of the p53 SUMOylation site or of the SUMOylation consensus (p53K386R or p53E388A) were not stabilized upon PML IV expression (Fig. 2C), demonstrating that PML IV-triggered p53 stabilization requires its SUMOylation. PML IV-induced senescence was proposed to rely on enhanced acetylation of p53 on K382 (10, 17, 20). In our experimental conditions, PML IV-enhanced K382 acetylation paralleled p53 stabilization (Fig. 2C). Conversely, mutation of the K382 acetylation site did not affect p53 SUMOylation (Fig. 2D) or stabilization by PML IV.
To further assess the SUMO paralog specificity of PML IV-enhanced p53 activation, we cotransduced PML IV in combination with SUMO paralogs in WI38 cells. PML IV-dependent p53 stabilization was modestly enhanced upon SUMO-1 coexpression, leading to massive p21 induction (Fig. 2E). Strikingly, overexpression of SUMO-2 antagonized PML IV-enhanced p53 stabilization and p21 activation, unraveling a strict SUMO paralog selectivity of this process. Collectively, PML IV enhances p53 SUMO-1 conjugation, resulting in its stabilization and activation.
PML IV Enhances Global SUMOylation.
Most p53 signaling partners shuttle onto PML NBs (16, 32, 33). To examine the impact of PML IV on p53 regulators, we first investigated SUMO modification of HDMX and HDM2. In HeLa cells or H1299 cells stably expressing His–SUMO-1 or -2, ARF sharply stabilized either HDMX or HDM2 and enhanced their SUMO-1 or SUMO-2 conjugation, as expected (23, 34) (Fig. 2F and Fig. S3A). Importantly, PML IV (but not IV∆C or IV3A) selectively triggered SUMO-1 modification of HDMX but had no effect on HDM2 (Fig. 2F and Fig. S3A).
Fig. S3.
PML IV increases global SUMO-1 conjugation but retains target selectivity. (A) PML IV does not affect HDM2 SUMOylation. HDM2 was expressed in combination with empty vector (–), PML I, PML IV, PML IV∆C, or ARF in HeLa cells stably expressing his-SUMO paralogs. ARF induces HDM2 stabilization (inputs) and SUMO conjugation with the two SUMO paralogs (His purification). (B and C) Empty vector (Ctrl), PML IV, or PML IV∆C was transiently expressed in H358 cells (B) or H1299 cells stably expressing his-SUMO paralogs (C). Global SUMO conjugation was assessed using SUMO-1 or SUMO-2 antibodies.
Moreover, in several human cell lines (WI38 primary fibroblasts, H1299 and H358 cell lines), PML IV, but not PML IV∆C, enhanced global SUMOylation (Fig. 2G and Fig. S3 B and C). This was observed for both endogenous and stably overexpressed SUMO-1 and much less or not at all for SUMO-2. Thus, PML IV enhances global SUMO-1 conjugation but likely retains some target selectivity, most likely for NB partners.
PML IV Specifically Interacts with ARF.
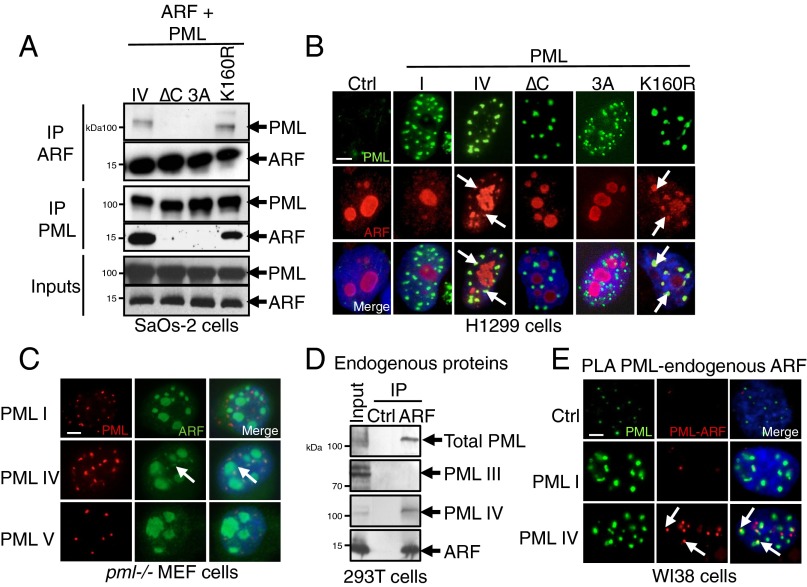
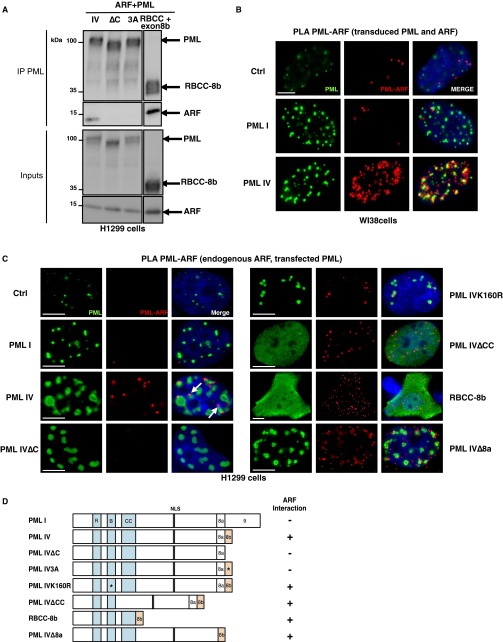
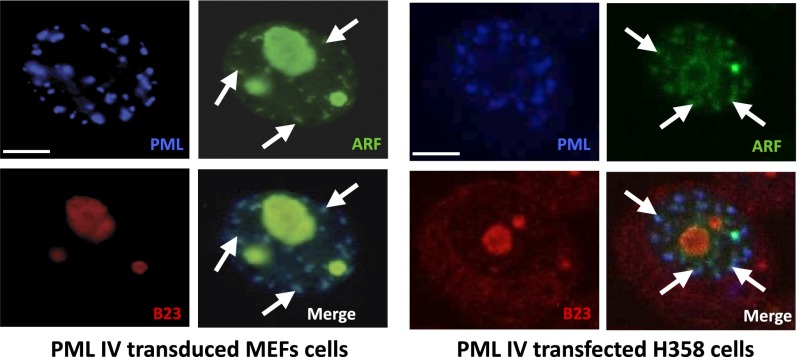
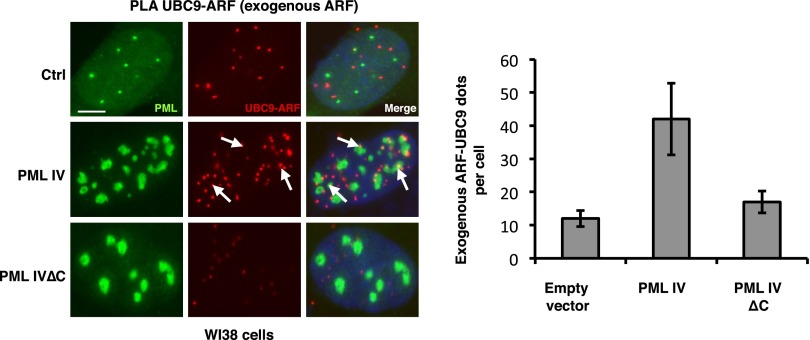
We then investigated NB’s association of partners that could facilitate SUMO-1 conjugation by PML IV. Human ARF was proposed to promote SUMOylation and to localize into NBs in virus-infected cells (35, 36). We thus investigated a possible interaction between PML IV and ARF. By immunoprecipitation, overexpressed PML IV and ARF were shown to interact (Fig. 3A). This PML–ARF coimmunoprecipitation was observed with all mutants expressing exon 8b (Fig. S4 A–D). This interaction led to a partial relocalization of ARF to PML NBs (Fig. 3B). Interestingly, PML IV K160R, a SUMOylation mutant that fails to recruit most NBs partners (4), still recruits ARF (Fig. 3 A and B). In stable PML transfectants derived from primary pml−/− mouse embryo fibroblasts (MEFs), only PML IV, but not other isoforms (PML I or V), was able to recruit ARF from the nucleolus into NBs (Fig. 3C). Localization of other nucleolar proteins like B23/NPM was unaffected by PML IV (Fig. S5). Using isoform-specific PML antibodies (6), we demonstrated endogenous PML IV/ARF interaction by coimmunoprecipitation in 293T cells (Fig. 3D). Existence of this PML IV/ARF interaction was also substantiated by a significantly increased PLA in PML IV-transduced WI38 cells (Fig. 3E), PML IV/ARF-cotransduced WI38 cells (Fig. S6B), or H1299-transfected cells (Fig. S6C). Collectively, these results demonstrate that PML IV specifically interacts with ARF through its C terminus.
Fig. 3.
PML IV specifically interacts with ARF through its C terminus. (A) ARF or PML immunoprecipitations followed by PML or ARF Western blot in SaOs-2 cells. Inputs are shown. (B) PML (green) and ARF (red) immunostaining in H1299 cells transfected as indicated. Arrows point to NBs where the two proteins colocalize. (Scale bar, 5 µm.) (C) Primary pml−/− MEFs were stably transduced by expression vectors encoding PML I, IV, or V isoforms and ARF. Cells were labeled with anti-PML antibody (red) and anti-ARF antibody (green). (Scale bar, 5 µm.) Arrows denote PML/ARF colocalization. (D) Interaction of endogenous PML IV and ARF was tested by coimmunoprecipitation in 293T cells. Control serum (Ctrl) or anti-ARF antibody was used for immunoprecipitation. PML was revealed either with pan-PML antibody or isoform-specific antibodies (6). (E) Empty vector (Ctrl), PML I, or PML IV was transduced in WI38 cells. PLA was assessed between PML and endogenous ARF (red dots), and PML was immunostained (green). (Scale bar, 5 µm.)
Fig. S4.
The PML IV/ARF interaction requires PML IV C terminus. (A) H1299 cells were transfected with ARF and wild type, mutant PML IV, and RBCC-8b. ARF and PML immunoprecipitations are followed by Western blot. (B) ARF and empty vector (Ctrl), PML I, PML IV, or PML IV∆C were transduced in WI38 cells. PLA was assessed between PML and ARF (red dots), and PML was immunostained (green). (Scale bar, 5 µm.) (C) PLA between endogenous ARF and exogenous PML constructs (PML I, PML IV, PML IV∆C, K160R, ∆CC, RBCC-8b, and ∆8a mutants) expressed in H1299 cells. (Scale bar, 5 µm.) RBCC-8b lacking the NLS sequence is localized in the nucleus and cytoplasm. (D) Schematic representation of the PML mutants explored and their ability to interact with ARF. NLS, nuclear localization signal; RBCC, RING-finger, B-boxes, coiled-coil domain.
Fig. S5.
PML IV recruits endogenous ARF and not B23/NPM nucleolar proteins. Immunofluorescence of B23/NPM (red), PML (blue), and ARF (green) in MEF and H358 cells expressing PML IV. ARF but not B23/NPM is partially relocalized into PML IV NBs. (Scale bar, 5 µm.) See Fig. 3 for details.
Fig. S6.
PML IV specifically enhances ARF–UBC9 interaction. (Left) Empty vector (Ctrl), PML IV, or PML IV∆C vectors were transduced in combination with ARF in WI38 cells. PLA was assessed between endogenous UBC9 and exogenous ARF. (Scale bar, 5 µm.) (Right) Quantification of ARF-UBC9 PLA dots. See Fig. 4 for details.
ARF Binds to and Stabilizes UBC9.
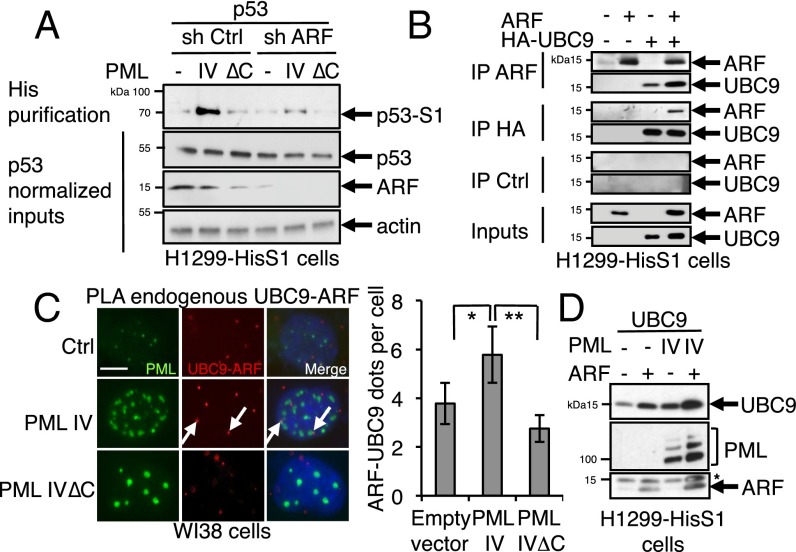
PML IV-induced p53 SUMOylation was dampened upon ARF extinction, suggesting that ARF could be implicated in PML IV-enhanced SUMOylation of p53 (Fig. 4A). Global ARF-enhanced SUMOylation was suggested to rely on its ability to interact with UBC9 (26). Indeed, UBC9 protein and ARF coimmunoprecipitated (Fig. 4B). The association between endogenous ARF and UBC9 was further demonstrated by PLA in WI38 cells (Fig. 4C) and was significantly enhanced by PML IV, but not PML IVΔC, expression (Fig. 4C and Fig. S6). Moreover, we observed a sharp stabilization of transfected UBC9 upon PML IV or ARF expression, with a synergistic impact for PML IV–ARF combination, which is suggestive for complex formation (Fig. 4D). PML binds UBC9 through its RING domain and ARF through its C terminus, which could favor formation of a tripartite complex that could explain their mutual stabilization in an environment with active proteolysis (37, 38).
Fig. 4.
PML IV/ARF interaction promotes UBC9 stabilization and p53 SUMOylation. (A) H1299-HisS1 cells were transfected with p53 and PML IV-expressing constructs in combination with control or ARF shRNA. His purification was followed by p53 Western blot. Samples were normalized for p53 expression to compare SUMOylation efficiency. (B) H1299-HisS1 cells were transfected as indicated. ARF or HA immunoprecipitation is analyzed by Western blot. IP Ctrl, immunoprecipitation with nonrelevant serum. (C) Empty vector (Ctrl), PML IV, or PML IV∆C expression vectors were transduced in WI38 cells. PLA was assessed between both endogenous UBC9 and ARF (Left), and dots per cell were quantified (means from 50 cells) (Right). *P < 0.05; **P < 0.01. (D) UBC9 protein is stabilized by ARF or PML IV in H1299-HisS1 cells. The remaining UBC9 signal is indicated (*).
NB Recruitment of Partners Is Essential for PML IV-Driven Senescence.
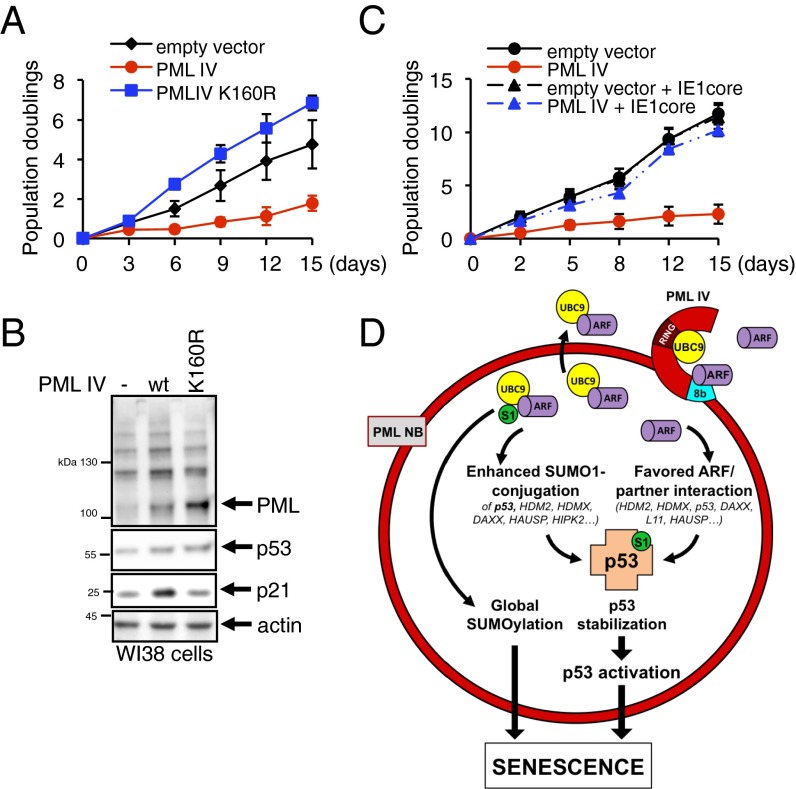
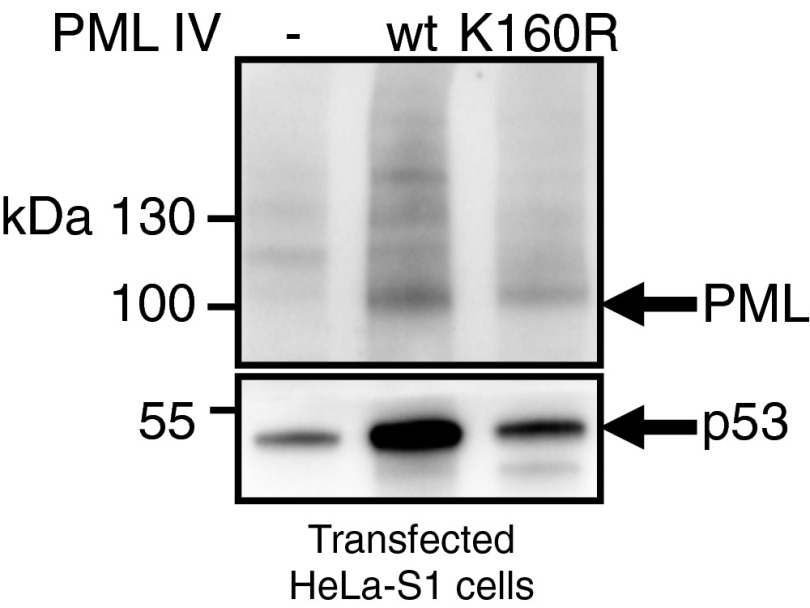
Our studies suggest that ARF recruitment within NBs enhances activity of UBC9, notably on p53 and its modulators, and drives PML IV-initiated senescence. However, a previous study argued that PML IV does not need to form NBs and/or recruit a partner to initiate senescence. A single SUMOylation site in PML, K160, is required for recruitment of partner proteins through their own SUMO-interacting motif (SIM) (4, 5). We therefore assessed whether PML IV K160R would induce senescence. Remarkably, PML IV K160R did not affect the growth of WI38 cells (Fig. 5A) or p53 activation, as shown by p21 stabilization 48 h postinfection (Fig. 5B). Absence of p53 stabilization upon expression of PML IV K160R was also observed upon transfection of HeLa-S1 (Fig. S7).
Fig. 5.
NB recruitment of partners is essential for PML IV-driven senescence. (A) Proliferation of primary human WI38 fibroblasts infected by lentiviruses expressing the indicated constructs. (B) Western blot analysis of PML, p53, p21, and actin in WI38 cell extracts 2 d after transduction. (C) Proliferation of primary human WI38 fibroblasts transduced by PML IV-expressing lentiviruses in combination with the IE1 core (39). (D) PML IV/ARF-controlled senescence model. Although UBC9 is recruited through the PML RBCC domain (5), PML IV specifically interacts with ARF, enhancing formation of an ARF/UBC9 complex within PML NBs. This leads to an increase of SUMO-1 conjugation of p53 and some of its partners. ARF enrichment into PML NBs also enhances ARF-mediated HDM2 inhibition, leading to p53 stabilization. Finally, enhanced global SUMOylation could also promote senescence. RING, RING-finger domain; S1, SUMO-1.
Fig. S7.
PML IV K160R does not stabilize p53. Empty vector (Ctrl), PML IV, or PML IV K160R vectors were transfected in HeLa-S1 cells, and the p53 level was assessed by Western blot.
We also cotransduced PML IV with CMV IE1, a protein disrupting PML NBs or its variant IE1core, which spares NB structure and only impedes partner recruitment (39). Critically, coexpression of either IE1 constructs with PML IV completely reversed growth arrest, whereas these viral proteins alone did not significantly alter cell proliferation (Fig. 5C). Therefore, PML IV-driven senescence relies on its ability to recruit partners, such as ARF and p53, into NBs (Fig. 5D).
Discussion
We unravel a specific interaction between two key senescence drivers, PML IV and ARF. This interaction likely accounts for PML IV control over p53 stability, activation, and subsequent senescence induction. These unexpected results shed a new light on the relationship between PML, ARF, or p53 and further link PML NBs to regulation of global SUMOylation.
Most studies assessing the role of PML on p53 function used PML IV, the only isoform that stabilizes p53 and induces senescence. However, to date, the molecular basis for this striking selectivity remained poorly understood. Here, we demonstrate that the C terminus of PML IV is required for ARF binding, p53 SUMOylation, stabilization, and activation. Although ARF notoriously engages in nonspecific binding when overexpressed, the specific interaction between endogenous ARF and PML IV (Fig. 3 C–E) is supportive for a physiologic relevance. When expressed in pml−/− MEFs, PML IV may be observed surrounding the nucleolus, where ARF is normally located and fusion to GFP of an exon 8b-containing peptide enforced its nucleolar localization (40). Recruitment of ARF in the p53-enriched PML NB environment can activate p53 signaling by multiple mechanisms. First, PML IV/ARF-dependent p53 SUMO-1 conjugation is critical for p53 stabilization (Fig. 2C). Note that P53 SUMO-1 conjugation was implicated in apoptotic control or senescence (11, 41) and that the unexpected SUMO-1 paralog specificity for p53 stabilization and p21 activation deserves further investigation. Second, ARF directly interacts with several NB-associated p53 regulators, such as HDM2 (Fig. 5D). Third, NB-associated p53 regulators (HIPK2, HAUSP, DAXX, CBP, HDM2, SIRT1, Chk2, MageA2, or MOZ) may all be SUMOylated. PML and/or ARF-enhanced SUMOylation could alter their functions, as proposed for HDMX (34). Note that NB-enhanced p53 signaling is not always associated with p53 stabilization (Fig. 5B) (12), suggesting that PML-enhanced PTMs may actually suffice for p53 activation and senescence induction.
As proposed for DNA damage domains, recruitment of the SUMOylation machinery into PML NBs targets a functionally defined group, rather than an individual protein (42). NBs appear as scaffolds that facilitate interactions of ARF, UBC9, and multiple p53 regulators to favor their reciprocal SUMOylation and interactions, ultimately activating p53 signaling. However, that PML IV, physiologically a minor isoform (6), fails to trigger senescence in pml−/− cells (8) stresses the importance of the other, more abundant, isoforms. These recruit p53 and HDM2 (16, 32, 33) and could enforce efficient NB recruitment of other p53 modifiers by SUMO/SIM interactions (5). A previous study had argued that PML IV-initiated senescence does not require NBs (8). Technical differences in the lentiviral expression vector used and culture protocols that result in higher PML expression levels may explain these discrepancies. NBs facilitate a number of PTMs by a scaffolding effect, concentrating enzymes and protein substrates within a restricted domain (1). With high levels of PML expression, NB formation may no longer be required for p53 activation.
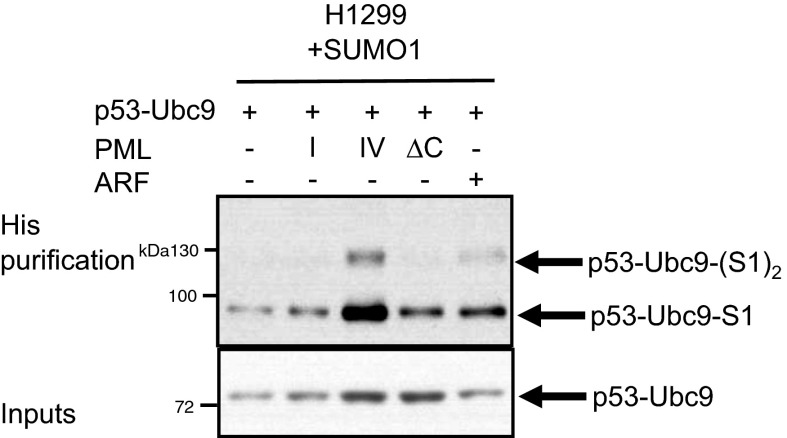
ARF tumor-suppressive activities are only partially p53-dependent and were proposed to also rely on enhanced global SUMO-1 conjugation. ARF interacts with recombinant UBC9 in vitro and favors global SUMO-1 conjugation in cell lines (26). Our results suggest that PML IV favors endogenous ARF/UBC9 interaction, at least in part by recruiting them within NBs (Fig. 4C). The ARF/UBC9 interaction stabilizes the proteins (Fig. 4 B and D) and could also enhance the intrinsic activity of UBC9. In that respect, PML IV sharply enhanced SUMO-1 conjugation of a p53–UBC9 fusion (Fig. S8). These enhanced ARF/UBC9 interactions could explain global PML IV-mediated hyper–SUMO-1 conjugation (Fig. 2G), which might contribute to PML IV-driven senescence independently of p53 activation (Fig. 5D).
Fig. S8.
PML IV specifically induces SUMO-1 conjugation of a p53–UBC9 fusion protein. The P53–UBC9 construct was transfected alone or in combination with indicated vectors in H1299-S1 cells. After 48 h, cells were harvested and the SUMO-1–conjugated p53–UBC9 level was assessed by His purification followed by Western blot.
Our findings have important implications for cancer biology, as they directly link two major senescence drivers and upstream activators of p53. These results highlight the importance of PML or ARF loss in tumors (1, 43) and that of SUMOylation in cancer progression (44, 45).
Experimental Procedures
Plasmids, Transfection, and Transduction.
ARF, PML isoforms I and IV, and PML IV mutants were expressed from the pSG5 vector. Oncogenic Ras was cloned in the pLPC vector, p53 in pCMV, HDM2 and HDMX in pcDNA3.1(+), and ARF shRNA in pSUPER (kindly provided by S. Lain, Karolinska Institutet, Stockholm). PML IV mutants (∆C, 3A, K160, ∆CC, and ∆8a) or p53 K386R and E388A mutants were obtained by site-directed mutagenesis (Quick Change Kit, Stratagene). HA-tagged UBC9 expression vector was obtained from Addgene (p3258, hCMV huUBC9-HA). PML RING-finger, B-boxes, coiled-coil domain (RBCC) fused to exon 8b (RBCC-8b) was obtained by assembly PCR. For lentiviral transduction, the pWPI expression vector containing GFP cDNA and either PML isoforms ARF or His-SUMO cDNAs was used, and the pTRIP vector was used for p53 extinction. For retroviral transduction, all of the constructs were expressed from MSCV-based retroviral vectors. Stable H1299 and HeLa cells expressing His–SUMO-1 or -2 were obtained by lentiviral transduction and GFP sorting. Transient transfections were performed with CaCl2 or Effectene Transfection Reagent (Qiagen) and WI38 nucleofection with Amaxa technology (Lonza).
Cell Culture, β-Galactosidase Staining, and BrdU Labeling.
H1299, SaOs-2, HEK293T, HeLa, and MEF cell lines were grown in 10% FCS-supplemented DMEM (GIBCO). WI38 primary cells were cultured in Eagle’s minimal essential medium (EMEM) (ATCC) supplemented with 10% FCS and essential amino acids (GIBCO). WI38 population doublings were analyzed as previously described (8). SA-β-Gal activity was detected according to the manufacturer’s instructions (Senescence β-Galactosidase Staining Kit, Cell Signaling).
Protein Purification, Immunoprecipitation, and Western Blot.
His-tagged protein purification was performed after lysis into buffer (6 M guanidium–HCl, 0.1 M NaH2PO4/Na2HPO4, 10 mM imidazole pH 8). NiNTA resin from Invitrogen was incubated for 2 h, washes were performed with decreasing amounts of guanidium–HCl, and elutions were performed in Laemmli buffer with 200 mM imidazole. For PML or ARF immunoprecipitation, cells were lysed (lysis buffer, 10 mM Tris·HCl pH 7.5, 120 mM NaCl, 1 mM EDTA, 0.5% Nonidet P-40, 1 mM PMSF, 0.1% SDS, 1 mM DTT, protease inhibitor Complete Mini Roche). Immunoprecipitation was performed using our own designed PML rabbit polyclonal antibody or ARF monoclonal antibody (Calbiochem, clone 14PO2) and protein A agarose beads (Roche). Western blots were performed using rabbit polyclonal PML isoform antibodies (6), chicken polyclonal PML antibody, p53 monoclonal DO-1 antibody (Santa Cruz, sc-126), Acetyl-p53 K382 polyclonal antibody (Cell Signaling, 2525S), ARF polyclonal antibody (Biosource, clone AHZ0472), actin monoclonal antibody (Sigma, 20–33), HDM2 monoclonal antibody (Santa Cruz, SMP14), HDMX monoclonal antibody (Bethyl, A300-287A), SUMO-1 monoclonal antibody kindly provided by M. J. Matunis, Johns Hopkins Bloomberg School of Public Health, Baltimore, SUMO-2 polyclonal antibody (Invitrogen, 51–9100), GFP polyclonal antibody (Santa Cruz, sc-8334), UBC9 polyclonal antibody (Santa Cruz, clone H-81), and anti-His Tag monoclonal antibody (Novagen, 71941). Secondary antibodies were coupled to peroxidase (Jackson Immunoresearch).
Immunostaining, PLA, and Microscopy Image Acquisition.
Prepermeabilized (0.1% Triton-X100) cells were fixed in 4% paraformaldehyde and were immunolabeled with the indicated antibodies in 0.1% Triton-X100 and 1% BSA. Primary antibodies using p53 (DO-1, Santa-Cruz, sc-126), chicken PML, ARF (Biosource, AHZ0472), and SUMO-1 polyclonal antibody (Cell Signaling, 4930) were detected by secondary antibodies (from Jackson Immuno-Research) coupled to fluorochromes [Alexa Fluor 488 or 594 or aminomethylcoumarin acetate (AMCA)] with Mowiol mounting medium. In situ PLAs were performed using the Duolink technology (Olink Bioscience). Confocal analyses were performed with a LSM510 meta-laser microscope (Carl Zeiss Micro-Imaging, Inc.) with a Plan-Apochromat 63× N.A. 1.4 oil immersion objective at 20 °C. Images were acquired with LSM510 software (Carl Zeiss).
Statistical Analyses.
Bilateral Student’s t tests were used to assess the statistical significance of observed differences. All error bars are SD.
Note Added in Proof.
ARF was similarly shown to cooperate with TRIM28, a PML family member, to promote NPM1 SUMOylation (46).
Acknowledgments
We thank T. Stamminger for the kind gift of IE1 expression vectors. We thank N. Setterblad for imaging; C. Larsen for advice; and V. Lallemand-Breitenbach, D. Ribet, C. Esnault, and M. Ogrunc for critical reading of the manuscript. The H.d.T. laboratory is supported by the Ligue Nationale contre le Cancer, INSERM, CNRS, University Paris Diderot, Institut National du Cancer, the Association pour la Recherche contre le Cancer (Prix Griffuel), European Research Council Senior Grant 268729–STEMAPL (to H.d.T.), and French National Research Agency “Investissements d’Avenir” Programs ANR-11-PHUC-002 and ANR-10-IHUB-0002. L.I. is supported by a fellowship from the Ligue Nationale contre le Cancer.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1507540112/-/DCSupplemental.
References
- 1.Lallemand-Breitenbach V, de Thé H. PML nuclear bodies. Cold Spring Harb Perspect Biol. 2010;2(5):a000661. doi: 10.1101/cshperspect.a000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Thé H, et al. The PML-RAR alpha fusion mRNA generated by the t(15;17) translocation in acute promyelocytic leukemia encodes a functionally altered RAR. Cell. 1991;66(4):675–684. doi: 10.1016/0092-8674(91)90113-d. [DOI] [PubMed] [Google Scholar]
- 3.Ishov AM, et al. PML is critical for ND10 formation and recruits the PML-interacting protein daxx to this nuclear structure when modified by SUMO-1. J Cell Biol. 1999;147(2):221–234. doi: 10.1083/jcb.147.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lallemand-Breitenbach V, et al. Role of promyelocytic leukemia (PML) sumolation in nuclear body formation, 11S proteasome recruitment, and As2O3-induced PML or PML/retinoic acid receptor alpha degradation. J Exp Med. 2001;193(12):1361–1371. doi: 10.1084/jem.193.12.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sahin U, et al. Oxidative stress-induced assembly of PML nuclear bodies controls sumoylation of partner proteins. J Cell Biol. 2014;204(6):931–945. doi: 10.1083/jcb.201305148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Condemine W, et al. Characterization of endogenous human promyelocytic leukemia isoforms. Cancer Res. 2006;66(12):6192–6198. doi: 10.1158/0008-5472.CAN-05-3792. [DOI] [PubMed] [Google Scholar]
- 7.Cuchet D, et al. PML isoforms I and II participate in PML-dependent restriction of HSV-1 replication. J Cell Sci. 2011;124(Pt 2):280–291. doi: 10.1242/jcs.075390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bischof O, et al. Deconstructing PML-induced premature senescence. EMBO J. 2002;21(13):3358–3369. doi: 10.1093/emboj/cdf341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferbeyre G, et al. PML is induced by oncogenic ras and promotes premature senescence. Genes Dev. 2000;14(16):2015–2027. [PMC free article] [PubMed] [Google Scholar]
- 10.Pearson M, et al. PML regulates p53 acetylation and premature senescence induced by oncogenic Ras. Nature. 2000;406(6792):207–210. doi: 10.1038/35018127. [DOI] [PubMed] [Google Scholar]
- 11.Marcos-Villar L, et al. SUMOylation of p53 mediates interferon activities. Cell Cycle. 2013;12(17):2809–2816. doi: 10.4161/cc.25868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ablain J, et al. Activation of a promyelocytic leukemia-tumor protein 53 axis underlies acute promyelocytic leukemia cure. Nat Med. 2014;20(2):167–174. doi: 10.1038/nm.3441. [DOI] [PubMed] [Google Scholar]
- 13.Bieging KT, Mello SS, Attardi LD. Unravelling mechanisms of p53-mediated tumour suppression. Nat Rev Cancer. 2014;14(5):359–370. doi: 10.1038/nrc3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Möller A, et al. PML is required for homeodomain-interacting protein kinase 2 (HIPK2)-mediated p53 phosphorylation and cell cycle arrest but is dispensable for the formation of HIPK domains. Cancer Res. 2003;63(15):4310–4314. [PubMed] [Google Scholar]
- 15.Kim EJ, Park JS, Um SJ. Identification of Daxx interacting with p73, one of the p53 family, and its regulation of p53 activity by competitive interaction with PML. Nucleic Acids Res. 2003;31(18):5356–5367. doi: 10.1093/nar/gkg741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei X, et al. Physical and functional interactions between PML and MDM2. J Biol Chem. 2003;278(31):29288–29297. doi: 10.1074/jbc.M212215200. [DOI] [PubMed] [Google Scholar]
- 17.Langley E, et al. Human SIR2 deacetylates p53 and antagonizes PML/p53-induced cellular senescence. EMBO J. 2002;21(10):2383–2396. doi: 10.1093/emboj/21.10.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Louria-Hayon I, et al. The promyelocytic leukemia protein protects p53 from Mdm2-mediated inhibition and degradation. J Biol Chem. 2003;278(35):33134–33141. doi: 10.1074/jbc.M301264200. [DOI] [PubMed] [Google Scholar]
- 19.Peche LY, Scolz M, Ladelfa MF, Monte M, Schneider C. MageA2 restrains cellular senescence by targeting the function of PMLIV/p53 axis at the PML-NBs. Cell Death Differ. 2012;19(6):926–936. doi: 10.1038/cdd.2011.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rokudai S, et al. MOZ increases p53 acetylation and premature senescence through its complex formation with PML. Proc Natl Acad Sci USA. 2013;110(10):3895–3900. doi: 10.1073/pnas.1300490110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin N, et al. Physical and functional interaction between PML and TBX2 in the establishment of cellular senescence. EMBO J. 2012;31(1):95–109. doi: 10.1038/emboj.2011.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weber JD, et al. p53-independent functions of the p19(ARF) tumor suppressor. Genes Dev. 2000;14(18):2358–2365. doi: 10.1101/gad.827300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xirodimas DP, Chisholm J, Desterro JM, Lane DP, Hay RT. P14ARF promotes accumulation of SUMO-1 conjugated (H)Mdm2. FEBS Lett. 2002;528(1-3):207–211. doi: 10.1016/s0014-5793(02)03310-0. [DOI] [PubMed] [Google Scholar]
- 24.Chen L, Chen J. MDM2-ARF complex regulates p53 sumoylation. Oncogene. 2003;22(34):5348–5357. doi: 10.1038/sj.onc.1206851. [DOI] [PubMed] [Google Scholar]
- 25.Tago K, Chiocca S, Sherr CJ. Sumoylation induced by the Arf tumor suppressor: A p53-independent function. Proc Natl Acad Sci USA. 2005;102(21):7689–7694. doi: 10.1073/pnas.0502978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rizos H, Woodruff S, Kefford RF. p14ARF interacts with the SUMO-conjugating enzyme Ubc9 and promotes the sumoylation of its binding partners. Cell Cycle. 2005;4(4):597–603. doi: 10.4161/cc.4.4.1597. [DOI] [PubMed] [Google Scholar]
- 27.Vivo M, et al. Downregulation of DeltaNp63alpha in keratinocytes by p14ARF-mediated SUMO-conjugation and degradation. Cell Cycle. 2009;8(21):3545–3551. doi: 10.4161/cc.8.21.9954. [DOI] [PubMed] [Google Scholar]
- 28.Choi YH, Bernardi R, Pandolfi PP, Benveniste EN. The promyelocytic leukemia protein functions as a negative regulator of IFN-gamma signaling. Proc Natl Acad Sci USA. 2006;103(49):18715–18720. doi: 10.1073/pnas.0604800103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim YE, Ahn JH. Positive role of promyelocytic leukemia protein in type I interferon response and its regulation by human cytomegalovirus. PLoS Pathog. 2015;11(3):e1004785. doi: 10.1371/journal.ppat.1004785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kahyo T, Nishida T, Yasuda H. Involvement of PIAS1 in the sumoylation of tumor suppressor p53. Mol Cell. 2001;8(3):713–718. doi: 10.1016/s1097-2765(01)00349-5. [DOI] [PubMed] [Google Scholar]
- 31.Kwek SS, Derry J, Tyner AL, Shen Z, Gudkov AV. Functional analysis and intracellular localization of p53 modified by SUMO-1. Oncogene. 2001;20(20):2587–2599. doi: 10.1038/sj.onc.1204362. [DOI] [PubMed] [Google Scholar]
- 32.Guo A, et al. The function of PML in p53-dependent apoptosis. Nat Cell Biol. 2000;2(10):730–736. doi: 10.1038/35036365. [DOI] [PubMed] [Google Scholar]
- 33.Bernardi R, et al. PML regulates p53 stability by sequestering Mdm2 to the nucleolus. Nat Cell Biol. 2004;6(7):665–672. doi: 10.1038/ncb1147. [DOI] [PubMed] [Google Scholar]
- 34.Ghosh M, Weghorst K, Berberich SJ. MdmX inhibits ARF mediated Mdm2 sumoylation. Cell Cycle. 2005;4(4):604–608. [PubMed] [Google Scholar]
- 35.Kashuba E, et al. EBV-encoded EBNA-5 associates with P14ARF in extranucleolar inclusions and prolongs the survival of P14ARF-expressing cells. Int J Cancer. 2003;105(5):644–653. doi: 10.1002/ijc.11124. [DOI] [PubMed] [Google Scholar]
- 36.Kashuba E, Mattsson K, Klein G, Szekely L. p14ARF induces the relocation of HDM2 and p53 to extranucleolar sites that are targeted by PML bodies and proteasomes. Mol Cancer. 2003;2:18. doi: 10.1186/1476-4598-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lallemand-Breitenbach V, et al. Arsenic degrades PML or PML-RARalpha through a SUMO-triggered RNF4/ubiquitin-mediated pathway. Nat Cell Biol. 2008;10(5):547–555. doi: 10.1038/ncb1717. [DOI] [PubMed] [Google Scholar]
- 38.Fang NN, et al. Rsp5/Nedd4 is the main ubiquitin ligase that targets cytosolic misfolded proteins following heat stress. Nat Cell Biol. 2014;16(12):1227–1237. doi: 10.1038/ncb3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scherer M, et al. Crystal structure of cytomegalovirus IE1 protein reveals targeting of TRIM family member PML via coiled-coil interactions. PLoS Pathog. 2014;10(11):e1004512. doi: 10.1371/journal.ppat.1004512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Condemine W, Takahashi Y, Le Bras M, de Thé H. A nucleolar targeting signal in PML-I addresses PML to nucleolar caps in stressed or senescent cells. J Cell Sci. 2007;120(Pt 18):3219–3227. doi: 10.1242/jcs.007492. [DOI] [PubMed] [Google Scholar]
- 41.Mauri F, et al. Modification of Drosophila p53 by SUMO modulates its transactivation and pro-apoptotic functions. J Biol Chem. 2008;283(30):20848–20856. doi: 10.1074/jbc.M710186200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Psakhye I, Jentsch S. Protein group modification and synergy in the SUMO pathway as exemplified in DNA repair. Cell. 2012;151(4):807–820. doi: 10.1016/j.cell.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 43.Koken MH, et al. The PML growth-suppressor has an altered expression in human oncogenesis. Oncogene. 1995;10(7):1315–1324. [PubMed] [Google Scholar]
- 44.Bischof O, Dejean A. SUMO is growing senescent. Cell Cycle. 2007;6(6):677–681. doi: 10.4161/cc.6.6.4021. [DOI] [PubMed] [Google Scholar]
- 45.Ivanschitz L, De Thé H, Le Bras M. PML, SUMOylation, and senescence. Front Oncol. 2013;3:171. doi: 10.3389/fonc.2013.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neo SH, et al. TRIM28 is an E3 ligase for ARF-mediated NPM1/B23 SUMOylation that represses centrosome amplification. Mol Cell Biol. 2015;35(16):2851–2863. doi: 10.1128/MCB.01064-14. [DOI] [PMC free article] [PubMed] [Google Scholar]