Significance
The problem of how the brain undertakes multiple tasks concurrently is one of the oldest in psychology and neuroscience. Although successful negotiation of the rich sensory world clearly requires the ongoing management of multiple tasks, humans show substantial multitasking impairments in the laboratory and everyday life. Fortunately, training facilitates multitasking. However, until now, the neural mechanisms driving this functional adaptation were not understood. Here, in a large-scale human brain imaging study, we apply an individual differences approach and pattern analysis of brain imaging data to reveal that training segregates individual task representations in the capacity limited processor that constitutes the frontoparietal–subcortical (FP-SC) network. Therefore, the brain separates the neural representations of constituent tasks to conquer multitasking.
Keywords: multitasking, cognitive training, frontoparietal–subcortical, MVPA, executive function
Abstract
Negotiating the information-rich sensory world often requires the concurrent management of multiple tasks. Despite this requirement, humans are thought to be poor at multitasking because of the processing limitations of frontoparietal and subcortical (FP-SC) brain regions. Although training is known to improve multitasking performance, it is unknown how the FP-SC system functionally changes to support improved multitasking. To address this question, we characterized the FP-SC changes that predict training outcomes using an individual differences approach. Participants (n = 100) performed single and multiple tasks in pre- and posttraining magnetic resonance imaging (fMRI) sessions interspersed by either a multitasking or an active-control training regimen. Multivoxel pattern analyses (MVPA) revealed that training induced multitasking improvements were predicted by divergence in the FP-SC blood oxygen level-dependent (BOLD) response patterns to the trained tasks. Importantly, this finding was only observed for participants who completed training on the component (single) tasks and their combination (multitask) and not for the control group. Therefore, the FP-SC system supports multitasking behavior by segregating constituent task representations.
It is thought that humans are poor at multitasking because frontoparietal and subcortical (FP-SC) brain regions both serve a broad range of mental functions (1, 2) and are limited information processors (3). Thus, performing multiple tasks concurrently exceeds the capability of the system, and performance impairments are incurred. Fortunately, these performance costs can be largely overcome with training: training improves multitasking ability (4) and typically leads to reduced activity in FP-SC brain regions (5, 6).
One explanation for these effects is that training diverts task performance away from the capacity limited FP-SC system (5, 6), toward an unmediated sensory–motor association. According to this account, referred to here as the “redistribution account,” the FP-SC system contributes minimally to trained task performance. Therefore, after training, any task representations in this system should be dissociated from behavioral performance. A less considered alternative is that training differentiates the FP-SC response between trained tasks (7), thereby reducing intertask competition between neurons that were initially recruited by both tasks (2) and expanding the capacity for concurrent task processing. According to this framework, referred to here as the “divergence account,” the separation of task representations in these regions should predict training benefits. Thus, the “redistribution” and “divergence” theories make distinct predictions regarding the relationship between FP-SC task representations and improved multitasking abilities.
We conducted a large-scale magnetic resonance imaging (MRI) study to test these opposing accounts, capitalizing on an underused information source: interindividual variability in the blood oxygen level-dependent (BOLD) signal. A key characteristic of multitasking is that large and meaningful individual differences have been observed for both the behavioral response to training (4) and the FP response to tasks typically used to study multitasking (8). Thus, analysis of interindividual variability may reveal hitherto unknown aspects of brain function that predict multitasking improvements. To ensure sufficient statistical power for the analysis of interindividual variability, sample sizes much larger than those typically used for fMRI studies (∼N = 16–32) (9) are required. To achieve 80% statistical power (10) to detect medium sized correlations between behavior and the BOLD signal within each group (r = 0.4), we recruited a total of 100 participants (training group, n = 50; control group, n = 50).
Because each voxel potentially captures the activity of over a million neurons (11), the spatial resolution obtained by averaging BOLD activity across voxels is insufficient to assess task representations within brain regions. To examine how training alters task representations in FP-SC areas, we instead applied multivoxel pattern analysis (MVPA). This method uses a classification algorithm to decode the degree to which patterns of brain activity measured across voxel ensembles in a brain region carry task specific information, given that each voxel contains a nonuniform distribution of neural selectivity (12). Higher decoding accuracies reflect increased levels of task-relevant information being represented within a given brain area. Therefore, changes in task decoding accuracies from pre- to posttraining can provide insights into task representation changes in the FP-SC system. To preview the results, we observed that multitasking improvements were predicted by decoding accuracy increases in the FP-SC response to the constituent tasks. In support of the divergence account, this demonstrates that enhanced multitasking behavior is supported by the segregation of task-representations in the FP-SC system.
Results
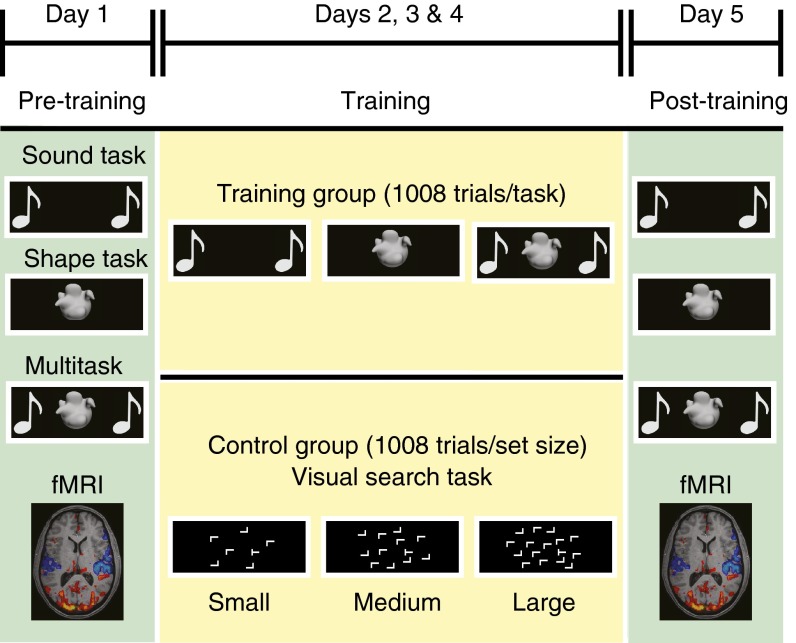
Multitasking ability and concurrent functional brain activity were assessed using a slow event-related design (Fig. 1). These measurements were taken both before and after a multisession training regimen (3,024 trials over 3 days) that focused either on the single- and multitasking paradigm (training group) or visuospatial skills (13) (visual search paradigm; control group; Fig. 1). The use of an active control design alleviated extraneous factors between groups (e.g., motivational differences) that have been prominent confounds in previous studies examining how training for multitasking influences brain function (7, 14). For the measure of multitasking ability, participants performed two simple sensorimotor tasks in isolation and together (multitask condition). Participants used the first two fingers of one hand to press a button that was paired with one of two shapes (shape task), and/or the first two fingers of the other hand to press a button associated with one of two sounds (sound task). Multitasking costs were quantified as the response time (RT) difference between single-task and multitask trials (i.e., the cost incurred as a consequence of performing both tasks concurrently). Improvements in multitasking ability were quantified as the reduction of multitasking costs between pre- and posttraining sessions.
Fig. 1.
Training protocol and tasks. Overview of the study protocols for the training and control groups. Familiarization sessions took place on the Friday afternoon preceding the week of participation. Pretraining and posttraining MRI sessions (in green) were held on Mondays and Fridays at the same time for each participant. Measurements of multitasking behavior and concurrent brain activity were recorded using a slow event-related design. In the intervening days, three training sessions lasting ∼45–60 min were completed. Participants trained either on the multitasking paradigm (training group) or on a visual-search task (control group).
Multitasking Behavior and Training-Induced Improvements.
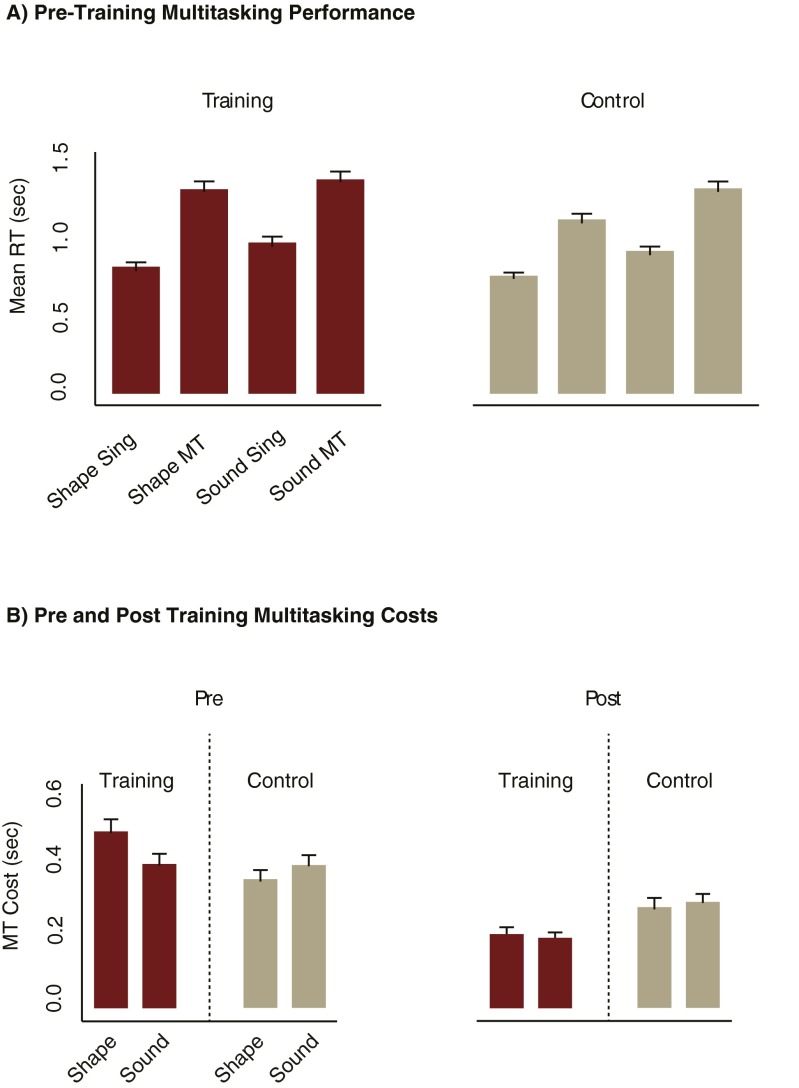
At pretraining, both groups showed large multitasking RT costs [main effect of single-task vs. multitask; F(1, 98) = 688.74, mean square error (MSE) = 0.026, P < 0.001, = 0.88; Fig. S1A]. Importantly, these were reduced more for the training group than for the control group at the posttraining session [session (pre vs. post) × condition (single-task vs. multitask) × group (training vs. control) interaction; F(1, 98) = 31.12, MSE = 0.01, P < 0.001, = 0.24; Fig. S1B], even though both groups showed large improvements in performing their respective training tasks (Fig. S2). Therefore, training on both the single- and multitasks was effective for improving multitask performance for the training group (training also improved single-task performance in this group, Fig. S2).
Fig. S1.
Pre- and posttraining performance on the multitasking paradigm. Both groups (each n = 50) showed increased RTs to the shape and sound task under multitask relative to single-task conditions during the pretraining session (A). Multitasking costs were calculated as the difference between multitask and single-task RTs and were comparable between the training and control groups at the pretraining session and were reduced more for the training group at the posttraining session (B). Importantly, this pattern of results was also found when the shape and sound tasks were examined individually; therefore multitasking performance was improved in the training group when performing both of the component tasks. MT, multitask; s, seconds; sing, single-task. Error bars reflect the SEM.
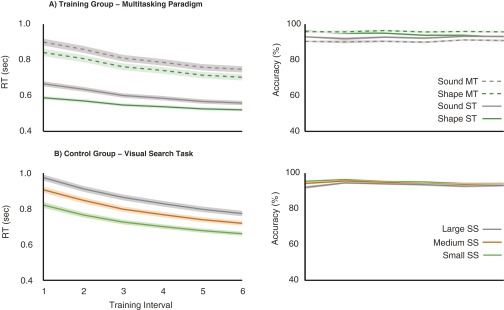
Fig. S2.
Improvement on respective training tasks. Both groups (each n = 50) showed high accuracy and a training-related reduction in RTs whether training on the multitasking paradigm (A) or the visual search task (B) (each training interval represents 504 trials for each condition). MT, multitask; s, seconds; SS, set size; ST, single-task. Shaded areas represent the SEM.
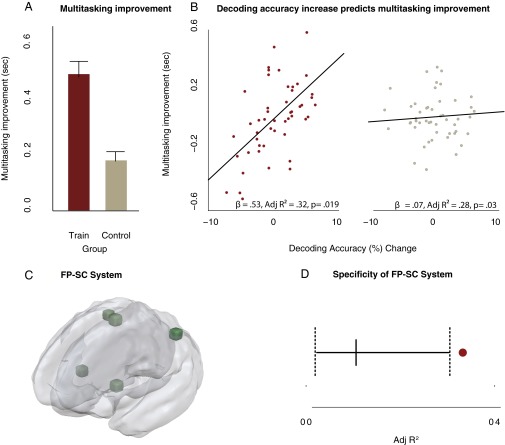
We sought to capture these training-induced multitasking benefits as a single measure that could be correlated with functional changes in the FP-SC system. Therefore, we summed multitasking costs across the shape and sound tasks for both the pre- and posttraining sessions and took the difference between the two sessions [(pretraining shape RT cost + pretraining sound RT cost) − (posttraining shape RT cost + posttraining sound RT cost); Fig. 2A]. Positive values reflect greater multitasking improvements.
Fig. 2.
Increased decoding accuracy predicts improvements in multitasking. (A) Behavioral multitasking improvements were larger for the training group than for the control group (training group, n = 50; control group, n = 50), and these improvements were correlated with decoding accuracy changes (posttraining accuracy − pretraining accuracy) in the training group. (B) Partial regression plots, between decoding accuracy changes and multitasking improvements after controlling for the influence of single-task response times and head motion. Decoding accuracy predicted multitasking improvements in the training group and not the control group. To ensure this predictive relationship was specific to the FP-SC system (C), a Monte Carlo analysis was conducted; five regions were randomly sampled from the ROIs initially demonstrated as being sensitive to multitasking demands (Fig. 2 and Table S1). The resulting average decoding change was substituted into the multiple regression model. This was repeated over 1,000 iterations. (D) The adjusted R2 observed for the model when the FP-SC system decoding changes were included as a regressor was significantly higher than the adjusted R2 values that were obtained from the Monte Carlo analysis (solid vertical line represents the mean adjusted R2 from the Monte Carlo analysis, and dotted lines reflect the upper and lower bounds of the 95% confidence interval). The maroon dot represents the adjusted R2 value of the multiple regression model that includes decoding changes from the FP-SC regions). s, seconds. Error bars represent SEM.
FP-SC Regions Influenced by Training.
Next, we identified the FP-SC regions of interest (ROIs) for which training-induced functional changes would be characterized. To be included, a given region had to meet the following criteria after adjustment for false discovery rate (15) (q < 0.05); first, to find regions that support multiple mental functions, we identified FP-SC areas that showed increased activity in response to both the shape and the sound single-tasks relative to baseline activity (conjunction analysis) for all 100 participants in the pretraining session. This analysis implicated a wide range of frontal, parietal, and subcortical regions (Table S1). The same analysis on the posttraining data identified the same ROIs. The subtraction of the summed single-task activity from multitask trial activity did not identify any further ROIs when using the pretraining or posttraining data. Therefore, our subsequent results are not dependent on either the session or the contrast used to define them.
Table S1.
Talaraich coordinates for brain regions identified by the regions of interest analysis
| Region | TAL, x, y, z | Training, N | Control, N |
| Caudal ACC | −2, 18, 35 | 49 | 47 |
| Dorsal ACC | −1, 2, 45 | 49 | 50 |
| L. DLPFC | −33, 39, 28 | 37 | 36 |
| R. DLPFC | 29, 42, 29 | 37 | 43 |
| L. Premotor | −30, −12, 53 | 43 | 47 |
| R. Premotor | 29, −11, 53 | 42 | 43 |
| L. pLPFC | −48, 8, 29 | 39 | 40 |
| R. pLPFC | 43, 10, 30 | 42 | 42 |
| L. IFG | −50, 11, 1 | 47 | 46 |
| R. IFG | 51, 12, 5 | 47 | 46 |
| L. IPL | −36, −52, 41 | 47 | 50 |
| R. IPL | 36, −38, 42 | 48 | 48 |
| L. Insula | −35, 11, 10 | 47 | 50 |
| R. Insula | 35, 12, 7 | 49 | 50 |
| L. TPJ | −51, −39, 20 | 41 | 44 |
| R. TPJ | 45, −44, 12 | 44 | 37 |
| SMFC | −7, −8, 55 | 49 | 49 |
| PCC | −3, −21, 31 | 44 | 43 |
| R. SMG | 56, −39, 24 | 47 | 47 |
| L. Cerebellum | −35, −52, −28 | 48 | 47 |
| R. Cerebellum | 29, −53, −26 | 47 | 48 |
| Putamen | −24, 5, 4 | 47 | 47 |
| 19, 8, 4 | |||
| L. MFG | −44, −20, 48 | 47 | 43 |
| R. MFG | 41, −20, 47 | 37 | 40 |
| Thalamus | −14, −18, 10 | 50 | 50 |
| 9, −17, 9 |
Across all of the univariate analysis, any participants showing < mean 0.2% signal change, calculated across single-task and multitask conditions at the pretraining session, were excluded from the analysis for that ROI (Univariate MRI Analysis). ACC, anterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex; IFG, inferior frontal gyrus; MFG, medial frontal gyrus; PCC, posterior cingulate cortex; pLPFC, posterior lateral prefrontal cortex; Region: brain region; SMFC, superior medial frontal cortex; SMG, supramarginal gyrus; TAL: Talairach coordinates; TPJ, temporal parietal junction; Training, N/Control, N, number remaining in training and control groups after excluding participants showing <0.2% signal change.
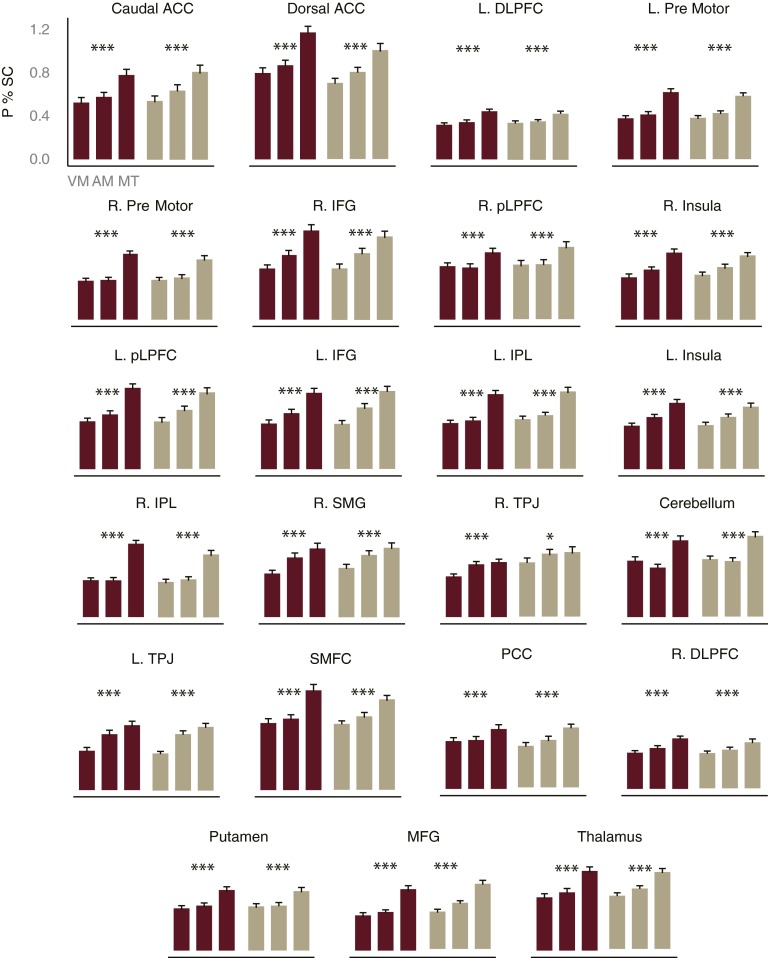
Second, we identified the FP-SC regions that displayed increased activity in response to multitasking demands at the pretraining session, by comparing extracted time course data between the multitask condition and the average of the two single-task conditions. To ensure the reliable detection of FP-SC regions that are sensitive to multitasking demands, we performed this analysis separately for the training and control groups (internal replication). All of the FP-SC regions identified by the first criterion showed reliable sensitivity to multitasking demands (Fig. S3), suggesting that regions coding various aspects of multitasking are more widespread than has previously been hypothesized (3, 16).
Fig. S3.
Peak percent signal change for multitask and single-task conditions. We first identified FP-SC brain regions that showed increased BOLD activity for the shape single-task and for the sound single-task (conjunction analysis), as would be expected of a task-general brain area. These ROIs were defined across all participants (n = 100) in the pretraining session. This analysis implicated a wide range of frontal, parietal, and subcortical regions. To examine which of these task- general ROIs were involved in multitasking, we identified the areas that showed a larger peak percent signal change for the multitask condition relative to the mean of the two single-task conditions, using extracted time course data. To ensure that evidence for any region’s multitasking involvement replicated across both groups of participants (internal replication), this analysis was performed separately for both the training (n = 50) and control (n = 50) groups at the pretraining session. All of the task-general ROIs showed evidence for involvement in multitasking, suggesting that neurons coding various aspects of the concurrent task performance are more widespread throughout the brain than has been previously hypothesized (3, 20). Error bars reflect SEM. ACC, anterior cingulate cortex; DLPFC, dorsal lateral prefrontal cortex; IFG, inferior frontal gyrus; IPL, inferior parietal lobule; L, left; MFG, medial frontal gyrus; P % SC, peak percent signal change; PCC, posterior cingulate cortex; pLPFC, posterior lateral prefrontal cortex; R, right; SMFC, superior medial prefrontal cortex; SMG, superior medial gyrus; TPJ, temporal parietal junction. Talaraich coordinates are presented in Table S1. *P < 0.02; ***P < 0.001.
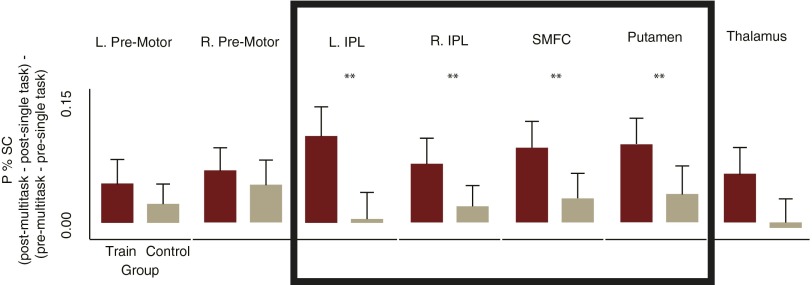
Third, to isolate FP-SC regions influenced by training, we tested for those areas that showed, within the training group, reduced differences between single-tasks and multitasks at the post- relative to the pretraining session (Fig. S4). Lastly, to rule out any regions that showed pre- to posttraining changes not specific to the influence of training on single- and multitasks, we retained only those areas that showed larger changes for the training group compared with the control group (Fig. S4, boxed region). These four criteria isolated the superior medial frontal cortex (SMFC), the left and right inferior parietal lobule (L/R IPL), and the putamen (Fig. 2C). These areas have all been previously implicated in multitasking and other tasks tapping executive function (1, 3, 17).
Fig. S4.
The FP-SC system. Of the seven brain regions that showed an influence of training [peak percentage signal change session × condition (mean single-task vs. multitask) interaction for the training group], only the regions within the boxed area [left and right inferior parietal lobules (L/R IPL), superior medial frontal cortex (SMFC); putamen] showed changes that were significantly larger for the training group (n = 50) relative to the control group (n = 50). Bar plots show the amount that the regions sensitivity to multitasking (peak percent difference between single and multitasks) reduced from pre- to posttraining [(post-multitask − post-single-task) − (pre-multitask − pre-single-task)], for each group separately. Error bars reflect SEM. IPL, inferior parietal lobule; P % SC, peak percent signal change; SMFC, superior medial frontal cortex. **P < 0.035.
The Influence of Training on Task Representations in the FP-SC System.
To test between the redistribution and divergence accounts, we examined whether training induced changes in decoding accuracy, in the FP-SC ROIs for the two single-tasks, could predict improved multitasking behavior. Because the reliability of a measure determines the degree to which variance is available to correlate with other measures (18), we first determined the reliability of the BOLD signal elicited by single-tasks by correlating pre- and posttraining measures within the control group while factoring out any variance that could be attributed to head motion. All of the BOLD measures showed statistically significant reliability (Table S2). Therefore, the measures of BOLD activity were amenable to an individual differences approach.
Table S2.
Reliability analysis of the BOLD signal across regions
| Region | Shape single-task | Sound single-task |
| Caudal ACC* | 0.74 | 0.76 |
| Dorsal ACC* | 0.74 | 0.57 |
| L. DLPFC† | 0.45 | 0.53 |
| R. DLPFC‡ | 0.55 | 0.48 |
| L. Premotor§ | 0.69 | 0.61 |
| R. Premotor§ | 0.74 | 0.69 |
| L. pLPFC | 0.48 | 0.44 |
| R. pLPFC§ | 0.67 | 0.64 |
| L. IFG* | 0.50 | 0.57 |
| R. IFG§ | 0.56 | 0.68 |
| L. IPL§ | 0.61 | 0.54 |
| R. IPL§ | 0.75 | 0.75 |
| L. Insula§ | 0.59 | 0.73 |
| R. Insula* | 0.50 | 0.50 |
| L. TPJ† | 0.49 | 0.41 |
| R. TPJ§ | 0.72 | 0.82 |
| SMFC‡ | 0.50 | 0.55 |
| PCC | 0.12 | 0.47 |
| R. SMG§ | 0.66 | 0.67 |
| L. Cerebellum* | 0.60 | 0.45 |
| R. Cerebellum* | 0.73 | 0.75 |
| Putamen (L, R)† | 0.43 | 0.34 |
| L. MFG§ | 0.65 | 0.58 |
| R. MFG§ | 0.72 | 0.75 |
| Thalamus* | 0.50 | 0.51 |
Pearson’s partial correlation r values are presented for each region; correlations were conducted between pre- and posttraining sessions for the control group (n = 50), controlling for head motion. The symbols denote the threshold for the lowest P value across the two trial types for that region. ACC, anterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex; IFG, inferior frontal gyrus; IPL, inferior parietal lobule; L, left; MFG, medial frontal gyrus; PCC, posterior cingulate cortex; pLPFC, posterior lateral prefrontal cortex; R, right; SMFC, superior medial frontal cortex; SMG, supramarginal gyrus; TPJ, temporal parietal junction. *P < 0.005; †P < 0.05; ‡P < 0.01; §P < 0.0001.
We quantified FP-SC task representation changes as the difference between post- and pretraining MVPA decoding accuracy, averaged across the key ROIs (SMFC, L/R IPL, L/R putamen). Positive values indicated increases in decoding accuracy, and therefore an increase in the separation of task representations in the FP-SC system. We then assessed whether increases in decoding accuracy predicted multitasking improvements (redistribution vs. divergence account) using a simultaneous multiple regression analysis. To control for the influence of single-task performance, we included mean RTs from the pre- and post-shape and -sound single-tasks as regressors. Furthermore, we controlled for the potentially confounding influence of head motion on decoding accuracy (19) by including the pre- and posttraining mean translation and rotation parameters as regressors in the model. Both models significantly accounted for variation in multitasking improvements (training: adjusted R2 = 0.32, P = 0.019; control, adjusted R2 = 0.28, P = 0.03). Crucial to the divergence hypothesis, that separation of task representations in the FP-SC system strongly predicted multitasking improvements: a comparison of the beta coefficients showed that increased decoding accuracies within the FP-SC system predicted multitasking improvements to a greater extent for the training group than for the control group (β = 0.53 vs. β = 0.07, z = 2.86, P = 0.004; Fig. 2B).
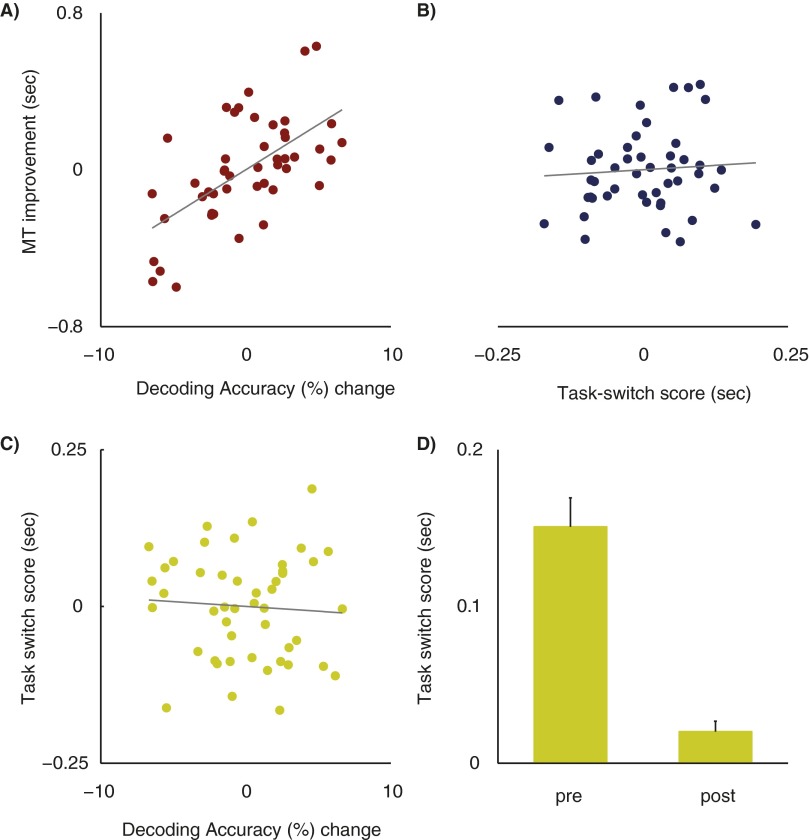
If segregation of FP-SC task representations is important for improving multitasking ability, then decoding accuracy increases should predict variance in multitasking improvement above and beyond that predicted by single-task RTs. Thus, we next tested the predictive value of decoding accuracy changes in each group using a hierarchal regression analysis, while also controlling for variance accounted for by head-movements and single-task RTs. This analysis showed that in the training group, decoding accuracy increases predicted 27.4% [change in adjusted R2 (ΔadjR2)] of the variance in multitasking improvement, which was a statistically significant increase relative to that predicted by single-task RTs and head movement measures [F(1, 32) = 14.25; P = 0.0007]. For the control group, decoding accuracy increases reduced the predicted multitasking variance by 1.73% (ΔadjR2), a value that was not statistically different from that predicted by single-task RTs and head movements [F(1, 32) = 0.2; P = 0.65]. Importantly, decoding accuracy increases predicted multitasking improvements to a greater extent for the training group compared with the control group. Both groups were directly compared in a simultaneous multiple regression analysis that also included a group interaction term. The slope coefficients were significantly different between the two groups [t(80) = 2.19; P = 0.03], showing directly that decoding accuracy increases were more predictive of training-induced improvements for the training group than for the control group. This strongly supports the divergence account: separation of task representations in the FP-SC system is a substantial predictor of improvements in multitasking ability (also see Fig. S5 for a demonstration that decoding accuracy increases uniquely predict multitasking improvements and not improved task-switching ability induced by training).
Fig. S5.
Role of task-switching in multitasking improvements for the training group. Partial regression plots showing the relationship between decoding accuracy changes and training benefits (A), task-switching scores and training benefits (B), and decoding accuracy changes and task-switching changes (C). Task-switching costs were significantly larger pretraining than posttraining (D). s, seconds. Error bars represent SEM.
Specificity of the FP-SC System.
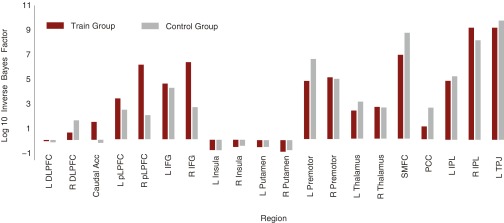
Finally, we wanted to ensure that the relationship between decoding accuracy increases and multitasking improvements were specific to the FP-SC system. Therefore, the critical test was to determine whether decoding accuracies from randomly selected ROIs could also predict multitasking improvements. We randomly sampled five brain regions previously identified as sensitive to multitasking (Fig. S3) from the training group data and evaluated the extent to which decoding accuracy increases predicted multitasking improvements, while simultaneously controlling for head motion and single-task RTs (see Fig. S6 for baseline decoding performance for all brain regions). This was repeated over 1,000 iterations. The predictive relationship between FP-SC decoding accuracy increases and multitasking improvements (adjusted R2 = 0.32; β = 0.53) was larger than any that was observed when regressing decoding changes in randomly sampled regions against multitasking improvements [mean adjusted R2 = 0.09 (95% CI, 0.01–0.29); mean β = −0.11 (95% CI, −0.36 to −0.11); Fig. 2D]. Therefore, the predictive relationship between divergence in task representations and multitasking improvement is specific to the FP-SC system.
Fig. S6.
Pretraining session MVPA results across task-general regions for the training (n = 50) and control (n = 50) groups, showing log-inverse Bayes factors assessing the ratio of evidence in favor of the alternative hypothesis (“region does code task modality”) relative to evidence in favor of the null hypothesis (“region does not code task modality”) in FP-SC regions (Materials and Methods). ACC, anterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex; IFG, inferior frontal gyrus; IPL, inferior parietal lobule; L, Left; PCC, posterior cingulate cortex; pLPFC, posterior lateral prefrontal cortex; R, right; TPJ, temporal parietal junction.
Discussion
We examined the neural mechanisms that underlie training induced enhancements in multitasking performance. In accordance with the divergence account (7), we found that the fractionation of neural response patterns to single-tasks predicted multitasking improvements, and that this relationship was specific to the FP-SC system. This challenges the long held assumption that training redistributes information away from brain systems that support multiple mental functions (5, 6). Indeed, the present results demonstrate that to meet the computational demands of multitasking, the FP-SC system employs a “divide and conquer” strategy, separating out representations of task-specific information.
These findings illustrate that a training-induced reduction in a region’s averaged BOLD signal need not be interpreted as a redistribution of information-flow away from that area, as has been assumed previously not only for multitasking (17) but also for memory and selective-attention (5, 6). Rather, we show here that a divergence of response patterns to trained tasks occurs in concert with reductions in FP-SC activity, suggesting that training refines the neural code that contributes to task performance. More specifically, response properties of individual FP-SC neurons may adapt with training, so that the response profile across neurons becomes more dissociable between trained tasks.
How exactly neural responses to constituent tasks adapt with training remains to be characterized. It may be that training reduces noise, thereby decreasing overlap in the neural response for each task. Single-unit work in monkeys has previously shown that fewer neurons respond to a repeated image in brain areas showing topographic stimulus representations (20). It may be that training causes a similar change to the neural response in brain systems consisting of distributed task representations, such as the FP-SC system. Alternately, training may specialize neurons to fire for one task over the other, analogous to the experience driven selectivity of neurons for people or objects in the medial temporal lobe (21), thereby driving spatially distinct task-representations. Regardless of the exact mechanisms that drive the underlying neural changes, the current data provide the first demonstration that task-representations can become more distinct within the FP-SC system after training, a network that has been largely characterized for its generality and contribution to multiple mental operations (1).
Materials and Methods
Participants.
Participants were recruited if they were aged 18 y or over, had normal or corrected-to-normal vision, and reported no history of psychiatric or neurological illness, injury or disorder, or the use of psychoactive medications. In total, 111 participants were recruited for the study. Of these participants, six were excluded because of excessive head motion (>5 mm/° in any translational direction or rotation), two because of a failure of the sound presentation equipment in the pretraining session, one because of responding incorrectly to the sound task across all six runs, one because of a technical error in the first training session, and one dropped out midway through participation. The remaining 100 participants were pseudorandomly allocated to the training (n = 50) or the control group (n = 50). The two groups were well matched for age (training group = 24.3 y, SD 6.2; control group = 24.6, SD 5.5), years of education (training group = 16.3 y, SD 2.4; control group = 16.8, SD 2.8), sex (training group, 15 males; control group, 11 males), and handedness (training group, three left handers; control group, four left handers).
All participants received 10 AUD per hour for participation. Participants were also able to earn bonus dollars across the three training sessions. Bonus dollars were accrued for high accuracy and for beating RT deadlines (∼20 AUD per participant). The University of Queensland Human Research Ethics Committee approved the study as being within the guidelines of the National Statement on Ethical Conduct in Human Research, and all participants gave informed, written consent.
Experimental Overview.
Participants attended six experimental sessions: a familiarization session, two MRI sessions and three behavioral training sessions. Familiarization sessions were conducted the Friday before the week of participation, where participants learned the stimulus-response mappings and completed two short runs of the task. The MRI sessions were conducted to obtain pretraining (Monday session) and posttraining (Friday session) measures. These sessions were held at the same time of day for each participant. Between the two MRI sessions, participants completed three behavioral training sessions, where they either trained upon the multitasking paradigm (training group) or the control task (control group). Participants typically completed one training session per day, although on occasion two training sessions occurred on the same day to accommodate participants’ schedules (when this occurred, the two sessions were administered with a minimum of an hours break between them). Participants also completed an online battery of questionnaires that formed part of a different study.
Behavioral Tasks.
All tasks were programmed using Matlab R2010a (Mathworks) and the Psychophysics Toolbox v3.0.9 extension (22). The familiarization and behavioral training sessions were conducted with a 21-inch, Sony Trinitron CRT monitor and a Macintosh 2.5 GHz Mini computer.
Multitasking Paradigm.
For each trial of the multitasking paradigm, participants performed either one (single-task condition) or two (multitask condition) sensorimotor tasks. Both involved a two-alternative discrimination (2-AD), mapping the two stimuli to two responses. For one task, participants were presented with one of two white shapes that were distinguishable in terms of their smooth or spikey texture, presented on a black screen and subtending ∼6° of visual angle. The shapes were created using digital sculpting software (Scluptris Alpha 6) and Photoshop CS6. Participants were required to make the appropriate manual button press to the presented shape, using either the first or index finger of either the left or right hand (task/hand assignment was counterbalanced across participants). For the other task, participants responded to one of two sounds using the first or index finger of the hand that was not assigned to the shape task. Both sounds were complex tones, selected from the set used by (16). The sounds were selected to be easily discriminable from one another. Across both the single-task and multitask trial types, stimuli were presented for 200 ms, and on multitask trials, were presented simultaneously.
Familiarization Session.
During the familiarization session, participants completed two runs of the experimental task. Task runs consisted of 18 trials, divided equally between the three trial types (shape single-task, sound single-task, and multitask trials). The order of trial-type presentation was pseudorandomized. The first run had a short intertrial-interval (ITI) and the trial structure was as follows; an alerting fixation dot, subtending 0.5° of visual angle was presented for 400 ms, followed by the stimulus/stimuli that was presented for 200 ms. Subsequently a smaller fixation dot, subtending 0.25° of visual angle, was presented for 1,800 ms, during which participants were required to respond. Participants were instructed to respond as accurately and quickly as possible to all tasks. For the familiarization session only, performance feedback was then presented until the participant hit the space bar to continue the task. For example, if the participant had completed the shape task correctly, they were presented with the message “You got the shape task right.” If they performed the task incorrectly, the message “Oh no! You got the shape task wrong” was displayed. On multitask trials, feedback was presented for both tasks. If participants failed to achieve at least five of six trials correct for each trial type, they repeated the run until this level of accuracy was attained.
The second run familiarized participants with the timing of the paradigm to be used during the MRI sessions—a slow event-related design with a long ITI. The alerting fixation was presented for 2,000 ms, followed by the 200 ms stimulus presentation, 1,800 ms response period and feedback. Subsequently, an ITI, during which the smaller fixation dot remained on screen, was presented for 12,000 ms.
MRI Sessions.
Participants completed six long ITI runs in the scanner, with 18 trials per run (6 of each trial type, pseudorandomly ordered for each run), for a total of 108 trials for the session. Trial presentation was identical to the long ITI run presented at the familiarization session, except that feedback was not presented at the end of each trial.
Training Sessions.
All participants were informed that they were participating in a study examining how training improves attention, with the intention that both the training and control groups would expect their training regimen to improve performance. The first training session began with an overview of the goals of the training regimen; participants were informed that they were required to decrease their RT, while maintaining a high level of accuracy. The second and third sessions began with visual feedback in the form of a line graph, plotting RT performance from the previous training sessions.
For each session, participants completed 56 blocks of 18 trials, for a total of 1,008 trials, resulting in 3,024 training trials overall. To ensure that participants retained familiarity with the timings of the task as presented in the scanner, between two and four of the blocks in each session used long ITI timings.
The training group performed the multitasking paradigm, as described above (see Familiarization Session), except that performance feedback was not displayed after each trial. Over the course of training, participants from this group performed 1,008 trials of each trial type (shape single-task, sound single-task, multitask). Participants in the control group went through the identical procedures to the training group, except that they completed a visual search task instead of the multitasking paradigm. Participants searched for a “T” target among 7, 11, or 15 rotated “L’s” (to either 90° or 270°). Participants indicated whether the target was oriented to 90° or 270°, using the first two fingers of their left or right hand (depending upon handedness). Over the course of the three training sessions, participants completed 1,008 trials for each set size.
For both groups, performance feedback showed mean RT (collapsed across the two single-tasks for the training group, and over the three set-sizes for the control group) and accuracy for the previous eight blocks, total points scored, and the RT target for the subsequent eight blocks. If participants met their RT target for over 90% of trials, and achieved greater than 90% accuracy, a new RT target was calculated by taking the 75th percentile of RTs recorded over the previous eight blocks. Furthermore, two points were awarded. If participants did not beat their RT target for over 90% trials but did maintain greater than 90% accuracy, one point was awarded.
MRI Data Acquisition.
Images were acquired using a 3T Siemens Trio MRI scanner housed at the Centre for Advanced Imaging at The University of Queensland. Participants lay supine in the scanner and viewed the visual display via rear projection onto a mirror mounted on a 12-channel head coil. A T1-weighted anatomic image was collected after the fourth experimental run of the scanning session [repetition time (TR), 1.9 s; echo time (TE), 2.32 ms; flip angle (FA), 9°; field of view (FOV), 192 × 230 × 256 mm; resolution, 1 mm3]. Functional T2*-weighted images were acquired parallel to the anterior commissure–posterior commissure plane using a gradient echo–echo planar imaging (GRE EPI) sequence (TR, 2 s; TE, 35 ms; FA, 79 °; FOV, 192 × 192 mm; matrix, 64 × 64; in-pane resolution, 3 × 3 mm). Each volume consisted of 29 slices (thickness, 3 mm; interslice gap, 0.5 mm), providing whole brain coverage. We synchronized the stimulus presentation with the acquisition of functional volumes. Diffusion tensor imaging (DTI) was also conducted after all T1 and T2* data had been acquired (TR, 9.5 s; TE, 116 ms; FOV, 300 × 300 mm; matrix, 128 × 128 mm; in-pane resolution, 2.3 × 2.3 × 2.5 mm; 60 slices; thickness, 3 mm; interslice gap, 0.5 mm). DTI images were collected for another study.
MRI Data Analysis.
Image analysis was performed using Brain Voyager QX 2.6 (Brain Innovation) and custom Matlab scripts (Mathworks). Data preprocessing included 3D motion correction, slice scan time correction, and linear trend removal. All functional data were aligned to the first localizer run, and anatomical T1-weighted data were transformed into standardized Talairach space (23).
Univariate MRI Analysis.
A group (n = 100) statistical parametric map (SMP) analysis was conducted for the pretraining session data. Regressors were defined for the shape single-task, sound single-task, and the multitask trials. Brain regions that were significantly activated by both the shape single- and sound single-tasks (i.e., conjointly activated by the shape single open contrast and the sound single open contrast) were identified as FP-SC regions supporting multiple mental functions.
ROIs were defined using the pretraining SPMs. Cubic ROIs were defined around the peak voxel of activated foci up to a size of 10 mm3 (37 voxels). A total of 25 ROIs were defined. We collapsed data across the left and right ROIs for medial structures (putamen and thalamus), reducing the final ROI count to 23. Following this procedure, time courses for each participant were extracted from the isolated ROIs and percent signal change was calculated relative to the two time points preceding stimulus onset for each trial. Peak activation values of a time course were defined as the maximum percent signal change occurring between 4–8 s relative to stimulus onset and were used as the dependent variable for standard parametric approaches (i.e., ANOVA and t tests). For these analyses, a FDR correction (15) of q < 0.05 was again applied to control for multiple comparisons. Because examination of the individual time courses indicated that not all participants showed activation in all of the ROIs, individuals who scored <0.2% signal change for any given ROI were excluded from analyses involving that ROI (but their data were retained for any ROIs where >0.2% signal change was observed).
Multivoxel Pattern Classification Analysis.
ROIs were centered on the Talairach coordinates for those regions that were deemed as sensitive to multitasking demands. The total number of ROIs included in the analysis was selected by iteratively comparing the coordinates of all ROIs to determine the maximum number that could be retained without spatial overlap (see Fig. S6 for the included FP-SC ROIs). Where overlap occurred, regions that had previously been demonstrated to play a role in multitask processing were selected. This resulted in a total of 20 ROIs. As is typical for decoding analyses, spherical ROIs were defined at two sizes of 11 mm per side for 1,331 mm3 and 15 mm per side for 3,375 mm3 (24). However, for the insula and putamen regions, only the 11-mm ROIs were defined as otherwise these areas overlapped with voxels of other regions. For the areas where both the 11-mm and the 15-mm ROI could be defined, results were consistent across both sizes (only the results from the larger ROI analysis are presented).
MVPA was implemented using custom Matlab (Mathworks) software and a linear support vector machine binary algorithm (25). For each voxel in a given ROI, we extracted the average peak percent signal change for each trial (4–8 s poststimulus onset). Before each MVPA, data for each voxel in an ROI were z-transformed and mean-centered by subtracting the condition mean for the entire ROI from the response in each individual voxel. This controlled for overall differences in signal amplitude between conditions. We trained a series of binary classifiers to discriminate between patterns of activity associated with the shape and sound single-tasks, using the leave-one-out cross validation method. In each fold, one run was used to test the classifiers generalization performance, and the remaining five runs were used to train the classifier. Decoding accuracy for each ROI was averaged across each cross-validation loop.
Reliability Analysis.
We assessed the reliability of BOLD measures by correlating observations between the pretraining and posttraining sessions for the control group (Table S2). This demonstrated that task-evoked BOLD activity was reliable within participants; the range of reliability scaled from r = 0.34–0.82. We also determined the reliability of the pre- and posttraining decoding accuracies (while factoring out any variance that could be attributed to head motion), and the reliability of the behavioral multitasking costs. Mean decoding accuracy for the key multitasking regions showed good reliability (r = 0.59, n = 50, P < 0.0001), as did the measure of multitasking costs (r = 0.62, n = 50, P < 0.001).
Task-Switching Analysis
Whether multitasking and task-switching overlap in terms of underlying mechanisms, or even reflect the same cognitive operation, has long been discussed (26). To assess whether a training induced task-switching improvement could account for the current results, we reasoned that if the training group is learning to efficiently task-switch, then a measure of task-switching costs should predict training benefits. Furthermore, if the decoding accuracy changes reflect changes to the neural underpinnings of task-switching, then decoding accuracy changes should predict changes to task-switching costs. As a measure of task-switching change, we calculated the RT difference between single-task trials where the preceding single-task trial was from the same condition (i.e., tn shape task, tn-1 shape task or tn sound task, tn-1 sound task) or from the other condition (tn shape task, tn-1 sound task or tn sound task, tn-1 shape task). We took the resulting task-switch costs and subtracted the posttraining cost from the pretraining cost to calculate a task-switch score for each participant (see Fig. S4D). Larger values represent a larger decrease in task-switching costs across training. (Note: we present here the task-switch costs from the scan sessions; however, using task-switch costs taken from the first and last training sessions revealed the same pattern of results.) Because only the training group practiced multitasking/task-switching, we only include data from the training group in this analysis; however, three participants were excluded from the analysis because of an absence of scores from one condition, which occurred as a consequence the randomization procedures. Therefore, we cannot directly test whether this model is statistically different to the model used to predict multitasking improvements).
To see whether task-switch scores predicted variance in training benefits over and above that already accounted for by the current data, we added task-switch scores as a regressor in a hierarchal multiple regression analysis. Task-switch scores predicted an extra 5% (ΔadjR2) of the variance in multitasking improvement; however the regression coefficient did not achieve significance (β = 0.18 (SE = 0.47), t(28) = 0.38, P = 0.71, see Fig. S4B).
We then tested whether decoding accuracy changes predicted changes in task-switching by entering task-switch scores as the dependent variable into a multiple regression model, using the same regressors as that used to predict training benefits (The Influence of Training on Task Representations in the FP-SC System). Although this model accounted for 21.6% (adjusted R2) of the variance in task-switch scores, the model itself did not achieve statistical significance [F(17, 29) = 1.75, P = 0.09, see Fig. 1C]. In contrast, when decoding accuracy changes were used to predict training benefits, 32% of the variance was accounted for (adjusted R2) and the model achieved significance (P = 0.019).
These results suggest that although changes in task-switching may predict some unique variance (∼5%) in training benefits, task-switching is distinct from the multitasking behavior changes that are associated with changes in decoding accuracy.
Acknowledgments
We thank Luke Hearne and Amy Taylor for data collection assistance and Chris Asplund, Jason Mattingley, John Serences, and Thomas Yeo for helpful comments on earlier versions of the manuscript. Neuroimaging was performed at The University of Queensland Centre for Advanced Imaging facility. This work was supported by Australian Research Council Future Fellowship FT120100033 (to P.E.D.), The University of Queensland Foundation Research Excellence Award (to P.E.D.), and the Australian Research Council–Special Research Initiative (ARC-SRI) Science of Learning Research Centre Grant SR120300015 (to P.E.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper has been deposited in UQ eSpace, espace.library.uq.edu.au (ID code 370251).
See Commentary on page 14127.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1511423112/-/DCSupplemental.
References
- 1.Duncan J. The multiple-demand (MD) system of the primate brain: Mental programs for intelligent behaviour. Trends Cogn Sci. 2010;4(4):172–179. doi: 10.1016/j.tics.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Watanabe K, Funahashi S. Neural mechanisms of dual-task interference and cognitive capacity limitation in the prefrontal cortex. Nat Neurosci. 2014;17(4):601–611. doi: 10.1038/nn.3667. [DOI] [PubMed] [Google Scholar]
- 3.Sigman M, Dehaene S. Brain mechanisms of serial and parallel processing during dual-task performance. J Neurosci. 2008;28(30):7585–7598. doi: 10.1523/JNEUROSCI.0948-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schumacher EH, et al. Virtually perfect time sharing in dual-task performance: Uncorking the central cognitive bottleneck. Psychol Sci. 2001;12(2):101–108. doi: 10.1111/1467-9280.00318. [DOI] [PubMed] [Google Scholar]
- 5.Kelly AMC, Garavan H. Human functional neuroimaging of brain changes associated with practice. Cereb Cortex. 2005;15(8):1089–1102. doi: 10.1093/cercor/bhi005. [DOI] [PubMed] [Google Scholar]
- 6.Chein JM, Schneider W. The brain’s learning and control architecture. Curr Dir Psychol Sci. 2012;21(2):78–84. [Google Scholar]
- 7.Dux PE, et al. Training improves multitasking performance by increasing the speed of information processing in human prefrontal cortex. Neuron. 2009;63(1):127–138. doi: 10.1016/j.neuron.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crittenden BM, Duncan J. Task difficulty manipulation reveals multiple demand activity but no frontal lobe hierarchy. Cereb Cortex. 2014;24(2):532–540. doi: 10.1093/cercor/bhs333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friston K. Ten ironic rules for non-statistical reviewers. Neuroimage. 2012;61(4):1300–1310. doi: 10.1016/j.neuroimage.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 10.Cohen J. Statistical Power Analysis for the Behavioural Sciences. 2nd Ed Lawrence Erlbaum Associates, Inc; Hillsdale, NJ: 1988. [Google Scholar]
- 11.Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453(7197):869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- 12.Kamitani Y, Tong F. Decoding the visual and subjective contents of the human brain. Nat Neurosci. 2005;8(5):679–685. doi: 10.1038/nn1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolfe JM, Horowitz TS. What attributes guide the deployment of visual attention and how do they do it? Nat Rev Neurosci. 2004;5(6):495–501. doi: 10.1038/nrn1411. [DOI] [PubMed] [Google Scholar]
- 14.Erickson KI, et al. Training-induced functional activation changes in dual-task processing: An FMRI study. Cereb Cortex. 2007;17(1):192–204. doi: 10.1093/cercor/bhj137. [DOI] [PubMed] [Google Scholar]
- 15.Benjamin Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Ser A Stat Soc. 1995;57(1):289–300. [Google Scholar]
- 16.Dux PE, Ivanoff J, Asplund CL, Marois R. Isolation of a central bottleneck of information processing with time-resolved FMRI. Neuron. 2006;52(6):1109–1120. doi: 10.1016/j.neuron.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erickson KI, et al. Neural correlates of dual-task performance after minimizing task-preparation. Neuroimage. 2005;28(4):967–979. doi: 10.1016/j.neuroimage.2005.06.047. [DOI] [PubMed] [Google Scholar]
- 18.Cohen J, West SG, Aiken L, Cohen P. Applied Multiple Regression/Correlation Analysis for the Behavioural Sciences. Routledge; London, UK: 2002. [Google Scholar]
- 19.Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59(1):431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller EK, Desimone R. Parallel neuronal mechanisms for short-term memory. Science. 1994;263(5146):520–522. doi: 10.1126/science.8290960. [DOI] [PubMed] [Google Scholar]
- 21.Waydo S, Kraskov A, Quian Quiroga R, Fried I, Koch C. Sparse representation in the human medial temporal lobe. J Neurosci. 2006;26(40):10232–10234. doi: 10.1523/JNEUROSCI.2101-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brainard DH. The psychophysics toolbox. Spat Vis. 1997;10(4):433–436. [PubMed] [Google Scholar]
- 23.Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. 3-Dimensional Proportional System: An Approach to Cerebral Imaging. Thieme; Stuttgart: 1988. [Google Scholar]
- 24.Spiridon M, Kanwisher N. How distributed is visual category information in human occipito-temporal cortex? An fMRI study. Neuron. 2002;35(6):1157–1165. doi: 10.1016/s0896-6273(02)00877-2. [DOI] [PubMed] [Google Scholar]
- 25.Chang CC, Lin CJ. LIBSVM: A library for support vector machines. ACM Trans Intell Syst Technol. 2011;2(3):1–27. [Google Scholar]
- 26. Pashler (2000). Task switching and multitask performance. Attention and Multitask Performance XVIII: Control of Mental Processes, eds Monsell S, Driver J (MIT Press, Cambridge, MA), 275–307.