Significance
Restoration is rapidly becoming a key conservation tool to counteract coastal degradation and enhance biodiversity and the provisioning of ecosystem services. This paper shows that the success of wetland restorations can be significantly enhanced through simple, no-cost design changes that harness positive species interactions. Although 96% of restoration organizations we surveyed plant propagules in dispersed arrangements that seek to reduce competition, our cross-continent study using clumped arrangements to harness facilitation challenges this conventional theory. Harnessing previously untapped, but naturally occurring, facilitation through small adjustments in design resulted in significantly higher yields and survivorship with no additional cost. These results highlight the value for the use of facilitation theory in restoration designs and call for integration of this theory into practice.
Keywords: shoreline defense, facilitation, coastal wetlands, wetland restoration
Abstract
Restoration has been elevated as an important strategy to reverse the decline of coastal wetlands worldwide. Current practice in restoration science emphasizes minimizing competition between out-planted propagules to maximize planting success. This paradigm persists despite the fact that foundational theory in ecology demonstrates that positive species interactions are key to organism success under high physical stress, such as recolonization of bare substrate. As evidence of how entrenched this restoration paradigm is, our survey of 25 restoration organizations in 14 states in the United States revealed that >95% of these agencies assume minimizing negative interactions (i.e., competition) between outplants will maximize propagule growth. Restoration experiments in both Western and Eastern Atlantic salt marshes demonstrate, however, that a simple change in planting configuration (placing propagules next to, rather than at a distance from, each other) results in harnessing facilitation and increased yields by 107% on average. Thus, small adjustments in restoration design may catalyze untapped positive species interactions, resulting in significantly higher restoration success with no added cost. As positive interactions between organisms commonly occur in coastal ecosystems (especially in more physically stressful areas like uncolonized substrate) and conservation resources are limited, transformation of the coastal restoration paradigm to incorporate facilitation theory may enhance conservation efforts, shoreline defense, and provisioning of ecosystem services such as fisheries production.
Degradation of coastal ecosystems is occurring worldwide (1). Human-generated threats such as overharvesting, eutrophication, climate change, habitat destruction, and pollution have threatened these valuable ecosystems at local, regional and global scales (2–6). As these threats have intensified and combined, substantial declines in overall habitat coverage have occurred in almost all major coastal ecosystems, including those generated by key habitat-forming foundation species. For example, oyster reefs have declined by at least ∼85% (7), coral reefs by ∼19% (8), seagrasses by ∼29% (9), North American salt marshes by ∼42% (10), and mangroves by ∼35% (1). Because these ecosystems generate some of the richest biodiversity hotspots on Earth (11, 12), and provide critical services for human populations, including storm protection (13), fisheries production (2, 14, 15), and carbon storage (16, 17), conservation resources totaling over 1 billion US dollars have been spent globally in an attempt to halt and reverse the decline of foundation species in the coastal realm (18, 19).
A number of strategies have been used to conserve coastal ecosystems, including threat reduction, marine protected areas, buffer establishment, and international treaties. Habitat restoration, although in existence for many decades, has only recently been elevated as a global strategy for stemming coastal habitat loss (20, 21). The call for increasing investment in restoration efforts has emerged with significant advances in propagule rearing of habitat-forming organisms (e.g., oysters and corals; refs. 22 and 23) and with increased restoration resources allocated by governments and/or large corporations aiming, for example, to: (i) fix past landscape engineering efforts that had unintended consequences (e.g., water diversions in the Everglades and Mississippi Delta; refs. 24 and 25), (ii) provide jobs for those unemployed during economic downturns (coral and oyster reef restoration; ref. 23), (iii) restore ecosystems destroyed by natural disasters and stressors (e.g., mangroves after the Indian Ocean Tsunami, coral reefs following warming events; refs. 25 and 26), (iv) increase coastal defense in response to increased frequency of intense storms (e.g., after Hurricane Katrina and Sandy; ref. 14), and/or (v) compensate for pollution-driven habitat degradation (e.g., salt marshes and BP-Deepwater Horizon Oil Spill). One widely adopted technique in coastal restoration is planting habitat-forming species, especially in areas where natural recolonization is a rare event or limited by poor dispersal (27).
Planting designs for restoration of coastal wetlands are influenced by forestry science, which emphasizes designs that minimize competition between out-planted propagules by planting them at a constant and dispersed distance from each other (e.g., plantation-style designs) (28, 29). As such, restoration configuration plans for seagrasses, mangroves, corals and salt marshes (Fig. 1) have focused on maintaining empty space between out-planted propagules to ostensibly reduce negative effects they could have on one another (29). We surveyed 25 restoration organizations (Table S1) across 14 states located on both coasts of the United States and found a high degree of entrenchment of this paradigm (χ2 test, χ2 = 20.17, P < 0.0001; Fig. 1G). All but one of the organizations surveyed plant propagules at restoration sites with, rather than without, spacing. This focus on planting designs that minimize the potential for negative interactions among outplants has persisted despite over two decades of ecological research showing positive interactions (e.g., mutualisms, facilitations) play a critical role in controlling the structure and function of ecological communities, especially under conditions of elevated physical stress (30–32) where neighboring plants can ameliorate physical stress for each other, including anoxic stress and wave-induced erosion stress in seagrasses, marshes, and mangroves (33–35). Indeed, a global metaanalysis of over 700 studies revealed that positive species interactions are most important for organism success and community persistence and recovery as physical stress increases (32), a scenario that mirrors intense abiotic stress conditions that are found on bare substrate after a coastal ecosystem has been degraded, which then is often targeted for restoration efforts (29, 31).
Fig. 1.
Dispersed configurations are widely used in wetland restorations globally (A–F) and in the United States (G). (A) Marsh restoration in Jamaica Bay, NY. Reprinted with permission from Don Riepe/American Littoral Society. (B) Tidal wetland restoration in Maryland. Reprinted with permission from Griff Evans/Ecological Restoration and Management, Inc. (C) Salt marsh restoration in Beaufort, NC. Reprinted with permission from Tess Malijenovsky. (D) Wetland restoration on a site previously used for agriculture in Australia (Creative Commons license 3.0 noncommercial attribution; photography by Nick Carson). (E) Mangrove restoration project in Puerto Rico. Reprinted with permission from Kyle Wicomb. (F) Seagrass planting in Little Narragansett Bay, CT. Reprinted with permission from Cornell Corporative Extension of Suffolk County's Eelgrass Restoration Program. (G) Survey of planting designs of US salt marsh restoration organizations. Twenty-five organizations from 14 states were asked to provide estimates of the minimum and maximum spacing of salt marsh grass plugs in restoration projects, and to classify their planting project designs as either clumped (spacing < 6 in) or dispersed. A list of the surveyed organizations is provided in Table S1.
Table S1.
List of the surveyed salt marsh restoration organizations in the United States
| Organization (State) | Configuration type | Min. spacing, in | Max. spacing, in |
| NC Coastal Federation - Headquarters (NC) | Dispersed | 6 | 12 |
| NC Coastal Federation - Northeast Office (NC) | Dispersed | 6 | 12 |
| The Nature Conservancy (SC) | Clumped | 1 | 2 |
| Galveston Bay Foundation (TX) | Dispersed | 12 | 36 |
| Save the Bay - Narragansett (RI) | Dispersed | 18 | 18 |
| Texas Parks and Wildlife (TX) | Dispersed | 12 | 36 |
| Marine Discovery Center (FL) | Dispersed | 48 | 48 |
| Littoral Society - Jamaica Bay (NY) | Dispersed | 24 | 24 |
| Wetlands Institute (NJ) | Dispersed | 12 | 12 |
| Northeast Florida Aquatic Preserves (FL) | Dispersed | 18 | 18 |
| Northwest Florida Aquatic Preserve (FL) | Dispersed | 12 | 12 |
| Earth Corps (WA) | Dispersed | NA | NA |
| Army Corps of Engineers (NY) | Dispersed | 18 | 18 |
| Coalition to Restore Coastal Louisiana (LA) | Dispersed | 72 | 72 |
| Save the Bay - San Francisco (CA) | Dispersed | 12 | 12 |
| NOAA Restoration Center (RI) | Dispersed | 12 | 36 |
| Tampa Bay Watch (FL) | Dispersed | 18 | 36 |
| Anchor QEA LLC (WA) | Dispersed | 24 | 24 |
| Ducks Unlimited (MA) | Dispersed | 24 | 36 |
| MA Department of Fish and Game (MA) | Dispersed | 12 | 12 |
| NJ DEP (NJ) | Dispersed | 24 | 36 |
| NYC Parks (NY) | Dispersed | 12 | 18 |
| Georgia DNR (GA) | Dispersed | 6 | 12 |
| Maryland DNR (MD) | Dispersed | 18 | 36 |
In this study, we experimentally test whether a restoration design that is focused on maximizing positive interactions among out-planted propagules instead of the current paradigm of minimizing potential competitive interactions can increase a project’s yield with no added cost or resource input. We tested our idea on mudflats on the coasts of both Florida and The Netherlands and did so for the following reasons. First, in both locations, salt marshes once dominated protected shorelines but have recently experienced intense die-off due to human-induced stressors (36, 37). Second, salt marsh restoration is a primary focus of nongovernmental and governmental organizations in both locations, so experiments can inform local efforts for coastal conservation. Finally, we wanted to examine if results are general across two systems that contrast in abiotic and biotic variables, including temperature, flooding, and dominant marsh grass species. At both sites, we tested whether salt marsh propagules planted in clumped vs. dispersed configurations experienced higher growth and expansion rates and how those responses varied across marsh elevations. Although theoretical papers have called for the inclusion of positive interactions into restoration designs (27–29) and many studies have shown that increased propagule size or density can increase recovery and/or restoration success (38, 39), few restoration projects or studies to date have tested impacts of designs that harness positive interactions on restoration success while holding the overall number of propagules constant [i.e., conservation resources are constant; but see O’Brien and Zedler (40) for a study that assessed impacts of propagule spacing, with closest spacing being 10 cm]. We accomplished this by arranging the same number (i.e., 9) of out-planted propagules in either a dispersed or clumped (i.e., no space between outplants) configuration in the same plot area (1.5 × 1.5 m; Fig. 2) and comparing plot-level plant performance between these two different configurations.
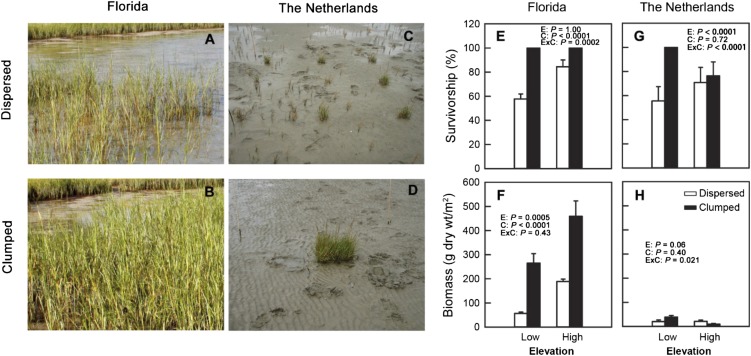
Fig. 2.
Impacts of elevation and configuration on marsh-plant plug survival and biomass in Florida and The Netherlands. (A–D) Representative photos of dispersed and clumped Spartina plantings in low elevation plots. The pictures were taken about one growing season after initial planting. (E–H) Marsh-plant plug survival and biomass in each treatment. Error bars represent SE (Florida n = 5, The Netherlands n = 8). Test results for main (E, elevation; C, configuration) and interactive (ExC) effects are shown with P values.
Our idea that restoration propagules planted in clumps will mutually benefit each other is based on the fact that positive interactions in marsh plants can occur due to alleviation of physical stress by immediately neighboring plants, such as anoxia and erosion. Firstly, plants in coastal wetlands shunt oxygen to their roots to reduce anoxia stress in sediments caused by waterlogging (33–35). When plants are in close proximity to one another, there is a shared group benefit as oxygen “leaks” from shallow roots into sediments that then becomes available to neighboring plants (41, 42). Secondly, grasses planted closely together can mitigate erosion stress generated by waves or high currents (43, 44). This facilitative interaction occurs because belowground plant material on the edge of marsh culms or established marshes absorbs most of the wave and/or current stress and thus reduces erosion around more interior marsh plants (43, 44).
Results
We found that clumped configuration positively affected both survival and growth parameters of out-planted marsh propagules (i.e., biomass, stem density, and expansion), particularly at low elevations where physical stress was higher. In Florida, survivorship of transplanted plugs was significantly affected by configuration (z = −6.76, P < 0.0001) and its interaction with elevation (z = −3.78, P = 0.0002), but not by elevation alone (z = 0.00, P = 1.00) (Fig. 2E). At low elevations, average survivorship in dispersed treatments was 58%, and increased to 100% in clumped treatments, whereas at high elevations average survivorship in dispersed treatments was 84%, and increased to 100% in clumped treatments. In The Netherlands, survivorship of transplanted plugs was significantly affected by elevation (z = 6.65, P < 0.0001) and its interaction with configuration (z = −4.54, P < 0.0001), but not by configuration alone (z = −3.78, P = 0.72) (Fig. 2G). At low elevations, average survivorship in dispersed treatments was 56%, and increased to 100% in clumped treatments, whereas at high elevations clumping had little effect.
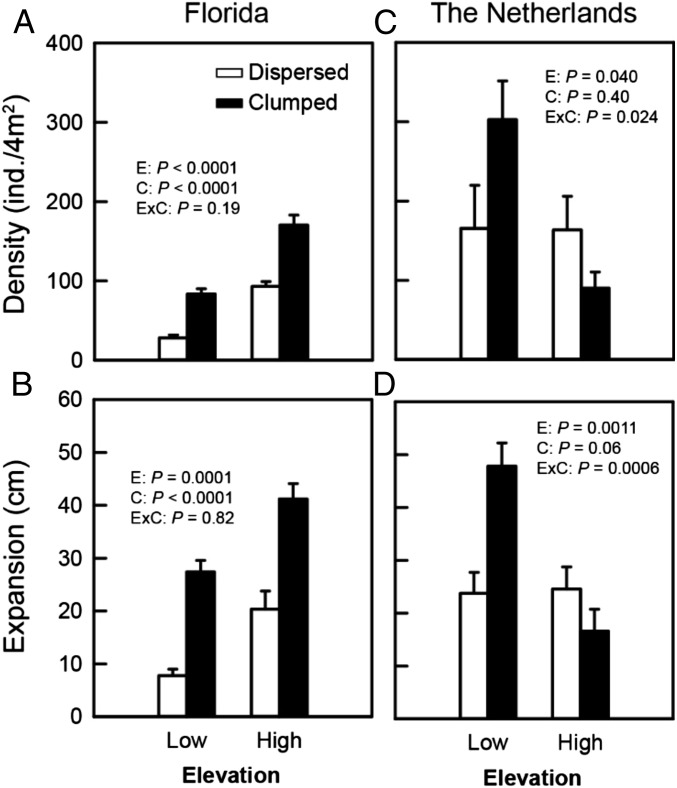
In Florida, plot-averaged aboveground biomass (Fig. 2F) and stem density (including all stems in original plantings and emergent runners and thus a measurement of clump expansion in the 2 × 2 m plot; Fig. 3A), and maximal runner length (reflecting dispersal potential; Fig. 3B) were all lower at low vs. high elevations, and were enhanced in the clumped compared with the dispersed configuration, although no significant interactions between the two factors were detected (P > 0.19 in all cases). Stem density was 109% higher (F1,16 = 91.1, P < 0.0001), biomass 196% higher (F1,16 = 18.6, P = 0.0005), and maximal runner length 43% higher (F1,16 = 26.2, P = 0.0001) at high relative to low elevations. Clumping increased stem density by 80% (F1,16 = 69.1, P < 0.0001), aboveground biomass by nearly 200% (F1,16 = 40.1, P < 0.0001) and maximal runner length by 143% (F1,16 = 61.4, P < 0.0001). In The Netherlands (Figs. 2G and 3 C and D), however, we found a significant interaction between elevation and configuration for all three variables (F1,28 = 6.0, P = 0.021 for biomass; F1,28 = 5.7, P = 0.024 for stem density; and F1,28 = 14.8, P = 0.0006 for maximal runner length). At low elevations, clumping increased biomass by 94%, stem density by 83%, and maximal runner length by 102%; but, at high elevations, clumping decreased biomass by 52%, stem density by 45%, and expansion by 32%.
Fig. 3.
Impacts of elevation and configuration on marsh-plant plug mean total plot density (A and B) and maximum expansion (C and D) in Florida and The Netherlands. Error bars represent SE (Florida n = 5, The Netherlands n = 8). Test results for main (E, elevation; C, configuration) and interactive (ExC) effects are shown with P values.
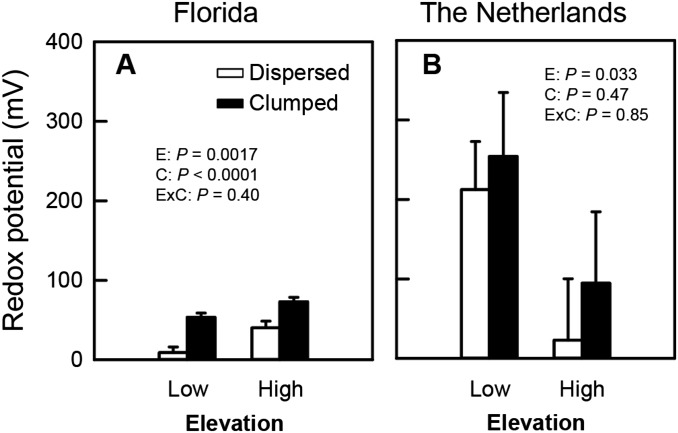
Measurements of physical stresses helped explain the possible mechanisms underlying the impacts of clumped vs. dispersed configurations on plant performance. In Florida, soil redox was significantly affected by elevation (F1,16 = 14.2, P = 0.0017) and configuration (F1,16 = 32.8, P < 0.0001), but not by their interaction (F1,16 = 0.74, P = 0.40) (Fig. S1). Soil redox potential was 81% higher at high than at low elevations, and clumping increased soil redox potential by >150%. In The Netherlands, soil redox potential was affected only by elevation (F1,28 = 5.0, P = 0.033), and it was higher at low compared with high elevations (Fig. S1). Edge erosion stress was significantly affected by configuration (F1,28 = 4.38, P = 0.046), but not by elevation (F1,28 = 1.76, P = 0.20) or the interaction between elevation and configuration (F1,28 = 1.47, P = 0.24). On average across both elevations in The Netherlands, clumping reduced erosion on the edge of individual outplants by ∼53% (Fig. S2). Although there was not a significant interaction between elevation and configuration, there was a nonsignificant trend of increased benefits of clumping at low elevations, where clumping reduced erosion on the edge of individual outplants by ∼70%, whereas at high elevations it reduced edge erosion by ∼25%.
Fig. S1.
Impacts of elevation and configuration on mean redox potential in Florida (A) and The Netherlands (B). Probability values from two-way ANOVAs testing for main (E, elevation; C, configuration) and interactive (ExC) effects are shown. Error bars represent SE (Florida n = 5, The Netherlands n = 8).
Fig. S2.
Impacts of elevation and configuration on mean edge erosion in The Netherlands. Probability values from two-way ANOVAs testing for main (E, elevation; C, configuration) and interactive (ExC) effects are shown. Error bars represent SE (n = 8).
Discussion
Results from our cross-continent, comparative experiment reveal significant increases in restoration yields (density and aboveground biomass) with simple design changes that harness previously untapped positive interactions. This effect of positive interactions on restoration success was evident for multiple measures of the performance of transplanted grasses, from survival, to plot-averaged density and biomass, to maximal runner length, and was observed in both Florida and The Netherlands. Combined with our survey results (Fig. 1) showing the current paradigm in coastal wetland restoration emphasizes minimizing competition between out-planted propagules, the findings of this paper question the widespread use of this dogma and call for incorporation of facilitation into both coastal restoration theory and design.
That clumped configuration increases success of transplanted grasses in our Florida site is likely due in part due to alleviation of anoxia stress by closely planted propagules. In low-elevation areas, anoxia stress was reduced significantly in clumped treatments, ostensibly because closely planted Spartina plugs shared oxygen shunted and leaked through roots. The idea that closely placed outplants, by sharing oxygen in the root layer, facilitate rather than suppress each other agrees with the general theory and empirical data that illustrate that plant survival and growth in low marsh zones are often limited by anoxia stress, and when in close proximity marsh plants can decrease anoxia stress by passively diffusing oxygen into the substrate that can be used by neighbors (41, 45). We also observed evidence for this mechanism in the high elevation areas of our Florida site. Although anoxia stress at higher elevations is generally thought to play a lesser role in limiting plant performance (46), our high-elevation site in Florida was located just above the high marsh–low marsh border, flooded frequently, and had significant anoxia stress compared with the low marsh (Fig. S1) and thus positive interactions that ameliorate anoxia stress were likely to emerge at this elevation as well.
In The Netherlands study site, reduced edge erosion stress was likely a more important mechanism underlying the emergence of facilitation in clumped configurations. Geomorphologic studies in this area (44, 47, 48) have shown that small outplants like we used here can die from erosion stress induced by high flow environments typical of these heavily channelized estuaries (47, 48). These studies also revealed that larger-sized clumps experienced greater growth and survival, ostensibly because of reduced erosion stress relative to overall patch size (47, 48). We saw a similar pattern in our restoration study where individual transplants in clumped treatments experienced less edge erosion stress and had increased survivorship and growth. This similarity in pattern and process suggests that a primary mechanism of intraspecific facilitation in The Netherlands in clumped treatments was mitigation of edge erosion stress for individual outplants by adjacent neighbors (i.e., each outplant in clumped treatments had two or more of its edges protected by another outplant).
Although our measurements of physical stress suggest that clumping lowered anoxia stress in Florida and decreased edge erosion stress in The Netherlands, use of this configuration design at this and other restoration sites may also provide additional benefits. Clumping may reduce porewater salinity at other sites, as shading provided by neighbors can reduce evaporation and salt accumulation in the soil (30, 49), and/or increase survival in areas characterized by intense herbivory due to associational defenses (50). Hence, the mechanism underlying the positive effects of clumping during restoration are likely to be multifaceted and vary from site to site. Indeed, in The Netherlands, interactions shifted from facilitative to competitive with decreasing stress from low to high elevation. This agrees with the stress-gradient hypothesis that the importance of positive species interactions increases with increasing physical stress (30, 32). The applied implications for these findings are that clumping designs are likely to enhance restoration designs in high stress areas, such as the low intertidal for marshes, but could be counterproductive in more benign habitats, such as in the high intertidal. Therefore, it is important to take into account potential within- and across-site variations in the strength of facilitation when incorporating positive interactions into current coastal restoration designs.
The differences in the effects of clumping and the mechanisms of facilitation between Florida and The Netherlands also suggest that the extent to which clumping may enhance marsh restoration success varies across regions. In natural communities, positive interactions have been shown to be more prevalent in arid and tropical regions where drought and heat stresses are often high (32). Moreover, natural, self-organized patchiness is most prevalent in ecosystems in dry (51, 52), cold (52), or intertidal regions (52, 53), where there are significant environmental constraints on organism growth and establishment (54). We therefore suggest that restoration experiments from a broader range of ecosystems are required to further determine the regions where the effects of clumped configuration are likely to be beneficial to restoration practices (e.g., in salt marshes in arid environments, such as southern California, alleviation of salt stress may emerge as the primary mechanism underlying the positive effects of clumping).
Our experiments provide a proof of concept for using facilitation in restoration designs. As with many experimental tests of ecological theory and/or dogma, the experiments were, however, limited in size and time. Thus, although our study reveals clumping of outplants can increase restoration yields at the plot scale (2 × 2 m), it does not provide an exact practical design for larger scale applications (i.e., the site scale). Experiments further investigating the quantitative effects of clumping on restoration efforts on a larger, whole-site scale (i.e., dispersed vs. clumped treatments should be allocated to separate sites and sites then replicated) are still required to optimize practical applications. However, given that clumping, on average, doubled growth and yields in our experiments and in low elevations in Florida marshes increased it by ∼3×, we expect that a well tuned design can significantly reduce the number of outplants needed per site to still achieve whole marsh restoration over the same time period. Moreover, aggregated designs may more closely follow the observed, natural patchiness that is apparent in many emerging estuarine ecosystems, which in turn improves ecosystem resilience (55).
Although to our knowledge few previous studies have examined how positive interactions can be harnessed by configuration (and not density) in restoration efforts [but see O’Brien and Zedler (40), who tested for the effects of closely spaced, but not touching, outplants], the importance of facilitation in restoration has been increasingly recognized (28, 29, 56), in broad agreement with our study. For example, restoration of seagrass populations often relies on seagrass transplantation, and it has been found that survival of seagrasses is higher when propagules are planted in higher densities, especially where hydrodynamic stress is high (39). Restoration of oyster populations has been found to be more successful with construction of reefs with higher oyster density (57). Likewise, densely planted mangroves can achieve higher survival rates in comparison with those loosely planted, especially at sites susceptible to sea-level rise (28, 58). Although future experiments are required to test how facilitation in a clumped configuration can enhance the success of restoration of these coastal habitats, those past works together with ours suggest that harnessing positive interactions may significantly enhance those restoration efforts.
Our experimental study has important implications for coastal restoration, and calls for integration of facilitation theory (facilitation, its underlying mechanisms and factors driving its variation) into restoration practice. Current coastal restoration programs in the United States for the large part focus on reducing threats or negative species interactions, rather than enhancing positive ones (29) (Fig. 1). Our findings suggest that intentional harnessing of positive interactions can increase efficacy of conservation efforts. The yield enhancements generated from incorporating positive species interactions are not trivial, as billions of US dollars will be spent in the near future as coastal defenses shift from ones that are man-made only to ones that integrate protection generated by both natural and engineered systems (59, 60). For instance, as part of the Gulf Coast cleanup initiative following the Deep Water Horizon Oil Spill and the recent US Restoration Act, considerable effort will likely be undertaken to restore and expand degraded marshes through plantings in mudflat areas. Because restoration is rapidly becoming a key conservation tool to enhance both ecosystem services and biodiversity worldwide, we suggest that expanding the current paradigm to incorporate facilitation theory into restoration designs has real potential to enhance the scale and success of conservation investments.
Methods
Fieldwork was conducted in the Gulf of Mexico at Fort DeSoto, Tampa, Florida (N 27°36′56″, W 82°44′09″) from February to October 2009, and in Baarland, The Netherlands (N 51°24′30″, E 3°53′6″) from April to September 2011. Marshes at both sites were characterized by visibly sandy soils (i.e., more sandy than is typical for a fully mature salt marsh that has high concentrations of organic matter). Salinities in surrounding waters averaged ∼24–28 ppt. In Florida, the tidal range was ∼1.20 m, whereas in The Netherlands the range was much larger at ∼2 m.
At each site, we manipulated the configuration of marsh grass transplants (clumped vs. dispersed) in two marsh elevations (low vs. high) using a 2 × 2 factorial design. This design generated four treatment combinations: low dispersed, low clumped, high dispersed, and high clumped. Low and high elevations at both sites were determined by comparison with high and low marsh environments in nearby marshes as identified by plant species. Plots were established at elevations that were midway in the elevation range of the nearby low marsh (low elevation plots) and just above the border between the high and low marsh areas (high elevations). In Florida, the difference in elevation between elevations was ∼40 cm; in The Netherlands, the difference in elevation between elevations was ∼70 cm. Both elevations at both sites were flooded daily by the tides. Clumped treatments allowed propagules to be in contact with each other and thus interact, whereas dispersed designs represented the current restoration paradigm and did not allow interaction among transplants. Replicate 2 × 2 m plots of unvegetated mudflats were located and marked in both the high and low elevations (n = 5 per treatment in Florida; n = 8 per treatment in Baarland). Transplant plugs were 10 × 10 × 10 cm and consisted of the dominant marsh grass Spartina alterniflora in Florida, obtained from Tampa Bay Watch, and Spartina anglica in Baarland, obtained on-site. Plots in each elevation were randomly assigned as either clumped or dispersed. In clumped treatments, nine transplant plugs were planted in the middle of each plot so that all plugs were touching. In dispersed treatments, nine plugs were planted at equal distances from each other (50 cm in all directions).
Survival of each marsh transplant plug and stem density in each plot were quantified at the end of the experiment. Survivorship of plugs was assessed by noting presence or absence of each live plug. As a first measure of plot-level yield and colonization success, plot-averaged density was assessed by counting all stems present in each 2 × 2 m plot. We estimated aboveground plant biomass in each plot using stem height to stem biomass regressions combined with stem density measurements and stem height frequency distributions (61). To assess treatment impacts on expansion rates, we measured maximum lateral extension of runners from all plugs in each plot. To do so, we measured the maximum distance that any surviving transplant in a plot (either dispersed or clumped) grew clonally and recorded it as a single datum point for that plot. We also measured sediment redox potential, a proxy for soil oxygen levels (42, 62), with a soil redox probe (Orion Redox/ORP Electrode) in August following the methods of Silliman and Zieman (63). We measured redox potential at 10-cm depth in the middle of the root zone. We also measured the edge erosion stress experienced by the marsh transplants in The Netherlands, where edge erosion stress was quickly apparent in all replicates and is typically experienced by colonizing Spartina clumps in this area due to its relatively higher currents (44). This heightened erosion around the edges of establishing Spartina causes stress and, at times, death to the plants (44). To assess total area of erosive edge on the side of each transplanted plug and how that varies with our treatments, we measured the height of all escarped edges of each transplant in each plot at the beginning and end of the experiment. The difference between these measurements was the depth of the erosive edge. If an edge was erosive, we also measured the total length of the erosive edge. We then computed the total area of erosive edge for each transplant by multiplying the average depth of the edge for that plot by the total length of erosive edges. Edge erosion stress was not measured in the Florida study site, where erosive edges never formed.
The effects of configuration and elevation on each response variable were analyzed using two-way Analysis of Variance (ANOVA) in R version 3.0.2 (64). Density, biomass, and edge erosion stress measurements from The Netherlands were square root, third root, and log transformed, respectively, to satisfy the assumption of normality for ANOVAs. Beta regression analysis was applied to survivorship data in both sites, where ANOVA assumptions of normality could not be met (65).
Acknowledgments
We thank Andrew Altieri, Jim van Belzen, Tjeerd Bouma Bas Koutstaal, and Jos van Soelen for helping with the field work. Comments from two anonymous reviewers improved the manuscript. This work was supported in part by a National Science Foundation CAREER Grant (BIO-OCE; 1056980; to B.R.S.), and a visiting professorship from The Netherlands Royal Society of Arts and Sciences to B.R.S. Q.H. is the Stolarz Conservation Fellow and supported in part by the Edward Stolarz Foundation. T.v.d.H. was funded in part by Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO) Veni Grant 863.12.003. E.S. was funded by the National Science Foundation Research Fellowship Program (DGE; 1106401), the Garden Club of America Fellowship, and the Joe Ramus Fund.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1515297112/-/DCSupplemental.
References
- 1.Millennium Ecosystem Assessment . Ecosystems and Human Well-being. Island Press; Washington, DC: 2005. [Google Scholar]
- 2.Burke LM, Reytar K, Spalding M, Perry A. Reefs at Risk Revisited. World Resources Institute; Washington, DC: 2011. [Google Scholar]
- 3.Diaz RJ, Rosenberg R. Spreading dead zones and consequences for marine ecosystems. Science. 2008;321(5891):926–929. doi: 10.1126/science.1156401. [DOI] [PubMed] [Google Scholar]
- 4.Hoegh-Guldberg O, Bruno JF. The impact of climate change on the world’s marine ecosystems. Science. 2010;328(5985):1523–1528. doi: 10.1126/science.1189930. [DOI] [PubMed] [Google Scholar]
- 5.Shahidul Islam M, Tanaka M. Impacts of pollution on coastal and marine ecosystems including coastal and marine fisheries and approach for management: a review and synthesis. Mar Pollut Bull. 2004;48(7-8):624–649. doi: 10.1016/j.marpolbul.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Silliman BR, Bertness MD. Shoreline development drives invasion of Phragmites australis and the loss of plant diversity on New England salt marshes. Conserv Biol. 2004;18(5):1424–1434. [Google Scholar]
- 7.Beck MW, et al. Oyster reefs at risk and recommendations for conservation, restoration, and management. Bioscience. 2011;61(2):107–116. [Google Scholar]
- 8.Wilkinson C. Status of Coral Reefs of the World: 2008. Global Coral Reef Monitoring Network and Reef and Rainforest Research Centre; Townsville, Australia: 2008. [Google Scholar]
- 9.Waycott M, et al. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc Natl Acad Sci USA. 2009;106(30):12377–12381. doi: 10.1073/pnas.0905620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gedan KB, Silliman BR. Patterns of salt marsh loss within coastal regions of North America: Presettlement to present. In: Silliman BR, Grosholz ED, Bertness MD, editors. Human Impacts on Salt Marshes: A Global Perspective. University of California Press; Berkeley: 2009. pp. 253–266. [Google Scholar]
- 11.Roberts CM, et al. Marine biodiversity hotspots and conservation priorities for tropical reefs. Science. 2002;295(5558):1280–1284. doi: 10.1126/science.1067728. [DOI] [PubMed] [Google Scholar]
- 12.De Fontaubert AC, Downes DR, Agardy TS. Biodiversity in the Seas: Implementing the Convention on Biological Diversity in Marine and Coastal Habitats. IUCN; Gland, Switzerland: 1996. [Google Scholar]
- 13.Barbier EB, et al. The value of estuarine and coastal ecosystem services. Ecol Monogr. 2011;81(2):169–193. [Google Scholar]
- 14.Boesch DF, Turner RE. Dependence of fishery species on salt marshes: the role of food and refuge. Estuaries. 1984;7(4):460–468. [Google Scholar]
- 15.Moberg F, Folke C. Ecological goods and services of coral reef ecosystems. Ecol Econ. 1999;29(2):215–233. [Google Scholar]
- 16.Mcleod E, et al. A blueprint for blue carbon: toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front Ecol Environ. 2011;9(10):552–560. [Google Scholar]
- 17.Fourqurean JW, et al. Seagrass ecosystems as a globally significant carbon stock. Nat Geosci. 2012;5(7):505–509. [Google Scholar]
- 18.Schrack E, Beck M, Brumbaugh R, Crisley K, Hancock B. Restoration Works: Highlights from a Decade of Partnership Between The Nature Conservancy and the National Oceanic and Atmospheric Administration’s Restoration Center. Nature Conservancy; Arlington, VA: 2012. [Google Scholar]
- 19.Pendleton L. Measuring and Monitoring the Economic Effects of Habitat Restoration: A Summary of a NOAA Blue Ribbon Panel. Nicholas Institute for Environmental Policy Solutions, Duke University; Durham, NC: 2010. [Google Scholar]
- 20.Aronson J, Clewell AF, Blignaut JN, Milton SJ. Ecological restoration: A new frontier for nature conservation and economics. J Nat Conserv. 2006;14(3):135–139. [Google Scholar]
- 21.Zedler JB. Progress in wetland restoration ecology. Trends Ecol Evol. 2000;15(10):402–407. doi: 10.1016/s0169-5347(00)01959-5. [DOI] [PubMed] [Google Scholar]
- 22.Grabowski JH, et al. Restoring oyster reefs to recover ecosystem services. In: Cuddington K, Byers JE, Wilson WG, Hastings A, editors. Ecosystem Engineers: Plants to Protists. Academic Press; Waltham, MA: 2007. pp. 281–298. [Google Scholar]
- 23.Johnson ME, Lustic C, Bartels E. Caribbean Acropora Restoration Guide: Best Practices for Propagation and Population Enhancement. The Nature Conservancy; Arlington, VA: 2011. [Google Scholar]
- 24.Sklar FH, et al. The ecological-societal underpinnings of Everglades restoration. Front Ecol Environ. 2005;3(3):161–169. [Google Scholar]
- 25.Ferrario F, et al. The effectiveness of coral reefs for coastal hazard risk reduction and adaptation. Nat Commun. 2014;5:3794. doi: 10.1038/ncomms4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagelkerken I, et al. How important are mangroves and seagrass beds for coral-reef fish? The nursery hypothesis tested on an island scale. Mar Ecol Prog Ser. 2002;244:299–305. [Google Scholar]
- 27.Zedler JB, Kercher S. Wetland resources: Status, trends, ecosystem services, and restorability. Annu Rev Environ Resour. 2005;30:39–74. [Google Scholar]
- 28.Gedan KB, Silliman BR. Using facilitation theory to enhance mangrove restoration. Ambio. 2009;38(2):10–9. doi: 10.1579/0044-7447-38.2.109. [DOI] [PubMed] [Google Scholar]
- 29.Halpern BS, Silliman BR, Olden JD, Bruno JP, Bertness MD. Incorporating positive interactions in aquatic restoration and conservation. Front Ecol Environ. 2007;5(3):153–160. [Google Scholar]
- 30.Bertness MD, Callaway R. Positive interactions in communities. Trends Ecol Evol. 1994;9(5):191–193. doi: 10.1016/0169-5347(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 31.Angelini C, Altieri AH, Silliman BR, Bertness MD. Interactions among foundation species and their consequences for community organization, biodiversity, and conservation. Bioscience. 2011;61(10):782–789. [Google Scholar]
- 32.He Q, Bertness MD, Altieri AH. Global shifts towards positive species interactions with increasing environmental stress. Ecol Lett. 2013;16(5):695–706. doi: 10.1111/ele.12080. [DOI] [PubMed] [Google Scholar]
- 33.Smith RD, Dennison WC, Alberte RS. Role of seagrass photosynthesis in root aerobic processes. Plant Physiol. 1984;74(4):1055–1058. doi: 10.1104/pp.74.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gleason ML, Zieman JC. Influence of tidal inundation on internal oxygen supply of Spartina alterniflora and Spartina patens. Estuar Coast Shelf Sci. 1981;13(1):47–57. [Google Scholar]
- 35.Scholander P, Van Dam L, Scholander SI. Gas exchange in the roots of mangroves. Am J Bot. 1955;42(1):92–98. [Google Scholar]
- 36.Silliman BR, van de Koppel J, Bertness MD, Stanton LE, Mendelssohn IA. Drought, snails, and large-scale die-off of southern U.S. salt marshes. Science. 2005;310(5755):1803–1806. doi: 10.1126/science.1118229. [DOI] [PubMed] [Google Scholar]
- 37.Cox R, Wadsworth R, Thomson A. Long-term changes in salt marsh extent affected by channel deepening in a modified estuary. Cont Shelf Res. 2003;23(17):1833–1846. [Google Scholar]
- 38.Teas HJ. Ecology and restoration of mangrove shorelines in Florida. Environ Conserv. 1977;4(01):51–58. [Google Scholar]
- 39.Bos AR, Van Katwijk MM. Planting density, hydrodynamic exposure and mussel beds affect survival of transplanted intertidal eelgrass. Mar Ecol Prog Ser. 2007;336:121–129. [Google Scholar]
- 40.O’Brien EL, Zedler JB. Accelerating the restoration of vegetation in a southern California salt marsh. Wetlands Ecol Manage. 2006;14(3):269–286. [Google Scholar]
- 41.Howes BL, Dacey JWH, Goehringer DD. Factors controlling the growth form of Spartina alterniflora: feedbacks between above-ground production, sediment oxidation, nitrogen and salinity. J Ecol. 1986;74(3):881–898. [Google Scholar]
- 42.Howes BL, Howarth RW, Teal JM, Valiela I. Oxidation‐reduction potentials in a salt marsh: Spatial patterns and interactions with primary production. Limnol Oceanogr. 1981;26(2):350–360. [Google Scholar]
- 43.Silliman BR, et al. Degradation and resilience in Louisiana salt marshes after the BP-Deepwater Horizon oil spill. Proc Natl Acad Sci USA. 2012;109(28):11234–11239. doi: 10.1073/pnas.1204922109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Balke T, et al. Conditional outcome of ecosystem engineering: A case study on tussocks of the salt marsh pioneer Spartina anglica. Geomorphology. 2012;153:232–238. [Google Scholar]
- 45.Bertness MD. Zonation of Spartina patens and Spartina alterniflora in New England salt marsh. Ecology. 1991;72(1):138–148. [Google Scholar]
- 46.Pennings SC, Bertness MD. Salt marsh communities. In: Bertness MD, Gaines SD, Hay M, editors. Marine Community Ecology. Sinauer Associates; Sunderland, MA: 2001. pp. 289–316. [Google Scholar]
- 47.Bouma T, et al. Density‐dependent linkage of scale‐dependent feedbacks: A flume study on the intertidal macrophyte Spartina anglica. Oikos. 2009;118(2):260–268. [Google Scholar]
- 48.van Wesenbeeck BK, van de Koppel J, Herman PM, Bouma TJ. Does scale‐dependent feedback explain spatial complexity in salt‐marsh ecosystems? Oikos. 2008;117(1):152–159. [Google Scholar]
- 49.Bertness MD, Yeh SM. Cooperative and competitive interactions in the recruitment of marsh elders. Ecology. 1994;75(8):2416–2429. [Google Scholar]
- 50.Alberti J, Escapa M, Iribarne O, Silliman B, Bertness M. Crab herbivory regulates plant facilitative and competitive processes in Argentinean marshes. Ecology. 2008;89(1):155–164. doi: 10.1890/07-0045.1. [DOI] [PubMed] [Google Scholar]
- 51.Klausmeier CA. Regular and irregular patterns in semiarid vegetation. Science. 1999;284(5421):1826–1828. doi: 10.1126/science.284.5421.1826. [DOI] [PubMed] [Google Scholar]
- 52.Rietkerk M, Dekker SC, de Ruiter PC, van de Koppel J. Self-organized patchiness and catastrophic shifts in ecosystems. Science. 2004;305(5692):1926–1929. doi: 10.1126/science.1101867. [DOI] [PubMed] [Google Scholar]
- 53.van de Koppel J, Rietkerk M, Dankers N, Herman PM. Scale-dependent feedback and regular spatial patterns in young mussel beds. Am Nat. 2005;165(3):E66–E77. doi: 10.1086/428362. [DOI] [PubMed] [Google Scholar]
- 54.Rietkerk M, van de Koppel J. Regular pattern formation in real ecosystems. Trends Ecol Evol. 2008;23(3):169–175. doi: 10.1016/j.tree.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 55.Liu Q-X, et al. Pattern formation at multiple spatial scales drives the resilience of mussel bed ecosystems. Nat Commun. 2014;5:5234. doi: 10.1038/ncomms6234. [DOI] [PubMed] [Google Scholar]
- 56.Padilla FM, Pugnaire FI. The role of nurse plants in the restoration of degraded environments. Front Ecol Environ. 2006;4(4):196–202. [Google Scholar]
- 57.Schulte DM, Burke RP, Lipcius RN. Unprecedented restoration of a native oyster metapopulation. Science. 2009;325(5944):1124–1128. doi: 10.1126/science.1176516. [DOI] [PubMed] [Google Scholar]
- 58.Kumara MP, Jayatissa LP, Krauss KW, Phillips DH, Huxham M. High mangrove density enhances surface accretion, surface elevation change, and tree survival in coastal areas susceptible to sea-level rise. Oecologia. 2010;164(2):545–553. doi: 10.1007/s00442-010-1705-2. [DOI] [PubMed] [Google Scholar]
- 59.Peyronnin N, et al. Louisiana’s 2012 coastal master plan: overview of a science-based and publicly informed decision-making process. J Coast Res. 2013;67(sp1):1–15. [Google Scholar]
- 60.Duarte CM, Losada IJ, Hendriks IE, Mazarrasa I, Marbà N. The role of coastal plant communities for climate change mitigation and adaptation. Nat Clim Chang. 2013;3(11):961–968. [Google Scholar]
- 61.Morris JT, Haskin B. A 5-yr record of aerial primary production and stand characteristics of Spartina alterniflora. Ecology. 1990;71(6):2209–2217. [Google Scholar]
- 62.DeLaune R, Smith C, Patrick W. Relationship of marsh elevation, redox potential, and sulfide to Spartina alterniflora productivity. Soil Sci Soc Am J. 1983;47(5):930–935. [Google Scholar]
- 63.Silliman BR, Zieman JC. Top-down control of Spartina alterniflora production by periwinkle grazing in a Virginia salt marsh. Ecology. 2001;82(10):2830–2845. [Google Scholar]
- 64.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2014. [Google Scholar]
- 65.Cribari-Neto F, Zeileis A. Beta regression in R. J Stat Softw. 2009;34(2):1–24. [Google Scholar]