Significance
Secondary organic aerosol (SOA) plays a pivotal role in climate and air quality. Characterizing the molecular makeup of SOA has been a major research goal for several decades, yet the chemical dynamics of most anthropogenic and biogenic SOA systems remain poorly resolved. We report here the time-resolved molecular characterization of SOA derived from the canonical α-pinene system, one of the most abundant biogenic emissions in the troposphere. We reveal distinct features of SOA components in terms of molecular structure, abundance, growth rates, evolution patterns, and responses to variations in temperature, relative humidity, and oxidant type. Our findings provide a comprehensive analysis of processes governing α-pinene SOA formation and aging.
Keywords: secondary organic aerosol, particulate matter, air quality, climate
Abstract
Much of our understanding of atmospheric secondary organic aerosol (SOA) formation from volatile organic compounds derives from laboratory chamber measurements, including mass yield and elemental composition. These measurements alone are insufficient to identify the chemical mechanisms of SOA production. We present here a comprehensive dataset on the molecular identity, abundance, and kinetics of α-pinene SOA, a canonical system that has received much attention owing to its importance as an organic aerosol source in the pristine atmosphere. Identified organic species account for ∼58–72% of the α-pinene SOA mass, and are characterized as semivolatile/low-volatility monomers and extremely low volatility dimers, which exhibit comparable oxidation states yet different functionalities. Features of the α-pinene SOA formation process are revealed for the first time, to our knowledge, from the dynamics of individual particle-phase components. Although monomeric products dominate the overall aerosol mass, rapid production of dimers plays a key role in initiating particle growth. Continuous production of monomers is observed after the parent α-pinene is consumed, which cannot be explained solely by gas-phase photochemical production. Additionally, distinct responses of monomers and dimers to α-pinene oxidation by ozone vs. hydroxyl radicals, temperature, and relative humidity are observed. Gas-phase radical combination reactions together with condensed phase rearrangement of labile molecules potentially explain the newly characterized SOA features, thereby opening up further avenues for understanding formation and evolution mechanisms of α-pinene SOA.
Secondary organic aerosol (SOA), comprising a large number of structurally different organic oxygenates, is a dominant constituent of submicrometer atmospheric particulate matter (1). Molecular characterization of SOA has been a major research goal in atmospheric chemistry for several decades (2), owing to the importance of organic aerosol in air quality and Earth’s energy budget. Both biogenic (e.g., isoprene, monoterpenes) and anthropogenic (e.g., aromatics, large alkanes) organic compounds are well-established precursors to SOA. Knowledge of the SOA molecular composition is crucial for elucidation of its underlying formation mechanisms.
The most abundant monoterpene in the troposphere is α-pinene (3). The oxidation of α-pinene by ozone has become a canonical SOA system (4–12). Identification of multifunctional particle-phase products has been reported, including monomers with carboxylic acid moieties (4, 6) and high-molecular-weight compounds (7, 8, 12), although molecular structures and formation pathways of oligomers remain uncertain (5). Recently, a class of extremely low-volatility gas-phase organic compounds (ELVOCs) has been identified as an important component in the α-pinene ozonolysis chemistry (13). Identification of the ELVOCs in the particle phase and elucidation of the mechanism of their formation remain key missing pieces in closing the α-pinene SOA system (14).
We report here, for the first time, to our knowledge, time-resolved molecular characterization of the abundance, formation, and evolution of organic species in α-pinene-derived SOA. Identified classes of species account for (∼58–72) ± (∼34–39)% of the overall α-pinene SOA mass, with volatilities spanning from the semivolatile to extremely low-volatility range and molecular structures characterized as multifunctionalized monomers and dimers. These organic species exhibit distinct characteristics in terms of oxidation states, chemical structures, initial growth rates, evolution patterns, and responses to variations in temperature (T), relative humidity (RH), and oxidant type.
Results and Discussion
Distribution of α-pinene SOA Constituents.
The α-pinene-derived SOA was generated in the Caltech Environmental Chamber; see Materials and Methods. The Particle-into-Liquid Sampler (PILS) integrated with Ultra Performance Liquid Chromatography/Electrospray Ionization Quadrupole Time-of-Flight Mass Spectrometry (UPLC/ESI-Q-ToFMS) is used to characterize temporal profiles of particulate molecular constituents (15). A spectrum of monomers and dimers, with molecular formulas C8–10H12–16O3–6 and C14–19H24–28O5–9, respectively, is observed in α-pinene SOA. Chemical structure elucidation of these organic molecules is based on the interpretation of chromatographic and spectrometric behaviors of the corresponding ions upon electrospray ionization (SI Appendix, Molecular Structure Elucidation). Based on the fragmentation pattern of the relevant parent ions upon collision-induced dissociation in MS/MS spectra, monomers are generally (di)-carboxylic acids. Ester-containing structures are prevalent in dimers, with C8H11O4 (m/z 171) and C9H13O4 (m/z 185) as primary building blocks.
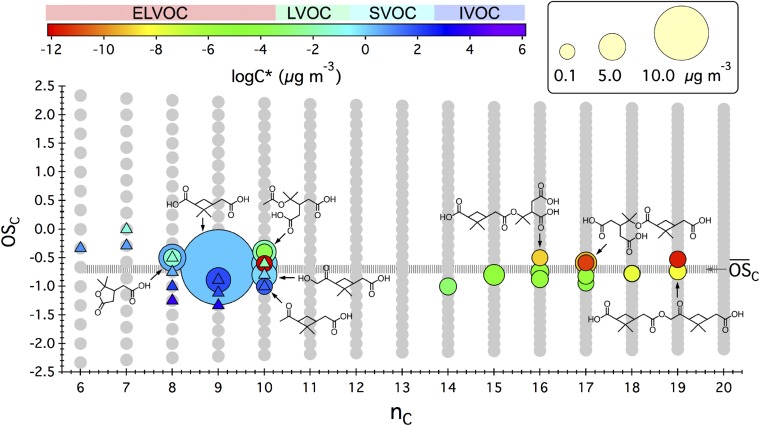
Fig. 1 summarizes identified gas- and particle-phase products mapped onto the carbon number versus oxidation state () space at the point when >99% α-pinene is consumed during ozonolysis in the absence of an OH scavenger at 298 K and <5% relative humidity. Products with mass saturation concentration (C*) in the range of <3 × 10−4, 3 × 10−4 to 0.3, 0.3–300, and 300 to 3 × 106 µg m−3 are designated as extremely low-volatility, low-volatility, semivolatile, and intermediate-volatility organic compounds (ELVOCs, LVOCs, SVOCs, and IVOCs), respectively. A number of monomers in the SVOC category (C8H12O4, C8H14O5, C9H14O3, C9H14O4, C10H16O3, and C10H16O4) are detected both in gas and particle phases. Two monomers (C10H14O5 and C10H16O6) fall in the LVOC range, whereas all of the dimers are categorized as ELVOCs. At the completion of the oxidation of α-pinene, mass concentrations of individual oxidation products, represented by the size of the circular symbols in Fig. 1, range from ∼0.1 μg m−3 to ∼26 μg m−3 and account for 58 ± 34% of the total organic particulate mass. The mean carbon oxidation state of the identified SOA molecular constituents (−0.68 ± 0.27) agrees essentially identically with the average level derived from the Aerosol Mass Spectrometer (AMS) measurement (−0.72 ± 0.43). Note that the monomer and dimer units have comparable O:C ratios.
Fig. 1.
Distribution of gas/particle-phase products from the ozonolysis of α-pinene in the absence of an OH scavenger in the oxidation state versus carbon number () space. The gray circles represent all possible combinations of OSC and nC for stable organic molecules. Experimental details are given in SI Appendix, Table S1. Chemical structures of particle-phase species, as denoted by filled circles, are given in SI Appendix, Table S2. Here, structures are shown only for compounds that have been reported in the literature. Particle-phase mass concentration of each compound (micrograms per cubic meter), as denoted by the marker size, is a 5-min average quantification at the point when >99% of α-pinene is consumed via reaction with O3 and, to a lesser extent, OH radicals. Molecular formulas and potential structures of gas-phase species, as denoted by filled triangles, are given in SI Appendix, Table S3. The average carbon oxidation state () derived from AMS measurement is represented by the horizontal gray line. The saturation mass concentration of each species (log C*) is estimated via an empirical model developed by Donahue et al. (34).
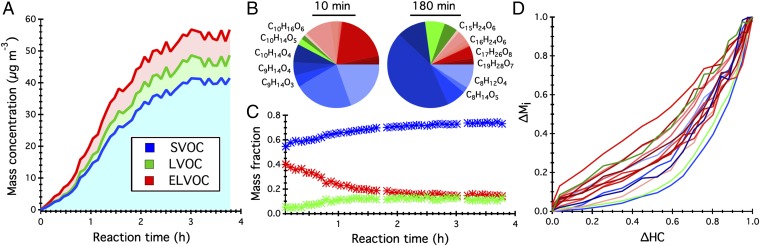
Fig. 2 shows the temporal profiles of the three volatility categories, characterized as the mass loading and fraction (Fig. 2 A and C), as well as the growth dynamics of individual species (Fig. 2 B and D). At first glance, SVOC products dominate the α-pinene SOA, whereas the LVOC and ELVOC products in total account for less than 45% of the overall organic mass. The growth rates of ELVOCs are comparable to or even exceed those for SVOCs and LVOCs. As α-pinene oxidation proceeds, ELVOC production is inhibited, whereas SVOC and LVOC accumulation is favored. By examining the growth dynamics of individual oxidation products, key processes that significantly contribute to α-pinene SOA formation and evolution can be deduced. Prompt formation of ELVOCs at the beginning of the ozonolysis of α-pinene is crucial to initiate organic particle growth. The observation that most ELVOC dimers grow faster than SVOCs and LVOCs indicates that there is no intrinsic kinetic barrier to the ELVOC dimer formation (16). The role of aging in the α-pinene SOA evolution is reflected, to a certain degree, by the slow but continuous growth of LVOCs over the 4-h course of an experiment.
Fig. 2.
Temporal profiles of identified organic molecules lumped as SVOCs (blue hues), LVOCs (green hues), and ELVOCs (red hues) in α-pinene SOA. (A) Particle-phase mass concentration of three volatility categories as a function of reaction time. (B) A 2D pie chart representing the mass fraction of 23 molecules in the particle phase after 10 min and 180 min of α-pinene reaction with O3. Note that molecular formulas are shown for compounds with mass fractions exceeding 5%. (C) Mass fraction of three volatility categories in the particle phase as a function of reaction time. (D) Growth rates of individual species in the particle phase, defined as the ratio of the mass of each species normalized by its highest mass value to the reacted mass of α-pinene normalized by its highest mass value after ∼83 min of reaction.
Effect of Temperature, Relative Humidity, and Oxidant.
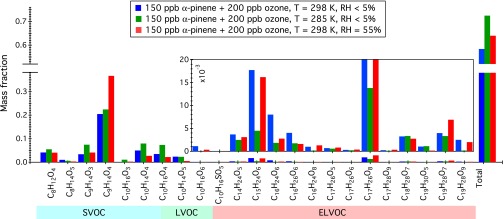
Current understanding regarding the effect of temperature on SOA yield is that lower temperature favors partitioning into the particle phase and hence enhances organic aerosol production owing to decreasing compound vapor pressure. For instance, mass fractions of terpenylic acid (C8H12O4), pinalic acid (C9H14O3), pinic acid (C9H14O4), oxopinonic acid (C10H14O4), and hydroxy-pinonic acid (C10H16O4), increase by 33%, 119%, 10%, 59%, and 111%, respectively, after 3 h of reaction (>99% of α-pinene is consumed) when the chamber temperature is decreased from 25 °C to 12 °C; see Fig. 3 and SI Appendix, Fig. S6. The particle-phase mass fractions of most dimers, on the other hand, decrease at lower temperature, indicating that additional pathways that are temperature-sensitive govern dimer production other than purely gas–particle partitioning. We discuss this observation in more detail subsequently.
Fig. 3.
Effect of temperature and relative humidity on the mass fraction of particle-phase components (defined as the ratio of PILS + UPLC/ESI-Q-ToFMS characterized mass concentration of individual species to the SMPS measured total particulate organic mass) when >99% α-pinene is reacted (predominantly with O3). Corresponding temporal profiles of these compounds are given in SI Appendix, Figs. S6 and S7.
The impact of RH on the growth and evolution of individual oxidation products is shown in Fig. 3 and SI Appendix, Fig. S7. As RH increases, SOA yield increases slightly, potentially as a result of decreasing the particle-phase diffusion timescale and the mean molecular weight of the organic particulate matter. Prompt and significant increase in the mass concentration of certain compounds in the aerosol is also observed, potentially a result of enhanced reactive uptake of hydrophilic compounds and/or reactions involving water. However, the presence of liquid water can lead to a decrease in the mass yield of certain products. As shown in SI Appendix, Fig. S7, the continuous dynamics observed for species C10H16O6 (m/z 231) and C19H29O9 (m/z 399) over the course of SOA formation reflects the competition between production and removal pathways governing the accumulation of these two compounds in the particle phase.
Significant differences in the product distribution and abundances are observed in the SOA generated from the O3-initiated vs. OH-initiated oxidation of α-pinene. The identities of carboxylic acid monomers in both systems are quite similar; see SI Appendix, Fig. S1. Gas-phase products with peroxide functionalities are prevalent in the OH system (SI Appendix, Table S3), which is expected because the RO2 + HO2 pathway is dominant under the current experimental conditions. Importantly, none of the covalent ester dimers is observed in the OH system, although the major building blocks, i.e., C8H11O4 (m/z 171) and C9H13O4 (m/z 185), are still present. This provides strong evidence that these dimers are not a product of particle-phase esterification (17).
Mechanism of Extremely Low-Volatility Dimer Formation.
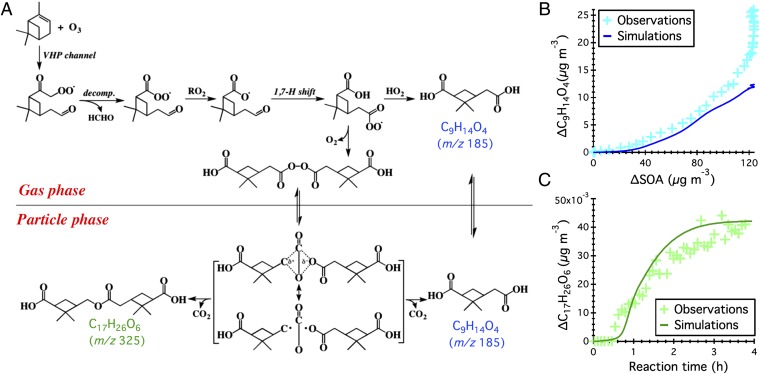
We propose that a combination of two well-established mechanisms, that is, combination of acetylperoxy radicals yielding diacyl peroxides and their subsequent decomposition in the condensed phase after partitioning, is an important pathway for the production of ELVOC dimers. This mechanism is illustrated for the product, C17H26O6 (m/z 325), in Fig. 4A. Addition of ozone to the double bond of α-pinene produces two carbonyl-substituted Criegee biradicals, which either are collisionally stabilized or isomerize via 1,4-H-shift, yielding an alkenoxy radical (C10H15O4⋅). The C10H15O4⋅ radical reacts further with an RO2 radical and undergoes subsequent 1,7-H shift, due to the presence of a labile H atom at the aldehydic carbon, producing an acetylperoxy radical (C9H13O5⋅). The homogeneous recombination of two C9H13O5⋅ radicals via elimination of O2 produces a covalently bound diacyl peroxide. Note that this reaction pathway was proposed as a radical chain termination step from oxidation of aldehydes in both gas and liquid phases (18, 19). The covalent dimer homologs have been observed in the gas phase from the ozonolysis of α-pinene ranging from ∼500 parts per trillion (ppt) to ∼30 parts per billion (ppb) (13, 20). Alternatively, the C9H13O5⋅ radical reacts with RO2/HO2, yielding pinic acid.
Fig. 4.
(A) Proposed gas- and particle-phase mechanism for the formation of the dimer C17H26O6 and pinic acid (C9H14O4). Model simulations (solid line) and experimental observations (plus sign) of the growth dynamics of (B) C9H14O4 and (C) C17H26O6.
The decomposition of diacyl peroxides in the condensed phase generally proceeds through the formation of ion/radical pair intermediates, which undergo decarboxylation, with an ester as a primary product, as well as carboxylic acids and alcohols (21–27). The ester yield could be increased markedly by increasing the solvent polarity and the addition of common ions (25–27). The e-folding lifetime of diacyl peroxides with respect to the first-order decomposition ranges from essentially instantaneous to several hours at room temperature (23, 24, 27), and the decomposition rate is accelerated with increasing solvent polarity and temperature (22–24, 27). Based on measurement of decomposition rates of secondary/tertiary alkyl and phenyl diacyl peroxides in pure organic solvents (e.g., acetone), we expect that the decomposition of diacyl peroxide homologs produced in the α-pinene+O3 system proceeds rapidly (shorter than the SOA formation timescale) in the organic, water, and ammonium sulfate aerosol mixture.
Processes Altering the α-pinene SOA Nature.
The gas-phase production of diacyl peroxides via acetylperoxy radical self/cross-combination and its subsequent decomposition upon solvation can rationalize a series of important experimental observations. First, it has been suggested that the highly functionalized and extremely low-volatility vapors (O:C > 0.7) produced via the gas-phase autooxidation of peroxy radicals contribute, on average, >10% of the total α-pinene SOA and dominate the organic mass at the onset of the SOA growth (13). However, the AMS measured average O:C ratio ranges from ∼0.45 to ∼0.55 over the entire course of α-pinene SOA formation; see SI Appendix, Fig. S5. This indicates that processes involving the loss of oxygen significantly alter the oxygenated nature of compounds contributing to α-pinene SOA. Here the RO2 + RO2 → ROOR reaction and decomposition of diacyl peroxides result in a net loss of four oxygen atoms, substantially decreasing the O:C ratio of the ELVOC dimer produced in the particle phase. Second, as shown in Fig. 3 and SI Appendix, Figs. S6 and S7, the yield of ester dimers increases as the aerosol water content and temperature increase, providing further evidence that the decomposition of diacyl peroxide is temperature and solvent polarity sensitive, consistent with previous observations (24–27). Third, none of the ELVOC dimers is observed from the OH-initiated oxidation of α-pinene, although the monomeric building blocks, such as pinic acid, are present in the OH system. This demonstrates that traditionally cited particle-phase esterification is not important in dimer production. It is also consistent with the general α-pinene degradation chemistry in the gas phase, where the diacyl peroxide precursors, acetylperoxy radicals, are one of the major products from the ozonolysis vinylhydroperoxide channel, but rather limited from the OH oxidation pathway (6). Additionally, the mass fraction of the ELVOC dimers decreases significantly as the ozonolysis reaction proceeds (Fig. 2 B and C), because the continuous production of free radicals enhances their collision probability, thus decreasing the lifetime of free radicals and inhibiting the isomerization channel. Fourth, gas-phase production coupled with gas–particle partitioning cannot completely explain the observed behavior of pinic acid, which is the dominant monomeric product and accounts for up to ∼23% of the overall α-pinene SOA mass. As shown in Fig. 4B and SI Appendix, Fig. S8, a continuous growth of pinic acid is observed after >99% of α-pinene is consumed, indicating an additional production pathway, which will be discussed in SOA Formation Kinetics in the a-pinene System.
SOA Formation Kinetics in the α-pinene System.
Successive chemical reactions yielding one covalent ester dimer (C17H26O6), as listed in SI Appendix, Table S4, are incorporated into the Vapor–Particle Dynamics Model, which accounts for a series of comprehensive processes during SOA formation in chamber experiments (SI Appendix, Vapor–Particle Dynamics Model). By adjusting free parameters in the model framework, simulation of the temporal profile of the C17H26O6 ester dimer agrees well with the experimental observation (see Fig. 4C), confirming that the mechanism involving diacyl peroxide as an intermediate is a plausible explanation for the formation of the identified ELVOC dimers in α-pinene SOA. Optimal molar yield of the C18H26O8 diacyl peroxide from C9H13O5⋅ radical combination is 2%. Despite a small yield, the irreversible partitioning of diacyl peroxides onto aerosols makes these species an important contributor to α-pinene SOA. The best-fit first-order decomposition rate of the C18H26O8 diacyl peroxide in the condense phase is 10−1 s−1. This timescale needs to be rather short to match the rapid growth of the C17H26O6 ester dimer in the particle phase.
Although our model framework captures the observed rapid growth of the C17H26O6 ester dimer in the particle phase, the predicted pinic acid concentration is up to ∼50% lower than that measured; see Fig. 4B. This is expected because, in the current mechanism, only one diacyl peroxide decomposition reaction is considered, whereas 14 structurally different ELVOC dimers are identified. Note that the model captures the initial growth trend of pinic acid, reflecting the role of gas-phase production coupled with gas–particle partitioning. The later gap between simulations and observations potentially represents an upper bound of the contribution of diacyl peroxide decomposition to pinic acid formation. Although other potential reaction channels for the continuous production of pinic acid may exist, the fact that the pinic acid concentration continues to increase after α-pinene is consumed strongly suggests an additional particle-phase production pathway.
Optimal fitting of model simulations to the observed temporal profiles of individual particulate species yields the effective vapor–particle accommodation coefficient (αp,i). Here “effective” indicates that αp,i represents gas–particle interfacial mass transfer as well as particle bulk phase diffusion. The best-fit value of αp,i ranges from 0.01 to 0.1, indicative of rather slow diffusion in the particle phase, which is consistent with recent observations reporting a semisolid phase state of α-pinene SOA (28). This provides insights into the limiting timescale that ultimately controls the aerosol growth.
To assess the effect of the relatively high experimental α-pinene mixing ratio of 150 ppb, we simulated the C17H26O6 ester dimer formation under more atmospherically relevant mixing ratios of 10 ppb α-pinene and 20 ppb O3. The predicted dimer yield increases by ∼6 times at ∼30 μg m−3 total SOA mass loadings (SI Appendix, Fig. S9). Although the timescale with respect to the decomposition of diacyl peroxide depends on ambient conditions (e.g., RH and T), changes in initial reactant concentrations impact the lifetime of free radicals. At low α-pinene levels, the longer lifetime of radicals favors the isomerization pathway and consequently leads to enhanced ELVOC dimer production.
Atmospheric Implications
The identified SVOC/LVOC monomers and ELVOC dimers, with carboxylic acids and esters as the prevalent functionalities, are found to account for a significant fraction of total α-pinene SOA mass (∼58–72%). The remaining carbon mass likely comprises highly oxidized multifunctional organic compounds (HOMs) that have been successfully characterized in the gas phase, with an O:C ratio of >0.7 and a molar yield of 7% (13). HOMs production is ultimately controlled by two competing processes, i.e., RO2 autooxidation via H shift vs. reaction with RO2/HO2. The initial α-pinene mixing ratio in the present chamber experiment was ∼150 ppb, which favors RO2 radical combination rather than self-isomerization, compared with more atmospherically relevant conditions (a few parts per billion). This increase in α-pinene level is estimated to lead to a negligible to moderate decrease in HOMs yield (SI Appendix, Fig. S10). The PILS+UPLC/ESI-Q-ToFMS technique used here is sensitive to relatively polar compounds, whereas the HOMs are mostly carbonyl-/peroxide-containing compounds. That these HOMs might undergo rapid chemical transformation after partitioning into particles is supported by the AMS measured average O:C ratio of α-pinene SOA, ranging from 0.45 to 0.55 over the course of 4-h ozonolysis.
Prompt and significant production of the covalent ester dimers accounts for up to 40% of the α-pinene SOA mass at the early stage of particle growth. We propose these dimers are produced from the particle-phase decomposition of diacyl peroxide, which has been suggested as an important nucleating agent of aerosol formed from reactions of cyclic alkenes and ozone (29). Although the role of organic peroxides in SOA production in pristine atmospheres has been recognized, their actual characterization in aerosols is attended by substantial difficulties due to their thermally labile nature. We provide here indirect evidence for the abundance of organic peroxides and their rapid transformation to covalent esters upon solvation in organic aerosol mixtures. Significant and continuous production of SVOC monomers underscores the importance of gas-phase photochemistry coupled with gas–particle partitioning, as well as particle-phase reaction pathways, e.g., diacyl peroxide decomposition. Although the importance of accretion reactions in oligomer formation has been widely acknowledged, the role of rapid rearrangement/decomposition of large labile molecules in SOA growth and evolution is established here.
Materials and Methods
Experiments were carried out in the 24-m3 Teflon reactor in the Caltech Environmental Chamber. The α-pinene (∼150 ppb) was oxidized by O3 (∼200 ppb) or OH radicals (∼2 × 106 molecules cm−3) in the presence of ammonium sulfate seed particles at concentrations of NOx typical of pristine conditions. The ozonolysis experiments were conducted in the absence of an OH scavenger, resulting in an initial OH molar yield of 0.74 (30). As a consequence, ∼20% of the α-pinene mass is expected to react with OH, and first-generation ozonolysis products are subject to oxidation by OH as well; see SI Appendix, Chamber Experiments. The gas-phase composition of oxidation products was monitored by a Chemical Ionization Mass Spectrometer. The particle size distribution and number concentration were characterized using a custom-built Scanning Mobility Particle Sizer (SMPS). The particle-phase elemental composition was measured by an Aerodyne High Resolution Time-of-Flight AMS; see SI Appendix, Instrument Operation and Data Analysis Protocols.
Temporal profiles of α-pinene SOA components were characterized by using the PILS+UPLC/ESI-Q-ToFMS technique (15). The overall PILS collection efficiency toward α-pinene-derived SOA is estimated to be >85%, based on an empirical correlation of water solubility and average O:C ratio of the aerosol ensemble (15). The time resolution of the PILS+UPLC/ESI-Q-ToFMS technique is 5 min, thus providing information on the particle-phase dynamics during ∼4 h of SOA formation experiments. Mass concentrations of organic molecules in the particle phase (micrograms per cubic meter) are calculated based on mass conservation balance upon phase transfer (SI Appendix, Particle-Phase Components Mass Concentration Retrieval). The negative ESI sensitivity of pinonic acid product in the PILS collected liquid sample is calculated based on the counts/concentration calibration curve of a commercially available standard (98% purity; Sigma-Aldrich). Owing to the lack of authentic standards, the relative ionization efficiency of other identified products toward that of cis-pinonic acid in the ESI negative mode is calculated using a linear model with the acid dissociation constant at logarithmic scale (pKa) of individual compounds, pH of the mobile phase, and weighted average positive sigma of different molecules as the input (31). These three parameters are computed using the Conductor-like Screening Model for Real Solvents (32) implemented into the Amsterdam Density Functional (ADF 2014.07) molecular modeling suite (33). Computational details are given in SI Appendix, Electrospray Ionization Efficiency Estimation.
Supplementary Material
Acknowledgments
We thank John Crounse and Paul Wennberg for useful discussions. This work was supported by National Science Foundation Grant AGS-1523500.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1517742112/-/DCSupplemental.
References
- 1.Jimenez JL, et al. Evolution of organic aerosols in the atmosphere. Science. 2009;326(5959):1525–1529. doi: 10.1126/science.1180353. [DOI] [PubMed] [Google Scholar]
- 2.Hallquist M, et al. The formation, properties and impact of secondary organic aerosol: Current and emerging issues. Atmos Chem Phys. 2009;9(14):5155–5236. [Google Scholar]
- 3.Guenther A, et al. A global model of natural volatile organic compound emissions. J Geophys Res. 1995;100(D5):8873–8892. [Google Scholar]
- 4.Claeys M, et al. Terpenylic acid and related compounds from the oxidation of α-pinene: Implications for new particle formation and growth above forests. Environ Sci Technol. 2009;43(18):6976–6982. doi: 10.1021/es9007596. [DOI] [PubMed] [Google Scholar]
- 5.Hall WA, Johnston MV. Oligomer content of α-pinene secondary organic aerosol. Aerosol Sci Technol. 2011;45(1):37–45. [Google Scholar]
- 6.Jenkin ME, Shallcross DE, Harvey JN. Development and application of a possible mechanism for the generation of cis-pinic acid from the ozonolysis of α- and β-pinene. Atmos Environ. 2000;34(18):2837–2850. [Google Scholar]
- 7.Kristensen K, et al. Dimers in α-pinene secondary organic aerosol: Effect of hydroxyl radical, ozone, relative humidity and aerosol acidity. Atmos Chem Phys. 2014;14(8):4201–4218. [Google Scholar]
- 8.Muller L, Reinnig MC, Warnke J, Hoffmann T. Unambiguous identification of esters as oligomers in secondary organic aerosol formed from cyclohexene and cyclohexene/α-pinene ozonolysis. Atmos Chem Phys. 2008;8(5):1423–1433. [Google Scholar]
- 9.Pathak RK, et al. Ozonolysis of α-pinene: Parameterization of secondary organic aerosol mass fraction. Atmos Chem Phys. 2007;7(14):3811–3821. [Google Scholar]
- 10.Presto AA, Donahue NM. Investigation of α-pinene + ozone secondary organic aerosol formation at low total aerosol mass. Environ Sci Technol. 2006;40(11):3536–3543. doi: 10.1021/es052203z. [DOI] [PubMed] [Google Scholar]
- 11.Shilling JE, et al. Loading-dependent elemental composition of α-pinene SOA particles. Atmos Chem Phys. 2009;9(3):771–782. [Google Scholar]
- 12.Yasmeen F, et al. Terpenylic acid and related compounds: Precursors for dimers in secondary organic aerosol from the ozonolysis of α- and β-pinene. Atmos Chem Phys. 2010;10(19):9383–9392. [Google Scholar]
- 13.Ehn M, et al. A large source of low-volatility secondary organic aerosol. Nature. 2014;506(7489):476–479. doi: 10.1038/nature13032. [DOI] [PubMed] [Google Scholar]
- 14.Mutzel A, et al. Highly oxidized multifunctional organic compounds observed in tropospheric particles: A field and laboratory study. Environ Sci Technol. 2015;49(13):7754–7761. doi: 10.1021/acs.est.5b00885. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, et al. Time-resolved molecular characterization of organic aerosols by PILS + UPLC/ESI-Q-TOFMS. Atmos Environ. 2015 doi: 10.1016/j.atmosenv.2015.08.049. [DOI] [Google Scholar]
- 16.Heaton KJ, Dreyfus MA, Wang S, Johnston MV. Oligomers in the early stage of biogenic secondary organic aerosol formation and growth. Environ Sci Technol. 2007;41(17):6129–6136. doi: 10.1021/es070314n. [DOI] [PubMed] [Google Scholar]
- 17.DePalma JW, Horan AJ, Hall WA, Johnston MV. Thermodynamics of oligomer formation: Implications for secondary organic aerosol formation and reactivity. Phys Chem Chem Phys. 2013;15(18):6935–6944. doi: 10.1039/c3cp44586k. [DOI] [PubMed] [Google Scholar]
- 18.Clinton NA, Kenley RA, Traylor TG. Autoxidation of acetaldehyde. 3. Oxygen-labeling studies. J Am Chem Soc. 1975;97(13):3757–3762. [Google Scholar]
- 19.McDowell CA, Sifniades S. Oxygen-18 tracer evidence for termination mechanism in photochemical oxidation of acetaldehyde. Can J Chem. 1963;41(2):300–307. [Google Scholar]
- 20.Rissanen MP, et al. Effects of chemical complexity on the autoxidation mechanisms of endocyclic alkene ozonolysis products: From methylcyclohexenes toward understanding α-pinene. J Phys Chem A. 2015;119(19):4633–4650. doi: 10.1021/jp510966g. [DOI] [PubMed] [Google Scholar]
- 21.Cooper RA, Ward HR, Lawler RG. Mechanistic implications of chemically induced dynamic nuclear polarization spectra derived from radical-pair interactions in aliphatic diacyl peroxide decompositions. J Am Chem Soc. 1972;94(2):545–552. [Google Scholar]
- 22.Lamb RC, Pacifici JG, Ayers PW. Organic peroxides. 4. Kinetics and products of decompositions of cyclohexaneformyl and isobutyryl peroxides. BDPA as a free-radical scavenger. J Am Chem Soc. 1965;87(17):3928–3935. [Google Scholar]
- 23.Leffler JE, More AA. Decomposition of bicyclo [2.2.2]-1-formyl and pivaloyl peroxides. J Am Chem Soc. 1972;94(7):2483–2487. [Google Scholar]
- 24.Linhardt RJ, Murr BL. Mechanism of decomposition for diacyl peroxides and related carboxy inversion compounds in aqueous acetone. Tetrahedron Lett. 1979;20(12):1007–1010. [Google Scholar]
- 25.Linhardt RJ, Murr BL, Montgomery E, Osby J, Sherbine J. Mechanism for diacyl peroxide decomposition. J Org Chem. 1982;47(12):2242–2251. [Google Scholar]
- 26.Taylor KG, Govindan CK, Kaelin MS. Radical and ionic reaction pathways in the thermal decomposition of diacyl peroxides. J Am Chem Soc. 1979;101(8):2091–2099. [Google Scholar]
- 27.Walling C, Waits HP, Milovanovic J, Pappiaonnou CG. Polar and radical paths in decomposition of diacyl peroxides. J Am Chem Soc. 1970;92(16):4927–4932. [Google Scholar]
- 28.Kidd C, Perraud V, Wingen LM, Finlayson-Pitts BJ. Integrating phase and composition of secondary organic aerosol from the ozonolysis of α-pinene. Proc Natl Acad Sci USA. 2014;111(21):7552–7557. doi: 10.1073/pnas.1322558111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ziemann PJ. Evidence for low-volatility diacyl peroxides as a nucleating agent and major component of aerosol formed from reactions of ozone with cyclohexene and homologous compounds. J Phys Chem A. 2002;106(17):4390–4402. [Google Scholar]
- 30.Aumont B, Szopa S, Madronich S. Modelling the evolution of organic carbon during its gas-phase tropospheric oxidation: Development of an explicit model based on a self generating approach. Atmos Chem Phys. 2005;5(9):2497–2517. [Google Scholar]
- 31.Kruve A, Kaupmees K, Liigand J, Leito I. Negative electrospray ionization via deprotonation: Predicting the ionization efficiency. Anal Chem. 2014;86(10):4822–4830. doi: 10.1021/ac404066v. [DOI] [PubMed] [Google Scholar]
- 32.Klamt A. Conductor-like screening model for real solvents: A new approach to the quantitative calculation of solvation phenomena. J Phys Chem. 1995;99(7):2224–2235. [Google Scholar]
- 33.Pye CC, Ziegler T, van Lenthe E, Louwen JN. An implementation of the conductor-like screening model of solvation within the Amsterdam density functional package - Part II. COSMO for real solvents. Can J Chem. 2009;87(7):790–797. [Google Scholar]
- 34.Donahue NM, Epstein SA, Pandis SN, Robinson AL. A two-dimensional volatility basis set: 1. Organic-aerosol mixing thermodynamics. Atmos Chem Phys. 2011;11(7):3303–3318. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.