Significance
Loss of telomere repeats leads to cellular senescence and the secretion of inflammatory cytokines. How telomere dysfunction is linked to this inflammatory phenotype and its role in aging and cancer is not yet understood. We show here that noncoding telomere RNA transcripts [telomeric repeat-containing RNA (TERRA)] are secreted into the extracellular environment in exosome vesicle fractions. This cell-free TERRA (cfTERRA) is shorter and more stable than intracellular TERRA, is associated with histone proteins, and can induce inflammatory cytokines in responsive cells. These findings suggest that TERRA can have a cell extrinsic function and provide a mechanism through which telomere dysfunction can lead to the activation of inflammatory cytokine signals in the tissue microenvironment through the signaling capacity of cfTERRA.
Keywords: TERRA, telomere, exosome, innate immunity, cytokine
Abstract
Telomeric repeat-containing RNA (TERRA) has been identified as a telomere-associated regulator of chromosome end protection. Here, we report that TERRA can also be found in extracellular fractions that stimulate innate immune signaling. We identified extracellular forms of TERRA in mouse tumor and embryonic brain tissue, as well as in human tissue culture cell lines using RNA in situ hybridization. RNA-seq analyses revealed TERRA to be among the most highly represented transcripts in extracellular fractions derived from both normal and cancer patient blood plasma. Cell-free TERRA (cfTERRA) could be isolated from the exosome fractions derived from human lymphoblastoid cell line (LCL) culture media. cfTERRA is a shorter form (∼200 nt) of cellular TERRA and copurifies with CD63- and CD83-positive exosome vesicles that could be visualized by cyro-electron microscopy. These fractions were also enriched for histone proteins that physically associate with TERRA in extracellular ChIP assays. Incubation of cfTERRA-containing exosomes with peripheral blood mononuclear cells stimulated transcription of several inflammatory cytokine genes, including TNFα, IL6, and C-X-C chemokine 10 (CXCL10) Exosomes engineered with elevated TERRA or liposomes with synthetic TERRA further stimulated inflammatory cytokines, suggesting that exosome-associated TERRA augments innate immune signaling. These findings imply a previously unidentified extrinsic function for TERRA and a mechanism of communication between telomeres and innate immune signals in tissue and tumor microenvironments.
Telomeres are the repetitive and dynamic DNA structures that play a critical role in controlling cellular replicative capacity and cancer suppression (1, 2). Human telomeric DNA contains 4- to 15-kb double-stranded DNA with a sequence of TTAGGG repeats that are bound by a telomere-specific protein complex, referred to as shelterin (3). Telomere repeats are lost by attrition during DNA replication due to the end-replication problem, and critically short telomeres elicit a DNA damage-associated cell cycle arrest and replicative senescence (3, 4). Telomere repeat loss is thought to be part of a somatic cell senescence program that restricts cellular proliferation and regulates tissue homeostasis. Specialized telomere elongation mechanisms, including activation of the reverse transcriptase telomerase or alternative lengthening of telomeres (ALTs) through recombination, can overcome telomere repeat loss-induced cellular senescence. Telomere dysfunction occurs when abnormally short telomeres fail to induce senescence and is an early hallmark of human cancer. Cells with telomere dysfunction are also known to secrete distinct types of inflammatory cytokines (5, 6), but how telomeres are linked to this phenotype is not well characterized.
Telomere repeat DNA can be transcribed in response to developmental changes and cellular stress conditions (7, 8). Telomeric repeat-containing RNA (TERRA) has been implicated in telomere length regulation and DNA damage signaling (9, 10). TERRA can be found in complexes containing nuclear proteins, including hnRNP1, Pot1, RPA, and HP1 (11, 12) and forms stable RNA-DNA hybrids at telomere DNA repeats (13, 14). TERRA may also form foci in cells that can colocalize with the inactive X chromosome (15, 16) or form aggregates in some cancer cells and tissues (17). TERRA can also form highly stable G-quadruplex structures (18), and these structures have been implicated in telomere length regulation (19). Whether TERRA has additional functions distinct from telomere end regulation is not yet known.
Structured nucleic acids, like TERRA, can have potent effects on innate immune sensing pathways (20). Extracellular forms of repetitive DNA fragments, including telomeric DNA, have been shown to modulate inflammatory cytokine production (21). Furthermore, cell-free nucleic acids can be used as a biomarkers for various diseases, including autoimmunity and cancer (22). Cell-free nucleic acid has been identified in stable protein complexes, as well as encapsulated in microvesicles and exosomes (23–25). Exosomes are small (50–100 nm) vesicles that carry a unique composition of proteins (26), lipids (27), mRNA (28), and miRNA (29). Exosomes form in the endosomal multivesicular bodies of the cytoplasm of various cell types and are secreted into body fluids, including blood plasma (30). Depending on their cellular origin and conditions, exosomes exhibit differential enrichment of components, allowing for specialized functions (23). Exosomes have been implicated in regulation of the immune response (31), gene expression by transmission of miRNA (32), and pathogen spreading (33). Tumor-derived exosomes promote tumor progression at many levels, either by suppressing antitumor immune responses (34) or by incorporating oncogenic materials (35). Whether telomeres and their derived RNA are involved in intercellular communication through exosome transport has not been studied.
Here, we report the identification of a previously unidentified, small form of TERRA found in the cell-free environment of mouse normal and tumor tissue, human blood plasma, and cell culture medium. This cell-free TERRA (cfTERRA) was highly enriched in exosome fractions that also induced transcription of inflammatory cytokines. These findings reveal a previously unrecognized extracellular localization of TERRA and provide a molecular mechanism through which telomere dysfunction may impact the tissue microenvironment.
Results
Identification of cfTERRA in the Fraction of Exosomes.
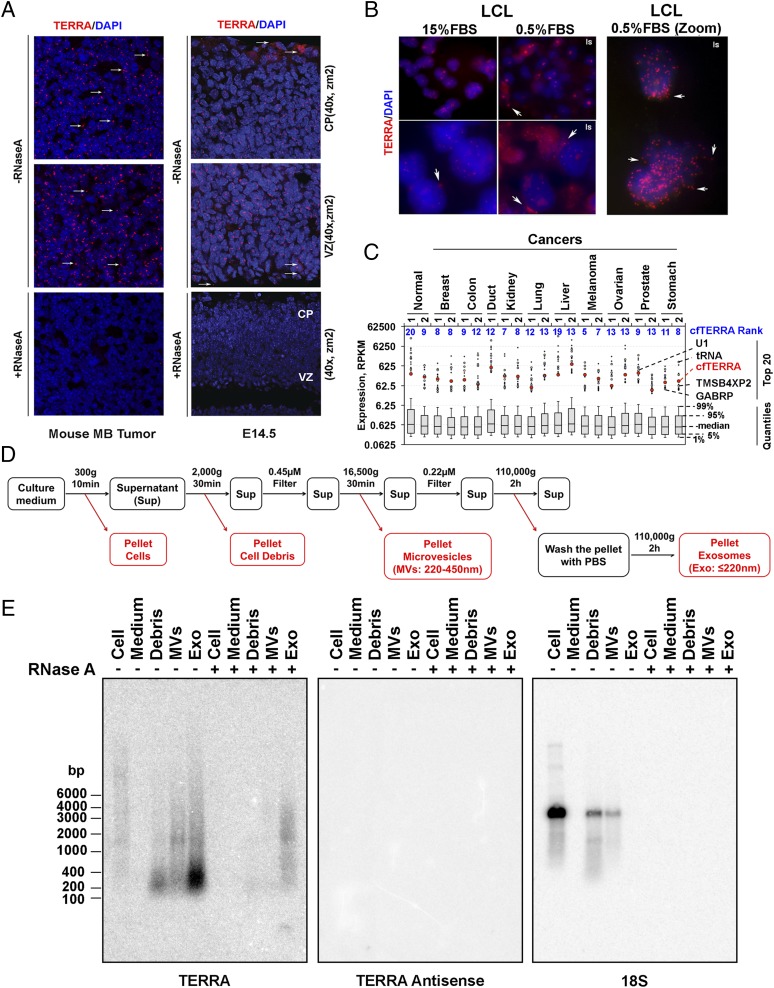
In a previous study (17) we observed that TERRA formed discrete foci in the nuclear compartment of highly proliferating cells in mouse embryonic cerebellum and brain tumors. We now report that a significant number of TERRA foci localize outside of the nuclear and cellular compartments in tissue sections of a mouse model of medulloblastoma (Fig. 1A, Left), as well as in developing embryonic brain (Fig. 1A, Right). Many of these foci were sensitive to RNase treatment, indicating they are mostly telomeric RNA and not DNA fragments (Fig. 1A, Lower). We also observed TERRA foci forming outside of nuclear compartments in human tissue culture cells, especially in serum-starved human lymphoblastoid cell lines (LCLs) (Fig. 1B). Consistent with this, we found that serum-starved LCLs produced higher levels of a shorter form of TERRA (Fig. S1 A and B). We next asked whether TERRA RNA could be detected in RNA-seq analyses from cell-free RNA derived from plasma samples of normal or cancer patients (Fig. 1C). TERRA RNA, as defined by a least six telomere repeats, was detected at relatively high abundance in all samples. RNA with 2 or 3 UUAGGG-repeats were found at much lower read counts, suggesting that most TERRA RNA was derived from longer repeat transcripts (Table S1). While no significant differences between cancer and normal patients were found, read counts for TERRA ranked in the top 20 most frequent transcripts for all RNA-seq reads of extracellular RNA (Fig. 1C). These findings indicate that extracellular TERRA is a relatively abundant component of the cell free RNA from human blood plasma.
Fig. 1.
Identification of cfTERRA in exosome fractions. (A) RNA-FISH analysis of TERRA expression on mouse medulloblastoma tumor (Left) and embryonic E14.5 brain tissue sections by confocal microscopy. TERRA was stained with (CCCTAA)3 PNA probe in red, and nuclei were counterstained with DAPI in blue on mouse medulloblastoma tissue (Left) or cerebral cortex section of E14.5 WT mouse embryo (Right). Cortical plate (CP) and ventricular Zone (VZ) are indicated. RNase A treatment eliminates all signals of TERRA (Lower). Arrows indicate TERRA signals found outside of nuclei. Images were taken with 40× lens at zoom 2. (B) TERRA foci were found outside of nuclei in human lymphoblastoid cell lines grown under normal serum (15%) or serum starved (0.5%) conditions for 24 h before fixation. (Right) Zoom image of the same LCL samples. (C) RNA-seq analysis of cell-free DNA from various normal and cancer blood plasma samples. TERRA (as defined by six tandem UUAGGG repeats) and its ranks in read counts relative to all other genes. Whisker plots demonstrate distribution of gene expression levels that had at least 10 aligned RNA-seq reads. Dots represent RPKM values for the top 20 expressed genes. Among those, highlighted are cfTERRA and 6 other known genes that appear in the top 20 genes across all samples the most. (D) Flowchart for fractionation of culture medium by differential centrifugation. The conditions of each centrifugation or filtering are indicated above the black arrows. Pellets highlighted in red are used for analysis of RNA or proteins. (E) Northern blot analysis of TERRA levels in extracellular fractions from LCL culture medium. RNA was isolated from pellets of the differential centrifugation as shown in C. Equal mass amounts of RNA (1 µg) were either mock treated (−) or treated with RNase A (100 µg/mL) for 30 min at 37 °C before Northern blot analysis. The blot is hybridized with 32P-labeled probes for TERRA, antisense TERRA, or 18S RNA, or indicated under the blot. Numbers on the left show the position of RNA markers in base pairs.
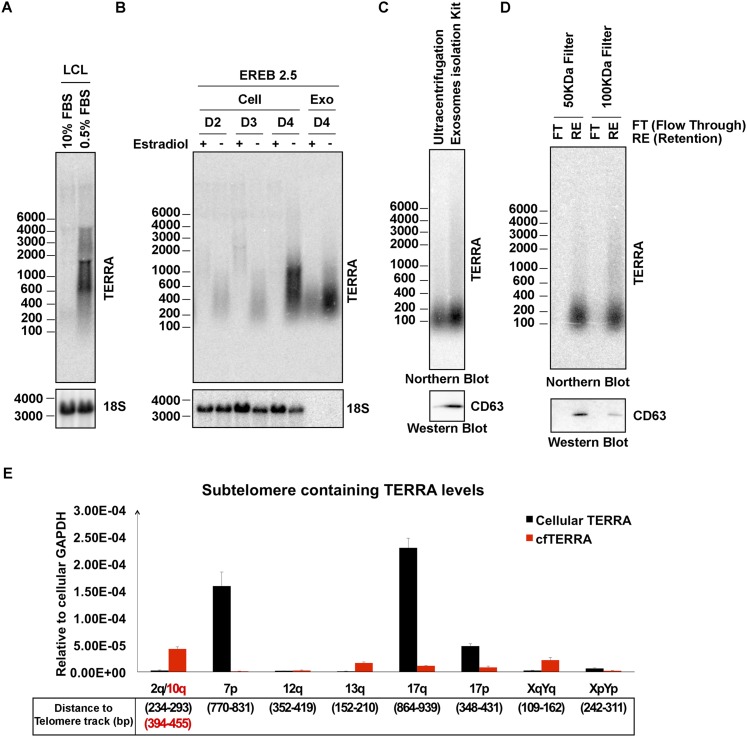
Fig. S1.
Growth arrest induces cfTERRA production in LCLs. (A) Northern blot showing total cellular TERRA levels in LCLs grown in 15% or 0.5% serum for 24 h. (B) Northern blot showing total cellular TERRA or exosome fraction for cfTERRA in EREB LCLs with or without estradiol treatment required for EBV EBNA2 expression and cell proliferation. (C) Comparison of two exosomes isolation methods to purify cfTERRA complex. Exosomes were isolated from equal volume of LCL culture medium (10 mL) by either ultracentrifugation or exosomes isolation kit (Invitrogen). RNAs were isolated from 80% of recovered exosomes and analyzed for cfTERRA by Northern blotting. The remaining exosomes (20%) were assayed by Western blotting with CD63 antibody. (D) Comparison of the recovery of cfTERRA complex by filters with different molecular weight cutoff. Equal amount of exsomes was loaded on the centrifugal filters with either 50- or 100-kDa cutoff. RNA were isolated from either flow through (FT) or retention (RE), and analyzed for cfTERRA by Northern blotting. Proteins of each fraction were TCA precipitated and assayed by Western blotting with CD63 antibody. (E) qRT-PCR for quantification of subtelomere containing TERRA levels in 1 µg of total RNA from LCL cells and exosomes. The subtelomere containing TERRA levels were determine using ∆CT method relative to cellular internal control GAPDH. The distances of the primers to specific telomere track were indicated below.
Table S1.
RNA-seq analysis for TERRA
| Sample | 5/6 reps | 2 rep at 5′/3′ | % of 5/6 | Unique |
| Normal 1 | 1,177 | 21 | 1.8 | 13 |
| Normal 2 | 957 | 32 | 3.3 | 22 |
| Breast 1 | 672 | 14 | 2.1 | 14 |
| Breast 2 | 1,189 | 29 | 2.4 | 25 |
| Colon 1 | 893 | 17 | 1.9 | 16 |
| Colon 2 | 1,001 | 22 | 2.2 | 15 |
| Duct | 1,951 | 24 | 1.2 | 21 |
| Kidney 1 | 728 | 18 | 2.5 | 11 |
| Kidney 2 | 704 | 27 | 3.8 | 19 |
| Liver 1 | 1,129 | 14 | 1.2 | 14 |
| Liver 2 | 2,094 | 18 | 0.9 | 15 |
| Lung 1 | 427 | 21 | 4.9 | 18 |
| Lung 2 | 846 | 25 | 3.0 | 20 |
| Melanoma 1 | 1,743 | 23 | 1.3 | 21 |
| Melanoma 2 | 1,829 | 33 | 1.8 | 16 |
| Ovarian 1 | 399 | 24 | 6.0 | 22 |
| Ovarian 2 | 2,633 | 36 | 1.4 | 20 |
| Prostate 1 | 1,128 | 17 | 1.5 | 15 |
| Prostate 2 | 328 | 15 | 4.6 | 13 |
| Stomach 1 | 783 | 28 | 3.6 | 24 |
| Stomach 2 | 789 | 23 | 2.9 | 23 |
We searched all of the samples for reads with no more than two telomeric repeats at the 5′ or 3′ end of the read. Those full reads were then aligned against complete human genome and the number of reads mapped to unique sites are indicated (unique). The full counts data are presented in the table. 5/6 reps, number of reads with 5/6 telomeric repeats; 2 at 5′/3′, total number of reads with two, but no more than two, telomeric repeats at the 3′ or 5′ end; % of 5/6, percent of two-repeat reads over 5/6 telomeric repeats; unique, number of unique reads (unique sequence) that had no more than two telomeric repeats.
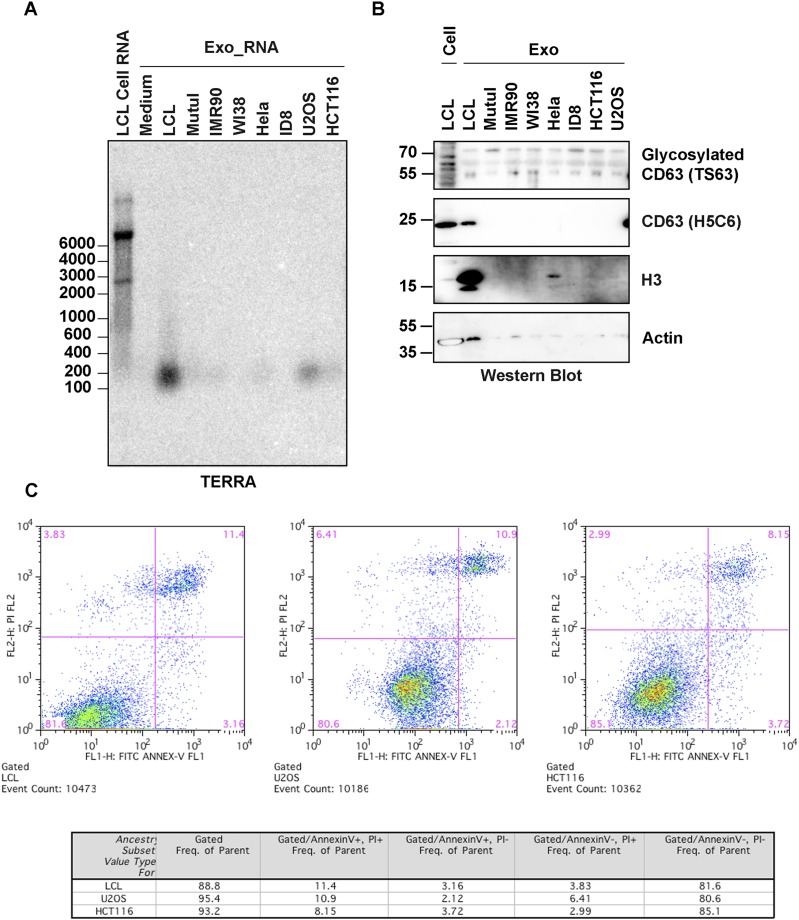
To investigate the possibility that TERRA was exported to the extracellular compartment, we isolated the microvesicle and exosomal fractions from LCL culture media using differential centrifugation (Fig. 1D). We then assayed the total cellular RNA, cellular debris, microvesicle fraction, and exosome fractions for TERRA RNA by Northern blot (Fig. 1E). We found that a smaller form of TERRA migrating at ∼200 nt was highly enriched in the exosome fraction. Identical forms of TERRA were identified when exosomes were isolate by ultrafiltration or exosome precipitation reagent (Fig. S1 C and D). Quantitative RT-PCR (qRT-PCR) with primers situated close (<300 nt) to the subtelomere–telomere junction showed enrichment in exosome fractions relative to total cellular TERRA (Fig. S1E). Similar forms of TERRA could be isolated from different cell types, although LCLs produced the highest amounts among the cells tested (Fig. S2A). TERRA production correlated with higher levels of fast migrating CD63-positive exosomes (Fig. S2B) and did not correlate with cell death or apoptosis (Fig. S2C). This form of TERRA (referred to as cfTERRA) was partly resistant to RNase A treatment, forming a diffuse and slower migrating signal on Northern blot (Fig. 1D). We did not detect any antisense TERRA, suggesting that this is mostly G-rich single-stranded RNA. The control 18S probe identified 18S RNA in cellular debris and microvesicles, but not in the exosome fraction. These results indicate that cfTERRA is enriched in exosome-like fractions from human LCLs.
Fig. S2.
cfTERRA was released with different levels in other cell lines. (A) Comparison of cfTERRA levels across several cell lines in exosomes. Exosomes were isolated from indicated cell lines by ultracentrifugation. RNAs were purified from exosomes with equal amount of proteins and analyzed for cfTERRA by Northern blotting. (B) Western blot analysis of exosomes as shown in A. Equal amount of exosomal proteins were analyzed by Western blotting with CD63, H3, or Actin antibodies. (C) Apoptosis analysis of cell lines used for exosome isolation. After 48-h culture of the indicated cell lines in conditional medium, cells were stained with FITC-Annexin V and PI and analyzed by flow cytometry. Flow cytometry profile present as FITC-Annexin V staining in the x axis and PI in the y axis. The number in the upper right quadrant represents the percentage of late apoptotic cells in each cell line.
cfTERRA Was Protected by a Structure with Similar Density as Exosomes.
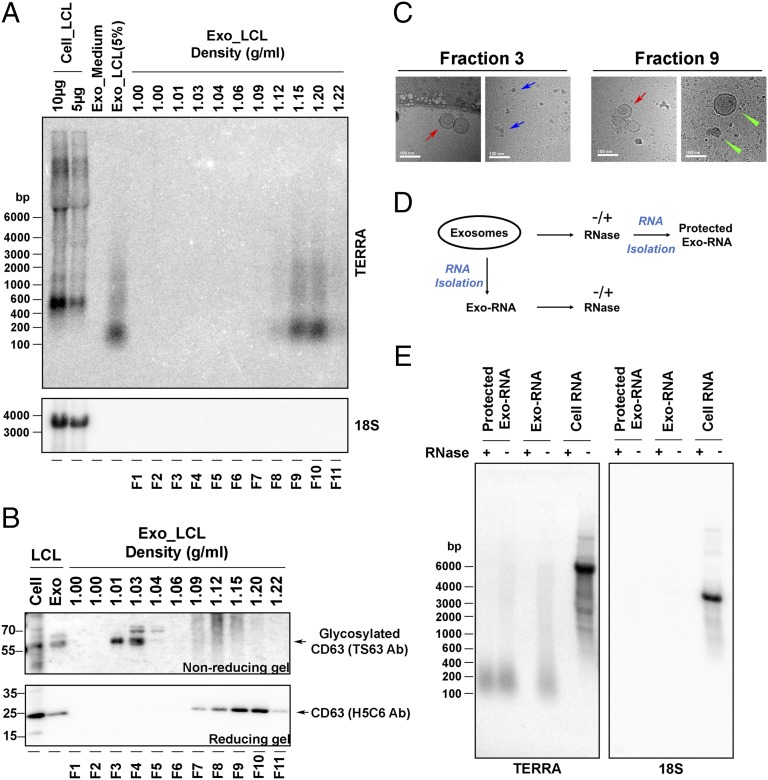
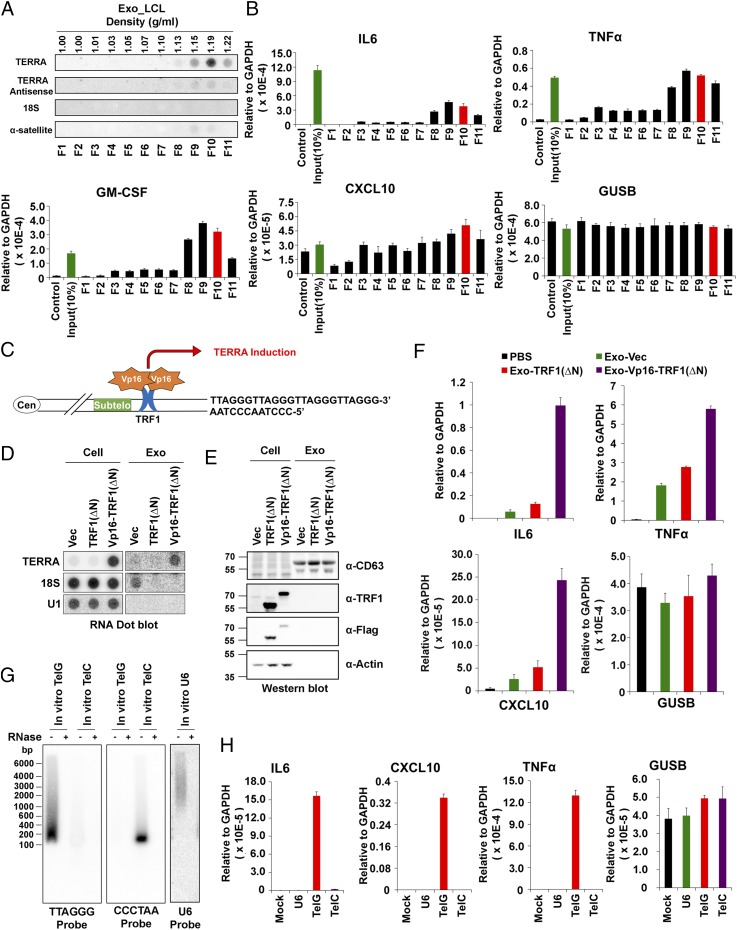
To better characterize cfTERRA, we fractionated extracellular vesicles on sucrose gradients using tetraspanin CD63 as a marker for exosomes (36) (Fig. 2). We observed that cfTERRA cofractionated with the faster migrating form of CD63+ through the sucrose gradient centrifugation (Fig. 2 A and B). We examined these fractions by electron cryo-microscopy and observed that most of the spherical exosomes (red arrows) comigrated with cfTERRA and fast migrating CD63 in fraction 9 (density, 1.15 g/mL), along with some other membrane vesicles (green arrows). Although fraction 3 contained the slower mobility (and presumably glycosylated) form of CD63 typically associated with exosomes, there were few exosome structures and many large macromolecular complexes presumably of protein composition (blue arrows) in this fraction. To investigate whether cfTERRA was within exosomes, we compared the RNase sensitivity of cellular TERRA with exosome fraction of cfTERRA (Fig. 2D). Although cellular TERRA was efficiently degraded by RNase mixture treatment, cfTERRA was protected from RNase activity when the exosome structure was intact. In contrast, purified cfTERRA from denatured exosomes were mostly degraded by RNase mixture treatment (Fig. 2D). Exosome fractions did not contain detectable amounts of control 18S RNA. These findings indicate that cfTERRA cofractionates with the nongylcosylated CD63+ exosome fraction where it remains resistant to RNase treatment either by encapsulation within the exosome or its association with other factors that copurify with exosomes.
Fig. 2.
cfTERRA copurifies with exosomes. (A) Northern blot analysis of total cellular RNA (10 and 5 μg) or RNA isolated from total exosome fractions from either fresh media or LCL extracellular media or exosomes that were fractionated on a continuous sucrose gradient (fractions 1–11) were probed for TERRA (Upper) or 18S RNA (Lower). (B) Western blot analysis of sucrose fractions (as shown in A) with CD63 antibody using nonreducing (Upper) or reducing (Lower) SDS/PAGE. The unmodified and glycosylated CD63 mobilities are shown as indicated. (C) Electron cryo-microscopy analysis of sucrose fractions 3 and 9. Exosomes are indicated with red arrows, whereas other vesicle structures are indicated with green arrows. The blue arrows indicate a presumed protein macromolecular complexes found in fraction 3. (Scale bars, 100 nm.) (D) Schematic of RNase protection assay used in E. Exosomes were treated with or without RNase mixture (Protected Exo-RNA) or exosome RNA was first isolated and then treated with or without RNase mixture (Exo-RNA). (E) Northern blot of RNA isolated from LCLs or LCL-derived exosomes. Exosomes were pretreated without (−) or with (+) RNase mixture before RNA isolation (Protected Exo-RNA) or treated after RNA isolation (Exo-RNA) and cellular RNA. The isolated RNA was analyzed by Northern blotting and hybridized with 32P-labeled probes for TERRA or 18S RNA as indicated.
cfTERRA Is Bound by Histones in Exosome Fraction.
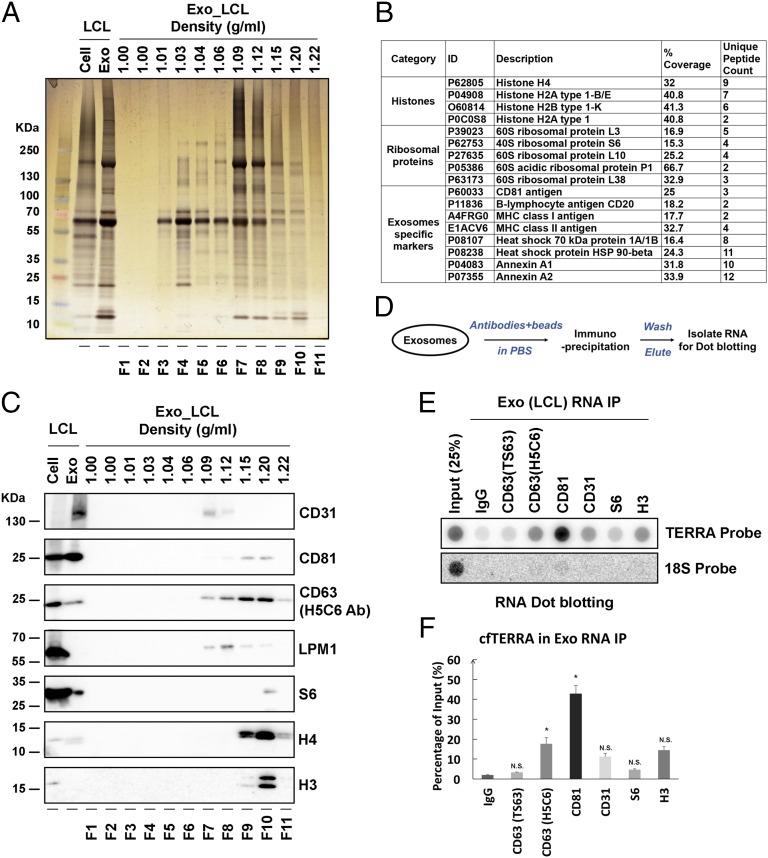
Sucrose gradient fractions enriched in TERRA (fraction 9) and CD63 were analyzed by silver staining of SDS/PAGE and then by liquid chromatography-tandem mass spectrometry (LC-MS/MS) to identify the protein composition (Fig. 3 A and B, Fig. S3, and Dataset S1). MS revealed histones and ribosomal proteins, as well as many known exosome components (Fig. S3), including CD81, CD20, and annexin A1 (Fig. 3B) (37). Western blot confirmed the enriched levels of histone H3 and H4, as well as CD81, CD63, and LMP1 in the sucrose fractions containing TERRA (Fig. 3B). To determine whether cfTERRA is associated with any of the protein components of the exosome fraction, we performed immunoprecipitation assays on these exosomes (Fig. 3 D–F). We found exosome-associated TERRA could be immunopurified with antibodies to exosome membrane constituents CD81 and to a lesser extent with CD63. TERRA could also be detected in immunoprecipitation (IP) with H3 antibody, suggesting some cfTERRA may associate with chromatin components outside of exosomes (Fig. 3 E and F). Exosome-associated TERRA was detected at higher levels than 18S RNA relative to total cellular amounts, suggesting that TERRA is selectively enriched in exosomes in LCLs.
Fig. 3.
cfTERRA is associated with exosomes. (A) Sucrose fractions collected in Fig. 2 were assayed by SDS/PAGE and visualized by silver staining. Molecular weight of the marker was indicated on the left in kilodaltons. (B) Summary of LC/MS/MS data from sucrose fractions F8 and F9. Proteins identified by MS from the major categories of histone, ribosomal protein, or exosome component are shown. Percent coverage and unique peptide counts are indicated. Full list of MS identified peptides is provided in Table S2. (C) Western blotting of sucrose factions using antibodies specific for CD31, CD81, CD63, LMP1, S6, histone H4, and H3 antibodies. (D) Schema of exosome immunoprecipitation and RNA isolation method used in D and E. (E) Exo RNA IP using antibodies to CD63 (TS63), CD63 (H5C6), CD81, CD31, S6, H3, or control IgG. Isolated RNA was then assayed by dot blotting with TERRA or 18S-specific probes. (F) Quantification of three independent replicates of Exo RNA IP as represented in E. Error bars, SD.
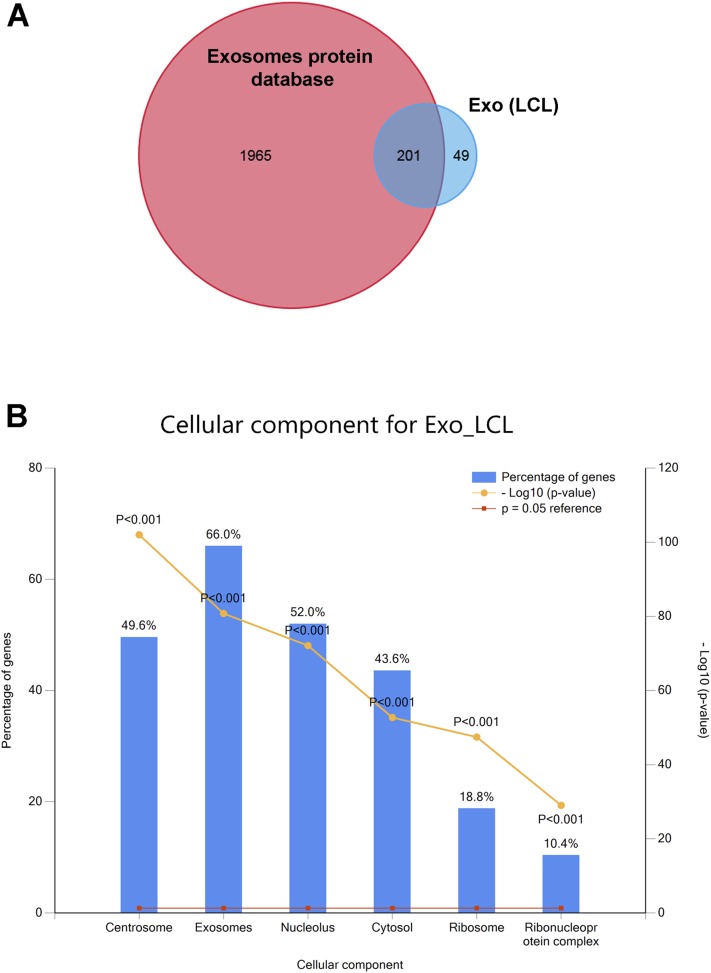
Fig. S3.
Functional enrichment analysis of proteins identified by LC-MS/MS. (A) The Venn diagram shows 201 proteins of the top 250 proteins identified in LCL exosomes (Exo_LCL) overlap with that found in 12 other studies of exosome proteins in the Vesiclepedia database. (B) Enrichment of cellular component for LCL exosomes (Exo_LCL). The percentages of genes that function in different cellular components are shown of the top 250 proteins identified by LC-MS/MS.
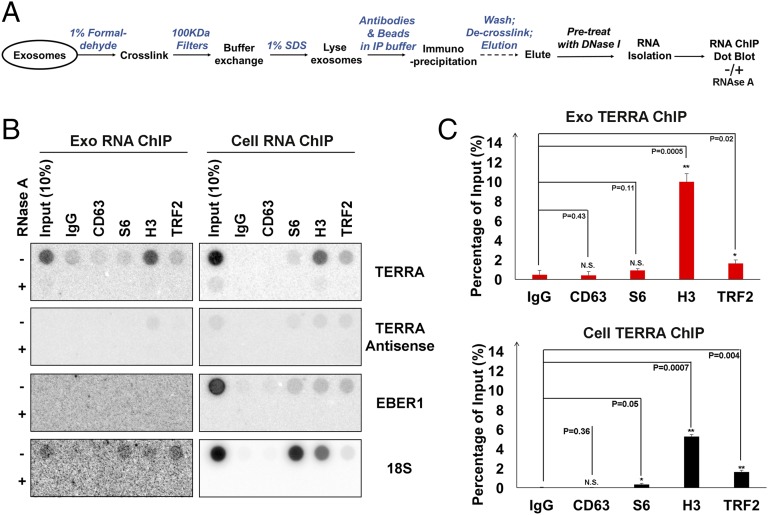
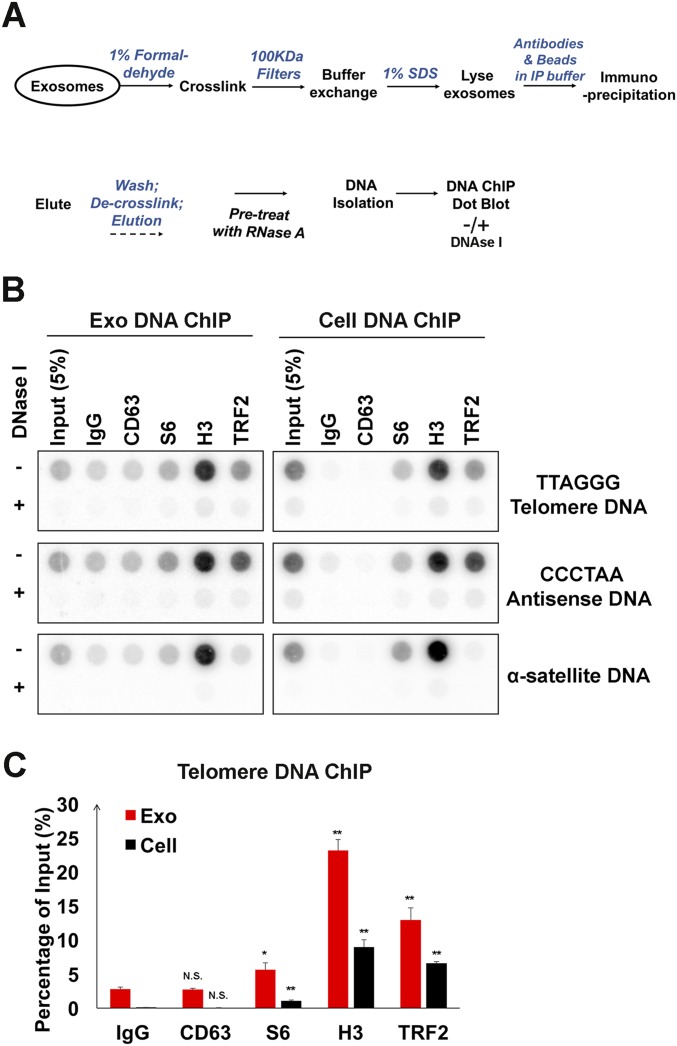
To determine whether TERRA was physically associated with any protein constituents found in exosome fractions, we performed extracellular ChIP (Exo RNA-ChIP) assays using formaldehyde cross-linking before exosome lysis (Fig. 4A). We found that TERRA was significantly enriched in histone H3 Exo RNA-ChIP and to a much lesser extent with TRF2 (Fig. 4 B and C). In contrast, 18S RNA was enriched with S6 and H3 cellular RNA-ChIP, but not detectable in Exo RNA-ChIP. We also performed Exo DNA-ChIP on total extracellular fractions (Fig. S4). We found that telomere repeat DNA (both sense and antisense) could be detected in both H3 and TRF2 Exo DNA-ChIPs, whereas α-satellite DNA was enriched only in the H3 ChIP (Fig. S4 B and C). These findings indicate that chromatin-associated DNA fragments enriched with telomeric and α-satellite DNA fragments can be found in extracellular fractions.
Fig. 4.
cfTERRA is associated with histones. (A) Schema of Exo RNA ChIP assay. (B) RNA ChIP assays were performed with exosomes (Exo) or cellular (Cell) LCLs using antibodies specific for CD63, S6, H3, TRF2, or control IgG. Isolated RNA was treated with either mock (−) or RNase A and then assayed by hybridization with probes for TERRA, TERRA-antisense, EBER1, or 18S, as indicated. (C) Quantification of at least three independent TERRA RNA ChIP assays, a representative shown in B. Bar graphs represent mean values with SDs. P values were calculated by two-tailed Student t test: *P < 0.05, **P < 0.01.
Fig. S4.
Exosomes contain chromatin-associated DNA. (A) Schema for Exo DNA ChIP assays. (B) DNA dot blots for Exo DNA ChIP assays. ChIP was performed with antibodies to CD63, S6, H3, TRF2, or control IgG. (C) Quantification of three independent DNA ChIP assays for telomere DNA from exosomes (red) or cells (black). Bar graphs represent mean values with SDs. P values were calculated by two-tailed Student t test: *P < 0.05, **P < 0.01.
cfTERRA Modulates the Transcription of Inflammatory Cytokines in Recipient Cells.
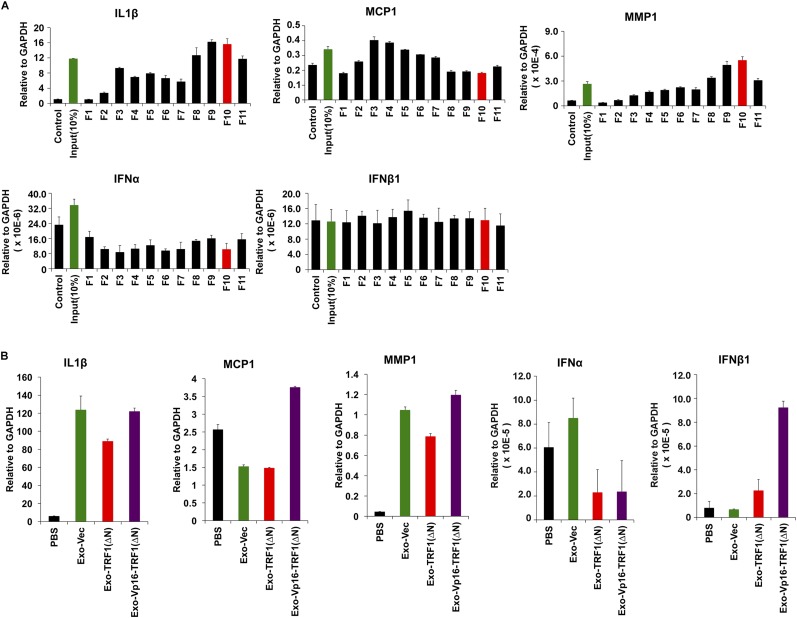
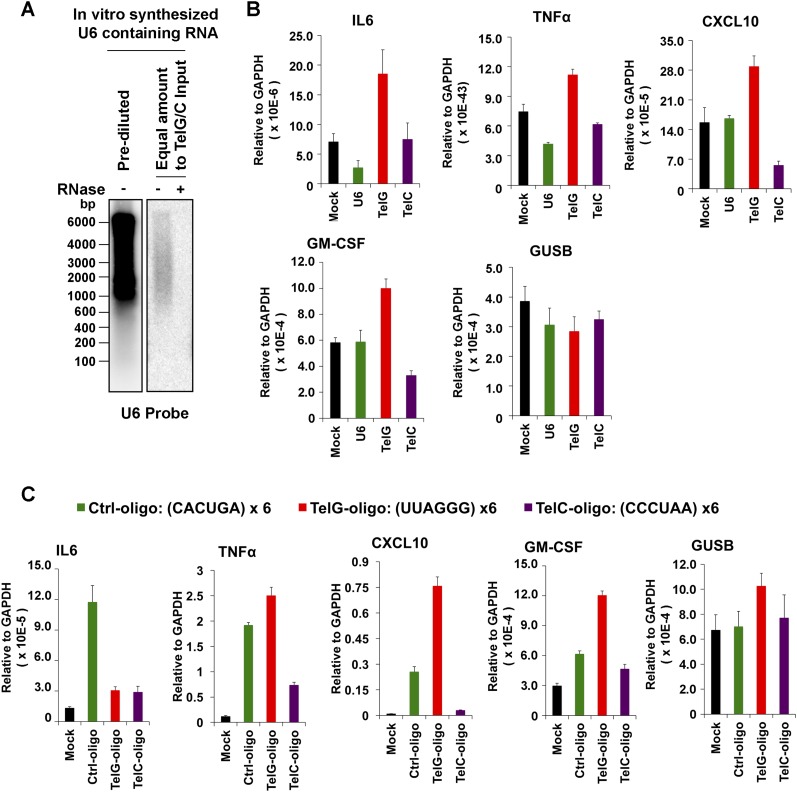
Exosomes have been implicated in various types of intercellular communications, including the modulation of inflammatory cytokines and the innate immune signaling (38). We therefore tested whether exosomes from LCLs enriched with cfTERRA could induce transcription for various cytokines and chemokines. We found that cfTERRA-enriched exosome fractions efficiently induced transcription of several cytokines, including IL6, TNFα, GMCSF, and C-X-C chemokine 10 (CXCL10) (Fig. 5 A and B and Fig. S5A). To determine whether cfTERRA levels in exosomes correlated with cytokine activation, we isolated exosomes from cells engineered to produce elevated TERRA levels (Fig. 5C). Exosomes were isolated from HCT116 cells transduced with ectopic TRF1(ΔN) or TRF1(ΔN) fused to the transcription activation domain of VP16 [VP16-TRF1(ΔN)]. We validated by Northern blot that VP16-TRF1(ΔN) induced high levels of cellular and exosome-associated TERRA relative to vector and TRF1ΔN only (Fig. 5D), suggesting TERRA was induced by the VP16 domain instead of ectopic expression of TRF1(∆N). Although some 18S RNA was detected in exosomes from vector control samples, no 18S was detected in TRF1(ΔN) or VP16-TRF1(ΔN), and U1 RNA was not detected in any exosome fraction (Fig. 5D). Protein levels of cellular TRF1 and exosomal CD63 were monitored by Western blot (Fig. 5E). Exosomes normalized by CD63 expression levels were then incubated with peripheral blood mononuclear cells (PBMCs) and assayed for cytokine induction (Fig. 5F). We found that exosomes from VP16-TRF1ΔN containing the highest levels of cfTERRA induced the highest levels of cytokine mRNA, including IL6, TNFα, and CXCL10 while having no significant effect on control GUSB mRNA levels (Fig. 5F and Fig. S5B). To determine whether TERRA alone is capable of stimulating inflammatory cytokine transcription on recipient cells, we expressed and purified sense or antisense TERRA-containing RNA transcripts, as well as equimolar U6 transcripts, and delivered these in liposomes to either PBMCs (Fig. S6A) or IMR90 fibroblasts (Fig. 5 G and H). We found that synthetic TERRA-containing liposomes selectively stimulated IL6, CXCL10, and TNFa in IMR90 cells (Fig. 5H) and to a lesser extent in PBMCs (Fig. S6A). We also found that short synthetic oligonucleotides (36 bp) containing TERRA could partially induce some cytokine production, although not fully recapitulating endogenous exosomes (Fig. S6B). Taken together, these findings suggest that exosome-associated cfTERRA may function to modulate cytokine production in recipient cells.
Fig. 5.
Exosome-associated TERRA stimulates inflammatory cytokines. (A) RNA dot blot analysis of sucrose gradient fractionation of LCL-derived exosomes probed for TERRA (Upper), TERRA antisense, 18S rRNA, or alpha-satellite RNA (Lower). (B) Total exosomes (input) or sucrose gradient fractions were incubated with PBMCs for 3 h and then assayed by qRT-PCR for expression of IL6, TNFα, GM-CSF, CXCL10, or control GUSB mRNA. Bar graphs represent qRT-PCR values relative to gapdh mRNA (mean ± SD) from three independent experiments. (C) Schema of VP16-TRF1(ΔN) activation of TERRA. (D) RNA dot blot for TERRA, 18S, or U1 RNA from HCT116 cells (Left) or exosomes (Right) transduced with vector, TRF1(ΔN), or VP16-TRF1(ΔN). (E) HCT116 cells transduced as in D were assayed by Western blot for CD63, TRF1, FLAG, and Actin. (F) qRT-PCR for expression of IL6, TNFα, CXCL10, or control GUSB mRNA for PBMCs treated with exosomes derived from HCT116 cells transduced with vector control (green), TRF1(ΔN) (red), VP16-TRF1(ΔN) (purple), or PBS control (black). (G) Northern blot of in vitro transcribed TelG, TelC, or U6 RNA treated with control or RNaseA and probed for TERRA (Left), TERRA antisense (Center), or U6 (Right). (H) IMR90 cells were treated with liposomes containing TelG or TelC RNA for 24 h and then assayed by qRT-PCR for IL6, TNFα, CXCL10, or control GUSB mRNA. Bar graphs represent qRT-PCR values relative to gapdh mRNA (mean ± SD) from three independent experiments.
Fig. S5.
Exosome-associated TERRA stimulates inflammatory cytokines. (A) Experiments described in Fig. 5B where sucrose fractions from LCL exosomes were used to treat PBMCs and then assayed for induction of cytokine gene transcription, including mRNA for IL1B, MCP1, MMP1, IFNα, and IFNβ1. (B) Experiments described in Fig. 5 C–E include addition cytokine genes IL1B, MCP1, MMP1, IFNα, and IFNβ1.
Fig. S6.
Synthetic TERRA stimulates cytokine production. (A) In vitro transcribed U6 RNA is shown by Northern blot in undiluted and diluted amounts used for molar comparison with TelG or TelC as shown in Fig. 5 G and H. (B) PBMCs treated with liposomes containing mock, U6, TelG or TelC in vitro transcribed RNA were assayed by qRT-PCR for activation of cytokines IL6, TNFα, CXCL10, GM-CSF, or control GUSB. (C) Synthetic 36-nt RNA oligonucleotides for TERRA (TelG), antisense TERRA (TelC), or control (CACUGA)6 were delivered in liposomes to PBMCs and assayed for IL6, TNFα, CXCL10, GM-CSF, or control GUSB mRNA expression.
Discussion
Telomeres have been implicated in the cell intrinsic regulation of senescence (39), as well as in more complex functions, including tissue homeostasis (40) and organismal aging (41). Telomere-associated changes are known to occur in cancerous and precancerous lesions (42), and many of these lesions are known to have a senescence-associated secretory phenotype (SASP) that can drive carcinogenesis (43). Cells with short telomeres produce a distinct pattern of cytokines that has been referred to as a telomere-associated secretory phenotype (TASP), which is distinct from SASP (5, 44). The mechanism through which telomere dysfunction produces extracellular signals relevant to tissue microenvironment, inflammation, and cancer is not completely understood.
Here, we demonstrate that TERRA-derived RNA fragments can be found in the extracellular fraction of mouse tumor and normal embryonic tissue, human blood plasma, and human cell lines in culture. cfTERRA from human LCLs copurified with CD63+ and CD81+ exosome fractions and coprecipitated with histone H3, suggesting that cfTERRA forms a chromosomal-like ribonucleoprotein particle within or associated with exosomes. We showed that exosome fractions enriched in cfTERRA induced inflammatory cytokines from human PBMCs. We also found that synthetic TERRA could induce a similar inflammatory response in human fibroblasts. We conclude that cfTERRA is a component of exosome fractions that can modulate the inflammatory response.
TERRA Is Deregulated in Cancer and Stress Response.
TERRA expression can be regulated by developmental and stress-related signals, including DNA damage and viral infection (7, 45–47). Telomere shortening may also increase TERRA expression (48), but it is not clear that senescent cells show a global increase in TERRA levels. We found that TERRA can be enriched in some cancer tissues (17) and is highly induced in cells after infection by herpes simplex virus 1 (HSV1) (47). TERRA has been shown to have several functions at telomeres, including recruitment of telomerase (48), inhibition of telomerase (49), assembly of DNA damage repair proteins (50), and maintenance of telomeric heterochromatin (12). However, TERRA has not yet been implicated in TASP or other related telomere-extrinsic functions.
Telomeres and Immunological Response.
Several lines of evidence suggest that telomeric events can impact the innate immune response and tissue microenvironment. Although telomere shortening and dysfunction can limit immunological function by restricting proliferation of immune cells, telomere shortening appears to also increase systemic inflammation, including that associated with lupus erythematosus, rheumatoid arthritis, and granulomatous diseases (51). Individuals with short telomeres in leukocytes were found to have elevated biomarkers for systemic inflammation (52). TERC−/− mice with shortened telomeres undergo immune inflammatory response in bone marrow macrophages due to a TLR4-depenent activation of IL6 and TNFα (53). Perhaps related is the finding that telomere shortening in aged human macrophages resulted in impaired STAT5 signaling (54). Telomere uncapping was found to be associated with cellular senescence and inflammation in human arteries (55). Furthermore, malignant cells with elevated TRF2 levels had a decrease in natural killer (NK) cell infiltration in the tumor microenvironment (56). These findings suggest that telomeres contribute directly or indirectly to inflammatory signaling.
Immunological Effects of Telomere Repeat DNA.
Synthetic oligonucleotides containing CpG-DNA are known to be potent agonists of innate immunity through activation of Toll-like receptors (TLRs) (57). This activity is thought to reflect the innate immune response to foreign viral and bacterial DNA. Interestingly, synthetic telomere repeat DNA was found to suppress the production of cytokines induced by CpG DNA, as well as by other TLR agonists, including lipopolysaccharides (LPSs) (58) and various polyclonal activators (21, 59). Molecular targets for TTAGGG-repeat oligonucleotides have included STAT1 and STAT4 (60) and the lupus autoantigen Ku (61). Additionally, native DNA from telomerase-deficient mice had reduced capacity to inhibit inflammation compared to that of the control DNA (62), supporting the hypothesis that telomere-rich DNA is immunomodulatory. There have been fewer studies on the immunological effects of telomeric RNAs. However, a recent report showed that telomere RNA forming G-quadruplex structures can induce global changes in gene expression, including suppression of innate immune sensing genes (63).
Components of the Exosome Code.
The complex combination of factors that comprise exosomes and the type of recipient cells that sense the exosomes may determine the nature of the signal and response. Specific signaling through exosomes depends on the cell source of the exosomes, as well as the recipient cell receptors. Exosome coding information is provided by the lipid, protein, and nucleic acid composition. Although we did not detect full-length TERRA molecules in exosomes, the smaller processed forms of TERRA are highly enriched in exosomes from various cell types, especially LCLs (Fig. S2). This smaller, processed form of TERRA was also found to be associated with histones, which were also a major protein component of the inflammatory exosome fraction from human LCLs. Although exosomes containing higher levels of TERRA elicited greater cytokine response and purified TERRA molecules can stimulate cytokines, it is not yet clear whether the endogenous cfTERRA in exosomes is the primary immunomodulator in these microvesicles. Nevertheless, we propose that processed cfTERRA associated with histones constitutes an important telomere-derived component of inflammatory exosomes with potential to modulate signaling capacity. Thus, cfTERRA may constitute an important component of a complex, yet incompletely understood exosome code.
Materials and Methods
Plasmids for TERRA Induction.
TRF1∆N (44-439) was cloned from pBSK-hTRF1 (a gift from T. de Lange, Rockefeller University, New York) and inserted either in control Lentivirus vector pLU-CMV-Flag (Protein Expression Facility, Wistar Institute) or Vp16 domain-containing vector pLU-CMV-Flag-Vp16.
Culture Medium Fractionation and Exosome Isolation.
The supernatant of the LCL culture was fractionated and prepared for exosomes isolation by differential centrifugation as previously described (64), with some modifications (SI Materials and Methods).
ChIP Assays.
Cellular ChIP assays were performed as previously described (65).
PBMC Isolation and Cytokine Stimulation.
PBMCs were isolated from fresh donated human blood by density gradient centrifugation with Lymphoprep in SepMate-50 tubes (Stemcell Technologies). Liposomes were prepared by incorporating RNA from in vitro transcription or synthesized oligos (IDT) into Lipofectamine 2000 as previously described (66).
Additional methods are included in SI Materials and Methods.
SI Materials and Methods
Cell Culture.
LCLs were grown in RPMI medium 1640. Human colon cancer cell line HCT116 (ATCCVA) was grown in McCoy’ 5A medium. Human fibroblast IMR90 cells (ATCC) were grown in DMEM. All these mediums were supplemented with 10% (vol/vol) FBS and 1× penicillin-streptomycin. All cells were cultured in a 5% CO2 incubator at 37 °C. Serum starvation stress was induced by growth in serum containing 0.5% FBS for 24 h. When preparing cultures for exosomes purification, cells were washed twice with PBS and cultured in conditional medium. The conditional medium replaced the normal FBS with 10% exosome-depleted FBS, which was subjected to ultracentrifugation at 150,000 × g for 18 h to deplete exosomes in FBS.
Plasmids for TERRA Induction.
TRF1∆N (44-439) was cloned from pBSK-hTRF1 (a gift from T. de Lange, Rockefeller University, New York), and inserted either in control Lentivirus vector pLU-CMV-Flag (Protein Expression Facility, Wistar Institute) or Vp16 domain-containing vector pLU-CMV-Flag-Vp16. Lentivirus was produced from 293T cells by cotransfecting the constructs with viral packaging vectors PMD2.G and psPAX2 and harvested 48 h after transfection. For TERRA induction, 5 × 106 HCT116 cells were infected with 10 mL Lentivirus overnight in the presence of 2 µg/mL Polybrene (Sigma). Infected cells were selected by 2.5 µg/mL Puromycin (Sigma) 48 h after infection. After 2 d of selection, cells were then washed twice with 1× PBS and cultured 3 d in conditional medium for exosomes purification.
Culture Medium Fractionation and Exosomes Isolation.
The supernatant of LCL culture was fractionated and prepared for exosomes isolation by differential centrifugation as previously described (64) with some modifications. Briefly, LCLs were grown in conditional medium for 3 d with cell density maintained around 0.5 × 106 cells/mL. Cells were harvested by centrifugation at 300 × g for 10 min. The supernatant was collected and centrifuged at 2,000 × g for 30 min to pellet cell debris. The supernatant was subsequently filtered through a 0.45-µm filter (Millipore) and centrifuged at 16,500 × g for 30 min to pellet large microvesicles. The supernatant was further filtered through a 0.22-µm filter (Millipore) and subjected to ultracentrifugation at 110,000 × g (T45i rotor; Beckman) for 2 h to pellet exosomes. To remove potential contaminated proteins, the exosome pellet was washed once with PBS and repelleted by ultracentrifugation at 110,000 × g for 2 h. All pellets were resuspended in 100 µL PBS and kept at −80 °C until ready for use. All of the centrifugations were performed at 4 °C. The same procedures were used in preparing exosomes from culture medium of HCT116 cells.
Sucrose gradient separation of exosomes was performed as previously described (64) with some modifications. The sucrose gradient was poured 1 d before use to generate a continuous 0.25–2 M sucrose solution in 20 mM Hepes buffer (pH 7.4) at 4 °C. Exosomes were isolated from 800 mL LCL culture and resuspended in 2 mL of 20 mM Hepes buffer (pH 7.4). After loaded on the top of sucrose gradient, exosomes were ultracentrifuged at 210,000 × g for 18 h at 4 °C. After the ultracentrifugation, 1-mL fractions were collected from the top, and the density of each fraction was determined by weight. Particles were pelleted from each fraction by centrifugation at 110,000 × g for 2 h in 4 °C, resuspended in 100 µL PBS, and kept at −80 °C until ready to use.
ChIP Assays.
Cellular ChIP assays were performed as previously described (65). Exosome ChIP assays (ExChIP) were developed based on cellular ChIP assays with some modifications. For exosome RNA ChIP assays, exosomes were isolated from 800 mL LCL culture and resuspended in 4 mL PBS. Cross-linking was performed by adding formaldehyde to a final concentration of 1% to exosomes, followed by 125 mM glycine to quench cross-linking. To remove the cross-linking reagents, exosomes were subjected to buffer exchange by 100 kDa MWCO Amicon Ultra 4 mL device (Millipore) with 5 volumes of non-SDS buffer B [50 mM Tris⋅HCl (pH 8.1), 10 mM EDTA] and concentrated to 1 mL for 10 ChIP materials. After buffer exchange, exosomes were added with protease inhibitor mixture and 50 U/mL SUPERasein (Ambion), and lysed by SDS to a final concentration of 1%. The lysates were diluted 10-fold into IP buffer [0.01% SDS, 1.1% Triton X-100, 1.2 mM EDTA, 16.7 mM Tris (pH 8.1), 167 mM NaCl, protease inhibitor mixture, 50 U/mL SUPERasein] for an IP reaction at 4 °C overnight.
Each immunoprecipitated complex was pulled down by magnetic protein A/G beads (Pierce) by rotating at 4 °C for 2 h and washed five times (1-mL wash, 10 min each) in ChIP-related wash buffer at 4 °C. The immunoprecipitates were eluted by addition of 200 µL elution buffer (1% SDS, 0.1 M NaHCO3, SUPERasein) and rotated for 15 min at room temperature. Elutes were placed at 65 °C for 2 h to reverse cross-linking and subjected to RNA isolation with TRIzol reagent as describe for cell-free RNA isolation. For exosome DNA ChIP assays, the same procedures were performed without adding SUPERasein, and ChIP DNA was isolated by phenol-chloroform extraction and treated with RNase A as previously described (67). Antibodies used in this study include rabbit IgG (Santa Cruz Biotechnology), CD63 antibody (H5C6; Developmental Studies Hybridoma Bank), ribosomal protein S6 antibody (C-8; Santa Cruz Biotechnology), histone H3 antibody (catalog no. 39163; Active Motif), and TRF2 antibody (12). All ChIP assays were done in triplicate to obtain the SD.
ChIP DNA or RNA was analyzed by dot blotting. The samples were mix with denaturing solution (50% formamide, 7% formaldehyde, 1× SSC), incubated at 65 °C for 20 min, and followed immediately by cooling down on ice. The denatured samples were mixed with ice-cold 20× SSC to a final concentration of 10× and loaded onto GeneScreen Plus blotting membranes using a dot-blotting apparatus. The membrane was cross-linked and hybridized with a 32P-labeled probe with the procedures same as described in Northern blotting. Radioactive signals were quantified using ImageQuant 5.2 software (Molecular Dynamics).
Exosomes RNA Immunoprecipitation.
Exosome RNA immunoprecipitation was performed by incubating 100 µg LCL exosomes with 5 µg of each antibody and beads complex in isolation buffer (1× PBS, 0.1%BSA) by rotating overnight at 4 °C. Each immunoprecipitated complex was washed three times (1 mL wash, 10 min each) in isolation buffer. The immunoprecipitates were eluted by addition of 100 µL elution buffer (1× RIPA, SUPERasein) and rotated for 15 min at room temperature. RNA was isolated by adding 900 µL TRIzol reagent to elutes and analyzed by dot blotting as described above.
The antibodies used in this study included mouse IgG (Santa Cruz Biotechnology), CD63 antibody (H5C6; Developmental Studies Hybridoma Bank), CD63 antibody (TS63; Invitrogen), CD81 antibody (M38; Invitrogen), CD31 antibody (P2B1; Developmental Studies Hybridoma Bank), ribosomal protein S6 antibody (C-8; Santa Cruz Biotechnology), and histone H3 antibody (catalog no. 39163; Active Motif). The antibodies were pre–cross-linked with N-hydroxysuccinimide (NHS)-activated magnetic beads (Pierce) per the manufacturer’s instruction.
Blood Plasma Cell-Free RNA Isolation and RNA-seq Analysis.
The blood plasma was obtained from 2 normal subjects and 19 cancer patients, consisting of 10 different cancers that include breast, prostate, colon, duct, kidney, liver, melanoma, ovarian, and stomach. Total cell-free RNA was isolated from blood plasma with the formalin-fixed, paraffin-embedded (FFPE) RNA purification kit (Norgen Biotek) per the manufacturer’s instructions. The RNA-seq library was constructed by the Script-Seq strand-specific total RNA sample preparation kit (Illumina) per the manufacturer’s instructions and sequenced by illumina genome analyzer IIx. Reads from 36-bp RNA-seq runs were aligned against human genome hg19 using the Tophat 2.0 algorithm, and RPKM (reads per kilobase of transcript per million mapped reads) values for all transcripts from the University of California, Santa Cruz (UCSC) database were estimated.
Additionally, all 36-bp reads were tested for maximum number of telomeric motif repeats in sense orientation. Only reads containing five and six (of six possible) perfectly matched motif repeats were considered. RPKM values were calculated assuming average cfTERRA length of 200 bp. All genes with at least 10 reads (on average, ∼28,000 per sample) were ranked by RPKM values of their most expressed transcript. The cfTERRA ranks were reported along with visual representation of the 1st, 5th, 50th, 95th, and 99th quantiles for gene distribution of RPKM expression values for every sample.
RNA in Vitro Transcription.
The in vitro transcription of TERRA was performed using the MEGAscript T7 kit (Ambion) using linearized plasmids containing 250-bp telomeric repeats with a T7 promoter at either the 3′ end for sense TelG RNA or at the 5′ end for antisense TelC RNA (a gift from H. C. Riethman, Wistar Institute, Philadelphia). U6-containing RNA was synthesized using the linearized pU6-neo-TET plasmid (Addgene plasmid #51286) as a template. Plasmid templates were removed by Turbo DNase I after the transcription reaction. RNA was gel purified, and 50 ng was used in RNase digestion for Northern blot.
PBMC Isolation and Cytokine Stimulation.
PBMCs were isolated from fresh donated human blood by density gradient centrifugation with Lymphoprep in SepMate-50 tubes (Stemcell Technologies). Briefly, the blood was placed on top of Lymphoprep in SepMate-50 tubes and centrifuged at 1,200 × g for 10 min. PBMCs from the top layer were harvested and washed twice with PBS. Cells were resuspended in serum-free RPMI-1640 medium supplemented with 1 mM sodium pyruvate, 10 mM Hepes (pH 7.25), 1× Glutamax, and penicillin-streptomycin. For experiments, cells were seeded in 24-well nonadhering plates at a density of 1.5 × 106 cells/mL in each well and stimulated with exosomes or RNA-containing liposomes. These liposomes were prepared by incorporating RNA from in vitro transcription or synthesized oligos (IDT) into Lipofectamine 2000 as previously described (65). After stimulated for 3 h, PBMCs were collected for RNA isolation by TRIzol reagent as described earlier. The stimulation of IMR90 cells was performed in the same way as that in PBMCs, except that IMR90 cells were seeded in six-well plates at a density of 0.5 × 106 cells per well and cultured overnight in conditional medium with 10% exosome-depleted FBS. RNA oligos used in this study are 5′-CACUGACACUGACACUGACACUGACACUGACACUGA-3′ as control oligos, 5′-UUAGGGUUAGGGUUAGGGUUAGGGUUAGGGUUAGGG-3′ as TelG RNA oligos, and 5′-CCCUAACCCUAACCCUAACCCUAACCCUAACCCUAA-3′ as TelC RNA oligos.
Electron Microscopy.
After sucrose gradient separation of exosomes, fractions were submitted to the Electron Microscopy Resource Laboratory (EMRL) at University of Pennsylvania for electron microscopy imaging. Three microliters of each fraction was applied to lacey carbon films on 400-mesh copper transmission electron microscopy (TEM) grids. Excess liquid was blotted away with filter paper until a thin vicinal film remained. The liquid containing the fractions contents was vitrified in liquid nitrogen and cooled with liquid ethane by plunge freezing. Samples were observed at −180 °C using a Gatan 626 cryo-transfer holder on a FEI Tecnai 12 transmission electron microscope operating at 120 KeV. Images were collected digitally on a Gatan US1000 CCD bottom-mounted camera.
Telomeric RNA FISH on Tissue Sections.
Telomeric RNA FISH on tissue sections (7–12 µm) was prepared as previously described (17). Briefly, fresh frozen sections were fixed in 4% paraformaldehyde (PFA), followed by acetylation. For RNase A treatment, the slides were incubated in PBST with 100 mg/mL RNase A at 37 °C for 45 min and washed three times with PBS. All slides were prehybridized in hybridization buffer (50% formamide, 5× SSC, 5× Denhardts, 25 mg/mL yeast RNA, 0.5 mg/mL salmon sperm DNA) for 1 h at 37 °C and followed by hybridization with probe overnight at 37 °C. The probe used for TERRA detection is a Tamra-(CCCTAA)3 PNA probe (Panagene). After hybridization, slides were washed in the following sequence for 5 min at each step with gentle shaking: 2× SSC, 50% formamide for three times at 39 °C; 2× SSC for three times at 39 °C; 1× SSC once for 10 min at room temperature; and 4× SSC once at room temperature. Cellular nucleus DNA was counterstained with 0.1 g/mL DAPI in 4× SSC and 0.1% Tween-20, washed in 4× SSC, and mounted with mounting media. Slides were visualized by a Nikon E600 Upright microscope (Nikon Instruments) with ImageProPlus software (Media Cybemetrics) and Adobe PhotoShop CS5 for image processing.
RNA Isolation.
Cellular and cell-free RNA was isolated with TRIzol reagent (Invitrogen) per the manufacturer’s instructions. For cellular RNA, the isolation was performed as previously described. For cell-free RNA, 50 µL of each culture fraction (cell debris, microvesicles, or exosomes) was mixed with 450 µL TRIzol, followed by adding 100 µL chloroform. The mix was centrifuged at 12,000 × g for 15 min at 4 °C, and the aqueous phase was collected. RNA was precipitated at −20 °C for at least 1 h by mixing the aqueous phase with an equal volume of isopropanol precipitation and 1.5 µL GlycoBlue (Ambion) and collected by centrifugation at 20,000 × g for 30 min. RNA precipitates were washed with 75% ethanol, air dried, and resuspended in Nuclease-free water. These samples were treated with 1 U DNase I for 30 min at 37 °C, followed by DNase I inactivation in the presence of EDTA at 65 °C for 5 min. RNA concentration was determined by Nanodrop-2000 (Thermo Fisher). The same procedures were used in isolating cell-free RNA from sucrose fractions, RNA ChIP elute, in vitro transcribed RNA, and exosomes of HCT116 cells. For RNase treatment, RNA was incubated with the indicated RNase at 37 °C for 1 h. The RNase mixture used in this study was a combination of 100 ng/µL RNase A, 1 U/µL RNase H, and 1 U/µL RNase T1.
Northern Blot and RNA Detection.
RNA was electrophoresed on an agarose formamide gel and transferred onto a nylon membrane for Northern blotting analysis. The amounts of RNA loaded were normalized based on the purpose of each experiment as indicated in the figure legends. Briefly, each RNA sample was denatured in NorthernMax formaldehyde load dye (Ambion) for 15 min at 65 °C and separated by 1.2% agarose formamide gel in 1× Mops buffer (Ambion) at 5 V/cm. As the size of cfTERRA is ∼200 bp, electrophorese was stopped when bromophenol blue dye reached 5 cm of a 9 cm agarose formamide gel. After electrophorese, RNA samples were transferred to GeneScreen Plus blotting membranes (Perkin-Elmer) with 10× SSC and UV cross-linked onto membrane at 125 mJ in UV Stratalinker 2400 (Stratagene). For RNA detection, the blot was hybridized with a 32P-labeled probe using Church buffer (0.5 N Na-phosphate, pH 7.2, 7% SDS, 1 mM EDTA, 1% BSA) for 18 h at 50 °C. The blot was washed twice in 0.2 N Na-phosphate, 2% SDS, and 1 mM EDTA at room temperature and once in 0.1 N Na-phosphate, 2% SDS, and 1 mM EDTA at 50 °C. Radioactive signals were collected by phosphor-imager (Amersham Biosciences). Images were visualized with a Typhoon 9410 Imager (GE Healthcare). To reprobe the blot, the membrane was stripped in 1% SDS, 0.1× SSC, and 40 mM Tris⋅HCl (pH 7.5) and hybridized with another probe under the same conditions.
The probes were 5′ end-labeled with 32P by T4 polynucleotide kinase (NEB) per the manufacturer’s instructions. The probe sequences are 5′-TAACCCTAACCCTAACCCTAACCC-3′ for TERRA, 5′-TTAGGGTTAGGGTTAGGGTTAGGG-3′ for antisense, 5′-CCATCCAATCGGTAGTAGCG-3′ for 18S, and 5′-GCAGGGGCCATGCTAATCTTCTCTGTATCG -3′ for U6. The alpha satellite probe was a mixture of three oligos: 5′-CATTAAAACAAGACAGAAGCATTCTCAGAAACTCCTTTAGATGTCTGCA-3′, 5′-TGGACATTTGGAGCTCTTTTAGGCTATCGGTTGAAAAGGAAGTATCTTCA-3′, and 5′-TTTCTTTTGATAGTGCAGTTTTGAAACATTCTTTTTAAAAAATCTGCAG-3′. The EREB1 probe was a mixture of two oligos: 5′-CGATAAGCTTAAAACATGCGGACCACC-3′ and 5′-AAGCAGAGTCTGGGAAGACAACCA-3′.
Protein Analysis.
For cellular proteins, cells were lysed in RIPA buffer containing protease inhibitor mixture by rotating at 4 °C for 30 min and centrifuged at 14,000 × g at 4 °C for 15 min. Exosomal proteins were obtained by lysing the membrane with 1% SDS in the presence of the protease inhibitor mixture. The protein concentration was determined by the Bradford assay (BioRad). Total proteins were separated on a 8–16% Tris-Glycine gel (Invitrogen).
For Western blotting, the gel was transferred to a nitrocellulose membrane (BioRad). The membrane was first stained with Ponceau S to reveal the relative loading in each lane and blocked in 5% nonfat dry milk in Tris-buffered saline with Tween-20 (TBST). Then, it was probed with primary antibodies against CD63 (H5C6 for nongylcosylated form), CD63 [MEM-259 (Abcam) for the gylcosylated form], histone H4 (L64C1; Cell Signaling Tech), H3, S6, and TRF2 as indicated. After washing three times with TBST, the blot was incubated with HRP-coupled secondary antibody and developed with Luminata HRP detection reagent (Milipore).
Before submitting for MS, the gel was stained by Silver Stain Plus (BioRad). The samples corresponding to cfTERRA signals were digested in solution with trypsin. The digested protein sample was analyzed by LC-MS/MS on a Q Exactive Plus mass spectrometer. MS/MS spectra generated from the MS were searched against a human database using SEQUEST. The proteins identified by LC-MS/MS were sorted by normalized spectra count (spectra count/molecular weight) to determine which proteins are unique and abundant as shown in Dataset S1. The top 250 proteins in the LCL exosomes were analyzed by the FunRich program and compared with exosome proteins in the Vesiclepedia database.
RT-PCR Analysis of RNA.
RT-PCR experiments were performed as previously described (17) with some modifications. Briefly, 1 µg RNA was reversed transcribed with random hexamers by Super Script IV First-Strand Synthesis System (Invitrogen) in a 20-µL reaction. Synthesized cDNA was diluted with 80 µL Nuclease-free water and analyzed by real-time PCR with a SYBR green probe with the QuantStudio 6 Flex System (Life Technologies) using the standard curve method. Relative RT-PCR was determined using the ∆CT method relative to the internal control GAPDH.
Primer sequences used for real-time PCR are listed in Table S2.
Table S2.
List of oligonucleotides used for qRT-PCR and ChIP-qPCR
| Gene name | Primer sequences |
| GAPDH | Forward primer 5′-TGGGCTACACTGAGCACCAG-3′ |
| Reverse primer 5′-GGGTGTCGCTGTTGAAGTCA-3′ | |
| IL6 | Forward primer 5′-CTTTTGGAGTTTGAGGTATACCTAG-3′ |
| Reverse primer 5′-GCTGCGCAGAATGAGATGAGTTGTC-3′ | |
| TNFα | Forward primer 5′-CAGCCTCTTCTCCTTCCTGAT-3′ |
| Reverse primer 5′-GCCAGAGGGCTGATTAGAGA-3′ | |
| IL1β | Forward primer 5′-TCAGCCAATCTTCATTGCTCAA-3′ |
| Reverse primer 5′-TGGCGAGCTCAGGTACTTCTG-3′ | |
| CXCL10 | Forward primer 5′-GAAAGCAGTTAGCAAGGAAAGGT-3′ |
| Reverse primer 5′-GACATATACTCCATGTAGGGAAGTGA-3′ | |
| GM-CSF | Forward primer 5′-TCTCAGAAATGTTTGACCTCCA-3′ |
| Reverse primer 5′-GCCCTTGAGCTTGGTGAG-3′ | |
| IFNα | Forward primer 5′-CCGTGCTGGTGCTCAGCTA-3′ |
| Reverse primer 5′-TGGGTCTGAGGCAGATCACA-3′ | |
| IFNβ1 | Forward primer 5′-ACTGCCTCAAGGACAGGATG-3′ |
| Reverse primer 5′-AGCCAGGAGGTTCTCAACAA-3′ | |
| MCP1 | Forward primer 5′-AGTCTCTGCCGCCCTTCT-3′ |
| Reverse primer 5′-GTGACTGGGGCATTGATTG-3′ | |
| MMP1 | Forward primer 5′-GCTAACCTTTGATGCTATAACTACGA-3′ |
| Reverse primer 5′-TTTGTGCGCATGTAGAATCTG-3′ | |
| 2q (234-293) | Forward primer 5′-GCCTTGCCTTGGGAGAATCT-3′ |
| 10q (396-455) | Reverse primer 5′-AAAGCGGGAAACGAAAAGC-3′ |
| 7p (770-831) | Forward primer 5′-GGAGGCTGAGGCAGGAGAA-3′ |
| Reverse primer 5′-CAATCTCGGCTCACCACAATC-3′ | |
| 12q (352-419) | Forward primer 5′-ATTTCCCGTTTTCCACACTGA-3′ |
| Reverse primer 5′-CTGTTTGCAGCGCTGAATATTC-3′ | |
| 13q (152-210) | Forward primer 5′-GCACTTGAACCCTGCAATACAG-3′ |
| Reverse primer 5′-CCTGCGCACCGAGATTCT-3′ | |
| 17q (864-939) | Forward primer 5′-AGCTACCTCTCTCAACACCAAGAAG-3′ |
| Reverse primer 5′-GTCCATGCATTCTCCATTGATAAG-3′ | |
| 17p (348-431) | Forward primer 5′-GGGACAGAAGTGGATAAGCTGATC-3′ |
| Reverse primer 5′-GATCCCACTGTTTTTATTACTGTTCCT-3′ | |
| XqYq (109-162) | Forward primer 5′-CCCCTTGCCTTGGGAGAA-3′ |
| Reverse primer 5′-GAAAGCAAAAGCCCCTCTGA-3′ | |
| XpYp (243-311) | Forward primer 5′-CCACAACCCCACCAGAAAGA-3′ |
| Reverse primer 5′-GCGCGTCCGGAGTTTG-3′ |
Statistics.
Statistical analyses were carried out by paired Student t tests unless otherwise stated. P values and significance levels are annotated in the figures and described in the figure legends.
Supplementary Material
Acknowledgments
We thank Andreas Wiedmer for technical assistance, Harold C. Riethman for plasmids, and the Wistar Cancer Center Cores for Genomics and Proteomics. This work was supported by funding from National Institutes of Health, National Cancer Institute (NCI) Grant CA RO1CA140652 (to P.M.L.), NCI Cancer Center Core Grant P30 CA10815, and the Commonwealth Universal Research Enhancement Program, PA Department of Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1505962112/-/DCSupplemental.
References
- 1.Blackburn EH, Greider CW, Szostak JW. Telomeres and telomerase: The path from maize, Tetrahymena and yeast to human cancer and aging. Nat Med. 2006;12(10):1133–1138. doi: 10.1038/nm1006-1133. [DOI] [PubMed] [Google Scholar]
- 2.Cech TR. Beginning to understand the end of the chromosome. Cell. 2004;116(2):273–279. doi: 10.1016/s0092-8674(04)00038-8. [DOI] [PubMed] [Google Scholar]
- 3.Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu Rev Genet. 2008;42:301–334. doi: 10.1146/annurev.genet.41.110306.130350. [DOI] [PubMed] [Google Scholar]
- 4.O’Sullivan RJ, Karlseder J. Telomeres: Protecting chromosomes against genome instability. Nat Rev Mol Cell Biol. 2010;11(3):171–181. doi: 10.1038/nrm2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braig M, et al. A ‘telomere-associated secretory phenotype’ cooperates with BCR-ABL to drive malignant proliferation of leukemic cells. Leukemia. 2014;28(10):2028–2039. doi: 10.1038/leu.2014.95. [DOI] [PubMed] [Google Scholar]
- 6.Fumagalli M, et al. Telomeric DNA damage is irreparable and causes persistent DNA-damage-response activation. Nat Cell Biol. 2012;14(4):355–365. doi: 10.1038/ncb2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schoeftner S, Blasco MA. Developmentally regulated transcription of mammalian telomeres by DNA-dependent RNA polymerase II. Nat Cell Biol. 2008;10(2):228–236. doi: 10.1038/ncb1685. [DOI] [PubMed] [Google Scholar]
- 8.Azzalin CM, Reichenbach P, Khoriauli L, Giulotto E, Lingner J. Telomeric repeat containing RNA and RNA surveillance factors at mammalian chromosome ends. Science. 2007;318(5851):798–801. doi: 10.1126/science.1147182. [DOI] [PubMed] [Google Scholar]
- 9.Azzalin CM, Lingner J. Telomere functions grounding on TERRA firma. Trends Cell Biol. 2015;25(1):29–36. doi: 10.1016/j.tcb.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Maicher A, Lockhart A, Luke B. Breaking new ground: Digging into TERRA function. Biochim Biophys Acta. 2014;1839(5):387–394. doi: 10.1016/j.bbagrm.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Flynn RL, et al. TERRA and hnRNPA1 orchestrate an RPA-to-POT1 switch on telomeric single-stranded DNA. Nature. 2011;471(7339):532–536. doi: 10.1038/nature09772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng Z, Norseen J, Wiedmer A, Riethman H, Lieberman PM. TERRA RNA binding to TRF2 facilitates heterochromatin formation and ORC recruitment at telomeres. Mol Cell. 2009;35(4):403–413. doi: 10.1016/j.molcel.2009.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arora R, et al. RNaseH1 regulates TERRA-telomeric DNA hybrids and telomere maintenance in ALT tumour cells. Nat Commun. 2014;5:5220. doi: 10.1038/ncomms6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balk B, et al. Telomeric RNA-DNA hybrids affect telomere-length dynamics and senescence. Nat Struct Mol Biol. 2013;20(10):1199–1205. doi: 10.1038/nsmb.2662. [DOI] [PubMed] [Google Scholar]
- 15.Schoeftner S, et al. Telomere shortening relaxes X chromosome inactivation and forces global transcriptome alterations. Proc Natl Acad Sci USA. 2009;106(46):19393–19398. doi: 10.1073/pnas.0909265106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang LF, et al. Telomeric RNAs mark sex chromosomes in stem cells. Genetics. 2009;182(3):685–698. doi: 10.1534/genetics.109.103093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng Z, et al. Formation of telomeric repeat-containing RNA (TERRA) foci in highly proliferating mouse cerebellar neuronal progenitors and medulloblastoma. J Cell Sci. 2012;125(Pt 18):4383–4394. doi: 10.1242/jcs.108118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu Y, Komiyama M. Structure, function and targeting of human telomere RNA. Methods. 2012;57(1):100–105. doi: 10.1016/j.ymeth.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 19.Takahama K, et al. Regulation of telomere length by G-quadruplex telomere DNA- and TERRA-binding protein TLS/FUS. Chem Biol. 2013;20(3):341–350. doi: 10.1016/j.chembiol.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 20.Barbalat R, Ewald SE, Mouchess ML, Barton GM. Nucleic acid recognition by the innate immune system. Annu Rev Immunol. 2011;29:185–214. doi: 10.1146/annurev-immunol-031210-101340. [DOI] [PubMed] [Google Scholar]
- 21.Gursel I, et al. Repetitive elements in mammalian telomeres suppress bacterial DNA-induced immune activation. J Immunol. 2003;171(3):1393–1400. doi: 10.4049/jimmunol.171.3.1393. [DOI] [PubMed] [Google Scholar]
- 22.Mansour H. Cell-free nucleic acids as noninvasive biomarkers for colorectal cancer detection. Front Genet. 2014;5:182. doi: 10.3389/fgene.2014.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Théry C, Zitvogel L, Amigorena S. Exosomes: Composition, biogenesis and function. Nat Rev Immunol. 2002;2(8):569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 24.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14(3):195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Théry C. Exosomes: Secreted vesicles and intercellular communications. F1000 Biol Rep. 2011;3:15. doi: 10.3410/B3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ji H, et al. Proteome profiling of exosomes derived from human primary and metastatic colorectal cancer cells reveal differential expression of key metastatic factors and signal transduction components. Proteomics. 2013;13(10-11):1672–1686. doi: 10.1002/pmic.201200562. [DOI] [PubMed] [Google Scholar]
- 27.Laulagnier K, et al. Mast cell- and dendritic cell-derived exosomes display a specific lipid composition and an unusual membrane organization. Biochem J. 2004;380(Pt 1):161–171. doi: 10.1042/BJ20031594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomasoni S, et al. Transfer of growth factor receptor mRNA via exosomes unravels the regenerative effect of mesenchymal stem cells. Stem Cells Dev. 2013;22(5):772–780. doi: 10.1089/scd.2012.0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valadi H, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 30.Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Geuze HJ. Exosome: From internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci. 2000;113(Pt 19):3365–3374. doi: 10.1242/jcs.113.19.3365. [DOI] [PubMed] [Google Scholar]
- 31.Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9(8):581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 32.Umezu T, Ohyashiki K, Kuroda M, Ohyashiki JH. Leukemia cell to endothelial cell communication via exosomal miRNAs. Oncogene. 2013;32(22):2747–2755. doi: 10.1038/onc.2012.295. [DOI] [PubMed] [Google Scholar]
- 33.Dreux M, et al. Short-range exosomal transfer of viral RNA from infected cells to plasmacytoid dendritic cells triggers innate immunity. Cell Host Microbe. 2012;12(4):558–570. doi: 10.1016/j.chom.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lundholm M, et al. Prostate tumor-derived exosomes down-regulate NKG2D expression on natural killer cells and CD8+ T cells: Mechanism of immune evasion. PLoS One. 2014;9(9):e108925. doi: 10.1371/journal.pone.0108925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atay S, et al. Oncogenic KIT-containing exosomes increase gastrointestinal stromal tumor cell invasion. Proc Natl Acad Sci USA. 2014;111(2):711–716. doi: 10.1073/pnas.1310501111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caby MP, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Int Immunol. 2005;17(7):879–887. doi: 10.1093/intimm/dxh267. [DOI] [PubMed] [Google Scholar]
- 37.Verweij FJ, et al. LMP1 association with CD63 in endosomes and secretion via exosomes limits constitutive NF-κB activation. EMBO J. 2011;30(11):2115–2129. doi: 10.1038/emboj.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beninson LA, Fleshner M. Exosomes: An emerging factor in stress-induced immunomodulation. Semin Immunol. 2014;26(5):394–401. doi: 10.1016/j.smim.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 39.Suram A, Herbig U. The replicometer is broken: Telomeres activate cellular senescence in response to genotoxic stresses. Aging Cell. 2014;13(5):780–786. doi: 10.1111/acel.12246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Armanios M, Blackburn EH. The telomere syndromes. Nat Rev Genet. 2012;13(10):693–704. doi: 10.1038/nrg3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Falandry C, Bonnefoy M, Freyer G, Gilson E. Biology of cancer and aging: A complex association with cellular senescence. J Clin Oncol. 2014;32(24):2604–2610. doi: 10.1200/JCO.2014.55.1432. [DOI] [PubMed] [Google Scholar]
- 42.Reddel RR. Telomere maintenance mechanisms in cancer: Clinical implications. Curr Pharm Des. 2014;20(41):6361–6374. doi: 10.2174/1381612820666140630101047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Velarde MC, Demaria M, Campisi J. Senescent cells and their secretory phenotype as targets for cancer therapy. Interdiscip Top Gerontol. 2013;38:17–27. doi: 10.1159/000343572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coppé JP, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6(12):2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Porro A, et al. Functional characterization of the TERRA transcriptome at damaged telomeres. Nat Commun. 2014;5:5379. doi: 10.1038/ncomms6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caslini C, Connelly JA, Serna A, Broccoli D, Hess JL. MLL associates with telomeres and regulates telomeric repeat-containing RNA transcription. Mol Cell Biol. 2009;29(16):4519–4526. doi: 10.1128/MCB.00195-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deng Z, et al. HSV-1 remodels host telomeres to facilitate viral replication. Cell Reports. 2014;9(6):2263–2278. doi: 10.1016/j.celrep.2014.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cusanelli E, Romero CA, Chartrand P. Telomeric noncoding RNA TERRA is induced by telomere shortening to nucleate telomerase molecules at short telomeres. Mol Cell. 2013;51(6):780–791. doi: 10.1016/j.molcel.2013.08.029. [DOI] [PubMed] [Google Scholar]
- 49.Schoeftner S, Blasco MA. Chromatin regulation and non-coding RNAs at mammalian telomeres. Semin Cell Dev Biol. 2010;21(2):186–193. doi: 10.1016/j.semcdb.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 50.Porro A, Feuerhahn S, Lingner J. TERRA-reinforced association of LSD1 with MRE11 promotes processing of uncapped telomeres. Cell Reports. 2014;6(4):765–776. doi: 10.1016/j.celrep.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 51.Georgin-Lavialle S, et al. The telomere/telomerase system in autoimmune and systemic immune-mediated diseases. Autoimmun Rev. 2010;9(10):646–651. doi: 10.1016/j.autrev.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 52.Wong JY, De Vivo I, Lin X, Fang SC, Christiani DC. The relationship between inflammatory biomarkers and telomere length in an occupational prospective cohort study. PLoS One. 2014;9(1):e87348. doi: 10.1371/journal.pone.0087348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bhattacharjee RN, Banerjee B, Akira S, Hande MP. Telomere-mediated chromosomal instability triggers TLR4 induced inflammation and death in mice. PLoS One. 2010;5(7):e11873. doi: 10.1371/journal.pone.0011873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sebastián C, et al. Telomere shortening and oxidative stress in aged macrophages results in impaired STAT5a phosphorylation. J Immunol. 2009;183(4):2356–2364. doi: 10.4049/jimmunol.0901131. [DOI] [PubMed] [Google Scholar]
- 55.Morgan RG, et al. Age-related telomere uncapping is associated with cellular senescence and inflammation independent of telomere shortening in human arteries. Am J Physiol Heart Circ Physiol. 2013;305(2):H251–H258. doi: 10.1152/ajpheart.00197.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Biroccio A, et al. TRF2 inhibits a cell-extrinsic pathway through which natural killer cells eliminate cancer cells. Nat Cell Biol. 2013;15(7):818–828. doi: 10.1038/ncb2774. [DOI] [PubMed] [Google Scholar]
- 57.Ishii KJ, Akira S. Innate immune recognition of, and regulation by, DNA. Trends Immunol. 2006;27(11):525–532. doi: 10.1016/j.it.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 58.Yagci FC, et al. Mammalian telomeric DNA suppresses endotoxin-induced uveitis. J Biol Chem. 2010;285(37):28806–28811. doi: 10.1074/jbc.M110.125948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lenert PS. Classification, mechanisms of action, and therapeutic applications of inhibitory oligonucleotides for Toll-like receptors (TLR) 7 and 9. Mediators Inflamm. 2010;2010:986596. doi: 10.1155/2010/986596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shirota H, Gursel M, Klinman DM. Suppressive oligodeoxynucleotides inhibit Th1 differentiation by blocking IFN-gamma- and IL-12-mediated signaling. J Immunol. 2004;173(8):5002–5007. doi: 10.4049/jimmunol.173.8.5002. [DOI] [PubMed] [Google Scholar]
- 61.Bianchi A, de Lange T. Ku binds telomeric DNA in vitro. J Biol Chem. 1999;274(30):21223–21227. doi: 10.1074/jbc.274.30.21223. [DOI] [PubMed] [Google Scholar]
- 62.Klinman DM, Tross D, Klaschik S, Shirota H, Sato T. Therapeutic applications and mechanisms underlying the activity of immunosuppressive oligonucleotides. Ann N Y Acad Sci. 2009;1175:80–88. doi: 10.1111/j.1749-6632.2009.04970.x. [DOI] [PubMed] [Google Scholar]
- 63.Hirashima K, Seimiya H. Telomeric repeat-containing RNA/G-quadruplex-forming sequences cause genome-wide alteration of gene expression in human cancer cells in vivo. Nucleic Acids Res. 2015;43(4):2022–2032. doi: 10.1093/nar/gkv063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thery C, Amigorena S, Raposo G, Clayton A. 2006. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Current Protocols in Cell Biology, ed Bonifacino JS (John Wiley & Sons, Somerset, NJ), Chap 3. [DOI] [PubMed]
- 65.Deng Z, et al. A role for CTCF and cohesin in subtelomere chromatin organization, TERRA transcription, and telomere end protection. EMBO J. 2012;31(21):4165–4178. doi: 10.1038/emboj.2012.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rigby RE, et al. RNA:DNA hybrids are a novel molecular pattern sensed by TLR9. EMBO J. 2014;33(6):542–558. doi: 10.1002/embj.201386117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dahl JA, Collas P. MicroChIP: Chromatin immunoprecipitation for small cell numbers. Methods Mol Biol. 2009;567:59–74. doi: 10.1007/978-1-60327-414-2_4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.