Significance
Eukaryotic cells contain many different organelles between which vesicles traffic material. COPI-coated vesicles mediate essential, evolutionarily conserved retrograde trafficking pathways from the Golgi to the endoplasmic reticulum (ER) and within the Golgi. We have determined structures of the μ-homology domain (MHD) of the COPI δ-COP subunit in complex with tryptophan-based motifs from the ER-resident vesicle-docking/tethering complex Dsl1, giving a mechanistic description of a tether/coat interaction; furthermore we demonstrate that this interaction plays a role in facilitating COPI-coated vesicle transport in vivo. Our work demonstrates the structure of each eukaryotic cell MHD and shows that MHDs are adaptable scaffolds that can interact specifically with a range of proteins and phospholipids at different positions.
Keywords: δ-COP μ homology domain-binding motifs, coatomer, COPI, vesicle coat, membrane trafficking
Abstract
Coatomer consists of two subcomplexes: the membrane-targeting, ADP ribosylation factor 1 (Arf1):GTP-binding βγδζ-COP F-subcomplex, which is related to the adaptor protein (AP) clathrin adaptors, and the cargo-binding αβ’ε-COP B-subcomplex. We present the structure of the C-terminal μ-homology domain of the yeast δ-COP subunit in complex with the WxW motif from its binding partner, the endoplasmic reticulum-localized Dsl1 tether. The motif binds at a site distinct from that used by the homologous AP μ subunits to bind YxxΦ cargo motifs with its two tryptophan residues sitting in compatible pockets. We also show that the Saccharomyces cerevisiae Arf GTPase-activating protein (GAP) homolog Gcs1p uses a related WxxF motif at its extreme C terminus to bind to δ-COP at the same site in the same way. Mutations designed on the basis of the structure in conjunction with isothermal titration calorimetry confirm the mode of binding and show that mammalian δ-COP binds related tryptophan-based motifs such as that from ArfGAP1 in a similar manner. We conclude that δ-COP subunits bind Wxn(1–6)[WF] motifs within unstructured regions of proteins that influence the lifecycle of COPI-coated vesicles; this conclusion is supported by the observation that, in the context of a sensitizing domain deletion in Dsl1p, mutating the tryptophan-based motif-binding site in yeast causes defects in both growth and carboxypeptidase Y trafficking/processing.
COPI vesicles mediate retrograde trafficking from the Golgi to the endoplasmic reticulum (ER) and within the Golgi (reviewed in refs. 1–3). The COPI vesicle coat consists of two major components, coatomer and the small GTPase ADP ribosylation factor 1 (Arf1). Coatomer is an ∼600 kDa heteroheptameric complex consisting of two linked subcomplexes, the βγδζ-COP F-subcomplex and the αβ’ε-COP B-subcomplex, all conserved from yeast to humans.
Recent studies have led to a model for COPI-coated vesicle formation in which the F-subcomplex functions as a traditional Arf1:GTP effector but with two membrane-attached Arf1:GTP molecules binding to quasi-equivalent sites on a single F-subcomplex, recruiting F-subcomplex and its associated B-subcomplex onto the membrane en bloc (4). Once the vesicle has budded from its donor membrane, an Arf GTPase-activating protein (ArfGAP) catalyzes the hydrolysis of GTP on Arf1, and the Arf1:GDP dissociates from the membrane, followed later by the dissociation of the coatomer complex that was bound to the transport vesicle (5). ArfGAPs also have been proposed to function at different stages in vesicle biogenesis, both as terminators and, during an earlier cargo-editing step, as effectors (reviewed in depth in ref. 6). In the final stages of its life, a COPI-coated vesicle docks with the ER via the Dsl1-tethering complex, completes uncoating, and finally undergoes SNARE-mediated fusion with the ER (7–9).
The two main ArfGAPs associated with COPI-dependent retrograde transport in yeast are Gcs1p and Glo3p. These proteins can substitute for one another, at least partially, in COPI vesicle-mediated transport, although Glo3p, which binds the γ-COP appendage domain, has been proposed to play the more significant role (10–12). Cassel and coworkers (13) have demonstrated, for mammalian ArfGAP1, that a di-tryptophan motif (WxxxxW) located within the unstructured C terminus of the protein can bind to the mammalian δ-COP C-terminal μ-homology domain (MHD). Intriguingly, this tryptophan motif resembles a proposed yeast δ-COP–binding motif termed “δL,” which was identified by yeast two-hybrid screening and then discovered in a cytosolic protein which at that time was of uncertain function in COPI vesicle trafficking (14). Subsequently similar motifs were identified in the central low complexity region (the so-called “lasso”) of the Dsl1p subunit of the yeast Dsl1 tethering complex (7–9, 15). Here we reveal the structure of Saccharomyces cerevisiae δ-COP MHD and the basis for its binding to di-tryptophan–based motifs from Dsl1p and also demonstrate that a related WxxF motif from the extreme C terminus of Gcs1p binds at the same site on δ-COP. The functional relevance of this binding site on δ-COP is indicated by the observation that mutations in the site that abolish motif binding can affect CPY trafficking/processing and cause growth phenotypes.
Results
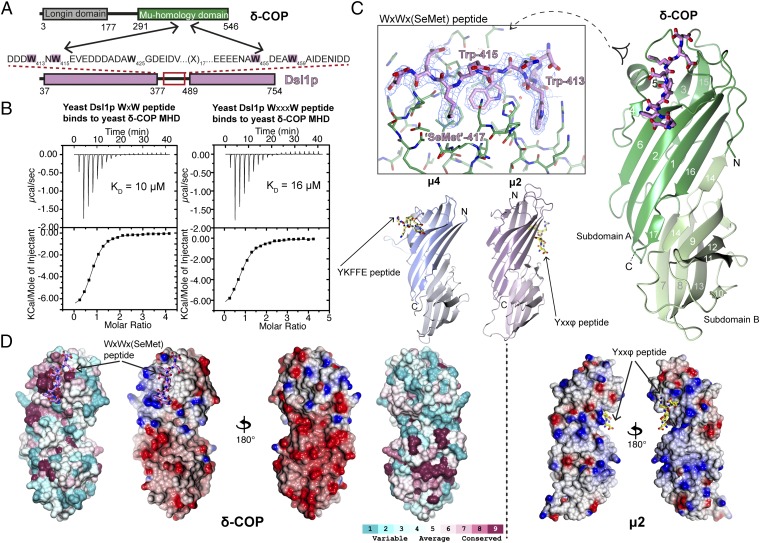
Structure of the δ-COP MHD in Complex with the Dsl1p WxW Motif.
Isothermal titration calorimetry (ITC) was used to test the ability of bacterially expressed yeast δ-COP MHD (residues 282–546) (Fig. 1A) to bind di-tryptophan peptides derived from the yeast Dsl1p lasso (residues 378–488). Low micromolar KDs (∼10 μM) were observed for the binding of δ-COP MHD to peptides with spacing WxW and WxxxW (Fig. 1B). Di-tryptophan peptides with four, five, or six residues between the tryptophans also bound, with KDs between 10 and 50 μM (Fig. S1). These results suggest that the presence of two relatively closely spaced tryptophan side chains is the main determinant for binding.
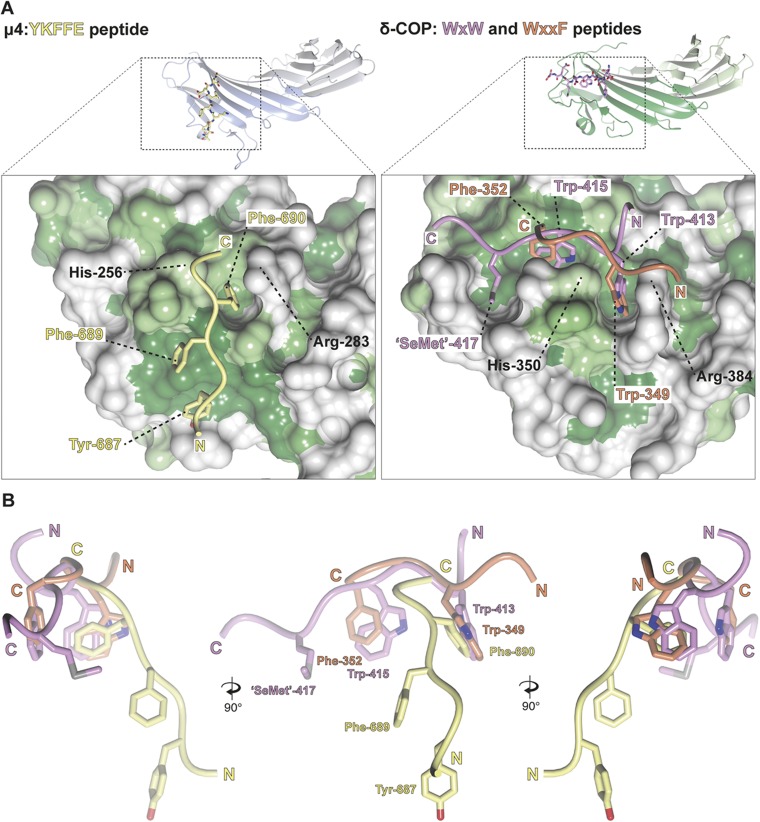
Fig. 1.
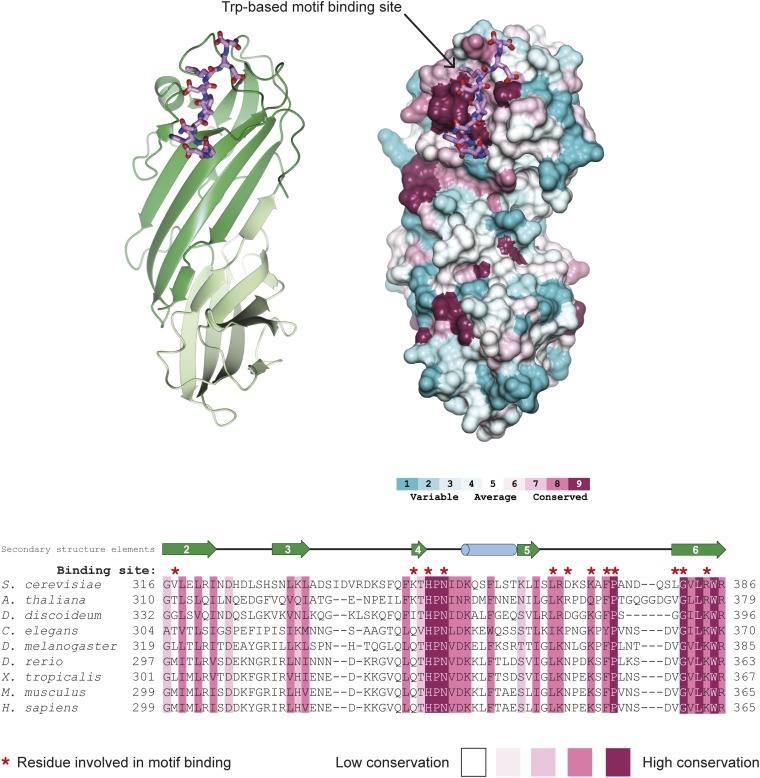
Structure of yeast δ-COP MHD with the yeast Dsl1p WxWxϕ motif. (A) Schematic representation of interactions between δ-COP and Dsl1p. (B) ITC experiments showed that yeast δ-COP MHD (residues 282–546) binds to di-tryptophan peptides corresponding to the WxW (DDW413NW415EVED) and WxxxW (ENAW455DEAW459AIDEC) motifs in yeast Dsl1p with KDs of 11 ± 2 and 16 ± 1 μM (SD, n = 3) respectively, at a 1:1 stoichiometric ratio. (C) Crystal structure of yeast δ-COP MHD with a SeMet-substituted Dsl1p WxW peptide [DDWNWE(SeMet)ED] showing that di-tryptophan motifs bind to δ-COP at a position similar to that at which the YKFFE sequence of APP binds to the μ4 MHD (PDB ID code 3L81) (17) but at a completely different site from the binding of Yxxϕ signals to AP MHDs (PDB ID code 1BXX) (16, 18, 19). The final refined 2mFO-DFC density contoured at 1.2σ and mFO-DFC difference density at ±3.0σ for the peptide are shown in the Inset. The total buried area of the interface between δ-COP and the WxWx(SeMet) peptide is 1,354 Å2, as calculated by the PISA server (www.ebi.ac.uk/pdbe/pisa/). (D) The Consurf server (consurf.tau.ac.il/) was used to create a surface representation of the evolutionary conservation of residues in δ-COP MHD based on an alignment from yeast to humans using ClustalO (see also Fig. S3). The tryptophan-based motif-binding site is the outstanding feature of this conservation surface representation. Surface representations of the electrostatic potential of δ-COP MHD and μ2 MHD, highlight that δ-COP MHD is largely negative [contoured from −0.5 V (red) to +0.5 V (blue)]. The di-tryptophan motif-binding site on δ-COP MHD is relatively positive compared with the remainder of the domain. The conservation surface representation shows that the negatively charged surface on the backside of subdomain B is also highly conserved.
Fig. S1.
δ-COP MHD binds to W(x)nW motifs where n = 4, 5, or 6. (A) ITC experiments showed that yeast δ-COP MHD (residues 282–546) (W404A) binds to W(x)nW peptides (TDDGWDNQNW, TDDGWDNSQNW, and TDDGWDNSSQNW) where n = 4, 5, or 6 with KDs of 10, 21 ± 2, and 38 ± 2 μM (SD, n = 3), respectively, at a 1:1 stoichiometric ratio. (B) ITC showing that δ-COP MHD (residues 282–546) (W404A) binds to a peptide [DDWNWE(SeMet)ED] corresponding to the WxW motif in the Dsl1p lasso, in which the valine in the native sequence was replaced by a SeMet to help identify the peptide orientation. The peptide bound with an affinity similar to that of the native peptide [Kd = 4 ± 1 μM vs. 11 ± 2 μM (SD); n = 3)] (Fig. 1B) at a 1:1 stoichiometric ratio. The slightly higher affinity of the SeMet-derived peptide presumably results from additional van der Waals interactions of the methionine side chain with hydrophobic pocket 3, as compared with the native valine side chain (Fig. S2). (C) ITC showed that a combination of H330A and K363S mutations in human δ-COP (homologous to the H350A and R384S mutations in yeast δ-COP; see Fig. S3) abolishes (Kd >300 μM) the interaction of the human ArfGAP1 WxxxxW peptide (TDDGWDNQNW) (red squares) with δ-COP MHD (residues 272–511) (W381A) (black squares). The lack of peptide binding shown by the H330A/K363S shows that the human ArfGAP1 WxxxxW peptide (TDDGWDNQNW) binds to the human δ-COP MHD at the same site as the Gcs1p WxxF peptide binds to the yeast δ-COP MHD. Data for the H330A K363S W381A mutant are offset by +0.25 μcal/s for clarity.
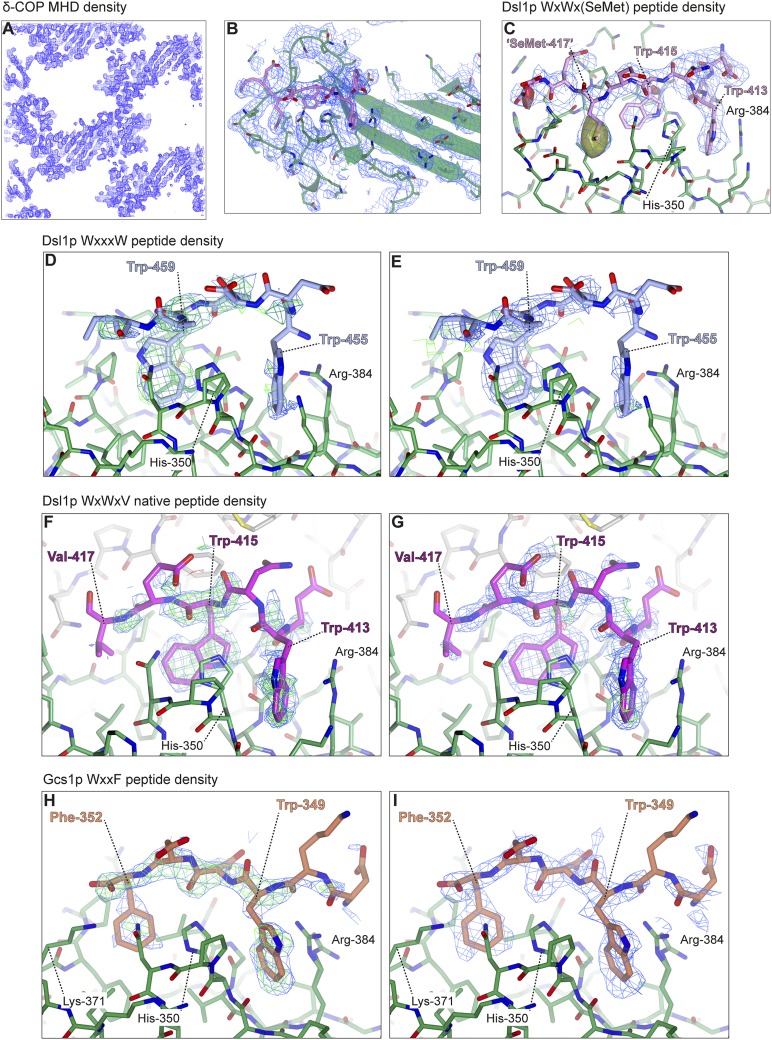
Yeast δ-COP MHD was cocrystallized with the strongest-binding peptide, corresponding to the WxW motif in the Dsl1p lasso [DDWNWE(SeMet)ED]. [N.B.: The valine in the native sequence was replaced by a selenomethionine (SeMet) to help identify the peptide orientation; this peptide bound with an affinity similar to that of a native sequence peptide (Fig. S1).] The structure was determined at 2.8-Å resolution by SeMet multiwavelength anomalous dispersion (MAD) methods (see Materials and Methods, Fig. S2, and Table S1 for detailed descriptions of structure determination).
Fig. S2.
Structural overview of δ-COP MHD binding to tryptophan-based motifs: electron density from δ-COP MHD in complex with the Dsl1p WxWx(SeMet) peptide (A–C), with the Dsl1p WxxxW peptide (D and E), with the Dsl1p WxWxV native peptide (F and G), and with the Gcs1p WxxF peptide (H and I). (A–C) Part of the experimentally phased, solvent-flattened electron density map from autoSHARP (44) highlighting the clear protein–solvent boundary (A and B) and the clear density for the peptide (B and C) (1.0σ). In C an anomalous difference map (solid) (±3.0σ; yellow = positive; red = negative) (clipped around peptide) shows the large positive peak representing the SeMet in the WxWx(SeMet) peptide. (D and E) 2mFO-FC (0.8σ) and mFO-FC (±2.5σ) density maps representing the WxxxW peptide before (D) and after (E) peptide model building, showing that the peptide occupancy is low, and the density is difficult to interpret. However the peptide most likely binds in the same orientation as the other peptides. (F and G) 2mFO-FC (0.8σ) and mFO-FC (±3.0σ) density maps representing the native Dsl1p WxWxV peptide before (F) and after (G) peptide model building, showing the clear density for the two tryptophans. (H and I) 2mFO-FC (1.0σ) and mFO-FC (±3.0σ) density maps representing the Gcs1p WxxF peptide before (H) and after (I) peptide model building, showing the clear density for the tryptophan and phenylalanine.
Table S1.
Data collection and refinement statistics for δ-COP MHD complexed with WxWx(SeMet) and WxxF peptides
| δ-COP MHD W404A SeMet with Dsl1p WxWx(SeMet) peptide | δ-COP MHD W404A SeMet with Gcs1p WxxF peptide | |||
| High remote | Inflection | Peak | ||
| Data collection | ||||
| Beamline | Diamond I03 | Diamond I03 | Diamond I03 | Diamond I04-1 |
| Space group | P1 | P1 | P1 | P212121 |
| Wavelength, Å | 0.97631 | 0.97961 | 0.97949 | 0.9173 |
| Cell dimensions | ||||
| a, b, c, Å | 68.4, 79.4, 163.4 | 68.4, 79.4, 163.4 | 68.4, 79.4, 163.4 | 70.1, 84.9, 148.6 |
| α, β, γ, ° | 79.7, 87.5, 83.6 | 79.7, 87.5, 83.6 | 79.7, 87.5, 83.6 | 90.0, 90.0, 90.0 |
| Resolution range, Å* | 78–2.80 (2.85–2.80) | 78–2.90 (2.90–2.97) | 78–2.80 (2.85–2.80) | 55.9–2.45 (2.55–2.45) |
| Rmerge*,† | 0.126 (1.208) | 0.090 (1.076) | 0.128 (1.855) | 0.105 (0.990) |
| Rmeas*,‡ | 0.149 (2.340) | 0.127 (1.574) | 0.151 (2.214) | 0.114 (1.086) |
| CC1/2*,§ | 0.996 (0.537) | 0.996 (0.532) | 0.996 (0.504) | 0.998 (0.508) |
| Mean I/σI* | 12.9 (1.1) | 10.4 (1.0) | 11.5 (1.0) | 12.5 (1.9) |
| Completeness, %* | 97.8 (97.4) | 83.7 (93.0) | 97.9 (97.4) | 99.9 (99.9) |
| Multiplicity* | 7.0 (7.2) | 3.7 (3.7) | 7.0 (7.2) | 6.5 (5.8) |
| Wilson <B>, Å2 | 86 | 55.7 | ||
| Anomalous completeness, %* | 96.6 (96.6) | 77.6 (87.5) | 96.7 (96.9) | 99.7 (98.4) |
| CC1/2 on Δanom (inner bin)* | 0.191 (0.000) | 0.183 (0.011) | 0.461 (0.031) | 0.205 (0.021) |
| Phasing | ||||
| Rcullis isomorphous | — | 0.705 | 0.713 | |
| Rcullis anomalous | 0.945 | 0.962 | 0.879 | |
| Overall phasing power isomorphous | — | 0.470 | 0.352 | |
| Overall phasing power anomalous | 0.525 | 0.387 | 0.775 | |
| Figure of merit (before/after solvent flattening) | 0.293/0.887 | 0.293/0.887 | 0.293/0.887 | |
| Refinement | ||||
| Resolution range, Å | 77.6–2.80 | 77.6–2.80 | 77.6–2.80 | 55.9–2.45 |
| No. of reflections | 76,847 | 76,847 | 76,847 | 31880 |
| Rwork/Rfree | 0.189/0.234 | 0.189/0.234 | 0.189/0.234 | 0.193/0.258 |
| Number of atoms | ||||
| Protein | 16,445 | 16,445 | 16,445 | 6,156 |
| Ligand/ion | 696 | 696 | 696 | 110 |
| Water | 554 | 554 | 554 | 257 |
| B-factors, Å2 | 92.3 | 92.3 | 92.3 | 32.4 |
| Rmsd from ideal values | ||||
| Bond lengths, Å | 0.012 | 0.012 | 0.012 | 0.013 |
| Bond angles, ° | 1.526 | 1.526 | 1.526 | 1.596 |
| Ramachandran plot | ||||
| Favored region, % | 97.4 | 97.4 | 97.4 | 97.5 |
| Allowed, % | 1.8 | 1.8 | 1.8 | 2.1 |
| Outliers, % | 0.8 | 0.8 | 0.8 | 0.4 |
| Rotamer outliers, % | 2.9 | 2.9 | 2.9 | 4.8 |
| C-beta outliers | 4 | 4 | 4 | 1 |
| PDB ID code | 5FJW | 5FJW | 5FJW | 5FJX |
Values in parentheses are for the highest-resolution shell.
Rmerge = Σ(Ihl - <Ih>)/Σ(Ihl) where <Ih> is the mean intensity of unique reflection h, summed over all reflections for each observed intensity Ihl.
Rmeas = Σ(n/n – 1)1/2 (Ihl - <Ih>)/Σ(Ihl) where n is the number of observations for unique reflection h with mean intensity <Ih>, summed over all reflections for each observed intensity Ihl.
CC1/2 is the correlation coefficient on <I> between random halves of the dataset. Δanom, anomalous difference I+ − I−.
As predicted, yeast δ-COP MHD resembled μ1–4 adaptins and Syp1p, being constructed of 17 β-strands arranged in two interlinked β-sandwich subdomains (Fig. 1C) (16–20). One striking feature of the δ-COP MHD structure is its large negative electrostatic surface potential, which is conserved across species and therefore presumably is functionally important (Fig. 1D). This global feature is in marked contrast to the MHDs of AP1 and especially AP2, which are extremely positively charged (Fig. 1D). The role of these positively charged patches in AP MHDs is to mediate binding to negatively charged, organelle-specific phosphatidylinositol phosphates (PIPs): PtdIns(4,5)P2 in the case of adapter protein 2 (AP2) at the plasma membrane and presumably PtdIns(4)P in the case of AP1 at the trans-Golgi network (TGN) (21–23). By analogy the δ-COP MHD also could be involved in the specificity of COPI membrane recruitment and/or of COPI-coated vesicle docking (see Discussion).
All nine residues of the Dsl1p lasso peptide were clearly visible in electron density maps, binding at a site comprising δ-COP MHD strands 4 and 6 and the loop between strands 5 and 6. The identities of the residues and length of the interstrand loop at this site are well conserved from yeast to mammals (Fig. 1D and Fig. S3). The WxW peptide-binding motif occurs at a position adjacent to and slightly overlapping with the binding site of the YKFFE sequence of APP (amyloid precursor protein) to the μ4 MHD (17) but at a site completely different from and in fact on a face orthogonal to the binding of YxxΦ signals to AP MHDs (Fig. 1C) (16).
Fig. S3.
Conservation of the tryptophan-based motif-binding site in δ-COP MHD. The Consurf server (consurf.tau.ac.il/) was used to create a surface representation of evolutionary conservation of residues in the δ-COP MHD (also shown in Fig. 1D), based on an alignment from yeast to humans using ClustalO (shown here). The region that forms the tryptophan-based motif-binding site in this alignment is shown here. Residues directly involved in the binding site are highlighted above the alignment in red. The tryptophan-based motif-binding site is the outstanding feature of the conservation surface representation, indicating its importance. The Dsl1p WxW peptide is shown. Note the conservation of the length of the loop between strands 5 and 6.
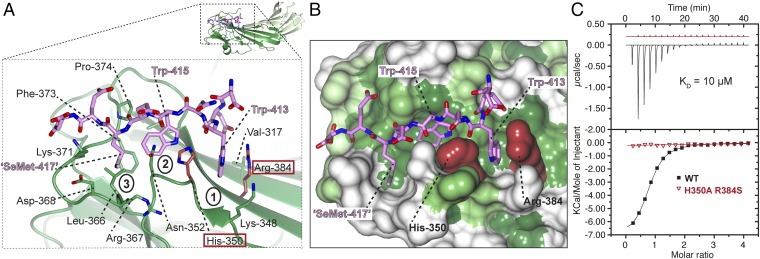
The WxW peptide binds in an extended conformation with the three hydrophobic residues binding into three complementary hydrophobic pockets (Fig. 2A and Fig. S2). The N-terminal tryptophan of the WxW motif in the Dsl1p lasso, Trp-413, is deeply buried in pocket 1 (Fig. 2B). Structure-based superposition shows that pocket 1 in δ-COP is equivalent to the pocket in μ4 in which Phe-690 (YKFFE) in APP is buried (Fig. S4) (17). The C-terminal tryptophan, Trp-415, is deeply buried in a hydrophobic pocket (pocket 2) created by the loop between strands 5 and 6, which is significantly longer than in other MHDs (15 residues vs. 4 in μ2, for example). The SeMet in the DDWNWE(SeMet)ED) peptide (SeMet replacing valine in the native sequence) is buried in the third hydrophobic pocket (pocket 3), which could be considered an extension of pocket 2. Aspartate residues, often found adjacent to the tryptophan residues in δ-COP MHD-binding sequences, may serve to funnel ligands toward the binding site, which is the only significant non-negatively charged patch on the otherwise negatively charged δ-COP MHD surface (Fig. 1D).
Fig. 2.
Structural details of Dsl1p WxW peptide bound to δ-COP MHD. (A) The Dsl1p WxWx(SeMet) peptide [DDWNWE(SeMet)ED] binds to the δ-COP MHD in an extended conformation with the three hydrophobic residues binding into three complementary pockets (labeled). (B) Surface representation of the di-tryptophan motif-binding site in the δ-COP MHD colored from high (dark green) to low (white) hydrophobicity. The three hydrophobic pockets into which the hydrophobic residues in the peptide bind can be seen clearly. (C) ITC showing that a combination of H350A and R384S mutations (highlighted in dark red in A and B) abolish (Kd >300 μM) (red triangles) the interaction of the Dsl1p WxW peptide (DDWNWEVED) with δ-COP MHD (black squares) (Kd = 11 ± 2 μM) (SD, n = 3). Data for the mutant are offset for clarity.
Fig. S4.
The δ-COP MHD binds to tryptophan-based motifs at a site similar to that at which μ4 binds to the YKFFE motif. (A) Surface representations of the YKFFE-binding site in the μ4 MHD (PDB ID code 3L81) (17) and the tryptophan-based motif-binding site in δ-COP MHD colored from high (dark green) to low (white) hydrophobicity with the relevant peptides shown. This representation shows that pocket 1 (the pocket between His-350 and Arg-384) in δ-COP is equivalent to the pocket in μ4 in which Phe-690 (YKFFE) in the APP motif is buried, as is evident from the superposition of the μ4 and δ-COP MHDs. (B) Three different angles of the superposed peptides are shown.
We used this structure as a molecular replacement model to solve at 1.8-Å resolution a different (and merohedrally twinned) crystal form of yeast δ-COP MHD in complex with the equivalent non-SeMet peptide DDWNWEVED (Table S2). The structures were essentially identical, with the peptides bound in the same way (Fig. S2). We also were able to solve another cocrystal structure of yeast δ-COP MHD in complex with a peptide corresponding to the W455xxxW459 motif in Dsl1p (Table S2). In this structure, the motif also bound at the same site and most likely in the same orientation, although low occupancy of the peptide prevented detailed analysis of the binding (Fig. S2). A final crystal form of yeast δ-COP MHD, crystallized without peptide, was determined by molecular replacement (Table S2). This structure showed that the binding of peptide had no discernable effect on the structure (Fig. S5); i.e., binding occurs to an effectively rigid template.
Table S2.
Data collection and refinement statistics for δ-COP MHD complexed with WxWxV and WxxxW peptides and without peptide
| δ-COP MHD W404A native with Dsl1p WxWxV peptide merohedrally twinned | δ-COP MHD WT native | δ-COP MHD W404A SeMet with Dsl1p WxxxW peptide | |
| Data collection | |||
| Beamline | Diamond I03 | Diamond I02 | Diamond I04-1 |
| Space group | P3121 | P212121 | P3121 |
| Wavelength, Å | 0.96863 | 1.0 | 0.91732 |
| Cell dimensions | |||
| a, b, c, Å | 72.8, 72.8, 343.3 | 86.9, 148.6, 222.7 | 72.9, 72.9, 340.9 |
| α, β, γ, ° | 90.0, 90.0, 120.0 | 90.0, 90.0, 90.0 | 90.0, 90.0, 120.0 |
| Resolution range, Å* | 36.4–1.90 (1.94–1.90) | 48.4–3.00 (3.16–3.00) | 85.2–2.75 (2.9–2.75) |
| Rmerge*,† | 0.129 (0.713) | 0.098 (0.869) | 0.117 |
| Rmeas*,‡ | 0.137 (0.755) | 0.115 (1.020) | 0.219 (0.598) |
| CC1/2*,§ | 0.994 (0.643) | 0.997 (0.448) | 0.974 (0.577) |
| Mean I/σI* | 11.3 (3.4) | 8.5 (1.7) | 6.1 (2.8) |
| Completeness, %* | 98.3 (98.3) | 99.8 (99.9) | 97.8 (95.9) |
| Multiplicity* | 9.1 (9.2) | 3.6 (3.6) | 5.5 (4.7) |
| Wilson <B>, Å2 | 19.1 | 95.1 | |
| No. of twin domains | 2 | ||
| Twin fractions, %, h,k,l/-h,-k,l | 0.59/0.41 | ||
| Refinement | |||
| Resolution range, Å | 36.4–1.90 | 48.4–3.00 | |
| No. of reflections | 78,953 | 554,31 | |
| Rwork/Rfree | 0.136/0.189 | 0.195/0.233 | |
| Number of atoms | |||
| Protein | 8,217 | 16,523 | |
| Ligand/ion | 240 | 2 | |
| Water | 589 | 288 | |
| B-factors, Å2 | 28.0 | 92.6 | |
| Rmsd from ideal values | |||
| Bond lengths, Å | 0.020 | 0.010 | |
| Bond angles, ° | 2.11 | 1.38 | |
| Ramachandran plot | |||
| Favored region, % | 95.9 | 95.8 | |
| Allowed, % | 3.4 | 3.4 | |
| Outliers, % | 0.7 | 0.8 | |
| Rotamer outliers, % | 2.1 | 2.1 | |
| C-beta outliers | 8 | 8 | |
| PDB ID code | 5FJZ | 5FK0 | |
Values in parentheses are for the highest resolution shell.
Rmerge = Σ(Ihl - <Ih>)/Σ(Ihl) where <Ih> is the mean intensity of unique reflection h, summed over all reflections for each observed intensity Ihl.
Rmeas = Σ(n/n – 1)1/2 (Ihl - <Ih>)/Σ(Ihl) where n is the number of observations for unique reflection h with mean intensity <Ih>, summed over all reflections for each observed intensity Ihl.
CC1/2 is the correlation coefficient on <I> between random halves of the dataset. Δanom, anomalous difference I+ − I−.
Fig. S5.
Binding of tryptophan-based motifs to the δ-COP MHD through a lock-and-key mechanism. Superposition of the δ-COP MHD bound to peptide (dark green) [of the δ-COP: Dsl1p WxWx(SeMet) peptide structure] with a molecule not bound to peptide (light green) shows that the binding of peptide has no discernable effect on the structure. The only clear difference is the different conformation of the Leu-380 side chain, creating a larger hydrophobic pocket into which the second tryptophan binds (pocket 2).
Mutagenesis of the Tryptophan-Based Motif-Binding Site on δ-COP.
Most (8 of 12) of the S. cerevisiae δ-COP residues that contact the di-tryptophan peptide motif, including His-350 and Arg-384, are conserved across species (Fig. S3). We were able to validate the peptide-binding mode observed in the crystal structure experimentally by using a double point mutant (H350A, R384S) that, while fully folded as determined by circular dichroism and gel filtration, eliminates the cation-π interactions between His-350 and Arg-384 and the indole rings of Trp-415 and Trp-413, respectively. As predicted, the double point mutant no longer bound the WxW peptide (Kd >300 μM) (Fig. 2C). In addition, mammalian δ-COP MHD-bearing mutations corresponding to yeast H350A, R384S (H330A K363S) no longer bound to the WxxxxW binding peptide from its ligand ArfGAP1, confirming that the mode of di-tryptophan motif binding is conserved from yeast to mammals (Fig. S1).
Studying the Binding of Tryptophan-Based Motifs to δ-COP in Vivo.
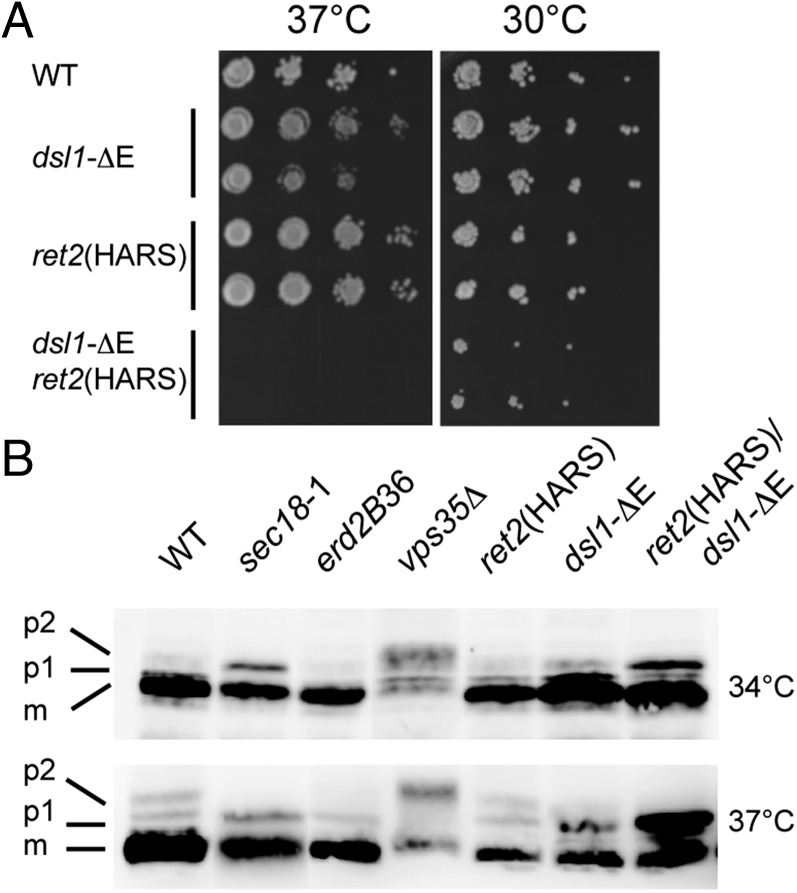
The yeast δ-COP H350A, R384S (HARS) mutant provided us with a tool for assessing the functional role of the δ-COP:Dsl1p lasso interaction in vivo. When wild-type δ-COP was replaced by the HARS double mutant to abolish the δ-COP:Dsl1p interaction, yeast grew normally. This result is not unexpected, given the previous finding that the Dsl1p lasso is functionally redundant with another region of Dsl1p, the highly conserved C-terminal E domain (9, 24), for COPI-mediated transport: Yeast containing a deletion of either the entire lasso region or of the E-domain grow normally (24). Strikingly, yeast lacking the Dsl1p E domain—and therefore rendered dependent on the Dsl1 lasso for COPI-dependent transport and hence viability—displayed marked growth-rate defects when lasso binding was compromised by the δ-COP HARS mutations (Fig. 3A). This growth defect was accompanied by a major defect in carboxypeptidase Y (CPY) trafficking/processing at 37 °C and to a lesser degree at 34 °C (Fig. 3B), suggesting that δ-COP MHD is involved in an interaction that is important for maintaining correct vesicular trafficking between the Golgi and ER.
Fig. 3.
ret2(HARS)/dsl1-ΔE mutant cells show defects in growth and in CPY trafficking. (A) Isogenic strains bearing mutations at either the DSL1 or RET2 (δ-COP) gene loci, labeled as dsl1-ΔE and ret2(HARS), were serially diluted, spotted, and grown at the indicated temperatures for 48 h. The double mutant harboring both the ret2(HARS) and dsl1-ΔE allele was lethal at 37 °C (no colonies formed after 1 wk) but was viable at lower temperatures, although with a slight growth defect. All combinations are shown in biological duplicate. (B) Cells of the indicated strains were grown to midlog at 30 °C in yeast extract peptone dextrose (YEPD) medium and then were shifted for 2 h to either 34 °C or 37 °C. Glass bead extracts of collected cells were separated by 7.5% SDS-PAGE and probed with α-CPY antibodies (40). Positions of p1 (ER), p2 (Golgi), and mature (m, vacuolar) forms of CPY are indicated. Note that p1 CPY accumulates in ret2(HARS) dsl1-ΔE double-mutant cells at 34 °C and more strongly at 37 °C but does not accumulate in single-mutant cells.
Structure of the δ-COP μ-Homology Domain with a WxxF Motif from Yeast Gcs1p.
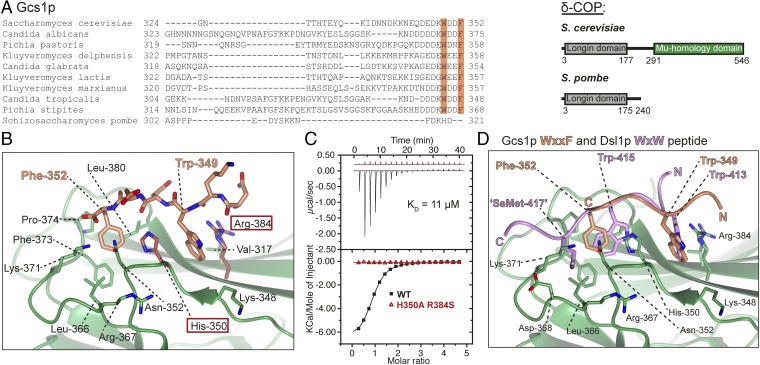
Curiously, mammalian and yeast δ-COP MHDs are thought to bind different di-tryptophan–containing ligands. In mammals, ArfGAP1 but not the Dsl1 (also called “NRZ”) complex is proposed to be the functionally relevant δ-COP MHD ligand; indeed, the mammalian Dsl1p homolog ZW10 lacks a tryptophan-rich lasso. In yeast, Dsl1p but not the ArfGAP1 ortholog Gcs1p has been proposed as the δ-COP MHD ligand. In light of our results, we reexamined the Gcs1p sequence from S. cerevisiae and found a WxxF motif at the very C terminus that is conserved in most yeasts (Fig. 4A).
Fig. 4.
Structural details of Gcs1p WxxF peptide bound to δ-COP MHD. (A, Left) Alignment of the C-terminal region of Gcs1p from different yeasts showing that the WxxF motif at the extreme C terminus is conserved in all except S. pombe. (Right) δ-COP in S. pombe does not contain an MHD; the lack of selective pressure means the WxxF in the Gcs1p homolog in S. pombe has been lost. (B) Gcs1p WxxF peptide (DEDKWDDF) binds to δ-COP MHD at the same site where the Dsl1p WxW peptide binds with Trp-349 in pocket 1 and Phe-352 in pocket 2. (C) ITC showing that a combination of H350A and R384S mutations (highlighted in dark red in B) also abolish (Kd >300 μM) (red triangles) the interaction of the Gcs1p WxxF peptide (DEDKWDDF) with the δ-COP MHD (black squares) (Kd = 11 ± 1 μM at a 1:1 stoichiometric ratio; n = 3). Data for mutant are offset for clarity. (D) Superposition of the two δ-COP MHD tryptophan-based peptide cocrystal structures showing that the N-terminal tryptophan of both peptides binds into pocket 1, and the C-terminal tryptophan/phenylalanine into pocket 2. Superposition is within the tryptophan motif-binding site (residues 348–355 and 363–384). For clarity only the model of δ-COP is shown from the Gcs1p WxxF peptide cocrystal structure, and only the hydrophobic residue side chains within the peptides are shown.
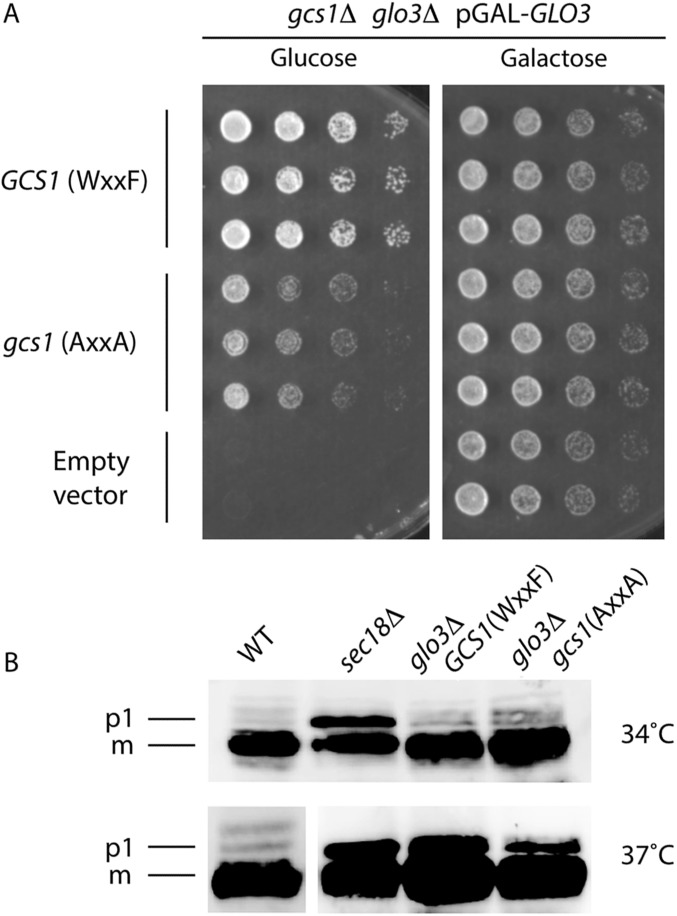
A WxxF peptide from Gcs1p (residues 345–352; DEDKWDDF) bound to the δ-COP MHD with an affinity similar (Kd = ∼10 μM) to that of di-tryptophan peptides (Fig. 4C). Moreover, a complex of δ-COP MHD and the same WxxF peptide crystallized in a different crystal form that diffracted to 2.5 Å (Table S1). Electron density representing D347KWDDF352 residues in the peptide was clearly visible, showing that the peptide binds at the same site and in the same orientation as the Dsl1p WxW peptide (Fig. 4B and Fig. S2). As expected, the C-terminal Phe residue occupies pocket 2, and the HARS mutation in δ-COP MHD abrogates WxxF peptide binding (Fig. 4C). However, the WxxF peptide is slightly displaced relative to the di-tryptophan peptides (Fig. 4D), partly to accommodate the different number of intervening residues but also to allow the formation of a salt bridge between the C terminus of the peptide and the side chain of Lys-371 (Fig. 4B). In the absence of Glo3p, cells expressing Gcs1p with its WxxF motif mutated grew more slowly than cells expressing wild-type Gcs1p, implying an in vivo role for the WxxF motif (Fig. S6A); however, CPY sorting was compromised to a similar extent when either wild-type or mutant Gcs1p with its terminal WDDF motif mutated to ADDA was expressed in the absence of Glo3p (Fig. S6B).
Fig. S6.
Compromised Gcs1p binding to the δ-COP MHD impairs yeast cell growth. (A) Yeast cells carrying simultaneous chromosomal deletions of the ArfGAP genes GCS1 and GLO3 are not viable unless the expression of one of these ArfGAPs is driven from a plasmid. Galactose-dependent expression of Glo3p from the GAL10 promoter can keep double-mutant cells growing in medium containing galactose but not in galactose-free (i.e., glucose-containing) medium. Individual plasmid transformants were serially diluted, spotted onto glucose-containing plates, and incubated at 30 °C for 48 h. Note that the mutant Gcs1-AA protein (with the W349A, F352A substitutions) cannot sustain optimal yeast colony growth in galactose-free medium, in comparison with and in contrast to wild-type Gcs1p. (B) Cells of the indicated strains (the RET2 gene encodes δ-COP) were grown to midlog phase in YEPD medium at 30 °C and then were shifted to 34 °C or 37 °C for 2 h. Glass bead extracts of collected cells were separated by 7.5% SDS-PAGE and were probed with anti-CPY antibodies (see ref. 40). Positions of p1 (ER) and mature (m, vacuolar) forms of CPY are indicated. glo3Δ/gcs1-WF single- and the glo3Δ/gcs1-AA double-mutant cells display a strong defect in ER-to-Golgi transport of CPY at 37 °C but not at 34 °C. Note that the mutants do not differ noticeably in their CPY-processing ability. Extensive attempts to demonstrate the physical interaction between coatomer and Gcs1p in vivo have proved technically impossible, because the levels of detergent needed to reduce nonspecific background binding to acceptable levels [>1% (vol/vol) IGEPAL; Sigma-Aldrich] abrogate the interaction in vitro. This lack of binding in the presence of <1% IGEPAL likely indicates that the interaction is mediated mainly by hydrophobic side-chain interactions that are readily outcompeted by detergent molecules.
Discussion
We have described the structure of the S. cerevisiae δ-COP MHD and the basis for its binding to di-tryptophan motifs found in Dsl1p (WxW and WxxxW; first reported in ref. 7) and to a newly identified WxxF motif found at the C terminus of the ArfGAP protein Gcs1p. Interestingly in the fission yeast Schizosaccharomyces pombe the δ-COP gene is actually missing its MHD altogether, and this is the one yeast that lacks a WxxF sequence at the very C terminus of its Gcs1p homolog (Fig. 4A) and also lacks a Dsl1p homolog (25), perhaps reflecting the loss of the evolutionary pressure to maintain one half of an interacting pair of sites when the other is absent.
Unlike AP MHD ligands, Gcs1p and Dsl1p are not transmembrane proteins but are vesicle coat assembly/disassembly factors, indicating a fundamentally different role for δ-COP MHD than for transmembrane cargo-sorting AP MHDs. This observation is in line with the fact that a third yeast protein proposed as a tryptophan-based motif containing δ-COP MHD ligand, originally termed “δL” and now named “Cex1p” (14), has a mammalian homolog, Scyl1, that now has been shown to be a cytosolic protein implicated in Golgi-to-ER transport (26). Because binding of similar strength can be detected to WxxxxW, WxxxxxW, and WxxxxxxW constructs (Fig. S1; note, however, that the last of these binds less tightly, with a Kd of ∼50 µM), the δ-COP MHD-binding motif can be redefined as Wxn(1–6)[WF], which may aid in identifying new accessory factors for COPI-coated vesicle formation/disassembly. Our in vivo data indicate that growth defects and CPY trafficking/processing defects can be brought about by disrupting δ-COP MHD Wxn(1–6)[WF] motif binding, but only in a sensitized system (e.g., in which the Dsl1p E-domain is deleted or Glo3p is absent). The need for multiple mutations to reveal phenotypic alterations likely reflects high levels of redundancy in this important cellular transport route, such as the binding of the other ArfGAP Glo3p by the F-subcomplex γ-COP appendage (12) and the binding of the Dsl1p lasso by α-COP (7).
By analogy with AP2 and AP1 (21, 27, 28), membrane recruitment of the coatomer might be expected to trigger an Arf1:GTP-driven conformational change in the F-subcomplex from an inactive closed form to an open form (4). However, the open form of F-subcomplexes attached to a membrane in a coat recently has been demonstrated to be even more extreme than the open conformation of AP complexes (27, 28) and now is referred to as “hyper-open” (29). The hyper-open conformation of coatomer, in which δ-COP MHD is on the very outside of the coat away from the membrane, would be maintained in the vesicle coat as long as coatomer was bound to Arf1:GTP. After nucleotide hydrolysis and Arf1:GDP disengagement, the F-subcomplex likely would revert to a more closed form; however, because of the COPI B-subcomplex binding to KKxx and KxKxx transmembrane cargoes (29–31), the coat should not dissociate from the vesicle surface immediately (5). Assuming that the F-subcomplex adopts a closed conformation similar to that of a cytosolic AP complex, modeling suggests that the Wxn(1–6)[WF] binding site on δ-COP should be accessible when the F-subcomplex is closed (Fig. S7). This situation contrasts with the unavailability of YxxΦ motif-binding site on the μ subunits of closed AP complexes (21, 32). EM tomography suggests that the δ-COP MHD Wxn(1–6)[WF] binding site remains accessible in the hyper-open membrane-associated conformation, indicating that it possesses no switching mechanism to regulate ligand binding. The lack of a switching mechanism likely reflects the different roles of the MHDs in COPI and AP complexes. APs must bind YxxΦ motifs only when the APs are on the membrane so they do not bind erroneously to YxxΦ motifs in cytosolic proteins. By contrast, COPI needs to bind cytosolic, vesicle-coat assembly/disassembly factors throughout a COPI-coated vesicle’s life cycle: Gcs1p during the early stages of COPI-coated vesicle assembly for a possible role in cargo-selection editing, Cex1p at an undefined stage, and Dsl1p in the latter stages for docking and ultimately fusion with target membranes.
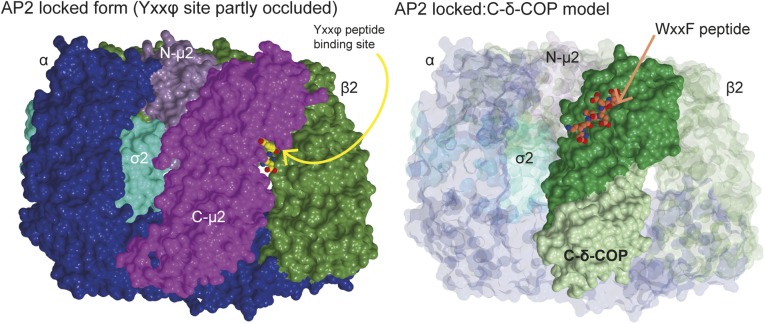
Fig. S7.
Docking of the δ-COP MHD structure into the AP2 locked/closed form. Surface representation of the AP2 locked form (PDB ID code 2VGL) (21) and docking of the δ-COP MHD (C-δ-COP)-Gcs1p WxxF peptide cocrystal structure into the AP2 locked form based on the superposition of C-δ-COP with C-μ2. The Gcs1p WxxF peptide is shown as spheres. Note the significant occlusion of the Yxxϕ binding site in μ2 in the AP2 structure and the fully accessible WxxF binding site in δ-COP.
The remarkable electronegativity of δ-COP MHD surfaces could, by analogy to the homologous AP MHD surfaces that target APs to PIP-rich and hence negatively charged membranes, function in membrane recruitment specificity. However, because the cis- and medial-Golgi membranes are not positively charged but are much less negatively charged than the late Golgi, such a role would need to be achieved by inhibiting recruitment to the more negatively charged membranes of the late Golgi and TGN. The reduction in negative charge of the cis- and medial-Golgi relative to the TGN and late Golgi is the result of two factors. First, the negatively charged PtdIns(4)P is concentrated in the later Golgi because of the constant cycling of the PtdIns(4)P phosphatase Sac1 between the cis-Golgi and ER (33, 34). Second, phosphatidylserine, another major negatively charged phospholipid, is located mostly in the inner leaflet of the ER and cis-Golgi membranes but in the outer leaflet of compartment membranes later in the secretory pathway (35). Interestingly the β’-COP (30) and α-COP (31) juxtamembrane surfaces are similarly highly electronegative and thus also could contribute to selective membrane recruitment of coatomer.
However, in a recent ground-breaking electron tomographic reconstruction of COPI-coated vesicles (29), Dodonova et al. suggested that δ-COP MHD is actually on the outside of coat, being the furthest part of the COPI coat from the membrane surface. Therefore one intriguing possibility is that the high global negative charge of δ-COP may contribute to the specificity of target membrane fusion of COPI-coated vesicles that in δ-COP could inhibit close apposition and hence fusion with more negatively charged membranes such as the late Golgi/TGN, but not inhibit contact with the comparatively uncharged membranes of the cis-Golgi and ER. Such a mechanism could impart retrograde directionality to COPI vesicle transport through the Golgi toward the ER and also might involve the α-COPCTD/ε–COP subcomplex, which likewise is both highly negatively charged (36, 37) and located on the outside of the COPI coat (29). It should be noted that for such a scenario to operate, a significant proportion of coatomer must indeed remain associated with vesicles right up to their docking step to allow Dsl1 complex-mediated tethering of COPI-coated vesicles to occur (5, 15).
Finally, a comparison of the structures of MHDs of the μ subunits in AP complexes and the δ-COP subunit of coatomer indicates that the Wxn(1–6)[WF] motif binding is unique to δ-COP because both the residues and the lengths of loops involved in Wxn(1–6)[WF] motif binding in δ-COP are not significantly conserved in the MHDs of AP complexes or TSET (38). Further, the proteins containing those motifs are cytosolic coat assembly/disassembly factors rather than membrane-embedded cargo, and as such δ-COP MHDs may play a role more analogous to that of the MHDs of muniscin family members such as Syp1p and its mammalian homologs FCHo1 and FCHo2 (39), than to the role of the MHDs of AP complexes in clathrin-coated vesicle formation.
Materials and Methods
Protein Expression and Purification.
The δ-COP μ-homology domain (residues 282–546) from S. cerevisiae was expressed in BL21(DE3)plysS (for native protein), or in B834(DE3)plysS (for SeMet-substituted protein) for 18–20 h at 20 °C after induction with 0.5 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) as a GST-fusion protein. GST-δ-COP-MHD was affinity purified using glutathione Sepharose. Following washing with 20 mM Hepes (pH 7.5), 700 mM NaCl, and 5 mM β-mercaptoethanol (β-ME), δ-COP MHD was eluted through overnight cleavage of the GST-fusion protein with PreScission protease (GE Healthcare) at 4 °C. δ-COP-MHD was purified further by size-exclusion chromatography using a Superdex SD75 preparative column in 20 mM Hepes (pH 7.5), 200 mM NaCl, and 5 mM DTT.
ITC.
All ITC experiments were carried out at 20 °C, with proteins and peptides in 50 mM Hepes (pH 7.5), 200 mM NaCl, and 5 mM β-ME with δ-COP MHD at 100 μM. All experiments were carried out at least three times, with appropriate SDs reported.
Structure Determination of the δ-COP MHD with Dsl1p WxW and Gcs1p WxxF Peptides.
The Dsl1p SeMet-substituted WxW peptide [DDWNWE(SeMet)ED] was mixed with SeMet-substituted δ-COP (residues 282–546) W404A (30 mg/mL) at a 2:1 molar ratio and was crystallized in in 0.1 M Hepes-Na (pH 7.0), 0.15 M (NH4)2SO4, and 21% (wt/vol) PEG-3350. Crystals were cryoprotected with 15% glycerol, and a three-wavelength MAD dataset was collected at the selenium edge. The best crystal diffracted to 2.8 Å and belonged to space group P1. The data were processed and the structure was solved as described in SI Materials and Methods and Tables S1 and S2.
The Gcs1p WxxF peptide (DEDKWDDF) was mixed with SeMet-substituted δ-COP (residues 282–546) W404A (20 mg/mL) at a 2:1 molar ratio and was crystallized in 0.1 M Tris⋅HCl (pH 8.5), 0.15 M magnesium chloride, and 26% (wt/vol) PEG-4000. Crystals were cryoprotected with 15% glycerol, and data were collected to 2.5 Å (space group P212121). The structure was solved by molecular replacement using one molecule of the previously determined δ-COP MHD structure [in complex with the DDWNWE(SeMet)ED peptide].
Yeast Methods.
Heterozygous DSL1/dsl1H701Stop::natMX (24) was transformed with a linearized plasmid containing ret2H350A,R384S::URA3. Diploid cells were sporulated and dissected to obtain the double mutant strain. Growth assays were performed as previously described (8). CPY secretion assays and immunoblots testing CPY trafficking/processing were performed as previously described (40, 41), using rabbit α-CPY antibodies generously provided by Karin Römisch, Saarland University, Saarbrucken, Germany. The following yeast mutants were used as positive controls to assay for trafficking phenotypes: sec18-1 for p1-CPY accumulation (i.e., an ER block) and vps35Δ for possible vacuolar protein-sorting defects (i.e., p2-CPY accumulation and loss of mCPY).
Note.
While this manuscript was under review a paper describing the structure of the unliganded bovine δ-COP MHD was published (42).
SI Materials and Methods
Cloning and Mutagenesis.
Initially δ-COP (residues 282–546) from S. cerevisiae was ligated into pQE-30 (Qiagen) using BamHI and PstI sites, incorporating an N-terminal 6× histidine (His6) tag. Initial crystals of the His-tagged wild-type protein were obtained but could not be solved by experimental phasing or molecular replacement. (These crystals subsequently were solved by molecular replacement using the δ-COP-W404A structure; see Crystallization of Wild-Type δ-COP MHD below). Subsequently δ-COP-282–546 (residues 272–511 for human) was subcloned into pGEX6P.1 (GE Healthcare) using BamHI and NotI sites incorporating an N-terminal PreScission protease (GE Healthcare)-cleavable GST tag. Purification using the GST tag increased the purity of δ-COP MHD. The W404A and nonbinding mutants were created using site-directed mutagenesis by overlap PCR using complementary primers with the relevant mutation(s) across the site of interest. All constructs were sequenced by Beckman Coulter Genomics before use.
Protein Expression and Purification.
All constructs were expressed at high levels in BL21(DE3)plysS cells (Novagen, Merck KGaA). Five-milliliter starter cultures were used to inoculate 1-L flasks containing 2× tryptone yeast medium and appropriate antibiotics. Cells were grown at 37 °C to an OD600 between 0.6–1.0; then the temperature was lowered to 20 °C for 1 h. After cooling, proteins were expressed overnight at 20 °C following induction with 0.5 mM IPTG. After ∼20 h of expression cells were harvested and resuspended in 20 mM Hepes (pH 7.5), 500 mM NaCl, 5 mM β-ME, plus EDTA-free protease inhibitor tablets (1 tablet per 50 mL buffer) (Roche Diagnostics GmbH).
SeMet-substituted proteins were expressed in a methionine-auxotroph Escherichia coli strain, B834(DE3)plysS (Novagen). Cells were grown in M9 medium supplemented with MgSO4 at 0.24 g/L (anhydrous), FeSO40.7H2O at 0.025 g/L, glucose at 0.4% (wt/vol), vitamins (riboflavin, niacinamide, pyridoxine monohydrochloride, and thiamine at a 10:10:1:10 mass ratio) at 0.0031 g/L, amino acids (without methionine) at 0.78 g/L [Complete Supplement Mixture Drop-Out: −Met (Formedium)], and seleno-l-methionine at 0.04 g/L plus appropriate antibiotics. All solutions added to media were sterile filtered; however, amino acids were added directly as powder. SeMet-substituted proteins were expressed as were native proteins, but the cultures grew more slowly.
Cells were lysed using a disruptor (Constant Systems Limited). Lysate containing the His6-δ-COP MHD was incubated for 1 h at 4 °C with 50% (vol/vol) Ni-NTA agarose (Qiagen) in buffer, and GST-tagged proteins were incubated with 50% (vol/vol) glutathione Sepharose (GE Healthcare) in buffer. Protein bound to either matrix was washed extensively with >500 mL of buffer [20 mM Hepes (pH 7.5), 700 mM NaCl, and 5 mM β-ME]. For the His6-δ-COP MHD, the wash buffer included 20 mM imidazole to remove contaminants that bind nonspecifically to Ni-NTA. The His6-δ-COP MHD was eluted in batch in buffer containing 300 mM imidazole. GST-tagged proteins were cleaved overnight with human rhinovirus 3C protease (PreScission protease; GE Healthcare) at 4 °C and were eluted in batch in buffer. Following elution, all proteins were concentrated for gel filtration on a Hi Load Superdex 75 16/60 prep grade size-exclusion column (GE Healthcare) preequilibrated in the appropriate buffer: 20 mM Hepes (pH 7.5), 200 mM NaCl, and 5 mM DTT if the protein was to be used for crystallization or 50 mM Hepes (pH 7.5), 200 mM NaCl, and 5 mM β-ME for ITC.
The δ-COP-H350A-R384S-W404A mutant (H330A-K363S-W381A for human) was determined to be folded as judged by circular dichroism and by identical gel filtration elution profiles compared with W404A.
ITC.
ITC experiments were conducted on a VP-ITC-200 isothermal titration calorimeter (GE Healthcare) at 20 °C, using 20 injections of the sample in the syringe into the cell, with a 120-s interval between injections. Each of these injections had a volume of 2 μL except for the first one, which consisted of only 0.5 μL. All components were in 50 mM Hepes (pH 7.5), 200 mM NaCl, and 5 mM β-ME. For all experiments the peptide was loaded into the syringe and the δ-COP MHD into the cell. A peptide-into-buffer run was subtracted from all runs to take into account the effect of peptide dilution. For all runs the δ-COP MHD was at 50–150 μM; the concentration of the peptide was adjusted to ensure a significant number of points were on the binding curve and that saturation was reached. Because of the presence of tryptophans, both protein and peptide concentrations were measured at 280 nm. Samples in both the cell and syringe were filtered using a Proteus Clarification Mini Spin Column (Generon) before loading. Titration data were analyzed in ORIGIN to obtain values for stoichiometry (N), equilibrium dissociation constant (Kd), and enthalpy of binding. All experiments were carried out at least three times with appropriate SDs reported. (N.B.: Curves labeled as “WT” in the main text were experiments done with W404A mutant. Likewise, H350A R384S also contained the W404A mutation. “W404A” label was removed to avoid adding complication in the main text.)
General Data Collection, Data Processing, and Structure Determination Methods.
Programs included in or affiliated with the CCP4 software package (43) were used for most steps during data processing and structure solution. The collected datasets were indexed and integrated using Mosflm or Xia2, the Laue and point group were determined with Pointless, and the data were scaled, merged, and assessed using Scala or Aimless (43). Data were cut when the correlation coefficient (CC1/2) between half datasets equaled 0.5. autoSHARP (44) was used to search for heavy atoms and phasing. Solvent flipping was used to determine the hand of the substructure and to improve the phases. The structures could be built using the automated model building software Buccaneer (45). Phaser (46) was used for all molecular replacement work. The refinement of the models was done using iterative steps of model building in Coot (47) and in Refmac5 (48). Final model validation was undertaken using MolProbity (molprobity.biochem.duke.edu/). Images of the refined structures were prepared with the molecular graphic programs CCP4MG.
Crystallization of Wild-Type δ-COP MHD.
Initially crystallization trials were set up with the native N-terminally His-tagged wild-type protein alone and in the presence of the Dsl1p WxxxW peptide. Only crystals of the protein alone were obtained; the best crystal was obtained at a protein concentration of 10 mg/mL in 0.1 M Hepes (pH 7.0), 0.05 M CaCl20.2H2O, 2.2% (wt/vol) PEG-8000, and 20% (vol/vol) ethylene glycol by vapor diffusion. This crystal was cryoprotected in 30% (vol/vol) ethylene glycol and was flash cooled in liquid nitrogen. Data were collected to 3.0 Å and belonged to space group P212121 (Table S2). This crystal form could not be solved by molecular replacement using other known MHD structures, and, despite soaking with various heavy atoms, no anomalous signal was visible to enable structure solution by experimental phasing.
This crystal form was solved eventually by molecular replacement using the δ-COP-W404A structure as a model (see below). It has eight molecules in the asymmetric unit packed in a helical arrangement via the interaction between Trp-404 in one molecule with pocket 1 in the tryptophan-based motif-binding site of another molecule. The final structure was refined to Rwork/Rfree values of 0.195/0.233 in Refmac5 using local non-crystallographic symmetry (NCS) restraints. Densities representing residues 284–546 of δ-COP are visible in all eight molecules in the asymmetric unit. Two calcium ions appear to be present (from the crystallization condition), both of which bridge neighboring molecules in the crystal.
Crystallization of Native δ-COP-W404A with the Dsl1p WxW Peptide.
δ-COP MHD had a tendency to aggregate into larger oligomers, and binding to di-tryptophan peptides prevented this aggregation (highlighted during purification of protein–motif fusion constructs). Modeling studies suggested that W404 in the δ-COP MHD was exposed to solvent and might be causing aggregation, so we mutated W404 to alanine, and this mutation appeared to prevent oligomerization as assessed by gel filtration. The native δ-COP-W404A protein crystallized in the presence of the Dsl1p WxW peptide (DDWNWEVED) with the protein at 10 mg/mL and the peptide at a 2:1 molar ratio by vapor diffusion, in 0.1 M Hepes (pH 6.6) and 2.8 M ammonium sulfate, and was cryoprotected in 4 M sodium formate, 0.05 M Hepes (pH 6.6), 1.4 M ammonium sulfate, and 1 mM DDWNWEVED peptide. Data were collected to 1.8-Å resolution at Diamond Light Source beamline I03 (Table S2). The L test and cumulative intensity distributions suggested that all crystals were perfect merohedral twins. All crystals belonged to apparent point group P622, with unit cell dimensions of a = 73 Å, b = 73 Å, c = 343 Å, α = 90°, β = 90°, γ = 120°, with an estimated two molecules in the asymmetric unit (44% solvent) or four molecules for a hemihedral twin. Because reflections along the 0,0,l axis were present only when l = 3n, the true space group would be P62, P64, P3121, P3221, P3112, or P3212. Molecular replacement was tried in all six possible space groups, using other known MHD structures as search models; however, no believable solution was found. We failed to solve the structure by experimental phasing with Hg, presumably because of the twinning. Microseeding failed to produce untwinned crystals.
This merohedrally twinned crystal form was solved eventually by molecular replacement using the δ-COP-W404A structure, solved by SeMet MAD (see below), as a model in space group P3121. Initially molecular replacement with Phaser (46) was carried out in all six possible space groups searching for one molecule (of an expected four), and plausible solutions were found in both the P3121 and P3112 space groups. Second and third molecules were found in both of these space groups, but a fourth molecule was found only after a clashing loop (residues 459–465) was removed. The structure was refined with restrained amplitude-based twin refinement using Refmac (48): After one round the R factors clearly discriminated between space groups P3121 (Rwork;Rfree = 0.27; 0.33) and P3112 (Rwork;Rfree = 0.34; 0.40).
During refinement it became clear that the loop between strands 11 and 12 adopts a different conformation because of contacts with a neighboring molecule in the crystal. This different conformation explained the failure of the molecular replacement to find all the molecules using the initial unmodified model. It is not clear whether this conformational change has any functional role. Following refinement, the mFO-DFC difference map showed a clear electron density map representing the two tryptophans in the peptide (Fig. S2 F and G). The DDW413NW415EVED peptide was built (density for DW413NW415EV residues was visible) in the same orientation as the SeMet Dsl1p DDWNWE(SeMet)ED peptide, with the valine binding into pocket 3; however, the density for Val-417 is poor and makes little contribution to binding, unlike the SeMet. The interactions between δ-COP and the native peptide are largely the same as with the SeMet-derivative peptide, preserving the tryptophan orientations and the hydrogen bond interactions with side chains His-350, Asn-352, and Gly-381. The final structure was refined to Rwork/Rfree values of 0.136/0.189 using amplitude-based twin refinement and map sharpening in Refmac5 (48). Residues 412–417 of the peptide and residues 285–546 of δ-COP of the four molecules in the asymmetric unit are visible; however, the density for two loops is poor in some molecules (residues 409–412 in chains A and D and residues 447–452 in chains B and D), and there are two breaks in the main chain at D/409 and B/451–452.
Crystallization of SeMet-Substituted δ-COP-W404A Bound to the Dsl1p SeMet-WxW Peptide.
SeMet-substituted δ-COP-W404A was crystallized with a Dsl1p WxW peptide with SeMet replacing valine [DDWNWE(SeMet)ED] to help identify the orientation of the peptide. This peptide bound with an affinity similar to that of the native peptide (Fig. S1). Crystals were obtained by vapor diffusion with 30 mg/mL protein (2:1 molar ratio peptide:protein) in 0.1 M Hepes-Na (pH 7.0), 0.15 M (NH4)2SO4, and 21% (wt/vol) PEG-3350. Crystals grew after 2–3 d before degrading, eventually disappearing after ∼7 d and therefore were flash-cooled on days 3–4. Crystals were cryoprotected in 15% glycerol, 0.1 M Hepes-Na (pH 7.0), 0.15 M (NH4)2SO4, 22% (wt/vol) PEG-3350, and 1.5 mM DDWNWE(SeMet)ED peptide, and a three-wavelength MAD dataset was collected at 2.8-Å resolution at the selenium K edge (Table S1). After MAD phasing, the solvent content was optimized to 56% with eight molecules in the asymmetric unit (Fig. S2). The initial atomic model was built automatically using Buccaneer (45) and was refined against the high remote dataset to 2.8-Å resolution using local NCS restraints, experimental phase restraints, and map sharpening in Refmac5 (48), with rebuilding in Coot (47). The two tryptophans on either side of His-350 and the SeMet in pocket 3 of the peptide were clear even in the experimental-phase maps before model building and refinement (Fig. S2 B and C), and the SeMet in the peptide was clear from the anomalous difference map (Fig. S2C). The final structure was refined to final Rwork/Rfree values of 0.189/0.234. All nine residues of the peptide and residues 285–546 of δ-COP are visible in all eight molecules in the asymmetric unit. However, because of crystal contacts the density for four of the eight molecules (chains A–D) is better than for the other four (chains E–H); the density for some or all of residues 406–415, 437–466, and 482–493 (chain H) in these chains (E–H) is poor.
Crystallization of SeMet-Substituted δ-COP-W404A with the Dsl1p WxxxW Peptide.
SeMet-substituted δ-COP-W404A protein crystallized in the presence of the Dsl1p WxxxW peptide (ENAWDEAWAIDEC) with the protein at 15 mg/mL and the peptide at a 2:1 molar ratio by vapor diffusion in 0.1 M bicine (pH 9.0) and 3.2 M ammonium sulfate and were cryoprotected in 4 M sodium formate, 0.05 M bicine (pH 9.0), 1.7 M ammonium sulfate, and 2 mM ENAWDEAWAIDEC peptide. Data were collected at 2.8-Å resolution at Diamond Light Source, beamline I03 (Table S2). These crystals belonged to space group P3121, the same space group as the native Dsl1p WxW peptide, but were not twinned. Following refinement the density representing the peptide was discontinuous, presumably because of low occupancy of the peptide; however, the motif clearly binds at the same site and most likely in the same orientation (Fig. S2 D and E).
Crystallization of SeMet-Substituted δ-COP-W404A with the Gcs1p WxxF Peptide.
Following the observation that a WxxF peptide (DEDKWDDF) corresponding to the WxxF at the extreme C terminus of Gcs1p binds to δ-COP MHD (Fig. 4C), crystals of SeMet-substituted δ-COP-MHD W404A were grown with 20 mg/mL protein with the Gcs1p WxxF peptide (DEDKWDDF) at a 2:1 molar ratio in 0.1 M Tris⋅HCl (pH 8.5), 0.15 M magnesium chloride, and 26% (wt/vol) PEG-4000. The SeMet-substituted protein allowed the use of the experimental-phase information in refinement, which improved the connectivity of the density (particularly of the peptides). Crystals were obtained by vapor diffusion. Crystals were cryoprotected with 15% glycerol, and data were collected to 2.5-Å resolution. These crystals belonged to space group P212121, (Table S1). This crystal form was solved by molecular replacement using one molecule of the previously determined structure and had three molecules in the asymmetric unit. Following refinement the mFO-DFC difference map showed clear electron density representing the tryptophan and phenylalanine in the peptide (Fig. S2 H and I). The DEDKWDDF peptide binds in the same orientation as the Dsl1p WxWxϕ peptides, with the tryptophan binding into pocket 1 and the phenylalanine binding into pocket 2. The tryptophan motif-binding site is accessible in all three molecules in the asymmetric unit; however, the electron density was better in one molecule (chain B) than in the other two because of interactions of the peptide with a neighboring molecule. Despite lower occupancy of the peptide bound to the other two molecules, the peptide appeared to bind in the same orientation. The structure was refined to final Rwork/Rfree values of 0.193/0.258 using TLS refinement, local NCS restraints, the single-wavelength anomalous dispersion data directly, and map sharpening in Refmac5 (48). In the best molecule, all except the first two residues (DE) of the peptide could be built.
Yeast Strains and Growth Media.
The S. cerevisiae strains Y7092 (MATa, his3Δ1 leu2Δ0 ura3Δ0 met15Δ0) and MY15059 (MATα, can1Δ::pSTE2-Sp_HIS5 his3∆1 lyp1Δ his3Δ1 leu2Δ0 ura3Δ0 met15Δ0 dsl1ΔE-natMX) (24) were used to study the δ-COP:Dsl1p interaction in vivo. Yeast strain PPY17:168 (gcs1::URA3 glo3::HIS3 ura3 his3 leu2 trp1 ade2) harboring plasmids pGAL-GLO3 and pPP16:100-5-GCS1-WF (i.e., wild-type GCS1), pPP16:100-12-gcs1-AA (the gcs1 mutant carrying the W349A and F352A mutations), or empty-vector pRS315 was used to study the δ-COP:Gcs1p interaction in vivo. Yeast cells were grown aerobically at 30 °C unless otherwise noted. YEPD-rich medium contained yeast extract (1% wt/vol), peptone (2% wt/vol), and glucose (2% wt/vol). Sporulation medium contained potassium acetate (0.3% wt/vol) and raffinose (0.02% wt/vol) and was supplemented with histidine, leucine, methionine, and uracil (each 0.03% wt/vol). YEPD+Nat medium contained 0.1 g/L nourseothricin (clonNAT).
Acknowledgments
We thank the beamline scientists at the Diamond Light Source and Mike Lewis [Medical Research Council (MRC) Laboratory of Molecular Biology], Gerry Johnston (Dalhousie University), and Mark Rose (Princeton University) for helpful discussions and technical advice. R.J.S. and D.J.O. were funded by Wellcome Trust Fellowship 090909 (to D.J.O.) and funding from Wellcome Trust Strategic Award 100140. P.P.P. was funded by the Canadian Institute of Health Research. R.D. received support from the German Research Foundation Clusters of Excellence “Inflammation and Interfaces” ECX306 and the University of Lubeck. S.M.T. and F.M.H. were supported by NIH Grant GM071574. P.R.E. was supported by MRC Grant U105178845.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.H.H. is a guest editor invited by the Editorial Board.
Data deposition: Crystallography, atomic coordinates, and structure factors reported in this paper have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 5FJW, 5FJX, 5FJZ, and 5FK0).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1506186112/-/DCSupplemental.
References
- 1.Banfield DK. Mechanisms of protein retention in the Golgi. Cold Spring Harb Perspect Biol. 2011;3(8):a005264. doi: 10.1101/cshperspect.a005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Popoff V, Adolf F, Brügger B, Wieland F. COPI budding within the Golgi stack. Cold Spring Harb Perspect Biol. 2011;3(11):a005231. doi: 10.1101/cshperspect.a005231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szul T, Sztul E. COPII and COPI traffic at the ER-Golgi interface. Physiology (Bethesda) 2011;26(5):348–364. doi: 10.1152/physiol.00017.2011. [DOI] [PubMed] [Google Scholar]
- 4.Yu X, Breitman M, Goldberg J. A structure-based mechanism for Arf1-dependent recruitment of coatomer to membranes. Cell. 2012;148(3):530–542. doi: 10.1016/j.cell.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Presley JF, et al. Dissection of COPI and Arf1 dynamics in vivo and role in Golgi membrane transport. Nature. 2002;417(6885):187–193. doi: 10.1038/417187a. [DOI] [PubMed] [Google Scholar]
- 6.East MP, Kahn RA. Models for the functions of Arf GAPs. Semin Cell Dev Biol. 2011;22(1):3–9. doi: 10.1016/j.semcdb.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andag U, Schmitt HD. Dsl1p, an essential component of the Golgi-endoplasmic reticulum retrieval system in yeast, uses the same sequence motif to interact with different subunits of the COPI vesicle coat. J Biol Chem. 2003;278(51):51722–51734. doi: 10.1074/jbc.M308740200. [DOI] [PubMed] [Google Scholar]
- 8.Reilly BA, Kraynack BA, VanRheenen SM, Waters MG. Golgi-to-endoplasmic reticulum (ER) retrograde traffic in yeast requires Dsl1p, a component of the ER target site that interacts with a COPI coat subunit. Mol Biol Cell. 2001;12(12):3783–3796. doi: 10.1091/mbc.12.12.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ren Y, et al. A structure-based mechanism for vesicle capture by the multisubunit tethering complex Dsl1. Cell. 2009;139(6):1119–1129. doi: 10.1016/j.cell.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frigerio G, Grimsey N, Dale M, Majoul I, Duden R. Two human ARFGAPs associated with COP-I-coated vesicles. Traffic. 2007;8(11):1644–1655. doi: 10.1111/j.1600-0854.2007.00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis SM, Poon PP, Singer RA, Johnston GC, Spang A. The ArfGAP Glo3 is required for the generation of COPI vesicles. Mol Biol Cell. 2004;15(9):4064–4072. doi: 10.1091/mbc.E04-04-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watson PJ, Frigerio G, Collins BM, Duden R, Owen DJ. Gamma-COP appendage domain - structure and function. Traffic. 2004;5(2):79–88. doi: 10.1111/j.1600-0854.2004.00158.x. [DOI] [PubMed] [Google Scholar]
- 13.Rawet M, Levi-Tal S, Szafer-Glusman E, Parnis A, Cassel D. ArfGAP1 interacts with coat proteins through tryptophan-based motifs. Biochem Biophys Res Commun. 2010;394(3):553–557. doi: 10.1016/j.bbrc.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 14.Cosson P, Lefkir Y, Démollière C, Letourneur F. New COP1-binding motifs involved in ER retrieval. EMBO J. 1998;17(23):6863–6870. doi: 10.1093/emboj/17.23.6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zink S, Wenzel D, Wurm CA, Schmitt HD. A link between ER tethering and COP-I vesicle uncoating. Dev Cell. 2009;17(3):403–416. doi: 10.1016/j.devcel.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 16.Owen DJ, Evans PR. A structural explanation for the recognition of tyrosine-based endocytotic signals. Science. 1998;282(5392):1327–1332. doi: 10.1126/science.282.5392.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burgos PV, et al. Sorting of the Alzheimer’s disease amyloid precursor protein mediated by the AP-4 complex. Dev Cell. 2010;18(3):425–436. doi: 10.1016/j.devcel.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jia X, et al. Structural basis of evasion of cellular adaptive immunity by HIV-1 Nef. Nat Struct Mol Biol. 2012;19(7):701–706. doi: 10.1038/nsmb.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mardones GA, et al. Structural basis for the recognition of tyrosine-based sorting signals by the μ3A subunit of the AP-3 adaptor complex. J Biol Chem. 2013;288(13):9563–9571. doi: 10.1074/jbc.M113.450775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reider A, et al. Syp1 is a conserved endocytic adaptor that contains domains involved in cargo selection and membrane tubulation. EMBO J. 2009;28(20):3103–3116. doi: 10.1038/emboj.2009.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collins BM, McCoy AJ, Kent HM, Evans PR, Owen DJ. Molecular architecture and functional model of the endocytic AP2 complex. Cell. 2002;109(4):523–535. doi: 10.1016/s0092-8674(02)00735-3. [DOI] [PubMed] [Google Scholar]
- 22.Höning S, et al. Phosphatidylinositol-(4,5)-bisphosphate regulates sorting signal recognition by the clathrin-associated adaptor complex AP2. Mol Cell. 2005;18(5):519–531. doi: 10.1016/j.molcel.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 23.Wang YJ, et al. Phosphatidylinositol 4 phosphate regulates targeting of clathrin adaptor AP-1 complexes to the Golgi. Cell. 2003;114(3):299–310. doi: 10.1016/s0092-8674(03)00603-2. [DOI] [PubMed] [Google Scholar]
- 24.Rogers JV, McMahon C, Baryshnikova A, Hughson FM, Rose MD. ER-associated retrograde SNAREs and the Dsl1 complex mediate an alternative, Sey1p-independent homotypic ER fusion pathway. Mol Biol Cell. 2014;25(21):3401–3412. doi: 10.1091/mbc.E14-07-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmitt HD. Dsl1p/Zw10: Common mechanisms behind tethering vesicles and microtubules. Trends Cell Biol. 2010;20(5):257–268. doi: 10.1016/j.tcb.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Burman JL, Hamlin JN, McPherson PS. Scyl1 regulates Golgi morphology. PLoS One. 2010;5(3):e9537. doi: 10.1371/journal.pone.0009537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackson LP, et al. A large-scale conformational change couples membrane recruitment to cargo binding in the AP2 clathrin adaptor complex. Cell. 2010;141(7):1220–1229. doi: 10.1016/j.cell.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ren X, Farías GG, Canagarajah BJ, Bonifacino JS, Hurley JH. Structural basis for recruitment and activation of the AP-1 clathrin adaptor complex by Arf1. Cell. 2013;152(4):755–767. doi: 10.1016/j.cell.2012.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dodonova SO, et al. VESICULAR TRANSPORT. A structure of the COPI coat and the role of coat proteins in membrane vesicle assembly. Science. 2015;349(6244):195–198. doi: 10.1126/science.aab1121. [DOI] [PubMed] [Google Scholar]
- 30.Jackson LP, et al. Molecular basis for recognition of dilysine trafficking motifs by COPI. Dev Cell. 2012;23(6):1255–1262. doi: 10.1016/j.devcel.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma W, Goldberg J. Rules for the recognition of dilysine retrieval motifs by coatomer. EMBO J. 2013;32(7):926–937. doi: 10.1038/emboj.2013.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heldwein EE, et al. Crystal structure of the clathrin adaptor protein 1 core. Proc Natl Acad Sci USA. 2004;101(39):14108–14113. doi: 10.1073/pnas.0406102101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheong FY, et al. Spatial regulation of Golgi phosphatidylinositol-4-phosphate is required for enzyme localization and glycosylation fidelity. Traffic. 2010;11(9):1180–1190. doi: 10.1111/j.1600-0854.2010.01092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wood CS, et al. Local control of phosphatidylinositol 4-phosphate signaling in the Golgi apparatus by Vps74 and Sac1 phosphoinositide phosphatase. Mol Biol Cell. 2012;23(13):2527–2536. doi: 10.1091/mbc.E12-01-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bigay J, Antonny B. Curvature, lipid packing, and electrostatics of membrane organelles: Defining cellular territories in determining specificity. Dev Cell. 2012;23(5):886–895. doi: 10.1016/j.devcel.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 36.Hsia KC, Hoelz A. Crystal structure of alpha-COP in complex with epsilon-COP provides insight into the architecture of the COPI vesicular coat. Proc Natl Acad Sci USA. 2010;107(25):11271–11276. doi: 10.1073/pnas.1006297107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee C, Goldberg J. Structure of coatomer cage proteins and the relationship among COPI, COPII, and clathrin vesicle coats. Cell. 2010;142(1):123–132. doi: 10.1016/j.cell.2010.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirst J, et al. Characterization of TSET, an ancient and widespread membrane trafficking complex. eLife. 2014;3:e02866. doi: 10.7554/eLife.02866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Umasankar PK, et al. A clathrin coat assembly role for the muniscin protein central linker revealed by TALEN-mediated gene editing. eLife. 2014;3:3. doi: 10.7554/eLife.04137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duden R, et al. Yeast beta- and beta’-coat proteins (COP). Two coatomer subunits essential for endoplasmic reticulum-to-Golgi protein traffic. J Biol Chem. 1994;269(39):24486–24495. [PubMed] [Google Scholar]
- 41.Wuestehube LJ, et al. New mutants of Saccharomyces cerevisiae affected in the transport of proteins from the endoplasmic reticulum to the Golgi complex. Genetics. 1996;142(2):393–406. doi: 10.1093/genetics/142.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lahav A, Rozenberg H, Parnis A, Cassel D, Adir N. Structure of the bovine COPI Δ subunit μ homology domain at 2.15 Å resolution. Acta Crysttallagr D Biol Crystallogr. 2015;71(Pt 6):1328–1334. doi: 10.1107/S1399004715006203. [DOI] [PubMed] [Google Scholar]
- 43.Winn MD, et al. Overview of the CCP4 suite and current developments. Acta Crystallogr D Biol Crystallogr. 2011;67(Pt 4):235–242. doi: 10.1107/S0907444910045749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vonrhein C, Blanc E, Roversi P, Bricogne G. Automated structure solution with autoSHARP. Methods Mol Biol. 2007;364:215–230. doi: 10.1385/1-59745-266-1:215. [DOI] [PubMed] [Google Scholar]
- 45.Cowtan K. The Buccaneer software for automated model building. 1. Tracing protein chains. Acta Crystallogr D Biol Crystallogr. 2006;62(Pt 9):1002–1011. doi: 10.1107/S0907444906022116. [DOI] [PubMed] [Google Scholar]
- 46.McCoy AJ, et al. Phaser crystallographic software. J Appl Cryst. 2007;40(Pt 4):658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Emsley P, Cowtan K. Coot: Model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60(Pt 12 Pt 1):2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 48.Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr. 1997;53(Pt 3):240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]