Significance
The kinase MEKK3 (MAP3K) regulates cellular functions from proliferation to maintenance of cell identity, and plays an important role in cardiovascular development, yet little is known about how it is regulated. Loss-of-function mutations in CCM1, CCM2, or CCM3 cause cerebral cavernous malformations characterized by dilated, leaky blood vessels. CCM proteins form a complex and regulate the signal strength of several pathways. We demonstrate that CCM2 and CCM2-like (CCM2L), a recently described paralog, strongly prevented the activation of MEKK3 in vitro. In zebrafish, ccm2 and ccm2l genetically interacted to control cardiovascular development and body axis patterning during embryogenesis, and these were regulated by MEKK3. Therefore, two homologous CCM proteins regulate MEKK3 activity, and thus may modulate the strength of key signaling pathways.
Keywords: cerebral cavernous malformation, signaling, MAP kinase, expression, endothelium
Abstract
Three genes, CCM1, CCM2, and CCM3, interact genetically and biochemically and are mutated in cerebral cavernous malformations (CCM). A recently described member of this CCM family of proteins, CCM2-like (CCM2L), has high homology to CCM2. Here we show that its relative expression in different tissues differs from that of CCM2 and, unlike CCM2, the expression of CCM2L in endothelial cells is regulated by density, flow, and statins. In vitro, both CCM2L and CCM2 bind MEKK3 in a complex with CCM1. Both CCM2L and CCM2 interfere with MEKK3 activation and its ability to phosphorylate MEK5, a downstream target. The in vivo relevance of this regulation was investigated in zebrafish. A knockdown of ccm2l and ccm2 in zebrafish leads to a more severe “big heart” and circulation defects compared with loss of function of ccm2 alone, and also leads to substantial body axis abnormalities. Silencing of mekk3 rescues the big heart and body axis phenotype, suggesting cross-talk between the CCM proteins and MEKK3 in vivo. In endothelial cells, CCM2 deletion leads to activation of ERK5 and a transcriptional program that are downstream of MEKK3. These findings suggest that CCM2L and CCM2 cooperate to regulate the activity of MEKK3.
Cerebral cavernous malformations (CCMs) are characterized by endothelial cell channels in low-blood flow venous capillaries with poor coverage of pericytes and smooth cells and poorly developed tight and adherens junctions, resulting in increased permeability, hemorrhage, and subsequent neurologic deficits. Germline loss-of-function mutations in any one of three CCM genes—CCM1 (Krit1), CCM2 (OSM), or CCM3 (PDCD10)—cause the familial and sporadic forms of CCMs. The proteins encoded by these genes interact in a cytosolic complex. Critical insights into their in vivo functions have been obtained from genetic manipulation in fish and mice.
In zebrafish, null mutations in either ccm1 (santa, san) or ccm2 (valentine, vtn) result in an enlarged heart (“big heart”) and dilation of the subintestinal vessels and posterior cardinal vein, and morpholino (MO) studies indicate that ccm1 and ccm2 are in the same pathway during zebrafish cardiovascular development (1, 2). The contribution of ccm3 or its downstream effectors in the ccm1/2 pathway in zebrafish is less clear (3, 4). CCM proteins have been shown to regulate the signal strength of several pathways acting both as down-regulators and as activators. Reduced expression of CCM1, CCM2, or CCM3 increases RhoA activation (5). CCM2 and CCM3 siRNAs increase phosphorylation of AKT and the MAP kinases p38 and ERK1/2 (6). CCM1 promotes NOTCH activation (7) and deficiency in CCM proteins leads to endothelial–mesenchymal transition (EMT) by up-regulating TGF-β/BMP signaling (8). ccm1 and ccm2 deletion in zebrafish leads to the up-regulation of β1-integrin signaling and klf2 expression that results in a proangiogenic program (9). Ccm2-like (Ccm2l), a recently described paralog of Ccm2, was shown to have an antagonistic function to Ccm2 in cardiovascular development in mice and failed to rescue the phenotype in ccm2 null zebrafish (10). However, in another study, ccm2l, like ccm2, was required for cardiovascular development in zebrafish, suggesting redundant functions for these two proteins (11).
CCM2 has been shown to interact with the kinase MEKK3 (MAP3K3) (12), and MEKK3 is known to activate several signaling pathways via the formation of specific signaling modules. The MEKK3-MEK5-ERK5 kinase module promotes cellular functions from EMTs to cell proliferation (13) and, in vivo, represents an important signaling axis in early cardiovascular development in mice (14, 15).
Here we report differential regulation of CCM2 and CCM2L expression in endothelial cells, an interaction of these proteins with MEKK3′s N-terminal regulatory domain that interferes with its activation in vitro. Furthermore, genetic studies in zebrafish and cultured endothelial cells demonstrate MEKK3 dysregulation in the absence of these proteins.
Results and Discussion
CCM2L Tissue Expression and Regulation and Its Interaction with CCM1.
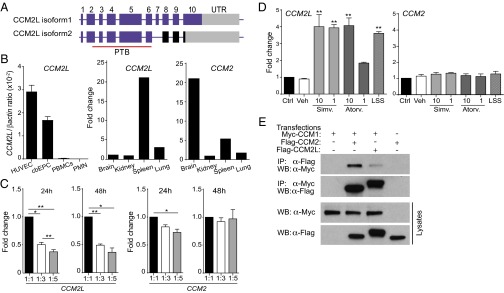
Several CCM2L isoforms exist (UniProt database). The canonical isoform 1 (long, 62 kD) and isoform 2 (short, 46 kD) retain a functional PTB domain (Fig. 1A), implicated in CCM2 interaction with CCM1 and other signaling molecules (2). Quantitative RT-PCR (qRT-PCR) primers that detect both isoforms 1 and 2 were used to evaluate their relative expression in vivo. mRNAs were detected in human umbilical vein endothelial cells (HUVEC) and endothelial progenitor cells (Fig. 1B). qRT-PCR primers that specifically detect only the long or short isoform indicated that the long isoform accounts for more than 90% of the mRNA for this gene product in all cells analyzed. The CCM2L isoforms were not detected in mononuclear cell fractions or neutrophils (Fig. 1B), suggesting selective expression in endothelial lineages versus hematopoietic cells.
Fig. 1.
CCM2L expression and interaction with CCM1. (A) Exons with identical amino acid sequences for canonical isoform 1 (62 kD) and shorter isoform 2 (46 kD) are in blue. Sequences only in isoform 2 are in black. The PTB domain is underlined, and the gray boxes represent the 3′ UTR. (B) CCM2L and CCM2 mRNA analysis by qRT-PCR using primers for CCM2 and both CCM2L isoforms in endothelial cells (HUVEC), cord blood endothelial progenitor cells (cbEPC), peripheral blood mononuclear cells (PBMCs), neutrophils (PMN), or indicated samples of human tissue. (C) HUVEC were plated at confluency (1:1) or at indicated dilutions for 24 or 48 h. mRNA levels were evaluated by qRT-PCR. (D) HUVEC were treated with vehicle control, simvastatin, or atorvastatin at indicated µM concentrations, or subjected to shear stress (LSS) for 24 h, followed by analysis of CCM2L and CCM2 mRNA levels by qRT-PCR. (E) HEK293 cells were transfected with Myc-tagged CCM1, Flag-tagged CCM2L, or Flag-tagged CCM2 as indicated. Cell lysates were subjected to IP with anti-tag antibodies, followed by Western blot analysis (WB) with indicated antibodies. CCM1 coimmunoprecipitated with CCM2L and CCM2. n = 3 independent experiments.
There is emerging evidence of an endothelial autonomous function for the CCM genes (5, 16, 17). Here we analyzed CCM2L expression in endothelial cells under various biological settings. Several genes, such as claudin-5 (18), are regulated by cell density. Confluent HUVEC monolayers replated at lower densities exhibited reduced CCM2L levels, suggesting that cell–cell contacts may be required to maintain optimal CCM2L levels (Fig. 1C), whereas CCM2 levels were largely unaffected, indicating distinct regulation of these two genes. Hemodynamic forces (shear stress) are strong modulators of the endothelial phenotype, and patterns of gene expression induced by flow can be recapitulated by statin treatment (19). HUVEC monolayers subjected to shear stress or treated with statins showed up-regulation of CCM2L, but not CCM2, mRNA (Fig. 1D). Thus, whereas CCM2L levels are dynamically regulated, CCM2 levels are not similarly responsive to cues that modulate the endothelial phenotype. All subsequent analyses of CCM2L were conducted in heterologous cells owing to the observed variability of expression in endothelial cells.
Mutations that impair CCM1–CCM2 interactions result in vascular malformations. The ability of CCM2L to interact with CCM1 was examined in transient transfection assays. As expected, CCM2 interacted with CCM1. Similarly, both CCM2L isoforms were found in complex with CCM1 (Fig. 1E), suggesting regulation of CCM2L by canonical CCM pathways.
CCM2 and CCM2L Interact with the MEKK3/MEK5 Complex and Regulate MEKK3 Activity.
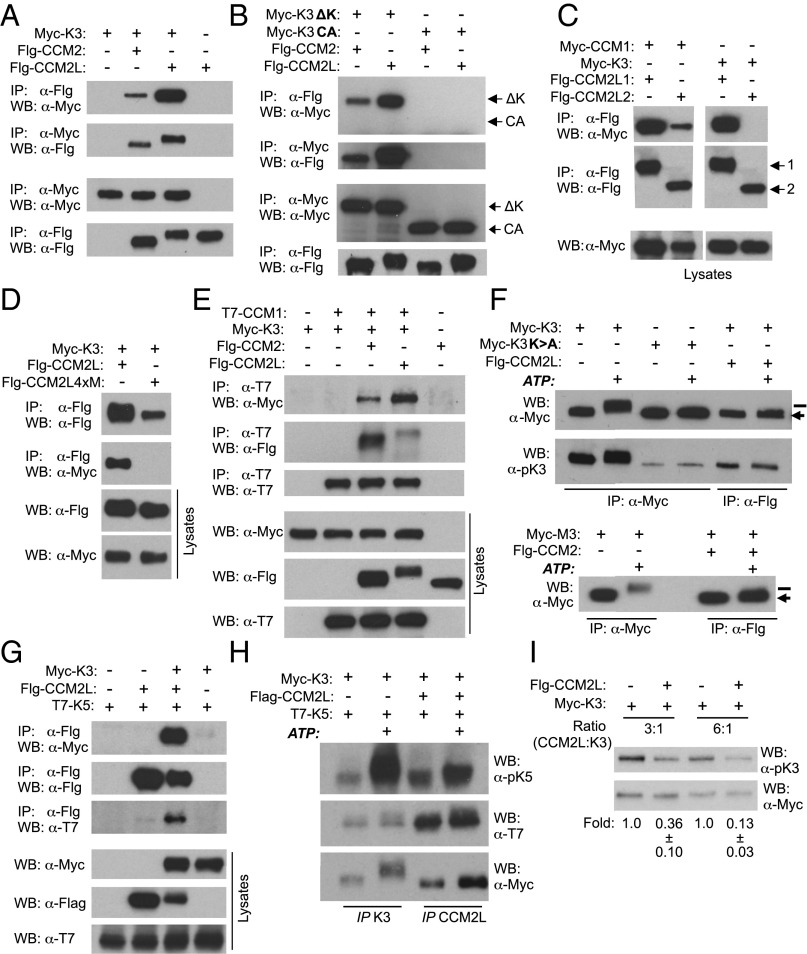
CCM2 has been identified as a scaffold protein for hypertonicity-induced MEKK3-p38 activation (12). Here we show an interaction of CCM2 and CCM2L with MEKK3 (Fig. 2A) that did not involve the catalytic domain of the kinase, as they bound MEKK3 lacking this region and failed to interact with the kinase domain alone (Fig. 2B). The binding of MEKK3 with CCM2L was consistently more robust than that with CCM2 (Fig. 2A). Similarly, transfected CCM2L interacted with endogenous MEKK3 in HUVEC as assessed in immunoprecipitation (IP) assays (Fig. S1A). The region of CCM2L mediating interaction with MEKK3 mapped to the C terminus (amino acids 357–571) of the canonical CCM2L, as isoform 2, which lacks these sequences, failed to interact with MEKK3 while retaining binding to CCM1 (Fig. 2C). A recent study localized the region of interaction of CCM2 with MEKK3 to the harmonin homology domain of the CCM2L C terminus (20). Based on these data, we mutated four hydrophobic amino acids in the predicted second α-helix of this domain in CCM2L, because in CCM2, the α1 and α2 helices form a hydrophobic cleft that accommodates the N-terminal α-helix of MEKK3 (20). We found that this mutant did not bind MEKK3 (Fig. 2D). CCM2L and CCM2 were also present in a ternary complex with CCM1 and MEKK3, because CCM1 immunoprecipitates contained MEKK3 only in the presence of CCM2L or CCM2 (Fig. 2E).
Fig. 2.
CCM2L and CCM2 interact with MEKK3 and prevent its autophosphorylation. HEK293 cells were transfected with the indicated Myc-, Flag (Flg)-, or T7-tagged constructs alone or in combination. Anti-tag immunoprecipitated proteins and cell lysates were analyzed by Western blot (WB). (A) CCM2L and CCM2 interact with MEKK3 (K3). (B) CCM2L and CCM2 interact with only the N-terminal domain of MEKK3 (K3ΔK, lacking the kinase domain), and not with the kinase domain (K3CA, catalytically active kinase domain). (C) CCM2L isoform 1 (-1) and isoform 2 (-2) interact with CCM1, but only isoform 1 (-1) binds MEKK3. (D) CCM2L, but not CCM2L4xM (with mutations in four hydrophobic residues), interacts with MEKK3. (E) MEKK3 is in a complex with CCM1 only when CCM2L or CCM2 are present. (F) CCM2L (Upper) and CCM2 (Lower) prevent MEKK3 autophosphorylation. Immunoprecipitated MEKK3 and kinase dead MEKK3 (K3K > A) alone or with CCM2L or CCM2 were subjected to an in vitro kinase assay (+ATP). MEKK3 p-Ser526 status was analyzed by Western blot analysis. MEKK3, but not MEKK3K > A, shows a slower migrating band, indicating autophosphorylated MEKK3 (bar). MEKK3 present in CCM2L or CCM2 immunoprecipitates is unable to autophosphorylate (arrow), and MEKK3 complexed with CCM2L has less p-Ser526. (G) MEKK3 (K3), MEK5 (K5), and CCM2L are present in a complex. Anti-CCM2L immunoprecipitates contain MEK5 only when MEKK3 is also cotransfected. (H) MEK5 phosphorylation by MEKK3 is reduced in the presence of CCM2L. In vitro kinase assays of indicated immunoprecipitates were followed by WB using anti-tag antibodies and p-MEK5–specific antibody (Ser311/Thr315). (I) Cells were transfected with MEKK3 with and without CCM2L at the indicated plasmid ratios. Aliquots of cell lysates containing similar amounts of MEKK3 (Lower) were evaluated for MEKK3 p-Ser526 (Upper). Densitometric analyses of WBs are reported as fold reduction in CCM2L-containing samples. n = 3–4 independent experiments for each panel.
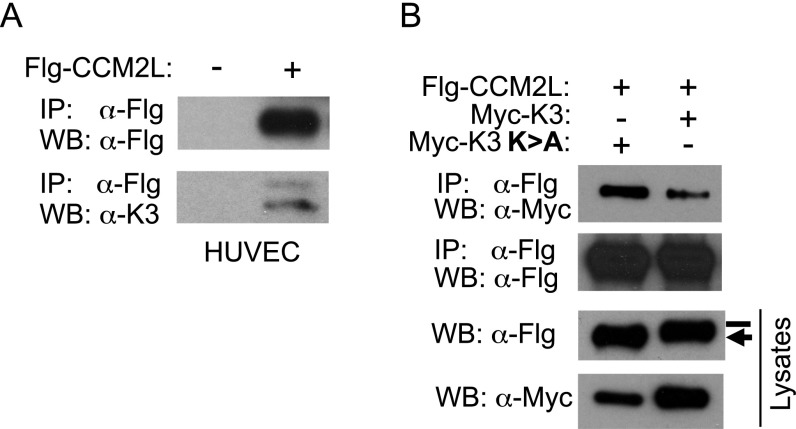
Fig. S1.
(A) HUVEC were transduced with adenoviral control or adenovirus expressing FLAG (Flg)-tagged CCM2L and harvested 48 h later. Western blot analysis of anti-Flag immunoprecipitates show that endogenous MEKK3 interacts with CCM2L. (B) CCM2L was transiently transfected together with MEKK3 (K3) or MEKK3 kinase dead (K3 K > A). MEKK3 kinase dead is more highly represented than MEKK3 in the CCM2L immunoprecipitates. CCM2L phosphorylation specifically by active MEKK3 results in reduced mobility in SDS gels (bar).
We next evaluated whether CCM2/CCM2L interacting with MEKK3 altered its function. Unlike other MAPKs, MEKK3 undergoes spontaneous activation in overexpression studies owing to its ability to autophosphorylate critical residues necessary for its kinase activity (21). Endogenous MEKK3 is not constitutively active in the cell without the appropriate stimulus, indicating that self-activation is tightly regulated to avoid excessive kinase activation. Cotransfections of MEKK3 with CCM2L or CCM2 were performed, followed by MEKK3 pull-down and an in vitro kinase assay (Fig. 2F). In this assay, on the addition of ATP, MEKK3 autophosphorylation results in reduced mobility of the kinase in SDS/PAGE gels (22). Interestingly, the ability of CCM2L-bound MEKK3 to undergo autophosphorylation was substantially impaired; that is, the significant shift in mobility in the wild-type (WT) kinase did not occur in the presence of CCM2L and indeed was similar to that of an MEKK3 kinase inactive mutant (Fig. 2F). Phosphorylation of Ser526 (p-Ser526) in the activation loop of MEKK3 plays a critical role in its activation (21). MEKK3 complexed to CCM2L exhibited a significant reduction in p-Ser526, and the signal did not increase after the addition of ATP (Fig. 2F). Like CMM2L, CCM2-bound MEKK3 showed diminished autophosphorylation (Fig. 2F). Dimerization of MEKK2 is important for its activation by transphosphorylation (23). We propose that the interaction with CCM2L or CCM2 prevents MEKK3 dimerization and thus its spontaneous activation. This may restrict MEKK3’s activity and/or enable its activation in a stimulus-specific manner. This hypothesis predicts that the lack of this controlled activity will result in an unregulated population of MEKK3 under homeostatic conditions.
MEK5, a kinase that interacts with MEKK3 and an important downstream effector, was also present in CCM2L immunoprecipitates, but only when MEKK3 was coexpressed (Fig. 2G). We found that CCM2L in the MEKK3/MEK5 complex significantly attenuated MEKK3-mediated MEK5 phosphorylation (MEK5 p-Ser311/p-Thr315) in its activation loop (Fig. 2H). The strong interaction of MEKK3 and CCM2L would predict a greater amount of inactive MEKK3 in the presence of CCM2L in total cell lysates. In agreement with this, cells containing MEKK3 and CCM2L showed a significant reduction in p-Ser526 compared with cells with MEKK3 alone (Fig. 2I).
In summary, CCM2L and CCM2 interact with MEKK3, with CCM2L demonstrating greater binding, and both proteins prevent MEKK3 activity. Other MEKK3-interacting proteins also reportedly regulate kinase activation in in vitro assays. The interaction of TAK1 kinase with MEKK3 prevents its basal activation, and dissociation of the complex increases MEKK3 activity (22). Moreover, the interaction of MEKK3 and MEKK2 with 14-3-3 proteins limits their ability to dimerize and autophosphorylate in the activation loop (24, 25). The interaction of CCM2L with MEKK3 may prevent a feed-forward loop of MEKK3 activation by transphosphorylation, but might not in itself interfere with MEKK3’s ability to phosphorylate all of its downstream targets. In this case, CCM2L may serve as a scaffold, as has been shown for CCM2. When CCM2L interacts with inactive MEKK3, it forms a stable complex (Fig. S1A) that keeps MEKK3 inactive and prevents its activation by autophosphorylation (Fig. 2F), whereas the interaction with active MEKK3 results in CCM2L phosphorylation, as demonstrated by its reduced mobility in SDS gels (Fig. S1A). Whether phosphorylated CCM2L dissociates from this complex and allows dimerization and further kinase activation is an area of interest for future investigation. Thus, the regulation of MEKK3 likely is related to local amounts of CCM2L, whose dynamic expression may serve this purpose. The finding that the total pool of active MEKK3 in cell lysates is markedly reduced in the presence of CCM2L highlights the significance of this regulation.
Genetic Interaction of ccm2l with ccm2 and ccm1 in Zebrafish.
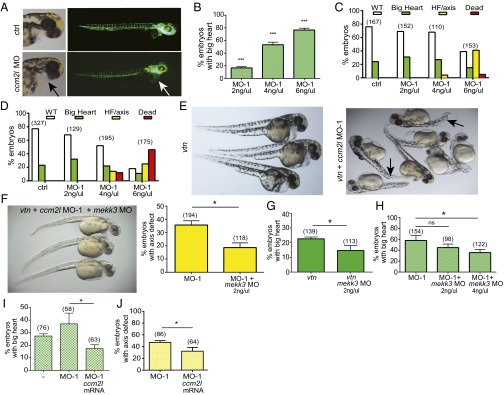
We exploited the zebrafish model to address whether ccm2 and ccm2l are in the same pathway and regulate mekk3 function in vivo. Mutations in the zebrafish orthologs of CCM proteins result in distinct phenotypes that include a “big heart,” characterized by an abnormally enlarged heart cavity, lack of circulation, and disruption of intersomitic vessels (26). A MO designed to knock down ccm2l was injected into one-cell stage embryos of zebrafish expressing GFP in endothelial cells (flk-GFP) to visualize the vasculature. At 48 h postfertilization, ccm2l MO-injected fish displayed an enlarged atrium, ventricle, and pericardial cavity (big heart) (Fig. 3A), associated with mild reductions in heart rate [mean, 156 ± 8 bpm in controls (n = 6) vs. 140 ± 9 bpm in morphants (n = 11); P < 0.005] and cardiac output [44 ± 7 nL/min in controls (n = 6) vs. 32 ± 10 nL/min in morphants (n = 11); P < 0.05]. Vasculogenesis appeared grossly unaffected. This phenotype was dose-dependent; increasing amounts of ccm2l MO-1 resulted in more embryos with the heart enlargement/big heart phenotype (Fig. 3B), which is consistent with a previous report (11).
Fig. 3.
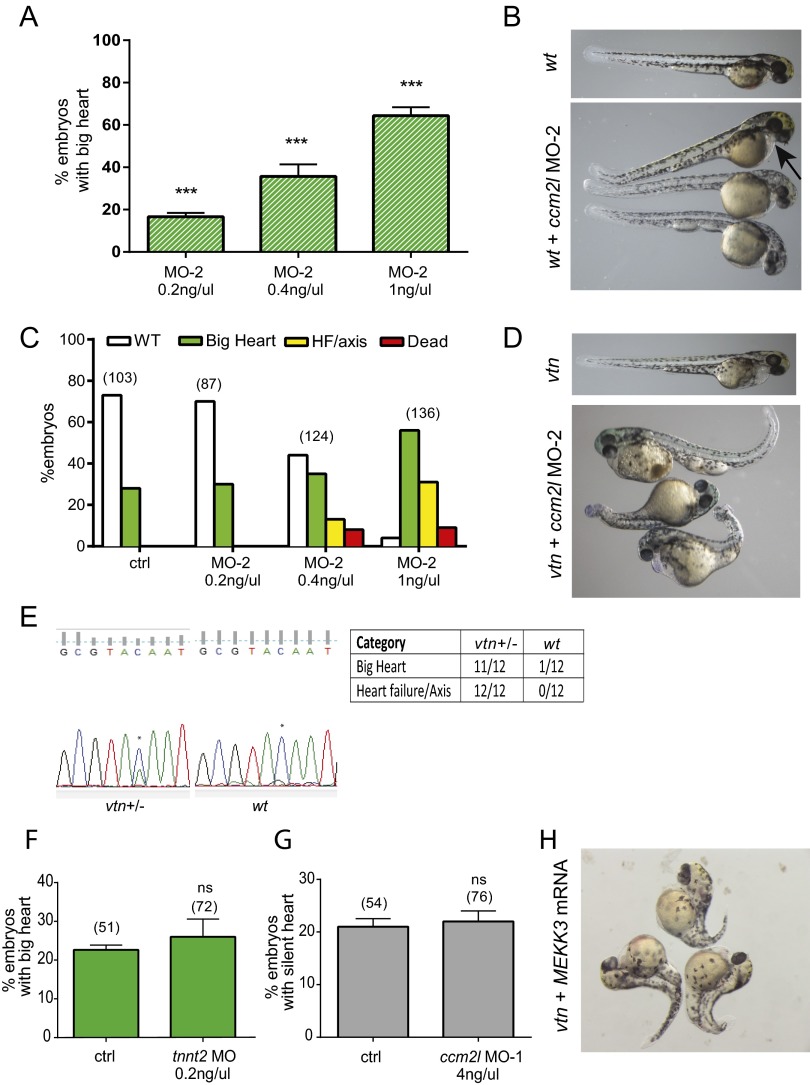
Genetic interaction of ccm2l with ccm1 and ccm2 and modulation of mekk3 activity in zebrafish. (A) Single-cell flk-GFP embryos were injected with control (ctrl) or ccm2l MO (MO-1), and images were obtained at 48 h postfertilization. The arrow indicates cardiac enlargement/big heart. (B) The big heart phenotype was evaluated with increasing doses of ccm2l MO-1. (C–E) Different amounts of ccm2l MO-1 or ctrl MO were injected into embryos from san/ccm1 (C) and vtn/ccm2 (D) heterozygous crosses. The vtn/ccm2 zebrafish with ccm2l knockdown show further enlargement of the heart in combination with heart failure (HF) and tail/axis defects (arrows). Phenotypes were classified as WT (no phenotype), big heart, HF/axis (HF and axis/tail defects), or dead (lack of survival). (E) Representative pictures of vtn/ccm2 mutants without (Left) or with ccm2l MO-1 injected (Right). (F) Rescue of the axis phenotype in ccm2/ccm2l-deficient fish with 4 ng/µL mekk3 MO. Embryos from vtn/ccm2 heterozygous crosses were injected with ccm2l alone or in combination with ctrl or mekk3 MO, and the axis defect phenotype was scored. (G) Rescue of the big heart phenotype in vtn/ccm2 mutant fish (vtn) with ctrl or 2 ng/µL mekk3 MO. (H) Rescue of big heart in WT plus 4 ng/µL ccm2l MO-1 injection with ctrl MO or indicated concentrations of mekk3 MO. (I and J) Rescue of big heart (I) and tail axis defects (J) in vtn + ccm2l MO-1 zebrafish, with human CCM2L mRNA. The big heart phenotype in vtn alone (I, −) is shown for comparison. *P < 0.05, unpaired t test. Graphs represent data pooled from three independent experiments. Error bars represent SEM.
Injections of ccm2l MO into santa (san/ccm1) (Fig. 3C) and valentine (vtn/ccm2) (Fig. 3D) heterozygous pair crosses demonstrated a strong genetic interaction between ccm2l and ccm1 or ccm2, which was more prominent with vtn/ccm2, as evidenced by a decrease in survival rate, a complete heart failure (HF) phenotype, and appearance of a markedly distorted body axis (Fig. 3 D and E). Here 14% of vtn/ccm2 mutants sensitized with the ccm2l MO-1 (4 ng/μL) had a severe HF/axis phenotype, and 12% died. This was even more pronounced with the higher-dose injections (6 ng/μL), where 25% of vtn/ccm2 mutants showed HF/axis defects and 46% died. In similarly sensitized san/ccm1 mutants, 4% had the HF/axis defect and 0% died at 4 ng/μL ccm2l MO, and 41% had the HF/axis defect and 5% died at higher MO concentrations.
To further confirm the specificity of this phenotype and the genetic interaction with the CCM pathway, we injected a second MO (MO-2) against ccm2l in WT embryos and embryos generated by vtn/ccm2 intercrosses. We observed the same big heart phenotype in the injected WT embryos (Fig. S2 A and B). Moreover, MO-2 injection in vtn/ccm2 embryos (Fig. S2 C and D) again resulted in a decrease in survival rates, and embryos with the HF/axis phenotype. These experiments were performed with lower doses of the MO-2, because the higher doses produced toxicity and poor survival.
Fig. S2.
(A) Dose–response curve of ccm2l MO-2 injected into embryos from WT. ***P < 0.005, ANOVA. (B) Representative pictures of WT embryos (Upper) with and without ccm2l MO-2 (Lower). The arrow indicates big heart. (C and D) Injection of MO-2 in vtn/ccm2 heterozygous crosses results in heart failure and severe tail/axis defects. Quantitation of phenotypes in C: WT, no phenotype; big heart; HF/axis, heart failure and axis/tail defects; dead, lack of survival. Representative images are shown in D. (E) Genotyping. Chromatograms representing sequence results from vtn heterozygous crosses injected with ccm2l MO-1 (Upper). vtn heterozygous embryos show a double peak (C and A, asterisks), inferring the presence of a vtn (A) and an ortholog WT (C) gene, whereas WT controls show a single peak (C). vtn homozygous embryos were not detected in the surviving embryos in this cross, which may reflect lower survival rates in this group after ccm2l MO injection (Fig. 2D). (F and G) Injections of a cardiac-specific MO (troponinT, tnnt2) in vtn mutants (F) and ccm2l MO-1 injections in silent heart mutant (G) did not phenocopy the ccm2l MO-1 axis defect results (ns, not significant, unpaired t test). Graphs represent data pooled from three independent experiments. The number of embryos scored is in parentheses. Error bars represent SEM. (H) Representative picture of mekk3 mRNA-injected vtn/ccm2 embryos showing more severe tail axis defects and cardiac failure.
Individual, differently classified zebrafish embryos from experiments were genotyped. The embryos with the axis defects correlated with embryos containing a mutant vtn allele in the presence of ccm2l MO, whereas no WT fish were detected in this group (Fig. S2E). Control injections using a cardiac-specific MO (troponinT; tnnt2) in vtn/ccm2 mutants and the injection of ccm2l MO-1 into the silent heart mutants (sih/tnnt2) resulted in the expected big heart and silent phenotype, respectively, but with no additional tail axis defects (Fig. S2 F and G). This indicates that the distorted axis/tail phenotype is specific to the ccm2l/ccm2 interaction, and that a defect in heart function is not responsible for the observed phenotype. These results suggest perturbed body axis patterning (27), a phenotype not previously reported in ccm mutant lines (11, 26, 28) or in WT fish injected with ccm2l MO alone (11).
Role for MEKK3 in a CCM Genetic Pathway in Zebrafish.
Our biochemical results prompted us to investigate the role of MEKK3 in the body axis phenotype observed in the vtn/ccm2l morphants. Overexpression of MEKK3 in the vtn/ccm2l morphants by injection of in vitro translated MEKK3 mRNA resulted in more severe tail axis defects and cardiac failure (Fig. S2H); however, MOs directed to mekk3 efficiently rescued the tail axis defect (Fig. 3F). Because rescue experiments usually require careful dosing of MOs, we chose to use the milder ccm2l MO 1 (MO-1) to rescue the CCM signaling loss-of-function–specific phenotypes. In this experiment, 33% of vtn/ccm2 embryos receiving only the ccm2l MO-1 showed a pronounced axis defect, compared with 19% receiving coinjection with the mekk3 MO (Fig. 3F). Thus, mekk3 knockdown decreased the severity of the axial phenotypes, indicating that ccm2 and ccm2l modulation of mekk3 activity may be an important regulator of body axis patterning. Ccm2 and Ccm2l play antagonistic functions in cardiovascular development in mice (10), whereas in zebrafish, ccm2l deficiency enhances ccm1 and ccm2 mutant phenotypes in heart development, as shown here by our experiments as well as in a previous study (11).
Injection of the mekk3 MO into embryos generated by vtn/ccm2 intercrosses also reduced the prevalence of the big heart phenotype from 23% to 15% in mekk3 MO-injected embryos (Fig. 3G), consistent with a recent report (29). Similarly, the mekk3 MO reduced the big heart phenotype in WT fish injected with ccm2L MO (Fig. 3H). The rescue of the body axis patterning phenotype in ccm2/ccm2l-deficient zebrafish and the big heart phenotype in single gene deficiency with mekk3 MOs demonstrates the importance of MEKK3 regulation by these two CCM-related proteins. Notably, ccm2l mRNA is located in the tail of embryos both in the cell mass ventral to the notochord and in the presumptive notochord (11), an important structure in vertebral column development. ccm2 is also present in the ventral cell mass, whereas ccm1 mRNA is present in the notochord (26). Moreover, MAPK pathways play key roles in notochord differentiation, with interference in this pathway leading to distortion in the tail axis (30). Finally, using in vitro transcribed human CCM2L, we found that CCM2L isoform 1 significantly rescued the greater heart and tail axis defects observed in zebrafish mutants (Fig. 3 I and J). This suggests that the defects observed in ccm2l MO-injected zebrafish are specific and not due to off-target effects.
CCM2 Deletion Leads to Activation of an MEKK3-Dependent Pathway.
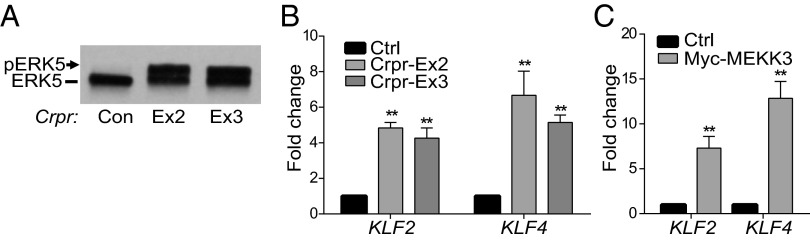
In endothelial cells, activation of ERK5, a downstream effector of MEKK3, up-regulates Kruppel-like transcription factors (KLFs), which contribute to endothelial cell homeostasis. To examine whether CCM2 in endothelial cells regulates this pathway, we genetically deleted CCM2 using CRISPR/Cas9 lentiviral approaches in HUVEC. Knockdown of CCM2 using two independent guide RNAs led to ERK5 hyperactivation and up-regulation of KLF2 and KLF4. In agreement with the role of MEKK3 in this pathway, overexpression of this kinase in endothelial cells led to significant increases in KLF2 and KLF4 expression (Fig. 4).
Fig. 4.
ERK5 activation in CCM2 deleted endothelial cells. (A and B) HUVEC transduced with control or CCM2 exons 2 or 3 CRISPR/Cas9 (Crpr) lentivirus were analyzed for ERK activation by Western blot (A) or for KLF2 or KLF4 mRNA by qRT-PCR (B). Activation of ERK5 is detected by the appearance of a slower migrating form indicating phosphorylation (arrow). (C) HUVEC were transduced with lentiviral particles to express Myc-tagged MEKK3 and harvested for qRT-PCR after 48 h, and KLF2 and KLF4 mRNA levels were evaluated. ***P < 0.05. n = 4 experiments. Error bars represent SEM.
In conclusion, hyperactivation of MEKK3 in the absence of the CCM proteins CCM2 and/or CCM2L interferes with heart development and body axis patterning in zebrafish. This correlates in vitro with interference of MEKK3 hyperactivation by these CCM proteins. Although loss-of-function mutations in the newly identified CCM2L in human CCM lesions have not yet been reported, we predict that CCM2L regulation of MEKK3 can modify the severity of CCM disease. Because CCM2L expression is regulated by flow, low-flow conditions in CCM lesions may result in a reduction in CCM2L expression and subsequent exacerbation of MEKK3 activation, the tight regulation of which is required for formation of a normal vasculature. Genetic inactivation of members of the MEKK3 pathway results in embryonic lethality, characterized by defects in angiogenesis and early cardiovascular development (14, 15). Our data in zebrafish are informative of the molecular mechanism of action of CCM2L; however, the link with the CCM disease in humans remains unclear, given that the phenotypes observed with a deficiency of CCM2L or other CCM proteins are not recapitulated in CCM patients. Nevertheless, features of the phenotypes reported in zebrafish, such as dilated vessels and defective tight junctions, are observed in CCM lesions.
Materials and Methods
All experiments were performed in Tuebingen AB zebrafish or Danio fish obtained from EkkWill Waterlife Resources. Zebrafish were bred and maintained at 28.5 °C with 14-h light and 10-h dark exposure (day/night cycle), in accordance with the protocol approved by the Harvard Subcommittee for Animal Research. Detailed information on cDNA constructs and experimental procedures is provided in SI Materials and Methods.
SI Materials and Methods
Cell Culture.
Cord blood endothelial progenitor cells were isolated as described previously (31) and cultured on fibronectin-coated plates with Endothelial Basal Medium (Lonza) supplemented with 20% FBS and SingleQuot (Lonza) and 1× PSF (antibiotic-antimycotic solution; CellGro Mediatech). Then 293T cells, cultured in DMEM supplemented with 10% FCS, were transfected using Lipofectamine 2000 (Life Technologies). HUVEC were isolated as described previously (32) and cultured in M199 supplemented with 20% FBS, 10 μg/mL heparin, 5 μg/mL endothelial cell growth supplement (BD Biosciences), and antibiotics. For statin treatments, HUVEC were incubated for 16 h in growth medium with simvastatin, atorvastatin, or DMSO (vehicle) control.
For analysis of cell density effects, confluent HUVEC monolayers (75–80,000 cells/cm2) were trypsinized and replated at the indicated dilutions and harvested at the indicated times. Human neutrophils and mononuclear cells were isolated from whole blood drawn from healthy volunteers and used as described previously (32). Blood draw protocols for protection of human subjects were approved by the Brigham and Women's Hospital Institutional Review Board.
Shear Stress Treatment.
For this treatment, 60,000 cells/cm2 were plated in a 3.5-cm-diameter dish in 2.5 mL of Complete Medium. After 10 h, cells were exposed to shear for 24 h using an orbital shaker (33, 34). At 170 rpm, the shear stress at the periphery of the plate was calculated as 12 dynes/cm2 according to published procotols (33, 34), which is within the range of physiological arterial shear stress (∼6–40 dynes/cm2). Cells in the inner circle within a radius of 0.9 cm were scraped off and excluded, because they were subjected to lower shear. HUVEC from the same passage served as the static control.
RNA Isolation and qRT-PCR.
Total RNA was isolated using the Qiagen RNAeasy Mini Kit. Human tissue samples were homogenized using the Tissulyzer LT System (Qiagen) before RNA isolation and used for cDNA synthesis with the Maxima First-Strand cDNA Synthesis Kit for qRT-PCR (Thermo Scientific). qRT-PCR was performed using Power SYBR Green Master Mix and analyzed using the 2-ΔCt method. The expression level of CCM2L in different cell lines was normalized with β-actin. For relative quantification, gene expression levels were normalized with the control GAPDH in the same sample, and differences were expressed as mean ± SEM fold change compared with controls, which were set at 1. For human tissues, mean fold change was compared with levels in the kidney samples, which were set at 1. Primers for CCM2, CCM2L, GAPDH, and β-actin were obtained from Qiagen.
cDNA Constructs.
The cDNAs for the CCM2L splice variant isoform 2 (Uniprot Q9NUG4-2) and canonical isoform 1 (Uniprot Q9NUG4-1) (Open Biosystems) were N-terminally tagged with a 3× FLAG epitope by qRT-PCR and cloned into a mammalian expression vector (CMV promoter). CCM2 cDNA (Open Biosystems) was cloned into pEGFPC1 (Clontech), and the GFP cDNA sequence was replaced by a 3× FLAG tag. CCM1 cDNA was cloned into the expression vector PRK5-Myc-tag (Addgene). This construct was also used to generate T7-tagged CCM1 by replacing the tag sequences. MEKK3 cDNA was obtained by qRT-PCR from HUVEC mRNA and cloned into PRK5-Myc-tag plasmid. The lentiviral construct to express Myc-MEKK3 was obtained by cloning the cDNA into pWPXL (Addgene). Site-directed mutagenesis of the ATP-binding site, lysine 391 to alanine (K391A), generated inactive MEKK3 (MEKK3 K > A). To delete the kinase domain (MEKK3ΔK), a stop codon was introduced after Asn-342. The kinase domain [amino acids 341–626 (35)] was also obtained by qRT-PCR and cloned into PRK5-Myc-tag. Human MEK5 cDNA was obtained by qRT-PCR from HUVEC mRNA and cloned into PRK5-T7-Tag. FLAG-tagged CCM2L4x mutant (F434R, A435S, L437K, and L438K) was generated by PCR. FLAG-tagged CCM2L was cloned into Adeno-pCMV DEST vector (Gateway System; Life Technologies) and transfected into 293 cells to generate adenovirus supernatant.
Antibodies.
Antibodies used in these experiments included anti-Myc, anti-T7 tag, and anti-ERK5 (Cell Signaling); anti-Flag tag (M2; Sigma-Aldrich); anti-phospho-MEK5 (S311/T315; Santa Cruz Biotechnology); anti-MEK5 (BD Biosciences); and anti-phospho-MEKK3 (S526; a generous gift from R. Vaillancourt, University of Arizona).
IP and Western Blot Analyses.
Transfected cells were washed with ice-cold PBS and lysed in Nonidet P-40 lysis buffer with protease and phosphatase inhibitors (Sigma-Aldrich) on ice. Cell extracts were boiled in Laemmli buffer and subjected to SDS/PAGE and Western blot analysis. For IP of tagged proteins, lysates were incubated with 20 μL of protein G beads (Dynalbeads) and 1 μg of the indicated anti-tag antibody.
Kinase Assays.
Beads containing immunopecipitated proteins were washed with lysis buffer and then kinase buffer (25 mM Tris⋅HCl pH 7.5, 5 mM β-glycerophosphate, 2 mM DTT, 0.1 mM Na3VO4, and 10 mM MgCl2). Kinase assays were performed at 25 °C for 30 min in 50 μL of kinase buffer supplemented with 200 pμM ATP. Beads were prepared for SDS/PAGE and Western blot analysis.
MO Experiments.
MOs (Gene Tools) were resuspended in sterile water (1 mM) and diluted to 10–100 µM with 1× Danieau's buffer [58 mM NaCl, 0.7 mM KCl, 0.4 mM MgSO4, 0.6 mM Ca(NO3)2, and 0.5 mM Hepes, pH 7.6]. The MOs were diluted as indicated (μg/mL), and 1 nL was injected at the single-cell stage. For each experimental MO, a control MO (4 ng/μL) was injected as a control.
MO sequences were as follows: ccm2l MO-1 ex4SA, 5′-AGCTTCTACAGGCATACAGAAATGT-3′; ccm2l MO-2 ex2SA, 5′-AATCCCTGAGAGAAGAATTGGTGTA-3′; mekk3 ex2 MO, 5′-AAATGTGTTTTACCAATCTGTTGGA-3′; ctrl MO, 5′-CCTCTTACCTCAGTTACAATTTATA-3′; TnT/sih MO, 5′-CATGTTTGCTCTGATCTGACACGCA-3′.
For mRNA preparations, a full-length human MEKK3 construct or human CCM2L isoform 1 construct containing Sp6 sites was linearized, and capped mRNA was transcribed in vitro using the mMessage mMachine Kit (Ambion). For the MEKK3 mRNA injection experiments, doses (50, 100, and 200 ng/μL) of capped mRNA were coinjected with ccm2l MO-1. Zebrafish cardiac physiology was performed as described previously (36). For the 50 ng/μL dose, hCCM2L mRNA was injected. In all experiments, data were pooled from three independent experiments.
Zebrafish Genotyping.
Individual embryos were collected in labeled PCR strips in 100 μL of 50 mM NaOH, incubated for 20 min at 95 °C, and cooled to 4 °C, after which 10 μL of Tris⋅HCL pH 7.5 was added. A two-step phusion PCR was performed, and purified PCR fragments were sequenced to detect the vtn/ccm2 mutation.
CRISPR/Cas Deletion of CCM2, Adenoviral Transduction of CCM2L, and Analysis in HUVEC.
Designed guide RNAs to CCM2 exon 2 or 3 or control guide were cloned into lentiCRISPRv2 (Addgene) to generate VSV-G pseudotyped lentiviral particles using 293 T cells. Transduced HUVEC were selected at 48 h after infection with puromycin (1 μg/mL) for 3 d, and lysates were analyzed by Western blot using anti-ERK5 antibody and by qRT-PCR using KLF2, KLF4, and β-actin primers (Qiagen). At 48 h after transduction with Adeno-CCM2L, HUVEC lysates were subjected to FLAG IP and Western blot analysis using anti-MEKK3 (Cell Signaling Technology).
Acknowledgments
We thank Dr. Dan Milner (Brigham and Women’s Hospital) for providing human tissue samples, Dr. Joyce Bischoff (Boston Children’s Hospital) for providing cord blood endothelial precursor cells, and Kay Case (Brigham and Women’s Hospital) for providing cultured HUVEC. This work was supported by National Institutes of Health Grants P01 HL036028 (to T.N.M. and X.C.) and R01 HL109264 (to C.A.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1510495112/-/DCSupplemental.
References
- 1.Fischer A, Zalvide J, Faurobert E, Albiges-Rizo C, Tournier-Lasserve E. Cerebral cavernous malformations: From CCM genes to endothelial cell homeostasis. Trends Mol Med. 2013;19(5):302–308. doi: 10.1016/j.molmed.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Fisher OS, Boggon TJ. Signaling pathways and the cerebral cavernous malformation proteins: Lessons from structural biology. Cell Mol Life Sci. 2014;71(10):1881–1892. doi: 10.1007/s00018-013-1532-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng X, et al. CCM3 signaling through sterile 20-like kinases plays an essential role during zebrafish cardiovascular development and cerebral cavernous malformations. J Clin Invest. 2010;120(8):2795–2804. doi: 10.1172/JCI39679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoruk B, Gillers BS, Chi NC, Scott IC. Ccm3 functions in a manner distinct from Ccm1 and Ccm2 in a zebrafish model of CCM vascular disease. Dev Biol. 2012;362(2):121–131. doi: 10.1016/j.ydbio.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Richardson BT, Dibble CF, Borikova AL, Johnson GL. Cerebral cavernous malformation is a vascular disease associated with activated RhoA signaling. Biol Chem. 2013;394(1):35–42. doi: 10.1515/hsz-2012-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu Y, et al. Differential angiogenesis function of CCM2 and CCM3 in cerebral cavernous malformations. Neurosurg Focus. 2010;29(3):E1. doi: 10.3171/2010.5.FOCUS1090. [DOI] [PubMed] [Google Scholar]
- 7.Wüstehube J, et al. Cerebral cavernous malformation protein CCM1 inhibits sprouting angiogenesis by activating DELTA-NOTCH signaling. Proc Natl Acad Sci USA. 2010;107(28):12640–12645. doi: 10.1073/pnas.1000132107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maddaluno L, et al. EndMT contributes to the onset and progression of cerebral cavernous malformations. Nature. 2013;498(7455):492–496. doi: 10.1038/nature12207. [DOI] [PubMed] [Google Scholar]
- 9.Renz M, et al. Regulation of β1 integrin-Klf2–mediated angiogenesis by CCM proteins. Dev Cell. 2015;32(2):181–190. doi: 10.1016/j.devcel.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 10.Zheng X, et al. Dynamic regulation of the cerebral cavernous malformation pathway controls vascular stability and growth. Dev Cell. 2012;23(2):342–355. doi: 10.1016/j.devcel.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosen JN, Sogah VM, Ye LY, Mably JD. ccm2-like is required for cardiovascular development as a novel component of the Heg-CCM pathway. Dev Biol. 2013;376(1):74–85. doi: 10.1016/j.ydbio.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uhlik MT, et al. Rac-MEKK3-MKK3 scaffolding for p38 MAPK activation during hyperosmotic shock. Nat Cell Biol. 2003;5(12):1104–1110. doi: 10.1038/ncb1071. [DOI] [PubMed] [Google Scholar]
- 13.Drew BA, Burow ME, Beckman BS. MEK5/ERK5 pathway: The first fifteen years. Biochim Biophys Acta. 2012;1825(1):37–48. doi: 10.1016/j.bbcan.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang J, et al. Mekk3 is essential for early embryonic cardiovascular development. Nat Genet. 2000;24(3):309–313. doi: 10.1038/73550. [DOI] [PubMed] [Google Scholar]
- 15.Rose BA, Force T, Wang Y. Mitogen-activated protein kinase signaling in the heart: Angels versus demons in a heart-breaking tale. Physiol Rev. 2010;90(4):1507–1546. doi: 10.1152/physrev.00054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boulday G, et al. Tissue-specific conditional CCM2 knockout mice establish the essential role of endothelial CCM2 in angiogenesis: Implications for human cerebral cavernous malformations. Dis Model Mech. 2009;2(3-4):168–177. doi: 10.1242/dmm.001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boulday G, et al. Developmental timing of CCM2 loss influences cerebral cavernous malformations in mice. J Exp Med. 2011;208(9):1835–1847. doi: 10.1084/jem.20110571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taddei A, et al. Endothelial adherens junctions control tight junctions by VE-cadherin–mediated up-regulation of claudin-5. Nat Cell Biol. 2008;10(8):923–934. doi: 10.1038/ncb1752. [DOI] [PubMed] [Google Scholar]
- 19.Gimbrone MA, Jr, Garcia-Cardena G. Vascular endothelium, hemodynamics, and the pathobiology of atherosclerosis. Cardiovasc Pathol. 2013;22(1):9–15. doi: 10.1016/j.carpath.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, et al. Structural insights into the molecular recognition between cerebral cavernous malformation 2 and mitogen-activated protein kinase kinase kinase 3. Structure. 2015;23(6):1087–1096. doi: 10.1016/j.str.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Fritz A, et al. Phosphorylation of serine 526 is required for MEKK3 activity, and association with 14-3-3 blocks dephosphorylation. J Biol Chem. 2006;281(10):6236–6245. doi: 10.1074/jbc.M509249200. [DOI] [PubMed] [Google Scholar]
- 22.Di Y, Li S, Wang L, Zhang Y, Dorf ME. Homeostatic interactions between MEKK3 and TAK1 involved in NF-kappaB signaling. Cell Signal. 2008;20(4):705–713. doi: 10.1016/j.cellsig.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng J, et al. Dimerization through the catalytic domain is essential for MEKK2 activation. J Biol Chem. 2005;280(14):13477–13482. doi: 10.1074/jbc.M414258200. [DOI] [PubMed] [Google Scholar]
- 24.Matitau AE, Gabor TV, Gill RM, Scheid MP. MEKK2 kinase association with 14-3-3 protein regulates activation of c-Jun N-terminal kinase. J Biol Chem. 2013;288(39):28293–28302. doi: 10.1074/jbc.M113.511352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matitau AE, Scheid MP. Phosphorylation of MEKK3 at threonine 294 promotes 14-3-3 association to inhibit nuclear factor kappaB activation. J Biol Chem. 2008;283(19):13261–13268. doi: 10.1074/jbc.M801474200. [DOI] [PubMed] [Google Scholar]
- 26.Mably JD, et al. santa and valentine pattern concentric growth of cardiac myocardium in the zebrafish. Development. 2006;133(16):3139–3146. doi: 10.1242/dev.02469. [DOI] [PubMed] [Google Scholar]
- 27.Hammerschmidt M, Mullins MC. Dorsoventral patterning in the zebrafish: Bone morphogenetic proteins and beyond. In: Solnica-Krezel L, editor. Pattern Formation in Zebrafish (Results and Problems in Cell Differentiation) Springer; Berlin, Germany: 2002. [DOI] [PubMed] [Google Scholar]
- 28.Mably JD, Mohideen MA, Burns CG, Chen JN, Fishman MC. heart of glass regulates the concentric growth of the heart in zebrafish. Curr Biol. 2003;13(24):2138–2147. doi: 10.1016/j.cub.2003.11.055. [DOI] [PubMed] [Google Scholar]
- 29.Zhou Z, et al. The cerebral cavernous malformation pathway controls cardiac development via regulation of endocardial MEKK3 signaling and KLF expression. Dev Cell. 2015;32(2):168–180. doi: 10.1016/j.devcel.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hawkins TA, Cavodeassi F, Erdélyi F, Szabó G, Lele Z. The small molecule Mek1/2 inhibitor U0126 disrupts the chordamesoderm-to-notochord transition in zebrafish. BMC Dev Biol. 2008;8:42. doi: 10.1186/1471-213X-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu X, et al. Tissue-engineered microvessels on three-dimensional biodegradable scaffolds using human endothelial progenitor cells. Am J Physiol Heart Circ Physiol. 2004;287(2):H480–H487. doi: 10.1152/ajpheart.01232.2003. [DOI] [PubMed] [Google Scholar]
- 32.Bevilacqua MP, Pober JS, Wheeler ME, Cotran RS, Gimbrone MA., Jr Interleukin 1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes, and related leukocyte cell lines. J Clin Invest. 1985;76(5):2003–2011. doi: 10.1172/JCI112200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dardik A, et al. Differential effects of orbital and laminar shear stress on endothelial cells. J Vasc Surg. 2005;41(5):869–880. doi: 10.1016/j.jvs.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 34.dela Paz NG, Walshe TE, Leach LL, Saint-Geniez M, D’Amore PA. Role of shear-stress–induced VEGF expression in endothelial cell survival. J Cell Sci. 2012;125(Pt 4):831–843. doi: 10.1242/jcs.084301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ellinger-Ziegelbauer H, Brown K, Kelly K, Siebenlist U. Direct activation of the stress-activated protein kinase (SAPK) and extracellular signal-regulated protein kinase (ERK) pathways by an inducible mitogen-activated protein kinase/ERK kinase kinase 3 (MEKK) derivative. J Biol Chem. 1997;272(5):2668–2674. doi: 10.1074/jbc.272.5.2668. [DOI] [PubMed] [Google Scholar]
- 36.Asimaki A, et al. Identification of a new modulator of the intercalated disc in a zebrafish model of arrhythmogenic cardiomyopathy. Sci Transl Med. 2014;6(240):240ra74. doi: 10.1126/scitranslmed.3008008. [DOI] [PMC free article] [PubMed] [Google Scholar]