Significance
Emerging evidence indicates patients who benefit from antiangiogenic therapies have improved vessel function. To determine how bevacizumab modulates vessel morphology to improve vessel function we conducted a phase II trial of preoperative bevacizumab followed by bevacizumab combined with chemotherapy in HER2-negative breast cancer patients. Our results suggest that the clinical response to bevacizumab may occur through an increase in the extent of vascular normalization primarily in patients with a high baseline tumor microvessel density. If validated, these observations suggest approaches to improve antiangiogenic therapy and to identify patients likely to benefit.
Keywords: antiangiogenic therapy, circulating and tissue biomarkers, PAM50 gene signature, cellular proliferation
Abstract
Preoperative bevacizumab and chemotherapy may benefit a subset of breast cancer (BC) patients. To explore potential mechanisms of this benefit, we conducted a phase II study of neoadjuvant bevacizumab (single dose) followed by combined bevacizumab and adriamycin/cyclophosphamide/paclitaxel chemotherapy in HER2-negative BC. The regimen was well-tolerated and showed a higher rate of pathologic complete response (pCR) in triple-negative (TN)BC (11/21 patients or 52%, [95% confidence interval (CI): 30,74]) than in hormone receptor-positive (HR)BC [5/78 patients or 6% (95%CI: 2,14)]. Within the HRBCs, basal-like subtype was significantly associated with pCR (P = 0.007; Fisher exact test). We assessed interstitial fluid pressure (IFP) and tissue biopsies before and after bevacizumab monotherapy and circulating plasma biomarkers at baseline and before and after combination therapy. Bevacizumab alone lowered IFP, but to a smaller extent than previously observed in other tumor types. Pathologic response to therapy correlated with sVEGFR1 postbevacizumab alone in TNBC (Spearman correlation 0.610, P = 0.0033) and pretreatment microvascular density (MVD) in all patients (Spearman correlation 0.465, P = 0.0005). Moreover, increased pericyte-covered MVD, a marker of extent of vascular normalization, after bevacizumab monotherapy was associated with improved pathologic response to treatment, especially in patients with a high pretreatment MVD. These data suggest that bevacizumab prunes vessels while normalizing those remaining, and thus is beneficial only when sufficient numbers of vessels are initially present. This study implicates pretreatment MVD as a potential predictive biomarker of response to bevacizumab in BC and suggests that new therapies are needed to normalize vessels without pruning.
Ten drugs that target VEGF or its receptors have been approved for the treatment of various malignant diseases (1). However, bevacizumab, an anti-VEGF antibody, and other antiangiogenic agents (AAs) that target the VEGF pathway have failed to provide an overall survival benefit to metastatic breast cancer (BC) patients (2). Preoperative (neoadjuvant) therapy is an effective way of treating certain BC patients, because this strategy leads to survival rates similar to those from postoperative therapy (3) while reducing the extent of surgery. Moreover, a favorable pathologic response to neoadjuvant therapy is associated with longer disease-free survival (4, 5). Recent studies report significant increases in the percentage of patients with no detectable residual disease—referred to as pathologic complete response (pCR)—with the addition of bevacizumab to neoadjuvant chemotherapy in human epidermal growth factor receptor 2 (HER2)-negative BC. The GeparQuinto, the CALGB 40603, and the ARTemis trials demonstrated a significant increase in pCR with the addition of bevacizumab in patients with triple-negative BC (TNBC) (6–8). However, the National Surgical Adjuvant Breast and Bowel Project B-40 study demonstrated a higher pCR rate in hormone receptor-positive BC (15.1% without bevacizumab vs. 23.2% with bevacizumab, P = 0.007) but no statistically significant difference in TNBC (9). Moreover, two postoperative (adjuvant) trials of bevacizumab, BEATRICE and E5103, demonstrated no improvement in disease-free survival with the addition of bevacizumab to standard anthracycline- and taxane-based chemotherapy (2, 10). These inconsistent results underscore the need to identify mechanistic biomarkers of response to bevacizumab therapy.
There are two major hypotheses concerning AAs’ mechanism of action in tumors: (i) starving the tumor by blocking its blood supply and (ii) alleviating hypoxia by normalizing the function of tumor vasculature (1). Emerging functional imaging data in glioblastoma, nonsmall-cell lung cancer, and BC patients suggests that improved vascular function and the resulting increase in tumor oxygenation are associated with response to AAs (1, 11–15). However, there are no structural features of tumor vessels that can be used to predict the response (16), and the vascular changes induced by AAs to increase vessel function remain unclear. To complement the previous functional imaging studies with histological analysis of in situ changes in vascular structure in response to AA, we conducted a phase II trial of HER2-negative BC to investigate the neoadjuvant use of bevacizumab combined with standard of care dose-dense chemotherapy, which consists of doxorubicin and cyclophosphamide followed by paclitaxel (ACP). Neoadjuvant treatment enables pre- and posttherapy biopsies to explore potential mechanisms of action and biomarkers that may help select patients likely to benefit from AAs. In this exploratory correlative study, we evaluated biomarkers in serial biopsies, blood samples, and a functional marker of vascular normalization—tumor interstitial fluid pressure (IFP)—before and after a single dose of bevacizumab. Our results suggest that a high baseline microvascular density (MVD) in breast tumors may be necessary to benefit from bevacizumab-induced vascular normalization.
Results
Bevacizumab Combined with ACP Showed Superior Antitumor Activity in TNBC Compared with Hormone Receptor-Positive BC.
A total of 104 patients were registered on study between November 2007 and June 2011 (Table S1). One patient did not initiate study treatment and was removed from the analysis; another patient withdrew consent; one was ineligible after rediagnosis with HER2+ BC; and two other patients were not evaluable for response because they did not complete therapy as per protocol due to toxicity. Therefore, 99 patients represent the efficacy population. A total of 91 patients (88%) completed all protocol therapy (Fig. S1). Of the 103 patients who initiated study treatment, 52 patients (50.5%) experienced grade ≥3 adverse events. The toxicities observed in this study were consistent with that observed in prior studies of similar bevacizumab/chemotherapy regimens (Table S2). pCR was observed in 16 of 99 patients, with greater responses seen within the TNBC cohort [52%, 95% confidence interval (CI): 30–74%] than within the hormone receptor-positive BC (HRBC) cohort (6%, 95% CI: 2–14%). This represents a 15-fold increase in the odds of pCR in TNBC over HRBC (P < 0.0001). Similar differences were seen in residual cancer burden (RCB) (P < 0.0001) and Miller–Payne (MP) scores (P = 0.0005).
Table S1.
Patient characteristics
| Safety population (n = 103) | Efficacy population (n = 99) | |||
| Characteristics | N | % | N | % |
| Median age, y (range) | 48 (23–67) | 48 (23–67) | ||
| Race | ||||
| White | 96 | 92 | 92 | 93 |
| Black or African American | 4 | 4 | 4 | 4 |
| Asian | 3 | 3 | 3 | 3 |
| Grade | ||||
| 1 | 5 | 5 | 4 | 4 |
| 2 | 42 | 41 | 40 | 40 |
| 3 | 53 | 51 | 52 | 53 |
| Unknown/not done | 3 | 3 | 3 | 3 |
| Tumor size by MRI, median (range) | 3.2 (0.3–12) | 3.2 (0.3–12) | ||
| Tumor size by clinical examination, median (range) | 4 (0–12) | 4 (0–12) | ||
| Receptor status | ||||
| ER-positive | 80 | 78 | 76 | 77 |
| PR-positive | 70 | 68 | 66 | 67 |
| HER2-positive | 1 | 1 | 0 | 0 |
| Protocol therapy completion | ||||
| Completed | 91 | 88 | 91 | 92 |
| Not completed | 12 | 12 | 8 | 8 |
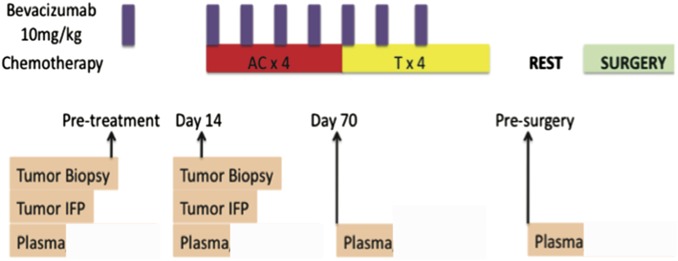
Fig. S1.
Study schema. Schedule for treatment with bevacizumab with adriamycin (A) /cyclophosphamide (C)/paclitaxel (T) chemotherapy, tumor tissue biopsies, and measurements of IFP and circulating blood (plasma) biomarkers.
Table S2.
Adverse events after neoadjuvant bevacizumab with dose-dense chemotherapy in BC patients
| Grade 2 | Grade 3 | Grade 4 | ||||
| Adverse event | N | % | N | % | N | % |
| Fatigue | 36 | 35 | 4 | 4 | 1 | 1 |
| Hyperglycemia | 34 | 33 | 7 | 7 | 0 | 0 |
| Alopecia | 36 | 35 | 1 | 1 | 0 | 0 |
| Lymphopenia | 23 | 22 | 6 | 6 | 1 | 1 |
| Hemoglobin | 22 | 21 | 2 | 2 | 1 | 1 |
| Nausea | 21 | 20 | 2 | 2 | 0 | 0 |
| Head/headache | 18 | 17 | 3 | 3 | 0 | 0 |
| Muscle pain | 14 | 14 | 4 | 4 | 0 | 0 |
| Hematologic, other | 16 | 16 | 0 | 0 | 0 | 0 |
| Hypertension | 13 | 13 | 3 | 3 | 0 | 0 |
| Leukopenia | 7 | 7 | 5 | 5 | 3 | 3 |
| Neuropathy, sensory | 13 | 13 | 2 | 2 | 0 | 0 |
| Joint pain | 11 | 11 | 2 | 2 | 0 | 0 |
| Neutrophils | 7 | 7 | 4 | 4 | 2 | 2 |
| Diarrhea without prior colostomy | 7 | 7 | 3 | 3 | 0 | 0 |
| Dyspepsia | 8 | 8 | 2 | 2 | 0 | 0 |
| Maximum grade by patient | 48 | 47 | 45 | 44 | 7 | 7 |
Gene Expression Profile Analysis Showed Differential Response in BC Subtypes.
PAM50 gene signature, which measures expression profiles for 50 genes and classifies tumors into four intrinsic subtypes (luminal A, luminal B, HER2-enriched, and basal-like), was available for 70 patients in the efficacy population (Table S3). Within this group, there were 13 pCRs (19%). The distribution of responses did not differ between subjects with and without PAM50 data available. Of the 13 pCRs, 11 were seen within the basal-like subset, 1 within the luminal A subset, and 1 within the luminal B subset (Table S3). Overall, responses varied by subtype in terms of pCR (P < 0.0001), MP (P = 0.0001), and RCB (P < 0.0001). Within the HR-positive subset with PAM50 data (n = 54), there was insufficient power to contrast pCR among luminal tumors (one pCR each, luminal A and B). However, even within these HRBCs, basal-like subtype was significantly associated with pCR (P = 0.007; Fisher exact test).
Table S3.
Pathologic response by PAM50 subtype
| Relationship of residual cancer burden to pam50 subtype | |||||
| Residual cancer burden | Luminal A | Luminal B | HER2-enriched | Basal-like | Total |
| pCR | 1 | 1 | 0 | 11 | 13 |
| I | 3 | 1 | 0 | 5 | 9 |
| II | 12 | 7 | 2 | 5 | 26 |
| III | 16 | 5 | 0 | 1 | 22 |
| Total | 32 | 14 | 2 | 22 | 70 |
| Relationship of MP score to PAM50 subtype in all patients | |||||
| MP | |||||
| 5 | 1 | 1 | 0 | 11 | 13 |
| 4 | 2 | 6 | 1 | 7 | 16 |
| 3 | 23 | 3 | 1 | 1 | 28 |
| 2 | 0 | 1 | 0 | 2 | 3 |
| 1 | 6 | 3 | 0 | 1 | 10 |
| Total | 32 | 14 | 2 | 22 | 70 |
| Relationship of MP score to PAM50 subtype in HRBC patients | |||||
| MP | |||||
| 5 | 1 | 1 | 0 | 3 | 5 |
| 4 | 2 | 6 | 1 | 2 | 11 |
| 3 | 23 | 3 | 1 | 0 | 27 |
| 2 | 0 | 1 | 0 | 0 | 1 |
| 1 | 6 | 3 | 0 | 1 | 10 |
| Total | 32 | 14 | 2 | 6 | 54 |
Bevacizumab Treatment Exerts Effects Consistent with Vascular Normalization in BC.
In patients with available biopsy and/or plasma samples, we examined the effects of bevacizumab treatment on biomarker levels related to vascular normalization (Tables S4–S6). We quantified all vessels in two biopsy sections per patient with custom software that automatically segmented CD31+ endothelial cells, αSMA+ perivascular cells (pericytes), and vessel lumen, combined them into vascular structures, and allowed manual confirmation of every selection (Fig. S2 and SI Methods). Bevacizumab reduced the intratumoral MVD (number of vessels per square millimeter; Fig. 1A), but not the density of mature vessels [pericyte-covered MVD (PC-MVD) and number of αSMA+ vessels per square millimeter; Fig. 1B and Table S4], indicating pruning of immature vessels, which lack pericytes, but not of mature vessels (Fig. S2 A–C). Pruning of immature vessels likely increases the fraction of vessels that are pericyte-covered (number of αSMA+ vessels per number of vessels), but VEGF blockade may also promote maturation of nascent vessels through active pericyte recruitment, thereby increasing PC-MVD (17, 18). Through both mechanisms, bevacizumab increased the average proportion of vessel perimeter associated with pericytes (αSMA+ vessel surface length per total vessel length; Fig. 1C), leading to a reduction in IFP (Fig. 1D), presumably due to a decrease in vascular permeability (19). However, biomarkers of functional normalization such as fraction of proliferative cells (Ki67-positive) and fraction of tissue area positive for endogenous hypoxia marker HIF-1α did not significantly change (Fig. 1 E and F). The only biomarker that differed between subtypes significantly in opposite directions was Ki67, because the fraction of proliferative cells increased in TNBC and decreased in HRBC (Fig. S3E and Table S4). Of note, bevacizumab also tended to increase cellular proliferation in lesions of colorectal cancer patients (20).
Table S4.
In situ biomarker levels at baseline and on-treatment changes in HRBC and TNBC patients
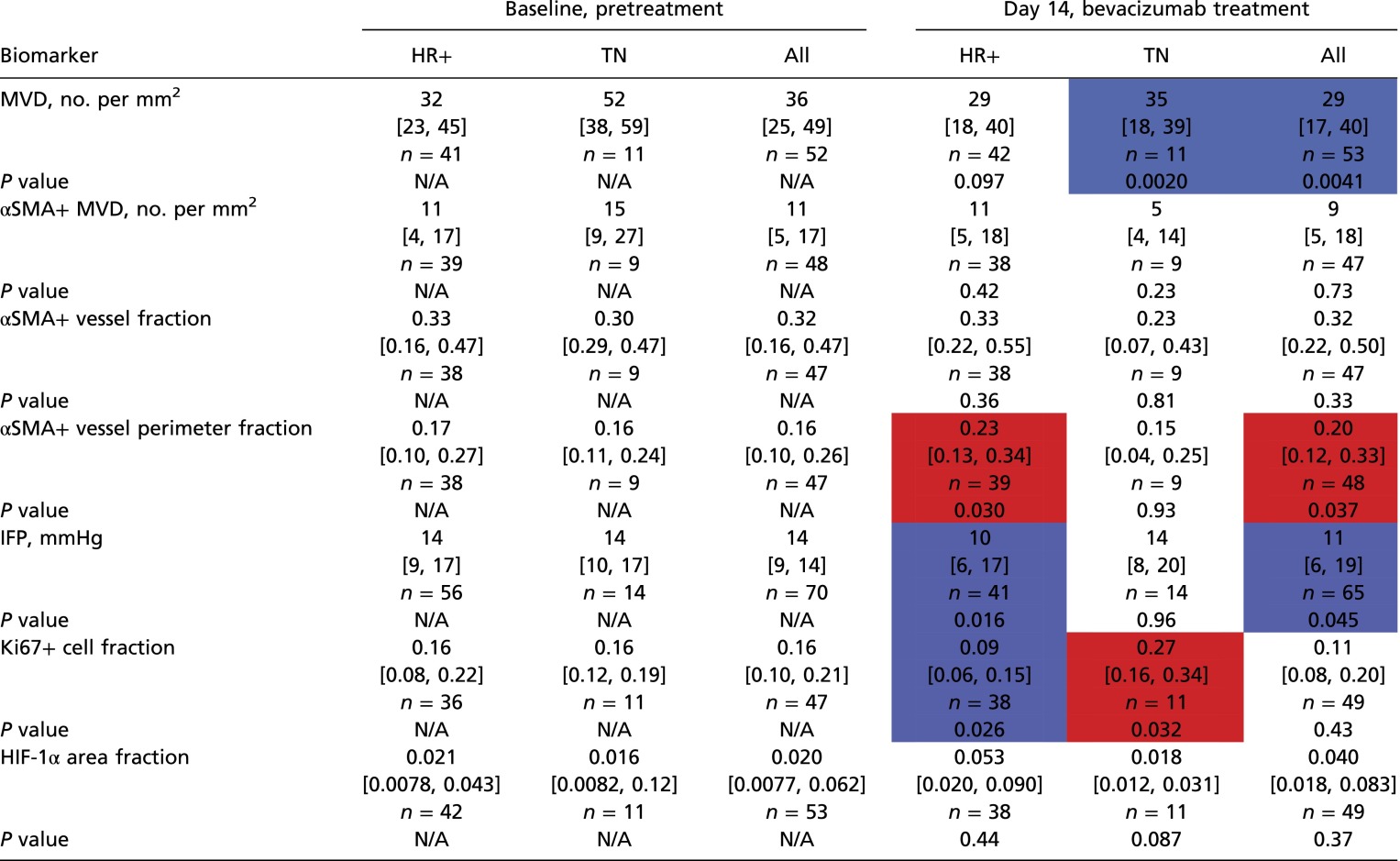 |
Values are shown as median and interquartile ranges for biomarker values at baseline and after treatment with bevacizumab alone (day 14). In red, significant increase; in blue, significant decrease. N/A, not available.
Table S6.
Blood circulating biomarker levels during and after bevacizumab plus chemotherapy in HRBC and TNBC patients
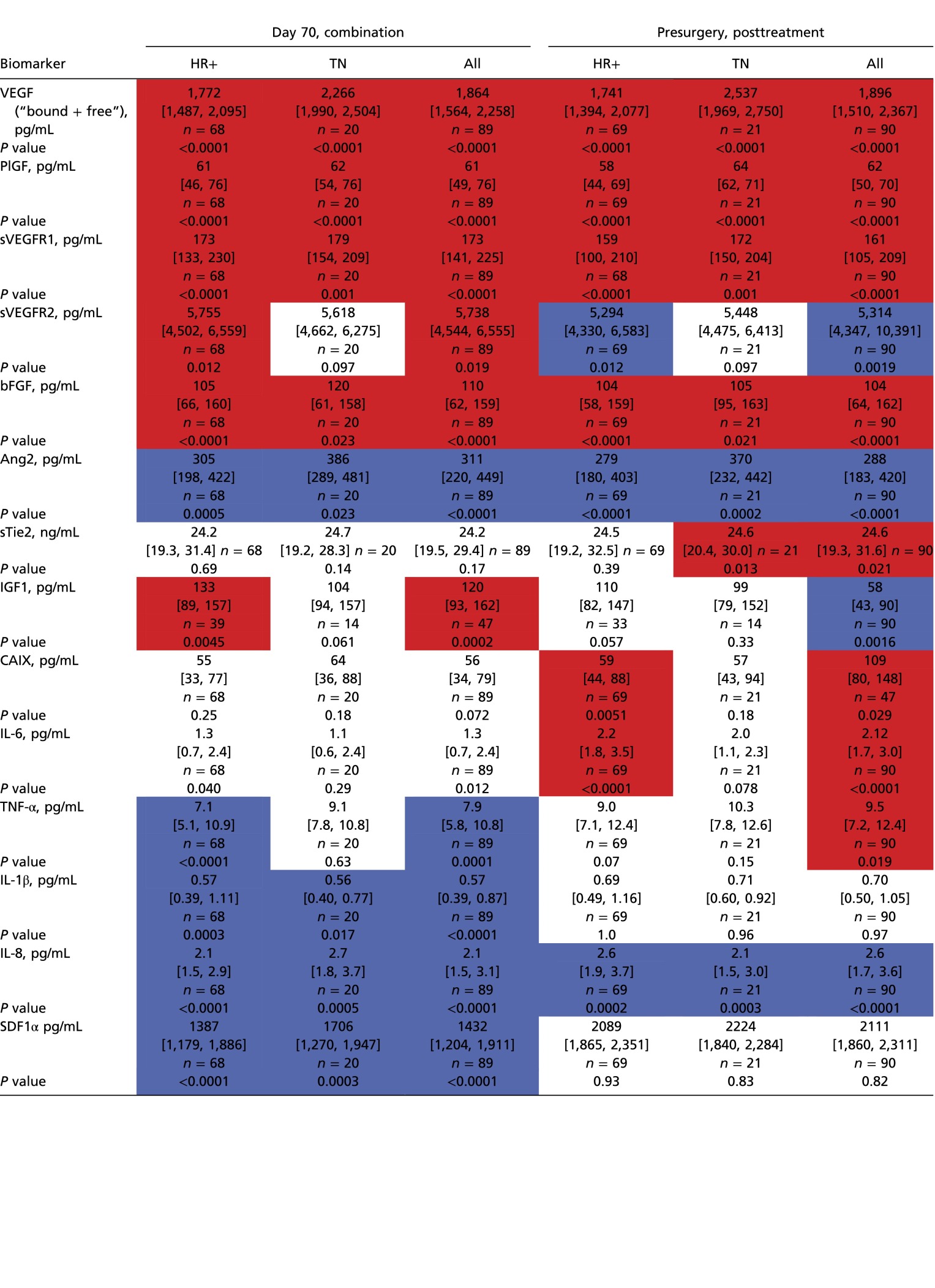 |
Values are shown as median and interquartile ranges for absolute plasma concentration values during (day 70) and after (presurgery) bevacizumab with dose-dense chemotherapy. In red significant increase; in blue significant decrease. P value is from Wilcoxon sign rank test for percent change after treatment.
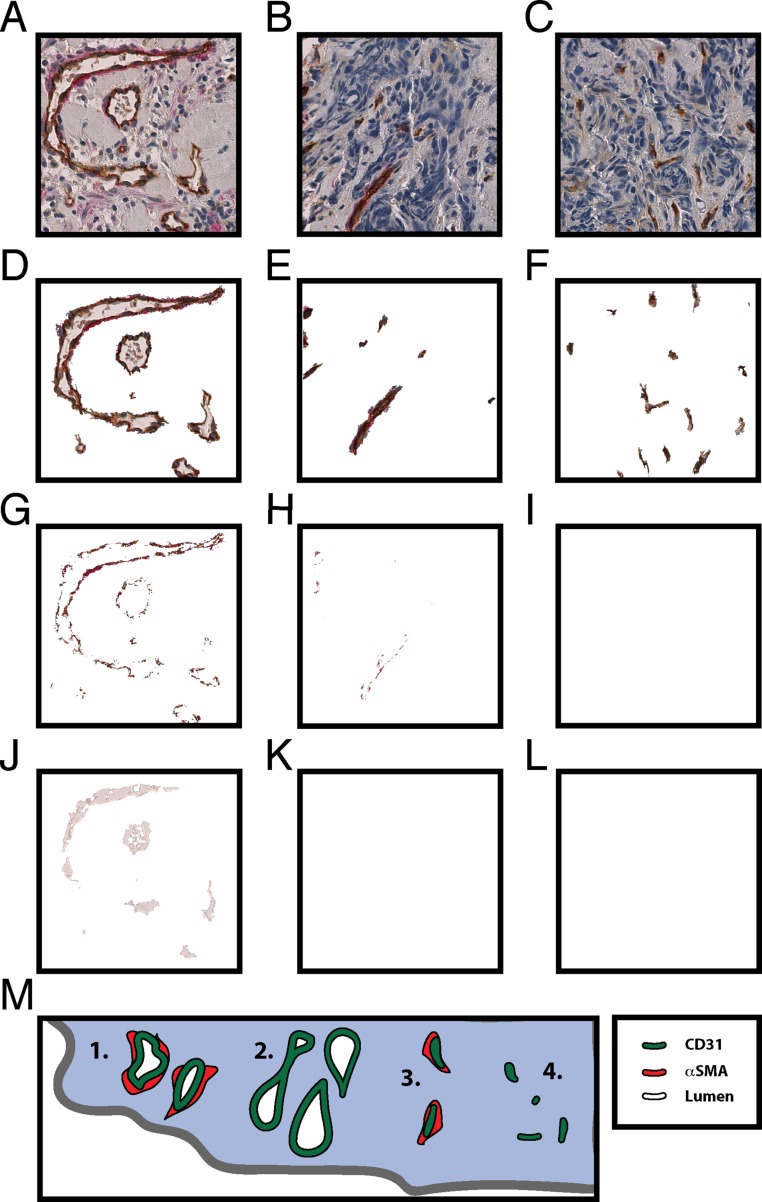
Fig. S2.
Representative images of vessel segmentation of immature, pericyte-covered, and patent vessels. We segmented endothelial cell staining (CD31), pericytes (αSMA), and lumen to identify the total density of microvessels and the density of pericyte-covered and patent vessels. We analyzed every vessel within two entire tissue biopsy sections separated by 100 μm. To depict the types of vessels and how the stains were segmented, we selected from a single biopsy representative fields of tumor regions with vascular dense regions of (A) pericyte-covered patent vessels, (B) pericyte-covered compressed vessels, and (C) immature vessels without pericytes. (D–F) Based on staining intensity and criteria (Methods), we used a custom computer application to identify vessels, (G–I) pericytes, and (J–L) lumen. There was no detectable pericyte stain in I and no visible lumen visible in K and L. (M) Schematic showing the possible classes of vessels: (1) pericyte-covered patent vessels (shown in A), (2) patent vessels lacking pericytes, (3) pericyte-covered compressed vessels (shown in B), and (4) immature vessels that lack pericytes (shown in C).
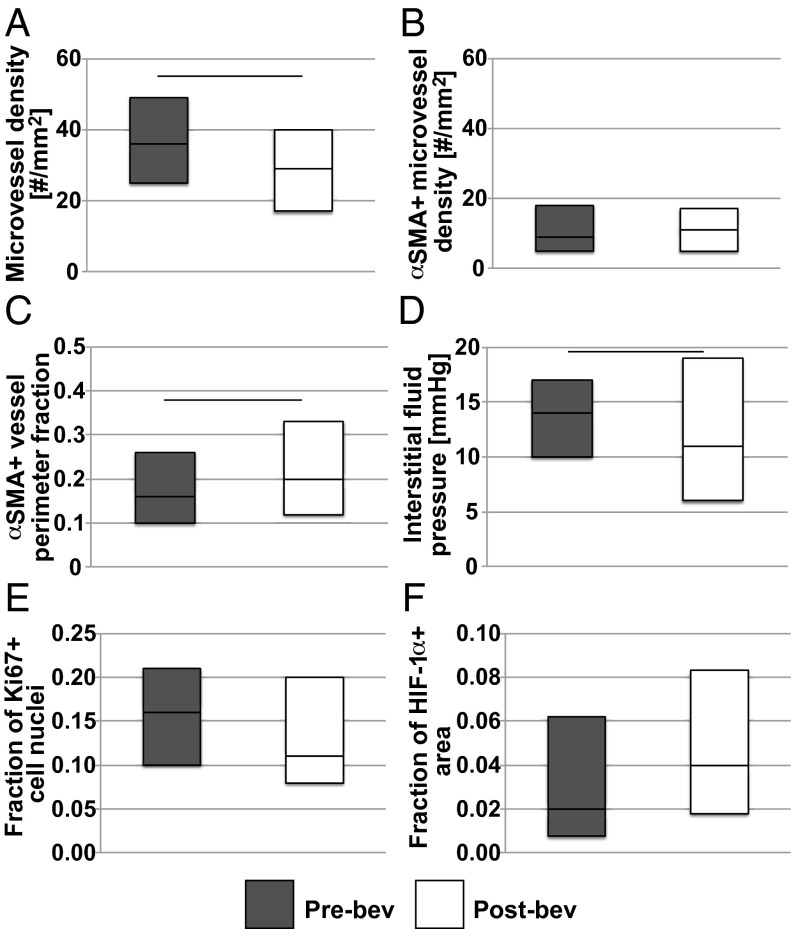
Fig. 1.
Effect of a single injection of bevacizumab on structural and functional markers of vascular normalization. Box plots depict median and interquartile ranges for biomarker values pre- (gray) and postbevacizumab alone (white). Horizontal lines between bars pre- and postbevacizumab monotherapy mark changes with a P value less than 0.05. (A) Microvessel density decreased (P = 0.0041, Student’s t test, n = 52 and 53). (B) Bevacizumab did not affect the density of mature vessels. (C) Fraction of vessel perimeter associated with pericytes (αSMA+ cells), a marker that distinguishes between poorly and completely covered vessels, increased (P = 0.037, Student’s t test, n = 47 and 48). (D) Interstitial fluid pressure, which is a functional measurement of vessel leakiness and lymphatic vessel dysfunction, decreased (P = 0.045, Student’s t test, n = 70 and 65). (E and F) Histological markers of functional vascular normalization, Ki67 for proliferation (n = 47 and 49) and HIF-1α (n = 53 and 49), did not change significantly.
Fig. S3.
In situ biomarker levels pre- and postbevacizumab monotherapy in HRBC (red) and TNBC (blue). Bars are shown as averages with error bars depicting the SEM. Horizontal lines between bars pre- and postbevacizumab monotherapy mark changes with a P value less than 0.05. Table S3 contains this data tabulated. (A) Microvessels were marked throughout the entire tissue area of two parallel sections 100 μm apart and the microvessel densities of the two sections were averaged. Bevacizumab monotherapy pruned microvessels in TNBC (P = 0.0020, Student’s t test). (B) The association of pericytes and the vascular wall was assessed as the fraction of vessel perimeter associated with pericytes. This value was measured for every vessel scored in A and averaged for each section and then each patient. Bevacizumab monotherapy increased the fraction of vessel perimeter associated with pericytes in HRBC (P = 0.030, Student’s t test). (C) Tumor interstitial fluid pressure was measured in patients using the wick-in-needle technique. IFP was reduced in HRBC (P = 0.016, Student’s t test). (D) The area fraction of tissue stained positive for HIF-1α was assessed using immunofluorescence and did not change with bevacizumab monotherapy in either subtype (TNBC P = 0.087, Student’s t test). (E) The fraction of nuclei stained positive for Ki67 protein, which is a cellular marker of proliferation, decreased in HRBC (P = 0.026, Student’s t test) and increased in TNBC (P = 0.032, Student’s t test).
Among circulating biomarkers, bevacizumab therapy alone decreased plasma angiopoietin 2 (Ang-2), soluble Tie2 receptor (sTie2), and IL-1β and increased plasma VEGF, placental growth factor (PlGF), and carbon anhydrase (CA)IX (Table S5). During combination therapy, plasma Ang-2, TNF-α, IL-1β, IL-8, and stromal-derived factor (SDF)1α decreased, and plasma VEGF, PlGF, soluble (s) sVEGFR1 and sVEGFR2, basic fibroblast growth factor (bFGF), and IGF1 increased (Table S6). These changes after bevacizumab alone or combination therapy were consistent between BC subtypes, with the exception of sVEGFR2 (which did not increase in TNBC patients) and TNF-α (which did not decrease in HRBC patients) following combination therapy. After completion of neoadjuvant therapy, plasma Ang-2, IGF1, sVEGFR2, and IL-8 decreased whereas plasma VEGF, PlGF, sVEGFR1, bFGF, sTie2, IL-6, TNF-α, and CAIX increased (Table S6). Once again, the changes were largely consistent between BC subtypes, with the exception of sTie2 (not decreased in HRBC patients) and IGF1 (not increased in TNBC patients).
Table S5.
Blood circulating biomarker levels at baseline and after bevacizumab treatment in HRBC and TNBC patients
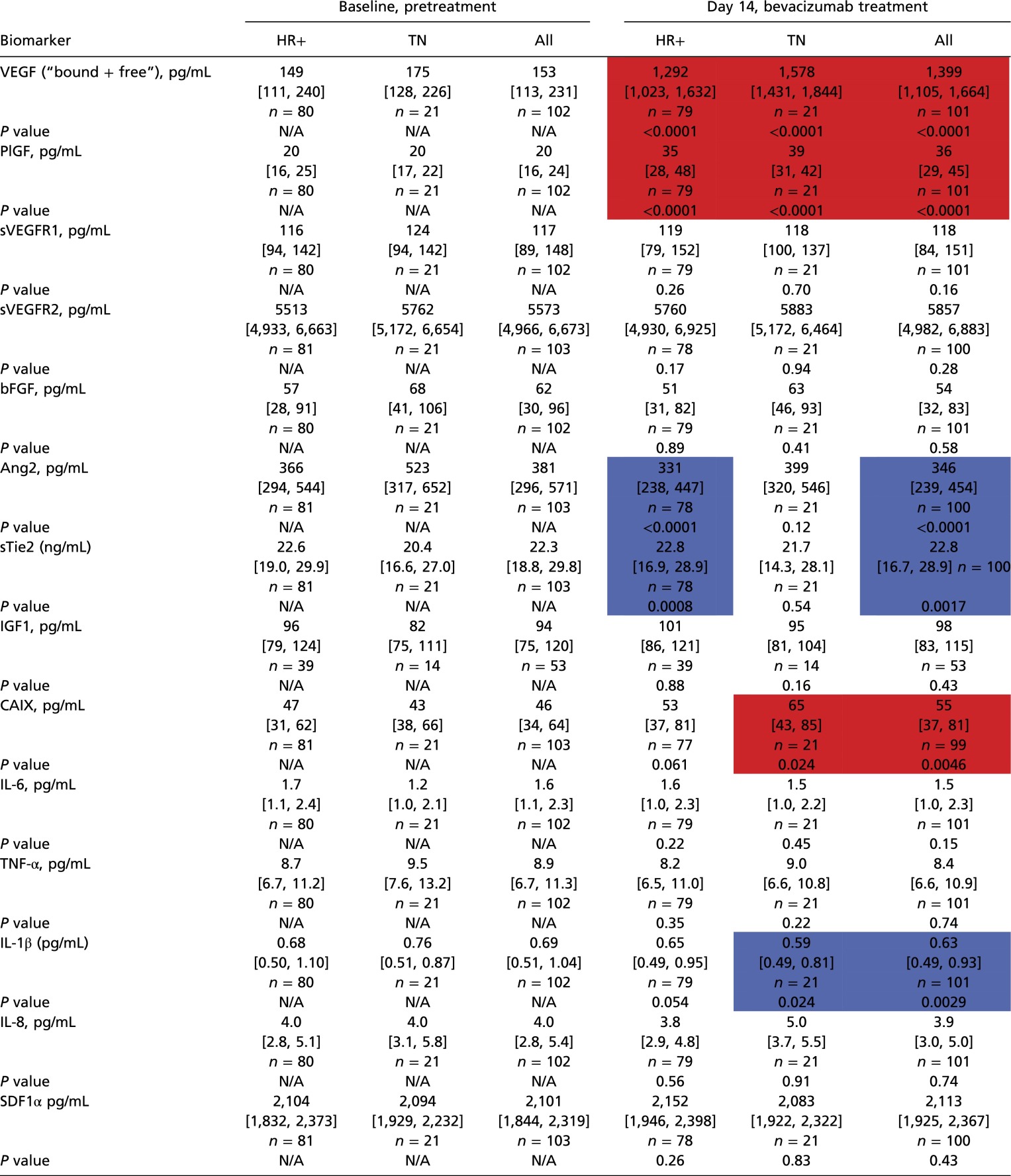 |
Values are shown as median and interquartile ranges for absolute plasma concentration values at baseline and after treatment with bevacizumab alone (day 14). In red significant increase; in blue significant decrease. P value is from Wilcoxon Sign Rank test for percent change after treatment. N/A, not available.
Baseline MVD, but Not Markers of Normalization, Associates with Pathologic Response in All Patients.
Although the sample size precluded rigorous inferential statistical analysis, we explored the association between biomarkers and tumor regression in this patient population to generate hypotheses about potential mechanisms of action. Of all biomarkers, only baseline tumor MVD of both total and patent (i.e., with open lumen) vessels associated with both the RCB and MP tumor regression scores in all patients with evaluable samples (n = 52, Table 1). Postbevacizumab monotherapy, the increase in the fraction of cells positive for the proliferation marker Ki67 correlated with RCB (more relevant to prognosis after neoadjuvant therapy) but not MP (more relevant to neoadjuvant therapy’s effectiveness in killing cancer cells in primary tumors) regression scores (n = 45, Table 1).
Table 1.
Correlations between in situ biomarker levels and MP pathologic regression score and RCB after neoadjuvant bevacizumab with dose dense chemotherapy
| Biomarker | Subtype | Time point | MP score | RCB |
| MVD | All | Baseline | 0.465 | –0.364 |
| P value | 0.0005 | 0.0079 | ||
| Patent MVD | All | Baseline | 0.507 | –0.426 |
| P value | 0.0001 | 0.0016 | ||
| Proliferation (Ki67) | All | Fold change after bevacizumab | 0.181 | –0.416 |
| P value | 0.258 | 0.0068 | ||
| PC-MVD | TN | Fold change after bevacizumab | 0.879 | –0.852 |
| P value | 0.0091 | 0.015 | ||
| HIF-1α area fraction | TN | Baseline | –0.663 | 0.649 |
| P value | 0.026 | 0.031 |
Data are shown as Spearman's ρ values; significant correlations are highlighted in bold. Higher MP scores indicate better pathologic response. Lower RCB scores indicate better pathologic response. Positive values of Spearman's ρ indicate a direct correlation between MP/RCB score with higher biomarker levels; P values are from the test of ρ = 0.
In TNBC, the baseline HIF-1α area fraction (n = 11) and the postbevacizumab increase in PC-MVD (n = 7) were associated with both tumor regression scores (Table 1). Among circulating biomarkers in TNBC, tumor regression scores were associated with high plasma VEGF (at baseline) and low sVEGFR1 and PlGF levels (postbevacizumab monotherapy) (n = 21, Table S7). In TNBC at baseline, sVEGFR1 levels were associated with the fraction of the average proportion of vascular perimeter associated with pericytes (Spearman’s rho = 0.857, P = 0.024, n = 7). No other proangiogenic or inflammatory factor demonstrated any association with pathologic response.
Table S7.
Correlations between circulating biomarkers levels before cytotoxic therapy and tumor regression
| Baseline (pretreatment) | Day 14 (after bevacizumab alone) | |||
| Biomarker/time point | MP score | RCB | MP score | RCB |
| VEGF | 0.419 | –0.492 | −0.388 | 0.339 |
| P value | 0.058 | 0.023 | 0.082 | 0.13 |
| sVEGFR1 | −0.362 | 0.309 | –0.609 | 0.610 |
| P value | 0.11 | 0.17 | 0.0034 | 0.0033 |
| PlGF | −0.173 | 0.157 | –0.546 | 0.508 |
| P value | 0.45 | 0.50 | 0.010 | 0.019 |
MP pathologic regression score and RCB after neoadjuvant bevacizumab with dose-dense chemotherapy in TNBC patients (n = 21). Data are shown as Spearman's ρ values; significant correlations are highlighted in bold. Higher MP scores indicate better pathologic response. Lower RCB scores indicate better pathologic response. Positive values of Spearman's ρ indicate a direct correlation between MP/RCB score with higher biomarker levels; P values are from the test of ρ = 0.
Response to Bevacizumab in BC May Depend on Extent of Vascular Normalization Only in Patients with High Baseline MVD.
The only biomarker of vascular normalization that correlated with response was change in PC-MVD after bevacizumab monotherapy, but the association held only in the 7 TNBC patients (Table 1) and not the 32 HRBC patients with data available at both baseline and postbevacizumab alone. We reasoned that baseline MVD could be an important distinguishing factor between TNBC and HRBC, because baseline MVD was 62.5% greater in TNBC (Fig. S3A) and correlated with RCB and MP scores in all patients (Table 1). Thus, we hypothesize that low baseline MVD might be related to the lack of association of PC-MVD and tumor regression scores in HRBC.
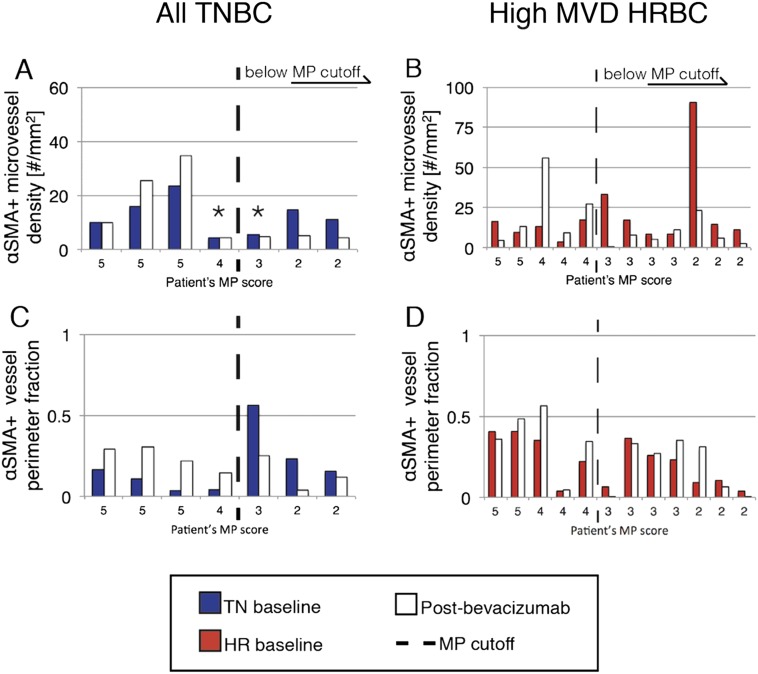
To investigate this hypothesis, we classified high MVD HRBC patients as those with a baseline MVD greater than or equal to the lowest baseline MVD of a TNBC patient with pCR (MP = 5). We then used a standard cutoff point (MP >3) to differentiate patients with the greatest tumor response (reduction in tumor cellularity) postcombination therapy (SI Methods). Using MP score rather than the RCB score focused the analysis on the effect of combination therapy on the primary tumor. We evaluated whether PC-MVD increased in 19 patients (all 7 TNBC and the 12 high MVD HRBC). Seventeen of 19 patients followed the pattern of stable or increased PC-MVD postbevacizumab alone in tumors with MP >3 and decreased PC-MVD in tumors with MP ≤3 (Fig. S4 A and B and Table S8). Stable or increased PC-MVD implies pericyte recruitment to vessels. In contrast, fewer than half (8 of 20) of HRBC patients with low MVD followed this pattern (Table S8), which suggests that increased PC-MVD might not be related to tumor regression scores in patients with low baseline MVD. The fraction of pericyte-covered vessel perimeter in TNBC and high MVD HRBC patients also followed this pattern (Fig. S4 C and D), whereas functional normalization biomarkers did not, nor was there an apparent connection between structural and functional biomarkers (Fig. S5).
Fig. S4.
Biomarkers of vascular normalization in individual TNBC and high MVD HRBC patients. Bars represent single patients and are shown in the same order in each column of panels (A, C and B, D) from left to right in descending order of MP for all TNBC and high MVD HRBC patients, which have PC-MVD data available pre- and postbevacizumab alone. Each patient’s MP scores are written on the horizontal axis. Asterisks in A denote TNBC patients with baseline MVD below the threshold. Dashed vertical lines represent MP tumor regression score cutoff values. MP >3 corresponds to a good tumor response (Methods). Baseline TNBC and HRBC biomarker values are depicted in blue and red, respectively. Postbevacizumab alone biomarker values are charted in white. Data include the density of αSMA+ vessels (PC-MVD, A and B) and fraction of αSMA+ vessel perimeter (C and D).
Table S8.
Direction of change of PC-MVD after the first cycle of bevacizumab between better and worse MP pathologic regression scores within TNBC, high MVD HRBC, and low MVD HRBC patient groups
| TNBC | High MVD HRBC | Low MVD HRBC | ||||
| Direction of change | MP >3 | MP ≤3 | MP >3 | MP ≤3 | MP >3 | MP ≤3 |
| Increased or maintained PC-MVD, no. of patients | 4 of 4 | 0 of 3 | 4 of 5 | 1 of 7 | 2 of 4 | 10 of 16 |
| Decreased PC-MVD, no. of patients | 0 of 4 | 3 of 3 | 1 of 5 | 6 of 7 | 2 of 4 | 6 of 16 |
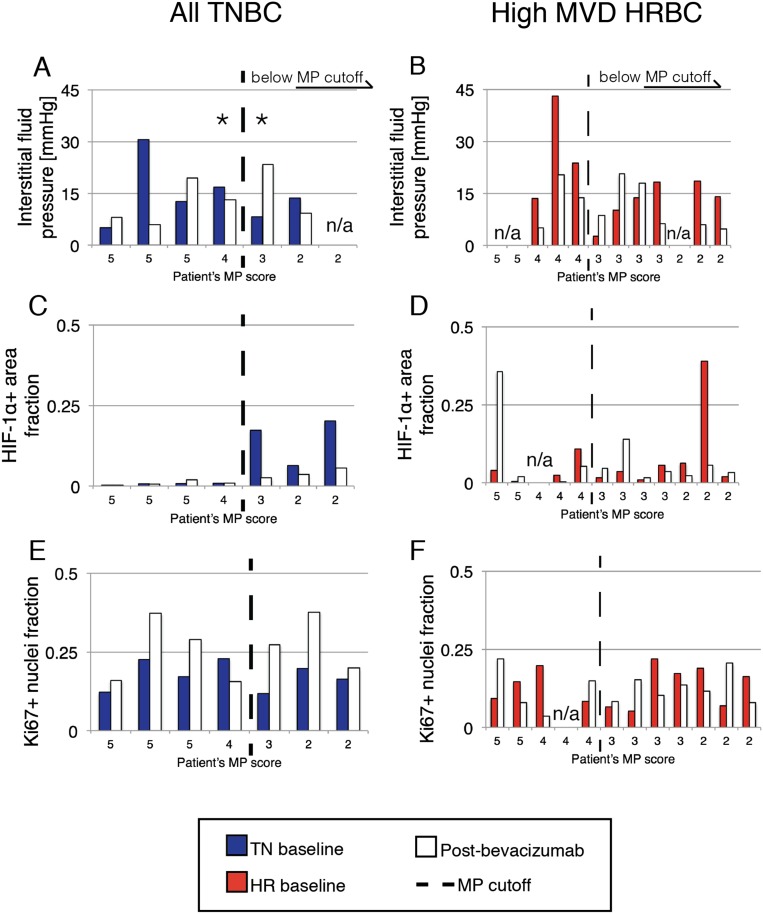
Fig. S5.
Functional biomarkers of individual TNBC and high MVD HRBC patients. Bars are shown in decreasing order of MP and in the same order as patients with PC-MVD data available pre- and postbevacizumab (Fig. 2). Each patient’s MP score is written on the horizontal axis. When data for a particular biomarker were unavailable, “n/a” is written in place of the bars. Baseline TNBC and HRBC are depicted in blue and red, respectively. Postbevacizumab monotherapy values are in white. Dashed vertical lines represent MP tumor regression score cutoff values. MP >3 corresponds to a good tumor response (Methods). Data include a functional measurement of vessel leakiness to fluid (interstitial fluid pressure in millimeters of mercury, A and B), histological assessment of a hypoxia-induced marker (HIF-1α+ fraction of total biopsy area, C and D), and histological assessment of the cellular proliferation marker (Ki67+ nuclei fraction, E and F) for TNBC and HRBC, respectively.
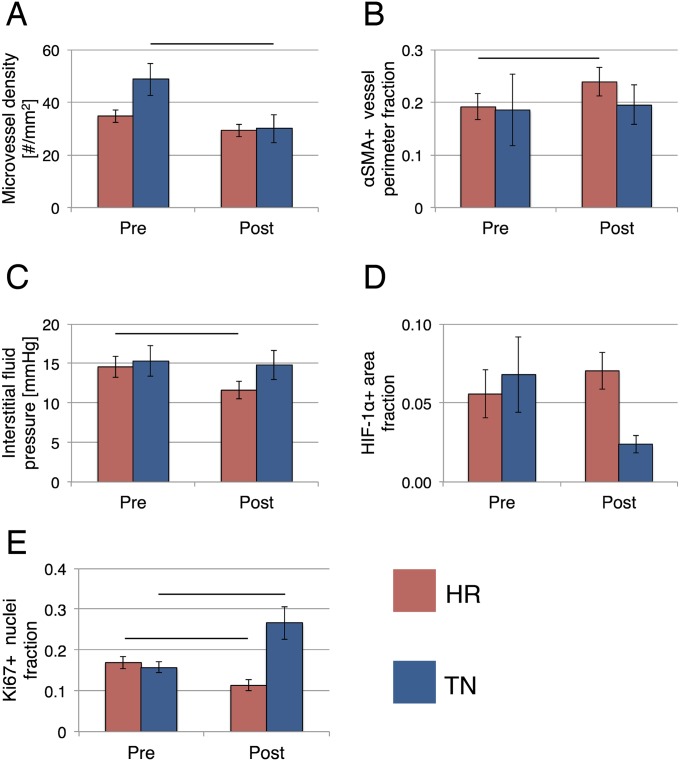
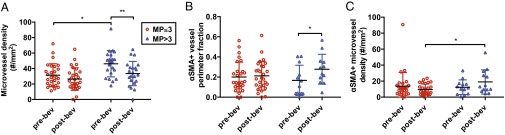
Averages of these vascular biomarkers in all patients, regardless of baseline MVD, within the same groups of good (MP >3) or poor (MP ≤3) response postcombination therapy were consistent with these results. At baseline, the tumor MVD was significantly higher in patients with MP >3 (Fig. 2A and Table S9). In these patients’ tumors, bevacizumab significantly reduced the MVD and increased the fraction of pericyte-covered vessel perimeter (Fig. 2 A and B and Table S9). Despite the vascular pruning, PC-MVD trended toward an increase in patients with MP >3, suggesting pericyte recruitment contributed to the increased pericyte coverage (Table S9). Indeed, PC-MVD postbevacizumab monotherapy was significantly higher in patients with MP >3 than in those with MP ≤3 (Fig. 2C and Table S9). Unlike the association between fold change of PC-MVD and tumor regression, which only occurred in TNBC (Table 1), in HRBC patients the pericyte-covered vessel perimeter significantly increased postbevacizumab in MP >3 tumors and PC-MVD was also significantly higher in MP >3 than in MP ≤3 tumors (Table S9). These results in HRBC are consistent with our hypothesis that low baseline MVD contributes to the lack of association between change in PC-MVD and tumor regression scores. Hence, in TNBC and high MVD HRBC patients with improved pathologic response postcombination therapy, bevacizumab induced vascular remodeling, which led to a higher density of normalized vessels (Fig. 3).
Fig. 2.
(A) The MVD at baseline is significantly higher (P = 0.0024) in patients with MP >3. In these patients, bevacizumab significantly reduced the MVD (P = 0.009). (B) Postbevacizumab the fraction of vessel perimeter covered by pericytes is significantly higher (P = 0.001) in patients with MP >3. (C) Postbevacizumab PC-MVD is significantly higher (P = 0.008) in patients with MP >3.
Table S9.
Average values of in situ biomarkers pre- and postbevacizumab alone in TNBC and HRBC patients divided into good (MP >3, less cellularity) and poor (MP ≤3, more cellularity) response groups
| All patients | TNBC | HRBC | |||||||
| Response | Day 0 | Day15 | P value | Day 0 | Day15 | P value | Day 0 | Day15 | P value |
| HIF-1α area fraction | |||||||||
| Good response | 0.03 (19) | 0.05 | NS | 0.04 (8) | 0.02 | NS | 0.03 (12) | 0.08 | 0.075 |
| Poor response | 0.08 (28) | 0.06 | NS | 0.15 (3) | 0.04 | NS | 0.07 (25) | 0.07 | NS |
| P value | 0.066 | NS | 0.036 | 0.097 | NS | NS | |||
| HIF-1αintensity, arbitrary units | |||||||||
| Good response | 15.9 (20) | 19.1 | NS | 14.1 (8) | 17.3 | NS | 18.4 (12) | 21.3 | 0.062 |
| Poor response | 19.0 (28) | 21.2 | NS | 23.5 (3) | 19.9 | NS | 17.1 (25) | 20.3 | NS |
| P value | 0.08 | NS | 0.06 | NS | NS | NS | |||
| MVD, no. per mm2 | |||||||||
| Good response | 45.9 (21) | 35.1 | 0.009 | 52.3 (7) | 37.2 | 0.003 | 42.7 (14) | 34.0 | NS |
| Poor response | 30.9 (27) | 27.3 | NS | 39.4 (3) | 23.4 | NS | 29.9 (24) | 27.8 | NS |
| P value | 0.0024 | NS | NS | NS | 0.01 | NS | |||
| αSMA+ vessel perimeter fraction | |||||||||
| Good response | 0.17 (12) | 0.28 | 0.001 | 0.09 (4) | 0.24 | 0.006 | 0.21 (8) | 0.30 | 0.039 |
| Poor response | 0.20 (27) | 0.21 | NS | 0.32 (3) | 0.14 | NS | 0.19 (24) | 0.22 | NS |
| P value | NS | NS | NS | NS | NS | NS | |||
| αSMA MVD, no. per mm2 | |||||||||
| Good response | 12.4 (13) | 19.0 | 0.084 | 13.4 (4) | 18.6 | NS | 11.9 (9) | 19.2 | NS |
| Poor response | 13.8 (28) | 9.5 | NS | 10.3 (3) | 4.61 | NS | 14.2 (25) | 10.1 | NS |
| P value | NS | 0.008 | NS | NS | NS | 0.021 | |||
| Ki67+ nuclei fraction | |||||||||
| Good response | 0.16 (20) | 0.17 | NS | 0.16 (8) | 0.26 | NS | 0.17 (12) | 0.11 | NS |
| Poor response | 0.17 (25) | 0.13 | NS | 0.16 (3) | 0.28 | NS | 0.17 (22) | 0.11 | 0.038 |
| P value | NS | NS | NS | NS | NS | NS | |||
| IFP, mmHg | |||||||||
| Good response | 16.4 (25) | 12.6 | NS | 16.9 (10) | 13.7 | NS | 16.1 (15) | 12.0 | NS |
| Poor response | 13.6 (35) | 12.2 | NS | 11.5 (4) | 17.8 | NS | 13.9 (31) | 11.5 | NS |
| P value | NS | NS | NS | NS | NS | NS | |||
Comparisons were tested using paired and unpaired, two-sided Student’s t tests. Sample sizes are presented in parentheses at day 0. P values less than 0.10 are presented with those less than 0.05 bolded. P values greater than 0.10 are denoted as NS.
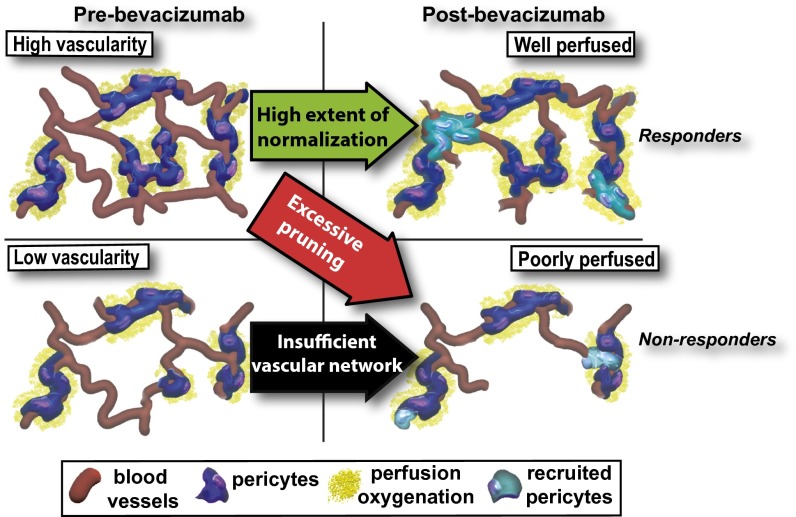
Fig. 3.
A schematic of the proposed mechanism of action of benefit from antiangiogenic therapy. The left two quadrants depict tumor vessels (red) at baseline before bevacizumab monotherapy, and the two right quadrants depict the vasculature following bevacizumab monotherapy. In the top left quadrant, there is a higher vascular density (MVD) than in the bottom left quadrant. Tumors with insufficient baseline MVD do not respond to antiangiogenic therapy (bottom left to bottom right quadrants), because the increase in functional vessels resulting from the recruitment of pericytes (teal) cannot overcome the paucity of vessels. In contrast, tumors with high baseline vascularity that recruit pericytes respond to bevacizumab combined with chemotherapy (top left quadrant to top right quadrant) better than tumors that undergo excessive pruning (top left quadrant to bottom right quadrant). Responders: MP scores of 4–5; nonresponders: MP scores of 1–3.
SI Methods
Patients.
Enrollment required a pathological diagnosis of adenocarcinoma of the breast. Two cohorts of patients were eligible: patients with HRBCs and patients with TNBCs. For eligibility, an HRBC diagnosis was required [i.e., expression of estrogen (ER) and/or progesterone receptors (PR) by standard immunohistochemical methods]. Eligible TNBCs had to be negative for ER, PR and HER2. Low positive (<10%) hormone receptor status was considered HRBC. Patients with HRBC were eligible if they had high-risk disease, defined as either having clinically positive axillary lymph nodes by pathological analysis, with a primary tumor ≥1.5 cm or no evidence of axillary lymph node involvement with a high grade tumor ≥1.5 cm, or a low/intermediate grade tumor with a primary tumor ≥2.5 cm. Patients could not have evidence of metastatic disease. Patients with bilateral cancers were eligible provided that at least one cancer met the eligibility requirements. Patients with TNBC were required to have a tumor ≥1.5 cm. Patients with a clinically negative axilla were required to have a sentinel lymph node biopsy performed either before starting preoperative therapy or at the time of definitive surgery. For patients with a clinically positive axilla, a needle aspiration or core biopsy was required before initiation of therapy. Patients with a positive sentinel node or needle biopsy at baseline were required to undergo a level I and II axillary lymph node dissection at the time of definitive surgery. Other requirements included adequate hematopoietic, hepatic, and renal function and a left ventricular ejection fraction ≥50%. Patients were ineligible if they had HER2-positive disease defined as HER2-amplified by FISH or immunohistochemistry (IHC) 3+, a history of prior myocardial infarction, uncontrolled hypertension, neuropathy greater than or equal to grade 2, significant bleeding within 6 mo of study entry, or urine protein:creatinine ratio ≥1.0.
Treatment Regimen.
Treatment consisted of a single dose of bevacizumab 10 mg/kg, followed 2 wk later by doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 with bevacizumab 10 mg/kg every 2 wk × 4, followed by paclitaxel 175 mg/m2 with bevacizumab 10 mg/kg every 2 wk × 3, followed by paclitaxel 175 mg/m2 × 1. Primary breast surgery was performed no less than 4 wk and no more than 8 wk after the last dose of bevacizumab. Patients could undergo either lumpectomy or modified radical mastectomy. All surgical specimens and diagnostic specimens were reviewed by a central pathologist and response was determined by both the MP scale and the RCB scale (50, 51). pCR was defined as no residual invasive cancer in the breast.
Patients and physicians were cautioned about proceeding with immediate reconstruction given potential wound healing complications with bevacizumab exposure. Appropriate adjuvant chemotherapy, hormonal therapy, and radiation therapy was determined by the patient’s treating physician.
Tumor Genomic Analyses.
All enrolled patients consented to undergo a research core biopsy at baseline and after one cycle of bevacizumab (SI Methods). Five to eight cores were obtained; one core was fixed in formalin and all other cores were frozen in optimum cutting temperature compound. Gene expression profiles were generated by mRNA sequencing using the Illumina TruSeq Kit to create libraries for paired-end sequencing on an Illumina HiSEq 2000 and data were aligned and genes quantified as previously described (52). The predictor of Parker et al. (53) was used for subtype categorizations using a nearest-centroid procedure and the centroid with the largest correlation value and returned as a five-level classifier (basal-like, luminal a, luminal b, HER2-enriched, and normal-like).
Interstitial Fluid Pressure Measurements.
In brief, to measure the IFP, a 23-gauge needle with a 2-mm side hole at 5 mm from the tip was used (54, 55). The needle and tubing filled with sterile heparinized saline were connected to a disposable pressure transducer and an electronic data acquisition and recording system (AdInstruments Inc.). The needle and tubing were gas-sterilized. The calibration of the pressure transducer was verified before IFP measurements in each patient. The needle was then inserted under ultrasound guidance into the tumor center and the IFP was recorded. Stable IFP measurements with a good fluid communication between the tumor interstitial space and needle were considered valid. The IFP was measured in two or three different locations within the tumor.
Tissue Biomarkers Evaluation.
Following the completion of the IFP measurements, research core tumor biopsies were obtained. Five-micrometer-thick sections were cut from formalin-fixed and paraffin-embedded tissue blocks. A double immunostaining procedure was performed with CD31 (N1596; Dako) and αSMA (M0850; Dako) antibodies. In brief, the CD31 antibody was incubated at room temperature for 1 h. Slides were then washed and incubated in secondary antibody (K4007, EnVision anti-mouse; Dako) for 30 min and developed with 3,3′-diaminobenzidine. Slides were then blocked with EnVision doublestain block for 5 min and incubated overnight with the αSMA antibody. After washes, slides were incubated in secondary antibody (Doublestain AP Polymer; Dako) for 30 min, washed, and developed with Fast Red. Slides were counterstained with hematoxylin and coverslipped with Faramount (Dako). To determine the percentage of proliferating cancer cells and hypoxia induced factor (HIF)-1α fraction, immunostaining was performed with a Ki67 antibody (N1633; Dako) and a HIF-1α antibody (610958; BD Biosciences), respectively.
Histological Image Analysis.
Bright-field or fluorescent digital images of stained slides were acquired with a Hamamatsu NanoZoomer slide scanner. Images were reviewed before analysis and slides with clear nonspecific staining or tissue folding were excluded. Microvessel metrics and proliferation analyses were performed semiautomatically using custom software from Visiopharm. The software automatically detected the tissue area, areas positive for Ki67 or CD31, areas stained positive for αSMA/pericytes, and areas of vessel lumen. The automatic selection was checked and modified by an investigator. MVD was counted for vessels that displayed at least two of the following three characteristics: positive CD31 stain, visible lumen, and elongated endothelial cell nuclei. Two entire biopsy sections, separated by 100 μm and excluding areas of fatty tissue and nonspecific staining, were quantified and averaged for each measurement to account for spatial heterogeneity. To confirm the MVD results from the computer-assisted analysis, manual counting was also performed by two trained investigators and correlated with the computer analysis (spearman’s rho = 0.63, P = 0.0016). The correlation between the two manual counts was also tested (spearman’s rho = 0.74, P = 8.5 × 10−9). The vessel perimeter fraction was calculated as the mean ratio of vessel wall length associated with αSMA-positive perivascular cells to the total length of the vessel wall. Values were obtained for every vessel and vessel values were averaged for each sample. Proliferation was assessed as the fraction of nuclei positive for Ki67 staining. For the immunofluorescent stain of HIF-1α, analysis was performed with custom algorithms in MATLAB (MathWorks). Tissue selection was performed semiautomatically based on DAPI counterstaining, and selection of positively stained areas was performed automatically based on intensity and size thresholds. The intensity threshold was selected based on the maximum nonspecific staining intensity measured in three to five negative control slides that were incubated with only secondary antibodies. The area fraction is the fraction of tissue area positive for the stain, and the intensity is the mean signal intensity of positively stained area. The investigators who performed the analyses were blinded from patient, treatment, and time point.
Plasma Biomarkers Analysis.
Peripheral blood was obtained from all patients enrolled and evaluated as previously described (56). In brief, blood samples were collected in EDTA-containing tubes before any treatment (baseline), after bevacizumab treatment alone (day 14), during dose-dense chemotherapy with bevacizumab (day 70), and before surgery (posttreatment). Plasma samples were separated by centrifugation then aliquoted and stored at –80 °C until they were used for multiplex array and single analyte ELISA measurements. Measurements were carried out for circulating VEGF, sVEGFR-1 (sFLT1), PlGF, and bFGF using the Human Angiogenesis Kit (K15190D), for IL-1β, IL-6, IL-8, and TNF-α using the Human ProInflammatory Panel kit (K15049G) from Meso-Scale Discovery, and for SDF1α, CAIX, and insulin-like growth factor (IGF)-1 using ELISA kits from R&D Systems. All samples were run in duplicate.
Statistical Analysis.
The primary objectives were to determine pCR rates in the HRBC and TNBC cohorts and to test the hypothesis that the luminal B subtype is more responsive to preoperative therapy than luminal A subtype among HR+ patients. The study was designed to have 85% power to detect a difference of 10% pCR in luminal A and 40% pCR in luminal B under the assumption the two subgroups are equally represented in the HR+ cohort. Rates of pCR in IHC and molecular subtypes are reported with binomial 95% CIs, and odds ratios significance was assessed using Fisher’s exact tests. Response by MP was evaluated on an ordinal scale using Cochran–Mantel–Haenszel χ2 tests, and by RCB on a continuous scale using Kruskal–Wallis ranked tests. Changes of biomarkers within groups and comparisons between groups were tested with paired and unpaired, two-sided Student’s t tests. To separate patients with more and less tumor regression we used a cutoff value of the MP grading scale of residual cellularity in resected primary lesions following neoadjuvant therapy. Similar to other studies (57, 58), we selected MP >3 as a cutoff for a better response, which includes grade 4 (≥90% reduction in tumor cells from baseline) and grade 5 (no invasive cancer cells remaining). For each biomarker, patients were only included if there was data available both pre- and postbevacizumab therapy alone.
Discussion
BC is a complex disease composed of several biologically distinct subtypes (21). For patients with HRBC, only 5–10% of patients will achieve a pCR to preoperative chemotherapy. Recent neoadjuvant phase III trials showed that bevacizumab increases rates of pCR in HER2-negative BC, yet the results were inconsistent regarding which subtype benefits (6–9). Our study found that even with the addition of bevacizumab to dose-dense ACP chemotherapy, only 6.4% of HRBC patients achieved a pCR, in contrast to 52% of TNBC patients. The study was not powered to compare pCR rates between luminal A vs. luminal B BCs. However, among patients with either HRBC or TNBC, PAM50 data suggested an increase in response in those with the basal-like subtype, relative to other subtypes.
Serial biopsies enabled exploratory investigation of bevacizumab’s effects and mechanism of action in BC. Consistent with other tumor types, in BC bevacizumab pruned immature vasculature, induced maturation of vessels (as evidenced by increased pericyte coverage), reduced IFP, and changed levels of circulating biomarkers PlGF, VEGF, and Ang-2. Nonetheless, the magnitude of the decrease in IFP was less than that seen in rectal cancer (20, 22), which may indicate that either BC vessels are less sensitive to VEGF blockade or bevacizumab distribution in BC tissue is limited.
The effects of bevacizumab, though consistent with vascular normalization, did not correlate with pathologic response in all patients, because baseline MVD was the only biomarker to correlate with both RCB and MP scores in the 52 patients with data available. Although this patient cohort is too small for rigorous statistical analysis, these results suggest that a sufficiently high baseline MVD might be necessary for bevacizumab to aid primary tumor regression. A phase II trial in 20 BC patients also noted an association between expression of the endothelial cell marker CD31 and tumor regression (23). In patients with other tumor types treated with bevacizumab and cytotoxic therapy, correlations between baseline MVD and response have also been documented (24, 25), whereas other studies have found no relationship between MVD or MVD surrogates and response (26, 27). The exploratory nature of our current analysis notwithstanding, the association between MVD and tumor regression scores is consistent with the notion that vascular remodeling postbevacizumab can convert some nonfunctional vessels into functional ones, but it cannot create new vessels. Thus, the function of the vascular network in tumors with high MVD and redundant vasculature (Fig. 3, top left) might benefit from the pruning of certain vessels and increased function of the remaining, normalized vessels (Fig. 3, top right). In contrast, tumors with low MVD (Fig. 3, bottom left) might have their already limited number of vessels pruned, which would outweigh the benefit of increased function of normalized vessels (Fig. 3, bottom right). In this context, our results also offer a potential explanation for the failure of antiangiogenesis therapy postoperatively (2), because micrometastatic lesions might have very low MVD and lack angiogenesis (28). Additionally, a significant fraction of BCs are desmoplastic (29, 30), which might cause vessel compression and reduce the patency of vessels (31–33). In our current study, patent MVD had a stronger association with tumor regression than total MVD. Therefore, because a fraction of BCs are likely hypoperfused, the response to normalizing therapy might be more sensitive to baseline MVD.
The results of our correlative analyses suggested that the fold increase in PC-MVD might be associated with tumor regression in TNBCs (which are highly vascularized) (Table 1), but not in HRBCs (which tend to be poorly vascularized). Nonetheless, we reasoned that HRBC patients with baseline MVD greater than or equal to that of TNBC patients with pCR might follow a pattern similar to that of TNBC patients. Of note, this high MVD threshold in HRBC is lower than the lowest MVD of all patients assessed in a recent colorectal study (27). In this descriptive analysis of high baseline tumor MVD in the HRBC cohort, the majority of patients with a good response in the primary tumor after combination therapy (MP >3) had maintained or increased PC-MVD (Table S8 and Fig. 3, top left to top right), whereas patients with a poor response to therapy (MP ≤3) had decreased PC-MVD (Table S8 and Fig. 3, top left to bottom right). This pattern only held in about half of low MVD HRBC patients (Table S8 and Fig. 3, bottom left to bottom right), which suggests no association between changes in PC-MVD and tumor regression. The averaging of the biomarkers of all patients followed the trend of higher baseline MVD, increased pericyte-covered vessel perimeter after bevacizumab, and higher postbevacizumab monotherapy PC-MVD in patients with MP >3 (Fig. 2). Thus, our exploratory findings support the concept that bevacizumab-induced increase in PC-MVD is necessary but not sufficient for chemotherapy-induced tumor regression.
The evidence of PC-MVD increases in patients with better responses to combination therapy indicates that, rather than vascular pruning, VEGF blockade is inducing pericyte recruitment to immature vessels to increase the fraction of pericyte-covered vessel perimeter. To our knowledge this is the first clinical evidence of antiangiogenic therapy-induced pericyte recruitment, which has previously only been demonstrated preclinically (18). One limitation of the present study is the lack of functional imaging to confirm the significance of the structural vascular changes. Because HIF-1α at best weakly correlates with oxygen electrode measurements, our HIF-1α measurements may not reflect functional changes accurately (34–36). Although there are no clinical studies investigating the relationship between PC-MVD and tumor oxygenation, preclinical studies demonstrated that increased PC-MVD is associated with increased vascular function and oxygenation (37, 38). Our result of increased PC-MVD correlating with tumor regression is consistent with a cediranib study in glioblastoma, which identified a “vascular normalization index” as a measure of the extent of vascular normalization (14). This index is proportional to a reduction in vessel permeability, which is related to high levels of pericyte coverage in preclinical models (39), and an increase in cerebral blood volume, which is indicative of increased MVD. As a result, this functional imaging index mirrors our histological assessment of increased PC-MVD in BC patients with good response to combination therapy. Similar imaging studies of cediranib in glioblastoma also linked improved survival with treatment-induced increases in perfusion and oxygenation (11, 15). Although our study’s design with paired biopsies separated by 2 wk did not allow examination of a normalization window over an extended time period, our findings are consistent with these other studies’ functional associations with tumor regression, as early changes—not absolute values—of normalization markers associated with regression. Thus, our results indicate that tumor regression from VEGF blockade might be restricted to tumors with a sufficiently high MVD and might occur through pericyte recruitment rather than vascular pruning, leading to an increased extent of vascular normalization.
We found several biomarkers that associated with tumor regression in TNBC, including high VEGF levels at baseline and low sVEGFR1 and PlGF levels before combination treatment (Table S7). Changes of sVEGFR1 levels—a factor linked with “vascular normalization” (37) that we proposed as a resistance biomarker to neoadjuvant bevacizumab in rectal cancer (40)—was directly associated with pericyte coverage and inversely associated with response. Two phase III randomized trials of bevacizumab have shown that specific VEGFR1 SNPs correlated with high VEGFR1 expression and poor outcome (41). In addition, we have reported inverse associations between plasma sVEGFR1 and treatment outcomes after anti-VEGF therapies in patients with metastatic colorectal (vandetanib plus cetuximab/irinotecan), hepatocellular carcinoma (cediranib monotherapy), sarcoma (sorafenib), and lung cancer (bevacizumab plus chemotherapy) (12, 42–44). Finally, although plasma PlGF (a growth factor not blocked by bevacizumab) consistently increased in these BC patients, the extent of this increase was associated with lower tumor regression scores in TNBC patients. This association with less regression warrants further exploration of this potential mechanism of resistance to anti-VEGF therapy in TNBC.
In summary, bevacizumab induced changes in vascular structure and levels of circulating biomarkers indicative of vascular normalization in BC, although these changes were small compared with other tumor types. Baseline MVD was associated with tumor regression, indicating that many BCs may be insufficiently vascularized to yield improvement in perfusion or oxygenation because anti-VEGF therapy-induced pruning of immature vessels may outweigh the number of normalized vessels, leaving an insufficient number of functional vessels. In patients with relatively high baseline MVD, response may result from adequate number of normalized vessels, as evidenced by increased PC-MVD. However, the potential mechanistic link between sufficient MVD and changes in PC-MVD with tumor regression needs to be confirmed through simultaneous biopsies and functional imaging studies in larger clinical studies (45). The observed association of circulating biomarkers with regression in TNBC is consistent with previous studies in BC and other tumor types and reveals a potential connection between sVEGFR1 and vascular maturity. Together, these results suggest that in poorly perfused BCs and other hypoperfused tumors (e.g., pancreatic ductal adenocarcinoma), strategies that increase perfusion without pruning—targeting alternative angiogenic pathways (46), directly inducing differentiation of intratumoral sources of pericyte progenitors (47), and increasing vessel patency by reducing solid stress (31–33) or enhancing lumen formation (48)—should be explored (1, 49).
Methods
Patients and Treatment Regimen.
Enrollment required a pathological diagnosis of adenocarcinoma of the breast. Two cohorts of patients were eligible: patients with HRBCs and patients with TNBCs. Additional information about patient eligibility and ineligibility requirements, and treatment regimen are provided in SI Methods. This study was approved by the Dana–Farber/Harvard Cancer Center Institutional Review Board. Written informed consent was required for enrollment.
Tumor Genomic and Correlative Analyses.
The experimental procedures for IFP measurements, analysis of tissue and circulating biomarkers, and statistical analysis are described in SI Methods.
Acknowledgments
The authors thank Drs. James Baish, Vikash Chauhan, Kyrre Emblem, Dai Fukumura, Joao Incio, and Triantafyllos Stylianopoulos for their helpful comments on the manuscript and Carolyn Smith for technical assistance. The clinical trial was supported by a Genentech grant (to S.M.T., I.E.K., and E.P.W.) and AVON National Cancer Institute Progress for Patients Program Grant 2P50 CA089393-08S2 (to Dr. Dick J. Iglehart). Correlative studies were funded through Department of Defense Breast Cancer Research Innovator Award W81XWH-10-1-0016 (to R.K.J.), National Cancer Institute Grant R01CA098706 (to Y.B.), Susan G. Komen Foundation Fellowship PDF14301739 (to G.S.), and Associazione Italiana per la Ricerca sul Cancro Fellowship 13604 (to G.S.).
Footnotes
Conflict of interest statement: Y.B. served as consultant for Xtuit. D.G.D. received research grants from Merrimack Pharma and HealthCare Pharma. S.G. served on the advisory board of Lilly. J.L. is an employee of Merrimack Pharmaceuticals. S.J.I. served as consultant for Myriad Genetics. M.G. served on the advisory board of Lightpoint Medical. R.K.J. received consulting fees from Enlight, Ophthotech, SPARC, and SynDevRx; owns equity in Enlight, Ophthotech, SynDevRx, and XTuit; and serves on the board of directors of XTuit and board of trustees of Tekla Healthcare Investors, Tekla Life Sciences Investors, Tekla Healthcare Opportunities Fund, and Tekla World Healthcare Fund. No reagents or funding from these companies was used in these studies. Therefore these authors do not have any conflict of interest related to this study.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1518808112/-/DCSupplemental.
References
- 1.Jain RK. Antiangiogenesis strategies revisited: From starving tumors to alleviating hypoxia. Cancer Cell. 2014;26(5):605–622. doi: 10.1016/j.ccell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sledge GW. Anti-vascular endothelial growth factor therapy in breast cancer: Game over? J Clin Oncol. 2015;33(2):133–135. doi: 10.1200/JCO.2014.58.1298. [DOI] [PubMed] [Google Scholar]
- 3.Fisher B, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16(8):2672–2685. doi: 10.1200/JCO.1998.16.8.2672. [DOI] [PubMed] [Google Scholar]
- 4.Kuerer HM, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999;17(2):460–469. doi: 10.1200/JCO.1999.17.2.460. [DOI] [PubMed] [Google Scholar]
- 5.Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant systemic treatment in breast cancer: A meta-analysis. J Natl Cancer Inst. 2005;97(3):188–194. doi: 10.1093/jnci/dji021. [DOI] [PubMed] [Google Scholar]
- 6.von Minckwitz G, et al. German Breast Group; Arbeitsgemeinschaft Gynäkologische Onkologie–Breast Study Groups Neoadjuvant chemotherapy and bevacizumab for HER2-negative breast cancer. N Engl J Med. 2012;366(4):299–309. doi: 10.1056/NEJMoa1111065. [DOI] [PubMed] [Google Scholar]
- 7.Sikov WM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance) J Clin Oncol. 2015;33(1):13–21. doi: 10.1200/JCO.2014.57.0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Earl HM, et al. ARTemis Investigators Efficacy of neoadjuvant bevacizumab added to docetaxel followed by fluorouracil, epirubicin, and cyclophosphamide, for women with HER2-negative early breast cancer (ARTemis): An open-label, randomised, phase 3 trial. Lancet Oncol. 2015;16(6):656–666. doi: 10.1016/S1470-2045(15)70137-3. [DOI] [PubMed] [Google Scholar]
- 9.Bear HD, et al. Bevacizumab added to neoadjuvant chemotherapy for breast cancer. N Engl J Med. 2012;366(4):310–320. doi: 10.1056/NEJMoa1111097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cameron D, et al. Adjuvant bevacizumab-containing therapy in triple-negative breast cancer (BEATRICE): Primary results of a randomised, phase 3 trial. Lancet Oncol. 2013;14(10):933–942. doi: 10.1016/S1470-2045(13)70335-8. [DOI] [PubMed] [Google Scholar]
- 11.Batchelor TT, et al. Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation. Proc Natl Acad Sci USA. 2013;110(47):19059–19064. doi: 10.1073/pnas.1318022110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heist RS, et al. Improved tumor vascularization after anti-VEGF therapy with carboplatin and nab-paclitaxel associates with survival in lung cancer. Proc Natl Acad Sci USA. 2015;112(5):1547–1552. doi: 10.1073/pnas.1424024112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Foncillas J, et al. 2012. Dynamic contrast-enhanced MRI versus 18F-misonidazol-PET/CT to predict pathologic response in bevacizumab-based neoadjuvant therapy in breast cancer. J Clin Oncol 30(Suppl):10512 (abstr)
- 14.Sorensen AG, et al. A “vascular normalization index” as potential mechanistic biomarker to predict survival after a single dose of cediranib in recurrent glioblastoma patients. Cancer Res. 2009;69(13):5296–5300. doi: 10.1158/0008-5472.CAN-09-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sorensen AG, et al. Increased survival of glioblastoma patients who respond to antiangiogenic therapy with elevated blood perfusion. Cancer Res. 2012;72(2):402–407. doi: 10.1158/0008-5472.CAN-11-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lambrechts D, Lenz H-J, de Haas S, Carmeliet P, Scherer SJ. Markers of response for the antiangiogenic agent bevacizumab. J Clin Oncol. 2013;31(9):1219–1230. doi: 10.1200/JCO.2012.46.2762. [DOI] [PubMed] [Google Scholar]
- 17.Greenberg JI, et al. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature. 2008;456(7223):809–813. doi: 10.1038/nature07424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winkler F, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: Role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6(6):553–563. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 19.Jain RK, Tong RT, Munn LL. Effect of vascular normalization by antiangiogenic therapy on interstitial hypertension, peritumor edema, and lymphatic metastasis: Insights from a mathematical model. Cancer Res. 2007;67(6):2729–2735. doi: 10.1158/0008-5472.CAN-06-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Willett CG, et al. Surrogate markers for antiangiogenic therapy and dose-limiting toxicities for bevacizumab with radiation and chemotherapy: Continued experience of a phase I trial in rectal cancer patients. J Clin Oncol. 2005;23(31):8136–8139. doi: 10.1200/JCO.2005.02.5635. [DOI] [PubMed] [Google Scholar]
- 21.Zardavas D, Irrthum A, Swanton C, Piccart M. Clinical management of breast cancer heterogeneity. Nat Rev Clin Oncol. 2015;12(7):381–394. doi: 10.1038/nrclinonc.2015.73. [DOI] [PubMed] [Google Scholar]
- 22.Willett CG, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10(2):145–147. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang SX, et al. Gene expression profile and angiogenic marker correlates with response to neoadjuvant bevacizumab followed by bevacizumab plus chemotherapy in breast cancer. Clin Cancer Res. 2008;14(18):5893–5899. doi: 10.1158/1078-0432.CCR-07-4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gasparini G, et al. A phase II study of neoadjuvant bevacizumab plus capecitabine and concomitant radiotherapy in patients with locally advanced rectal cancer. Angiogenesis. 2012;15(1):141–150. doi: 10.1007/s10456-011-9250-0. [DOI] [PubMed] [Google Scholar]
- 25.Foernzler D, et al. 2010. Tumor tissue based biomarker analysis in NO16966: A randomized phase III study of first-line bevacizumab in combination with oxaliplatin-based chemotherapy in patients with mCRC. ASCO Gastrointestinal Cancers Symposium (abstr 374). Available at meetinglibrary.asco.org/content/2207-72.
- 26.Vasudev NS, et al. Changes in tumour vessel density upon treatment with anti-angiogenic agents: Relationship with response and resistance to therapy. Br J Cancer. 2013;109(5):1230–1242. doi: 10.1038/bjc.2013.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verstraete M, et al. Combining bevacizumab and chemoradiation in rectal cancer. Translational results of the AXEBeam trial. Br J Cancer. 2015;112(8):1314–1325. doi: 10.1038/bjc.2015.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeong H-S, et al. Investigation of the lack of angiogenesis in the formation of lymph node metastases. J Natl Cancer Inst. 2015;107(9):djv155. doi: 10.1093/jnci/djv155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rønnov-Jessen L, Petersen OW, Bissell MJ. Cellular changes involved in conversion of normal to malignant breast: Importance of the stromal reaction. Physiol Rev. 1996;76(1):69–125. doi: 10.1152/physrev.1996.76.1.69. [DOI] [PubMed] [Google Scholar]
- 30.de Kruijf EM, et al. Tumor-stroma ratio in the primary tumor is a prognostic factor in early breast cancer patients, especially in triple-negative carcinoma patients. Breast Cancer Res Treat. 2011;125(3):687–696. doi: 10.1007/s10549-010-0855-6. [DOI] [PubMed] [Google Scholar]
- 31.Stylianopoulos T, et al. Causes, consequences, and remedies for growth-induced solid stress in murine and human tumors. Proc Natl Acad Sci USA. 2012;109(38):15101–15108. doi: 10.1073/pnas.1213353109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chauhan VP, et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat Commun. 2013;4:2516. doi: 10.1038/ncomms3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chauhan VP, et al. Compression of pancreatic tumor blood vessels by hyaluronan is caused by solid stress and not interstitial fluid pressure. Cancer Cell. 2014;26(1):14–15. doi: 10.1016/j.ccr.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hutchison GJ, et al. Hypoxia-inducible factor 1α expression as an intrinsic marker of hypoxia: Correlation with tumor oxygen, pimonidazole measurements, and outcome in locally advanced carcinoma of the cervix. Clin Cancer Res. 2004;10(24):8405–8412. doi: 10.1158/1078-0432.CCR-03-0135. [DOI] [PubMed] [Google Scholar]
- 35.Lehmann S, et al. Longitudinal and multimodal in vivo imaging of tumor hypoxia and its downstream molecular events. Proc Natl Acad Sci USA. 2009;106(33):14004–14009. doi: 10.1073/pnas.0901194106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mayer A, et al. Lack of correlation between expression of HIF-1α protein and oxygenation status in identical tissue areas of squamous cell carcinomas of the uterine cervix. Cancer Res. 2004;64(16):5876–5881. doi: 10.1158/0008-5472.CAN-03-3566. [DOI] [PubMed] [Google Scholar]
- 37.Mazzone M, et al. Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell. 2009;136(5):839–851. doi: 10.1016/j.cell.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang Y, et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci USA. 2012;109(43):17561–17566. doi: 10.1073/pnas.1215397109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tong RT, et al. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 2004;64(11):3731–3736. doi: 10.1158/0008-5472.CAN-04-0074. [DOI] [PubMed] [Google Scholar]
- 40.Duda DG, et al. Plasma soluble VEGFR-1 is a potential dual biomarker of response and toxicity for bevacizumab with chemoradiation in locally advanced rectal cancer. Oncologist. 2010;15(6):577–583. doi: 10.1634/theoncologist.2010-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lambrechts D, et al. VEGF pathway genetic variants as biomarkers of treatment outcome with bevacizumab: An analysis of data from the AViTA and AVOREN randomised trials. Lancet Oncol. 2012;13(7):724–733. doi: 10.1016/S1470-2045(12)70231-0. [DOI] [PubMed] [Google Scholar]
- 42.Meyerhardt JA, et al. Phase I study of cetuximab, irinotecan, and vandetanib (ZD6474) as therapy for patients with previously treated metastastic colorectal cancer. PLoS One. 2012;7(6):e38231. doi: 10.1371/journal.pone.0038231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu AX, et al. Efficacy, safety, pharmacokinetics, and biomarkers of cediranib monotherapy in advanced hepatocellular carcinoma: A phase II study. Clin Cancer Res. 2013;19(6):1557–1566. doi: 10.1158/1078-0432.CCR-12-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raut CP, et al. Effects of sorafenib on intra-tumoral interstitial fluid pressure and circulating biomarkers in patients with refractory sarcomas (NCI protocol 6948) PLoS One. 2012;7(2):e26331. doi: 10.1371/journal.pone.0026331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andre F, Deluche E, Bonnefoi H. Bevacizumab: The phoenix of breast oncology? Lancet Oncol. 2015;16(6):600–601. doi: 10.1016/S1470-2045(15)70201-9. [DOI] [PubMed] [Google Scholar]
- 46.Goel S, et al. Effects of vascular-endothelial protein tyrosine phosphatase inhibition on breast cancer vasculature and metastatic progression. J Natl Cancer Inst. 2013;105(16):1188–1201. doi: 10.1093/jnci/djt164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patenaude A, et al. A novel population of local pericyte precursor cells in tumor stroma that require Notch signaling for differentiation. Microvasc Res. 2015;101:38–47. doi: 10.1016/j.mvr.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 48.Abraham S, et al. A Rac/Cdc42 exchange factor complex promotes formation of lateral filopodia and blood vessel lumen morphogenesis. Nat Commun. 2015;6:7286. doi: 10.1038/ncomms8286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rivera LB, Bergers G. CANCER. Tumor angiogenesis, from foe to friend. Science. 2015;349(6249):694–695. doi: 10.1126/science.aad0862. [DOI] [PubMed] [Google Scholar]
- 50.Symmans WF, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25(28):4414–4422. doi: 10.1200/JCO.2007.10.6823. [DOI] [PubMed] [Google Scholar]
- 51.Ogston KN, et al. A new histological grading system to assess response of breast cancers to primary chemotherapy: Prognostic significance and survival. Breast. 2003;12(5):320–327. doi: 10.1016/s0960-9776(03)00106-1. [DOI] [PubMed] [Google Scholar]
- 52.Network TCGA. Cancer Genome Atlas Network Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parker JS, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27(8):1160–1167. doi: 10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Less JR, et al. Interstitial hypertension in human breast and colorectal tumors. Cancer Res. 1992;52(22):6371–6374. [PubMed] [Google Scholar]
- 55.Boucher Y, Kirkwood JM, Opacic D, Desantis M, Jain RK. Interstitial hypertension in superficial metastatic melanomas in humans. Cancer Res. 1991;51(24):6691–6694. [PubMed] [Google Scholar]
- 56.Batchelor TT, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11(1):83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen S, et al. Clinical and pathological response to neoadjuvant chemotherapy based on primary tumor reduction is correlated to survival in hormone receptor-positive but not hormone receptor-negative locally advanced breast cancer. Ann Surg Oncol. 2015;22(1):32–39. doi: 10.1245/s10434-014-3894-0. [DOI] [PubMed] [Google Scholar]
- 58.Zhu Q, et al. Pathologic response prediction to neoadjuvant chemotherapy utilizing pretreatment near-infrared imaging parameters and tumor pathologic criteria. Breast Cancer Res. 2014;16(5):456. doi: 10.1186/s13058-014-0456-0. [DOI] [PMC free article] [PubMed] [Google Scholar]