Abstract
The human gut contains a microbial community composed of tens of trillions of organisms that normally assemble during the first 2–3 y of postnatal life. We propose that brain development needs to be viewed in the context of the developmental biology of this “microbial organ” and its capacity to metabolize the various diets we consume. We hypothesize that the persistent cognitive abnormalities seen in children with undernutrition are related in part to their persistent gut microbiota immaturity and that specific regions of the brain that normally exhibit persistent juvenile (neotenous) patterns of gene expression, including those critically involved in various higher cognitive functions such as the brain’s default mode network, may be particularly vulnerable to the effects of microbiota immaturity in undernourished children. Furthermore, we postulate that understanding the interrelationships between microbiota and brain metabolism in childhood undernutrition could provide insights about responses to injury seen in adults. We discuss approaches that can be used to test these hypotheses, their ramifications for optimizing nutritional recommendations that promote healthy brain development and function, and the potential societal implications of this area of investigation.
Keywords: childhood undernutrition, gut microbiota development, brain development, brain metabolism
In 2001, Raichle et al. published a paper in PNAS describing the default mode network (DMN) (1). This network consists of discrete, bilateral, and symmetrical areas in parietal, prefrontal, and temporal cortices of the human, nonhuman primate, feline, and rodent brain (2). The DMN consistently decreases its activity during the performance of novel, attention-demanding, non–self-referential tasks compared with quiet repose and more automatic activities. Another unique feature of the DMN is that it exhibits an overall high metabolic rate. The discovery of the DMN with its unique constellation of features has reignited a longstanding interest in the significance of the brain’s ongoing or intrinsic activity (currently dubbed the brain’s “resting state”) (3), which accounts for the vast majority of its enormous energy cost. We believe it is timely to provide a Perspective that challenges us to consider how this cost is being met and how new knowledge gleaned from studying the development of our gut microbial communities can be leveraged to build healthy brains and minds through better nutrition.
The importance of adequate nutrition for infants and children routinely commands our attention because of its personal and societal importance. Not only is severe malnutrition/undernutrition (see Box for definitions) a threat to life but chronic undernutrition results in impaired cognitive abilities that are often not evident until the second or third decade, including effects on behaviors, such as self-control (4), that are of critical importance for a successful and productive life. Nutritional status in infancy and childhood is typically defined based on anthropometric measurements (the extent of deviation of height-for-age, weight–for-age, or weight-for-height scores from mean values established by the World Health Organization for a multinational, multiethnic healthy cohort). Using these metrics, numerous studies have documented how childhood undernutrition represents a pervasive global public health challenge and have shown that its origins are not attributable to food insecurity alone but rather reflect complex and still poorly understood interactions between factors that operate within and across generations (5, 6).
Box—Definition of Terms
Malnutrition.
Broadly defined as a biological state reflective of the underrepresentation or overrepresentation of nutrients. In this Perspective, we refer to childhood undernutrition as a pathologic state reflecting the interactions between a number of factors including, but not limited to, the inadequate representation of macro- and micronutrients in the diet. Nutritional status in infancy and childhood is typically defined based on anthropometric measurements (height-for-age, weight–for-age, or weight-for-height). The values for a given individual or individuals are compared with the mean and SDs of a World Health Organization (WHO) reference cohort of 8,440 individuals living in six countries representing diverse geographic and cultural features who manifested healthy growth phenotypes. The WHO standards are designed to depict normal early childhood growth under optimal environmental conditions and can be used to assess children everywhere, regardless of ethnicity, socioeconomic status, and type of feeding.
Transcriptional Neoteny.
Neoteny refers to the persistence of juvenile traits in adults. Transcriptional neoteny refers to the persistence of juvenile gene expression patterns in the adult. Regions of the adult brain that exhibit neoteny include the default mode, control, and dorsal attention networks. They demonstrate persistent heightened expression of genes related to synaptic growth and turnover and exhibit elevated levels of aerobic glycolysis. A hypothesis arising from these observations is that the neotenous regions may be vulnerable to childhood undernutrition due to their dependence on high rates of biosynthesis and aerobic glycolysis. Accordingly, childhood undernutrition may affect higher cognitive functions associated with neotenous regions more so than it affects primary functions associated with, for example, the visual cortex or cerebellum.
Gnotobiotic Animals.
Animals born sterile (germfree) and either maintained in a germfree state in specially designed isolators for the duration of their lives, or at some specified time point colonized with intact uncultured microbial communities harvested from various sources, including the intestines of children with and without undernutrition living in various geographic locations representing different cultural traditions, or with one or more cultured microbial species/strains. Animals can be fed various diets, sterilized by irradiation or other means, including diets representative of those consumed by human microbiota donors, so that interactions among foods, gut microbes, and microbial/host metabolism can be delineated. Colonizing recipient germfree mice with (human) donor microbiota represents a preclinical test of whether that microbial community is causally related to various donor phenotypes and the extent to which those features are diet-dependent.
Microbiota.
The collection of organisms that together form a microbial community occupying a given habitat (e.g., the gut). These organisms can represent all three domains of life: Bacteria, Archaea and Eukarya (and their viruses), but are dominated by Bacteria.
Microbiome.
The collection of microbial genes present in the genomes of members of a given microbial community.
A common practice in understanding the origins, effects, and effectiveness of various treatment/prevention strategies for childhood undernutrition has been to focus on the “first 1,000 d,” which begins with conception and ends approximately 2 y after birth (7). Here, we call for an expanded view beyond these 1,000 d, particularly in relation to the brain, and a broader cellular, metabolic, and genetic view of our developmental biology that encompasses our gut microbial community (microbiota) and its genes (microbiome) (Fig. 1A and Box). We propose that understanding the relationship between brain development, metabolism, and assembly of this gut “microbial organ,” with its capacity to transform the foods that we consume into valuable cellular building blocks and energy, will provide new insights about the determinants, definitions, and optimization of brain nutrition. A corollary is that adequate brain nutrition and metabolism is required not only to build a healthy mind but also to support its adaptations and redevelopment throughout life.
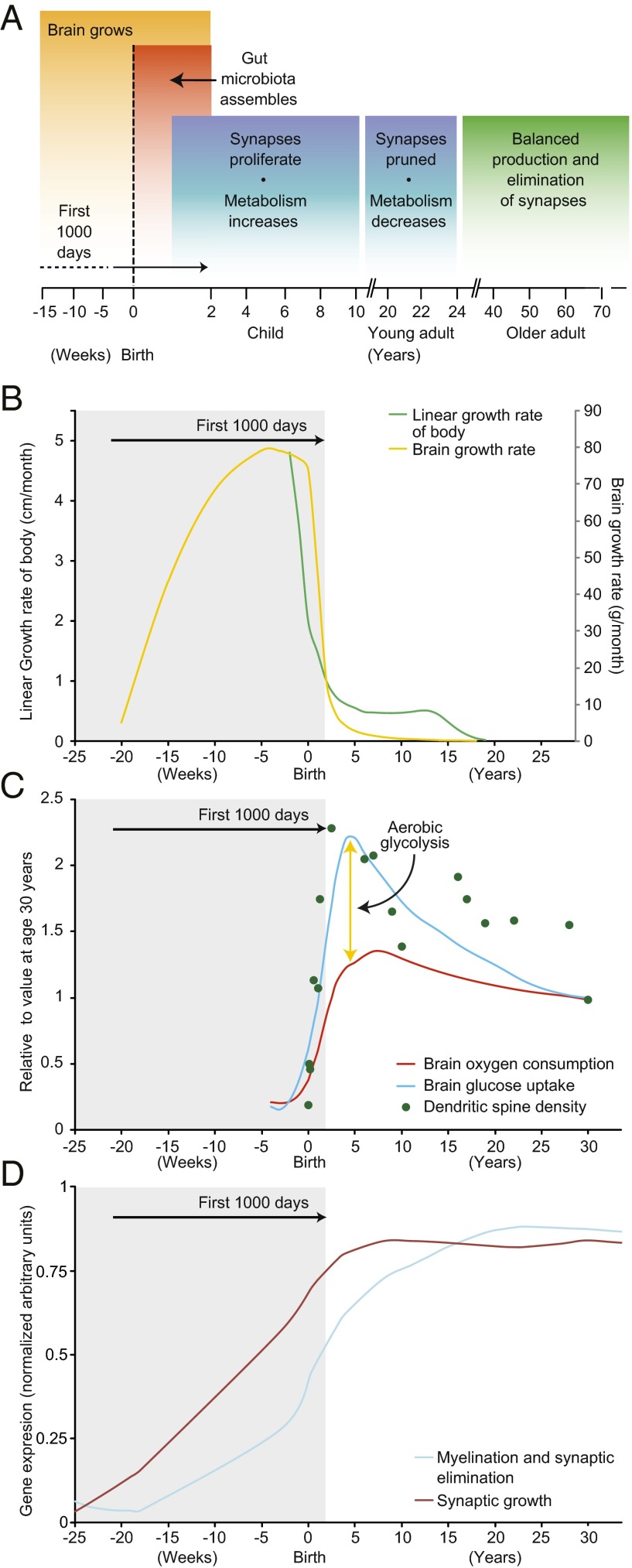
Fig. 1.
Development of the brain extends beyond the first 1,000 d. (A) An overview. Note that the gut microbiota assembles during the first 2–3 y after birth. (B) The growth rates of the brain (yellow line), and human body (green line) increase most rapidly during the first 1,000 d after conception (i.e., up to the end of the second year of postnatal life). (C) Dendritic spine density (green dots) and brain glucose uptake (blue line) are sustained well after the first 1,000 d. Although the brain’s oxygen consumption (red line) is also higher during this period, it is not nearly as high as the increase in glucose uptake, suggesting that much of the glucose is metabolized via aerobic glycolysis, in keeping with the high levels of synaptic growth and turnover during this time. Adapted from ref. 11. Note that the data shown are based on an invasive method (modified Kety–Schmidt technique for repeated measures of cerebral blood flow and metabolism, which requires continuous sampling of arterial and venous blood) and on PET imaging, which involves exposure to radiation; because these approaches are unlikely to be generally used in studies of children, there is an impetus to apply MRI-based methods to characterize some features of brain metabolism during development. (D) Cortical expression of human genes that control synaptic proliferation persists well beyond the period of synaptic “pruning” during adolescence. Stable synaptic density in the adult brain results from a balance between the expression of genes that control synaptic proliferation and elimination. Adapted from ref. 52.
Brain Metabolism in Children and Adults
The resource demands of the human brain are astonishing. The adult brain represents 2% of the body weight yet consumes ∼20% of the body’s energy (8, 9). The brain is even more expensive in childhood. At approximately age 10, a child’s brain represents 5–10% of body mass, consumes twice the glucose and 1.5 times the oxygen per gram of tissue compared with an adult’s brain, and accounts for up to 50% of the total basal metabolic rate of the body (10–12).
By age 2 (i.e., the end of the first 1,000 d), the brain is 75% of its adult size (Fig. 1B) (13). However, the explosive growth of synapses has yet to occur, with synaptic proliferation peaking a few years later. Synaptic pruning follows until a stable synaptic density is reached sometime in the third decade of life. These features underscore a key point: When factoring in synaptic proliferation and associated metabolism, the “initial” development of the human brain extends well into the second decade of life, far beyond the first 1,000 d postconception (14) (Fig. 1 A–D). Moreover, critical constituents of the adult brain turn over continuously (15). Thus, the brain may be vulnerable to poor nutrition not only during its initial development but also during its ongoing remodeling and as it recovers from various forms of damage.
There is growing evidence that postpubescent psychiatric illnesses such as schizophrenia have their roots in early brain development (16). Even minor effects on self-control and anxiety may have profound implications for families, communities, and societies where the prevalence of childhood undernutrition is high. Of course, brain development is not simply reliant on optimal nutrition: Nurture and education clearly contribute to ensuring that the brain receives the resources it needs to grow and develop optimally (17, 18).
Given the brain’s voracious appetite, the vast majority of studies of brain glucose metabolism have focused on the generation of energy in the form of ATP from glycolysis and oxidative phosphorylation (Fig. 2A). However, glycolysis is also critical for biosynthesis of cellular macromolecules (e.g., the lipids required for membrane maintenance) (19, 20), and likely for other functions (e.g., powering membrane channels and transporters). The amount of glucose devoted to these additional functions is revealed by the discrepancy between the amount of glucose metabolized by the brain and the amount of oxygen used to convert it to carbon dioxide, water, and energy. In adults, the amount of glucose devoted to these functions accounts for 10–12% of the total glucose metabolized by the brain (21–23) [note that these values may be underestimates (24)]. The process by which glucose is metabolized to lactate despite oxygen availability has been referred to as “aerobic glycolysis” or the “Warburg effect” after its discovery as a metabolic signature of cancer (25). This term has since expanded to encompass the intermediary pathways that arise from glycolysis and fuel cellular anabolic demands (20, 25–30) (Fig. 2A).
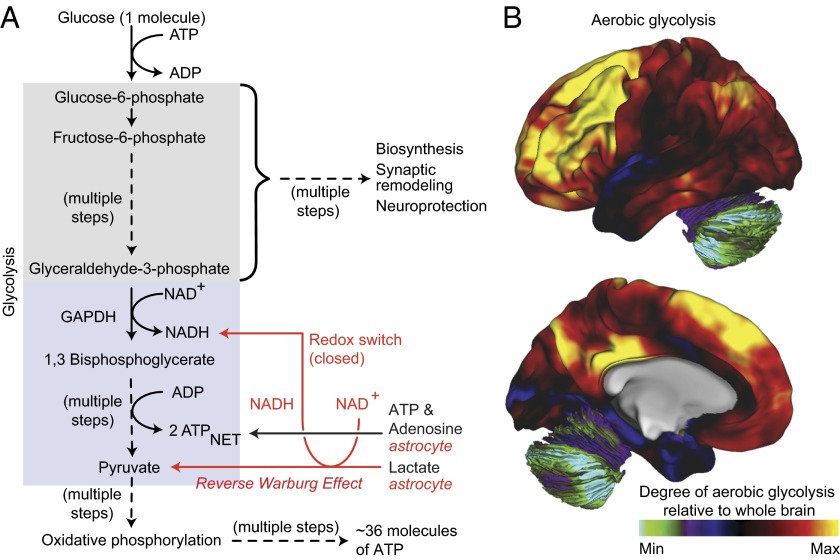
Fig. 2.
Aerobic glycolysis in the brain. (A) In many cell types, glucose is efficiently catabolized to generate ATP via oxidative phosphorylation in mitochondria. However, under conditions where oxygen is limiting, glucose is converted to lactate via anaerobic glycolysis. In contrast, certain unicellular organisms and mammalian cell lineages (e.g., fibroblasts, lymphocytes, and those obtained from various cancers) preferentially convert glucose to lactate regardless of the availability of oxygen. This nonoxidative process is referred to as aerobic glycolysis and is thought to occur primarily to increase production of intermediary metabolites from glycolysis that can enter biosynthetic pathways. In addition, neurons consume lactate released by astrocytes, presumably as a substrate for energy generation akin to a similar process found in some cancer cells (reverse Warburg effect). This neuronal lactate consumption would further be predicted to alter the redox potential of neurons (redox switch) and thereby redirect glycolysis to support other biosynthetic and neuroprotective pathways (i.e., biosynthesis and neuroprotection). The colored boxes denote the elements of glycolysis involved in biosynthesis and neuroprotection (gray), and those involved in energy generation (blue). (B) Regional variation in the levels of aerobic glycolysis in the lateral and medial surfaces of 33 healthy adult human brains (31). The numbers on the color scale indicate the glycolytic index: This metric represents the degree of aerobic glycolysis normalized to whole brain.
Aerobic glycolysis has a number of distinguishing features that reveal its role in brain function. One of the most striking is its nonuniform distribution in the brain (Fig. 2B) and its spatial overlap with cortical regions responsible for many higher-level cognitive functions such as the DMN (2, 31). Another important feature of aerobic glycolysis in the adult human brain is its correlation with persistent expression of genes associated with earlier phases of brain development (“transcriptional neoteny”) (Box) (11). Regions of the brain with the highest levels of aerobic glycolysis preferentially express genes related to synapse formation and dendritic spine growth whereas regions high in oxidative glucose metabolism preferentially express genes related to synaptic transmission. Furthermore, aerobic glycolysis in the brain is substantially increased during early childhood, occurring precisely during the period of peak neuronal arborization (Fig. 1C) (11). Finally, aerobic glycolysis reflects the “symbiotic” relationship between neurons and astrocytes (32) as well as oligodendrocytes (33). In this relationship, glutamate in the synaptic cleft is taken up by astrocytes in a sodium-dependent manner where it triggers aerobic glycolysis and the production and release of lactate. Lactate is subsequently taken up by the neuron where it profoundly influences allocation of metabolic resources in favor of biosynthesis (Fig. 2A), such as that required for long-term memory formation (34).
A critical element in this orchestration of neuronal metabolism is the nucleotide nicotinamide-adenine dinucleotide (NAD+) and its reduced form NADH (Fig. 2A) whose precursors are derived from the diet (35). NAD+ is a central coenzyme in biochemical pathways with far-reaching effects on health and responses to stress (36). NAD+ is essential for carrying out glycolysis and thus the production of basic cellular building blocks from glucose (Fig. 2A). In addition, NAD+ is consumed by a number of important enzymes: e.g., the sirtuins that link metabolism to a variety of cellular processes including aging, the poly- and mono-ADP ribosylases that participate in cell death pathways, and other enzymes that promote metabolic homeostasis via monitoring NAD+ levels (37).
The integrity of the axon is critical for maintaining proper functional connectivity in the brain. Degeneration of injured or unhealthy axons is an active, self-destructive process. This degenerative process is initiated by decreases in axonal levels of NAD. This role for NAD was first uncovered during studies of the wlds mutant mouse in which axonal degeneration is delayed due to overexpression of the NAD+ biosynthetic enzyme nicotinamide nucleotide adenyltransferase 1 (Nmnat1) (38, 39). Later studies showed that a related short-lived protein, Nmnat2, is transported down the axon and is responsible for maintaining axonal NAD levels (40). The sterile alpha- and armadillo-motif-containing protein-1 (Sarm1), a Toll-like receptor adaptor protein, also plays a key role in axon maintenance (41, 42); upon activation, it mediates a massive rapid loss of NAD+ in the axon, which promotes axon degeneration (43). The importance of NAD homeostasis in maintaining brain circuitry suggests that the NAD metabolic pathways of the gut microbiota, which are diverse and different from those in humans, are likely to influence the development, maintenance, and function of the human brain.
Given the high metabolic needs of the brain during development, it is not surprising that poor nutritional status in childhood is associated with cognitive impairments later in life (44–49). Thus far, two longitudinal, randomized, controlled trials suggest that early-life nutritional interventions may improve cognitive outcomes although much more work in this area is needed to determine the effect size as a function of the severity of undernutrition at the time of diagnosis, the type and timing of the nutritional interventions, and diet type and diversity (including during the period of complementary feeding) (50, 51).
The brain undergoes remodeling at multiple time scales and levels of organization throughout life (15). Notably, synaptic “pruning” during adolescence may not be accomplished merely by a reduction in the activity of synaptic proliferation genes but rather via the increased expression of synaptic elimination genes (52). Although genes that control synaptic proliferation are expressed well into adulthood, increased rates of myelination and synaptic elimination could maintain stable synaptic densities (Fig. 1C). Intriguingly, transcriptional networks underlying brain development and growth are redeployed in adulthood to assist recovery in murine models of stroke (53, 54). This postinjury regenerative growth process may require metabolic and nutritional resources similar to those used during development (55, 56). Thus, efforts to understand these needs in the developing brains of healthy and undernourished children may enhance our ability to treat an injured adult brain (57, 58).
Development of the Gut Microbiota in Healthy Versus Undernourished Children
The gut microbiota, composed of tens of trillions of microbes belonging to all three domains of life (Bacteria, Archaea, and Eukarya) but dominated by members of Bacteria, is responsible for myriad tasks related to nutrient metabolism. Assembly of the microbiota begins at birth (Figs. 1A and 3). Studies of infants and children living in diverse geographic areas representing distinct cultural traditions have shown that it takes 2–3 y for the gut microbiota to mature to a configuration resembling that of adults (59).
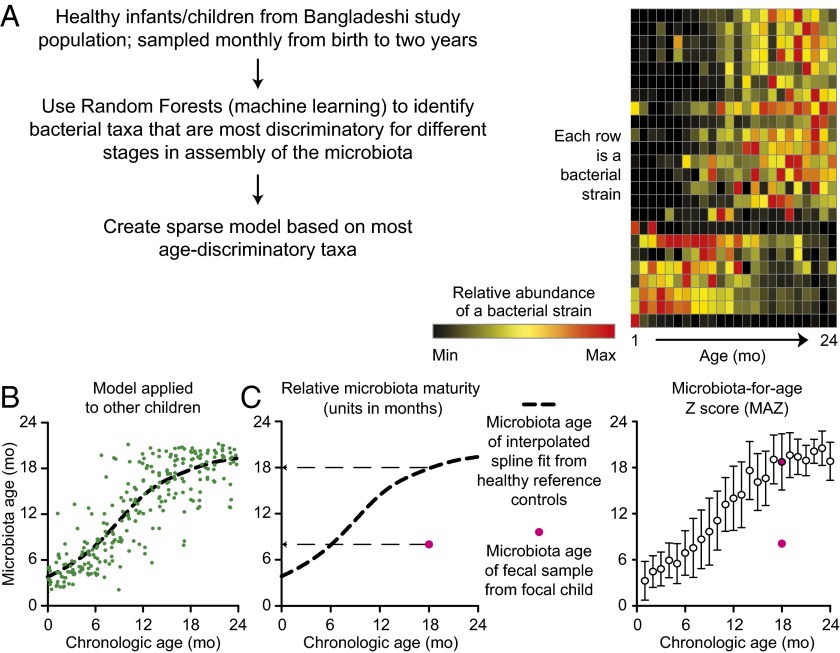
Fig. 3.
Maturation of the gut microbiota. The gut microbiota is acquired beginning at birth and attains a configuration similar to that of adults during the first 2–3 y of postnatal life, during which time the brain’s synaptic density and rate of glucose uptake are quickly rising. (A) The approach used to identify age-discriminatory bacterial strains in the microbiota of members of a birth cohort living in Bangladesh with healthy growth phenotypes, as defined by anthropometry. A sparse 24-strain Random Forests model, comprising the most age-discriminatory organisms, provides a microbial signature for defining a postnatal developmental program of microbiota assembly shared across biologically unrelated infants and children. Each row represents one of the 24 bacterial strains. Each column represents the postnatal month where fecal samples were obtained. The different colors represent relative abundances of each bacterial strain in the microbiota as a function of the infant/child’s chronological age. (B) Using the model described in A, a “microbiota age” is assigned to individual fecal samples collected from healthy children of various ages. Each circle is a separate fecal sample collected from members of the birth cohort over time. The dashed line represents a spline fit of the data. (C) Two metrics describing postnatal development of the microbiota can be defined using the model: relative microbiota maturity and microbiota-for-age Z score. The latter represents the extent of deviation of a given individual’s microbiota from the median ± SD of the reference healthy cohort. Red circles in the plots represent an immature gut microbiota from an 18-mo-old child with severe acute undernutrition. Adapted from ref. 60.
Recent studies have disclosed an order to this process of community assembly (maturation) that is shared across biologically unrelated individuals living in different parts of the world, with age-indicative sets of bacterial species/strains represented at different relative abundances at different time points during this process (60) (Fig. 3A). Metrics (“relative microbiota maturity” and “microbiota-for-age Z score”) have been developed using this signature set of indicative bacterial species that can be used to define the state of maturation of an individual’s microbiota relative to that of a reference group of healthy infants/children of similar chronological age (Fig. 3 B and C). Applying these metrics to children with undernutrition revealed that they harbor gut microbiota with delayed development: i.e., their microbiota have configurations that are younger than that of chronologically age-matched individuals who manifest healthy growth phenotypes (Fig. 3C). Moreover, the gut microbiota of undernourished children are not durably repaired with current therapeutic interventions; they revert to immature configurations after cessation of treatment (60, 61). In other words, these individuals have a persistent developmental abnormality.
Transplanting immature microbiota from children with severe undernutrition and mature microbiota from healthy controls to germfree mice, fed diets representative of those consumed by the microbiota donors, has shown that perturbations in gut community development are causally related to a number of the metabolic and immunologic manifestations of undernutrition and are not simply an effect of undernutrition (61, 62).
Comparative metabolic studies have been performed in the prefrontal cortex of adult conventionally raised mice (i.e., animals that have acquired microbes from environmental sources, including their mothers, beginning at birth) and their germfree counterparts. The results suggest that at least in this region of the brain there is increased glycolysis and oxidative phosphorylation in conventionally raised animals with an established microbiota (63).
Hypotheses About the Interrelationship Between Development of a Healthy Gut Microbiota and Development of a Healthy Brain
Given the very large postnatal metabolic needs of the developing human brain and the importance of the gut microbiota in energy harvest and nutrient metabolism, we postulate that gut microbiota immaturity is causally related to the neurological abnormalities associated with undernutrition. This concept can be restated in the form of several interrelated and testable hypotheses. (i) Normal development of the gut microbiota is required to support the metabolic activities of the brain during and after critical windows of neurodevelopment (Fig. 1A). A corollary is that the metabolic output of the gut microbiota might include compounds that directly influence brain development and physiology. It is known that i.v. infusion of d-lactate induces panic attacks in certain individuals (64, 65) whose susceptibility is associated with a significant asymmetry in cerebral blood flow in the parahippocampal gyri at rest (66). Intriguingly, the microbiota produces d-lactate and might mediate similar effects (note that the host produces l-lactate). Moreover, neurotransmitters produced either by microbial enzymes (67) or by stimulation of enteroendocrine cells by the gut microbiota (68, 69) can influence the enteric nervous system. (ii) The coordinated development of the brain and gut microbiota reflects, in part, an underlying bidirectional signaling between these two organs mediated by innervation of the gut, by the products of the gut epithelium’s enteroendocrine cell lineage, by the metabolic effector circuits located in the hypothalamus and elsewhere in the brain (e.g., the circuits that control feeding behavior and energy storage) (70), and by yet-to-be-defined mechanisms. (iii) Perturbations in normal gut microbiota development result in disruption of various aspects of brain metabolism, globally and/or in specific regions, interfere with the formation and function of specific neural circuits, and may affect synaptic development or myelination. Effects on brain and gut microbiota development are likely reciprocal, resulting in a self-reinforcing pathogenetic cascade. Indeed, there is evidence that neural circuits impinge directly upon acetylcholine-producing T cells to control innate immune responses (71). A corollary hypothesis is that auto-regulatory mechanisms that act to preserve and maintain brain blood flow and meet metabolic needs and that are especially active in childhood may not be sufficient to fully correct for glucose and energy deficiencies. (iv) Regions of the brain that have high levels of gene expression related to growth and axonal projection may be particularly vulnerable to undernutrition and the effects of gut microbiota immaturity during brain development and during recovery from brain injury.
Testing the Hypotheses
At this moment, when so much attention and exciting technical innovation is enabling advances in both basic and clinical science, it is timely to consider ways in which tests of these hypotheses can be conceived and executed. The urgency of doing so is underscored by the interrelated global challenges of rapid population growth, threats to sustainable agriculture, the need to identify more affordable and more nutritious food sources, the slow progress in combating the already enormous problem of childhood undernutrition, and the long-term effects of undernutrition on cognition and behavioral disorders.
Gnotobiotic animals (Box) represent one way of simulating diet-by-microbiota interactions that occur in humans. Methods have been developed that allow a previously frozen human fecal sample, obtained from a donor representing a chronologic age, cultural tradition, geographic region, and physiologic or disease state, to be efficiently transplanted to recipient germfree animals (typically mice) (61, 72, 73). Once acquired, these communities can be transmitted across generations of animals as long as the animals are maintained in gnotobiotic isolators, thereby allowing the impact of the human gut community to be examined during pregnancy [the female gnotobiotic mouse (dam) is colonized with the human donor’s microbiota], the suckling period (as the dam’s human microbiota is transferred to her pups), as well as during and after weaning. Given improvements in embryo transfer-based methods for rederiving conventionally raised mice as germfree (74), these experiments can be conducted in mice representing a variety of genotypes.
Gnotobiotic animals colonized with a human donor’s microbiota can be fed diets whose composition and method of preparation are representative of those presently consumed by the donor population (72); if the representative diet is deficient in macro- or micronutrients, additional reference control diets can be fashioned that are supplemented to adequately meet the nutritional requirements of humans and mice (62). Recipients of microbiota from undernourished (and healthy control) donors can also be given diets composed of ingredients envisioned to represent more affordable and more nutritious food sources in the future, or they can be given therapeutic foods to reenact current nutritional interventions (75). The latter option allows studies to be performed that are not possible in humans: for example, overcoming potentially confounding bias and variables by “enrolling” a given child (via his/her microbiota) in multiple treatment arms to directly compare their efficacy. Alternatively, these preclinical models can be used to develop new nutritional interventions, whose effects can then be examined in humans.
Gnotobiotic models also offer an opportunity to examine the effects of gut microbiota from infants and children (representing different human populations and varying degrees of undernutrition/growth faltering, in various diet contexts) on the biotransformation of food ingredients (including nutritional supplements), as well as brain metabolism and gene expression as a function of (i) region, (ii) cell type (e.g., neurons, astrocytes, oligodendrocytes, microglia, and their progenitors), (iii) age, and (iv) genetic background of the animal. Additional parameters that can be measured include immune function and vascular permeability in the brain; dendritic spine growth/turnover; social, exploratory, and innate behaviors; and functional connectivity.
If transplantation of an intact uncultured microbiota is shown to mediate an effect (or effects) on brain biology, follow-up studies can be performed using collections of cultured bacterial strains recovered from that microbiota sample (62, 76, 77). These “personal” culture collections, containing microbes that have evolved together in a given individual exposed to (i) the microbial reservoirs present in a given geographic locale and (ii) the selection imposed by the dietary practices of the population, can be archived in a clonally arrayed format, with each well in a multiwell plate containing a single bacterial strain. Efficient methods for mining these personal culture collections for strains that effect or modulate host processes/phenotypes of interest have been developed that overcome the large combinatorial challenge inherent in determining which set of microbe–microbe interactions in a multistrain collection are responsible (62, 76, 78).
Little is currently known about the extent to which being germfree alters brain metabolism and development, raising questions about how to best use gnotobiotic models for defining causal relationships between the microbiota and brain biology. The approaches proposed above are based on comparative analyses, where germfree animals are not used as controls per se; rather, the effects of healthy versus undernourished donor microbiota are compared when transplanted into groups of germfree animals with identical genetic backgrounds, ages, and diets.
The Interdisciplinary Opportunities and Challenges That These Preclinical Studies Present
Characterizing these preclinical models requires an inherently interdisciplinary approach and should, out of necessity, catalyze a number of experimental and computational advances. For example, characterizing variations in functional connectivity by blood oxygen level-dependent functional MRI (fMRI) is challenging given that the small size of the mouse brain requires exceptionally high signal-to-noise and spatial resolutions that are prohibitively difficult to obtain with current MRI techniques. Functional connectivity optical intrinsic signal (fcOIS) imaging, which has recently been adapted to investigate cognitive defects in children with undernutrition (79, 80), represents one potential way of addressing these problems in mice because the instrumentation can be maintained within a typical gnotobiotic isolator. Longitudinal PET and magnetic resonance (MR) spectroscopy imaging studies of brain metabolism also present their own set of challenges. The good news is that protected transport devices have been developed for moving animals out of their gnotobiotic isolators into various imaging instruments without exposure to environmental microbes.
Defining neural circuits responsible for particular behaviors affected by the gut microbiota may be facilitated through the use of engineered, cell type-specific expression of optogenetic proteins, such as channelrhodopsin and halorhodopsin; this approach has allowed the activities of individual neurons in conventionally raised mouse models to be monitored and manipulated in vivo. The coupling of genetically engineered ultrasensitive calcium indicator proteins to two-photon microscopy could enable the activity of thousands of neurons to be imaged at the resolution of individual dendritic spines (81) in models where nutritional status and microbiota are deliberately manipulated. Coupled with installation of standardized behavior tests in gnotobiotic isolators and the use of 3D virtual-reality environments, these approaches could offer an unprecedented view of brain circuitry in living animals that are computing sensory inputs, thinking creatively, and making decisions.
Nonetheless, the inherent complexity, modularity, and plasticity of neural circuits make the task of correlating specific regions, cell types, and networks of the brain with behavior notoriously difficult although notable progress is being made in mouse models of human neurodevelopmental abnormalities such as autism (82). Advancing these characterizations of neural circuits to include assessments of their metabolic activities represents an inspiring but daunting challenge. An aspirational goal will be to apply the analytic methods and lessons learned from conventionally raised mouse models, including those that have been used to explore interactions between the microbiota and brain (83–86), to animals rederived as germfree so that the effects of deliberate manipulations of their gut microbial community membership and diets can be determined.
Translation to Humans
Movement from preclinical models to clinical studies should be viewed as bidirectional, with each informing the other. At a time when “big data” multimodal neuroimaging initiatives are including children and adults to survey brain development across the human lifespan, it is timely to consider how the resulting datasets and the underlying protocols for imaging can be used to inform and interpret studies of the effects of disrupted development of the gut microbiota on the structure and functional connectivity of the developing brain. Studies of twins in Malawi have revealed high incidence of discordance for moderate and severe acute undernutrition during the first 3 y of life (i.e., one cotwin in the pair presents with disease whereas the other has a healthy growth phenotype). Remarkably, the incidence of discordance (43% in a study of 317 twin pairs) was not significantly different between mono- and dizygotic pairs (61). In addition, the standard of care in Malawi for discordant twin pairs is to treat both members of the pair with therapeutic foods. Imaging studies of discordant pairs and control concordant healthy pairs, living in a variety of populations where childhood undernutrition is a pressing and pervasive problem, represent one attractive way of minimizing potentially confounding variables in defining the effects of microbiota immaturity on brain structure and function. Establishing MRI capability at sites in such low-income countries can be extremely challenging due to constraints imposed by inadequate infrastructure and/or lack of experienced personnel. Novel approaches, such as fcOIS imaging, may help overcome these limitations. Malnutrition is also prevalent among premature infants in nations where advanced MRI of the neonatal brain is routinely performed (87), providing another avenue for studying the role of nutrition and gut microbiota maturation in shaping brain development.
The gut microbiota and diets of human subjects exhibiting phenotypic extremes in microbiota maturity and brain imaging biomarkers could then be further characterized using gnotobiotic animal models of the type described above to test for a causal relationship between their microbiota and brain development and to delineate underlying mechanisms. Similarly, imaging, metabolomic, and gene expression datasets, emanating from preclinical models where microbiota immaturity and brain metabolism and development are being characterized, should sponsor efforts to create databases, bioinformatic tools, and statistical methods for identifying significant correlations between the different data types and discriminatory biomarkers. These preclinical databases and tools could help inform the design, assembly, and mining of analogous human databases (and vice versa; existing human databases and algorithms can help with regard to the preclinical databases). Together, such efforts should establish whether preclinical proof-of-concept, if established in gnotobiotic animal models, can translate to humans. The path forward will be challenging, but the rewards could be great.
Societal Implications
Ultimately, results emanating from these types of studies could have a large number of societal implications that need to be addressed through an open, proactive, and sustained public discussion. This discussion should be accompanied by a factual, sober, and ongoing educational initiative designed to inform the public about the purpose of such studies and the meaning of scientific advances. Given the global nature of childhood undernutrition, this educational initiative will have to build a vocabulary and a narrative that is sensitive to the widely ranging scientific literacy and disparate cultural traditions of the varied populations to which it is directed (88, 89). Examples of the potential implications of this work range from a better understanding of brain development and repair to a more general consideration of the biological, ethical, regulatory, and societal impact of dietary and/or microbe-based interventions early in postnatal life that affect various aspects of cognition and behavior.
We humans live in a microbe-dominated planet and have benefited from the “invention,” early in metazoan evolution, of a gut that harbors microbial resources with the capacity to adaptively support metabolic activities not represented in the host genome. Looking ahead, understanding how feeding our gut satisfies the needs of our developing brain should help determine, and hopefully ultimately facilitate, our continued evolution as a species.
Acknowledgments
Work from our laboratories cited in this Perspective was supported by grants from the Bill & Melinda Gates Foundation (to J.I.G.) and NIH Grants R37DK30292 (to J.I.G.), RO1AG013730 (to J.M.), and P01NS080675 (to M.E.R.).
Footnotes
Conflict of interest statement: J.I.G. is a cofounder of Matatu, Inc., a company characterizing the role of diet-by-microbiota interactions in animal health.
This article is a PNAS Direct Submission.
References
- 1.Raichle ME, et al. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raichle ME. The brain’s default mode network. Annu Rev Neurosci. 2015;38:433–447. doi: 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- 3.Raichle ME. The restless brain: How intrinsic activity organizes brain function. Philos Trans R Soc Lond B Biol Sci. 2015;370(1668):20140172. doi: 10.1098/rstb.2014.0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moffitt TE, et al. A gradient of childhood self-control predicts health, wealth, and public safety. Proc Natl Acad Sci USA. 2011;108(7):2693–2698. doi: 10.1073/pnas.1010076108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed T, et al. An evolving perspective about the origins of childhood undernutrition and nutritional interventions that includes the gut microbiome. Ann N Y Acad Sci. 2014;1332:22–38. doi: 10.1111/nyas.12487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Black RE, et al. Maternal and Child Undernutrition Study Group Maternal and child undernutrition: Global and regional exposures and health consequences. Lancet. 2008;371(9608):243–260. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- 7.Prentice AM, et al. Critical windows for nutritional interventions against stunting. Am J Clin Nutr. 2013;97(5):911–918. doi: 10.3945/ajcn.112.052332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clarke DD, Sokoloff L. Circulation and energy metabolism of the brain. In: Siegel GJ, Agranoff BW, Albers RW, Fisher SK, Uhler MD, editors. Basic Neurochemistry: Molecular, Cellular and Medical Aspects. Lippincott-Raven; Philadelphia: 1999. pp. 637–670. [Google Scholar]
- 9.Raichle ME, Mintun MA. Brain work and brain imaging. Annu Rev Neurosci. 2006;29:449–476. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- 10.Chugani HT. A critical period of brain development: Studies of cerebral glucose utilization with PET. Prev Med. 1998;27(2):184–188. doi: 10.1006/pmed.1998.0274. [DOI] [PubMed] [Google Scholar]
- 11.Goyal MS, Hawrylycz M, Miller JA, Snyder AZ, Raichle ME. Aerobic glycolysis in the human brain is associated with development and neotenous gene expression. Cell Metab. 2014;19(1):49–57. doi: 10.1016/j.cmet.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kennedy C, Sokoloff L. An adaptation of the nitrous oxide method to the study of the cerebral circulation in children: Normal values for cerebral blood flow and cerebral metabolic rate in childhood. J Clin Invest. 1957;36(7):1130–1137. doi: 10.1172/JCI103509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blinkov SM, Glezer II . The Human Brain in Figures and Tables: A Quantitative Handbook. Basic; New York: 1968. [Google Scholar]
- 14.Dewey KG, Begum K. Long-term consequences of stunting in early life. Matern Child Nutr. 2011;7(Suppl 3):5–18. doi: 10.1111/j.1740-8709.2011.00349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marder E, Goaillard JM. Variability, compensation and homeostasis in neuron and network function. Nat Rev Neurosci. 2006;7(7):563–574. doi: 10.1038/nrn1949. [DOI] [PubMed] [Google Scholar]
- 16.Insel TR. Rethinking schizophrenia. Nature. 2010;468(7321):187–193. doi: 10.1038/nature09552. [DOI] [PubMed] [Google Scholar]
- 17.Blakemore SJ. The developing social brain: Implications for education. Neuron. 2010;65(6):744–747. doi: 10.1016/j.neuron.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luby J, et al. The effects of poverty on childhood brain development: The mediating effect of caregiving and stressful life events. JAMA Pediatr. 2013;167(12):1135–1142. doi: 10.1001/jamapediatrics.2013.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Locasale JW, Cantley LC. Metabolic flux and the regulation of mammalian cell growth. Cell Metab. 2011;14(4):443–451. doi: 10.1016/j.cmet.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lunt SY, Vander Heiden MG. Aerobic glycolysis: Meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 2011;27:441–464. doi: 10.1146/annurev-cellbio-092910-154237. [DOI] [PubMed] [Google Scholar]
- 21.Boyle PJ, et al. Diminished brain glucose metabolism is a significant determinant for falling rates of systemic glucose utilization during sleep in normal humans. J Clin Invest. 1994;93(2):529–535. doi: 10.1172/JCI117003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Powers WJ, et al. Selective defect of in vivo glycolysis in early Huntington’s disease striatum. Proc Natl Acad Sci USA. 2007;104(8):2945–2949. doi: 10.1073/pnas.0609833104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raichle ME, Posner JB, Plum F. Cerebral blood flow during and after hyperventilation. Arch Neurol. 1970;23(5):394–403. doi: 10.1001/archneur.1970.00480290014002. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez-Rodriguez P, Fernandez E, Bolaños JP. Underestimation of the pentose-phosphate pathway in intact primary neurons as revealed by metabolic flux analysis. J Cereb Blood Flow Metab. 2013;33(12):1843–1845. doi: 10.1038/jcbfm.2013.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8(6):519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bryant C, Voller A, Smith MJ. The incorporation of radioactivity from (14C)glucose into the soluble metabolic intermediates of malaria parasites. Am J Trop Med Hyg. 1964;13:515–519. doi: 10.4269/ajtmh.1964.13.515. [DOI] [PubMed] [Google Scholar]
- 27.Chang CH, et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013;153(6):1239–1251. doi: 10.1016/j.cell.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Munyon WH, Merchant DJ. The relation between glucose utilization, lactic acid production and utilization and the growth cycle of L strain fibroblasts. Exp Cell Res. 1959;17(3):490–498. doi: 10.1016/0014-4827(59)90069-2. [DOI] [PubMed] [Google Scholar]
- 29.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang T, Marquardt C, Foker J. Aerobic glycolysis during lymphocyte proliferation. Nature. 1976;261(5562):702–705. doi: 10.1038/261702a0. [DOI] [PubMed] [Google Scholar]
- 31.Vaishnavi SN, et al. Regional aerobic glycolysis in the human brain. Proc Natl Acad Sci USA. 2010;107(41):17757–17762. doi: 10.1073/pnas.1010459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bélanger M, Allaman I, Magistretti PJ. Brain energy metabolism: Focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14(6):724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 33.Fünfschilling U, et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature. 2012;485(7399):517–521. doi: 10.1038/nature11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suzuki A, et al. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. 2011;144(5):810–823. doi: 10.1016/j.cell.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gossmann TI, et al. NAD(+) biosynthesis and salvage: A phylogenetic perspective. FEBS J. 2012;279(18):3355–3363. doi: 10.1111/j.1742-4658.2012.08559.x. [DOI] [PubMed] [Google Scholar]
- 36.Bogan KL, Brenner C. Nicotinic acid, nicotinamide, and nicotinamide riboside: A molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu Rev Nutr. 2008;28:115–130. doi: 10.1146/annurev.nutr.28.061807.155443. [DOI] [PubMed] [Google Scholar]
- 37.Nikiforov A, Kulikova V, Ziegler M. The human NAD metabolome: Functions, metabolism and compartmentalization. Crit Rev Biochem Mol Biol. 2015;50(4):284–297. doi: 10.3109/10409238.2015.1028612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Araki T, Sasaki Y, Milbrandt J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science. 2004;305(5686):1010–1013. doi: 10.1126/science.1098014. [DOI] [PubMed] [Google Scholar]
- 39.Sasaki Y, Milbrandt J. Axonal degeneration is blocked by nicotinamide mononucleotide adenylyltransferase (Nmnat) protein transduction into transected axons. J Biol Chem. 2010;285(53):41211–41215. doi: 10.1074/jbc.C110.193904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gilley J, Coleman MP. Endogenous Nmnat2 is an essential survival factor for maintenance of healthy axons. PLoS Biol. 2010;8(1):e1000300. doi: 10.1371/journal.pbio.1000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gerdts J, Summers DW, Sasaki Y, DiAntonio A, Milbrandt J. Sarm1-mediated axon degeneration requires both SAM and TIR interactions. J Neurosci. 2013;33(33):13569–13580. doi: 10.1523/JNEUROSCI.1197-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Osterloh JM, et al. dSarm/Sarm1 is required for activation of an injury-induced axon death pathway. Science. 2012;337(6093):481–484. doi: 10.1126/science.1223899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gerdts J, Brace EJ, Sasaki Y, DiAntonio A, Milbrandt J. SARM1 activation triggers axon degeneration locally via NAD+ destruction. Science. 2015;348(6233):453–457. doi: 10.1126/science.1258366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crosby L, Jayasinghe D, McNair D. Food for Thought: Tackling Child Malnutrition to Unlock Potential and Boost Prosperity. Save the Children; London: 2013. [Google Scholar]
- 45.Grantham-McGregor SM, Powell CA, Walker SP, Himes JH. Nutritional supplementation, psychosocial stimulation, and mental development of stunted children: The Jamaican study. Lancet. 1991;338(8758):1–5. doi: 10.1016/0140-6736(91)90001-6. [DOI] [PubMed] [Google Scholar]
- 46.Hoddinott J, Maluccio JA, Behrman JR, Flores R, Martorell R. Effect of a nutrition intervention during early childhood on economic productivity in Guatemalan adults. Lancet. 2008;371(9610):411–416. doi: 10.1016/S0140-6736(08)60205-6. [DOI] [PubMed] [Google Scholar]
- 47.Scrimshaw NS. Malnutrition, learning and behavior. Am J Clin Nutr. 1967;20(5):493–502. doi: 10.1093/ajcn/20.5.493. [DOI] [PubMed] [Google Scholar]
- 48.Udani PM. Protein energy malnutrition (PEM), brain and various facets of child development. Indian J Pediatr. 1992;59(2):165–186. doi: 10.1007/BF02759978. [DOI] [PubMed] [Google Scholar]
- 49.Victora CG, et al. Maternal and Child Undernutrition Study Group Maternal and child undernutrition: Consequences for adult health and human capital. Lancet. 2008;371(9609):340–357. doi: 10.1016/S0140-6736(07)61692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Isaacs EB, et al. The effect of early human diet on caudate volumes and IQ. Pediatr Res. 2008;63(3):308–314. doi: 10.1203/PDR.0b013e318163a271. [DOI] [PubMed] [Google Scholar]
- 51.Maluccio JA, et al. The impact of improving nutrition during early childhood on education among Guatemalan adults. Econ J. 2009;119(537):734–763. [Google Scholar]
- 52.Goyal MS, Raichle ME. Gene expression-based modeling of human cortical synaptic density. Proc Natl Acad Sci USA. 2013;110(16):6571–6576. doi: 10.1073/pnas.1303453110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carmichael ST. Gene expression changes after focal stroke, traumatic brain and spinal cord injuries. Curr Opin Neurol. 2003;16(6):699–704. doi: 10.1097/01.wco.0000102621.38669.77. [DOI] [PubMed] [Google Scholar]
- 54.Carmichael ST, et al. Growth-associated gene expression after stroke: Evidence for a growth-promoting region in peri-infarct cortex. Exp Neurol. 2005;193(2):291–311. doi: 10.1016/j.expneurol.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 55.Dávalos A, et al. Effect of malnutrition after acute stroke on clinical outcome. Stroke. 1996;27(6):1028–1032. doi: 10.1161/01.str.27.6.1028. [DOI] [PubMed] [Google Scholar]
- 56.Heiss WD, Emunds HG, Herholz K. Cerebral glucose metabolism as a predictor of rehabilitation after ischemic stroke. Stroke. 1993;24(12):1784–1788. doi: 10.1161/01.str.24.12.1784. [DOI] [PubMed] [Google Scholar]
- 57.Aquilani R, Sessarego P, Iadarola P, Barbieri A, Boschi F. Nutrition for brain recovery after ischemic stroke: An added value to rehabilitation. Nutr Clin Pract. 2011;26(3):339–345. doi: 10.1177/0884533611405793. [DOI] [PubMed] [Google Scholar]
- 58.Murphy TH, Corbett D. Plasticity during stroke recovery: From synapse to behaviour. Nat Rev Neurosci. 2009;10(12):861–872. doi: 10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]
- 59.Yatsunenko T, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Subramanian S, et al. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature. 2014;510(7505):417–421. doi: 10.1038/nature13421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith MI, et al. Gut microbiomes of Malawian twin pairs discordant for kwashiorkor. Science. 2013;339(6119):548–554. doi: 10.1126/science.1229000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kau AL, et al. Functional characterization of IgA-targeted bacterial taxa from undernourished Malawian children that produce diet-dependent enteropathy. Sci Transl Med. 2015;7(276):276ra24. doi: 10.1126/scitranslmed.aaa4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matsumoto M, et al. Cerebral low-molecular metabolites influenced by intestinal microbiota: A pilot study. Front Syst Neurosci. 2013;7:9. doi: 10.3389/fnsys.2013.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gorman JM, et al. Sodium D-lactate infusion of panic disorder patients. Neuropsychopharmacology. 1990;3(3):181–189. [PubMed] [Google Scholar]
- 65.Pitts FN, Jr, McClure JN., Jr Lactate metabolism in anxiety neurosis. N Engl J Med. 1967;277(25):1329–1336. doi: 10.1056/NEJM196712212772502. [DOI] [PubMed] [Google Scholar]
- 66.Reiman EM, Raichle ME, Butler FK, Herscovitch P, Robins E. A focal brain abnormality in panic disorder, a severe form of anxiety. Nature. 1984;310(5979):683–685. doi: 10.1038/310683a0. [DOI] [PubMed] [Google Scholar]
- 67.Williams BB, et al. Discovery and characterization of gut microbiota decarboxylases that can produce the neurotransmitter tryptamine. Cell Host Microbe. 2014;16(4):495–503. doi: 10.1016/j.chom.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reigstad CS, et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015;29(4):1395–1403. doi: 10.1096/fj.14-259598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yano JM, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161(2):264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zuker CS. Food for the brain. Cell. 2015;161(1):9–11. doi: 10.1016/j.cell.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 71.Rosas-Ballina M, et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334(6052):98–101. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ridaura VK, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341(6150):1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Turnbaugh PJ, et al. The effect of diet on the human gut microbiome: A metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1(6):6ra14. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Faith JJ, et al. Creating and characterizing communities of human gut microbes in gnotobiotic mice. ISME J. 2010;4(9):1094–1098. doi: 10.1038/ismej.2010.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Subramanian S, et al. Cultivating healthy growth and nutrition through the gut microbiota. Cell. 2015;161(1):36–48. doi: 10.1016/j.cell.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Faith JJ, Ahern PP, Ridaura VK, Cheng J, Gordon JI. Identifying gut microbe-host phenotype relationships using combinatorial communities in gnotobiotic mice. Sci Transl Med. 2014;6(220):220ra11. doi: 10.1126/scitranslmed.3008051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goodman AL, et al. Extensive personal human gut microbiota culture collections characterized and manipulated in gnotobiotic mice. Proc Natl Acad Sci USA. 2011;108(15):6252–6257. doi: 10.1073/pnas.1102938108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ahern PP, Faith JJ, Gordon JI. Mining the human gut microbiota for effector strains that shape the immune system. Immunity. 2014;40(6):815–823. doi: 10.1016/j.immuni.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lloyd-Fox S, et al. Measuring brain function in newborns in rural Gambia. FASEB J. 2015;29:899.5. [Google Scholar]
- 80.Papademetriou MD, et al. Optical imaging of brain activation in Gambian infants. Adv Exp Med Biol. 2014;812:263–269. doi: 10.1007/978-1-4939-0620-8_35. [DOI] [PubMed] [Google Scholar]
- 81.Chen TW, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499(7458):295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rothwell PE, et al. Autism-associated neuroligin-3 mutations commonly impair striatal circuits to boost repetitive behaviors. Cell. 2014;158(1):198–212. doi: 10.1016/j.cell.2014.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Braniste V, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6(263):263ra158. doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bravo JA, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA. 2011;108(38):16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Diaz Heijtz R, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA. 2011;108(7):3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hsiao EY, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155(7):1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Joosten KF, Hulst JM. Malnutrition in pediatric hospital patients: Current issues. Nutrition. 2011;27(2):133–137. doi: 10.1016/j.nut.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 88.Benezra A, DeStefano J, Gordon JI. Anthropology of microbes. Proc Natl Acad Sci USA. 2012;109(17):6378–6381. doi: 10.1073/pnas.1200515109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gordon JI. Honor thy gut symbionts redux. Science. 2012;336(6086):1251–1253. doi: 10.1126/science.1224686. [DOI] [PubMed] [Google Scholar]