Significance
Polycystic ovary syndrome (PCOS) is the leading cause of anovulatory infertility characterized by excessive androgen secretion. PCOS women are at an increased risk of developing depression and anxiety disorders. Although the etiology of PCOS is unclear, it is proposed to originate during fetal development because of maternal androgen excess. We describe here, in rodent models reflecting the anxiety phenotype of PCOS, evidence for disordered androgen receptor function in the amygdala, together with changes in estrogen receptor-α, serotonergic and GABAergic genes in the amygdala, and hippocampus. These findings define a previously unknown mechanism that may be critical in understanding how maternal androgen excess has the potential to increase the risk of developing anxiety disorders in daughters and sons of PCOS mothers.
Keywords: maternal androgen excess, anxiety, behavior, polycystic ovary syndrome, amygdala
Abstract
During pregnancy, women with polycystic ovary syndrome (PCOS) display high circulating androgen levels that may affect the fetus and increase the risk of mood disorders in offspring. This study investigated whether maternal androgen excess causes anxiety-like behavior in offspring mimicking anxiety disorders in PCOS. The PCOS phenotype was induced in rats following prenatal androgen (PNA) exposure. PNA offspring displayed anxiety-like behavior in the elevated plus maze, which was reversed by flutamide [androgen receptor (AR) blocker] and tamoxifen [selective estrogen receptor (ER) modulator]. Circulating sex steroids did not differ between groups at adult age. The expression of serotonergic and GABAergic genes associated with emotional regulation in the amygdala was consistent with anxiety-like behavior in female, and partly in male PNA offspring. Furthermore, AR expression in amygdala was reduced in female PNA offspring and also in females exposed to testosterone in adult age. To determine whether AR activation in amygdala affects anxiety-like behavior, female rats were given testosterone microinjections into amygdala, which resulted in anxiety-like behavior. Together, these data describe the anxiety-like behavior in PNA offspring and adult females with androgen excess, an impact that seems to occur during fetal life, and is mediated via AR in amygdala, together with changes in ERα, serotonergic, and GABAergic genes in amygdala and hippocampus. The anxiety-like behavior following testosterone microinjections into amygdala demonstrates a key role for AR activation in this brain area. These results suggest that maternal androgen excess may underpin the risk of developing anxiety disorders in daughters and sons of PCOS mothers.
Polycystic ovary syndrome (PCOS) is a heterogeneous disorder characterized by excessive androgen secretion and abnormal insulin activity and affects up to 17% of women worldwide (1). Women with PCOS are at an increased risk of developing symptoms of anxiety and depression. In fact, over 60% of women with PCOS are diagnosed with at least one psychiatric disorder, such as depression, anxiety, or an eating disorder (2). Suicide attempts have also been shown to be seven times more common in women with PCOS than in healthy controls (3). The mechanisms underlying the development of PCOS are poorly understood. Although a genetic basis for PCOS has been suggested, the intrauterine milieu might also affect the reproductive/endocrine function of a child born to a PCOS mother in a manner that is independent of genetic inheritance or sex. It is also known that daughters of mothers with PCOS are at increased risk of developing the syndrome and that sons tend to suffer from obesity and insulin resistance (4). Thus, it has been proposed that PCOS originates during fetal development and that this might be, in part, a result of maternal androgen excess (5).
Maternal testosterone levels in humans have been shown to affect brain morphology and function (6) and to be correlated to neural development and mental function (7). There is evidence for a crucial role of the hippocampus and the amygdala in the development of anxiety and depression, and that these neural circuits are affected by fluctuations in sex steroids in humans and in rodents (8). We have previously demonstrated that continuous exposure to dihydrotestosterone (DHT) from puberty until adulthood in female rats down-regulates androgen receptor (AR) expression in the hypothalamus and induces anxiety-like behavior in female rats (9). The increased rates of anxiety disorders and disruptive behavioral disorders among children with genetically induced hyperandrogenism further indicate that androgen excess may contribute to a higher risk of psychopathology (10).
During pregnancy, androgens are metabolized to estrogens by the placenta in women and by the ovaries in rodents. Thus, the effects of testosterone on pregnancy are partly mediated by estrogen (11). Women with PCOS exhibit high circulating androgen levels during pregnancy, which hypothetically could be related to the increased risk of mood disorders in their offspring (12).
Here, we tested the hypothesis that an excess of androgens in dams during pregnancy may cause anxiety-like behavior in adult female and male offspring. We used the prenatal androgen (PNA) model, which mimics the elevation of androgens in women with PCOS during pregnancy (13). The phenotype of the PNA model in mice (14) and in rats (12) reflects reproductive and metabolic characteristics of lean women with PCOS. However, whether it reflects symptoms of anxiety (2) is unknown. We demonstrated that female PNA offspring exhibited increased anxiety-like behavior, which was prevented by blocking the AR during pregnancy, implicating AR-mediated signaling in mediating the altered behavior of PNA offspring. To understand the neuroanatomical distribution of sites affected by the PNA treatment we evaluated the gene expression of key steroid receptors [Ar, estrogen receptor-α (Erα), Erβ, and G protein-coupled estrogen receptor (Gper)] in the hypothalamus, hippocampus, and amygdala, brain areas known to be involved in the regulation of mood behavior in female offspring. The expression of the AR gene was selectively altered in the amygdala of the PNA offspring. We further show that subchronic testosterone exposure in adult females also reduced Ar expression in the amygdala. Because the amygdala is known to be involved in the regulation of mood behavior, we hypothesized that testosterone exerts an anxiogenic effect in the amygdala. We obtained support for this hypothesis by demonstrating that intra-amygdala testosterone microinjections resulted in anxiety-like behavior.
Materials and Methods
Detailed materials and methods are provided in SI Materials and Methods.
Experiments 1 and 2.
Animals.
Pregnant Wistar rats (Charles River) arrived on gestational day (GD) 8 and were housed in individual cages on a 12-h light/dark cycle, with a temperature of 21–22 °C at 55–65% humidity. They received ad libitum access to water and standard chow (no. 2016; Harlan Winkelmann). All studies were carried out with ethical permissions from the Animal Ethics Committee of the University of Gothenburg (Ethical no: 53-2013 and 195-2013), in accordance with legal requirements of the European Community (Decree 86/609/EEC).
PNA treatment.
Pregnant dams were randomly assigned to one of four groups and treated with daily subcutaneous injections [from GD 15–19 (n = 5 per group)] of: (i) vehicle; (ii) testosterone (T): T 0.5 mg⋅kg⋅d; (iii) testosterone + flutamide (T + Flut): T 0.5 mg⋅kg⋅d with flutamide 7.5 mg⋅kg⋅d; and (iv) testosterone + tamoxifen (T + Tam): T 0.5 mg⋅kg⋅d with tamoxifen 10 µg⋅kg⋅d.
Experiment 1.
On GD21, dams were anesthetized and maternal and placental samples were collected. Blood from dams was centrifuged, and the serum was frozen at –80 °C for subsequent measurement of sex steroids.
Experiment 2.
Phenotyping of female offspring.
The estrous cycle phase was determined by vaginal smears. Ovaries were dissected and fixed for ovarian morphology. Euglycemic hyperinsulinemic clamp was performed to evaluate insulin sensitivity.
Postnatal weight development and food intake in offspring.
Pups were weighed from day 4 until adult age. Sex was confirmed by genotyping and female and male offspring were separated from dams at day 21. Body weight and food intake were measured weekly until behavioral testing or clamp.
Behavioral testing.
For details see SI Materials and Methods. To investigate the presence of anxiety-like behavior in male and female offspring of PCOS dams the elevated plus maze (EPM) test was performed. Locomotor activity was tested immediately after the EPM for 30 min in photo-cell equipped activity boxes (Kungsbacka Mätoch Reglerteknik).
Tissue collection and sex-steroid and corticosterone analyses.
After the last behavioral test and the euglycemic-hyperinsulinmic clamp, rats were killed by decapitation and tissues dissected and snap-frozen.
RNA isolation and mRNA expression.
Tissues from the hypothalamus, hippocampus and amygdala were analyzed in female and male offspring of PCOS dams. TaqMan probe sets for target genes and reference genes were chosen from the online catalog (Table S1). Gene expression was quantified relative to the housekeeping genes β-actin and Gapdh. Genes examined were selected because of their role in the regulation of steroidal hormones that may affect the development of PCOS as well as genes with a role in anxiety-like behavior in these brain areas. Table S2 shows mean of target gene CT value.
Table S1.
Genes evaluated in this study
| Gene symbol | Assay ID | Gene name | Reference sequence | Alias |
| Ar | Rn00560747_m1 | Androgen receptor | NM_012502.1 | Andr; Tfm |
| Esr1 | Rn01640372_m1 | Estrogen receptor-α | NM_012689.1 | ERα; Esr; RNESTROR |
| Esr2 | Rn00562610_m1 | Estrogen receptor-β | NM_012754.1 | ERβ; Erb2 |
| Gper1 | Rn01643280_s1 | G protein-coupled estrogen receptor 1 | NM_133573.1 | GPR41; Gpr30 |
| Htr1a | Rn00561409_s1 | 5-hydroxytryptamine (serotonin) receptor 1A | NM_012585.1 | 5HT1A; RAT5HT1A |
| Htr2c | Rn00562748_m1 | 5-hydroxytryptamine (serotonin) receptor 2C | NM_012765.3 | 5-HT2C;5-HTR2C; 5HT-1C |
| Gad1 | Rn00690300_m1 | Glutamate decarboxylase 1 | NM_017007.1 | GAD67 |
| Gad2 | Rn00561244_m1 | Glutamate decarboxylase 2 | NM_012563.1 | GAD65 |
| Gabbr1 | Rn00578911_m1 | γ-Aminobutyric acid (GABA) B receptor 1 | NM_031028.3 | |
| Actb | Rn00667869_m1 | Actin-β | NM_031144.2 | Actx |
| Gapdh | Rn01775763_g1 | Glyceraldehyde-3-phosphate dehydrogenase | NM_017008.3 | Gapd |
Table S2.
The mean of target gene CT value in the hypothalamus, hippocampus and amygdala
| Gene | Female | Male | ||||
| Hypothalamus | Hippocampus | Amygdala | Hypothalamus | Hippocampus | Amygdala | |
| Ar | 28.65 | 29.71 | 28.31 | 28.83 | 29.58 | 30.16 |
| Esr1 | 29.29 | 33.93 | 31.63 | 30.79 | 34.43 | 32.38 |
| Esr2 | 31.54 | 34.52 | 32.71 | 32.44 | 35.63 | 33.23 |
| Gper1 | 30.89 | 32.17 | 30.57 | 30.65 | 31.66 | 31.53 |
| Htr1a | NA | 27.24 | 28.73 | NA | 27.97 | 29.95 |
| Htr2c | NA | 30.81 | 28.64 | NA | 32.42 | 28.30 |
| Gad1 | NA | 28.52 | 27.93 | NA | 28.67 | 25.72 |
| Gad2 | NA | 26.22 | 25.19 | NA | 26.57 | 27.48 |
| Gabbr1 | NA | 24.94 | 25.53 | NA | 25.23 | 25.63 |
| Actb | 20.65 | 20.71 | 20.12 | 22.10 | 21.79 | 22.82 |
| Gapdh | 19.93 | 20.30 | 19.87 | 21.36 | 21.35 | 21.89 |
NA, not applicable.
Experiment 3.
Animals and brain cannula surgery.
Adult female Wistar rats (7–8 wk of age) (Charles River) were housed in individual cages in a 12-h light/dark cycle with free access to chow and water. Brain cannula surgery was performed as described in ref. 15.
Behavioral testing and intra-amygdala microinjections.
Details of intra-amygdala microinjection of testosterone including dosing and behavioral testing is described in SI Materials and Methods.
Experiment 4.
Adult female Wistar rats (8–9 wk of age) were treated with daily subcutaneous injection of: (i) vehicle (n = 12); (ii) T 0.5 mg⋅kg⋅d (n = 14). After 4 d animals were tested in the diestrus phase for 5 min on the EPM and again 8 d of treatment on the EPM and in the open field, followed by dissection of amygdala and mRNA expression analyses.
Statistical Analysis.
All data are presented as mean ± SEM. The Kruskal–Wallis test followed by Mann–Whitney U test or one-way ANOVA followed by Dunnett’s post hoc test was used in Exp. 1 and 2. Two-way ANOVA was used to analyze behavior in Exp. 2. The independent-samples t test was used for group comparisons of behavioral activity and relative gene expression, one-way ANOVA repeated measurement was used for body weight change and food intake in Exp. 3 and 4 (SPSS version 21.0; SPSS, and Prism GraphPad version 6.0, GraphPad Software). P values lower than 0.05 were considered statistically significant.
SI Materials and Methods
Animal Ethics.
All studies were carried out with ethical permissions from the Animal Ethics Committee of the University of Gothenburg (Ethical no: 53-2013 and 195-2013), in accordance with legal requirements of the European Community (Decree 86/609/EEC). All efforts were made to minimize suffering.
Drugs.
Testosterone propionate (T, T1875), flutamide (an AR antagonist; Flut, F9397-1G) and tamoxifen (SERM; Tam, T5648-1G) were purchased from Sigma. In Exp. 1, all injected substances were dissolved in a 2:1 mixture of sesame oil (S3547) and benzyl benzoate (B6630). In Exp. 2, DMSO (D2650-5) was used as the vehicle and solvent for testosterone for central microinjection. All vehicles were purchased from Sigma.
Experiments 1 and 2.
PNA treatment.
The testosterone dose used for PNA treatment has previously been shown to have a masculinizing effect on the offspring (39) and to mimic the human PCOS from a maternal perspective (13, 39).
Experiment 1.
Maternal and placenta characteristics.
On GD21, dams were anesthetized with thiobutabarbital sodium (130 mg/kg, i.p., Inactin; Sigma) and killed, and maternal and placental samples were collected. Blood from dams was obtained by heart puncture and centrifuged, and the serum was frozen at –80 °C for subsequent measurement of sex steroids. Placentas were dissected and cleaned to remove umbilical cord, and weighed. Placentas were washed in cold Tris-saline buffer, placed in buffer D containing 10 mM Tris-Hepes, 250 mM sucrose, 1 mM EDTA, protease, and phosphatase inhibitors (P8340, P5726, and P0044; Sigma), and homogenized on ice with a Polytron. Homogenates were snap-frozen and stored at –80 °C until Western blot analyses.
Protein preparation.
Homogenates from rat placentas were centrifuged at 10,000 × g for 15 min to remove debris. Supernatants were collected, and protein concentration was determined with a spectrometer (Direct Detect).
Western blot.
For Western blot analyses, we used antibodies against total STAT3 (#9139, Cell Signaling) and phosphorylated (P) fractions of STAT3 (Tyr-705; #9145, Cell Signaling). Protein signal intensity was quantified by densitometry with MultiGauge Software v3.0 or Image Lab Software (Bio-Rad). β-actin was used as a loading control and for normalization. For each protein target, all individual density values for controls and treated subjects were expressed relative to the mean density of the controls.
Experiment 2.
Phenotyping of female offspring.
Euglycemic-hyperinsulinemic clamp.
Insulin sensitivity was evaluated by euglycemic-hyperinsulinemic clamp in female and male PNA offspring. Briefly, rats were anesthetized with thiobutabarbital sodium (Inactin; Sigma). Insulin (Actrapid; Novo Nordisk) diluted in 10 mL of saline plus 0.2 mL of albumin was infused at 8 mU⋅min·kg. Plasma glucose was analyzed every 5 min with a OneTouch Ultra 2 Meter (LifeScan) and maintained at 6.0 mM by administration of 20% (wt/vol) glucose in saline. At steady-state, 50-µL blood samples were taken to determine plasma insulin concentrations (Mercodia). The mean glucose infusion rate was normalized to body weight. An insulin sensitivity index (M/Iclamp) was calculated by the mean glucose infusion rate/plasma insulin levels at steady-state.
Ovarian morphology.
Ovaries were excised, fixed in neutral buffered 4% formaldehyde for 24 h, placed in 70% ethanol, dehydrated, and embedded in paraffin. For assessment of ovarian morphology, two 4-μm sections of each ovary were taken 40 μm apart at the largest diameter, mounted on a glass slide, stained with H&E, and analyzed by conventional light microscopy.
Behavioral testing.
Anxiety-like behavior and locomotor activity testing was carried out at 53–59 d of age. Female offspring were tested in the diestrus phase of the ovarian cycle, which was confirmed by vaginal smears.
Elevated plus maze.
To investigate the presence of anxiety-like behavior in male and female offspring of PCOS dams, the EPM test was performed. The EPM is a well-established rodent model used to characterize anxiety-like behavior. The maze is comprised of two open and two closed arms (Med Associates). The rats were placed in the junction area and their movements were measured for 5 min using infrared beams installed on each arm and automatically registered by the MED_PC software (Med Associates) for further analysis.
Locomotor activity was tested immediately after the EPM for 30 min in photo-cell equipped activity boxes (Kungsbacka Mätoch Reglerteknik).
Tissue collection and sex-steroid measurements.
In diestrus phase, the hypothalamus, hippocampus, and amygdala were quickly dissected on ice using a brain matrix, the tissue was then frozen in liquid nitrogen and stored in −80 °C for mRNA expression analyses after performing euglycemic-hyperinsulinmic clamp. Adrenal gland was dissected and weighed. Blood was collected to assess corticosterone and a comprehensive sex-steroid profile in serum in diestrus phase after the last behavior test. Circulating corticosterone was analyzed by ELISA (Catalog #80554, Chrystal Chem). Sex-steroids: E2, estrone, testosterone, DHT, progesterone, and androstenedione were analyzed by using the highly sensitive GC-MS/MS method recently evaluated in rodents (40). The limit of quantitation defined as lowest level that can be detected with a CV < 20% for E2, estrone, testosterone, DHT, progesterone, and androstenedione were 0.5, 0.5, 8, 2.5, 74, and 12 pg/mL, respectively.
RNA isolation and mRNA expression.
Individual samples were homogenized in Qiazol lysis reagent (Qiagen) using TissueLyzer (Qiagen). Total RNA was extracted with RNeasy Lipid Tissue Mini Kit (Qiagen) according to the manufacturer’s protocol. RNA quantification and quality were assessed by spectrophotometric measurements (Nanodrop 1000, NanoDrop Technologies) and labchip microfluidic technology (Experion automated electrophoresis system; Bio-Rad). cDNA synthesis was carried out using the iScript cDNA synthesis kit (Bio-Rad). Quantitative real-time PCR was performed using TaqMan Custom Arrays (Applied Biosystems). TaqMan probe sets for target genes and reference genes were chosen from the online catalog (Table S1). The arrays were run according to the manufacturer’s protocol with Biomek FX robot and QuantStudio 12K Flex Real-Time PCR System (Applied Biosystem).
The relative gene expression was measured using the comparative critical threshold (Ct) method (Table S2). Gapdh and Actb were used as endogenous controls and identified by ExpressionSuite Software v1.0.4 (Life Technologies).
Experiment 3.
Brain cannula surgery.
Adult female rats, age 7–8 wk old, were anesthetized with a mixture of ketamine (90 mg/kg) and xylazine (2.7 mg/kg) and implanted with a guide cannula targeting the amygdala (26 gauge; Plastics One). The following coordinates were chosen for the amygdala: −2.0 mm posterior to bregma, ±4.2 mm from the midline, and −7.2 mm from the skull surface on which it was based. Cannulae were attached to the skull with dental acrylic and jeweler’s screws and closed with an obturator. Cannula placement was verified histologically postmortem by injection of 0.5 μL of India ink (volume matched drug delivery in the experiments). Rats whose dye injections were not located in the amygdala were excluded from the data analysis.
Behavioral testing.
Elevated plus maze.
All behavioral tests were performed in the diestrus phase at 9–10 wk of age. One hour and 24 h after the first intra-amygdala microinjection of testosterone (10 µg/0.5 µL) (n = 13) or vehicle (0.5 µL DMSO) (n = 12), animals were tested for 5 min on the EPM. After the first testing day, microinjections of testosterone (10 µg/0.5 µL) or vehicle were repeated daily until the following diestrus phase (∼4–5 d), behavioral testing was then repeated. During the second estrus cycle, the dose of daily microinjections was increased to 20 µg/0.5 µL testosterone followed by behavioral testing in the following diestrus phase.
Open-field test.
Immediately following the EPM the rats were tested in an open-field arena for 30 min (MED-OFA-RS, Med Associates). Animals were placed in the center of an open field and exploration was assessed for 30 min. The dimensions of the arena were 40 cm × 40 cm, of which the peripheral 10 cm were considered as the peripheral zone and the central 20 cm were considered as the central zone. The open-field test was repeated during the following diestrus phase subsequent to EPM testing.
Results for Experiment 2.
Phenotyping of female offspring.
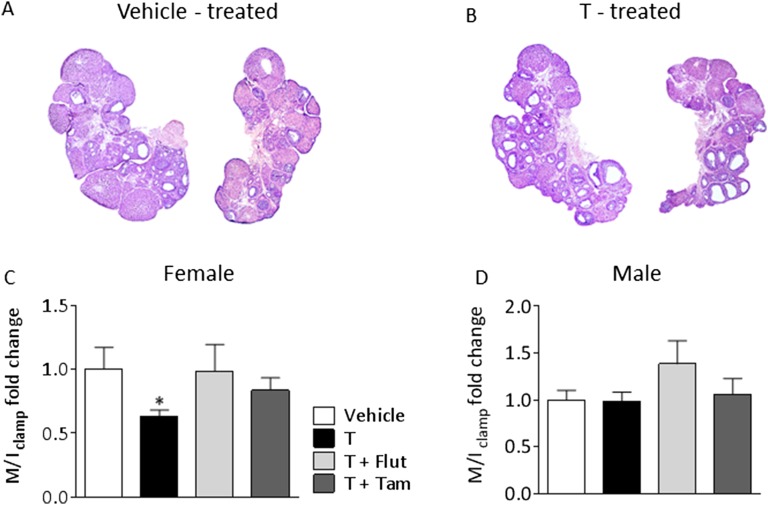
Female offspring of T-treated dams had irregular cycles (vehicle 3.90 ± 0.10 vs. testosterone 4.33 ± 0.14, P = 0.022) and polycystic ovary morphology (Fig. S2 A and B). Circulating E2, estrone, testosterone, DHT, progesterone, and androstenedione did not differ between the groups with one exception; DHT was lower in female PNA offspring receiving AR blocker (Table S4). Female, but not male, offspring of T-treated dams were insulin-resistant, as measured by euglycemic hyperinsulinemic clamp compared with vehicle offspring (Fig. S2 C and D). Thus, the model mimics the human PCOS, and reproduces the irregular cycles and PCO morphology found in PCOS patients.
Fig. S2.
Ovarian morphology and insulin sensitivity of offspring. (A) Two representative pictures of ovaries from vehicle-treated and (B) two from T-treated offspring. The 4-μm sections of each ovary were taken 40 μm apart at the largest diameter, mounted on a glass slide, stained with H&E, and analyzed by conventional light microscopy (5× magnification). The insulin sensitivity index (M/I clamp) in (C) female and (D) male offspring of T-treated dams obtained during the euglycemic-hyperinsulinemic clamp. Values represent means ± SEM, *P < 0.05 vs. vehicle analyzed by Student t test. (Graphpad prism 6). Females: vehicle, n = 6; T, n = 9; T+Flut, n = 8; T+Tam, n = 11, and males vehicle, n = 10; T, n = 9; T+Flut, n = 9; T+Tam, n = 9.
Table S4.
Circulating sex steroid in female PNA offspring collected in diestrus phase
| Steroid | Vehicle (n = 5) | T (n = 7) | T + Flut (n = 6) | T + Tam (n = 6) | P value | ||
| T vs. vehicle | T+Flut vs. vehicle | T+Tam vs. vehicle | |||||
| Estrone (pg/mL) | 2.86 ± 0.79 | 2.30 ± 0.23 | 2.07 ± 0.30 | 3.55 ± 1.13 | 0.884 | 0.761 | 0.824 |
| Estradiol (pg/mL) | 5.62 ± 1.99 | 3.53 ± 0.38 | 3.40 ± 0.44 | 5.92 ± 2.33 | 0.617 | 0.599 | 0.998 |
| Progesterone (ng/mL) | 18.54 ± 1.57 | 19.14 ± 0.78 | 24.93 ± 1.70 | 17.36 ± 4.23 | 0.996 | 0.202 | 0.974 |
| Testosterone (pg/mL) | 51.64 ± 8.72 | 33.34 ± 2.76 | 41.30 ± 7.38 | 44.55 ± 13.99 | 0.347 | 0.759 | 0.900 |
| Androstenedione (pg/mL) | 197.60 ± 30.78 | 108.43 ± 11.11 | 144.67 ± 18.51 | 161.17 ± 40.86 | 0.072 | 0.401 | 0.668 |
| DHT (pg/mL) | 13.46 ± 1.02 | 10.90 ± 0.83 | 7.68 ± 0.88* | 11.08 ± 1.89 | 0.343 | 0.013 | 0.424 |
Values are mean ± SEM; *significant differences (P < 0.05). P values were determined with one-way ANOVA followed by Dunnett’s post hoc test. Bold entries indicate significant values.
Postnatal body weight, food intake, adrenal weight, and circulating corticosterone.
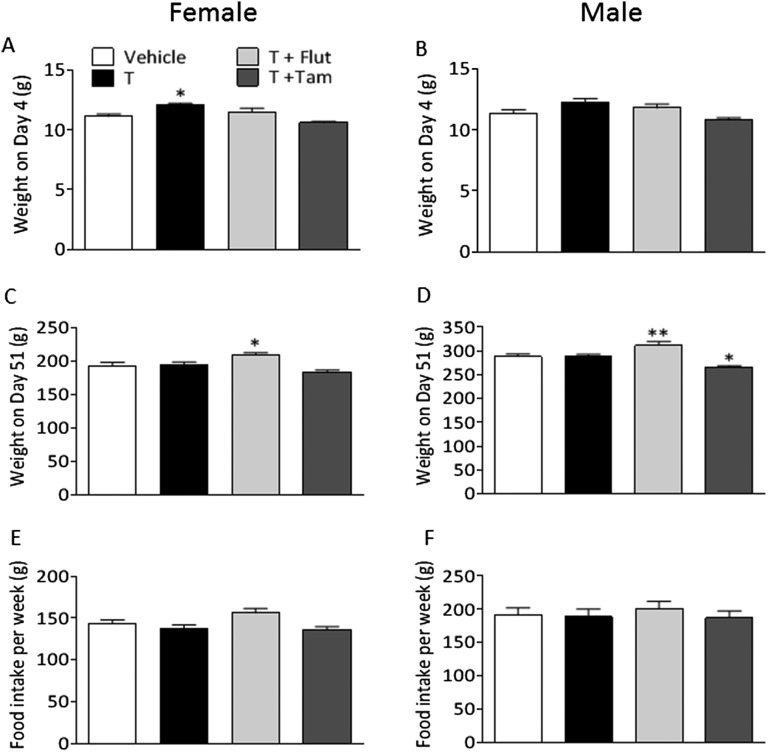
Female PNA offspring had an increased body weight 4 d after birth with no difference in male offspring (Fig. S3 A and B). However, at the time of behavioral testing, female and male offspring of T-treated dams did not differ in body weight compared with the vehicle-treated group (Fig. S3 C and D). Both female and male offspring of T+Flut-treated dams weighed significantly more than the vehicle-treated group (Fig. S3 C and D). Male offspring of T+Tam-treated dams weighed significantly less than vehicle-treated pups (Fig. S3D). Food intake did not differ between the groups (Fig. S3 E and F).
Fig. S3.
Body weight and food intake of female and male offspring of T-treated dams. Body weight on day 4 in (A) female and (B) male offspring; body weight on day 51 before behavioral tests in (C) female and (D) male offspring. (E) Female and (F) male offspring food intake measured weekly per rat from weaning until the behavioral test. Values are means ± SEM; *P < 0.05, **P < 0.01, vs. vehicle, analyzed by one-way ANOVA followed by Dunnett’s post hoc test. Females: vehicle, n = 9; T, n = 9; T+Flut, n = 8; T+Tam, n = 9; and males: vehicle, n = 12; T, n = 12; T+Flut, n = 10; T+Tam, n = 10.
Adrenal weight did not differ between the groups in female and male PNA offspring: female vehicle 43.4 ± 2.30 vs. T 46.0 ± 3.23, (P = 0.541); male vehicle 28.6 ± 1.89 vs. T 33.5 ± 2.56, (P = 0.132). Similarly, circulating corticosterone levels were not different in female and male offspring: female vehicle 129.9 ± 24.81 vs. T 89.68 ± 14.15 (P = 0.178); male vehicle 47.46 ± 14.99 vs. T 85.26 ± 15.36 (P = 0.097).
Anxiety-like behavior (continued).
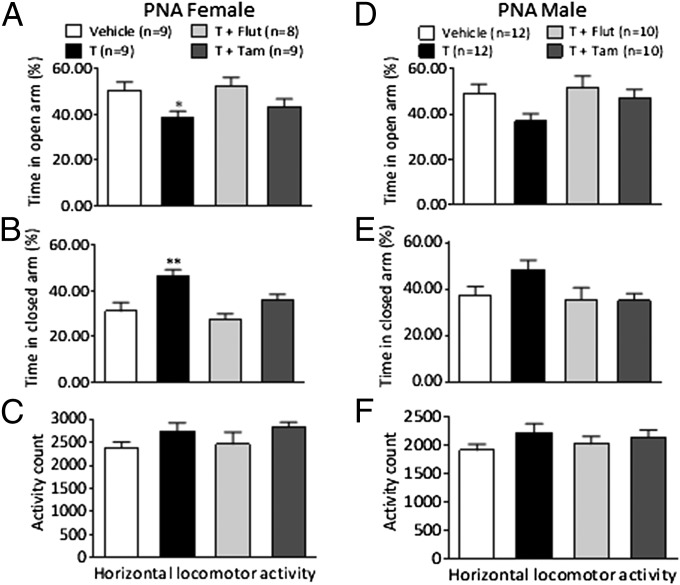
When data from male and female offspring are analyzed together by two-way ANOVA (sex and treatment) there was a main effect of treatment on both time in the open and closed arm (F3,71 = 5.270, P = 0.002; F3,71 = 6.539, P = 0.001, respectively) but no effect for sex (F1,71 = 0.001, P = 0.971; F1,71 = 1.953, P = 0.167, respectively), and no interaction between the two factors (F3,71 = 0.209, P = 0.890; F3,71 = 0.495, P = 0.687, respectively). Post hoc Dunnett’s tests indicated the offspring spend less time in the open arm (P = 0.006) and more time in the closed arm (P = 0.003). This effect was reversed by the AR blockade (time in open arm and closed arm P = 0.883 and P = 0.838, respectively) and the SERM (time in open arm and closed arm P = 0.547 and P = 0.992, respectively). Thus, the PNA effect on anxiety may not be restricted to female offspring. The total number of open-arm entries were not different (female: vehicle 15.33 ± 2.87 vs. T 13.89 ± 2.47; male: vehicle 11.00 ± 1.27 vs. T 14.17 ± 2.45).
Gene-expression analysis.
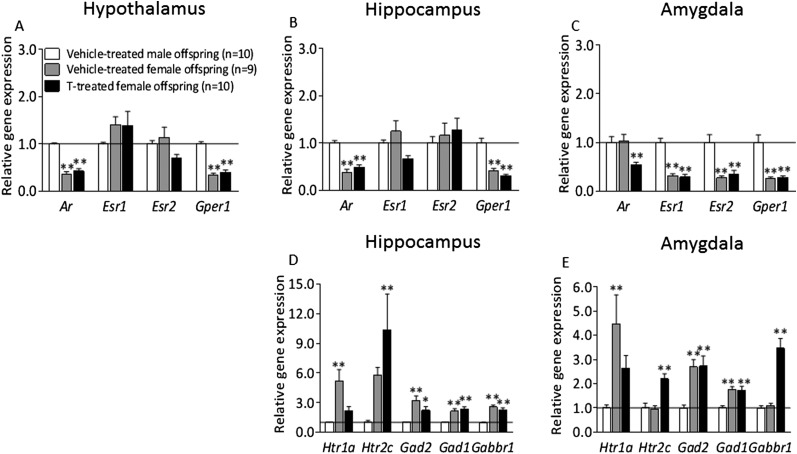
To address whether prenatal testosterone masculinizes the female brain, vehicle-treated male offspring was compared with vehicle-treated females. The expression of Htr1a in hippocampus and amygdala was higher in vehicle-treated females compared with vehicle-treated male offspring, whereas the expression in T-treated female offspring did not differ from vehicle-treated male offspring (Fig. S4 D and E). These data indicate that the maternal testosterone dose used masculinizes the brain in female offspring. The Ar and Gper1 mRNA expression in the hypothalamus, hippocampus and amygdala in vehicle-treated females differed from vehicle-treated male offspring and T-treated female offspring followed the same pattern as control females (Fig. 4 A–C).
Fig. S4.
Gene expression of androgen and estrogen receptors in the (A) hypothalamus, (B) hippocampus, and (C) amygdala in vehicle-treated male and female offspring and T-treated female offspring. Expression of serotonergic and GABAergic genes in the amygdala (D) and hippocampus (E) and T-treated female offspring. The gene expression was quantified by quantitative RT-PCR relative to the housekeeping genes β-actin and Gapdh. Results represent relative gene expression in vehicle-treated male offspring compared with T-treated female rats. Values are means ± SEM; *P < 0.05; **P < 0.01 vs. the vehicle-treated male group analyzed using one-way ANOVA followed by Dunnett’s post hoc test.
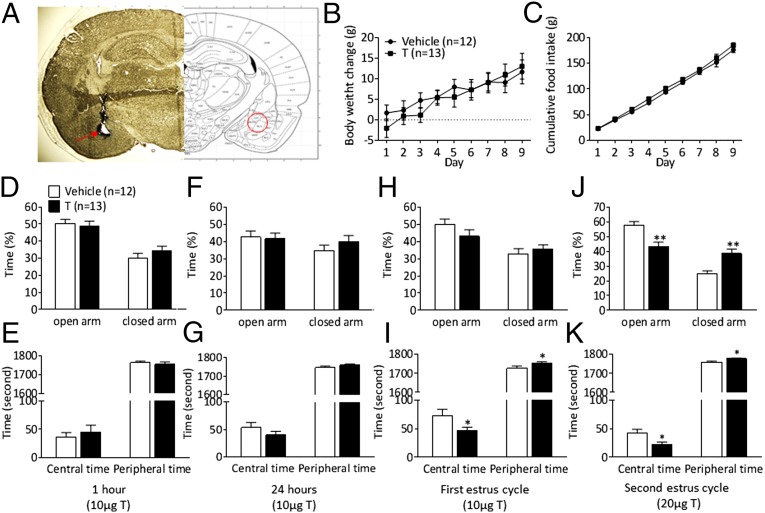
Fig. 4.
Intra-amygdala testosterone application produces anxiety-like behavior in adult females (Exp. 3). EPM and open-field test were performed after subchronic daily testosterone microinjections into the amygdala during two estrus cycles. (A) Histological verification of the location of the cannula in the amygdala. (Right) Photomicrograph of a 40-μm coronal section of a rat brain at the level of bregma −2.80 mm (i.e., microinjection site). (Left) Schematic representation of the amygdala. Body weight change (B) and cumulative daily food intake (C) during microinjections of testosterone or vehicle into the amygdala. EPM and open-field test 1 h (D and E) and 24 h (F and G) after vehicle (n = 12) or testosterone (n = 13) microinjections. The EPM and open-field test during the first (H and I) and second diestrus phase (J and K). Time spent in the open and closed arms during the EPM test (D, F, H, J). Time spent in central and peripheral area in the open field test (E, G, I, K). Values are expressed as means ± SEM; *P < 0.05, **P < 0.01 vs. the vehicle-treated group.
Results
Experiment 1.
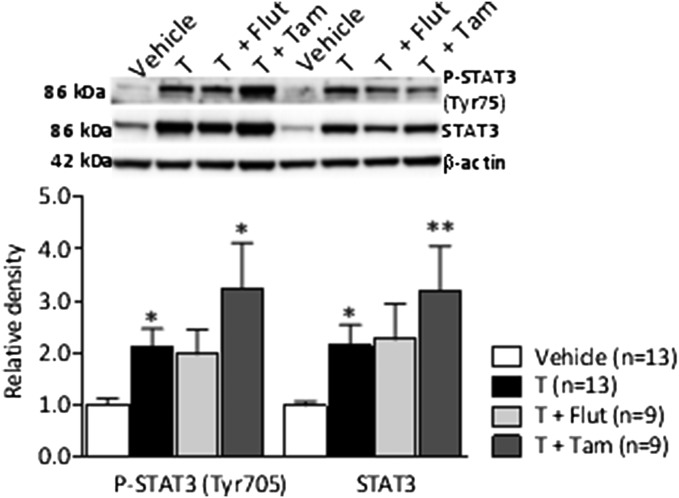
Testosterone-treated pregnant dams had elevated circulating testosterone and DHT and decreased placental weight (Table S3). Placentas from testosterone-treated dams displayed the same pattern as in women with PCOS characterized by increased phosphorylation and total STAT3 protein expression compared with vehicle-treated dams (Fig. S1). Tamoxifen further increased pSTAT3 and STAT3. Thus, the animal model used here displays a lean PCOS-like feature from a maternal and placental perspective.
Table S3.
Maternal circulating sex steroids and placental characteristics
| Sex steroids and plcacental characteristics | Vehicle (n = 13) | T (n = 13) | T+Flut (n = 10) | T+Tam (n = 9) | P value | ||
| T vs. vehicle | T+Flut vs. vehicle | T+Tam vs. vehicle | |||||
| Sex steroids | |||||||
| Estrone (pg/mL) | 43.06 ± 11.19 | 34.14 ± 5.50 | 44.34 ± 4.80 | 45.44 ± 5.12 | 0.607 | 0.315 | 0.258 |
| Estradiol (pg/mL) | 26.12 ± 5.19 | 24.24 ± 3.32 | 28.51 ± 2.28 | 30.40 ± 2.64 | 0.689 | 0.211 | 0.161 |
| Testosterone (pg/mL) | 268.68 ± 76.50 | 727.24 ± 64.55* | 734.61 ± 73.24* | 927.70 ± 132.15* | 0.005 | 0.001 | <0.001 |
| Androstenedione (pg/mL) | 495.31 ± 81.24 | 370.40 ± 52.66 | 437.14 ± 63.06 | 442.88 ± 51.79 | 0.456 | 0.842 | 0.796 |
| DHT (ng/mL) | 10.63 ± 1.83 | 18.40 ± 1.65* | 25.94 ± 4.61* | 23.23 ± 5.04* | 0.018 | 0.001 | 0.040 |
| Placental characteristics | |||||||
| Placental weight (g) | 0.60 ± 0.02 | 0.53 ± 0.02* | 0.56 ± 0.01 | 0.65 ± 0.03 | 0.029 | 0.115 | 0.324 |
| Fetal/placental weight ratio | 7.37 ± 0.35 | 7.83 ± 0.30 | 7.19 ± 0.25 | 7.07 ± 0.42 | 0.125 | 1.000 | 0.601 |
Values are mean ± SEM; *significant differences (P < 0.05). P values were determined with the nonparametric Kruskal–Wallis test followed by Mann–Whitney U test. Bold entries indicate significant values.
Fig. S1.
Phosphorylation and total protein expression of placental signal transducer and activator of transcription 3 (STAT3) (Tyr-705). Values represent mean ± SEM; *P < 0.05, **P < 0.01, vs. vehicle calculated by Kruskal–Wallis followed by Mann–Whitney U test.
Experiment 2.
Phenotyping of female offspring.
The phenotypic presentation of female offspring of T-treated dams is presented in SI Materials and Methods, with Fig. S2 and Table S4 demonstrating that the model, in part, mimics human PCOS, and reproduces the irregular cycles and PCO morphology, albeit not elevated circulating testosterone.
Postnatal body weight and food intake.
Body weight development and food intake of female and male offspring of T-treated dams is presented in Fig. S3.
Anxiety-like behavior.
Female offspring of T-treated dams spent significantly less time in the open arm (P < 0.05) and more time in the closed arm (P < 0.01) in the EPM compared with vehicle-treated animals (Fig. 1 A and B). This behavior was reversed by AR blockade and selective ER modulator (SERM) because time spent in open and closed arms in T+Flut and T+Tam-treated rats did not differ from vehicle-treated female rats (Fig. 1 A and B). These effects were observed without changes in total horizontal locomotor activity (Fig. 1C). One-way ANOVA indicated no significant changes in behavior in male PNA rats (Fig. 1 D–F). However, as shown in detail in SI Materials and Methods, when data from male and female offspring are analyzed together by two-way ANOVA (sex and treatment), there was a main effect of treatment on both time in the open and closed arm and this effect was reversed by the AR blockade and the SERM. Thus, the PNA effect on anxiety may not be restricted to female offspring.
Fig. 1.
Anxiety-like behavior in female and male offspring of T-treated dams (Exp. 2). Time (%) spent in (A and D) open arms and (B and E) closed arms in the EPM in female offspring (A and B) and male offspring (D and E). Horizontal locomotor activity in females (C) and males (F). Values expressed as means ± SEM; *P < 0.05, **P < 0.01 compared with vehicle-treated group.
Gene expression analysis.
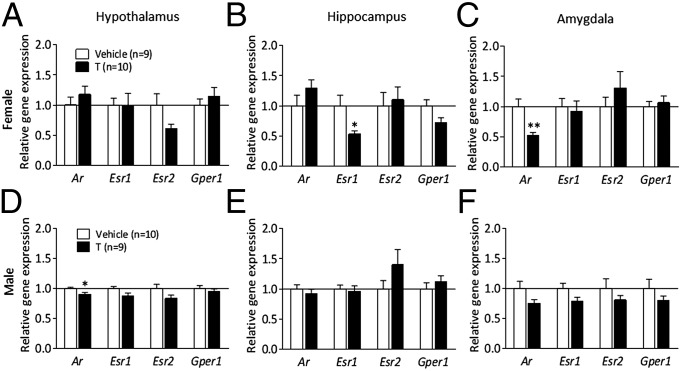
Because of anxiety-like behavior exhibited by female and possibly male PNA offspring, we analyzed gene expression in the hypothalamus, hippocampus, and amygdala, three important areas in the regulation of anxiety-like behavior, as gene expression of AR and ER in these brain nuclei may play an important role in emotionality and energy balance regulation. No significant differences were found in the mRNA expression of Ar, Esr1 (ERα), Esr2 (ERβ), and Gper1 in the hypothalamus between PNA and vehicle-treated female offspring (Fig. 2A). The hypothalamic mRNA expression of Ar was slightly lower in male PNA offspring than in vehicle-treated (Fig. 2D). There was a 50% reduction of Esr1 mRNA expression in the hippocampus (P < 0.05); in contrast, the expression of Gper1, Ar, and Esr2 were not different in female PNA offspring compared with control animals (Fig. 2B). Ar mRNA expression in the amygdala was decreased by ∼50% in female PNA rats compared with vehicle-treated offspring (P < 0.01), but there were no significant differences in the expression of Esr1, Esr2, or Gper1 compared with vehicle-treated rats (Fig. 2C). There were no differences in the gene expression of AR and ERs in the hippocampus and amygdala of male PNA offspring (Fig. 2 E and F).
Fig. 2.
Gene expression of androgen and estrogen receptors (Exp. 2) in (A) hypothalamus, (B) hippocampus, and (C) amygdala in female PNA offspring, and in (D) hypothalamus, (E) hippocampus, and (F) amygdala in male PNA offspring. Values represent means ± SEM; *P < 0.05, **P < 0.01 vs. the vehicle-treated group.
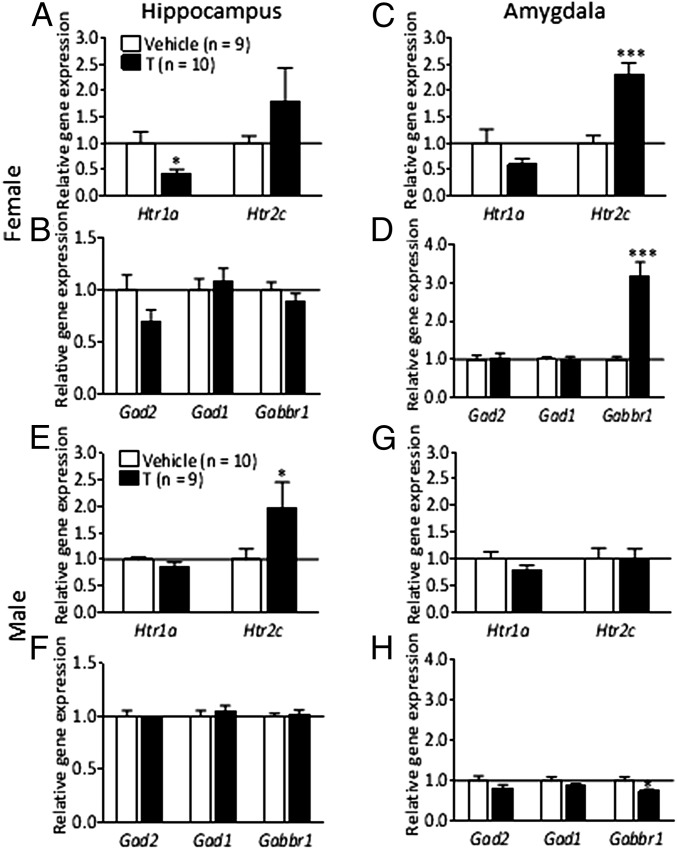
As the initial analysis revealed significant differences in steroid-related gene expression in the amygdala and the hippocampus, we next investigated whether serotonergic and GABAergic receptors and enzymes involved in anxiety-like behavior were affected in these areas in PNA offspring. The mRNA expression of 5-hydroxytryptamine (serotonin) receptor 2C (Htr2c) was more than twofold increased in the amygdala of PNA female rats compared with control rats (P < 0.001), whereas Htr1a did not differ (Fig. 3C). There were no changes in serotonin receptor gene expression in male PNA offspring (Fig. 3G). There was a marked reduction in the expression of Htr1a in the hippocampus (P < 0.05), with no difference in Htr2c expression in female PNA offspring in this brain area (Fig. 3A). There was a pronounced increase in Htr2c expression in the hippocampus of PNA male rats compared with controls (Fig. 3E). Next, we analyzed the expression of genes related to GABAergic signaling and synthesis as they were previously shown to be crucial in the modulation of anxiety behavior. The amygdala and hippocampus mRNA expression of glutamate decarboxylase genes, Gad1 and Gad2, enzymes necessary for the production of the inhibitory neurotransmitter GABA, was not affected by PNA treatment in female (Fig. 3 B and D) or male offspring (Fig. 3 F and H). GABAB receptors have been reported to be involved in the modulation of anxiety-like behavior (16). The Gabbr1 expression was increased threefold in the amygdala of female PNA rats (P < 0.001) (Fig. 3D); in male PNA rats, a much less-pronounced decrease was detected (P < 0.05) (Fig. 3H).
Fig. 3.
Expression of serotonergic and GABAergic genes (Exp. 2) in the hippocampus (A and B) and amygdala (C and D) of female offspring, and in the hippocampus (E and F) and the amygdala (G and H) of male offspring. Values are means ± SEM; *P < 0.05, ***P < 0.001 vs. vehicle-treated group.
In addition, we compared hypothalamus, hippocampus, and amygdala gene expression of vehicle-treated male and female offspring with testosterone-treated female offspring. With this analysis we demonstrate that the expression of a serotonergic receptor, Htr1a, found at higher levels in control female offspring compared with control male offspring, is reduced in females by maternal testosterone treatment, indicating that the maternal testosterone dose used may masculinize the brain in female offspring (Fig. S4 D and E).
Experiment 3.
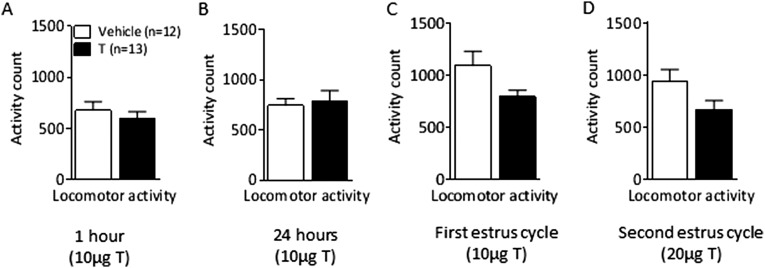
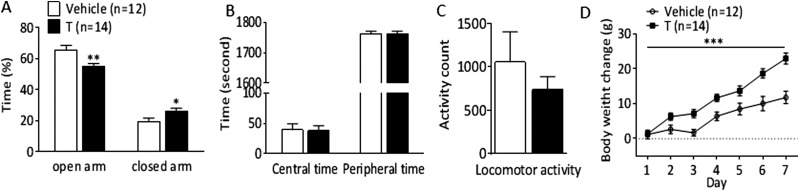
In light of the more pronounced anxiety-like behavior exhibited by female PNA offspring and the critical role that the amygdala plays in the regulation of anxiety combined with the profound changes in Ar, Htr2c, and Gabbr1 expression in this area induced by PNA treatment in females but not males, we set out to determine whether AR activation, specifically in the amygdala, induces anxiety-like behavior in females. Intra-amygdala microinjections of testosterone were centered at the basolateral part of the amygdala (Fig. 4A). There were no changes in anxiety-like behavior 1 and 24 h after intra-amygdala microinjections of 10 µg testosterone (Fig. 4 D–G). Anxiogenic effects of the intra-amygdala testosterone treatment (10 µg daily) emerged after 4–5 d of treatment, and persisted throughout the second estrus cycle (8–10 treatments), where the dose was increased to 20 µg. Intra-amygdala testosterone-treated female rats spent significantly less time in the open arm and longer time in the closed arm of the EPM compared with vehicle-treated rats (Fig. 4J). In the open-field test, testosterone-treated rats spent significantly less time in the central area and more time in the periphery than control females (Fig. 4 I and K), without differences in locomotor activity (Fig. S5 A–D). There was no difference in body weight gain or food intake between control and testosterone-treated females receiving daily microinjections into the amygdala (Fig. 4 B and C). The intra-amygdala testosterone injection in adult female rats did not significantly affect the cycle length.
Fig. S5.
Locomotor activity after microinjection of testosterone into the amygdala in adult females. (A) Locomotor activity count 1 h, (B) 24 h, (C) during the first and (D) during second diestrus phase. Values are means ± SEM, T-injected vs. vehicle-treated group analyzed by Student t test.
Experiment 4.
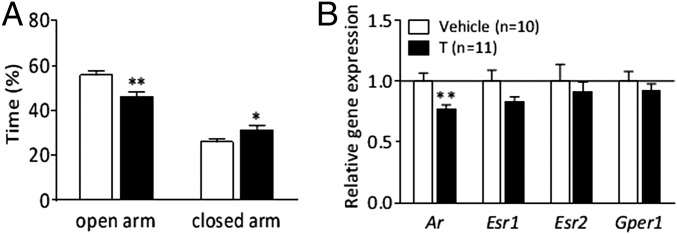
To link the anxiety-like behavior in female PNA offspring and in adult females exposed for intra-amygdala testosterone, we further investigated whether adult testosterone exposure alters amygdala AR expression. Adult female rats were subcutaneously injected with testosterone for 8 d. Testosterone-treated female rats spent significantly less time in the open arm (P < 0.01), and longer time in the closed arm (P < 0.05), with no differences in locomotor activity (Fig. 5A and Fig. S6 A–C). The Ar mRNA expression in the amygdala was decreased by ∼30% in testosterone-treated adult females compared with controls (P < 0.01), with no differences in Esr1, Esr2, or Gper1 expression (Fig. 5D). Testosterone-treated females had a disrupted estrus cycle after 4–5 d of testosterone exposure and gained more weight than controls during the treatment (Fig. S6D).
Fig. 5.
Anxiety-like behavior and amygdala steroid receptor expression in adult female rats treated with 0.5 mg⋅kg⋅d testosterone subcutaneously during 8 d (Exp. 4). Time (%) spent in open arms and closed arms in the EPM at (A) second diestrus phase. Gene expression of androgen and estrogen receptors (B). Values expressed as means ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001 compared with vehicle-treated group. Ar, androgen receptor; Esr1, ERα; Esr2, ERβ; Gper1, G protein-coupled estrogen receptor 1; T, testosterone.
Fig. S6.
Anxiety-like behavior during subcutaneous testosterone (0.5 mg⋅kg⋅d) treatment. Time (%) spent in open arms and closed arms in the EPM at first diestrus phase (A). Time spent in central and peripheral area (B) and locomotor activity (C) in the open-field test at second diestrus phase, and (D) body weight change. Values are means ± SEM; * P < 0.05; **P < 0.01, *** P < 0.001, vs. vehicle-treated group analyzed by Student t-test and repeated measure ANOVA.
Discussion
These findings reveal a brain mechanism potentially underpinning anxiety disorder in women with PCOS. We find that maternal androgen excess results in anxiety-like behavior in female PNA offspring, which is associated with decreased gene expression of Ar and increased expression of GABAergic and serotoninergic receptors in the amygdala, consistent with an increased anxiety-like behavior. We further show that subchronic exposure of adult females to testosterone also reduces amygdala Ar expression. Activation of AR restricted to the amygdala, by intra-amygdala microinjections of testosterone, was sufficient to induce anxiety-like behavior in females, demonstrating a new role for amygdala AR. These experiments suggest that the anxiety-like behavior in female PNA offspring may be mediated by direct effects of testosterone on fetal amygdala. Although no changes in behavior in male PNA rats were observed, when taking sex and treatment into consideration, both female and male PNA offspring display anxiety-like behavior.
Women with PCOS have an increased prevalence of anxiety and depression symptoms (2, 17), which is independent of body mass index. Clinical studies have previously shown that the levels of circulating free testosterone are lower in women with symptoms of depression, but no associations with symptoms of anxiety were observed (18). Although low, rather than high, circulating testosterone levels are associated with more symptoms of depression, testosterone was higher in these individuals than in women without PCOS (18, 19). Importantly, other investigators have reported higher testosterone concentrations in women with severe clinical depression (20), suggesting that testosterone may cause mood disturbances. Furthermore, there seems to be a link between estrogens and symptoms of depression, although no differences were found between serum estradiol levels in women with and without depression (21). Although clinical data are inconsistent, there are indications that androgens play a crucial role in behavior and mood regulation in females. Of note, women with PCOS are not only hyperandrogenic, many are also hyperestrogenic (19). By using testosterone we wanted to closely mimic the elevated testosterone levels in women with PCOS that could potentially result in activation of both androgenic and estrogenic pathways. Although our approach cannot separate the androgenic effect alone, it better represents the pathophysiology of PCOS.
Women with PCOS, and their daughters, have an increased risk of metabolic dysfunction, impaired quality of life, and infertility. Whether these women suffer from psychiatric-ill health is not well established. In an experimental setting, where autonomic activity and mood were assessed in healthy women exposed to testosterone or placebo, women exposed to testosterone exhibited an elevated anxiety-prone response (22). Chronic exposure to testosterone also leads to increased anxiety in female mice, an effect associated with increased levels of corticotropin-releasing factor (23). In the present study, female offspring of dams treated with testosterone during pregnancy, as well as adult females treated with intra-amygdala testosterone, displayed increased anxiety-like behavior. Importantly, the anxiety-like behavior in female PNA offspring was independent of changes in body weight, food intake, and circulating corticosterone levels.
Studies on the link between testosterone and anxiety behavior in males have generated inconsistent results (24, 25). In our study, male PNA offspring displayed anxiety-like behavior pattern similar—although less prominent—to female offspring, mirroring previous human and animal studies (26).
Several possibilities exist for a potential mechanism by which high circulating testosterone in the mother ultimately changes emotionality behavior in the offspring. One possible explanation may be an effect of maternal testosterone exposure on the organization of the brain, which may permanently alter brain morphology and subsequently give rise to altered behavioral responses (27). Another possibility is that high testosterone in the mother alters steroid production in the placenta (12), which may affect the fetus. We found that placental weight was reduced in androgenized rats. The increase in placental total and phosphorylated STAT3 in pregnant rats exposed to testosterone or testosterone + SERM indicate an androgenic effect, which is not mediated by estrogen receptors. The consequences of increased placental STAT3 phosphorylation remain to be established, but may include activation of key placental amino acid transporters (28). It is interesting to note that STAT3 activation is increased in placenta from women with PCOS and in obese women (13). In both conditions the offspring develops insulin resistance and metabolic disturbances in the adult life (4), as in the present study.
If maternal androgen excess with altered placenta function results in offspring with altered testosterone levels, it could be the main culprit behind the increased anxiety. To our knowledge, our study is the first to use the highly sensitive and specific gas chromatography (GC)-MS/MS to analyze circulating sex steroids in female rat PNA offspring. We found no differences between the experimental groups. These results are in line with a recent study in the offspring of female PNA mice that used liquid chromatography tandem (LC)-MS/MS (29). In contrast, higher circulating testosterone has previously been reported in female rat PNA offspring (12). This discrepancy may be a result of the higher doses of maternal testosterone (5 mg) used in the previous study compared with the present study (0.5 mg). The association between circulating testosterone and mood disorders has been suggested to be U-shaped, in which too-high or too-low levels cause behavioral dysfunction (30). Although the anxiety-like behavior observed in the female PNA offspring in the present study cannot be directly explained by high circulating androgens, the reduced AR expression in the amygdala suggests a compensatory response to the high prenatal testosterone exposure, a result implicating the amygdala as the CNS site underlying the changes in anxiety in the PNA offspring. This idea is further strengthened by our experiment showing that subchronic testosterone exposure into amygdala is sufficient to produce anxiety-like behavior in adult females. Furthermore, we also show that a compensatory reduction of amygdala Ar expression can also be driven by exposure of adult females to subchronic peripheral testosterone injections.
Increased anxiety-like behavior in PNA-females was prevented by flutamide or tamoxifen administration, indicating that both AR and ER receptors are involved in mediating the effect of PNA on anxiety. Whether these changes are in part mediated via aromatization of testosterone and therefore an estrogenic action remains to be established. Our findings demonstrate that anxiety-related behavior in female PNA offspring is associated with a decrease in mRNA expression of Esr1 in the hippocampus. This finding is consistent with previous observations where ER antagonists injected into the hippocampus, but not amygdala, increase anxiety-like behavior in female rats (31).
Previous findings have indicated that testicular feminization, through mutations of the AR in male rats, causes anxiety-like behavior, further supporting the involvement of AR in anxiety-like behavior (32). In the present study, Ar expression was decreased in the hypothalamus of male PNA rats, with no changes in the expression of any steroid receptors measured in the amygdala.
In the present study, hypothalamic Ar expression was not altered in female PNA offspring, suggesting AR outside the hypothalamus may underlie emotionality responses. AR are also ubiquitously expressed in the limbic system, including in the amygdala (32). Ar expression was altered only in amygdala of the female PNA rats, indicating that AR in this area are uniquely sensitive to the increased exposure of maternal androgens. These results are in stark contrast to those obtained in males and indicate a differential response to prenatal androgen exposure in male versus female offspring. To understand testosterone’s role in the regulation of anxiety-like behavior, testosterone was microinjected into this brain area and anxiety-like behavior was measured. Females given daily microinjections of testosterone into the amygdala displayed increased anxiety-like behavior in both the EPM and the open-field test. The onset of the anxiogenic effect required several days of treatment, indicating that synthesis of new proteins or even synaptic reorganization may be required for the anxiogenic effect. Of note, peripheral testosterone injections in adult females resulted in anxiety-like behavior already after 4 d of testosterone administration; increased anxiety persisted through the 8 d of treatment. Even in adult females, 8 d of testosterone exposure was sufficient to reduce Ar expression in the amygdala. Thus, both in utero and adult elevation of testosterone can impact on the amygdala and increase anxiety-like behavior.
Central serotonin and GABA signaling are well established for their role in the regulation of anxiety-like behavior, and both may be affected in PNA female offspring. Htr1a and Htr2c are two receptors that are included in a subfamily of serotonin receptors that are associated with mood regulation. Pharmacological blockade of Htr1a receptors can be anxiolytic because antagonists to these receptors have been shown to relieve anxiety and depression (33). Htr2c receptors are distributed throughout the central nervous system and have been linked to several behavioral responses, including depression, anxiety, and feeding behavior (34). In this study we found an increase of ∼50% of Htr2c expression in the amygdala and a reduction of Htr1a expression in the hippocampus. These observations are in line with previous studies demonstrating that activation of the Htr2c and depletion of Htr1a results in anxiety-like behavior in mice (35). Thus, the increase in Htr2c receptor expression in the amygdala of female PNA offspring is highly consistent with an increased anxiety profile. Of note, this was not replicated in males. In fact, in males Htr2c receptor expression was reduced in the hippocampus, a change more in line with an improved emotionality profile (36). That the maternal testosterone dose used can have a masculinizing effect on the female brain is supported by the finding that the amygdala and hippocampus expression of Htr1a is similar in control males and T-treated female offspring, but higher in control females.
Supraphysiological doses of androgens cause anxiety-like behavior in female mice and enhance presynaptic release of GABA (37). Anxiety-related behavior has also been associated with altered GABAergic transmission in the amygdala, including altered expression of GABA-synthesizing enzymes GAD65 (Gad2), GAD67 (Gad1), and GABA receptors. In the present study, amygdala Gabbr1 mRNA expression was elevated in PNA female offspring. Previous studies demonstrated that GABAB receptor activation might provide a useful strategy in the treatment of anxiety-like behavior (16), thus we can only speculate that the change detected here may be in response to lower activity at this receptor in female PNA rats. Alterations in GABAergic receptors may also be expected because testosterone metabolites act as GABA receptor agonists (38). Taken together, these findings indicate that maternal androgen excess may cause anxiety-like behavior in daughters and sons of PCOS mothers, and that it may be because of altered amygdala AR, GABAergic, and serotonergic signaling.
In conclusion, maternal testosterone exposure causes anxiety-like behavior in female, and to a lesser extent male offspring, an effect that seems to occur during fetal life and to be mediated via AR in the amygdala, together with changes in ERα and in the serotonergic and GABAergic pathways in the amygdala and hippocampus of female PNA rats. To our knowledge, this is also the first study to show that testosterone has an anxiogenic role in females at the level of the amygdala. Collectively, our results suggest that maternal androgen excess may contribute to a greater risk of developing anxiety disorders in women with PCOS.
Acknowledgments
We thank the Experimental Biomedicine and Genomics Core Facility at the Sahlgrenska Academy, University of Gothenburg, for animal housing and the use of technical equipment and support. M.M. thanks the Becas Chile Program (Chile) and University of Chile for financial support through a postdoctoral fellowship. The work was supported by the Swedish Medical Research Council Project 2014-2775 (to E.S.-V.) and Project 2014-2945 (to K.P.S.); the Jane and Dan Ohlsson Foundation (E.S.-V.); the Wilhelm and Martina Lundgrens’s Science Fund (E.S.-V. and M.H.); the Hjalmar Svensson Foundation (E.S.-V. and M.H.); the Adlerbert Research Foundation (E.S.-V.); the Swedish federal government under the LUA/ALF agreement ALFGBG-429501 (E.S.-V.); the Novo Nordisk Foundation (E.S.-V. and K.P.S.); the Ragnar Söderberg Foundation (K.P.S.); The Longjiang Scholar of Chair Professorship (E.S.-V. and X.W.); and The Innovative Team of Science and Technology of Heilongjiang Province Universities Project2011TD006 (to M.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1507514112/-/DCSupplemental.
References
- 1.Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet. 2007;370(9588):685–697. doi: 10.1016/S0140-6736(07)61345-2. [DOI] [PubMed] [Google Scholar]
- 2.Dokras A, Clifton S, Futterweit W, Wild R. Increased prevalence of anxiety symptoms in women with polycystic ovary syndrome: Systematic review and meta-analysis. Fertil Steril. 2012;97(1):225-230.e2. doi: 10.1016/j.fertnstert.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 3.Månsson M, et al. Women with polycystic ovary syndrome are often depressed or anxious—A case control study. Psychoneuroendocrinology. 2008;33(8):1132–1138. doi: 10.1016/j.psyneuen.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Coviello AD, Sam S, Legro RS, Dunaif A. High prevalence of metabolic syndrome in first-degree male relatives of women with polycystic ovary syndrome is related to high rates of obesity. J Clin Endocrinol Metab. 2009;94(11):4361–4366. doi: 10.1210/jc.2009-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abbott DH, Tarantal AF, Dumesic DA. Fetal, infant, adolescent and adult phenotypes of polycystic ovary syndrome in prenatally androgenized female rhesus monkeys. Am J Primatol. 2009;71(9):776–784. doi: 10.1002/ajp.20679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chura LR, et al. Organizational effects of fetal testosterone on human corpus callosum size and asymmetry. Psychoneuroendocrinology. 2010;35(1):122–132. doi: 10.1016/j.psyneuen.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Lombardo MV, et al. Fetal programming effects of testosterone on the reward system and behavioral approach tendencies in humans. Biol Psychiatry. 2012;72(10):839–847. doi: 10.1016/j.biopsych.2012.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McEwen BS. Stress, sex, and neural adaptation to a changing environment: Mechanisms of neuronal remodeling. Ann N Y Acad Sci. 2010;1204(Suppl):E38–E59. doi: 10.1111/j.1749-6632.2010.05568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng Y, et al. Effects of androgen and leptin on behavioral and cellular responses in female rats. Horm Behav. 2011;60(4):427–438. doi: 10.1016/j.yhbeh.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Rasgon NL, et al. Depression in women with polycystic ovary syndrome: Clinical and biochemical correlates. J Affect Disord. 2003;74(3):299–304. doi: 10.1016/s0165-0327(02)00117-9. [DOI] [PubMed] [Google Scholar]
- 11.Padmanabhan V, Veiga-Lopez A. Developmental origin of reproductive and metabolic dysfunctions: Androgenic versus estrogenic reprogramming. Semin Reprod Med. 2011;29(3):173–186. doi: 10.1055/s-0031-1275519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun M, et al. Maternal androgen excess reduces placental and fetal weights, increases placental steroidogenesis, and leads to long-term health effects in their female offspring. Am J Physiol Endocrinol Metab. 2012;303(11):E1373–E1385. doi: 10.1152/ajpendo.00421.2012. [DOI] [PubMed] [Google Scholar]
- 13.Maliqueo M, et al. Placental STAT3 signaling is activated in women with polycystic ovary syndrome. Hum Reprod. 2015;30(3):692–700. doi: 10.1093/humrep/deu351. [DOI] [PubMed] [Google Scholar]
- 14.Moore AM, Prescott M, Marshall CJ, Yip SH, Campbell RE. Enhancement of a robust arcuate GABAergic input to gonadotropin-releasing hormone neurons in a model of polycystic ovarian syndrome. Proc Natl Acad Sci USA. 2015;112(2):596–601. doi: 10.1073/pnas.1415038112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderberg R, Anefors C, Bergquist F, Nissbrandt H, Skibicka KP. Dopamine signaling in the amygdala, increased by food ingestion and GLP-1, regulates feeding behavior. Physiology & Behavior. 2014;136:135–144. doi: 10.1016/j.physbeh.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 16.Mombereau C, et al. Genetic and pharmacological evidence of a role for GABA(B) receptors in the modulation of anxiety- and antidepressant-like behavior. Neuropsychopharmacology. 2004;29(6):1050–1062. doi: 10.1038/sj.npp.1300413. [DOI] [PubMed] [Google Scholar]
- 17.Jedel E, et al. Anxiety and depression symptoms in women with polycystic ovary syndrome compared with controls matched for body mass index. Hum Reprod. 2010;25(2):450–456. doi: 10.1093/humrep/dep384. [DOI] [PubMed] [Google Scholar]
- 18.Jedel E, et al. Sex steroids, insulin sensitivity and sympathetic nerve activity in relation to affective symptoms in women with polycystic ovary syndrome. Psychoneuroendocrinology. 2011;36(10):1470–1479. doi: 10.1016/j.psyneuen.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Stener-Victorin E, et al. Are there any sensitive and specific sex steroid markers for polycystic ovary syndrome? J Clin Endocrinol Metab. 2010;95(2):810–819. doi: 10.1210/jc.2009-1908. [DOI] [PubMed] [Google Scholar]
- 20.Weber B, Lewicka S, Deuschle M, Colla M, Heuser I. Testosterone, androstenedione and dihydrotestosterone concentrations are elevated in female patients with major depression. Psychoneuroendocrinology. 2000;25(8):765–771. doi: 10.1016/s0306-4530(00)00023-8. [DOI] [PubMed] [Google Scholar]
- 21.Harlow BL, Wise LA, Otto MW, Soares CN, Cohen LS. Depression and its influence on reproductive endocrine and menstrual cycle markers associated with perimenopause: The Harvard Study of Moods and Cycles. Arch Gen Psychiatry. 2003;60(1):29–36. doi: 10.1001/archpsyc.60.1.29. [DOI] [PubMed] [Google Scholar]
- 22.Hermans EJ, et al. Exogenous testosterone attenuates the integrated central stress response in healthy young women. Psychoneuroendocrinology. 2007;32(8-10):1052–1061. doi: 10.1016/j.psyneuen.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Costine BA, et al. Chronic anabolic androgenic steroid exposure alters corticotropin releasing factor expression and anxiety-like behaviors in the female mouse. Psychoneuroendocrinology. 2010;35(10):1473–1485. doi: 10.1016/j.psyneuen.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frye CA, Edinger KL. Testosterone’s metabolism in the hippocampus may mediate its anti-anxiety effects in male rats. Pharmacol Biochem Behav. 2004;78(3):473–481. doi: 10.1016/j.pbb.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 25.Ambar G, Chiavegatto S. Anabolic-androgenic steroid treatment induces behavioral disinhibition and downregulation of serotonin receptor messenger RNA in the prefrontal cortex and amygdala of male mice. Genes Brain Behav. 2009;8(2):161–173. doi: 10.1111/j.1601-183X.2008.00458.x. [DOI] [PubMed] [Google Scholar]
- 26.Van den Bergh BR, Van Calster B, Smits T, Van Huffel S, Lagae L. Antenatal maternal anxiety is related to HPA-axis dysregulation and self-reported depressive symptoms in adolescence: A prospective study on the fetal origins of depressed mood. Neuropsychopharmacology. 2008;33(3):536–545. doi: 10.1038/sj.npp.1301450. [DOI] [PubMed] [Google Scholar]
- 27.van Wingen GA, et al. Testosterone increases amygdala reactivity in middle-aged women to a young adulthood level. Neuropsychopharmacology. 2009;34(3):539–547. doi: 10.1038/npp.2008.2. [DOI] [PubMed] [Google Scholar]
- 28.Jones HN, et al. High-fat diet before and during pregnancy causes marked up-regulation of placental nutrient transport and fetal overgrowth in C57/BL6 mice. FASEB J. 2009;23(1):271–278. doi: 10.1096/fj.08-116889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caldwell AS, et al. Haplosufficient genomic androgen receptor signaling is adequate to protect female mice from induction of polycystic ovary syndrome features by prenatal hyperandrogenization. Endocrinology. 2015;156(4):1441–1452. doi: 10.1210/en.2014-1887. [DOI] [PubMed] [Google Scholar]
- 30.Rohr UD. The impact of testosterone imbalance on depression and women’s health. Maturitas. 2002;41(Suppl 1):S25–S46. doi: 10.1016/s0378-5122(02)00013-0. [DOI] [PubMed] [Google Scholar]
- 31.Frye CA, Rhodes ME. Enhancing effects of estrogen on inhibitory avoidance performance may be in part independent of intracellular estrogen receptors in the hippocampus. Brain Res. 2002;956(2):285–293. doi: 10.1016/s0006-8993(02)03559-x. [DOI] [PubMed] [Google Scholar]
- 32.Zuloaga DG, Poort JE, Jordan CL, Breedlove SM. Male rats with the testicular feminization mutation of the androgen receptor display elevated anxiety-related behavior and corticosterone response to mild stress. Horm Behav. 2011;60(4):380–388. doi: 10.1016/j.yhbeh.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parks CL, Robinson PS, Sibille E, Shenk T, Toth M. Increased anxiety of mice lacking the serotonin1A receptor. Proc Natl Acad Sci USA. 1998;95(18):10734–10739. doi: 10.1073/pnas.95.18.10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pockros-Burgess LA, Pentkowski NS, Der-Ghazarian T, Neisewander JL. Effects of the 5-HT2C receptor agonist CP809101 in the amygdala on reinstatement of cocaine-seeking behavior and anxiety-like behavior. Int J Neuropsychopharmacol. 2014;17(11):1751–1762. doi: 10.1017/S1461145714000856. [DOI] [PubMed] [Google Scholar]
- 35.Li Q, Luo T, Jiang X, Wang J. Anxiolytic effects of 5-HT₁A receptors and anxiogenic effects of 5-HT₂C receptors in the amygdala of mice. Neuropharmacology. 2012;62(1):474–484. doi: 10.1016/j.neuropharm.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Husain BF, Nanavaty IN, Marathe SV, Rajendran R, Vaidya VA. Hippocampal transcriptional and neurogenic changes evoked by combination yohimbine and imipramine treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2015;61:1–9. doi: 10.1016/j.pnpbp.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 37.Oberlander JG, Henderson LP. Corticotropin-releasing factor modulation of forebrain GABAergic transmission has a pivotal role in the expression of anabolic steroid-induced anxiety in the female mouse. Neuropsychopharmacology. 2012;37(6):1483–1499. doi: 10.1038/npp.2011.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bitran D, Kellogg CK, Hilvers RJ. Treatment with an anabolic-androgenic steroid affects anxiety-related behavior and alters the sensitivity of cortical GABAA receptors in the rat. Horm Behav. 1993;27(4):568–583. doi: 10.1006/hbeh.1993.1041. [DOI] [PubMed] [Google Scholar]
- 39.Sathishkumar K, et al. Prenatal testosterone-induced fetal growth restriction is associated with down-regulation of rat placental amino acid transport. Reprod Biol Endocrin. 2011;9:110. doi: 10.1186/1477-7827-9-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nilsson ME, et al. Measurement of a comprehensive sex steroid profile in rodent serum by high-sensitive gas chromatography-tandem mass spectrometry. Endocrinology. 2015;156(7):2492–2502. doi: 10.1210/en.2014-1890. [DOI] [PubMed] [Google Scholar]