Significance
Sharp-wave–ripple (SPW-R) episodes observed in the electrical activity of mammalian hippocampus are traditionally associated to memory consolidation during sleep but have been recently observed during active behavior. Their involvement in various cognitive functions suggests the existence of SPW-R subtypes engaged in distinct neuronal activity patterns at multiple scales. We use concurrent electrophysiological and functional MRI (fMRI) recordings in macaque monkeys to investigate this hypothesis. We discover several subtypes of SPW-R with distinct electrophysiological properties. Importantly, fMRI recordings reveal differences between the large-scale signatures of SPW-R subtypes, indicating differentiated interactions with neocortex, and contributions of neuromodulatory pathways to the SPW-R phenomenon. Understanding the detailed properties of hippocampal SPW-Rs at multiple scales will provide new insights on the function of memory systems.
Keywords: hippocampus, memory, in vivo electrophysiology, fMRI, local field potential
Abstract
Sharp-wave–ripple (SPW-R) complexes are believed to mediate memory reactivation, transfer, and consolidation. However, their underlying neuronal dynamics at multiple scales remains poorly understood. Using concurrent hippocampal local field potential (LFP) recordings and functional MRI (fMRI), we study local changes in neuronal activity during SPW-R episodes and their brain-wide correlates. Analysis of the temporal alignment between SPW and ripple components reveals well-differentiated SPW-R subtypes in the CA1 LFP. SPW-R–triggered fMRI maps show that ripples aligned to the positive peak of their SPWs have enhanced neocortical metabolic up-regulation. In contrast, ripples occurring at the trough of their SPWs relate to weaker neocortical up-regulation and absent subcortical down-regulation, indicating differentiated involvement of neuromodulatory pathways in the ripple phenomenon mediated by long-range interactions. To our knowledge, this study provides the first evidence for the existence of SPW-R subtypes with differentiated CA1 activity and metabolic correlates in related brain areas, possibly serving different memory functions.
Memory processes require mechanisms for large-scale integration of neuronal activity, in which information processing is precisely coordinated at multiple scales. A prominent example of such phenomenon is the replay of specific sequences of action potentials of hippocampal and neocortical neurons, reflecting previous experiences during wakefulness (1–7). Sharp-wave–ripple (SPW-R) complexes observed in the hippocampal CA1 local field potential (LFP) mark the reactivation of these sequences by the simultaneous occurrence of two distinct but related phenomena: a strong LFP deflection, known as sharp wave (SPW), and a high-frequency oscillation known as ripple (8, 9). SPW-R episodes are thought to reflect brain-wide processes mediating memory consolidation (10–13). However, the large-scale cooperative mechanisms associated to these episodes and their relationship to the observed SPW-R electrical signature remain largely unknown. Investigating this relationship is critical for understanding memory processes at a system level and may provide new insights into the mechanisms of pathological fast ripples observed during epilepsy (14).
Although they were initially thought to occur during slow-wave sleep and quiescence periods, later on SPW-R and sequence replay were also observed during or shortly after active behavior (15–17). Moreover, many SPW-Rs occur at path choice points (18, 19), which are also locations where vicarious trial and error are reported (20). Thus, reactivation of memory sequences during SPW-Rs could provide a convenient mechanism not only for consolidation of long-term memory (4) but also for quickly recalling memories during awake state, serving various cognitive functions (21). The generation of SPW-R complexes in these various contexts likely involves brain-wide network mechanisms, and as a consequence, SPW-R–related brain dynamics may vary, reflecting different types of interactions with cortical and subcortical systems. These interactions may in turn affect the underlying dynamics of hippocampal circuits, thus modifying the observed SPW-R–associated functional activity at multiple scales.
In this work, we investigate how the LFP signature of SPW-R events varies during ongoing activity at a given CA1 recording site, and whether these variations reflect differences in the coordination of neural activity at multiple scales, possibly related to different functions. We test our hypothesis by studying the SPW-R correlates at mesoscopic and macroscopic scales. Specifically, using multisite hippocampal LFP recordings and functional MRI (fMRI) in anesthetized rhesus monkeys (Macaca mulatta), we examine the spatiotemporal properties of SPW-R complexes with a multivariate clustering approach. This approach revealed that recorded multisite SPW-R LFP activity can be classified in four subtypes differing in the temporal SPW-to-ripple coupling and low-frequency SPW pattern. On the one hand, SPW-R–triggered fMRI statistical maps suggest that ripples synchronized to the positive peak of their SPWs mark enhanced neocortical activations. On the other hand, ripples occurring at the trough of their SPWs reveal weak or absent down-regulation in subcortical structures, especially in two neuromodulatory structures: dorsal raphe nucleus and locus coeruleus. Altogether, our results show that hippocampal episodes displaying specific SPW temporal patterns and distinct couplings between SPWs and ripples may instantiate cortico- and subcortico-hippocampal interactions of different nature, possibly associated with specific memory-related functions.
Results
Four Subtypes of SPW-R Complexes.
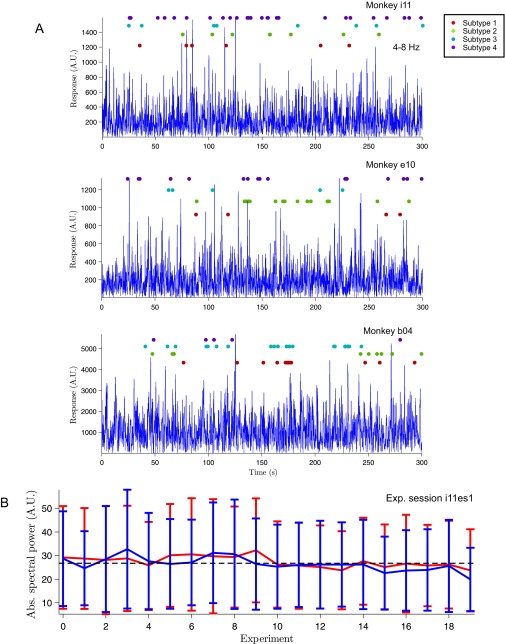
To study the variability of SPW-R complexes, we analyzed extracellular recordings and characterized the temporal and frequency profile of LFPs of the macaque hippocampus. Multicontact recording electrodes were positioned in CA1 on the basis of high-resolution structural MRI scans and on-line tracking of stereotypical hippocampal neuronal response profiles (see Materials and Methods for details). Fig. 1 depicts a schematic representation of the recording configuration and typical electrode penetration (Fig. 1A), together with an example of a typical SPW-R complex signature across multiple electrode tips (Fig. 1 B and C). SPW-R amplitude, duration (∼100 ms) (Fig. 1B), current source density (CSD) profile (Fig. 1D), and spectrogram (Fig. 1E) are consistent with previous studies in macaque monkeys (22). To check the stability of the level of anesthesia, we monitored the power of hippocampal LFP in the theta frequency band (4–8 Hz) (23). Each experiment of a given session was divided into two blocks (5 min each). The distribution of theta power in these two blocks was assessed by randomly choosing time intervals of 1 min (for a total of 50 time intervals) within the block and computing its theta power using Morlet-wavelet spectrograms (see Materials and Methods). Our analysis revealed no significant differences between the first block and second block of each experiment (n = 242 experiments; P > 0.69, paired-samples permutation t test). Theta activity was thus stable across experiments of single-recording sessions, reflecting no putative fluctuations on the anesthesia levels (Fig. S1 A and B).
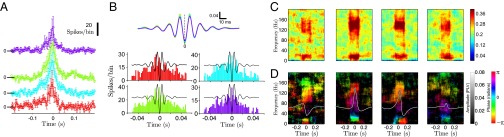
Fig. 1.
Electrode position, recordings in the hippocampus, and SPW-Rs. (A) Schematic representation of our hippocampal recordings. The diagram of the electrode position is superimposed on an Inset of the MRI histology atlas (49) indicating the approximate thickness of the pyramidal cell layer in the CA1 field. (B) Raw multisite LFP traces (0.5–250 Hz). Labels indicate the site targeted by each electrode tip: “sr” for stratum radiatum, “pl” for pyramidal layer. (C) LFP traces depicted in B filtered in the ripple band (80–180 Hz). (D) Averaged current source density (CSD) maps (50) for SPW (Left) and ripple (Right) for a typical experimental session (n = 1,020, monkey i11). In the CSD plots, warm colors (red) indicate sources, whereas cold colors (blue) indicate sinks. (E) Averaged ripple-triggered Morlet-wavelet spectrogram for a typical recording session (n = 1,020, monkey i11).
Fig. S1.
Theta LFP activity during different states. (A) Hilbert-rectified theta (4–8 Hz) frequency band LFP amplitude (blue) with superimposed SPW-R raster plots. Different colors indicate different SPW-R event subtypes as clustered using the procedure showed in Fig. 2. Two anesthetized animals (monkey i11 and monkey e10, Top and Middle) show comparable traces and SPW-R raster plots to one unanesthetized animal (monkey b04, Bottom). (B) Absolute average spectral power in the theta band as computed using wavelet-Morlet spectrograms is stable over time. Only one example experimental session (monkey i11) is shown. Error bars indicate SD.
To study the dynamics of SPW-Rs, we have initially identified candidate events using the process described in ref. 24, coupled with several refinements. Briefly, oscillatory events were initially detected as peaks in the envelope of the broadband LFP (10–250 Hz). Candidate events were clustered on the basis of their spectral signatures, and only events exhibiting increases in their spectra above 80 Hz were identified as ripples. Detection of ripples was further refined by quantifying the Z-scored power in the ripple frequency band (80–180 Hz) and ripple localization in time. Only events satisfying stringent localization and power profile criteria were taken into account for further processing steps (see full procedure in SI Section A).
To quantify the variability in hippocampal LFP during SPW-R events, we first compared their spatiotemporal signature. We asked whether this variability supported the existence of well-differentiated SPW-R event types, possibly reflecting distinct microcircuit dynamics and functional roles. As a preliminary step, we first aligned perievent waveforms with respect to the averaged ripple power peak across recording sites. We next used a two-step procedure to cluster the detected and aligned SPW-R events as illustrated in Fig. S2. The cluster analysis procedure was performed for each experimental session separately. First, we grouped the spatiotemporal SPW-R series into 200 representative signals using a growing neural gas (GNG) algorithm (25). Each representative signal is the average of a group of raw perievent signals with similar time courses (Fig. S2 A and B). It is worth noting that GNG does not extract particular features of the signals but represents them solely based on their corresponding time courses in an unsupervised manner. In the second step (Fig. S2C), we clustered these representative signals based on their cosine similarity using the normalized cuts algorithm (26) (see SI Section A for details).
Fig. S2.
Diagram of the SPW-R clustering methodology. (A) Multichannel, raw perievent SPW-Rs are represented by squares. Groups of contiguous colored squares represent ripple events with similar time courses (Insets). (B) Stage 1 of the clustering procedure builds an intermediate representation of the multichannel data points by averaging over the groups exemplified in A. (C) Stage 2 of the clustering procedure consists in grouping the representative signals into four SPW-R subtypes based on pairwise cosine similarities (see Fig. S3 for clustering quality analysis). Dotted, thin black lines mark the center of each exemplary SPW-R, defined as the peak of power of the ripple-band LFP trace (80–180 Hz).
We applied this two-step algorithm to 12 experimental sessions (a total of ∼11,000 detected SPW-R complexes). The results of the clustering procedure were consistent across all experimental sessions and animals, and did not vary significantly due to recording channel selection (the algorithm was amenable to one or multiple recording channels). However, it was difficult to determine whether SPW-R complexes were truly clustered or they only existed as part of some continuum. To address this question, we devised two procedures: clustering quality analysis, and cluster consistency analysis (see SI Section A for methodological details, and Fig. S3). The first analysis revealed that the population of SPW-R complexes in the CA1 field is best represented by four LFP signatures. Furthermore, the second procedure revealed that clustering consistency across experimental sessions was significantly higher than chance (P < 10−6, t test; mean value with 95% confidence interval for clustering consistency, 74.17% ± 1.63%).
Fig. S3.
Clustering quality measures and 2D projection of SPW-Rs. (A) Normalized cuts index (NCI) for different cluster partitions. The optimal clustering partition is four, corresponding to the maximum NCI. Results are computed for 200 runs of the clustering algorithm across all experimental sessions; shaded area indicates SE across experimental sessions. (B) Four-cluster partition given by the GNG and normalized graph cuts compared with normalized graph cuts alone and to randomly assigned clusters. Clustering quality is evaluated by various measures. (Left) Silhouette index. (Middle) Davies–Bouldin (DB) index. (Right) C index. Asterisks indicate paired significant differences, according to a two-sample Kolmogorov–Smirnov test (KS test). On each box, the top and bottom are the 25th and 75th percentiles of the samples, respectively; the line in the middle of each box is the sample median; the dashed lines extending below and above each box are drawn from the ends of the interquartile ranges to the furthest observation (extreme points); crosses (if any) in the diagrams are the outliers of the samples. (C) Cosine similarity matrix of a representative GNG, sorted according to the cluster labels (1–4) for a single experimental session. Spots that pop-out denote connected (neighboring) SPW-R complexes (GNG representative signals). Note that SPW-Rs of a given subtype remain closely connected to each other, whereas connections between clusters remain very sparse. (D) Two-dimensional projection of the representative GNG in C using Laplacian Eigenmaps. Here, SPW-R complexes (GNG representative signals) are represented by open circles of different colors. Note that clusters remain relatively well separated in the projection, despite the high dimensionality of the real data. Ripple-triggered LFP averages are indicated schematically by arrows. (E) Confusion matrix of the cluster consistency procedure. Note that most of the SPW-Rs are classified consistently across experimental sessions (only test datasets are reported).
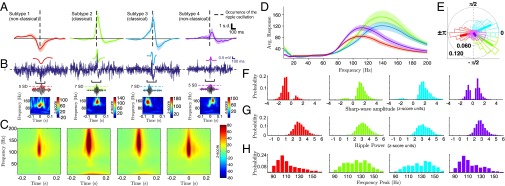
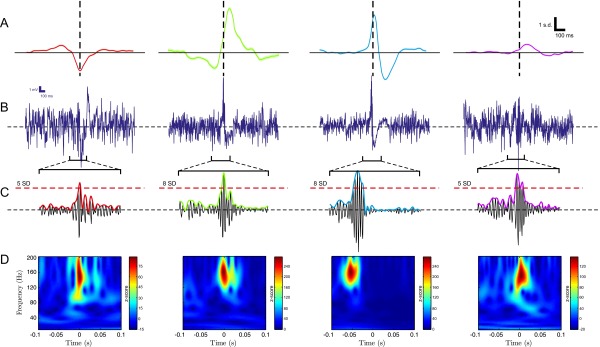
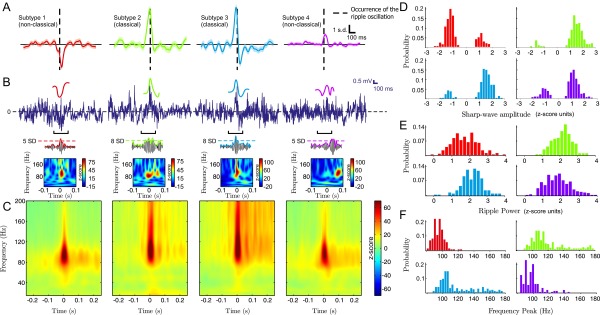
Fig. 2A depicts the SPW-R LFP grand averages of the resulting clusters (n = 12 experimental sessions, 4 animals), showing that ripple oscillations may follow (subtype 1), precede (subtype 2), or be located at the peak of dendritic depolarization (subtype 3). Additionally, ripples may be deprived from a clear SPW depolarization signature (subtype 4). For brevity, we refer to ripple oscillations located near the peak of the SPW (subtypes 2 and 3) as “classical” (SPW-R patterns with the strongest visual resemblance to those first reported in ref. 22). The remaining SPW-Rs (subtypes 1 and 4) will be referred to as “nonclassical.” In correspondence with each SPW-R subtype, typical raw signal traces (0.5–300 Hz) are illustrated in Fig. 2B (Top) (monkey i11), together with their SPW (0–20 Hz) and ripple (80–180 Hz) filtered traces (Fig. 2B, Top and Middle, respectively), a colored dotted line shows the SD threshold for the event in the ripple band. Finally, single-event (broadband) Z-scored Morlet-wavelet spectrograms for every example event are given in Fig. 2B (Bottom), displaying significant and localized ripple-band power increases. We found that results were very consistent from one experimental session to another. As additional evidence, Fig. S4 shows results from another anesthetized experimental session (monkey e10). As previous results have shown, single-event ripple band traces and spectrograms show that nonclassical ripples appear localized in line with our selection criteria (Materials and Methods), and in good agreement with ripple events reported in the literature (9, 27). It is worth noting that each SPW-R subtype occurred concomitantly across all recording sites, displaying little depth-dependent differences. Furthermore, SPW-R episodes usually maintained the same polarity across all channels.
Fig. 2.
Classification of SPW-R complexes across 12 experimental sessions in anesthetized macaque monkeys. (A) Grand averages of ripple power-triggered SPW-R field potential signatures, from one representative stratum radiatum recording site. Occurrence of the ripple oscillation is marked by dashed lines. Shaded areas indicate SEM. (B) Representative single-trial events for each subtype. (Top) SPW and related raw signals of each event subtype (monkey i11). Note that shapes correspond to the average patterns presented in A. (Middle) Filtered, Z-scored LFP in the ripple frequency range (80–180 Hz), illustrating that all SPW-R classes present a significant power increase in this range. Dashed lines indicate ripple amplitude in SD units. (Bottom) Z-scored single-event spectrogram of the broadband signal presented in the top rows. (C) Spectrogram grand averages, in correspondence with each of the SPW-R signatures depicted in A. Averages are computed across all recording sites. (D) Averaged clusterwise spectra. Shaded areas indicate SEM. (E) Phase coupling of the ripple to the SPW of dendritic depolarization for each cluster. Thick lines indicate the circular mean of the phase-coupling values. (F–H) Empirical distribution of SPW and ripple subtypes properties. (F) SPW amplitude. (G) Ripple power. (H) Ripple frequency peak. Colors indicate SPW-R subtype.
Fig. S4.
Raw SPW-R complexes with different signatures in time and frequency domain from an example anesthetized animal (monkey e10). (A) Average SPW waveform for each subtype for this monkey, computed from one representative stratum radiatum recording site. (B) Randomly chosen representative raw signals (0–300 Hz) of each event subtype. (C) Filtered, Z-scored LFP in the ripple frequency range (80–180 Hz), illustrating that all SPW-R classes present a “genuine” power increase in this range, above event detection threshold. Dashed lines indicate ripple amplitude in SD units. (D) Z-scored single-event time–frequency spectrum of the broadband signal (0.5–330 Hz) presented in the top rows. Spectra have been Z-scored with respect to random baseline events.
In addition to average waveforms, we asked whether SPW-R subtypes showed differences in the frequency content of perievent LFP. Population complex Morlet-wavelet spectrograms corresponding to each SPW-R type are depicted in Fig. 2C (n = 12 experimental sessions, 4 animals). This time–frequency analysis shows a clearer low-frequency peak for classical SPW-Rs, suggesting they have higher-power sharp waves than nonclassical SPWs. In addition, the spectral profiles at ripple onset extracted from the middle vertical line of each spectrogram (Fig. 2D) show that classical ripples have higher frequency peaks [mean values with 95% confidence intervals, , , , and , respectively; pairwise bootstrapped Kolmogorov–Smirnov (KS) test, P < 10−6, for the difference between frequency peaks, Bonferroni corrected] and spectral power (SI Section B and SI Section C, and Fig. S5; specifically note that Fig. S5H shows a clear bimodal empirical distribution of the ripple frequency peaks) compared with nonclassical ripples.
Fig. S5.
Distribution of SPW-R field potential signatures’ properties. (A) Normalized absolute SPW amplitude. (B) Ripple power. (C) Number of oscillations above 50% of the maximum ripple oscillation peak. (D) Frequency peak in the ripple band (80–180 Hz). (E) Empirical probability density functions for frequency peak in the ripple band (80–180 Hz) vs. normalized absolute SPW amplitude; and ripple power vs. normalized absolute SPW amplitude. Histograms were taken from Fig. 2 F and H and illustrate the empirical probability density functions for each feature. (F) Empirical probability density functions in E, but in absolute SPW Z-scored values. Correlation values are given at the Top. (G) Empirical probability density function of SPW-to-ripple coupling phase: Raw, mixed density function (Top); density functions computed for each SPW-R subtype separately (Bottom). (H) Empirical probability density function of ripple frequency peak: raw density function (Left); density functions computed for each SPW-R subtype. Note that all raw distributions present multiple modes, corresponding to the discovered SPW-R subtypes. Colors indicate the SPW-R subtypes shown in Fig. 2; asterisks indicate significant differences between clusters for a given feature, according to a pairwise bootstrapped Kolmogorov–Smirnov test (KS test). On each box, the top and bottom are the 25th and 75th percentiles of the samples, respectively; the red dot of each box is the sample median; the dashed lines extending below and above each box are drawn from the ends of the interquartile ranges to the furthest observation (extreme points); crosses (if any) in the diagrams are the outliers of the samples.
A characteristic of our results is the different coupling of average SPW waveforms to the ripple onset. To quantify precisely this relationship in individual events, we measured the phase of the sharp wave (0–20 Hz) at each ripple occurrence. The results, grouped by SPW-R subtypes, are shown as polar histograms on Fig. 2E. Classical ripple signatures and only one nonclassical signature (subtype 1, depicted in red) presented statistically significant coupling to the sharp wave (mean values with 95% confidence interval, , , and ; P < 10−10, permuted KS test). In contrast, the nonsignificant coupling of the last cluster (subtype 4) (mean value with 95% confidence interval, ; P > 0.8, permuted KS test) is attributable to the lack of clear SPW signature. Notably, ripple-to-SPW coupling was statistically different among all SPW-R subtypes (P < 0.0001, permuted KS test; see also Fig. S5E, where histograms show clear multimodality). In line with this analysis, the empirical distributions illustrated in Fig. 2F show mostly positive SPW amplitude distributions for classical SPW-Rs, whereas nonclassical events show negative distribution or distribution around zero. As illustrated by Fig. 2 G and H, all SPW-R subtypes have their main spectral support in the frequency range of 80–180 Hz and have similar ripple power profiles (Fig. S5). Altogether, these results suggest that SPW-R subtypes are distinct in several ways, likely reflecting a discrete set of modes for the underlying hippocampal dynamics (e.g., mediated by differentiated inputs to CA1), rather than reflecting a continuum of variations of the same phenomenon. Furthermore, these differences observed at local scales are further supported by subsequent analyses on recording sessions from one unanesthetized animal (SI Section B and Fig. S6).
Fig. S6.
Classification of SPW-R complexes across four experimental sessions in unanesthetized macaque monkeys. (A) Grand averages of ripple power-triggered SPW-R field potential signatures, from one representative stratum radiatum recording site. Occurrence of the ripple oscillation is marked by dashed lines. Shaded areas indicate SEM. (B, Top) Representative SPWs and related raw signals of each event type. (B, Middle) Filtered, Z-scored LFP in the ripple frequency range (80–180 Hz). Dashed lines indicate ripple amplitude in SD units. (B, Bottom) Z-scored single-event time–frequency spectrum of the broadband signal presented in the top rows. (C) Spectrogram grand averages, in correspondence with each of the SPW-R signatures depicted in A. Averages are computed across all recording sites. (D–F) SPW and ripple subtypes statistics. (D) SPW amplitude. (E) Ripple power. (F) Ripple frequency peak. Colors denote the distinct SPW-R signatures.
Some of the SPW-R patterns described here are in close correspondence with signatures reported in a previous macaque monkey study (22). Our results extend previous observations on the variability of the time course of SPW-R complexes across species (9, 22, 28). In fact, Skaggs et al. (22) suggested that ripple oscillations come before the largest deflection of SPW in macaque monkeys. Here, we demonstrate that SPWs and ripples exhibit a variety of couplings. Moreover, our findings show that SPWs also come in different shapes. In SI Section B, we further characterize SPWs and ripples of distinct type. In particular, we report comparable ripple power, and higher ripple peak frequency for classical SPW-Rs with respect to their nonclassical counterparts (see the overall ripple and SPW statistics across experimental sessions in Fig. S5). These differences may be related to changes in neuronal synchrony (recruitment of pyramidal neurons and interneurons) and modifications of the E–I balance resulting in a higher neuronal excitability during classical SPW-Rs. Interestingly, we also report that, although subtypes have comparable rates and appear at similar timescales (Fig. S7 A and B), classical sharp waves have larger autocorrelation density (P < 0.01; t test; n = 12 experimental sessions), suggesting larger burstiness for these subtypes (Fig. S7C and SI Section B), and consistent with previous studies reporting that ripples tend to occur at comparable timescales, in time windows of increased multiunit spiking activity (29). We now investigate the coupling of SPW-R signatures to multiunit spiking activity.
Fig. S7.
SPW-R events timing dynamics. (A) Typical SPW-R event subtype raster plots. (B) Mean event rate (in events per experiment) for each of the reported SPW-R clusters. No significant differences were found between clusters, according to a paired Kolmogorov–Smirnov test (KS test). For a detailed description of the box plots, see legend of Fig. S5. (C) Autocorrelation functions corresponding to each type of SPW-R. Events associated to strong sharp waves (2, 3) exhibit more autocorrelation than other event types (P < 0.01, t test).
Spike-Field Coherence Reflects Differences in Population Synchrony During SPW-R Subtypes.
Ripples are correlated with single-unit and multiunit spiking activity from the CA1 pyramidal layer (9, 27). We asked whether this was the case for the identified SPW-R subtypes. Using the recorded multiunit spiking activity, we computed perievent time histograms (5-ms bins) for each SPW-R subtype across experimental sessions. For such a purpose, we limited our analysis to the electrode tip with the largest ripple oscillation power located in stratum pyramidale (see SI Section A for details). Our first analysis revealed a significant increase in spiking activity from baseline (Fig. 3A; n = 12 experimental sessions) concomitant with the ripple occurrence. Broadly, spiking activity of all SPW-R event types peaked at the same time.
Fig. 3.
Relationship between SPW-R complex subtypes and neuronal spiking activity. (A) Perievent time histograms of multiunit spiking activity using bins of size 5 ms. Histograms are computed with respect to random point processes of the same rate. (B) Cross-correlogram (Top) and ripple-trough perievent time histograms (Bottom). (C) Maps of absolute SFC for each SPW-R subtype defined in Fig. 3. (D) Spike-field phase-locking maps corresponding to SFC map displayed in C.
Ripple events have a characteristic phase relationship to the firing of participating units (8). We thus asked whether this relationship was the same for differentiated SPW-R subtypes. We assessed precisely the relationship between multiunit spikes and LFP phase using cross-correlation. For this analysis, the SPW-R signal was bandpass filtered in the ripple range (80–180 Hz), and we used the location of the largest trough of the oscillation as event onset reference for the cross-correlation. We then computed the ripple trough-triggered perievent time histograms (bins of 2 ms; n = 12 experimental sessions). Our results not only indicated that neuronal assemblies increase their discharge probability (Fig. 3A) during the occurrence of SPW-Rs but also revealed that unit discharges occur preferentially at the negative peaks of the ripple oscillations (ripple trough) (Fig. 3B), in agreement with previous studies in rats (8, 9). This finding was consistent across all SPW-R event subtypes, displaying virtually identical cross-correlograms (Fig. 3B, Top). In addition, the average ripple oscillation signature was almost identical among SPW-R subtypes.
Differences in the relationship between multiunit spikes and LFP across SPW-R subtypes may also span over the entire frequency axis, instead of remaining localized to a particular frequency band (such as the ripple range). Hence we further studied the LFP–spike relationship of SPW-R subtypes using spike-field coherence (SFC) of each SPW-R cluster across all frequencies in the [0–200 Hz] range (30) (see SI Section A). We computed this quantity for the four SPW-R signatures independently, across all experimental sessions. SFC group results are shown in Fig. 3C for each SPW-R subtype (n = 12 experimental sessions). Absolute values reported in Fig. 3C reveal coherency peaks both in the ripple and sharp-wave frequency bands. Classical SPW-Rs have the highest coherence values. Furthermore, the ripple-band peak of coherency of nonclassical events was consistently below that of classical SPW-Rs, in agreement with previous analyses (Fig. 2 and Fig. S6).
We further investigated whether spikes had a consistent phase relationship to LFP at different frequencies. Phase-locking values and circular mean phase of the coherence maps are reported in Fig. 3D. Consistent with our preliminary analysis, we found that multiunit spikes were phase-locked approximately to the trough of the ripple oscillation (corresponding to a phase of radians or 180°; Fig. 3D, middle map plots; mean phase with 95% circular confidence interval −2.84 ± 0.04 radians or 197.28 ± 2.29° for subtype 2; −2.62 ± 0.02 radians or 209.78 ± 1.25° degrees for subtype 3). However, in our SFC result, this relationship holds more precisely for classical ripples (nonclassical ripples mean phase with 95% circular confidence interval −2.32 ± 0.04 radians or 227.07 ± 2.29°; −2.52 ± 0.05 radians or 215.61 ± 2.86°; P < 10−3, paired Kuiper test for the difference between phase couplings of all ripple subtypes, Bonferroni corrected). This finding most likely reflects different degrees of neuronal population synchrony 50 ms around the occurrence of the ripple oscillation for different types of SPW-R complexes, which may explain the difference between our preliminary results and those with the SFC. Furthermore, this finding also suggests that nonclassical episodes represent the activity of a less synchronous neuronal population, in agreement with results in Fig. 3C.
Finally, SFC analysis shows that multiunit spiking activity is significantly locked to the gamma rhythm. A previous study showed that a gamma rhythm is ubiquitous in the SPW-R phenomenon (31). Notably, the results of this study suggest that SWR-related gamma coordinates CA3 and CA1 assemblies, and could coordinate the reactivation of stored memories. It is worth noting that, in the present work, we only selected “pure” ripple events taking into account a signal-to-noise ratio, thus assuring an almost unimodal ripple band power profile (24) (SI Section C). To fully account for the possible influence of putative perievent gamma oscillations, we considered all detected events, namely pure and “nonpure” events in two separate groups. In SI Section C, we show that detailed examination of SPW-R episode-related gamma oscillations across all detected nonpure events reveal neither new SPW-R LFP signatures nor distinct electrophysiological features compared with pure events (see Fig. S8 A–D). Interestingly, we found a transient increase of power over gamma frequencies concomitant with the occurrence of pure, nonpure, and all SPW-R subtypes (Fig. S8E). These oscillations spread over the slow- and high-gamma range of 25–75 Hz, with a unimodal distribution of instantaneous frequencies peaking at ∼50 Hz (see Fig. S8C, Insets). We found that individual SPW-R episodes can be predicted—to a certain extent—from this transient increase in gamma power, but not their specific subtype (Fig. S8F). Furthermore, in line with our SFC analysis, the relationship between gamma LFP and multiunit spikes displays significant differences in phase-coupling and phase-locking value among SPW-R subtypes, further supporting our hypothesis that these episode subtypes are functionally different (Fig. S8G). Importantly, these results demonstrate that our observations are invariant to SPW-R event detection protocols, and most likely reflect the intricate hippocampal dynamics. Furthermore, in line with ref. 31, our data suggest that macaque SPW-Rs are also mediated by a gamma rhythm (see SI Section C and SI Section D for further discussion).
Fig. S8.
Classification of SPW-R complexes across 12 experimental sessions in anesthetized macaque monkeys (nonpure events). (A) Grand averages of ripple power-triggered SPW-R field potential signatures, from one representative stratum radiatum recording site. Occurrence of the ripple oscillation is marked by dashed lines. Shaded areas indicate SEM. (B) Normalized cuts index (NCI) for different cluster partitions. The optimal clustering partition is four, corresponding to the maximum NCI. Results are computed for 200 runs of the clustering algorithm across all experimental sessions; shaded area indicates SE across experimental sessions. (C) Overlaid averaged clusterwise spectra for pure (continuous lines) and nonpure (dashed lines) SPW-Rs. Note that gamma instantaneous frequency distributions are unimodal and virtually identical (Insets). Shaded areas indicate SEM. (D) Phase coupling of the ripple to the SPW of dendritic depolarization for each cluster. Thick lines indicate the circular mean of the phase coupling values. (E) SPW-R–triggered gamma power (25–75 Hz) of pure (Top) and nonpure (Bottom) events reveals nonsignificant differences between SPW-R subtypes. (F) Accuracy of a linear SVM classifier in predicting the occurrence of a SPW-R complex by its underlying gamma-power signal. Note that performance is significantly above chance. (G) Perievent time histograms of multiunit spiking activity using bins of 2 ms. Histograms are computed taking the maximum perievent gamma-trough as a reference. Colors indicate SPW-R subtype. Box plots: top and bottom are the 25th and 75th percentiles of the samples, respectively; the red dot of each box is the sample median; the dashed lines extending below and above each box are drawn from the ends of the interquartile ranges to the furthest observation (extreme points); crosses (if any) in the diagrams are the outliers of the samples.
Brain-Wide Signatures During Distinct SPW-R Episodes.
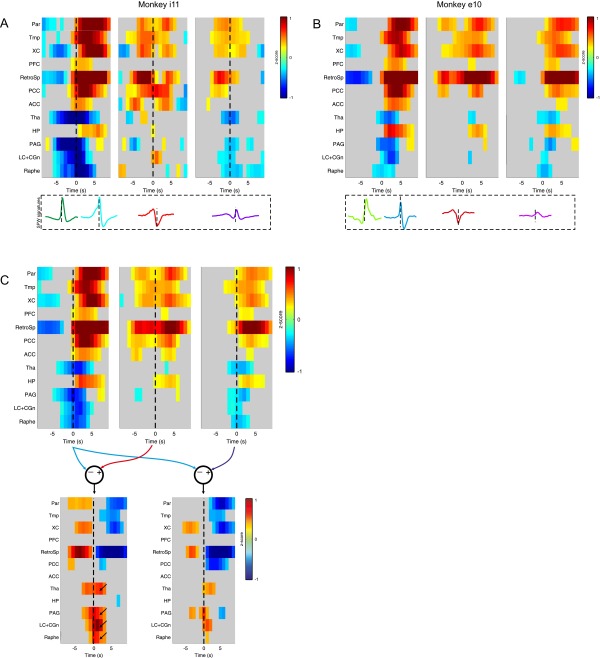
To investigate the differentiated signature of each SPW-R subtype on brain-wide activity, we measured neural event-triggered fMRI (NET-fMRI) for a set of regions of interest (ROIs) (24). The occurrence of each SPW-R complex was used as a reference (trigger) to align and average the time course of the blood oxygenation level-dependent (BOLD) fMRI signal (see Materials and Methods). We defined the ROIs according to previously established functional specificity criteria (24). We analyzed the averaged Z-scored BOLD maps for each experimental session, across four animals. Because classical SPW-R (subtypes 2 and 3) have virtually identical ripple-triggered fMRI signature, they were averaged together. First, we tracked qualitative differences in brain-wide activations for the three remaining SPW-R subtypes (subtype 1, subtype 2–3, and subtype 4).
As shown in the population analysis across all experimental sessions (n = 12 experimental sessions, 4 monkeys, Fig. 4A; see Fig. S9 A and B for examples in individual animals), the overall NET-fMRI time courses of all subtypes matches previously reported observations: cortical up-regulation and subcortical down-regulation. However, BOLD responses related to classical SPW-Rs exhibit the largest cortical up-regulations and subcortical down-regulations. In agreement with these observations, significant differences between BOLD activations of classical and nonclassical subtypes are observed in both neocortical and subcortical domains (KS test, P < 0.02; Fig. 4B), with larger effect over subcortical domains in the case of event subtype 1 (Fig. S9C).
Fig. 4.
Ripple-triggered NET-fMRI associated with SPW-R subtypes. (A) Absolute averaged BOLD full-width at half absolute maximum, illustrating that significant activation related to subtype 1 occurs almost exclusively for neocortex. Event subtypes 2 and 4 present heterogeneous activations across all brain domains (Top). Averaged BOLD time courses show differentiated neocortical activations and subcortical deactivations for distinct event subtypes in Z-score units across 12 experimental sessions, 4 animals. The gray regions in the previous plots represent activations below a 0.3-Z-score-units threshold to ease visualization (Bottom). (B) Averaged cortical and subcortical BOLD responses computed using the first singular-value decomposition (SVD) component (Fig. S9).
Fig. S9.
Ripple-triggered NET-fMRI associated with SPW-R subtypes. Averaged BOLD time courses showing differentiated neocortical activations and subcortical deactivations for distinct event types in Z-score units: (A) Only monkey i11. (B) Only monkey e10. (C) Across 12 experimental sessions, 4 animals. Gray regions in the previous plots represent activations below a 0.3-Z-score-units threshold. Population contrast maps (Bottom) show differences between subtypes in both neocortical and subcortical domains. Note that subtype 1 relates to the lowest subcortical down-regulations, whereas subtype 4 presents down-regulation comparable to that of classical SPW-Rs. In A–C, listed ROIs are as follows: Parietal (Par), temporal (Tmp), prefrontal (PFC) cortical areas, extrastriate occipital areas (XC), posterior and anterior cingulate cortices (PCC, ACC, respectively), retrosplenial area (RetroSp), thalamus (Tha), hippocampus (HP), periaqueductal gray (PAG), locus coeruleus (LC), and raphe.
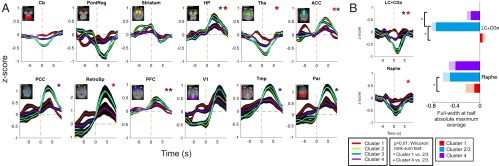
We found no significant deactivation of subcortical structures associated with SPW-R subtype 1 (t test, Bonferroni-corrected, P > 0.36), with very low amplitude or no negative deviation from zero. Interestingly, SPW-R subtype 1 was related to increased neocortical BOLD responses compared with SPW-R subtype 4, the last being associated with weak cortical up-regulation, and subcortical down-regulations qualitatively comparable to that of subtypes 2–3. The grand average NET-fMRI results are shown in Fig. 5A for a large number of ROIs. We assessed how subtype 1 and subtype 4 differed from classical SPW-Rs (subtypes 2–3) in each ROI. Because ROI-associated NET-fMRI responses exhibit similar shapes across subtypes, presenting differences mostly in magnitude, we extracted the average NET-fMRI response over the full width at half absolute maximum and compared the magnitude of up- or down-regulation for each ROI across subtypes with univariate statistical tests corrected for multiple comparisons. We found significantly higher up-regulation during classical SPW-Rs than during nonclassical SPW-Rs within the hippocampal formation, and in cortical associative areas (posterior and anterior cingulate cortex, retrosplenial area, prefrontal, temporal, and parietal cortices) (Fig. 5A).
Fig. 5.
Population ripple-triggered NET-fMRI time course in ROIs for each SPW-R subtype. (A) Average BOLD response show differentiated activations (all experimental sessions) of hippocampus (HP), thalamus (Tha), posterior and anterior cingulate cortices (PCC, ACC), retrosplenial area (RetroSp), and prefrontal (PFC) and parietal (Par) cortical areas (significant differences are indicated by asterisks). Black-shaded areas indicate SEM. (B) Average NET-fMRI responses (full width at half-maximum) show differentiated contributions of two neuromodulatory structures, dorsal raphe nucleus (serotonergic) and locus coeruleus (noradrenergic), to the SPW-R phenomenon (Fig. S10).
In support of previous analyses, nonclassical SPW-Rs gave rise to differences between subcortical down-regulation profiles. Detailed statistical analysis of all subcortical ROIs showed that differentiated BOLD activations occur in periaqueductal gray (PAG), thalamus, mesencephalon, locus coeruleus (LC), and dorsal raphe nucleus (Fig. S10B). The last two areas are neuromodulatory structures known to exert control on the overall brain state, and may influence the emergence of SPW-Rs. Notably, we found that, compared with classical SPW-Rs, subtype 1 (Fig. 5B, red curves) was associated with weaker deactivation of LC and raphe [P < 0.002, pairwise Wilcoxon rank-sum test, false-discovery rate (FDR) corrected with qFDR < 0.05] (Fig. 5B, red bars). In contrast, during the occurrence of SPW-R subtype 4, deactivation of the raphe was comparable to that of classical SPW-Rs (P > 0.1, Wilcoxon rank-sum test, FDR corrected with qFDR < 0.05), but LC down-regulation was significantly weaker (P < 0.002, pairwise Wilcoxon rank-sum test, FDR corrected with qFDR < 0.05) (Fig. 5B, purple bars).
Fig. S10.
Statistical analysis of subcortical structures’ NET-fMRI results. (A) Average over the full width at half-minimum of all subcortical regions, related to classical (pooled subtypes 2 and 3) and nonclassical (subtypes 1 and 4) SPW-Rs (grayed areas indicate SE). (B) P values from the pairwise Wilcoxon rank-sum test (note logarithmic scale), showing that differences in subcortical activations relate to locus coeruleus, dorsal raphe nucleus, thalamus, and periaqueductal gray (PAG). In contrast, modest differences were observed in lateral geniculate nucleus (LGN), ventral tegmental area (VTA), substantia nigra (SN), globus pallidus (GP), striatum (putamen and caudate nucleus), diagonal band of Broca and medial septum (DB+MS), inferior and superior colliculus (InfCol, SC), pons (PontReg), cerebellum (Cb), and brainstem. The dashed red line is drawn to indicate a FDR-corrected significance threshold of P = 0.005 (PFDR < 0.05).
SI Section A: Neural Data Analysis Methods
Estimate of Changes in the Level of Anesthesia.
The theta LFP band was used to estimate changes in the level of anesthesia during individual experimental sessions in anesthetized animals (23, 51). Raw data experiments were divided into two parts (5 min each). Signals were filtered in the theta frequency band (4–8 Hz) and were then rectified using the Hilbert transform and averaged across recording sites (only for plotting purposes). Spectral power, on the other hand, was estimated using the nonfiltered signal (0.5–300 Hz) across 15 frequencies in this band using Morlet-wavelet spectrograms. Power estimates from 50 randomly chosen time windows of 1 min were compared statistically using a paired-samples permutation t test. This statistical test was performed in 242 experiments separately. A P value of 0.05 was considered a statistically significant difference.
Selection of SPW-R Events.
Following a methodology presented in Logothetis et al. (24), we examined changes of power in the broadband signal (10–250 Hz). In addition to anatomical criteria, we classified the electrode recording tips into stratum radiatum (SR) and stratum pyramidale (PL) tips, based on visual tracking of oscillations with distinct frequency contents (complex spike features, ripple or gamma-like high-frequency events and low-frequency sharp waves) and observing synchronous activity across recording sites. The broadband signal was rectified, low-pass filtered at 20 Hz, and then normalized. Candidate events were detected as epochs during which the signal exceeded a 3.5 SD threshold. Because increases in power may result from oscillations occurring in different frequency bands, we clustered the spectra using nonnegative matrix factorization (NNMF), an unsupervised algorithm that creates data decompositions for a user-defined number of components. Stable representation of the data was achieved using three components, corresponding to increases over different frequency bands, namely sigma (8–22 Hz), gamma (25–75 Hz), and ripple (80–180 Hz).
Ripple events clustered using the NNMF procedure were considered “candidate ripples.” All candidate ripple events must be highly localized in time and distinguish themselves from any spurious baseline ripple-band activity or brief oscillatory episode. To ensure that all selected events were genuine ripples, a higher threshold was applied for ripple event selection. To this end, all candidate ripples were filtered in the ripple band (80–180 Hz) with a fourth-order Butterworth bandpass filter. We selected ripples exceeding a threshold of 5 SD, a higher threshold than that reported in previous research in rats and monkeys (16, 22, 27, 28, 52). We further refined the procedure with a “ripple time-localization criterion”: a Gaussian function was fitted to the envelope of the signal filtered in the ripple band using nonlinear least squares (53). Only events with a fitted width of and greater than 0.6 () were taken into account for further processing.
Clustering of SPW-R LFP Time Series.
Cluster analysis was applied to each experimental session separately. Perievent SPW signal time courses were used for clustering. After low-pass filtering with a cutoff frequency of 20 Hz, distortion of waveforms induced by hardware filtering was corrected by numerical temporal integration of the wave form (given that our hardware filtering acted as a temporal derivative in this frequency band). For the first stage of the clustering approach, the spatiotemporal SPW-R series were grouped in 200 representative signals using a growing neural gas (GNG) algorithm (25) (see SI Section B for mathematical details). Each representative signal (also called “node”) corresponds to the average of a group of raw perievent signals with similar time courses (reduced pairwise Euclidean distance). Because representative signals are easier to cluster due to noise reduction, in the second step, we clustered these representative signals based on their pairwise cosine similarity matrix using the normalized cuts algorithm, a clustering technique that creates partitions in the data by maximizing the overall similarity of the signals to be included in a given cluster (26). SPW-Rs were sorted and then averaged across sessions.
This procedure was performed for SPW-R data from each experimental session (∼11,000 SPW-R complexes) using up to 14 cluster partitions and 200 repetitions. We chose the optimal number of cluster partitions based on how well separated the distinct clusters were. We devised a cluster validity measure to have an objective measure of the clustering quality across clustering partitions. This measure quantifies the ratio of the pairwise intracluster similarity to the pairwise intercluster similarity, in the following referred to as the normalized cuts index (NCI). In addition, we used well-known clustering quality measures that typically compare the distances between signals belonging to a given cluster to those belonging to distinct clusters. Because all clustering quality values are normalized measures, across-sessions statistics were computed by pulling together the values obtained for each session (n = 12 experimental sessions). Finally, we devised a consistency procedure to validate the results of the clustering (see details below).
The GNG Network.
The GNG, originally proposed by Fritzke (25, 54), is an incremental self-organizing network of “codebook” vectors that fits an arbitrary topology, incorporating a growing mechanism. Connections between the vectors in the network are developed dynamically as the number of vectors in the network grows over time.
The network topology is initialized with randomly generated codebook vectors connected by edges and covering the input space (matching the number of dimensions of the input data). During one iteration of the network’s training stage, a data point is randomly selected and the closest codebook vector is iteratively updated following a simple learning rule:
| [S1] |
where is the closest codebook vector to the μth input data point and is a constant learning rate.
The topological arrangement of the network is represented by edges connecting codebook vectors, thus defining “topological neighborhoods.” A connection between two nodes is created (or “strengthen”) if the two nodes happen to be the nearest to a given input signal. These topological neighborhoods allow us to cluster the data more easily, because data points with similar time courses tend to be connected.
GNG is a graph of connected data points (representative vectors) that grows over time. The insertion policy of the GNG based on tracking representative vectors with large error variables along with the establishment of a topological neighborhood adaptation is somewhat equivalent to a stochastic gradient descent rule: vectors move in the input space together with their topological neighbors, and new vectors are inserted over large-error areas, i.e., over areas where the network has not yet fitted adequately the input data (55).
Clustering the GNG (Connection to Normalized Cuts Criterion).
The codebook vectors obtained by the GNG are clustered as follows: let be a neural network forming a graph, with codebook vector as vertices in the set V and edges in the set E. If we add a weight on each edge, denoted as a function of the cosine similarity between the codebook vectors i and j being linked by the edge, we may denote the weighted graph as . The normalized cuts criterion seeks to partition the set of codebook vectors (vertices of the graph) into several sets, such that we maximize the fraction of total weights within a group with respect to the total weights of all member nodes in the graph (26, 56). Thus, given a partition of , , the objective function to maximize is:
| [S2] |
In practice, an N-by-K partition matrix X is used to represent , where and if , i.e., if the codebook vector i belongs to the cluster ; otherwise . We can notice that there is an implicit exclusion constraint on , where denotes a N-by-1 vector of all 1’s. The neural network graph clustering problem is then formulated as follows:
| [S3] |
where is the degree matrix, i.e., gathering on its diagonal the total weight of connections of each codebook vector. The computational solution of the above problem reduces to a generalized eigenvalue system.
Validation of the Clustering Procedure.
To help determine whether SPW-R complexes were truly clustered, or they only existed as part of some continuum, we used two procedures: (i) clustering quality analysis, and (ii) clustering consistency analysis (see details below).
i) After running the clustering algorithm for several numbers of clusters (for up to 14 clusters), we compared the quality of the outcome quantitatively and qualitatively using clustering quality indices, together with low-dimensional projections of the original datasets (via Laplacian Eigenmaps, described in detail in ref. 57), respectively.
To assess the quality of the outcome of the Normalized Cut approach, we use the NCI, which corresponds to the value of the objective function described in Eq. S3 at its optimum (when the Normalized Cut Algorithm has converged). NCI is used to estimate the quality of the clustering for different numbers of clusters.
We also assessed the clustering quality using the following classical indices: silhouette index, Davies–Bouldin (DB) index, and C index (58). Briefly, the silhouette for each cluster (or across clusters) is a measure defined in the range [−1, 1] and the best clustering partition is achieved when the silhouette index approaches 1. Furthermore, the DB index is a classical index suited to compact and well-separated clusters; we note that good clustering partitions are obtained as , because this measure is the ratio of the intracluster distances to the distances between the clusters’ barycenters. Finally, the C index compares the total sum of the pairwise distances in each cluster and the smallest pairwise distances in the data against an equivalent number of the largest pairwise distances. An optimal cluster partition is obtained when .
ii) To check the stability of our clustering results, we devised an internal consistency procedure amenable to single experimental sessions, and several repetitions per session. To this end, 75% of each SPW-R dataset was taken as training set, and the remaining 25% was taken as test set. Training and test groups were balanced on the basis of the original cluster labels established a priori for the whole dataset, so that the marginal probabilities of ripple types remained equal. Training sets were further divided in two halves to train two GNGs independently, for each session. Clustering consistency of both GNGs was then measured on remaining test set, using the following equation:
| [S4] |
where and are the ith cluster labels of the µth repetition, N is the total number of signals in the test set, and is described by the following function:
| [S5] |
Thus, when the sets cluster labels and are identical, the consistency takes value 1 (or 100% consistency).
SPW-R Features and Morlet-Wavelet Spectrograms.
Four different features were computed for each of the SPW-R signatures from the previous clustering procedure, namely normalized SPW amplitude, ripple power, number of oscillations exceeding 50% of the peak of the ripple oscillation, and frequency peak in the ripple band. Ripple power was computed as the average of the squared, filtered signal in the ripple band (80–180 Hz). In addition, spectral analysis was performed using Morlet-wavelet spectrograms. The spectrograms were Z scored with respect to spectrograms computed using the same number of events with randomized interevent intervals. Ripple-band frequency peaks for each event were extracted using this spectral technique. Barring the number of oscillations feature, all features and spectra were Z scored with respect to baseline activity. In addition, we measured correlations between the aforementioned features using the standard Spearman rank correlation coefficient; a value of P < 0.05 was considered a statistically significant correlation.
Multiunit Spikes Analysis.
Multiunit spike times were detected by threshold crossing (3 SD) of the high-pass–filtered extracellular signal (1,000-Hz cutoff frequency). Single units were not isolated due to constraints of the recording hardware. Spikes around each perievent SPW-R signal were pooled together and perievent time histograms were computed (bin size, 5 and 2 ms, as indicated in main text) with respect to random point processes of the same rate. Thus, only above-baseline activity is reported in the main text of this work. Perievent cross-correlation analysis between LFPs and spikes was performed for each event subtype using the cumulative number of multiunit spike events (9).
Spike-Field Coherence.
Spike-field coherence (SFC) was computed for all recording sites located in the CA1 stratum pyramidale, across all SPW-R broadband signal events using a tapering window duration of 200 ms with an increment of 10 ms, using the Chronux toolbox available at chronux.org (59). This process outputs a complex-valued matrix , where refers to the length of the perievent time windows, F is the number of frequencies (regularly spaced between 0 and 200 Hz), and M is the total number of ripple events. Complex-valued coefficients of this matrix account for the entrainment between LFP and spikes at particular frequency and time points. Average absolute SFCs values within the same cluster label were averaged, resulting in four absolute coherence matrices. To study the phase-locking properties of spikes to the LFP, the complex SFC coefficients were also normalized to an absolute value of 1 and the circular mean of the normalized maps was computed for each cluster subtype, resulting in phase-locking maps described by their magnitude [phase-locking value (PLV)] and phase (in the range radians).
fMRI Data Processing.
Preprocessing of the MRI data has been described in a previous study (24). Briefly, linear trends were removed and bandpass filtering (0.01–0.4 Hz) was applied for temporal noise reduction. Due to the slow nature of the BOLD signal, we excluded events with overlapped BOLD time courses in a time window of [−5, 5] s. Maps from different sessions were computed using Hotelling’s T2 statistic. BOLD activations were subsequently averaged and compared across triggering SPW-R events. For qualitative analysis, contrast maps were computed as the average difference between activations associated with paired event types across experimental sessions. Statistical significance for the differences between full-width at half-maximum BOLD activations was tested later on using the Wilcoxon rank-sum test. All statistical tests were FDR corrected.
SI Section B: Supporting Results on SPW-R Complexes Subtypes
Putative Fluctuations on the Anesthesia Level.
To check the influence of the fluctuations of the level of anesthesia on the occurrence of event subtypes, event raster plots were superimposed to the rectified theta-band activity, showing no relationship between the occurrence of different types of SPW-R episodes and changes in the theta activity, used as a marker of the level of anesthesia (Fig. S1A, first two panels for anesthetized animals and third panel for one unanesthetized animal) (see also main text).
Clustering Quality Analysis.
To measure the quality of the SPW-R clustering partition, we computed four clustering quality measures: NCI, silhouette index, DB index, and C index. Clustering quality analysis measures were consistent across experimental sessions and were put together for population analysis. NCI, on the one hand, was computed as a quality measure for the GNG graph clustering procedure across up to 14 partitions to quantify the optimal number of clusters present in the data. Based on this analysis, we concluded that the optimal number of clusters is 4 (Fig. S3A). On the other hand, silhouette, DB and C indexes were used to assess the clustering quality of the raw data points and compare the quality of the two-stage clustering procedure to the quality of a single-stage clustering procedure, i.e., clustering with no neural network as preprocessing stage.
Fig. S3B (Left) illustrates the silhouette indices for our two-stage clustering methodology, in comparison with the normalized graph cuts alone and to randomly permuted clusters. Positive silhouette values are suggestive of good clustering quality, whereas negative values indicate the opposite. Our two-stage methodology largely outperforms the normalized graph cuts (P < 10−4, pairwise Bonferroni-corrected KS test). No significant differences were found across recording sites. Fig. S3B (Middle) illustrates the clustering quality assessment using DB indices. As in the previous case, we compared the two-stage methodology to the normalized graph cuts alone and to randomly permuted clusters. DB-index values close to zero indicate a good clustering quality and clusters are assumed compact and well separated. The two-stage methodology again outperforms the normalized graph cuts (P < 10−5, pairwise Bonferroni-corrected KS test). No significant differences were found across recording sites. Finally, Fig. S3B (Right) illustrates the C-indices for our two-stage clustering methodology, in comparison with the normalized graph cuts alone and to randomly permuted clusters. C-index values close to zero indicate a good clustering quality, whereas higher values indicate the opposite. The two-stage methodology was deemed better than normalized graph cuts alone (P < 10−5, pairwise Bonferroni-corrected KS test). In all of the previous cases, no significant differences were found across recording sites (P < 0.1, P < 0.07, and P < 0.4, respectively).
Finally, a typical GNG similarity matrix is depicted in Fig. S3C; the matrix is sorted according to the cluster label of each SPW-R representative. The matrix illustrates the pairwise cosine similarity between SPW-R representatives. Note that SPW-Rs of the same type remain heavily connected and bear strong similarity (greater than 0.7; brightest spots in the matrix account for connections between SPW-R representatives), whereas very few, sparse connections remain between SPW-Rs of distinct type. In addition, Fig. S3D depicts the Laplacian Eigenmap (58) (2D representation) of a typical GNG network, where each vertex corresponds to a SPW-R representative. Edges computed by the GNG algorithm (SI Section A) are shown as solid black lines. Note that clusters remain well separated, despite their high dimensionality. This result suggests that it is unlikely that distinct SPW-R signatures are a continuum of variations of the same phenomenon.
Consistency of the Clustering Procedure.
Clustering consistency was then measured for each experimental session, for a total of 100 repetitions. Session results were averaged individually, and results were pooled together for population statistics. We found that clustering consistency across experimental sessions was significantly higher than chance (P < 10−6, t test; mean value with 95% confidence interval for clustering consistency across 12 experimental sessions, 74.17 ± 1.63%).
We next examined the classification changes underlying the performance of the clustering consistency procedure. More specifically, we asked which SPW-R categories most of the inconsistently classified SPW-Rs were associated to. We addressed this question by constructing a “confusion matrix,” averaged across cross-validations. Our results show that SPW-R subtypes 2 and 3 were inconsistently classified slightly more often than other subtypes, likely due to their similar time courses (Fig. S3E). Likewise, the least inconsistently clustered subtype was subtype 1, probably because its time course is anticorrelated with respect to the time course of other SPW-R signatures.
Variations in Amplitude, Frequency Content, and Statistics of SPW-R Events.
We compared the four SPW-R types on the basis of normalized absolute sharp-wave amplitude (Fig. S5A), ripple power (defined as the average of the squared, filtered signal) (Fig. S5B), number of oscillations above 20% of the ripple oscillation peak (Fig. S5C), and frequency peak of the ripple (band, 80–180 Hz) (Fig. S5D).
This analysis revealed statistically significant differences across clusters for the Z-scored sharp-wave amplitude (mean values in Z-score units with 95% confidence intervals, , , , , respectively; pairwise bootstrapped KS test, P < 10−6, Bonferroni corrected) and frequency peak of the ripple (mean values with 95% confidence intervals, , , , and , respectively; pairwise bootstrapped KS test, P < 10−6, Bonferroni corrected). In contrast, no significant differences were observed for ripple power (mean values in Z-score units with 95% confidence intervals, , , , and , respectively; pairwise bootstrapped KS test, P > 0.05, Bonferroni corrected) and the number of oscillations above 50% of the ripple oscillation peak (mean values with 95% confidence intervals, , , , and , respectively; pairwise bootstrapped KS test, P < 0.1).
SPWs and ripples have correlated time courses and correlated features (28, 31, 60, 61). In particular, we studied the relationship between ripple power and sharp-wave amplitude and also between ripple frequency peak and sharp-wave amplitude. We conducted regression analyses to quantify such relationships. Consistent with our spectral analysis reported in the main text, we found that ripple power correlated positively to sharp-wave amplitude (R = 0.4534; P = 0.0001) and this relationship is observed for each type of SPW-R (Fig. S5 E and F). Analogously, frequency peak in the ripple band (80–180 Hz) correlated positively to sharp-wave amplitude (R = 0.3548; P = 0.0001). In all subtypes, both the ripple-band frequency peak and ripple power remained relatively localized (Fig. S5 E and F).
In addition to their specific amplitude and frequency properties, a vast body of evidence suggests that SPW-R events do not happen randomly (4, 27, 62). First, we examined how different event types occurred over time. Fig. S7A shows two typical event raster plots across different experiments of two different experimental sessions. SPW-R events tend to happen with no apparent clustering along the time course of an experimental session. To examine potential differences in “bursting” behavior, we computed the autocorrelation density of each event type (Fig. S7C) and found that classical sharp-wave ripples are autocorrelated to a significantly higher extent than other types of sharp-wave ripples. It is noteworthy that the rate of each event subtype across experiments is comparable (Fig. S7B).
SPW-R Subtypes in LFP Recordings from One Unanesthetized Animal.
We asked whether the same SPW-R classification would apply for drug-free animals. In correspondence with each SPW-R pattern in Fig. 2A, Fig. S6A shows equivalent SPW-R patterns in one unanesthetized (drug-free) animal (monkey b04), recorded during quiet wakefulness. Single-event broadband (0.5–300 Hz) traces show remarkable similarity with those detected in anesthetized animals. Single-event ripples and broadband spectral profiles (Fig. S6B, third and bottom panels, respectively) display significant power increase in the ripple band. Both SPW and ripple features were encountered in good agreement with the anesthetized data (Fig. S6 D and E). Notably, both positive and negative SPW deflections were prevalent and the spectra of fast oscillations were localized in the ripple frequency range. Classical ripples had higher frequency peaks and power as in their anesthetized counter parts (mean values in hertz with 95% confidence intervals, , , , and , respectively; pairwise bootstrapped KS test, P < 0.001, Bonferroni corrected). However, in this case, both classical and nonclassical ripples had more localized ripple-band spectral distributions (Fig. S6F). This effect could be explained by state-dependent differences between ripples (31). In this case, quiet wakefulness ripples present lower frequency peak in the ripple frequency band, compared with off-line–state ripples.
Predicting SPW-R Subtypes from BOLD fMRI Time Courses.
To assess how different these responses were between subtypes, we first used a multivariate predictive approach. We trained a multiclass linear SVM classifier (63) to predict the SPW-R subtype using the time course of all ROIs averaged over a few experiments. The classifier trained in this way could not distinguish between the BOLD responses related to subtypes 2 and 3, which have virtually identical ripple-triggered fMRI signature. We thus pooled them together for further comparison. We next predicted SPW-R subtype 1, against subtype 4, against pooled SPW-R subtypes 2 and 3, which resulted in an average of 72.87 ± 6.19% (mean with 95% confidence interval) cross-validation accuracy. We then quantified the discriminative power of each ROI by counting the fraction of time points from each ROI that were attributed a large coefficient by the classifier for distinguishing between all subtypes. We found that largest differences between the classical and nonclassical SPW-R subtypes mostly involve associative cortical areas and neuromodulatory systems. Furthermore, the largest differences between subtype 1 and subtype 4 involve solely associative cortical areas, consistent with previous analysis (Results in the main text).
It is worth noting that the SVM classifier trained in this way is not in a one-to-one correspondence to predicting the occurrence of SPW-R complexes from ongoing BOLD fMRI activity. Addressing such a question goes beyond the scope of the present work and shall be part of a future investigation.
SI Section C: Supporting Results on SWR-Related Gamma Oscillations
Since ripple oscillations from CA3 and CA1 are largely incoherent (61), it is unlikely that synchronization in this band coordinates the activity in both subfields during memory replay. Instead, gamma oscillations during SPW-R have been recently suggested to play this role in a recent study (31). In the following sections, we address whether such oscillations are present in our monkey recordings, and how they relate to specific SPW-R subtypes, possibly reflecting differentiated levels of coordination between CA3 and CA1.
SPW-R Detection Procedure, “Pure” and “Nonpure” Events.
In the present work, detection of oscillatory events is first performed using the following procedure [following Logothetis et al. (24)]. Candidate events are detected using the smoothed envelope of the broad-band LFP signal (10–250 Hz) (we set 3.5 SD as threshold). Events’ spectral signatures were clustered using the NMF algorithm as explained in SI Section A, thus establishing three event groups with distinct spectra. We examined how well a single factor of the NMF could explain the observed power spectrum of individual events. For this, we computed a signal-to-noise (SNR) ratio, defined as the ratio of the sum of the squared power spectrum values to the sum of the squared difference of the spectrum, from its projection on a single factor. Events with unimodal (or close to unimodal spectral distribution) associated to a factor SNR > 3 were selected as pure events. These events are the ones reported in the main text of this work, in correspondence with those reported in ref. 24. However, there are not only ripple events with SNR ≤ 3 but also sigma and gamma episodes with SNR ≤ 3 that may have substantial power in the ripple frequency band (80–180 Hz), even at or above a 5 SD threshold and well localized in time according to our previous ripple selection criteria. The compound of all detected events with SNR ≤ 3 that had substantial power in the ripple band are henceforth referred to as nonpure SPW-R events and were analyzed separately in the present section of Supporting Information.
Nonpure SPW-Rs Also Come in Four Subtypes.
Following the event detection procedure, we applied the stringent criteria for SPW-R detection described previously in SI Section A. We next used our two-step methodology to cluster nonpure SPW-R episodes time series. We applied the algorithm to the nonpure SPW-Rs of 12 experimental sessions (a total of ∼10,000 nonpure SPW-R complexes were in compliance with our stringent ripple selection criteria). The results of the clustering were consistent across experimental sessions and animals. Furthermore, our clustering quality analysis revealed that nonpure SPW-R complexes are best represented by four LFP signatures (Fig. S8 A and B).
Fig. S8A depicts the nonpure SPW-R LFP grand averages, in close correspondence with pure SPW-R LFP signatures (n = 12 experimental sessions, 4 animals). Population Morlet-wavelet spectra for each pure and nonpure SPW-R subtype are depicted in Fig. S8C. Both pure and nonpure SPW-Rs presented enhanced power profiles over gamma frequencies (25–75 Hz), where nonpure events displayed the largest power over such frequency range. However, spectral profiles of pure and nonpure events were remarkably consistent over the SPW and ripple frequency bands. We found that nonpure classical ripples had higher frequency peaks (mean values with 95% confidence intervals, 106.90 ± 1.0900, 119.4697 ± 1.0260, 118.2110 ± 1.0985, and 105.1701 ± 0.4481, respectively; pairwise bootstrapped KS test, P < 0.0007 for the difference between frequency peaks, Bonferroni corrected). Notably, nonpure SPW-R complexes presented virtually the same coupling of the SPW wave form to the ripple onset as their pure counterparts (see polar histograms on Fig. S8D). We found that classical ripple signatures and only one nonclassical signature (subtype 1, depicted in red) presented statistically significant coupling to the SPW (mean values with 95% confidence interval, −2.9406 ± 0.0292, −0.2535 ± 0.0137, 0.1091 ± 0.0065, and −1.0074 ± 0.1613; and P < 10−6 and P > 0.05 permuted KS test for the phase coupling, respectively). Moreover, ripple-to-SPW coupling was statistically different among all nonpure SPW-R subtypes (P < 10−6, permuted KS test). We now investigate the fine properties of nonpure events and the relation of pure and nonpure SPW-R signatures to the observed gamma component.
Variations in Amplitude, Frequency Content, and Statistics of Nonpure SPW-R Events.
As in our analysis for pure events, we compared the four SPW-R types on the basis of the normalized absolute SPW amplitude, ripple power, and number of oscillations above 20% of the ripple oscillation peak.
This analysis revealed statistically significant differences across clusters for the Z-scored SPW amplitude (mean values in Z-score units with 95% confidence intervals, −0.5537 ± 0.0968, 1.4038 ± 0.0664, 1.2835 ± 0.0831, and 0.0808 ± 0.0341, respectively; pairwise bootstrapped KS test, P < 10−10, Bonferroni corrected). However, no significant differences were observed for ripple power (mean values in Z-score units with 95% confidence intervals, 2.3832 ± 0.0660, 2.6662 ± 0.0470, 2.6976 ± 0.0535, and 2.2102 ± 0.0267, respectively; pairwise bootstrapped KS test, P > 0.05, noncorrected) and the number of oscillations above 50% of the ripple oscillation peak (mean values with 95% confidence intervals, 4.9098 ± 0.1042, 5.0149 ± 0.0868, 4.8820 ± 0.0887, and 4.9462 ± 0.0455, respectively; pairwise bootstrapped KS test, P > 0.2).
Next, we conducted regression analyses to quantify relationships between ripple power and SPW amplitude and also between ripple frequency peak and SPW amplitude. In line with the quantitative analysis performed for pure events, we found that ripple power and frequency peak in the ripple band (80–180 Hz) correlated positively to SPW amplitude (R = 0.4534, P = 0.0001; R = 0.3548, P = 0.0001, respectively).
Transient Increase of Gamma Power (25–75 Hz) During Pure and Nonpure SPW-R Events.
Ripple-triggered spectrograms show that both pure and nonpure SPW-Rs present somewhat enhanced power profiles over gamma frequencies (25–75 Hz), in addition to the expected increases in SPW and ripple frequency bands (<20 and 80–180 Hz, respectively). To identify the increased gamma band, we followed the procedure reported in ref. 31 with a slight modification. We filtered the perievent pure and nonpure SPW-R signals in the range 15–80 Hz with a fourth-order Butterworth filter, i.e., covering all of the gamma frequencies until the beginning of the ripple band. We estimated the instantaneous frequency by measuring the inverse of the time between the peaks of this signal in a [−0.1 0.1]-s time window around the occurrence of each event. From this somewhat broadband signal, we could expect one or various peaks to emerge (e.g., in the low-gamma band [25–49 Hz], or high-gamma band [50–79 Hz]) (24). However, we found that pure, nonpure, and all SPW-R event subtypes presented virtually identical gamma-band distributions with peaks around 50 Hz (in between low-gamma and high-gamma; P > 0.4, pairwise permutation KS test for the comparison between gamma instantaneous frequency distributions). Notably, pure and nonpure gamma distributions were largely unimodal (P > 0.4, Hartigan’s dip test) and ranged from 25 to 80 Hz, approximately (Fig. S8C, Insets). To avoid conflating gamma with ripple-band oscillations, we performed all following gamma-band analyses in the 25- to 75-Hz frequency band, which captures ∼99% of the gamma empirical probability density function.
Altogether, these results suggest that concomitant with the occurrence of the SPW-R complexes, there is an increase in power over gamma frequencies, in correspondence with previously published data (31). We discover, however, that the gamma rhythm is in the range of 25–75 Hz in macaques, covering the slow- and high-gamma ranges simultaneously. We now investigate whether this increase in power is exclusive to SPW-R complexes or it is simply an effect of ongoing baseline activity intermingled with the occurrence of such events.
Occurrence of Pure and Nonpure SPW-R Events Can Be Predicted by Gamma Power Increases.
We asked how systematic is the relationship between gamma-power increases and SPW-R complexes in single trials. To partly address this question, we computed SWR-triggered gamma-power averages, by bandpass filtering the raw LFP perievent signal in the range of 25–75 Hz, and then computed the absolute value of its Hilbert transform. Gamma power increased significantly above baseline concomitant during both pure and nonpure SPW-R episodes, and presented its largest increase at the ripple power peak (Fig. S8E). This analysis revealed that the gamma-power increase was largely transient, decaying to baseline in about 100 ms from the ripple peak. Notably, the peak of gamma power was not significantly different among pure and nonpure events (P > 0.1, KS test). Furthermore, “classical” SPW-R subtypes presented higher average peak perievent gamma power compared with “nonclassical” SPW-Rs; however, this difference was not significant (P > 0.4, pairwise KS test, noncorrected). Thus, increases in gamma power were transient, concomitant with the occurrence of SPW-R episodes, and spanning the duration of a single episode. These results demonstrate that gamma is concurrent with SPW and does not reflect putative fluctuations of baseline activity, nor a gamma tail following SPW-R occurrence.
We next asked whether a power increase over the gamma frequency range (25–75 Hz) was predictive of the occurrence of a SPW-R episode. We learned linear SVM classifiers to predict the presence (or absence) of a SPW-R complex (pure, nonpure, and SPW-R subtypes were analyzed separately for each experimental session). To this end, periripple gamma-power signals were intended to be discriminated against surrogate (randomly chosen) baseline events in time windows of [−0.1 0.1] s around the event. The classifiers performed significantly above chance, with 66.89 ± 4.03% and 64.22 ± 3.19% (mean with 95% confidence interval, 10-fold cross-validation) prediction accuracy for pure and nonpure events, respectively (P > 0.3, two-sample t test, for the comparison between pure and nonpure SPW-R prediction accuracy). We found no significant difference in the prediction accuracy among SPW-R subtypes (Fig. S8F), in line with our previous analysis. Furthermore, individual SPW-R subtypes could not be discriminated from their underlying gamma-power profiles, resulting in ∼25% prediction accuracy.
These results suggest that gamma oscillations reflect an intrinsic mechanism of SPW-R episodes, common to all SPW-Rs subtypes discovered in this work. This phenomenon is invariant to event detection protocols, suggesting that it can only be attributed to network mechanisms underlying SPW-R episodes themselves. We speculate, in line with observations from a previous study in rats (31), that SPW-Rs may occur when CA3 gamma rhythm entrains CA1 assemblies in the macaque hippocampus.
Gamma Rhythm Modulates Local Multiunit Spiking Activity.
The transient increase in gamma power during SPW-R complexes covered both slow- and high-gamma frequency ranges (25–75 Hz). If gamma is an intrinsic network rhythm of SPW-R episodes as our results suggest, it is expected that gamma rhythm modulates the spiking activity of participating neuronal ensembles during such events within CA1. Indeed, our SFC analysis suggests that spiking activity is locked not only to the ripple and SPW bands but also to the gamma range. To quantify this relationship, we filtered the perievent SPW-R signals in the range of 25–75 Hz using a fourth-order bandpass Butterworth filter. For this analysis, we referred to the recording tip with the largest gamma power. We computed the largest-gamma-trough–triggered perievent histograms (bins of 2 ms; n = 12 experimental sessions). Our results revealed that multiunit spiking activity was phase-locked to the gamma rhythm underlying pure and nonpure SPW-R complexes. Notably, neuronal discharges occur preferentially at the falling phase or rising phase of gamma (Fig. S8G). These findings were consistent across all SPW-R subtypes. Importantly, SPW-R subtype-related multiunit spikes showed significantly different gamma-rhythm phase preference (mean values with 95% confidence intervals, 2.7941 ± 0.06; 4.5972 ± 0.0882; 3.4514 ± 0.1162; 4.8442 ± 0.0830 radians; P < 10−4, pairwise bootstrapped KS test). Furthermore, subtypes 2 and 3 presented the largest PLVs, suggesting that multiunit spikes are more consistently entrained by the gamma rhythm at a given phase in such cases (P < 0.0001, pairwise KS test for the comparison between classical and nonclassical SPW-R subtypes PLV).
SI Section D: Supporting Discussion
SPW-R episodes may be initiated over relatively localized neuronal circuitry in hippocampus, presenting different spatial extents and propagation features. Hence, it is expected that SPW-R phenomena would present some variability. Indeed, a previous study has reported that SPW-R events propagate along the septo-temporal axis of the CA1 of rats (28), such that the events detected at a given location may be initiated in another region of this subfield. SPW-R complexes may be deprived of a clear SPW pattern, which may indicate a more local spread of neuronal activity in the hippocampus. Patterns with disparate SPW polarity, however, cannot be fully explained in light of the observations of Patel et al. (28). The similarity of the SPW-R waveforms as they propagate reported in their work further supports this view. The patterns observed in the present work may indicate different localizations of the current sources in the first place (32); possibly reflecting a different repartition of the activity over the underlying cell groups.
Previous studies (34, 64) suggest that CA1 pyramidal neurons from deep and superficial layers form two functionally distinct groups. A subgroup of CA1 pyramidal cells (located in deep layers) presents differentiated coupling to intrinsic LFP rhythms such as theta and gamma. Notably, the coupling of modulated cells strongly depends on the behavioral state (namely, REM sleep or awake exploration), suggesting that pyramidal cells from functionally distinct sublayers can influence targets jointly or differentially in a brain state-dependent way. Although modulated cells during REM sleep respond more strongly to CA3 inputs (34, 64), a parsimonious explanation to our results is that distinct CA1 sublayers may be active during different SPW-R subtypes. Although this question remains open, in this work we show that SPW-R subtype-related multiunit spiking activity synchronizes differently to ripple- and gamma-band LFP oscillations, suggesting that activity of CA1 subcircuits is differentially organized (64). These changes are a possible indication of differentiated afferent inputs from upstream structures originating in both cortical and subcortical domains. For instance, entorhinal cortex may drive more strongly some of the discovered SPW-R patterns, thus shifting the coupling between LFP gamma and spikes, which in turn may be fired mostly by deep sublayer cells (i.e., REM-modulated cells). The fact that deep and superficial CA1 sublayers have different targets as suggested by anatomical data (65) is in line with the hypothesis that SPW-R subtypes may arise from the population activity of distinct local sublayers, and further supports our hypothesis that SPW-R subtypes are functionally distinct as pointed out by our fMRI data.
Discussion
In the present study, we identified—for the first time (to the best of our knowledge)—distinct subtypes of in vivo SPW-R patterns in a single recording site, displaying qualitative and quantitative electrophysiological differences. Our procedure reliably clusters the perievent time series into a number of event subtypes; examines their characteristic properties, such as their frequency content, amplitude, and frequency of occurrence; and maps the topology and dynamics of brain-wide metabolic activity at the time of event occurrence.
This approach revealed the existence of four hippocampal SPW-R subtypes with differentiated mesoscopic and brain-wide dynamics. Subtype identification relied on the presence of a SPW pattern in the LFP trace and its shape. The characteristics of SPW patterns and ripple oscillations in the hippocampal CA1 subfield suggested differences in subthreshold activity of the underlying neural populations. Importantly, such differences at the mesoscopic level were complemented by differences in brain-wide signatures mapped with NET-fMRI. Our results demonstrate significant modulation of two brain-wide networks linked to hippocampal-dependent memory functions: one involving associative neocortex, and another one involving subcortical and specifically neuromodulatory structures.
It is unlikely that our results are an effect of fluctuations in the levels of anesthesia (in the case of the four anesthetized animals), because the low-frequency LFP activity over the theta range was stable over time for each experimental session. Furthermore, the occurrences of SPW-R complex subtypes showed no apparent clustering in time for different event types and were comparable to those observed in experimental sessions from one drug-free animal, recorded during quiet wakefulness.
Identification of Four Subtypes of the SPW-R Phenomenon.
We have demonstrated that hippocampal LFP dynamical variability during SPW-Rs can be summarized into four typical signatures. Each signature corresponds to a specific relationship between SPWs and ripple oscillations: high-frequency oscillations preceding, following, or coinciding with the peak of the SPW of dendritic depolarization, as well as high-frequency oscillations with no clear SPW signature. Such highly structured variability suggests differentiated underlying neural mechanisms, possibly associated with different memory-related functions.
Some SPW-R subtypes reported in the present work are in agreement with recently reported SPW-R signatures in slice models (32, 33). However, Hofer et al. (32) report only two event classes based on the SPW polarity in CA3. In contrast, Reichinnek et al. (33) have also observed two SPW-R event classes, differing only in SPW amplitude. The discrepancy between previously reported results and ours may be due to the chosen SPW-R detection protocol, solely based on SPW amplitude and visual inspection (33), and also to the limitations inherent to slice models, unable to reproduce the effect of long-range brain interactions.
The observed variability of LFP profiles during ripples suggests a different spatiotemporal repartition of extracellular currents in the CA1 microcircuits, possibly resulting from the differences in the recruitment of—and interactions between—the underlying neuronal subgroups. Indeed, several inhibitory and excitatory subgroups of neurons interact during SPW-R episodes, and their nonhomogenous spatial configuration can affect the spatial distribution of extracellular activity. For instance, Mizuseki et al. (34) report differential involvement of the two CA1 pyramidal sublayers, having distinct phase shifting with respect to the theta rhythm during rapid eye movement (REM) sleep, and they further indicate that REM shifting cells were more strongly associated with SPW-R activity, compared with that of non-REM shifting cells. Whether these two sublayers are differentially involved during SPW-R subtypes and are at the origin of the observed LFP characteristics remains an open question.
Another candidate for modulating the physiological properties of SPW-R is the influence of CA3. In particular, gamma oscillations during SPW-R are assumed to play a central role in coordinating CA3 and CA1 cell assemblies during memory replay (31). We discovered that a gamma rhythm is ubiquitous in all SPW-R subtypes. Specifically, we found a transient increase in gamma power concomitant with the occurrence of SPW-R episodes, and barring the detailed single-unit analysis, we found similar SWR-gamma correlates as those reported in ref. 31. Thus, gamma-related mechanisms mediating CA3–CA1 interactions during SPW-R episodes seem to be independent from the mechanisms generating the SPW-R diversity.
Additional discussion about the possible influence of the recording locations and the local circuits in stratum pyramidale within CA1 in relation to our results can be found in SI Section D. Although the above comments discuss modifications of hippocampal neural activity that might explain our LFP observations, we hypothesize that these changes may in turn result from the influence of other brain structures.
SPW-R Subtypes Relate to Differentiated Neuromodulatory Activities.
Modulations in amplitude of the BOLD responses in subcortical domains suggest the involvement of two neuromodulatory structures directly connected to hippocampus in the SPW-R phenomenon, namely dorsal raphe nucleus and LC. Due to the transient nature of the examined neuronal events, the lack of clustering of the SPW-R subtypes, and their NET-fMRI correlates, it is likely that our study reflects short-duration “phasic” changes, rather than tonic neuromodulatory effects spanning over 10 s or larger timescales.
A dense noradrenergic and serotonergic innervation is present in the hippocampus proper, exerting modulatory effects in the activity of microcircuits. For instance, LC deactivation is involved in switching cortex and hippocampus to inactive states (35). However, LC neurons exhibit also phasic modes of activity, with bursts of activity of 15- to 70-ms duration, followed by a 300- to 700-ms period of suppression (36). Experimental evidence suggests that the timescales of interactions between LC and hippocampus (∼1–2 s) (37) are compatible with the scale of occurrence of individual ripple episodes. Although the hypothesis that differentiated SPW-R complexes emerge partly as a result of LC inputs remains to be tested using more direct methods, it has been shown that decision making under uncertainty involves LC (38). Moreover, SPW-Rs are more frequent after task modifications, such as exposing an animal to a second novel environment (18).
In addition, nonclassical SPW-R subtypes may mark a differentiated involvement of the ascending serotonergic system. Notably, raphe projects to several subtypes of hippocampal GABAergic interneurons and may exert control on the E–I balance over local circuitry engaged during ripples via fast synapses (39). Moreover, the capacity of raphe projections to selectively influence well-segregated groups of hippocampal parvalbumin (PV) expressing interneurons has been recently stressed (40), raising the possibility of serotonergic control of neuronal circuits over timescales spanning the occurrence of single ripples (∼200 ms). This view is further supported by a recent study where groups of the three major PV cell classes are differentially modulated during ripples, suggesting that fast network episodes involve distinct inhibitory circuits (41), modulated at timescales at which raphe–hippocampal modulations may occur.
Hippocampal–Neocortical Interactions May Contribute to SPW-R Variability.
We showed that SPW-R subtypes 1 and 4 (nonclassical SPW-Rs) in the hippocampus consistently led to lesser activation across different cortical domains compared with SPW-R subtypes 2 and 3 (classical SPW-Rs). Because our experiments were performed in anesthetized macaque monkeys without any previous behavioral training, the robustness of our results across sessions suggests that these differentiated signatures reflect different types of brain-wide dynamical processes involving memory traces, not necessarily accounting for differences in memory content per se.
Differentiated activations were detected in the posterior and anterior cingulate (PCC, ACC) (42), PFC (43), and parietal cortices (44). One possible explanation for these results is that increased cortical activity marks enhanced recurrent interactions between neocortex and hippocampus during classical SPWs. Taking into account hippocampal connectivity, two recurrent mechanisms might account for differentiated SPW-R patterns. First, entorhinal cortex (EC) input may modulate CA1 activity by episodes of persistent activity that occur concomitantly with neocortical up-down state transitions (45). These neocortical–EC episodes develop at timescales similar to those of a typical burst of ripples, i.e., series of ripples with relatively short delays. Second, reentry may also be achieved at the scale of a single SPW-R event, through projections of medial entorhinal cortex (MEC) to CA1 stratum lacunosum moleculare (SLM) (46). Layer-specific activation of MEC would be followed by a local depolarization of SLM with the delay of a single synapse, thus influencing the time course of single SPW-Rs (46). In both scenarios, hippocampal activity could be controlled by recurrent activity within the local microcircuits and through cortico-hippocampal dialogue. These mechanisms might serve many purposes, such as volitional reactivation of a set of related memory traces or controlling the consolidation of new memories according to their relevance to preexisting memories and their reward value.
A Repertoire of Memory-Related Brain-Wide Events.
To the best of our knowledge, the present study shows for the first time the existence of in vivo SPW-R subtypes occurring in the same recording site, and reflecting qualitative differences in brain activity within hippocampus and across cortical and subcortical domains. Processes such as memory reactivation, retrieval, and consolidation could exploit these brain-wide differences to fulfill different functions. Locally, the described SPW-R signatures may be the result of selective interactions between neuronal groups, involving varying degrees of network synchrony and dynamical E–I balance. This balance may be mediated by cortico-parahippocampal-hippocampal and subcortico-hippocampal interactions. Our results suggest these dynamical properties of local microcircuits are strongly intertwined with brain-wide interactions during SPW-R, possibly establishing distinct modes of selective processing and routing of information across multiple scales (SI Section D). The presented pattern classification will enable the characterization of SPW-Rs in behaving and natural sleep preparations and establish the functional role of each subtype. Finally, our approach could be used to better understand the underlying mechanisms of epileptiform events and more generally pathological phenomena (see ref. 47 for a perspective).
Materials and Methods
Surgical Procedures, Electrophysiology, and fMRI Recordings.
Experimental and surgical procedures have been detailed in a previous study (27). In summary, a total of 242 experiments (10 min each) spread over 12 sessions were carried out in anesthetized male rhesus monkeys (Macaca mulatta). Head holders and recording chambers were located stereotaxically based on high-resolution anatomical MRI scans. Recordings were conducted in the anterior part of the hippocampus in the right hemisphere of each animal. All recording hardware, including the electrodes and amplifiers for simultaneous fMRI and multisite electrophysiological recordings, was developed at the Max Planck Institute for Biological Cybernetics. Custom-made, multicontact, NMR-compatible recording electrodes were made from a carbon fiber composite baton with 500-µm diameter (R&G). The 2.6-mm-long tip grounded down to a diameter of 250 µm. Electrodes contained 10 contacts spaced 150 µm apart, with 6–10 targeting the structure of interest. Recording electrodes were positioned around the pyramidal layer of the hippocampal CA1 subfield (8–14 mm anterior of the interaural line). Fine adjustment of the recording electrode was achieved by intermediate MRI anatomical scans. Functional imaging was carried out in a vertical 4.7-T scanner with a 40-cm-diameter bore (BioSpec 47/40v; Bruker BioSpin), in which each animal was positioned in a custom-made chair [see Logothetis et al. (24) for details on the gradient coil]. Typically, 22 axial slices were acquired, covering the entire brain (voxel size, 0.75 × 0.75 × 2 mm3). BOLD activity was acquired at a resolution of 2 s with two-shot gradient-echo echo-planar imaging images (repetition time/echo time, 1,000/20 ms; bandwidth, 150 kHz; flip angle, 53°; field of view, 96 × 96 mm; matrix, 96 × 96; 2-mm slice thickness). MRI data were analyzed off-line. During all experiments, anesthesia was maintained with remifentanil (0.5–2 µg⋅kg−1⋅min−1) in combination with a fast-acting paralytic mivacurium chloride (5–7 mg⋅kg−1⋅h−1), known to only mildly affect the magnitude and time course of neural and vascular responses (24, 48). All experimental and surgical procedures were approved by the local authorities (Regierungspraesidium, Tübingen Referat 35, Veteriärwesen) and were in full compliance with the guidelines of the European Community (EUVD 86/609/EEC) for the care and use of laboratory animals.
Processing and Analysis of Neural Data.
Analyses of electrophysiology and fMRI data were performed using MATLAB (The MathWorks). Signal denoising, electromagnetic interference elimination, and frequency band isolation procedures have been described in detail in a previous study (24). In SI Section A, we describe the main signal processing and neuronal data analysis performed on the denoised broadband extracellular signals (0.05 Hz to 7 kHz).
Acknowledgments
We acknowledge A. Ramírez-Cárdenas and D. Zaldivar for insightful comments and discussions on previous versions of this manuscript. J. Werner and M. Schnabel provided excellent technical support during the development of this work. This research was supported by the Max Planck Society.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1518257112/-/DCSupplemental.
References
- 1.Euston DR, Tatsuno M, McNaughton BL. Fast-forward playback of recent memory sequences in prefrontal cortex during sleep. Science. 2007;318(5853):1147–1150. doi: 10.1126/science.1148979. [DOI] [PubMed] [Google Scholar]
- 2.Johnson LA, Euston DR, Tatsuno M, McNaughton BL. Stored-trace reactivation in rat prefrontal cortex is correlated with down-to-up state fluctuation density. J Neurosci. 2010;30(7):2650–2661. doi: 10.1523/JNEUROSCI.1617-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kudrimoti HS, Barnes CA, McNaughton BL. Reactivation of hippocampal cell assemblies: Effects of behavioral state, experience, and EEG dynamics. J Neurosci. 1999;19(10):4090–4101. doi: 10.1523/JNEUROSCI.19-10-04090.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee AK, Wilson MA. Memory of sequential experience in the hippocampus during slow wave sleep. Neuron. 2002;36(6):1183–1194. doi: 10.1016/s0896-6273(02)01096-6. [DOI] [PubMed] [Google Scholar]
- 5.Nádasdy Z, Hirase H, Czurkó A, Csicsvari J, Buzsáki G. Replay and time compression of recurring spike sequences in the hippocampus. J Neurosci. 1999;19(21):9497–9507. doi: 10.1523/JNEUROSCI.19-21-09497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skaggs WE, McNaughton BL. Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science. 1996;271(5257):1870–1873. doi: 10.1126/science.271.5257.1870. [DOI] [PubMed] [Google Scholar]
- 7.Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265(5172):676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- 8.Buzsáki G, Horváth Z, Urioste R, Hetke J, Wise K. High-frequency network oscillation in the hippocampus. Science. 1992;256(5059):1025–1027. doi: 10.1126/science.1589772. [DOI] [PubMed] [Google Scholar]
- 9.Ylinen A, et al. Sharp wave-associated high-frequency oscillation (200 Hz) in the intact hippocampus: Network and intracellular mechanisms. J Neurosci. 1995;15(1 Pt 1):30–46. doi: 10.1523/JNEUROSCI.15-01-00030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ego-Stengel V, Wilson MA. 2010. Disruption of ripple-associated hippocampal activity during rest impairs spatial learning in the rat. Hippocampus 20(1):1–10.
- 11.Gerrard JL, Burke SN, McNaughton BL, Barnes CA. Sequence reactivation in the hippocampus is impaired in aged rats. J Neurosci. 2008;28(31):7883–7890. doi: 10.1523/JNEUROSCI.1265-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Girardeau G, Benchenane K, Wiener SI, Buzsáki G, Zugaro MB. Selective suppression of hippocampal ripples impairs spatial memory. Nat Neurosci. 2009;12(10):1222–1223. doi: 10.1038/nn.2384. [DOI] [PubMed] [Google Scholar]
- 13.Nakashiba T, Buhl DL, McHugh TJ, Tonegawa S. Hippocampal CA3 output is crucial for ripple-associated reactivation and consolidation of memory. Neuron. 2009;62(6):781–787. doi: 10.1016/j.neuron.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karlócai MR, et al. Physiological sharp wave-ripples and interictal events in vitro: What’s the difference? Brain. 2014;137(Pt 2):463–485. doi: 10.1093/brain/awt348. [DOI] [PubMed] [Google Scholar]
- 15.Foster DJ, Wilson MA. Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature. 2006;440(7084):680–683. doi: 10.1038/nature04587. [DOI] [PubMed] [Google Scholar]
- 16.Singer AC, Carr MF, Karlsson MP, Frank LM. Hippocampal SWR activity predicts correct decisions during the initial learning of an alternation task. Neuron. 2013;77(6):1163–1173. doi: 10.1016/j.neuron.2013.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dupret D, O’Neill J, Pleydell-Bouverie B, Csicsvari J. The reorganization and reactivation of hippocampal maps predict spatial memory performance. Nat Neurosci. 2010;13(8):995–1002. doi: 10.1038/nn.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karlsson MP, Frank LM. Awake replay of remote experiences in the hippocampus. Nat Neurosci. 2009;12(7):913–918. doi: 10.1038/nn.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jadhav SP, Kemere C, German PW, Frank LM. Awake hippocampal sharp-wave ripples support spatial memory. Science. 2012;336(6087):1454–1458. doi: 10.1126/science.1217230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muenzinger KF. Vicarious trial and error at a point of choice: I. A general survey of its relation to learning efficiency. Pedagog Semin J Genet Psychol. 1938;53(1):75–86. [Google Scholar]
- 21.Carr MF, Jadhav SP, Frank LM. Hippocampal replay in the awake state: A potential substrate for memory consolidation and retrieval. Nat Neurosci. 2011;14(2):147–153. doi: 10.1038/nn.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skaggs WE, et al. EEG sharp waves and sparse ensemble unit activity in the macaque hippocampus. J Neurophysiol. 2007;98(2):898–910. doi: 10.1152/jn.00401.2007. [DOI] [PubMed] [Google Scholar]
- 23.Bland BH, et al. Effect of halothane on type 2 immobility-related hippocampal theta field activity and theta-on/theta-off cell discharges. Hippocampus. 2003;13(1):38–47. doi: 10.1002/hipo.10044. [DOI] [PubMed] [Google Scholar]
- 24.Logothetis NK, et al. Hippocampal–cortical interaction during periods of subcortical silence. Nature. 2012;491(7425):547–553. doi: 10.1038/nature11618. [DOI] [PubMed] [Google Scholar]
- 25.Fritzke B. A growing neural gas network learns topologies. Adv Neural Inf Process Syst. 1995;7:625–632. [Google Scholar]
- 26.Shi J, Malik J. Normalized cuts and image segmentation. IEEE Trans Pattern Anal Mach Intell. 2000;22(8):888–905. [Google Scholar]
- 27.Csicsvari J, Hirase H, Mamiya A, Buzsáki G. Ensemble patterns of hippocampal CA3-CA1 neurons during sharp wave–associated population events. Neuron. 2000;28(2):585–594. doi: 10.1016/s0896-6273(00)00135-5. [DOI] [PubMed] [Google Scholar]
- 28.Patel J, Schomburg EW, Berényi A, Fujisawa S, Buzsáki G. Local generation and propagation of ripples along the septotemporal axis of the hippocampus. J Neurosci. 2013;33(43):17029–17041. doi: 10.1523/JNEUROSCI.2036-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci. 2007;10(1):100–107. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]
- 30.van der Meer MA, Redish AD. Low and high gamma oscillations in rat ventral striatum have distinct relationships to behavior, reward, and spiking activity on a learned spatial decision task. Front Integr Neurosci. 2009;3:9. doi: 10.3389/neuro.07.009.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carr MF, Karlsson MP, Frank LM. Transient slow gamma synchrony underlies hippocampal memory replay. Neuron. 2012;75(4):700–713. doi: 10.1016/j.neuron.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hofer KT, et al. The hippocampal CA3 region can generate two distinct types of sharp wave-ripple complexes, in vitro: Hippocampal sharp-waves in vitro. Hippocampus. 2015;25(2):169–186. doi: 10.1002/hipo.22361. [DOI] [PubMed] [Google Scholar]
- 33.Reichinnek S, Künsting T, Draguhn A, Both M. Field potential signature of distinct multicellular activity patterns in the mouse hippocampus. J Neurosci. 2010;30(46):15441–15449. doi: 10.1523/JNEUROSCI.2535-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mizuseki K, Diba K, Pastalkova E, Buzsáki G. Hippocampal CA1 pyramidal cells form functionally distinct sublayers. Nat Neurosci. 2011;14(9):1174–1181. doi: 10.1038/nn.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981;1(8):876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: Adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28(1):403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- 37.Yavich L, Jäkälä P, Tanila H. Noradrenaline overflow in mouse dentate gyrus following locus coeruleus and natural stimulation: Real-time monitoring by in vivo voltammetry. J Neurochem. 2005;95(3):641–650. doi: 10.1111/j.1471-4159.2005.03390.x. [DOI] [PubMed] [Google Scholar]
- 38.Payzan-LeNestour E, Dunne S, Bossaerts P, O’Doherty JP. The neural representation of unexpected uncertainty during value-based decision making. Neuron. 2013;79(1):191–201. doi: 10.1016/j.neuron.2013.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Freund TF, Gulyás AI, Acsády L, Görcs T, Tóth K. Serotonergic control of the hippocampus via local inhibitory interneurons. Proc Natl Acad Sci USA. 1990;87(21):8501–8505. doi: 10.1073/pnas.87.21.8501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Varga V, et al. Fast synaptic subcortical control of hippocampal circuits. Science. 2009;326(5951):449–453. doi: 10.1126/science.1178307. [DOI] [PubMed] [Google Scholar]
- 41.Varga C, et al. Functional fission of parvalbumin interneuron classes during fast network events. Elife. 2014;3:e04006. doi: 10.7554/eLife.04006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maddock RJ, Garrett AS, Buonocore MH. Remembering familiar people: The posterior cingulate cortex and autobiographical memory retrieval. Neuroscience. 2001;104(3):667–676. doi: 10.1016/s0306-4522(01)00108-7. [DOI] [PubMed] [Google Scholar]
- 43.Wierzynski CM, Lubenov EV, Gu M, Siapas AG. State-dependent spike-timing relationships between hippocampal and prefrontal circuits during sleep. Neuron. 2009;61(4):587–596. doi: 10.1016/j.neuron.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salazar RF, Dotson NM, Bressler SL, Gray CM. Content-specific fronto-parietal synchronization during visual working memory. Science. 2012;338(6110):1097–1100. doi: 10.1126/science.1224000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Isomura Y, et al. Integration and segregation of activity in entorhinal-hippocampal subregions by neocortical slow oscillations. Neuron. 2006;52(5):871–882. doi: 10.1016/j.neuron.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 46.Kloosterman F, van Haeften T, Lopes da Silva FH. Two reentrant pathways in the hippocampal-entorhinal system. Hippocampus. 2004;14(8):1026–1039. doi: 10.1002/hipo.20022. [DOI] [PubMed] [Google Scholar]
- 47.Kerber K, et al. Differentiation of specific ripple patterns helps to identify epileptogenic areas for surgical procedures. Clin Neurophysiol. 2014;125(7):1339–1345. doi: 10.1016/j.clinph.2013.11.030. [DOI] [PubMed] [Google Scholar]
- 48.Goense JBM, Logothetis NK. Neurophysiology of the BOLD fMRI signal in awake monkeys. Curr Biol. 2008;18(9):631–640. doi: 10.1016/j.cub.2008.03.054. [DOI] [PubMed] [Google Scholar]
- 49.Saleem KS, Logothetis NK. 2007. A Combined MRI and Histology Atlas of the Rhesus Monkey Brain in Stereotaxic Coordinates (Academic, London)
- 50.Łęski S, et al. Inverse current source density method in two dimensions: Inferring neural activation from multielectrode recordings. Neuroinformatics. 2011;9(4):401–425. doi: 10.1007/s12021-011-9111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kortelainen J, Väyrynen E, Seppänen T. Depth of anesthesia during multidrug infusion: Separating the effects of propofol and remifentanil using the spectral features of EEG. IEEE Trans Biomed Eng. 2011;58(5):1216–1223. doi: 10.1109/TBME.2010.2103560. [DOI] [PubMed] [Google Scholar]
- 52.Battaglia FP, Sutherland GR, McNaughton BL. Hippocampal sharp wave bursts coincide with neocortical “up-state” transitions. Learn Mem. 2004;11(6):697–704. doi: 10.1101/lm.73504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seber GAF, Wild CJ. Nonlinear Regression. Wiley; Hoboken, NJ: 1989. Computational methods for nonlinear least squares; pp. 619–660. [Google Scholar]
- 54.Fritzke B. Growing cell structures—a self-organizing network for unsupervised and supervised learning. Neural Netw. 1994;7(9):1441–1460. [Google Scholar]
- 55.Martinetz TM, Berkovich SG, Schulten KJ. Neural-gas’ network for vector quantization and its application to time-series prediction. IEEE Trans Neural Netw. 1993;4(4):558–569. doi: 10.1109/72.238311. [DOI] [PubMed] [Google Scholar]
- 56.Yu SX, Shi J. Proceedings of the Ninth IEEE International Conference on Computer Vision. IEEE; Washington, DC: 2003. Multiclass spectral clustering; pp. 313–319. [Google Scholar]
- 57.Smola AJ, Kondor R. Kernels and regularization on graphs. In: Schölkopf B, Warmuth MK, editors. Learning Theory and Kernel Machines, Lecture Notes in Computer Science. Vol 2777. Springer; Berlin: 2003. pp. 144–158. [Google Scholar]
- 58.Desgraupes B. 2013. Clustering Indices. Available at https://cran.r-project.org/web/packages/clusterCrit/vignettes/clusterCrit.pdf. Accessed February 17, 2014.
- 59.Bokil H, Andrews P, Kulkarni JE, Mehta S, Mitra PP. Chronux: A platform for analyzing neural signals. J Neurosci Methods. 2010;192(1):146–151. doi: 10.1016/j.jneumeth.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hájos N, et al. Input-output features of anatomically identified CA3 neurons during hippocampal sharp wave/ripple oscillation in vitro. J Neurosci. 2013;33(28):11677–11691. doi: 10.1523/JNEUROSCI.5729-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sullivan D, et al. Relationships between hippocampal sharp waves, ripples, and fast gamma oscillation: Influence of dentate and entorhinal cortical activity. J Neurosci. 2011;31(23):8605–8616. doi: 10.1523/JNEUROSCI.0294-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Buzsáki G, et al. Hippocampal network patterns of activity in the mouse. Neuroscience. 2003;116(1):201–211. doi: 10.1016/s0306-4522(02)00669-3. [DOI] [PubMed] [Google Scholar]
- 63.Schölkopf B, Smola AJ. Learning with Kernels: Support Vector Machines, Regularization, Optimization, and Beyond. MIT; Cambridge, MA: 2001. [Google Scholar]
- 64.Lee S-H, et al. Parvalbumin-positive basket cells differentiate among hippocampal pyramidal cells. Neuron. 2014;82(5):1129–1144. doi: 10.1016/j.neuron.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sørensen JC, Tønder N, Slomianka L. Zinc-positive afferents to the rat septum originate from distinct subpopulations of zinc-containing neurons in the hippocampal areas and layers. A combined fluoro-gold tracing and histochemical study. Anat Embryol (Berl) 1993;188(2):107–115. doi: 10.1007/BF00186245. [DOI] [PubMed] [Google Scholar]