Recently, we provided broad evidence for a negative association between community diversity and the abundance of human and wildlife parasites (1), supporting the dilution effect hypothesis (2). Salkeld et al. (3) point out that our meta-analysis reached a different conclusion than their previous meta-analysis on the dilution effect (4), which focused solely on 13 effect sizes from six species of human parasites. Salkeld et al. (3) argue that their smaller sample size is justified because only field studies capture the ecological realism necessary to test the dilution effect hypothesis. However, field studies can have confounding variables that can generate false-positive or false-negative relationships between diversity and disease. Although laboratory studies often more confidently capture cause–effect relationships, they can be contrived. Hence, given these costs and benefits of field and laboratory studies, we included both in our meta-analysis and found that each strongly supported the dilution effect hypothesis. Nevertheless, we reanalyzed the data in our publicly available dataset using the inclusion criteria (i.e., field studies on human parasites at certain scales) of Salkeld et al. (4) and, once again, found a significant negative relationship between biodiversity and the abundance of human parasites (random effects meta-analysis: n = 22 effect sizes and 12 species as a random effect, mean Hedges’ d = −0.75, P = 0.018). Hence, the difference between the marginally nonsignificant finding in the meta-analysis of Salkeld et al. (mean Fisher’s Z = −0.23, 95% confidence interval: −0.470 to 0.008) (4) and our significant finding can be explained by the increased statistical power associated with the inclusion of nine more recent studies on six human parasites. These results further support the robustness of the negative relationship between biodiversity and infectious disease using our dataset.
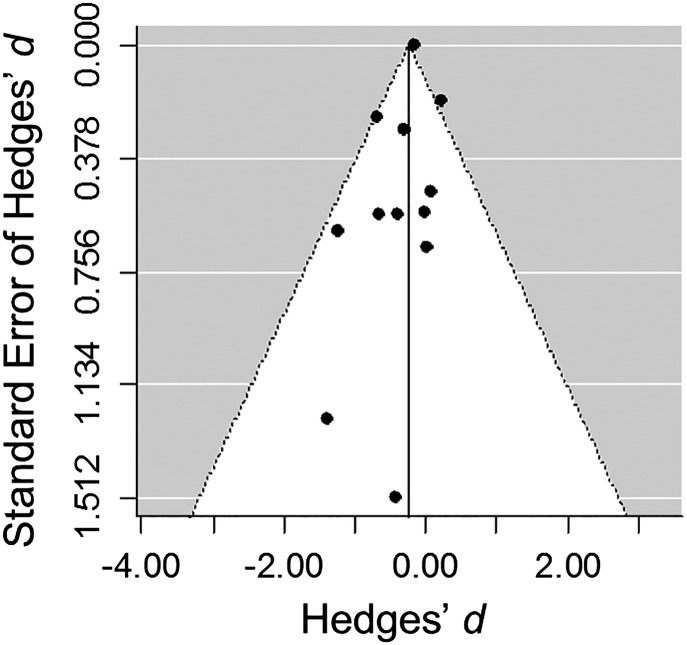
Salkeld et al. (3) also suggest that publication bias might influence the biodiversity-disease literature, and we agree that this bias is possible. We emphasized (1) that we did not test for publication bias because the currently available diagnostic tools are invalidated when effect sizes share some underlying replicates, come from the same larger experiment, or are on the same species [i.e., are not independent (5)]. These sources of dependence are also present in the dataset of Salkeld et al. (4), compromising their original tests of publication bias. Despite this caveat, we here explore for signs of bias using the weighted means and variances of Hedges’ d for each human parasite species, again using the selection criteria of Salkeld et al. (n = 12) (4). Neither the funnel plot (Fig. 1) nor diagnostic statistical tests suggest publication bias (rank correlation test: Kendall’s τ = −0.24, P = 0.31; Egger’s regression test: z = −1.05, P = 0.29) (6).
Fig. 1.
Funnel plot for supplementary analysis of mean effect sizes for the relationship between biodiversity and parasite abundance for 12 human zoonotic parasites. There is no evidence for funnel plot asymmetry, a potential indicator of publication bias, using either the rank correlation test or Egger’s regression test implemented in the metafor package in R (6).
We reiterate that the evidence supporting the dilution effect hypothesis is robust to study design, meta-analysis selection criteria, and publication bias. However, the dilution effect is a heterogeneous phenomenon; therefore, in some instances, increasing biodiversity could increase rather than decrease disease (1). Hence, when the goal is to manage a known problematic disease, obtaining system-specific information might improve the success of disease control over blindly conserving biodiversity. In contrast, if the goal is to minimize disease emergence/abundance without species-specific knowledge, conserving biodiversity may be a useful strategy.
Footnotes
The authors declare no conflict of interest.
References
- 1.Civitello DJ, et al. Biodiversity inhibits parasites: Broad evidence for the dilution effect. Proc Natl Acad Sci USA. 2015;112(28):8667–8671. doi: 10.1073/pnas.1506279112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostfeld RS, Keesing F. Effects of host diversity on infectious disease. Annu Rev Ecol Evol Syst. 2012;43(1):157–182. [Google Scholar]
- 3.Salkeld DJ, Padgett KA, Jones JH, Antolin MF. Public health perspective on patterns of biodiversity and zoonotic disease. Proc Natl Acad Sci USA. 2015;112:E6261. doi: 10.1073/pnas.1517640112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salkeld DJ, Padgett KA, Jones JH. A meta-analysis suggesting that the relationship between biodiversity and risk of zoonotic pathogen transmission is idiosyncratic. Ecol Lett. 2013;16(5):679–686. doi: 10.1111/ele.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lau J, Ioannidis JPA, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ. 2006;333(7568):597–600. doi: 10.1136/bmj.333.7568.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1–48. [Google Scholar]