Significance
Platelets are known to be both numerically and functionally altered in some patients with cancer. However, structural differences in the platelets from these patients have not been studied. Here we use electron cryotomography to reveal that, compared with control donors, the microtubule system and the mitochondria of platelets from patients diagnosed with ovarian cancer are significantly different. This finding suggests the potential of electron cryotomography as a technology to detect structural biomarkers of diseases affecting platelets.
Keywords: electron, cryotomography, platelet, microtubule, cancer
Abstract
Thrombocytosis and platelet hyperreactivity are known to be associated with malignancy; however, there have been no ultrastructure studies of platelets from patients with ovarian cancer. Here, we used electron cryotomography (cryo-ET) to examine frozen-hydrated platelets from patients with invasive ovarian cancer (n = 12) and control subjects either with benign adnexal mass (n = 5) or free from disease (n = 6). Qualitative inspections of the tomograms indicate significant morphological differences between the cancer and control platelets, including disruption of the microtubule marginal band. Quantitative analysis of subcellular features in 120 platelet electron tomograms from these two groups showed statistically significant differences in mitochondria, as well as microtubules. These structural variations in the platelets from the patients with cancer may be correlated with the altered platelet functions associated with malignancy. Cryo-ET of platelets shows potential as a noninvasive biomarker technology for ovarian cancer and other platelet-related diseases.
Platelets are small anucleate multifunctional cells derived from megakaryocytes. Platelets circulate in the bloodstream and respond to vascular lesions (1). In their resting state, they adopt a discoidal shape and have an average lifespan of 5–7 d in humans (2). Platelets have increasingly been recognized as playing an important role in tumor growth and metastasis, in addition to their traditional roles in hemostasis (3–6). Thrombocytosis (platelet count >450,000/μL) is found in 31% of patients with ovarian cancer and is associated with a poor clinical prognosis (7, 8). In addition, platelets in patients with cancer are functionally altered, and often adopt a hyperreactive state (9). This platelet hyperreactivity helps explain the higher thrombosis risk in these patients (10). Furthermore, compelling preclinical and clinical data demonstrate that platelets actively promote tumor growth and metastasis through multiple pathways. Platelets form a physical shield to protect tumor cells from natural killer cell-mediated lysis; they facilitate the adhesion of tumor cells to the endothelium, allowing the critical extravasation step in the metastatic cascade to occur; and they release a plethora of bioactive molecules, including growth factors and cytokines stored in their secretory granules, that promote angiogenesis and tumor cell growth (11). It is shown in various experimental models that disruption of platelet–tumor interactions abolishes these effects (5, 9), improving clinical outcomes of patients.
The relatively high incidence of thrombocytosis in these patient populations (12), which is related to cytokine signaling from both tumor and nontumor tissue (8), and the implication of platelets in cancer pathology (6, 8) suggested the possibility of ultrastructural perturbations in the platelets of patients with ovarian cancer. These perturbations could provide structural clues to improve our understanding of cancer-related hyperreactivity, thrombocytosis, or as-yet-unknown platelet defects, and could serve as potential biomarkers for screening for ovarian cancer. Prior attempts at examining platelet ultrastructure in diseases have been largely limited by methods that used plastic embedding and chemical fixation (13–15). The present investigation overcomes these technical limitations by using electron cryotomography (cryo-ET) to visualize human platelets in their naturally occurring pathophysiological states without chemical fixation or staining (16).
Results
After approval by the institutional review boards for research on human subjects, we prospectively collected peripheral blood samples from patients with newly diagnosed invasive ovarian cancer or benign adnexal masses (Table S1) before any chemotherapy or surgical treatment. Age-matched healthy females were recruited as controls. None of the participants had a primary platelet disorder or a coexisting inflammatory condition, and none was taking medications known to interfere with platelet function. Average platelet counts and mean platelet volume of the healthy control subjects were 279.6 × 103/μL and 7.9 fL, respectively. Blood samples (anticoagulant: 0.38% sodium citrate final concentration) were collected from the patients and healthy subjects after informed consent was provided. Platelet-rich plasma (PRP) was obtained by centrifugation and vitrified for cryo-ET. From these frozen-hydrated samples, a total of 338 tilt series of tomography images of individual platelets were generated. Each image series was then reconstructed into a 3D volume called a tomogram. One hundred twenty tomograms with adequate contrast were selected for filtering, feature annotation, structural measurements, and statistical analysis. For the quantitative analysis, we required at least five annotatable tomograms from each subject. In addition, platelets from these subjects were analyzed for aggregation induced by ADP.
Table S1.
Patient characteristics
| ID | Age, y | Stage | Pathology | Platelet count | Mean platelet volume | Hemoglobin | WBC |
| C001 | 58 | 3C | High-grade serous | 339 | 10.0 | 11.0 | 12.5 |
| C002 | 70 | 3C | High-grade serous | 328 | 9.4 | 12.8 | 6.7 |
| C003 | 69 | 3C | High-grade serous | 687 | 8.8 | 8.9 | 11.9 |
| C004 | 55 | 2 | Endometrioid | 436 | 9.1 | 13.1 | 13.8 |
| C005 | 76 | 3C | Low-grade serous | 303 | 10.2 | 11.4 | 10.2 |
| C006 | 38 | 3 | Granulosa cell tumor | 353 | 7.4 | 9.2 | 6.6 |
| C007 | 46 | 4 | Mixed serous and endometrioid | 335 | 9.2 | 11.1 | 110 |
| C008 | 59 | 3C | High-grade serous | 468 | 10.2 | 10.5 | 9.5 |
| C009 | 74 | 2 | Endometrioid | 231 | 9.5 | 14.0 | 7.7 |
| C010 | 64 | 1 | Endometrioid | 328 | 9.4 | 11.6 | 6.4 |
| C011 | 62 | 3 | Endometrioid | 335 | 10.2 | 13.0 | 4.3 |
| C012 | 53 | 4 | Endometrioid | 244 | 9.5 | 12.5 | 8.5 |
| B001 | 81 | — | Serous cystadenoma | 218 | 11.7 | 11.8 | 6.9 |
| B002 | 52 | — | Serous cystadenoma | 255 | 11.4 | 14.9 | 7.6 |
| B003 | 64 | — | Mature cystic teratoma | 203 | 12.3 | 12.6 | 4.5 |
| B004 | 79 | — | Fibroma | 258 | 10.8 | 12.1 | 6.9 |
| B005 | 26 | — | Corpus luteum cyst | 311 | 8.4 | 9.9 | 5.4 |
WBC, white blood cell.
Cryo-ET of Platelets from Healthy Control Subjects.
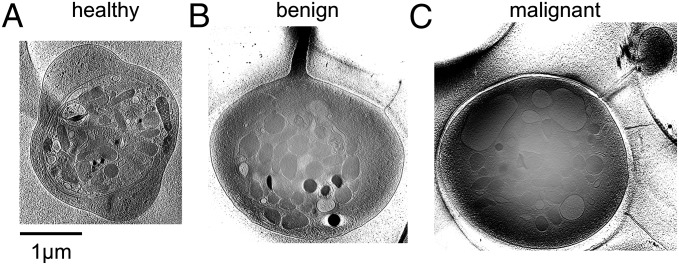
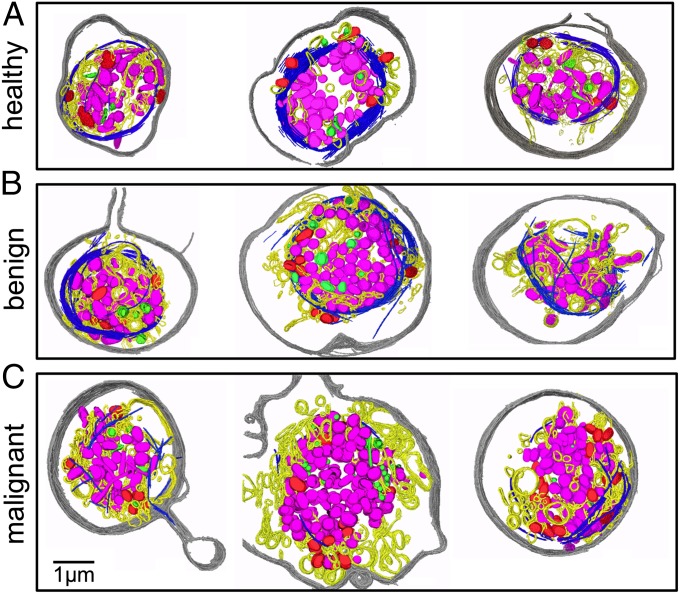
We first examined platelets from the healthy controls (n = 6). Fig. 1A shows an example slice from a 3D tomogram of a typical platelet of a healthy subject (Movie S1). Fig. 2A shows the annotated structural features of three platelets randomly selected from this healthy group, including the plasma membrane, circumferentially coiled microtubule (marginal band), α and dense granules, mitochondria, and low-contrast vacuole-like (LCV) features. A fraction of the LCV was seen as empty vacuole-like structures (Figs. 1A and 2A and Movie S1). The rest of LCV exists as narrow and tortuous invaginations of the surface membrane, the area of which was impractical to estimate. We interpret this structural feature to be the platelet surface-connected open canalicular system. Most platelets from healthy subjects share a similar structural pattern. The lack of a significant number of pseudopods indicates that the observed platelets are mostly in the resting state. These structural features are consistent with the morphology of platelets from healthy subjects obtained by conventional 2D thin-section, plastic-embedded electron microscopy (2, 13).
Fig. 1.
Platelets from control subjects and patients with cancer, raw cryo-electron tomogram. (A) Slice of a tomogram of a human blood platelet from a healthy female donor. This platelet has a circumferential microtubule ring and numerous secretory granules inside. The general shape is discoidal. (B) Slice of a tomogram of a human blood platelet from a patient with a benign tumor in the ovary. This platelet shares similar cytoplasmic structures with the platelet in A, with the exception of the presence of an extended pseudopod. (C) Slice of a tomogram of a human blood platelet from a patient with invasive ovarian cancer. This platelet has more enlarged LCV structures and an extended pseudopod. In contrast to the platelets in A and B, this platelet has fragmented and fewer microtubules.
Fig. 2.
Platelets from control subjects and patients with cancer, annotated tomogram. (A) Three randomly selected annotated platelets from healthy donors. All of them have an intact marginal band of microtubules (blue) enclosing most of their granules (α pink, dense green) and mitochondria (red) inside. The plasma membrane is gray and the LCV is yellow. (B) Three randomly selected annotated platelets from patients with benign masses. Their structures are similar to those shown in A. (C) Three randomly selected annotated platelets from patients with invasive ovarian cancer. Their morphologies appear to be more heterogeneous compared with the six platelets in the other two panels. They seem to have more LCV, fewer and shorter microtubule filaments, and more mitochondria.
Cryo-ET of Platelets from Patients.
Next, we examined platelets obtained from women with either benign adnexal mass (n = 5) or invasive ovarian cancer (n = 12). Qualitatively, platelets from patients with benign masses (Figs. 1B and 2B) had a similar appearance to those from the healthy subjects, including a relatively intact microtubule ring and abundant secretory granules.
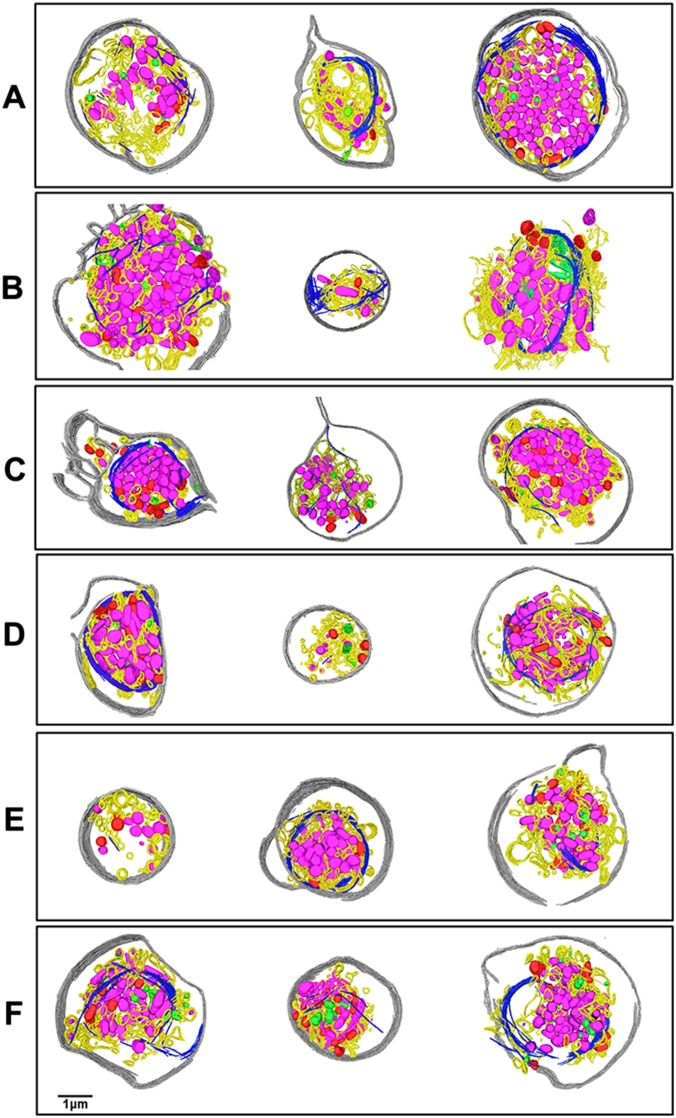
In contrast, platelets from patients with an invasive ovarian cancer (Figs. 1C and 2C and Movie S2) had a strikingly different appearance compared with those from either healthy subjects (Figs. 1A and 2A) or patients with benign masses (Figs. 1B and 2B). In many of these platelets from patients with invasive cancer, the microtubule marginal bands seen in the benign and control platelets were severed and depolymerized to various degrees (Figs. 1C and 2C). Also unique to patients with invasive cancer was marked visual and quantitative heterogeneity among individual platelets, even those from the same patient (Fig. S1).
Fig. S1.
Platelets from patients with ovarian cancer are heterogeneous. (A–F). The three platelets from the same panel are from the same ovarian cancer patient. The 18 platelets shown in this figure show strong structural heterogeneity, both inter- and intrapatient. The shapes of the platelets, plasma membrane integrity, microtubule organization and length, numbers of mitochondria and granules, and size of the platelets are also very different. A few tiny “platelets” with very little microtubule and a few granules and mitochondria in them are seen (second one in D, first one in E). These might actually be platelet microparticles. Cryo-ET of platelets can capture those structural variations and provide quantifiable morphological information on a single-cell basis. The color annotations are the same as those used in Fig. 2.
Quantification of Platelet Tomograms.
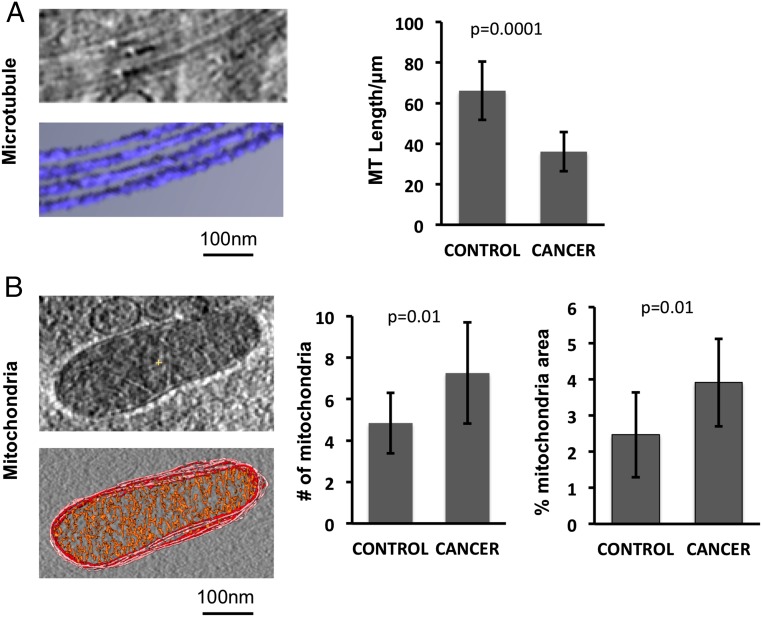
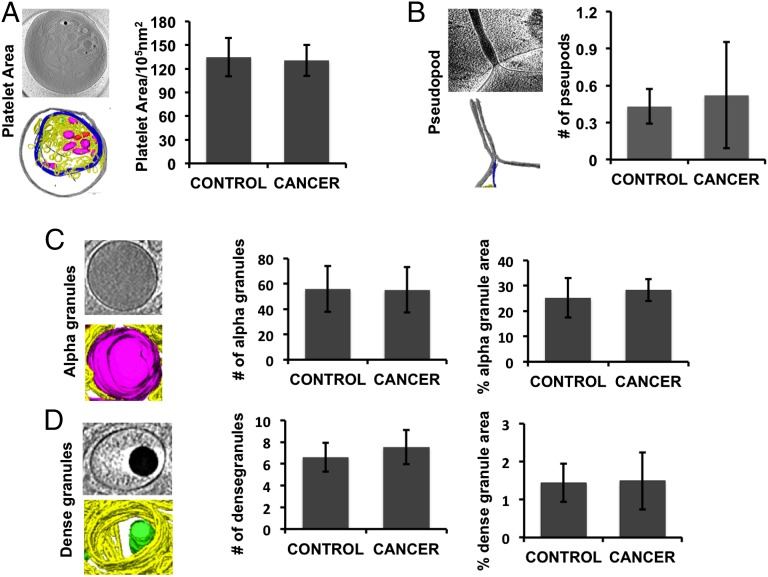
To quantitate the structural features of each platelet, we measured nine parameters (Figs. 3 and 4), including number of α granules, number of dense granules, number of mitochondria, percentage of total platelet area occupied by each of these three structures, microtubule length, pseudopod number, and total platelet area. Tomograms with insufficient contrast were excluded from further analysis. Platelets from individual patients with ovarian cancer are heterogeneous (Fig. S1), which warrants the decision to measure these parameters from at least five platelets per subject. These nine parameters were chosen because they are associated with platelet structural integrity and functions and are easily recognized in tomograms. For instance, α and dense granules in our tomograms have relatively large size and characteristic scattering densities because of their different biochemical contents. The clinical utility of this study lies in the distinction between cancer and noncancer groups. Therefore, we combined the data from healthy donors and patients with benign masses to form the control group for subsequent analyses because we found that many of these measurements exhibited the same pattern for both healthy and benign samples, as expected from qualitative analysis (Figs. 1 and 2). Measurements from this control group (n = 11) were compared with those from patients with invasive ovarian cancer (n = 12). Among these measurements, three of the nine parameters were found to show significant differences between control and cancer subjects: microtubule length, mitochondria number, and percentage of platelet total area occupied by mitochondria (Fig. 3). Again, these three parameters did not differ significantly between the benign and healthy donors.
Fig. 3.
Statistical analysis shows microtubule and mitochondria are discriminating parameters. (A) Microtubule strands are clearly seen in the subtomogram of the platelet. Microtubule lengths are measured and compared between the control group (healthy donors plus patients with benign mass) and the cancer group (patients with ovarian cancer). The cancer group has significantly fewer and shorter microtubules in their platelets than the control group. (B) Mitochondria appear as enclosed double-membrane organelles; the inner membrane forms the characteristic cristae structure inside. Mitochondria numbers are counted, and the percentage of the whole platelet area covered by mitochondria is measured. The cancer group has significantly more mitochondria in their platelets compared with the control group. They also have a higher percentage of area covered by mitochondria.
Fig. 4.
The six other measured platelet parameters are found not to be significantly different between the control and cancer groups. These are (A) platelet area, (B) number of pseudopods, (C) number of α granules and percentage of granule area, and (D) number of dense granules and percentage of granule area.
Our quantification shows that the mean microtubule length was significantly shorter in platelets from patients with invasive ovarian cancer compared with those from the control group (36.08 ± 9.70 μm vs. 66.17 ± 14.42 μm; P = 0.0001; Fig. 3A). Qualitatively, microtubules of the patients were fragmented, instead of forming intact marginal band rings as in the control group (Fig. 2). Because all the quantifications were performed by the same investigator, it is likely that the analysis was self-consistent, but not necessarily unbiased. However, subjectivity is reduced because we chose tomograms having unequivocally good contrast for analysis.
We also found differences in the number of mitochondria and the percentage of the whole platelet area covered by the mitochondria. Platelets from patients with cancer had more mitochondria than platelets from the control group (Fig. 3B) (7.26 ± 2.44 vs. 4.85 ± 1.46 mitochondria per platelet; P = 0.01). The absolute number of mitochondria per platelet in the control group is consistent with previous reports (17). The mitochondria from patients with cancer occupied a larger fraction of the area of the platelet (3.91 ± 1.21 vs. 2.47 ± 1.18% of platelet area; P = 0.009). Most of the differences in total mitochondria area among platelets can be explained by differences in the number of mitochondria per cell (R2 = 0.80; n = 120).
The other six quantitated parameters did not show statistically significant differences (Fig. 4).
Predictive Modeling.
To assess whether cryo-ET quantification provides enough information to predict whether a subject has a malignancy, and to assess whether the sample size in this study was sufficient to provide predictive power, we performed discriminant analysis with leave-one-out cross validation (18). Prediction of malignancy was made using only the number of mitochondria and the microtubule length. In 20 of 23 cases, the malignancy status was correctly predicted, yielding an accuracy of 87% in this study.
Platelet Aggregation Assay.
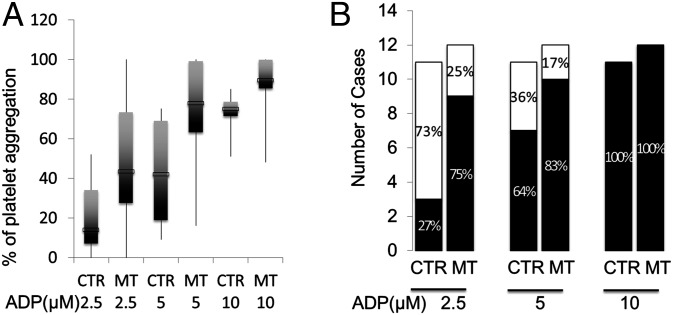
Platelet–platelet and platelet–leukocyte aggregates are often detected in peripheral blood of patients with inflammatory conditions. These aggregates are formed among primed and activated platelets, and their presence is widely recognized as a risk for thrombosis. In addition to the detectable structural differences between control and cancer platelets, we also investigated their functional consequences by measuring platelet aggregation induced by ADP at 2.5, 5, and 10 μM. Using lower concentrations of ADP allows us to detect platelet hyperreactivity, which was defined as ≥30% aggregation induced by 2.5 μM ADP. In a previous study of 359 healthy subjects, ADP at 1.5–2.5 μM was used to differentiate hypo- from hyperreactive platelets (19). This concentration of ADP does not induce significant platelet aggregation in most healthy subjects. Our data show that mean levels of platelet aggregation induced by 2.5 and 5 μM were significantly higher in the patients with ovarian cancer compared with controls (Fig. 5A). We also found that 75% of patients with ovarian cancer and 27% of our controls have hyperreactive platelets, as defined by the above criterion (Fig. 5B), indicating an enhanced response of cancer platelets to the lowest dose of ADP. At the highest concentration of ADP (10 μM), the two groups become indistinguishable in their aggregation, suggesting the platelets from patients with ovarian cancer are not activated before the addition of the agonist.
Fig. 5.
Platelet aggregation assay shows a correlation between hyperactivity and malignancy. (A) The mean level of platelet aggregation is significantly higher in patients with invasive ovarian cancer compared with control subjects (Student’s t test, n = 23). In comparison, there is no significant difference in maximal aggregation between the two groups. (B) There are significantly more patients with invasive ovarian cancer with hyperreactive platelets (black bar vs. normal platelets indicated by white bar), as defined by aggregation at 2.5 μM ADP (χ2 test, P = 0.018), but not at 5 and 10 μM of ADP compared with control subjects. CTR, control group; MT, metastatic tumor.
Discussion
Thin-section electron microscopy has revealed the morphologic features of platelets associated with a variety of (patho)physiological conditions (15), but this imaging method lacks 3D information of the subcellular contents in a platelet close to its native state (16). Cryo-ET has emerged as a viable technology to study well-preserved ultrastructure of mammalian and bacterial cells in 3D (20–22). Therefore, we used cryo-ET to examine the structures of individual platelets from patients with ovarian tumors. The acquired tomograms of platelets are rich in 3D structural information, allowing the subcellular arrangement of various components in different physiological states to be seen for the first time to our knowledge.
The platelets from the healthy subjects studied here are mostly in the resting state, as evidenced by the paucity (0.4 ± 0.7 per platelet) of extended pseudopods called filopodia, the presence of which is a structural landmark of an activated platelet. Because the platelet is crowded with numerous subcellular components, we only annotated the most recognizable features in this study. Platelets from patients with cancer share many visually identifiable subcellular features with platelets from control subjects (Fig. 4). More than 70% of α granules in all platelets were nearly spherical; the rest were irregularly shaped and morphologically heterogeneous (23). The mean diameter of the near-spherical α granules was 221.34 ± 56.03 nm, in agreement with previously reported sizes of 200–500 nm by other imaging technologies (24). The numbers of α and dense granules per tomogram for the control platelets were 43.0 ± 22.9 and 5.5 ± 1.9, respectively, which is consistent with reported counts of 50–80 and 3–9 per platelet (25). Platelets from patients with cancer appeared qualitatively to have a higher percentage area covered by enlarged LCV structures, which are likely to be either enlarged open canalicular system or empty granules, compared with those from healthy subjects and patients with benign masses (Figs. 1C and 2C).
To quantify the visual differences of the platelets, we measured the length, area, and/or number of the observed subcellular features. Each tomogram has a missing wedge resulting from limited specimen tilt angles, resulting in inaccuracy in the measurements along the direction of the electron beam. Therefore, our size quantifications of the identified features are made from their central areas instead of the volumes. Our analysis shows the mean platelet area did not differ significantly between control subjects and patients with cancer. However, three other quantitative measures showed significant difference between control and cancer platelets including microtubule length, the number of mitochondria, and the percentage of the total platelet area occupied by mitochondria.
Platelets contain fully functional mitochondria that are active in ATP production, regulation of redox signals, and platelet apoptosis (26). With cryo-ET, mitochondria are easily distinguishable in the crowded cytosol from other membranous organelles because of their double membrane and internal cristae. A significant increase in the number of, and percentage of platelet area covered by, mitochondria (Fig. 3B) suggest they may serve as one of the structural signatures to differentiate platelets of patients with invasive cancer from those of the control subjects. The number of mitochondria per platelet is elevated by about 50% among patients with malignancies, with only a small increase (<15%) in the mean size of a mitochondrion.
The biological cause for the increase in mitochondria remains further to be investigated. Activated platelets can release mitochondria in the form of microparticles (17), and mitochondria may replicate in circulating platelets (27). We occasionally observed small “platelets,” which may represent platelet microparticles, whose size can approach that of a true platelet (Fig. S1). Nevertheless, it is likely that platelet mitochondrial number is largely determined in the megakaryocyte during thrombopoiesis. Prothrombopoietic factors affecting the megakaryocyte are emitted by tumor and host tissue during ovarian malignancy. This dysregulation can cause thrombocytosis. However, thrombocytosis is observed in only a third of patients at initial diagnosis, which limits its diagnostic potential (8). Platelet mitochondrial number warrants further investigation as a potentially more sensitive marker of megakaryocytic dysregulation, and because platelet activity affects disease progression in an animal model (8).
In addition, we also detect a significant reduction in the length of microtubules. Microtubules are critical for maintaining the platelet’s discoidal shape and participate in platelet shape changes and granule movements upon platelet activation (2).
Platelet hyperreactivity, which has been reported in cancer and other diseases of inflammation but has not been morphologically explained, could be caused by the disintegration of microtubules (Fig. 3A), and in particular the disruption of the marginal band (Fig. 2 and Fig. S1). Platelet hyperreactivity means platelets are primed to activation: they form aggregates when exposed to lower concentrations of agonists than normal platelets, which require higher concentrations of agonists to activate. Hyperreactive platelets are thus not activated platelets, but they become activated more easily upon stimulation. This hyperreactivity of platelets from patients with cancer is supported by the aggregation assay (Fig. 5A). Nine of our patients with cancer (75.0%) were identified as having hyperreactive platelets (Fig. 5B) compared with three of our control subjects (27.3%; P = 0.039). There may be an underlying mechanism of cytoskeleton dysregulation in ovarian cancer causing both hyperreactivity and reduction in microtubule length that requires further study.
Abnormal platelet number (thrombocytosis) and hyperreactivity of platelets have been reported in cancer (8, 28), but have not been useful as screening tools because of their low positive predictive values. Our cryo-ET analysis demonstrates that the cancer group has more fragmented microtubules and more mitochondria in their platelets compared with the control group. In this study, the presence or absence of a malignancy was predicted with 87% accuracy solely from information contained within the cryotomograms of subjects’ platelets. These findings suggest a structural basis for the altered platelet function associated with malignancy, and indicate that platelet ultrastructure could be useful as a biomarker for ovarian cancer detection.
To further substantiate our proof-of-principle observations before becoming a clinical tool, larger patient numbers and a higher-throughput protocol will be required. Recent advances in cryo-ET suggest it can be used as a high-throughput method in a research laboratory (29–31), raising the possibility of its use in clinical laboratory. The increase in cell tomogram contrast using the Zernike phase plate technology in cryo-ET will likely ease the recognition and quantification of the observed subcellular features (32). Software algorithms are being actively developed for automatic feature extraction of filamentous features (33). Therefore, the ability of cryo-ET to measure fine ultrastructure aberrations opens the possibility for this technology to be used for large-scale biomarker development in this and other disease states.
Methods
Cryo-ET of Human Platelets.
PRP was separated from drawn blood samples and then vitrified for cryo-ET. Women with benign adnexal masses had a final pathological diagnosis of ovarian cystadenoma/fibroma (n = 4) or corpus luteum cyst (n = 1). Protocol was approved by Baylor College of Medicine Institutional Review Board, protocol no. H-12278. Patients with ovarian cancer had stage III–IV high-grade serous ovarian cancer or other ovarian malignancies (n = 12). All patients provided written informed consent for participation in this study. All samples were obtained preoperatively, before any chemotherapy treatment. Blood (2.5 mL) was drawn using a 21-gauge needle Vacutainer brand blood collection set (Becton Dickinson) into 3.8% (wt/vol) sodium citrate polyethylene tubes. The blood was immediately centrifuged at 150 × g for 20 min, and the PRP was collected. Blood and PRP were maintained at room temperature during manipulation. Quantifoil holey carbon supported transmission electron microscope grids with 3.5-µm circular holes (Quantifoil Micro Tools GmbH) pretreated with colloidal gold (fiducial tracer, 15 nm) were glow-discharged (15 s) before use. PRP (3 μL) was applied to the grid and then blotted with calcium-free blotting paper, using a Vitrobot (Mark IV, FEI Corp), and immediately plunged into liquid ethane at liquid nitrogen temperature to vitrify the platelets (1 blot, 3-s wait, or 2 blot, 2-s wait).
All platelet samples were vitrified for cryo-ET study within 1 h of blood draw to prevent any potential activation that may disturb the platelet ultrastructure.
The frozen grids with platelets were then transferred at liquid nitrogen temperature into JEOL electron cryomicroscopes (JEM 2100, JEM 2200FS, or JEM 3200FSC; JEOL Ltd.) for imaging. Low-dose conditions were used to preserve the structural integrity. For JEM2200FS and JEM3200FSC microscopes, an in-column energy filter (slit = 15 eV) was applied to enhance the image contrast by zero loss imaging. Using the SerialEM package (34), tilt series were recorded at 200 keV/300 keV on a Gatan 4,096 × 4,096 pixel CCD camera (Gatan Inc.). Images were collected at a defocus range of ∼8–15 μm and microscope magnification range of 8–12,000×. Total electron dose per tomogram was ∼75 electrons/Å2, as typically used for cryo-ET (35). Each tilt series has 62–66 CCD frames with a fixed 2-degree increment.
A total of 338 tilt series of individual platelets were recorded in this study. For each individual under study, 10–20 platelets at suitable places for imaging (e.g., not close to the grid bar) were selected without a particular bias, imaged, and processed. Platelets were selected for imaging on the basis of their positions on the microscopy grid, and not on internal features that are not visible before 3D reconstruction. Of these 10–20 platelet tomograms, at least five tomograms had adequate contrast to clearly visualize microtubules and to distinguish double-membrane mitochondria from single-membrane α granule. In general, quantifiable tomograms were randomly selected for quantification. Some subjects yielded fewer than five visually good tomograms, and were not included for further tomographic reconstruction and statistical analysis. The final data set of 120 tomograms was selected from healthy female controls (n = 6), women with benign adnexal masses (n = 5), and patients with newly diagnosed invasive ovarian cancer (n = 12).
Platelet Tomogram Processing and Quantification.
Image stacks were aligned with gold tracers, using the IMOD (version 4.0.27) package (36), yielding 3D reconstructed tomograms (voxel 4k × 4k × 1k, ∼11–13 Å per pixel sampling). To enhance the contrast, tomograms were averaged by two and filtered by denoising tools such as low-pass and nonlinear anisotropic diffusion (37) in the EMAN2 (38) and IMOD packages. Structural features such as α granules, dense granules, mitochondria, microtubules, and membrane systems were identified and manually annotated using Amira (version 5.2; Visage Imaging Inc.). Microtubules were clearly seen as a coiled circumferential marginal band in the control group and as partially fragmented filaments in some platelets in the cancer group. Granules with very dark densities were annotated as dense granules. Single membrane-bound granules with gray densities were annotated as α granules. Organelles with double membranes were annotated as mitochondria. The inner membrane of mitochondria was seen to form cristae-like structures.
Structural features (microtubule length, α granule number, α granule area, dense granule number, dense granule area, mitochondria number, mitochondria area, pseudopod number) were manually quantified for each individual platelet tomogram, using Amira. The number of pseudopods, α and dense granules, and mitochondria was counted with IMOD. The 3D tomogram of the whole platelet was projected in Z direction, and the area of the whole platelet enclosed by the plasma membrane in the projection image was measured as platelet area. A small fraction of platelets in the cancer group had part of their platelet area beyond the CCD frame. For these platelets, the area of the polygon was measured as an approximation for the whole platelet area. For mitochondria, the area of their central slice was measured individually in each tomogram and summed up as the area of mitochondria. The mitochondria area was then divided by the whole platelet area to estimate the percentage of mitochondria area. α and dense granule areas were measured in the same way. A small fraction of α and dense granules adopt an irregular shape compared with the spherically shaped majority. Irregularly shaped granules were projected in the Z direction first, and their areas in the projection image were measured. For microtubules, a mask was drawn in their central slice and then was corrected by an algorithm to remove redundant annotation (i.e., measurement of the same microtubule on consecutive sections of the tomogram; Movie S3), and the total area of the mask was measured in Amira. The algorithm to remove redundant microtubule mask is a Python script using two functions that was applied offline to the manually selected 3D mask that was produced by AMIRA. This area was then divided by 250 Å (the microtubule diameter) to estimate the microtubule length (Fig. 3). No change in significance was observed for any comparison when the redundancy-removing algorithm was omitted. All the quantification was performed by the same researcher to maintain the consistency for the entire study.
Statistical Analysis of Quantified Platelet Tomograms.
Unpaired, two-tailed Student t test was performed to test for statistically significant differences in the measured features in the platelet tomograms between the two groups (control group that comprises healthy donors and patients with benign mass; cancer group that comprises patients with ovarian cancer), with a P < 0.05 considered to be significant.
Predictive Modeling.
We evaluated the potential of cryo-ET to distinguish between subjects with or without malignancy. Each subject’s disease state was coded as malignant (n = 12) or nonmalignant (n = 11). Number of mitochondria and total microtubule length were selected as predictors for discriminant function analysis based on the qualitative observations of the individual who performed the segmentation (R.W.). Although each predictor was measured about five times per subject, we collapsed these subsamples into one mean per subject per predictor, which is a conservative approach. Because the patient sample size is small, we used leave-one-out cross validation (18). A discriminant function is built from all but one of the subjects and used to predict the malignancy status of the omitted subject. This procedure is repeated 23 times, leaving out one subject each time. Calculations were performed in JMP version 11.1 (SAS Institute).
Platelet Aggregation Assay.
Blood samples were collected, using 0.38% sodium citrate as the anticoagulant (final concentration) from control subjects and patients with invasive ovarian cancer under an approved institutional review board protocol. They were centrifuged at 150 × g for 15 min at 25 °C to collect PRP. An aliquot of PRP was loaded into a microcuvette and incubated for 10 min at 37 °C. Platelet aggregation was initiated by adding ADP (2.5, 5, and 10 μM) and monitored for 10 min in an eight-channel optical aggregometer (PAP8; Bio/Data Corp.). ADP-induced platelet aggregation was tested at multiple doses to detect platelet hyperreactivity, which was defined as ≥30% of aggregation by subthreshold concentrations of the agonist (2.5 and 5 μM), compared with the standard dose of 10 μM that induces maximal aggregation. Platelet aggregation assays were completed within 2 h after blood collection to prevent spontaneous platelet aggregation.
For the aggregation assay, data were analyzed by pair comparisons to compare levels of platelet aggregation between control subjects and patients, and by χ2 test to compare the numbers of subjects who were considered to have hyperactive platelets between patients and controls.
Supplementary Material
Acknowledgments
This research has been supported by NIH Grants P41GM103832, HL071895, HL085769, HL081613, and CA177909; Department of Defense Grants OC120547 and OC093416; an Ovarian Cancer Research Fund Program Project Development Grant; the Bettyann Asche Murray Distinguished Professorship; and a Baylor College of Medicine and MD Anderson Cancer Center Collaborative Award.
Footnotes
The authors declare no conflict of interest.
Data deposition: Representative tomograms of platelets in three different pathophysiological states reported in this paper have been deposited in the EMDataBank, www.emdatabank.org/ (accession nos. EMD-6471, EMD-6472, and EMD-6473).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1518628112/-/DCSupplemental.
References
- 1.Leslie M. Cell biology. Beyond clotting: The powers of platelets. Science. 2010;328(5978):562–564. doi: 10.1126/science.328.5978.562. [DOI] [PubMed] [Google Scholar]
- 2.Patel-Hett S, et al. Visualization of microtubule growth in living platelets reveals a dynamic marginal band with multiple microtubules. Blood. 2008;111(9):4605–4616. doi: 10.1182/blood-2007-10-118844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11(2):123–134. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemost. 2011;9(2):237–249. doi: 10.1111/j.1538-7836.2010.04131.x. [DOI] [PubMed] [Google Scholar]
- 5.Borsig L. The role of platelet activation in tumor metastasis. Expert Rev Anticancer Ther. 2008;8(8):1247–1255. doi: 10.1586/14737140.8.8.1247. [DOI] [PubMed] [Google Scholar]
- 6.Holmes CE, Levis JE, Ornstein DL. Activated platelets enhance ovarian cancer cell invasion in a cellular model of metastasis. Clin Exp Metastasis. 2009;26(7):653–661. doi: 10.1007/s10585-009-9264-9. [DOI] [PubMed] [Google Scholar]
- 7.Levin J, Conley CL. Thrombocytosis Associated with Malignant Disease. Arch Intern Med. 1964;114:497–500. doi: 10.1001/archinte.1964.03860100079008. [DOI] [PubMed] [Google Scholar]
- 8.Stone RL, et al. Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med. 2012;366(7):610–618. doi: 10.1056/NEJMoa1110352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20(5):576–590. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jurasz P, Alonso-Escolano D, Radomski MW. Platelet--cancer interactions: Mechanisms and pharmacology of tumour cell-induced platelet aggregation. Br J Pharmacol. 2004;143(7):819–826. doi: 10.1038/sj.bjp.0706013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goubran HA, Stakiw J, Radosevic M, Burnouf T. Platelet-cancer interactions. Semin Thromb Hemost. 2014;40(3):296–305. doi: 10.1055/s-0034-1370767. [DOI] [PubMed] [Google Scholar]
- 12.Gungor T, Kanat-Pektas M, Sucak A, Mollamahmutoglu L. The role of thrombocytosis in prognostic evaluation of epithelial ovarian tumors. Arch Gynecol Obstet. 2009;279(1):53–56. doi: 10.1007/s00404-008-0673-9. [DOI] [PubMed] [Google Scholar]
- 13.White JG, Krumwiede M. Some contributions of electron microscopy to knowledge of human platelets. Thromb Haemost. 2007;98(1):69–72. [PubMed] [Google Scholar]
- 14.White JG. Electron microscopy methods for studying platelet structure and function. Methods Mol Biol. 2004;272:47–63. doi: 10.1385/1-59259-782-3:047. [DOI] [PubMed] [Google Scholar]
- 15.White JG. Use of the electron microscope for diagnosis of platelet disorders. Semin Thromb Hemost. 1998;24(2):163–168. doi: 10.1055/s-2007-995836. [DOI] [PubMed] [Google Scholar]
- 16.Lucić V, Leis A, Baumeister W. Cryo-electron tomography of cells: Connecting structure and function. Histochem Cell Biol. 2008;130(2):185–196. doi: 10.1007/s00418-008-0459-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boudreau LH, et al. Platelets release mitochondria serving as substrate for bactericidal group IIA-secreted phospholipase A2 to promote inflammation. Blood. 2014;124(14):2173–2183. doi: 10.1182/blood-2014-05-573543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shao J. Linear Model Selection by Cross-Validation. J Am Stat Assoc. 1993;88(422):486–494. [Google Scholar]
- 19.Yee DL, Sun CW, Bergeron AL, Dong JF, Bray PF. Aggregometry detects platelet hyperreactivity in healthy individuals. Blood. 2005;106(8):2723–2729. doi: 10.1182/blood-2005-03-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tocheva EI, Li Z, Jensen GJ. Electron cryotomography. Cold Spring Harb Perspect Biol. 2010;2(6):a003442. doi: 10.1101/cshperspect.a003442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobs MR. In vivo veritas: In vitro macrolide resistance in systemic Streptococcus pneumoniae infections does result in clinical failure. Clin Infect Dis. 2002;35(5):565–569. doi: 10.1086/341980. [DOI] [PubMed] [Google Scholar]
- 22.Jensen GJ, Briegel A. How electron cryotomography is opening a new window onto prokaryotic ultrastructure. Curr Opin Struct Biol. 2007;17(2):260–267. doi: 10.1016/j.sbi.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 23.van Nispen tot Pannerden H, et al. The platelet interior revisited: Electron tomography reveals tubular alpha-granule subtypes. Blood. 2010;116(7):1147–1156. doi: 10.1182/blood-2010-02-268680. [DOI] [PubMed] [Google Scholar]
- 24.Blair P, Flaumenhaft R. Platelet alpha-granules: Basic biology and clinical correlates. Blood Rev. 2009;23(4):177–189. doi: 10.1016/j.blre.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charafeddine AH, et al. Platelet-derived CD154: Ultrastructural localization and clinical correlation in organ transplantation. Am J Transplant. 2012;12(11):3143–3151. doi: 10.1111/j.1600-6143.2012.04241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zharikov S, Shiva S. Platelet mitochondrial function: From regulation of thrombosis to biomarker of disease. Biochem Soc Trans. 2013;41(1):118–123. doi: 10.1042/BST20120327. [DOI] [PubMed] [Google Scholar]
- 27.Schwertz H, et al. Anucleate platelets generate progeny. Blood. 2010;115(18):3801–3809. doi: 10.1182/blood-2009-08-239558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holmes CE, Ramos-Nino ME, Littenberg B. An association between anti-platelet drug use and reduced cancer prevalence in diabetic patients: Results from the Vermont Diabetes Information System Study. BMC Cancer. 2010;10:289. doi: 10.1186/1471-2407-10-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu J, Wright ER, Winkler H. 3D visualization of HIV virions by cryoelectron tomography. Methods Enzymol. 2010;483:267–290. doi: 10.1016/S0076-6879(10)83014-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suloway C, et al. Fully automated, sequential tilt-series acquisition with Leginon. J Struct Biol. 2009;167(1):11–18. doi: 10.1016/j.jsb.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nickell S, et al. TOM software toolbox: Acquisition and analysis for electron tomography. J Struct Biol. 2005;149(3):227–234. doi: 10.1016/j.jsb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 32.Dai W, et al. Visualizing virus assembly intermediates inside marine cyanobacteria. Nature. 2013;502(7473):707–710. doi: 10.1038/nature12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rigort A, et al. Automated segmentation of electron tomograms for a quantitative description of actin filament networks. J Struct Biol. 2012;177(1):135–144. doi: 10.1016/j.jsb.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 34.Mastronarde DN. Automated electron microscope tomography using robust prediction of specimen movements. J Struct Biol. 2005;152(1):36–51. doi: 10.1016/j.jsb.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 35.Iancu CV, Wright ER, Heymann JB, Jensen GJ. A comparison of liquid nitrogen and liquid helium as cryogens for electron cryotomography. J Struct Biol. 2006;153(3):231–240. doi: 10.1016/j.jsb.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Kremer JR, Mastronarde DN, McIntosh JR. Computer visualization of three-dimensional image data using IMOD. J Struct Biol. 1996;116(1):71–76. doi: 10.1006/jsbi.1996.0013. [DOI] [PubMed] [Google Scholar]
- 37.Frangakis AS, Hegerl R. Noise reduction in electron tomographic reconstructions using nonlinear anisotropic diffusion. J Struct Biol. 2001;135(3):239–250. doi: 10.1006/jsbi.2001.4406. [DOI] [PubMed] [Google Scholar]
- 38.Tang G, et al. EMAN2: An extensible image processing suite for electron microscopy. J Struct Biol. 2007;157(1):38–46. doi: 10.1016/j.jsb.2006.05.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.