The unprecedentedly devastating Ebola epidemic in West Africa brought international attention to the challenges faced by resource-constrained nations in curtailing outbreaks. As the epidemic tapers in Sierra Leone and Guinea, the focus of epidemiologists has shifted from emergency response toward retrospection. Lessons learned from this outbreak will be fundamental for establishing preparedness strategies and for averting future epidemics. In a masterful data-driven modeling study in PNAS, Kucharski et al. (1) quantified the extent to which the international effort to provide more treatment beds prevented new infections across the 12 districts of Sierra Leone, as well as the incremental benefit that could have been achieved if the provision had been earlier in the epidemic.
Approximately US $1.6 billion, corresponding to 70% of the funds committed by the interagency response plan for Liberia, Sierra Leone, and Guinea, was allocated between October 2014 and June 2015 (2). The response involved provision of personal protective equipment, training of health workers regarding infection prevention, hazard compensation to frontline workers, vehicles, door-to-door surveillance, and outreach efforts (3). However, the majority of funding was designated for construction and maintenance of Ebola holding centers (EHCs), community care centers (CCCs), and Ebola treatment units (ETUs). Such funding commitments were made months into the outbreak, and actual construction of the majority of treatment centers was likewise delayed. There has thus been considerable debate surrounding the contribution of case isolation beds to controlling the outbreak, as well as the repercussions of the delay.
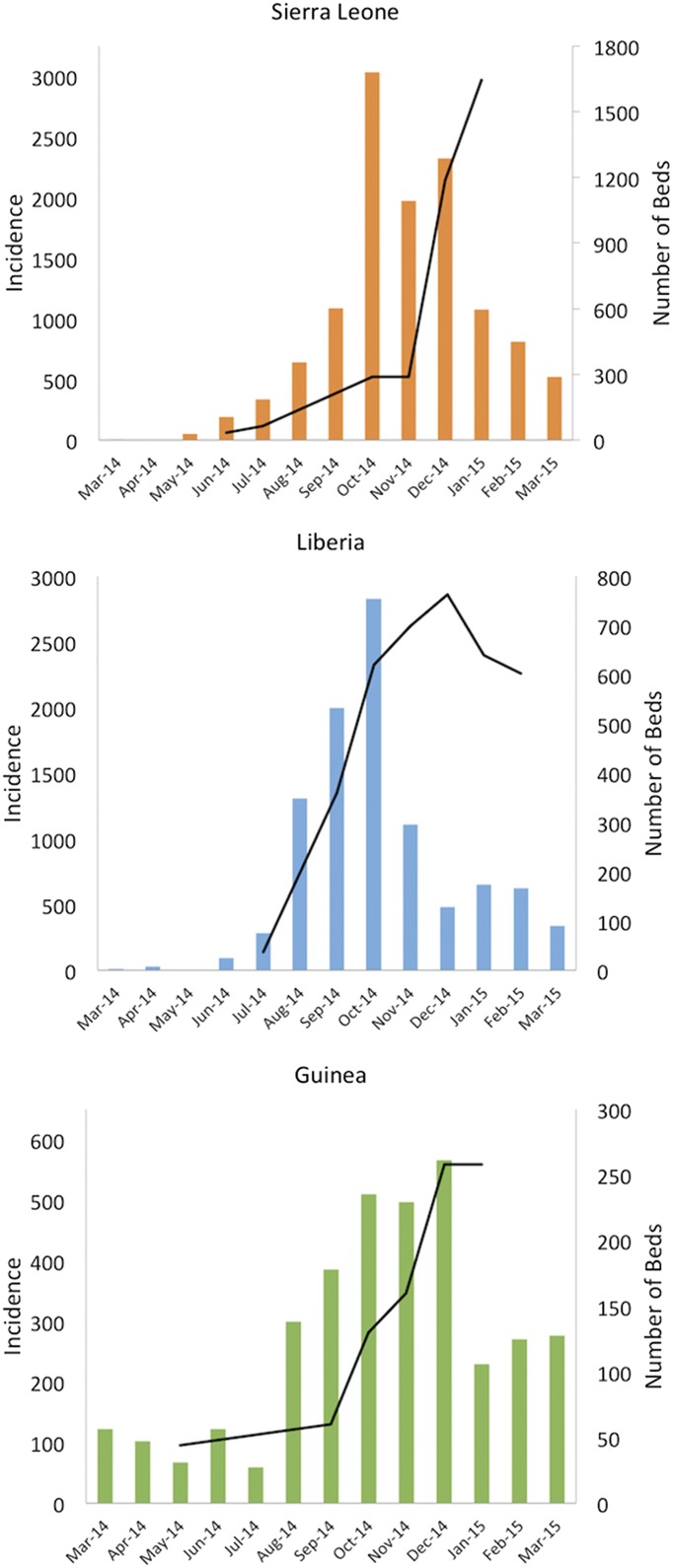
In each of the three West African nations most affected by the epidemic, Ebola incidence was observed to diminish shortly after the majority of beds was provided (Fig. 1). Although these temporal correlations are informative about general associations between bed capacity and incidence, they do not demonstrate causation. They cannot disentangle myriad other potentially confounding factors. For instance, as beds were being made available, endogenous behavioral changes (4) and rollout of other interventions were concomitantly having an impact on the transmission rate. To incorporate this geographic and temporal variation while simultaneously deciphering the impact of the international effort to supply beds, Kucharski et al. (1) applied a sophisticated time-varying rate that captures heterogeneity in transmission beyond the heterogeneity that is explained by the introduction of beds.
Fig. 1.
Bed capacity and monthly incidence of Ebola in Sierra Leone, Liberia, and Guinea. Significant increases in bed capacity were observed through early 2015 in all three countries (9–13), with incidence peaking in or before December 2014 (14).
Kucharski et al. (1) have elegantly executed an anthem of data-driven mathematical modeling. Reality is complex, yet no model can be expected to foresee or even retrospectively capture all effects on the system. Accordingly, Kucharski et al. (1) avoided relying on too many assumptions (5) but still constructed a model specific enough to evaluate the importance of the factor under consideration: isolation beds. Rather than specify a parameter-heavy model that attempts to distinguish exhaustively between many behaviors and interventions, they used a flexible sigmoid function to represent temporal changes in transmission intensity in each district of Sierra Leone. Capturing these changes was vital: Although some districts were able to extinguish transmission relatively rapidly, others remained hotspots for much longer. An interplay between behavioral changes and response measures likely accounted for considerable variation in the rapidity of Ebola control among districts. Over the course of the epidemic, transmission intensity varied in response to increasingly cautious patterns of human contact because of heightened awareness and risk perceptions, as well as responding to diminishing time to case isolation. In Liberia, for example, the probability of transmission occurring within households appears to have increased from July through September, the months of most intense transmission, and other contact types with people outside the household declined, suggesting that people were spending more time at home. Additionally, time from symptom onset to hospitalization peaked in July and then consistently decreased through November in Liberia—as it did in Sierra Leone (1). As interesting as individual-level shifts in behavior might be, explicitly incorporating such details would add complexity, yet would be unlikely to be informative with regard to evaluating the effectiveness of increased bed capacity. Instead, to quantify the impact of the internationally supplied beds in EHCs, CCCs, and ETUs, Kucharski et al. (1) used a Bayesian approach that accounts for spatial and temporal changes in transmission without relying on assumptions that are challenging to parameterize or difficult to represent in a computationally feasible way.
Using this Bayesian approach, Kucharski et al. (1) demonstrate that the provision of beds saved many lives in Sierra Leone even as the epidemic began to dwindle, a finding that will be reassuring to many governments and nongovernmental organizations that contributed to these efforts, as well as instructive for preparedness plans to mitigate future outbreaks. They diligently assessed the uncertainty inherent to their model fit to data and examined the sensitivity of their results to a key parameter that has plagued modeling efforts: the degree of underreporting. There remains considerable uncertainty regarding how many sick individuals stayed at home, died without being diagnosed, or otherwise evaded surveillance measures. To understand why underreporting is so important to the effectiveness of contact tracing, case isolation, and the impact of increased bed capacity, consider two extremes: zero reporting would mean these measures would be completely ineffective, whereas perfect reporting would provide the greatest opportunity for intervention, given adequate resources to do so. In the classic mathematical modeling rubric of “known knowns,” “known unknowns,” and “unknown unknowns,” underreporting during the Ebola outbreak is a known unknown: its existence is widely recognized, but its extent is elusive to quantify (6), as well as being highly geographically and temporally variable (7). To address this known unknown, Kucharski et al. (1) performed a sensitivity analysis spanning three plausible values. They show that while underreporting affects the cumulative incidence of the outbreak, provision of beds always significantly reduced the death toll.
The degree of dependence of case isolation on active surveillance is another known unknown that closely relates to underreporting, because people could be underusing beds if surveillance teams were unaware of cases or could not coordinate an efficient response. Where an integrated intervention approach with culturally sensitive messaging and community-led initiatives was applied, hotspots were revealed that had been
Kucharski et al. avoided relying on too many assumptions but still constructed a model specific enough to evaluate the importance of the factor under consideration: isolation beds.
hidden by community members as a consequence of distrust in the Ebola response (8). Once beds became available, field-based identification of infected individuals was often led by grassroots organizations. Differences in the implementation and effectiveness of community efforts to identify and report sick individuals likely contributed to how the endgame has varied between and even within the three most affected countries.
Kucharski et al. (1) did incorporate computational complexity where it is important. Their use of a stochastic approach addresses the potentially highly variable epidemic spread that is characteristic of an outbreak with relatively low prevalence and socially clustered transmission. The many lives that were demonstrated to be saved by increased availability of beds are consistent with the high social clustering of Ebola transmission: manifest in that when an index case transmits disease to a second individual, and a second transmits disease to a third, then that third individual is highly likely to be a social contact of the first. A consequence of clustering of social transmission is that community surveillance, contact tracing, and case isolation can be extraordinarily effective in curbing epidemic spread, because social contacts obtained from the ill are then highly meaningful predictors of who will likely be the next victims of disease. Early in the West African epidemic, it was extremely challenging for severely resource-constrained governments in West Africa to mobilize an effective contact-tracing program rapidly, and nearly impossible to provide effective case isolation. Once contact tracing became more rapid and effective, and case isolation beds became available, disease spread was much more effectively curtailed.
The results of Kucharski et al. (1) resolve the controversy that has been percolating the last several months regarding the value of expansion of bed capacity, and thus provide a critical piece to our retrospective understanding of the 2014–2015 Ebola outbreak, demonstrating that international efforts to supply treatment beds to the afflicted nation of Sierra Leone saved many lives. The finding that the availability of more beds earlier could have averted a further 13,000 cases highlights the importance of sustainable capacity building within West Africa to mitigate future resurgence of Ebola and other emerging diseases.
Footnotes
The authors declare no conflict of interest.
See companion article on page 14366.
References
- 1.Kucharski AJ, et al. Measuring the impact of Ebola control measures in Sierra Leone. Proc Natl Acad Sci USA. 2015;112:14366–14371. doi: 10.1073/pnas.1508814112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Financial Tracking Service (FTS) Tracking Global Humanitarian Aid Flows (2014) Ebola Virus Outbreak-WEST AFRICA-April 2014. Available at: https://fts.unocha.org/pageloader.aspx?page=emerg-emergencyDetails&emergID=16506. Accessed September 23, 2015.
- 3. World Bank (2015) World Bank Group Ebola Response Fact Sheet. Available at www.worldbank.org/en/topic/health/brief/world-bank-group-ebola-fact-sheet. Accessed October 2, 2015.
- 4.Funk S, Knight GM, Jansen VAA. Ebola: The power of behaviour change. Nature. 2014;515(7528):492. doi: 10.1038/515492b. [DOI] [PubMed] [Google Scholar]
- 5.May RM. Uses and abuses of mathematics in biology. Science. 2004;303(5659):790–793. doi: 10.1126/science.1094442. [DOI] [PubMed] [Google Scholar]
- 6.Scarpino SV, et al. Epidemiological and viral genomic sequence analysis of the 2014 ebola outbreak reveals clustered transmission. Clin Infect Dis. 2015;60(7):1079–1082. doi: 10.1093/cid/ciu1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atkins KE, et al. Under-reporting and case fatality estimates for emerging epidemics. BMJ. 2015;350:h1115. doi: 10.1136/bmj.h1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fallah F, et al. Interrupting Ebola transmission in Liberia through community-based initiatives. Ann Intern Med. 2015 doi: 10.7326/M15-1464. in press. [DOI] [PubMed] [Google Scholar]
- 9. Humanitarian Data Exchange (2015) Ebola Treatment Centers or Units (ETCs or ETUs). Available at https://data.hdx.rwlabs.org/dataset/ebola-treatment-centers. Accessed October 2, 2015.
- 10. World Health Organization (2015) Ebola Situation Reports, 5 September 2014–14 January, 2015. Available at: apps.who.int/ebola/ebola-situation-reports. Accessed October 1, 2015.
- 11.Nyenswah T, et al. Centers for Disease Control and Prevention (CDC) Ebola epidemic--Liberia, March-October 2014. MMWR Morb Mortal Wkly Rep. 2014;63(46):1082–1086. [PMC free article] [PubMed] [Google Scholar]
- 12.Doctors Without Borders 2014 The Race To Control Ebola in Sierra Leone. Available at www.doctorswithoutborders.org/news-stories/field-news/race-control-ebola-sierra-leone. Accessed October 2, 2015.
- 13.World Health Organization 2015 The Guinean town that overcame Ebola. Available at www.who.int/features/2014/telimele-ebola-free/en/. Accessed October 2, 2015.
- 14. Rivers CM (2015) Data for the 2014 ebola outbreak in West Africa. GitHub. Available at: https://github.com/cmrivers/ebola. Accessed October 2, 2015.