Abstract
Background
Patients with left ventricular (LV) systolic dysfunction, coronary artery disease (CAD), and angina are often thought to have a worse prognosis and a greater prognostic benefit from coronary artery bypass graft (CABG) surgery than those without angina.
Objectives
We investigated whether: 1) angina is associated with a worse prognosis; 2) angina identified patients who had a greater survival benefit from CABG; and 3) whether CABG improved angina in patients with LV systolic dysfunction and CAD.
Methods
We performed an analysis of the Surgical Treatment for Ischemic Heart Failure trial, in which 1,212 patients with an ejection fraction ≤35% and CAD were randomized to CABG or medical therapy. Multivariable Cox and logistic models were used to assess long-term clinical outcomes.
Results
At baseline, 770 patients (64%) reported angina. Amongst patients assigned to MED, all-cause mortality was similar in patients with and without angina (HR: 1.05; 95% CI: 0.79 to 1.38). The effect of CABG was similar whether the patient had angina (HR: 0.89; 95% CI: 0.71 to 1.13) or not (HR: 0.68; 95% CI: 0.50 to 0.94) (p interaction = 0.14). Patients assigned to CABG were more likely to report improvement in angina than those assigned to medical therapy alone (OR: 0.70; 95% CI: 0.55 to 0.90; p < 0.01).
Conclusions
Angina does not predict all-cause mortality in medically treated patients with LV systolic dysfunction and CAD, nor does it identify patients who have a greater survival benefit from CABG. However, CABG does improve angina to a greater extent than medical therapy alone.
(Comparison of Surgical and Medical Treatment for Congestive Heart Failure and Coronary Artery Disease [STICH]: NCT00023595)
Keywords: Coronary Artery Bypass Grafting, Coronary Artery Disease, Heart Failure, Mortality
Introduction
Coronary artery bypass grafting (CABG) is recommended in patients with angina (1), coronary artery disease (CAD), and left ventricular (LV) systolic dysfunction (2,3). However, compelling evidence that the presence or absence of angina should guide decisions about revascularization is lacking (4). Tests of myocardial viability or stress-induced ischemia have failed, so far, to identify a subset of patients with heart failure who have more to gain from CABG compared to medical therapy alone in randomized controlled trials (5,6).
Clinical guidelines identify angina as an important consideration when deciding whether patients with heart failure and reduced LV function should have CABG (7). Angina pectoris signals the presence of viable myocardium that is prone to ischemia and at risk of infarction, and may therefore confer an adverse outcome (8). Patients with heart failure and CAD who do not have angina might have less jeopardized myocardium at risk of ischemia, indicating a better prognosis, or have cardiac denervation rendering myocardial ischemia silent, or a substantial volume of myocardium affected by hibernation or replaced by scar, which could signal an adverse prognosis (9,10). Surprisingly, information on the prognostic significance of angina in patients with heart failure who are known to have CAD has not been reported. Accordingly, we conducted an analysis of the Surgical Treatment for Ischemic Heart Failure (STICH) trial data to address 3 questions: 1) Does the presence of angina influence outcomes in patients with heart failure and CAD managed without resort to revascularization? 2) Does angina identify patients who will experience a greater survival benefit from CABG? 3) Does CABG improve angina in this population?
Methods
Study population
The rationale and design of the STICH trial were published previously (11). STICH was a prospective, multicenter, randomized trial sponsored by the National Heart, Lung and Blood Institute (NHLBI) that recruited patients with CAD and an LV ejection fraction of 35% or less between 2002 and 2007 (NCT00023595) (4). For the present study, 1,212 participants enrolled in the surgical revascularization hypothesis were included. This part of the STICH trial assessed whether CABG combined with optimal medical therapy improved survival compared with optimal medical therapy alone. The inclusion and exclusion criteria and the requirements for ensuring high-quality surgical revascularization have been described (12). The NHLBI and the ethics committee at each recruiting institution approved the study protocol. All patients provided written informed consent. All authors have read and agreed to the paper as written.
Study outcomes
A blinded clinical events committee adjudicated all deaths using pre-specified criteria (4,11). Angina status was assessed using the Canadian Cardiovascular Society (CCS) classification at baseline and at each follow-up visit. Angina relief was primarily defined as an improvement of ≥1 CCS angina class.
Statistical analysis
Continuous variables are presented using the median (interquartile range) and compared using Wilcoxon rank-sum tests. Discrete variables are presented as counts (percentages), and compared using either Pearson’s chi-square or the Fisher exact test, as appropriate. For time-to-event analysis, Kaplan–Meier survival estimates were used to estimate event rates over the long-term follow-up for patients who did and did not receive CABG surgery (13). Statistical significance was determined at the 2-sided α = 0.05 level. No adjustments were made for multiple comparisons, as this analysis should be considered exploratory, rather than definitive. Data analyses were performed with SAS software package (version 9.4, SAS Institute, Cary, North Carolina).
Modeling of outcomes
For each research question, a multivariable model was developed to adjust for known or expected confounding variables. For each model, candidate variables were pre-specified, using either clinical experience or previously reported risk factors (4,5,14). The primary analysis was on the basis of the intention-to-treat principle. We repeated the analyses according the treatments actually received (rather than assigned) to ascertain the influence of crossover outcomes.
The first analysis ascertained the relationship between angina and all-cause mortality (primary endpoint), and the composite of all-cause mortality or all-cause hospitalization (secondary endpoint) exclusively in patients assigned to medical therapy alone. The second analysis compared the effect of CABG versus medical therapy alone on all-cause mortality and the composite of all-cause mortality or hospitalization in patients with and without angina, and sought potential interactions between the presence of angina and randomly assigned group (see Online Supplementary Methods). For both analyses, adjustment for baseline risk factors was implemented by utilizing a multivariable Cox proportional hazards model to estimate the adjusted hazard ratio and its 95% confidence interval (CI) (15). In both models, the presence of angina was dichotomized as no angina (CCS = 0) versus angina (CCS = 1 or above). However, to assess the stability of our models across the spectrum of angina severity, we conducted alternate analyses after stratifying angina as absent (CCS = 0), mild (CCS = 1), and moderate or severe (CCS >1).
The third analysis investigated the relationship between randomization to CABG or medical therapy alone and the relief of angina. For the purpose of this analysis, we modeled a multivariable logistic regression in which the primary endpoint was defined as improvement of ≥1 CCS angina class from baseline to last available follow-up. The candidate independent predictors were identical to previous analyses, with the addition of change in beta-blocker and nitrate use from baseline and at follow-up. The robustness of the model was tested in a series of sensitivity analyses (see Online Supplementary Methods).
The authors reviewed the data, participated in the analyses, and wrote the manuscript, and assume responsibility for the completeness and accuracy of the data and the analyses, and for the fidelity of the study to the trial protocol.
Results
Study population
Of 1,212 patients enrolled, 770 patients (63.5%) reported angina at baseline (187 in CCS class I, 525 in CCS class II, and 58 in CCS class III/IV) and 442 patients (36.5%) reported having no angina. In each angina group, the proportion of patients assigned to CABG or medical therapy alone was similar (Table 1). Final follow-up status for all-cause mortality and all-cause hospitalization was ascertained for 1,207 patients (99.6%) over a median follow-up time of 56 months.
Table 1.
Baseline Characteristics by Presence of Angina in the STICH Trial
| Characteristics | Angina (n = 770) |
No angina (n = 442) |
p Value |
|---|---|---|---|
| Treatment Group | 0.52 | ||
| Medical therapy alone | 377 (49.0%) | 225 (50.9%) | |
| CABG + medical therapy | 393 (51.0%) | 217 (49.1%) | |
| Demographics | |||
| Age, median (IQR), yrs | 59 (53, 66) | 61 (54–69) | <0.001 |
| Sex, male, number (%) | 671 (87.1%) | 393 (88.9%) | 0.37 |
| BMI, median (IQR), kg/m2 | 27 (24, 30) | 27 (24–30) | 0.76 |
| Race or ethnic group, number (%) | <0.001 | ||
| White | 494 (64.2%) | 333 (75.3%) | |
| Hispanic, Latino, or nonwhite | 276 (35.8%) | 109 (24.7%) | |
| Medical history, number (%) | |||
| Previous myocardial infarction | 633 (82.2%) | 301 (68.1%) | <0.001 |
| Diabetes mellitus | 278 (36.1 %) | 200 (45.2 %) | <0.01 |
| Hypertension | 454 (59.0 %) | 274 (62.0 %) | 0.30 |
| Hyperlipidemia | 436 (56.6 %) | 294 (66.5 %) | <0.001 |
| Previous coronary artery bypass surgery | 24 (3.1 %) | 12 (2.7 %) | 0.69 |
| Previous percutaneous coronary intervention | 97 (12.6 %) | 59 (13.3 %) | 0.71 |
| Chronic renal insufficiency* | 44 (5.7 %) | 50 (11.3 %) | <0.001 |
| Previous stroke | 48 (6.2 %) | 44 (10.0 %) | 0.02 |
| Atrial flutter/fibrillation | 83 (10.8%) | 70 (15.8%) | 0.01 |
| Peripheral vascular disease | 109 (14.2%) | 75 (17.0%) | 0.19 |
| Presenting characteristics | |||
| Systolic blood pressure, median (IQR), mm Hg | 120 (110, 130) | 120 (110–130) | 0.002 |
| CSS class, number (%) | |||
| 0 | –– | 442 (100%) | |
| I | 187 (24.3%) | –– | |
| II | 525 (68.2%) | –– | |
| III | 48 (6.2%) | –– | |
| IV | 10 (1.3%) | –– | |
| NYHA class, number (%) | <0.001 | ||
| I | 261 (33.9%) | 174 (39.6%) | |
| II | 373 (48.4%) | 181 (41.2%) | |
| III | 112 (14.5%) | 69 (15.7%) | |
| IV | 24 (3.1%) | 15 (3.4%) | |
| Hemoglobin, median (IQR), g/dl | 14 (13, 15) | 14 (13–15) | 0.23 |
| Creatinine, median (IQR), mg/dl | 1.09 (0.94, 1.24) | 1.10 (0.94–1.30) | 0.04 |
| Left ventricular ejection fraction, median (IQR), % | 28 (22, 34) | 27 (22–33) | 0.06 |
| Left ventricular end-systolic volume indexed | 80 (61, 108) | 83 (62–109) | 0.44 |
| Myocardial viability† | 285 (78.1%) | 202 (85.6%) | 0.02 |
| Mitral regurgitation, number (%) | 0.11 | ||
| none/trace (1+) | 261 (33.9%) | 174 (39.6%) | |
| mild (2+) | 373 (48.4%) | 181 (41.2%) | |
| moderate (3+) | 112 (14.5%) | 69 (15.7%) | |
| severe (4+) | 24 (3.1%) | 15 (3.4%) | |
| Angiographic information | |||
| Duke CAD index, median (IQR) | 65 (39, 77) | 52 (39, 77) | 0.29 |
| Left main disease | 19 (2.5%) | 13 (2.9%) | 0.62 |
| 1-vessel disease | 76 (9.9%) | 36 (8.1%) | 0.32 |
| 2-vessel disease | 230 (29.9%) | 136 (30.8%) | 0.74 |
| 3-vessel disease | 463 (60.1%) | 270 (61.1%) | 0.74 |
| Medications, number (%) | |||
| Beta-blockers | 662 (86.0%) | 374 (84.6%) | 0.52 |
| Long-acting nitrates | 484 (62.9%) | 162 (36.7%) | <0.001 |
Defined as creatinine >1.5: mg/dl.
A total of 601 patients underwent viability assessment in the trial.
BMI = body mass index; CABG = coronary artery bypass grafting; CAD = coronary artery disease; CCS = Canadian Cardiovascular Society; IQR = interquartile range; NYHA = New York Heart Association
Baseline characteristics
The baseline characteristics and the burden of CAD of patients who did or did not report angina were similar, with some exceptions. Patients of Caucasian origin reported less angina. Patients who reported angina were younger, were more likely to have experienced a previous myocardial infarction (MI) and to be treated with long-acting nitrates, but were less likely to have a history of atrial fibrillation, renal dysfunction, diabetes mellitus, stroke, or hyperlipidemia (Table 1). There was no significant difference in angina status at baseline between treatment arms (p = 0.52).
Angina and outcomes in patients assigned to medical therapy alone
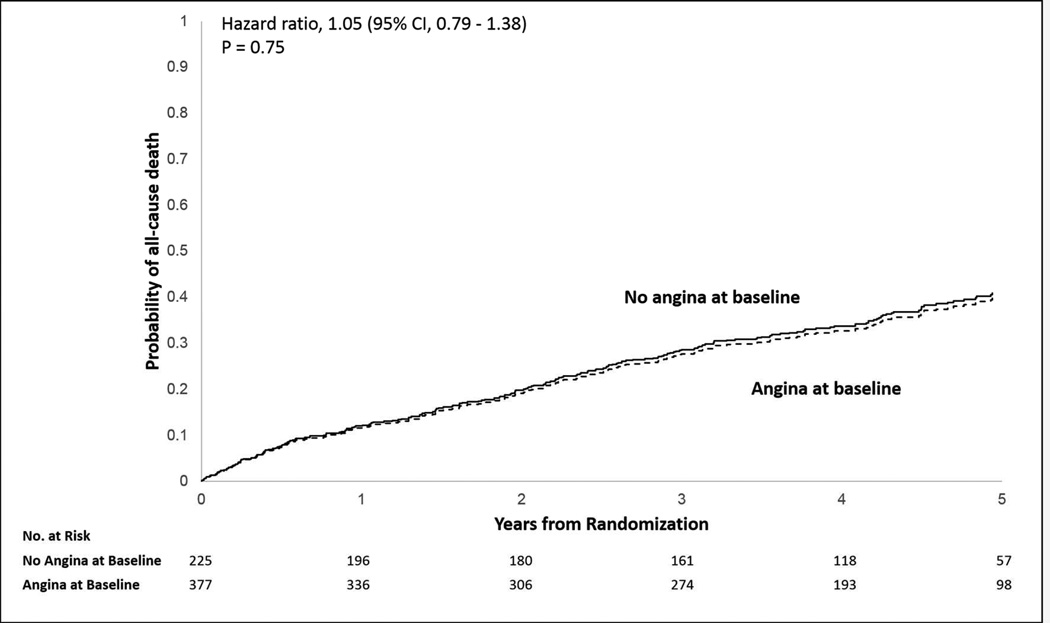
Baseline characteristics of patients assigned to medical therapy alone with and without angina are compared in Online Table 1. Among patients assigned to medical therapy alone (n = 602), the presence or absence of angina at baseline was not associated with all-cause mortality (HR: 1.05; 95% CI: 0.79 to 1.38; p = 0.74, for absence of angina) (Figure 1, and Online Table 2A). However, after stratification, moderate to severe angina (CCS ≥2) as compared with no angina was associated with all-cause mortality (HR: 1.27; 95% CI: 1.04 to 1.57; p = 0.02) (Online Table 2B). Angina was not associated with a greater risk of the composite of all-cause mortality or hospitalization (HR: 1.05; 95% CI: 0.86 to 1.28; p = 0.64).
Figure 1. Kaplan-Meier Estimates: Cumulative Incidence of All-Cause Mortality in Medically Treated Patients With and Without Angina.
Amongst patients assigned to medical therapy, all-cause mortality was similar in patients with and without angina (HR: 1.05; 95% CI: 0.79 to 1.38).
Influence of angina on the impact of CABG on all-cause death, hospitalizations, and cardiovascular endpoints
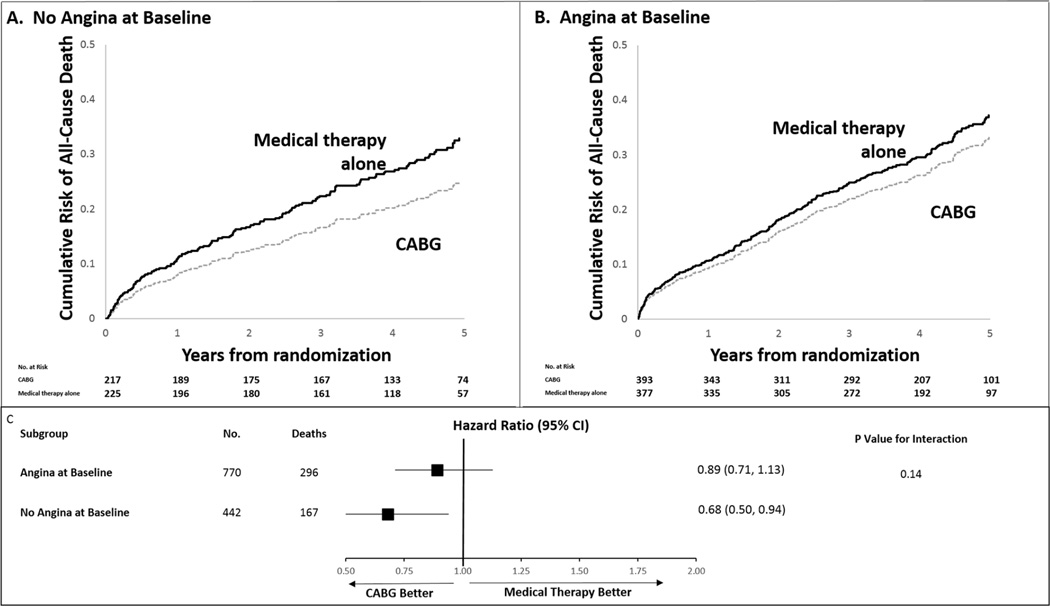
In patients with angina, all-cause mortality was similar among patients assigned to CABG or medical therapy alone (37.4% vs. 39.5%; adjusted HR: 0.89; 95% CI: 0.71 to 1.13; p = 0.34). In patients without angina, mortality was lower in patients assigned to CABG (32.7% vs. 42.7%; adjusted HR: 0.68; 95% CI: 0.50 to 0.94; p = 0.02) (Table 2, Figure 2). However, the interaction between the presence of angina and the effect of CABG versus medical therapy on survival was not significant (p = 0.14). The composite of all-cause mortality or hospitalizations was reduced to a similar extent in patients assigned to CABG, whether they had angina or not (HR: 0.78; 95% CI: 0.66 to 0.93, and HR: 0.80; 95% CI: 0.64 to 1.00, respectively) (Table 2).
Table 2.
Stratified Outcomes Analysis for Medical Therapy Alone Versus CABG by Presence of Angina at Baseline
| Event | Medical Therapy Alone (n = 602) |
CABG + Medical Therapy (n = 610) |
Adjusted Hazard Ratio for CABG + Medical Therapy (95% CI)*† |
p Value |
|---|---|---|---|---|
| No angina at baseline (n = 442) | (n = 225) | (n = 217) | ||
| All-cause mortality | 0.68 (0.50–0.94) | 0.02 | ||
| Number of events (crude event rate, %) | 96 (42.7%) | 71(32.7%) | ||
| KM estimate of 5-yr event rate (%) | 41.0% | 31.9% | ||
| Adjusted Cox proportional hazard estimates of cumulative risk of all-cause death at 5 yrs (%) | 32.9% | 25.0% | ||
| All-cause mortality or all-cause hospitalization | 0.80 (0.64–1.00) | 0.05 | ||
| Number of events (crude event rate, %) | 172 (76.4%) | 149 (68.7%) | ||
| KM estimate of 5-yr event rate (%) | 79.0% | 68.1% | ||
| Angina at baseline (n =770) | (n = 377) | (n = 393) | ||
| All-cause mortality | 0.89 (0.71–1.13) | 0.34 | ||
| Number of events (crude event rate, %) | 149 (39.5%) | 147 (37.4%) | ||
| KM estimate of 5-year event rate (%) | 39.8% | 36.8% | ||
| Adjusted Cox proportional hazard estimates of cumulative risk of all-cause death at 5 yrs (%) | 37.2% | 33.2% | ||
| All-cause mortality or all-cause hospitalization | 0.78 (0.66–0.93) | <0.001 | ||
| Number of events (crude event rate, %) | 270 (71.6%) | 250 (63.6%) | ||
| KM estimate of 5-year event rate (%) | 72.2% | 63.8% | ||
Of 1,212 patients randomized in STICH, 1,205 with known angina status at baseline were included in the multivariable statistical analysis. Implantation of a left ventricular assist device during follow-up was considered as equivalent to death (n = 1).
All-cause death analysis was adjusted for LVEF, age, BMI (<35), log of creatinine (0 to 0.4), peripheral vascular disease, mitral regurgitation, beta-blocker at baseline, and atrial fibrillation/flutter. The p interaction for angina and treatment (medical therapy alone or CABG) = 0.14
All-cause death plus all-cause hospitalization analyses were adjusted for treatment group (CABG vs. medical therapy alone), LVEF, age, white race, log of creatinine (< 0.4), hemoglobin, mitral regurgitation, and NYHA classification. The p interaction for angina and treatment (medical therapy alone or CABG) = 0.99.
The following values were assigned to baseline covariates for adjusted event rates: age = 60 years; LVEF = 28; BMI = 27; log2(creatinine) = 0.14; PVD = 0.15; mitral valve regurgitation (moderate/severe) = 0.18; beta-blocker = 0.85; and atrial fibrillation/flutter = 0.13.
KM = Kaplan-Meier; LVEF = left ventricular ejection fraction. Other abbreviations as in Table 1.
Figure 2. Adjusted Cox Proportional Hazards Estimates of the Cumulative Risk of All-Cause Mortality According to Angina Status and Treatment Arm.
The effect of CABG was similar whether the patient had angina (HR: 0.89; 95% CI: 0.71 to 1.13) or not (HR: 0.68; 95% CI: 0.50 to 0.94) (p interaction = 0.14). Analyses adjusted for LVEF, age, BMI (above or below 35), log of creatinine (0 to 0.4), peripheral vascular disease, mitral regurgitation, beta-blockers at baseline, atrial fibrillation/flutter. BMI = body mass index; CABG = coronary artery bypass graft; CI = confidence interval; LVEF = left ventricular ejection fraction.
Further stratifying patients according to severity of angina yielded similar results. Mortality rates in those assigned to CABG rather than to medical therapy alone, were similar for patients in CCS I (34.4% vs. 38.5%; HR: 0.83; 95% CI: 0.49 to 1.39; p = 0.47) and CCS >1 (39.9% vs. 38.4%; HR: 0.94; 95% CI: 0.73 to 1.23; p = 0.66) (Online Table 3). When crossovers from one treatment arm to another were considered, mortality was lower in patients with and without angina if they had CABG (HR: 0.67; 95% CI: 0.53 to 0.85, and HR: 0.63; 95% CI: 0.47 to 0.88, respectively) (Online Figure 1, Online Table 4). Data for other cardiovascular endpoints is presented in Online Table 5.
Effect of CABG and medical therapy on relief of angina
Eighty-five patients died in hospital after CABG or in the interval before the first follow-up outpatient visit at 4 months. Of the remaining 1,127 patients, an evaluation of angina was available in 1,089 (97%), with a median follow-up time of 52 months (Online Figure 2).
Of the 1,089 patients with information on angina available at follow-up, 494 (45.4%) reported an improvement in angina: 50% of those assigned to the CABG + medical therapy and 41% of those assigned to medical therapy alone. Patients reporting angina at baseline were less likely to have worsening CCS angina class if assigned to CABG rather than medical therapy alone (odds ratio: 0.70; 95% CI: 0.55 to 0.90; p < 0.01). The positive association between CABG and relief of angina persisted in all sensitivity analyses (Table 3).
Table 3.
Odds Ratios of Worsening Angina for Patients Treated with CABG Compared With Patients Treated with Medical Therapy Alone
| Models | OR (95% CI) | p Value |
|---|---|---|
| Unadjusted data | 0.70 (0.55–0.89) | < 0.01 |
| Third principal model* | 0.70 (0.55–0.90) | < 0.01 |
| Sensitivity analyses | ||
| 1. Proportional odds model (angina as 3-level categorical variable)† | 0.69 (0.55–0.87) | < 0.01 |
| 2. Third principal model + adjusted for ntiangina medication post-randomization | 0.78 (0.60–1.00) | 0.05 |
| 3. Worst-case scenario | 0.77 (0.61–0.98) | 0.03 |
Of the 1,212 patients randomized in STICH, 1,089 with angina assessed at least once after randomization were included in the statistical model
The model is the final adjusted binary logistic regression model. The model remained stable after internal validation (OR: 0.69; 95% CI: 0.54 to 0.89), and after crossover patients were taken into consideration (OR: 0.57; 95% CI: 0.45 to 0.74; p < 0.01).
Proportional odds model defines angina relief as an ordinal 3-level categorical variable with values representing worsening angina, stable angina, and angina relief. The odds ratio supplied is predicting the probability of angina relief averaging over worsening angina and stable angina.
Discussion
These analyses of the STICH trial are the first to investigate the impact of angina on outcomes in a randomized controlled study evaluating the benefits of CABG in patients with CAD, heart failure, and LV systolic dysfunction. Angina does not predict prognosis in medically treated patients with known CAD, nor does the presence or absence of angina identify patients with LV dysfunction and CAD who have a greater benefit from CABG (Central Illustration). Although mortality was lower in patients assigned to CABG who did not have angina, the interaction between assigned treatment and angina at baseline was not significant. When crossovers were considered, the observed reduction in mortality with CABG was significant and of similar magnitude in patients with and without angina. Finally, as may be expected, CABG improves angina compared with medical therapy alone. These findings have important clinical implications, given the paucity of prior evidence to guide decision-making in patients with angina and LV systolic dysfunction.
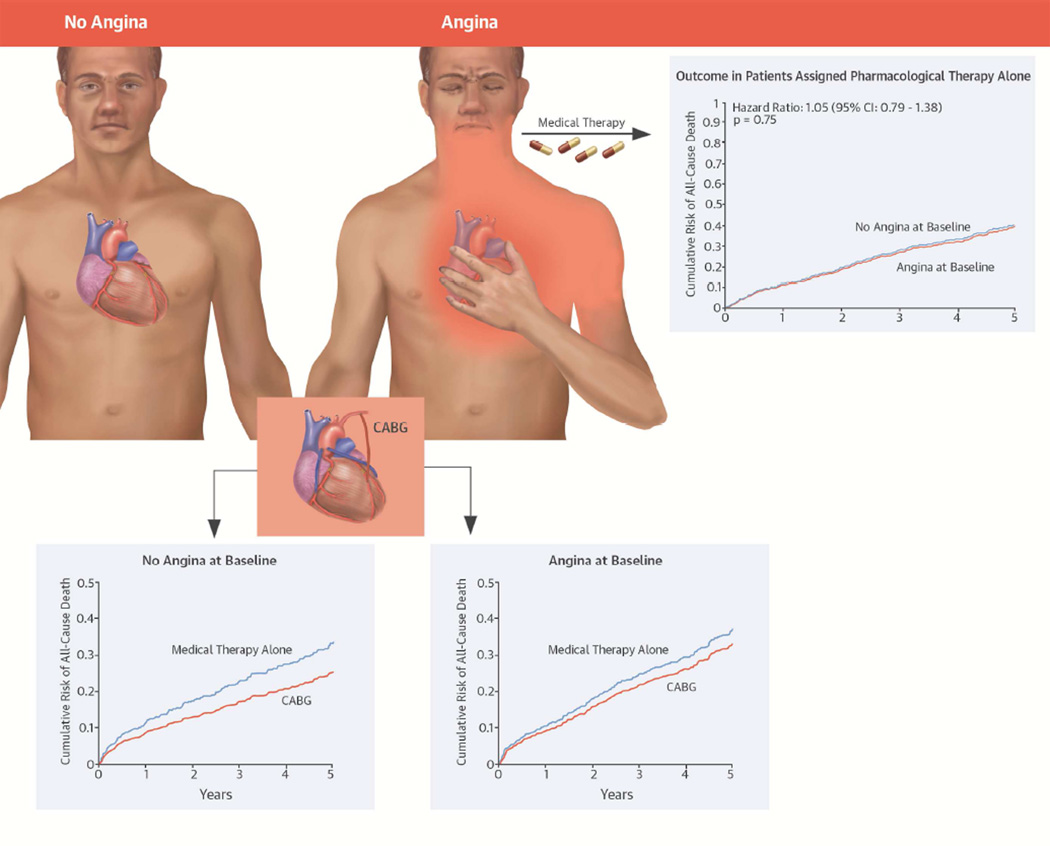
Central Illustration. The STICH Angina Substudy: The Interaction of Angina, Revascularization, and Outcomes in Patients With LV Systolic Dysfunction and Coronary Artery Disease.
The presence of angina does not confer a markedly poorer prognosis in medically treated patient. By intention-to-treat, mortality rates are similar in patients assigned to CABG or to medical therapy, whether angina is present or not. Patients treated with CABG had greater improvement in CCS angina class compared to patients treated with medical therapy only, but the treatment effect diminishes over time. Angina, unless recalcitrant to medical therapy, does not appear useful in selecting patients with CAD, heart failure and LV systolic dysfunction for CABG. CABG = coronary artery bypass graft; CAD = coronary artery disease; CCS = Canadian Cardiology Society; LV = left ventricular.
Among patients with severe LV dysfunction, CABG is associated with an early risk of serious complications, including death (16). Findings from this study challenge the perceived benefit–risk balance for CABG in patients with and without angina. Although patients with heart failure and angina recalcitrant to pharmacological therapy should be considered for revascularization for symptom relief, this analysis suggests that insofar as subsequent prognosis is concerned, the presence or absence of angina should not be used as a discriminating factor to decide for or against revascularization as an initial treatment strategy. These findings conflict with the opinion expressed in several clinical practice guidelines, which infer that angina is both a marker of a poorer prognosis and of a greater likelihood of prognostic benefit from revascularization. The European Society of Cardiology recommends coronary revascularization when angina persists despite treatment with 2 anti-anginal drugs; revascularization is not recommended in patients without angina and viable myocardium (1,17). The American practice guidelines do not directly address the question of angina. Instead, the decision to proceed with either CABG or PCI in patients with CAD and LV systolic dysfunction should be on the basis of clinical judgment after a multidisciplinary consideration of the coronary anatomy (including single vs. multiple coronary lesions), the presence of severe comorbid conditions, and the severity of LV systolic dysfunction (18,19).
By design, all patients in this study had CAD, which is well known to be associated with an adverse prognosis in patients with heart failure. Other analyses, in populations with less robust evidence of CAD, suggest that angina is associated with a worse outcome (14), but this may be because angina is a marker confirming the presence of CAD. The reasons why angina does not predict a mortality benefit from CABG are uncertain, and cannot be determined from the current study. Results of this analysis are consistent with previous findings from STICH and the Heart Failure Revascularization Trial (HEART) (20), suggesting that neither myocardial viability (5) nor reversible myocardial ischemia (6) helps predict which patients benefit from CABG.
There are no ongoing trials of CABG versus medical therapy in heart failure to confirm or refute our findings. The Study of Efficacy and Safety of Percutaneous Coronary Intervention to Improve Survival in Heart Failure (REVIVED-BCIS2) will address the effects of revascularization in heart failure, albeit by percutaneous coronary intervention (21), in patients with and without concomitant angina.
The findings of this analysis of the STICH trial should be applied to other types of patients with CAD with caution. CABG should be considered, whenever feasible, in patients with angina refractory to medical therapy, whether LV systolic function is reduced or not. However, the relationship between CAD, angina, and ischemic burden is complex, due, in part, to the great improvement in the contemporary medical therapy of heart failure and CAD. In the COURAGE (Clinical Outcomes Using Revascularization and Aggressive DruG Evaluation) (22), and BARI 2D (Bypass Angioplasty Revascularization Investigation 2 Diabetes) trials (23,24), both of which included very few patients with LV systolic dysfunction, the ischemic burden failed to predict the effect of revascularization on clinical endpoints. However, regardless of the presence of angina or ischemic burden, the severity of CAD (25) and the extent of myocardial dysfunction and scar do appear to influence the benefits of CABG.
Limitations
The present study is a retrospective analysis of a randomized trial, and unknown confounders and biases may have affected the complex relation between angina, CABG, and outcomes. To minimize this, we adjusted for key variables known to affect survival and performed sensitivity analyses. With few exceptions (20,26), there is no other prospective dataset besides the STICH trial, which assesses the effect of CABG on outcomes in patients with LV systolic dysfunction. This precludes any external validation of our findings with an independent trial population. Patients enrolled in the STICH trial were mostly men, white, and relatively young. Many patients and investigators may have elected to proceed to revascularization if the coronary anatomy was thought to be associated with a particularly adverse prognosis or was highly amenable to percutaneous coronary intervention, rather than enroll the patient in this study. This might be particularly true for patients with angina. For these reasons, extrapolation of our results to broader populations should be made with caution. The identification and classification of angina in patients enrolled in STICH was at the discretion of study-site investigators using established clinical guidelines. Even so, there may be local variability in the angina definitions employed; hence, our findings should be interpreted with caution in groups (women and older patients) (27) who present with atypical symptoms. Patients with markedly limiting angina (CCS class III or IV) were under-represented in the study. For this reason, it was not possible to fully explore a dose-response effect across the complete spectrum of angina severity.
Conclusions
Our study suggests that among patients with CAD, heart failure, and LV systolic dysfunction, the presence of angina does not confer a markedly worse prognosis or the potential for a greater benefit from revascularization by CABG. However, CABG does improve angina compared with medical therapy alone. These findings may influence clinical practice by diminishing the relevance of the role of angina for treatment decisions and prognostication in patients with ischemic cardiomyopathy.
Supplementary Material
PERSPECTIVES.
Competency in Medical Knowledge
The occurrence of angina pectoris is not a predictor or mortality in medically treated patients with coronary artery disease (CAD) and left ventricular (LV) systolic dysfunction. Bypass surgery (CABG) is more effective than medical therapy in relieving angina and lowers mortality to a similar extent in those with and without angina.
Translational Outlook
Additional studies are needed to clarify the mechanisms underlying the interaction between angina, revascularization and clinical cardiovascular outcomes.
Acknowledgments
Funding: This work was supported by grants U01HL69015 and U01HL69013 from the National Heart, Lung and Blood Institute, National Institutes of Health, Bethesda, Maryland. This work is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or National Institutes of Health. Dr. Jolicoeur is supported by research grants from les Fonds la Recherche du Québec en santé (FRQS), the Canadian Institutes for Health Research (CIHR), and by la Fondation de l’Institut de Cardiologie de Montréal.
Abbreviations
- BMI
body mass index
- CABG
coronary artery bypass graft
- CAD
coronary artery disease
- CCS
Canadian Cardiovascular Society
- CI
confidence interval
- HR
hazard ratio
- IQR
interquartile range
- LVEF
left ventricular ejection fraction
- MI
myocardial infarction
- OR
odds ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Authors/Task Force Members; McMurray JJ, Adamopoulos S, et al. ESC Committee for Practice Guidelines (CPG), Document Reviewers. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012. Eur Heart J. 2012;33:1787–1847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 2.Serruys PW, Morice MC, Kappetein AP, et al. SYNTAX Investigators. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961–972. doi: 10.1056/NEJMoa0804626. [DOI] [PubMed] [Google Scholar]
- 3.Park SJ, Kim YH, Park DW, et al. Randomized trial of stents versus bypass surgery for left main coronary artery disease. N Engl J Med. 2011;364:1718–1727. doi: 10.1056/NEJMoa1100452. [DOI] [PubMed] [Google Scholar]
- 4.Velazquez EJ, Lee KL, Deja MA, et al. STICH Investigators. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med. 2011;364:1607–1616. doi: 10.1056/NEJMoa1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonow RO, Maurer G, Lee KL, et al. STICH Trial Investigators. Myocardial viability and survival in ischemic left ventricular dysfunction. N Engl J Med. 2011;364:1617–1625. doi: 10.1056/NEJMoa1100358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panza JA, Holly TA, Asch FM, et al. Inducible myocardial ischemia and outcomes in patients with coronary artery disease and left ventricular dysfunction. J Am Coll Cardiol. 2013;61:1860–1870. doi: 10.1016/j.jacc.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 8.Warren J. Remarks on angina pectoris. N Engl J Med. 1:1–11. doi: 10.1056/NEJM196201042660101. [DOI] [PubMed] [Google Scholar]
- 9.Ekman I, Cleland JG, Swedberg K, et al. Symptoms in patients with heart failure are prognostic predictors: insights from COMET. J Card Fail. 2005;11:288–292. doi: 10.1016/j.cardfail.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Mentz RJ, Fiuzat M, Shaw LK, et al. Comparison of clinical characteristics and long-term outcomes of patients with ischemic cardiomyopathy with versus without angina pectoris (from the Duke Databank for Cardiovascular Disease) Am J Cardiol. 2012;109:1272–1277. doi: 10.1016/j.amjcard.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 11.Velazquez EJ, Lee KL, O'Connor CM, et al. STICH Investigators. The rationale and design of the Surgical Treatment for Ischemic Heart Failure (STICH) trial. J Thorac Cardiovasc Surg. 2007;134:1540–1547. doi: 10.1016/j.jtcvs.2007.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones RH, Velazquez EJ, Michler RE, et al. STICH Hypothesis 2 Investigators. Coronary bypass surgery with or without surgical ventricular reconstruction. N Engl J Med. 2009;360:1705–1717. doi: 10.1056/NEJMoa0900559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 14.Badar AA, Perez-Moreno AC, Jhund PS, et al. Relationship between angina pectoris and outcomes in patients with heart failure and reduced ejection fraction: an analysis of the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA) Eur Heart J. 2014;35:3426–3433. doi: 10.1093/eurheartj/ehu342. [DOI] [PubMed] [Google Scholar]
- 15.Mentz RJ, Broderick S, Shaw LK, et al. Persistent angina pectoris in ischaemic cardiomyopathy: increased rehospitalization and major adverse cardiac events. Eur J Heart Fail. 2014;16:854–860. doi: 10.1002/ejhf.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wrobel K, Stevens SR, Jones RH, et al. Influence of baseline characteristics, operative conduct and postoperative course on 30-day outcomes of coronary artery bypass grafting among patients with left ventricular dysfunction: results from the Surgical Treatment for Ischemic Heart Failure (STICH) Trial. Circulation. 2015;132:720–730. doi: 10.1161/CIRCULATIONAHA.114.014932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Guidelines on myocardial revascularization. Eur J Cardiothorac Surg. 2010;38(Suppl):S1–S52. doi: 10.1016/j.ejcts.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 18.Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60:e44–e164. doi: 10.1016/j.jacc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 19.Hillis LD, Smith PK, Anderson JL, et al. 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the American Association for Thoracic Surgery, Society of Cardiovascular Anesthesiologists, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011;58:e123–e210. doi: 10.1016/j.jacc.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Cleland JG, Calvert M, Freemantle N, et al. The Heart Failure Revascularisation Trial (HEART) Eur J Heart Fail. 2011;13:227–233. doi: 10.1093/eurjhf/hfq230. [DOI] [PubMed] [Google Scholar]
- 21.King’s College, London. Study of Efficacy and Safety of Percutaneous Coronary Intervention to Improve Survival in Heart Failure (REVIVED-BCIS2) [Accessed August 30, 2015];2015 Available at: http://clinicaltrials.gov/show/NCT01920048.
- 22.Mancini GB, Hartigan PM, Shaw LJ, et al. Predicting outcome in the COURAGE trial (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation): coronary anatomy versus ischemia. J Am Coll Cardiol Intv. 2014;7:195–201. doi: 10.1016/j.jcin.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 23.BARI 2D Study Group. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360:2503–2515. doi: 10.1056/NEJMoa0805796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaw LJ, Cerqueira MD, Brooks MM, et al. Impact of left ventricular function and the extent of ischemia and scar by stress myocardial perfusion imaging on prognosis and therapeutic risk reduction in diabetic patients with coronary artery disease: results from the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. J Nucl Cardiol. 2012;19:658–669. doi: 10.1007/s12350-012-9548-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Panza JA, Velazquez EJ, She L, et al. Extent of coronary and myocardial disease and benefit from surgical revascularization in ischemic LV dysfunction [Corrected] J Am Coll Cardiol. 2014;64:553–561. doi: 10.1016/j.jacc.2014.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beanlands RS, Nichol G, Huszti E, et al. PARR-2 Investigators. F-18-fluorodeoxyglucose positron emission tomography imaging-assisted management of patients with severe left ventricular dysfunction and suspected coronary disease: a randomized, controlled trial (PARR-2) J Am Coll Cardiol. 2007;50:2002–2012. doi: 10.1016/j.jacc.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 27.Canto JG, Fincher C, Kiefe CI, et al. Atypical presentations among Medicare beneficiaries with unstable angina pectoris. Am J Cardiol. 2002;90:248–253. doi: 10.1016/s0002-9149(02)02463-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.