SUMMARY
Endophthalmitis remains a rare but serious cause of visual loss. Over time, changes have been noted in endophthalmitis in terms of predominant causes, infecting organisms, and antibiotic susceptibilities. There is controversy regarding the use of intracameral prophylactic antimicrobials during cataract surgery. Alternatively, there appears to be increasing evidence against using routine topical antibiotics for intravitreal injections. There are also increasing reports of multidrug-resistant organisms causing endophthalmitis, but the combination of vancomycin and ceftazidime appears effective for the vast majority of cases. Future trends may involve increasing utilization of polymerase chain reaction for diagnosis, and possibly office-based pars plana vitrectomy for treatment of endophthalmitis.
Keywords: Antibiotics, Endophthalmitis, Intravitreal injection, Pars plana vitrectomy, Polymerase chain reaction
Endophthalmitis, characterized by marked inflammation of intraocular tissues and fluids, remains a rare but potentially serious cause of visual loss. Outcomes may be poor, even with prompt diagnosis and treatment. Endophthalmitis may be classified by the cause of the infection, the timing of signs and symptoms, and other predisposing factors (Table) [1]. This classification helps predict the most likely causative organisms and guides treatment decisions.
TABLE.
Categories of endophthalmitis
| Category | Common Organisms |
|---|---|
| Acute-onset postoperative | Coagulase-negative staphylococci |
| Staphylococcus aureus | |
| Streptococcus spp. | |
| Gram-negative organisms | |
| Delayed-onset (chronic) postoperative | Propionobacterium acnes |
| Coagulase-negative staphylococci | |
| Candida parapsilosis and other fungi | |
| Post-glaucoma filtering bleb | Haemophilus influenza |
| Streptococcus spp. | |
| Staphylococcus spp. | |
| Post-traumatic | Bacillus cereus |
| Staphylococcus spp. | |
| Streptococcus spp. | |
| Endogenous | Candida albicans |
| Aspergillus spp. | |
| Staphylococcus aureus | |
| Gram-negative organisms | |
| Post-microbial keratitis | Gram-negative organisms |
| Staphylococcus aureus | |
| Fusarium spp. | |
| Post-intravitreal injection | Coagulase-negative staphylococci |
| Streptococcus spp. |
Adapted from Schwartz SG, Flynn HW Jr, Scott IU. Endophthalmitis: classification and current management. Expert Rev. Ophthalmol. 2(3):385–396 (2007) [1].
Over the past few years, there has been new information related to causes, predisposing factors, treatment, and prevention of endophthalmitis. In addition, two particular clinical situations have assumed increasing importance in recent years. First, the marked increase in the use of office-based intravitreal injections has resulted in a corresponding increase in endophthalmitis following these injections. And second, some surgeons, especially in Europe, are using intracameral antibiotics after cataract surgery in an attempt to reduce rates of endophthalmitis, which remains controversial in the US.
Endophthalmitis After Pars Plana Vitrectomy (PPV) and Intravitreal Injections
There appears to be some evolution in terms of which categories of endophthalmitis are most prevalent. Acute-onset postoperative endophthalmitis, which occurs within 6 weeks of intraocular surgery, has traditionally been considered the major category (Figure 1). This category has predominantly consisted of infections following cataract surgery, but the increasing use of pars plana vitrectomy (PPV) has drawn increasing attention to post-PPV endophthalmitis, which may be quite severe [2]. Notably, a systematic review reported no difference in endophthalmitis rates between patients treated with 23- or 25-gauge surgery versus 20-gauge surgery [3].
Figure 1.
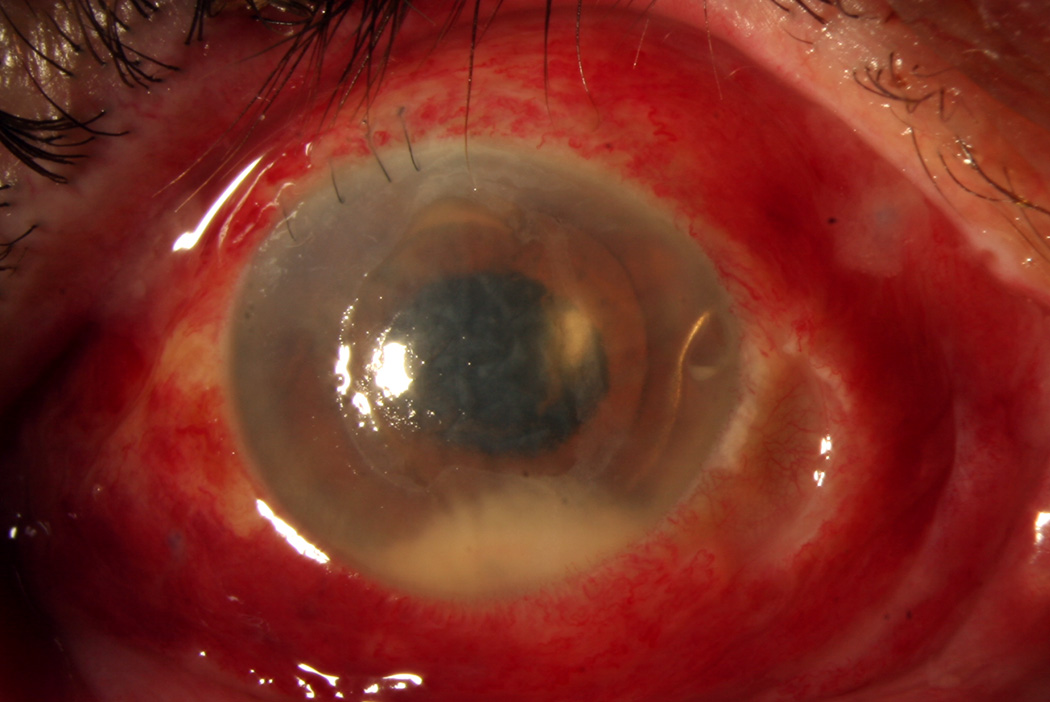
1A. External photograph, right eye. A 73-year-old man presented with endophthalmitis following complicated cataract surgery. Visual acuity was light perception. The patient was treated with immediate pars plana vitrectomy and injection of intravitreal vancomycin, ceftazidime, and dexamethasone. Vitreous cultures were positive for coagulase-negative staphylococcus.
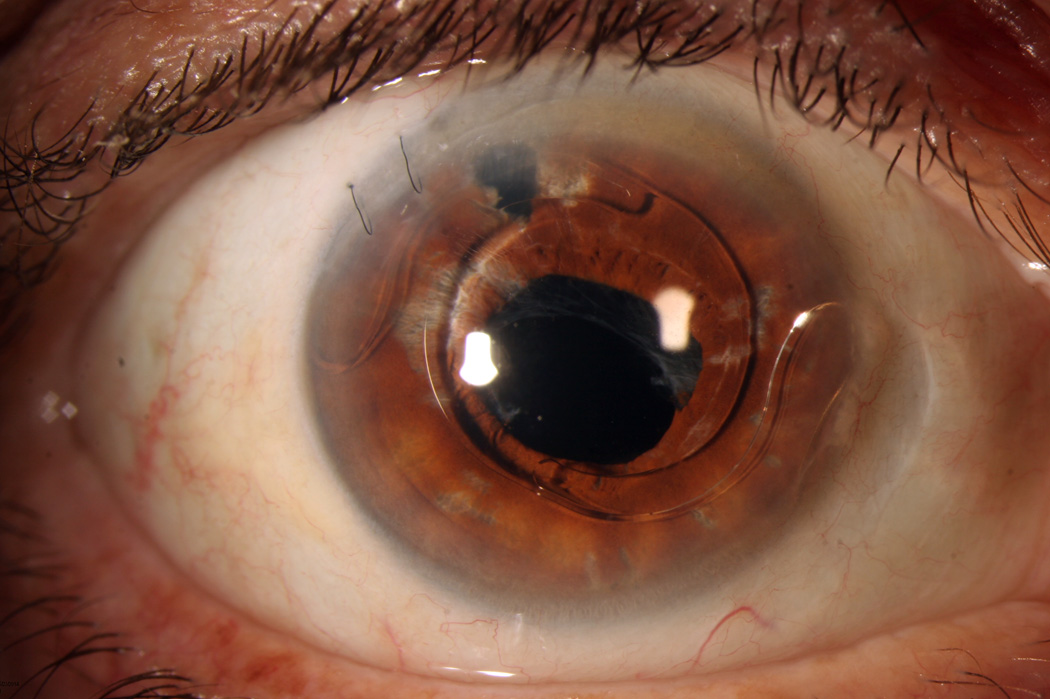
1B. External photograph, right eye. Following successful treatment, visual acuity 5 months after presentation was 20/50, limited by chronic cystoid macular edema. Note the distortion of the iris and the anterior chamber intraocular lens present.
Similarly, the increasing use of intravitreal injectable pharmacotherapy has led to increasing numbers of patients with post-injection endophthalmitis. One 2014 single-center series reported that the primary cause of endophthalmitis in that institution changed from post-surgical in 2003 to post-injection in 2010 [4]. There is growing understanding of the additional risks posed by the use of off-label medications and surgical adjuncts prepared by compounding pharmacies, with several recent reported series, including Streptococcus mitis/oralis endophthalmitis following injection of bevacziumab (Avastin, Genentech, South San Francisco, CA) [5], Bipolaris hawaiiensis endophthalmitis following injection of triamcinolone [6], Bipolaris hawaiiensis endophthalmitis following injection of combined bevacizumab and triamcinolone [7], and Fusarium incarnatum-equiseti species complex endophthalmitis following the use of Brilliant Blue G dye during PPV [8].
Changes in Microbiology and Antibiotic Susceptibilities
There also appear to be changes over time with respect to the relative prevalence of various infecting organisms and their antimicrobial susceptibilities. A single-center review reported an increase in Gram-negative endophthalmitis, but a decrease in fungal endophthalmitis, from 1999 through 2013 [9]. Klebsiella pneumoniae appears to be increasing in prevalence and incidence as a cause of endogenous endophthalmitis, especially in Asia [10]. Further, Klebsiella endophthalmitis may be associated with poor outcomes, including high rates of enucleation and evisceration [11].
Antibiotic susceptibilities may also change over time. A single-center review of 911 eyes over 25 years reported a trend toward increasing resistance to methicillin and cephalosporins, but decreasing resistance to aminoglycosides and imipenem [12]. Another single-center review of 63 eyes with streptococcal endophthalmitis over 11 years reported increasing minimal inhibitory concentrations for ceftriaxone and levofloxacin over time, suggesting increasing resistance to these agents [13].
The use of polymerase chain reaction (PCR) may allow faster and/or more accurate diagnosis of infecting organisms [14–16]. For example, PCR has been reported to identify Prevotella species [17], Mycobacterium abscessus [18], Curvularia lunata [19]and Eschericia fergusonii [20], as well as multiple cases of post-filtering bleb endophthalmitis [21].
Newer Approaches to Endophthalmitis Prevention
Endophthalmitis probably cannot be completely prevented, regardless of prophylaxis methods. The rates and major risk factors for endophthalmitis are well documented. For example, the reported incidence of acute-onset postoperative endophthalmitis following cataract surgery is in the range of 0.028% [22,23]. The incidence of post-intravitreal injection endophthalmitis has been estimated at about 1 in 3000 injections of anti-vascular endothelial growth factor (VEGF) agents, but perhaps slightly higher with intravitreal corticosteroids [24].
The preferred method of reducing the incidence of endophthalmitis remains undetermined. In 2007, the European Society of Cataract and Refractive Surgeons reported that intracameral cefuroxime at the conclusion of cataract surgery was associated with a significantly decreased rate of acute-onset postoperative endophthalmitis [25]. A 2013 follow-up to this study reported zero cases of endophthalmitis in 13,390 cataract surgeries using intracameral cefuroxime [26]. Despite these reports, there remains no consensus on the use of intracameral antibiotics for cataract surgery, and in fact the ESCRS guidelines are not universally followed even in Europe [27]. Intracameral antibiotics are not without risk, and anaphylaxis has been reported following the use of intracameral cefuroxime [28]. Notably, a Cochrane systematic review, which included the original ESCRS study, concluded: “it is unlikely that additional clinical trials will be conducted to evaluate currently available prophylaxis” and advised surgeons to “rely on current evidence to make informed decisions regarding prophylaxis choices [29].”
Approaches vary from center to center, and favorable results have been reported with the use of intracameral moxifloxacin [30,31] and with vancomycin and gentamicin in the irrigating solution [32]. Alternatively, a 2014 case-control study reported that the rate of post-cataract surgery endophthalmitis was significantly reduced with the use of postoperative topical fluoroquinolones and increased with the use of topical timolol at the end of the procedure [33].
Similarly, there is no consensus regarding risk reduction with other procedures. Pretreatment with antibiotics has been reported ineffective in reducing the rates of post-PPV endophthalmitis [34]. Further, there is mounting evidence that routine topical antibiotics following intravitreal injections are not beneficial and may in fact be harmful [35], perhaps by altering normal conjunctival flora [36]. Povidone-iodine antisepsis, rather than antibiotics, are preferred in this situation [37].
In large retrospective studies including all categories, post-traumatic endophthalmitis was less common than most other categories of endophthalmitis [38]. A series of 108 patients with retained intraocular foreign bodies reported endophthalmitis on presentation in 6.4% [39]. Because of the infrequency of these cases, it may be relatively more difficult to design clinical trials to study this category. Nevertheless, a randomized clinical trial of 346 penetrating eye injuries compared the use of intracameral or intravitreal gentamicin and clindamycin versus balanced salt solution and reported a significant decrease in rates of endophthalmitis among the subgroup of patients with retained intraocular foreign bodies that received intraocular antibiotics [40]. A more recent randomized clinical trial of patients with similar injuries reported no significant increase in endophthalmitis with the use of intravitreal injection of balanced salt solution during the primary repair [41].
Newer Approaches to Endophthalmitis Treatment
The Endophthalmitis Vitrectomy Study (EVS) results were first reported in 1995 [42] and, in general, these guidelines are generally followed today [43], with important caveats. The EVS only recruited patients with acute-onset postoperative endophthalmitis following cataract surgery or secondary intraocular lens implantation. Therefore, these results are not necessarily applicable to other categories of endophthalmitis.
It may not be medically practical to perform “immediate” PPV (defined by the EVS as within 6 hours of presentation) in patients reported to benefit from this treatment. Prompt tap and inject, followed by close observation and subsequent PPV, has been reported as an alternative [44]. Also, the EVS reported no difference in outcomes with or without the use of systemic amikacin and ceftazidime. However, systemic gatifloxacin [45] and moxifloxacin [46] have been reported to achieve intraocular drug levels even in the noninflamed eye. However, systemic gatifloxacin is no longer available because of associated risks of dysglycemia [47].
Of note, in the EVS, 10 patients developed endophthalmitis despite the use of antibiotics in the irrigating solution. The exact drugs were not reported, but this study was the first report of endophthalmitis developing in this clinical setting [48].
Corticosteroids have a theoretical role in reducing inflammation-related ocular damage associated with endophthalmitis. All patients enrolled in the EVS were treated with systemic corticosteroids, which are infrequently used today in this context. The use of intravitreal corticosteroids in the initial treatment of acute-onset postoperative endophthalmitis is somewhat controversial but has many potential benefits. Various animal models of bacterial endophthalmitis have reported improved outcomes with the use of intravitreal dexamethasone in addition to intravitreal antibiotics alone [49,50], although other studies have not shown a benefit [51]. In a pilot series of 5 patients with endophthalmitis, the use of intravitreal triamcinolone acetonide 48–72 hours after initial treatment with antibiotics (and confirmation that isolates were sensitive to the antibiotics used) was associated with favorable clinical outcomes [52].
Most surgeons are still using empiric broad-spectrum combination therapy, which generally is very effective. Pharmacokinetic data on antibiotics inside the eye are relatively limited, but decades of empirical experience have led to the common use of vancomycin and a third-generation cephalosporin, typically ceftazidime or ceftriaxone. In a US single-center 10-year review of endophthalmitis isolates, no single agent would have provided effective treatment for all isolated microbes, although vancomycin provided 100% coverage for Gram-positive organisms and both ceftazidime and levofloxacin provided 100% coverage for Gram-negative organisms during this time period [53]. However, acute-onset postoperative endophthalmitis with multidrug-resistant organisms does occur rarely [54–56]. Alternative intravitreal agents, such as moxifloxacin [57] and imipenem [58], have been reported useful in these situations.
Antibiotic resistance remains an important clinical challenge. A review of microbiological records of 111 coagulase-negative staphylococci isolates recovered over 15 years in a single center reported increasing resistance to fluoroquinolones. During this time frame, susceptibility to gatifloxacin and moxifloxacin declined from 96.6% to 65.4% [59].
Newer approaches might involve the use of encapsulated antimicrobials to improve ocular penetration and/or duration of effect. Intravitreal liposomal amphotericin B has been reported effective in the treatment of Candida endophthalmitis [60], but this approach has not been widely adapted. Nanoparticles loaded with daptomycin have been formulated as a potential topical therapy for bacterial endophthalmitis [61], but again this approach has not been reported clinically at this time. An investigational foldable capsular vitreous body loaded with levofloxacin was investigated in a rabbit model [62], but not in clinical management.
EXPERT COMMENTARY
Almost 20 years after the first publication of the EVS results, in general the investigators’ recommendations remain relevant today. However, uncertainties remain as to the optimal management of many patients with endophthalmitis. The relative frequencies of certain categories of endophthalmitis, such as following intravitreal injections and PPV, appear to be increasing. In addition, even within the same category of endophthalmitis, there may be changes in the relative frequencies of various causative organisms. PCR may offer faster diagnosis than traditional culture techniques. As case reports and small case series reporting the benefits of PCR accumulate, interest in this diagnostic technique continues to increase. Nevertheless, the benefits of PCR will have to be balanced against increased costs and the need for specialized equipment and support staff, which is available only in major centers. For these reasons, traditional culture techniques will likely remain standard practice for the near and intermediate terms.
Similarly, increasing reports of multidrug-resistant organisms suggest a rationale for further clinical research on alternative intravitreal antibiotics, including fluoroquinolones and imipenem. However, it is noted that a 10-year series from a large US eye hospital reported that 100% of bacterial isolates were susceptible to the combination of vancomycin and ceftazidime [53]. Therefore, it appears that this combination is still appropriate as empiric first-line therapy in patients with presumed bacterial infections. In general, the authors typically use intravitreal dexamethasone at the time of initial treatment in patients with acute-onset postoperative endophthalmitis judged likely to be caused by bacteria.
FIVE-YEAR VIEW
In spite of the reported dramatic reductions in endophthalmitis rates associated with the use of intracameral antibiotics during cataract surgery, using either ESCRS or other protocols, many surgeons have not adapted prophylactic intracameral antimicrobials. Because of the difficulties associated with designing another randomized clinical trial enrolling a sufficient number of patients, it seems more likely that a preferred technique will evolve gradually, as centers from around the world report results with differing strategies. There is more of a rationale to use prophylactic antibiotics during a “one-time” procedure, such as cataract surgery or PPV, than there is for intravitreal injection, which is often repeated at regular intervals for months or for years.
The potential impact of in-office PPV also bears consideration. Office-based small-gauge PPV has been reported to be effective for select patients [63], and recent advances in instrumentation appear to make this technique more viable at present [64], although there have been no randomized clinical trials regarding this technique. To our knowledge, office-based 3-port PPV in the treatment of endophthalmitis has not been reported. This approach may be beneficial in remote offices and locations physically distant from specialized vitrectomy equipment, but at this time it remains more of a theoretical concept than a viable clinical option.
KEY ISSUES.
Endophthalmitis remains an important cause of ocular morbidity
Due to changing practice patterns, endophthalmitis following PPV and intravitreal injections are increasingly encountered
There is controversy in the use of intracameral antibiotics during cataract surgery, and there remains no consensus regarding their use
There is increasing evidence in support of PCR for faster and/or more accurate isolation of microorganisms, although there remains no consensus regarding its use
At this time, the results of the EVS remain broadly applicable to patients with acute-onset endophthalmitis after cataract surgery
Future research may focus on alternative intravitreal antimicrobials and the possible use of office-based PPV in the treatment of endophthalmitis
Acknowledgments
The work was partially supported by NIH Center Core Grant P30EY014801, an unrestricted grant from Research to Prevent Blindness and the Department of Defense (DOD Grant #W81XWH-09-1-0675). SG Schwartz has served on advisory boards for Bausch + Lomb and Santen, and has received lecture fees from ThromboGenics.
Footnotes
Financial and competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
REFERENCES
- 1.Schwartz SG, Flynn HW, Jr, Scott IU. Endophthalmitis: classification and current management. Expert Rev. Ophthalmol. 2007;2(3):385–396. [Google Scholar]
- 2.Park JC, Ramasamy B, Shaw S, Ling RH, Prasad S. A prospective and nationwide study investigating endophthalmitis following pars plana vitrectomy: clinical presentation, microbiology, management and outcome. Br. J. Ophthalmol. 2014 Mar 31; doi: 10.1136/bjophthalmol-2013-304486. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3.Govetto A, Virgili G, Menchini F, Lanzetta P, Menchini U. A systematic review of endophthalmitis after microincisional versus 20-gauge vitrectomy. Ophthalmology. 2013;120(11):2286–2291. doi: 10.1016/j.ophtha.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 4.Kessner R, Golan S, Barak A. Changes in the etiology of endophthalmitis from 2003 to 2010 in a large tertiary medical center. Eur. J. Ophthalmol. 2014 May 1; doi: 10.5301/ejo.5000473. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Matthews JL, Dubovy SR, Goldberg RA, Flynn HW., Jr Histopathology of Streptococcus mitis/oralis endophthalmitis after intravitreal injection with bevacizumab: a report of 7 patients. Ophthalmology. 2014;121(3):702–708. doi: 10.1016/j.ophtha.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 6.Minckler D, Small KW, Walsh TJ. Clinical and pathologic features of Bipolaris endophthalmitis after intravitreal triamcinolone. JAMA Ophthalmol. 2014 Mar 13; doi: 10.1001/jamaophthalmol.2014.257. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Sheyman AT, Cohen BZ, Friedman AH, Ackert JM. An outbreak of fungal endophthalmitis after intravitreal injection of compounded combined bevacizumab and triamcinolone. JAMA Ophthalmol. 2013;131(7):864–869. doi: 10.1001/jamaophthalmol.2013.88. [DOI] [PubMed] [Google Scholar]
- 8.Mikosz CA, Smith RM, Kim M, et al. Fungal endophthalmitis associated with compounded products. Emerg. Infect. Dis. 2014;20(2):248–256. doi: 10.3201/eid2002.131257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jindal A, Pathengay A, Mithal K, et al. Microbiologic spectrum and susceptibility of isolates in acute postcataract surgery endophthalmitis: are they same as they were more than a decade ago? Br. J. Ophthalmol. 2014;98(3):414–416. doi: 10.1136/bjophthalmol-2013-304289. [DOI] [PubMed] [Google Scholar]
- 10.Kashani AH, Eliott D. The emergence of Klebsiella pneumoniae endogenous endophthalmitis in the USA: basic and clinical advances. J. Ophthalmic Inflamm Infect. 2013;3(1):28. doi: 10.1186/1869-5760-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sridhar J, Flynn HW, Jr, Kuriyan AE, Dubovy S, Miller D. Endophthalmitis caused by Klebsiella species. Retina. 2014 May 5; doi: 10.1097/IAE.0000000000000162. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gentile RC, Shukla S, Shah M, et al. Microbiological spectrum and antibiotic sensitivity in endophthalmitis: a 25-year review. Ophthalmology. 2014 Apr 2; doi: 10.1016/j.ophtha.2014.02.001. [Epub ahead of print] • Single-center review of 988 isolates from 911 consecutive eyes over 25 years.
- 13.Kuriyan AE, Weiss KD, Flynn HW, Jr, et al. Endophthalmitis caused by streptococcal species: clinical settings, microbiology, management, and outcomes. Am. J. Ophthalmol. 2014;157(4):774–780. doi: 10.1016/j.ajo.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bharathi MJ, Rameshkumar G, Ramakrishnan R, et al. Comparative evaluation of uniplex, nested, semi-nested, multiplex and nested multiplex PCR methods in the identification of microbial etiology of clinically suspected infectious endophthalmitis. Curr. Eye Res. 2013;38(5):550–562. doi: 10.3109/02713683.2013.772205. [DOI] [PubMed] [Google Scholar]
- 15.Sugita S, Ogawa M, Shimizu N, et al. Use of a comprehensive polymerase chain reaction system for diagnosis of ocular infectious diseases. Ophthalmology. 2013;120(9):1761–1768. doi: 10.1016/j.ophtha.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 16.Cornut PL, Boisset S, Romanet JP, et al. Principles and applications of molecular biology techniques for the microbiological diagnosis of acute post-operative endophthalmitis. Surv. Ophthalmol. 2014;59(3):286–303. doi: 10.1016/j.survophthal.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Amissah-Arthur KN, Farooq TA, Dhillon N, Cunliffe IA, Bansal A. Rare case of post-cataract-surgery Prevotella endophthalmitis diagnoses by polymerase chain reaction DNA sequencing. J. Cataract Refract. Surg. 2013;39(3):463–466. doi: 10.1016/j.jcrs.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 18.Rolfe NE, Garcia C, Widen RH, Taylor SP. Rapid diagnosis of Mycobacterium abscessus endophthalmitis. J. Med. Microbiol. 2013;62(Pt 7):1089–1091. doi: 10.1099/jmm.0.051771-0. [DOI] [PubMed] [Google Scholar]
- 19.Alex D, Li D, Calderone R, Peters SM. Identification of Curvularia lunata by polymerase chain reaction in a case of fungal endophthalmitis. Med. Mycol. Case Rep. 2013;2:137–140. doi: 10.1016/j.mmcr.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gokhale VV, Therese KL, Bagyalakshmi R, Biswas J. Detection of Escherichia fergusonii by PCR-based DNA sequencing in a case of delayed-onset chronic endophthalmitis after cataract surgery. J. Cataract Refract. Surg. 2014;40(2):327–330. doi: 10.1016/j.jcrs.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 21.Brillat-Zaratzian E, Bron A, Aptel F, et al. FRIENDS Group: clinical and microbiological characteristics of post-filtering surgery endophthalmitis. Graefes Arch. Clin. Exp. Ophthalmol. 2014;252(1):101–107. doi: 10.1007/s00417-013-2503-4. [DOI] [PubMed] [Google Scholar]
- 22.Wykoff CC, Parrott MB, Flynn HW, Jr, et al. Nosocomial acute-onset postoperative endophthalmitis at a university teaching hospital (2002–2009) Am. J. Ophthalmol. 2010;150(3):392–398. doi: 10.1016/j.ajo.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 23.Galor A, Goldhardt R, Wellik SR, et al. Management strategies to reduce risk of postoperative infections. Curr. Ophthalmol. Rep. 2013;1(4) doi: 10.1007/s40135-013-0021-5. no pages listed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz SG, Flynn HW., Jr Endophthalmitis associated with intravitreal anti-vascular endothelial growth factor injections. Curr. Ophthalmol. Rep. 2014;2(1):1–5. doi: 10.1007/s40135-013-0033-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Endophthalmitis Study Group. European Society of Cataract and Refractive Surgeons. Prophylaxis of postoperative endophthalmitis following cataract surgery: results of the ESCRS multicenter study and identification of risk factors. J. Cataract Refract. Surg. 2007;33(6):978–988. doi: 10.1016/j.jcrs.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 26. Beselga D, Campos A, Castro M, et al. Postcataract surgery endophthalmitis after introduction of the ESCRS protocol: a 5-year study. Eur. J. Ophthalmol. 2014;24(4):516–519. doi: 10.5301/ejo.5000417. • Series of 15,689 eyes undergoing cataract surgery by 9 surgeons, before and after institution of prophylactic intracameral antibiotics.
- 27.Behndig A, Cochener B, Guell JL, et al. Endophthalmitis prophylaxis in cataract surgery: overview of current practice in 9 European countries. J. Cataract Refract. Surg. 2013;39(9):1421–1431. doi: 10.1016/j.jcrs.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 28.Moisseiev E, Levinger E. Anaphylactic reaction following intracameral cefuroxime injection during cataract surgery. J. Cataract Refract. Surg. 2013;39(9):1432–1434. doi: 10.1016/j.jcrs.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 29.Gower EW, Lindsley K, Nanji AA, Leyngold I, McDonnell PJ. Perioperative antibiotics for prevention of acute endophthalmitis after cataract surgery. Cochrane Database Syst. Rev. 2013 Jul 15;7:CD006364. doi: 10.1002/14651858.CD006364.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsuura K, Miyoshi T, Suto C, Akura J, Inoue Y. Efficacy and safety of prophylactic intracameral moxifloxacin injection in Japan. J. Cataract Refract. Surg. 2013;39(11):1702–1706. doi: 10.1016/j.jcrs.2013.05.036. [DOI] [PubMed] [Google Scholar]
- 31.Galvis V, Tello A, Sanchez MA, Camacho PA. Cohort study of intracameral moxifloxacin in postoperative endophthalmitis prophylaxis. Ophthalmol. Eye Dis. 2014;6:1–4. doi: 10.4137/OED.S13102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asencio MA, Huertas M, Carranza R, et al. A case-control study of post-operative endophthalmitis diagnosed at a Spanish hospital over a 13-year-period. Epidemiol. Infect. 2014 Mar 11; doi: 10.1017/S095026881400034X. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rudinsky CJ, Wan D, Weis E. Antibiotic choice for the prophylaxis of post-cataract extraction endophthalmitis. Ophthalmology. 2014;121(4):835–841. doi: 10.1016/j.ophtha.2013.08.046. [DOI] [PubMed] [Google Scholar]
- 34.Shi XY, Zhao HS, Wei WB. Analysis of post-operative endophthalmitis after pars plana vitrectomy: a 10-year experience at a single center. Chin. Med. J. (Engl) 2013;126(15):2890–2893. [PubMed] [Google Scholar]
- 35.Storey P, Dollin M, Pitcher J, et al. The role of topical antibiotic prophylaxis to prevent endophthalmitis after intravitreal injection. Ophthalmology. 2014;121(1):283–289. doi: 10.1016/j.ophtha.2013.08.037. [DOI] [PubMed] [Google Scholar]
- 36.Yin VT, Weisbrod DJ, Eng KT, et al. Antibiotic resistance of ocular surface flora with repeated use of a topical antibiotic after intravitreal injection. JAMA Ophthalmol. 2013;131(4):456–461. doi: 10.1001/jamaophthalmol.2013.2379. [DOI] [PubMed] [Google Scholar]
- 37.Ahmed Y, Scott IU, Pathengay A, Bawdekar A, Flynn HW., Jr Povidone-iodine for endophthalmitis prophylaxis. Am. J. Ophthalmol. 2014;157(3):503–504. doi: 10.1016/j.ajo.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 38.Puliafito CA, Baker AS, Haaf J, Foster CS. Infectious endophthalmitis: review of 36 cases. Ophthalmology. 1982;89(8):921–929. [PubMed] [Google Scholar]
- 39.Parke DW, 3rd, Pathengay A, Flynn HW, Jr, Albini T, Schwartz SG. Risk factors for endophthalmitis and retinal detachment with retained intraocular foreign bodies. J. Ophthalmol. 2012;2012:758526. doi: 10.1155/2012/758526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soheilian M, Rafati N, Mohebbi MR, et al. Prophylaxis of acute posttraumatic bacterial endophthalmitis: a multicenter, randomized clinical trial of intraocular antibiotic injection, report 2. Arch. Ophthalmol. 2007;125(4):460–465. doi: 10.1001/archopht.125.4.460. [DOI] [PubMed] [Google Scholar]
- 41.Rafati N, Azarmina M, Zaeri F, et al. Rate of post-traumatic endophthalmitis with or without injection of balanced salt solution. J. Ophthalmic Vis. Res. 2013;8(3):237–243. [PMC free article] [PubMed] [Google Scholar]
- 42.Endophthalmitis Vitrectomy Study Group. Results of the Endophthalmitis Vitrectomy Study: a randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Arch. Ophthalmol. 1995;113(12):1479–1496. [PubMed] [Google Scholar]
- 43.Flynn HW, Jr, Scott IU. Legacy of the Endophthalmitis Vitrectomy Study (EVS) Arch. Ophthalmol. 2008;126(4):559–561. doi: 10.1001/archopht.126.4.559. [DOI] [PubMed] [Google Scholar]
- 44.Javey G, Schwartz SG, Moshfeghi AA, Asrani S, Flynn HW., Jr Methicillin-resistant Staphylococcus epidermidis isolation from the vitrectomy specimen four hours after initial treatment with vancomycin and ceftazidime. Clin. Ophthalmol. 2010;4:101–104. doi: 10.2147/opth.s9206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hariprasad SM, Mieler WF, Holz ER. Vitreous and aqueous penetration of orally administered gatifloxacin in humans. Arch. Ophthalmol. 2003;121(3):345–350. doi: 10.1001/archopht.121.3.345. [DOI] [PubMed] [Google Scholar]
- 46.Hariprasad SM, Shah GK, Mieler WF, et al. Vitreous and aqueous penetration of orally administered moxifloxacin in humans. Arch. Ophthalmol. 2006;124(2):178–182. doi: 10.1001/archopht.124.2.178. [DOI] [PubMed] [Google Scholar]
- 47.Park-Wyllie LY, Juurlink DN, Kopp A, et al. Outpatient gatifloxacin therapy and dysglycemia in older adults. N. Engl. J. Med. 2006;354(13):1352–1361. doi: 10.1056/NEJMoa055191. [DOI] [PubMed] [Google Scholar]
- 48.Han DP, Wisniewski SR, Wilson LA, et al. Spectrum and susceptibilities of microbiologic isolates in the Endophthalmitis Vitrectomy Study. Am. J. Ophthalmol. 1996;122(1):1–17. doi: 10.1016/s0002-9394(14)71959-2. [DOI] [PubMed] [Google Scholar]
- 49.Liu F, Kwok AK, Cheung BM. The efficacy of intravitreal vancomycin and dexamethasone in the treatment of experimental Bacillus cereus endophthalmitis. Curr. Eye Res. 2008;33(9):761–768. doi: 10.1080/02713680802344690. [DOI] [PubMed] [Google Scholar]
- 50.De Kaspar HM, Ta CN, Engelbert M, et al. Effects of intravitreal corticosteroid in the treatment of Staphylococcus aureus-induced experimental endophthalmitis. Retina. 2008;28(2):326–332. doi: 10.1097/IAE.0b013e3181237cf8. [DOI] [PubMed] [Google Scholar]
- 51.Pollack JS, Beecher DJ, Pulido JS, Lee Wong AC. Failure of intravitreal dexamethasone to diminish inflammation or retinal toxicity in an experimental model of Bacillus cereus endophthalmitis. Curr. Eye Res. 2004;29(4–5):253–259. doi: 10.1080/02713680490516701. [DOI] [PubMed] [Google Scholar]
- 52.Pathengay A, Shah GY, Das T, Sharma S. Intravitreal triamcinolone acetonide in the management of exogenous bacterial endophthalmitis. Am. J. Ophthalmol. 2006;141(5):938–940. doi: 10.1016/j.ajo.2005.11.043. [DOI] [PubMed] [Google Scholar]
- 53. Schimel AM, Miller D, Flynn HW., Jr Endophthalmitis isolates and antibiotic susceptibilities: a 10-year review of culture-proven cases. Am. J. Ophthalmol. 2013;156(1):50–52.e1. doi: 10.1016/j.ajo.2013.01.027. • Single-center review of 448 isolates over 10 years.
- 54.Khera M, Pathengay A, Jindal A, et al. Vancomycin-resistant Gram-positive bacterial endophthalmitis: epidemiology, treatment options, and outcomes. J. Ophthalmic Inflamm. Infect. 2013;3(1):46. doi: 10.1186/1869-5760-3-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Won JY, Kim M. Vancomycin-resistant Staphylococcus hominis endophthalmitis following cataract surgery. Clin. Ophthalmol. 2013;7:1193–1195. doi: 10.2147/OPTH.S46792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bhat SS, Undrakonda V, Mukhopadhyay C, Parmar PV. Outbreak of multidrug-resistant acute postoperative endophthalmitis due to Enterobacter aerogenes. Ocul. Immunol. Inflamm. 2014;22(2):121–126. doi: 10.3109/09273948.2013.830752. [DOI] [PubMed] [Google Scholar]
- 57.Jacobs DJ, Grube TJ, Flynn HW, Jr, et al. Intravitreal moxifloxacin in the management of Ochrobactrum intermedium endophthalmitis due to metallic intraocular foreign body. Clin. Ophthalmol. 2013;7:1727–1730. doi: 10.2147/OPTH.S44212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jindal A, Pathengay A, Khera M, et al. Combined ceftazidime and amikacin resistance among Gram-negative isolates in acute-onset postoperative endophthalmitis: prevalence, antimicrobial susceptibilities, and visual acuity outcome. J. Ophthalmic Inflamm Infect. 2013;3(1):62. doi: 10.1186/1869-5760-3-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miller D, Flynn PM, Scott IU, et al. In vitro fluoroquinolone resistance in staphylococcal endophthalmitis isolates. Arch. Ophthalmol. 2006;124(4):479–483. doi: 10.1001/archopht.124.4.479. [DOI] [PubMed] [Google Scholar]
- 60.Koc A, Onal S, Yenice O, Kazokoglu H. Pars plana vitrectomy and intravitreal liposomal amphotericin B in the treatment of Candida endophthalmitis. Ophthalmic Surg. Lasers Imgaging. 2010 Mar 9; doi: 10.3928/15428877-20100215-35. [DOI] [PubMed] [Google Scholar]
- 61.Silva NC, Silva S, Sarmento B, Pintado M. Chitosan particles for daptomycin delivery in ocular treatment of bacterial endophthalmitis. Drug Deliv. 2013 Nov 25; doi: 10.3109/10717544.2013.858195. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang T, Huang X, Gao Q, et al. A preliminary study to treat severe endophthalmitis via a foldable capsular vitreous body with sustained levofloxacin release in rabbits. Invest. Ophthalmol. Vis. Sci. 2013;54(1):804–812. doi: 10.1167/iovs.12-9695. [DOI] [PubMed] [Google Scholar]
- 63.Hilton GF, Josephberg RG, Halperin LS, et al. Office-based sutureless transconjunctival pars plana vitrectomy. Retina. 2002;22(6):725–732. doi: 10.1097/00006982-200212000-00007. [DOI] [PubMed] [Google Scholar]
- 64.Shevchenko L, Westhouse SJ, Aaberg TM., Jr When office-based vitrectomy makes sense. Retinal Physician. 2014;11:23–27. 52. [Google Scholar]