Abstract
Class IB phosphoinositide 3-kinases (PI3Kγ) are second-messenger-generating enzymes downstream of signalling cascades triggered by G-protein-coupled-receptors (GPCRs). PI3Kγ variants have one catalytic p110γ subunit that can form two different heterodimers by binding to one of a pair of non-catalytic subunits, p87 or p101. Growing experimental data argue for a different regulation of p87-p110γ and p101-p110γ allowing integration into distinct signalling pathways. Pharmacological tools enabling distinct modulation of the two variants are missing. The ability of an anti-p110γ monoclonal antibody (mAb(A)p110γ) to block PI3Kγ enzymatic activity attracted us to characterize this tool in detail using purified proteins. In order to get insight into the antibody-p110γ-interface, hydrogen-deuterium exchange coupled to mass spectrometry measurements were performed demonstrating binding of the monoclonal antibody to the C2 domain in p110γ, which was accompanied by conformational changes in the helical domain harbouring the Gβγ-binding site. We then studied the modulation of phospholipid vesicles association of PI3Kγ by the antibody. p87-p110γ showed a significantly reduced Gβγ-mediated phospholipid recruitment as compared with p101-p110γ. Concomitantly, in the presence of mAb(A)p110γ Gβγ did not bind to p87-p110γ. These data correlated with the ability of the antibody to block Gβγ-stimulated lipid kinase activity of p87-p110γ 30 times more potently than p101-p110γ. Our data argue for differential regulatory functions of the non-catalytic subunits and a specific Gβγ-dependent regulation of p101 in PI3Kγ activation. In this scenario, we consider the antibody as a valuable tool to dissect the distinct roles of the two PI3Kγ variants downstream of GPCRs.
Keywords: Gβγ, G-protein, p101, p87, PI3Kγ, signal transduction
INTRODUCTION
Class I phosphoinositide 3-kinases (PI3Ks) are lipid kinases that transduce extracellular signals to trigger phosphatidylinositol 3,4,5-trisphosphate (PtdIns(3,4,5)P3) synthesis, an essential second-messenger at the plasma membrane. PtdIns(3,4,5)P3, together with its metabolites, PtdIns(3,4)P2 and PtdIns(3,5)P2, play fundamental roles in the regulation of basic cellular processes, such as proliferation, differentiation, growth and chemotaxis [1–8]. Class I PI3Ks are heterodimers composed of a catalytic (p110) and a non-catalytic subunit of the p85- or p101-type. Based on their interaction with non-catalytic subunits and their specific modes of regulation, class I PI3Ks can be further subdivided into class IA and class IB [2,3,9–12]. Class IA is characterized by heterodimers consisting of a catalytic p110α, p110β or p110δ subunit associated with a p85-type non-catalytic subunit, which has dual roles acting as an adaptor and a regulator [11,13–16]. Although the p85-type subunit is indispensable for class IA PI3K stability and regulation, the p110 catalytic subunit determines the signalling specificity [17–24].
The class IB PI3Ks are represented by two enzymes consisting of one catalytic p110γ subunit associated with either a p101 or a p87 (also known as p87PIKAP or p84) non-catalytic subunit [25–29]. Both PI3Kγ variants, i.e. p87-p110γ and p101-p110γ, are stimulated by Gβγ-heterodimers (Gβγ) released upon G-protein-coupled receptor activation and by active Ras proteins [25–39]. The former view of p87 and p101 being redundant adapters in Gβγ-mediated recruitment of PI3Kγ variants to the membrane compartment [27–29] has been challenged by recent data showing a different contribution of Gβγ and Ras on the two PI3Kγ variants [38]. In particular, distinct Gβγ-binding affinities of the non-catalytic subunits for p110γ are intriguing [38,40,41]. These findings support data showing that PI3Kγ variants integrate into different and independent signalling cascades [39,42–44]. We have recently reported specific features for p87 and p101, such as diverse spatial and temporal distribution in human tissues and a different regulatory impact on p110γ activity, which may contribute to the differential regulation of the PI3Kγ variants [40,41]. These findings, in combination with the fact that only a single class IB catalytic subunit is present in cells led us to postulate that p87 and p101 serve as signal-discriminating regulatory subunits defining specific functions for both p87-p110γ and p101-p110γ variants [41]. However, the exact molecular mechanisms that maintain the specificity and selectivity of the two PI3Kγ variants are still unknown.
In the present study, we have identified and characterized a functional monoclonal anti-p110γ antibody that specifically inhibits the Gβγ-induced p87-p110γ enzymatic activity via contacting the C2 domain of p110γ. Our results point to a differential impact of the non-catalytic subunits thereby revealing a specific Gβγ-dependent regulatory role of p101 in PI3Kγ activation.
EXPERIMENTAL
Cell cultures and expression plasmids
HEK293 cells (German Resource Centre for Biological Materials) were cultured and transfected with expression plasmids encoding p101 and p110γ as described previously [27,37,38]. HL-60 cells were grown in RPMI-1640 supplemented with 10% fetal calf serum, 1% non-essential amino acids, 2 mM L-glutamine, 40 µg/ml folic acid and antibiotics, 100 U/ml penicillin, and 100 µg/ml streptomycin. For preparation of whole cell lysates, cells were directly lysed by adding 1 × Laemmli sample buffer [45].
Expression and purification of recombinant proteins
Sf9 cells (Fall Armyworm Ovary; Invitrogen) were cultured and infected as described previously [40]. Recombinant baculoviruses for expression of Gβ1γ2, PI3Kγ and PI3Kβ subunits as well as their expression in Sf9 cells and purification of hexahistidine (His)6-tagged recombinant Gβ1(His)6γ2, (His)6p110γ, p87-(His)6p110γ, p101-(His)6p110γ, and p85-(His)6p110β have been described elsewhere [38,40,41,46–48]. The pFastBac™ HTb baculovirus transfer vector (Invitrogen) was used to generate human full-length N-terminally (His)6-tagged H-Ras using BamHI/XhoI cloning site. H-Ras was produced in Sf9 insect cells and isolated using the Triton X-114 partition method as described previously [48,49]. The posttranslational processing and lipidation of the protein was verified by MS analysis. Purified proteins were quantified by Coomassie Brilliant Blue staining after SDS/PAGE (10% acrylamide) with BSA as the standard. The proteins were stored at −80 °C.
Hydrogen-deuterium exchange coupled to mass spectrometry measurements
Hydrogen-deuterium exchange coupled to mass spectrometry (HDX-MS) analyses of PI3Kγ in the presence and absence of mAb(A)p110γ were performed following a similar protocol as described previously [21,48]. The rate of exchange of full length p110γ(His)6 alone and in the presence of a 3-fold molar excess of mAb(A)p110γ were compared. Reactions were initiated by mixing 10 µl of protein solution with 40 µl of deuterated buffer containing 20 mM Hepes, pH 7.2, 50 mM NaCl and 0.5 mM EGTA. Deuteration reactions were run for 3, 30, 300 and 3000 s of on-exchange at 23 °C, before being quenched by addition of 20 µl of a 2 M guanidine-HCl and 1.2% formic acid solution. Final deuterium concentration during the reaction was of 78%. Every time point and state was a unique experiment, and every HDX-MS experiment was repeated twice. Samples were immediately frozen in liquid nitrogen and stored at −80 °C for less than a week.
Analysis of p110γ deuteration level was done as described previously [48], by sequentially digesting the protein with pepsin, separating the fragments on a C18 column and measuring the masses of peptides on a LTQ Orbitrap XL mass spectrometer. Manually selected peptides were then examined for deuterium incorporation by the HD-examiner software (Sierra Analytics). Results are presented as relative levels of deuteration with no correction for back exchange.
Gel electrophoresis, immunoblotting, and antibodies
Generation and characterization of the antiserum against the Gβ1 subunit are detailed elsewhere [31,50]. Specific antibodies against p87 and p101 were generous gifts from Michael Schaefer (Leipzig, Germany) and Len Stephens (Cambridge, U.K.), respectively. Monoclonal anti-p110γ antibody, mAb(A)p110γ and mAb(B)p110γ, were raised against full-length human p110γ using mouse hybridome cells and characterized earlier [37]. Large scale preparations of mAb(A)p110γ were generated in cooperation with BioGenes, Berlin, Germany. mAb(B)p110γ was described earlier [31,40,41]. Generation and characterization of monoclonal anti-p110γ antibody, mAb(C)p110γ, raised against the N-terminal 210 amino acids of catalytic p110γ was detailed earlier [43]. Anti-Ras antibody was purchased from BD Biosciences (#610002). Anti-p110β antibody was purchased from Cell Signaling (#3011S). Proteins were fractionated by SDS/PAGE (10% acrylamide) and transferred to nitrocellulose membranes (Hybond™-C Extra, GE Healthcare). Visualization of specific antisera was performed using the ECL chemiluminescence system (GE Healthcare) or the SuperSignal® West Pico Chemiluminescent Substrate (Pierce) according to the manufacturers’ instructions. Chemiluminescence signals were estimated using the VersaDoc™ 4000 MP imaging system (Bio-Rad).
Immunoprecipitation of PI3K
Purified recombinant p110γ, p87-p110γ, and p101-p110γ, and p85α-p110β variants were subjected to immunoprecipitation (IP) using monoclonal anti-p110γ antibodies, mAb(A)p110γ, mAb(B)p110γ or mAb(C)p110γ. IP experiments were performed as detailed previously [41] with some modifications. In brief, Protein A-Sepharose CL-4B beads (GE Healthcare) were preincubated with or without antibody, washed, incubated overnight with cleared cell lysates or purified proteins, and washed again. Proteins bound to beads were either tested for their lipid kinase activity or eluted by adding 1 × Laemmli sample buffer [45] and subjected to SDS/PAGE.
Analysis of PI3K enzymatic activity
The lipid kinase activity of PI3Kγ, and analysis of Gβ1γ2, H-Ras, and PI3Kγ association with phospholipid vesicles were performed as described previously [32,34,40,41,46].
Analytical ultracentrifugation analyses
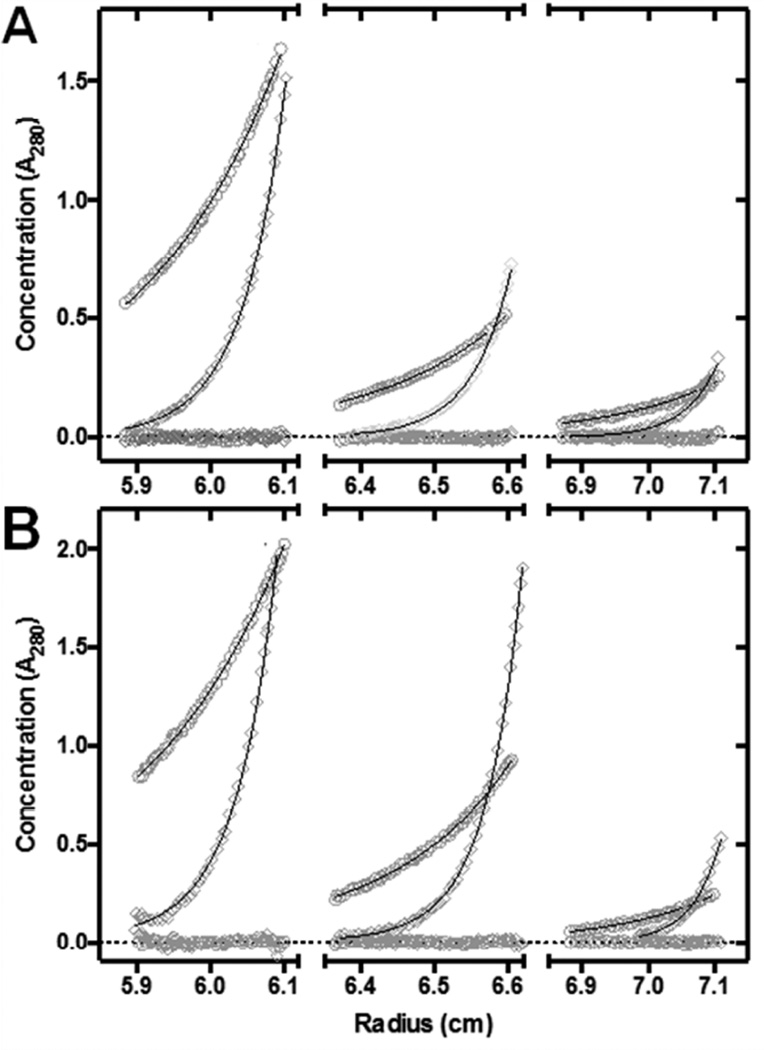
Molecular weight and complex stability of purified p87-p110γ and p110-p110γ heterodimers were analyzed by sedimentation equilibrium analysis using a Beckman Optima XL-I centrifuge using the AN-60Ti rotor with the absorption optics set to 280 nm. Analyses were conducted in a buffer containing 20 mM Tris/HCl, pH 8.0, 150 mM NaCl, 2 mM DTT, and 0.033% polyoxyethylene-10-lauryl ether (C12E10) at 10 °C. Sample and buffer (120 µl each) were loaded into six-channel cell assemblies. Replicate scans were taken following a 24 h equilibration at 6,000 rpm and then following a second 24 h equilibration at 11,000 rpm. Scans were also taken at 22 h at each speed so that equilibration could be confirmed. The equilibrium protein concentration distributions were globally analyzed using the program HeteroAnalysis ver. 1.1.58 [51,52]. Sednterp version 20120828 Beta (http://sednterp.unh.edu) was used to calculate the partial specific volume of the proteins from their sequence and the density of the buffer from its composition neglecting the contribution of the detergent. The sedimentation parameters were corrected to standard conditions (20, w) using these values. The 280 nm extinction coefficients calculated from each protein’s sequence were used to calculate the concentrations of the protein complexes (http://web.expasy.org/protparam/).
Statistical analysis
Results (mean ± S.E.) were analyzed using Student’s t test (*, p ≤ 0.05; **, p ≤ 0.01).
RESULTS
Inhibition of monomeric p110γ by a monoclonal anti-p110γ antibody, mAb(A)p110γ
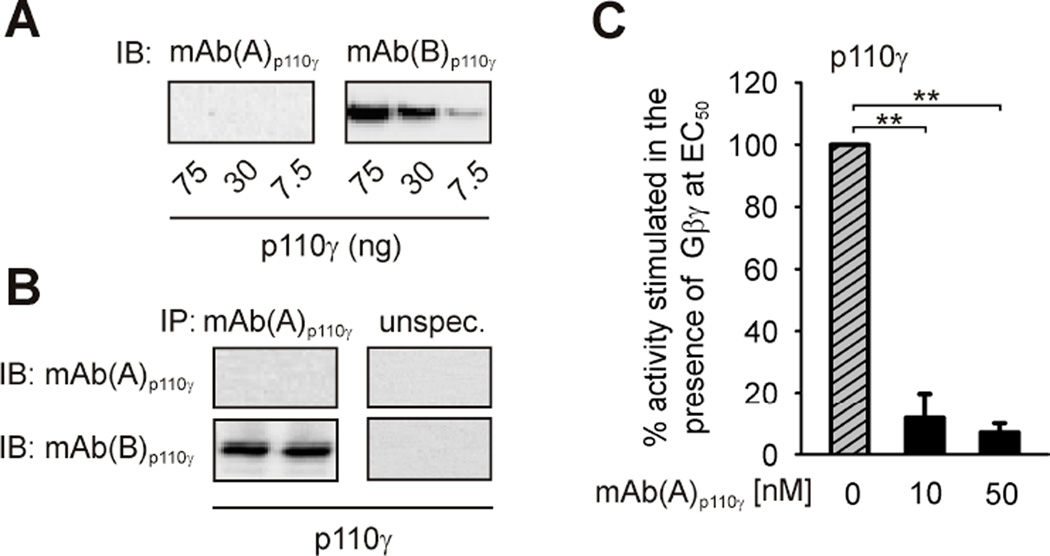
A monoclonal anti-p110γ antibody (mAb(A)p110γ) raised against full-length human catalytic p110γ subunit used earlier in immunoprecipitation (IP) experiments [37] displayed interesting features attracting our attention. mAb(A)p110γ failed to visualize p110γ in immunoblots (Figure 1A), however, it was able to interact with the intact protein in solution enabling IP experiments (Figure 1B). The feature of recognizing native p110γ made it worthwhile to test whether mAb(A)p110γ interferes with p110γ activity. As shown in Figure 1C, incubation with mAb(A)p110γ led to a drastic reduction of p110γ lipid kinase activity stimulated by Gβ1γ2, defining mAb(A)p110γ as a putative PI3Kγ inhibitor.
Figure 1. mAb(A)p110γ inhibits enzymatic activity of monomeric p110γ.
(A) mAb(A)p110γ does not interact with denatured catalytic p110γ subunit in immunoblots. Monomeric p110γ was expressed in and purified from Sf9 cells. Different amounts of the protein were subjected to SDS/PAGE (10% acrylamide) followed by immunoblotting (IB) using monoclonal anti-p110γ antibodies, mAb(A)p110γ, mAb(B)p110γ. (B) mAb(A)p110γ binds intact p110γ. Purified recombinant p110γ was subjected to immunoprecipitation (IP) using mAb(A)p110γ p110γ-unspecific antibody as detailed in the Experimental section. Duplicates of immunoprecipitates were separated by SDS/PAGE (10% acrylamide) followed by immunoblotting (IB) with mAb(A)p110γ or mAb(B)p110γ. (C) mAb(A)p110γ was tested for its ability to affect Gβ1γ2-induced lipid kinase activity of purified recombinant monomeric p110γ. The lipid kinase activity of enzyme (1.5 nM) was measured in the presence of 300 nM Gβ1γ2 (EC50 value) and in the absence or presence of increased concentrations of mAb(A)p110γ. The data shown here are mean values ± S.E. (n = 3).
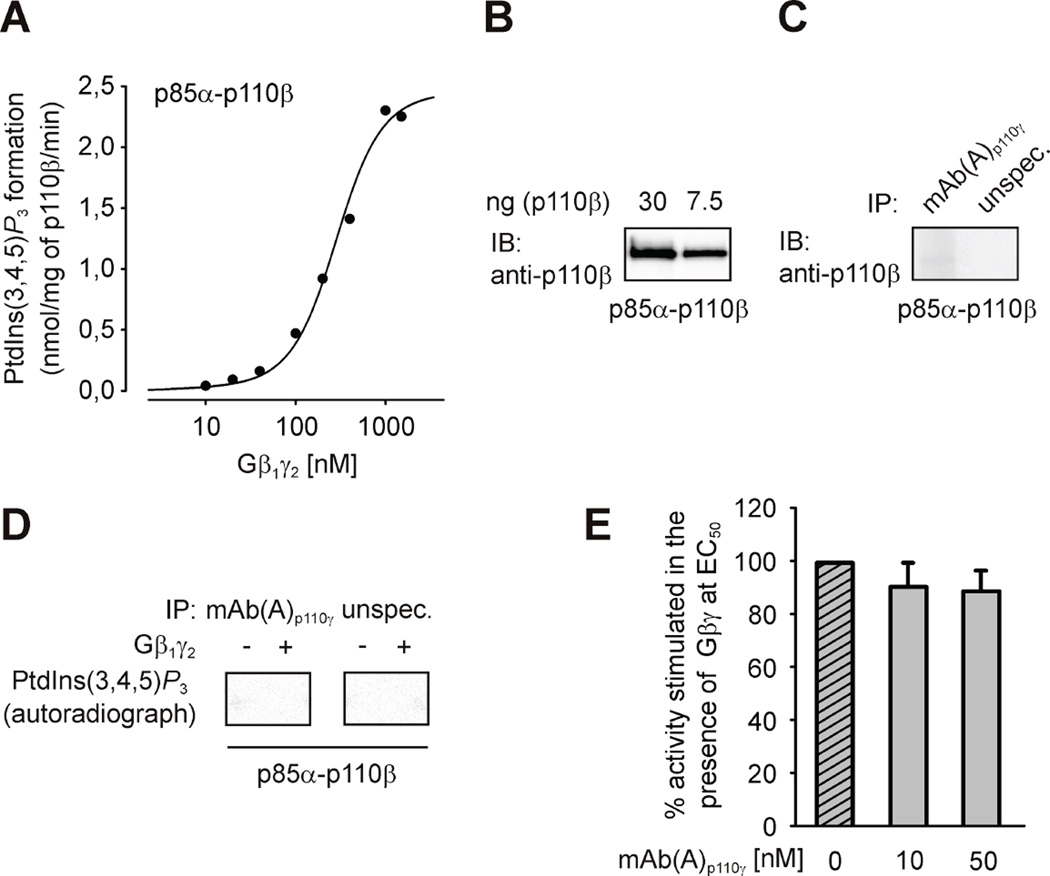
In order to test the selectivity of the mAb(A)p110γ antibody, we measured its effect on the activity of the class IA PI3Kβ, another Gβγ-sensitive PI3K. Recombinant and functionally active Gβγ-sensitive p85α-p110β was purified following heterologous expression in Sf9 cells (Figures 2A and 2B). IP experiments (Figure 2C) as well as analysis of the immunoprecipitates in the lipid kinase assays (Figure 2D) showed complete lack of interaction between mAb(A)p110γ and p85α-p110β. Correspondingly, mAb(A)p110γ did not inhibit lipid kinase activity of purified p85α-p110β (Figure 2E).
Figure 2. mAb(A)p110γ does not interact with Gβγ-sensitive PI3Kβ.
(A) Stimulation of recombinant class IA PI3Kβ (p85α-p110β) lipid kinase activity in response to increasing concentrations of Gβ1γ2. The data shown here represent the average of three independent experiments. (B) Different amounts of purified recombinant p85α-p110β were subjected to SDS/PAGE (10% acrylamide) followed by immunoblotting (IB) using specific anti-p110β antibody. (C) and (D) Purified p85α-p110β was immunoprecipitated (IP) using mAb(A)p110γ or p110β-unspecific antibody as described in the Experimental section. Obtained immunoprecipitates were analyzed by immunoblotting using specific anti-p110β antibody (shown in C) and tested in the lipid kinase assay in the absence or presence of 120 nM Gβ1γ2 (shown in D). Shown here are one typical immunoblot and autoradiograph out of three independent experiments. (E) mAb(A)p110γ was tested for its ability to affect Gβ1γ2-induced lipid kinase activity of purified recombinant p85α-p110β. The lipid kinase activity of enzyme (1.5 nM) was measured in the presence of 300 nM Gβ1γ2 (EC50 value) and in the absence or presence of increased concentrations of mAb(A)p110γ. The data shown here are mean values ± S.E. (n = 3).
Mapping of p110γ regions affected by interaction with mAb(A)p110γ
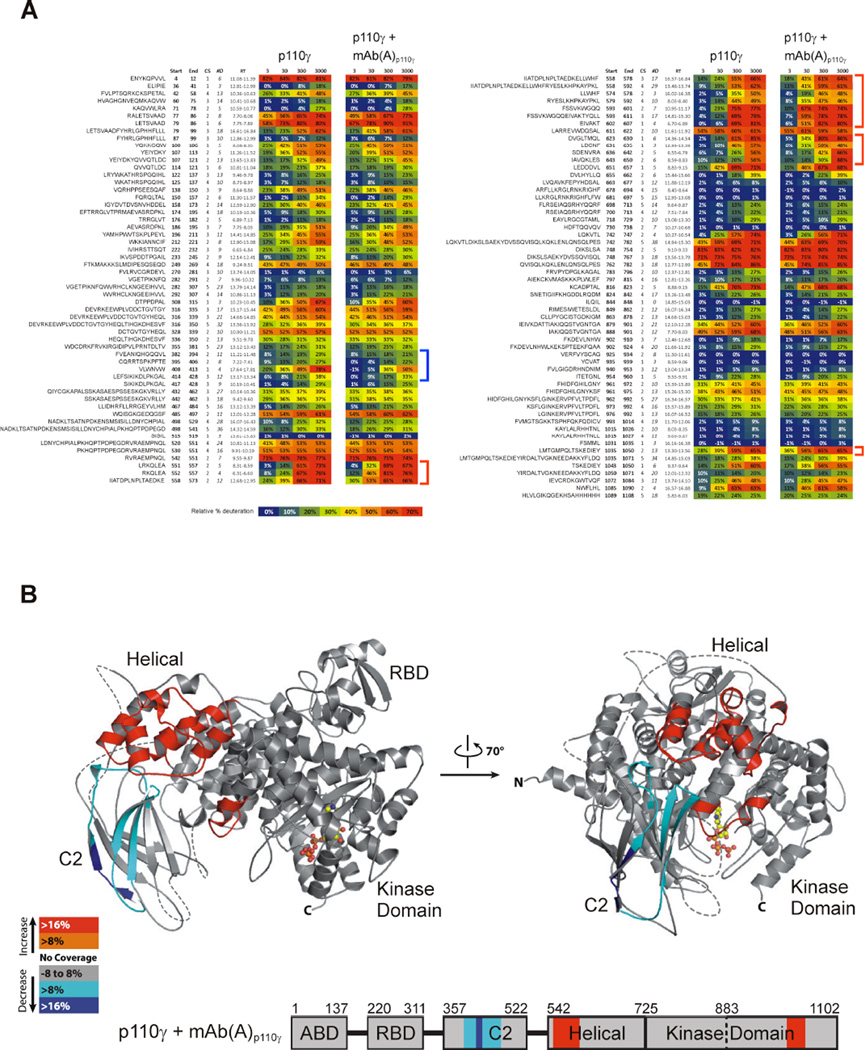
Since mAb(A)p110γ was generated by an immunization and selection protocol using full-length human catalytic p110γ subunit, the epitope of p110γ targeted by mAb(A)p110γ was unknown. To determine the p110γ epitope recognized by mAb(A)p110γ, we used hydrogen-deuterium exchange (HDX) coupled to mass spectrometry (MS). HDX-MS is a powerful technique that can map protein-protein and protein-lipid interaction, as well as provide useful information on the dynamics of proteins [53,54]. The technique is based on the differences in exchange rate of amide protons from a protein with solvents, a reaction that is influenced by secondary structure and solvent exposure.
To map the regions in p110γ that are affected by the interaction with mAb(A)p110γ, we compared the HDX rates of p110γ in solution and when in a complex with the mAb(A)p110γ. A large proportion of the C2 domain shows reduced HDX rate in the p110γ-mAb(A)p110γ complex, suggesting that the antibody binds this region of p110γ (Figures 3A and 3B). More precisely, the most solvent-exposed part of the C2 domain, spanning residues 382–413, has a strongly reduced dynamics, probably stabilizing the beta-strand underneath (residues 414–428). Interestingly, binding of mAb(A)p110γ seems to induce allosteric changes in p110γ, as increased HDX rates are observed in two distinct domains of p110γ, the helical and kinase domains (Figure 3B). The increased dynamics in the p110γ helical domain (551–607, 622–630, 636–650) overlaps with the previously identified Gβγ binding site (546–607) [48]. The two helices within the kinase domain that show increased dynamics (1035–1050) correspond to a region essential for inhibition of p110α activity by its regulatory subunit [55].
Figure 3. Binding of the of mAb(A)p110γ to the C2 domain of p110γ promotes allosteric changes in distinct domains.
(A) Global hydrogen-deuterium exchange (HDX) in p110γ were analyzed for the following states: p110γ alone and p110γ associated to mAb(A)p110γ. The HDX percentage for each p110γ peptide is shown at 3, 30, 300, and 3000 s. The beginning and ending residues for each peptide are illustrated along with the charge state (CS), number of amide deuterons (#D), and retention time (RT). Peptides in p110γ showing reduced (blue) and increased (red) HDX rate after incubation with mAb(A)p110γ are indicated with brackets. (B) Mapping of the changes in deuteration levels between free p110γ and p110γ bound to mAb(A)p110γ are visualized on p110γ crystal structure (top, PDB ID 1e8x) and on a schematic representation of p110γ sequence (bottom). Peptides with significant changes are coloured on p110γ model according to the color scheme shown (red and orange indicate increased exposure on binding, and cyan and blue represent decreased exposure).
In summary, HDX-MS experiments revealed that mAb(A)p110γ associates with the C2 domain of p110γ and induces conformational changes in the helical and kinase domains. Since both domains are important for PI3Kγ regulation, binding of mAb(A)p110γ to p110γ might affect kinase enzymatic activity.
Effect of mAb(A)p110γ on p87-p110γ and p101-p110γ heterodimers activity
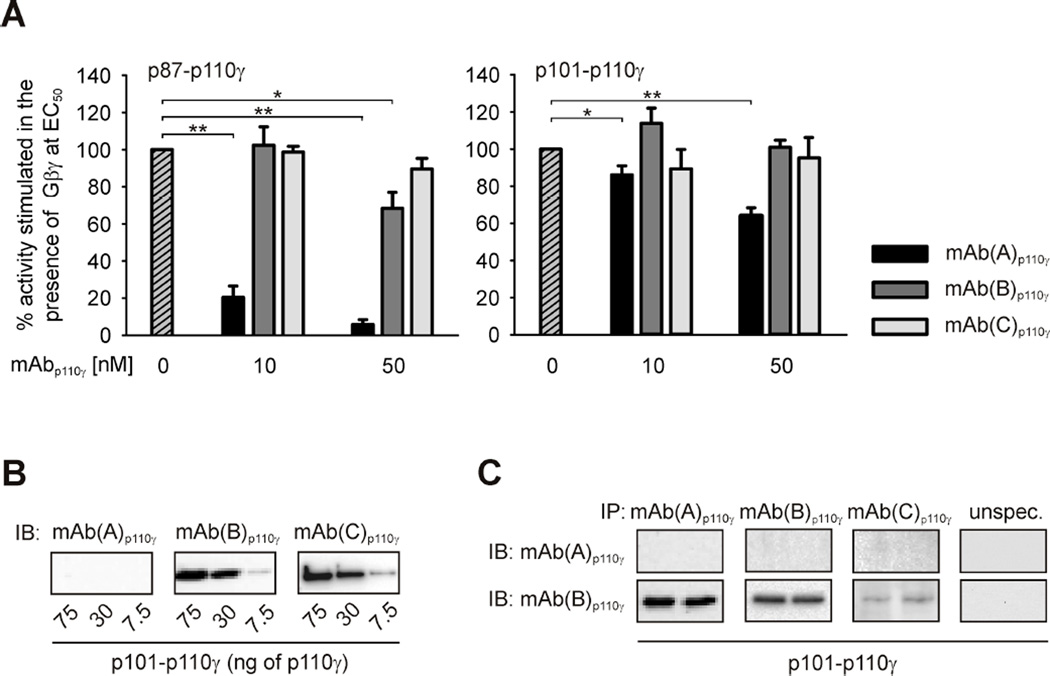
Class IB PI3Kγ is present as two distinct functional p87-p110γ and p101-p110γ heterodimers in vivo [26,38,41,42]. We tested how mAb(A)p110γ affects enzymatic activities of these two PI3Kγ variants stimulated by Gβ1γ2. Two additional monoclonal antibodies raised against full-length human catalytic p110γ subunit (mAb(B)p110γ) and N-terminal amino acids 1–210 of p110γ (mAb(C)p110γ) were also included in order to validate the specificity of interactions. As depicted in Figure 4A significant differences in the ability of the antibodies to affect lipid kinase activities of the two PI3Kγ variants became apparent. While incubation of p87-p110γ with mAb(A)p110γ resulted in drastic reduction of Gβ1γ2-stimulated lipid kinase activity, inhibition of p101-p110γ activity by this antibody, in the concentrations tested, was weak. In contrast, mAb(B)p110γ and mAb(C)p110γ were ineffective in inhibiting enzymatic activity of either PI3Kγ variant under the identical experimental conditions (Figure 4A). The intriguing finding of differential mAb(A)p110γ-mediated effect on the two PI3Kγ variants showing only weak inhibition of p101-p110γ as compared with strong inhibition of p87-p110γ prompted us to check whether mAb(A)p110γ was able to interact with p110γ when associated with p101. Comparable to monomeric p110γ (Figure 1A), immunoblotting analysis revealed that mAb(A)p110γ does not recognize denatured p101-p110γ complex (Figure 4B). In contrast, mAb(B)p110γ and mAb(C)p110γ recognize p110γ in immunoblots (Figure 4B). Nonetheless, the capability of mAb(A)p110γ to directly bind to p110γ when complexed to p101 could be verified by IP (Figure 4C).
Figure 4. Different impact of monoclonal anti-p110γ antibodies on enzymatic activity of heterodimeric PI3Kγ variants.
(A) Monoclonal anti-p110γ antibodies, mAb(A)p110γ, mAb(B)p110γ and mAb(C)p110γ, were tested for their ability to affect Gβ1γ2-induced lipid kinase activity of purified recombinant p87-p110γ and p101-p110γ. PI3Kγ variants (1.5 nM) were stimulated by Gβ1γ2 at EC50 values, i.e. 300 nM for p87-p110γ and 30 nM for p101-p110γ. The data shown here are mean values ± S.E. (n = 3). (B) mAb(A)p110γ does not interact with catalytic p110γ subunit of denatured p101-p110γ in immunoblots. Heterodimeric enzyme was expressed in and purified from Sf9 cells. Different amounts of the recombinant protein were subjected to SDS/PAGE (10% acrylamide) followed by immunoblotting (IB) using monoclonal anti-p110γ antibodies, mAb(A)p110γ, mAb(B)p110γ or mAb(C)p110γ. (C) mAb(A)p110γ binds catalytic subunit of intact p101-p110γ. Purified recombinant p101-p110γ was subjected to immunoprecipitation (IP) using mAb(A)p110γ, mAb(B)p110γ, mAb(C)p110γ or p110γ-unspecific antibody as described in the Experimental section. Duplicates of immunoprecipitates were separated by SDS/PAGE (10% acrylamide) followed by immunoblotting (IB) with mAb(A)p110γ or mAb(B)p110γ.
Taken together, mAb(A)p110γ inhibits Gβγ-stimulated lipid kinase activity of p87-p110γ more potently than of p101-p110γ
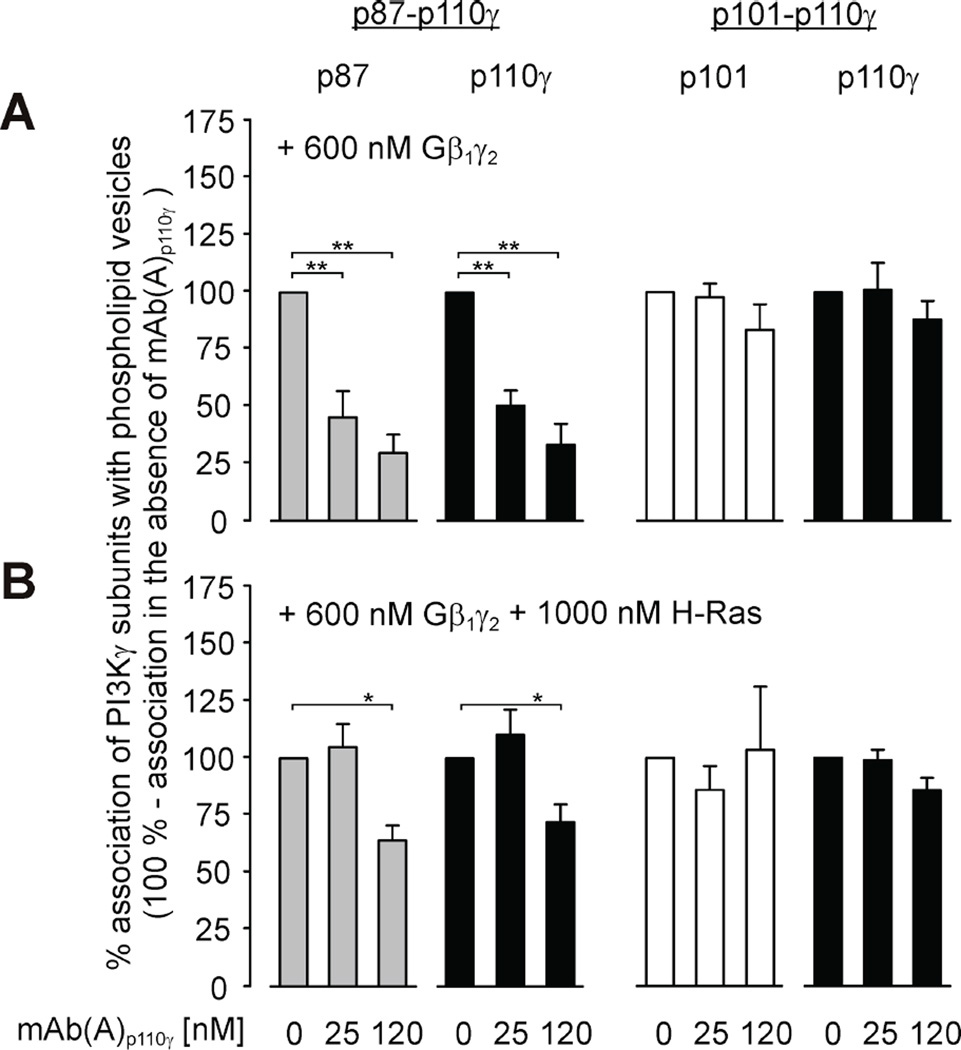
Interaction of p87-p110γ or p101-p110γ heterodimers with phospholipid vesicles
The HDX-MS data demonstrate binding of mAb(A)p110γ to the C2 domain of p110γ (Figure 3B). The C2 domain of p110γ, similarly to other C2 domains, is considered to mediate protein-lipid interactions [56–58]. This encouraged us to check whether mAb(A)p110γ interferes with Gβ1γ2-mediated association of p87-p110γ or p101-p110γ to phosholipid vesicles, in the absence and presence of another known PI3Kγ regulator, i.e. H-Ras. Strikingly, mAb(A)p110γ differently affected Gβ1γ2-mediated phospholipid vesicle association of PI3Kγ variants. Whereas mAb(A)p110γ strongly reduced Gβ1γ2-mediated vesicle association of p87-p110γ in a concentration-dependent manner, association of p101-p110γ remained unchanged (Figure 5A). mAb(A)p110γ did not change binding of p101-p110γ to phospholipid vesicles upon exposure to both regulators, Gβ1γ2 and H-Ras (Figure 5B). However, concomitant incubation with Gβ1γ2 and prenylated H-Ras partially rescued phospholipid vesicle association of p87-p110γ in the presence of mAb(A)p110γ. Nonetheless, membrane association was impaired by high concentrations of mAb(A)p110γ (Figure 5B). It should be pointed out that in these experiments p87, p101, and p110γ were found in ratios corresponding the starting condition suggesting that the stoichiometry of the PI3Kγ variants bound to phospholipid vesicles was not affected by mAb(A)p110γ (Figure 5, grey or white bars vs. black bars). Control experiments excluded that the association of Gβγ or H-Ras to phospholipid vesicles was significantly affected by mAb(A)p110γ (Table 1). High complex stability was supported by equilibrium analytical ultracentrifugation showing Kd values of ≤ 0.2 µM for p87-p110γ and ≤ 0.1 µM for p101-p110γ (Figure 6).
Figure 5. Effect of mAb(A)p110γ on association of PI3Kγ variants with phospholipid vesicles.
mAb(A)p110γ was tested for its ability to affect Gβ1γ2-mediated association (600 nM Gβ1γ2) of purified recombinant PI3Kγ variants (28 nM) with phospholipid vesicles in the absence (A) or presence of 1000 nM H-Ras (B). Aliquots of supernatants and sedimented phospholipid vesicles were subjected to SDS/PAGE (10% acrylamide). Association of each PI3Kγ subunits with phospholipid vesicles was analyzed by immunoblotting using mAb(A)p110γ and antibodies specific against p87 or p101. Chemiluminescence signals were estimated with a VersaDoc™ 4000 MP imaging system (Bio-Rad). For calculation of phospholipid vesicle-associated subunits of PI3Kγ variants, signal intensities in the sedimented phospholipid vesicles and their supernatants were added and considered as 100 %. Shown here are the mean values ± S.E. of at least three separate experiments.
Table 1. mAb(A)p110γ does not change association of Gβ1γ2 and H-Ras with phospholipid vesicles.
Recombinant purified Gβ1γ2 dimers (600 nM) and H-Ras (1000 nM) were mixed with 28 nM p87-p110γ or p101-p110γ and incubated with phospholipid vesicles in the absence or presence of 25 nM or 120 nM mAb(A)p110γ. Aliquots of sedimented phospholipid vesicles and their supernatants were subjected to SDS/PAGE (10 % acrylamide) followed by immunoblotting using antibodies specific for Gβ1–4 and H-Ras proteins. Chemiluminescence signals were estimated with a VersaDoc™ 4000 MP imaging system (Bio-Rad). For calculation of phospholipid vesicle-associated proteins, signal intensities in the sedimented phospholipid vesicles and their supernatants were added and considered as 100 %. Shown here are the mean values ± S.E. of at least three separate experiments.
| Incubation with PI3Kγ variants |
mAb(A)p110γ, [nM] |
Association with lipid vesicles | |
|---|---|---|---|
| Gβ1γ2 | H-Ras | ||
| % | |||
| p87-p110γ | 0 | 29.2 ± 5.3 | 35.2 ± 6.9 |
| 25 | 24.7 ± 5.2 | 34.1 ± 7.3 | |
| 120 | 23.7 ± 4.5 | 34.8 ± 8.1 | |
| p101-p110γ | 0 | 31.4 ± 4.8 | 32.2 ± 7.2 |
| 25 | 30.2 ± 7.9 | 28.2 ± 8.1 | |
| 120 | 32.1 ± 7.9 | 29.3 ± 5.9 | |
Figure 6. Comparable complex stability of p87-p110γ and p101-p110γ measured by analytical sedimentation equilibrium.
Three concentrations (0.5, 2.0, and 4.0 µM) of p87-p110γ (A) and p101-p110γ (B) were centrifuged and analyzed to yield the equilibrium concentration distributions of the protein complexes (measured by their absorption at 280 nm) as a function of the radial distance from the center of the rotor at 6,000 rpm (○) and 11,000 rpm (◊) for each of the three sample channels. The solid lines depict the best nonlinear least squares fit of the heteroassociation model to each complex. The residuals of the fits are shown at the bottom of each channel along the dotted line at 0.0. Sedimentation equilibrium analysis yielded weight-average molecular weights (196.8 ± 7.8 kDa for p87-p110γ and 206.6 ± 10.2 kDa for p101-p110γ) which were slightly less than the values calculated from the sequences of the proteins (210.7 kDa for p87-p110γ and 223.8 kDa for p101-p110γ), assuming a 1:1 stoichiometry for the complexes. The Kd values determined from these data are presented in the “Results” section.
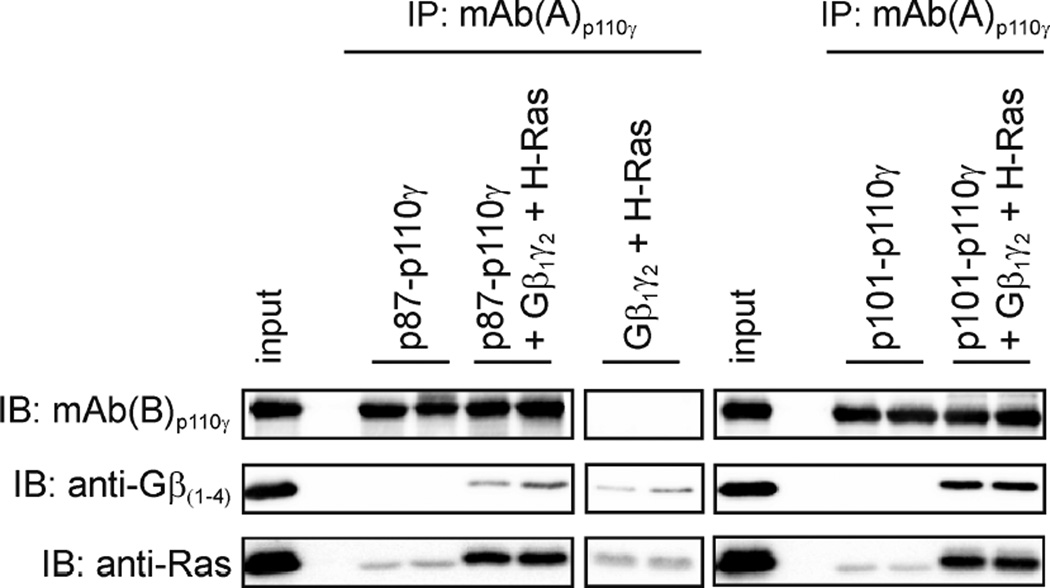
The interference of mAb(A)p110γ with Gβγ-binding was tested by co-immunoprecipitation of p87-p110γ or p101-p110γ with Gβ1γ2 and H-Ras (Figure 7). In the case of p87-p110γ a reduction of Gβ1γ2 monitored by Gβ1-immunoreactivity was evident whereas H-Ras-levels remained unaffected (Figure 7). Taken together the data show a mAb(A)p110γ-dependent inhibition of Gβ1γ2-induced recruitment of p87-p110γ to the lipid compartment. Next, we asked for consequences on enzymatic activity.
Figure 7. mAb(A)p110γ affects binding of Gβ1γ2 to p87-p110γ.
Purified recombinant p87-p110γ or p101-p110γ (0.375 µg of catalytic p110γ subunit) in the absence or presence of Gβ1γ2 (1.25 µg) and H-Ras (1.25 µg) were subjected to immunoprecipitation (IP) using mAb(A)p110γ as described in the Experimental section. Duplicates of immunoprecipitates were separated by SDS/PAGE (10% acrylamide) followed by immunoblotting (IB) with mAb(B)p110γ. Co-immunoprecipitated Gβ1γ2 and H-Ras were visualized using specific anti-Gβ(1–4) and anti-Ras antibodies. Weak unspecific chemiluminescence signals detected by anti-Ras antibody in PI3Kγ immunoprecipitates in the absence of Gβ1γ2 and H-Ras are caused by light chains of mAb(A)p110γ.
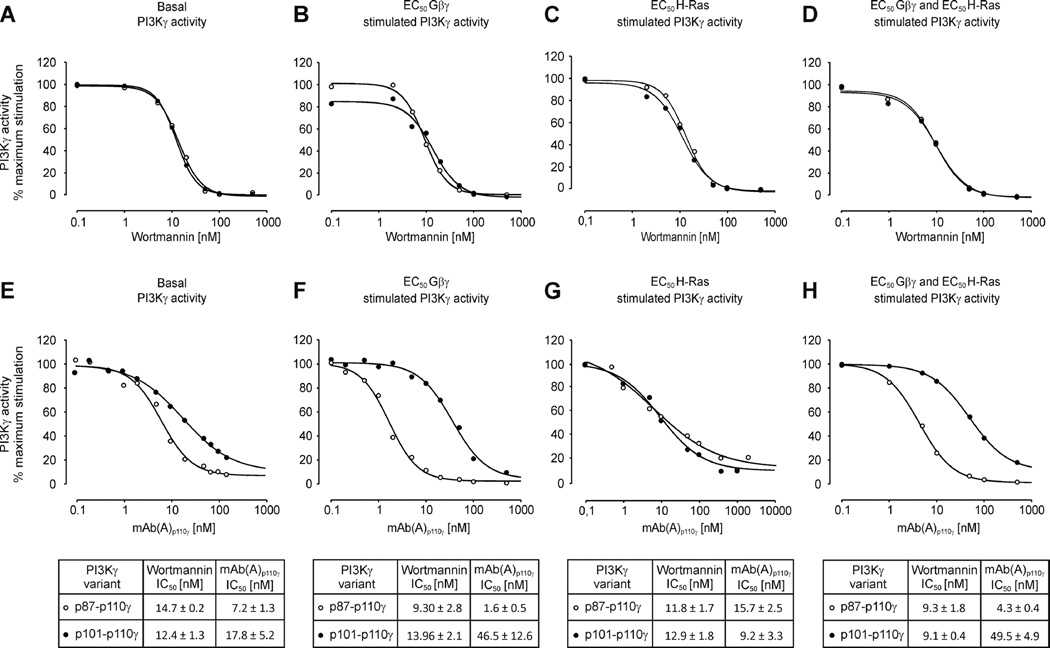
Concentration-dependent inhibition of PI3Kγ variants by mAb(A)p110γ
We studied concentration-dependent inhibition of variously stimulated lipid kinase activities of p87-p110γ and p101-p110γ in the presence of increasing concentrations either of the pan-PI3K inhibitor wortmannin (Figures. 8A – 8D) or mAb(A)p110γ (Figures 8E – 8H). Wortmannin, which blocks all class I PI3Ks by covalent binding to a lysine residue in the ATP binding pocket of p110 isoforms [59], inhibited both PI3Kγ variants at similar IC50 concentrations under all conditions tested and failed to differentiate between the two PI3Kγ variants.
Figure 8. Discriminative inhibition of heterodimeric PI3Kγ variants by mAb(A)p110γ.
The activities of p87-p110γ or p101-p110γ either in the basal condition or in the presence of Gβ1γ2, Ras and Gβ1γ2 together with Ras were measured in the presence of increasing concentrations of pan-PI3K inhibitor wortmannin (A–D) or mAb(A)p110γ (E–H). (A) and (E) The activities of PI3Kγ variants were measured under basal conditions with 7 nM (n=2) or 14 nM (n=1) of kinase in the assay. (B) and (F) The activities of PI3Kγ variants (1.5 nM) were measured in the presence of EC50 concentrations of Gβ1γ2 (300 nM for p87-p110γ and 30 nM for p101-p110γ). (C) and (G) The activities of PI3Kγ enzymes (7 nM) were measured in the presence of EC50 concentrations of Ras (450 nM for p87-p110γ and 850 nM for p101-p110γ). (D) and (H) The activities of PI3Kγ variants (1.5 nM) were measured in the presence of EC50 concentrations of Ras (450 nM for p87-p110γ and 850 nM for p101-p110γ) and EC50 concentrations of Gβ1γ2 (300 nM for p87-p110γ and 30 nM for p101-p110γ). The data shown in graphs and in tables are the mean values ± S.E. of at least three separate experiments.
In the presence of mAb(A)p110γ, basal lipid kinase activities of the two PI3Kγ variants were inhibited in a concentration-dependent manner with IC50 values of 7.2 ± 1.3 nM and 17.8 ± 5.2 nM for p87-p110γ and p101-p110γ, respectively (Figure 8E). Strikingly, the Gβ1γ2-stimulated activity of p87-p110γ was inhibited about 30 times more potently as compared to the p101-p110γ counterpart (IC50: 1.6 ± 0.5 nM vs. IC50: 46.5 ± 12.6 nM; Figure 8F). In contrast, mAb(A)p110γ inhibition of H-Ras-stimulated variants was indistinguishable (Figure 8G). When the enzymes were co-stimulated by Gβ1γ2 and H-Ras, p87-p110γ was 10 times more potently inhibited as compared to p101-p110γ by mAb(A)p110γ (IC50: 4.3 ± 0.4 nM vs. IC50: 49.5 ± 4.9 nM; Figure 8H). Thus, mAb(A)p110γ represents not only a valuable experimental tool to understand the different regulation of PI3Kγ variants but also serves to selectively intervene into Gβγ-induced p87-p110γ lipid kinase activity.
DISCUSSION
We recently described p87-p110γ as a constitutively and ubiquitously expressed class IB PI3Kγ variant [41]. In contrast, p101-p110γ appeared as an inducible counterpart which is up-regulated upon activation and expressed in various tissues side-by side with p87-p110γ. In line with this view, growing experimental evidence indicate a divergent function and regulation of the two class IB PI3Kγ variants [38,39,42–44]. Unfortunately, pharmacological tools discriminating between the two variants are not available [60]. Here, we identified a monoclonal antibody mAb(A)p110γ as a potent inhibitor of PI3Kγ isoforms acting at low nanomolar concentrations. mAb(A)p110γ blocked basal lipid kinase activities of either p87-p110γ or p101-p110γ with potencies comparable to that of wortmannin, an inhibitor acting at the ATP-binding site. Interestingly, enzymatic activities were differentially inhibited with a significant preference for p87-p110γ following stimulation by Gβγ. This preferential inhibition of p87-p110γ activity by mAb(A)p110γ persisted even in experiments stimulating the PI3Kγ variants simultaneously with Ras and Gβγ.
The monoclonal antibody mAb(A)p110γ was generated using full-length human p110γ protein for immunization und selection procedure and, therefore, the exact antibody-p110γ-interaction site was unknown [37,61]. Hydrogen-deuterium exchange coupled to mass spectrometry (HDX-MS), an approach that has provided inside into PI3K regulation at the membrane and by regulatory partners [21,48,62], identified dynamic changes within three domains of p110γ upon association with mAb(A)p110γ. Residues 382–428 in the C2 domain of p110γ were protected from HDX-MS exchange, most likely due to binding of the antibody to this region. In addition, antibody-p110γ-interaction induced increased dynamics in both the helical and the kinase domain of p110γ, probably as a result of allosteric modifications.
Generally, C2 domains have been associated with membrane interactions. The C2 domain of p110γ was also proposed to be involved in the interaction of p110γ with the plasma membrane [58]. However, recent data looking at lipid binding sites of class I PI3Ks have identified the C-terminal helix of the kinase domain rather than the C2 domain to be involved in binding to lipids [21,48,63]. Our data obtained in phospholipid pull-down assays are in agreement with these recent data. The necessity of the C2 domain of p110γ to act as the membrane interaction module in the regulation of PI3Kγ was not hitherto experimentally validated. Although Kirsch et al. [64] have shown that the phospholipid binding of a p110γ fragment comprising amino acids 740–1068 was significantly lower than the binding of full-length p110γ, this truncation construct lacked more than just the C2 domain (comprising residues 357–522). In addition to phospholipid-binding, C2 domains have been reported to exhibit additional functions. In p110α, the C2 domain seems to be crucial for the inhibitory function of p85 on p110, whereas the C2 domain of p110β harbours a nuclear localization signal motif mediating translocation into the nucleus [11,15,65].
Our data argue for a different impact of mAb(A)p110γ on Gβγ-mediated stimulation of p87-p110γ and p101-p110γ. HDX-MS analyses indicate that binding of mAb(A)p110γ to p110γ C2 domain induces allosteric changes in the helical domain. Since the helical domain is responsible for Gβγ-binding [48], it is possible that the conformational changes directly affect the affinity of Gβγ for p110γ. Additionally, the different potencies by which mAb(A)p110γ inhibits Gβγ stimulation of PI3Kγ variants may be a consequence of a distinct impact of the two non-catalytic subunits, i.e. p87 and p101, on PI3Kγ activity (Figure 9).
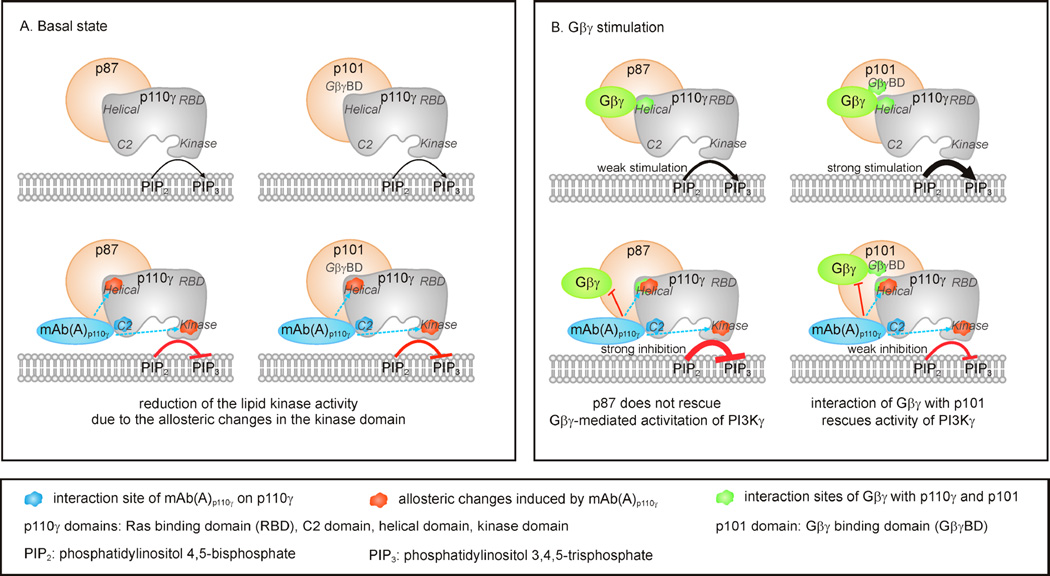
Figure 9. Schematic representation of putative molecular mechanisms induced by mAb(A)p110γ resulting in discriminative inhibition of the PI3Kγ variants.
(A) Effect of mAb(A)p110γ on the basal states of the PI3Kγ variants. Binding of mAb(A)p110γ to the C2 domain mediates allosteric modulation of residues 551–650 in the helical domain and residues 1035–1050 located in the helices kα9 and kα10 of the C-terminal lobe of the kinase domain. These helices play important role in allosteric activation of p110γ, as it was shown in the case of Ras stimulation [35]. mAb(A)p110γ -induced structural change of the kinase domain may interfere and reduce the basal lipid kinase activities of p87-p110γ and p101-p110γ. Slight protection of p101-p110γ basal lipid kinase activity from the inhibitory effect of mAb(A)p110γ is in line with the previous data showing stimulatory modulation of p110γ by p101 independently of its Gβγ adaptor function [41]. (B) Effect of mAb(A)p110γ on the PI3Kγ variants stimulated by Gβ1γ2. Binding of mAb(A)p110γ to the C2 domain of p110γ causes allosteric exposure of a region (residues 551–650) in the helical domain which also includes crucial amino acids involved in interaction with Gβγ, Arg552 and Lys553 [48]. This results in allosteric interference of mAb(A)p110γ with Gβγ binding to p110γ. p101 was shown to be also involved in interaction with Gβγ via putative Gβγ binding domain (GβγBD) located in the C-terminal region of p101 [48]. In contrast to p101, p87 contributed much lesser (if at all) to Gβγ interaction [28,38,41,48]. In the scenario of discriminative inhibition, mAb(A)p110γ disrupts p110γ-Gβγ interaction in a similar way for each PI3Kγ variant, whereas unaltered Gβγ binding capacity of p101 still allows effective translocation of p101-p110γ and regulatory activity. In contrast, p87-p110γ showed reduced capability to interact with Gβγ in the presence of mAb(A)p110γ resulting in drastic reduction of enzymatic activity.
Indicated are phosphatidylinositol 4,5-bisphosphate (PIP2), phosphatidylinositol 3,4,5-trisphosphate (PIP3), the Ras binding domain (RBD, residues 220–311), the C2 domain (residues 357–522), the helical domain (residues 545–725), the kinase domain (residues 726–1092) of p110γ [58], and putative Gβγ binding domain (GβγBD) of p101 [48].
Ample evidence suggests that p101 acts as a Gβγ adaptor [26,32,37,38]. Since p101 is able to rescue the stimulatory effect of Gβ1 mutants deficient in stimulating p110γ [40] and enhances Gβγ-induced stimulation of lipid-associated p110γ [41] we characterize p101 as a Gβγ-dependent regulator of PI3Kγ enzymatic activity. HDX-MS analysis on the p101-p110γ complex has identified two regions within the C-terminal part of p101 to mediate PI3Kγ activation by Gβγ [48]. In contrast, whether p87 functionally interacts with Gβγ remains an open question. Although p87 exhibits significant degree of homology with p101 at the C-terminal region [27–29], up to now we could not find any evidence that it displays a Gβγ-adapter function or serves as a Gβγ-dependent regulator [38,40,41]. Therefore, we suppose that in the presence of Gβγ, mAb(A)p110γ induces structural alterations in the helical domain that result in more drastic consequences for p87-p110γ than for p101-p110γ on phospholipid vesicle recruitment and enzymatic activation. An alternative mechanism of discriminative inhibition of PI3Kγ variants would be that mAb(A)p110γ binding induces the allosteric effect in monomeric p110γ and p87-p110γ, while p101 prevents the binding-induced change in p110γ. However, the molecular dynamics of deuterium exchange from p110γ were indistinguishable when complexed with p87 or p101 [48,66].
Taken together, we have characterized the inhibitory action of the monoclonal anti-p110γ antibody, mAb(A)p110γ, mapped the antibody-p110γ interface and present new structure-functions insights of PI3Kγ activity. Specific features of mAb(A)p110γ to differentially block Gβγ-mediated association of p87-p110γ and p101-p110γ and, hence, their enzymatic activities provide the basis for a selective inhibition of Gβγ-initiated hormonal pathways of PI3Kγ variants and argues for a specific Gβγ-dependent regulatory role of p101 in PI3Kγ activation. This supports the idea of a differential regulatory impact of p87 and p101 on PI3Kγ activation.
ACKNOWLEDGEMENTS
The expert technical assistance of Renate Riehle and Rosi Maier is greatly appreciated. We thank all members of the Nürnberg laboratory previously located in Düsseldorf and in Tübingen. We thank John Burke, Olga Perisic, Mark Skehel, Sarah Maslen, Farida Begum and Sew-Yeu Peak-Chew for help with the HDX-MS setup.
FUNDING
This work was supported in part by the Deutsche Forschungsgemeinschaft. Emilio Hirsch is supported by an AIRC grant. Oscar Vadas was supported by a Swiss National Science Foundation fellowship (grant No PA00P3_134202), and a European Commission fellowship (FP7-PEOPLE-2010-IEF, N°275880). Roger Williams is funded by the Medical Research Council (file reference U10518430).
ABBREVIATIONS
- C12E10
polyoxyethylene-(10)-lauryl ether
- Gβγ
βγ dimer of the heterotrimeric G-protein
- GPCR
G-protein-coupled receptor
- HDX-MS
hydrogen-deuterium exchange coupled to mass spectrometry
- His6
hexahistidine tag
- IB
immunoblotting
- IP
immunoprecipitation
- PI3K
phosphatidylinositol 3-kinase
- PtdIns(3,4,5)P3
phosphatidylinositol 3,4,5-trisphosphate
Footnotes
AUTHOR CONTRIBUTION
Aliaksei Shymanets, Christian Harteneck and Bernd Nürnberg designed the study. Aliaksei Shymanets, Prajwal, Oscar Vadas, Cornelia Czupalla, Jaclyn LoPiccolo, Alessandra Ghigo, and Eberhard Krause performed the experiments. Aliaksei Shymanets, Oscar Vadas, Michael Brenowitz, Eberhard Krause, Emilio Hirsch, Reinhard Wetzker, Roger L. Williams, Christian Harteneck and Bernd Nürnberg analysed, and interpreted the data, and wrote the paper.
REFERENCES
- 1.Bunney TD, Katan M. Phosphoinositide signalling in cancer: beyond PI3K and PTEN. Nat. Rev. Cancer. 2010;10:342–352. doi: 10.1038/nrc2842. [DOI] [PubMed] [Google Scholar]
- 2.Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat. Rev. Mol. Cell Biol. 2010;11:329–341. doi: 10.1038/nrm2882. [DOI] [PubMed] [Google Scholar]
- 3.Balla T. Phosphoinositides: tiny lipids with giant impact on cell regulation. Physiol. Rev. 2013;93:1019–1137. doi: 10.1152/physrev.00028.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klempner SJ, Myers AP, Cantley LC. What a tangled web we weave: emerging resistance mechanisms to inhibition of the phosphoinositide 3-kinase pathway. Cancer Discov. 2013;3:1345–1354. doi: 10.1158/2159-8290.CD-13-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Megison ML, Gillory LA, Beierle EA. Cell survival signaling in neuroblastoma. Anticancer Agents Med. Chem. 2013;13:563–575. doi: 10.2174/1871520611313040005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagai S, Kurebayashi Y, Koyasu S. Role of PI3K/Akt and mTOR complexes in Th17 cell differentiation. Ann. N. Y. Acad. Sci. 2013;1280:30–34. doi: 10.1111/nyas.12059. [DOI] [PubMed] [Google Scholar]
- 7.Okkenhaug K. Signaling by the phosphoinositide 3-kinase family in immune cells. Annu. Rev. Immunol. 2013;31:675–704. doi: 10.1146/annurev-immunol-032712-095946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat. Rev. Drug Discov. 2014;13:140–156. doi: 10.1038/nrd4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balla T. Finding partners for PI3Kγ: when 84 is better than 101. Sci. Signal. 2009;2:pe35. doi: 10.1126/scisignal.274pe35. [DOI] [PubMed] [Google Scholar]
- 10.Fruman DA. Regulatory subunits of class IA PI3K. Curr. Top. Microbiol. Immunol. 2010;346:225–244. doi: 10.1007/82_2010_39. [DOI] [PubMed] [Google Scholar]
- 11.Vadas O, Burke JE, Zhang X, Berndt A, Williams RL. Structural basis for activation and inhibition of class I phosphoinositide 3-kinases. Sci. Signal. 2011;4:re2. doi: 10.1126/scisignal.2002165. [DOI] [PubMed] [Google Scholar]
- 12.Jean S, Kiger AA. Classes of phosphoinositide 3-kinases at a glance. J. Cell Sci. 2014;127:923–928. doi: 10.1242/jcs.093773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu J, Zhang Y, McIlroy J, Rordorf-Nikolic T, Orr GA, Backer JM. Regulation of the p85/p110 phosphatidylinositol 3'-kinase: stabilization and inhibition of the p110α catalytic subunit by the p85 regulatory subunit. Mol. Cell. Biol. 1998;18:1379–1387. doi: 10.1128/mcb.18.3.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geering B, Cutillas PR, Nock G, Gharbi SI, Vanhaesebroeck B. Class IA phosphoinositide 3-kinases are obligate p85-p110 heterodimers. Proc. Natl. Acad. Sci. U. S. A. 2007;104:7809–7814. doi: 10.1073/pnas.0700373104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dbouk HA, Pang H, Fiser A, Backer JM. A biochemical mechanism for the oncogenic potential of the p110β catalytic subunit of phosphoinositide 3-kinase. Proc. Natl. Acad. Sci. U. S. A. 2010;107:19897–19902. doi: 10.1073/pnas.1008739107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X, Vadas O, Perisic O, Anderson KE, Clark J, Hawkins PT, Stephens LR, Williams RL. Structure of lipid kinase p110β/p85β elucidates an unusual SH2-domain-mediated inhibitory mechanism. Mol. Cell. 2011;41:567–578. doi: 10.1016/j.molcel.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta S, Ramjaun AR, Haiko P, Wang Y, Warne PH, Nicke B, Nye E, Stamp G, Alitalo K, Downward J. Binding of Ras to phosphoinositide 3-kinase p110α is required for Ras-driven tumorigenesis in mice. Cell. 2007;129:957–968. doi: 10.1016/j.cell.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 18.Okkenhaug K, Ali K, Vanhaesebroeck B. Antigen receptor signalling: a distinctive role for the p110δ isoform of PI3K. Trends Immunol. 2007;28:80–87. doi: 10.1016/j.it.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ciraolo E, Iezzi M, Marone R, Marengo S, Curcio C, Costa C, Azzolino O, Gonella C, Rubinetto C, Wu H, Dastru W, Martin EL, Silengo L, Altruda F, Turco E, Lanzetti L, Musiani P, Rückle T, Rommel C, Backer JM, Forni G, Wymann MP, Hirsch E. Phosphoinositide 3-kinase p110β activity: key role in metabolism and mammary gland cancer but not development. Sci. Signal. 2008;1:ra3. doi: 10.1126/scisignal.1161577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beer-Hammer S, Zebedin E, von Holleben M, Alferink J, Reis B, Dresing P, Degrandi D, Scheu S, Hirsch E, Sexl V, Pfeffer K, Nürnberg B, Piekorz RP. The catalytic PI3K isoforms p110γ and p110δ contribute to B cell development and maintenance, transformation, and proliferation. J. Leukoc. Biol. 2010;87:1083–1095. doi: 10.1189/jlb.0809585. [DOI] [PubMed] [Google Scholar]
- 21.Dbouk HA, Vadas O, Shymanets A, Burke JE, Salamon RS, Khalil BD, Barrett MO, Waldo GL, Surve C, Hsueh C, Perisic O, Harteneck C, Shepherd PR, Harden TK, Smrcka AV, Taussig R, Bresnick AR, Nürnberg B, Williams RL, Backer JM. G protein-coupled receptor-mediated activation of p110β by Gβγ is required for cellular transformation and invasiveness. Sci. Signal. 2012;5:ra89. doi: 10.1126/scisignal.2003264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luk SK, Piekorz RP, Nürnberg B, Tony To SS. The catalytic phosphoinositol 3-kinase isoform p110δ is required for glioma cell migration and invasion. Eur. J. Cancer. 2012;48:149–157. doi: 10.1016/j.ejca.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Fritsch R, de Krijger I, Fritsch K, George R, Reason B, Kumar MS, Diefenbacher M, Stamp G, Downward J. RAS and RHO families of GTPases directly regulate distinct phosphoinositide 3-kinase isoforms. Cell. 2013;153:1050–1063. doi: 10.1016/j.cell.2013.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burke JE, Williams RL. Synergy in activating class I PI3Ks. Trends Biochem. Sci. 2015 doi: 10.1016/j.tibs.2014.12.003. in press. [DOI] [PubMed] [Google Scholar]
- 25.Stoyanov B, Volinia S, Hanck T, Rubio I, Loubtchenkov M, Malek D, Stoyanova S, Vanhaesebroeck B, Dhand R, Nürnberg B, Gierschik P, Seedorf K, Hsuan JJ, Waterfield MD, Wetzker R. Cloning and characterization of a G protein-activated human phosphoinositide-3 kinase. Science. 1995;269:690–693. doi: 10.1126/science.7624799. [DOI] [PubMed] [Google Scholar]
- 26.Stephens LR, Eguinoa A, Erdjument-Bromage H, Lui M, Cooke F, Coadwell J, Smrcka AS, Thelen M, Cadwallader K, Tempst P, Hawkins PT. The Gβγ sensitivity of a PI3K is dependent upon a tightly associated adaptor, p101. Cell. 1997;89:105–114. doi: 10.1016/s0092-8674(00)80187-7. [DOI] [PubMed] [Google Scholar]
- 27.Voigt P, Brock C, Nürnberg B, Schaefer M. Assigning functional domains within the p101 regulatory subunit of phosphoinositide 3-kinase γ. J. Biol. Chem. 2005;280:5121–5127. doi: 10.1074/jbc.M413104200. [DOI] [PubMed] [Google Scholar]
- 28.Suire S, Coadwell J, Ferguson GJ, Davidson K, Hawkins P, Stephens L. p84, a new Gβγ-activated regulatory subunit of the type IB phosphoinositide 3-kinase p110γ. Curr. Biol. 2005;15:566–570. doi: 10.1016/j.cub.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 29.Voigt P, Dorner MB, Schaefer M. Characterization of p87PIKAP, a novel regulatory subunit of phosphoinositide 3-kinase γ that is highly expressed in heart and interacts with PDE3B. J. Biol. Chem. 2006;281:9977–9986. doi: 10.1074/jbc.M512502200. [DOI] [PubMed] [Google Scholar]
- 30.Rubio I, Rodriguez-Viciana P, Downward J, Wetzker R. Interaction of Ras with phosphoinositide 3-kinase γ. Biochem. J. 1997;326(Pt 3):891–895. doi: 10.1042/bj3260891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leopoldt D, Hanck T, Exner T, Maier U, Wetzker R, Nürnberg B. Gβγ stimulates phosphoinositide 3-kinase-γ by direct interaction with two domains of the catalytic p110 subunit. J. Biol. Chem. 1998;273:7024–7029. doi: 10.1074/jbc.273.12.7024. [DOI] [PubMed] [Google Scholar]
- 32.Maier U, Babich A, Nürnberg B. Roles of non-catalytic subunits in Gβγ-induced activation of class I phosphoinositide 3-kinase isoforms β and γ. J. Biol. Chem. 1999;274:29311–29317. doi: 10.1074/jbc.274.41.29311. [DOI] [PubMed] [Google Scholar]
- 33.Rubio I, Wittig U, Meyer C, Heinze R, Kadereit D, Waldmann H, Downward J, Wetzker R. Farnesylation of Ras is important for the interaction with phosphoinositide 3-kinase γ. Eur. J. Biochem. 1999;266:70–82. doi: 10.1046/j.1432-1327.1999.00815.x. [DOI] [PubMed] [Google Scholar]
- 34.Maier U, Babich A, Macrez N, Leopoldt D, Gierschik P, Illenberger D, Nürnberg B. Gβ5γ2 is a highly selective activator of phospholipid-dependent enzymes. J. Biol. Chem. 2000;275:13746–13754. doi: 10.1074/jbc.275.18.13746. [DOI] [PubMed] [Google Scholar]
- 35.Pacold ME, Suire S, Perisic O, Lara-Gonzalez S, Davis CT, Walker EH, Hawkins PT, Stephens L, Eccleston JF, Williams RL. Crystal structure and functional analysis of Ras binding to its effector phosphoinositide 3-kinase γ. Cell. 2000;103:931–943. doi: 10.1016/s0092-8674(00)00196-3. [DOI] [PubMed] [Google Scholar]
- 36.Suire S, Hawkins P, Stephens L. Activation of phosphoinositide 3-kinase γ by Ras. Curr. Biol. 2002;12:1068–1075. doi: 10.1016/s0960-9822(02)00933-8. [DOI] [PubMed] [Google Scholar]
- 37.Brock C, Schaefer M, Reusch HP, Czupalla C, Michalke M, Spicher K, Schultz G, Nürnberg B. Roles of Gβγ in membrane recruitment and activation of p110γ/p101 phosphoinositide 3-kinase γ. J. Cell Biol. 2003;160:89–99. doi: 10.1083/jcb.200210115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kurig B, Shymanets A, Bohnacker T, Prajwal, Brock C, Ahmadian MR, Schaefer M, Gohla A, Harteneck C, Wymann MP, Jeanclos E, Nürnberg B. Ras is an indispensable coregulator of the class IB phosphoinositide 3-kinase p87/p110γ. Proc. Natl. Acad. Sci. U. S. A. 2009;106:20312–20317. doi: 10.1073/pnas.0905506106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmid MC, Avraamides CJ, Dippold HC, Franco I, Foubert P, Ellies LG, Acevedo LM, Manglicmot JR, Song X, Wrasidlo W, Blair SL, Ginsberg MH, Cheresh DA, Hirsch E, Field SJ, Varner JA. Receptor tyrosine kinases and TLR/IL1Rs unexpectedly activate myeloid cell PI3Kγ, a single convergent point promoting tumor inflammation and progression. Cancer Cell. 2011;19:715–727. doi: 10.1016/j.ccr.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shymanets A, Ahmadian MR, Kössmeier KT, Wetzker R, Harteneck C, Nürnberg B. The p101 subunit of PI3Kγ restores activation by Gβ mutants deficient in stimulating p110γ. Biochem. J. 2012;441:851–858. doi: 10.1042/BJ20111664. [DOI] [PubMed] [Google Scholar]
- 41.Shymanets A, Prajwal, Bucher K, Beer-Hammer S, Harteneck C, Nürnberg B. p87 and p101 subunits are distinct regulators determining class IB phosphoinositide 3-kinase (PI3K) specificity. J. Biol. Chem. 2013;288:31059–31068. doi: 10.1074/jbc.M113.508234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bohnacker T, Marone R, Collmann E, Calvez R, Hirsch E, Wymann MP. PI3Kγ adaptor subunits define coupling to degranulation and cell motility by distinct PtdIns(3,4,5)P3 pools in mast cells. Sci. Signal. 2009;2:ra27. doi: 10.1126/scisignal.2000259. [DOI] [PubMed] [Google Scholar]
- 43.Perino A, Ghigo A, Ferrero E, Morello F, Santulli G, Baillie GS, Damilano F, Dunlop AJ, Pawson C, Walser R, Levi R, Altruda F, Silengo L, Langeberg LK, Neubauer G, Heymans S, Lembo G, Wymann MP, Wetzker R, Houslay MD, Iaccarino G, Scott JD, Hirsch E. Integrating cardiac PIP3 and cAMP signaling through a PKA anchoring function of p110γ. Mol. Cell. 2011;42:84–95. doi: 10.1016/j.molcel.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brazzatti JA, Klingler-Hoffmann M, Haylock-Jacobs S, Harata-Lee Y, Niu M, Higgins MD, Kochetkova M, Hoffmann P, McColl SR. Differential roles for the p101 and p84 regulatory subunits of PI3Kγ in tumor growth and metastasis. Oncogene. 2012;31:2350–2361. doi: 10.1038/onc.2011.414. [DOI] [PubMed] [Google Scholar]
- 45.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 46.Czupalla C, Culo M, Müller EC, Brock C, Reusch HP, Spicher K, Krause E, Nürnberg B. Identification and characterization of the autophosphorylation sites of phosphoinositide 3-kinase isoforms β and γ. J. Biol. Chem. 2003;278:11536–11545. doi: 10.1074/jbc.M210351200. [DOI] [PubMed] [Google Scholar]
- 47.Shymanets A, Ahmadian MR, Nürnberg B. Gβγ-copurified lipid kinase impurity from Sf9 cells. Protein Pept. Lett. 2009;16:1053–1056. doi: 10.2174/092986609789055340. [DOI] [PubMed] [Google Scholar]
- 48.Vadas O, Dbouk HA, Shymanets A, Perisic O, Burke JE, Abi Saab WF, Khalil BD, Harteneck C, Bresnick AR, Nürnberg B, Backer JM, Williams RL. Molecular determinants of PI3Kγ-mediated activation downstream of G-protein-coupled receptors (GPCRs) Proc. Natl. Acad. Sci. U. S. A. 2013;110:18862–18867. doi: 10.1073/pnas.1304801110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Porfiri E, Evans T, Bollag G, Clark R, Hancock JF. Purification of baculovirus-expressed recombinant Ras and Rap proteins. Methods Enzymol. 1995;255:13–21. doi: 10.1016/s0076-6879(95)55004-6. [DOI] [PubMed] [Google Scholar]
- 50.Leopoldt D, Harteneck C, Nürnberg B. G proteins endogenously expressed in Sf 9 cells: interactions with mammalian histamine receptors. Naunyn. Schmiedebergs Arch. Pharmacol. 1997;356:216–224. doi: 10.1007/pl00005044. [DOI] [PubMed] [Google Scholar]
- 51.Cole JL. Analysis of heterogeneous interactions. Methods Enzymol. 2004;384:212–232. doi: 10.1016/S0076-6879(04)84013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cole JL, Lary JW, Moody TP, Laue TM. Analytical ultracentrifugation: sedimentation velocity and sedimentation equilibrium. Methods Cell Biol. 2008;84:143–179. doi: 10.1016/S0091-679X(07)84006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cao J, Burke JE, Dennis EA. Using hydrogen/deuterium exchange mass spectrometry to define the specific interactions of the phospholipase A2 superfamily with lipid substrates, inhibitors, and membranes. J. Biol. Chem. 2013;288:1806–1813. doi: 10.1074/jbc.R112.421909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pirrone GF, Iacob RE, Engen JR. Applications of Hydrogen/Deuterium Exchange MS from 2012 to 2014. Anal. Chem. 2015;87:99–118. doi: 10.1021/ac5040242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miller MS, Schmidt-Kittler O, Bolduc DM, Brower ET, Chaves-Moreira D, Allaire M, Kinzler KW, Jennings IG, Thompson PE, Cole PA, Amzel LM, Vogelstein B, Gabelli SB. Structural basis of nSH2 regulation and lipid binding in PI3Kα. Oncotarget. 2014;5:5198–5208. doi: 10.18632/oncotarget.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bittova L, Sumandea M, Cho W. A structure-function study of the C2 domain of cytosolic phospholipase A2. Identification of essential calcium ligands and hydrophobic membrane binding residues. J. Biol. Chem. 1999;274:9665–9672. doi: 10.1074/jbc.274.14.9665. [DOI] [PubMed] [Google Scholar]
- 57.Verdaguer N, Corbalan-Garcia S, Ochoa WF, Fita I, Gomez-Fernandez JC. Ca2+ bridges the C2 membrane-binding domain of protein kinase Cα directly to phosphatidylserine. EMBO J. 1999;18:6329–6338. doi: 10.1093/emboj/18.22.6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walker EH, Perisic O, Ried C, Stephens L, Williams RL. Structural insights into phosphoinositide 3-kinase catalysis and signalling. Nature. 1999;402:313–320. doi: 10.1038/46319. [DOI] [PubMed] [Google Scholar]
- 59.Wymann MP, Bulgarelli-Leva G, Zvelebil MJ, Pirola L, Vanhaesebroeck B, Waterfield MD, Panayotou G. Wortmannin inactivates phosphoinositide 3-kinase by covalent modification of Lys-802, a residue involved in the phosphate transfer reaction. Mol. Cell. Biol. 1996;16:1722–1733. doi: 10.1128/mcb.16.4.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Freitag A, Prajwal P, Shymanets A, Harteneck C, Nürnberg B, Schächtele C, Kubbutat M, Totzke F, Laufer SA. Development of First Lead Structures for Phosphoinositide 3-Kinase-C2γ Inhibitors. J. Med. Chem. 2015;58:212–221. doi: 10.1021/jm5006034. [DOI] [PubMed] [Google Scholar]
- 61.Bondev A, Bondeva T, Wetzker R. Regulation of phosphoinositide 3-kinase γ protein kinase activity in vitro and in COS-7 cells. Signal Transduction. 2001;1:79–85. [Google Scholar]
- 62.Wales TE, Engen JR. Hydrogen exchange mass spectrometry for the analysis of protein dynamics. Mass Spectrom. Rev. 2006;25:158–170. doi: 10.1002/mas.20064. [DOI] [PubMed] [Google Scholar]
- 63.Burke JE, Perisic O, Masson GR, Vadas O, Williams RL. Oncogenic mutations mimic and enhance dynamic events in the natural activation of phosphoinositide 3-kinase p110α (PIK3CA) Proc. Natl. Acad. Sci. U. S. A. 2012;109:15259–15264. doi: 10.1073/pnas.1205508109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kirsch C, Wetzker R, Klinger R. Anionic phospholipids are involved in membrane targeting of PI 3-kinase γ. Biochem. Biophys. Res. Commun. 2001;282:691–696. doi: 10.1006/bbrc.2001.4623. [DOI] [PubMed] [Google Scholar]
- 65.Kumar A, Redondo-Munoz J, Perez-Garcia V, Cortes I, Chagoyen M, Carrera AC. Nuclear but not cytosolic phosphoinositide 3-kinase β has an essential function in cell survival. Mol. Cell. Biol. 2011;31:2122–2133. doi: 10.1128/MCB.01313-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Walser R, Burke JE, Gogvadze E, Bohnacker T, Zhang X, Hess D, Kuenzi P, Leitges M, Hirsch E, Williams RL, Laffargue M, Wymann MP. PKCβ phosphorylates PI3Kγ to activate it and release it from GPCR control. PLoS Biol. 2013;11:e1001587. doi: 10.1371/journal.pbio.1001587. [DOI] [PMC free article] [PubMed] [Google Scholar]