Abstract
Background
We planned to investigate the relationship of thrombus burden with SYNTAX score in patients with ST elevation myocardial infarction (STEMI).
Material/Methods
We retrospectively enrolled 780 patients who underwent PPCI in our clinic due to STEMI. Clinical, laboratory, and demographic properties of the patients were recorded. Angiographic coronary thrombus burden was classified using thrombolysis in myocardial infarction (TIMI) thrombus grades.
Results
Patients with high thrombus burden were older, with higher diabetes prevalence longer pain to balloon time, higher leukocyte count, higher admission troponin, and admission CK-MB concentrations. SYNTAX score was higher and myocardial perfusion grades were lower in patients with high thrombus burden. Multivariate logistic regression analysis revealed SYNTAX score as the strongest predictor of thrombus burden. ROC analysis demonstrated a sensitivity of 75.5%, specificity of 61.2%, and cut-off value of >14 (area under the curve (AUC): 0.702; 95% confidence interval [CI]: 0.773–0.874;P<0.001) for high thrombus burden.
Conclusions
SYNTAX score may have additional value in predicting higher thrombus burden besides being a marker of coronary artery disease severity and complexity.
MeSH Keywords: Myocardial Infarction, Percutaneous Coronary Intervention, Thrombosis
Background
ST segment elevation myocardial infarction (STEMI) is still an important cause of cardiovascular mortality, and morbidity. Without doubt, coronary thrombosis is the main physiopathologic mechanism of STEMI. Moreover, higher thrombotic burden in infarct related artery (IRA) is related to stent thrombosis, distal embolization, no-reflow phenomenon, and long-term mortality [1–4].
No-reflow phenomenon poses as a major problem in patients with STEMI undergoing primary percutaneous coronary intervention (primary PCI, PPCI). Although the thrombotic lesion in IRA is successfully stented, tissue level perfusion remains low with increased cardiovascular morbidity, and mortality [5,6]. Thus, identification of factors that could increase no-reflow and potential remedies of this phenomenon is clinically significant.
SYNTAX (Synergy between Percutaneous Coronary Intervention with TAXUS and Cardiac Surgery) score is an angiographic scoring system, which grades coronary artery disease severity and complexity [7]. Even though SYNTAX score is primary utilized for selection of elective revascularization strategies, there is growing data implicating additional clinical value in STEMI. Recent studies revealed that pre-PPCI SYNTAX score is associated with no-reflow phenomenon and long-term mortality in STEMI [8–11]. Moreover, SYNTAX score independently predicted angiographically visible distal embolization in patients undergoing PPCI [12]. These results implicate an association between pre-PPCI thrombus burden and SYNTAX score. Therefore, we planned to investigate this relationship in patients with STEMI.
Material and Methods
Study population
We retrospectively enrolled 780 patients who underwent PPCI in our clinic due to STEMI between April 2009 and February 2015. Our study was approved by the local ethics board.
Our study included patients who had the diagnosis of STEMI according to recent STEMI guideline, and underwent PPCI [13]. The clinical, demographic, and laboratory data of the patients were obtained using patient records and registries. Heart rate, Killip class, previous medications, pain to balloon time and door to balloon time, serum creatinine, lipid panel, and hematological indices of all patients were recorded during hospital admission. Data regarding coronary angiography were gathered through re-evaluation of stored media. The patients were treated according to relevant guidelines [13,14]. Tirofiban was used at a dose of 10 mg/kg bolus and 0.15 mg/kg/min intravenous infusion if preferred by the physician. Experienced operators performed all PPCI procedures.
We excluded cases that underwent fibrinolytic therapy (n=15), emergent cardiovascular bypass surgery (n=18), and patients with end stage renal failure (n=11), malignancy (n=3), culprit left main coronary lesion (n=3), and spontaneous coronary dissection (n=1).
SYNTAX score was calculated using SYNTAX Score Calculator 2.11 software downloaded from http://www.syntaxscore.com [7,15].
Clinical definitions
Hypertension was defined as systemic blood pressure >140/90 mmHg or the use of antihypertensive medication. Hypercholesterolemia was accepted if a serum total cholesterol level >200 mg/dL, or with the use of a cholesterol lowering agent. Diabetes mellitus was acknowledged as an HbA1c >6.5%, a plasma glucose level ≥126 mg/dL (7.0 mmol/L) after an overnight fast, or the use of antidiabetic medications. Positive family history of coronary artery disease was defined as documented evidence of premature coronary artery disease in a first degree relative (men <55 and women <65 years of age).
Killip Classification was noted as follows: Class I: No evidence of heart failure. Class II: Findings of mild to moderate heart failure (S3 gallop, rales < half-way up lung fields or elevated jugular venous pressure). Class III: Pulmonary edema. Class IV: Cardiogenic shock defined as systolic blood pressure <90 and signs of hypoperfusion such as oliguria, cyanosis, and sweating.
Angiographic definitions
Three experienced investigators who were blinded to clinical parameters of the patients’ carefully reviewed coronary angiograms. The TIMI flow grades were determined by the consensus of the three investigators. Angiographic thrombus burden was classified as follows: Grade 0: no thrombus, Grade 1: Possible thrombus, Grade 2: the thrombus’ greatest dimension is <1/2 vessel diameter, Grade 3: Greatest dimension >1/2 to <2 vessel diameters, Grade 4: Greatest dimension >2 vessel diameters, Grade 5: total vessel occlusion due to thrombus [3]. The patients were stratified into low thrombus burden (Grades 1, 2 and 3) and high thrombus burden groups (4 and 5) according to final thrombus score.
Postprocedural final thrombolysis in myocardial infarction (TIMI) flow grade, TIMI myocardial perfusion grade (TMPG), corrected TIMI frame count (cTFC), and TMPG were noted as previously defined. [16–18]. TIMI flow grade <3, and final myocardial blush grade <2 were described as angiographic no-reflow.[19].
Statistical analyses
Continuous variables are expressed as mean ± standard deviation, whereas categorical variables are expressed as percentage. Comparison between groups was made using the Student t test, Mann-Whitney U test or chi-square tests, as appropriate. Multiple logistic regression analysis was performed to identify the independent predictors of high thrombus burden using variables. Two-tailed P values <.05 were considered to indicate statistical significance. Statistical analyses were performed using SPSS, version 18.0 for Windows. In order to predict cutoff value of SYNTAX score, receiver operating characteristics (ROC) curve analysis was performed by MedCalc statistic software (version 13.2.0, Mariakerke, Belgium).
Results
The study population consisted of 780 patients with STEMI (mean age 56±11, 52.8% male). The mean SYNTAX score was 18±9.5. We formed two groups according to the final TIMI thrombus grade; 299 (%38.3) patients had low thrombus burden whereas 481 subjects (%61.7) had high thrombus burden. The comparisons of basic clinical and laboratory findings between groups thrombus burden were presented in Table 1. Patients with high thrombus burden were older (57.1 vs. 55.4 p=0.033), with higher diabetes prevalence (30.4% vs. 23.7% p=0.049), longer pain to balloon time (280±502 min vs. 220±121 min p=0.042), higher leukocyte count (9.7±2.5×103/μL vs. 9.2±2.5×103/μL p=0.013), higher baseline troponin (2.3±1.5 mg/L vs. 2.1±1.5 mg/L P=0.047), and baseline CK-MB concentrations (37.1±15.1 IU/L vs. 35±13.7 IU/L p=0.048). Comparison of the baseline angiographic characteristics and postprocedural findings of the groups based on thrombus burden were detailed in Table 2. Tirofiban administration (42.8% vs. %68.6%, p<.001), and direct stenting (8.9% vs. 12.4%, P=0.008) were less frequent, and SYNTAX score was higher (20.7± 9.1 vs. 13.8±8.6) in patients with high thrombus burden. Although epicardial perfusion parameters were better (82.1% vs. 72.9% for TIMI flow III, p=.003 and 25.2±21 vs. 22.1±6.2 for cTFC, p=.013), myocardial perfusion grades were lower (58% vs. 75.3%, p<0.001) in patients with high thrombus burden.
Table 1.
Baseline clinical and laboratory characteristics according to thrombus burden.
| Variable | Low thrombus burden (n=299) | High thrombus burden (n=481) | P value |
|---|---|---|---|
| Age, years | 55.4±10.8 | 57.1±11 | .033 |
| Sex, male% | 43.5 | 49.5 | .105 |
| Diabetes,% | 23.7 | 30.4 | .049 |
| Hypertension,% | 42.5 | 43.2 | .882 |
| Smoking,% | 42.1 | 44.9 | .459 |
| Dyslipidemia,% | 43.5 | 48 | .237 |
| Previous history of CAD,% | 11 | 14 | .297 |
| Family history of CAD | 37.4 | 43.2 | .116 |
| Pain-balloon time, min | 220±121 | 280±502 | .042 |
| Door-balloon time, min | 30.8±10.9 | 30.4±10.2 | .623 |
| Killip status (≥II) | 9 | 13.3 | .085 |
| Heart rate, /min | 76.5±16 | 74.7±16 | .145 |
| Hemoglobin, g/dL | 13.3±1.4 | 13.9±1.3 | .732 |
| White blood cell count ×103/μL | 9.2±2.5 | 9.7±2.5 | .013 |
| Platelet count, ×103/μL | 216±76 | 218±78 | .734 |
| Baseline troponin I, mg/L | 2.1±1.5 | 2.3±1.5 | .047 |
| Baseline CK-MB, IU/L | 35±13.7 | 37.1±15.1 | .048 |
| LDL cholesterol, mg/dL | 150±32 | 155±26 | .188 |
| HDL cholesterol, mg/dL | 29±14 | 28±11 | .913 |
| Triglyceride, mg/dL | 188±91 | 201±102 | .061 |
| EF,% | 48±9.8 | 47.7±8.1 | .595 |
| Previous medications,% | |||
| Aspirin | 14.7 | 13.1 | .523 |
| Statin | 16.7 | 17.0 | .922 |
| ACE inhibitors/ARB | 26.8 | 29.7 | .415 |
| β-blocker | 8.7 | 6.0 | .195 |
| Clopidogrel | 1 | 2.7 | .124 |
| CCB | 16.7 | 17.9 | .699 |
| Antidiabetic medications | 9.6 | 7.8 | .104 |
CAD – coronary artery disease; LDL – low-density lipoprotein; HDL – high-density lipoprotein; ACE – angiotensin converting enzyme; ARB – angiotensin receptor blocker; CCB – calcium channel blocker; OAD – oral antidiabetic drug. The normal cut-off value of troponin in our laboratory <0.04 mg/L.
Table 2.
Baseline angiographic and postprocedural characteristics according to final thrombus grade.
| Variables | Low thrombus burden (n=299) | High thrombus burden (n=481) | P Value |
|---|---|---|---|
| Infarct-related artery,% | |||
| LAD | 49.5 | 42.4 | |
| LCx | 18.4 | 24.5 | .070 |
| RCA | 29.8 | 31.8 | |
| Tirofiban administration,% | 68.6 | 42.8 | <.001 |
| Procedure,% | |||
| Direct stenting | 12.4 | 8.9 | |
| PTCA + stenting | 86 | 85.2 | .008 |
| Only PTCA | 1.7 | 5.8 | |
| Postprocedural TIMI flow (≥III)% | 82.1 | 72.9 | .003 |
| IRA-cTFC | 22.1±6.2 | 25.2±21 | .013 |
| TMPG (≥II),% | 75.3 | 58 | <.001 |
| No reflow% | 5.5 | 14.4 | <.001 |
LAD – left anterior descending; LCx – left circumflex; RCA – right coronary artery; LMCA – left main coronary artery; IRA – infarct related artery; PTCA – percutaneous transluminal coronary angioplasty; TIMI – thrombolysis in myocardial infarction; cTFC – corrected TIMI frame count; TMPG – TIMI myocardial perfusion grade; ECG – electrocardiography.
The discriminatory value of SYNTAX score for high thrombus burden was assessed by ROC analysis and revealed a sensitivity of 75.5%, specificity of 61.2%, and cut-off value of >14 (area under the curve (AUC): 0.702; 95% confidence interval [CI]: 0.773–0.874; P<0.001) (Figure 1).
Figure 1.
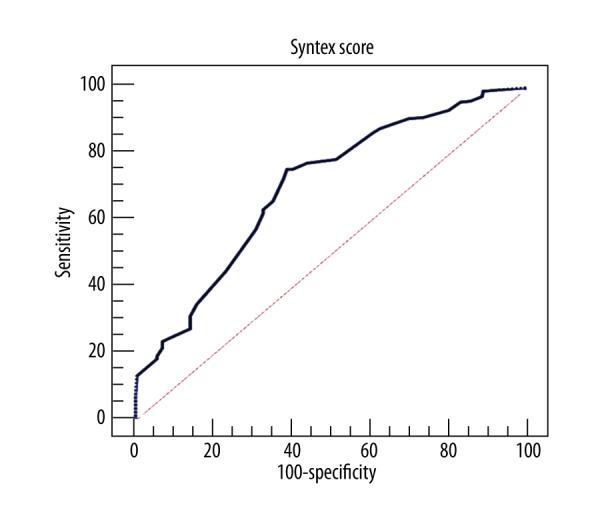
The discriminatory value of Syntax score for high thrombus burden was assessed by ROC analysis.
Multivariate logistic regression analysis was performed in order to determine the independent predictors of high thrombus burden. SYNTAX score (odds ratio: 2.45,95% confidence interval: 1.36–4.39, P <0.001), age (odds ratio: 1.01,95% confidence interval: 1.00–1.03, P <0.01), DM (odds ratio: 0.58, 95% confidence interval: 0.58–0.40, P <0.004), WBC (odds ratio: 1.11,95% confidence interval: 1.04–1.18, P<0.001), baseline troponin levels (odds ratio: 1.16, 95% confidence interval: 1.05–1.29, P<0.004) were found as significant independent predictors for HTB in patients with STEMI (Table 3).
Table 3.
Multivariate logistic regression analyses to detect the independent predictors of high TIMI thrombus burden.
| Variables | Multivariate OR, 95% CI | Multivariate P value |
|---|---|---|
| Age | 1.01 (1.00–1.03) | 0.019 |
| Diabetes | 0.58 (0.58–0.40) | 0.004 |
| Pain-balloon time | 1.00 (1.00–1.00) | 0.102 |
| White blood cell count | 1.11 (1.04–1.18) | 0.001 |
| Baseline troponin | 1.16 (1.05–1.29) | 0.004 |
| Baseline CK-MB | 1.00 (0.99–1.02) | 0.104 |
| Syntax score | 2.45 (1.36–4.39) | <0.001 |
OR – odds ratio; CI – confidence interval; CK-MB – creatine kinase-MB. Boldface values indicate the variables entered to multivariate model.
Discussion
We revealed that SYNTAX is independently related to TIMI thrombus burden in addition to presence of DM, baseline leukocytes and troponin concentration, and age. Our study is the first to show the higher thrombus burden with increasing SYNTAX scores.
The basic pathophysiologic event that initiates atherosclerotic myocardial infarction is plaque rupture, ensued by intracoronary thrombus generation [20]. Following thrombus generation several mechanisms, one of which is nitric oxide (NO) secretion from endothelial cells counteracts in order to limit thrombus propagation. Endothelial dysfunction is closely related to decreased NO secretion and predisposition to vasoconstriction [21]. A recent study documented that coronary artery disease severity and complexity documented by SYNTAX score is associated with worsening grades of endothelial dysfunction [22]. Therefore, increased SYNTAX score with higher endothelial dysfunction may cause decreased NO secretion and result in higher thrombus burden. We think that the same mechanism may also apply in diabetes mellitus and advanced age.
Inflammatory reaction due to atherosclerosis may be another possible mechanism. Atherosclerosis is a chronic inflammatory disease of the vessel wall [23]. Inflammation has both local and systemic effects, which results in plaque rupture or erosion, and thrombocyte activation [24,25]. It is well known that the thrombogenicity of the ruptured material increase with higher inflammatory mediator and cell content [24–26]. Systemic inflammatory response may have additional influence on thrombogenesis [27]. Barron et al. documented that higher leukocyte count was an independent predictor of thrombus burden in patients with STEMI [28], highlighting the importance of systemic inflammation in intracoronary thrombogenesis. We think that higher SYNTAX scores reflect higher atherosclerotic burden and increased systemic inflammation, proved by elevated leukocyte counts, may cause higher thrombus burden in our patients with STEMI. Both increased inflammation and endothelial dysfunction may justify the link between SYNTAX score and thrombus burden in our study.
A recent study by Tanboğa and co-workers identified red cell distribution width as the only independent predictor of thrombus burden in STEMI [29]. Interestingly, DM and advanced age were not predictors in this study. This study and ours share similar results, one of which is that increased troponin concentrations were related to higher thrombus burden. Similarly, angiographic no-reflow was more frequent in patients with high thrombus burden and pre-procedural tirofiban infusion lowered thrombus burden in both studies. Although pain-balloon time was longer in high thrombus burden group, this was not an independent predictor.
DM is an important cardiovascular risk factor. Several mechanisms are responsible for cardiovascular morbidity and mortality in DM, one of which is increased thrombogenicity [30]. We found DM as an independent prognosticator of initial thrombus burden in patients undergoing PPCI. Similar to our study, Wang et al. demonstrated that admission glucose concentration was independently related to no-reflow phenomenon [31].
Several studies identified that SYNTAX score has important prognostic value. Higher SYNTAX values indicate high thrombus burden, no-reflow risk and higher mortality. Although there is not any definitive proof in the current medical literature, higher SYNTAX scores may warrant additional treatment options like glycoprotein IIb-IIIa inhibitors, thrombus aspiration [32–34]. High SYNTAX scores help the interventionalist additionally by identifying patients with more complex coronary anatomy, larger ischemic territory, and possibility of peripheral arterial disease. High SYNTAX score following PPCI is associated with increased long-term morbidity and mortality [35,36].
Limitations
The main limitation of our study is the retrospective design. We could not assess inflammation by using additional markers such as high-sensitive C reactive protein. Even though we utilized the TIMI thrombus grade, a well-known and widely used classification, this grading may not be the perfect and ideal. Lastly, we did not utilize thrombus aspiration in our study.
Conclusions
Age, diabetes mellitus, admission troponin and leukocyte concentrations, and SYNTAX score are independently associated with TIMI thrombus burden in patients undergoing PPCI for STEMI. Angiographic no-reflow is more frequent in patients with high thrombus burden. SYNTAX score may have additional value in predicting higher thrombus burden besides being a marker of coronary artery disease severity and complexity.
Footnotes
Source of support: Departmental sources
References
- 1.Iwakura K, Ito H, Kawano S, et al. Predictive factors for development of the no-reflow phenomenon in patients with reperfused anterior wall acute myocardial infarction. J Am Coll Cardiol. 2001;38:472–77. doi: 10.1016/s0735-1097(01)01405-x. [DOI] [PubMed] [Google Scholar]
- 2.Sianos G, Papafaklis MI, Daemen J, et al. Angiographic stent thrombosis after routine use of drug-eluting stents in ST-segment elevation myocardial infarction: the importance of thrombus burden. J Am Coll Cardiol. 2007;50:573–83. doi: 10.1016/j.jacc.2007.04.059. [DOI] [PubMed] [Google Scholar]
- 3.Sianos G, Papafaklis MI, Serruys PW. Angiographic thrombus burden classification in patients with ST-segment elevation myocardial infarction treated with percutaneous coronary intervention. J Invasive Cardiol. 2010;22:6B–14B. [PubMed] [Google Scholar]
- 4.Fukuda D, Tanaka A, Shimada K, et al. Predicting angiographic distal embolization following percutaneous coronary intervention in patients with acute myocardial infarction. Am J Cardiol. 2003;91:403–7. doi: 10.1016/s0002-9149(02)03233-2. [DOI] [PubMed] [Google Scholar]
- 5.Iijima R, Shinji H, Ikeda N, et al. Comparison of coronary arterial finding by intravascular ultrasound in patients with “transient no-reflow” versus “reflow” during percutaneous coronary intervention in acute coronary syndrome. Am J Cardiol. 2006;97:29–33. doi: 10.1016/j.amjcard.2005.07.104. [DOI] [PubMed] [Google Scholar]
- 6.Ndrepepa G, Tiroch K, Fusaro M, et al. 5-year prognostic value of no-reflow phenomenon after percutaneous coronary intervention in patients with acute myocardial infarction. J Am Coll Cardiol. 2010;55:2383–89. doi: 10.1016/j.jacc.2009.12.054. [DOI] [PubMed] [Google Scholar]
- 7.Sianos G, Morel MA, Kappetein AP, et al. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. Eurointervention. 2005;1:219–27. [PubMed] [Google Scholar]
- 8.Capodanno D, Di Salvo ME, Cincotta G, et al. Usefulness of the SYNTAX score for predicting clinical outcome after percutaneous coronary intervention of unprotected left main coronary artery disease. Circ Cardiovasc Interv. 2009;2:302–8. doi: 10.1161/CIRCINTERVENTIONS.108.847137. [DOI] [PubMed] [Google Scholar]
- 9.Capodanno D, Capranzano P, Di Salvo ME, et al. Usefulness of SYNTAX score to select patients with left main coronary artery disease to be treated with coronary artery bypass graft. JACC Cardiovasc Interv. 2009;2:731–38. doi: 10.1016/j.jcin.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Magro M, Nauta ST, Simsek C, et al. Usefulness of the SYNTAX score to predict “no reflow” in patients treated with primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. AmJ Cardiol. 2012;109:601–6. doi: 10.1016/j.amjcard.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 11.Brown AJ, McCormick LM, Gajendragadkar PR, et al. Initial SYNTAX score predicts major adverse cardiac events after primary percutaneous coronary intervention. Angiology. 2014;65:408–12. doi: 10.1177/0003319713483542. [DOI] [PubMed] [Google Scholar]
- 12.Biyik I, Akturk IF, Ozturk D, et al. Can syntax score predict angiographically visible distal embolization during primary percutaneous coronary intervention? Minerva Cardioangiol. 2015 [Epub ahead of print] [PubMed] [Google Scholar]
- 13.O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362–425. doi: 10.1161/CIR.0b013e3182742cf6. [DOI] [PubMed] [Google Scholar]
- 14.Kushner FG, Hand M, Smith SC, Jr, et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009;54:2205–41. doi: 10.1016/j.jacc.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 15.Serruys PW, Onuma Y, Garg S, et al. Assessment of the SYNTAX score in the Syntax study. Eurointervention. 2009;5:50–56. doi: 10.4244/eijv5i1a9. [DOI] [PubMed] [Google Scholar]
- 16.Gibson CM, Cannon CP, Daley WL, et al. TIMI frame count: a quantitative method of assessing coronary artery flow. Circulation. 1996;93:879–88. doi: 10.1161/01.cir.93.5.879. [DOI] [PubMed] [Google Scholar]
- 17.Gibson CM, Murphy SA, Rizzo MJ, et al. Relationship between TIMI frame count and clinical outcomes after thrombolytic administration. Thrombolysis In Myocardial Infarction (TIMI) Study Group. Circulation. 1999;99:1945–50. doi: 10.1161/01.cir.99.15.1945. [DOI] [PubMed] [Google Scholar]
- 18.Gibson CM, Cannon CP, Murphy SA, et al. Relationship of TIMI myocardial perfusion grade to mortality after administration of thrombolytic drugs. Circulation. 2000;101:125–30. doi: 10.1161/01.cir.101.2.125. [DOI] [PubMed] [Google Scholar]
- 19.Gibson CM, Cannon CP, Murphy SA, et al. Relationship of the TIMI myocardial perfusion grades, flow grades, frame count, and percutaneous coronary intervention to long-term outcomes after thrombolytic administration in acute myocardial infarction. Circulation. 2002;105:1909–13. doi: 10.1161/01.cir.0000014683.52177.b5. [DOI] [PubMed] [Google Scholar]
- 20.Davies MJ, Thomas A. Thrombosis and acute coronary-artery lesions in sudden cardiac ischemic death. N Engl J Med. 1984;310:1137–40. doi: 10.1056/NEJM198405033101801. [DOI] [PubMed] [Google Scholar]
- 21.Endemann DH, Schiffrin EL. Endothelial dysfunction. J Am Soc Nephrol. 2004;15:1983–92. doi: 10.1097/01.ASN.0000132474.50966.DA. [DOI] [PubMed] [Google Scholar]
- 22.Woo JS, Jang WS, Kim HS, et al. Comparison of peripheral arterial tonometry and flow-mediated vasodilation for assessment of the severity and complexity of coronary artery disease. Coron Artery Dis. 2014;25:421–26. doi: 10.1097/MCA.0000000000000094. [DOI] [PubMed] [Google Scholar]
- 23.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–43. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 24.Barlis P, Serruys PW, Devries A, Regar E. Optical coherence tomography assessment of vulnerable plaque rupture: predilection for the plaque ‘shoulder’. Eur Heart J. 2008;29:2023. doi: 10.1093/eurheartj/ehn085. [DOI] [PubMed] [Google Scholar]
- 25.Katritsis DG, Pantos J, Efstathopoulos E. Hemodynamic factors and atheromatic plaque rupture in the coronary arteries: from vulnerable plaque to vulnerable coronary segment. Coron Artery Dis. 2007;18:229–37. doi: 10.1097/MCA.0b013e328012a93d. [DOI] [PubMed] [Google Scholar]
- 26.Wu AH. Early detection of acute coronary syndromes and risk stratification by multimarker analysis. Biomark Med. 2007;1:45–57. doi: 10.2217/17520363.1.1.45. [DOI] [PubMed] [Google Scholar]
- 27.Melamed KH, Goldhaber SZ. Inflammation and myocardial infarction. Circulation. 2014;130:e334–36. doi: 10.1161/CIRCULATIONAHA.114.010614. [DOI] [PubMed] [Google Scholar]
- 28.Barron HV, Cannon CP, Murphy SA, et al. Association between white blood cell count, epicardial blood flow, myocardial perfusion, and clinical outcomes in the setting of acute myocardial infarction: a thrombolysis in myocardial infarction 10 substudy. Circulation. 2000;102:2329–34. doi: 10.1161/01.cir.102.19.2329. [DOI] [PubMed] [Google Scholar]
- 29.Tanboga IH, Topcu S, Aksakal E, et al. Determinants of angiographic thrombus burden in patients with ST-segment elevation myocardial infarction. Clin Appl Thromb Hemost. 2014;20:716–22. doi: 10.1177/1076029613483169. [DOI] [PubMed] [Google Scholar]
- 30.Vazzana N, Ranalli P, Cuccurullo C, Davi G. Diabetes mellitus and thrombosis. Thromb Res. 2012;129:371–77. doi: 10.1016/j.thromres.2011.11.052. [DOI] [PubMed] [Google Scholar]
- 31.Wang CH, Chen YD, Yang XC, et al. A no-reflow prediction model in patients with ST-elevation acute myocardial infarction and primary drug-eluting stenting. Scand Cardiovasc J. 2011;45:98–104. doi: 10.3109/14017431.2011.558209. [DOI] [PubMed] [Google Scholar]
- 32.De Luca G, Suryapranata H, Stone GW, et al. Abciximab as adjunctive therapy to reperfusion in acute ST-segment elevation myocardial infarction: a meta-analysis of randomized trials. JAMA. 2005;293:1759–65. doi: 10.1001/jama.293.14.1759. [DOI] [PubMed] [Google Scholar]
- 33.De Luca G, Navarese E, Marino P. Risk profile and benefits from Gp IIb-IIIa inhibitors among patients with ST-segment elevation myocardial infarction treated with primary angioplasty: a meta-regression analysis of randomized trials. Eur Heart J. 2009;30:2705–13. doi: 10.1093/eurheartj/ehp118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Costopoulos C, Gorog DA, Di Mario C, Kukreja N. Use of thrombectomy devices in primary percutaneous coronary intervention: a systematic review and meta-analysis. Int J Cardiol. 2013;163:229–41. doi: 10.1016/j.ijcard.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 35.Sebastianski M, Narasimhan S, Graham MM, et al. Usefulness of the ankle-brachial index to predict high coronary SYNTAX scores, myocardium at risk, and incomplete coronary revascularization. Am J Cardiol. 2014;114:1745–49. doi: 10.1016/j.amjcard.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 36.Magro M, Nauta S, Simsek C, et al. Value of the SYNTAX score in patients treated by primary percutaneous coronary intervention for acute ST-elevation myocardial infarction: The MI SYNTAXscore study. Am Heart J. 2011;161:771–81. doi: 10.1016/j.ahj.2011.01.004. [DOI] [PubMed] [Google Scholar]


